Abstract
Purpose
Nipple-sparing mastectomy (NSM) includes various techniques, including conventional or endoscopic mastectomies. Since the introduction of robot-assisted NSM (RANSM) in 2015, 2 main methods have been used: gasless and gas-inflated techniques. The aim of this study was to compare clinicopathologic characteristics, surgical outcomes, and postoperative complications between patients treated with gasless RANSM and those treated with gas-inflated RANSM.
Methods
We conducted a retrospective study of women who underwent gasless or gas-inflated RANSM with immediate breast reconstruction between November 2016 and May 2019. The indications for RANSM were early breast cancer, interstitial mastopathy, or BRCA1/2 mutation carriers. Clinicopathologic characteristics, surgical outcomes, and postoperative complications were analyzed. The severity of complications was graded using the Clavien-Dindo system.
Results
A total of 58 RANSM procedures were performed in 46 women: 15 cases of gasless RANSM and 43 cases of gas-inflated RANSM. The proportion of node-negative disease was higher in the gas-inflated group (97.1%) than in the gasless group (69.2%, p = 0.016). Adjuvant radiotherapy was administered in 30.6% of the cases in the gasless group and only 5% of the cases in the gas-inflated group. Other clinicopathological factors were not significantly different between the groups. Regarding surgical outcomes, the initial incision was 1 cm longer in the gasless group (5.17 ± 0.88 cm) than that in the gas-inflated group (4.20 ± 1.05 cm; p = 0.002). The final incision was also longer in the gasless group (5.17 ± 0.88 cm) than that in the gas-inflated group (4.57 ± 1.07 cm; p = 0.040). Operation time, complication rate, and complication grade were not significantly different between the 2 groups.
Conclusion
In this study, there were no significant differences in surgical outcomes or postoperative complications between gasless and gas-inflated RANSM, except for a longer incision with the gasless technique. Both techniques are reasonable options for RANSM followed by immediate reconstruction.
Keywords: Breast neoplasms, Mastectomy, Prophylactic mastectomy, Robotic surgical procedures
INTRODUCTION
Nipple-sparing mastectomy (NSM) is a popular and effective treatment option in patients requiring breast surgery without skin or nipple-areolar complex (NAC) involvement and with a plan for immediate reconstruction. However, this procedure requires incisions on the breast, especially near the NAC, which can affect the shape of the breast after reconstruction.
Endoscopic surgery has emerged as a strategy for NSM that uses minimal or hidden incisions [1]. While this type of minimally invasive surgery, which has been suggested to reduce incision size and minimize damage to the breast envelope, has produced satisfactory outcomes, the procedure is technically difficult [2]. Robot-assisted NSM (RANSM) was introduced in 2015 and has several technical advantages, including flexible robotic arms with a high-resolution 3-dimensional (3D) camera, ergonomic consoles, compensation for hand shaking, and detailed movements by robotic arms with high degrees of freedom [3]. Therefore, RANSM can reduce the technical barriers associated with endoscopic surgery.
Various methods of RANSM have been used by surgeons worldwide [4,5,6,7]. These techniques share similarities, such as the use of a single or hidden axillary incision, use of a tumescent solution, and preservation of skin envelopes, which are the general characteristics of robotic and endoscopic breast surgery. However, these methods differ in many ways. Toesca et al. [3] described the use of gas-inflated RANSM with a single-port device, whereas Sarfati et al. [5] reported the use of a gas-inflated RANSM without a single-port device. Park et al. [6] described a gasless RANSM technique using a self-retractor. In general, RANSM procedures can be categorized as gas-inflated or gasless based on the method of dissection of the subcutaneous flaps.
The majority of previous studies involved the use of gas-inflated techniques using a single-port device introduced by Toesca et al. [3,7,8,9]. Gas-inflated techniques for robotic surgery have become popular in various surgical fields [10,11,12]. Gasless robotic surgery is widely used in thyroid surgery [13]. The gasless technique for RANSM uses a self-retractor called Chung's retractor, which was originally introduced for robotic thyroid surgery [6]. This method was performed using a single hidden axillary incision. After manual dissection of the working space, the fixed self-retractor raises subcutaneous flaps to enable dissection of the remaining superficial layer of the superficial fascia and retromammary space. The gasless technique does not require a single-port device generally used for gas-inflated techniques.
Although many authors have reported their experience with gas-inflated or gasless techniques for RANSM, no published study has directly compared the 2 methods. Therefore, we conducted the present study to analyze and compare these 2 robotic breast surgery techniques.
METHODS
Patients
A total of 58 RANSM procedures (cases) were performed in 46 women at our institution (Severance Hospital) between November 2016 and May 2019, and were included in this retrospective study. When a patient was treated for both breasts, each breast corresponded to one case. RANSM using a gasless technique was developed at our institution. Therefore, the first 10 RANSM cases were performed using the gasless technique. After recognizing that the previous studies applied the gas-inflated technique for robotic breast surgery, we decided to adopt the gas-inflated technique as well [3,5]. The gas-inflated technique was used as an alternative to the gasless technique based on the surgeon's preference. The indications for RANSM were similar to those for NSM: early breast cancer without invasion of the NAC or skin, interstitial mastopathy caused by paraffin injection, or prophylactic mastectomy because of a BRCA gene mutation.
