Abstract
Prior epidemiological studies have found that in utero exposure to gestational diabetes mellitus (GDM) is associated with increased risk for neurodevelopmental disorders. However, brain alterations associated with GDM are not known. The hippocampus is pivotal for cognition and emotional regulation. Therefore, we assessed relationships between in utero exposure to GDM and hippocampal morphology and subfield structure during childhood. One hundred seventeen children aged 7–11 years (57% girls, 57% exposed to GDM), born at Kaiser Permanente Southern California, participated in the BrainChild Study. Maternal GDM status was determined from electronic medical records. Children underwent brain magnetic resonance imaging. Freesurfer 6.0 was used to measure hippocampal and individual hippocampal subfield gray matter volume (mm3). Morphological analyses on the hippocampal surface were carried out using shape analysis. GDM‐exposed children exhibited reduced radial thickness in a small, spatially‐restricted portion of the left inferior body of the hippocampus that corresponds to the CA1 subfield. There was a significant interaction between GDM‐exposure and sex on the right hippocampal CA1 subfield. GDM‐exposed boys had reduced right CA1 volume compared to unexposed boys, but this association was no longer significant after controlling for age. No significant group differences were observed in girls. Our results suggest that GDM‐exposure impacts shape of the left hippocampal CA1 subfield in both boys and girls and may reduce volume of right hippocampal CA1 only in boys. These in‐depth findings illuminate the unique properties of the hippocampus impacted by prenatal GDM‐exposure and could have important implications for hippocampal‐related functions.
Keywords: developmental programming, gestational diabetes, hippocampal shape analysis, hippocampal subfield analysis
We sought to investigate the influence of in utero exposure to gestational diabetes mellitus (GDM) on hippocampal morphology and subfield volume in a cohort of children between the ages of 7 and 11 years. We found GDM exposure was associated with reduced radial thickness of the left inferior hippocampal body. We also found a significant interaction between GDM exposure and sex in the right whole hippocampus and CA1 subfield volumes.
1. INTRODUCTION
Gestational diabetes mellitus (GDM) is the most common complication in pregnancy and affects approximately 10% of expecting mothers (DeSisto, Kim, & Sharma, 2014). GDM is defined as a state of glucose intolerance and hyperglycemia with initial diagnosis during pregnancy (Nold & Georgieff, 2004). GDM constitutes an altered fetal environment and can induce hyperglycemia, hyperinsulinemia, iron deficiency (Verner et al., 2007) and neuroinflammation (Esposito et al., 2002) in the developing fetus. Consequently, children exposed to GDM in utero are at an increased risk for acquiring neurodevelopmental disorders including ASD (Xiang et al., 2015) and ADHD (Nomura et al., 2012; Xiang et al., 2018). Therefore, understanding the nature of brain alterations in children born to mothers with GDM and long‐term consequences of such exposure is of clinical importance.
The hippocampus is a subcortical structure within the medial temporal lobe critical for learning, memory and emotion regulation (Squire & Zola‐Morgan, 1991) and is particularly vulnerable to early insults due to its protracted developmental profile (Lynch, Shi, Toga, & Clark, 2018). Additionally, the hippocampus exhibits a high metabolic demand (Jabès, Lavenex, Amaral, & Lavenex, 2011; Kirschen, Kéry, & Ge, 2018), which makes it susceptible to perturbations in vital nutrients, such as glucose, iron and oxygen. Moreover, prior neuroimaging studies have shown that prenatal exposure to alcohol or maternal obesity preferentially impacts hippocampal volume of offspring, most notably in a sex‐specific manner (Alves et al., 2020; Treit et al., 2017). Collectively, prior studies provide evidence that the hippocampus may be particularly sensitive to in utero insults. Deficits in hippocampal‐dependent behaviors have been described in GDM‐exposed infants and toddlers, including auditory (DeRegnier, Nelson, Thomas, Wewerka, & Georgieff, 2000), and cross‐modal recognition memory impairments (Nelson, Wewerka, Borscheid, DeRegnier, & Georgieff, 2003). Identifying the structural changes that accompany these differences in memory performance in GDM‐exposed children is crucial for understanding cognitive and neurodevelopmental outcomes.
The hippocampus is composed of several distinct subfields with unique functional and cytoarchitectonic features including the cornu ammonis 1–4 (CA1‐4), dentate gyrus (DG), and subiculum (SUB) (Duvernoy, Cattin, & Risold, 2013). In addition to the cellular diversity observed cross‐sectionally, the hippocampus also exhibits a functional gradient along its anterior–posterior axis (Strange, Witter, Lein, & Moser, 2014). Animal models replicating adverse prenatal metabolic conditions show early iron deficiency affects dendritic arborization (Fretham, Carlson, & Georgieff, 2011) and perinatal hypoxia differentially impacts intra‐hippocampal connectivity (Nyakas, Buwalda, & Luiten, 1996). In a rodent model of GDM, where maternal hyperglycemia was induced through a high‐fat and sucrose diet, offspring had selective reductions in hippocampal CA1 neuronal density and synaptic integrity (Vuong et al., 2017). In other rodent models of GDM, where pregnant rats were treated with Streptozotocin to induce beta‐cell toxicity, GDM‐exposed offspring had reduced CA1 and CA3 neuronal density compared to unexposed offspring, which further suggests that the GDM prenatal environment adversely affects hippocampal structure, particularly the CA1 and CA3 subfields (Golalipour, Kafshgiri, & Ghafari, 2012; Lotfi, Hami, Hosseini, Haghir, & Haghir, 2016). However, the limited evidence from a single human study did not find a significant relationship between hippocampal volume and prenatal GDM‐exposure in 10‐year‐old children (Jabès, Thomas, Langworthy, Georgieff, & Nelson, 2015).
This apparent discrepancy may be attributed to the lack of granularity typically afforded by in vivo volumetric neuroimaging studies. Because GDM selectively and differentially impacts hippocampal structure in animal models, whole volumetric measures may provide crude approximations that obscure structural differences on the cellular level and thus underestimate the extent to which structures are affected. Due to the cytoarchitectonic and functional diversity of the hippocampus, in vivo MRI approaches sensitive to regional differences along the multidimensional spatial gradient of the hippocampus may help to elucidate specific structural alterations in children with prenatal GDM‐exposure.
