Upon inhalation of spores from the fungus Blastomyces dermatitidis from the environment, humans and animals can develop the disease blastomycosis. Based on disease epidemiology, B. dermatitidis is known to be endemic in the United States and Canada around the Great Lakes and in the Ohio and Mississippi River Valleys but is starting to emerge in other areas. B. dermatitidis is extremely difficult to culture from the environment, so little is known about the environmental reservoirs for this pathogen.
KEYWORDS: mycosis, mycoses, human fungal pathogen, soil, nonculturable, modeling, GIS, dog, animal, veterinary, canine, endemic mycoses, modelling, soil microbiology, veterinary pathogens
ABSTRACT
Outbreaks of blastomycosis, caused by the fungus Blastomyces dermatitidis, occur in areas of endemicity in the United States and Canada, but the geographic range of blastomycosis is expanding. Previous studies inferred the location of B. dermatitidis through epidemiologic data associated with outbreaks because culture of B. dermatitidis from the environment is often unsuccessful. In this study, we used a culture-independent, PCR-based method to identify B. dermatitidis DNA in environmental samples using the BAD1 promoter region. We tested 250 environmental samples collected in Minnesota, either associated with blastomycosis outbreaks or environmental samples collected from regions of high and low endemicity to determine the basal prevalence of B. dermatitidis in the environment. We identified a fifth BAD1 promoter haplotype of B. dermatitidis prevalent in Minnesota. Ecological niche analysis identified latitude, longitude, elevation, and site classification as environmental parameters associated with the presence of B. dermatitidis. Using this analysis, a random forest model predicted the presence of B. dermatitidis in basal environmental samples with 75% accuracy. These data support the use of culture-independent, PCR-based environmental sampling to track spread into new regions and to characterize the unknown B. dermatitidis environmental niche.
IMPORTANCE Upon inhalation of spores from the fungus Blastomyces dermatitidis from the environment, humans and animals can develop the disease blastomycosis. Based on disease epidemiology, B. dermatitidis is known to be endemic in the United States and Canada around the Great Lakes and in the Ohio and Mississippi River Valleys but is starting to emerge in other areas. B. dermatitidis is extremely difficult to culture from the environment, so little is known about the environmental reservoirs for this pathogen. We used a culture-independent PCR-based assay to identify the presence of B. dermatitidis DNA in soil samples from Minnesota. By combining molecular data with ecological niche modeling, we were able to predict the presence of B. dermatitidis in environmental samples with 75% accuracy and to define characteristics of the B. dermatitidis environmental niche. Importantly, we showed the effectiveness of using a PCR-based assay to identify B. dermatitidis in environmental samples.
INTRODUCTION
Infection by fungal pathogens in the Blastomyces dermatitidis complex (B. dermatitidis and B. gilchristii) causes blastomycosis that is predominantly observed in humans and canines (1–4). While uncommon globally, disease rates in areas of hyperendemicity can exceed 100 per 100,000 humans (5, 6). Untreated blastomycosis can be serious and is often misdiagnosed as bacterial pneumonia, with mortality rates over 10% (3, 7). Outbreaks of blastomycosis are frequently documented but can be sporadic, even in regions of endemicity (1–3, 6, 7).
Based on disease prevalence, the B. dermatitidis complex is endemic in the Mississippi and Ohio River Valleys and around the Great Lakes, with the highest infection rates occurring in Minnesota, Ontario, and Wisconsin (3, 8, 9). In Minnesota, most infections occur in the north or along the St. Croix River but can occur throughout the state (7). However, the geographic range of B. dermatitidis is expanding; blastomycosis is now frequently reported in New York state and Saskatchewan (10, 11). Two additional species, Blastomyces helicus and Blastomyces percursus, cause human disease similar to blastomycosis, but epidemiologic studies suggest these fungi are endemic to western North America and Africa, respectively (12–14).
The environmental niche of B. dermatitidis remains unknown. To date, the environmental location of B. dermatitidis has been determined through epidemiologic data associated with outbreaks. Patients in one Wisconsin outbreak had explored a beaver dam, and two other outbreaks had evidence of transmission from riverbank soil (15, 16). These and other epidemiologic outbreak data led to the suggestion that the ecological niche for B. dermatitidis involves decayed wood and/or moist soil (9, 17). Epidemiologic data also indicate increased risk from behaviors that increase outdoor exposure (7, 15, 16, 18).
B. dermatitidis is a dimorphic fungus that grows as a filamentous mold and sporulates at 25°C but grows as budding yeasts in humans at 37°C (1, 3, 19). Yeasts are readily isolated from infected individuals, and B. dermatitidis can be propagated in the laboratory as both mold and yeast, but isolation from the environment through culture-based methods has remained elusive (15, 20–23). The only successful environmental isolation of B. dermatitidis was from a single woodpile sample (22) and has not been replicated. The most effective method of isolating B. dermatitidis from the environment is through animal passage, but this suffers from reproducibility and animal welfare issues, is labor-intensive, and is not well suited for large-scale studies (20, 23).
A PCR-based method to identify the presence of B. dermatitidis DNA in environmental samples was recently developed. Burgess et al. used PCR amplification of the BAD1 virulence gene (3, 24) to detect B. dermatitidis DNA in five soil samples (25). The purpose of this study was to use this culture-independent PCR-based assay to identify B. dermatitidis DNA in 250 environmental samples collected in Minnesota. We used the resulting data to build a random forest model that was 75% effective at predicting the environmental presence of B. dermatitidis.
RESULTS
Epidemiologic data.
The environmental location of B. dermatitidis has historically been tracked by determining the probable point of exposure in humans and animals presenting with blastomycosis (7). Counties with the highest rates of blastomycosis were in northeastern Minnesota (Table 1). This region is a popular vacation destination, and thus rates of blastomycosis within the population differ when calculated based on county of residence and county of probable exposure. For example, most residents of the urban Hennepin and Ramsey counties diagnosed with blastomycosis reported a probable county of exposure in northeastern Minnesota. Importantly, the rates of blastomycosis based on county of probable exposure do not take into account the number of transient visitors to the area; thus, it is unclear if probable county of exposure rates accurately reflect the level of infection and subsequent disease that occurs from environmental exposures within that county.
TABLE 1.
Minnesota counties with the highest rates of blastomycosis per capita
| Countya | Populationb | Annual rate of blastomycosisb per 100,000 individuals by: |
|
|---|---|---|---|
| County of residence | Probable county of exposure | ||
| Hennepinc | 1,235,478 | 0.3 | 0.1 |
| Ramseyc | 541,493 | 0.4 | 0.1 |
| St. Louis | 200,080 | 3.4 | 3.1 |
| Crow Wing | 63,855 | 0.5 | 0.9 |
| Itasca | 45,203 | 7.5 | 7.3 |
| Cass | 29,022 | 4.9 | 7.1 |
| Hubbard | 20,862 | 2.4 | 2.7 |
| Roseau | 15,462 | 3.6 | 3.3 |
| Koochiching | 12,644 | 4.8 | 2.4 |
| Lake | 10,569 | 0.5 | 3.8 |
| Cook | 5,311 | 5.7 | 4.7 |
| Lake of the Woods | 3,809 | 5.3 | 11.8 |
Counties where basal environmental samples or outbreak samples were collected are indicated in bold.
