Abstract
Higher impulsivity may arise from neurophysiological deficits of cognitive control in the prefrontal cortex. Cognitive control can be assessed by time‐frequency decompositions of electrophysiological data. We aimed to clarify neuroelectric mechanisms of performance monitoring in connection with impulsiveness during a modified Eriksen flanker task in high‐ (n = 24) and low‐impulsive subjects (n = 21) and whether these are modulated by double‐blind, sham‐controlled intermittent theta burst stimulation (iTBS). We found a larger error‐specific peri‐response beta power decrease over fronto‐central sites in high‐impulsive compared to low‐impulsive participants, presumably indexing less effective motor execution processes. Lower parieto‐occipital theta intertrial phase coherence (ITPC) preceding correct responses predicted higher reaction time (RT) and higher RT variability, potentially reflecting efficacy of cognitive control or general attention. Single‐trial preresponse theta phase clustering was coupled to RT in correct trials (weighted ITPC), reflecting oscillatory dynamics that predict trial‐specific behavior. iTBS did not modulate behavior or EEG time‐frequency power. Performance monitoring was associated with time‐frequency patterns reflecting cognitive control (parieto‐occipital theta ITPC, theta weighted ITPC) as well as differential action planning/execution processes linked to trait impulsivity (frontal low beta power). Beyond that, results suggest no stimulation effect related to response‐locked time‐frequency dynamics with the current stimulation protocol. Neural oscillatory responses to performance monitoring differ between high‐ and low‐impulsive individuals, but are unaffected by iTBS.
Keywords: impulsivity, intertrial phase coherence, performance monitoring, single‐trial phase behavior coupling, time‐frequency analysis, transcranial magnetic stimulation
We aimed to clarify neuroelectric mechanisms of performance monitoring in connection with impulsiveness during a modified Eriksen flanker task in high‐ and low‐impulsive subjects. Performance monitoring was associated with time‐frequency patterns reflecting cognitive control (parieto‐occipital theta ITPC, theta weighted ITPC) as well as differential action planning/execution processes linked to trait impulsivity (frontal low beta power). Beyond that, results suggest no stimulation effect related to response‐locked time‐frequency dynamics with the current stimulation protocol.
1. INTRODUCTION
Cognitive control involves a multitude of processes such as allocation of attention, suppressing irrelevant information, and inhibition of inappropriate cognitive processes or actions that may interfere with the correct execution of an intended action (Diamond, 2013). Poor inhibitory control as part of these processes is associated with impulsivity (Brevet‐Aeby, Brunelin, Iceta, Padovan, & Poulet, 2016). The complex construct of impulsivity involves, inter alia, deficits in inhibitory response, impaired decision‐making processes, as well as deficits in planning capacities (Barratt, 1985; Dalley, Everitt, & Robbins, 2011). Impulsivity occurs in healthy individuals as well as in various psychiatric conditions such as attention deficit/hyperactivity disorder (ADHD). To study cognitive control processes in well‐controlled laboratory settings, variations of the Eriksen flanker task have been used in healthy participants, ADHD, and other disorders to assess behavioral as well as neurophysiological aspects of cognitive control functions, including selective attention, response conflict, or performance monitoring (e.g., Franken, Luijten, van der Veen, & van Strien, 2017; Herrmann et al., 2010; McDermott, Wiesman, Proskovec, Heinrichs‐Graham, & Wilson, 2017; Mullane, Corkum, Klein, & McLaughlin, 2009). Supposedly, performance monitoring comprises one process for the detection of current errors (monitoring step) as well as another process responsible for the avoidance of future errors (posterror adjustments; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004), both reflected on a behavioral level (Wessel, 2018)—for example, reaction time slowing, higher accuracy in trials after errors (Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001)—as well as on a functional level as described in the following section with a focus on error monitoring.
Research on electrophysiological correlates of cognitive response control has revealed particular patterns of brain oscillations in the prefrontal cortex. Referring to performance monitoring, erroneous responses have been shown to be associated with immediately following enhanced theta power at mid‐frontal and frontal lateral sites (reflecting error detection processes) as well as enhanced fronto‐central beta 600–800 ms posterror (reflecting motor inhibition). Furthermore, a time‐frequency (TF) analysis revealed that delta (1.5–3.5 Hz) band‐specific activity was associated with error detection at the level of performance monitoring (Yordanova, Falkenstein, Hohnsbein, & Kolev, 2004). In contrast to and independently from oscillatory power, TF intertrial phase clustering (ITPC) reflects the consistency of band‐specific oscillations (i.e., consistency of frequency‐specific phases across trials), thus providing a direct measure of cortical synchrony related to the stimulus or response (Cohen, 2014). Lower theta ITPC during performance monitoring was related to higher reaction time variability (Papenberg, Hämmerer, Müller, Lindenberger, & Li, 2013) presumably reflecting impaired error processing and subsequent behavior adaption (Yordanova et al., 2011). Further, preresponse theta ITPC were reported to be involved in response selection and could further predict endogenous conflict as reflected by reaction times (Cohen & Cavanagh, 2011). Importantly, alterations of cognitive control as found in ADHD reduce theta ITPC as compared to normal developing adolescents (Gonen‐Yaacovi et al., 2016; Groom et al., 2010).
To probe the causal role of neuronal oscillations in cognitive control, repetitive transcranial magnetic stimulation (repetitive TMS, rTMS) can be applied over dlPFC because it modulates rhythmic brain activity in healthy populations in relatively widespread oscillation at rest, in terms of—for example—increases in delta power over frontal, central, and parietal sites (Griškova, Rukšėnas, Dapšys, Herpertz, & Höppner, 2007) as well as increases in prefrontal theta (Schutter, van Honk, d'Alfonso, Postma, & de Haan, 2001). In the present study, we applied rTMS combined with transcranial direct current stimulation (tDCS) in a subclinical population of high‐impulsive subjects. We aimed at temporarily facilitating right dlPFC activation and investigated TMS effects on neurophysiological (via simultaneous functional near‐infrared spectroscopy and electroencephalography) and behavioral processes during a flanker task. Thus, we used the highly effective theta‐burst stimulation (TBS; Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005) that—even after a short stimulation interval—induces sustained effects in the cortex. Specifically, we implemented an excitatory intermittent TBS (iTBS) protocol to temporally increase activation in the right dlPFC in high‐impulsive volunteers. To optimize stimulation effects, we first applied a cathodal tDCS protocol as it has been shown to induce an optimal state for the following rTMS protocol in cortical tissue (cf., Lang et al., 2004). tDCS is a noninvasive electrostimulation method that delivers weak electric currents in localized cortical regions altering their excitability as well as in connected neuronal networks (e.g., Nitsche & Paulus, 2001). In a recent review, the right‐hemisphere was hypothesized to be causally linked to impulsivity because noninvasive brain stimulation techniques facilitating right PFC activity mostly reduced impulsive behavior (Brevet‐Aeby et al., 2016).
In the present study, we investigated high‐impulsive individuals as a sub‐clinical model population of ADHD sharing many of the same characteristics as patients diagnosed with ADHD (e.g., Biederman et al., 2018; Herrmann et al., 2009; T. Li et al., 2019). This has also led to an at least partly continuous understanding of the disorder, where diagnosed patients cluster at the extreme end of certain traits that are continuously distributed among the general population (e.g., Levy, Hay, McStephen, Wood, & Waldman, 1997). Therefore, we expected these participants to exhibit frontal lobe dysfunction typically observed in patients with ADHD (albeit to a possibly lesser extent). 1
In order to substantiate general executive function deficits in high‐impulsive subjects, we additionally included a control group of low‐impulsive healthy volunteers. In this manner, we aimed at investigating principal neural characteristics of a high‐impulsive model population as well as mechanisms underlying prefrontal interventions. A better knowledge of the basic mechanisms of interventions targeting frontal hypofunction might help to optimize neuromodulation approaches in this cortical region of interest (ROI) in a clinical population (e.g., ADHD).
In the current study, we used EEG TF decompositions to investigate the underlying spatio‐temporal dynamics during performance monitoring based on previous research and additional explorative analyses. We focused our investigation on the oscillatory changes induced by correct, erroneous, or slow responses (as a possible strategy to avoid errors). Several cognitive control processes are involved in task execution here, including allocation of attention, visual perception of the cue, decision on the appropriate reaction, and finally the motor response, which will be evaluated afterward. We additionally investigated whether these spatio‐temporal dynamics are modified by verum versus sham stimulation of the right dlPFC.
To investigate whether cognitive control functions modulate neural oscillations and to investigate whether TBS normalizes impaired cognitive control functions, we measured response‐locked neuronal oscillations in a Flanker task in high‐ versus low‐impulsive individuals after verum or sham TBS. Because we specifically investigated error monitoring as part of performance monitoring in the current study, all neural oscillations were analyzed in a response‐locked manner (i.e., relative to participants' behavioral response in the flanker task). We investigated the effect of correct, erroneous, and slow responses on three TF parameters of neural oscillations locked to subjects' responses: Regarding hypotheses, first, we expected that the response type would alter TF beta power over prefrontal scalp sites and sensorimotor areas since there is evidence for a close link between frontal beta oscillations and movement preparation/execution as well as motor inhibition/control (central sites: movement preparation/execution; fronto‐central sites: motor inhibition/control; Alegre et al., 2004; Liang, Bressler, Ding, Truccolo, & Nakamura, 2002; Perri, Berchicci, Lucci, Spinelli, & Di Russo, 2016). Second, we expected enhanced theta ITPC to be associated with improved performance monitoring (Yordanova et al., 2011). Third, we expected altered weighted ITPC indicating the influence of trial‐to‐trial reaction time on oscillatory phases (Cohen, 2014). Further, we expected effects of TBS and impulsivity on response‐related TF parameters, in terms of both error‐specific increases in delta, theta, and beta power as well as higher theta intertrial phase coherence after verum versus sham stimulation, specifically in high‐impulsive participants.
2. METHODS AND MATERIALS
2.1. Participants
We enrolled 31 adult high‐impulsive and 33 low‐impulsive participants in the study. Questionnaire cut‐offs for categorization of participants into the high‐ or low‐impulsive group are shown in Table 1. Exclusion criteria for both high‐ and low‐impulsive subjects were axis I disorders as assessed by the German Structured Clinical Interview for DSM‐IV (SCID; Wittchen, Zaudig, & Fydrich, 1997), with the exception of mild to moderate depression (Beck Depression Inventory score <28; Beck, Steer, & Brown, 2009) and specific phobias. Antisocial and borderline personality disorders were also excluded based on SCID‐II (Wittchen et al., 1997) and a short version of the Borderline Symptom List (cutoff score >32; Bohus et al., 2009). All participants gave written informed consent. In the present study, we investigated high‐impulsive individuals as a model population of ADHD; thus, it may be expected that some participants fulfill an ADHD diagnosis but as we only screened participants without in‐depth diagnostics, we cannot be sure (according to ADHS‐SB and WURS‐K, all but one of the participants screened positive for ADHD which, however, does not justify an actual ADHD diagnosis). The study was carried out in accordance with the Declaration of Helsinki in its latest revision and approved by the local ethics committee (University Hospital of Tübingen). All participants gave written informed consent.
TABLE 1.
Questionnaire cutoffs for categorization of impulsivity
| Questionnaire | Low‐impulsive | High‐impulsive |
|---|---|---|
| ADHS‐SB | <18 | ≥18 |
| ASRS | <15 | ≥15 |
| WURS‐K | <30 | <30 |
| BSL | <47 | <47 |
| BIS | ≤55 | ≥70 |
Abbreviations: ADHS‐SB, German ADHD self‐rating scale for symptoms in adulthood (Rösler, Retz‐Junginger, Retz, & Stieglitz, 2008); ASRS, German Adult ADHD Self‐Report Scale (Kessler et al., 2005); BIS, Barratt Impulsiveness Scale (Hartmann, Rief, & Hilbert, 2011); BSL, Borderline Symptom List (Bohus et al., 2009); WURS‐K, short version of the German Wender Utah Rating Scale for ADHD childhood symptoms (Retz‐Junginger et al., 2002).