Clinicopathologic characteristics of the enrolled patients were obtained by reviewing their medical records. Patient characteristics included age, body mass index, smoking habit, bilaterality, BRCA mutation status, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, Ki-67 index, TNM stage, tumor grade, and neoadjuvant or adjuvant treatments. Adjuvant therapies for women with breast cancer were in accordance with the national guidelines and were administered based on physician or patient preferences [14]. Women with BRCA1/2 mutations received pre- and post-test genetic counseling by certified genetic counselors at the Cancer Prevention Center of Yonsei Cancer Center.
Two breast surgeons and 3 plastic surgeons performed RANSM and immediate breast reconstruction, respectively. Breast surgeon 1 performed 57 cases and breast surgeon 2 performed 1 case of RANSM. Plastic surgeons 1, 2, and 3 performed 49, 7, and 2 cases of immediate breast reconstruction, respectively.
Surgical techniques
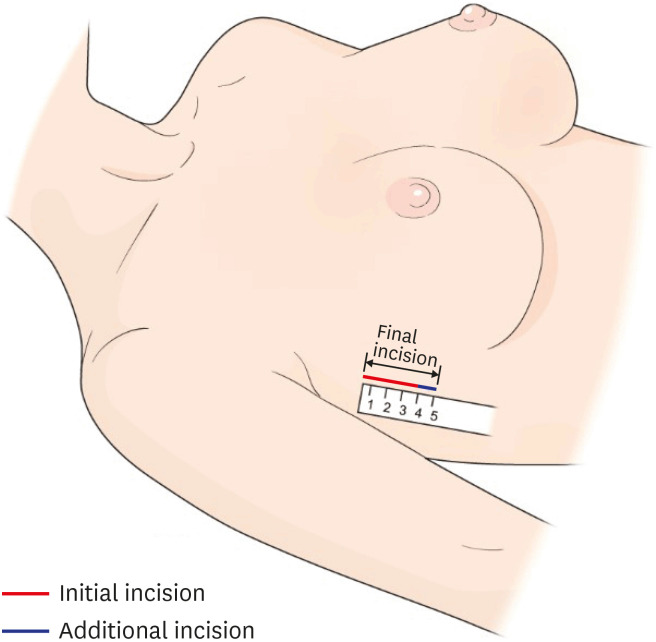
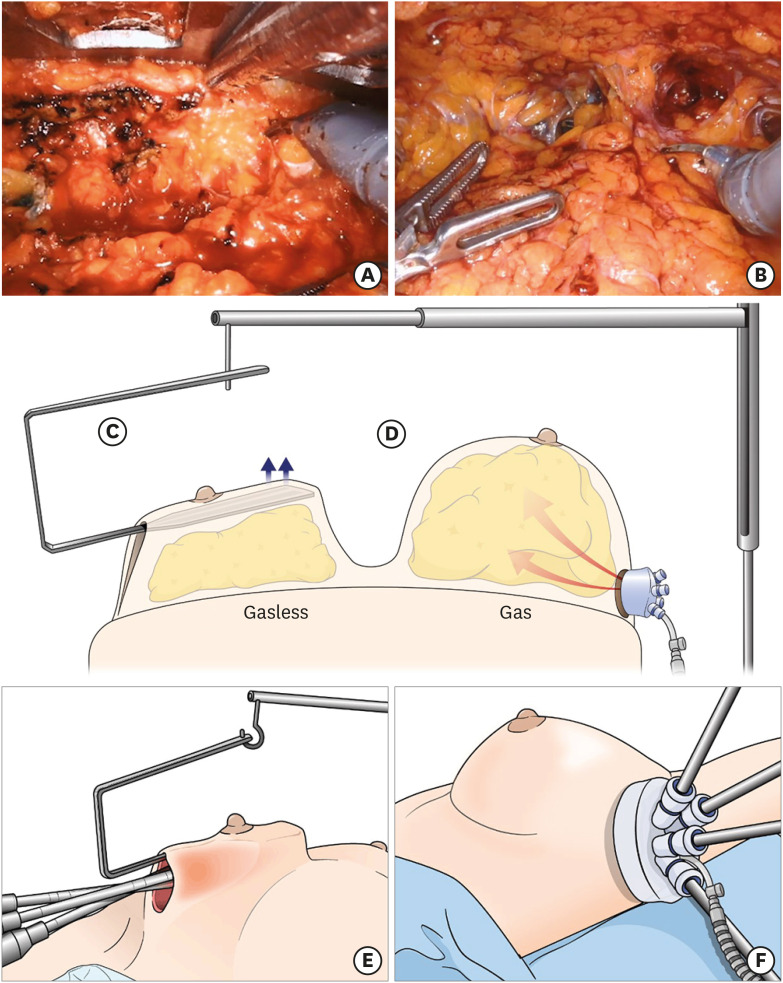
Both techniques used a similar location for a single incision between the upper anterior and the mid-axillary lines. Surgery was performed through a single mid-axillary incision (Figure 1). The main difference between the 2 techniques was the manner in which the subcutaneous flaps were raised (Figure 2). The gas-inflated technique used gas insufflation to separate the subcutaneous flaps and parenchyma. In contrast, the gasless technique used a self-retractor (Chung's retractor) to raise the subcutaneous flaps. The gasless technique required manipulation of the self-retractor during the operation, such as moving the retractor back and forth and changing its angle. Details of the surgical techniques have been described previously [15]. After RANSM was complete, the breast was reconstructed with either a tissue expander (TE) or an implant (i.e., direct-to-implant [DTI] reconstruction).
