Abstract
Purpose
The aim of this study was to assess the association between the clinical status of rheumatoid arthritis (RA) and periodontitis (PD) in patients diagnosed with PD and to evaluate the impact of RA treatment on the severity of PD.
Methods
The study included 148 participants with PD, of whom 64 were also diagnosed with RA (PD+RA group), while 84 age-matched participants were rheumatologically healthy (PD-only group). PD severity was assessed by the following periodontal parameters: clinical attachment loss, probing pocket depth (PPD), bleeding on probing (BOP), alveolar bone loss, and number of missing teeth. RA disease characteristics and impact of disease were evaluated by the Disease Activity Score 28 using C-reactive protein, disease duration, RA treatment, the RA Impact of Disease tool, and the Health Assessment Questionnaire. Outcome variables were compared using parametric and non-parametric tests and associations were evaluated using regression analysis with the calculation of odds ratios (ORs).
Results
Participants in the PD+RA group had higher mean PPD values (2.81 ± 0.59 mm vs. 2.58 ± 0.49 mm, P=0.009) and number of missing teeth (6.27±4.79 vs. 3.93±4.08, P=0.001) than those in the PD-only group. A significant association was found between mean PPD and RA (OR, 2.22; 95% CI, 1.16–4.31; P=0.016). Within the PD+RA group, moderate to severe periodontal disease was significantly more prevalent among participants with higher RA disease activity (P=0.042). The use of biologic disease-modifying antirheumatic drugs (bDMARDs) was associated with a lower BOP percentage (P=0.016).
Conclusions
In patients with PD, RA was associated with a higher mean PPD and number of missing teeth. The severity of PD was affected by the RA disease clinical activity and by treatment with bDMARDs, which were associated with a significantly lower mean BOP percentage.
Keywords: Epidemiology, Periodontitis, Rheumatoid arthritis
Graphical Abstract

INTRODUCTION
Periodontitis (PD) is a chronic infectious inflammatory disease that affects tooth-supporting tissues and leads to gradual destruction of the alveolar bone [1]. PD is one of the most prevalent chronic inflammatory diseases, with the severe form of PD alone estimated to affect 11.2% of the global adult population [2]. Significant oral health-related adverse effects of PD (higher risk of tooth loss, masticatory dysfunction, etc.) coincide with a negative impact on overall health [3]. Pathogenic bacteria residing in periodontal pockets constantly interact with host cells, causing innate and adaptive immunity activation and the consequent release of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 [4,5]. Additionally, bacteria and various virulence factors may enter the blood circulation through damaged blood vessels in the periodontium and induce an inflammatory response further away from the periodontium [6]. A long-lasting inflammatory load, usually associated with severe PD, is related to an increased risk of other chronic inflammatory diseases, including type 2 diabetes, atherogenic cardiovascular disease, chronic kidney disease, and rheumatoid arthritis (RA) [3].
RA is a systemic inflammatory autoimmune disease characterized by chronic inflammation and joint tissue destruction [7]. PD and RA share common risk factors, including a genetic predisposition (the HLA-DRB1 allele of major histocompatibility class II) and smoking [8]. Moreover, the diseases are similar in terms of pathogenesis, as they involve chronic inflammation fueled by proinflammatory cytokines, connective tissue breakdown, and bone erosion [8]. Emerging evidence suggests possible PD involvement in the initiation and progression of RA through periodontal pathogen Porphyromonas gingivalis (Pg)–induced citrullination of proteins and subsequent autoantibody production [9,10]. A substantial amount of epidemiological studies reported significant correlations between PD and RA with regard to prevalence and/or severity [11]. The assumption that PD and RA are associated because of shared risk factors was recently assessed by Rodriguez-Lozano and colleagues, who reported that the association between PD severity and RA disease activity was independent of other confounders such as age, sex, and smoking habit [12]. However, other recent studies did not find correlations between RA disease activity and PD severity, especially when only patients with PD were included in the study [13,14]. It is likely that the association between PD and RA may be influenced by multiple factors, including the effects of disease-modifying antirheumatic drugs (DMARDs) [15].
The main aim of the present study was to assess the association between the clinical status of RA and PD in patients diagnosed with PD and to evaluate the impact of RA treatment on the severity of PD.