2.2. Paradigm
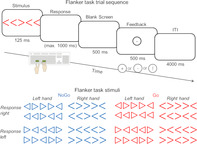
We applied a modified Eriksen flanker task according to Yordanova et al. (2011) as well as studies carried out in our group (Ehlis et al., 2011; Ehlis, Deppermann, & Fallgatter, 2018). The experiment was programmed in Presentation software (Neurobehavioral Systems Inc.). Subjects were presented for 125 ms either with an arrow or a triangle pointing to the left or right, prompting participants to respond as quickly and accurately as possible via a left or right button press, respectively. The shape of the stimuli (arrow vs. triangle) further determined the response hand, resulting in four response options. The target stimuli were flanked by two more stimuli of the same type on each side, providing incongruent information by pointing either completely (all four flankers) or partly (two of four flankers) in the opposite direction. The resulting eight types of flanker stimuli were presented in randomized order. Additionally, we included a Go/NoGo instruction which was implemented by the color of the prompts (50% blue vs. 50% red). Overall, we applied a 2 (response direction: L vs. R) × 2 (response hand: L vs. R) × 2 (flanker number: 2 vs. 4) × 2 (Go vs. NoGo) design yielding 16 stimulus conditions (Figure 1). We switched the assignment of stimulus shape to response hand (left or right) and color to instruction (Go or NoGo) for each participant between two blocks of the paradigm, and counterbalanced the sequence of events across subjects. After each button press, participants received direct visual feedback on their response (correct, incorrect, and correct but too slow). To individually adjust the level of difficulty, we determined the reaction time (RT) threshold for feedback based on a pretest including 40 trials. We used the individual median of RTs for correct responses during this pretest (discarding the first 10 trials) as a boundary between sufficiently fast responses and slow responses (cf., Ehlis et al., 2011; Schneider et al., 2013).
FIGURE 1.
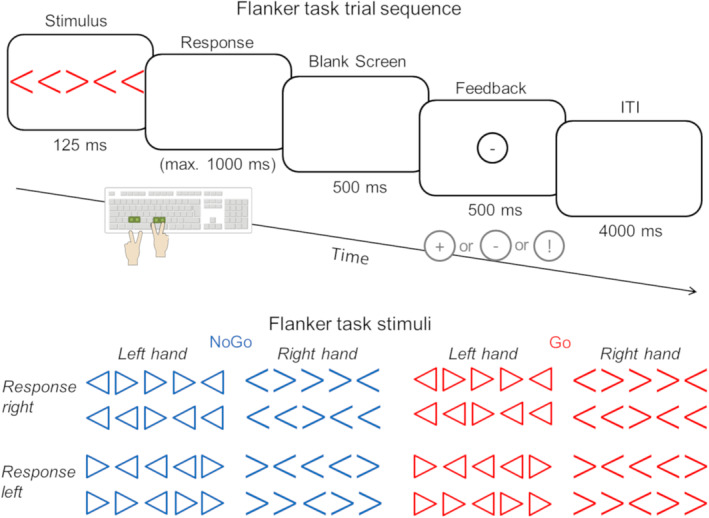
Task design of the modified Eriksen flanker task with Go/NoGo instruction and illustration of the corresponding stimuli (modified from Ehlis et al., 2018). Subjects were presented with a central arrow/triangle and two flanking stimuli on either side. We instructed participants to indicate the direction of the central arrow/triangle (left or right) via key press while ignoring the flanking stimuli. The feedback screen was presented exactly 1,625 ms after stimulus onset, with a fixed response window of 1,000 ms. The four response keys on the computer keyboard are colored in green. Stimuli indicated the action (Go or NoGo, i.e., red vs. blue), the response hand (left or right, i.e., triangle vs. arrow) and the response finger (left or right, i.e., direction of the central arrow/triangle). We changed the mapping of response hand to stimulus type and Go/NoGo instruction to color between the two experimental blocks within participants and counterbalanced the sequence of the mappings across subjects
Participants underwent 400 trials separated into two blocks. The intertrial interval was set to 4,000 ms and the response window lasted for max. 1,000 ms. Participants received their feedback exactly 1,625 ms after the onset of the stimulus. If participants made less than 10 errors over all 400 trials, the number of trials was increased up to 600 trials (n = 3 in high‐impulsive, n = 9 in low‐impulsive subjects). Post hoc analyses of the number of trials presented revealed that the groups differed significantly with respect to the total number of trials (p = .015) with a higher number of trials in low‐impulsive subjects.
2.3. tDCS and transcranial magnetic stimulation
TMS is a noninvasive neuromodulation (or brain stimulation) technique that induces electrical currents in the area under the stimulation site as well as connected neural networks in the cortex using pulsed magnetic fields on the head (Camprodon, 2016). Participants were tested twice in a controlled double‐blind crossover study design to assess the acute effects of an excitatory rTMS intervention on brain oscillations in high‐impulsive volunteers as a model population for ADHD compared to a low‐impulsive control population. The decision to facilitate right dlPFC activation was based on asymmetry findings that show patients with ADHD to exhibit stronger relative left‐hemispheric cortical activity than controls (Keune et al., 2011; Shaw et al., 2009). For the preceding tDCS (DC Stimulator MC, NeuroConn, Germany), we placed self‐adhesive sponge electrods (35 mm2) over the positions F4 and Fp1. The direct current (1 mA) was applied continuously for 10 min, and is expected to lead to changes of regional cortical excitability lasting for minutes up to hours after the stimulation phase (Iyer et al., 2005; Nitsche et al., 2003; Nitsche & Paulus, 2001). To reduce tingling under the electrodes during switch‐on and switch‐off, we steadily increased the current strength during the first 20 s of stimulation and tapered the current strength the same way at the end of stimulation. This tapering procedure was applied in sham tDCS as well to avoid tactile perceptual differences between the conditions. However, stimulation was stopped immediately after the 20‐s ramp‐up period so that it only lasted 20 s in the sham condition.
To allow for double‐blind rTMS application, we attached two self‐adhesive gelled stimulation electrodes centered on position F4 (according to 10–20) with an interelectrode distance of 1.5 cm and used a placebo‐verum TMS coil (Cool B65 A/P placebo verum coil, MagPro X100 Stimulator, MagVenture, Denmark). F4 was chosen as stimulation point (presumably located over the dlPFC) based on previous publications localizing 10–20 standard positions to underlying brain areas (Herwig, Satrapi, & Schönfeldt‐Lecuona, 2003). In one of the two sessions, as part of a verum stimulation, we first implemented a cathodal tDCS protocol followed by an excitatory (facilitating) iTBS protocol (cf., Huang et al., 2005; Huang & Rothwell, 2004) positioning the coil over the stimulation locus at 45° over the right dlPFC after the tDCS electrodes had been removed. The iTBS protocol consisted of a burst of three pulses (50 Hz) repeated every 200 ms within an interval of 2 s with a 10 s break for a total of 600 pulses and a total duration of 190 s. The intensity of stimulation was adjusted to 80% of participants' motor threshold. To determine the motor threshold, the subjects were asked to sit comfortably and relaxed on the chair with their legs at a 90° angle. The hands should be placed on the thighs with the back of the hand and relaxed. The motor threshold was determined between sites Cz and C4. The coil was placed on the scalp at a 45° angle to the sagittal. To determine the individual resting motor threshold, we used visual observation, whereby the threshold was defined as the highest setting at which 0 out of 5 stimuli resulted in any observable movement of the thumb (interstimulus interval ≥5 s).
In the sham session, the same protocol was applied, however, only sham tDCS was administered and skin afferences under the electrodes were stimulated in TMS. After the stimulation, the EEG cap was applied. The sequence of stimulation protocols (verum vs. sham in session 1 vs. 2) was balanced across participants (cf., Huang et al., 2005). The mean time span between iTBS and the beginning of EEG recordings was 30 min (range 22–95 min). The mean time interval between sessions was 6.69 days (range 1–62 days). Hence, the range of time span between iTBS and the beginning of EEG recordings was relatively high. To ensure that longer time intervals did not impact results concerning stimulation effects on TF data, we conducted a sub‐analysis including only subjects with a preparation time lower than 45 min (n = 17 high‐impulsive; n = 14 low‐impulsive).
2.4. Recording
We conducted EEG measurements using a 32‐channel DC‐amplifier (BrainVision Recorder, Brain Products, Germany) and 23 Ag/AgCl ring electrodes placed according to the international 10/20 system, with three additional electrooculography electrodes. We used a fronto‐central electrode position (FCz) as reference electrode (all impedances below 5 kΩ, sampling rate 1,000 Hz; online filter 0.1–100 Hz). Simultaneously, we recorded near‐infrared spectroscopy data using the ETG‐4000 (Hitachi Medical Co., Japan), the results of which will be reported elsewhere.
2.5. EEG preprocessing and analyses
We preprocessed and analyzed EEG data using the MATLAB (R2017a, The MathWorks, USA) software toolbox FieldTrip (Oostenveld, Fries, Maris, & Schoffelen, 2011) and custom‐made analysis scripts. We re‐referenced EEG raw data to linked mastoids and bandpass filtered (0.5–40 Hz) data to remove line noise and high frequency noise (cf., Figee et al., 2013). We then epoched data 2,000 ms preresponse to 2,000 ms postresponse and baseline corrected data in the time domain (−200 ms until response). We removed excessive noise (muscle artifacts, artifacts due to disconnected electrodes) via manual inspection and afterward resampled EEG data to 250 Hz. We ran an independent component analysis to remove EOG and muscle artifacts using the “runica” algorithm implemented in FieldTrip. After automatic threshold artifact rejection (± 70 μV), we manually inspected data once more to make sure all artifacts had been removed. We performed a TF analysis with a Hanning taper and frequency‐dependent window length (for frequencies 2–30 Hz; 3 cycles per time window) within a time window of 800 ms preresponse to 850 ms postresponse (in order to exclude feedback‐related responses) in time steps of 20 ms and frequency steps of 1 Hz. In our task design, preresponse baseline windows may include stimulus‐related activity that could thus be carried over to response‐related activity. Therefore, we baseline corrected data for between‐subject comparisons in the TF domain using a whole trial event baseline (800 ms preresponse until 850 ms postresponse). This alternative baseline approach is sensitive to detect phasic changes whereas longer changes over the trial period are more difficult to be detected (Cohen, 2014; p. 233). Potentially, response‐locked activity is confounded with stimulus‐related activity if reaction times differ systematically between stimulus conditions. Thus, to address potentially systematic contributions of differential stimulus‐related activity to response‐related activity, we used a trial wise linear regression method to dissect relationships between frequency power and reaction times (Hu, Xiao, Zhang, Mouraux, & Iannetti, 2014).
We calculated ITPC for any given channel, time and frequency time‐locked to response onset. ITPC reflects the extent to which oscillation phase values are consistent over trials at that point in TF space. ITPC can take values between zero (random phase distribution) and 1 (perfect phase synchronization). To reduce the number of multiple comparisons in TF‐channel space and, thereby, increase statistical power, we focused on specific channels and frequency bands: Based on previous studies, we clustered the electrodes into a fronto‐central ROI (F7, F3, Fz, F4, F8, C3, Cz, and C4) for band‐specific analyses of delta (1.5–3.5 Hz), low beta (13–20 Hz), and theta (4–7.5 Hz) (Kolev, Falkenstein, & Yordanova, 2005; Yordanova et al., 2004), and a parieto‐occipital (P7, P3, Pz, P4, P8, O1, Oz, and O2) ROI for band‐specific analyses of alpha (8–12.5 Hz). Likewise, we adjusted the time windows of interest for the respective frequencies of interest based on previous literature. We selected the time window of interest for band‐specific analysis of alpha as 750 ms preresponse (O'Connell et al., 2009) until response and from response to 850 ms postresponse (Mazaheri, Nieuwenhuis, van Dijk, & Jensen, 2009), for delta from response to 850 ms postresponse (Beste et al., 2010; Yordanova et al., 2004), for theta 250 ms preresponse to 500 ms postresponse (Luu, Tucker, & Makeig, 2004; Mazaheri et al., 2009; Trujillo & Allen, 2007), and for low beta from 500 ms preresponse (Grent‐'t‐Jong, Oostenveld, Jensen, Medendorp, & Praamstra, 2013) to 850 ms postresponse (Marco‐Pallarés, Camara, Münte, & Rodríguez‐Fornells, 2008; Mazaheri et al., 2009).