Figure 1. Illustration of the axillary incision.
Figure 2. Schematic comparison of the gasless and gas-inflated methods. (A) Console view of the gasless technique. (B) Console view of the gas-inflated technique. (C) Schematic view of the gasless technique: Chung's retractor raises the flap and is fixed to the operating table. (D) Schematic view of the gas-inflated technique. (E) Outside view of the gasless technique. (F) Outside view of the gas-inflated technique.
Pathology
ER and PR status were evaluated by immunohistochemistry (IHC), as previously described, with positivity defined as more than 1% staining of the surgically resected specimen. HER2 positivity was defined as 3+ expression on IHC or amplification of HER2 during fluorescence in situ hybridization or silver in situ hybridization studies. The Ki-67 index was calculated using the Roche iScan and Ki-67 (30-9) antibody, as described in a previous study [6]. Anatomic staging was based on the 8th American Joint Committee on Cancer TNM staging system. The histologic tumor grade was based on the modified Bloom Richardson grading system.
Surgical outcomes
Nipple margins were evaluated by examination of intraoperative frozen biopsies, if necessary. Other margins, including superficial or basal tumor margins, for patients with breast cancer were evaluated during the permanent pathology examinations.
Initial incision length was measured using a ruler with the breast in the anatomic position. The final incision length was the sum of the initial incision length and the length of any incision extension (additional incision) required for specimen retrieval. Breast volume was measured before surgery using a 3D breast image scanner (AX3 Technologies, Miami, USA).
The operation time was divided into mastectomy and reconstruction times. Mastectomy time was defined as the time from the initial incision to the start of reconstruction. It was divided into 4 phases: pre-docking time, docking time, console time, and time for specimen retrieval and preparation for reconstruction (TSPR). Pre-docking time was defined as the time from incision to insertion of the single-port device. Docking time was defined as the time required for docking the robotic system into the working space. Console time was defined as the time from the beginning to the end of the operation using the console. TSPR was defined as the time from the end of the console to the initiation of reconstruction.
All cases were categorized according to the time period during which the RANSM was performed. Using the median number of cases as the cutoff value, the first 29 cases were included in the early period and the subsequent 29 cases were included in the late period.
Postoperative complications
The Clavien-Dindo classification was used to grade postoperative complications [16]. Complications occurring within 30 days after surgery, including ischemia or necrosis of the NAC or flap, infection, or implant removal, were identified by reviewing the medical records and postoperative pictures maintained by the plastic surgeons. We dichotomized postoperative complications into grades ≤ II and ≥ III for statistical analysis. Nipple ischemia and necrosis were defined according to the grading system published in a previous study [17].
Statistics
The data are reported as mean ± standard deviation, unless indicated otherwise. Categorical variables were examined using the χ2 or Fisher's exact tests, as appropriate. Continuous variables were analyzed using the Student's t-test. Statistical significance was set at p < 0.05. All tests were 2-sided. Statistical analyses were performed using SPSS software, version 25 (SPSS Inc., Armonk, USA).
Ethics statement
This study was approved by the Institutional Review Board of Severance Hospital (No. 4-2020-0284). The requirement for informed consent was waived because of the retrospective nature of this study.
RESULTS
Clinicopathologic characteristics
Of the 58 RANSM procedures, 43 were gas-inflated and 15 were gasless. The mean age of all patients was 45 ± 10 years. The proportion of node-negative disease (pN stage = 0) was higher in the gas-inflated group (97.1%) than in the gasless group (69.2%; p = 0.017). Adjuvant radiotherapy was administered to 30.8% of the patients in the gasless group and only 5.6% of the patients in the gas-inflated group (p = 0.036). Other clinicopathological characteristics were not statistically different between the 2 groups (Table 1).
