Abstract
Purpose
Endoscopic breast surgery for patients with breast cancer was introduced for its superior cosmetic outcomes; it was initially studied in the field of breast-conserving surgery and, more recently, in robotic-assisted nipple-sparing mastectomy (NSM). The main purpose of this study was to investigate the feasibility and safety of endoscopic NSM (E-NSM) in patients with breast cancer by comparing E-NSM and conventional NSM (C-NSM).
Methods
Between May 2017 and October 2020, we retrieved the records of 45 patients who underwent NSM with permanent silicone implants and divided them into the E-NSM group (20 patients) and the C-NSM group (25 patients), depending on the use of the endoscopic device. We also analyzed demographic information, pathology, operative time, and complications.
Results
No significant differences were observed between the 2 groups based on demographic information, postoperative pathological data, mean length of hospital stay, and total number of complications. The mean preparation time for surgery was comparable between both groups. Compared to the C-NSM group, the E-NSM group had a significantly longer mean operative time and, subsequently, a significantly longer mean total operative time and number of complications.
Conclusion
The results showed that E-NSM was feasible and safe with a more inconspicuous incision in patients with breast cancer.
Keywords: Breast neoplasms, Endoscopic surgical procedure, Mastectomy, Reconstructive surgery, Robot-assisted surgery
INTRODUCTION
Nipple-sparing mastectomy (NSM) was initially reported in the 1960s by Freeman et al. [1,2]. It was known to achieve oncological safety comparable to standard mastectomy, with psychological benefits and improved cosmetic results for patients with breast cancer [3,4]. Various types of incisions have been used for NSM, including radial, periareolar, elliptical, and inframammary incisions [5,6]; however, further studies on the approach of NSM have been extensively conducted to explore ways to create a more inconspicuous and efficient incision.
With the development of minimally invasive devices, laparoscopic or endoscopic surgeries have been developed in many areas. Endoscopic breast surgery for patients with breast cancer was also introduced for its superior cosmetic effects. It was initially studied in the field of breast-conserving surgery [7,8,9] and, more recently, in robotic-assisted NSM [10,11,12,13,14].
To the best of our knowledge, few studies have compared single incision endoscopic and conventional methods in patients with breast cancer undergoing NSM with immediate reconstruction using a permanent silicone implant. Thus, the main purpose of this study was to investigate the feasibility and safety of endoscopic NSM (E-NSM) in patients with breast cancer by comparing E-NSM and conventional NSM (C-NSM) in terms of pathology, operative time, and outcome.
METHODS
Study population
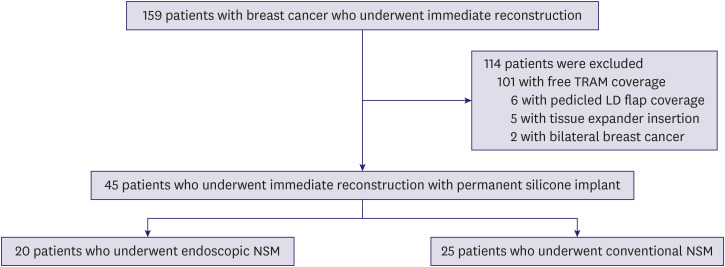
We retrieved the records of 159 consecutive patients with breast cancer who underwent skin-sparing mastectomy with immediate reconstruction between May 2017 and October 2020. Among these, 101 patients who underwent free transverse rectus abdominus myocutaneous (TRAM) coverage, 6 patients who underwent pedicled latissimus dorsi flap coverage, and 5 patients who underwent tissue expander insertion were excluded. Two patients with bilateral breast cancer were also excluded from the analysis in order to focus only on unilateral breast cancer. The finalized pool of eligible participants comprised 45 patients who underwent NSM with permanent silicone implants, who were divided into 2 groups depending on the use of endoscopic devices. There were 20 patients in the E-NSM group and 25 patients in the C-NSM group (Figure 1).
Figure 1. Flow chart of the study population.
