Abstract
Cellular senescence is characterized by an irreversible cell cycle arrest as well as a pro‐inflammatory phenotype, thought to contribute to aging and age‐related diseases. Neutrophils have essential roles in inflammatory responses; however, in certain contexts their abundance is associated with a number of age‐related diseases, including liver disease. The relationship between neutrophils and cellular senescence is not well understood. Here, we show that telomeres in non‐immune cells are highly susceptible to oxidative damage caused by neighboring neutrophils. Neutrophils cause telomere dysfunction both in vitro and ex vivo in a ROS‐dependent manner. In a mouse model of acute liver injury, depletion of neutrophils reduces telomere dysfunction and senescence. Finally, we show that senescent cells mediate the recruitment of neutrophils to the aged liver and propose that this may be a mechanism by which senescence spreads to surrounding cells. Our results suggest that interventions that counteract neutrophil‐induced senescence may be beneficial during aging and age‐related disease.
Keywords: aging, neutrophils, senescence, telomeres
Subject Categories: Cell Cycle, Immunology, Molecular Biology of Disease
Oxidative damage caused by inflammatory neutrophils contributes to neighbouring cell senescence and liver injury.

Introduction
Neutrophils play a critical role in the process of microbial killing, which they achieve through the actions of a variety of neutrophil‐expressed microbicidal factors including proteolytic enzymes and reactive oxygen species (ROS). In addition, neutrophils are rapidly recruited to sites of tissue damage in response to damage‐associated molecular patterns (DAMPs) and their induction of neutrophil chemoattractants which include C‐X‐C chemokines, calprotectins, and lipid mediators important in the sterile immune response to tissue damage (Peiseler & Kubes, 2019). In acute resolving injuries, neutrophils stimulate wound repair by removing injured cells, clearing cellular remnants, and releasing growth factors and pro‐angiogenic molecules. In this context, neutrophils are important for resolution of inflammation and enabling the subsequent regenerative response. Where damage is chronic, neutrophils fail to effectively resolve inflammation and can instead exacerbate cellular stress and injury through their release of matrix metalloproteinases and ROS. Less well understood is the degree to which neutrophilic inflammation may induce changes in tissues that impact on longer‐term health and susceptibility to disease.
Cellular senescence refers to an irreversible cell cycle arrest, which can be triggered by a number of stressors, including oxidative stress and telomere dysfunction among many others (Gorgoulis et al, 2019). Senescence is associated with a number of distinctive phenotypes, including the development of a pro‐inflammatory response, commonly known as the senescence‐associated secretory phenotype (SASP). The SASP is thought to communicate with the immune system and facilitate immune surveillance, stimulating the clearance of senescent or pre‐malignant cells (Ovadya et al, 2018). However, chronic exposure to the SASP can lead to age‐associated tissue dysfunction (Jurk et al, 2014; Xu et al, 2018). Consistent with this idea, senescent cells have been shown to accumulate in many tissues with age and at etiological sites in multiple chronic diseases (Ogrodnik et al, 2019a). Importantly, clearance of senescent cells either genetically or by using “senolytic” drugs has been shown to alleviate the onset of a number of pathologies during aging and disease (Zhu et al, 2015; Baker et al, 2016; Farr et al, 2017; Ogrodnik et al, 2017; Bussian et al, 2018; Musi et al, 2018; Anderson et al, 2019; Ogrodnik et al, 2019b).
Senescent cells have been shown to accumulate during aging in murine liver (Wang et al, 2009; Hewitt et al, 2012) and in the context of age‐related liver disease in humans (Ogrodnik et al, 2017). The pathophysiological impacts of senescence are exemplified by the aging liver where regeneration and capacity to overcome injuries wanes in older animals and humans. Consequently, aging is associated with a higher incidence of acute liver failure and chronic liver disease. Consistent with a role for senescent cells in liver pathology, it has been shown that clearance of senescent cells reduces liver steatosis in aged, obese, and diabetic mice (Ogrodnik et al, 2017). However, the underlying mechanisms driving senescence in the liver are not completely understood. Here, we investigated the hypothesis that neutrophils recruited from the circulation in response to damage can act as drivers of cellular senescence and as such contribute to organ dysfunction during aging and disease.
We first observed that short‐term co‐culture of neutrophils with human primary fibroblasts accelerates the rate of telomere shortening and induces premature replicative senescence in the latter, a process which is dependent on ROS and requires cell‐to‐cell contact. Importantly, we found that ectopic expression of the catalytic subunit of telomerase (hTERT) prevents neutrophil‐induced telomere shortening and premature senescence. Ex vivo studies involving precision‐cut liver slices (PCLS) obtained from human subjects confirm that hepatic infiltration by neutrophils induces telomere dysfunction and senescence markers in liver hepatocytes. Further investigation involving mouse PCLS showed that neutrophils from transgenic mice overexpressing human catalase in mitochondria (MCAT) did not induce paracrine telomere dysfunction or expression of p21 as opposed to wild‐type neutrophils. In vivo, we found that depletion of neutrophils reduces telomere dysfunction and senescence in a model of acute liver injury. Finally, we observed that during liver aging, neutrophils accumulate in the hepatic parenchyma and are found in close proximity to hepatocytes containing dysfunctional telomeres. Genetic clearance of p16Ink4a‐positive cells in aged INK‐ATTAC mice reduces the number of neutrophil infiltrates. In contrast, stimulation of neutrophil recruitment to the liver enhances hepatocyte senescence. Altogether, our findings suggest that neutrophils are both recruited by senescent cells and can be drivers of senescence. Therapies which counteract neutrophil‐induced senescence may be beneficial during aging and age‐related disease.
Results
1‐Neutrophils induce paracrine senescence
In order to test the hypothesis that neutrophils contribute to cellular senescence, we began by co‐culturing human MRC5 fibroblasts for 3 days with freshly isolated neutrophils from similarly aged healthy donors (Figs 1A, and EV1A and B). To avoid the confounding factor of neutrophil cell‐death (which we observed to occur after 24 h), we replaced neutrophils daily and cultured them at 3% O2. In order to test the role of ROS as a mediator of neutrophil‐induced senescence, neutrophils and fibroblasts were co‐cultured in the presence or absence of extracellular recombinant catalase for 3 days. After 3 days of co‐culture, neutrophils were removed (as well as the catalase) and MRC5 fibroblasts were passaged until they reached replicative senescence (Fig 1A).
Figure 1. Neutrophils induce paracrine senescence.
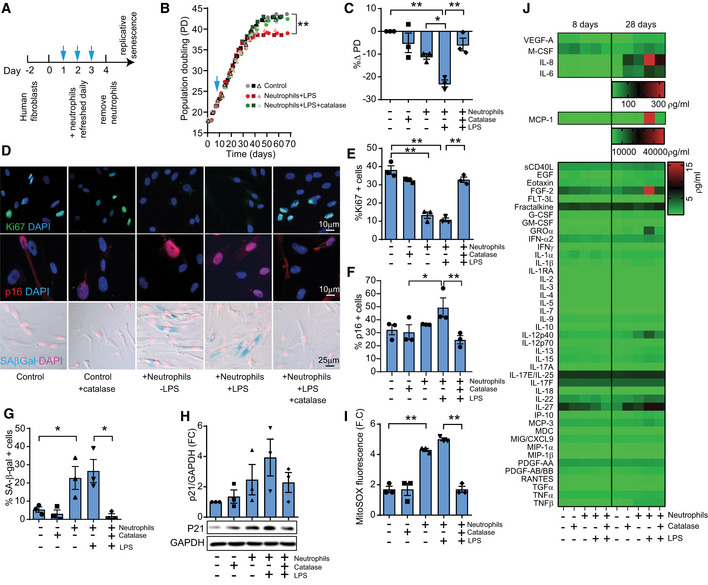
-
ASchematic depicting experimental design.
-
BEffect of neutrophils and recombinant catalase on population doublings (data are from three independent cultures are shown). Blue arrow indicates time point in which neutrophils were added to culture for consecutive 3 days.
-
C% Δ Population Doublings (PD) following neutrophil co‐culture.
-
DRepresentative images of Ki67 and p16INK4A immunofluorescence and SA‐β‐Gal staining.
-
E% of Ki67‐positive cells (28 days after co‐culture).
-
F% of p16INK4A‐positive cells (28 days after co‐culture).
-
G% of SA‐β‐Gal‐positive cells (28 days after co‐culture).
-
HWestern blotting for p21 and quantification.
-
IMitoSOX fluorescence.
-
JHeat map depicting expression of 48 human cytokines in conditioned media at 8 and 28 days following co‐culture.
Data information: Data are mean ± SEM of three independent experiments. Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
Source data are available online for this figure.
Figure EV1. Neutrophils induce paracrine senescence.
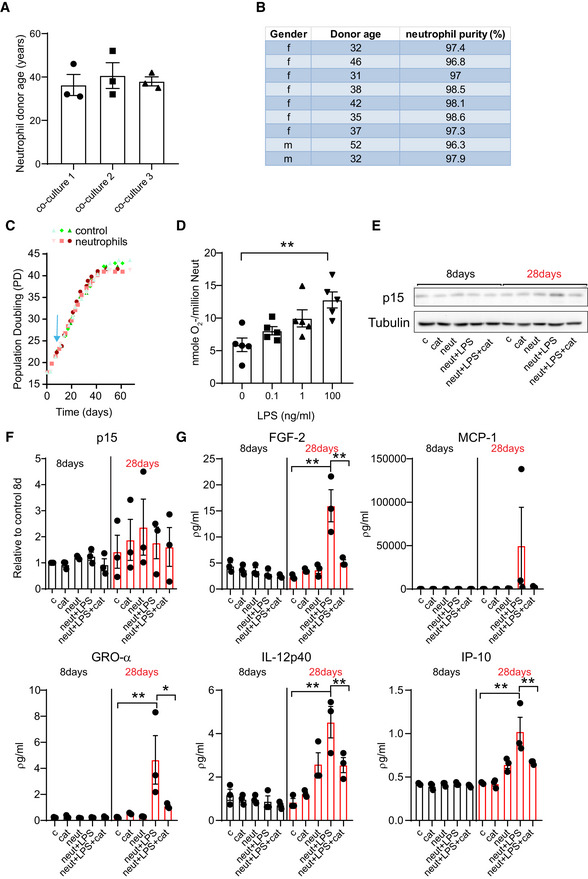
-
AAge of neutrophil healthy donors used in independent co‐culture experiments. Data are mean ± SEM of n = 3.
-
BTable summarizing gender and donor age as well as % of neutrophil purity.
-
CGrowth curves comparing population doublings in controls and MRC5 co‐cultured with non‐primed neutrophils. Blue arrow indicates time point in which neutrophils were added to culture for consecutive 3 days.
-
DSuperoxide anion levels in neutrophils following treatment with different concentrations of LPS. The highest increase was observed after 1 h treatment with 100 ng/ml. Data are mean ± SEM of n = 5 independent experiments.
-
ERepresentative Western blot of p15 and tubulin expression 8 and 28 days after neutrophil co‐culture.
-
FQuantification of p15 expression relative to tubulin. Data are mean ± SEM of three independent experiments.
-
GExpression of selected secreted factors (from cytokine array depicted in Figure 1j) at 8 and 28 days after neutrophil co‐culture. Data are mean ± SEM of three independent experiments.
Data information: Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
Growth curves revealed that co‐culture with neutrophils did not acutely affect the proliferation rate of MRC5 fibroblasts; however, instead it led to a premature growth arrest at later stages in culture, which could be prevented by pre‐incubation with catalase (Fig 1B and C). We also observed that when neutrophils were LPS‐primed the effects on replicative lifespan were more pronounced (Figs 1C and EV1C), which may be due to neutrophil activation and an associated stimulation of ROS (Fig EV1D).
Analysis of the proliferation marker Ki67 after 28 days in culture confirmed that fibroblasts co‐cultured with neutrophils displayed reduced proliferation (Fig 1D and E). In contrast, senescent markers p16INK4A (Fig 1D and F) and SA‐β‐Gal (Fig 1D and G) were elevated in co‐cultured fibroblasts at 28 days and these effects were prevented by treatment with catalase. p21 expression showed a trend for an increase with activated neutrophils and reduction with catalase treatment (Fig 1H). We observed in one experiment that neutrophil co‐culture led to an increase in p15 expression at the 28‐day time point and a reduction with catalase (Fig EV1E) but this finding was not reproducible in independent experiments (Fig EV1F).