Shape analysis and subfield volumetric analysis overcomes the limitations of whole volumetric approaches by enabling the quantification of regional measures that describe morphological surface features and investigating individual subregions within the hippocampus. Shape analysis quantifies regional hippocampal morphology using geometric surface features that describe local measures of surface topology. In the present study, we use metric optimization for computational anatomy (MOCA) (Shi et al., 2014) to quantify radial thickness features distributed across equidistant vertices on the hippocampal surface, which reflect regional indices of cross‐sectional thickness (Shi, Morra, Thompson, & Toga, 2009). Hippocampal shape analysis with MOCA has been used to identify regional morphological alterations of the hippocampus in several applications, including multiple sclerosis (Gold et al., 2014) and child development (Lynch et al., 2018). In addition to investigating hippocampal morphology, we used Freesurfer 6.0 to identify differences in hippocampal subfield volume and whole hippocampal volume. Based on advances in identifying hippocampal subfield boundaries with improved accuracy by Iglesias et al. (2015), FreeSurfer 6.0 is commonly used to assess hippocampal subfields, and has been shown to be as reliable as manual segmentation methods, while proving to be more efficient (Cover, van Schijndel, Bosco, Damangir, & Redolfi, 2018). Therefore, for the first time in humans, we investigated if children exposed to GDM in utero differ in hippocampal shape and individual hippocampal subfields when compared to unexposed children. Additionally, because of known sex differences in the effects of maternal metabolic disorders during pregnancy on the developing hippocampus (Alves et al., 2020; Zhu et al., 2018), we assessed whether there are sex differences in associations between GDM‐exposure and hippocampal morphology in children.
2. METHODS
2.1. Study overview
Children between the ages of 7–11 years old were recruited from Kaiser Permanente Southern California (KPSC), to participate in the BrainChild Study. BrainChild studies the impact of intrauterine exposure to metabolic disorders on brain pathways during childhood (Page et al., 2019). Additionally, the BrainChild cohort is a unique cohort of children whose mothers had well‐documented glucose levels during their pregnancies. KPSC is a large health care organization that uses an integrated electronic medical record (EMR) system. All children were born at KPSC. Children with the following characteristics were also excluded: had a history of premature birth (<37 weeks gestation), born to mothers diagnosed with diabetes pre‐existing pregnancy, had neurological, psychiatric, or other significant medical disorders, including diabetes; use of medications known to alter metabolism (i.e., glucocorticoids), or had contraindications to magnetic resonance imaging (MRI) (i.e., permanent metal, claustrophobia) or left‐handedness. Each participating Institutional Review Board approved this study (University of Southern California [USC] # HS‐14‐00034 and KPSC # 10282). Participants' parents gave written informed consent and children provided written informed assent. Children came in for a study visit at the Dana & David Dornsife Cognitive Neuroimaging Center at USC, where a brain MRI was collected. Children's height (in cm) and weight (in kg) were measured by trained staff to determine body mass index (BMI) and BMI z‐scores (BMI standard deviations scores, adjusted for child age and sex) (CDC, 2018).
From the electronic medical records (EMR), each mother's GDM status was determined. Diagnosis of GDM was based on laboratory glucose values confirming a plasma glucose level ≥ 200 mg/dl from a 50‐g glucose challenge tests or at least two plasma glucose values meeting or exceeding the following values on the 100‐g or 75‐g oral glucose tolerance test: fasting, 95 mg/dL; 1 hr, 180 mg/dl; 2 hr, 155 mg/dl; and 3 hr, 140 mg/dl (American Diabetes Association, 2012). Maternal pre‐pregnancy BMI (kg/m2) was calculated from maternal height (cm) and weight (kg) measurements closest to last menstrual period within 180 days, using EMR.
2.2. MRI methods
After a mock scanner training session, a brain MRI was performed using a Siemens MAGNETOM Prismafit 3 T MRI scanner (Siemens Medical Systems) with a 20‐channel phased array coil. The MRI session started with a localizer scan. A high‐resolution MRI scan was then acquired using a T1‐weighted three‐dimensional magnetization prepared rapid gradient echo (MP‐RAGE) sequence with the parameters: 256 × 256 × 176‐matrix size with 1 × 1 × 1‐mm3 resolution; inversion time = 900 ms; repetition time (TR) = 1950 ms; echo time (TE) = 2.26 ms; flip angle = 90°; total scan duration was 4 min and 14 s.
2.3. Hippocampal shape analysis
Automated shape analysis was performed using Metric Optimization for Computational Anatomy (MOCA) software, RRID:SCR_015524 (Shi et al., 2014). Hippocampal morphological maps are generated using Laplace‐Beltrami (LB) eigen‐functions to compute isometry‐invariant conformal maps between subcortical surfaces. First, hippocampal volumes are segmented using FSL FIRST, RRID:SCR_002823 (Patenaude, Smith, Kennedy, & Jenkinson, 2011). Segmentations were manually inspected to ensure the surface boundaries aligned with the hippocampal anatomy. Hippocampal volumes are then converted to a triangulated mesh in native space through iterative deformations that removes outliers, avoids shrinkage and preserves surface topology (Shi et al., 2010). Each mesh was re‐meshed to contain 2000 equally spaced across the surface. Radial distance (RD) surface features were computed using the Reeb graph of the first order LB eigen‐functions (Shi et al., 2009). The RD features reflect local hippocampal thickness and is defined as the shortest distance from a given vertex to the longitudinal core of the hippocampus.
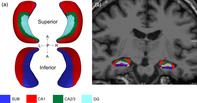
To enable one‐to‐one correspondence, meshes in native space were registered to an average hippocampal surface template with 2000 vertices generated in a large normative pediatric dataset described in (Lynch et al., 2018). The features of each subject surface were directly mapped onto the average template by pulling back the average mesh structure onto each subject mesh using linear interpolation, enabling anatomical alignment for each vertex. See Figure 1a for example.
FIGURE 1.