TRAM = transverse rectus abdominis muscle; LD = latissimus dorsi; E-NSM = endoscopic nipple-sparing mastectomy; C-NSM = conventional nipple-sparing mastectomy.
This retrospective study was approved by the Institutional Review Board of Korea University Medical Center, Ansan (approval number: 2020AS0322). A waiver of informed consent was requested and was approved.
Surgical procedures of endoscopic NSM
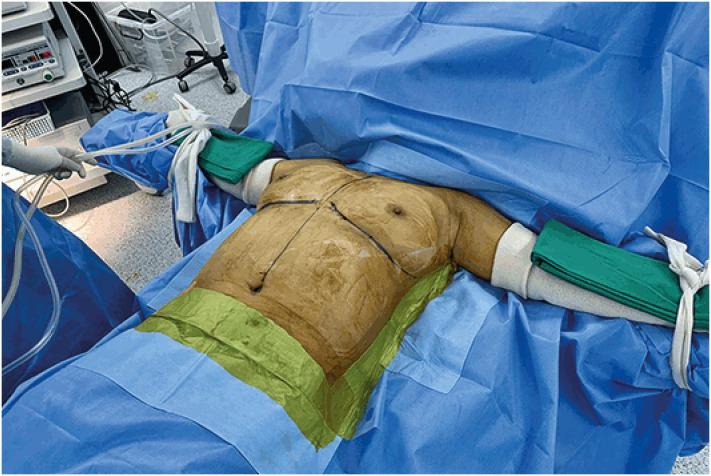
Patients were placed in a supine position with both upper limbs on arm boards abducted at 90° (Figure 2). An incision of 5 cm was made along the anterior axillary line starting from the inferior mammary fold. The sentinel lymph node, which was dyed with a technetium-99m-labeled nanocolloid, was excised through this incision. A workspace for the insertion of a Glove Port (Nelis Corporation, Bucheon, Korea) was created within a radius of 3 cm from the incision, and the subcutaneous tissue flaps were raised. Approximately 250 mL of tumescent solution was infiltrated into the subcutaneous fat layer of the breast with a Veress needle through the workspace, and blunt dissection was performed using a straight tunneler before the insertion of the Glove Port.
Figure 2. The patient's position for endoscopic nipple-sparing mastectomy.
The Glove Port was inserted into the incision site. Upon lifting the lateral breast to approach the retromammary space, carbon dioxide (CO2) gas was insufflated, and the pressure was maintained at approximately 6 mmHg. The retromammary space was dissected with caution to avoid dissecting the interpectoral space, using an energy device and an endoscopic grasper guided by a flexible endoscope (ENDOEYE FLEX 10 mm, LTF-S190-10; Olympus Corporation, Tokyo, Japan). The breast was elevated laterally to the edge of the latissimus dorsi muscle, inferiorly to the thoracoabdominal aponeurosis, superiorly to the level of the clavicle, and medially to the edge of the sternum.
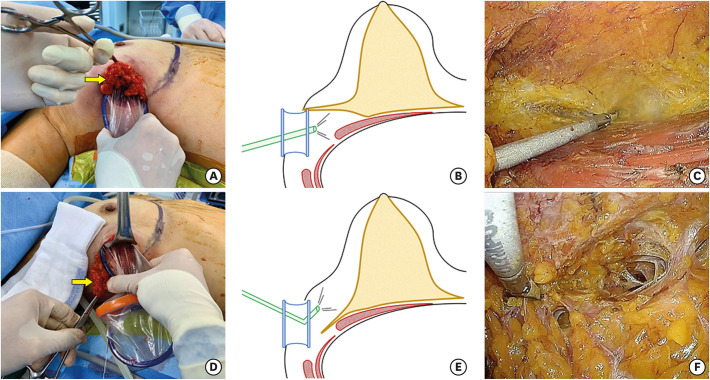
After dissecting the retromammary fat plane, the Glove Port was relocated to approach the subcutaneous space, which had already been dissected bluntly with a straight tunneler. Structures remaining after blunt dissection were cut off using an energy device, and the duct beneath the nipple was cut off using endoscopic scissors. The subcutaneous flap was completely dissected along the boundaries of the breast, and the entire breast was removed (Figure 3).