MATERIALS AND METHODS
Participants
The study participants were enrolled from Vilnius University Hospital Santaros and Zalgirio Clinics. A group of 64 participants who fulfilled the 2010 American College of Rheumatology and European League Against Rheumatism rheumatoid arthritis classification criteria [16] were invited to participate in the study and referred for periodontal examinations. Only participants diagnosed with PD according to the 2018 American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) guidelines for the classification of periodontal diseases were included in the study [17]. Additionally, a group of 84 interval age-matched participants with diagnosed PD and without autoimmune rheumatic disease were enrolled. Participants who had fewer than 8 teeth remaining, were pregnant, had diabetes mellitus, or were taking medication known to affect periodontal tissues (calcium channel blockers, antiepileptic drugs) were excluded from the research in order to eliminate any sources of bias.
The study was approved by the Vilnius regional biomedical research ethics committee (approval No. 158200-18-922-500). Written informed consent was obtained from all participants before enrollment in the study. The research was carried out in accordance with the principles of the Declaration of Helsinki of 1975, revised in 2013.
Periodontal examinations
Clinical periodontal status assessment was performed by 1 researcher, who performed full-mouth probing at 6 points and an X-ray examination. The intra-examiner agreement of the clinical assessment was calculated by means of the kappa coefficient, and a value of 0.86 was obtained for clinical attachment loss (CAL) with a difference of ±1 mm. The following measurements were recorded: 1) CAL, 2) probing pocket depth (PPD), 3) bleeding on probing (BOP), 4) alveolar bone loss (BL), and 5) the number of missing teeth. Third molars and dental implants were excluded. Based on these periodontal outcome parameters, patients were categorized using the 2018 AAP/EFP definitions of PD cases and staging of periodontal disease [17,18]. Accordingly, the presence of PD was defined as having PD-related detectable interdental CAL at ≥2 non-adjacent teeth or having a buccal or oral CAL ≥3 mm with pockets of >3 mm detectable at ≥ 2 teeth. Patients were categorized into 4 stages: stage I (initial), stage II (moderate), stage III (severe), and stage IV (advanced severe PD).
The radiographic evaluation of mean BL values followed the standardized protocol presented by Rydén and colleagues [19]. Distal and mesial sites of first and second molars were analyzed. Prior to the examinations, the image quality of the tooth sites was categorized into 3 groups (excellent, acceptable, and unacceptable) according to the modified California Dental Association (1977) guidelines [20]. Only sites with acceptable or excellent image quality, as well as only teeth with visible crowns, were assessed. Alveolar bone loss was determined as the proportion of the distance from the cementoenamel junction to the alveolar crest compared to the root length.
A questionnaire was used to record anthropometric and sociodemographic variables including age, sex, body mass index (BMI), education, smoking status, alcohol consumption, and oral health-related behaviors (the frequency of tooth brushing, approximal tooth cleaning, and annual dental prophylaxis).
Rheumatological evaluation
The clinical status of RA was assessed by an expert rheumatologist using the Disease Activity Score 28 using C-reactive protein (DAS28-CRP), which involved counting 28 tender and swollen joints using a visual analogue scale (100 mm) and measuring CRP concentrations (mg/L) [21]. Disease duration as well as current RA treatment with glucocorticoids (prednisolone or methylprednisolone), synthetic disease-modifying antirheumatic drugs (sDMARDs), such as methotrexate or sulfasalazine, and/or biologic disease-modifying anti-rheumatic drugs (bDMARDs), such as TNF-α inhibitors, IL-6 receptor antagonist, and anti-CD20 monoclonal antibodies, were recorded. Additionally, the rheumatoid arthritis impact of disease (RAID) tool and Health Assessment Questionnaire were used to assess the impact of RA on patients' quality of life and disease itself [22,23]. Seropositivity of RA (positive rheumatoid factor and/or anti-citrullinated protein antibodies [ACPA]) and CRP concentrations were recorded from patients' medical records.
Statistical analysis
For the sample size calculation, CAL was considered as the primary endpoint, with an expected difference of 1.0 mm and a standard deviation of 1.5 mm between groups. For the desired statistical power of 90% and an α error probability of 0.05, a minimum sample size of 49 participants per group was required.
Quantitative data were presented as mean±standard deviation. Inter-group comparisons of continuous variables were carried out by performing the Shapiro-Wilk normality test, followed by the Student's t-test or Mann-Whitney U test as appropriate. The χ2 test was used for categorical variables. To assess multivariate associations of RA diagnosis with periodontal outcome parameters, logistic regression was used and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. The analysis was adjusted for possible covariates including age and sex. P values less than 0.05 were considered to indicate statistical significance. All data were analyzed using SPSS version 25.0 (IBM, Armonk, NY, USA).