County population data based on United States Census Bureau data (http://www.census.gov/). Average annual blastomycosis rates per county for the years 1999 to 2018 (7).
Hennepin and Ramsey counties are the most populous in Minnesota, containing Minneapolis and St. Paul, and are included for reference only.
Basal sampling.
B. dermatitidis is an important human fungal pathogen, but very little is known about its environmental niche, due in part to difficulty in culturing it from the environment. The location of B. dermatitidis was historically tracked using disease data, but tracking the location of B. dermatitidis using only disease data has disadvantages. First, it is impossible to determine with certainty the location at which the infection occurred. Additionally, epidemiologic data also do not include individuals who were infected but asymptomatic or did not seek medical aid. To identify more precise environmental information about B. dermatitidis location, we performed environmental sampling to determine the basal presence of B. dermatitidis in the environment. Human cases are diagnosed year-round, but peak diagnosis occurs in September (https://www.health.state.mn.us/diseases/blastomycosis/statistics.html), suggesting that environmental exposure likely occurs between June and September. Thus, environmental samples were collected from June through September in nine regions in Minnesota. Six regions were selected for basal environmental sampling at random GPS coordinates between 2014 and 2017 (Fig. 1A). Five regions were located in northeastern Itasca (2014 and 2017), St. Louis (2014 and 2017), and Lake (2017) counties. Each of these five regions was visited a single time to collect samples. For an additional region in Hennepin county (2014), samples were collected weekly over a period of 6 weeks. An additional three regions were associated with outbreaks or clusters of blastomycosis cases, and environmental samples from the suspected environmental source of the blastomycosis infection were collected (Fig. 1B).
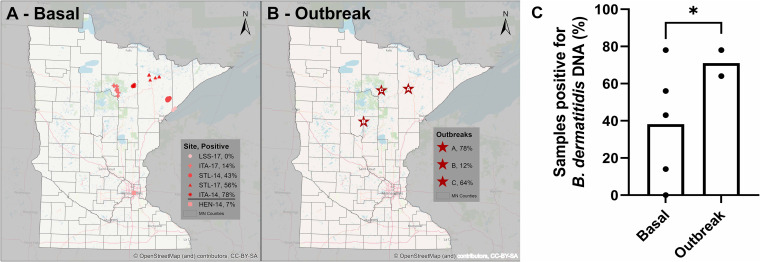
FIG 1.
Environmental sampling locations tested for the presence of Blastomyces dermatitidis DNA in Minnesota. The percentage of detectable B. dermatitidis DNA is indicated for each location. (A) Basal environmental sampling locations. Environmental samples were collected at randomly generated GPS coordinates at the designated regions in the Blastomyces-endemic Itasca (ITA-14 and ITA-17) and St. Louis (STL-14 and STL-17) counties, as well as in Lake (LSS-17) and Hennepin (HEN-14) counties. (B) Outbreak sampling locations. Environmental samples were collected at sites associated with human or animal blastomycosis cases within the previous year. Maps were generated using base map data from OpenStreetMap. (C) A trend toward a higher percentage of samples with detectable B. dermatitidis DNA is observed in outbreak environmental nonflooding samples compared to basal environmental samples. *, P = 0.017 using a t test with Welch’s correction for unequal variances.
The BAD1 gene is specific to B. dermatitidis and is known to be a virulence determinate (24). Total DNA was isolated from the samples, and the BAD1 promoter region was PCR amplified and sequenced, as described by Burgess et al. (25), to determine the presence of B. dermatitidis in the environmental samples (Fig. 2). Following DNA purification, some samples contained contaminants that inhibited the PCR. To balance detectable DNA and reduced PCR inhibition, the samples were diluted 1:1, 1:5, 1:10, or 1:100, and PCR was performed on the dilutions (Fig. 2). To confirm DNA isolation from all samples, PCR with fungal universal internal transcribed spacer (ITS) primers (26) was used as a positive control. Amplification of purified B. dermatitidis DNA was used to determine the limit of detection for the PCR assay (Fig. 2). PCRs that yielded DNA fragments between 300 and 700 bp (the target BAD1 promoter region is 368 bp in B. dermatitidis and 663 bp for B. gilchristii) were sequenced and compared to the promoter region of B. dermatitidis and B. gilchristii BAD1 sequences using a BLAST search, with ≥99% sequence similarity considered confirmatory for B. dermatitidis or B. gilchristii. Unlike previous studies, where the presence of Blastomyces was determined following infection of animals by soil samples, samples with detectable B. dermatitidis DNA were consistently and reproducibly positive, and samples that were negative for the presence of BAD1 DNA were always negative.
FIG 2.
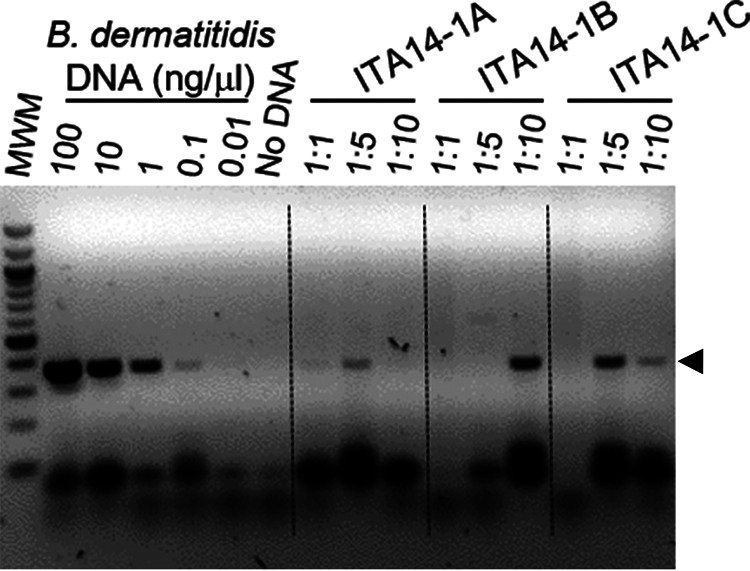
Detection of Blastomyces dermatitidis DNA from environmental samples. B. dermatitidis DNA was amplified by PCR using BAD1-specific primers (25) with total soil DNA dilutions of 1:1, 1:5, or 1:10 for each environmental soil DNA sample. Purified B. dermatitidis DNA was serially diluted to determine the limit of detection (lanes 2 to 7). The PCR products were loaded and visualized on a 1.5% agarose gel using ethidium bromide and image inversion (dark bands on light background). PCR product bands in environmental samples of equivalent size to the purified B. dermatitidis control DNA BAD1 promoter fragment, indicated by black arrowhead, were purified and sequenced, and a BLAST search of the amplicon was used to determine sequence identity. Three positive environmental soil samples from ITA-14 are shown.