Table 1. Clinicopathologic characteristics.
| Characteristics | Gasless | Gas | p-value | |
|---|---|---|---|---|
| Age group (yr) | 0.198 | |||
| ≤ 50 | 13 (86.7) | 30 (69.8) | ||
| > 50 | 2 (13.3) | 13 (30.2) | ||
| Smoking History | ||||
| Never | 13 (86.7) | 39 (90.7) | ||
| Past | 2 (13.3) | 2 (4.7) | 0.380 | |
| Current | 0 (0.0) | 2 (4.7) | ||
| Histology | 0.741 | |||
| In situ carcinoma | 3 (20.0) | 9 (20.9) | ||
| Invasive cancer | 10 (66.7) | 27 (62.8) | ||
| BRCA mutation | 0 (0.0) | 3 (7.0) | ||
| Interstitial mastopathy | 2 (13.3) | 4 (9.3) | ||
| Bilaterality | 0.179 | |||
| Unilateral | 11 (73.3) | 23 (53.5) | ||
| Bilateral | 4 (26.7) | 20 (46.5) | ||
| BRCA1 | 0.551 | |||
| Negative/VOUS/not tested | 15 (100.0) | 42 (97.7) | ||
| positive | 0 (0.0) | 1 (2.3) | ||
| BRCA2 | 0.127 | |||
| Negative/VOUS/not tested | 15 (100.0) | 37 (86.0) | ||
| positive | 0 (0.0) | 6 (14.0) | ||
| ER | 0.895 | |||
| Negative | 2 (15.4) | 5 (13.9) | ||
| Positive | 11 (84.6) | 31 (86.1) | ||
| PR | 0.256 | |||
| Negative | 5 (38.5) | 8 (22.2) | ||
| Positive | 8 (61.5) | 28 (77.8) | ||
| HER2 | 0.331 | |||
| Negative | 7 (53.8) | 25 (69.4) | ||
| Positive | 6 (46.2) | 11 (30.6) | ||
| Ki-67 index | 0.603 | |||
| ≤ 14% | 4 (30.8) | 14 (38.9) | ||
| > 14% | 9 (69.2) | 22 (61.1) | ||
| pT stage | 0.957 | |||
| T0 | 3 (23.1) | 8 (24.2) | ||
| T1 | 8 (61.5) | 21 (63.6) | ||
| T2 | 2 (15.4) | 4 (12.1) | ||
| pN stage | 0.016 | |||
| N0 | 9 (69.2) | 33 (97.1) | ||
| N1 | 2 (15.4) | 1 (2.9) | ||
| N2/N3 | 2 (15.4) | 0 (0.0) | ||
| Grade | 0.110 | |||
| G1 | 0 (0.0) | 8 (22.2) | ||
| G2 | 9 (69.2) | 23 (63.9) | ||
| G3 | 4 (30.8) | 5 (13.9) | ||
| Neoadjuvant CTx | 0.544 | |||
| No | 13 (100.0) | 35 (97.2) | ||
| Yes | 0 (0.0) | 1 (2.8) | ||
| Adjuvant CTx | 0.057 | |||
| No | 6 (46.2) | 27 (75.0) | ||
| Yes | 7 (53.8) | 9 (25.0) | ||
| Adjuvant RTx | 0.017 | |||
| No | 9 (69.2) | 34 (94.4) | ||
| Yes | 4 (30.8) | 2 (5.6) | ||
| Adjuvant HTx | 0.476 | |||
| No | 2 (15.4) | 9 (25.0) | ||
| Yes | 11 (84.6) | 27 (75.0) | ||
| Adjuvant TTx | 0.442 | |||
| No | 10 (76.9) | 31 (86.1) | ||
| Yes | 3 (23.1) | 5 (13.9) | ||
Values are presented as number (%).
CTx = chemotherapy; ER = estrogen receptor; HER2 = human epithelial growth factor receptor 2; HTx = hormonal therapy; PR = progesterone receptor; RTx = radiotherapy; TTx = targeted therapy; VOUS = variant of unknown significance.
In situ or invasive breast cancer was the indication for 84.5% of all procedures included in this study. In the gas-inflated group, 3 BRCA mutation carriers with unilateral breast cancer underwent contralateral prophylactic mastectomy. Interstitial mastopathy secondary to paraffin injection was present in 2 patients (4 breasts) in the gas-inflated group and in 1 patient (2 breasts) in the gasless group. Only 1 patient received neoadjuvant therapy and was included in the gas-inflated group.
Da Vinci Xi® (Intuitive Surgical, Sunnyvale, USA) was the most commonly used robotic system in both groups: 12 of the 15 cases in the gasless group and 32 of the 43 cases in the gas-inflated group. The Da Vinci SP® system was used in 7 cases in the gas-inflated group. For reconstruction, TE was used more frequently in the gasless group (60% [9 cases]) than in the gas-inflated group (39.5% [17 cases]). Conversely, DTI reconstruction was more commonly performed in the gas-inflated group than in the gasless group. The difference in the types of reconstruction between the groups did not reach statistical significance (p = 0.17). Plastic surgeons decided on the type of reconstruction based on the state of the flap after mastectomy. In general, when the flap was promising or not damaged, the surgeons opted for DTI rather than TE. This conservative approach was because of their early experience with this technique.
Surgical outcomes
Surgical outcomes are summarized in Table 2. The initial incision was 1 cm longer in the gasless group (5.17 ± 0.88 cm) than in the gas-inflated group (4.20 ± 1.05 cm), which was a statistically significant difference (p = 0.002). The final incision was also significantly longer in the gasless group (5.17 ± 0.88 cm) than in the gas-inflated group (4.57 ± 1.07 cm; p = 0.040). In all cases, the mean breast volume was 314 ± 127 mL, and the mean specimen weight was 345 ± 149 g. There were no significant differences between the groups for either of these outcomes. Conversion to open surgery was not required in any case in either group.