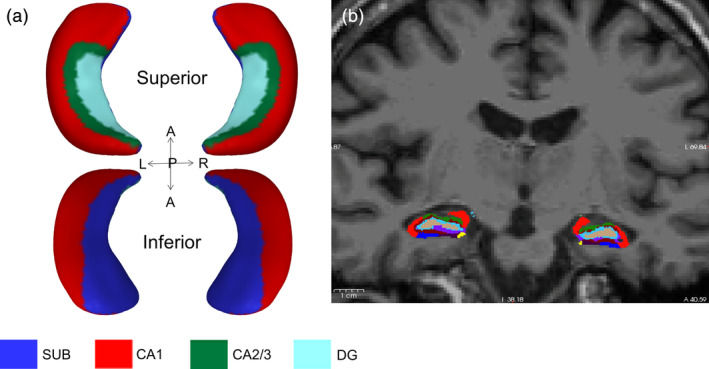
Example of hippocampal shape analysis (a) and hippocampal subfield volumetric segmentation overlaid on a T1‐weighted image in coronal view (b). Blue denotes subiculum, red denotes CA1, green denotes CA2/3 and turquoise denotes molecular layer of the dentate gyrus. L, left; R, right; A, anterior; P, posterior
2.4. Hippocampal subfield volumetric analysis
The T1 MP‐RAGE structural image was processed using the automated segmentation software, FreeSurfer version 6.0 hippocampal module (http://surfer.nmr.mgh.harvard.edu/, RRID:SCR_001847) to examine hippocampal gray matter volume and hippocampal subfield volumes. The procedure uses Bayesian inference and a probabilistic atlas of the hippocampal formation based on manual delineations of subfields in ultra‐high‐resolution MRI scans (Iglesias et al., 2015). See Figure 1b for example.
2.5. Quality assurance procedure
All MRI scans were reviewed by a neuroradiologist and participants with anatomical abnormalities were excluded from the study. Each child's T1‐weighted image was manually inspected for quality assurance purposes and scans with excess head motion were excluded from analyses. In total, 128 participants with T1‐weighted images and demographic information were originally considered for the study. Following quality assurance procedures, seven scans were excluded due to excessive head motion, one scan was excluded due to incomplete temporal lobe coverage, and three scans were excluded due to automated hippocampal segmentation failures. The present analyses utilized the T1‐weighted images and demographic information of 117 subjects (Table 1). Further rigorous quality check and segmentation details for the hippocampal subfield analysis can be found in the methods section of Alves et al. (2020).
TABLE 1.
Participant demographics, N = 117
| N (%) or mean (SD) | UnexposedN = 50 (100%) | GDM‐exposedN = 67 (100%) | t‐value |
|---|---|---|---|
| Child characteristics | |||
| Male | 23 (46.0%) | 27 (40.3%) | |
| Age (years) | 8.74 ± 1.11 | 8.40 ± 0.89 | 1.76 # |
| BMI z‐score | 0.72 ± 0.97 | 0.82 ± 1.16 | −0.55 |
| Maternal characteristics | |||
| Education | |||
| High school or less | 8 (16.0%) | 16 (23.9%) | |
| Some college | 14 (28.0%) | 19 (28.4%) | |
| College and above | 28 (56.0%) | 32 (47.8%) | |
| Income (USD) | 54,535 ± 24,377 | 57,382 ± 23,721 | −0.63 |
| Pre‐pregnancy BMI (kg/m2) | 29.42 ± 5.80 | 30.30 ± 7.66 | −0.71 |
p‐value <.10.
2.6. Statistical analysis
Participant characteristics were compared between GDM‐exposed and unexposed children using t‐test for continuous variables and chi‐square test for categorical variables. Groups did not differ in sex, BMI z‐score, socioeconomic status, or maternal pre‐pregnancy BMI however, GDM‐exposed children were slightly younger, so age was included as a covariate. General linear models (GLMs) were applied to each vertex in the group‐wise atlas to test if radial distance is significantly associated with main effect of GDM status, while controlling for age. Interactions between GDM status and sex were also tested. Because neighboring vertices are spatially correlated, methods such as Bonferroni multiple comparison correction may not be appropriate and can result in overly conservative significance thresholds (Lindquist & Mejia, 2015). In order to control for multiple comparisons across 2000 observations on the surface of the hippocampus, cluster‐wise thresholding of significant vertices was performed using random field theory (RFT). RFT uses Gaussian random fields to consider the magnitude and spatial correlation of smoothed statistical parameter maps of spatially correlated data points (Cao & Worsley, 1999). The corrected cluster‐wise p‐value is estimated using the Euler characteristic, which describes the search volume as a function of the image smoothness, and is derived from the vertex‐wise significance and number of resolution elements (resels) in the image (Friston, 1997). A supra‐threshold cluster level of p < .001 and a set level threshold of p < .05 was used for the height and spatial extent thresholds, respectively. Region of interest (ROI) post hoc analyses were then performed on significant clusters to determine the direction of significant associations in clusters using GLMs.
To limit multiple comparisons, volumetric analyses of group differences between GDM‐exposed and unexposed children were performed on the CA1 and CA3 subfields based on prior rodent studies exemplifying that in utero exposure to GDM preferentially impacts these subfields (Golalipour et al., 2012; Lotfi et al., 2016; Vuong et al., 2017). ANCOVA was also completed to compare hippocampal volume and hippocampal subfield volumes (CA1 and CA3) between GDM‐exposed and unexposed children. Linear regression was then used to test for interactions between GDM‐exposure and sex on child hippocampal volume (total) and hippocampal volume of subfields with age as a covariate. Sex‐stratified ANCOVA analyses were then completed for regions with significant interactions to test for group differences between GDM‐exposed and unexposed children. SAS 9.4 statistical software (SAS Institute, Cary, NC, USA) and RStudio (version 1.2.5033) were used for all statistical analyses. For volumetric analyses, a significance level of p < .05 was used.
3. RESULTS
Participant demographics are presented in Table 1. GDM‐exposed and unexposed groups did not differ by sex, BMI z‐score, maternal education, family income at birth or maternal pre‐pregnancy BMI. GDM‐exposed children tended to be younger. Age was not significantly different between girls and boys (t[115] = −1.01, p = .31). There were no significant differences in age between GDM‐exposed girls (Mean ± SD, 8.41 ± .89 years) and unexposed girls (8.55 ± .97 years; t[65] = .58, p = .57); however, GDM‐exposed boys were marginally younger (8.39 ± .92 years) than unexposed boys (8.97 ± 1.24 years; t[48] = 1.84, p = .07). Intracranial volume (ICV) was not significantly different between unexposed (1,540,367.0 ± 139,365.6 mm3) and GDM‐exposed children (1,520,900.0 ± 131,248.1 mm3, t[115] = 0.75, p = 0.45). ICV was significantly larger in boys (1,612,962.0 ± 133,515.6 mm3) compared to girls (1,467,591.0 ± 97,365.6 mm3, t[115] = 6.39, p = 0 < .001), and this effect was observed in both GDM‐exposed (t[65] = 3.88, p < .001) and unexposed children (t[48] = 5.18, p < .001).
