Abstract
Purpose
This study aimed to investigate the changes in microRNA-130a (miR-130a) and its correlation with cardiotoxicity during epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab (EC-D+T) adjuvant chemotherapy in human epidermal growth factor receptor-2-positive (HER2+) breast cancer patients.
Methods
A total of 72 HER2+ breast cancer patients who underwent resection and were scheduled to receive EC-D+T adjuvant therapy were consecutively enrolled. The expression of miR-130a and cardiotoxicity (defined as any of the following situations: 1) absolute decline of left ventricular ejection fraction (LVEF) ≥ 10% and LVEF < 53%; 2) heart failure; 3) acute coronary artery syndromes; and 4) fatal arrhythmia) were assessed every 3 months throughout the 15-month EC-D+T treatment.
Results
The accumulating cardiotoxicity rate was 12 (16.7%), of which the incidence of heart failure, acute coronary syndrome, life-threatening arrhythmias, ΔLVEF ≥ 10%, and LVEF < 53% was 0 (0.0%), 1 (1.4%), 0 (0.0%), and 12 (16.7%), respectively. Baseline miR-130a expression was negatively correlated with LVEF (%) and positively correlated with cardiac troponin I. The expression of miR-130a gradually increased in both cardiotoxicity and non-cardiotoxicity patients during EC-D+T treatment, while the increment of miR-130a was more obvious in cardiotoxicity patients compared with non-cardiotoxicity patients. Further logistic regression and receiver operating characteristic curve analysis indicated that miR-130a was an independent predictive factor for increased cardiotoxicity risk.
Conclusion
MiR-130a increases constantly and predicts high cardiotoxicity risk during EC-D+T adjuvant chemotherapy in HER2+ breast cancer patients.
Keywords: Breast neoplasms, Cardiotoxicity, Epirubicin, MicroRNA-130a, Trastuzumab
INTRODUCTION
Human epidermal growth factor receptor-2-positive (HER2+) breast cancer accounts for approximately 15% of all breast cancer cases [1,2]. For breast cancer patients, the amplification of the HER2 gene is a key prognostic factor, which is correlated with high recurrence and mortality, while it is also a therapeutic target for HER2-targeting agents [3]. As for the treatment, the current drugs specifically targeting the HER2 receptor, such as trastuzumab, lapatinib, pertuzumab, and neratinib, have improved the survival rates in both early stage and advanced HER2+ breast cancer patients. However, routine clinical use of HER2-targeting agents results in adverse cardiac events such as heart failure and decreased ejection fraction [4]. In clinical trials, the use of trastuzumab has been reported to correlate with high cardiotoxicity compared with breast cancer treatments without trastuzumab [5,6]. In addition, patients receiving adjuvant therapy need special attention because of the long-term occurrence of cardiotoxicity since they receive extended treatment [4]. For these specific breast cancer patients receiving adjuvant chemotherapy containing trastuzumab, cardiac monitoring is needed during pharmacological treatment to modify or cease the use of drugs.
MicroRNAs (miRNAs) are key regulators of biological functions as well as various pathological progressions [7]. Their roles in heart development and damage have been revealed in recent years, and a few studies have reported the predictive value of microRNAs on chemotherapy-induced cardiotoxicity in cancer treatment [8,9,10]. MiR-130a is abundantly expressed in the heart and lung and regulates cardiac pathology by regulating multiple genes and signaling pathways [7,8,11,12,13,14,15]. For instance, miR-130a overexpression is associated with the disease phenotype characteristic of arrhythmogenic cardiomyopathy by downregulating connexin43, and inhibition of miR-130a protects cardiomyocytes from hypoxia-triggered apoptosis by targeting Smad4 [7,11,14]. As for its value in drug-induced cardiotoxicity, downregulation of miR-130a has been reported to reduce anthracycline-induced cardiotoxicity in a mouse model [8], while the clinical implication of miR-130a in breast cancer regarding drug-related cardiotoxicity is rarely discussed. Therefore, in this study, we investigated the correlation of miR-130a with cardiotoxicity in HER2+ breast cancer patients who received epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab (EC-D+T) adjuvant chemotherapy.