We further determined weighted ITPC in order to explore phase modulation by reaction time. For statistical analyses of ITPC and weighted ITPC, we focused on the theta band based on previous literature (Cohen, 2014; Groom et al., 2010).
2.6. Statistical analysis
To avoid potential biases, we carried out blind statistical analyses (regarding verum vs. sham stimulation). We conducted between‐group comparisons using ᵡ 2‐tests for categorical variables (gender and handedness) or t‐tests for continuous variables (age and questionnaire data).
2.6.1. Statistical analyses of behavioral data
We analyzed behavioral measures including reaction times (RTs) for all trials, for error trials as well as for correct trials, standard deviation of reaction times (SD‐RTs) for all trials, and error rates. For analysis, we considered only correct and erroneous responses to Go stimuli (cf., Ehlis et al., 2018). We calculated posterror slowing (PES) by generating the difference between RTs following errors and RTs following correct responses (see Supporting Information). We further calculated error rates for postcorrect and posterror trials (see Supporting Information). We contrasted behavioral measures between groups using independent samples t‐tests or Mann–Whitney‐U‐tests in case of a non‐normal distribution. For within‐group comparisons, we applied paired t‐test or Wilcoxon signed rank test in case of non‐normality. We analyzed all behavioral data using IBM SPSS Statistics 25 (Armonk, NY) and Microsoft Excel 2010. Effect sizes were given as Cramer's V (× 2 ‐tests), Cohen's d (t‐tests), and r (Mann–Whitney‐U‐tests, Wilcoxon tests), respectively.
2.6.2. Statistical analyses of TF data
With regard to analysis of TF data, we also addressed electrophysiological dynamics associated with slow responses as a potential mean to prevent errors (in comparison to correct trials as a result of error prevention). We used nonparametric cluster‐based permutation tests on TF power data (cf., http://www.fieldtriptoolbox.org/tutorial/cluster_permutation_timelock/) contrasting high‐ and low‐impulsive participants (independent t‐test) as well as sham vs. verum stimulation (paired t‐test) for every channel‐frequency‐time‐triplet in error, correct and slow response trials. Triplets with a test statistic larger than the critical value (α = .05) were clustered in connected sets on the basis of temporal, spatial, and spectral adjacency (clusters were defined by one or more neighboring electrodes). The sum of t‐values in every cluster was calculated and the significance probability using the Monte Carlo method as an approximation to the reference distribution for a permutation test (5.000 permutations) was determined. The resulting Monte Carlo significance (p‐value) for each cluster was compared to an overall two‐tailed critical α = .05 (.025 in each tail). To deal with the multidimensional significant clusters and depict the relevant dimensions (cf., van Ede & Maris, 2016), we averaged TFRs across 1.5–3.5 Hz (delta), 4–7.5 Hz (theta), 8–12.5 Hz (alpha), and 13–20 Hz (low beta) in order to convert the data into a spatiotemporal structure. Cluster‐based permutation tests provide a model‐free approach solving the multiple comparison problem across multiple EEG channels, multiple frequencies, and multiple time points (Oostenveld et al., 2011).
First, we conducted nonparametric cluster‐based permutation tests on TF theta ITPC contrasting high‐ and low‐impulsive participants (independent t‐test) as well as sham versus verum stimulation (dependent t‐test) for every channel‐frequency‐time‐triplet in error, correct, and slow response trials.
2.6.3. Relation between theta ITPC and behavior (RT and RT variability)
To investigate brain‐behavior relations, we calculated independent samples regression coefficient t‐statistics with subject averages of TF theta ITPC associated with errors, correct, and slow responses as dependent variable and mean RTs of the same conditions as independent variable, separately for both groups and stimulation conditions from 250 ms preresponse to 500 ms postresponse, using the independent samples regression t‐test implemented in FieldTrip. Additionally, the relation of theta ITPC with SD‐RTs was estimated by means of independent samples regression t‐tests separately for both groups and stimulation conditions.
2.6.4. Single‐trial phase‐behavior relations
Further, we analyzed weighted theta ITPC to investigate trial‐specific phase‐behavior relations (Cohen & Cavanagh, 2011). wITPC was computed as the ITPC (i.e., vector length) of phase angles across trials after weighting each vector length by RT. Therefore, values of weighted ITPC are not restricted to values between 0 and 1 because they scale with RT per trial (Cohen & Cavanagh, 2011). To create a null distribution, we randomly shuffled (5.000×) reaction time‐phase pairings across trials in each permutation before weighted ITPC was computed. Subsequently, we subtracted the average of the permuted wITPC values from the observed weighted ITPC values and divided by the standard deviation of the permuted wITPC values, creating a standard Z‐score for each subject (Cohen & Cavanagh, 2011). Besides group and stimulation comparisons, we also contrasted correct and error trials as well as correct and slow trials using cluster‐based permutation tests.
Because of the multiple cluster‐based permutation tests, we applied Bonferroni correction. We applied two‐sided testing throughout. Significance thresholds in cluster‐based permutation testing were cluster‐corrected.
3. RESULTS
3.1. Sample characteristics
We excluded six participants in the high‐impulsive group, and seven participants in the low‐impulsive group due to a remaining number of <10 error, slow or correct trials after EEG preprocessing. We excluded one participant in the high‐impulsive group due to circulatory problems and—due to a recruitment error—two participants from the low‐impulsive group yielding too high questionnaire scores in ASRS or WURS‐K, respectively. We excluded another low‐impulsive participant due to early termination of measurement. We further excluded one participant in the low‐impulsive group who only completed the first assessment and one participant due to low data quality. Overall, we included a sample size of n = 24 in the high‐impulsive and n = 21 in the low‐impulsive group for statistical analyses. The groups did not differ significantly with respect to age, distribution of gender, handedness, or IQ (Table 2). As expected, high‐impulsive subjects had significantly higher scores on the hyperactivity and inattention subscale of the German Adult ADHD Self‐Report Scale (ASRS; Kessler et al., 2005) as well as on the Barratt Impulsiveness Scale (Hartmann et al., 2011), the short version of the German Wender Utah Rating Scale for ADHD childhood symptoms (WURS‐K; Retz‐Junginger et al., 2002) and German ADHD self‐rating scale for symptoms in adulthood (ADHS‐SB; Rösler et al., 2008). Results on sub‐analyses of TF data were not substantially different from the main analyses reported below (cf., Supporting Information). To test the blinding success, all subjects were asked to indicate which stimulation they thought they had received. × 2‐comparisons demonstrated statistical significance (× 2 = 4.46; p = .035) meaning that subjects were able to identify the correct stimulation condition.
TABLE 2.
Sample characteristics
| Characteristic | High‐impulsive (n = 24) | Low‐impulsive (n = 21) | Test statistic; effect size (group comparison) |
|---|---|---|---|
| Age, year, mean ± SD |
25.88 ± 8.75 [range 20–58] |
25.95 ± 6.67 [range 20–50] |
Z = −0.73; p = .464; r = −.109 |
| IQ, mean ± SD a | 116.75 ± 12.96 | 114.05 ± 11.34 |
Z = − 0.82; p = .414; r = −.122 |
| Sex, female/male, no. | 18/6 | 15/6 |
ᵡ 2 = 0.07; p = .787; V = 0.040 |
| Handedness, right/left, no. | 23/1 | 19/2 |
ᵡ 2 = 0.54; p = .765; V = 0.109 |
| ASRS hyperactivity/impulsivity, mean ± SD b | 24.41 ± 5.61 | 12.10 ± 1.64 |
Z = − 5.40; p <.001; r = −.823 |
| WURS‐K, mean ± SD b | 39.05 ± 7.21 | 27.48 ± 4.85 |
t 36.92 = −6.20; p <.001; d = −1.604 |
| ADHS‐SB, mean ± SD b | 36.73 ± 5.30 | 20.90 ± 1.76 |
Z = −5.63; p <.001; r = −.858 |
| BSL, mean ± SD b | 32.14 ± 8.55 | 22.38 ± 2.99 |
Z = −4.10; p <.001; r = −.624 |
| BIS, mean ± SD b | 70.64 ± 5.75 | 61.14 ± 5.81 |
Z = −4.46; p <.001; r = −.681 |
| I7 impulsivity, mean ± SD | 10.79 ± 3.45 | 3.71 ± 2.00 |
Z = −5.58; p <.001; r = −.832 |
Abbreviations: ADHS‐SB, German ADHD self‐rating scale for symptoms in adulthood (Rösler et al., 2008); ASRS, German Adult ADHD Self‐Report Scale (Kessler et al., 2005); BIS, Barratt Impulsiveness Scale (Hartmann et al., 2011); BSL, Borderline Symptom List (Bohus et al., 2009); SD, standard deviation; WURS‐K, short version of the German Wender Utah Rating Scale for ADHD childhood symptoms (Retz‐Junginger et al., 2002).
IQ was assessed based on the Mehrfachwahl–Wortschatz Intelligenz test (Lehrl, 2005).
Information missing for two participants.
3.2. Flanker task performance
Due to a very low error rate (M = 3.16; SD = 4.58) in NoGo trials (i.e., false alarms) in both groups and both stimulation conditions, we focused on correct and erroneous responses in Go trials for the analysis of behavioral and electrophysiological data. For the same reason, we did not analyze posterror trials as only a low number of postcorrect trials occurred on time (M = 3.02; SD = 2.88) and slow responses should be considered only partially correct (Coles, Scheffers, & Holroyd, 2001). Table 3 summarizes results of statistical analyses of flanker task performance. Groups did not differ significantly with respect to mean RTs or standard deviations of reaction times. However, individuals in the high‐impulsive group exhibited an increased number of errors after Go stimuli as compared to low‐impulsive subjects. Dependent t‐tests did not reveal any stimulation effect on the behavioral level.
TABLE 3.
Flanker task performance
| Verum | Sham | Verum versus sham | ||||||
|---|---|---|---|---|---|---|---|---|
| Measure, mean ± SD | High‐impulsive (n = 24) | Low‐impulsive (n = 21) | Test statistic with effect size (group comparison) | High‐impulsive (n = 24) | Low‐impulsive (n = 21) | Test statistic with effect size (group comparison) | Test statistic with effect size | |
| Overall RT (ms) | 459.88 ± 97.96 | 463.74 ± 79.51 | Z = −0.41; p = .682; r = −.061 | 449.28 ± 96.19 | 435.92 ± 118.52 | Z = −0.50; p = .617; r = −.075 | Z = −1.25; p = .212; r = −.186 | |
| Correct‐trial RT (ms) | 345.67 ± 53.26 | 356.72 ± 48.95 | t 43 = −0.72; p = .475; d = 0.215 | 343.20 ± 57.55 | 342.48 ± 90.42 | Z = −0.98; p = .328; r = .146 | Z = −0.51; p = .608; r = −.076 | |
| Error‐trial RT (ms) | 473.17 ± 117.87 | 470.51 ± 111.96 | t 43 = −0.08; p = .939; d = −0.023 | 450.03 ± 106.65 | 434.23 ± 123.13 | t 43 = 0.46; p = .647; d = 0.137 | t 44 = 1.66; p = .104; d = 0.257 | |
| Overall SD (ms) | 112.30 ± 25.07 | 114.39 ± 27.98 | t 43 = −0.27; p = .793; d = −0.081 | 115.05 ± 33.60 | 107.33 ± 36.05 | t 43 = 0.74; p = .461; d = 0.221 | t 44 = 0.36; p = .724; d = 0.060 | |
| Overall errors (no.) | 38.96 ± 19.01 | 28.38 ± 13.99 | Z = −2.13; p = .033; r = −0.318 | 41.75 ± 24.23 | 28.86 ± 20.45 | Z = −1.99; p = .046; r = −0.297 | Z = −0.06; p = .955; r = −0.009 | |
Abbreviations: d, effect size Cohen's d; r, effect size Mann–Whitney‐U‐test, Wilcoxon test; RT, reaction time; SD, standard deviation; Z, Mann–Whitney‐U‐test (group comparison), Wilcoxon test (stimulation comparison).
