Abstract
Metabolic syndrome (MetS) is a major public health burden worldwide and associated with brain abnormalities. Although insulin resistance is considered a pivotal feature of MetS, its role in the pathogenesis of MetS‐related brain alterations in the general population is unclear. Therefore, in 973 participants (mean age 52.5 years) of the population‐based Rhineland Study, we assessed brain morphology in relation to MetS and insulin resistance, and evaluated to what extent the pattern of structural brain changes seen in MetS overlap with those associated with insulin resistance. Cortical reconstruction and volumetric segmentation were obtained from high‐resolution brain images at 3 Tesla using FreeSurfer. The relations between metabolic measures and brain structure were assessed through (generalized) linear models. Both MetS and insulin resistance were associated with smaller cortical gray matter volume and thickness, but not with white matter or subcortical gray matter volume. Age‐ and sex‐adjusted vertex‐based brain morphometry demonstrated that MetS and insulin resistance were related to cortical thinning in a similar spatial pattern. Importantly, no independent effect of MetS on cortical gray matter was observed beyond the effect of insulin resistance. Our findings suggest that addressing insulin resistance is critical in the prevention of MetS‐related brain changes in later life.
Keywords: atrophy, cortical thickness, insulin resistance, magnetic resonance imaging, metabolic syndrome
Both MetS and insulin resistance were associated with smaller cortical gray matter volume and thickness, and related to cortical thinning in a similar spatial pattern. Importantly, no independent effect of MetS on cortical gray matter was observed beyond the effect of insulin resistance. Our findings suggest that addressing insulin resistance is critical in the prevention of MetS‐related brain changes in later life.
1. INTRODUCTION
Metabolic syndrome (MetS) is defined as a cluster of interconnected factors, including abdominal obesity, impaired glucose metabolism, elevated blood pressure, and dyslipidemia. It is associated with an increased risk of cardiovascular diseases, diabetes mellitus type 2, stroke as well as dementia (Lakka et al., 2002; Wilson, D'Agostino, Parise, Sullivan, & Meigs, 2005; Yates, Sweat, Yau, Turchiano, & Convit, 2012), and thereby poses a major public health threat. Although MetS is likely to result from a complex interplay among different metabolic, vascular, and inflammatory pathways, insulin resistance is thought to play a pivotal role in its pathogenesis (Eckel, Alberti, Grundy, & Zimmet, 2010).
MetS and insulin resistance have been associated with changes in brain structure as assessed by magnetic resonance imaging (MRI). Many studies have focused on different components of the MetS (Beauchet et al., 2013; Friedman et al., 2014; Yates et al., 2012), yet the number of studies assessing the relation between MetS, insulin resistance, and brain structural changes, especially in the general population, is limited. Most previous studies concerning MetS and insulin resistance enrolled patients in a clinical setting and mainly focused on macro‐ or micro‐vascular changes, that is, white matter hyperintensities, lacunes or stroke (Dearborn et al., 2015; Hishikawa et al., 2015; Zhang et al., 2010). The few community‐ or population‐based studies that investigated the relation between MetS or its components and brain structure either utilized relatively rough global volumetric measures such as total brain volume (TBV), gray matter volume (GMV), white matter volume (WMV), or brain parenchymal fraction (Sala et al., 2014; Tan et al., 2011; Tiehuis et al., 2008; Tiehuis et al., 2014), or exclusively focused on the medial temporal lobe structures, especially the hippocampus (den Heijer et al., 2003; Tan et al., 2011). Only a few cohort studies used voxel‐wise morphometry, but these studies included relatively small groups of participants, usually confined to a restricted age range (Benedict et al., 2012; Kotkowski et al., 2019; Willette et al., 2013).
Currently, it is thus still unclear whether and to what extent the reported effects of MetS on brain structure are due to insulin resistance per se, or should be regarded as sequelae of its other components including obesity, dyslipidemia, and hypertension. Elucidating this distinction at population level is important as it would imply a different prioritization of preventive and therapeutic strategies against the detrimental effects of MetS on the brain. Therefore, in this population‐based study, we aimed to (1) assess the relation of MetS and insulin resistance—as measured by fasting serum insulin (FSI) and homeostatic model assessment of insulin resistance (HOMA‐IR)—with structural brain changes, and (2) evaluate to what extent the pattern of structural brain changes seen in MetS overlap with those associated with insulin resistance.
2. MATERIALS AND METHODS
2.1. Study design
This study is based on the Rhineland Study, an on‐going population‐based cohort study that was initiated in 2016, and enrolls participants aged 30 years and above at baseline from Bonn, Germany (Breteler, Stöcker, Pracht, Brenner, & Stirnberg, 2014). The study was approved by the ethics committee of the University of Bonn, Medical Faculty. The study was carried out in accordance with the recommendations of the International Conference on Harmonization (ICH) Good Clinical Practice (GCP) standards (ICH‐GCP). We obtained written informed consent from all participants in accordance with the Declaration of Helsinki.
2.2. Assessment of metabolic syndrome
To determine lipid, glucose and insulin levels, venous blood samples were collected after an overnight fast, and if applicable, before taking any insulin or insulin‐sensitizing medication. Fasting plasma glucose (FPG) concentration was measured on the Nightingale platform (Nightingale Health, Helsinki, Finland) (Soininen, Kangas, Wurtz, Suna, & Ala‐Korpela, 2015). FSI, triglyceride, and high‐density lipoprotein (HDL) cholesterol were measured using standard methods at the local clinical chemistry laboratory of the University Hospital of Bonn. HOMA‐IR, which is highly correlated with direct estimates of insulin resistance as assessed by the hyperinsulinemic‐euglycemic clamp technique (Hermans, Levy, Morris, & Turner, 1999), was calculated using the following equation: HOMA‐IR = (fasting glucose in mmol/L × fasting insulin in mIU/L)/22.5 (Matthews et al., 1985). Waist circumference was measured in underwear with an anthropometric tape (SECA 201) by a trained technician to the nearest millimeter halfway between the 12th rib and the iliac crest. Blood pressure was measured three times with an oscillometric blood pressure device (OMRON 705 IT) in a semi‐recumbent position, and the average of the second and third measurements was calculated. The participants were asked to bring the medication that they used currently or during the last year for registration.
MetS was defined according to the revised National Cholesterol Education Program Adult Treatment Panel III (NCEP‐ATP III) criteria (Grundy et al., 2005). Participants were considered to have MetS if they met three or more of the following criteria: (1) waist circumference ≥102 cm in men and ≥88 cm in women, (2) triglyceride ≥150 mg/dL or treatment for hypertriglyceridemia, (3) HDL cholesterol <40 mg/dL in men and <50 mg/dL in women or treatment for reduced HDL cholesterol, (4) blood pressure ≥ 130/85 mmHg or on antihypertensive drug treatment, and (5) FPG ≥100 mg/dL or on drug treatment for elevated plasma glucose level.