Table 2. Surgical outcomes.
| Outcomes | Gasless | Gas | p-value |
|---|---|---|---|
| Pre-docking time (min) | 72.20 ± 18.71 | 83.60 ± 26.95 | 0.081 |
| Docking time (min) | 6.60 ± 3.54 | 7.28 ± 4.98 | 0.571 |
| Console time (min) | 56.33 ± 35.68 | 75.58 ± 47.28 | 0.110 |
| TSPR (min) | 59.93 ± 40.15 | 30.23 ± 23.53 | 0.001 |
| Mastectomy time (min) | 195.07 ± 44.85 | 196.66 ± 56.50 | 0.913 |
| Reconstruction time (min) | 136.93 ± 36.57 | 140.35 ± 52.33 | 0.784 |
| Total operation time (min) | 332.00 ± 51.87 | 337.02 ± 72.80 | 0.775 |
| Initial incision length (cm) | 5.17 ± 0.88 | 4.20 ± 1.05 | 0.002 |
| Final incision length (cm) | 5.17 ± 0.88 | 4.57 ± 1.07 | 0.040 |
| Specimen weight (g) | 303.67 ± 140.89 | 358.93 ± 150.49 | 0.219 |
| Breast volume (mL) | 276.40 ± 99.72 | 327.60 ± 133.87 | 0.181 |
Data are shown as mean ± standard deviation.
TSPR = time for specimen retrieval and preparation for reconstruction.
The mean total operation time was 320 ± 52 minutes in the gasless group and 317 ± 85 minutes in the gas-inflated group; these times were not significantly different (p = 0.863). The only time that differed significantly between groups was TSPR, which was longer in the gasless group (60 ± 40 minutes) than in the gas-inflated group (30 ± 24 minutes; p = 0.001).
Nipple margin involvement was not observed in any case in either group. However, further NAC excision was performed in one case in the gasless group because ductal carcinoma was identified in the subareolar mass near the sub-nipple area during permanent pathology examination. Superficial margin involvement was observed in one patient in the gas-inflated group. The patient was treated with post-mastectomy radiation therapy. One patient had an unknown superficial margin status. Superficial focal abutting of the specimen was noted intraoperatively and was treated with additional flap shaving after retrieval of the specimen. However, this additional flap was not evaluated during permanent pathology examination. In another case, the superficial margin abutted the tumor, which was attributed to epithelial cell displacement by the biopsy needle. The multidisciplinary team concluded that this did not represent true superficial margin involvement and, therefore, did not recommend adjuvant radiotherapy.
Complications
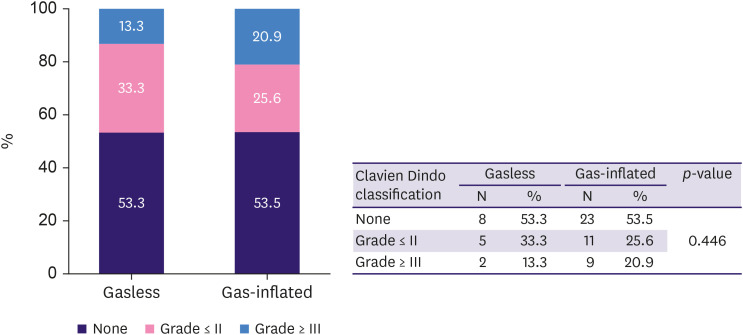
The complication rate in all cases was 46.5% (27/58 cases). Grade III and above complications, including NAC or flap necrosis and iatrogenic pneumothorax, occurred in 24.1% of all cases. Pneumothoraces in one patient were caused by the injection of blue dye while marking the breast border before incision. The patient had bilateral cancer and underwent gasless RANSM of the left breast and gas-inflated RANSM of the right breast. The overall complication rate was not significantly different between the gas-inflated and gasless groups (Table 3). The proportion of Clavien-Dindo grade ≥ 3 complications was higher in the gas-inflated group (20.9% [9/43 cases]) than in the gasless group (3.3% [2/15 cases]), but the difference between complication grades was not statistically significant (p = 0.446; Figure 3).
Table 3. Postoperative complications.
| Complications | Gasless | Gas-inflated | p-value | |
|---|---|---|---|---|
| Any complication | 0.992 | |||
| Yes | 7 (46.7) | 20 (46.5) | ||
| No | 8 (53.3) | 23 (53.5) | ||
| NAC partial ischemia | 0.788 | |||
| No | 10 (66.7) | 27 (62.8) | ||
| Yes | 5 (33.3) | 16 (37.2) | ||
| NAC total necrosis | 0.723 | |||
| No | 14 (93.3) | 42 (97.7) | ||
| Yes | 1 (6.7) | 1 (2.3) | ||
| Infection | 0.395 | |||
| No | 15 (100.0) | 41 (95.3) | ||
| Yes | 0 (0.0) | 2 (4.7) | ||
| Implant loss | 0.288 | |||
| No | 15 (100.0) | 39 (92.9) | ||
| Yes | 0 (0.0) | 3 (7.1) | ||
| Flap revision* | 0.395 | |||
| No | 15 (100.0) | 41 (95.3) | ||
| Yes | 0 (0.0) | 2 (4.7) | ||
| Other complications† | 0.428 | |||
| No | 14 (93.3) | 42 (97.7) | ||
| Yes | 1 (6.7) | 1 (2.3) | ||
NAC = nipple-areolar complex.