METHODS
Patients
During the period from July 2016 to September 2018, a total of 72 HER2+ breast cancer patients who underwent resection and were scheduled to receive EC-D+T adjuvant therapy in our hospital were consecutively enrolled in this study. The adult women with histologically and immunohistochemically confirmed HER2+ breast cancer were eligible if they met the following criteria: 1) underwent resection; 2) were scheduled to receive EC-D+T adjuvant chemotherapy; 3) left ventricular ejection fraction (LVEF) ≥ 55%; 4) Eastern Cooperative Oncology Group (ECOG) performance score ≤ 1; and 5) life expectancy ≥ 12 months. The major exclusion criteria were as follows: 1) history of neoadjuvant therapy; 2) obvious cardiac function abnormality (congenital heart defect, arrhythmia, heart failure, etc.); 3) complicated with or history of other malignancies; 4) severe infection; and 5) pregnancy or lactation. This study was approved by the ethics committee of our hospital (approval number: 2018090033). All patients signed informed consent forms.
Data collection
The basic characteristics of patients were recorded before initiation of adjuvant therapy (M0), which included age, body mass index (BMI), smoking status, hypertension, hyperlipidemia, diabetes mellitus, hyperuricemia, chromic kidney disease, ECOG performance score, LVEF level, cardiac troponin I (cTnI), and N-terminal pro-brain natriuretic peptide (NT-proBNP). In addition, LVEF was assessed based on the recommendations of the American Society of Echocardiography [16].
Adjuvant therapy
The EC-D+T regimen was administered to patients as follows 1) first 3 months: 100 mg/m2 epirubicin (E) and 600 mg/m2 cyclophosphamide (C) were intravenously infused every 3 weeks for 4 cycles, respectively; 2) 3–6 months: 75–100 mg/m2 docetaxel was intravenously infused every 3 weeks for 4 cycles; 4 mg/kg trastuzumab was intravenously infused at the first week concurrently with docetaxel, then a dose of 2 mg/kg weekly for a subsequent 11 weeks; 3) 6–15 months: 6 mg/kg trastuzumab was intravenously infused every 3 weeks.
Follow-up and sample collection
Intensive regular follow-up was conducted for 15 months after the initiation of adjuvant therapy. Peripheral blood samples were collected at M0, 3 months after initiation of adjuvant therapy (M3), 6 months after initiation of adjuvant therapy (M6), 9 months after initiation of adjuvant therapy (M9), 12 months after initiation of adjuvant therapy (M12), and 15 months after initiation of adjuvant therapy (M15). The plasma samples were separated from peripheral blood samples by centrifugation, and the level of miR-130a in plasma samples was detected by reverse transcription quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR
The expression of miR-130a was detected by RT-qPCR. In brief, RNA was extracted using the QIAamp RNA Blood Mini Kit (Qiagen, Duesseldorf, Nordrhein-Westfalen, Germany) and then reverse transcribed to cDNA template using ReverTra Ace® qPCR RT Master Mix (Toyobo, Osaka, Kansai, Japan) according to the manufacturer's instructions. The qPCR was performed using THUNDERBIRD® SYBR® qPCR Mix (Toyobo, Osaka, Kansai, Japan), and U6 was used as an internal reference. The primers used were as follows: miR-130a forward primer (5′→3′): ACACTCCAGCTGGGGCTCTTTTCACATTGT, reverse primer (5′→3′): TGTCGTGGAGTCGGCAATTC; U6 forward primer (5′→3′): CTCGCTTCGGCAGCACATATACTA, reverse primer (5′→3′): ACGAATTTGCGTGTCATCCTTGC. Relative expression of miR-130a was calculated using the 2−ΔΔCt method.
Cardiotoxicity assessment
Apart from M0, LVEF was also assessed at M3, M6, M9, M12, and M15, and the absolute decline of LVEF from M0 (ΔLVEF) was calculated for each visit. After initiation of adjuvant therapy, cardiotoxicity was assessed according to previous studies [17,18], which was defined as any of the following situations: 1) ΔLVEF ≥ 10% and LVEF < 53%; 2) heart failure (defined as LVEF < 40 or BNP > 35 ng/L plus NT-pro-BNP > 125 ng/L) [19]; 3) acute coronary artery syndromes; and 4) fatal arrhythmia.
