Highlights
-
•
Neutrophil-lymphocyte ratio is a promising prognostic marker for several cancers.
-
•
NLR is not useful as a marker of lung cancer survival in localized lung cancer.
-
•
NLR has potential as a marker of competing mortality risk in localized lung cancer.
-
•
NLR cutoff of 4.0 is proposed as a clinically useful cutoff point.
Abbreviations: NLR, Neutrophil-lymphocyte ratio; NSCLC, non-small cell lung cancer; SBRT, stereotactic body radiotherapy; VA, Veterans Affairs; OS, overall survival; LCS, lung cancer-specific survival; NCS, non-lung cancer survival
Keywords: Neutrophil-lymphocyte ratio, Prognostic factors, Non-small cell lung cancer, Stereotactic body radiotherapy, Veterans affairs (U.S.)
Abstract
Background and purpose
Neutrophil-lymphocyte ratio (NLR) has been associated with overall survival (OS) in non-small cell lung cancer (NSCLC). We aimed to assess the utility of NLR as a predictor of lung cancer-specific survival (LCS) and identify an optimal, pretreatment cutoff point in patients with localized NSCLC treated with stereotactic body radiotherapy (SBRT) within the Veterans Affairs’ (VA) national database.
Materials and methods
In the VA database, we identified patients with biopsy-proven, clinical stage I NSCLC treated with SBRT between 2006 and 2015. Cutoff points for NLR were calculated using Contal/O’Quigley’s and Cox Wald methods. Primary outcomes of OS, LCS, and non-lung cancer survival (NCS) were evaluated in Cox and Fine-Gray models.
Results
In 389 patients, optimal NLR cutoff was identified as 4.0. In multivariable models, NLR > 4.0 was associated with decreased OS (HR 1.44, p = 0.01) and NCS (HR 1.68, p = 0.01) but not with LCS (HR 1.32, p = 0.09). In a subset analysis of 229 patients with pulmonary function tests, NLR > 4.0 remained associated with worse OS (HR 1.51, p = 0.02) and NCS (HR 2.18, p = 0.01) while the association with LCS decreased further (HR 1.22, p = 0.39).
Conclusion
NLR was associated with worse OS in patients with localized NSCLC treated with SBRT; however, NLR was only associated with NCS and not with LCS. Pretreatment NLR, with a cutoff of 4.0, offers potential as a marker of competing mortality risk which can aid in risk stratification in this typically frail and comorbid population. Further studies are needed to validate pretreatment NLR as a clinical tool in this setting.
1. Introduction
Non-small cell lung cancer (NSCLC) continues to be a leading etiology of cancer-related deaths in the United States [1], [2]. Approximately 20–30% of NSCLC patients present with localized disease at diagnosis [3]. Although the long-standing, primary treatment for early stage lung cancer is surgical resection, a majority of patients are deemed ineligible for surgery owing to an amalgamation of poor baseline medical fitness (e.g. age, performance status) and concomitant comorbid conditions (e.g. COPD, heart disease) [4], [5], [6].
For patients that are medically inoperable or unwilling to undergo surgery, stereotactic body radiotherapy (SBRT) is an effective treatment option [7], [8], [9], [10]. However, distant recurrence is common (up to 33%) after SBRT [6], [11]. Therefore, identification of patients at high risk for lung cancer recurrence and death can potentially enable targeted treatment intensification with other modalities. Furthermore, patient selection remains important as SBRT is typically well tolerated but not completely devoid of risks [5]. Many patients with localized NSCLC may have alternative drivers for their demise, and thus treatment may not necessarily improve outcomes. Thus there are multiple motivations to identify additional prognostic markers to guide risk stratification and decision management in this population. Although there are many established patient and disease related prognostic markers for NSCLC, the SBRT patient population is primarily comprised of those who are medically inoperable and therefore have similarly poor factors across the board, and therefore prognostic markers in this population are lacking [4], [12], [13].
Chronic inflammation in the lungs is an established precursor state to NSCLC and other malignancies [14], [15]. One metric of cellular inflammation, readily obtained from routine labs indicated in the workup of numerous cancer types, is the neutrophil–lymphocyte ratio (NLR). However, previous studies have demonstrated that an elevated NLR is associated with poor outcomes in both non-oncologic, inflammatory conditions (e.g. coronary artery disease, end stage renal disease) and oncologic conditions (e.g. pancreatic cancer, mesothelioma) [16], [17], [18]. In the pulmonary realm, NLR has also been associated with lung function decline and inferior COPD outcomes [19].
Previous studies have demonstrated an association between NLR and overall survival (OS) in patients with localized NSCLC treated with SBRT [20], [21], [22]. However, they did not delineate the association of NLR with lung cancer-specific survival (LCS) and non-lung cancer survival (NCS); it remains unknown if NLR is predictive of oncologic (lung cancer risk) or non-oncologic (underlying lung disease, competing mortality risk) outcomes in this setting. Furthermore, these previous studies have been limited by small cohort sizes, lack of granular details (such as pulmonary function tests), and lack of time-dependent cutoff points. Here, we attempt to overcome these limitations. In patients with localized NSCLC treated with SBRT within the national Veterans’ Affairs (VA) database, we identify an optimal cutoff point for pretreatment NLR and perform competing risks analyses to delineate the prognostic potential of NLR on OS, LCS, and NCS individually.