3.3. TF power
Mean numbers of analyzable EEG segments after preprocessing are presented in Table 4.
TABLE 4.
Mean numbers of analyzable EEG segments after preprocessing
| Verum | Sham | |||
|---|---|---|---|---|
| Measure, mean ± SD (range) | High‐impulsive (n = 24) | Low‐impulsive (n = 21) | High‐impulsive (n = 24) | Low‐impulsive (n = 21) |
| Correct | 44.63 ± 32.82 (10–137) | 50.86 ± 19.77 (19–93) | 44.04 ± 25.96 (13–97) | 59.71 ± 27.19 (13–107) |
| Error | 32.63 ± 13.80 (10–71) | 26.05 ± 12.91 (10–56) | 38.25 ± 34.53 (12–119) | 26.67 ± 18.90 (10–90) |
| Slow | 77.33 ± 29.83 (30–140) | 97.62 ± 27.62 (41–146) | 81.38 ± 34.53 (23–146) | 90.10 ± 23.52 (51–137) |
For between‐group comparisons, we contrasted TF power in the delta, theta, alpha, and low beta range in high‐ versus low‐impulsive subjects in error, correct and slow trials after verum or sham stimulation (adjusted α = .05/24 = .002 for 24 comparisons). Within the a‐priori selected time windows and regions of interest, cluster‐based permutation tests revealed a frequency‐band specific difference in low beta between high‐ and low‐impulsive participants after sham stimulation in erroneous trials in the latency range from 500 ms preresponse to 850 ms postresponse (p <.001), indicating lower frontal beta power in high‐impulsive subjects (Figure 2). Within the a‐priori selected time windows and regions of interest, cluster‐based permutation tests revealed no significant differences between groups in delta (fronto‐central; 0–850 ms), theta (fronto‐central; −250 to 500 ms), or alpha band power (parieto‐occipital; −750–850 ms). After sham stimulation, cluster‐based permutation tests exhibited lower frontal low beta in the high‐ versus low‐impulsive group around correct (p = .005) and slow (p = .011) responses. Cluster‐based permutation tests identified lower frontal beta power preceding erroneous (p = .005) and slow responses (p = .008) on Go trials after verum stimulation in high‐impulsive subjects. However, none of these clusters survived Bonferroni correction for multiple comparisons.
FIGURE 2.
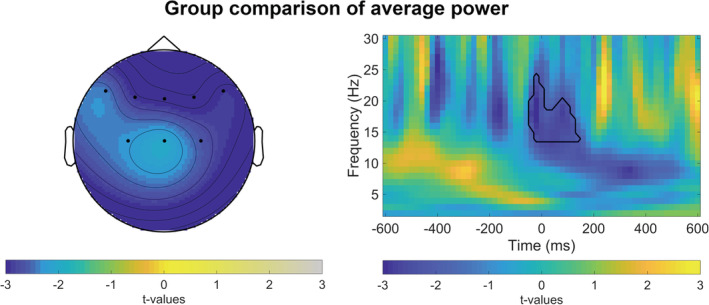
Time‐frequency representations of group comparisons time‐locked to erroneous stimulus reaction in Go trials after sham iTBS. Left side: t‐values for low beta band power (13–20 Hz) at frontal‐central electrodes averaged between 60 ms preresponse and 120 ms postresponse. Right side: t‐values for full time‐frequency (1–30 Hz) averaged over frontal‐central electrodes. Black lines highlight clusters in the data smaller than the prespecified threshold (α <.025) and smaller than Bonferroni‐adjusted α = .05/24 = .002)
For analysis of stimulation effects, we contrasted TF power in the delta, theta, alpha, and low beta range after verum versus sham stimulation in high‐ and low‐impulsive subjects in error, correct, and slow trials, respectively (adjusted α = .05/24 = .002 for 24 comparisons). Nonparametric cluster‐based permutation tests revealed no significant effect of stimulation on TF power.
3.4. TF intertrial phase coherence
Significance level for group comparisons of theta ITPC was set at α = .05, with number of repeated measures = 12 (verum vs. sham, high‐ vs. low‐impulsive, and correct vs. error vs. slow), which meant an adjusted α = .05/12 = .004. Nonparametric cluster‐based permutation tests revealed no group differences in frontal preresponse theta ITPC after Bonferroni correction. For analyses of stimulation effects we contrasted intertrial phase coherence in the theta range in high‐ versus low‐impulsive subjects in error, correct, and slow trials after verum or sham stimulation (adjusted α = .05/12 = .004 for 12 comparisons). Cluster‐based permutation tests did not reveal any effect of TMS on theta ITPC.
To investigate brain‐behavior relations, we regressed theta ITPC on reaction times in high‐ and low‐impulsive subjects in error, correct, and slow trials after verum or sham stimulation (with an adjusted α = .05/12 = .004 for 12 comparisons). Lower theta ITPC preceding stimulus response was related to higher flanker task reaction times in correct trials (p <.004; adjusted α = .05/12) and in slow responses (p <.004; adjusted α = .05/12) in the low‐impulsive group after sham stimulation as well as in slow responses after verum stimulation in the high‐impulsive group (Figure 3). None of the other RT‐theta ITPC relations for correct and slow responses was significant after this conservative Bonferroni correction.
FIGURE 3.
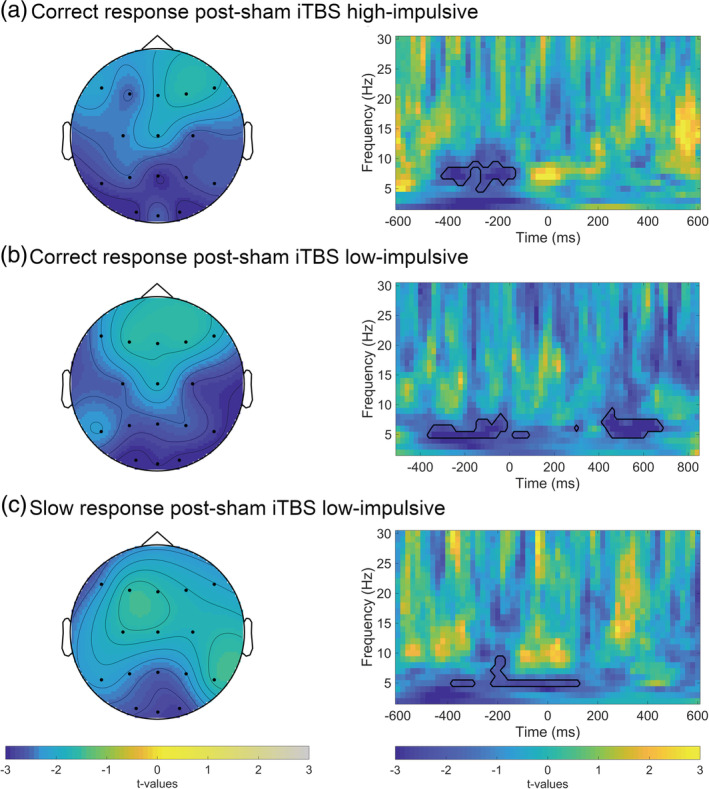
Response‐locked time‐frequency averages of brain‐behavior relations between theta (4–7.5 Hz) ITPC and flanker task reaction times in Go trials. The panel on the right depicts t‐values for full time‐frequency (1–30 Hz) averaged over parieto‐occipital electrodes. Black lines highlight clusters in the data smaller than the prespecified threshold (α <.025); t = 0 corresponds to the response. (a) Slow responses to Go stimuli after verum stimulation in the high‐impulsive group. Left side: t‐values for theta ITPC‐RT relations averaged between 480 and 160 ms preresponse. (b) Correct responses after sham stimulation in the low‐impulsive group. Left side: t‐values for theta ITPC‐RT relations averaged between 500 ms pre‐ and 280 ms postresponse. (c) Slow responses to Go stimuli after sham stimulation in the low‐impulsive group. Left side: t‐values theta ITPC‐RT relations averaged between 500 ms pre‐ and 140 ms postresponse
Furthermore, we regressed theta ITPC on reaction time variability in high‐ and low‐impulsive subjects over all trials after verum or sham stimulation (adjusted α = .05/4 = .013 for four comparisons). Cluster‐based regression analyses revealed negative relations between SD‐RT and theta ITPC in low‐impulsive participants after both sham and verum stimulation as well as in high‐impulsive participants after sham stimulation (p <.013; Figure 4).
FIGURE 4.
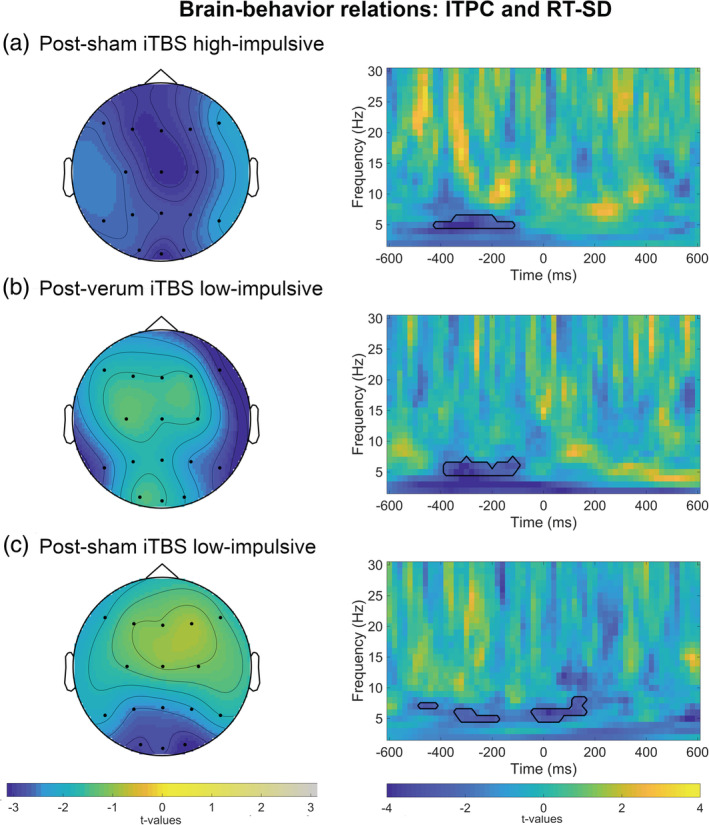
Response‐locked time‐frequency averages of brain‐behavior relations between theta (4–7.5 Hz) ITPC and flanker task reaction time variability (RT‐SD) in Go trials. The panel on the right depicts t‐values for full time‐frequency (1–30 Hz) averaged over parieto‐occipital electrodes. Black lines highlight clusters in the data smaller than the prespecified threshold (α <.025). t = 0 corresponds to the response. (a) Left side: t‐values for theta ITPC‐RT‐SD relations after sham stimulation in the high‐impulsive group averaged between 500 ms pre‐ and 380 ms postresponse. (b) Left side: t‐values for theta ITPC‐RT‐SD relations after verum stimulation in the low‐impulsive group averaged between 440 and 80 ms preresponse. (c) Left side: t‐values for theta ITPC‐RT‐SD relations after sham stimulation in the low‐impulsive group averaged between 500 ms pre‐ and 200 ms postresponse
3.5. Single‐trial phase reaction time coupling
Testing reaction time phase modulation in the theta range within the a‐priori selected time window, cluster‐based permutation tests identified no differences between groups or stimulation conditions (Bonferroni adjusted significance level α = .05/20 = .003). However, cluster‐based permutation tests revealed a significantly higher reaction time phase modulation in correct versus error trials as well as in correct versus slow trials in high‐impulsive individuals after both sham and verum stimulation (all p <.003). Likewise, cluster‐based permutation tests revealed a significantly higher reaction time phase modulation in correct versus error trials as well as in correct versus slow trials in low‐impulsive individuals after both sham and verum stimulation (all p <.003). As shown in Figures 5 and 6, the difference in theta reaction time phase modulation was largest among parieto‐occipital and frontal electrode sites.
FIGURE 5.