Statistical analysis
Data were processed and analyzed with the use of SPSS 24.0 software (IBM, Armonk, USA). Figures were plotted with the use of GraphPad Prism 7.01 software (GraphPad Software, La Jolla, USA). Based on their normality, quantitative data were displayed as the mean ± standard deviation (SD) or median with interquartile range (IQR). Qualitative data were expressed as counts and percentages. The correlation between quantitative data was estimated using Spearman's rank correlation test. The difference in miR-130a among different visits was analyzed by one-way analysis of variance (ANOVA) for repeated measurements. The difference in miR-130a between the two groups was determined by Wilcoxon rank sum test. A univariate logistic regression model was used to analyze the factors predicting cardiotoxicity risk, and a multivariate logistic regression model (conditional: forward) was used to screen independent factors predicting cardiotoxicity risk. The predictive performance of single independent factors or combined independent factors for cardiotoxicity risk was assessed by plotting the receiver operating characteristic (ROC) curve and calculating the area under the curve (AUC) with 95% confidence interval (CI). All tests were two-sided, and a p-value < 0.05 was considered significant.
RESULTS
Baseline characteristics of HER2+ breast cancer patients
Seventy-two patients with a mean age of 52.3 ± 7.6 years were enrolled, of whom 13 (18.1%), 13 (18.1%), 11 (15.3%), 5 (6.9%), 12 (16.7%), and 5 (6.9%) patients were smokers or complicated with hypertension, hyperlipidemia, diabetes mellitus, hyperuricemia, and chromic kidney disease, respectively (Table 1). In addition, 55 (76.4%) patients had an ECOG performance score of 0, and 17 (23.6%) patients had a score of 1. The LVEF, cTnl, and NT-proBNP levels were 68.6% ± 4.3%, 31.0 (10.3–66.3) pg/mL and 62.5 (52.0–88.5) ng/mL, respectively.
Table 1. Baseline characteristics.
| Items | Breast cancer patients (n = 72) | |
|---|---|---|
| Age (yr) | 52.3 ± 7.6 | |
| BMI (kg/m2) | 22.1 ± 2.1 | |
| Smoke | ||
| No | 59 (81.9) | |
| Yes | 13 (18.1) | |
| Hypertension | ||
| No | 59 (81.9) | |
| Yes | 13 (18.1) | |
| Hyperlipidemia | ||
| No | 61 (84.7) | |
| Yes | 11 (15.3) | |
| Diabetes mellitus | ||
| No | 67 (93.1) | |
| Yes | 5 (6.9) | |
| Hyperuricemia | ||
| No | 60 (83.3) | |
| Yes | 12 (16.7) | |
| Chromic kidney disease | ||
| No | 67 (93.1) | |
| Yes | 5 (6.9) | |
| ECOG performance score | ||
| 0 | 55 (76.4) | |
| 1 | 17 (23.6) | |
| LVEF (%) | 68.6 ± 4.3 | |
| cTnI (pg/mL) | 31.0 (10.3–66.3) | |
| NT-proBNP (ng/mL) | 62.5 (52.0–88.5) | |
Values are presented as mean ± standard deviation, number (%), or median (interquartile range).
BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; LVEF = left ventricular ejection fraction; cTnI = cardiac troponin I; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Correlation of miR-130a with cardiac function indexes
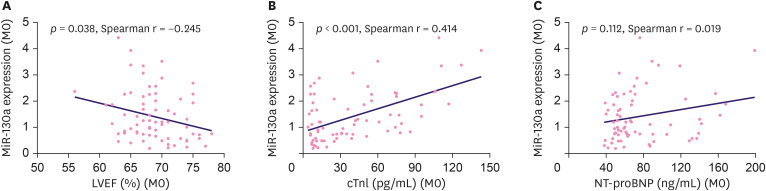
Baseline miR-130a expression was negatively correlated with LVEF (%) (p = 0.038, Spearman r = −0.245) (Figure 1A) and positively correlated with cTnl (p < 0.001, Spearman r = 0.414) (Figure 1B), but not with NT-proBNP (p = 0.112, Spearman r = 0.019) (Figure 1C) in HER2+ breast cancer patients.