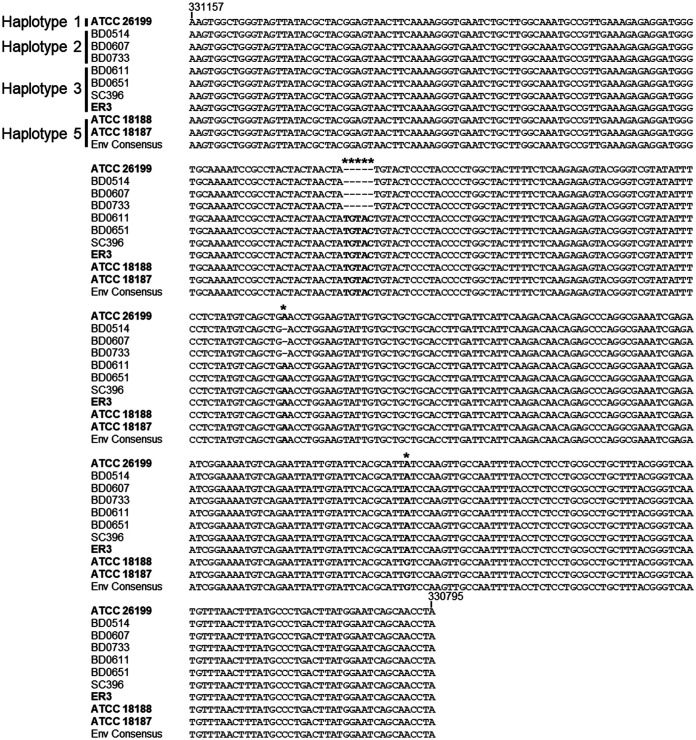
All of the sequence-positive samples identified had nucleotide polymorphisms in the sequenced region that differed from the ATCC 26199 reference sequence. Previous analysis of BAD1 promoter haplotypes by McTaggart et al. (27) had identified four BAD1 haplotypes. Haplotypes 1 to 3 were present in B. dermatitidis, whereas haplotype 4 contained two large insertions and was associated with B. gilchristii. Our environmental isolates had a novel haplotype not previously identified by the McTaggart study, although analysis of additional previously sequenced genomes of B. dermatitidis (28) revealed that this haplotype is present in other strains of B. dermatitidis, and thus, we classified it as BAD1 promoter haplotype 5 (Fig. 3).
FIG 3.
BAD1 sequences from the environmental samples belong to the newly defined haplotype 5. Representative B. dermatitidis BAD1 sequences from the previously identified haplotypes 1 to 3 (32), all published genomes (bolded), and the environmental isolate consensus sequence were aligned. Haplotype designations are indicated at the beginning of the sequences. Sequence numbering is based on the ATCC 26199 reference genome sequence but presented as the reverse complement because BAD1 is on the 3′ DNA strand. The. B. gilchristii haplotype 4 was not included in the alignment because it is substantially different from the BAD1 sequence of B. dermatitidis and contains two large insertions (32). The sequenced genomes from strains ATCC 18187, ATCC 18188, and the environmental consensus sequence (Env Consensus) did not match the previously identified haplotypes and were designated haplotype 5. Haplotype 5 BAD1 sequences contained an identical 5-base pair insertion as haplotype 3 but also had an additional A → G conversion at base 330708.
The number of samples with detectable B. dermatitidis DNA for each region with basal sampling is summarized in Table 2 and Fig. 1A and C. Overall, 38% of basal environmental samples had detectable B. dermatitidis DNA (no B. gilchristii was detected), but rates differed across regions. Only 14% of samples from ITA-17 had detectable B. dermatitidis DNA, but 78% of ITA-14 (collected in a different region of the same county) samples were positive. The sites in St. Louis county were more similar, with 56% of STL-17 and 43% of STL-14 sites positive for B. dermatitidis DNA. No B. dermatitidis DNA was detected at the sites in Lake county (LSS-17). Taken together, these data show that B. dermatitidis DNA is present in the environment in counties of high endemicity, even in the absence of an association with known human or animal blastomycosis.
TABLE 2.
Prevalence of Blastomyces dermatitidis DNA in basal soil samples collected from sites in Minnesota
| Sampling sitea | No. of samples with detectable B. dermatitidis DNA | Total no. of samples analyzed | No. of samples positive for B. dermatitidis DNA (%) |
|---|---|---|---|
| ITA-14 | 18 | 23 | 78 |
| ITA-17 | 4 | 28 | 14 |
| STL-14 | 29 | 67 | 43 |
| STL-17 | 9 | 16 | 56 |
| LSS-17 | 0 | 4 | 0.0 |
| HEN-14a | 6 | 73 | 8.2 |
| Avg ± SD | 38 ± 32 | ||
| Avg ± SD (excluding HEN-14 flooding site) | 33 ± 31 |
All sites were sampled one time except HEN-14, which was sampled every week for 6 weeks. Summary data are in bold.
Post-flood sampling.
The site in Hennepin county (HEN-14) was selected for two reasons: (i) this county has low blastomycosis prevalence (Table 1), and (ii) the region had undergone a recent flooding event prior to sample collection in 2014. Previous studies showed an association between flooding and blastomycosis outbreaks (16, 29–31). As depicted in Fig. 4A, samples at HEN-14 were collected at four randomly identified locations along the bank of a creek that had flooded the week prior to initiation of environmental sampling. Samples were collected each week for 6 weeks. B. dermatitidis DNA was not detected in any of the samples for the first 4 weeks (Fig. 4B). In week 5, 46% of the samples were positive for B. dermatitidis DNA, and at least one positive sample was detected at each location. At week 6, the samples no longer had detectable B. dermatitidis DNA. These data show that the presence of B. dermatitidis DNA in the environment was generally undetectable in this low-endemicity county but was transiently detected after a flooding event.
FIG 4.
Temporal sampling in Hennepin County along a creek identifies transient detectable Blastomyces dermatitidis DNA 5 weeks postflooding. (A) Four regions were randomly selected along a creek following a major flooding event in Hennepin County. Three random samples were taken from each region for 6 consecutive weeks after the flood. Circles indicate the presence of B. dermatitidis DNA; crosses indicate no detectable B. dermatitidis DNA. Map was generated using Minnesota GeoCommons data. (B) Detectable B. dermatitidis DNA was present only at week 5.
Outbreak data.
We investigated whether environmental samples collected at outbreak locations had higher rates of detectable B. dermatitidis DNA compared to basal environmental locations with no known link to blastomycosis cases. Samples were collected from three outbreaks locations (Fig. 1B). Outbreak A, in St. Louis county, was associated with soil excavation at a construction project. At the time of the outbreak in 1999, culture of B. dermatitidis from the environmental samples via in vitro culture or in vivo mouse bioassay culture was unsuccessful. However, both culture methods are known to have low success rates and suffer from lack of reproducibility (15, 20–23). Outbreak B was a cluster of canine cases along a river in Crow Wing county that occurred in the summer/fall of 2012 following a flooding event that spring. Outbreak C environmental samples were collected in 2017 from around and in a house in Itasca county where both human and animal inhabitants were diagnosed with blastomycosis.