2. Methods
2.1. Data source
The VA Informatics and Computing Infrastructure (VINCI) represents a comprehensive informatics platform that enables access to the database comprised of patient-level electronic health records and administrative data throughout the national VA health care system. Tumor registry data is uploaded by trained registrars in accordance with protocols issued from the American College of Surgeons, thereby capturing an estimated 90% of incident cancers within the VA system [23], [24]. Cause specific mortality (ICD-10 code C34 for lung cancer) information was obtained from the National Death Index (NDI) and then manually chart reviewed to fill in missing entries. Our protocol was approved by the San Diego VA Institutional Review Board.
2.2. Patient selection
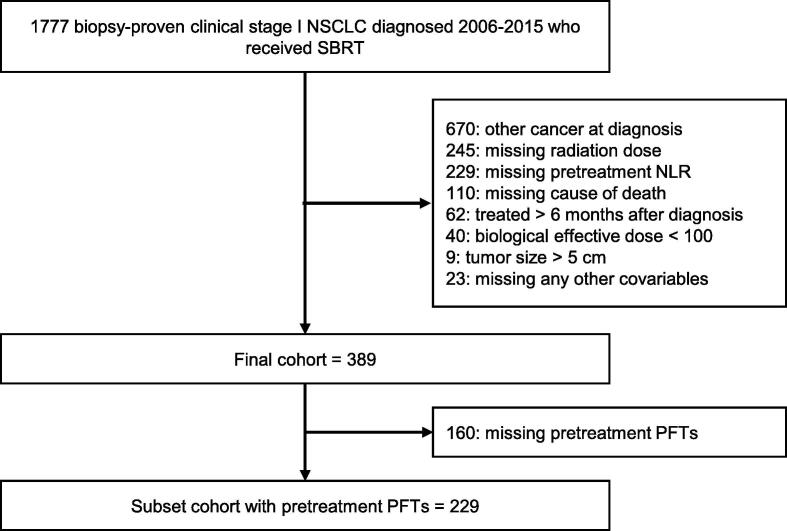
Of the veterans with biopsy-proven NSCLC diagnosed between January 1, 2006 and December 31, 2015 who were treated definitively with radiation, only those with clinical T1 or T2a (<5 cm in greatest dimension), N0 (no regional lymph node metastasis), and M0 (no distant metastasis) stages were included. Tumor staging was denoted by the 7th American Joint Committee on Cancer guidelines. Patients were excluded if they had a history of synchronous malignancy, treatment > 6 months after diagnosis, or missing covariables of interest. The final cohort was comprised of 389 patients with localized NSCLC treated with SBRT. The following text and Fig. 1 details the complete inclusion and exclusion criteria of this study’s final cohort.
Fig. 1.
Inclusion and exclusion criteria. (NSCLC = non-small cell lung cancer, SBRT = stereotactic body radiotherapy, PFTs = pulmonary function tests).
2.3. Patient, tumor, and treatment variables
SBRT was defined as delivery of one to five daily fractions of radiation directed at the lung. After identification of radiation receipt through the registry, we manually reviewed charts to extract radiation dose and fractionation information to ensure patients received definitive radiation directed at the lung. To account for the differences in dose and fractionation regimens, biologically equivalent dose was calculated for each patient with an α/β ratio of 10. Patients were excluded if they received a low biologically effective dose of radiation (<100 Gy10) [25].
The study covariables of interest were all derived from the VA cancer registry database. They include age at diagnosis, sex, race, tobacco history, histology, tumor size. Linked administrative data provided International Classification of Diseases-9 and 10 codes for comorbidities used to construct the Charlson comorbidity index score [26], [27]. Forced expiratory volume in one second (FEV1) values were obtained by searching clinical notes within one year from the start of treatment. FEV1 values were converted to percent predicted based on patient characteristics at diagnosis using standard reference equations [28]. Laboratory data was used to identify complete blood count (CBC) with differential test results. From this, NLR is calculated as the absolute neutrophil count (ANC) divided by the absolute lymphocyte count (ALC). Pretreatment NLR was defined as any value available within six months prior to starting treatment. If multiple values were present for a patient, that patient’s final pretreatment NLR was chosen to be the value closest to their treatment date. All patients were followed until death or last follow up with a VA provider before August 1, 2020.
2.4. Statistical analysis
In clinical data, transforming a continuous variable into a categorical variable to evaluate its predictive value on time dependent endpoints (such as survival) can make the model more interpretable and clinically useful. Although several techniques are commonly employed (e.g. median/quartiles, Receiver Operating Characteristic (ROC) tests), the appropriateness of such methods for time dependent endpoints have often been called into question [29], [30]. Therefore we chose to employ the Contal and O’Quigley cutpoint method that uses the log-rank test statistic [29], [31]. Using the methods described by Contal and O’Quigley and the SAS macro provided by Mandrekar et al., we were able to identify our continuous NLR variable to be eligible for dichotomization [31], [32]. Then, the Contal and O’Quigley statistic and Cox Wald statistic were identified and compared for a panel of candidate cutoff points to identify the optimal cutoff point(s) of pretreatment NLR on each survival endpoint.