3.1. Hippocampal shape analysis
Shape analysis revealed a significant group difference between GDM‐exposed and unexposed children in a cluster in the left inferior body (23 vertices, 0.67 resels, RFT‐corrected p = .011). Inclusion of age, BMI z‐score and ICV as covariates of no interest did not alter the relationship between GDM status and radial thickness of the cluster (18 vertices, 0.52 resels, corrected p = .022) (Figure 2). Post‐hoc analysis show radial distance in this cluster is reduced by 6.0% in GDM‐exposed children (Mean ± SD = 5.13 ± .40 mm) compared to unexposed controls (5.46 ± 0.41 mm; t[115] = −4.22, p < .001) (Figure 2c) and this effect remained significant after controlling for age, BMI z‐score and ICV (t[112] = −3.95, p < .001).
FIGURE 2.
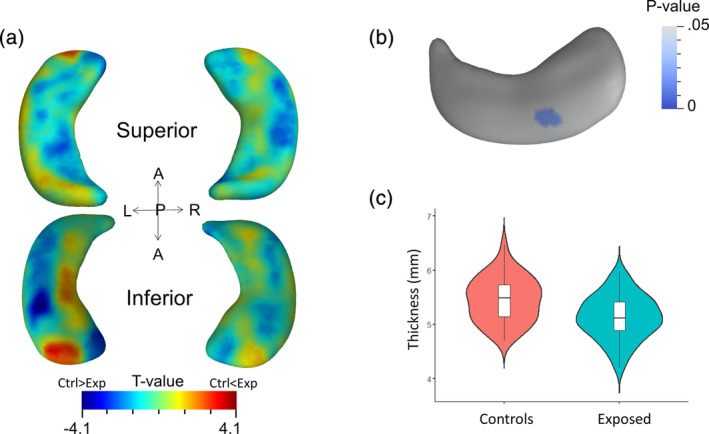
The main effect of GDM‐exposure on hippocampal morphology. (a) Corrected t‐value map shows vertex‐wise group differences on the whole hippocampal surface between GDM exposed children and unexposed children, where warm colors denote larger thickness in GDM exposed children compared to unexposed children and cool colors denote smaller thickness in GDM exposed children compared to unexposed children. (b) Significant group differences were observed in a cluster on the inferior body of the left hippocampus. The significance levels are shown with RFT‐corrected p‐values. (C) Post‐hoc analyses of the cluster reveal significantly reduced hippocampal thickness in children with GDM‐exposure compared to unexposed children. All statistical tests used age, BMI z‐score and ICV as covariates of no interest
We did not observe a significant interaction between GDM‐exposure and sex on hippocampal shape after controlling for age, BMI z‐score and ICV (Figure S1a). Significant radial thickness sex differences were observed in GDM‐exposed and unexposed children after controlling for age, BMI z‐score and ICV. Within the left hippocampus of GDM‐exposed children, a main effect of sex on radial thickness was observed in three clusters (lateral cluster: 53 vertices, 1.63 resels, corrected p < .001; inferior mesial cluster: 60 vertices, 1.41 resels, p < .001; inferior anterior cluster: 10 vertices, 0.52 resels, p = .02). Adjusted radial thickness in the lateral, inferior mesial and inferior anterior clusters were 6.0, 7.7, and 5.6% larger in exposed boys (lateral: 5.33 ± .06 mm; inferior mesial: 6.12 ± .08 mm; inferior anterior: 6.21 ± .07 mm) compared to exposed girls (lateral: 5.33 ± .05 mm; inferior mesial: 5.68 ± .06 mm; inferior anterior: 5.88 ± .05), respectively (Figure S1B, D). A main effect of sex was observed in a radial thickness cluster of the inferior anterior surface in unexposed children (59 vertices, 1.48 resels, corrected p < .001) where adjusted radial thickness was 9.0% larger in boys (5.44 ± .07 mm) compared to girls (4.99 ± .07 mm) (Figure S1C, E). We observed no significant sex differences in right hippocampal radial thickness within the GDM‐exposed group. Within unexposed subjects, a main effect of sex was observed in a portion of the right hippocampal head (338 vertices, 9.56 resels, corrected p < .001) where the adjusted radial thickness was 8.0% larger in boys (7.03 ± .06 mm) compared to girls (6.51 ± .06 mm).
3.2. Hippocampal subfield volumetric analysis
GDM exposure was not significantly associated with gray matter volume within the right whole hippocampus (GDM‐exposed: 3450.9 ± 39.9 mm3, unexposed: 3,520.8 ± 46.2 mm3, t[115] = −1.15, p = .25). GDM‐exposed children had marginally lower gray matter volume in the whole left hippocampus (3,368.8 ± 39.6 mm3) compared to unexposed children (3,481.6 ± 45.6 mm3; t[115] = −1.87, p = .06). No significant differences were observed within the gray matter volume of bilateral CA1 (right: t(115) = −.75, p = .45; left: t(115) = −.99, p = .32) and CA3 subfields (right: t(115) = −.2, p = .84; left: t(115) = .71, p = .48) between GDM‐exposed (right CA1: 648.1 ± 9.4 mm3; left CA1: 630.7 ± 10.2 mm3; right CA3: 218.4 ± 3.5 mm3; left CA3: 202.2 ± 3.7 mm3) and unexposed subjects (right CA1: 658.9 ± 10.9 mm3; left CA1: 646.1 ± 11.8 mm3; right CA3: 219.4 ± 4.0 mm3; left CA3: 198.2 ± 4.3 mm3). After adjusting for age, BMI z‐score and ICV, GDM exposure was not significantly associated with gray matter volume within the bilateral whole hippocampus, CA1 subfield or CA3 subfield (Table S1).