2.3. MRI acquisition
MRI scans were collected on 3 T Siemens MAGNETOM Prisma MRI scanners (Siemens Healthcare, Erlangen, Germany) equipped with 64‐channel head–neck coils in two examination sites in Bonn. The standardized protocol included a T1‐weighted multi‐echo magnetization‐prepared rapid gradient‐echo (MPRAGE) sequence (van der Kouwe, Benner, Salat, & Fischl, 2008) with 2D acceleration (Brenner, Stirnberg, Pracht, & Stocker, 2014) (acquisition time = 6.5 min, 4 echoes, repetition time = 2,560 ms, inversion time = 1,100 ms, flip angle = 7°, matrix size = 320 × 320 × 224, voxel size = 0.8 × 0.8 × 0.8 mm3), and a bandwidth‐matched T2‐weighted 3D Turbo‐Spin‐Echo (TSE) sequence using variable flip angles (Mugler & Brookeman, 2004) (SPACE, acquisition time = 5 min, repetition time = 2,800 ms, echo time = 405 ms, turbo factor = 282, matrix size = 320 × 320 × 224, voxel size = 0.8 × 0.8 × 0.8 mm3). Both sequences utilize elliptical sampling (Brenner et al., 2014) for faster acquisition.
2.4. MRI volume analysis
We performed volumetric segmentation with FreeSurfer image analysis suite version 6.0 (http://surfer.nmr.mgh.harvard.edu/) based on T1‐weighted images (Fischl, 2012; Fischl et al., 2002). In this study, we examined the associations of MetS and insulin resistance with global volumetric measures including TBV, WMV, subcortical, and cortical GMV. Each volumetric measure was adjusted for the Estimated Total Intracranial Volume (eTIV), an indicator of head size generated by FreeSurfer, by including it as a covariate in the model. One hundred and fifty‐six cases were selected and visually inspected by trained raters to check the anatomical accuracy of the FreeSurfer segmentations, which in all cases confirmed acceptable segmentation accuracy.
2.5. MRI surface analysis
We evaluated the association of MetS and insulin resistance with mean cortical thickness generated from FreeSurfer. In addition, cortical surface reconstructions were used for vertex‐wise analysis of cortical gray matter thickness. For this, the surface of each subject was registered to a spherical atlas based on folding patterns to match cortical geometry across the subjects and to construct a cortical parcellation following the Desikan‐Killiany‐Tourville (DKT) atlas (Klein & Tourville, 2012). Cortical thickness estimates were smoothed using a Gaussian kernel of 15‐mm full width at half‐ maximum. Then, we performed whole‐brain vertex‐wise analyses using generalized linear models (GLMs): (1) we assessed the effect of each predictor variable—that is, FSI, HOMA‐IR, and MetS—on cortical thickness, (2) we evaluated how those associations differ between men and women by including an interaction term between each predictor variable—that is, FSI, HOMA‐IR, and MetS—and sex, and (3) we examined to what extent the MetS‐related brain structural alteration could be explained by insulin resistance by additionally adjusting for FSI or HOMA‐IR in the GLM model of MetS. All GLMs were adjusted for age, a quadratic term for age (to account for potential non‐linear effects of age on cortical thickness), and sex. All GLM results were corrected for multiple comparisons using a false discovery rate (FDR) of 0.05 (Benjamini, Krieger, & Yekutieli, 2006). Only clusters larger than 200 mm2 were retained and labeled using the DKT atlas. Spatial information and cortical thickness estimates of vertices from FDR‐corrected significant clusters of different GLMs were extracted und used to identify overlapping regions. Final whole‐brain vertex‐wise analysis results were displayed on FreeSurfer's “fsaverage” brain surfaces using Freeview, a FreeSurfer visualization tool.
2.6. Study population
We used data from the first 2000 participants enrolled in the Rhineland Study, out of which 1,158 underwent structural MRI imaging. Participants with MRI scans were younger, had a lower waist circumference, had higher levels of HDL cholesterol but lower levels of fasting plasma glucose (FPG), FSI, and HOMA‐IR, compared to those without a scan (Table 1). Among the 1,158 participants, information for FSI, HOMA‐IR, and MetS was available for 1,059, 1,019, and 995 individuals, respectively, with complete data on insulin resistance as assessed by FSI and HOMA‐IR and MetS being available for 993 participants. Furthermore, 20 participants were excluded due to the presence of cerebral infarction or bleeding (n = 12), intracranial tumor (n = 5), or other parenchymal congenital or acquired defects (n = 3) on brain imaging. Therefore, the associations between MetS, insulin resistance, and brain structure were investigated using data from the remaining 973 participants.
TABLE 1.
Characteristics of the first 2000 participants of the Rhineland Study, with and without MRI
| Characteristic a | Without MRI | With MRI | Adjusted p value b |
|---|---|---|---|
| N | 842 | 1,158 | |
| Age, year | 57.6 (14.5) | 52.9 (13.6) | <.001 |
| Women, n (%) | 472 (56.1) | 662 (57.2) | .653 |
| Waist circumference, cm | 90.5 (14.8) | 86.6 (12.5) | <.001 |
| Triglyceride, mg/dL | 116.5 (75.1) | 111.5 (72.1) | .413 |
| HDL cholesterol, mg/dL | 62.7 (18.1) | 65.6 (18.9) | <.001 |
| Diastolic blood pressure, mmHg | 77.0 (9.8) | 76.9 (9.5) | .408 |
| Systolic blood pressure, mmHg | 129.1 (17.2) | 127.5 (15.9) | .201 |
| FPG, mmol/L | 4.2 (1.0) | 4.0 (0.6) | <.001 |
| FSI, mIU/L | 10.8 (7.8) | 9.7 (6.3) | .015 |
| HOMA‐IR, unit | 2.1 (1.9) | 1.8 (1.4) | .004 |
Note: Values are presented as mean (SD) for continuous variables and as n (%) for categorical variables.
Abbreviations: FPG, fasting plasma glucose; FSI, fasting serum insulin; HDL, high density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance.
Missing data in participants with and without MRI: waist circumference: 4 versus 9; triglyceride and HDL cholesterol: 98 versus 97; blood pressure: 17 versus 14; FPG: 58 versus 50; FSI: 99 versus 103; HOMA‐IR: 139 versus 131.
p‐value derived from linear regression model adjusted for age and sex.
2.7. Statistical analyses
Data are summarized as means (standard deviation, SD) or counts (percentage) for continuous and categorical variables, respectively. Differences between groups were compared using Student's t‐test or multivariable linear regression for continuous variables, and Pearson's chi‐square test, logistic or ordinal regression for categorical/ordinal variables. Separate multivariable linear regression models were used to examine the association between metabolic syndrome, insulin resistance and brain structural differences with adjustment for age, age‐squared, sex, and head size (only for volumetric measures). Additional sensitivity analyses were performed by adjusting the models for smoking status (i.e., current vs. non‐smoker) and education level (i.e., self‐reported highest education level was coded as low (lower secondary education or below), middle (upper secondary education to undergraduate university level) and high (postgraduate university study) according to the International Standard Classification of Education (ISCED 2011)) as a proxy for socioeconomic status. Using Bonferroni's method for multiple comparisons, for volumetric measures and mean cortical thickness statistical significance was set at a two‐tailed p < .0033 (=.05 divided by 15, that is, the number of different models applied, equaling the product of three different predictors [FSI, HOMA‐IR and MetS] and five different outcomes [TBV, WMV, subcortical and cortical GMV, and mean cortical thickness]). All statistical analyses were performed using R version 3.4 (R Core Team, 2017).