Enhanced mitochondrial ROS generation is both a characteristic of senescent cells (Correia‐Melo et al, 2016), but also can be a driver of senescence (Passos et al, 2007). We observed that fibroblasts that had been co‐cultured with neutrophils displayed increased MitoSOX fluorescence, which was significantly reduced in catalase‐treated co‐cultures (Fig 1I). An important phenotype of senescent cells is the development of the SASP. In order to characterize the SASP, we carried out a cytokine array in conditioned media (CM) collected from these cells after 8 and 28 days of culture. From the panel of 48 soluble proteins analyzed, we found that several factors were elevated in human fibroblasts that had been co‐cultured with activated neutrophils and that catalase was able to prevent this effect (Figs 1J and EV1G).
Altogether, our results suggest that exposure of a bystander cell to neutrophils, while being insufficient to acutely affect proliferation, can limit its replicative lifespan and that this effect is predominantly mediated by ROS.
2‐ Neutrophils induce paracrine senescence via telomere dysfunction
Telomere shortening is believed to be a major contributor to replicative senescence (Harley et al, 1990; Bodnar et al, 1998). Moreover, previous work has shown that oxidative stress accelerates the rate of telomere shortening (von Zglinicki, 2002) and that telomeres are particularly sensitive to oxidative modifications (Petersen et al, 1998). In order to investigate whether neutrophils can induce telomere dysfunction in neighboring cells, we co‐cultured neutrophils with human MRC5 fibroblasts as previously described and evaluated telomere length by Q‐FISH in metaphase spreads at different time points. We found that neutrophils (both primed or non‐primed) significantly reduced telomere FISH intensity over the course of 16 days, which is indicative of accelerated telomere shortening (Fig 2A–C). Consistent with a role for ROS in the process, 3‐day pre‐incubation with recombinant catalase was sufficient to prevent the accelerated telomere shortening.
Figure 2. Neutrophils induce paracrine senescence via telomere dysfunction.
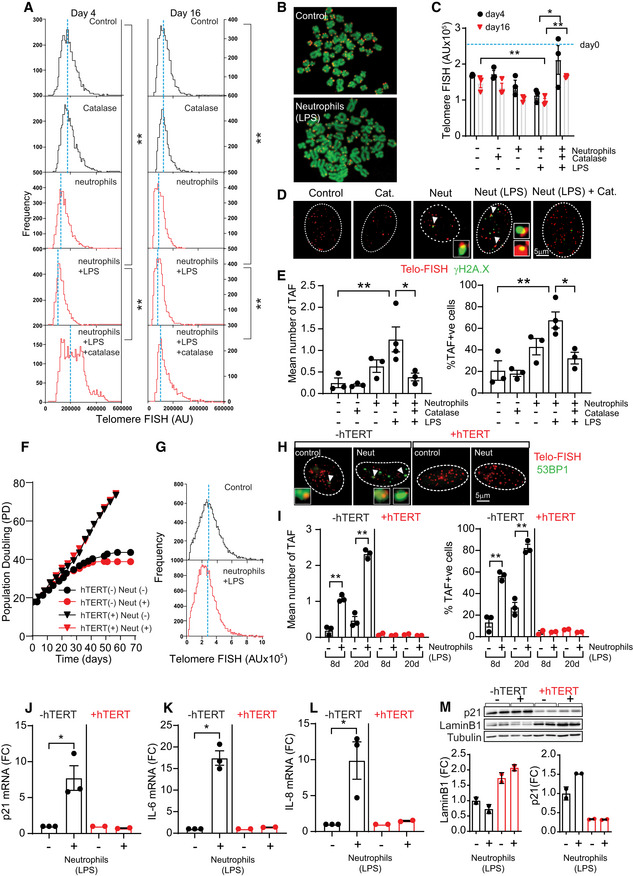
-
AHistograms representing distribution of individual telomere FISH intensities at day 4 and 16 following co‐culture with neutrophils. Dotted blue lines represent median telomere FISH fluorescence. Data are from n = 3 independent experiments.
-
BRepresentative micrographs of telomere FISH in metaphases.
-
CMean telomere FISH intensity at 4 and 16 days following co‐culture with neutrophils represented as mean ± SEM of three independent experiments.
-
DRepresentative Immuno‐FISH micrographs (using telomere‐specific (CCCTAA) peptide nucleic acid probe and anti‐γH2A.X antibody). Arrows indicate co‐localization between telomeres and γH2A.X.
-
EMean number and % of telomere‐associated DDR foci (TAF). Data are mean ± SEM of three independent experiments.
-
FPopulation doublings of hTERT‐expressing MRC5 fibroblasts or controls following co‐culture with primed neutrophils (data are representative of six independent experiments).
-
GHistograms representing distribution of individual telomere FISH intensities in MRC5 fibroblasts expressing hTERT 16 days following neutrophil co‐culture. Dotted blue lines represent median telomere FISH fluorescence.
-
HRepresentative Immuno‐FISH micrographs (using telomere‐specific (CCCTAA) peptide nucleic acid probe and anti‐53BP1 antibody) 20 days following co‐culture.
-
IQuantification of mean TAF and % TAF in MRC5 fibroblasts 8 and 20 days following neutrophil co‐culture. Data are mean ± SEM of three independent experiments.
-
J–LmRNA expression of p21 (J), IL‐6 (K), and IL‐8 (L) 20 days after neutrophil co‐culture; data are mean ± SEM of 2–3 independent experiments.
-
MWestern blot and quantification of p21 and Lamin B1 20 days after neutrophil co‐culture. Data are mean ± SEM of two independent experiments.
Data information: Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and Mann–Whitney test and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
Source data are available online for this figure.
Critically short telomeres induce senescence via the activation of a DNA damage response (DDR) (d'Adda di Fagagna et al, 2003). Furthermore, our previous work demonstrated that telomere DNA damage is irreparable and leads to a persistent DDR during cellular senescence (Fumagalli et al, 2012; Hewitt et al, 2012; Anderson et al, 2019). We therefore investigated the impact of neutrophils on telomere dysfunction in human fibroblasts by using Immuno‐FISH to quantify co‐localization between DDR proteins γH2A.X (or 53BP1) and telomeres, hereafter referred to as telomere‐associated foci (TAF). We found that neutrophils induced a significant increase in TAF, which could be prevented by catalase (Fig 2D and E). It should be noted that we also found an increase in the frequency of total γH2A.X foci (Fig EV2A). Similarly, DNA breaks measured by COMET assay increased in fibroblasts that had been co‐cultured with neutrophils, and the effect was rescued by catalase (Fig EV2B). However, suggesting a specific role for telomeres in the process, we found that MRC5 fibroblasts ectopically expressing the catalytic subunit of telomerase hTERT did not experience neutrophil‐induced premature growth arrest (Fig 2F) or telomere shortening (Fig 2G). Additionally, we found that hTERT‐expressing fibroblasts did not show increased TAF (Fig 2H and I), expression of senescent markers p21, IL‐6, and IL‐8 or decreased expression of Lamin B1 (Fig 2J–M), increased 53BP1 foci (Fig EV2C) or expression of p16 and p15 (Fig EV2D and E) 20 days after co‐culture with neutrophils. Previous work had shown that under conditions of stress, telomerase can be translocated from the nucleus to the mitochondria improving mitochondrial function and reducing ROS through non‐canonical roles (Ahmed et al, 2008). We did not find any evidence for translocation of telomerase to the cytosol as a result of neutrophil‐induced stress (Fig EV2F).
Figure EV2. Neutrophils induce a DNA damage response in bystander cells.
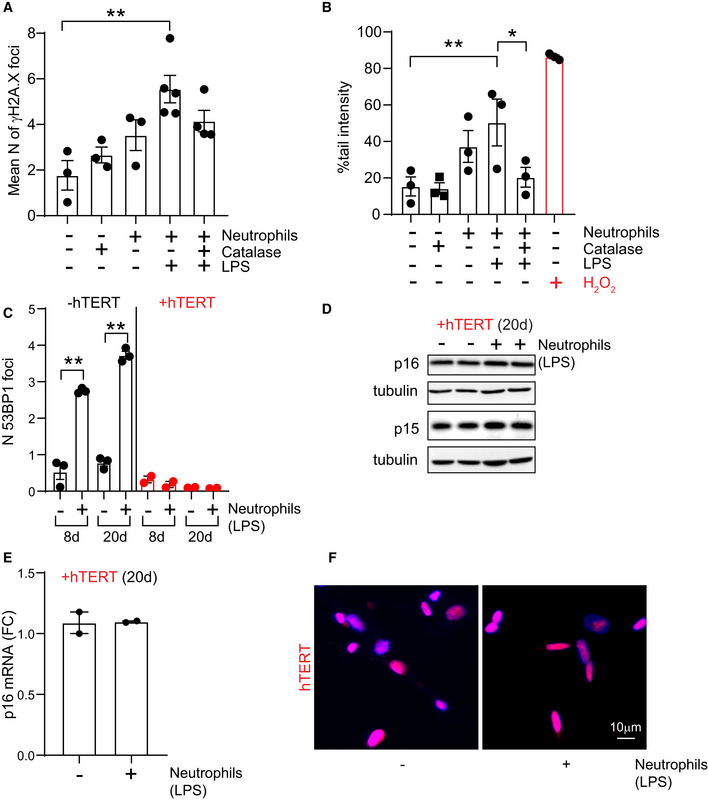
-
A, BEffect of neutrophil and recombinant catalase co‐culture on (A) mean number of γH2A.X foci per cell and (B) % tail intensity measured by Alkaline Comet assay. Data are mean ± SEM of n = 3 independent experiments.
-
C–EEffect of hTERT expression (8 and 20 days after primed neutrophil co‐culture) on (C) number of 53BP1 foci, (D) expression of p16INK4A and p15 and (E) p16INK4A mRNA levels. Data are mean ± SEM of n = 2–3 independent experiments.
-
FRepresentative micrograph showing nuclear localization of hTERT 4 days after co‐culture with primed neutrophils.
Data information: Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
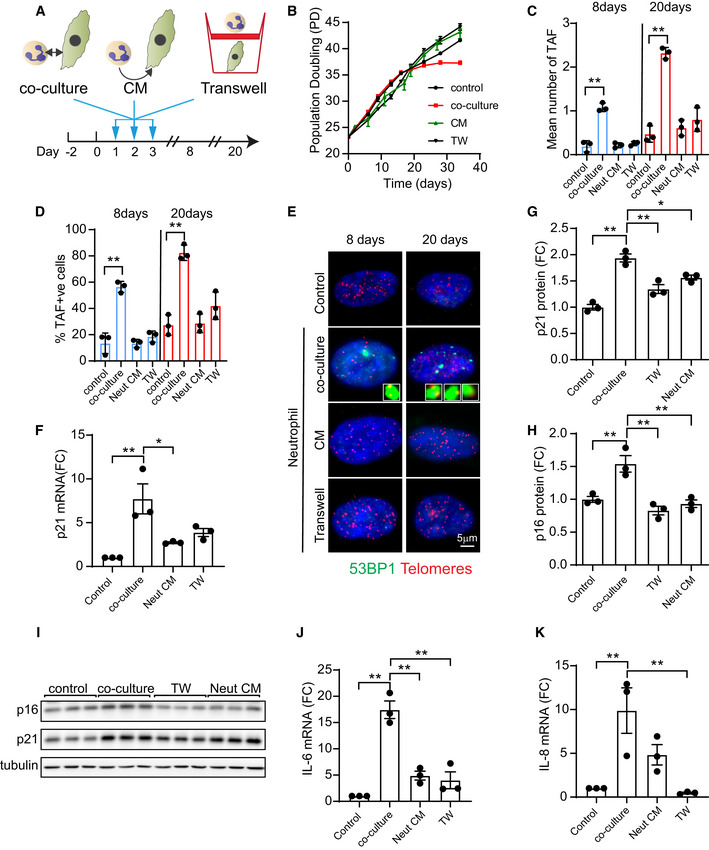
Following the observation that neutrophils can induce paracrine senescence in fibroblasts, we sought to investigate if direct cell‐to‐cell contact or if soluble factors released by neutrophils are required for this effect. In order to explore these hypotheses, we co‐cultured human fibroblasts with neutrophils for 3 days as described before and in parallel exposed human fibroblasts to conditioned media from neutrophils or co‐cultured neutrophils and fibroblasts in separate layers sharing the same medium using transwell inserts (Fig EV3A). We observed that human fibroblasts that had been in direct contact with neutrophils for 3 days experienced a premature growth arrest starting at 20 days of culture, while fibroblasts treated with conditioned medium or in transwells continued proliferation (Fig EV3B). Furthermore, our results indicate that direct cell‐to‐cell contact between neutrophils and fibroblasts is necessary to induce TAF (Fig EV3, EV4, EV5), p21 and p16 expression (Fig EV3, EV4, EV5) and expression of SASP factors IL‐6 and IL‐8 (Fig EV3J and K).
Figure EV3. Neutrophil‐induced paracrine senescence requires cell‐to‐cell contact.