RESULTS
Anthropometric and sociodemographic characteristics of the study participants
In total, 148 participants with PD were included in the study: 64 RA-diagnosed participants (55 women and 9 men; mean age, 55.5±9.57 years) and 84 rheumatologically healthy participants (63 women and 21 men; mean age, 52±10.95 years). Accordingly, participants were categorized into 2 groups: 1) PD+RA and 2) PD only.
The anthropometric and sociodemographic characteristics of all study participants are summarized in Table 1. There were no differences in age, sex, smoking status, education, tooth-brushing frequency, approximal teeth cleaning, or BMI between groups. A higher number of participants in the PD+RA group abstained from alcohol (50% vs. 13.1%, P<0.001) and had 1 or fewer annual dental prophylaxis visits (79.7% vs. 59.5%, P=0.013) as compared to participants in the PD-only group.
Table 1. Comparison of anthropometric and sociodemographic characteristics between participants with PD+RA and PD only.
| Characteristics | PD+RA (n=64) | PD only (n=84) | P value | Total (n=148) | |
|---|---|---|---|---|---|
| Sex | 0.148 | ||||
| Female | 55 (85.9) | 63 (75.0) | 118 (79.7) | ||
| Male | 9 (14.1) | 21 (25.0) | 30 (20.3) | ||
| Age (yr) | 55.5±9.57) | 52.7±10.95 | 0.104 | 53.9±10.44 | |
| Tobacco usage | 0.087 | ||||
| Never | 37 (57.8) | 42 (50.0) | 79 (53.4) | ||
| Former | 12 (18.8) | 29 (34.5) | 41 (27.7) | ||
| Current smoker | 15 (23.4) | 13 (15.5) | 28 (18.9) | ||
| Alcohol consumption | <0.001 | ||||
| Never | 32 (50.0) | 11 (13.1) | 43 (29.1) | ||
| Once per week | 3 (4.7) | 20 (23.8) | 23 (15.5) | ||
| Once per month | 29 (45.3) | 53 (63.1) | 82 (55.4) | ||
| Education | 0.098 | ||||
| Secondary | 16 (25.0) | 12 (14.3) | 28 (18.9) | ||
| Higher non-university | 17 (26.6) | 17 (20.2) | 34 (23.0) | ||
| University education | 31 (48.4) | 55 (65.5) | 86 (58.1) | ||
| Annual dental prophylaxis | 0.012 | ||||
| ≤Once per year | 51 (79.7) | 50 (59.5) | 101 (68.2) | ||
| ≥Two times per year | 13 (20.3) | 34 (40.5) | 47 (31.8) | ||
| Tooth brushing frequency | 0.262 | ||||
| ≤Once per day | 20 (31.2) | 19 (22.6) | 39 (26.4) | ||
| ≥Two times per day | 44 (68.8) | 65 (77.4) | 109 (73.6) | ||
| Approximal teeth cleaning | 0.593 | ||||
| Yes | 43 (67.2) | 60 (71.4) | 103 (69.6) | ||
| No | 21 (32.8) | 24 (28.6) | 45 (30.4) | ||
| BMI (kg/m2) | 25.3±4.37 | 25.6±3.85 | 0.579 | ||
| Underweight, <18.5 | 3 (4.7) | 1 (1.2) | 4 (2.7) | ||
| Normal, 18.5–24.99 | 31 (48.4) | 42 (50.0) | 73 (49.3) | ||
| Overweight, 25–29.99 | 21 (32.8) | 31 (36.9) | 52 (35.1) | ||
| Obesity class I, 30–34.99 | 9 (14.1) | 10 (11.9) | 19 (12.8) | ||
Values are presented as number (%) or mean±standard deviation. Significant P values are presented in bold.
PD: periodontitis, RA: rheumatoid arthritis, BMI: body mass index.
Comparison of the severity of PD between the PD+RA and PD-only groups
The severity of PD among all study participants varied from initial to severe. For a more accurate representation of PD severity, the stages of PD according to Tonetti's 2018 classification of periodontal outcome parameters (including mean CAL, PPD, BOP, BL, and number of missing teeth) were utilized [18]. The distribution of PD stages representing the severity of PD was not significantly different between participants with PD+RA and those with PD only. Almost half of the participants in both groups had severe periodontal disease (stage III and IV) (Table 2).