*Flap revision because of ischemia or necrosis; †Other complications were 2 cases of pneumothorax.
Figure 3. Proportions of complication grades in the gasless and gas-inflated groups.
The most common complication was nipple ischemia, which occurred in 21 cases (36.2% of all cases). The rate of nipple ischemia was not significantly different between the gasless group (33.3% [5/15 cases]) and the gas-inflated group (37.2% [16/43 cases]). NAC necrosis occurred in 1 case (6.7%) in the gasless group and 1 case (2.3%) in the gas-inflated group. Infection occurred in 2 cases (4.7%) in the gas-inflated group. Likewise, implant loss or removal due to ischemia or necrosis occurred in 3 cases (7.1%) in the gas-inflated group. Previous data showed that rate of NAC necrosis after DTI was higher compared to TE or other types of reconstruction [17]; however, other factors should be analyzed for this novel breast surgery. This is an interesting topic related to robotic breast surgery, which requires further investigation.
DISCUSSION
In this study, incision length was shorter with the gas-inflated technique than with the gasless technique. For the initial incision, the use of the self-retractor in the gasless technique almost always required an incision that was at least 4 cm in length, whereas the use of the single-port device in the gas-inflated technique generally required an incision that was at least 3 cm long. The use of the self-retractor or single-port device likely contributed to the different initial incision lengths. The final incision length can be determined by several factors. For example, an extended axillary incision is necessary for axillary lymph node dissection when nodal metastasis is identified in frozen sections. This may have influenced our results because patients who underwent gasless RANSM more frequently had node-positive disease than those who underwent gas-inflated RANSM. Other than incision length, however, there were no significant differences in outcomes between the 2 techniques, including margin positivity, postoperative complication rates, and grades of postoperative complications.
In the current study, adjuvant radiotherapy was more common in the gasless group than in the gas-inflated group. This was primarily due to the higher proportion of patients with node-positive disease in the gasless group. This incidental imbalance of clinicopathologic features occurred because of the study’s retrospective design, although there were no differences in clinical factors that might have influenced the choice of the surgical technique. Adjuvant radiotherapy after immediate DTI reconstruction may negatively affect cosmetic outcomes [18]; however, cosmetic outcome data assessed using standardized questionnaires were not available in the current study [19]. We plan to analyze the cosmetic outcomes after RANSM and DTI reconstruction in a future study.
The total operation time was approximately 5 hours: 3 hours for RANSM and 2 hours for reconstruction. The only operative time (total or individual components) that differed significantly between the groups was the TSPR. TSPR was 30 minutes longer in the gasless group than in the gas-inflated group. This difference could be at least partly attributed to the gasless technique being primarily performed during the early period of the study. In general, longer operation times are required for the initial cases of most new surgical procedures [20], and our plastic surgeons may have taken longer than usual time to prepare for the reconstructive procedure being performed through a small axillary incision with which they were unfamiliar. Thus, preparation for reconstruction may require a longer time in the early period of RANSM use than in the later period. Nevertheless, the 30-minute TSPR difference did not have a statistically or clinically significant effect on the total operation time.
Two cases of iatrogenic pneumothorax occurred in one patient. They were attributed to injury from a 19-gauge needle during breast border marking with blue dye before incision. We used a 19-gauge needle because it was convenient to inject jelly-mixed blue dye into the breast parenchyma. However, after pneumothoraces occurred, we switched to a short 26-gauge needle for border marking. No pneumothorax occurred when using this smaller needle. Therefore, caution is required when using a needle during breast border marking.
Other differences exist between the gas-inflated and gasless techniques, although they were not specifically analyzed in this study (Table 4). The potential advantages of the gas-inflated technique are related mainly to the use of a single-port device and gas insufflation. This technique minimizes the need for additional manipulations of setting the robotic arms when the expansion of the working space by gas inflation is appropriate. With the gas-inflated technique, subcutaneous flaps are dissected while maintaining the dome shape of the breast because the gas spreads evenly and expands into the subcutaneous tunneling space. This helps the surgeon gain an appreciation of the anatomy of the subcutaneous area and skin. Other technical advantages of the gas-inflated RANSM have been well described in a previous study by Toesca et al. [4]. For example, reduction of bleeding because of CO2 gas insufflation may improve visualization of the proper surgical dissection plane [4]. There are also some disadvantages of the gas-inflated technique. Visualization may be impaired because of the accumulation of smoke, which inevitably occurs with the use of electrocauterization in a closed space. However, this effect can be reduced by using an air filtration device (S-Port Filter; S-Medics Solution, Seoul, Korea). There is also a potential risk of a gas leak if the single-port device is not appropriately placed and used during the procedure. In addition, subcutaneous emphysema may occur, but it generally subsides with supportive treatment [21]. We did not observe any potential serious adverse events of the gas-inflated technique, such as hypercapnia and air embolism, in the current study.