-
ASchematic depicting experimental design. MRC5 fibroblasts were (i) co‐cultured with neutrophils; (ii) exposed to conditioned media from neutrophils; or (iii) co‐cultured with neutrophils in transwell inserts for 3 days (neutrophils were primed with LPS and replenished every 24 h).
-
BEffect of neutrophil co‐culture, conditioned media (CM) or transwell co‐culture (TW) on population doublings (Data are mean ± SEM of n = 3 independent experiments).
-
C, D(C) Mean number of TAF and (D) % TAF‐positive cells 8 and 20 days after neutrophil direct or indirect co‐culture.
-
ERepresentative Immuno‐FISH micrographs (telomere FISH and 53BP1).
-
F–H(F) p21 mRNA, (G) p21 protein expression and (H) p16 protein expression levels 20 days after neutrophil direct or indirect co‐culture.
-
IRepresentative Western blot detecting expression of p16INK4A, p21 and tubulin.
-
J, K(J) IL‐6 mRNA and (K) IL‐8 mRNA expression 20 days after neutrophil direct or indirect co‐culture.
Data information: Data are mean ± SEM of n = 3. Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
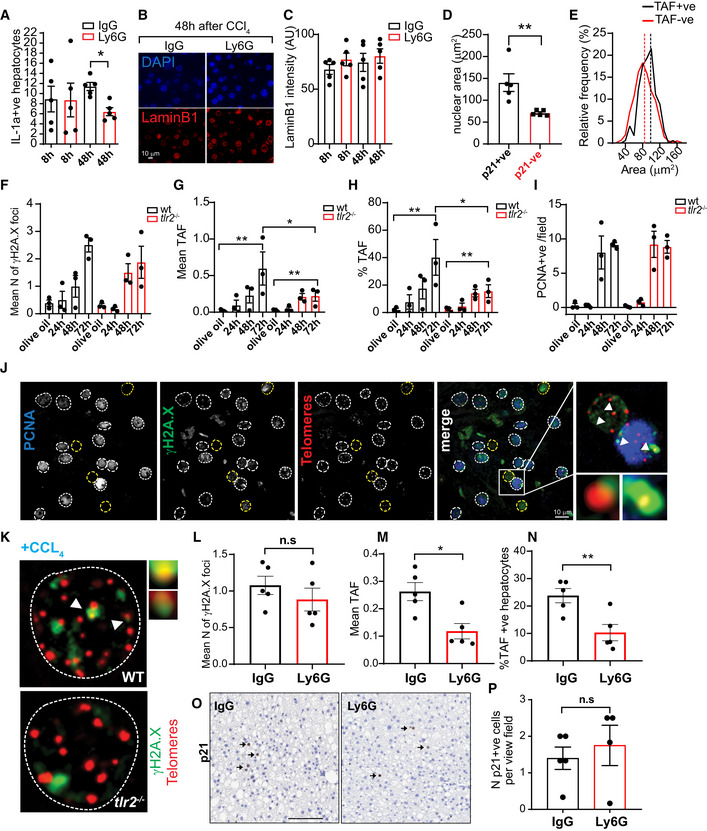
Figure EV4. Neutrophils induce telomere dysfunction and senescence‐associated pathways in vivo .
-
AIL‐1α mRNA expression in hepatocytes 8 and 48 h after injection of CCl4 pre‐treated with neutrophil neutralizing antibody against Ly6G (or with IgG control).
-
BRepresentative micrographs of Lamin B1 expression in hepatocytes 48 h after injection of CCl4.
-
CMean Lamin B1 intensity.
-
DNuclear area comparison of p21 negative (‐ve) or positive (+ve) hepatocytes 48 h after injection of CCl4.
-
EHistograms representing nuclear area distributions of TAF negative (−ve) or positive (+ve) hepatocytes 48 h after injection of CCl4. Wild‐type and Tlr2−/− mice were injected with a single intraperitoneal injection of CCl4 at a dose of 2 μl/g body weight and were sacrificed 24 h, 48 h and 72 h after injection.
-
F–IGraphs show quantification of (F) γH2A.X, (G) mean number of Telomere‐associated foci (TAF), (H) % of TAF‐positive hepatocytes (in PCNA negative hepatocytes) and (I) PCNA‐positive hepatocytes per field.
-
JRepresentative images showing Immuno‐FISH using antibodies against γH2A.X and PCNA and Cy‐3‐labeled telomere‐specific (CCCTAA) peptide nucleic acid probe.
-
KRepresentative image showing Immuno‐FISH comparison between wild‐type and tlr2−/− hepatocytes 72 h after CCl4. Arrows indicate co‐localization between γH2A.X and telomeres.
-
L–NAnalysis of (L) γH2A.X, (M) mean number of TAF and (N) % of TAF‐positive hepatocytes in mice under high‐fat diet treated with neutrophil neutralizing antibody against Ly6G (or with IgG control).
-
ORepresentative p21 immunohistochemistry in mice under high‐fat diet treated with neutrophil neutralizing antibody against Ly6G (or with IgG control). Scale bar = 100 μm.
-
PQuantification of p21‐positive cells per field of view.
Data information: Data are mean ± SEM of n = 3–5 mice per group. Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
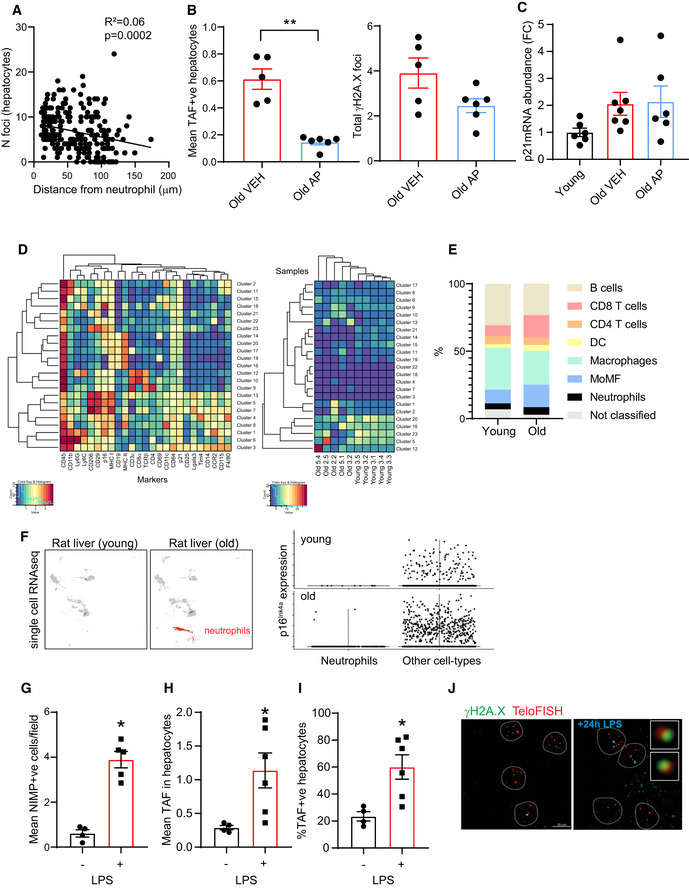
Figure EV5. Supporting data for Figure 6.
-
AQuantification of mean number of TAF in hepatocytes as a function of the distance from a Ly6G labeled neutrophil. Data are from five mice aged 29 months of age, line indicates linear regression (linear regression and F test: r2 = 0.06; P = 0.0002).
-
BMean number of TAF (left) and mean number of γH2A.X foci (right) in aged (28–29 months old) INK‐ATTAC mice treated with vehicle (VEH) or AP20187 (AP). Data are mean ± SEM of n = 5–6 mice per age group.
-
CComparison between p21 mRNA levels of young (3 months old) and old INK‐ATTAC mice (28–29 months old) treated with vehicle or AP20187. Data are mean ± SEM of n = 5–8 mice per age group.
-
D(left) Heat map demonstrating the distribution and relative intensity of the cell surface markers used in the clustering analysis in CyTOF experiment and (right) heat map showing the relative abundance of each cluster for each mouse (5 young and 5 old).
-
EGraph showing relative distribution of different intrahepatic leukocytes in young and old mice.
-
F(left) UMAP plots representing single‐cell RNA‐sequencing data obtained from (Ma et al, 2020) depicting different population in rat livers (5 and 27 months old). Neutrophils are labeled in red. (right) Violin plots show that p16Ink4a expression is very low in neutrophils at either age group.
-
G–JPure LPS was administered by intraperitoneal injection to wild‐type mice (8–10 weeks old; C57BL/6) at a dose of 300 µg/animal for 24 h: (G) Number of neutrophils infiltrates (NIMP+ve) in liver per field of view; (H) mean number of TAF and (I) % of TAF in hepatocytes 24 h following LPS injection; (J) representative images of Immuno‐FISH using antibodies against γH2A.X and Cy‐3‐labeled telomere‐specific (CCCTAA) peptide nucleic acid probe. Data are mean ± SEM of n = 4–5 mice per group.
Data information: Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
In summary, our data indicate that ROS produced by neutrophils can accelerate the rate of telomere shortening in bystander cells, thereby inducing premature senescence and that this requires direct cell‐to‐cell contact.
3‐ Neutrophils induce telomere dysfunction and senescence in precision‐cut liver slices
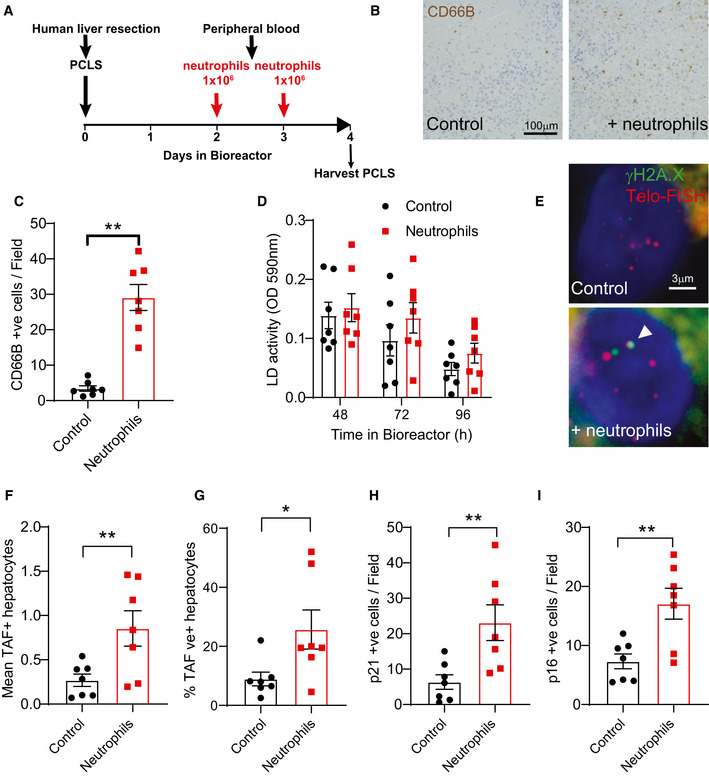
Precision‐cut liver slices (PCLS) are useful tools to investigate liver function, since they retain the structure and cellular composition of the native liver thereby being largely superior to two‐dimensional cultures. We have recently developed a bioreactor platform, which can maintain functional PCLS cultures for longer periods of time than other systems (up to 6 days). Using this methodology, we sought to investigate if neutrophils could induce telomere dysfunction and senescence in human livers.
In order to do this, we first isolated human liver tissue from the normal margins of colorectal metastasis resections from patients and generated PCLS. Slices were cultured in BioR plates in our patented bioreactor platform, as previously described (Paish et al, 2019). We then isolated human neutrophils from the peripheral blood of healthy donors and exposed PCLS to 1 × 106 neutrophils for 2 days (these were replenished every 24 h) (Fig 3A). We first confirmed that neutrophils infiltrated the 3D liver slice by immunohistochemistry against neutrophil marker CD66B (Fig 3B and C). We also monitored lactate dehydrogenase (LD) activity during the time of culture, which can be an indicator of cell‐death. There was a tendency for reduction of LD activity in all conditions over the time course of the experiment, which may reflect an adaptation to culture conditions, but no significant change following neutrophil addition (Fig 3D). Similarly, TUNEL staining revealed that neutrophils did not induce cell‐death in PCLS (Appendix Fig S1A).
Figure 3. Neutrophils induce telomere dysfunction and senescence in human precision‐cut liver slices (PCLS).
-
ASchematic depicting experimental design.
-
BRepresentative micrographs of neutrophil marker CD66B.
-
CQuantification of neutrophil counts per field.
-
DLactate dehydrogenase (LD) activity as a function of time in bioreactor.