After identification of the optimal cutoff point of pretreatment NLR, the cohort was dichotomized based on this value. Patient characteristics between these two NLR cohorts were compared using Chi Square test and Wilcoxon’s rank sum test for categorical and continuous variables, respectively. OS was assessed with Kaplan-Meier analysis for unadjusted models and with Cox proportional hazards analysis for multivariable models. Comparisons of LCS among groups were evaluated using a competing risk analysis framework to account for the competing risk of non-lung cancer mortality. Vice-versa logic was used to evaluate NCS. LCS and NCS were assessed with cumulative incidence analysis for unadjusted models and with Fine-Gray regression analysis for multivariable models. For all survival analysis, hazard ratios (HR) and 95% confidence intervals (CI) were reported. Throughout this study, all multivariable models incorporated variables chosen a priori – NLR batch, age at diagnosis, gender, race, Charlson score, tobacco history, histology, T stage, total biologically effective dose. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), with two-sided p-values < 0.05 considered statistically significant.
3. Results
3.1. Baseline characteristics and treatment exposure
The 389 patients in our final cohort were analyzed with a median post-treatment follow-up of 70 months. Median NLR of the cohort was 3.0 (range 0.4–42) obtained at a median of 41 days (range 1–174) before start of SBRT. The optimal cutoff pretreatment NLR value for our cohort was determined to be 4.0 using the Contal and O’Quigley cutpoint selection method (p = 0.006). This was validated to be an optimal cutoff point in the Cox Wald cutpoint selection method (p = 0.0002) as well. Therefore the cohort was split by this cutoff point into two batches – 275 patients with NLR ≤ 4.0, 114 patients with NLR > 4.0.
Table 1 depicts the breakdown of demographic and clinical variables for the overall cohort and each NLR cohort. The NLR cohorts were broadly similar, with the only significant difference being a larger proportion of Black patients in the lower NLR cohort (17% vs 7%). The median (and range) total dose, dose per fraction, number of fractions, and BED received for the entire cohort were 50 Gy (45–75 Gy), 12 Gy (7.5–20 Gy), 5 fractions (3–8 fractions), and 112.5 Gy (100–187.5 Gy) respectively. These radiation specific variables did not differ between the NLR cohorts.
Table 1.
Baseline patient, tumor, and treatment characteristics of the overall cohort stratified by NLR ≤ 4.0 and NLR > 4.0 groups.
| Variable | All Patients (N = 389) | NLR ≤ 4.0 (N = 275) | NLR > 4.0 (N = 114) | P value |
|---|---|---|---|---|
| Pretreatment NLR | <0.0001* | |||
| Median (range) | 3.0 (0.4–42) | 2.4 (0.4–4) | 5.3 (4.1–42) | |
| Age | 0.24 | |||
| Median (range) | 71 (49–93) | 71 (49–93) | 72 (53–90) | |
| Gender | 0.99 | |||
| Male | 378 (97.2%) | 267 (97.1%) | 111 (97.4%) | |