Table 4. Comparison between gasless and gas-inflated techniques.
| Features | Gasless | Gas-inflated | |
|---|---|---|---|
| Common features | Same patient position | ||
| Single hidden axillary incision | |||
| Same sentinel lymph node biopsy procedure | |||
| Differences | |||
| Size of the initial incision | ≥ 4 cm | Depends on the single port ring size; usually 2.5–3.5 cm | |
| Subcutaneous flap lifting | Self-retractor (Chung's) | Gas insufflation | |
| Working space area | Wide | Small space for port insertion | |
| Surgical field | Open | Closed | |
| Use of suction device | Easy | Difficult | |
| Use of a single-port device | No | Yes or no | |
| Nipple margin biopsy method | Manual | Robot-assisted | |
| Advantage | • Less fogginess because of open surgical field | • Possibly less bleeding because of CO2 gas insufflation | |
| • Steady elevation of subcutaneous flaps | • Less need for additional manipulations for setting the robotic arms | ||
| • No air leak | • Dissection of subcutaneous flaps while maintaining dome shape of the breast | ||
| • Surgeons with experience performing gasless thyroidectomy using a self-retractor are familiar with this technique | |||
| Disadvantages | • Inevitable need to manipulate the position of the self-retractor to maintain a proper surgical view | • Visual disturbance by smoke | |
| • Potential risk of gas leak | |||
| • Subcutaneous emphysema | |||
| • Hypercapnia | |||
| • Air embolism | |||
The gasless technique also has advantages, which are primarily related to the use of a self-retractor instead of a single-port device. The open surgical field with a self-retractor and an air-suction device can create a clearer visual field, as the air-suction device removes smoke more effectively than a gas filter in the gas-inflated technique. The self-retractor also steadily elevates the subcutaneous flaps, providing a stable surgical field during dissection. There is no gas leak to consider in the gasless technique. Furthermore, surgeons who have experience with gasless thyroidectomy using a self-retractor will be familiar with gasless RANSM. Nevertheless, there are also disadvantages to the gasless technique. It is necessary to change the position of the self-retractor during the operation to maintain a proper surgical view because the self-retractor has a long, rectangular shape and is fixed to the operation table, whereas the breast itself is dome-shaped.
The current study has some limitations. For example, this study included the earliest cases of RANSM, and gasless cases were predominantly performed during the early study period. The learning curve effect may have influenced the results. The learning curve and CUSUM (cumulative sum control chart) were analyzed for the initial 12 cases in a previous study [15]. In that study, the total operation time and console time were evaluated, but were not analyzed according to the operation technique, i.e., gasless vs. gas. In a future study with more cases enrolled, the learning curve should be analyzed according to various influencing factors, such as the disease entity, surgeon, operation method, or type of robotic system used. Other limitations due to the retrospective design of the study, including the small number of cases and unbalanced clinicopathologic features between the techniques, raise the possibility of selection bias, as discussed above. Despite these limitations, this study has several strengths. To our knowledge, this is the first study to compare gasless and gas-inflated RANSM. Although RANSM is a recently introduced technique, the study included more than 50 cases of RANSM. Nevertheless, a prospective study with a larger sample size should be conducted to allow more definitive conclusions and to provide additional information regarding the 2 surgical techniques.
In conclusion, the gasless technique requires an incision approximately 1 cm longer than that in the gas-inflated technique when used for RANSM. However, other surgical outcomes, as well as postoperative complications, were not significantly different between the 2 techniques. Both gasless and gas-inflated techniques are reasonable options for RANSM.
ACKNOWLEDGMENTS
The authors thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.
Footnotes
Funding: This work was supported by a faculty research grant from Yonsei University College of Medicine (6-2017-0072).
Conflict of Interest: Hyung Seok Park received honorarium for consultation from Intuitive Surgical Korea, Inc. Other authors declare that they have no competing interests.
- Conceptualization: Lee H, Lee J, Lee K, Kim JY, Park HS.
- Data curation: Lee H, Lee J, Lee K, Kim JY, Park HS.
- Formal analysis: Lee H, Park HS.
- Funding acquisition: Park HS.
- Investigation: Lee H, Lee J, Lee K, Kim JY, Park HS.
- Methodology: Lee H, Park HS.
- Project administration: Lee H, Park HS.
- Resources: Lee H, Lee J, Park HS.
- Software: Lee H.
- Supervision: Kim JY, Park HS.
- Validation: Lee J, Park HS.
- Visualization: Park HS.
- Writing - original draft: Lee H.
- Writing - review & editing: Lee H, Park HS.