3. RESULTS
3.1. General characteristics of the study population
Among the 973 participants, there were 354 (36.4%) with one, 159 (16.3%) with two, and 104 (10.7%) with three or more components of MetS, while 356 (36.6%) of the participants had none of the MetS components. Overall, elevated blood pressure and increased waist circumference were the two most frequent components of MetS: More than half (51.0%) of the participants had elevated blood pressure defined as ≥130/85 mmHg or were on antihypertensive drug treatment (43.7% in women and 60.4% in men), and 23.2% (25.8% in women and 19.9% in men) had increased waist circumference. As shown in Table 2, individuals with MetS were on average older (mean difference [95% CI]: 6.5 [4.0, 9.0] years), more often male (53.8% vs. 42.1%), and had higher levels of fasting insulin and HOMA‐IR (age‐ and sex‐adjusted difference [95% CI]: 8.2 [7.0, 9.3] mIU/L for FSI, and 1.8 [1.6, 2.1] unit for HOMA‐IR), and a worse metabolic status (age‐ and sex‐adjusted difference [95% CI]: 16.8 [14.8, 18.7] cm for waist circumference, 104.7 [92.1, 117.3] mg/dL for triglyceride levels, −0.3 [−0.4, −0.3] mg/dL for HDL cholesterol levels, 5.9 [4.0, 7.7] mmHg for diastolic blood pressure, 9.5 [6.6, 12.3] mmHg for systolic blood pressure, and 0.5 [0.4, 0.6] mmol/L for FPG levels) compared to those without MetS. Participants with MetS tended to have a lower education level, but similar smoking status when compared to those without (Table 2).
TABLE 2.
Characteristics of participants with and without metabolic syndrome (MetS)
| Characteristic | Overall | With MetS | Without MetS | Adjusted p value a |
|---|---|---|---|---|
| N | 973 | 104 | 869 | |
| Age, year | 52.5 (13.6) | 58.3 (12.1) | 51.8 (13.6) | <.001 |
| Men, n (%) | 422 (43.4) | 56 (53.8) | 366 (42.1) | .030 |
| Waist circumference, cm | 86.6 (12.5) | 103.7 (10.8) | 84.6 (11.1) | <.001 |
| Triglyceride, mg/dL | 111.1 (71.4) | 208.9 (108.1) | 99.4 (55.1) | <.001 |
| HDL cholesterol, mg/dL | 65.7 (18.8) | 47.8 (11.6) | 67.8 (18.4) | <.001 |
| Diastolic blood pressure, mmHg b | 77.3 (9.4) | 83.3 (9.6) | 76.5 (9.1) | <.001 |
| Systolic blood pressure, mmHg b | 127.9 (15.8) | 139.9 (14.0) | 126.5 (15.4) | <.001 |
| FPG, mmol/L | 4.0 (0.6) | 4.5 (0.8) | 3.9 (0.5) | <.001 |
| FSI, mIU/L | 9.6 (6.2) | 17.1 (9.4) | 8.8 (5.1) | <.001 |
| HOMA‐IR, unit | 1.8 (1.3) | 3.4 (2.0) | 1.6 (1.0) | <.001 |
| Current smoker, n (%) | 141 (14.5) | 18 (17.3) | 123 (14.2) | .281 |
| Education level, n (%) b | <.001 | |||
| Low | 13 (1.4) | 3 (2.9) | 10 (1.2) | |
| Middle | 380 (39.5) | 59 (56.7) | 321 (37.4) | |
| High | 569 (59.1) | 42 (40.4) | 527 (61.4) |
Note: Values are presented as mean (SD) for continuous variables and as n (%) for categorical variables. Participants were considered to have MetS if they met three or more of the following criteria: (1) waist circumference ≥102 cm in men and ≥88 cm in women, (2) triglyceride ≥150 mg/dL or treatment for hypertriglyceridemia, (3) HDL cholesterol <40 mg/dL in men and <50 mg/dL in women or treatment for reduced HDL cholesterol, (4) blood pressure ≥130/85 mmHg or on antihypertensive drug treatment, and (5) FPG ≥100 mg/dL or on drug treatment for elevated plasma glucose level.
Abbreviations: FPG, fasting plasma glucose; FSI, fasting serum insulin; HDL, high density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; MetS, metabolic syndrome.
p‐value derived from linear, logistic (in case of smoking) or ordinal (in case of education) regression models, adjusted for age and sex.
Missing data in participants with and without MetS: blood pressure: 2 versus 7; education level: 0 versus 11.
3.2. Global MRI measures
The regression‐based estimates of the associations of MetS and insulin resistance with global brain MRI measures are summarized in Table 3. Higher FSI and HOMA‐IR as well as the presence of MetS, were associated with a statistically significant reduction in cortical GMV and mean cortical thickness, and a statistically non‐significant reduction in TBV. There were no obvious associations between MetS and insulin resistance indices and subcortical GMV and WMV. Additional adjustment of the models for smoking status or education level did not materially change any of the findings (Table S1).
TABLE 3.
The association of insulin resistance and MetS with MRI global measures
| Difference (95% CI) in volume (mm3) or thickness (mm) | |||||
|---|---|---|---|---|---|
| TBV | WMV | Subcortical GMV | Cortical GMV | Mean cortical thickness | |
| FSI (per SD) |
−2,438 (−5,448, 572) |
1,363 (−544, 3,270) |
115 (−102, 332) |
−2944 * (−4,468, −1,419) |
−0.008 * (−0.014, −0.003) |
| HOMA‐IR (per SD) |
−2,824 (−6,110, 461) |
1,251 (−832, 3,333) |
124 (−113, 361) |
−3231 * (−4,895, −1,567) |
−0.010 * (−0.016, −0.004) |
| MetS: with versus without |
−9,606 (−18,202, −1,010) |
344 (−5,115, 5,802) |
59 (−563, 680) |
−9275 * (−13,627, −4,922) |
−0.032 * (−0.047, −0.016) |
Note: Values represent age‐ and sex‐adjusted beta (95% CI) values from linear regression models on associations between each metabolic measure and MRI global measures. Volumetric measures were additionally adjusted for head size assessed by the Estimated Total Intracranial Volume (eTIV).
Abbreviations: FSI, fasting serum insulin; GMV, gray matter volume; HOMA‐IR, homeostasis model assessment of insulin resistance; MetS, metabolic syndrome; TBV, total brain volume; WMV, white matter volume.
p < 0.0033, that is, the Bonferroni‐adjusted cut‐off value.