Figure 3. Illustrations of the flap dissections. (A) Images showing lifting of the lateral breast to approach the retromammary area (arrow, the lateral breast). (B) Schematic images showing the retromammary space dissection (yellow, glandular tissue; red, muscles in the axilla; blue, Glove Port; green, flexible endoscope). (C) Images showing the retromammary space dissection guided by a scope. (D) Images showing pulling of the lateral breast downward to approach the subcutaneous layer (arrow, the lateral breast). (E) Schematic images showing the subcutaneous flap dissection. (F) Images showing the subcutaneous flap dissection guided by a scope.
Axillary lymph node dissection (ALND) was performed in 2 patients in the E-NSM group who had metastatic sentinel lymph nodes; 1 patient underwent ALND using endoscopic devices and the other, directly through the anterior axillary incision.
Subsequently, the inferior origin of the pectoralis major was released, and an acellular dermal matrix sling was made for immediate reconstruction with a silicone implant. Surgery was performed after control of bleeding and drain insertion.
Data analysis
We analyzed demographic information and pathology obtained from medical records, including invasive tumor size, positive resection margin, status of the axillary lymph nodes, and tumor node metastasis classification according to the 8th American Joint Committee on Cancer [15]. Breast sagging was classified as follows based on the Regnault ptosis classification: grade 1, the nipple at the level of the inframammary fold; grade 2, the nipple below the level of the inframammary fold, but above the lower breast contour; grade 3, the nipple below the level of the inframammary fold and at the lower breast contour; pseudoptosis, the nipple above the level of the inframammary fold, but the breast hypoplastic and hanging below the fold [16].
The preparation time for the surgery was calculated (in minutes) from the induction of general anesthesia to the initial skin incision. Total operative time was defined from the initial skin incision to the closure of the wound, and it was divided into the time of NSM and of reconstruction based on the end of bleeding control after breast removal in all cases.
Complications were also analyzed. Cases requiring debridement and evacuation of a hematoma were considered as postoperative skin necrosis and hematoma, respectively. Postoperative implant infections requiring implant change were also identified.
Statistical analysis
All data were analyzed using IBM SPSS Statistics ver. 25 (IBM Corp., Armonk, USA). Continuous data are presented as means with standard deviations, and categorical data are presented as numbers with percentages. The t-test was used to compare continuous variables, and categorical variables were compared using the χ2 or Fisher's exact test to analyze the significance of differences. Differences were considered statistically significant at p < 0.05.
RESULTS
Clinical pathological characteristics
The study population included 20 patients in the E-NSM group and 25 in the C-NSM group. There were no significant differences between both groups in terms of mean age at operation (47.2 ± 9.5 years in E-NSM vs. 44.6 ± 9.6 years in C-NSM, p = 0.38) and body mass index (BMI) (24.1 ± 3.8 in E-NSM vs. 22.3 ± 3.6 in C-NSM, p = 0.11). The mean tumor extent and multicentricity based on preoperative radiological findings and the proportion of breast ptosis did not significantly differ between the 2 groups (p = 0.97).
Based on postoperative pathological data, no significant differences were observed in the mean size of the invasive tumor (0.92 ± 1.3 cm in E-NSM vs. 1.39 ± 1.2 cm in C-NSM, p = 0.21), the number of positive resection margin cases, and the mean number of metastatic (p = 0.93) and harvested (p = 0.26) lymph nodes. Tumor, node, metastasis staging did not significantly differ between the 2 groups (Table 1).