| Female | 11 (2.8%) | 8 (2.9%) | 3 (2.6%) | |
| Charlson Score | 0.46 | |||
| 0 | 83 (21.3%) | 61 (22.1%) | 22 (19.3%) | |
| 1 | 141 (36.2%) | 103 (37.5%) | 38 (33.3%) | |
| 2+ | 165 (42.5%) | 111 (40.4%) | 54 (47.4%) | |
| Race | 0.01* | |||
| White | 335 (86.1%) | 229 (83.3%) | 106 (93.0%) | |
| Black | 54 (13.9%) | 46 (16.7%) | 8 (7.0%) | |
| Smoker at Diagnosis | 0.74 | |||
| Yes | 167 (42.9%) | 120 (43.6%) | 47 (41.2%) | |
| No | 222 (57.1%) | 155 (56.4%) | 67 (58.8%) | |
| Histology | 0.42 | |||
| Adenocarcinoma | 162 (41.6%) | 120 (43.6%) | 42 (36.9%) | |
| Squamous | 156 (40.1%) | 105 (38.2%) | 51 (44.7%) | |
| Other/unknown | 71 (18.3%) | 50 (18.2%) | 21 (18.4%) | |
| T stage | ||||
| < 2 cm (T1a) | 166 (42.7%) | 118 (42.9%) | 48 (42.1%) | 0.57 |
| 2-3 cm (T1b) | 143 (36.8%) | 104 (37.8%) | 39 (34.2%) | |
| 3-5 cm (T2a) | 80 (20.5%) | 53 (19.3%) | 27 (23.7%) | |
| Total BED (Gy10) | 0.96 | |||
| Median (range) | 112.5 (100–187.5) | 112.5 (100–187.5) | 112.5 (100–180) | |
| White Blood Cell Count (K/μL) | <0.0001* | |||
| Median (range) | 8.0 (2.9–22.5) | 7.5 (2.9–22.5) | 9.1 (5–22.1) | |
| Absolute Neutrophil Count (K/μL) | <0.0001* | |||
| Median (range) | 5.1 (0.8–19.6) | 4.5 (0.8–12.1) | 6.7 (2.6–19.6) | |
| Absolute Lymphocyte Count (K/μL) | <0.0001* | |||
| Median (range) | 1.6 (0.1–13.5) | 1.8 (0.4–13.5) | 1.2 (0.1–12.5) | |
| Pretreatment FEV1 | (N = 229) | (N = 162) | (N = 67) | 0.96 |
| ≤ 30% | 22 (9.6%) | 15 (9.3%) | 7 (10.5%) | |
| 31-50% | 71 (31.0%) | 49 (30.2%) | 22 (32.8%) | |
| 51-80% | 103 (45.0%) | 74 (45.7%) | 29 (43.3%) | |
| > 80% | 33 (14.4%) | 24 (14.8%) | 9 (13.4%) |
3.2. Survival
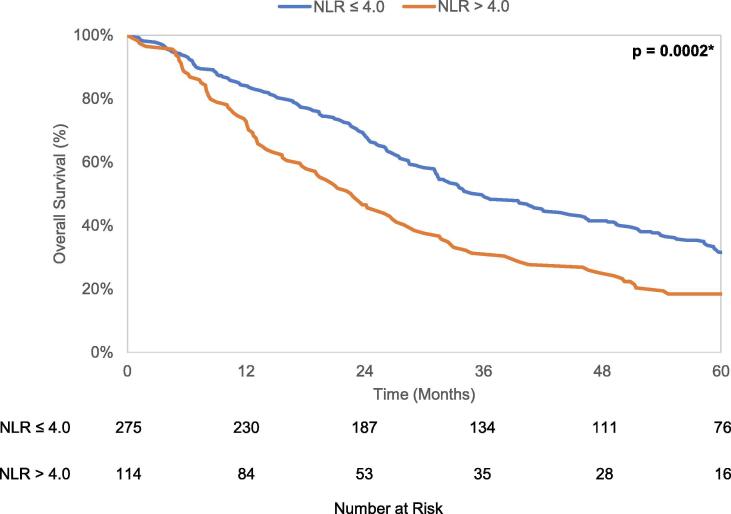
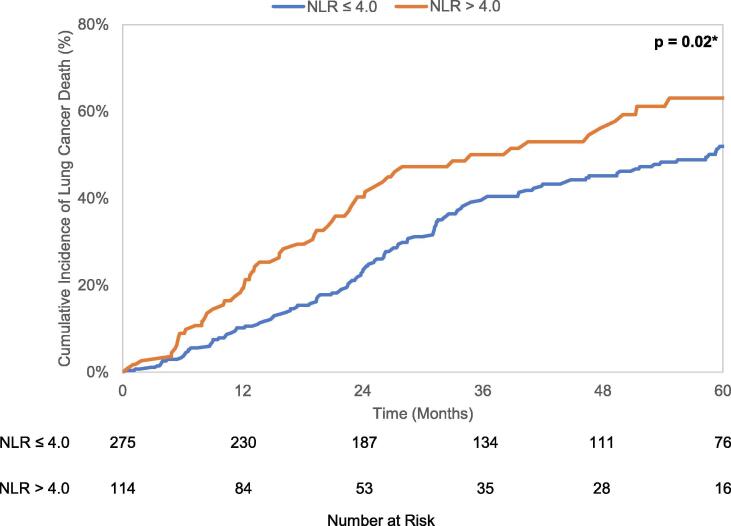
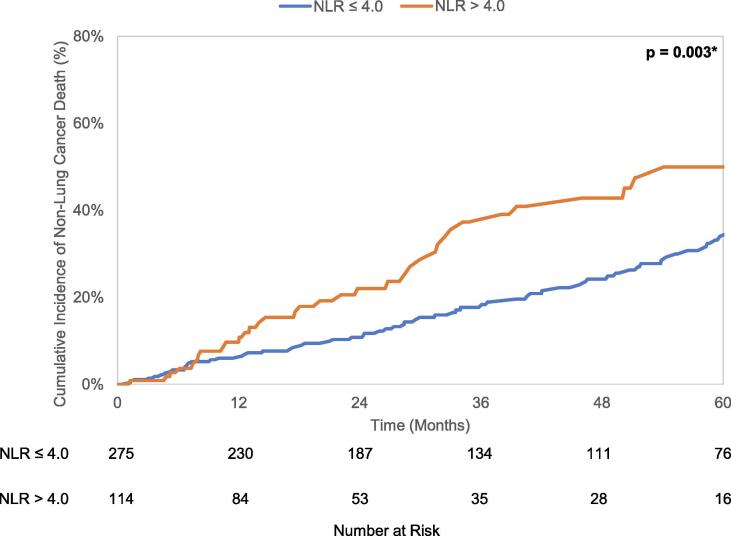
Median overall survival of the entire cohort was 31.5 months (95% CI 27.9–34.8 months). There were 304 deaths, of which 192 (63.2%) were attributed to lung cancer. The 5 year cumulative incidence of death from lung cancer and any non-lung cancer cause were 55.1% (95% CI 49.2–60.6%) and 38.2% (95% CI 31.8–44.6%), respectively. Kaplan-Meier analysis revealed a difference in OS (p = 0.0002) when stratified by the NLR cutoff, with median OS of 35.8 months (95% CI 31.3–43.8 months) in patients with NLR ≤ 4.0 and 22.7 months (95% CI 17.6–27.2 months) in patients with NLR > 4.0. Gray’s test found patients with NLR > 4.0 to have increased lung cancer mortality (LCM) (p = 0.02) and non-lung cancer mortality (NCM) (p = 0.003) compared to patients with NLR ≤ 4.0. These unadjusted OS curves and cumulative incidence plots depicting LCM and NCM are seen in Fig. 2a-c.
Fig. 2.
a. Kaplan-Meier curves for overall survival stratified by NLR cutoff value 4.0. b. Cumulative incidence curves for lung cancer-specific mortality stratified by NLR cutoff value 4.0. c. Cumulative incidence curves for non-lung cancer mortality stratified by NLR cutoff value 4.0.