Figure 1. MiR-130a's correlation with cardiac function. The correlation of baseline miR-130a with LVEF (A), cTnl (B), and NT-proBNP (C).
MiR-130a = microRNA 130a; LVEF = left ventricular ejection fraction; cTnI = cardiac troponin I; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Occurrence of cardiotoxicity
The accumulating occurrences of cardiotoxicity at different time points are listed in Table 2. In general, the accumulating occurrence of heart failure, acute coronary syndrome, life-threatening arrhythmias, ΔLVEF ≥ 10%, and LVEF < 53% were 0 (0.0%), 1 (1.4%), 0 (0.0%), and 12 (16.7%), respectively, during the whole EC-D+T treatment duration (M15), and the total cardiotoxicity was 12 (16.7%) (Table 2).
Table 2. Accumulating cardiotoxicity occurrence rate.
| Items | M0 | M3 | M6 | M9 | M12 | M15 |
|---|---|---|---|---|---|---|
| Heart failure | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Acute coronary syndrome | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| Life-threatening arrhythmias | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ΔLVEF ≥ 10% and LVEF < 53% | - | 0 (0.0) | 6 (8.3) | 9 (12.5) | 11 (15.3) | 12 (16.7) |
| Cardiotoxicity | - | 0 (0.0) | 6 (8.3) | 9 (12.5) | 11 (15.3) | 12 (16.7) |
Values are presented as number (%).
M0 = before initiation of adjuvant therapy; M3 = 3 months after initiation of adjuvant therapy; M6 = 6 months after initiation of adjuvant therapy; M9 = 9 months after initiation of adjuvant therapy; M12 = 12 months after initiation of adjuvant therapy; M15 = 15 months after initiation of adjuvant therapy; LVEF = left ventricular ejection fraction; ΔLVEF = absolute decline of LVEF from M0.
Correlation of miR-130a with cardiotoxicity
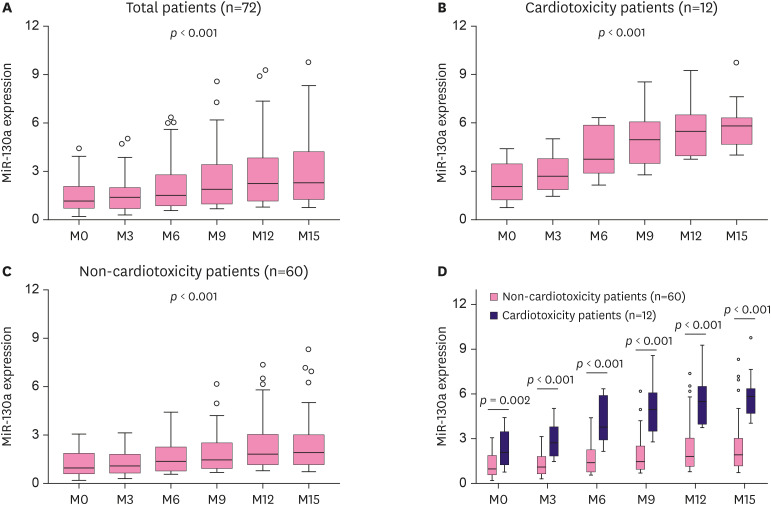
One-way ANOVA for repeated measurement illustrated that the miR-130a expression during EC-D+T treatment increased with time in all patients (p < 0.001) (Figure 2A), cardiotoxicity patients (p < 0.001) (Figure 2B), and non-cardiotoxicity patients (p < 0.001) (Figure 2C), among which the increment of miR-130a was greater in cardiotoxicity patients than in non-cardiotoxicity patients. Further, two-group comparison showed that miR-130a expression was higher in cardiotoxicity patients than in non-cardiotoxicity patients at each time point during EC-D+T treatment (all p < 0.05) (Figure 2D).
Figure 2. MiR-130a expression. MiR-130a expression at different time points in total patients. (A), cardiotoxicity patients (B), non-cardiotoxicity patients (C), and comparison of miR-130a between cardiotoxicity and non-cardiotoxicity patients at each time point throughout the treatment duration (D).
MiR-130a = microRNA-130a.
