Predation is an important survival strategy of the widespread myxobacteria, but it remains poorly understood on the mechanistic level. Without a basic understanding of how prey cell killing and consumption is achieved, it also remains difficult to investigate the role of predation for the complex myxobacterial lifestyle, reciprocal predator-prey relationships, or the impact of predation on complex bacterial soil communities.
KEYWORDS: bacterial behavior, bacteriolytic enzymes, glycoside hydrolase, outer membrane vesicles
ABSTRACT
Myxococcus xanthus kills other species to use their biomass as an energy source. Its predation mechanisms allow feeding on a broad spectrum of bacteria, but the identity of predation effectors and their mode of action remain largely unknown. We initially focused on the role of hydrolytic enzymes for prey killing and compared the activities of secreted M. xanthus proteins against four prey strains. Seventy-two secreted proteins were identified by mass spectrometry; among them is a family 19 glycoside hydrolase that displayed bacteriolytic activity in vivo and in vitro. This enzyme, which we name LlpM (lectin/lysozyme-like protein of M. xanthus), was not essential for predation, indicating that additional secreted components are required to disintegrate prey. Furthermore, secreted proteins lysed only Gram-positive, not Gram-negative, species. We thus compared the killing of different prey by cell-associated mechanisms: individual M. xanthus cells killed all four test strains in a cell contact-dependent manner but were only able to disintegrate Gram-negative, not Gram-positive, cell envelopes. Thus, our data indicate that M. xanthus uses different multifactorial mechanisms for killing and degrading different prey. Besides secreted enzymes, cell-associated mechanisms that have not been characterized so far appear to play a major role in prey killing.
IMPORTANCE Predation is an important survival strategy of the widespread myxobacteria, but it remains poorly understood on the mechanistic level. Without a basic understanding of how prey cell killing and consumption is achieved, it also remains difficult to investigate the role of predation for the complex myxobacterial lifestyle, reciprocal predator-prey relationships, or the impact of predation on complex bacterial soil communities. We study predation in the established model organism Myxococcus xanthus, aiming to dissect the molecular mechanisms of prey cell lysis. In this study, we addressed the role of secreted bacteriolytic proteins, as well as potential mechanistic differences in the predation of Gram-positive and Gram-negative bacteria. Our observation shows that secreted enzymes are sufficient for killing and degrading Gram-positive species but that cell-associated mechanisms may play a major role in killing Gram-negative and Gram-positive prey on fast timescales.
INTRODUCTION
Myxobacteria are Deltaproteobacteria that are ubiquitous in tropical and temperate aerobic soils. They display unique patterns of coordinated multicellular behavior, with individual cells building large clusters to glide on semidry surfaces and efficiently access nutrients. When nutrients become scarce, single cells coordinate their motility and aggregate into complex multicellular structures, called fruiting bodies, where they differentiate into spores (1, 2).
Myxobacteria preferably use amino acids or small peptides as a carbon and energy source. Unlike the majority of soil bacteria, myxobacteria not only live as saprophytes, they also show predatory behavior, i.e., they kill other bacteria to consume their biomass. Predation is a complex, multistep process: a group of myxobacteria invades a colony of prey cells, induces prey lysis from the outside, and consumes the released biomass (“epibiotic predation”) (3–5). Gliding motility enables Myxococcus xanthus to frequently encounter prey. Furthermore, cell reversal frequency can be adapted in response to prey (6, 7) such that a “rippling” pattern is induced, which may ensure a thorough sweeping of prey biomass by the predator population (8, 9). Cells that carry mutations affecting the S- or A-motility engines (e.g., pilA, cglB) or cell reversal regulation (e.g., frzE) are compromised in predation (6, 7, 10, 11).
Obligate, endobiotic predators, like Bdellovibrio species, are limited to prey on Gram-negative bacteria (12, 13), but myxobacteria can feed on a broad spectrum of microorganisms, including Gram-positive and Gram-negative bacteria and cyanobacteria as well as fungi (2, 4, 14). Differences in predation of Gram-positive and Gram-negative bacteria have been observed qualitatively in macroscopic assays (15), and differences in swarming rate of M. xanthus (16) and the maximum prey kill (17) have been reported. However, the molecular basis for the killing of different prey species remains largely unknown.