3.3. Vertex‐wise cortical thickness analysis
Estimates of whole brain vertex‐wise associations between cortical thickness and FSI, HOMA‐IR and MetS are summarized in Figure 1 and Tables 4, 5, 6. The effects on cortical thickness of FSI, HOMA‐IR, and MetS showed a very similar pattern: The largest effect sizes were mainly located in the precentral cortex, the transverse and superior temporal cortex, and the cuneus as well as its neighboring regions. Cortical thinning was especially observed in those brain regions in which the effects of FSI, HOMA‐IR and MetS overlapped (mean difference [95% CI] in cortical thickness between estimates in the overlapping and the non‐overlapping regions: −0.0005 [−0.0006, −0.0004] mm for FSI, −0.0041 [−0.0042, −0.0040] mm for HOMA‐IR, and −0.0078 [−0.0080, −0.0076] mm for MetS).
FIGURE 1.
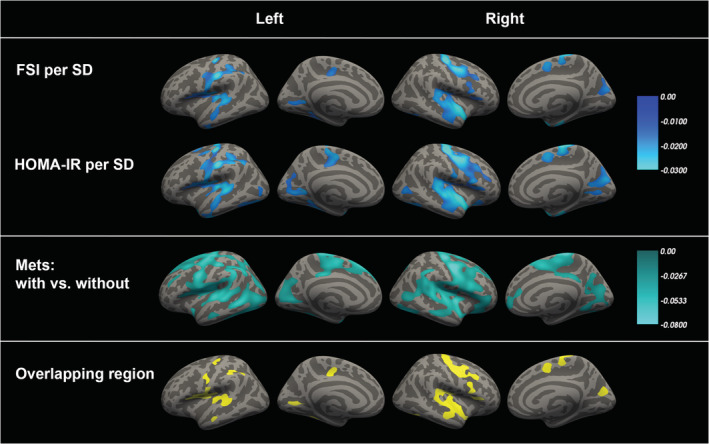
The relation between insulin resistance, metabolic syndrome and cortical thickness. Note: Age‐ and sex‐adjusted vertex‐based whole brain analysis in 973 participants (aged 30 years and above) demonstrated an inverse association between insulin resistance as represented by fasting serum insulin (FSI) and homeostasis model assessment of insulin resistance (HOMA‐IR) as well as metabolic syndrome (MetS) and cortical thickness in several brain regions. All clusters that survived statistical significance testing after multiple comparisons correction using FDR <0.05 and were larger than 200 mm2 are illustrated. The color scales indicate the age‐ and sex‐adjusted beta values per SD increase in FSI or HOMA‐IR or between participants with and without MetS. For further details, see Table 4, 5, 6
TABLE 4.
The association between FSI (per SD) and cortical thickness
| Hemisphere | Cluster size (mm2) | Anatomical region | Maximum difference in cortical thickness (mm) per SD increase in FSI | Talairach coordinates | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left | 3,183 | Postcentral | −0.031 | −53 | −18 | 33 |
| 1,406 | Transversetemporal | −0.025 | −43 | −24 | 10 | |
| 466 | Lingual | −0.020 | −22 | −53 | 6 | |
| 376 | Precentral | −0.033 | −34 | −21 | 50 | |
| 341 | Inferiortemporal | −0.022 | −48 | −14 | −32 | |
| 327 | Lingual | −0.019 | −26 | −45 | −3 | |
| 266 | Posteriorcingulate | −0.018 | −15 | −15 | 37 | |
| Right | 4,000 | Superiortemporal | −0.030 | 34 | 0 | −18 |
| 3,243 | Precentral | −0.038 | 35 | −19 | 52 | |
| 821 | Cuneus | −0.019 | 5 | −73 | 26 | |
| 380 | Parsopercularis | −0.019 | 50 | 14 | 15 | |
| 369 | Entorhinal | −0.031 | 23 | −14 | −25 | |
| 286 | Fusiform | −0.019 | 38 | −69 | −8 | |
| 266 | Superiorfrontal | −0.021 | 9 | 4 | 51 | |
| 224 | Parstriangularis | −0.018 | 44 | 23 | 7 | |
Note: Regions that survived a cluster‐wise correction for multiple comparisons using FDR < 0.05 and exceeding 200 mm2 are reported. Anatomical regions were labeled using the Desikan‐Killiany‐Tourville atlas.
TABLE 5.
The association between HOMA‐IR (per SD) and cortical thickness
| Hemisphere | Cluster size (mm2) | Anatomical region | Maximum difference in cortical thickness (mm) per SD increase in HOMA‐IR | Talairach coordinates | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left | 7,818 | Precentral | −0.038 | −34 | −21 | 50 |
| 1,204 | Lingual | −0.022 | −23 | −53 | 5 | |
| 1,147 | Lateraloccipital | −0.022 | −36 | −78 | −8 | |
| 659 | Paracentral | −0.023 | −9 | −22 | 54 | |
| 571 | Fusiform | −0.023 | −29 | −40 | −15 | |
| 328 | Superiortemporal | −0.031 | −35 | 5 | −27 | |
| 287 | Precentral | −0.020 | −35 | −6 | 45 | |
| 245 | Superiorfrontal | −0.020 | −20 | 7 | 53 | |
| 238 | Lateraloccipital | −0.017 | −41 | −76 | 1 | |
| Right | 10,599 | Precentral | −0.048 | 35 | −19 | 51 |
| 1,554 | Cuneus | −0.023 | 6 | −72 | 25 | |
| 680 | Fusiform | −0.023 | 40 | −68 | −9 | |
| 439 | Entorhinal | −0.032 | 24 | −14 | −26 | |
| 385 | Lingual | −0.019 | 25 | −52 | 4 | |
| 357 | Superiorfrontal | −0.023 | 9 | 4 | 50 | |
| 310 | Lateraloccipital | −0.019 | 45 | −70 | 7 | |
| 285 | Insula | −0.023 | 26 | 18 | −13 | |
| 246 | Postcentral | −0.021 | 64 | −8 | 14 | |
Note: Regions that survived a cluster‐wise correction for multiple comparisons using FDR < 0.05 and exceeding 200 mm2 are reported. Anatomical regions were labeled using the Desikan‐Killiany‐Tourville atlas.
TABLE 6.
The association between MetS and cortical thickness
| Hemisphere | Cluster size (mm2) | Anatomical region | Maximum difference in cortical thickness (mm) | Talairach coordinates | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left | 19,049 | Postcentral | −0.089 | −44 | −16 | 17 |
| 4,753 | Fusiform | −0.085 | −40 | −70 | −8 | |
| 2,240 | Pericalcarine | −0.057 | −14 | −73 | 7 | |
| 376 | Entorhinal | −0.078 | −26 | 1 | −29 | |
| 327 | Rostralmiddlefrontal | −0.045 | −39 | 39 | 22 | |
| 258 | Middletemporal | −0.051 | −61 | −16 | −14 | |
| Right | 19,966 | Precentral | −0.100 | 5 | −24 | 68 |
| 2,799 | Lingual | −0.074 | 19 | −54 | −4 | |
| 1,551 | Fusiform | −0.084 | 44 | −66 | −9 | |
| 1,074 | Superiorfrontal | −0.061 | 12 | 59 | −10 | |
| 231 | Superiorparietal | −0.049 | 18 | −64 | 44 | |
Note: Regions that survived a cluster‐wise correction for multiple comparisons using FDR < 0.05 and exceeding 200 mm2 are reported. Anatomical regions were labeled using the Desikan‐Killiany‐Tourville atlas.