The number of samples with detectable B. dermatitidis DNA in these environmental samples associated with outbreaks is summarized in Table 3 and Fig. 1B and C. The outbreak samples did not have significantly higher rates of detectable B. dermatitidis DNA compared to the basal samples (P = 0.09; t test with Welch’s correction for unequal variances), although a trend toward higher rates in the outbreak samples was observed when only nonflooding samples were compared (71% ± 10% versus 33% ± 31% for outbreak versus basal nonflooding samples) (Table 3; Fig. 1C). Unlike outbreaks A and C, but consistent with our temporal flood sampling, only 12% of the outbreak B environmental samples were positive for B. dermatitidis DNA (Table 3). These data might suggest increased B. dermatitidis DNA prevalence at outbreak locations but only transient association with flooding.
TABLE 3.
Soil samples collected from sites with previous blastomycosis human or canine outbreaks had high rates of detectable Blastomyces dermatitidis DNA
| Outbreak sitea | Yr sample collected | No. of samples with detectable B. dermatitidis DNA | Total no. of samples analyzed | No. of samples positive for B. dermatitidis DNA (%) |
|---|---|---|---|---|
| A | 1999 | 18 | 23 | 78 |
| B | 2012 | 5 | 43 | 12 |
| C | 2016 | 7 | 11 | 64 |
| Avg ± SD | 51 ± 35 | |||
| Avg ± SD (excluding B flooding site) | 71 ± 10 |
Summary data are in bold.
Ecological niche model.
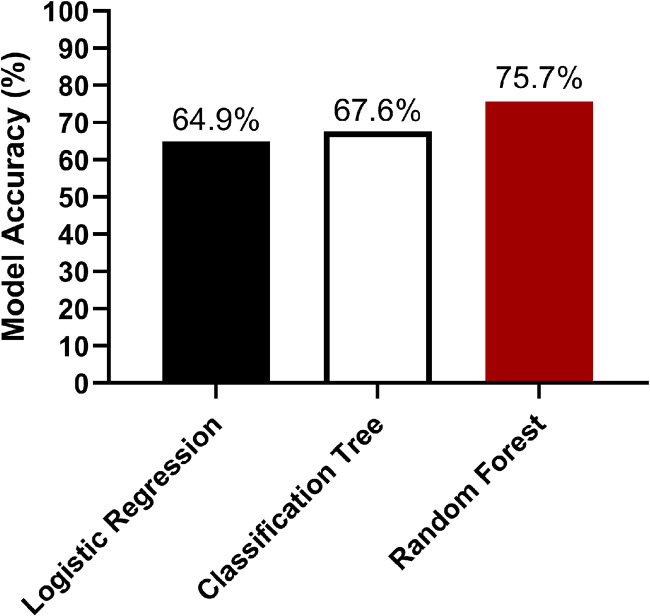
We used a modeling approach to identify characteristics of the B. dermatitidis environmental niche. Thirteen landscape characteristics were noted at each basal environmental sample collection site (see Table S1 in the supplemental material). The landscape characteristics and the presence/absence of detectable B. dermatitidis DNA for the 138 basal environmental samples (excluding HEN-14 with temporal sampling) were incorporated into three models—a simple logistic regression, a classification tree, and a random forest classification model (Fig. 5). The 138 samples were randomly split into training and test data sets with ∼75% (n = 101) of samples in the training set and ∼25% (n = 37) of samples in the test set.
FIG 5.
A random forest model predicts the presence of Blastomyces dermatitidis DNA in random environmental samples with 75% accuracy. The basal environmental sample data were split into training (n = 101) and test (n = 37) data sets and analyzed using logistic regression, classification tree, and random forest models to determine model accuracy at predicting the presence of B. dermatitidis DNA in the test data set using 13 environmental characteristics.
First, a simple logistic regression model was fit to predict whether B. dermatitidis was present or absent in a sample using the 13 landscape characteristics in the data set. An analysis of deviance was conducted using an analysis of variance (ANOVA) to compare the logistic regression model to the null model as each variable was added individually into the model. The ANOVA results are presented in Table S1 and showed that longitude, water classification, and nearby fecal matter had P values of <0.05, showing that adding these variables to the model resulted in the largest reduction in residual deviance. The predictive accuracy of the logistic regression model was tested by applying the model to the test data set to predict the presence/absence of B. dermatitidis compared to the known results. The logistic regression model accurately predicted the presence of B. dermatitidis in the samples only 64.9% of the time (Fig. 5).
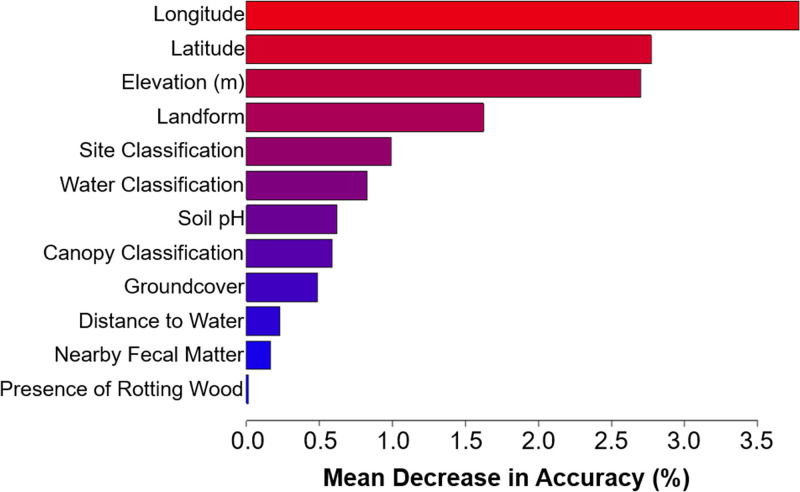
A classification tree for the B. dermatitidis sampling data set had 14 terminal nodes (Fig. S1) and had a misclassification error of 32.4% (Fig. 5). Pruning the tree to a depth of three in an attempt to improve the error rate did not improve the accuracy of the model. A random forest model is a more robust method in which a forest of bootstrapped classification trees is developed. A random subset of samples and predictors was included in each tree so that a forest of decorrelated trees was produced. The classification of each sample was determined by the most common results among the trees—a majority vote. A random forest classifier for the B. dermatitidis data set was constructed, and analysis of variable importance indicated that latitude, longitude, elevation, landform, and site classification were the most highly predictive variables resulting in the largest mean increase in accuracy (Fig. 6). Out of the models tested, the random forest model performed the best and was able to predict the presence of B. dermatitidis DNA in the test samples with 75.6% accuracy (Fig. 5).
FIG 6.
Landscape characteristics impact the accuracy of the random forest model to predict the presence of detectable Blastomyces dermatitidis DNA in environmental samples. Longitude, latitude, and elevation have the highest predictive power, while the presence of rotting wood, distance to water, and fecal matter had little impact on model accuracy.