A significant interaction was observed between GDM‐exposure and sex within the right whole hippocampus (interaction p = .03) and right CA1 hippocampal subfield (interaction p = .02). These interactions remained after adjusting for age, ICV and BMI z‐score (right whole hippocampus, p = .02; right CA1, p = .01) (Figure 3). Of the covariates included in the interaction models, ICV accounted for the most variance in both the right whole hippocampus (30.6%) and right CA1 subfield (20.5%) (Table S2). In GDM‐exposed stratified analyses, GDM‐exposed boys did not differ from GDM‐exposed girls in right whole hippocampal volume (3,533.9 ± 58.4 mm3 vs. 3,394.8 ± 47.9 mm3, p = .07) or right CA1 volume (663.8 ± 13.2 mm3 vs. 637.4 ± 10.8 mm3, p = .12). These group differences remained insignificant after adjusting for age, ICV and BMI z‐score (p > .05) (Table S3). However, significant sex differences were observed in unexposed subjects. In unexposed boys compared to girls, the right whole hippocampal volume (3,731.6 ± 61.0 mm3 vs. 3,341.3 ± 56.3 mm3, p < .001) and right CA1 volume (708.2 ± 15.4 mm3 vs. 616.8 ± 14.2 mm3, p < .001) were 11.7 and 14.8% larger, respectively. Further adjusting for age, ICV, and BMI z‐score did not change these significant associations within the right CA1 subfield (p = .04), however sex differences within the right whole hippocampal volume of unexposed subjects trended toward significance (p = .06) (Table S3). In unadjusted sex‐stratified analyses, the gray matter volume in the right hippocampus was reduced by 5.3% in GDM‐exposed compared to unexposed boys (3,533.9 ± 62.3 mm3 vs. 3,731.6 ± 67.5 mm3, respectively, p = .04). Additionally, the right CA1 subfield volume was 6.3% smaller in GDM‐exposed boys (663.8 ± 13.8 mm3) compared to unexposed boys (708.2 ± 14.9 mm3, p = .03). Adjusting for child age, BMI z‐score and ICV resulted in group differences that trended toward significance within boys in both the right hippocampus (GDM‐exposed: 3557.0 ± 50.5 mm3 vs. unexposed: 3704.4 ± 54.9 mm3, p = .06) and right CA1 subfield (GDM‐exposed: 668.8 ± 12.8 mm3 vs. unexposed: 702.4 ± 13.9 mm3, p = .09). There were no group differences in girls (Table S4).
FIGURE 3.
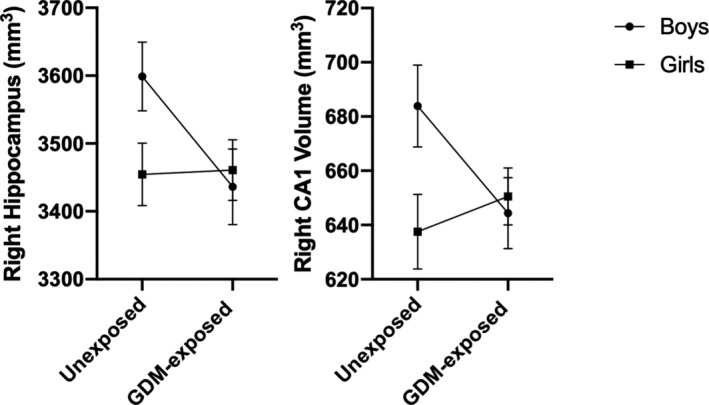
Interaction between GDM‐exposure and sex on right hippocampal volume and CA1 hippocampal subfield volume. LSmeans (SE) adjusted for child age, ICV and BMI z‐score between unexposed and GDM‐exposed children stratified by sex (boys in circles, girls in squares)
4. DISCUSSION
The present study used multiple segmentation and analytical techniques to investigate the relationship between hippocampal microstructure and prenatal GDM‐exposure in children aged 7–11 years old. We demonstrate that children exposed to GDM show localized cross‐sectional thickness reductions in the left inferior hippocampal body compared to unexposed children. Additionally, boys and girls showed differential associations between hippocampal volume and GDM‐exposure. Specifically, GDM‐exposed boys did not differ in hippocampal volume from GDM‐exposed girls, while unexposed boys had significantly greater hippocampal volume compared to unexposed girls, independent of age. Additionally, boys exposed to GDM in utero showed selective reductions in right hippocampal volume compared to unexposed boys, and this effect was driven by the CA1 subfield. However, these effects in boys were mitigated by age. Together, these results show prenatal exposure to GDM exerts a subtle, but regionally specific, influence on hippocampal morphology and may affect boys and girls differently in middle childhood.
Using shape analysis, we report that prenatal GDM‐exposure is significantly associated with reduced cross‐sectional thickness in the inferior body of the left hippocampus, independent of age and BMI z‐score. Radial thickness reductions in GDM‐exposed children were confined to a small cluster that co‐localizes with the anatomical location of the CA1 subfield (Figure 1). In our subfield volumetric analyses, however, neither whole hippocampal nor CA1 subfield volume were significantly associated with GDM exposure. The small spatial extent of our results suggest that the influence of GDM exposure on hippocampal structure is subtle in nature and may be related to localized alterations to specific cellular properties rather than modifications to global hippocampal features. Previous histological studies in animals have shown the biochemical sequelae associated with the adverse fetal environment in GDM, such as perinatal iron deficiency and hypoxia, preferentially affect CA1 pyramidal cell dendritic arborization and fiber density (Fretham et al., 2011; Nyakas et al., 1996). Additionally, microglial activation during chronic central inflammation due to maternal hyperglycemia is associated with reduced CA1 pyramidal cell layer density and decreased synaptic integrity in offspring (Vuong et al., 2017). Therefore, it is possible that exposure to GDM in utero may selectively alter morphological features of specific cell types in the CA1 subfield during critical periods of prenatal hippocampal development, specifically the pyramidal cell layer; however, future studies should further investigate the precise metabolic and neuroinflammatory mechanisms responsible for these changes.
Our finding of subtle radial thickness reductions in the left, but not right, hippocampus of children exposed to GDM in utero suggests small sub‐regions within the left hippocampus may be particularly vulnerable to the prenatal environment induced by GDM. One possible explanation for the asymmetric influence of GDM exposure on hippocampal morphology may be attributed to interhemispheric differences in hormone receptor expression in the hippocampus. A prior animal study showed that insulin receptors have differential distribution patterns between the left and right hippocampus in newborn rats (Hami, Kheradmand, & Haghir, 2014). Because maternal hyperglycemia is associated with chronic insulin resistance that influences the prenatal environment, alterations in insulin signaling due to prenatal exposure to GDM may influence the left and right hippocampus differently. Recent evidence has also shown that excess body weight in children is associated with selective volumetric and cross‐sectional thickness reductions in the left hippocampus during late childhood (Lynch et al., 2020); therefore, it is possible that the left hippocampus is uniquely susceptible to other neuroinflammatory metabolic stressors during development.