Vertex‐wise cortical thickness analysis did not reveal any robust sex‐specific effects of FSI or MetS. Only for HOMA‐IR, a sex‐specific effect on cortical thickness was observed in a region in the left rostral middle frontal cortex (cluster size = 236 mm2, peak estimate of HOMA‐IR × sex = 0.034, Talairach coordinate [X, Y, Z] = −32.8, 50.9, −6.8, Figure S1). However, the consistency of this finding remains unclear given that this region did not exhibit sex‐specific effects when either FSI or MetS were used as predictors (data not shown).
Importantly, there was no independent effect of MetS on cortical thickness when the estimates were additionally adjusted for insulin resistance, as assessed by either FSI or HOMA‐IR, in the whole brain analysis, that is, for all brain regions the effect estimates became statistically non‐significant after multiple comparison correction using FDR < 0.05. Conversely, the effect of MetS was independent of blood pressure, since the relation between MetS and cortical thickness did not change much after adjustment for elevated blood pressure (Figure S2). Additional adjustments of the vertex‐wise models for smoking status and education level did not materially change the findings (Tables S2–S4 and Figure S3).
4. DISCUSSION
In this large‐scale population‐based study, we found that both MetS and insulin resistance were associated with a reduced cortical GMV and mean cortical thickness but not with WMV or with volumes of subcortical gray matter structures. In addition, by applying vertex‐wise whole brain analysis, we demonstrated that the effects of MetS and insulin resistance on cortical thickness show a remarkable degree of similarity, with the most pronounced effects occurring in the precentral cortex, transverse and superior temporal cortex, as well as the cuneus and its neighboring regions.
A negative association of fasting insulin, HOMA‐IR or MetS with global measures of brain structural integrity, such as TBV and brain parenchymal fraction, has been described before in relatively small‐scaled studies (Tan et al., 2011; Tiehuis et al., 2014). However, only few studies investigated the effects of these metabolic indices on specific brain compartments, including gray matter, white matter, and cortical and subcortical structures in the general population. Studies in selected cohorts have so far yielded conflicting results: An inverse relation between HOMA‐IR and both GMV and WMV was reported in elderly adults (Benedict et al., 2012), while in elderly participants from long‐lived families no association was found between insulin sensitivity or MetS and gray and white matter volumes (Akintola et al., 2015; Sala et al., 2014). We found that in the general population, TBV tended to be smaller in those with MetS or higher FSI and HOMA‐IR values. This effect was mainly attributable to the influence on cortical gray matter, either assessed through volume or thickness, rather than on white matter or subcortical gray matter.
To pinpoint the cortical regions affected by MetS and insulin resistance more precisely, we applied whole‐brain vertex‐based morphometry, which demonstrated a considerable degree of similarity in the spatial patterns of cortical thinning in relation to MetS, FSI, and HOMA‐IR. Moreover, the areas in which the effects of MetS and insulin resistance overlapped—that is, the precentral cortex, transverse and superior temporal cortex, and cuneus and its neighboring regions—exhibited the largest reduction of cortical thickness and coincided with the regions that have been associated with HOMA‐IR previously (Benedict et al., 2012; Willette et al., 2013). To the best of our knowledge, the present study is the first to assess brain morphology in relation to both MetS and insulin resistance simultaneously in the general population. Given the substantial spatial overlap between the effects of MetS and insulin resistance on brain structure, as well as the fact that no independent effect of MetS remained on cortical thickness after adjustment for insulin resistance, our findings suggest that insulin resistance per se is responsible for MetS‐related brain changes. Although assessing the contribution of other metabolic parameters besides insulin resistance on the difference between individuals with and without MetS would also be interesting, previous studies have already found that other metabolic parameters like waist circumference are strongly related to insulin resistance, and in fact, may exert their effect through induction of insulin resistance (Bastien, Poirier, Lemieux, & Després, 2014; Farin, Abbasi, & Reaven, 2006). Similarly, in our study, waist circumference had a Pearson's correlation of 0.533 and 0.530 with HOMA‐IR and FSI, respectively. Therefore, it would be difficult to disentangle any independent effect of these other metabolic parameters from the effects of insulin resistance per se in mediating the effects of MetS on brain structure. Conversely, the effect of elevated blood pressure, another important component of MetS is unlikely to be mediated through insulin resistance (Pearson's correlation of elevated blood pressure with HOMA‐IR and FSI were 0.241 and 0.223, respectively). Indeed, we found that correction for elevated blood pressure did not affect the relation between MetS and cortical thickness, supporting our hypothesis that insulin resistance per se is likely to be the main driver of MetS‐related brain changes.
Many studies have indeed found a higher prevalence of deficits in multiple cognitive domains in subjects with insulin resistance and MetS (Ekblad et al., 2017; Yates et al., 2012; Young, Mainous 3rd, & Carnemolla, 2006). Interestingly, in our study, the pattern of cortical thinning associated with both insulin resistance and MetS approximately corresponded to the regions mainly involved in motor regulation and auditory and visual information processing. We found no association with structural changes in areas associated with higher cognitive functions as for example language processing or executive function. It remains unclear to what extent impairments in other functions that are required to perform most cognitive tests, including motor dexterity as well as hearing and visual function, might actually underlie the negative association between cognitive function and insulin resistance or MetS. The associations between central motor, auditory or visual processing and insulin resistance and MetS have received little study, especially in the general population (Poh, Mohamed Abdul, Lamoureux, Wong, & Sabanayagam, 2016; Seo, Lee, & Moon, 2016). Thus, our findings warrant further assessment of the role of motor function and auditory and visual processing as mediators of the well‐established association between cognitive function and insulin resistance and MetS.
There is an increasing awareness of potential sex differences in the relation between cardio‐metabolic disorders, cognitive decline, and dementia (Ferretti et al., 2018; Gerdts & Regitz‐Zagrosek, 2019). Although some previous studies have found that cortical thickness is more vulnerable to cardio‐metabolic risk factors in women as compared to men, particularly at an older age (Kim et al., 2019; Shin et al., 2020), in our participants aged from 30 to 95 years, we did not observe any robust sex‐specific effects of FSI or MetS on cortical thickness in the vertex‐based whole brain analysis. Only a small area of the left rostral middle frontal cortex demonstrated a sex difference in the association between HOMA‐IR and cortical thickness: men tended to have less cortical thinning than women in this region. This latter result is consistent with a previous study using a different imaging analysis approach, which reported an inverse association of obesity‐related insulin resistance with cortical thickness in several predefined frontal and parietal regions, including rostral middle frontal cortex, in women older than 50, but neither in younger women nor in men (Shin et al., 2020). Therefore, both a different imaging analysis approach as well as the fact that our study population consisted of a substantial group of relatively young individuals could account for the lack of robust sex‐specific differences in the relation between metabolic factors and cortical thickness in our study.