In univariable Cox analysis, pretreatment NLR > 4.0 was associated with worse overall survival (HR = 1.59, 95% CI 1.24–2.04, p = 0.0003). In multivariable analysis, NLR > 4.0 continued to be an independent association with worse overall survival (HR 1.44, 95% CI 1.12–1.86, p = 0.01). For the other endpoints, similar multivariable analysis demonstrated NLR > 4.0 to be independently associated with decreased NCS (HR 1.68, 95% CI 1.11–2.53, p = 0.01) but only trend towards significance with LCS (HR 1.32, 95% CI 0.95–1.82, p = 0.09). These results are summarized in Table 2.
Table 2.
Multivariable a priori regressions on overall survival (OS), lung cancer-specific survival (LCS), non-lung cancer survival (NCS) in the overall cohort.
| Variable | OS |
LCS |
NCS |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| NLR | ||||||
| ≤ 4.0 | 1.0 | 1.0 | 1.0 | |||
| > 4.0 | 1.44 (1.12–1.86) | 0.01* | 1.32 (0.95–1.82) | 0.09 | 1.68 (1.11–2.53) | 0.01* |
| Age | 1.03 (1.02–1.05) | 0.0001* | 1.03 (1.02–1.05) | 0.0004* | 1.03 (1.01–1.06) | 0.01* |
| Gender | ||||||
| Male | 1.0 | 1.0 | 1.0 | |||
| Female | 0.98 (0.59–1.62) | 0.93 | 1.11 (0.53–2.32) | 0.78 | 0.80 (0.31–2.08) | 0.65 |
| Charlson Score | ||||||
| 0 | 1.0 | 1.0 | 1.0 | |||
| 1 | 1.35 (0.98–1.86) | 0.07 | 1.56 (1.03–2.36) | 0.04* | 1.05 (0.62–1.78) | 0.85 |
| 2+ | 1.56 (1.13–2.16) | 0.01* | 1.50 (0.97–2.32) | 0.07 | 1.68 (1.02–2.78) | 0.04* |
| Race | ||||||
| White | 1.0 | 1.0 | 1.0 | |||
| Black | 0.73 (0.51–1.04) | 0.08 | 0.84 (0.56–1.28) | 0.43 | 0.57 (0.30–1.07) | 0.08 |
| Smoker at Diagnosis | ||||||
| Yes | 1.0 | 1.0 | 1.0 | |||
| No | 0.80 (0.63–1.02) | 0.07 | 0.89 (0.66–1.20) | 0.46 | 0.70 (0.45–1.02) | 0.06 |
| Histology | ||||||
| Adenocarcinoma | 1.0 | 1.0 | 1.0 | |||
| Squamous | 1.04 (0.80–1.33) | 0.79 | 1.23 (0.89–1.70) | 0.20 | 0.78 (0.52–1.18) | 0.24 |
| Other/unknown | 0.86 (0.59–1.24) | 0.41 | 1.05 (0.66–1.67) | 0.84 | 0.61 (0.33–1.15) | 0.13 |
| T stage | ||||||
| < 2 cm (T1a) | 1.0 | 1.0 | 1.0 | |||
| 2–3 cm (T1b) | 1.32 (1.02–1.72) | 0.04* | 1.41 (1.01–1.96) | 0.04* | 1.20 (0.77–1.87) | 0.42 |
| 3–5 cm (T2a) | 1.25 (0.91–1.70) | 0.16 | 1.23 (0.83–1.82) | 0.31 | 1.29 (0.79–2.11) | 0.31 |
| Total BED | 1.00 (0.99–1.00) | 0.49 | 1.00 (0.99–1.00) | 0.32 | 1.00 (0.99–1.01) | 0.92 |
3.3. Subset analysis with PFT data
In a subset analysis of 229 patients with PFT data available within one year of starting SBRT, 162 patients had pretreatment NLR ≤ 4.0 and 67 patients had pretreatment NLR > 4.0.
In multivariable analysis (including PFTs along with previously detailed covariables), NLR > 4.0 remained significantly associated with worse OS (HR 1.51, 95% CI 1.07–2.14, p = 0.02) and NCS (HR 2.18, 95% CI 1.24–3.84, p = 0.01) like in the overall cohort’s models. However, NLR was not associated with LCS (HR 1.22, 95% CI 0.77–1.93, p = 0.39). Table 1, Table 3 detail the breakdown of PFT data in this subset cohort and the subsequent multivariable analysis on each survival endpoint.
Table 3.
Multivariable a priori (including pulmonary function tests) regressions on overall survival (OS), lung cancer-specific survival (LCS), non-lung cancer survival (NCS) in the subset cohort.