-
ERepresentative Immuno‐FISH micrographs (using telomere‐specific (CCCTAA) peptide nucleic acid probe and anti‐γH2A.X antibody). Arrow indicates co‐localization between telomeres and γH2A.X.
-
FMean number of TAF in hepatocytes.
-
G% of TAF‐positive hepatocytes.
-
HNumber of p21‐positive hepatocytes per field.
-
INumber of p16Ink4a‐positive hepatocytes per field.
Data information: Data are mean ± SEM from liver biopsies obtained from seven male patients aged between 49 and 72 years. Neutrophils were isolated from healthy individuals aged between 21 and 30 years (with purity above 90%). Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
We then performed 3D Immuno‐FISH and Q‐FISH to evaluate TAF and telomere length, respectively. We found that the addition of neutrophils to PCLS resulted in increases in the mean number of TAF and % of TAF‐positive hepatocytes (Fig 3E–G). However, we failed to detect any effects on telomere length (Appendix Fig S1B). Finally, we found that exposure to neutrophils induced senescence markers p21 and p16INK4A in PCLS (Fig 3H and I).
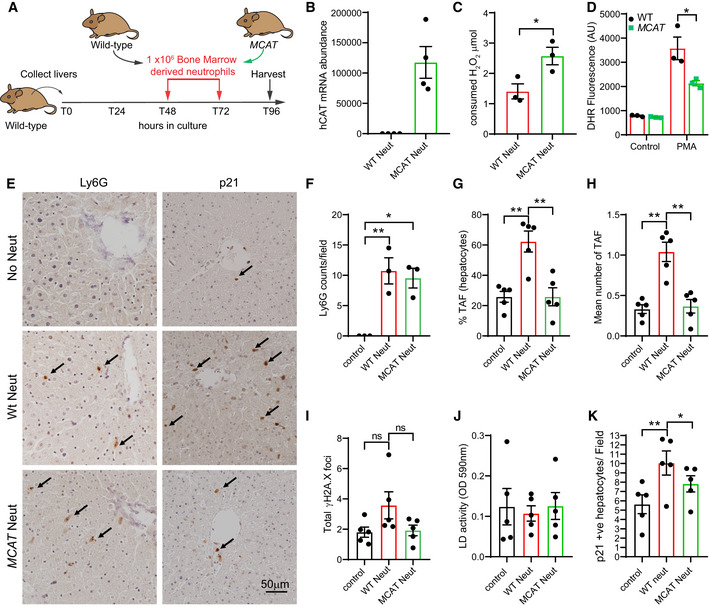
In order to further investigate the hypothesis that ROS derived from neutrophils contributes to bystander telomere dysfunction and senescence in liver, we isolated livers from 8‐ to 12‐week‐old male mice and generated PCLS as previously described (Paish et al, 2019). We then isolated neutrophils from the bone marrow of wild‐type and MCAT transgenic mice and added them to the PCLS (Fig 4A). MCAT mice have a CMV enhancer/chicken beta‐actin promoter driving the expression of human catalase gene in mitochondria and show reduced ROS in different tissues (Schriner et al, 2005). We first confirmed that neutrophils derived from MCAT mice express high levels of human catalase (hCAT) (Fig 4B), show increased catalase activity (Fig 4C), and show reduced DHR fluorescence following PMA stimulation (Fig 4D). After establishing that neutrophils from wild‐type and MCAT mice infiltrated PCLS at similar frequencies (Fig 4E and F), we proceeded to investigate their role in induction of paracrine telomere dysfunction. We found that the addition of wild‐type neutrophils to murine PCLS resulted in significant increases in the mean number of TAF and % of TAF‐positive hepatocytes, but this effect was abrogated when MCAT‐derived neutrophils were added (Fig 4G and H). We found no significant differences in total γH2A.X foci number or apoptosis (measured by LD activity) in any of the conditions (Fig 4I and J). Consistent with induction of senescence‐associated pathways, we found a significant increase in the p21‐positive cells following exposure to wild‐type but not MCAT‐derived neutrophils (Fig 4E and K).
Figure 4. Neutrophils induce telomere dysfunction in a ROS‐dependent manner in precision‐cut liver slices (PCLS).
-
ASchematic depicting experimental design.
-
BmRNA expression of hCAT in bone marrow‐derived neutrophils from wild‐type and MCAT mice.
-
CCatalase activity in bone marrow‐derived neutrophils from wild‐type and MCAT mice.
-
DDihydrorhodamine 123 fluorescence was assessed by flow cytometry in neutrophils from wild‐type and MCAT mice with or without stimulation with PMA.
-
ERepresentative micrographs of neutrophil marker Ly6G and senescent‐associated marker p21 (96 h).
-
FNumber of Ly6G‐positive cells per field (96 h).
-
G% of TAF‐positive hepatocytes (96 h).
-
HMean number of TAF in hepatocytes (96 h).
-
ITotal number of γH2A.X foci in hepatocytes (96 h).
-
JLactate dehydrogenase (LD) activity (96 h).
-
Kp21‐positive cells per field (96 h).
Data information: Data are mean ± SEM of 3–5 independent PCLS. Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
Altogether, these data further support our in vitro findings that neutrophils can induce bystander telomere dysfunction and senescence markers in the liver in a ROS‐dependent manner.
4‐ Neutrophils induce telomere dysfunction and senescence‐associated phenotypes in a model of acute liver injury in vivo
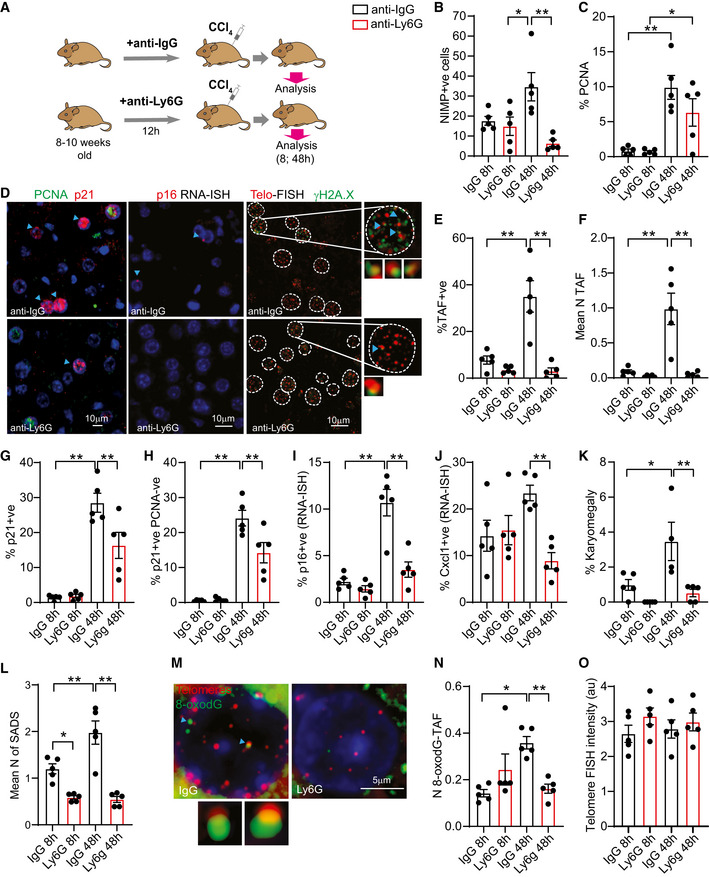
In order to investigate the role of neutrophils in the induction of senescence in vivo, we used the well‐established model of induction of acute liver injury and wound repair via the chemical carbon tetrachloride (CCl4). Mice were pre‐treated with neutrophil neutralizing antibody against Ly6G (or with anti‐IgG control) for 12 h before CCl4 injection (single intraperitoneal injection of CCl4 at a dose of 2 μl/g body weight) and were humanely killed 8 h and 48 h after injection (Fig 5A). As shown previously (Moles et al, 2014), livers exhibited increased neutrophil infiltrates (measured by NIMP1) at 48 h, which were significantly reduced by anti‐Ly6G (Fig 5B). We also found that hepatocytes positive for proliferation marker PCNA were increased 48 h after CCl4 injection, indicating increased compensatory proliferation and remodeling. However, neutrophil depletion did not affect significantly the frequency of PCNA‐positive hepatocytes (Fig 5C). Consistent with our previous data indicating that neutrophils can induce telomere dysfunction in neighboring cells, we found that the mean number of TAF and the % of hepatocytes positive for TAF increased after injury in IgG‐treated mice but were significantly reduced in the mice pre‐treated with anti‐Ly6G (Fig 5D–F). We also evaluated the % of p21‐positive hepatocytes and found that these increased significantly at 48 h, but were reduced by anti‐Ly6G (Fig 5D and G). Similar results were obtained when analyzing hepatocytes that were p21‐positive but negative for the proliferation marker PCNA, which may be a more reliable indication of senescence (Lawless et al, 2010) (Fig 5H). RNA‐ISH revealed that p16Ink4a mRNA levels were elevated 48 h after CCl4 and anti‐Ly6G‐mediated neutrophil depletion reversed this increase (Fig 5D and I). mRNA expression of SASP factors Cxcl1 and IL‐1α, detected by RNA‐ISH, also showed a significant decrease at 48 h with neutrophil depletion (Figs 5J and EV4A). Loss of Lamin B1, another marker of senescence, did not show any significant difference in any of the groups (Fig EV4B and C).
Figure 5. Neutrophils induce telomere dysfunction and senescence‐associated pathways in vivo in a model of acute liver injury.
-
ASchematic depicting experimental design. Mice were pre‐treated with neutrophil neutralizing antibody against Ly6G (or with IgG control) for 12 h before CCl4 injection (single intraperitoneal injection of CCl4 at a dose of 2 μl/g body weight) and were sacrificed 8 h and 48 h after injection.
-
BQuantification of neutrophils (NIMP+ve) in liver.
-
CQuantification of % of PCNA‐positive hepatocytes.
-
DRepresentative micrographs of PCNA and p21 immunofluorescence, p16 RNA in situ hybridization (RNA‐ISH) and Immuno‐FISH (telomere FISH and γH2A.X).
-
E% of TAF‐positive hepatocytes.
-
FMean number of TAF in hepatocytes.
-
Gp21‐positive hepatocytes.
-
H% p21‐positive PCNA negative hepatocytes.
-
I% p16 mRNA‐positive hepatocytes.
-
J% Cxcl1 mRNA‐positive hepatocytes.
-
K% of karyomegalic hepatocytes.
-
LMean number senescence‐associated distension of satellites (SADS).
-
MRepresentative Immuno‐FISH (telomere FISH and 8‐oxodG).
-
NMean number of 8‐oxodG co‐localizing with telomeres in hepatocytes.
-
OMean telomere FISH intensity in hepatocytes.
Data information: Data are mean ± SEM of five mice per group. Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
Another feature of hepatocyte senescence is karyomegaly. We analyzed hepatocyte nuclear size by morphometric analysis of 4,6‐diamidino‐2‐phenylindole (DAPI)‐stained liver sections and quantified the % of karyomegalic hepatocytes, as before (Ogrodnik et al, 2017). Consistent with data for TAF, p16Ink4a, and p21, we found an increase in the % of karyomegalic hepatocytes at 48 h and a significant reduction by neutrophil depletion (Fig 5K). Supporting the concept that senescent hepatocytes exhibit karyomegaly, we found that p21‐ and TAF‐positive hepatocytes had higher nuclear area than negative ones (Fig EV4D and E). We next analyzed senescence‐associated distension of satellites (SADS), an established marker of senescence and found that the mean number of SADS increased in hepatocytes 48 h after CCl4 and was reduced with anti‐Ly6G treatment (Fig 5L).
Our in vitro and ex vivo data suggest that oxidative stress is involved in neutrophil‐induced telomere dysfunction and senescence; thus, we evaluated by 3D Immuno‐FISH the presence of 8‐oxo‐2'‐deoxyguanosine (8‐oxodG) foci and its association with telomeres. Previous work has shown that telomeres are sensitive to ROS‐mediated 8‐oxodG formation that can contribute to telomere dysfunction (Fouquerel et al, 2019). We found that co‐localization between 8‐oxodG and telomeres was increased 48 h after CCl4 and reduced after treatment with anti‐Ly6G (Fig 5M and N) but total number of 8‐oxodG foci was not changed (Appendix Fig S2). Finally, we evaluated telomere length (by Q‐FISH) and found no statistically significant differences between groups (Fig 5O).
Previous work had demonstrated a specific role for Toll‐like receptor 2 (TLR2) in the recruitment of neutrophils after CCl4‐induced liver injury (Moles et al, 2014). Accordingly, we also observed reduced TAF in hepatocytes from Tlr2 −/− mice following CCl4, but no changes in the frequency of proliferating hepatocytes (Fig EV4, EV5). TLR2 has recently been implicated in the regulation of senescence arrest and the SASP; thus, it is entirely possible that reduced TAF in hepatocytes is a direct consequence of deletion of TLR2 rather than an indirect effect of impaired neutrophil recruitment (Hari et al, 2019; Jin et al, 2020).