Few studies have explored the influence of GDM exposure on hippocampal structure in children and adolescents. In one previous study, hippocampal volume was not significantly different between GDM‐exposed and unexposed 10‐year‐old children (Jabès et al., 2015). A number of factors may contribute to these discrepant findings. First, the subtle effects of GDM on hippocampal structure may require a more sensitive neuroimaging approach in order to detect the subtle and spatially restricted morphological differences observed in the present study. The prior study by Jabès et al. (2015) also had a smaller sample size and may not have been sufficiently powered to detect group differences. Lastly, participants in the present study were, on average, 2 years younger than those in Jabès et al. (2015). It is possible that structural differences attributed to GDM exposure may be observed earlier in development. The hippocampus undergoes dynamic structural changes across childhood and adolescence (Lynch et al., 2018) and perhaps GDM exposure results in alterations to the typical maturational trajectory of the hippocampus. While this hypothesis is outside the scope of the present study, future studies should explore how GDM influences brain structural changes longitudinally across a wider range of ages during development.
We observed that the relationship between GDM exposure and hippocampal gray matter volume significantly differed between boys and girls. While GDM‐exposed boys did not differ from GDM‐exposed girls in hippocampal volume, unexposed boys had significantly greater hippocampal volume compared to unexposed girls. However, this association was weakened after accounting for intracranial volume differences between boys and girls. This finding is unsurprising, given that ICV was significantly different between boys and girls and accounted for the largest proportion of variance in right whole hippocampal and CA1 subfield volumes. Nevertheless, even after accounting for intracranial volume differences, larger right CA1 volume remained in unexposed boys compared to girls, while GDM‐exposed boys did not differ from GDM‐exposed girls in right CA1 volume. Several other studies have shown that during childhood, boys exhibit greater hippocampal volume compared to girls, even after accounting for age or intracranial volume (Herting et al., 2018; Krogsrud et al., 2014; Wierenga et al., 2014). Therefore, GDM exposure may influence developmental sex differences in the hippocampus. Moreover, boys exposed to GDM in utero exhibited decreased gray matter volume in the right hippocampus and right CA1 subfield compared to unexposed boys, while this relationship was not observed in girls. Our findings are in line with other studies that have shown that the hippocampus of boys is particularly sensitive to in utero metabolic insults (Alves et al., 2020; Zhu et al., 2018). Importantly, while the interaction with GDM exposure and sex on child hippocampal volume and right CA1 subfield volume was independent of age and intracranial volume, sex stratified results in boys were no longer significant after adjusting for age and intracranial volume. These findings further suggest that age and intracranial volume may have important roles in the relationship between GDM exposure and hippocampal volume, thus exemplifying the need for longitudinal studies investigating GDM exposure on child hippocampal development. The diminished significance of group differences in boys when accounting for age, however, may also be attributed to age differences between GDM‐exposed and unexposed boys. Because the hippocampus continues to mature and expand through late adolescence (Lynch et al., 2018), older children will, on average, have larger hippocampal volumes than younger children. Therefore, larger hippocampal and CA1 volume observed in unexposed compared to GDM‐exposed boys may be driven by their marginally older age. However, a larger sample size is needed to determine if GDM exposure impacts boys differently after accounting for age differences. Interestingly, shape analysis did not reveal significant sex‐specific alterations to hippocampal morphology in prenatal GDM‐exposure, which suggests that the volumetric differences observed in the right CA1 subfield may not be associated with deformations of the hippocampal surface. It is also possible that the lack of convergence between the shape and volumetric analyses may be due in part to differences in hippocampal segmentation algorithms between Freesurfer and FSL.
5. LIMITATIONS
Our study had many strengths, including using electronic medical records to confirm GDM‐exposure through oral glucose testing results performed during pregnancy. However, there were some limitations to keep in mind. In‐vivo neuroimaging is an indirect approach for quantifying neuronal volume, and therefore cannot detail the cellular properties that are impacted by GDM‐exposure. Animal studies are required to characterize the precise cellular and molecular changes in the hippocampus that are induced by in utero GDM‐exposure (Vuong et al., 2017). Additionally, prior studies have observed poor neuropsychological outcomes among children exposed to GDM (Nelson et al., 2003; Nomura et al., 2012), and it will be important for future studies to ascertain whether the structural alterations observed in the hippocampus align with behavioral measures.
6. CONCLUSIONS
For the first time in humans, we found that children exposed to GDM in utero exhibit morphological and volumetric alterations in the hippocampus compared to unexposed children. Our findings suggest that GDM‐exposure exerts a subtle influence on hippocampal structure in children that may affect boys and girls differently. Shape analysis revealed reduced radial thickness in a spatially restricted portion of the left inferior hippocampus that corresponds to the CA1 subfield. Additionally, hippocampal subfield analysis revealed that the relationship between GDM exposure and right CA1 volume differed between boys and girls, with boys exposed to GDM exhibiting marginally reduced volume compared to unexposed boys, and girls having no group differences in right CA1 volume. Collectively, these results provide neuroanatomical evidence to support prior behavioral studies of hippocampal‐dependent alterations in offspring exposed to GDM in utero.
CONFLICT OF INTEREST
The authors have nothing to disclose.
Supporting information
Figure S1 Radial thickness sex differences in GDM exposure‐stratified data.
Table S1 Hippocampal gray matter volume measures in GDM‐exposed and unexposed children.
Table S2 Proportion of variance explained by variables included in the model testing for the interaction between sex and GDM exposure using partial omega‐squared (ω p 2) for the right whole hippocampus and CA1 subfield volumes.
Table S3 LSmeans of regions with interactions between sex and GDM‐exposure, stratified by GDM‐exposure.
Table S4 LSmeans of regions with interactions between sex and GDM‐exposure, stratified by sex.
ACKNOWLEDGMENTS
The authors would like to thank the families who participate in the BrainChild Study. The authors would also like to thank Ana Romero for managing the BrainChild study, Mayra Martinez and Janet Mora‐Marquez for recruiting volunteers, and the staff at Dana and David Dornsife Cognitive Neuroimaging Center at USC, particularly Dr's Bosco Tjan and J.C. Zhuang, and the staff at the USC Diabetes and Obesity Research Institute.
Lynch KM, Alves JM, Chow T, et al. Selective morphological and volumetric alterations in the hippocampus of children exposed in utero to gestational diabetes mellitus. Hum Brain Mapp. 2021;42:2583–2592. 10.1002/hbm.25390
Dr. Kirsten M. Lynch and Dr. Jasmin M. Alves should be considered joint first author.
Dr. Anny H. Xiang and Dr. Kathleen A. Page should be considered joint senior author.