Table 1. Clinical and pathological characteristics of breast cancer patients who underwent nipple-sparing mastectomy with immediate reconstruction with permanent silicone implants using endoscopic or conventional methods.
| Characteristics | E-NSM (n = 20) | C-NSM (n = 25) | p-value | |
|---|---|---|---|---|
| Age at operation (yr) | 47.2 ± 9.5 | 44.6 ± 9.6 | 0.38 | |
| Body mass index | 24.1 ± 3.8 | 22.3 ± 3.6 | 0.11 | |
| Tumor extent* (cm) | 4.1 ± 1.9 | 3.9 ± 2.1 | 0.68 | |
| Multicentricity | 12 (60.0) | 14 (56.0) | 0.79 | |
| Breast ptosis | 0.97 | |||
| Normal | 10 (50.0) | 13 (52.0) | ||
| Grade 1 | 6 (30.0) | 6 (24.0) | ||
| Grade 2 | 1 (5.0) | 2 (8.0) | ||
| Grade 3 | 1 (5.0) | 2 (8.0) | ||
| Pseudoptosis | 2 (10.0) | 2 (8.0) | ||
| Invasive size (cm) | 0.92 ± 1.3 | 1.39 ± 1.2 | 0.21 | |
| Positive resection margin | 0 (0.0) | 0 (0.0) | ||
| Metastatic LNs | 0.45 ± 1.2 | 0.48 ± 1.1 | 0.93 | |
| Harvested LNs | 4.40 ± 5.7 | 6.84 ± 8.1 | 0.26 | |
| TNM stage | 0.25 | |||
| 0 | 10 (50.0) | 6 (24.0) | ||
| IA | 3 (15.0) | 11 (44.0) | ||
| IIA | 5 (25.0) | 5 (20.0) | ||
| IIB | 1 (5.0) | 2 (8.0) | ||
| IIIA | 1 (5.0) | 1 (4.0) | ||
Values are presented as mean ± standard deviation or number (%).
E-NSM = endoscopic nipple-sparing mastectomy; C-NSM = conventional nipple-sparing mastectomy; LN = lymph node; TNM = tumor, node, metastasis.
*Based on preoperative radiological findings.
Operative details
ALND was performed in 2 patients in the E-NSM group and 4 patients in the C-NSM group (10.0% in E-NSM vs. 16.0% in C-NSM, p = 0.68), and contralateral balancing augmentation was performed in 3 patients in the E-NSM group and 2 patients in the C-NSM group (15.0% in E-NSM vs. 8.0% in C-NSM, p = 0.64). The weights of the removed breast tissues were comparable between the 2 groups (294.6 ± 116.7 g in E-NSM vs. 275.1 ± 150.7 g in C-NSM, p = 0.64). Regarding operative time, the mean preparation time for surgery was comparable between the 2 groups. However, the E-NSM group had a significantly longer mean total operative time (269.1 ± 39.6 minutes in E-NSM vs. 235.9 ± 43.8 minutes in C-NSM, p = 0.04) because the mean operative time for NSM was significantly longer in the E-NSM group (149.8 ± 37.7 minutes in E-NSM vs. 116.4 ± 28.2 minutes in C-NSM, p < 0.01). The mean time for reconstruction with the implant was comparable between the 2 groups (Table 2).
Table 2. Operative details of E-NSM and C-NSM groups.
| Characteristics | E-NSM (n = 20) | C-NSM (n = 25) | p-value | |
|---|---|---|---|---|
| ALND | 2 (10.0) | 4 (16.0) | 0.68 | |
| Balancing procedure | 3 (15.0) | 2 (8.0) | 0.64 | |
| Weight of the removed breast (g) | 294.6 ± 116.7 | 275.1 ± 150.7 | 0.64 | |
| Preparation time for surgery (min) | 54.1 ± 11.4 | 57.9 ± 13.5 | 0.32 | |
| Total operative time (min) | 269.1 ± 39.6 | 235.9 ± 43.8 | 0.04 | |
| Nipple-sparing mastectomy | 149.8 ± 37.7 | 116.4 ± 28.2 | < 0.01 | |
| Reconstruction with an implant | 112.0 ± 41.5 | 110.6 ± 39.4 | 0.91 | |
Values are presented as mean ± standard deviation or number (%).
E-NSM = endoscopic nipple-sparing mastectomy; C-NSM = conventional nipple-sparing mastectomy; ALND = axillary lymph node dissection.