Accumulating evidence suggests that MetS and insulin resistance are closely related to eating habits (Fabiani, Naldini, & Chiavarini, 2019; Tilles‐Tirkkonen et al., 2019), which might originate from their effects on brain structure and function (Kullmann et al., 2016). Previous studies, which were mainly conducted in obese individuals who are at a high risk of MetS and insulin resistance, reported functional alterations in several brain regions, including the striatum, prefrontal, temporal as well as insular cortex, following exposure to food cues (Scharmüller, Übel, Ebner, & Schienle, 2012; Val‐Laillet et al., 2015). Interestingly, many of these cortical regions correspond to the areas that exhibited an association with MetS and insulin resistance in our study. Furthermore, the relation between insulin resistance and food craving was shown to be mediated by activity in the striatum, insula, and thalamus in insulin‐resistant obese volunteers (Jastreboff et al., 2013). Therefore, insulin resistance might also contribute to altered eating behavior via its influence on brain function, which in turn could cause a vicious cycle that could further exacerbate insulin resistance and its detrimental effects on brain parenchyma.
The strengths of this study include its large sample size, the wide age range (i.e., 30–95 years), the population‐based design, the advanced brain imaging and the availability of data on both MetS and insulin resistance. However, our study also has some limitations. First, we used cross‐sectional observational data, which limits making inferences regarding the directionality of the effects between metabolic indices and brain structural measures. Nevertheless, based on known biological pathways, we consider it unlikely that cortical thinning would have induced metabolic disturbances. Second, we could not assess the effects of the duration of MetS or insulin resistance on brain structure as these data were not available. Finally, the prevalence of MetS was low (i.e., 10.7%) in our cohort as compared to previous studies (Carriere et al., 2014; Guallar‐Castillon et al., 2014; Scuteri et al., 2015). This likely resulted from the large proportion of young adult participants in the Rhineland Study and the required presence of brain imaging data. At early stages of the Rhineland Study, we applied extremely stringent contra‐indication criteria for MR imaging, which resulted in selection of relatively healthy people. However, we consider it unlikely that this has led to spurious results. Rather, the presence of strong effects of MetS and insulin resistance on brain structural measures in a largely asymptomatic, relatively healthy group of people underscores the importance of these findings and points to the potential of prevention of MetS‐related brain damage.
5. CONCLUSION
In this study, we found that both MetS and insulin resistance were linked to reduced cortical gray matter volume and thickness, especially in regions involved in motor regulation and auditory and visual signal processing, but not to volumetric changes in subcortical brain regions. Furthermore, we found that the effect of MetS on brain structure substantially overlapped with that of insulin resistance, implying that insulin resistance might underlie MetS‐related cortical brain atrophy. These findings suggest that screening for insulin resistance, as well as early interventions aimed at restoring insulin sensitivity, could be effective for preventing brain atrophy related to metabolic syndrome.
CONFLICT OF INTERESTS
No potential conflicts of interest relevant to this article were reported.
ETHICS APPROVAL STATEMENT AND CONSENT STATEMENT
The study was approved by the ethics committee of the University of Bonn, Medical Faculty. The study was carried out in accordance with the recommendations of the International Conference on Harmonization (ICH) Good Clinical Practice (GCP) standards (ICH‐GCP). We obtained written informed consent from all participants in accordance with the Declaration of Helsinki.
Supporting information
TABLE S1 The association of insulin resistance and MetS with MRI global measures
TABLE S2 The association between FSI (per SD) and cortical thickness
TABLE S3 The association between HOMA‐IR (per SD) and cortical thickness
TABLE S4 The association between MetS and cortical thickness
FIGURE S1 Sex difference in the association between HOMA‐IR and cortical thickness
FIGURE S2 The relation between metabolic syndrome and cortical thickness after correction for blood pressure
FIGURE S3 The relation between insulin resistance, metabolic syndrome and cortical thickness
ACKNOWLEDGMENT
The authors are grateful to the participants and staff of the Rhineland Study. The Rhineland Study is funded through the German Center for Neurodegenerative Diseases (DZNE) by the German Federal Ministry of Education and Research (BMBF) and the Ministry of Culture and Science of the German State of North Rhine‐Westphalia. R.L. was partially supported by a scholarship from China Scholarship Council (201506160104). Open Access funding enabled and organized by Projekt DEAL.
Lu R, Aziz NA, Diers K, Stöcker T, Reuter M, Breteler MMB. Insulin resistance accounts for metabolic syndrome‐related alterations in brain structure. Hum Brain Mapp. 2021;42:2434–2444. 10.1002/hbm.25377
Funding information China Scholarship Council, Grant/Award Number: 201506160104; The Rhineland Study is funded through the German Center for Neurodegenerative Diseases (DZNE) by the German Federal Ministry of Education and Research (BMBF) and the Ministry of Culture and Science of the German State of North Rhine‐Westphalia.
Contributor Information
N. Ahmad Aziz, Email: ahmad.aziz@dzne.de.
Monique M.B. Breteler, Email: monique.breteler@dzne.de.
DATA AVAILABILITY STATEMENT
The datasets for this manuscript are not publicly available because of data protection regulations. Access to data can be provided to scientists in accordance with the Rhineland Study's Data Use and Access Policy. Requests for further information or to access the datasets should be directed to RS‐DUAC@dzne.de. No applicable resources were generated or analyzed during the current study.