We next evaluated whether neutrophil depletion (using neutrophil neutralizing antibody against Ly6G) can reduce telomere dysfunction in the context of chronic liver damage induced by NASH (choline deficient high‐fat diet feeding). Notably, we found a significant reduction in TAF‐positive hepatocytes in neutrophil neutralizing antibody against Ly6G‐treated mice compared to controls (Fig EV4, EV5), however no significant changes in expression of p21 (Fig EV4O and P). Our data suggest that neutrophils can be drivers of senescence‐associated phenotypes in the liver.
5‐ p16Ink4a‐positive cells recruit neutrophils during aging
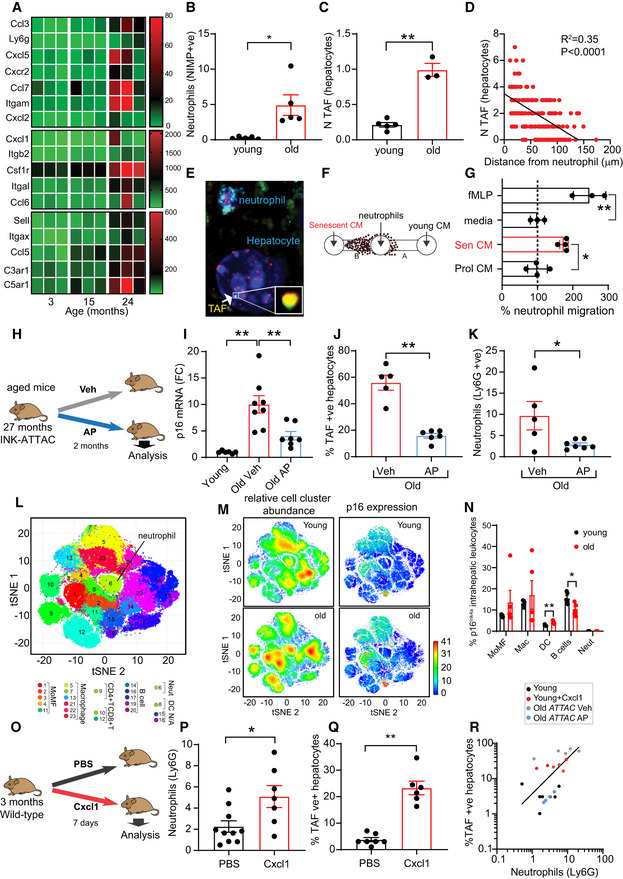
Analysis of RNA‐sequencing data from livers collected from 3‐, 15‐, and 24‐month‐old mice revealed that several genes associated with neutrophil recruitment were up‐regulated with age (Fig 6A). Interestingly, we found that both neutrophil infiltrates and TAF‐positive hepatocytes increased with age (Fig 6B and C). We also observed in aged mice that hepatocytes located in close proximity to neutrophils displayed higher TAF numbers and that TAF inversely correlated with distance from neutrophils (Fig 6D and E). A less pronounced decrease in total number of γH2A.X foci as a function of distance from neutrophils was also observed (Fig EV5A).
Figure 6. p16Ink4a‐expressing cells recruit neutrophils during aging.
-
ARNA‐sequencing data showing expression of genes associated with neutrophil recruitment during liver aging.
-
B, CQuantification of (B) neutrophil infiltrates (NIMP‐positive) and (C) mean number of TAF‐positive hepatocytes in liver of young (3 months) and old (24 months) mice. Data are mean ± SEM of 3–5 mice per group.
-
DQuantification of mean number of TAF in hepatocytes as a function of the distance from a Ly6G labeled neutrophil. Data are from five mice aged 29 months of age; line indicates linear relationship between mean TAF and distance (linear regression and F test: R 2 = 0.35; P < 0.0001).
-
ERepresentative micrograph showing hepatocyte from aged mouse containing a TAF located in close proximity to a neutrophil (labeled with Ly6G).
-
FSchematic depicting experimental design.
-
G% of neutrophil migration toward conditioned media (CM) obtained from young (early passage) and replicatively senescent human fibroblasts. Media alone and fMLP were used as negative and positive controls respectively. Data are mean ± SEM of three independent experiments.
-
HSchematic depicting experimental design for panels I–K.
-
IComparison between p16Ink4a mRNA levels of young (3 months old) and old INK‐ATTAC mice (28–29 months old) treated with vehicle or AP20187. Data are mean ± SEM of n = 5–8 mice per age group.
-
J% of TAF‐positive hepatocytes in old INK‐ATTAC mice (28–29 months old) treated with vehicle or AP20187. Data are mean ± SEM of n = 5–6 mice per group.
-
KLiver neutrophils (Ly6G) in old INK‐ATTAC mice (28–29 months old) treated with vehicle or AP20187. Data are mean ± SEM of n = 5–7 mice per group.
-
LIntrahepatic leukocyte profiling by mass CyTOF. CyTOF was performed on young (6 months) and old (24 months) INK‐ATTAC mice (n = 5 mice per group); 23 unique clusters of intrahepatic leukocytes were defined by a 24‐cell surface marker panel using the Rphenograph clustering algorithm and were visualized on a tSNE plot (t‐distributed stochastic neighbor embedding plot).
-
M(left) tSNE plots comparing the distribution of young and old intrahepatic leukocytes. Red indicates high frequency categorization of cells to a cluster while blue indicates low frequency; (right) tSNE plots comparing p16Ink4a expression in young and old intrahepatic leukocytes. Red indicates high p16Ink4a expression while blue indicates low expression. Data are from n = 5 mice per group.
-
N% of p16Ink4a‐positive cells (detected by CyTOF) in different intrahepatic leukocyte populations isolated from young and old mice. Data are mean ± SEM of n = 5 mice per group.
-
OSchematic depicting experimental design for panels P and Q. 3‐month‐old wild‐type mice were injected with murine Cxcl1 for 7 days.
-
PQuantification of liver neutrophils (Ly6G) in mice injected with vehicle or Cxcl1. Data are mean ± SEM of n = 7–10 mice per group.
-
QQuantification of the % of TAF‐positive hepatocytes in mice injected with vehicle or Cxcl1. Data are mean ± SEM of n = 6–7 mice per group.
-
RCorrelation between TAF‐positive hepatocytes and neutrophils (linear regression and F test: r 2 = 0.70; P = 0.0001).
Data information: Statistical analysis was performed using one‐way ANOVA (Holm–Sidak method) for multiple comparisons and two‐tailed t‐test for single comparisons. **P < 0.01; *P < 0.04.
This observation led us to propose the hypothesis that a vicious cycle occurs between neutrophil infiltrates and hepatic senescence. We hypothesize that senescent cells contribute to some extent to the recruitment of neutrophils during aging and that neutrophil recruitment possibly amplifies senescence in the liver. In order to investigate if senescent cells can recruit neutrophils, we first collected conditioned media from young (early PD) and replicatively senescent fibroblasts and measured the chemotaxis of neutrophils using the sub‐agarose method (Fig 6F). Our data indicate that neutrophils migrate preferentially toward senescent cells (Fig 6G). N‐Formylmethionyl‐leucyl‐phenylalanine (fMLP) chemoattractant was used as a positive control.
To further investigate this hypothesis in vivo, we used the INK‐ATTAC mouse model, in which a small molecule, AP20187 (AP), induces apoptosis through dimerization of FKBP‐fused Casp8, allowing clearance of p16Ink4a senescent cells (Baker et al, 2011). We aged INK‐ATTAC mice until they were 27 months old and treated them with AP for 2 months (Fig 6H). As expected, we found that aged INK‐ATTAC mice had increased mRNA levels of p16Ink4a compared to young mice and this was significantly reduced by addition of AP (Fig 6I). AP treatment of old mice also led to a significant reduction in the frequency of TAF‐positive hepatocytes (Figs 6J and EV5B) but no changes in p21 mRNA expression (Fig EV5C). Consistent with a role for senescent cells in the recruitment of neutrophils, we found that clearance of p16Ink4a cells reduced significantly the number of neutrophils infiltrating in the liver (Fig 6K). It remains a likelihood that AP is clearing p16Ink4a‐expressing neutrophils directly and this could explain our observations. In order to investigate this, we isolated intrahepatic leukocytes from young and old mouse livers and analyzed them by cytometry by time‐of‐flight (CyTOF), which allows for mapping and discriminating between different immune cell types (Figs 6L, and EV5D and E, and Appendix Fig S3). We found that while p16Ink4a was expressed in other immune cells such as monocyte‐derived macrophages, macrophages, B cells, and dendritic cells, it was not detectable in liver neutrophils (Fig 6M). In our experiment, we found that the % of p16Ink4a increased in dendritic cells and decreased in B cells with age (Fig 6N). Consistent with our results, analyses of published single‐cell RNA‐sequencing (scRNA‐seq) from aged rats (Ma et al, 2020) and mice (Mogilenko et al, 2020) revealed that while the frequency of neutrophils increased with age in the liver, these had negligible expression of p16Ink4a (Fig EV5F).
In order to further investigate the role of hepatic neutrophil recruitment in the induction of hepatocyte senescence, we injected 3‐month‐old mice with recombinant murine Cxcl1, a chemokine involved in neutrophil recruitment (Sawant et al, 2016) and the SASP (Coppé et al, 2010) (Fig 6O). We found that Cxcl1 injection increased the frequency of neutrophil infiltrates in the liver (Fig 6P) as well as the % of TAF‐positive hepatocytes (Fig 6Q). Similar results were obtained following injection with lipopolysaccharide (LPS), a potent activator and chemoattractant of neutrophils. LPS resulted in a significant increase in the mean number of neutrophils as well as TAF 24 h after injection (Fig EV5G–J). Interestingly, we found a positive correlation between TAF‐positive hepatocytes and neutrophil infiltrates (Fig 6R).
Altogether, our results support the hypothesis that while neutrophils can trigger telomere dysfunction in hepatocytes, once senescent cells are present in the liver we speculate that these may mediate further neutrophil recruitment and exacerbate telomere dysfunction.
Discussion
Neutrophils, because of their primary functions in the fight against pathogens, contain pore forming molecules, hydrolytic, and oxidative compounds that can inadvertently cause serious secondary damage to the microenvironment (Segal, 2005). Thus, augmented recruitment of neutrophils to sites of sterile inflammation can be detrimental and be a contributor to various diseases (Németh et al, 2020). However, the relationship between neutrophils and cellular senescence, a key driver of aging and disease, remains relatively understudied.
Studies have shown that senescent cells communicate with the immune system and mediate their own clearance. For instance, it was shown that during chronic liver damage, NK cells are involved in the clearance of senescent stellate cells (Krizhanovsky et al, 2008). In addition, it has been shown that pre‐malignant senescent hepatocytes mediate their own clearance via the action of CD4 (+) T cells, monocytes, and macrophages (Kang et al, 2011). Interestingly, depletion of neutrophils did not affect significantly senescence surveillance in this model (Kang et al, 2011). Thus, while neutrophils may be present in close proximity to senescent hepatocytes, the data available to date suggest that they are not involved directly in their clearance.
In this work, we hypothesized that neutrophil recruitment to sites of sterile inflammation could be a contributor to cellular senescence. Our in vitro and ex vivo data indicate that ROS released by neutrophils can accelerate telomere dysfunction and thus induce premature senescence. Furthermore, our data indicate that neutrophil‐induced paracrine senescence requires cell‐to‐cell contact, since human fibroblasts exposed to conditioned media from neutrophils or co‐cultured with neutrophils in transwells did not show premature senescence. These findings are reminiscent of a previous report, which suggested that paracrine senescence is ROS‐dependent and requires cell‐to‐cell contact through gap junctions (Nelson et al, 2012). Neutrophils are capable of producing and secreting a number of pro‐inflammatory factors, some of which have been implicated in the induction of senescence in a non‐autonomous fashion (Acosta et al, 2013). While our data indicate that it is unlikely that longer‐lived soluble factors released by neutrophils are major contributors to paracrine senescence, it remains a possibility that these may be transmitted via gap junctions or tunneling nanotubes (Ariazi et al, 2017).