Table 2. Comparison of PD severity according to PD stages and periodontal outcome parameters between participants with PD+RA and PD only.
| Variables | PD+RA (n=64) | PD only (n=84) | P value | Total (n=148) | |
|---|---|---|---|---|---|
| Periodontitis stages | 0.765 | ||||
| Stage I | 14 (21.9) | 20 (23.8) | 34 (23.0) | ||
| Stage II | 22 (34.4) | 24 (28.6) | 46 (31.1) | ||
| Stage III | 8 (12.5) | 15 (17.9) | 23 (15.5) | ||
| Stage IV | 20 (31.2) | 25 (29.8) | 45 (30.4) | ||
| Stage III+IV | 28 (43.7) | 40 (47.7) | 68 (45.9) | ||
| Periodontal outcome parameters | |||||
| CAL (mm) | 2.31±0.9 | 2.02±0.95 | 0.057 | 2.15±0.94 | |
| PPD (mm) | 2.81±0.59 | 2.58±0.49 | 0.009 | 2.68±0.55 | |
| BL (%) | 26.91±8.32 | 25.42±8.34 | 0.281 | 26.07±8.34 | |
| BOP (%) | 44.14±16.68 | 43.95±17.18 | 0.947 | 44.03±16.91 | |
| No. of missing teeth | 6.27±4.79 | 3.93±4.08 | 0.001 | 4.94±4.54 | |
Values are presented as number (%) or mean±standard deviation. Significant P values are presented in bold.
PD: periodontitis, RA: rheumatoid arthritis, CAL: clinical attachment loss, PPD: periodontal probing depth, BL: bone loss, BOP: bleeding on probing.
A more detailed analysis was performed comparing PD outcome parameters instead of PD stages between the participant groups. Univariate analysis revealed that PD+RA patients, as compared to participants with PD only, had a significantly higher mean PPD (2.81±0.59 vs. 2.58±0.49 mm, P=0.009) and number of missing teeth (6.27±4.79 vs. 3.93±4.08, P=0.001). No significant difference in mean CAL, BL, or BOP was observed between the groups. After adjusting for age and sex, multivariate analysis revealed that the OR of having RA was 2.22 times higher (95% CI, 1.16–4.31; P=0.016) for each 0.1-mm increase in the mean PPD.
Associations between the clinical status of RA and PD in the PD+RA group
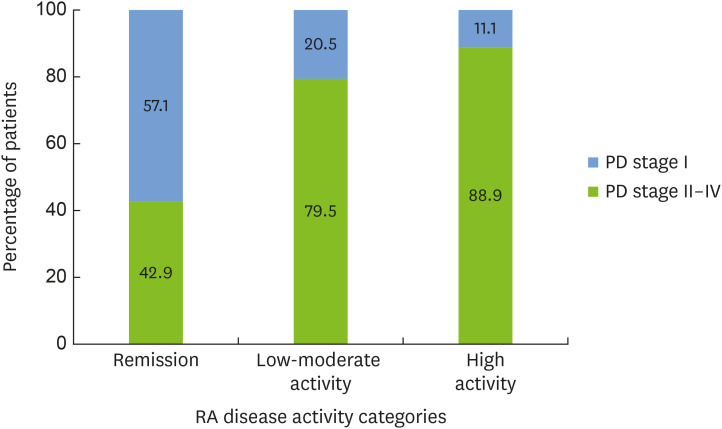
The clinical characteristics of RA patients are presented in Table 3. The majority of RA patients (89.1%) were seropositive (positive rheumatoid factor and/or ACPA). The mean disease duration was 11.47±8.59 years. RA disease activity (DAS28-CRP score) was associated with the severity of PD. Moderate to severe periodontal disease (stage II–IV) was observed in 88.9% of participants with high RA disease activity, as compared to 42.9% of patients in remission (P=0.042) (Figure 1). RA patients in remission, as compared to patients with higher clinical RA disease activity, had a lower mean CAL (1.67±0.78 vs. 2.39±0.89 mm, P=0.045). Similarly, the self-reported impact of RA disease activity represented by the RAID score was associated with CAL. A RAID value indicating remission as compared to higher disease activity was associated with a significantly lower mean CAL (1.87±0.8 vs. 2.43±0.9 mm, P=0.046).