Funding information American Diabetes Association, Grant/Award Number: 1‐14‐ACE‐36; National Institute of Biomedical Imaging and Bioengineering, Grant/Award Numbers: P41EB015922, U54EB020406; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: R01DK116858; National Institute of Mental Health, Grant/Award Numbers: F31MH115640, K01DK115638, R01MH094343; Southern California Clinical and Translational Science Institute, Grant/Award Number: UL1TR001855; Kaiser Permanente
DATA AVAILABILITY STATEMENT
Data is available upon reasonable request from the corresponding author.
REFERENCES
- Alves, J. M. , Luo, S. , Chow, T. , Herting, M. , Xiang, A. H. , & Page, K. A. (2020). Sex differences in the association between prenatal exposure to maternal obesity and hippocampal volume in children. Brain and Behavior, 10, e01522. 10.1002/brb3.1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association . (2012). Standards of medical care in diabetes—2012. Diabetes Care, 35(Suppl 1), S11–S63. 10.2337/dc12-s011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J. , & Worsley, K. J. (1999). The detection of local shape changes via the geometry of Hotelling's T2 fields. Annals of Statistics, 27(3), 925–942. [Google Scholar]
- CDC . (2018, October 24). About Child & Teen BMI|Healthy Weight. Retrieved from https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
- Cover, K. S. , van Schijndel, R. A. , Bosco, P. , Damangir, S. , & Redolfi, A. (2018). Can measuring hippocampal atrophy with a fully automatic method be substantially less noisy than manual segmentation over both 1 and 3 years? Psychiatry Research: Neuroimaging, 280, 39–47. 10.1016/j.pscychresns.2018.06.011 [DOI] [PubMed] [Google Scholar]
- DeRegnier, R. A. , Nelson, C. A. , Thomas, K. M. , Wewerka, S. , & Georgieff, M. K. (2000). Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. Journal of Pediatrics, 137(6), 777–784. 10.1067/mpd.2000.109149 [DOI] [PubMed] [Google Scholar]
- DeSisto, C. L. , Kim, S. Y. , & Sharma, A. J. (2014). Prevalence estimates of gestational diabetes mellitus in the United States, pregnancy risk assessment monitoring system (PRAMS), 2007‐2010. Preventing Chronic Disease, 11(12), 1–9. 10.5888/pcd11.130415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy, H. , Cattin, F. , & Risold, P.‐Y. (2013). The human hippocampus (4th ed.). Berlin, Germany: Springer Science+Business Media. [Google Scholar]
- Esposito, K. , Nappo, F. , Marfella, R. , Giugliano, G. , Giugliano, F. , Ciotola, M. , … Giugliano, D. (2002). Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation, 106(16), 2067–2072. 10.1161/01.CIR.0000034509.14906.AE [DOI] [PubMed] [Google Scholar]
- Fretham, S. J. B. , Carlson, E. S. , & Georgieff, M. K. (2011). The role of iron in learning and memory. Advances in Nutrition, 2(2), 112–121. 10.3945/an.110.000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. (1997). Testing for anatomically specified regional effects. Human Brain Mapping, 5, 133–136. [DOI] [PubMed] [Google Scholar]
- Golalipour, M. J. , Kafshgiri, S. K. , & Ghafari, S. (2012). Gestational diabetes induced neuronal loss in CA1 and CA3 subfields of rat hippocampus in early postnatal life. Folia Morphologica, 71(2), 7. [PubMed] [Google Scholar]
- Gold, S. M. , O'Connor, M. F. , Gill, R. , Kern, K. C. , Shi, Y. , Henry, R. G. , … Sicotte, N. L. (2014). Detection of altered hippocampal morphology in multiple sclerosis‐associated depression using automated surface mesh modeling. Human Brain Mapping, 35(1), 30–37. 10.1002/hbm.22154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hami, J. , Kheradmand, H. , & Haghir, H. (2014). Sex differences and laterality of insulin receptor distribution in developing rat hippocampus: An immunohistochemical study. Journal of Molecular Neuroscience, 54(1), 100–108. 10.1007/s12031-014-0255-1 [DOI] [PubMed] [Google Scholar]
- Herting, M. M. , Johnson, C. , Mills, K. L. , Vijayakumar, N. , Dennison, M. , Liu, C. , … Tamnes, C. K. (2018). Development of subcortical volumes across adolescence in males and females: A multisample study of longitudinal changes. NeuroImage, 172, 194–205. 10.1016/j.neuroimage.2018.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias, J. E. , Augustinack, J. C. , Nguyen, K. , Player, C. M. , Player, A. , Wright, M. , … Van Leemput, K. (2015). A computational atlas of the hippocampal formation using ex vivo, ultra‐high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage, 115, 117–137. 10.1016/j.neuroimage.2015.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabès, A. , Lavenex, P. B. , Amaral, D. G. , & Lavenex, P. (2011). Postnatal development of the hippocampal formation: A stereological study in macaque monkeys. The Journal of Comparative Neurology, 519(6), 1051–1070. 10.1002/cne.22549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabès, A. , Thomas, K. M. , Langworthy, S. , Georgieff, M. K. , & Nelson, C. A. (2015). Functional and anatomic consequences of diabetic pregnancy on memory in 10‐year‐old children. Journal of Developmental and Behavioral Pediatrics, 36(7), 529–535 110.1016/j.bbi.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen, G. W. , Kéry, R. , & Ge, S. (2018). The hippocampal Neuro‐Glio‐vascular network: Metabolic vulnerability and potential neurogenic regeneration in disease. Brain Plasticity, 3(2), 129–144. 10.3233/bpl-170055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsrud, S. K. , Tamnes, C. K. , Fjell, A. M. , Amlien, I. , Grydeland, H. , Sulutvedt, U. , … Walhovd, K. B. (2014). Development of hippocampal subfield volumes from 4 to 22 years: Development of hippocampal subfield volumes. Human Brain Mapping, 35(11), 5646–5657. 10.1002/hbm.22576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, M. A. , & Mejia, A. (2015). Zen and the art of multiple comparisons. Psychosomatic Medicine, 77(2), 114–125. 10.1097/PSY.0000000000000148.Zen [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi, N. , Hami, J. , Hosseini, M. , Haghir, D. , & Haghir, H. (2016). Diabetes during pregnancy enhanced neuronal death in the hippocampus of rat offspring. International Journal of Developmental Neuroscience, 51, 28–35. 