Outcome and morbidity details
The mean length of hospital stay showed no significant difference (14.4 ± 4.5 days in E-NSM vs. 12.0 ± 6.2 days in C-NSM, p = 0.16) between the groups. Regarding complications, in the C-NSM group, 2 (8.0%) patients experienced postoperative skin necrosis requiring debridement, and 2 (8.0%) developed hematomas. There was no skin necrosis or hematoma in the E-NSM group. Postoperative implant infection requiring an implant change was found in one patient (5.0%) in the E-NSM group. There was no significant difference in the total number of complications between the groups (Table 3). However, it is difficult to say that the statistical difference in surgical complications is absolute due to the small number of patients with events.
Table 3. Outcome and morbidity details of E-NSM and C-NSM groups.
| Characteristics | E-NSM (n = 20) | C-NSM (n = 25) | p-value | |
|---|---|---|---|---|
| Length of hospital stay (day) | 14.4 ± 4.5 | 12.0 ± 6.2 | 0.16 | |
| Complications | 1 (5.0) | 4 (16.0) | 0.36 | |
| Skin necrosis | 0 (0.0) | 2 (8.0) | ||
| Hematoma | 0 (0.0) | 2 (8.0) | ||
| Infection | 1 (5.0) | 0 (0.0) | ||
Values are presented as mean ± standard deviation or number (%).
E-NSM = endoscopic nipple-sparing mastectomy; C-NSM = conventional nipple-sparing mastectomy.
DISCUSSION
Comparing the positive resection margin rate between both groups helped to assess the feasibility of E-NSM, as a positive resection margin is a known risk factor for local recurrence in patients with breast cancer [17,18]. There were no cases of positive resection margins, including frozen and permanent pathologic findings, in both groups, demonstrating that the E-NSM method allowed for the complete and proper removal of the glandular and cancer tissue.
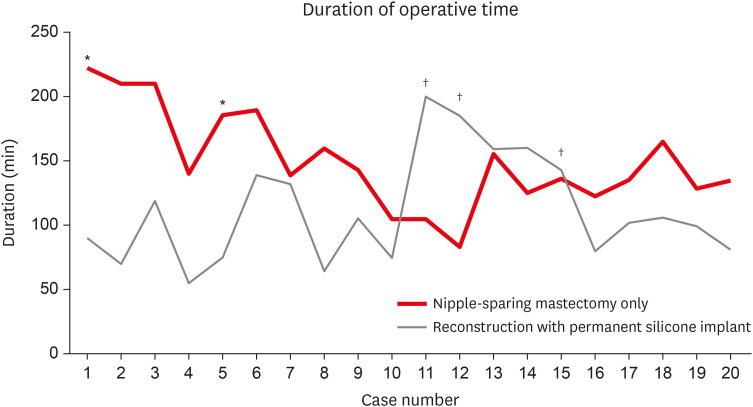
The results showed that the operative time for E-NSM was significantly longer than that for C-NSM. Despite a thorough preparation through previous studies on E-NSM, it took time to adapt to the new surgical view, endoscopic device, and position of the endoscope. The study by Hung et al. [19] on the learning curve of endoscopic total mastectomies showed that the inflection point occurred after 15 to 17 cases. In our cases, the operative time for NSM decreased sharply after approximately 10 cases (Figure 4). Blunt dissection of the subcutaneous flap using a straight tunneler and dissection of the subcutaneous flap after dissection of the retromammary flap reduced the operative time [20]. The preparation time for E-NSM was comparable to that for C-NSM. This was because, unlike in robotic-assisted NSM, which has recently been studied quite extensively, no further preparation for the patient's position was needed in E-NSM. In addition, as E-NSM was performed on the operating field, the preoperative injection of indigo carmine to check the borders of the breast through the scope was not needed [21,22].
Figure 4. The learning curve of endoscopic nipple-sparing mastectomy. Duration of total mastectomy only (red) and reconstruction with permanent silicone implant (gray).
*Performed with axillary lymph node dissection; †Performed with balancing reconstruction.