Factors predicting cardiotoxicity risk
Based on univariate logistic regression analysis, baseline miR-130a (p = 0.001, odds ratio [OR] = 3.287), age (p = 0.002, OR = 1.217), hyperuricemia (p = 0.021, OR = 9.667), cTnl (p = 0.001, OR = 1.032), and NT-proBNP (p = 0.006, OR = 1.023) were correlated with higher EC-D+T treatment-induced cardiotoxicity risk (Table 3). Further multivariate logistic regression analysis indicated that baseline miR-130a (p = 0.020, OR = 3.827), age (p = 0.038, OR = 1.182), diabetes mellitus (p = 0.016, OR = 27.771), and NT-proBNP (p = 0.048, OR = 1.025) were independent predictive factors for high EC-D+T treatment-induced cardiotoxicity risk in HER2+ breast cancer patients (Table 4). In addition, considering that hypertension medication might have a cardioprotective effect, we further analyzed the correlation of hypertension medication with cardiotoxicity risk, which indicated no correlation (Supplementary Table 1).
Table 3. Univariate logistic regression analysis of factors predicting cardiotoxicity risk.
| Items | Univariate logistic regression model | ||
|---|---|---|---|
| p-value | OR | 95% CI | |
| MiR-130a | 0.001 | 3.287 | 1.600–6.752 |
| Age | 0.002 | 1.217 | 1.072–1.382 |
| BMI | 0.077 | 1.304 | 0.972–1.751 |
| Smoke | 0.116 | 2.857 | 0.771–10.594 |
| Hypertension | 0.143 | 2.833 | 0.703–11.419 |
| Hyperlipidemia | 0.143 | 2.833 | 0.703–11.419 |
| Diabetes mellitus | 0.069 | 3.786 | 0.901–15.913 |
| Hyperuricemia | 0.021 | 9.667 | 1.414–66.071 |
| Chromic kidney disease | 0.402 | 1.889 | 0.427–8.351 |
| ECOG performance score | 0.171 | 3.800 | 0.562–25.693 |
| LVEF | 0.155 | 0.894 | 0.767–1.043 |
| cTnI | 0.001 | 1.032 | 1.012–1.052 |
| NT-proBNP | 0.006 | 1.023 | 1.007–1.040 |
OR = odds ratio; CI = confidence interval; MiR-130a = micro RNA-130a; BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; LVEF = left ventricular ejection fraction; cTnI = cardiac troponin I; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Table 4. Multivariate logistic regression analysis of factors independently predicting cardiotoxicity risk.
| Items | Multivariate logistic regression model (conditional: forward) | ||
|---|---|---|---|
| p-value | OR | 95% CI | |
| MiR-130a | 0.020 | 3.827 | 1.241–11.805 |
| Age | 0.038 | 1.182 | 1.009–1.385 |
| Diabetes mellitus | 0.016 | 27.771 | 1.870–412.356 |
| NT-proBNP | 0.048 | 1.025 | 1.000–1.051 |
OR = odds ratio; CI = confidence interval; MiR-130a = microRNA-130a; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Predictive model for cardiotoxicity risk by ROC curve
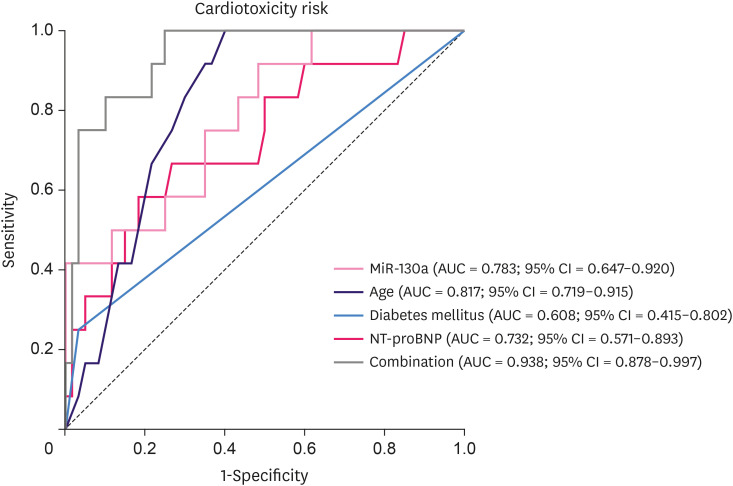
The independent predictive factors for cardiotoxicity risk in HER2+breast cancer patients receiving EC-D+T adjuvant chemotherapy were included in the following ROC curve analysis for construction of a predictive model. Baseline miR-130a (AUC = 0.783; 95% CI = 0.647–0.920), age (AUC = 0.817; 95% CI = 0.719–0.915), and NT-proBNP (AUC = 0.732; 95% CI = 0.571–0.893) could distinguish cardiotoxicity patients from non-cardiotoxicity patients, respectively, whereas diabetes mellitus (AUC = 0.608; 95% CI = 0.415–0.802) could not (Figure 3). The combination of these factors was of particularly good value in predicting EC-D+T treatment-induced cardiotoxicity in HER2+ breast cancer patients (AUC = 0.938; 95% CI = 0.878–0.997).