REFERENCES
- Akintola, A. A. , van den Berg, A. , Altmann‐Schneider, I. , Jansen, S. W. , van Buchem, M. A. , Slagboom, P. E. , … van der Grond, J. (2015). Parameters of glucose metabolism and the aging brain: A magnetization transfer imaging study of brain macro‐ and micro‐structure in older adults without diabetes. Age (Dordrecht, Netherlands), 37(4), 9802. 10.1007/s11357-015-9802-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien, M. , Poirier, P. , Lemieux, I. , & Després, J. (2014). Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in Cardiovascular Diseases, 56(4), 369–381. 10.1016/j.pcad.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Beauchet, O. , Celle, S. , Roche, F. , Bartha, R. , Montero‐Odasso, M. , Allali, G. , & Annweiler, C. (2013). Blood pressure levels and brain volume reduction: A systematic review and meta‐analysis. Journal of Hypertension, 31(8), 1502–1516. 10.1097/HJH.0b013e32836184b5 [DOI] [PubMed] [Google Scholar]
- Benedict, C. , Brooks, S. J. , Kullberg, J. , Burgos, J. , Kempton, M. J. , Nordenskjold, R. , … Schioth, H. B. (2012). Impaired insulin sensitivity as indexed by the HOMA score is associated with deficits in verbal fluency and temporal lobe gray matter volume in the elderly. Diabetes Care, 35(3), 488–494. 10.2337/dc11-2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , Krieger, A. M. , & Yekutieli, D. (2006). Adaptive linear step‐up procedures that control the false discovery rate. Biometrika, 93, 491–507. [Google Scholar]
- Brenner, D. , Stirnberg, R. , Pracht, E. D. , & Stocker, T. (2014). Two‐dimensional accelerated MP‐RAGE imaging with flexible linear reordering. Magma, 27(5), 455–462. 10.1007/s10334-014-0430-y [DOI] [PubMed] [Google Scholar]
- Breteler, M. M. , Stöcker, T. , Pracht, E. , Brenner, D. , & Stirnberg, R. (2014). MRI in the Rhineland study: A novel protocol for population neuroimaging. Alzheimer's & Dementia, 10(4), 92. [Google Scholar]
- Carriere, I. , Peres, K. , Ancelin, M. L. , Gourlet, V. , Berr, C. , Barberger‐Gateau, P. , … Akbaraly, T. (2014). Metabolic syndrome and disability: Findings from the prospective three‐city study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(1), 79–86. 10.1093/gerona/glt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn, J. L. , Schneider, A. L. , Sharrett, A. R. , Mosley, T. H. , Bezerra, D. C. , Knopman, D. S. , … Gottesman, R. F. (2015). Obesity, insulin resistance, and incident Small vessel disease on magnetic resonance imaging: Atherosclerosis risk in communities study. Stroke, 46(11), 3131–3136. 10.1161/STROKEAHA.115.010060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer, T. , Vermeer, S. E. , van Dijk, E. J. , Prins, N. D. , Koudstaal, P. J. , Hofman, A. , & Breteler, M. M. (2003). Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia, 46(12), 1604–1610. 10.1007/s00125-003-1235-0 [DOI] [PubMed] [Google Scholar]
- Eckel, R. H. , Alberti, K. G. , Grundy, S. M. , & Zimmet, P. Z. (2010). The metabolic syndrome. Lancet, 375(9710), 181–183. 10.1016/S0140-6736(09)61794-3 [DOI] [PubMed] [Google Scholar]
- Ekblad, L. L. , Rinne, J. O. , Puukka, P. , Laine, H. , Ahtiluoto, S. , Sulkava, R. , … Jula, A. (2017). Insulin resistance predicts cognitive decline: An 11‐year follow‐up of a nationally representative adult population sample. Diabetes Care, 40(6), 751–758. 10.2337/dc16-2001 [DOI] [PubMed] [Google Scholar]
- Fabiani, R. , Naldini, G. , & Chiavarini, M. (2019). Dietary patterns and metabolic syndrome in adult subjects: A systematic review and meta‐analysis. Nutrients, 11(9), 2056. 10.3390/nu11092056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin, H. , Abbasi, F. , & Reaven, G. (2006). Body mass index and waist circumference both contribute to differences in insulin‐mediated glucose disposal in nondiabetic adults. The American Journal of Clinical Nutrition, 83(1), 47–51. 10.1093/ajcn/83.1.47 [DOI] [PubMed] [Google Scholar]
- Ferretti, M. T. , Iulita, M. F. , Cavedo, E. , Chiesa, P. A. , Schumacher Dimech, A. , Santuccione Chadha, A. , … Hampel, H. (2018). Sex differences in Alzheimer disease—The gateway to precision medicine. Nature Reviews. Neurology, 14(8), 457–469. 10.1038/s41582-018-0032-9 [DOI] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- Friedman, J. I. , Tang, C. Y. , de Haas, H. J. , Changchien, L. , Goliasch, G. , Dabas, P. , … Narula, J. (2014). Brain imaging changes associated with risk factors for cardiovascular and cerebrovascular disease in asymptomatic patients. JACC: Cardiovascular Imaging, 7(10), 1039–1053.doi: 10.1016/j.jcmg.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Gerdts, E. , & Regitz‐Zagrosek, V. (2019). Sex differences in cardiometabolic disorders. Nature Medicine, 25(11), 1657–1666. 10.1038/s41591-019-0643-8 [DOI] [PubMed] [Google Scholar]
- Grundy, S. M. , Cleeman, J. I. , Daniels, S. R. , Donato, K. A. , Eckel, R. H. , Franklin, B. A. , … Blood, I. (2005). Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation, 112(17), 2735–2752. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- Guallar‐Castillon, P. , Perez, R. F. , Lopez Garcia, E. , Leon‐Munoz, L. M. , Aguilera, M. T. , Graciani, A. , … Rodriguez‐Artalejo, F. (2014). Magnitude and management of metabolic syndrome in Spain in 2008–2010: The ENRICA study. Revista Española de Cardiología, 67(5), 367–373. 10.1016/j.rec.2013.08.014 [DOI] [PubMed] [Google Scholar]
- Hermans, M. P. , Levy, J. C. , Morris, R. J. , & Turner, R. C. (1999). Comparison of tests of beta‐cell function across a range of glucose tolerance from normal to diabetes. Diabetes, 48(9), 1779–1786 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10480608 [DOI] [PubMed] [Google Scholar]
- Hishikawa, N. , Yamashita, T. , Deguchi, K. , Wada, J. , Shikata, K. , Makino, H. , & Abe, K. (2015). Cognitive and affective functions in diabetic patients associated with diabetes‐related factors, white matter abnormality and aging. European Journal of Neurology, 22(2), 313–321. 10.1111/ene.12568 [DOI] [PubMed] [Google Scholar]
- Jastreboff, A. , Sinha, R. , Lacadie, C. , Small, D. , Sherwin, R. , & Potenza, M. (2013). Neural correlates of stress‐ and food cue‐induced food craving in obesity: Association with insulin levels. Diabetes Care, 36(2), 394–402. 10.2337/dc12-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. E. , Lee, J. S. , Woo, S. , Kim, S. , Kim, H. J. , Park, S. , … Seo, S. W. (2019). Sex‐specific relationship of cardiometabolic syndrome with lower cortical thickness. Neurology, 93(11), e1045–e1057. 10.1212/wnl.0000000000008084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, A. , & Tourville, J. (2012). 101 labeled brain images and a consistent human cortical labeling protocol. Frontiers in Neuroscience, 6, 171. 10.3389/fnins.2012.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotkowski, E. , Price, L. R. , Franklin, C. , Salazar, M. , Woolsey, M. , DeFronzo, R. A. , … Fox, P. T. (2019). A neural signature of metabolic syndrome. Human Brain Mapping, 40(12), 3575–3588. 10.1002/hbm.24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann, S. , Heni, M. , Hallschmid, M. , Fritsche, A. , Preissl, H. , & Häring, H. (2016). Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiological Reviews, 96(4), 1169–1209. 10.1152/physrev.00032.2015 [DOI] [PubMed] [Google Scholar]