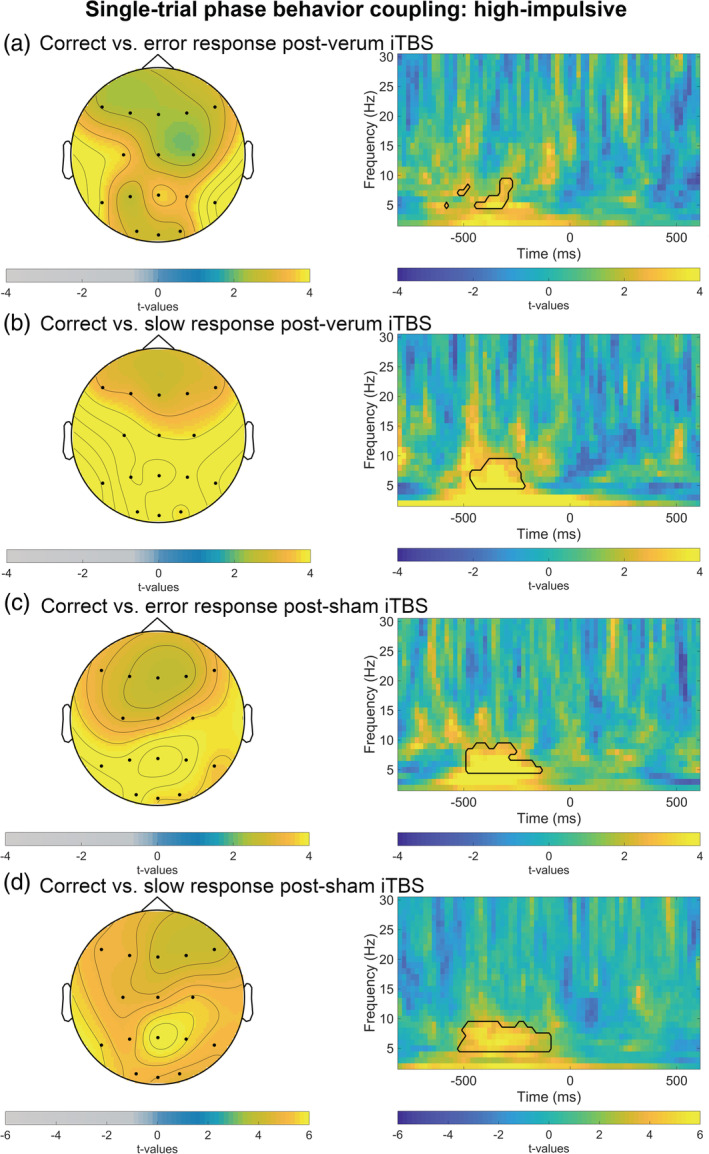
Averages of trial‐to‐trial brain‐behavior relations in high‐impulsive subjects: theta (4–7.5 Hz) ITPC weighted by flanker task reaction time (RT) in Go trials. The panel on the right depicts t‐values for full time‐frequency (1–30 Hz) from (c) parieto‐occipital and (a,b,d) frontal and parieto‐occipital electrodes. Black lines highlight clusters in the data smaller than the prespecified threshold (α <.025). t = 0 corresponds to the response. For visual clearness scales are optimized for each condition. (a) Left side: t‐values for theta wITPC in correct versus error trials after verum stimulation averaged between 500 and 80 ms preresponse. (b) Left side: t‐values for theta wITPC correct versus slow trials after verum stimulation averaged between 500 and 20 ms preresponse. (c) Left side: t‐values for theta wITPC in correct versus error trials after sham stimulation averaged between 500 and 0 ms preresponse. (d) Left side: t‐values for theta wITPC correct versus slow trials after sham stimulation averaged between 500 and 20 ms preresponse
FIGURE 6.
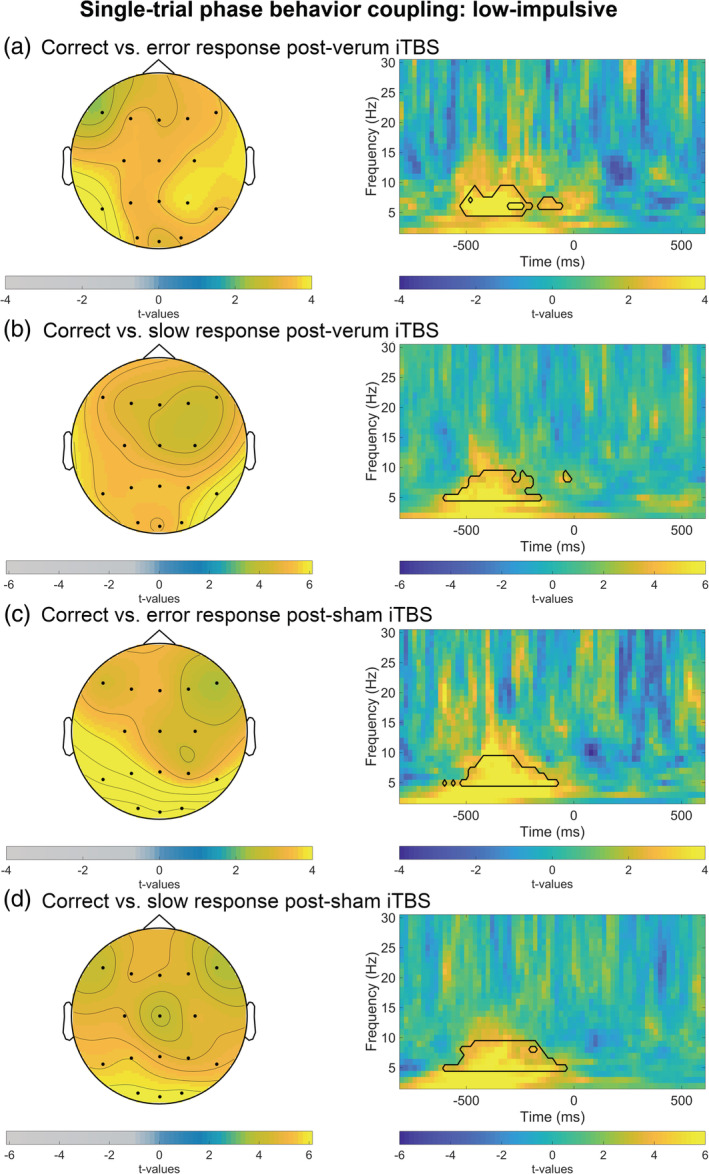
Averages of trial‐to‐trial brain‐behavior relations in low‐impulsive subjects: theta (4–7.5 Hz) ITPC weighted by flanker task reaction time (RT) in Go trials. The panel on the right depicts t‐values for full time‐frequency (1–30 Hz) from (a,c,d) parieto‐occipital and (b) frontal and parieto‐occipital electrodes. Black lines highlight clusters in the data smaller than the prespecified threshold (α <.025). t = 0 corresponds to the response. For visual clearness scales are optimized for each condition. (a) Left side: t‐values for theta wITPC in correct versus error trials after verum stimulation averaged between 500 and 60 ms preresponse. (b) Left side: t‐values for theta wITPC correct versus slow trials after verum stimulation averaged between 500 and 20 ms preresponse. (c) Left side: t‐values for theta wITPC in correct versus error trials after sham stimulation averaged between 500 and 20 ms preresponse. (d) Left side: t‐values for theta wITPC correct versus slow trials after sham stimulation averaged between 500 ms pre‐ and 40 ms postresponse
4. DISCUSSION
Here, we investigated response‐locked neurophysiological mechanisms underlying cognitive control in high‐ and low‐impulsive participants. We further scrutinized possible effects of facilitating iTBS applied over the right dorsolateral prefrontal cortex on these neurophysiological mechanisms.
Performance monitoring during flanker task execution was associated with prevailing activation patterns reflecting cognitive control over parieto‐occipital scalp sites (theta ITPC, theta wITPC), as well as action planning/execution over frontal electrode sites (low beta power). Analysis of the EEG TF spectrum revealed no significant effects of stimulation on any of the expected TF parameters. As the number of analyzable EEG segments was relatively low particularly for error trials, the interpretation and reliability of these results is limited. Thus, they should be considered as preliminary.
Cluster‐based permutation tests determined a frequency‐band specific difference in low beta power between the high‐ and low‐impulsive group, which became significant over frontal scalp sites only. When looking at the TF grand averages of the respective group, lower peri‐response beta power emerges in the high‐ as well as in the low‐impulsive group. However, in low‐impulsive subjects the power decrease around error responses is less pronounced (Figure S1). The timing around response (i.e., key press) as well as the decrease of power speak in favor of (movement‐related) beta desynchronization during preparation for a movement (e.g., Neuper, Wörtz, & Pfurtscheller, 2006). The topography does not exhibit any lateralization which is most probably due to the fact that reactions occurred with alternating hands. Conversely, it is fairly surprising that this group difference becomes only apparent in error trials and that it is most pronounced over frontal sites rather than over sensorimotor areas. Beta activity is an epiphenomenon in motor‐related tasks, yet, emerging during various motor and cognitive functions such as perceptual decisions (Donner et al., 2007), response preparation, and inhibition (Zhang, Chen, Bressler, & Ding, 2008), as well as motor planning and execution (Zaepffel, Trachel, Kilavik, & Brochier, 2013). Still, the precise cognitive role of beta rhythms is not entirely clear (Engel & Fries, 2010). In accordance with previous literature, we assume that the observed decrease in beta power over frontal sites around error responses reflects a state change in the motor system from rest to a state related to action execution (e.g., Rhodes, Gaetz, Marsden, & Hall, 2018). Our findings are to some extent in line with the results from Tzagarakis, Thompson, Rogers, and Pellizzer (2019) who found that the decrease of relative beta band power during the late stage of action planning over (left) fronto‐central scalp sites was more pronounced in a high‐impulsive group than in a low‐impulsive group. Likewise, trait impulsivity has been found to positively correlate with motor system excitability which has been hypothesized to be associated with less effective preparatory inhibition in trait impulsivity (Rossi et al., 2018). Accordingly, the more pronounced peri‐response beta band specific decrease could indicate less effective inhibition modulation processes and a reduced motor system efficiency (Rossi et al., 2018). High‐impulsive individuals might thus have more difficulty in adapting their action planning and execution commensurate with changing environmental requirements, for example, changes in motor plans (Tzagarakis et al., 2019). Efficiency in the motor system in high‐impulsive individuals might be impaired in a sense that stronger motor cortex beta suppression must occur in order to compensate for insufficient control (Heinrichs‐Graham & Wilson, 2016; Rossi et al., 2018).
Presumably, we only observed this group difference around erroneous responses because a lack of motor cortex beta suppression results in erroneous decisions. Our preliminary results indicate neural mechanisms through which impulsivity affects action planning and/or execution associated with errors. However, this hypothesis should be investigated in future studies of the role of beta‐band limited activity in impulsivity.
ITPC can be interpreted as a phenomenon underlying rather general cognitive functions time‐locked to the response. This trial‐to‐trial consistency of the theta ITPC is therefore an index of the variability in timing of the neural population level activity associated with the stimulus reaction (Cohen, 2014; p. 268 f.). In our study, we did not find any stimulation effect or group differences in theta ITPC. However, when relating ITPC to behavioral variables (i.e., mean RT and reaction time variability; RT‐SD), we could determine lower preresponse theta ITPC to be associated with higher RT and RT‐SD (cf., Cohen & Cavanagh, 2011; Papenberg et al., 2013) in correct trials and slow but correct trials in low‐impulsive participants. Likewise, analyses revealed lower preresponse theta ITPC to be associated with higher RT after verum stimulation in slow responses and higher RT‐SD in high‐impulsive participants after sham stimulation. This numerically negative relation suggests higher RT/RT‐SD with less pronounced theta ITPC only in trials without errors, that is, correct as well as slow trials.