Table 3. Rheumatoid arthritis clinical status and treatment in participants with PD+RA (n=64).
| Variables | Values | ||
|---|---|---|---|
| Rheumatoid factor/ACPA | |||
| Seropositive | 57 (89.1) | ||
| Duration of RA disease (yr) | 11.47±8.59 | ||
| Disease activity (DAS28-CRP score) | 4.37±1.27 | ||
| Remission | 7 (10.9) | ||
| Low activity | 3 (4.7) | ||
| Moderate activity | 36 (56.3) | ||
| High activity | 18 (28.1) | ||
| Self-reported impact of disease (RAID score) | 4.68±2.16 | ||
| Remission | 13 (20.3) | ||
| Low activity | 7 (10.9) | ||
| Moderate activity | 28 (43.8) | ||
| High activity | 16 (25.0) | ||
| HAQ score | 0.85±0.64 | ||
| Glucocorticoid therapy | |||
| No glucocorticoids | 21 (32.8) | ||
| Glucocorticoids | 43 (67.2) | ||
| Glucocorticoid dosage (mg/day) | 5.77±2.74 | ||
| Low dose, ≤7.5 | 28 (65.1) | ||
| Mean dose, >7.5 and ≤15 | 15 (34.9) | ||
| High dose, >15 | 0 (0.0) | ||
| Type of RA therapy | |||
| sDMARDs | 58 (90.63) | ||
| bDMARDs | 26 (40.63) | ||
| bDMARDs in combination with sDMARDs | 24 (37.5) | ||
| bDMARDs in combination with glucocorticoids | 18 (28.13) | ||
Values are presented as number (%) or mean±standard deviation.
PD: periodontitis, RA: rheumatoid arthritis, ACPA: anti-citrullinated protein antibodies, DAS28-CRP: Disease Activity Score 28 using C-reactive protein, RAID: Rheumatoid Arthritis Impact of Disease, HAQ: Health Assessment Questionnaire; RA: rheumatoid arthritis, sDMARDs: synthetic disease-modifying antirheumatic drugs; bDMARDs: biologic disease-modifying antirheumatic drugs.
Figure 1. Associations of RA disease activity with PD severity. Percentage of participants with moderate to severe (stage II–IV) as compared to initial (stage I) periodontitis among RA patients with varying disease activity categorized according to the DAS28-CRP (P=0.042).
RA: rheumatoid arthritis, PD: periodontitis, DAS28-CRP: Disease Activity Score 28 using C-reactive protein.
The associations of RA treatment (including bDMARDs, sDMARDs and glucocorticoids) with PD stages and periodontal outcome parameters were analyzed. Twenty-six participants received bDMARDs alone or in combination with sDMARDs or glucocorticoids. More than half (53.85%) of the participants receiving bDMARDs were treated with a TNF-α inhibitor (infliximab). The use of bDMARDs was related to overall lower scores for the mean CAL, PPD, BOP, BL, and number of missing teeth; however, it was only significant for BOP (Table 4). Glucocorticoid therapy and sDMARDs were not associated with PD stages and outcome parameters.
Table 4. Comparison of periodontal outcome parameters among participants with PD+RA with regard to bDMARDs treatment.
| Periodontal outcome parameters | Patients treated with bDMARDs (n=26) | Patients without bDMARDs (n=38) | P value |
|---|---|---|---|
| CAL (mm) | 2.11±0.8 | 2.45±0.95 | 0.133 |
| PPD (mm) | 2.72±0.43 | 2.88±0.67 | 0.269 |
| BL (%) | 24.85±7.05 | 28.33±8.9 | 0.101 |
| BOP (%) | 38.46±12.76 | 48.03±18.05 | 0.016 |
| No. of missing teeth | 5.5±3.76 | 6.79±5.37 | 0.294 |
Values are presented as mean±standard deviation. Significant P values are presented in bold.
PD: periodontitis, RA: rheumatoid arthritis, bDMARDs: biologic disease-modifying antirheumatic drugs, CAL: clinical attachment loss, PPD: periodontal probing depth, BL: bone loss, BOP: bleeding on probing.
DISCUSSION
The present study shows associations and complex interactions between the clinical status of PD and RA. In this study, the severity of PD was assessed based on PD stages according to Tonetti's 2018 guidelines and analysis of periodontal outcome parameters [18]. Despite the fact that the stages of PD were not significantly different between the PD+RA and PD-only groups, a more detailed analysis of periodontal outcome parameters yielded significant differences between the groups.