Figure 3. The predictive model for cardiotoxicity risk. ROC curve analysis for the value of miR-130a, age, diabetes mellitus, and NT-proBNP in distinguishing cardiotoxicity patients from non-cardiotoxicity patients.
MiR-130a = microRNA-130a; NT-proBNP = N-terminal pro-brain natriuretic peptide; ROC = receiver operating characteristic; AUC = area under the curve; CI = confidence interval.
DISCUSSION
Evidence shows that HER2+ breast cancer patients receiving the combination therapy of anthracyclines plus trastuzumab present with especially high cardiotoxicity risk [9]. The detailed mechanism of action may be that inhibition of the HER2 receptor diminishes pro-proliferation in cardiomyocytes by attenuating ATP or nitric oxide production and accelerating the pro-apoptotic process by increasing the cytochrome‐c release or intracellular calcium concentration [20]. In addition, during the metabolism of anthracyclines, large quantities of superoxide anion free radicals are produced, which causes oxidative stress to the cells, and anthracyclines also impair iron homeostasis, which produces reactive oxygen species and leads to cell damage [21]. RNA patterns are believed to facilitate clinical diagnosis and prognostic determination, especially in cancers, in which miRNAs are extensively studied. As for drug-related cardiotoxicity, a few RNA patterns have been revealed to play central roles. For instance, overexpression of miR-320a aggravates doxorubicin-induced cardiotoxicity via vessel homeostasis [10]. MiR-1 has been reported as an independent predictive factor for doxorubicin-induced cardiotoxicity in breast cancer patients [22]. As for HER2+ breast cancer patients who receive EC-D+T adjuvant chemotherapy, the predictive value of miRNAs for cardiotoxicity is still limited.
MiR-130a belongs to the miR-130 family that is situated on chromosomes 11 and 22 [23]. It is expressed in cardiac tissue, and its upregulation in a mouse model leads to delayed heart development with thinner ventricular muscle; the inhibition protects cardiac myocytes from hypoxia-triggered apoptosis [11,24]. In addition, miR-130a targets connexin43 and desmocollin 2 in cardiomyocytes, which results in cardiac arrhythmias [7,14]. These findings address the involvement of miR-130a in cardiac myopathy. In our study, we initially observed that baseline miR-130a was negatively correlated with LVEF and positively correlated with cTnl in HER2+ patients who underwent EC-D+T adjuvant chemotherapy, suggesting that miR-130a is associated with cardiac damage and reduced cardiac function. This was consistent with previous evidence in animal models that miR-130a contributes to a higher risk of cardiovascular events, which could be attributed to the fact that miR-130a regulates the cardiac gap junction protein connexin43 as well as desmocollin 2 and impaired cardiac rhythm [14]. Another explanation was that elevation of miR-130a increased cardiomyocyte apoptosis, reduced autophagy, and thereby promoted hypoxia/reoxygenation injury by targeting autophagy-related gene 14 [24]. Additionally, miR-130a was upregulated in patients with heart failure and contributed to Ang II‐induced cardiac fibrosis [25].