The second numerically negative relation indicates that less pronounced theta ITPC is associated with impaired behavior adaption (RT‐SD). Lower RT‐SD has been hypothesized to be related to more effective goal‐directed control (e.g., Bellgrove, Hester, & Garavan, 2004) with higher RT‐SD being associated with higher processing fluctuations. We did not detect any relations of theta ITPC and reaction time in error trials, indicating that it could reflect a crucial process for response accuracy (i.e., attending at just the right time to prepare the response). Most current studies report relations of RT to theta power rather than ITPC (for some examples see, e.g., Cohen & Cavanagh, 2011; Papenberg et al., 2013; Reteig, van den Brink, Prinssen, Cohen, & Slagter, 2019). Since ITPC is mostly independent of concurrent changes in power dynamics (Cohen, 2014; p. 251), conclusions can hardly be drawn from previous research on TF theta power. In line with very recent research on theta ITPC and its link to sustained attention, we suggest to interpret theta ITPC as a measure of attentional stability (Reteig et al., 2019). In their study, the authors also report theta ITPC over bilateral parieto‐occipital sites and assume it to constitute a strong correlate of later‐stage attentional or perceptual processes (Reteig et al., 2019). Based on this recent research, in our study, we link the ability to sustain attention (as quantified by RT‐SD) to the temporal consistency of neural responses across trials (as quantified by theta ITPC). If theta ITPC reflects sustained attention, our finding of a decrease in theta‐band cortical phase‐locking over parietal scalp sites associated with slower RT and higher RT‐SD could indicate mind wandering (Baird, Smallwood, Lutz, & Schooler, 2014).
Recent evidence suggests that theta oscillations in frontal and parietal regions are involved in proactive and reactive cognitive control (Cooper, Wong, McKewen, Michie, & Karayanidis, 2017). Furthermore, the strength of theta oscillations during proactive cognitive control was reported to be associated with efficiency of cognitive control (as indicated by higher RT‐SD; Cooper et al., 2017). Thus, the lack of theta ITPC relations to RT or RT‐SD in erroneous trials might indicate lower cognitive control or lower cognitive control efficiency resulting in worse performance. The brain‐behavior relations in correct and slow trials, however, indicate engaged cognitive control resulting in more efficient behavior. Lower theta ITPC associated with higher behavioral variability reflects less efficient cognitive control whereas lower theta ITPC associated with longer response latencies reflects response conflict between competing motor activations.
Our preliminary results further suggest altered cognitive control in high‐impulsive individuals as indicated by higher neuronal variability during cognitive control. The less pronounced theta ITPC relations to RT (only after verum stimulation) or RT‐SD (after sham stimulation) in high‐impulsive individuals might indicate lower cognitive control efficiency. This is in line with significantly larger theta ITPC in healthy controls compared to individuals with ADHD (Gonen‐Yaacovi et al., 2016). However, group comparisons between high‐ and low‐impulsive participants did not reveal significant differences between groups.
ITPC allows propositions on overall (i.e., rather general) response‐related phase consistencies whereas phase modulation (i.e., wITPC) is process‐specific and phase angles only need to be related to behavioral variables (Cohen & Cavanagh, 2011). The modulation of phase synchronization by RT analyzed in our study thus provides a different kind of information regarding neurocognitive processing and facilitates a more precise interpretation of results regarding specific cognitive processes. Hence, ITPC indicates whether the timing of band‐specific activity is related to the response whereas wITPC is specific to the trial‐wise behavior of interest (here: trial‐to‐trial RT) (Cohen & Cavanagh, 2011). Here, positive normalized theta wITPC values identify a theta phase modulation of RT, that is, the probability of specific RTs occurring at certain phase angles of the recorded oscillation are above chance. We found differences in wITPC preceding the response in correct versus error trials and in correct versus slow trials. Looking at the grand averages of wITPC (Figures S2 and S3), positive normalized theta wITPC can be shown in correct trials, however, not in error or slow trials. This indicates a dependence of reaction times on preresponse theta phase in correct trials. This finding reflects dynamics that are not phase‐locked to the response onset (as for ITPC), instead illustrating that the specific phases of neural oscillations predict behavior (Cohen & Donner, 2013).
Why did we not find any effect of TBS on EEG oscillation despite extensive explorations, partially motived by previous evidence? One limitation arises from the fact that we only analyzed the whole EEG recording after TBS. We did not analyze EEG power at different time points after stimulation (e.g., Schutter et al., 2001) which could have shown possible temporary effects. However, separating the whole session into subparts would have resulted in lower statistical power for TF analyses which would have particularly affected errors which occurred relatively rarely as compared to correct and slow reactions. Another important point to raise in the context of absent stimulation effects is a rather long time span between stimulation and EEG measurements, at least in some participants. Hence, at least for some participants, it is possible that the modulatory effect of stimulation has vanished. This could have had an impact on the absence of stimulation effects also at the group level. Yet, sub‐analyses only including subjects with a relatively low time span between stimulation and EEG recording did not reveal any stimulation effect either. Therefore, it is improbable that the time span between stimulation and EEG recording explains the lack of stimulation effects. Moreover, tDCS intensity was relatively low. However, with higher stimulation intensity participants were frequently able to correctly guess the stimulation conditions during the pilot phase of this study, which remained a problem even with this relatively low stimulation intensity. However, the tDCS protocol was implemented as a preceding stimulation to bring the cortical tissue into an optimal functional state with regard to the later rTMS effect. Since tDCS was not a stand‐alone intervention, it could at most have led to a lack of the supportive effect of tDCS on iTBS. And finally, cognitive control related to conflictual and erroneous responses can be subdivided into two sub‐processes: a monitoring component—with neuroanatomical correlates located predominantly in the medial PFC/ACC—and an “executive” component responsible for actual adjustments in cognitive control measures, which is strongly related to the lateral PFC, particularly the DLPFC (Ridderinkhof et al., 2004). With our stimulation procedure, we should have primarily reached the lateral PFC, that is, the executive component of cognitive control. It may well be, however, that the neurophysiological signatures captured here were (also) related to monitoring processes which would not have been affected by our tDCS/TBS intervention, which would in turn explain the presence of group differences in the absence of stimulation effects. A very recent study found site‐ and timing‐specific effects of online rTMS on cognitive performance (Beynel et al., 2020). Depending on the timing of stimulation and the associated brain‐state, rTMS could rather induce random noise by modulating endogenous task‐related oscillatory dynamics.
Considering findings on improved cognitive control after stimulation of the left dlPFC (Y. Li et al., 2017), for completeness, we could have compared stimulation between left and right dlPFC in a crossover design. However, from the evidence presented in the literature it is not entirely clear whether right hemisphere stimulation or left hemisphere stimulation results in stronger effects. Thus, to ensure a clearly arranged design, we made an informed decision regarding stimulation site.
Moreover, we focused on cognitive control processes with an emphasis on performance monitoring. TBS could conceivably have an impact on posterror adjustment rather than performance monitoring (Fusco et al., 2018) or on inhibition processes (i.e., NoGo trials).
Further, the partly exploratory nature of our analyses reduced statistical sensitivity for discovering subtle stimulation effects as compared to a purely hypothesis‐driven approach. Finally, the effect of iTBS can vary strongly between individuals (Tse et al., 2018).
A methodological limitation of our TF analyses is that ITPCs only stabilize with around 20 trials and are sensitive to the number of trials (Cohen, 2014; p. 246). However, in our study especially errors occurred relatively rarely. Adapting the number of trials accordingly (by adjusting the number of correct trials to the number of errors or by excluding subjects with less than 20 errors) would have led to a tremendous loss of statistical power. With that being said, our results should be interpreted carefully. Moreover, neurophysiological methods usually have to deal with a tradeoff between spatial and temporal resolution. TMS offers intermediate spatial and temporal resolution whereas EEG offers low spatial but high temporal resolution. Experiments using functional magnetic resonance imaging (fMRI)—an imaging method with a high spatial resolution—have shown that cognitive action control in the right dlPFC is characterized by structural and functional variability (Cieslik et al., 2013). However, using EEG, an accurate and reliable mapping without individual MRI data or source localization algorithms is not possible due to low spatial resolution and anatomical variability. With iTBS, on the other hand, one cannot predetermine the precise pattern of neuronal firing induced by the stimulation. Based on this, separation of the dlPFC into subparts would most probably not be reliable. So, while we have less precise topographical information here, we have precise information on the timescale of action monitoring processes.
5. CONCLUSIONS
We investigated response‐locked neurophysiological mechanisms underlying cognitive control in high‐ and low‐impulsive participants during performance monitoring. We further investigated effects of facilitating iTBS over the right dlPFC on these neurophysiological mechanisms. Performance monitoring during flanker task execution was associated with TF patterns reflecting attentional mechanisms (parieto‐occipital theta ITPC, theta wITPC) as well as differential action planning/execution processes linked to trait impulsivity (frontal low beta power). These preliminary findings significantly add to the understanding of peri‐response neurophysiological mechanisms of cognitive control, such as a lower efficiency of inhibitory processes in high‐impulsive individuals (frontal low beta power) as well as attentional stability associated with response accuracy (theta ITPC‐RT, theta ITPC‐RT‐SD relation in correct trials). We did not find any significant effect of stimulation on behavior or any of the expected TF parameters suggesting no modulation of performance monitoring via iTBS. Future studies could focus on the effects of rTMS on oscillatory activity related to stimulus processing rather than response‐related processes in the flanker task or on inhibitory control processes (i.e., NoGo trials).
CONFLICT OF INTEREST
Tim Rohe, Saskia Deppermann, Andreas Jochen Fallgatter, and Ann‐Christine Ehlis declare no commercial or financial relationships that could be construed as a potential conflict of interest. Beatrix Barth was paid for public speaking by the neuroCare Group. Where applicable, the authors declare that the present work is unrelated to the above‐mentioned relationships.
AUTHOR CONTRIBUTIONS
Beatrix Barth conceived the analysis design, performed the analysis, and drafted the manuscript. Ann‐Christine Ehlis conceived of the study design, participated in its coordination and contributed to the interpretation of the data as well as the drafting of the manuscript. Tim Rohe was involved in data analysis, contributed to the manuscript, and revised it critically for important intellectual content. Saskia Deppermann acquired data, participated in the design of the study, and contributed to the manuscript. Andreas Jochen Fallgatter contributed to the design and acquisition of the work and revised it critically for important intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Supporting information
Table S1 Flanker task performance
Figure S1. Time‐frequency grand averages of TF beta power
Figure S2. Time‐frequency grand averages of weighted intertrial phase clustering in high‐impulsive individuals
Figure S3. Time‐frequency grand averages of weighted intertrial phase clustering in low‐impulsive individuals
Figure S4. Sub‐analysis: time‐frequency grand averages of time‐frequency averages of brain‐behavior relations between theta ITPC and flanker task reaction times in Go trials
Figure S5. Sub‐analysis: time‐frequency grand averages of time‐frequency averages of brain‐behavior relations between theta ITPC and flanker task reaction time variability in Go trials
Figure S6. Sub‐analysis: time‐frequency grand averages of trial‐to‐trial brain‐behavior relations in high‐impulsive subjects: theta ITPC weighted by flanker task reaction time (RT) in Go trials
Figure S7. Sub‐analysis: time‐frequency grand averages of trial‐to‐trial brain‐behavior relations in low‐impulsive subjects: theta ITPC weighted by flanker task reaction time (RT) in Go trials
ACKNOWLEDGMENTS
This research was supported by IZKF Tübingen (Junior Research Group, grant 2115‐0‐0). TR is funded by Deutsche Forschungsgemeinschaft (DFG; grant number RO 5587/1‐1). The authors thank Martina Horakh, Ann‐Cathrin Valentin, Betti Schopp, and Ramona Täglich for their excellent work and their valuable support with the measurements. Open Access funding enabled and organized by Projekt DEAL.
Barth B, Rohe T, Deppermann S, Fallgatter AJ, Ehlis A‐C. Neural oscillatory responses to performance monitoring differ between high‐ and low‐impulsive individuals, but are unaffected by TMS . Hum Brain Mapp. 2021;42:2416–2433. 10.1002/hbm.25376
Funding information Deutsche Forschungsgemeinschaft, Grant/Award Number: RO 5587/1‐1; IZKF Tübingen, Grant/Award Number: 2115‐0‐0
Endnote
It may also be expected that some of our participants fulfill an ADHD diagnosis but as we only screened participants without in‐depth diagnostics, we cannot be entirely sure.