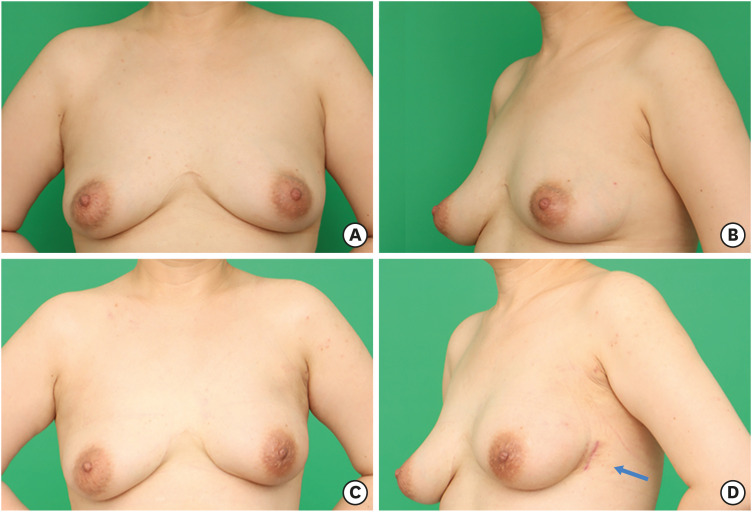
Only one incision, 5 cm along the anterior axillary line, was used in all patients in the E-NSM group. The incision was made starting on the midaxillary line; however, this made subcutaneous dissection more difficult because of the leverage effect of the pectoralis major muscle. Later, we used the anterior axillary line, approximately 2 cm above the midaxillary line ventrally; this allowed us to overcome the leverage effect without differences in cosmetic outcomes. The conventional method of NSM uses various incisions to remove the whole breast effectively and to hide the incision; however, conspicuous wounds from the front view are unavoidable. In contrast, the surgical wound was completely invisible and covered by the undergarment of the patients who underwent E-NSM, which improved patient satisfaction (Figure 5).
Figure 5. Cosmetic outcome of E-NSM in a 42-year old patient with breast cancer, 2 months after undergoing E-NSM with immediate permanent silicone implant reconstruction. (A) Preoperative anterior view. (B) Preoperative oblique view. (C) Postoperative anterior view. (D) Postoperative oblique view (arrow, the single incision made for E-NSM).
E-NSM = endoscopic nipple-sparing mastectomy.
Studies on endoscopic mastectomy using CO2 insufflation reported that the pressure was maintained at 8–10 mmHg without any issues related to CO2 [11,23]. In our cases, a constant CO2 pressure of 10 mmHg was used in the first patient who underwent E-NSM, and this patient experienced high end-tidal and arterial partial pressure of CO2 after bleeding while undergoing dissection of the retromammary fat plane. These levels were corrected to normal levels by lowering the CO2 pressure to 6 mmHg. After the event, a decreased CO2 pressure of 6 mmHg was used in all cases thereafter, and no complications occurred due to CO2. There were also no interruptions in the surgical view due to decreased pressure.
C-NSM requires a scrupulous surgical technique, as the entire breast has to be removed by narrowing the surgical view through a small incision [24]. The surgical view guided by a flexible endoscope was also narrow immediately after inserting the Glove Port in the patients of the E-NSM group; however, as flap dissection progressed, the surgical view widened without requiring excessive retraction of the skin. There was no blind spot in the operative field using the flexibility of the high-resolution scope, which made identification and control of bleeding easier.
Skin necrosis of the nipple-areolar complex (NAC) was found in 2 patients in the C-NSM group in the present study. Although our results showed no significant difference in skin necrosis requiring debridement, patients in the C-NSM group tended to experience more postoperative skin necrosis (0.0% vs. 8.0%). A previous study on E-NSM performed in 50 patients with breast cancer reported that the rate of NAC necrosis was approximately 2% [10]. A periareolar incision was not needed in patients who underwent E-NSM, which could have minimized injury to the blood supply of the NAC. In addition, energy devices, such as electrothermal bipolar and ultrasonic shears, were used to dissect the retromammary and subcutaneous flaps for all patients in the E-NSM group. These devices have lower lateral thermal spread and protect from skin necrosis during surgery [25].