The present study revealed that the mean PPD was higher in the PD+RA group than in the PD-only group. Increased PPD is associated with major micro-environmental changes in the periodontal pocket, which is favored by periodontal pathogenic bacteria, including Pg [24]. Kim et al. found that higher mean PPD values correlated with higher Pg quantity in periodontal pockets among patients with RA [25]. Pg expresses a unique virulence factor—the peptidylarginine deiminase enzyme, which deiminates C-terminal arginine residues in a process called citrullination [26]. As a result, new epitopes of host proteins found in periodontal pockets (such as fibrinogen and α-enolase) are presented to the immune system. Eventually, this process may lead to a breakdown of immune tolerance and production of autoantibodies [27]. To assess the possible influence of PPD on the likelihood of developing RA, a logistic regression model was constructed. After adjusting for age and sex, it was revealed that the OR of having RA was 2.22 times higher for each 0.1-mm increase in the mean PPD. None of the other measured periodontal outcome parameters (CAL, BOP, BL, or the number of missing teeth) were associated with a higher risk of developing RA, most likely due to the weaker connections with Pg quantity.
The study also found that the number of missing teeth was higher among participants with both PD and RA than among patients with PD only. PD and caries are the major reasons for teeth extraction, while other reasons such as trauma and comorbidities are less frequent [28]. The relative importance of these indications for teeth extraction may differ with regard to age, socioeconomic status, and cultural factors [28]. An analysis of the Korea National Health and Nutrition Examination Survey, which assessed 20,297 participants, revealed an association between RA and tooth loss independently of dental caries among participants younger than 60 years [29]. The results of this study and previous findings may imply that RA is associated with a higher incidence of tooth loss; however, the exact reasons behind this association still have not been fully explained.
The present study did not reveal significant differences in mean CAL, BL, and BOP between the groups. This aligns with the finding that PD stages were not significantly different between the groups, as the staging of PD is largely based on measurements of CAL and BL. In 2016, Fuggle and colleagues [11] published a systematic review and meta-analysis of 21 studies and reported that patients with RA showed a significantly higher risk of PD and higher mean CAL, PPD, BOP values than healthy participants. Indeed, the amount of evidence linking RA with PD is overwhelming and it is clear that these illnesses are closely interconnected [30]. However, the associations between some aspects of PD and RA may be elusive depending on the perspective. In 2020, a systematic review and meta-analysis published by Hussain and colleagues reported that there was insufficient evidence to conclude that RA has a significant effect on periodontal tissues, because RA did not worsen periodontal outcome parameters (including CAL and PPD) in RA patients with PD as compared to PD controls [31]. Similarly, as in the present study, the decision to compare a PD+RA group with a PD-only group seems to have contributed to the fact that only some of the periodontal outcome parameters were significantly different between groups. It is likely that comparisons with healthy participants would have yielded more obvious associations.
The secondary aim of this study was to assess intragroup associations between the clinical status of RA and PD and to evaluate the possible influence of RA treatment on the severity of PD. Within the PD+RA group, the prevalence of moderate and severe (stages II–IV) PD was more than 2 times higher among participants with high RA disease activity than among participants in remission. Similar findings were reported in other studies, indicating that RA disease activity is correlated with the severity of PD [12]. Regarding the influence of RA treatment on PD, the present study revealed that bDMARDs had a positive effect on all measured periodontal outcome parameters; however, only the differences between mean BOP values were significant. Several studies have reported similar results revealing that bDMARDs reduced inflammatory cytokine levels (including TNF-α and IL-1β) in gingival crevicular fluid and were associated with lower gingival inflammation and BOP values [32]. However, other studies failed to report significant relationships between the use of bDMARDs and the clinical status of PD [12,33]. There may be several reasons for these discrepancies, including differences in study designs, PD case definitions, and the regimen of RA treatment. In 2017, Ziebolz and colleagues [15] suggested that RA pharmaceutical therapy over a short period affected only recent inflammation, but not the overall destruction of bone. This assumption is in accordance with the findings of the current study, as BOP is the primary indicator of active inflammation in periodontal tissues. Meanwhile, other periodontal outcome parameters (such as CAL and BL) that represent previous bone destruction were not related to the use of bDMARDs. Another possible explanation for these inconsistent findings may be related to the use of multiple DMARDs. The majority of the participants in this study were receiving bDMARDs in combination with sDMARDs or glucocorticoids. The long-term use of glucocorticoids has been shown to impair bone metabolism and reduce bone density, and is associated with increased CAL and PPD [34]. In the present study, no significant associations were revealed between glucocorticoids or other conventional sDMARDs and the severity of PD. In summary, it is likely that the systemic effects of RA treatment affect the periodontium in one way or another; however, significant challenges (e.g., the use of multiple DMARDs, dosage, length of medication therapy, etc.) need to be addressed in order to clarify this relationship.