DATA AVAILABILITY STATEMENT
Data are available upon request.
REFERENCES
- Alegre, M. , Gurtubay, I. G. , Labarga, A. , Iriarte, J. , Valencia, M. , & Artieda, J. (2004). Frontal and central oscillatory changes related to different aspects of the motor process: A study in go/no‐go paradigms. Experimental Brain Research, 159(1), 14–22. 10.1007/s00221-004-1928-8 [DOI] [PubMed] [Google Scholar]
- Baird, B. , Smallwood, J. , Lutz, A. , & Schooler, J. W. (2014). The decoupled mind: Mind‐wandering disrupts cortical phase‐locking to perceptual events. Journal of Cognitive Neuroscience, 26(11), 2596–2607. 10.1162/jocn_a_00656 [DOI] [PubMed] [Google Scholar]
- Barratt, E. S. (1985). Impulsiveness defined within a systems model of personality. In Speilburger E. P. & Butcher J. N. (Eds.), Advances in personality assessment (pp. 113–132). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (2009). Beck‐Depressions‐Inventar (BDI‐II, dt. Version). In M. Hautzinger, F. Keller, & C. Kühner) (Vol. 2). Frankfurt: Pearson Assessment.
- Bellgrove, A. M. , Hester, R. , & Garavan, H. (2004). The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia, 42, 1910–1916. 10.1016/j.neuropsychologia.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Beste, C. , Domschke, K. , Kolev, V. , Yordanova, J. , Baffa, A. , Falkenstein, M. , & Konrad, C. (2010). Functional 5‐HT1a receptor polymorphism selectively modulates error‐specific subprocesses of performance monitoring. Human Brain Mapping, 31(4), 621–630. 10.1002/hbm.20892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynel, L. , Davis, S. W. , Crowell, C. A. , Dannhauer, M. , Lim, W. , Palmer, H. , … Appelbaum, L. G. (2020). Site‐specific effects of online rTMS during a working memory task in healthy older adults. Brain Sciences, 10(5), 255. 10.3390/brainsci10050255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman, J. , Fitzgerald, M. , Kirova, A. M. , Woodworth, K. Y. , Biederman, I. , & Faraone, S. V. (2018). Further evidence of morbidity and dysfunction associated with subsyndromal ADHD in clinically referred children. The Journal of Clinical Psychiatry, 79(5). 10.4088/JCP.17m11870 [DOI] [PubMed] [Google Scholar]
- Bohus, M. , Kleindienst, N. , Limberger, M. F. , Stieglitz, R. D. , Domsalla, M. , Chapman, A. L. , … Wolf, M. (2009). The short version of the Borderline Symptom List (BSL‐23): Development and initial data on psychometric properties. Psychopathology, 42, 32–39. 10.1159/000173701 [DOI] [PubMed] [Google Scholar]
- Brevet‐Aeby, C. , Brunelin, J. , Iceta, S. , Padovan, C. , & Poulet, E. (2016). Prefrontal cortex and impulsivity: Interest of noninvasive brain stimulation. Neuroscience & Biobehavioral Reviews, 71, 112–134. 10.1016/j.neubiorev.2016.08.028 [DOI] [PubMed] [Google Scholar]
- Camprodon, J. A. (2016). Transcranial magnetic stimulation. In Camprodon J., Rauch S., Greenberg B., & Dougherty D. (Eds.), Psychiatric Neurotherapeutics. Current clinical psychiatry. New York: Humana Press. [Google Scholar]
- Cieslik, E. C. , Zilles, K. , Caspers, S. , Roski, C. , Kellermann, T. S. , Jakobs, O. , … Eickhoff, S. B. (2013). Is there "one" DLPFC in cognitive action control? Evidence for heterogeneity from co‐activation‐based parcellation. Cerebral Cortex (New York, N.Y.: 1991), 23(11), 2677–2689. 10.1093/cercor/bhs256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M. X. (2014). Analyzing neural time series data: Theory and practice. Cambridge, MA: MIT Press. [Google Scholar]
- Cohen, M. X. , & Cavanagh, J. F. (2011). Single‐trial regression elucidates the role of prefrontal theta oscillations in response conflict. Frontiers in Psychology, 2(30). 10.3389/fpsyg.2011.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M. X. , & Donner, T. H. (2013). Midfrontal conflict‐related theta‐band power reflects neural oscillations that predict behavior. Journal of Neurophysiology, 110, 2752–2763. 10.1152/jn.00479.2013 [DOI] [PubMed] [Google Scholar]
- Coles, M. G. H. , Scheffers, M. K. , & Holroyd, C. B. (2001). Why is there an ERN/ne on correct trials? Response representations, stimulus‐related components, and the theory of error‐processing. Biological Psychology, 56(3), 173–189. 10.1016/S0301-0511(01)00076-X [DOI] [PubMed] [Google Scholar]
- Cooper, P. , Wong, A. , McKewen, M. , Michie, P. , & Karayanidis, F. (2017). Frontoparietal theta oscillations during proactive control are associated with goal‐updating and reduced behavioral variability. Biological Psychology, 129, 253–264. 10.1016/j.biopsycho.2017.09.008 [DOI] [PubMed] [Google Scholar]
- Dalley, J. W. , Everitt, B. J. , & Robbins, T. W. (2011). Impulsivity, compulsivity, and top‐down cognitive control. Neuron, 69(4), 680–694. 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner, T. H. , Siegel, M. , Oostenveld, R. , Fries, P. , Bauer, M. , & Engel, A. K. (2007). Population activity in the human dorsal pathway predicts the accuracy of visual motion detection. Journal of Neurophysiology, 98(1), 345–359. 10.1152/jn.01141.2006 [DOI] [PubMed] [Google Scholar]
- Ehlis, A. C. , Bauernschmitt, K. , Dresler, T. , Hahn, T. , Herrmann, M. J. , Röser, C. , … Renner, T. J. (2011). Influence of a genetic variant of the neuronal growth associated protein Stathmin 1 on cognitive and affective control processes: An event‐related potential study. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 156B(3), 291–302. 10.1002/ajmg.b.31161 [DOI] [PubMed] [Google Scholar]
- Ehlis, A. C. , Deppermann, S. , & Fallgatter, A. J. (2018). Performance monitoring and post‐error adjustments in adults with attention‐deficit/hyperactivity disorder: An EEG analysis. Journal of Psychiatry and Neuroscience, 43(5), 396–406. 10.1503/jpn.170118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, A. K. , & Fries, P. (2010). Beta‐band oscillations—Signalling the status quo? Current Opinion in Neurobiology, 20(2), 156–165. 10.1016/j.conb.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Figee, M. , Luigjes, J. , Smolders, R. , Valencia‐Alfonso, C.‐E. , van Wingen, G. , de Kwaasteniet, B. , … Denys, D. (2013). Deep brain stimulation restores frontostriatal network activity in obsessive‐compulsive disorder. Nature Neuroscience, 16, 386–387. 10.1038/nn.3344 [DOI] [PubMed] [Google Scholar]
- Franken, I. H. A. , Luijten, M. , van der Veen, F. M. , & van Strien, J. W. (2017). Cognitive control in young heavy drinkers: An ERP study. Drug and Alcohol Dependence, 175, 77–83. 10.1016/j.drugalcdep.2017.01.036 [DOI] [PubMed] [Google Scholar]
- Fusco, G. , Scandola, M. , Feurra, M. , Pavone, E. F. , Rossi, S. , & Aglioti, S. M. (2018). Midfrontal theta transcranial alternating current stimulation modulates behavioural adjustment after error execution. European Journal of Neuroscience, 48(10), 3159–3170. 10.1111/ejn.14174 [DOI] [PubMed] [Google Scholar]
- Gonen‐Yaacovi, G. , Arazi, A. , Shahar, N. , Karmon‐Presser, A. , Haar, S. , Meiran, N. , & Dinstein, I. (2016). Increased ongoing neural variability in ADHD. Cortex, 81, 63–81. 10.1016/j.cortex.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Grent‐'t‐Jong, T. , Oostenveld, R. , Jensen, O. , Medendorp, W. P. , & Praamstra, P. (2013). Oscillatory dynamics of response competition in human sensorimotor cortex. NeuroImage, 83, 27–34. 10.1016/j.neuroimage.2013.06.051 [DOI] [PubMed] [Google Scholar]
- Griškova, I. , Rukšėnas, O. , Dapšys, K. , Herpertz, S. , & Höppner, J. (2007). The effects of 10Hz repetitive transcranial magnetic stimulation on resting EEG power spectrum in healthy subjects. Neuroscience Letters, 419(2), 162–167. 10.1016/j.neulet.2007.04.030 [DOI] [PubMed] [Google Scholar]
- Groom, M. J. , Cahill, J. D. , Bates, A. T. , Jackson, G. M. , Calton, T. G. , Liddle, P. F. , & Hollis, C. (2010). Electrophysiological indices of abnormal error‐processing in adolescents with attention deficit hyperactivity disorder (ADHD). Journal of Child Psychology and Psychiatry, 51(1), 66–76. 10.1111/j.1469-7610.2009.02128.x [DOI] [PubMed] [Google Scholar]
- Hartmann, A. S. , Rief, W. , & Hilbert, A. (2011). Psychometric properties of the German version of the Barratt Impulsiveness Scale Version 11 (Bis–11) for adolescents. Perceptual and Motor Skills, 112(2), 353–368. [DOI] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , & Wilson, T. W. (2016). Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. NeuroImage, 134, 514–521. 10.1016/j.neuroimage.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, M. J. , Mader, K. , Schreppel, T. , Jacob, C. , Heine, M. , Boreatti‐Hümmer, A. , … Fallgatter, A. J. (2010). Neural correlates of performance monitoring in adult patients with attention deficit hyperactivity disorder (ADHD). The World Journal of Biological Psychiatry, 11, 457–464. 10.1080/15622970902977552 [DOI] [PubMed] [Google Scholar]
- Herrmann, M. J. , Saathoff, C. , Schreppel, T. J. , Ehlis, A. C. , Scheuerpflug, P. , Pauli, P. , & Fallgatter, A. J. (2009). The effect of ADHD symptoms on performance monitoring in a non‐clinical population. Psychiatry Research, 169(2), 144–148. 10.1016/j.psychres.2008.06.015 [DOI] [PubMed] [Google Scholar]
- Herwig, U. , Satrapi, P. , & Schönfeldt‐Lecuona, C. (2003). Using the international 10‐20 EEG system for positioning of transcranial magnetic stimulation. Brain Topography, 16(2), 95–99. 10.1023/B:BRAT.0000006333.93597.9d [DOI] [PubMed] [Google Scholar]
- Hu, L. , Xiao, P. , Zhang, Z. G. , Mouraux, A. , & Iannetti, G. D. (2014). Single‐trial time–frequency analysis of electrocortical signals: Baseline correction and beyond. NeuroImage, 84(84), 876–887. 10.1016/j.neuroimage.2013.09.055 [DOI] [PubMed] [Google Scholar]
- Huang, Y. Z. , Edwards, M. J. , Rounis, E. , Bhatia, K. P. , & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(2), 201–206. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- Huang, Y. Z. , & Rothwell, J. C. (2004). The effect of short‐duration bursts of high‐frequency, low‐intensity transcranial magnetic stimulation on the human motor cortex. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 115(5), 1069–1075. 10.1016/j.clinph.2003.12.026 [DOI] [PubMed] [Google Scholar]
- Iyer, M. B. , Mattu, U. , Grafman, J. , Lomarev, M. , Sato, S. , & Wassermann, E. M. (2005). Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology, 64(5), 872–875. 10.1212/01.WNL.0000152986.07469.E9 [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Adler, L. , Ames, M. , Delmer, O. , Faraone, S. , Hiripi, E. , … Walters, E. E. (2005). The World Health Organization adult ADHD self‐report scale (ASRS): A short screening scale for use in the general population. Psychological Medicine, 35(2), 245–256. 10.1017/s0033291704002892 [DOI] [PubMed] [Google Scholar]
- Keune, P. M. , Schönenberg, M. , Wyckoff, S. , Mayer, K. , Riemann, S. , Hautzinger, M. , & Strehl, U. (2011). Frontal alpha asymmetry in adults with attention deficit hyperactivity disorder: Replication and specification. Biological Psychology, 87(2), 306–310. 10.1016/j.biopsycho.2011.02.023 [DOI] [PubMed] [Google Scholar]