DISCUSSION
The use of culture-independent sampling methods to characterize microbial communities and localize specific microbes has become common in recent years. These methods allow for the identification of nonculturable organisms, as well as decreasing bias toward strains that grow better in the laboratory than the environment. For a recalcitrant disease-causing organism such as Blastomyces dermatitidis, where culturing from the environment ranges from extremely difficult to impossible (15, 20–23), molecular-based techniques offer a more consistent, reproducible, and less labor-intensive method of detection. This is supported by our detection of B. dermatitidis from the 1999 outbreak samples where previous in vitro and mouse bioassay cultures were unsuccessful. Using a PCR-based technique, we identified B. dermatitidis DNA in the environment in Minnesota and used those data to build a predictive ecological niche model for B. dermatitidis. Additionally, we detected only transient associations with flooding events.
Information about the environmental niche, and modeling of that niche, in B. dermatitidis was previously derived from epidemiological data because the organism could not be reproducibly cultured from the environment (9, 15, 20–23). Early studies focused on specific outbreaks, but more recently, studies utilizing patient location information have used global information system (GIS) data to examine associations between environments where individuals with blastomycosis reside and environmental factors (9). These modern GIS-linked analyses are invaluable tools to understand the epidemiology of blastomycosis but are limited by an increasingly mobile population. Our data highlight the difficulty in using patient-based epidemiological approaches because defining where patients acquire infections and infection rates in areas with seasonal populations is challenging (7).
Our DNA data show locations with high geographic prevalence of B. dermatitidis in the environment. Consistent with the epidemiologic data, when we collected samples from random GPS coordinates in high-endemicity counties, with the goal of identifying basal levels of B. dermatitidis in the environment, we detected B. dermatitidis DNA in 33% of samples. In contrast, Hennepin County, where levels of blastomycosis are low, had no detectable B. dermatitidis DNA except transiently after a flooding event. These data suggest that the basal presence of B. dermatitidis in the environment may be higher in high-endemicity areas.
We also observed environmental prevalence variability on multiple levels: (i) between regions in the same county, (ii) at sites collected in the same region, (iii) temporally at the Hennepin county site, and (iv) possibly between the basal and outbreak locations. We were able to link a spike in B. dermatitidis DNA detection with a flooding event at the Hennepin county site, and the short duration of this spike may explain the lack of reproducibility inherent in previous attempts to localize B. dermatitidis in the environment following flooding events and the much lower prevalence of B. dermatitidis DNA in the outbreak B site compared to the other outbreak sites.
There was variability in the number of samples collected and analyzed at each location due to differences in both the scale and the type of site being tested at the locations. These differences in number of samples at each location may impact the current modeling and should be taken into consideration when interpreting our data. For example, the association between the presence of B. dermatitidis and longitude could be due to the large number of samples collected in St. Louis County. Significantly larger-scale studies, with more diffuse environmental sampling strategies, will be needed to overcome this limitation and provide a better description of site-specific differences in B. dermatitidis environmental prevalence.
Our sampling technique was able to successfully detect the presence of the BAD1 gene, which is thought to be specific to B. dermatitidis, in environmental samples. However, this method does have limitations. Because the BAD1 PCR-based assay is culture-independent, the assay does not differentiate between DNA detected from living B. dermatitidis, dead B. dermatitidis, or a possible unknown species of Blastomyces that could also have the BAD1 gene. The fact that we observed a high percentage of positive environmental samples in areas of endemicity and from areas with previous outbreaks provides support that the assay is detecting virulent B. dermatitidis. However, additional studies confirming the viability and pathogenicity of B. dermatitidis would be beneficial. Additionally, this assay does not quantify the amount of B. dermatitidis at a given location.
Our study also identified a new BAD1 promoter haplotype not previously defined by Meece and colleagues (32). In their previous study, Meece et al. showed that B. gilchristii had two large insertions in the BAD1 promoter region (haplotype 4) compared to the haplotype present in the sequence reference strain ATCC 26199 (haplotype 1). In contrast, the two other previously identified B. dermatitidis haplotypes had relatively minor alterations in the promoter region, including a single nucleotide deletion (haplotype 2) and a small 5-bp insertion (haplotype 3). The new haplotype present in our environmental sequences, as well as two additional strains that were previously whole-genome sequenced (28), contain this same 5-bp insertion but also an additional A→G conversion. Interestingly, previous population genetic studies using microsatellites to analyze B. dermatitidis strains had identified four populations that were geographically distributed across North America, with Minnesotan strains identified in both populations 1 and 4 (36). The BAD1 haplotypes present in population 1 strains of B. dermatitidis are currently unknown, but both haplotypes 3 and 5 were identified in population 4 strains. Given this diversity in the BAD1 haplotype among the population 4 strains, and the levels of recombination/admixture detected between populations 1 and 4 previously observed (27), we hypothesize that BAD1 haplotype 5 could also be present in population 1 strains of B. dermatitidis and thus prevalent in Minnesota.
This study comprises the largest number of samples detecting the basal environmental presence of B. dermatitidis. We used these basal environmental samples to build three ecological niche models to better understand the unknown ecological niche by using 13 environmental characteristics for each sample. The logistic regression model indicated that longitude, water classification, and nearby fecal matter were significantly related to the presence of B. dermatitidis DNA, but the classification error was poor, with only 64.9% accuracy. Tree-based methods are used for analysis of ecological data because they can simultaneously handle both continuous and categorical variable types without the need for variable selection (33). The disadvantages of tree-based models include slightly lower predictive performance and a higher risk of overfitting the training data. In our case, the tree makes splits to maximize the purity of grouped sampling sites by the presence of B. dermatitidis DNA. Use of a random forest tree model improved prediction accuracy to 75.6%. The random forest model identified the latitude, longitude, elevation, landform, and site classification as major contributors to B. dermatitidis prevalence. Surprisingly, other factors previously associated with B. dermatitidis, such as distance to water, water classification, or decaying wood did not have a major influence on the model. Unfortunately, the error rate for this model is still too high to make definitive statements about the ecological niche or biology of B. dermatitidis.
However, the results provided by the random forest model did provide intriguing information to consider. While our model did not contain sufficient data to identify the defining characteristics of the ecological niche, it instead revealed general geographical data and site classification as surrogate information. Latitude and longitude were both associated with the presence of B. dermatitidis, with northern and northwestern sites showing a higher number of samples with detectible B. dermatitidis DNA. We performed sampling in areas of endemicity that tended to be in the northern latitudes of the state, so the observed associations may be based on the narrow sampling range. We do not yet know the role this geographic location plays in the presence of B. dermatitidis. Future studies, expanded to other locations, such as New York, Canada, and Wisconsin, will likely provide further insights into geographic associations. Based on the observation that B. dermatitidis is more prevalent in the Midwest and Northeast North America and not West and Southwest North America, it seems likely that geographic location is relevant in some way. Furthermore, once studies that are no longer limited to narrow geographic locations are performed, other environmental factors influencing site classification that explain the association with forested areas will likely emerge for evaluation. More samples in additional locations may also show that soil type, vegetation, or other unexpected environmental features not specifically coded for in our modeling are associated with the presence of B. dermatitidis. Elevation and the associated landform classification also had a significant association with the presence of B. dermatitidis, and this may be related to flooding risk or vegetation type. Surprisingly, factors previously associated with B. dermatitidis—such as rotting wood, distance to water, and water classification (9, 15–17)—did not show a positive association with the presence of B. dermatitidis, suggesting these might not be critical factors in the ecological niche. Our current model may not yet reveal the precise ecological niche of B. dermatitidis, but it provides an important first step and critical clues that lay the foundation for future studies to further expand our understanding of the ecological niche.