Several limitations of this study should be considered, including its retrospective design, which has a potential bias, such as information bias or selection bias. Because this was a retrospective study, the operator's opinion and the patient's choice may have influenced the selection of the surgical methods between the 2 groups. However, in this institution, patients with low BMI or small breasts underwent reconstruction using implant insertion, and the others underwent TRAM reconstruction. This indication was applied equally to both groups as E-NSM or C-NSM, which led to no significant difference between the demographic data of the 2 groups. This result (Table 1) showed that there was no significant difference between the 2 groups in the analysis of demographic data, such as BMI, tumor size, and age. Nevertheless, unequal numbers between the 2 groups and the small number of subjects could be insufficient to support our results. Relapse-free survival was not analyzed due to the short follow-up duration, because E-NSM was introduced relatively recently. Accordingly, the results must be interpreted with caution, and further randomized prospective studies with more cases and a longer follow-up period are needed to confirm the clinical role of E-NSM.
In conclusion, the results showed that E-NSM was feasible and safe with a more inconspicuous incision in patients with breast cancer. The positive resection margins and complication rates of E-NSM were comparable to those of C-NSM, although the operative time was longer.
Footnotes
Funding: This work was supported by grants from the Korea University Ansan Hospital Grant (K1811041) and Korea Breast Cancer Foundation (Q1809151).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Chang YW, Lee HY.
- Data curation: Chang YW, Lee HY, Yu DY, Kim DW
- Formal analysis: Chang YW, Lee HY.
- Investigation: Chang YW, Lee HY.
- Methodology: Chang YW, Lee HY, Yu DY, Lee TY, Kim DW.
- Supervision: Chang YW, Lee HY, Jung SP, Woo SU, Lee JB, Son GS.
- Validation: Chang YW, Lee HY, Jung SP, Woo SU, Lee JB, Son GS.
- Writing - original draft: Chang YW, Lee HY.
- Writing - review & editing: Chang YW, Lee HY.
References
- 1.Freeman BS. Complications of subcutaneous mastectomy with prosthetic replacement, immediate or delayed. South Med J. 1967;60:1277–1280. doi: 10.1097/00007611-196712000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull. 1962;30:676–682. doi: 10.1097/00006534-196212000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Orzalesi L, Casella D, Santi C, Cecconi L, Murgo R, Rinaldi S, et al. Nipple sparing mastectomy: surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast. 2016;25:75–81. doi: 10.1016/j.breast.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Gerber B, Krause A, Dieterich M, Kundt G, Reimer T. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg. 2009;249:461–468. doi: 10.1097/SLA.0b013e31819a044f. [DOI] [PubMed] [Google Scholar]
- 5.Blechman KM, Karp NS, Levovitz C, Guth AA, Axelrod DM, Shapiro RL, et al. The lateral inframammary fold incision for nipple-sparing mastectomy: outcomes from over 50 immediate implant-based breast reconstructions. Breast J. 2013;19:31–40. doi: 10.1111/tbj.12043. [DOI] [PubMed] [Google Scholar]
- 6.Cavalcante FP, Lima MV. Nipple-sparing mastectomy with periareolar incision and two-stage reconstruction: initial analysis of 31 cases. Breast J. 2018;24:940–943. doi: 10.1111/tbj.13114. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima H, Fujiwara I, Mizuta N, Sakaguchi K, Hachimine Y, Magae J. Video-assisted skin-sparing breast-conserving surgery for breast cancer and immediate reconstruction with autologous tissue: clinical outcomes. Ann Surg Oncol. 2009;16:1982–1989. doi: 10.1245/s10434-009-0429-1. [DOI] [PubMed] [Google Scholar]