Telomeres serve as protective structures at the ends of chromosomes, and previous work has revealed they are highly susceptible to oxidative stress mostly due to the high content of guanine residues, which can easily undergo oxidative modifications (Oikawa et al, 2001). Accordingly, mild oxidative stress has been shown to induce single‐stranded breaks preferentially at telomeres and accelerate the rate of telomere shortening (Petersen et al, 1998; von Zglinicki et al, 2000). Induction of 8‐doxoG (a common oxidative DNA lesion) specifically at telomere regions accelerates loss of telomeric repeats and reduces proliferation (Fouquerel et al, 2019). Oxidative stress may also disrupt the binding of certain shelterin proteins that protect telomeres (Opresko et al, 2005). Additionally, studies show that telomeres are less efficiently repaired than the bulk of the genome, mostly because shelterin components such as TRF2 inhibit DNA‐PL and Ligase‐IV‐mediated non‐homologous end joining (NHEJ) (Bae & Baumann, 2007; Fumagalli et al, 2012). Telomeres may also “sense” pro‐inflammatory factors. For instance, recent studies have shown that pro‐inflammatory factors released by senescent cells such as IP‐10 and TGF‐β activate signaling pathways that induce telomere dysfunction in neighboring cells (Razdan et al, 2018; Victorelli et al, 2019). Altogether, these studies support the hypothesis that telomeres are sensors of intrinsic and extrinsic stress and may therefore be particularly susceptible to the close proximity of neutrophils.
Supporting a role for telomeres in neutrophil‐induced paracrine senescence, we found that co‐culture of neutrophils with fibroblasts ectopically expressing hTERT did not affect telomere shortening, cell proliferation, or expression of senescence markers. We have however also observed that DNA damage foci present outside telomere regions are also significantly reduced, which could be suggestive of hTERT having non‐canonical effects uncoupled from its role in telomere maintenance (e.g., reducing DNA damage induction or improving overall DNA repair). Telomerase can shuttle from the nucleus to the mitochondria upon oxidative challenge, where it affects mitochondrial function and reduces ROS generation (Ahmed et al, 2008). While we did not observe any relocation of telomerase to the cytosol upon neutrophil co‐culture, we cannot discard the possibility that telomerase may be conferring resistance to neutrophil‐mediated paracrine damage via its non‐canonical roles. We have previously shown that upon telomere damage and activation of the DDR, cells produce high levels of ROS which contribute to secondary DNA damage (predominantly in non‐telomeric regions) (Passos et al, 2010). Thus, it is possible that hTERT, by preventing telomere shortening and induction of TAF, is also reducing non‐telomeric DNA damage induced by secondary ROS.
In the context of the liver, hepatocytes containing dysfunctional telomeres have been shown to increase during aging in mice (Hewitt et al, 2012) and also in alcoholic liver disease (ALD) (Wilson et al, 2015) and non‐alcoholic fatty liver disease (NAFLD) (Ogrodnik et al, 2017) patients. Furthermore, TAF in hepatocytes were shown to be enhanced by factors such as chronic inflammation (Jurk et al, 2014), obesity (Ogrodnik et al, 2017), oxidative stress (Jurk et al, 2014), and impaired autophagy (Cassidy et al, 2020). Importantly, genetic and pharmacological clearance of senescent cells has been shown to reduce TAF in hepatocytes, indicating that it is a robust indicator of cellular senescence. While our data support that neutrophils can induce TAF in hepatocytes, it is difficult to discern what is cause or consequence in the process. Chemotaxis assays show that neutrophils are preferentially recruited by senescent cells and clearance of p16Ink4a‐positive cells in aged livers from INK‐ATTAC mice reduces neutrophil infiltrates. However, our data do not allow us to conclusively identify the p16Ink4a‐positive cell types in the liver that contribute to neutrophil migration. In the case of INK‐ATTAC mice, AP kills cells based on high expression of p16Ink4a, which may include activated immune cells such as macrophages. We have however shown by CyTOF and scRNA‐seq analysis that infiltrating neutrophils do not express p16Ink4a in aged liver, suggesting that their reduction following AP treatment is most likely a consequence of elimination of other cell types. Injection of chemoattractant Cxcl1 or LPS led to neutrophil recruitment and induction of TAF; however, we acknowledge that this does not allow us to conclude a causal relationship between both, particularly since these factors may induce senescence directly or stimulate the recruitment of other immune cells apart from neutrophils that can conceivably induce paracrine effects.
Thus, while our results are consistent with a model in which the appearance of a few senescent cells may transmit senescence to otherwise healthy cells via the recruitment of neutrophils, this remains to be tested experimentally.
Our study supports the hypothesis of an evolutionary trade‐off between efficacy of the immune system and age‐related pathology. While neutrophils have evolved to play critical roles in microbial killing and inflammation, they may contribute to collateral damage and induction of cellular senescence which will be detrimental later in life.
Materials and Methods
Animals and procedures
Wild‐type mice were inbred C57BL/6 (Harlan, Blackthorn UK). Mice were housed in same‐sex cages in groups of 4–6 (56 × 38 × 18 cm, North Kent Plastics, Kent, UK) and individually identified by an ear notch. Mice were housed at 20 ± 2°C under a 12 h light/12 h dark photoperiod with lights on at 7.00 am. All work complied with the guiding principles for the care and use of laboratory animals and was licensed by the UK Home Office.
Acute liver injury using carbon tetrachloride (CCl4)
Single intraperitoneal injection of CCl4 at a dose of 2 μl (CCl4: olive oil, 1:1 [v:v])/g body weight was administered for 8 and 48 h to 8‐ to 10‐week‐old male littermates. Mice were pre‐treated with neutralizing antibody against Ly6G or IgG control antibody for 12 h before CCl4 injection. Animals were culled at 48 h post‐CCl4 injection.
p16Ink4a clearance
Experimental procedures were approved by the Institutional Animal Care and Use Committee at Mayo Clinic (protocol A26415). INK‐ATTAC transgenic mice were generated and genotyped as previously described (Baker et al, 2011) based on experimental strategies devised by J.L.K., T.T., J. van Deursen and D. Baker at Mayo Clinic. Briefly, INK‐ATTAC mice were produced and phenotyped at the Mayo Clinic. Controls for the INK‐ATTAC experiments were INK‐ATTAC‐null C57BL/6 background mice raised in parallel. Mice were housed 2–5 mice per cage, at 22 ± 0.5C on a 12–12 h day–night cycle and provided with food and water ad libitum. Cages and bedding (autoclaved Enrich‐o’Cobs (The Andersons Incorporated)) were changed once per week. In all the experiments, littermates of the same sex were randomly assigned to experimental groups. INK‐ATTAC mice were injected intraperitoneally (i.p.) with AP20187 (10 mg/kg) (MCE MedchemExpress; Cat: # HY‐13992/CS1953) or vehicle (4% ethanol, 10% PEG‐400, and 2% Tween‐20 in distilled water) for 3 days every 2 weeks for a total of 8–10 weeks.
I.P. Injection of recombinant Cxcl1
Recombinant CXCL1 (Peprotech, #250‐11) or vehicle (PBS) was administered to lean C57BL/6 via i.p. injection (5 mg/kg in PBS) daily for 7 days.
Mouse tissue collection and preparation
Livers were collected during necropsy and fixed with 4% formaldehyde aqueous solution (VWR; Cat. Number 9713.9010) and paraffin‐embedded for histochemical analysis, sectioned at 3 μm and mounted on Superfrost Plus glass slides. A fraction of the liver was snap‐frozen in liquid nitrogen and stored at −80°C for biochemical analyses.
Cell culture and treatments
Human embryonic lung MRC5 fibroblasts were obtained from ECACC (Europe Collection of Cell Culture) (Salisbury, UK). MRC5 fibroblasts were used for molecular and cellular biology analysis at a population doubling (PD) range of 16–25 for stress‐induced senescence or as a starting point for reaching replicative exhaustion (replicative senescence). MRC5 fibroblasts were transfected retro‐virally with the human catalytic subunit (TERT) of the enzyme telomerase. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% heat inactivated fetal bovine serum (FBS) (Biosera, Ringmer, UK), 100 μg/ml streptomycin, 100 units/ml penicillin and 2 mM l‐glutamine, incubated in a humidified atmosphere at 37°C with 95% air and 5% CO2 (complete medium, classic conditions).
Neutrophil isolation
Polymorphonuclear leukocytes (PMN) were isolated from peripheral blood of healthy donors. Briefly, after centrifugation of citrated whole blood at 300 g for 20 min and removal of platelet‐rich plasma, leukocytes were separated from erythrocytes by dextran sedimentation using 0.6% dextran T500. PMN were then separated from mononuclear leukocytes using discontinuous isotonic Percoll gradients. PMN leukocytes were 95–98% neutrophils using morphological criteria, and viability was assessed by trypan blue exclusion.
Murine neutrophil isolation
Polymorphonuclear leukocytes (PMN) were isolated from the bone marrow of 8 to 12‐week‐old male C57Bl6/J mice. Briefly, the bone marrow was flushed from the femur and tibia of both hind limbs using 5% FCS HBSS. The single‐cell suspension was then washed and placed onto a 62% Percoll Gradient and centrifuged at 1,000 g for 30 min. The pellet containing polymorphonuclear cells were collected, washed, and then counted. Neutrophil purity was assessed by Ly6G and CD11b (BioLegend) flow cytometry (BD FACS Canto II).
Human neutrophil and fibroblast direct co‐culture
MRC5 fibroblasts at an early PD (16–26) were used. Freshly isolated neutrophils were primed or not with 100 ng/ml LPS for 1 h at 37°C, 3% O2 in the dark. After 1 h incubation, neutrophils were centrifuged at 1,000 RPM to wash remaining LPS and 1 × 106 neutrophils were added to the MRC5 fibroblast culture at a ratio of 1 fibroblast for five neutrophils. Recombinant catalase (100 UI/ml) was added to the suspension for the duration of the co‐culture. Every 24 h, neutrophils were removed by aspiration followed by a wash with pre‐warmed media and then fresh neutrophils from a different healthy donor were added for a further 24‐h co‐incubation. This procedure was repeated for up to 3 days; then on last day, neutrophils were removed and the cells were transferred into new T150 flasks and cultured until replicative senescence. During the co‐culture, cells were cultivated at 3% O2 in order to better maintain neutrophil viability.
Indirect co‐culture
Neutrophils were first incubated for 1 h with 100 ng/ml LPS at 37°C, 3% O2 in the dark, then centrifuged at 1,000 RPM to wash remaining LPS and resuspended in media and co‐cultured with human MRC5 fibroblasts using a 3‐μm transwell insert (VWR® Tissue Culture Plate Inserts, Polyester (PET) Membrane, Sterilized, Standard Line) at a 1:5 ratio for 24 h. Every 24 h, fresh neutrophils from a different healthy donor were added to the transwell for a further 24 h (procedure was repeated three times—total 36 h). Separately, primed neutrophils were cultured in media, 3% O2 for 24 h; then, centrifuged and conditioned media (CM) was added to MRC5 fibroblasts. Every 24 h, CM from a different neutrophil healthy donor was added to MRC5 fibroblasts (procedure was repeated three times—total 36 h). Following 3 days of indirect co‐culture, cells were transferred into new T150 flasks and cultured until replicative senescence.
Neutrophil chemotaxis
Neutrophil chemotaxis was evaluated using the sub‐agarose method. As a positive control, formyl‐methionine‐leucine‐phenylalanine was used.
Metaphase spread generation
Metaphase spreads from MRC5 fibroblasts were prepared at different time points by treatment of subconfluent cells with 10 µg/ml colcemid for 24 h at 37°C, followed by 60 mM KCl for 15 min at RT and fixation in ethanol:acetic acid (3:1).
Quantitative RT–PCR
RNA was extracted using the RNeasy Mini Kit (Qiagen, 74106). Complementary DNAs were synthesized using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher, 4368814) following the manufacturer's instructions. Quantitative real‐time PCR was carried out using PerfeCTa qPCR ToughMix (Quantabio) in a C100TM Thermal Cycler, CFX96TM Real‐Time PCR System (Bio‐Rad), and Bio‐Rad CFX Manager software. Predesigned primers and probes from IDT PrimeTime. Mouse: p16: Mm.PT.58.42804808; p21: Mm.PT.58.5884610; TBP: Mm.PT.39a.22214839; Human: IL‐6: Hs.PT.58.40226675; IL‐8: Hs.PT.58.39926886.g; p21: Hs.PT.58.38492863.g; p16: Hs00923894_m1; TBP : Hs.PT.39a.22214825.
Immuno‐cyto and Immunohistochemistry
Cells grown on coverslips were fixed in 2% paraformaldehyde in PBS for 5 min. Cells were then permeabilized in PBG‐Triton (PBS, 0.4% Fish‐skin gelatin, 0.5% BSA, 0.5% Triton X‐100) for 45 min and incubated with primary antibody overnight at 4°C. Following PBS washes, cells were incubated with secondary antibody for 45 min and mounted onto glass microscope slides with ProLong Gold Antifade Mountant with DAPI (Invitrogen).