One of the strengths of this study was the homogeneity of the groups with regard to potential confounders of PD and RA, including smoking habit, age, and sex. However, there were some significant differences between the PD+RA and PD-only groups, notably the frequency of annual dental prophylaxis, which may have a significant impact on periodontal health. Another limitation is that the design of the present study addressed the correlation between PD and RA, rather than causality. Moreover, the participants in the RA group were receiving multiple DMARDs in combination with glucocorticoids. In order to better understand the exact effect of DMARDs on the severity of PD, it would be preferable to include participants receiving a uniform treatment.
In conclusion, in patients with PD, RA was associated with an increase in mean PPD and the number of missing teeth. The use of bDMARDs for RA treatment was related to a reduced mean BOP percentage. Still, the study showed the concerning finding that severe PD (stage III+IV) was very prevalent among RA patients, implying that further research is needed to explore the underlying reasons and possible preventive strategies.
Footnotes
Funding: The authors thank all the patients and their families. This project received funding from the European Regional Development Fund (project No. 01.2.2- LMT-K-718-01-0023) under a grant agreement with the Research Council of Lithuania (LMTLT).
- Conceptualization: Adomas Rovas, Alina Puriene, Egle Punceviciene, Irena Butrimiene, Kristina Stuopelyte, Sonata Jarmalaite.
- Data curation: Adomas Rovas, Egle Punceviciene, Kristina Stuopelyte.
- Formal analysis: Adomas Rovas, Alina Puriene, Egle Punceviciene, Irena Butrimiene, Kristina Stuopelyte, Sonata Jarmalaite.
- Funding acquisition: Alina Puriene, Irena Butrimiene, Sonata Jarmalaite.
- Investigation: Adomas Rovas, Egle Punceviciene.
- Methodology: Adomas Rovas, Alina Puriene, Egle Punceviciene, Irena Butrimiene, Kristina Stuopelyte, Sonata Jarmalaite.
- Project administration: Alina Puriene.
- Supervision: Alina Puriene, Irena Butrimiene, Sonata Jarmalaite.
- Visualization: Adomas Rovas.
- Writing - original draft: Adomas Rovas.
- Writing - review & editing: Alina Puriene, Egle Punceviciene, Irena Butrimiene, Kristina Stuopelyte, Sonata Jarmalaite.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapple IL. Time to take periodontitis seriously. BMJ. 2014;348:g2645. doi: 10.1136/bmj.g2645. [DOI] [PubMed] [Google Scholar]
- 4.Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75:7–23. doi: 10.1111/prd.12221. [DOI] [PubMed] [Google Scholar]
- 5.Cheng R, Wu Z, Li M, Shao M, Hu T. Interleukin-1β is a potential therapeutic target for periodontitis: a narrative review. Int J Oral Sci. 2020;12:2. doi: 10.1038/s41368-019-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agnihotri R, Gaur S. Rheumatoid arthritis in the elderly and its relationship with periodontitis: a review. Geriatr Gerontol Int. 2014;14:8–22. doi: 10.1111/ggi.12062. [DOI] [PubMed] [Google Scholar]
- 9.Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun Rev. 2015;14:490–497. doi: 10.1016/j.autrev.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Perricone C, Ceccarelli F, Saccucci M, Di Carlo G, Bogdanos DP, Lucchetti R, et al. Porphyromonas gingivalis and rheumatoid arthritis. Curr Opin Rheumatol. 2019;31:517–524. doi: 10.1097/BOR.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 11.Fuggle NR, Smith TO, Kaul A, Sofat N. Hand to mouth: a systematic review and meta-analysis of the association between rheumatoid arthritis and periodontitis. Front Immunol. 2016;7:80. doi: 10.3389/fimmu.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez-Lozano B, González-Febles J, Garnier-Rodríguez JL, Dadlani S, Bustabad-Reyes S, Sanz M, et al. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case-control study. Arthritis Res Ther. 2019;21:27. doi: 10.1186/s13075-019-1808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosgarea R, Tristiu R, Dumitru RB, Arweiler NB, Rednic S, Sirbu CI, et al. Effects of non-surgical periodontal therapy on periodontal laboratory and clinical data as well as on disease activity in patients with rheumatoid arthritis. Clin Oral Investig. 2019;23:141–151. doi: 10.1007/s00784-018-2420-3. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, Kido JI, Ishihara Y, Omori K, Ito S, Matsuura T, et al. The KCNQ1 gene polymorphism as a shared genetic risk for rheumatoid arthritis and chronic periodontitis in Japanese adults: a pilot case-control study. J Periodontol. 2018;89:315–324. doi: 10.1002/JPER.17-0412. [DOI] [PubMed] [Google Scholar]