In the cardiotoxicity assessment, the cumulative incidence of cardiotoxicity was 16.7%, and the level of miR-130a was higher in cardiotoxicity patients than in non-cardiotoxicity patients throughout the treatment period, which implied the positive correlation of miR-130a with cardiotoxicity in HER2+ breast cancer patients who underwent adjuvant EC-D+T. Moreover, further analysis validated that baseline miR-130a was an independent predictive factor for EC-D+T treatment-induced cardiotoxicity risk. The explanation was that, as a miRNA targeting peroxisome proliferator-activated receptor γ, miR-130a enhances myocardial oxidative stress and increases anthracycline-induced toxicity in cardiac cells [8]. In addition, as explained above, miR-130a was correlated with higher susceptibility to hypoxia injury, cardiomyocyte apoptosis, and cardiac fibrosis. Therefore, it could be speculated that patients with high miR-130a expression were at higher risk of cardiotoxicity [14,24,25]. In addition, the use of anthracyclines plus trastuzumab promoted oxidative stress as well as apoptosis of cardiomyocytes, which led to cardiotoxicity in HER2+ breast cancer patients [20,21]. It was also noted that the expression of miR-130a was gradually increased in both cardiotoxicity and non-cardiotoxicity HER2+ breast cancer patients who underwent adjuvant EC-D+T, and the increment of miR-130a was more obvious in cardiotoxicity patients than in non-cardiotoxicity patients. This could be because the chemotherapy drugs induce the production of superoxide anion free radicals and reactive oxygen species that cause oxidative stress and damage to cardiomyocytes, which increases the level of circulating miR-130a [26]. Besides, since miR-130a is associated with cardiac damage, cardiotoxicity patients might experience a greater increase in miR-130a levels.
Furthermore, we performed ROC curve analysis to evaluate the value of baseline miR-130a and constructed a predictive model (including independent predictive factors) for cardiotoxicity in HER2+ breast cancer patients who underwent adjuvant EC-D+T. It was displayed that baseline miR-130a, age, and NT-proBNP, individually, but not diabetes mellitus, were able to distinguish cardiotoxicity patients from non-cardiotoxicity patients, whereas the combination of these factors was of exceptionally good predictive value. This predictive model included accessible and easily detectable factors, which could potentially be used in clinical settings to monitor the risk of cardiotoxicity in HER2+ breast cancer patients receiving trastuzumab plus anthracyclines adjuvant chemotherapy.
In this study, we disclosed the correlation of miR-130a with cardiotoxicity in HER2+ breast cancer patients who underwent EC-D+T adjuvant chemotherapy, but some limitations need to be noted. Since this was a clinical study, the detailed mechanism of action of miR-130a in causing cardiac damage and facilitating drug-induced cardiotoxicity should be validated by cellular experiments. Regarding echocardiography, the measurement of LVEF could be different according to heart rate, loading condition, or sonographer, which might cause bias. In addition, the findings should be validated in a multi-center study with a larger sample size.
In conclusion, miR-130a constantly increases during EC-D+T adjuvant chemotherapy, and it correlates with poor cardiac function indexes as well as increased EC-D+T treatment-induced cardiotoxicity risk in HER2+ breast cancer patients.
Footnotes
Funding: This work was supported by Medical Science Research Project of Hebei Health and Health Commission Project Plan (20181670).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Feng Q, Ren Y.
- Data curation: Hou A, Guo J, Wang B, Bai Z.
- Formal analysis: Feng Q, Ren Y, Mao Z, Liu S, Hou X.
- Investigation: Hou A, Guo J, Wang B, Bai Z.
- Visualization: Feng Q, Ren Y.
- Writing - original draft: Feng Q, Hou A, Guo J, Wang B, Bai Z.
- Writing - review & editing: Ren Y, Mao Z, Liu S, Hou X.