- Lakka, H. M. , Laaksonen, D. E. , Lakka, T. A. , Niskanen, L. K. , Kumpusalo, E. , Tuomilehto, J. , & Salonen, J. T. (2002). The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA, 288(21), 2709–2716 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12460094 [DOI] [PubMed] [Google Scholar]
- Matthews, D. R. , Hosker, J. P. , Rudenski, A. S. , Naylor, B. A. , Treacher, D. F. , & Turner, R. C. (1985). Homeostasis model assessment: Insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 28(7), 412–419 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3899825 [DOI] [PubMed] [Google Scholar]
- Mugler, J. P. , & Brookeman, J. R. (2004). Efficient spatially‐selective single‐slab 3D turbo‐spin‐Echo imaging. Proceedings on International Society for Magnetic Resonance in Medicine, 11, 695. [Google Scholar]
- Poh, S. , Mohamed Abdul, R. B. , Lamoureux, E. L. , Wong, T. Y. , & Sabanayagam, C. (2016). Metabolic syndrome and eye diseases. Diabetes Research and Clinical Practice, 113, 86–100. 10.1016/j.diabres.2016.01.016 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Sala, M. , de Roos, A. , van den Berg, A. , Altmann‐Schneider, I. , Slagboom, P. E. , Westendorp, R. G. , … van der Grond, J. (2014). Microstructural brain tissue damage in metabolic syndrome. Diabetes Care, 37(2), 493–500. 10.2337/dc13-1160 [DOI] [PubMed] [Google Scholar]
- Scharmüller, W. , Übel, S. , Ebner, F. , & Schienle, A. (2012). Appetite regulation during food cue exposure: A comparison of normal‐weight and obese women. Neuroscience Letters, 518(2), 106–110. 10.1016/j.neulet.2012.04.063 [DOI] [PubMed] [Google Scholar]
- Scuteri, A. , Laurent, S. , Cucca, F. , Cockcroft, J. , Cunha, P. G. , Manas, L. R. , … Arteries Research, C. (2015). Metabolic syndrome across Europe: Different clusters of risk factors. European Journal of Preventive Cardiology, 22(4), 486–491. 10.1177/2047487314525529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, M. , Lee, Y. S. , & Moon, S. S. (2016). Association of hearing impairment with insulin resistance, beta‐cell dysfunction and impaired fasting glucose before onset of diabetes. Diabetic Medicine, 33(9), 1275–1282. 10.1111/dme.13096 [DOI] [PubMed] [Google Scholar]
- Shin, J. , Pelletier, S. , Richer, L. , Pike, G. B. , Gaudet, D. , Paus, T. , & Pausova, Z. (2020). Adiposity‐related insulin resistance and thickness of the cerebral cortex in middle‐aged adults. Journal of Neuroendocrinology, 32(12), e12921. 10.1111/jne.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen, P. , Kangas, A. J. , Wurtz, P. , Suna, T. , & Ala‐Korpela, M. (2015). Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circulation. Cardiovascular Genetics, 8(1), 192–206. 10.1161/CIRCGENETICS.114.000216 [DOI] [PubMed] [Google Scholar]
- Tan, Z. S. , Beiser, A. S. , Fox, C. S. , Au, R. , Himali, J. J. , Debette, S. , … Seshadri, S. (2011). Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle‐aged adults: The Framingham Offspring Study. Diabetes Care, 34(8), 1766–1770. 10.2337/dc11-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiehuis, A. M. , van der Graaf, Y. , Mali, W. P. , Vincken, K. , Muller, M. , Geerlings, M. I. , & Group, S. S . (2014). Metabolic syndrome, prediabetes, and brain abnormalities on MRI in patients with manifest arterial disease: The SMART‐MR study. Diabetes Care, 37(9), 2515–2521. 10.2337/dc14-0154 [DOI] [PubMed] [Google Scholar]
- Tiehuis, A. M. , van der Graaf, Y. , Visseren, F. L. , Vincken, K. L. , Biessels, G. J. , Appelman, A. P. , … Group, S. S . (2008). Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke, 39(5), 1600–1603. 10.1161/STROKEAHA.107.506089 [DOI] [PubMed] [Google Scholar]
- Tilles‐Tirkkonen, T. , Aittola, K. , Männikkö, R. , Absetz, P. , Kolehmainen, M. , Schwab, U. , … Karhunen, L. (2019). Eating competence is associated with lower prevalence of obesity and better insulin sensitivity in Finnish adults with increased risk for type 2 diabetes: The StopDia study. Nutrients, 12(1), 104. 10.3390/nu12010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val‐Laillet, D. , Aarts, E. , Weber, B. , Ferrari, M. , Quaresima, V. , Stoeckel, L. , … Stice, E. (2015). Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage, 24(8), 1–31. 10.1016/j.nicl.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe, A. J. W. , Benner, T. , Salat, D. H. , & Fischl, B. (2008). Brain morphometry with multiecho MPRAGE. NeuroImage, 40(2), 559–569. 10.1016/j.neuroimage.2007.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette, A. A. , Xu, G. , Johnson, S. C. , Birdsill, A. C. , Jonaitis, E. M. , Sager, M. A. , … Bendlin, B. B. (2013). Insulin resistance, brain atrophy, and cognitive performance in late middle‐aged adults. Diabetes Care, 36(2), 443–449. 10.2337/dc12-0922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, P. W. , D'Agostino, R. B. , Parise, H. , Sullivan, L. , & Meigs, J. B. (2005). Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation, 112(20), 3066–3072. 10.1161/CIRCULATIONAHA.105.539528 [DOI] [PubMed] [Google Scholar]
- Yates, K. F. , Sweat, V. , Yau, P. L. , Turchiano, M. M. , & Convit, A. (2012). Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(9), 2060–2067. 10.1161/ATVBAHA.112.252759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, S. E. , Mainous, A. G., 3rd , & Carnemolla, M. (2006). Hyperinsulinemia and cognitive decline in a middle‐aged cohort. Diabetes Care, 29(12), 2688–2693. 10.2337/dc06-0915 [DOI] [PubMed] [Google Scholar]
- Zhang, C. E. , van Raak, E. P. , Rouhl, R. P. , Lodder, J. , Staals, J. , Knottnerus, I. L. , & van Oostenbrugge, R. J. (2010). Metabolic syndrome relates to lacunar stroke without white matter lesions: A study in first‐ever lacunar stroke patients. Cerebrovascular Diseases, 29(5), 503–507. 10.1159/000297967 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 The association of insulin resistance and MetS with MRI global measures
TABLE S2 The association between FSI (per SD) and cortical thickness
TABLE S3 The association between HOMA‐IR (per SD) and cortical thickness
TABLE S4 The association between MetS and cortical thickness
FIGURE S1 Sex difference in the association between HOMA‐IR and cortical thickness
FIGURE S2 The relation between metabolic syndrome and cortical thickness after correction for blood pressure
FIGURE S3 The relation between insulin resistance, metabolic syndrome and cortical thickness
Data Availability Statement
The datasets for this manuscript are not publicly available because of data protection regulations. Access to data can be provided to scientists in accordance with the Rhineland Study's Data Use and Access Policy. Requests for further information or to access the datasets should be directed to RS‐DUAC@dzne.de. No applicable resources were generated or analyzed during the current study.