For FFPE tissues, sections were deparaffinized in 100% Histoclear, hydrated in 100, 90, and 70% ethanol and incubated twice for 5 min in distilled water. Antigen retrieval was performed by incubating sections in 0.01 M citrate buffer (pH 6.0) and heated until boiling for 10 min. Sections were allowed to cool down to room temperature followed by two washes in distilled water for 5 min. Next, sections were blocked in normal goat serum (1:60) in BSA/PBS for 30 min and incubated with primary antibody overnight at 4°C. Following three PBS washes, sections were then incubated with secondary antibody for 1 h, followed by PBS washes and mounted using ProLong Gold Antifade Mountant with DAPI (Invitrogen).
Antibodies
Primary antibodies used were as follows: mouse monoclonal anti‐p16 (dilution as provided by manufacturer, 9511; CINTec Histology, Roche) and rabbit polyclonal anti‐Ki67 antibody (1:1,000;ab15580; 4 μg/ml Abcam), Anti‐phospho‐Histone H2A.X (Ser139) Antibody, clone JBW301 (1:1,000), Anti‐PCNA antibody [PC10] (ab29) mouse (1:1,000, Abcam), NIMPR‐14 rat anti‐mouse monoclonal (1:200 Abcam); Anti‐p21 rat antibody [HUGO291] (ab107099); Anti‐p21 rabbit antibody (1:100 Abcam); Ly6g (BP0075‐1) rat monoclonal (1:100, BioXcell); 8oxoDG Anti‐8‐Oxoguanine Antibody, clone 483.15, Merck Millipore, MAB3560 (mouse 1:200); CD66B antibody (1:200, 305102—BioLegend); rabbit anti‐telomerase catalytic subunit (hTERT) (1:500, Rockland, 600‐401‐252S).
For Western blot
Rabbit Monoclonal (12D1) anti‐p21 Waf1 Cip1 (1:1,000, 2947S, Cell Signalling), Rabbit Monoclonal (D3W8G) anti‐p16 INK4A (1:1,000, 92803—Cell Signalling), Rabbit Polyclonal anti‐p15 CDKN2B (1:500, SAB4500078—Sigma‐Aldrich), Rabbit Polyclonal anti‐β‐Tubulin (1:1,000, 2146S—Cell Signalling ), Rabbit Polyclonal anti‐Lamin B1 (1:10,000, Abcam—ab16048).
TUNEL staining
TUNEL‐HRP‐DAB staining (ab206386) was performed as per manufacturer’s instructions.
Immuno‐FISH and Q‐FISH
For cells grown on coverslips, immunocytochemistry was performed as described above using mouse monoclonal anti‐γH2AX (1:200, 05‐636; Millipore) or rabbit anti‐53bp1 (1:500, # 4937S, Cell Signaling). Following secondary antibody incubation, cells were fixed in methanol: acetic acid (3:1 ratio) for 30 min followed by dehydration in graded cold ethanol solutions (70, 90, 100%) for 2 min each. Cells were incubated in PBS at 37°C for 5 min and fixed in 4% paraformaldehyde at 37°C for 2 min. Following a PBS wash, cells were dehydrated again with cold ethanol solutions (70, 90, 100%) for 2 min each and left to air‐dry. Coverslips were then placed onto glass slides containing 10 μl of PNA hybridization mix (70% deionized formamide (Sigma), 25 mM MgCl2, 1 M Tris pH 7.2, 5% blocking reagent (Roche) containing 2.5 μg/ml Cy‐3‐labeled telomere‐specific (CCCTAA) peptide nucleic acid probe or FAM‐labeled, CENPB‐specific (centromere) (ATTCGTTGGAAACGGGA) peptide nucleic acid probe (Panagene), and samples were denatured for 10 min at 80°C. Samples were incubated in a humidified chamber protected from light for 2 h at room temperature to allow hybridization to occur, followed by washes with 70% formamide in 2 × SSC (3 × 10 min each) and three washes in 0.05% TBS‐Tween‐20 for 5 min. Cells were then dehydrated in graded cold ethanol solutions (70, 90, 100%) and left to air‐dry before they were mounted onto glass microscope slides with ProLong Gold Antifade Mountant with DAPI (Invitrogen). Cells were imaged using in‐depth Z stacking (a minimum of 35 optical slices with 63× objective).
For FFPE tissues, immunohistochemistry was carried out as described above. Following an overnight incubation with rabbit monoclonal anti‐γH2AX (1:200, 9718; Cell Signalling) or 8oxoDG anti‐8‐oxoguanine antibody, clone 483.15, Merck Millipore, MAB3560 (mouse 1:200), sections were incubated with a goat anti‐rabbit biotinylated secondary antibody (1:200, PK‐6101; Vector Labs) for 30 min at room temperature. Following three PBS washes, tissues were incubated with fluorescein avidin DCS (1:500, A‐2011; Vector Labs) for another 30 min at room temperature. Sections were then washed three times in PBS and cross‐linked by incubation in 4% paraformaldehyde in PBS for 20 min. Sections were washed in PBS three times and then dehydrated in graded cold ethanol solutions (70, 90, 100%) for 3 min each. Tissues were then allowed to air‐dry prior to being denatured in 10 μl of hybridization mix (as described above) for 10 min at 80°C and then incubated for 2 h at room temperature in a dark humidified chamber to allow hybridization to occur. Sections were washed in 70% formamide in 2 × SCC for 10 min, followed by a wash in 2 × SSC for 10 min, and a PBS wash for 10 min. Tissues were then mounted using ProLong Gold Antifade Mountant with DAPI (Invitrogen). Sections were imaged using in‐depth Z stacking (a minimum of 40 optical slices with 63× objective) followed by Huygens (SVI) deconvolution.
Quantitative FISH (Q‐FISH) analysis of telomere FISH intensity was performed on fixed cells and FFPE tissue using the same protocol.
RNA in situ hybridization
RNA‐ISH was performed after RNAscope protocol from Advanced Cell Diagnostics Inc. (ACD). Paraffin sections were deparaffinized with Histoclear, rehydrated in graded ethanol (EtOH), and H2O2 was applied for 10 min at RT followed by two washes with water. Sections were placed in hot retrieval reagent and heated for 30 min. After washes in water and 100% EtOH, sections were air‐dried. Sections were treated with protease plus for 30 min at 40°C, washed with water and incubated with target probe (p16, Cxcl1, Il1‐α) for 2 h at 40°C. Afterward, slides were washed with water followed by incubation with AMP1 (30 min at 40°C) and then washed with wash buffer (WB) and AMP2 (15 min at 40°C), WB and AMP3 (30 min at 40°C), WB and AMP4 (15 min at 40°C), WB and AMP5 (30 min at RT) and WB and, finally, AMP6 (15 min at RT). Finally, RNAscope 2.5 HD Reagent kit‐RED was used for chromogenic labeling. Tissues were then mounted using ProLong Gold Antifade Mountant with DAPI (Invitrogen).
Human precision‐cut liver slices
Human liver tissue from the normal margins of colorectal metastasis resections were obtained with full ethical approval (H10/H0906/41) and CEPA biobank (17/NE/0070) and used subject to patients’ written consent. All experiments conformed to the principles set out in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. Precision‐cut liver slices were cut using a Leica VT1200S microtome (Leica Biosystems). Slices were then cultured for 4 days at 37°C supplemented with 5% CO2 in BioR plates in our patented bioreactor platform as previously described (Paish et al, 2019). Culture media were collected for analysis and replaced daily with fresh culture media. Human neutrophils were isolated from peripheral blood of healthy donors as described previously, and 1 × 106 neutrophils were added per tissue slices on days 2 and 3 post‐tissue slicing. Tissue slices were harvested at day 4, formalin‐fixed, and then paraffin‐embedded for future analysis.
Murine liver slice experiment details
Murine precision‐cut liver slices were generated and cultured as previously described (Paish et al, 2019). Briefly, livers of 8‐ to 12‐week‐old male C57Bl6/J mice were cored using an 8‐mm biopsy punch and embedded in agarose. Precision‐cut liver slices were cut using a Leica VT1200S microtome (Leica Biosystems) and cultured for 4 days at 37°C supplemented with 5% CO2 in BioR plates in our patented bioreactor platform. Cell culture medium was collected and replaced daily. Murine neutrophils were isolated as previously described, and 1 × 106 neutrophils were added per tissue slice on days 2 and 3 post‐tissue slicing. Tissue slices were harvested at day 4, formalin‐fixed, and then paraffin‐embedded for future analysis.
hCAT expression
RNA was extracted using the QIAGEN RNeasy Mini Kit according to manufacturer’s instructions from bone marrow‐derived neutrophils isolated from C57BL/6.mCAT mice (MCAT transgenic mice) and WT mice. cDNA was synthesized using the GoScript Reverse Transcription System (Promega). RT–PCR was performed using SYBR Green Jumpstart ready mix and the following primers; Forward—GAGCCTACGTCCTGAGTCTC, Reverse—ATCCCGGATGCCATAGTCAG.
Catalase activity assay
Catalase Activity Assay Kit (ab83464) was performed as per manufacturer’s instructions on bone marrow‐derived neutrophils isolated from WT and MCAT mice.
Lactate dehydrogenase assay
Lactate dehydrogenase (Thermo Fisher) assay kit was performed as per the manufacturer’s instructions.
RNA‐sequencing
RNA‐seq was carried out as previously described in (Ogrodnik et al, 2017). Briefly, strand‐specific paired‐end libraries for RNA‐seq were generated from DNAse‐treated total RNA using Ribozero and ScriptSeq systems (Epicentre/Illumina) and run on an Illumina 2500 sequencer to obtain 100 base pair paired‐end reads with four libraries multiplexed per flow cell lane using 100 bp paired‐end reads. This resulted in an average of 250 million reads per lane, with an average of 40 million reads per sample. Each individual library received a unique Illumina barcode. Low‐quality reads were filtered out using Kraken (Davis et al, 2013). The resulting filtered reads were mapped to the mouse genome version mm10 using STAR aligner (Dobin et al, 2013) followed by estimates of raw gene counts using HTSeq (Anders et al, 2015). Differential gene expression was analyzed using DESeq2 (Love et al, 2014). Statistical significance was expressed as a P value adjusted for a false discovery rate of 0.01 using the Benjamini–Hochberg correction for multiple testing.
Alkaline comet assay
Cells were trypsinized and frozen in 10% DMSO in fetal bovine serum and stored at −80°C prior to downstream processing. Cells were washed in cold PBS and resuspended in 0.7% Low Melting Point agarose (Sigma, A9414) at 37°C to a concentration of 2 × 105 cells/ml. Cell/agarose mix (70 μl) was placed on slides previously coated in 1% agarose. Slides were incubated in lysis buffer (2.5 M NaCl, 100 nM EDTA, 10 nM Tris, pH 10, 250 nM NaOH, 10% DMSO, 1% Triton X‐100) for 1 h at 4°C. Slides were then washed twice in cold PBS. Samples were subjected to electrophoresis for 40 min at 25 V at 4°C in alkaline buffer (300 mM NaOH, 1 mM EDTA). Slides were then washed twice in cold PBS and stained with Sybr Gold (Life Technologies, S11494) in Tris‐borate EDTA buffer for 40 min. Slides were washed twice in MilliQ water and allowed to dry. Samples were imaged using an Olympus BX51 widefield microscope with Olympus UPlanFL 20×/0.50 air objective. Comets were scored using Comet assay IV (Perceptive Instruments). For each sample, 100 randomly captured comets (50 cells on each of 2 comet slides) were quantified.
Superoxide anion release by neutrophils
The release of superoxide anions by freshly isolated neutrophils in response to fMLP was measured using cytochrome c reduction assay. Cells were pre‐incubated with LPS, PMA, or HBSS containing Ca2+ and Mg+2, or platelet‐activating factor (positive control) for 60 min, in the presence of 1% autologous serum, before stimulation with fMLP (100 nM). For each sample, a control with superoxide dismutase (200 U) was included to verify the specificity of cytochrome c reduction by superoxide anions. After 15 min incubation at 37°C, the reaction was stopped immediately by placing the cells on ice then centrifuged at 10,000 g for 3 min. The superoxide dismutase reduction of cytochrome c was then determined for each supernatant by measuring the absorbance at 550 nm using a BMG Fluostar 67. Optima plate reader (BMG Labtech Ltd, Aylesbury, UK). Results are expressed as nmole of O2.− per million neutrophils, using the extinction coefficient of 21 × 103 M−1 cm−1.
Dihydrorhodamine 123 (DHR)
The neutrophils were incubated in DHR final concentration 1 μM at 37°C for 15 min and then subsequently stimulated with platelet‐activating factor (PAF) at 100 nM for 10 min at 37°C. Median fluorescence intensity for DHR was assessed by flow cytometry (BD FACS Canto II).