| Variable |
OS |
LCS |
NCS |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| NLR | ||||||
| ≤ 4.0 | 1.0 | 1.0 | 1.0 | |||
| > 4.0 | 1.51 (1.07–2.14) | 0.02* | 1.22 (0.77–1.93) | 0.39 | 2.18 (1.24–3.84) | 0.01* |
| Age | 1.05 (1.03–1.07) | <0.0001* | 1.05 (1.02–1.08) | 0.0008* | 1.05 (1.01–1.10) | 0.03* |
| Gender | ||||||
| Male | 1.0 | 1.0 | 1.0 | |||
| Female | 1.22 (0.67–2.22) | 0.53 | 1.04 (0.37–1.93) | 0.94 | 1.55 (0.53–4.50) | 0.42 |
| Charlson Score | ||||||
| 0 | 1.0 | 1.0 | 1.0 | |||
| 1 | 1.41 (0.90–2.22) | 0.13 | 1.45 (0.83–2.55) | 0.20 | 1.39 (0.64–3.01) | 0.40 |
| 2+ | 1.65 (1.05–2.59) | 0.03* | 1.40 (0.77–2.53) | 0.27 | 2.30 (1.11–4.77) | 0.03* |
| Race | ||||||
| White | 1.0 | 1.0 | 1.0 | |||
| Black | 0.78 (0.49–1.24) | 0.30 | 0.99 (0.58–1.68) | 0.96 | 0.47 (0.20–1.12) | 0.09 |
| Smoker at Diagnosis | ||||||
| Yes | 1.0 | 1.0 | 1.0 | |||
| No | 0.71 (0.50–0.99) | 0.04* | 0.76 (0.50–1.15) | 0.19 | 0.63 (0.35–1.13) | 0.12 |
| Histology | ||||||
| Adenocarcinoma | 1.0 | 1.0 | 1.0 | |||
| Squamous | 1.18 (0.83–1.68) | 0.35 | 1.36 (0.85–2.15) | 0.20 | 0.96 (0.55–1.70) | 0.90 |
| Other/unknown | 0.85 (0.47–1.52) | 0.58 | 1.04 (0.51–2.10) | 0.92 | 0.62 (0.20–1.92) | 0.41 |
| T stage | ||||||
| < 2 cm (T1a) | 1.0 | 1.0 | 1.0 | |||
| 2-3 cm (T1b) | 1.31 (0.91–1.89) | 0.14 | 1.40 (0.88–2.23) | 0.15 | 1.15 (0.61–2.15) | 0.67 |
| 3-5 cm (T2a) | 1.21 (0.80–1.81) | 0.37 | 1.25 (0.73–2.13) | 0.42 | 1.15 (0.58–2.26) | 0.69 |
| Total BED | 1.00 (0.99–1.00) | 0.26 | 1.00 (0.99–1.00) | 0.16 | 1.00 (0.99–1.01) | 0.96 |
| Pretreatment FEV1 | ||||||
| < 30% | 1.0 | 1.0 | 1.0 | |||
| 31-50% | 0.53 (0.30–0.94) | 0.03* | 0.63 (0.29–1.33) | 0.23 | 0.37 (0.15–0.93) | 0.04* |
| 51-80% | 0.57 (0.33–0.99) | 0.04* | 0.60 (0.29–1.27) | 0.18 | 0.51 (0.22–1.20) | 0.12 |
| > 80% | 0.58 (0.30–1.11) | 0.10 | 0.62 (0.27–1.44) | 0.27 | 0.50 (0.18–1.38) | 0.18 |
3.4. Validation of previously proposed cutoff
Sebastian et al. proposed a pretreatment NLR cutoff of 3.6 identified by using the median pretreatment NLR value of their 156 patient cohort with localized NSCLC treated with SBRT [22]. They identified pretreatment NLR > 3.6 to be independently associated with overall mortality. Therefore we replicated our previous analysis using this previously proposed cutoff.
For our overall cohort of 389 patients, multivariable analysis revealed pretreatment NLR > 3.6 to not be associated with OS (HR 1.25, 95% CI 0.99–1.58, p = 0.06) or LCS (HR 1.12, 95% CI 0.84 – 1.51, p = 0.44). But it was associated with NCS (HR 1.52, 95% CI 1.03–2.24, p = 0.04). In our subset cohort of 229 patients with PFT data, multivariable analysis (incorporating PFTs) revealed pretreatment NLR > 3.6 to not be associated with OS (HR 1.31, 95% CI 0.65–1.47, p = 0.91), LCS (HR 0.98, 95% CI 0.65–1.47, p = 0.91), or NCS (HR 1.59, 95% CI 0.98–2.58, p = 0.08).
4. Discussion
In this hypothesis-generating study of patients with stage I NSCLC treated with SBRT in the VA system, we identified an optimal pretreatment NLR cutoff point of 4.0 and observed NLR to be independently associated with OS. However, this association was driven by the relationship between NLR and NCS rather than by the relationship between NLR and LCS. Subset analysis (incorporating PFT data which pose as a notable confounder) further evidenced our findings by demonstrating elevated NLR to have more pronounced associations with inferior OS and NCS. Although NLR’s association with LCS trended towards significance (p = 0.09) in the overall cohort, it was notably insignificant (p = 0.39) in the subset analysis. Our analysis suggests that NLR may not have potential as a marker of lung cancer-specific survival in this setting. However, it does suggest that NLR has strong potential as a marker for competing risk mortality in this population.
Previous studies have explored the associations between pre-SBRT NLR and survival outcomes but have presented a mix of results. Of these studies that have proposed a pre-SBRT NLR cutoff point, Cannon et al. (cutoff 2.98) and Luo et al. (cutoff 2.06) utilized a ROC approach while Sebastian et al. (cutoff 3.6) chose the cohort’s median as a cutoff point [20], [21], [22]. All of these cutoff points were evaluated to have no association with any survival outcomes in our dataset, but only data for the one with the largest cohort and strongest evidence (Sebastian et al.’s 3.6) was detailed in this paper. In the context of these previously proposed cutoff points yielding no associations in our dataset and given the time dependent nature of survival outcomes, we believe our study is unique in this domain to use statistically appropriate, time-dependent cutoff methods. Furthermore, our study is the largest cohort thus far with nearly 400 patients. Additionally, given that NLR has been associated with PFTs and that PFTs have been demonstrated to be an important factor in both lung function decline and NSCLC, we are able to account for a potentially notable confounding variable while most previous studies have not been able to [19], [33]. Finally, unlike most previous studies, we are able to delineate NLR’s impact on LCS and NCS individually. This enables a better understanding of the clinical utility NLR offers in this disease and population.