Historically, we had a very poor understanding of B. dermatitidis in the environment, as the organism is so difficult to isolate from the environment. The PCR-based assay provides a method to identify B. dermatitidis in the environment and can be combined with the environmental parameters associated with each sample. Unusual for an endemic mycosis, B. dermatitidis showed an almost ubiquitous presence in the regions of endemicity of Minnesota, with many environmental samples positive without a clear environmental source. In comparison, Coccidioides spp.—another dimorphic, environmentally associated, endemic mycosis found in North America—does not have a generalized environmental presence and instead is consistently found associated with rodent burrows (34). Unfortunately, because of the almost ubiquitous presence of B. dermatitidis in the basal and outbreak environmental samples, the current assay cannot be used to determine the likelihood of contracting blastomycosis in a given location within the region of endemicity, the source of exposure, or the potential risk of exposure. Instead, the trend toward outbreak samples being positive compared to the basal samples, and the temporal flooding samples showing changes in positivity over time, suggest that B. dermatitidis levels in the environment may fluctuate based on environmental conditions. Quantitative PCR assays with appropriate samples are needed to determine if these fluctuations impact blastomycosis. For example, very high basal levels of B. dermatitidis may be present in areas of hyperendemicity, leading to the higher rates of blastomycosis observed in these areas. There may also be temporary increases in B. dermatitidis due to environmental changes that result in outbreaks of blastomycosis. Such quantitative studies may also be able to predict a theoretical environmental infectious dose—a parameter that is completely unknown at this time.
This study is the most comprehensive analysis of the ecological niche of B. dermatitidis to date. Still, the study lacks sufficient environmental sampling data to train a model with a low enough error rate to reliably predict environmental presence of B. dermatitidis. In addition, an improved assay to quantify B. dermatitidis DNA levels in the soil will be necessary to differentiate risk, particularly in areas of endemicity where basal soil prevalence is high. Finally, the modeling performed in this study suggests that combining large-scale randomized environmental sampling in areas of endemicity, culture-independent PCR-based detection of B. dermatitidis, and analysis of environmental features has the potential to define the ecological niche for B. dermatitidis. Importantly, this unbiased approach allows modeling areas of future spread, such as the emergence of blastomycosis that is already being observed in New York and Saskatchewan (10, 11), as our climate continues to change.
MATERIALS AND METHODS
Epidemiologic data.
Confirmed human and animal cases were defined as a Minnesota resident with B. dermatitidis organisms cultured or visualized from tissue or body fluid or a positive urine or serum antigen test and compatible clinical signs and symptoms (7). Clusters of human and/or animal cases were investigated, and environmental samples were collected during three investigations. Samples from outbreak A were collected in September 1999 and stored at −80°C for 18 years prior to analysis. outbreak B samples were collected in September 2012, and outbreak C samples were collected in June 2017. Case data were entered into an Access database and analyzed using SAS version 9.4.
Sample collection.
Environmental samples were collected from 1999 to 2017 at locations in Itasca (ITA), St. Louis (STL), Lake (LSS), and Hennepin (HEN) counties in Minnesota between June and September. Thirteen landscape characteristics were recorded at each sampling location: latitude, longitude, elevation (m), site classification (urban, grassland, forest, agricultural, residential), groundcover, canopy coverage, distance to water, water classification (creek, river, ditch, pond, wetland), landform (flat, gentle slope, steep slope, hilltop, cliff, creek, valley, shoreline), nearby fecal matter, rotting wood present, sample type (soil, vegetative debris, gravel, sand), and sample pH. In total, 250 (138 basal + 73 temporal + 39 outbreak) environmental samples were collected. Approximately 100 g of sample was collected at each site using a metal garden trowel, which was cleaned repeatedly to remove residual dirt with detergent- or isopropanol-containing wipes and sterilized with 70% ethanol between samples. Loose vegetative debris on the ground surface was removed prior to sample collection. Samples were taken to a depth of two inches. Rotting wood or scat samples were obtained using the metal trowel to scrape/scoop samples into a collection container. All samples were immediately double-bagged. Samples were placed in dry ice within 2 h, placed at either 4°C or −20°C within 8 h, and then stored at −80°C within 72 h after collection until processing. For the 1999 outbreak, samples were collected in the same manner, but GPS coordinates were not determined. Instead, photographs of each site were taken at the time of collection, along with soil temperature, vegetation type, and pH measurements.
1999 in vitro culture-based and in vivo sample analysis.
Environmental samples were coded and split for in vitro culture, in vivo culture, and storage at −80°C. Samples were tested using the in vitro culture method developed by Baumgardner and Paretsky (22). Briefly, samples were cultured on Sabouraud dextrose agar with chloramphenicol and cycloheximide, on Remel buffered CYE selective agar, and on yeast extract phosphate agar (Smith’s medium) treated with concentrated NH4OH that was added after inoculation.
DNA analysis.
DNA was extracted from the samples using E.Z.N.A. soil DNA kits (Omega BioTek, GA). PCR was performed as described previously (25). Briefly, the primers described in Burgess et al. were used for amplification (P1: AAGTGGCTGGGTAGTTATACGCTAC, P2: TAGGTTGCTGATTCCATAAGTCAGG). PCRs were performed in 50 μl using TaKaRa Ex Taq polymerase and buffer (TaKaRa Bio, Inc., Japan), either 1.0 or 0.5 μl of the P1 and P2 primers, and 1 or 0.5 μl of sample, with the remaining reaction volume as nuclease-free water (Sigma-Aldrich, Missouri). The PCRs were performed as follows: either an initial 95°C for 3 min, followed by 35 cycles of 30 s at 95°C, 59°C for 30 s, and 68°C for 1 min, and a final 72°C for 5 min or an initial 94°C for 5 min, followed by 35 cycles of 30 s at 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min, with a final 72°C for 5 min. Fungal intergenic spacer region (ITS) universal primers were used as a positive control to verify the presence of DNA in each extraction (26). DNA extractions were diluted 1:1, 1:5, 1:10, and 1:100 with nuclease-free water and at least three dilutions used in the PCR. Bands of the correct approximate size (368 bp) on a 1.5% agarose gel upon electrophoresis were gel-extracted, sequenced, and compared to the B. dermatitidis BAD1 gene (GenBank accession number XM_002621752.1) by sequence alignment using Sequencher (Gene Codes Corporation, Michigan). For a subset of samples, processing was repeated from extraction to PCR analysis to determine reproducibility of the PCR-based assay.