- Kolev, V. , Falkenstein, M. , & Yordanova, J. (2005). Aging and error processing ‐ time frequency analysis of error‐related potentials. Journal of Psychophysiology, 19(4), 289–297. 10.1027/0269-8803.19.4.289 [DOI] [Google Scholar]
- Lang, N. , Siebner, H. R. , Ernst, D. , Nitsche, M. A. , Paulus, W. , Lemon, R. N. , & Rothwell, J. C. (2004). Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid‐rate transcranial magnetic stimulation and controls the direction of after‐effects. Biological Psychiatry, 56(9), 634–639. 10.1016/j.biopsych.2004.07.017 [DOI] [PubMed] [Google Scholar]
- Lehrl, S. (2005). Mehrfachwahl‐Wortschatz‐Intelligenztest MWT‐B. Balingen: Spitta Verlag. [Google Scholar]
- Levy, F. , Hay, D. A. , McStephen, M. , Wood, C. , & Waldman, I. (1997). Attention‐deficit hyperactivity disorder: A category or a continuum? Genetic analysis of a large‐scale twin study. Journal of the American Academy of Child & Adolescent Psychiatry, 36(6), 737–744. 10.1097/00004583-199706000-00009 [DOI] [PubMed] [Google Scholar]
- Li, T. , Mota, N. R. , Galesloot, T. E. , Bralten, J. , Buitelaar, J. K. , IntHout, J. , … Franke, B. (2019). ADHD symptoms in the adult general population are associated with factors linked to ADHD in adult patients. European Neuropsychopharmacology, 29(10), 1117–1126. 10.1016/j.euroneuro.2019.07.136 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Wang, L. , Jia, M. , Guo, J. , Wang, H. , & Wang, M. (2017). The effects of high‐frequency rTMS over the left DLPFC on cognitive control in young healthy participants. PLoS One, 12(6), e0179430. 10.1371/journal.pone.0179430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, H. , Bressler, S. L. , Ding, M. , Truccolo, W. A. , & Nakamura, R. (2002). Synchronized activity in prefrontal cortex during anticipation of visuomotor processing. Neuroreport, 13(16), 2011–2015. 10.1097/00001756-200211150-00004 [DOI] [PubMed] [Google Scholar]
- Luu, P. , Tucker, D. M. , & Makeig, S. (2004). Frontal midline theta and the error‐related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology, 115(8), 1821–1835. 10.1016/j.clinph.2004.03.031 [DOI] [PubMed] [Google Scholar]
- Marco‐Pallarés, J. , Camara, E. , Münte, T. F. , & Rodríguez‐Fornells, A. (2008). Neural mechanisms underlying adaptive actions after slips. Journal of Cognitive Neuroscience, 20(9), 1595–1610. 10.1162/jocn.2008.20117 [DOI] [PubMed] [Google Scholar]
- Mazaheri, A. , Nieuwenhuis, I. L. C. , van Dijk, H. , & Jensen, O. (2009). Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Human Brain Mapping, 30, 1791–1800. 10.1002/hbm.20763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, T. J. , Wiesman, A. I. , Proskovec, A. L. , Heinrichs‐Graham, E. , & Wilson, T. W. (2017). Spatiotemporal oscillatory dynamics of visual selective attention during a flanker task. NeuroImage, 156, 277–285. 10.1016/j.neuroimage.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane, J. C. , Corkum, P. V. , Klein, R. M. , & McLaughlin, E. (2009). Interference control in children with and without ADHD: A systematic review of flanker and Simon task performance. Child Neuropsychology, 15(4), 321–342. 10.1080/09297040802348028 [DOI] [PubMed] [Google Scholar]
- Neuper, C. , Wörtz, M. , & Pfurtscheller, G. (2006). ERD/ERS patterns reflecting sensorimotor activation and deactivation. In Neuper C. & Klimesch W. (Eds.), Progress in Brain Research (Vol. 159, pp. 211–222). Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis, S. , Ridderinkhof, K. R. , Blom, J. , Band, G. P. H. , & Kok, A. (2001). Error‐related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology, 38(5), 752–760. 10.1111/1469-8986.3850752 [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , Liebetanz, D. , Lang, N. , Antal, A. , Tergau, F. , & Paulus, W. (2003). Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical Neurophysiology, 114(11), 2220–2222 author reply 2222–2223. [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A. , & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899–1901. 10.1212/wnl.57.10.1899 [DOI] [PubMed] [Google Scholar]
- O'Connell, R. G. , Dockree, P. M. , Bellgrove, M. A. , Turin, A. , Ward, S. , Foxe, J. J. , & Robertson, I. H. (2009). Two types of action error: Electrophysiological evidence for separable inhibitory and sustained attention neural mechanisms producing error on go/no‐go tasks, 21(1), 93–104. 10.1162/jocn.2009.21008%M18476764 [DOI] [PubMed] [Google Scholar]
- Oostenveld, R. , Fries, P. , Maris, E. , & Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenberg, G. , Hämmerer, D. , Müller, V. , Lindenberger, U. , & Li, S.‐C. (2013). Lower theta inter‐trial phase coherence during performance monitoring is related to higher reaction time variability: A lifespan study. NeuroImage, 83, 912–920. 10.1016/j.neuroimage.2013.07.032 [DOI] [PubMed] [Google Scholar]
- Perri, R. L. , Berchicci, M. , Lucci, G. , Spinelli, D. , & Di Russo, F. (2016). How the brain prevents a second error in a perceptual decision‐making task. Scientific Reports, 6, 32058. 10.1038/srep32058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reteig, L. C. , van den Brink, R. L. , Prinssen, S. , Cohen, M. X. , & Slagter, H. A. (2019). Sustaining attention for a prolonged period of time increases temporal variability in cortical responses. Cortex, 117, 16–32. 10.1016/j.cortex.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Retz‐Junginger, P. , Retz, W. , Blocher, D. , Weijers, H. G. , Trott, G. E. , Wender, P. H. , & Rössler, M. (2002). Wender Utah rating scale. The short‐version for the assessment of the attention‐deficit hyperactivity disorder in adults. Nervenarzt, 73(9), 830–838. 10.1007/s00115-001-1215-x [DOI] [PubMed] [Google Scholar]
- Rhodes, E. , Gaetz, W. C. , Marsden, J. , & Hall, S. D. (2018). Transient alpha and Beta synchrony underlies preparatory recruitment of directional motor networks. Journal of Cognitive Neuroscience, 30(6), 867–875. 10.1162/jocn_a_01250 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof, K. R. , Ullsperger, M. , Crone, E. A. , & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306(5695), 443–447. 10.1126/science.1100301 [DOI] [PubMed] [Google Scholar]
- Rösler, M. , Retz‐Junginger, P. , Retz, W. , & Stieglitz, R. D. (2008). Homburger ADHS Skalen für Erwachsene (HASE). Göttingen: Hogrefe. [Google Scholar]
- Rossi, A. , Giovannelli, F. , Gavazzi, G. , Righi, S. , Cincotta, M. , & Viggiano, M. P. (2018). Electrophysiological activity prior to self‐initiated movements is related to impulsive personality traits. Neuroscience, 372, 266–272. 10.1016/j.neuroscience.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Schneider, S. , Bahmer, T. J. , Metzger, F. G. , Reif, A. , Polak, T. , Pfuhlmann, B. , … Ehlis, A. C. (2013). Quetiapine and flupentixol differentially improve anterior cingulate cortex function in schizophrenia patients: An event‐related potential study. The International Journal of Neuropsychopharmacology, 16(9), 1911–1925. 10.1017/S1461145713000540 [DOI] [PubMed] [Google Scholar]
- Schutter, D. J. , van Honk, J. , d'Alfonso, A. A. , Postma, A. , & de Haan, E. H. (2001). Effects of slow rTMS at the right dorsolateral prefrontal cortex on EEG asymmetry and mood. Neuroreport, 12(3), 445–447. 10.1097/00001756-200103050-00005 [DOI] [PubMed] [Google Scholar]
- Shaw, P. , Lalonde, F. , Lepage, C. , Rabin, C. , Eckstrand, K. , Sharp, W. , … Rapoport, J. (2009). Development of cortical asymmetry in typically developing children and its disruption in attention‐deficit/hyperactivity disorder. Archives of General Psychiatry, 66(8), 888–896. 10.1001/archgenpsychiatry.2009.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo, L. T. , & Allen, J. J. B. (2007). Theta EEG dynamics of the error‐related negativity. Clinical Neurophysiology, 118, 645–668. 10.1016/j.clinph.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Tse, N. Y. , Goldsworthy, M. R. , Ridding, M. C. , Coxon, J. P. , Fitzgerald, P. B. , Fornito, A. , & Rogasch, N. C. (2018). The effect of stimulation interval on plasticity following repeated blocks of intermittent theta burst stimulation. Scientific Reports, 8(1), 8526–8526. 10.1038/s41598-018-26791-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis, C. , Thompson, A. , Rogers, R. D. , & Pellizzer, G. (2019). The degree of modulation of Beta Band activity during motor planning is related to trait impulsivity. Frontiers in Integrative Neuroscience, 13(1). 10.3389/fnint.2019.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede, F. , & Maris, E. (2016). Physiological plausibility can increase reproducibility in cognitive neuroscience. Trends in Cognitive Sciences, 20(8), 567–569. 10.1016/j.tics.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Wessel, J. R. (2018). An adaptive orienting theory of error processing. Psychophysiology, 55(3), e13041. 10.1111/psyp.13041 [DOI] [PubMed] [Google Scholar]
- Wittchen, H. U. , Zaudig, M. , & Fydrich, T. (1997). SKID. Strukturiertes Klinisches Interview für DSM‐IV. Achse I und II. Handanweisung. Göttingen: Hogrefe. [Google Scholar]
- Yordanova, J. , Falkenstein, M. , Hohnsbein, J. , & Kolev, V. (2004). Parallel systems of error processing in the brain. NeuroImage, 22, 590–602. [DOI] [PubMed] [Google Scholar]
- Yordanova, J. , Kolev, V. , Albrecht, B. , Uebel, H. , Banaschewski, T. , & Rothenberger, A. (2011). May posterror performance be a critical factor for behavioral deficits in attentiondeficit/ hyperactivity disorder? Biological Psychiatry, 70(3), 246–254. 10.1016/j.biopsych.2011.02.026 [DOI] [PubMed] [Google Scholar]
- Zaepffel, M. , Trachel, R. , Kilavik, B. E. , & Brochier, T. (2013). Modulations of EEG Beta power during planning and execution of grasping movements. PLoS One, 8(3), e60060. 10.1371/journal.pone.0060060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Chen, Y. , Bressler, S. L. , & Ding, M. (2008). Response preparation and inhibition: The role of the cortical sensorimotor beta rhythm. Neuroscience, 156, 238–246. 10.1016/j.neuroscience.2008.06.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Flanker task performance
Figure S1. Time‐frequency grand averages of TF beta power
Figure S2. Time‐frequency grand averages of weighted intertrial phase clustering in high‐impulsive individuals
Figure S3. Time‐frequency grand averages of weighted intertrial phase clustering in low‐impulsive individuals
Figure S4. Sub‐analysis: time‐frequency grand averages of time‐frequency averages of brain‐behavior relations between theta ITPC and flanker task reaction times in Go trials
Figure S5. Sub‐analysis: time‐frequency grand averages of time‐frequency averages of brain‐behavior relations between theta ITPC and flanker task reaction time variability in Go trials
Figure S6. Sub‐analysis: time‐frequency grand averages of trial‐to‐trial brain‐behavior relations in high‐impulsive subjects: theta ITPC weighted by flanker task reaction time (RT) in Go trials
Figure S7. Sub‐analysis: time‐frequency grand averages of trial‐to‐trial brain‐behavior relations in low‐impulsive subjects: theta ITPC weighted by flanker task reaction time (RT) in Go trials
Data Availability Statement
Data are available upon request.