A large body of literature exists on the pathophysiology and interpretation of NLR in both oncologic and non-oncologic disease states. In various oncologic states, lymphocytes are hypothesized to primarily aid in the host response against cancer while neutrophils are hypothesized to primarily aid in the carcinogenesis response against the host [34], [35]. Similar immunologic principles and theories have implicated NLR to be associated with outcomes in several non-oncologic disease states [16], [17]. Therefore a major challenge to the use of NLR in the clinical setting is this hurdle of understanding what prognostic value it holds for each patient population in consideration. For localized NSCLC patients treated with SBRT, we hypothesize that NLR is predictive of competing mortality risk given our data. Giulani et al. reported similar findings of no association with lung cancer-specific outcomes when analyzing NLR as a continuous variable [36]. This suggests an underlying propensity for mortality in the elevated NLR cohort. However, this propensity cannot be simply explained by a difference in classic variables of age, Charlson scores, etc. in the elevated NLR cohort as Table 1 demonstrates the similarity in host and tumor characteristics for both cohorts. This is in line with the unmet need for additional risk stratification in localized NSCLC treated with SBRT as most of these patients possess unfavorable, classic risk stratification variables that typically preclude them from surgery.
Although our study overcomes important limitations of prior studies in this realm, there are still notable limitations present. Alongside the inherent drawbacks of a retrospective database study, detailed records of pro-inflammatory states (e.g. rheumatologic disorders, infections, etc.) and specific medications (e.g. corticosteroids, immunomodulators, etc.) that affect a patient’s immunologic state, and therefore likely their NLR, are not noted and raise concern for unaccounted confounding. Additionally, there is research demonstrating NLR to have differential prognostic capacity in different age and race populations [37]. Of note, Black patients in our cohort had higher NLR which possibly indicates an elevated, baseline inflammatory state in this population but this would need further research [38]. Although we hope the inclusion of these variables in multivariable models would account for this difference, the generalizability of our study to all populations consequently might be limited.
5. Conclusions
In summary, we identified an optimal pretreatment NLR cutoff of 4.0 and observed NLR to be independently associated with overall survival in patients with localized NSCLC who underwent SBRT. After accounting for clinical variables (notably including baseline lung function), this association was found to be driven by the relationship between NLR and non-lung cancer survival rather than by the statistically insignificant relationship between NLR and lung cancer-specific survival. Although there is a need for markers predictive of cancer-specific outcomes in this population, our analysis does not suggest NLR to have such potential. Instead, our hypothesis-generating study demonstrates strong potential for NLR as a marker for competing risk mortality in this setting. Given this population to typically be frailer and rife with comorbidities, this simple and cost-effective lab value with our proposed cutoff has potential to fill a need for further risk stratification and clinical decision making for these patients. Further studies are needed to validate these findings and investigate pretreatment NLR as a clinical tool in patients with localized NSCLC treated with SBRT.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.03.010.
Contributor Information
Nikhil V. Kotha, Email: nkotha@health.ucsd.edu.
Daniel R. Cherry, Email: dacherry@health.ucsd.edu.
Alex K. Bryant, Email: bralex@med.umich.edu.
Vinit Nalawade, Email: vnalawade@health.ucsd.edu.
Tyler F. Stewart, Email: tstewart@health.ucsd.edu.
Brent S. Rose, Email: bsrose@health.ucsd.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.v70.110.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Molina J.R., Yang P., Cassivi S.D., Schild S.E., Adjei A.A. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. Elsevier Ltd. 2008;83(5):584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019.