- 8.Park HS, Lee JS, Lee JS, Park S, Kim SI, Park BW. The feasibility of endoscopy-assisted breast conservation surgery for patients with early breast cancer. J Breast Cancer. 2011;14:52–57. doi: 10.4048/jbc.2011.14.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mok CW, Lai HW. Endoscopic-assisted surgery in the management of breast cancer: 20 years review of trend, techniques and outcomes. Breast. 2019;46:144–156. doi: 10.1016/j.breast.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Lai HW, Lin SL, Chen ST, Kuok KM, Chen SL, Lin YL, et al. Single-axillary-incision endoscopic-assisted hybrid technique for nipple-sparing mastectomy: technique, preliminary results, and patient-reported cosmetic outcome from preliminary 50 procedures. Ann Surg Oncol. 2018;25:1340–1349. doi: 10.1245/s10434-018-6383-z. [DOI] [PubMed] [Google Scholar]
- 11.Du J, Liang Q, Qi X, Ming J, Liu J, Zhong L, et al. Endoscopic nipple sparing mastectomy with immediate implant-based reconstruction versus breast conserving surgery: a long-term study. Sci Rep. 2017;7:45636. doi: 10.1038/srep45636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto N, Fukuma E, Higa K, Ozaki S, Sakamoto M, Abe S, et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg Oncol. 2009;16:3406–3413. doi: 10.1245/s10434-009-0661-8. [DOI] [PubMed] [Google Scholar]
- 13.Toesca A, Peradze N, Manconi A, Galimberti V, Intra M, Colleoni M, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017;31:51–56. doi: 10.1016/j.breast.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarfati B, Struk S, Leymarie N, Honart JF, Alkhashnam H, Tran de Fremicourt K, et al. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol. 2018;25:2579–2586. doi: 10.1245/s10434-018-6555-x. [DOI] [PubMed] [Google Scholar]
- 15.Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25:1783–1785. doi: 10.1245/s10434-018-6486-6. [DOI] [PubMed] [Google Scholar]
- 16.Regnault P. Breast ptosis. Definition and treatment. Clin Plast Surg. 1976;3:193–203. [PubMed] [Google Scholar]
- 17.Nishimura R, Akizuki M, Tashima R, Ootao R. Investigation of factors related to periods to ipsilateral breast tumor recurrence after breast-conserving surgery and measures for preventing recurrence in early breast cancer. Breast Cancer. 2006;13:152–158. doi: 10.2325/jbcs.13.152. [DOI] [PubMed] [Google Scholar]
- 18.Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–1620. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 19.Hung CS, Chang SW, Liao LM, Huang CC, Tu SH, Chen ST, et al. The learning curve of endoscopic total mastectomy in Taiwan: a multi-center study. PLoS One. 2017;12:e0178251. doi: 10.1371/journal.pone.0178251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai HW, Toesca A, Sarfati B, Park HS, Houvenaeghel G, Selber JC, et al. Consensus statement on robotic mastectomy-expert panel from International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg. 2020;271:1005–1012. doi: 10.1097/SLA.0000000000003789. [DOI] [PubMed] [Google Scholar]
- 21.Lai HW, Chen ST, Lin SL, Chen CJ, Lin YL, Pai SH, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2019;26:42–52. doi: 10.1245/s10434-018-6704-2. [DOI] [PubMed] [Google Scholar]
- 22.Park HS, Lee J, Lee H, Lee K, Song SY, Toesca A. Development of robotic mastectomy using a single-port surgical robot system. J Breast Cancer. 2020;23:107–112. doi: 10.4048/jbc.2020.23.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan LJ, Jiang J, Yang XH, Zhang Y, Li XG, Chen XC, et al. A prospective study comparing endoscopic subcutaneous mastectomy plus immediate reconstruction with implants and breast conserving surgery for breast cancer. Chin Med J (Engl) 2009;122:2945–2950. [PubMed] [Google Scholar]
- 24.Carlson GW, Bostwick J, 3rd, Styblo TM, Moore B, Bried JT, Murray DR, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225:570–575. doi: 10.1097/00000658-199705000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang YW, Kim HS, Jung SP, Woo SU, Lee JB, Bae JW, et al. Comparison of skin-sparing mastectomy using LigaSure™ Small Jaw and electrocautery. World J Surg Oncol. 2017;15:129. doi: 10.1186/s12957-017-1199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]