Sequences with 99% sequence identity were considered B. dermatitidis or B. gilchristii alleles and were compared to known BAD1 sequences to determine the haplotype. BAD1 promoter regions for all strain information deposited at NCBI as either nucleotide (ATCC 26911, BD0514, BD0607, BD0611, BD0622, BD0651, BD0733, BD0742, SC85, SC396, SC397) or genomic (ATCC 26911, ATCC 18187, ATCC 18188, ER-3, SLH1481) data were aligned with MEGA X version 10.2.0 (35). B. dermatitidis BAD1 haplotypes 1 to 4 were defined as previously described (32). Haplotype 5 was identified based on the genome sequences deposited in NCBI for strains ATCC 18187 and ATCC 18188.
Basal sampling sites.
Randomized GPS coordinates were produced using ArcGIS software (ESRI, California) within 6-square mile locations for ITA-14 and STL-14. ITA-17, STL-17, and LSS-17 were produced in the same manner, with randomized GPS coordinate sites, but were also previously used in a wetlands mapping project (36–39). Samples collected at each site included one at the randomly selected GPS coordinate and 1 to 2 additional samples with characteristics consistent with previous B. dermatitidis incidence (i.e., moist soil, rotting wood). HEN-14 samples were collected from four randomly generated sites near a creek starting 1 week after a major flooding event. Three samples were randomly collected (using the spinning pencil method) from each of the four sites each week for 6 weeks. During week 5, one additional sample was collected from a piece of rotting wood with visible mold.
Ecological niche model.
Data analysis was conducted using the R statistical software. The 13 landscape characteristics were divided into 2 continuous and 11 categorical variables. Dependent variables were coded as 0 if the PCR-based assay was negative and 1 if the assay was positive for B. dermatitidis DNA. The 138 basal environmental samples were randomly split into training (n = 101) and test (n = 37) data sets using the R command “set.seed(1234).” A simple logistic regression model was fit to predict the presence of B. dermatitidis DNA in a sample using the 13 landscape characteristics. The glm function was used with the options “family”=“binomial”(“link”=“logit”). An analysis of deviance was conducted using the “anova” function. A predictive analysis was performed by applying the test data set to the model and calculating the percentage of samples correctly predicted. A classification tree was developed to predict the presence of B. dermatitidis DNA using the Tree package (33, 40). The tuning parameter was determined using k-fold cross-validation (CV). Ten-fold CV was performed on the classification tree. A random forest classifier was constructed using the randomForest package with default parameters except “ntrees = 2000” (41). The tuneRF function was used to tune m to m = 2, where m is the number of variables randomly sampled as candidates at each split. R scripts are available at https://github.com/serina-robinson/Blastomyces-modeling/.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nathan Payne, Kyle Smith, Tory Whitten, Heather Fowler, Emily Gilbertson, and Emma Leof for help with environmental sampling.
Funding for this project was provided by the U.S. Centers for Disease Control and Prevention to the Minnesota Department of Health to J.S and K.N. This project was also supported by a University of Minnesota Academic Health Center BSL3 award. K.M.J. was funded in part by National Institutes of Health F31AI148047.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Castillo CG, Kauffman CA, Miceli MH. 2016. Blastomycosis. Infect Dis Clin North Am 30:247–264. doi: 10.1016/j.idc.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 2.McBride JS, Gauthier GM, Klein BS. 2017. Clinical manifestations and treatment of blastomycosis. Clin Chest Med 38:435–449. doi: 10.1016/j.ccm.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Martínez R, Méndéz-Tovar LJ. 2012. Blastomycosis. Clin Dermatol 30:565–572. doi: 10.1016/j.clindermatol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Sloss BL, Meece JK. 2013. Clinical and molecular epidemiology of veterinary blastomycosis in Wisconsin. BMC Vet Res 9:84. doi: 10.1186/1746-6148-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crampton TL, Light RB, Berg GM, Meyers MP, Schroeder GC, Hershfield ES, Embil JM. 2002. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis 34:1310–1316. doi: 10.1086/340049. [DOI] [PubMed] [Google Scholar]
- 6.Baumgardner DJ, Buggy BP, Mattson BJ, Burdick JS, Ludwig D. 1992. Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin Infect Dis 15:629–635. doi: 10.1093/clind/15.4.629. [DOI] [PubMed] [Google Scholar]
- 7.Ireland M, Klumb C, Smith K, Scheftel J. 2020. Blastomycosis in Minnesota, USA, 1999–2018. Emerg Infect Dis 26:866–875. doi: 10.3201/eid2605.191074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradsher RW, Chapman SW, Pappas PG. 2003. Blastomycosis. Infect Dis Clin North Am 17:21–40. doi: 10.1016/s0891-5520(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 9.Reed KD, Meece JK, Archer JR, Peterson AT. 2008. Ecologic niche modeling of Blastomyces dermatitidis in Wisconsin. PLoS One 3:e2034. doi: 10.1371/journal.pone.0002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohrenz S, Minion J, Pandey M, Karunakaran K. 2018. Blastomycosis in Southern Saskatchewan 2000–2015: unique presentations and disease characteristics. Med Mycol 56:787–795. doi: 10.1093/mmy/myx131. [DOI] [PubMed] [Google Scholar]
- 11.McDonald R, Dufort E, Jackson BR, Tobin EH, Newman A, Benedict K, Blog D. 2018. Notes from the field: blastomycosis cases occurring outside of regions with known endemicity: New York, 2007–2017. MMWR Morb Mortal Wkly Rep 67:1077–1078. doi: 10.15585/mmwr.mm6738a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz IS, Wiederhold NP, Hanson KE, Patterson TF, Sigler L. 2019. Blastomyces helicus, a new dimorphic fungus causing fatal pulmonary and systemic disease in humans and animals in western Canada and the United States. Clin Infect Dis 68:188–195. doi: 10.1093/cid/ciy483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dukik K, Muñoz JF, Jiang Y, Feng P, Sigler L, Stielow JB, Freeke J, Jamalian A, Gerrits van den Ende B, McEwen JG, Clay OK, Schwartz IS, Govender NP, Maphanga TG, Cuomo CA, Moreno LF, Kenyon C, Borman AM, de Hoog S. 2017. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 60:296–309. doi: 10.1111/myc.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz IS, Wiederhold NP, Kenyon CR, Jiang Y, Dukik K, De Hoog GS, Stubbe D, Maphanga TG, Govender NP, Munoz JF, Cuomo CA, Sigler L. 2018. Human and veterinary blastomycosis caused by Blastomyces helicus and B. percursus identified among global fungal collections. Med Mycol 56:S69. [Google Scholar]