10.1016/j.ijdevneu.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Lynch, K. M. , Page, K. A. , Shi, Y. , Xiang, A. H. , Toga, A. W. , & Clark, K. A. (2020). The effect of body mass index on hippocampal morphology and memory performance in late childhood and adolescence. Hippocampus, 31, 189–200. 10.1002/hipo.23280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, K. M. , Shi, Y. , Toga, A. W. , & Clark, K. A. (2018). Hippocampal shape maturation in childhood and adolescence. Cerebral Cortex, 29(9), 3651–3665. 10.1093/cercor/bhy244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, C. A. , Wewerka, S. S. , Borscheid, A. J. , DeRegnier, R. A. , & Georgieff, M. K. (2003). Electrophysiologic evidence of impaired cross‐modal recognition memory in 8‐month‐old infants of diabetic mothers. Journal of Pediatrics, 142(5), 575–582. 10.1067/mpd.2003.210 [DOI] [PubMed] [Google Scholar]
- Nold, J. L. , & Georgieff, M. K. (2004). Infants of diabetic mothers. Pediatric Clinics of North America, 51(3), 619–637 viii. [DOI] [PubMed] [Google Scholar]
- Nomura, Y. , Marks, D. J. , Grossman, B. , Yoon, M. , Loudon, H. , Stone, J. , & Halperin, J. M. (2012). Exposure to gestational diabetes mellitus and low socioeconomic status. Archives of Pediatrics & Adolescent Medicine, 166(4), 337–343. 10.1001/archpediatrics.2011.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakas, C. , Buwalda, B. , & Luiten, P. G. M. (1996). Hypoxia and brain development. Progress in Neurobiology, 49(1), 1–51. [DOI] [PubMed] [Google Scholar]
- Page, K. A. , Luo, S. , Wang, X. , Chow, T. , Alves, J. , Buchanan, T. A. , & Xiang, A. H. (2019). Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care, 42(8), 1473–1480. 10.2337/dc18-2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude, B. , Smith, S. M. , Kennedy, D. N. , & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage, 56(3), 907–922. 10.1016/j.neuroimage.2011.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Lai, R. , Morra, J. H. , Dinov, I. , Thompson, P. M. , & Toga, A. W. (2010). Robust surface reconstruction via Laplace‐Beltrami eigen‐projection and boundary deformation. IEEE Transactions on Medical Imaging, 29(12), 2009–2022. 10.1109/TMI.2010.2057441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Lai, R. , Wang, D. J. J. , Pelletier, D. , Mohr, D. , Sicotte, N. , & Toga, A. W. (2014). Metric optimization for surface analysis in the Laplace‐Beltrami embedding space. IEEE Transactions on Medical Imaging, 33(7), 1447–1463. 10.1109/TMI.2014.2313812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Morra, J. , Thompson, P. , & Toga, A. (2009). Inverse‐consistent surface mapping with Laplace‐Beltrami Eigen‐features. Information Processing in Medical Imaging, 21, 467–478. 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire, L. R. , & Zola‐Morgan, S. (1991). The medial temporal lobe memory system. Science, 253(5026), 1380–1386. [DOI] [PubMed] [Google Scholar]
- Strange, B. A. , Witter, M. P. , Lein, E. S. , & Moser, E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15(10), 655–669. 10.1038/nrn3785 [DOI] [PubMed] [Google Scholar]
- Treit, S. , Chen, Z. , Zhou, D. , Baugh, L. , Rasmussen, C. , Andrew, G. , … Beaulieu, C. (2017). Sexual dimorphism of volume reduction but not cognitive deficit in fetal alcohol spectrum disorders: A combined diffusion tensor imaging, cortical thickness and brain volume study. NeuroImage: Clinical, 15, 284–297. 10.1016/j.nicl.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner, A. M. , Manderson, J. , Lappin, T. R. J. , McCance, D. R. , Halliday, H. L. , & Sweet, D. G. (2007). Influence of maternal diabetes mellitus on fetal iron status. Archives of Disease in Childhood: Fetal and Neonatal Edition, 92(5), 399–401. 10.1136/adc.2006.097279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong, B. , Odero, G. , Rozbacher, S. , Stevenson, M. , Kereliuk, S. M. , Pereira, T. J. , … Kauppinen, T. M. (2017). Exposure to gestational diabetes mellitus induces neuroinflammation, derangement of hippocampal neurons, and cognitive changes in rat offspring. Journal of Neuroinflammation, 14(1), 1–13. 10.1186/s12974-017-0859-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga, L. , Langen, M. , Ambrosino, S. , van Dijk, S. , Oranje, B. , & Durston, S. (2014). Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage, 96, 67–72. 10.1016/j.neuroimage.2014.03.072 [DOI] [PubMed] [Google Scholar]
- Xiang, A. H. , Wang, X. , Martinez, M. P. , Getahun, D. , Page, K. A. , Buchanan, T. A. , & Feldman, K. (2018). Maternal gestational diabetes mellitus, type 1 diabetes, and type 2 diabetes during pregnancy and risk of ADHD in offspring. Diabetes Care, 41(12), 2502–2508. 10.2337/dc18-0733 [DOI] [PubMed] [Google Scholar]
- Xiang, A. H. , Wang, X. , Martinez, M. P. , Walthall, J. C. , Curry, E. S. , Page, K. , … Getahun, D. (2015). Association of maternal diabetes with Autism in offspring. JAMA, 313(14), 1425–1434. 10.1001/jama.2015.2707 [DOI] [PubMed] [Google Scholar]
- Zhu, C. , Han, T.‐L. , Zhao, Y. , Zhou, X. , Mao, X. , Qi, H. , … Zhang, H. (2018). A mouse model of pre‐pregnancy maternal obesity combined with offspring exposure to a high‐fat diet resulted in cognitive impairment in male offspring. Experimental Cell Research, 368(2), 159–166. 10.1016/j.yexcr.2018.04.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Radial thickness sex differences in GDM exposure‐stratified data.
Table S1 Hippocampal gray matter volume measures in GDM‐exposed and unexposed children.
Table S2 Proportion of variance explained by variables included in the model testing for the interaction between sex and GDM exposure using partial omega‐squared (ω p 2) for the right whole hippocampus and CA1 subfield volumes.
Table S3 LSmeans of regions with interactions between sex and GDM‐exposure, stratified by GDM‐exposure.
Table S4 LSmeans of regions with interactions between sex and GDM‐exposure, stratified by sex.
Data Availability Statement
Data is available upon reasonable request from the corresponding author.