References
- 1.Sakamoto N, Fukuma E, Higa K, Ozaki S, Sakamoto M, Abe S, et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg Oncol. 2009;16:3406–3413. doi: 10.1245/s10434-009-0661-8. [DOI] [PubMed] [Google Scholar]
- 2.Lai HW, Chen ST, Lin SL, Chen CJ, Lin YL, Pai SH, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2019;26:42–52. doi: 10.1245/s10434-018-6704-2. [DOI] [PubMed] [Google Scholar]
- 3.Toesca A, Peradze N, Manconi A, Galimberti V, Intra M, Colleoni M, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017;31:51–56. doi: 10.1016/j.breast.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toesca A, Peradze N, Galimberti V, Manconi A, Intra M, Gentilini O, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017;266:e28–30. doi: 10.1097/SLA.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 5.Sarfati B, Struk S, Leymarie N, Honart JF, Alkhashnam H, Kolb F, et al. Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: surgical technique. Plast Reconstr Surg. 2018;142:624–627. doi: 10.1097/PRS.0000000000004703. [DOI] [PubMed] [Google Scholar]
- 6.Park HS, Kim JH, Lee DW, Song SY, Park S, Kim SI, et al. gasless robot-assisted nipple-sparing mastectomy: a case report. J Breast Cancer. 2018;21:334–338. doi: 10.4048/jbc.2018.21.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houvenaeghel G, Bannier M, Rua S, Barrou J, Heinemann M, Knight S, et al. Robotic breast and reconstructive surgery: 100 procedures in 2-years for 80 patients. Surg Oncol. 2019;31:38–45. doi: 10.1016/j.suronc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Lai HW, Chen ST, Lin SL, Lin YL, Wu HK, Pai SH, et al. Technique for single axillary incision robotic assisted quadrantectomy and immediate partial breast reconstruction with robotic latissimus dorsi flap harvest for breast cancer: a case report. Medicine (Baltimore) 2018;97:e11373. doi: 10.1097/MD.0000000000011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai HW, Wang CC, Lai YC, Chen CJ, Lin SL, Chen ST, et al. The learning curve of robotic nipple sparing mastectomy for breast cancer: an analysis of consecutive 39 procedures with cumulative sum plot. Eur J Surg Oncol. 2019;45:125–133. doi: 10.1016/j.ejso.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Han DS, Suh YS, Ahn HS, Kong SH, Lee HJ, Kim WH, et al. Comparison of surgical outcomes of robot-assisted and laparoscopy-assisted pylorus-preserving gastrectomy for gastric cancer: a propensity score matching analysis. Ann Surg Oncol. 2015;22:2323–2328. doi: 10.1245/s10434-014-4204-6. [DOI] [PubMed] [Google Scholar]
- 11.Choi JY, Lee KE, Chung KW, Kim SW, Choe JH, Koo DH, et al. Endoscopic thyroidectomy via bilateral axillo-breast approach (BABA): review of 512 cases in a single institute. Surg Endosc. 2012;26:948–955. doi: 10.1007/s00464-011-1973-x. [DOI] [PubMed] [Google Scholar]
- 12.Levi Sandri GB, de Werra E, Mascianà G, Guerra F, Spoletini G, Lai Q. The use of robotic surgery in abdominal organ transplantation: a literature review. Clin Transplant. 2017;31:e12856. doi: 10.1111/ctr.12856. [DOI] [PubMed] [Google Scholar]
- 13.Kang SW, Kim MJ, Chung WY. Gasless, transaxillary robotic neck dissection: the technique and evidence. Gland Surg. 2018;7:466–472. doi: 10.21037/gs.2017.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano SH, Elias AD, Gradishar WJ. NCCN guidelines updates: breast cancer. J Natl Compr Canc Netw. 2018;16:605–610. doi: 10.6004/jnccn.2018.0043. [DOI] [PubMed] [Google Scholar]
- 15.Park HS, Lee J, Lee DW, Song SY, Lew DH, Kim SI, et al. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: an initial experience. Sci Rep. 2019;9:15669. doi: 10.1038/s41598-019-51744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 17.Ahn SJ, Woo TY, Lee DW, Lew DH, Song SY. Nipple-areolar complex ischemia and necrosis in nipple-sparing mastectomy. Eur J Surg Oncol. 2018;44:1170–1176. doi: 10.1016/j.ejso.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Hussien M, Salah B, Malyon A, Wieler-Mithoff EM. The effect of radiotherapy on the use of immediate breast reconstruction. Eur J Surg Oncol. 2004;30:490–494. doi: 10.1016/j.ejso.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Ahn SJ, Song SY, Park HS, Park SH, Lew DH, Roh TS, et al. Early experiences with robot-assisted prosthetic breast reconstruction. Arch Plast Surg. 2019;46:79–83. doi: 10.5999/aps.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pio L, Musleh L, Paraboschi I, Pistorio A, Mantica G, Clermidi P, et al. Learning curve for robotic surgery in children: a systematic review of outcomes and fellowship programs. J Robot Surg. 2020;14:531–541. doi: 10.1007/s11701-019-01026-w. [DOI] [PubMed] [Google Scholar]
- 21.Saggar VR, Singhal A, Singh K, Sharma B, Sarangi R. Factors influencing development of subcutaneous carbon dioxide emphysema in laparoscopic totally extraperitoneal inguinal hernia repair. J Laparoendosc Adv Surg Tech A. 2008;18:213–216. doi: 10.1089/lap.2007.0089. [DOI] [PubMed] [Google Scholar]