- 4.Datta D., Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123(6):2096–2103. doi: 10.1378/chest.123.6.2096. [DOI] [PubMed] [Google Scholar]
- 5.McDonald F., De Waele M., Hendriks L.E.L., Faivre-Finn C., Dingemans A.-M., Van Schil P.E. Management of stage I and II nonsmall cell lung cancer. Eur Respir J. 2017;49(1):1600764. doi: 10.1183/13993003.00764-201610.1183/13993003.00764-2016.Supp1. [DOI] [PubMed] [Google Scholar]
- 6.Choi J.I. Medically inoperable stage i non-small cell lung cancer: best practices and long-term outcomes. Transl Lung Cancer Res. 2018;8(1):32–47. doi: 10.21037/tlcr10.21037/tlcr.2018.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA J Am Med Assoc. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmerman R.D., Paulus R., Pass H.I., Gore E.M., Edelman M.J., Galvin J. Stereotactic body radiation therapy for operable early-stage lung cancer findings from the NRG oncology RTOG 0618 trial. JAMA Oncol. 2018;4(9):1263. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senthi S., Lagerwaard F.J., Haasbeek C.J.A., Slotman B.J., Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13(8):802–809. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 10.Bryant A.K., Mundt R.C., Sandhu A.P. Stereotactic body radiation therapy versus surgery for early lung cancer among US veterans. Ann Thorac Surg. 2018;105(2):425–431. doi: 10.1016/j.athoracsur.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Prezzano K.M., Ma S.J., Hermann G.M., Rivers C.I., Gomez-Suescun J.A., Singh A.K. Stereotactic body radiation therapy for non-small cell lung cancer: a review. World J Clin Oncol. 2019;10(1):14–27. doi: 10.5306/wjco.v10.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaguchi T., Takada M., Kubo A., Matsumura A., Fukai S., Tamura A. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5(5):620–630. doi: 10.1097/JTO.0b013e3181d2dcd9. [DOI] [PubMed] [Google Scholar]
- 13.Paesmans M. Prognostic and predictive factors for lung cancer. Breathe. 2012;9(2):113–122. doi: 10.1183/20734735.006911. [DOI] [Google Scholar]
- 14.Vermaelen K., Brusselle G. Exposing a deadly alliance: novel insights into the biological links between COPD and lung cancer. Pulm Pharmacol Ther. 2013;26(5):544–554. doi: 10.1016/j.pupt.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Valavanidis A., Vlachogianni T., Fiotakis K., Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013;10(9):3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah N., Parikh V., Patel N., Patel N., Badheka A., Deshmukh A. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: insights from the National Health and Nutrition Examination Survey-III. Int J Cardiol. 2014;171(3):390–397. doi: 10.1016/j.ijcard.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 17.Turkmen K., Erdur F.M., Ozcicek F., Ozcicek A., Akbas E.M., Ozbicer A. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17(3):391–396. doi: 10.1111/hdi.2013.17.issue-310.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 18.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106(6). doi:10.1093/jnci/dju124 [DOI] [PubMed]
- 19.Gao X.u., Coull B., Lin X., Vokonas P., Sparrow D., Hou L. Association of neutrophil to lymphocyte ratio with pulmonary function in a 30-year longitudinal study of US Veterans. JAMA Netw Open. 2020;3(7):e2010350. doi: 10.1001/jamanetworkopen.2020.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannon N.A., Meyer J., Iyengar P., Ahn C., Westover K.D., Choy H. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10(2):280–285. doi: 10.1097/JTO.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 21.Luo H., Ge H., Cui Y., Zhang J., Fan R., Zheng A. Systemic inflammation biomarkers predict survival in patients of early stage non-small cell lung cancer treated with stereotactic ablative radiotherapy - a single center experience. J Cancer. 2018;9(1):182–188. doi: 10.7150/jca.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastian N., Wu T., Bazan J., Driscoll E., Willers H., Yegya-Raman N. Pre-treatment neutrophil-lymphocyte ratio is associated with overall mortality in localized non-small cell lung cancer treated with stereotactic body radiotherapy. Radiother Oncol. 2019;134:151–157. doi: 10.1016/j.radonc.2019.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acs. FORDS Facility Oncology Registry Data Standards Revised for 2016; 2016.
- 24.Zullig LL, Jackson GL, Dorn RA, et al. Cancer Incidence Among Patients of the U.S. Veterans Affairs Health Care System. Mil Med 2012;177(6):693-701. doi:10.7205/MILMED-D-11-00434 [DOI] [PMC free article] [PubMed]
- 25.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J. Thor. Oncol; 2007. doi:10.1097/JTO.0b013e318074de34 [DOI] [PubMed]
- 26.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.-C. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Klabunde C.N., Potosky A.L., Legler J.M., Warren J.L. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/S0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 28.Quanjer P.H., Stanojevic S., Cole T.J., Baur X., Hall G.L., Culver B.H. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams BA, Mandrekar JN, Mandrekar SJ, et al. Finding Optimal Cutpoints for Continuous Covariates with Binary and Time-to-Event Outcomes.; 2006.
- 30.Kamarudin A.N., Cox T., Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17(1):53. doi: 10.1186/s12874-017-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contal C., O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30(3):253–270. doi: 10.1016/S0167-9473(98)00096-6. [DOI] [Google Scholar]
- 32.P MJ, N MJ, Clinic M. Cutpoint Determination Methods in Survival Analysis Using SAS ®: Updated %FINDCUT Macro.
- 33.Preoperative Pulmonary Function Tests (PFTs) and Outcomes from Resected Early Stage Non-small Cell Lung Cancer (NSCLC). http://ar.iiarjournals.org/content/38/5/2903.abstract. Accessed October 16, 2020. [DOI] [PubMed]
- 34.Gooden M.J.M., De Bock G.H., Leffers N., Daemen T., Nijman H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y., Zhao Q., Peng C., Sun L., Li X.F., Kuang D.M. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol. 2011;225(3):438–447. doi: 10.1002/path.2947. [DOI] [PubMed] [Google Scholar]
- 36.Giuliani M., Sampson L.R., Wong O., Gay J., Le L.W., Cho B.C.J. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes on outcomes in lung stereotactic body radiotherapy. Curr Oncol. 2016;23(4):362–368. doi: 10.3747/co.23.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howard R., Kanetsky P.A., Egan K.M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-56218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin C., Arevalo Y.A., Nanavati H.D., Lin D.M. Racial differences and an increased systemic inflammatory response are seen in patients with COVID-19 and ischemic stroke. Brain Behav Immun Heal. 2020;8:100137. doi: 10.1016/j.bbih.2020.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.