Mass cytometry by time‐of‐flight (CyTOF)
For mass cytometry experiment, we used 6‐month‐old (“young”) and 24‐month‐old (“old”) INK‐ATTAC mice. Liver tissue (1.6 g) was dissociated using mouse liver dissociation kit (Miltenyi, #130‐105‐807) according to the manufacturer’s instruction, including cell debris removal step (Miltenyi, #130‐109‐398). Intrahepatic leukocytes were purified by Percoll (Sigma‐Aldrich, #P1644) gradient centrifugation and stained as previously described in detail (Guo et al, 2019). CyTOF (Fluidigm) mass cytometry and data analysis were performed by the Immune Monitoring Core at Mayo Clinic as previously described (Guo et al, 2019). Visualization of different immune cell clusters was mapped by using a tSNE map. Relative marker intensity and cluster abundance for each sample were plotted using a heatmap. The CyTOF antibody panel used is listed in Appendix Table S1.
Statistical analysis
All statistical analyses were carried out using GraphPad Prism 8.01. For comparisons between two groups, a two‐tailed unpaired t‐test was used, and where the data were not normally distributed, a Mann–Whitney U‐test was performed. For multi‐group comparison, a one‐way ANOVA with Tukey's or Holm–Sidak's post hoc test was used.
Author contributions
AL performed the majority of the experiments; JL, MHRS, SV, PH, MO, AC, LH, SAV, HS, AH, MGV, SE, DG, KP, DD, MD, SG, SG, CW, SP, JB, BC, JC, NN, and CAM performed and evaluated individual experiments; TVZ, GS, HR, FO, SK, TT, JLK, MH, and DJ designed and supervised individual experiments; DAM and JFP designed and supervised the study; JFP and DAM wrote the manuscript with the contributions from all the authors.
Conflict of interest
FO and DAM are directors and shareholders in Fibrofind limited. JL is a shareholder in Fibrofind limited. Patents on INK‐ATTAC mice are held by Mayo Clinic and licensed to Unity Biotechnology. JLK and TT may gain financially from these patents and licenses. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. The remaining authors declare no competing financial interests.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Acknowledgements
The authors acknowledge support from NIH grants: R01 AG068048 (JFP), R01 AG068182‐01 (DJ), R37 AG013925 (JLK, TT), R01 AG050582 (NN) and P01 AG062413 (SK, JLK, DJ, TT, JFP), the Connor Fund (JLK, TT), Robert J. and Theresa W. Ryan (JLK, TT), and the Noaber Foundation (JLK, TT). F.O and D.A.M are supported by MRC program Grants MR/K0019494/1 and MR/R023026/1. D.A.M is supported by a CRUK program grant, reference C18342/A23390. JFP, DJ, AL, and SV would like to thank the Ted Nash Long Life Foundation, UL1 TR0002377 from the National Centre for Advancing Translational Sciences (NCATS) and BBSRC [grants BB/L502066/1; BB/K017314/1]. DG is supported by the Newcastle CRUK Clinical Academic Training Programme. AC is funded by the W.E. Harker Foundation. PH is supported by the AASLD Foundation. DJ and PH are supported by P30DK084567. CAM was supported by a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (Capes)—Finance Code 001. MCAT mice were a kind gift from Prof. Owen Samson at the Cancer Research UK Beatson Institute. We also thank the technical support provided by Dr. Glyn Nelson and the Newcastle University Bioimaging unit.
The EMBO Journal (2021) 40: e106048.
See also: MSJ Burbano et al (May 2021)
Contributor Information
Derek A Mann, Email: Derek.Mann@ncl.ac.uk.
João F Passos, Email: Passos.Joao@mayo.edu.
Data availability
The RNA‐seq data from this publication have been submitted to the European Nucleotide Archive https://www.ebi.ac.uk/ena/browser/home, accession numbers: PRJEB42908 and ERP126835.
References
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang T‐W, Lasitschka F, Andrulis M et al (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch‐Machin MA, von Zglinicki T, Saretzki G (2008) Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci 121: 1046–1053 [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high‐throughput sequencing data. Bioinformatics (Oxford, England) 31: 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D et al (2019) Length‐independent telomere damage drives post‐mitotic cardiomyocyte senescence. EMBO J 38: e100492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariazi J, Benowitz A, De Biasi V, Den Boer ML, Cherqui S, Cui H, Douillet N, Eugenin EA, Favre D, Goodman S et al (2017) Tunneling nanotubes and gap junctions‐their role in long‐range intercellular communication during development, health, and disease conditions. Front Mol Neurosci 10: 333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae NS, Baumann P (2007) A RAP1/TRF2 complex inhibits nonhomologous end‐joining at human telomeric DNA ends. Mol Cell 26: 323–334 [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM (2011) Clearance of p16Ink4a‐positive senescent cells delays ageing‐associated disorders. Nature 479: 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, A. Saltness R, Jeganathan KB, Verzosa GC, Pezeshki A et al (2016) Naturally occurring p16Ink4a‐positive cells shorten healthy lifespan. Nature 530: 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life‐span by introduction of telomerase into normal human cells. Science 279: 349–352 [DOI] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ (2018) Clearance of senescent glial cells prevents tau‐dependent pathology and cognitive decline. Nature 562: 578–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy LD, Young ARJ, Young CNJ, Soilleux EJ, Fielder E, Weigand BM, Lagnado A, Brais R, Ktistakis NT, Wiggins KA et al (2020) Temporal inhibition of autophagy reveals segmental reversal of ageing with increased cancer risk. Nat Commun 11: 307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J‐P, Desprez P‐Y, Krtolica A, Campisi J (2010) The senescence‐associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia‐Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM, Miwa S, Birch J, Merz A et al (2016) Mitochondria are required for pro‐ageing features of the senescent phenotype. EMBO J 35: 724–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay‐Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere‐initiated senescence. Nature 426: 194–198 [DOI] [PubMed] [Google Scholar]
- Davis MP, van Dongen S, Abreu‐Goodger C, Bartonicek N, Enright AJ (2013) Kraken: a set of tools for quality control and analysis of high‐throughput sequence data. Methods 63: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA‐seq aligner. Bioinformatics (Oxford, England) 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM et al (2017) Targeting cellular senescence prevents age‐related bone loss in mice. Nat Med 23: 1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquerel E, Barnes RP, Uttam S, Watkins SC, Bruchez MP, Opresko PL (2019) Targeted and persistent 8‐oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol Cell 75: 117–130.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM et al (2012) Telomeric DNA damage is irreparable and causes persistent DNA‐damage‐response activation. Nat Cell Biol 14: 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G et al (2019) Cellular senescence: defining a path forward. Cell 179: 813–827 [DOI] [PubMed] [Google Scholar]
- Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, Kabashima A, Pavelko KD, Madden B, Alhuwaish H et al (2019) Integrin β(1)‐enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol 71: 1193–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari P, Millar FR, Tarrats N, Birch J, Quintanilla A, Rink CJ, Fernández‐Duran I, Muir M, Finch AJ, Brunton VG et al (2019) The innate immune sensor Toll‐like receptor 2 controls the senescence‐associated secretory phenotype. Sci Adv 5: eaaw0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460 [DOI] [PubMed] [Google Scholar]
- Hewitt G, Jurk D, Marques FDM, Correia‐Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF (2012) Telomeres are favoured targets of a persistent DNA damage response in ageing and stress‐induced senescence. Nat Commun 3: 708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Zhang Y, Liu D, Wang SS, Ding Q, Rastogi P, Purvis M, Wang A, Elhadi S, Ren C et al (2020) Innate immune signaling contributes to tubular cell senescence in the Glis2 knockout mouse model of nephronophthisis. Am J Pathol 190: 176–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D, Wilson C, Passos JF, Oakley F, Correia‐Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R et al (2014) Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 5: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T‐W, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A et al (2011) Senescence surveillance of pre‐malignant hepatocytes limits liver cancer development. Nature 479: 547–551 [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless C, Wang C, Jurk D, Merz A, Tv Z, Passos JF (2010) Quantitative assessment of markers for cell senescence. Exp Gerontol 45: 772–778 [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol 15: 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Sun S, Geng L, Song M, Wang W, Ye Y, Ji Q, Zou Z, Wang S, He X et al (2020) Caloric restriction reprograms the single‐cell transcriptional landscape of rattus norvegicus aging. Cell 180: 984–1001.e22 [DOI] [PubMed] [Google Scholar]
- Mogilenko DA, Shpynov O, Andhey PS, Arthur L, Swain A, Esaulova E, Brioschi S, Shchukina I, Kerndl M, Bambouskova M et al (2020) Comprehensive profiling of an aging immune system reveals clonal GZMK(+) CD8(+) T cells as conserved hallmark of inflammaging. Immunity 54: 99–115.e12 [DOI] [PubMed] [Google Scholar]
- Moles A, Murphy L, Wilson CL, Chakraborty JB, Fox C, Park EJ, Mann J, Oakley F, Howarth R, Brain J et al (2014) A TLR2/S100A9/CXCL‐2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J Hepatol 60: 782–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME (2018) Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17: e12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin‐Ruiz C, von Zglinicki T (2012) A senescent cell bystander effect: senescence‐induced senescence. Aging Cell 11: 345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh T, Sperandio M, Mócsai A (2020) Neutrophils as emerging therapeutic targets. Nat Rev Drug Discovery 19: 253–275 [DOI] [PubMed] [Google Scholar]
- Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM et al (2017) Cellular senescence drives age‐dependent hepatic steatosis. Nat Commun 8: 15691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrodnik M, Salmonowicz H, Jurk D, Passos JF (2019a) Expansion and cell‐cycle arrest: common denominators of cellular senescence. Trends Biochem Sci 44: 996–1008 [DOI] [PubMed] [Google Scholar]
- Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Krüger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T et al (2019b) Obesity‐induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab 29: 1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa S, Tada‐Oikawa S, Kawanishi S (2001) Site‐specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry 40: 4763–4768 [DOI] [PubMed] [Google Scholar]
- Opresko PL, Fan J, Danzy S, Wilson DM 3rd, Bohr VA (2005) Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res 33: 1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A et al (2018) Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 9: 5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paish HL, Reed LH, Brown H, Bryan MC, Govaere O, Leslie J, Barksby BS, Garcia Macia M, Watson A, Xu X et al (2019) A bioreactor technology for modeling fibrosis in human and rodent precision‐cut liver slices. Hepatology 70: 1377–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birkett M, Harold G, Schaeuble K et al (2007) Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere‐dependent senescence. PLoS Biol 5: e110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A et al (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6: 347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiseler M, Kubes P (2019) More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Investig 129: 2629–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S, Saretzki G, von Zglinicki T (1998) Preferential accumulation of single‐stranded regions in telomeres of human fibroblasts. Exp Cell Res 239: 152–160 [DOI] [PubMed] [Google Scholar]
- Razdan N, Vasilopoulos T, Herbig U (2018) Telomere dysfunction promotes transdifferentiation of human fibroblasts into myofibroblasts. Aging Cell 17: e12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant KV, Poluri KM, Dutta AK, Sepuru KM, Troshkina A, Garofalo RP, Rajarathnam K (2016) Chemokine CXCL1 mediated neutrophil recruitment: role of glycosaminoglycan interactions. Sci Rep 6: 33123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H et al (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909–1911 [DOI] [PubMed] [Google Scholar]
- Segal AW (2005) How neutrophils kill microbes. Annu Rev Immunol 23: 197–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K, Chapman J, Birch J, Ogrodnik M, Meves A et al (2019) Senescent human melanocytes drive skin aging via paracrine telomere dysfunction. EMBO J 38: e101982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jurk D, Nelson G, Martin‐Ruiz C, von Zglinicki T (2009) DNA damage response and cellular senescence in aging mice. Aging Cell 8: 311–323 [DOI] [PubMed] [Google Scholar]
- Wilson CL, Jurk D, Fullard N, Banks P, Page A, Luli S, Elsharkawy AM, Gieling RG, Chakraborty JB, Fox C et al (2015) NFκB1 is a suppressor of neutrophil‐driven hepatocellular carcinoma. Nat Commun 6: 6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24: 1246–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Pilger R, Sitte N (2000) Accumulation of single‐strand breaks is the major cause of telomere shortening in human fibroblasts. Free Rad Biol Med 28: 64–74 [DOI] [PubMed] [Google Scholar]
- von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27: 339–344 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14: 644–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Data Availability Statement
The RNA‐seq data from this publication have been submitted to the European Nucleotide Archive https://www.ebi.ac.uk/ena/browser/home, accession numbers: PRJEB42908 and ERP126835.