- 15.Klein BS, Vergeront JM, Weeks RJ, Kumar UN, Mathai G, Varkey B, Kaufman L, Bradsher RW, Stoebig JF, Davis JP. 1986. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med 314:529–534. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- 16.Klein BS, Vergeront JM, DiSalvo AF, Kaufman L, Davis JP. 1987. Two outbreaks of blastomycosis along rivers in Wisconsin. Isolation of Blastomyces dermatitidis from riverbank soil and evidence of its transmission along waterways. Am Rev Respir Dis 136:1333–1338. doi: 10.1164/ajrccm/136.6.1333. [DOI] [PubMed] [Google Scholar]
- 17.Baumgardner DJ, Laundre B. 2001. Studies on the molecular ecology of Blastomyces dermatitidis. Mycopathologia 152:51–58. doi: 10.1023/a:1012438029997. [DOI] [PubMed] [Google Scholar]
- 18.Proctor ME, Klein BS, Jones JM, Davis JP. 2002. Cluster of pulmonary blastomycosis in a rural community: evidence for multiple high-risk environmental foci following a sustained period of diminished precipitation. Mycopathologia 153:113–120. doi: 10.1023/A:1014515230994. [DOI] [PubMed] [Google Scholar]
- 19.Kitchen MS, Reiber CD, Eastin GB. 1977. An urban epidemic of North American blastomycosis. Am Rev Respir Dis 115:1063–1066. [DOI] [PubMed] [Google Scholar]
- 20.Denton JF, McDonough ES, Ajello L, Ausherman RJ. 1961. Isolation of Blastomyces dermatitidis from soil. Science 133:1126–1127. doi: 10.1126/science.133.3459.1126. [DOI] [PubMed] [Google Scholar]
- 21.Bakerspigel A, Kane J, Schaus D. 1986. Isolation of Blastomyces dermatitidis from an earthen floor in southwestern Ontario, Canada. J Clin Microbiol 24:890–891. doi: 10.1128/JCM.24.5.890-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgardner DJ, Paretsky DP. 1999. The in vitro isolation of Blastomyces dermatitidis from a woodpile in north central Wisconsin, USA. Med Mycol 37:163–168. doi: 10.1080/j.1365-280X.1999.00214.x. [DOI] [PubMed] [Google Scholar]
- 23.Denton JF, DiSalvo AF. 1964. Isolation of Blastomyces dermatitidis from natural sites at Augusta, Georgia. Am J Trop Med Hyg 13:716–722. doi: 10.4269/ajtmh.1964.13.716. [DOI] [PubMed] [Google Scholar]
- 24.Brandhorst TT, Roy R, Wüthrich M, Nanjappa S, Filutowicz H, Galles K, Tonelli M, McCaslin DR, Satyshur K, Klein B. 2013. Structure and function of a fungal adhesin that binds heparin and mimics thrombospondin-1 by blocking T cell activation and effector function. PLoS Pathog 9:e1003464. doi: 10.1371/journal.ppat.1003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess JW, Schwan WR, Volk TJ. 2006. PCR-based detection of DNA from the human pathogen Blastomyces dermatitidis from natural soil samples. Med Mycol 44:741–748. doi: 10.1080/13693780600954749. [DOI] [PubMed] [Google Scholar]
- 26.White TJ, Bruns T, Lee S, Taylor JW, 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed). PCR protocols: a guide to methods and applications. Academic Press, New York, NY. [Google Scholar]
- 27.McTaggart LR, Brown EM, Richardson SE. 2016. Phylogeographic analysis of Blastomyces dermatitidis and Blastomyces gilchristii reveals an association with North American freshwater drainage basins. PLoS One 11:e0159396. doi: 10.1371/journal.pone.0159396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz JF, Gauthier GM, Desjardins CA, Gallo JE, Holder J, Sullivan TD, Marty AJ, Carmen JC, Chen Z, Ding L, Gujja S, Magrini V, Misas E, Mitreva M, Priest M, Saif S, Whiston EA, Young S, Zeng Q, Goldman WE, Mardis ER, Taylor JW, McEwen JG, Clay OK, Klein BS, Cuomo CA. 2015. The dynamic genome and transcriptome of the human fungal pathogen Blastomyces and close relative Emmonsia. PLoS Genet 11:e1005493. doi: 10.1371/journal.pgen.1005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman SW, Lin AC, Hendricks KA, Nolan RL, Currier MM, Morris KR, Turner HR. 1997. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect 12:219–228. [PubMed] [Google Scholar]
- 30.Saccente M, Woods GL. 2010. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev 23:367–381. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szeder V, Ortega-Gutierrez S, Frank M, Jaradeh SS. 2007. CNS blastomycosis in a young man working in fields after Hurricane Katrina. Neurology 68:1746–1747. doi: 10.1212/01.wnl.0000265229.31844.45. [DOI] [PubMed] [Google Scholar]
- 32.Meece JK, Anderson JL, Klein BS, Sullivan TD, Foley SL, Baumgardner DJ, Brummitt CF, Reed KD. 2010. Genetic diversity in Blastomyces dermatitidis: implications for PCR detection in clinical and environmental samples. Med Mycol. 48:285–290. doi: 10.3109/13693780903103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ripley BD. 1996. Pattern recognition and neural networks. Chapter 7. Cambridge University Press, Cambridge, UK. [Google Scholar]
- 34.Kollath DR, Teixeira MM, Funke A, Miller KJ, Barker BM. 2020. Investigating the role of animal burrows on the ecology and distribution of Coccidioides spp. in Arizona soils. Mycopathologia 185:145–159. doi: 10.1007/s11046-019-00391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corcoran J, Knight J, Brisco B, Kaya S, Cull A, Murnaghan K. 2011. The integration of optical, topographic, and radar data for wetland mapping in northern Minnesota. Can J Remote Sens (Basel) 37:564–582. doi: 10.5589/m11-067. [DOI] [Google Scholar]
- 37.Knight JF, Tolcser B, Corcoran J, Rampi L. 2013. The effects of data selection and thematic detail on the accuracy of high spatial resolution wetland classifications. Photogramm Eng Remote Sens (Basel) 79:613–623. doi: 10.14358/PERS.79.7.613. [DOI] [Google Scholar]
- 38.Rampi LP, Knight JF, Lenhart CF. 2014. Comparison of flow direction algorithms in the application of the CTI for mapping wetlands in Minnesota. Wetlands 34:513–525. doi: 10.1007/s13157-014-0517-2. [DOI] [Google Scholar]
- 39.Rampi LP, Knight JF, Pelletier KC. 2014. Wetland mapping in the Upper Midwest United States. Photogramm Eng Remote Sens (Basel) 80:439–449. doi: 10.14358/PERS.80.5.439. [DOI] [Google Scholar]
- 40.Ripley BD. 2002. Tree: classification and regression trees. (R package version 1.0-40) https://CRAN.R-project.org/package=tree. Retrieved 17 July 2020.
- 41.Liaw A, Wiener M. 2002. Classification and regression by randomForest. R News 2:18–22. https://cogns.northwestern.edu/cbmg/LiawAndWiener2002.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.