- 15.Ziebolz D, Rupprecht A, Schmickler J, Bothmann L, Krämer J, Patschan D, et al. Association of different immunosuppressive medications with periodontal condition in patients with rheumatoid arthritis: results from a cross-sectional study. J Periodontol. 2018;89:1310–1317. doi: 10.1002/JPER.17-0616. [DOI] [PubMed] [Google Scholar]
- 16.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 17.Caton JG, Armitage G, Berglundh T, Chapple IL, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(Suppl 20):S1–S8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 18.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 19.Rydén L, Buhlin K, Ekstrand E, de Faire U, Gustafsson A, Holmer J, et al. Periodontitis increases the risk of a first myocardial infarction: a report from the PAROKRANK study. Circulation. 2016;133:576–583. doi: 10.1161/CIRCULATIONAHA.115.020324. [DOI] [PubMed] [Google Scholar]
- 20.Ivanauskaite D, Lindh C, Rohlin M. Observer performance based on marginal bone tissue visibility in Scanora panoramic radiography and posterior bitewing radiography. Stomatologija. 2008;10:36–43. [PubMed] [Google Scholar]
- 21.van Riel PL, Renskers L. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:S40–4. [PubMed] [Google Scholar]
- 22.Gossec L, Paternotte S, Aanerud GJ, Balanescu A, Boumpas DT, Carmona L, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis. 2011;70:935–942. doi: 10.1136/ard.2010.142901. [DOI] [PubMed] [Google Scholar]
- 23.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Chaparro PJ, McCulloch JA, Mamizuka EM, Moraes AD, Faveri M, Figueiredo LC, et al. Do different probing depths exhibit striking differences in microbial profiles? J Clin Periodontol. 2018;45:26–37. doi: 10.1111/jcpe.12811. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Choi IA, Lee JY, Kim KH, Kim S, Koo KT, et al. Periodontal pathogens and the association between periodontitis and rheumatoid arthritis in Korean adults. J Periodontal Implant Sci. 2018;48:347–359. doi: 10.5051/jpis.2018.48.6.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laugisch O, Wong A, Sroka A, Kantyka T, Koziel J, Neuhaus K, et al. Citrullination in the periodontium--a possible link between periodontitis and rheumatoid arthritis. Clin Oral Investig. 2016;20:675–683. doi: 10.1007/s00784-015-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quirke AM, Lugli EB, Wegner N, Hamilton BC, Charles P, Chowdhury M, et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis. 2014;73:263–269. doi: 10.1136/annrheumdis-2012-202726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haworth S, Shungin D, Kwak SY, Kim HY, West NX, Thomas SJ, et al. Tooth loss is a complex measure of oral disease: determinants and methodological considerations. Community Dent Oral Epidemiol. 2018;46:555–562. doi: 10.1111/cdoe.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JW, Park JB, Yim HW, Lee J, Kwok SK, Ju JH, et al. Rheumatoid arthritis is associated with early tooth loss: results from Korea National Health and Nutrition Examination Survey V to VI. Korean J Intern Med (Korean Assoc Intern Med) 2019;34:1381–1391. doi: 10.3904/kjim.2018.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bingham CO, 3rd, Moni M. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol. 2013;25:345–353. doi: 10.1097/BOR.0b013e32835fb8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussain SB, Botelho J, Machado V, Zehra SA, Mendes JJ, Ciurtin C, et al. Is there a bidirectional association between rheumatoid arthritis and periodontitis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2020;50:414–422. doi: 10.1016/j.semarthrit.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Han JY, Reynolds MA. Effect of anti-rheumatic agents on periodontal parameters and biomarkers of inflammation: a systematic review and meta-analysis. J Periodontal Implant Sci. 2012;42:3–12. doi: 10.5051/jpis.2012.42.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Äyräväinen L, Leirisalo-Repo M, Kuuliala A, Ahola K, Koivuniemi R, Meurman JH, et al. Periodontitis in early and chronic rheumatoid arthritis: a prospective follow-up study in Finnish population. BMJ Open. 2017;7:e011916. doi: 10.1136/bmjopen-2016-011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beeraka SS, Natarajan K, Patil R, Manne RK, Prathi VS, Kolaparthi VS. Clinical and radiological assessment of effects of long-term corticosteroid therapy on oral health. Dent Res J (Isfahan) 2013;10:666–673. [PMC free article] [PubMed] [Google Scholar]