SUPPLEMENTARY MATERIAL
Correlation of hypertension therapy with the cardiotoxicity induced by chemotherapy
References
- 1.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 2.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol. 2019;11:1758835919833519. doi: 10.1177/1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerusalem G, Lancellotti P, Kim SB. HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Res Treat. 2019;177:237–250. doi: 10.1007/s10549-019-05303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balduzzi S, Mantarro S, Guarneri V, Tagliabue L, Pistotti V, Moja L, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;2014:CD006242. doi: 10.1002/14651858.CD006242.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Mazurek SR, Calway T, Harmon C, Farrell P, Kim GH. MicroRNA-130a regulation of desmocollin 2 in a novel model of arrhythmogenic cardiomyopathy. MicroRNA. 2017;6:143–150. doi: 10.2174/2211536605666161109111031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pakravan G, Foroughmand AM, Peymani M, Ghaedi K, Hashemi MS, Hajjari M, et al. Downregulation of miR-130a, antagonized doxorubicin-induced cardiotoxicity via increasing the PPARγ expression in mESCs-derived cardiac cells. Cell Death Dis. 2018;9:758. doi: 10.1038/s41419-018-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L, et al. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018;15:284–296. doi: 10.1016/j.redox.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Z, Zhao Y, Li H, Yan M, Zhou L, Chen C, et al. miR-320a mediates doxorubicin-induced cardiotoxicity by targeting VEGF signal pathway. Aging (Albany NY) 2016;8:192–207. doi: 10.18632/aging.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Du Y, Cao J, Gao Q, Li H, Chen Y, et al. MiR-130a inhibition protects rat cardiac myocytes from hypoxia-triggered apoptosis by targeting Smad4. Kardiol Pol. 2018;76:993–1001. doi: 10.5603/KP.a2018.0040. [DOI] [PubMed] [Google Scholar]
- 12.Zhou T, Qin G, Yang L, Xiang D, Li S. LncRNA XIST regulates myocardial infarction by targeting miR-130a-3p. J Cell Physiol. 2019;234:8659–8667. doi: 10.1002/jcp.26327. [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Wang X, Ha T, Hu Y, Liu L, Zhang X, et al. Attenuation of cardiac dysfunction and remodeling of myocardial infarction by microRNA-130a are mediated by suppression of PTEN and activation of PI3K dependent signaling. J Mol Cell Cardiol. 2015;89:87–97. doi: 10.1016/j.yjmcc.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osbourne A, Calway T, Broman M, McSharry S, Earley J, Kim GH. Downregulation of connexin43 by microRNA-130a in cardiomyocytes results in cardiac arrhythmias. J Mol Cell Cardiol. 2014;74:53–63. doi: 10.1016/j.yjmcc.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim GH, Samant SA, Earley JU, Svensson EC. Translational control of FOG-2 expression in cardiomyocytes by microRNA-130a. PLoS One. 2009;4:e6161. doi: 10.1371/journal.pone.0006161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Kitayama H, Kondo T, Sugiyama J, Kurimoto K, Nishino Y, Kawada M, et al. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer. 2017;24:774–782. doi: 10.1007/s12282-017-0778-8. [DOI] [PubMed] [Google Scholar]
- 19.Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association; Chinese Heart Failure Association of Chinese Medical Doctor Association; Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:760–789. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Nemeth BT, Varga ZV, Wu WJ, Pacher P. Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br J Pharmacol. 2017;174:3727–3748. doi: 10.1111/bph.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rochette L, Guenancia C, Gudjoncik A, Hachet O, Zeller M, Cottin Y, et al. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci. 2015;36:326–348. doi: 10.1016/j.tips.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Rigaud VO, Ferreira LR, Ayub-Ferreira SM, Ávila MS, Brandão SM, Cruz FD, et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget. 2017;8:6994–7002. doi: 10.18632/oncotarget.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang HD, Jiang LH, Sun DW, Li J, Ji ZL. The role of miR-130a in cancer. Breast Cancer. 2017;24:521–527. doi: 10.1007/s12282-017-0776-x. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Huan L, Yin J, Qin M, Zhang Z, Zhang Z, et al. Role of microRNA-130a in myocardial hypoxia/reoxygenation injury. Exp Ther Med. 2017;13:759–765. doi: 10.3892/etm.2016.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Bounds KR, Chatterjee P, Gupta S. MicroRNA-130a, a potential antifibrotic target in cardiac fibrosis. J Am Heart Assoc. 2017;6:e006763. doi: 10.1161/JAHA.117.006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Signorelli SS, Volsi GL, Pitruzzella A, Fiore V, Mangiafico M, Vanella L, et al. Circulating miR-130a, miR-27b, and miR-210 in patients with peripheral artery disease and their potential relationship with oxidative stress. Angiology. 2016;67:945–950. doi: 10.1177/0003319716638242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation of hypertension therapy with the cardiotoxicity induced by chemotherapy