Prey killing is mostly attributed to the secretion of hydrolytic enzymes and secondary metabolites, which presumably lyse surrounding bacteria (2, 5, 18–20). M. xanthus produces a multitude of hydrolytic enzymes, such as proteases, peptidases, lipases, and glycoside hydrolases (21), as well as bacteriostatic and bactericidal antibiotics (22, 23). For some of these components, a different efficacy against Gram-positive or Gram-negative bacteria has been documented: the peptide antibiotic myxoprincomide supports growth of M. xanthus on Bacillus subtilis but does not affect M. xanthus’s predation of Escherichia coli (24). On the other hand, the bactericidal antibiotic myxovirescin A (or “TA”), which targets signal peptidase II, is involved in the predation of growing, Gram-negative E. coli but not of Gram-positive Micrococcus luteus (25, 26). Moreover, bacteriolytic proteins with amidase and glucosaminidase activity but unknown identity were enriched from M. xanthus and Myxococcus virescens culture supernatants and shown to be active against several Gram-positive, but not Gram-negative, bacteria (27–29).
Several studies propose that secreted outer membrane vesicles (OMVs) are instrumental in the delivery of lytic factors onto prey cells. OMVs of M. xanthus are 30 to 100 nm in diameter and are secreted individually or are connected in long chains, forming a network within a group of cells (30, 31). Mass spectrometry revealed that OMVs contain multiple hydrolytic proteins and peptidases, as well as antibiotics, such as myxovirescin A and myxalamides (32–35). An inhibitory effect of isolated OMVs against E. coli cells has been reported (30, 33), with a partial activity of OMVs compared to that of intact M. xanthus cells (30).
Investigating predation as an important bacterial survival strategy might offer fascinating insights into the dynamics of bacterial communities. However, to address the impact of predation on both M. xanthus and prey bacteria, a more detailed understanding of the molecular basis of predation is necessary. In this study, we set out to investigate which mechanisms are used by M. xanthus to kill different prey species. We first compared the bacteriolytic activity of M. xanthus protein fractions and intact cells against M. luteus, B. subtilis, E. coli, and Agrobacterium tumefaciens. Secreted proteins killed live Gram-positive prey only, although hydrolysis of peptidoglycan from all four species was observed in vitro. Using a mass spectrometry-based approach, we identified MXAN_4534, which we named LlpM (lectin/lysozyme-like protein of M. xanthus), as one of the enzymes that likely contributes to this activity. OMV fractions showed bacteriostatic activity against E. coli. However, live-cell microscopy revealed that cell associated-mechanisms are very efficient in killing all four prey species on short timescales, whereas disintegration of the Gram-positive cell envelope additionally requires high concentrations of secreted proteins. Hence, cell-associated mechanisms, which have not been identified yet, may play a particularly important role for prey killing by M. xanthus.
RESULTS
Comparing the bacteriolytic properties of M. xanthus cells and protein fractions.
M. xanthus is able to utilize a broad variety of bacteria as prey. To investigate potential differences in the predation mechanisms used to target various prey bacteria, we compared the bacteriolytic activity of M. xanthus intact cells and isolated protein fractions against different species. For this we chose two Gram-positive strains, Micrococcus luteus DSM 20030 and Bacillus subtilis 168, and two Gram-negative strains, Escherichia coli DH5α and Agrobacterium tumefaciens C58.
To observe predation at a macroscopic scale, we spotted a cell suspension of M. xanthus wild-type DK1622 in close proximity to a dense suspension of prey bacteria on agar plates and documented the cocultures after 2 days of incubation (Fig. 1A). In this assay, the M. xanthus population showed similar behavior toward Gram-positive and Gram-negative prey species; the M. xanthus population spread radially from the inoculation spot and invaded the prey colony, thereby visibly clearing the prey cells. Rippling of M. xanthus, a form of motility coordination in the presence of bacterial prey (8, 9), was observed on all four prey strains (Fig. 1A).
FIG 1.
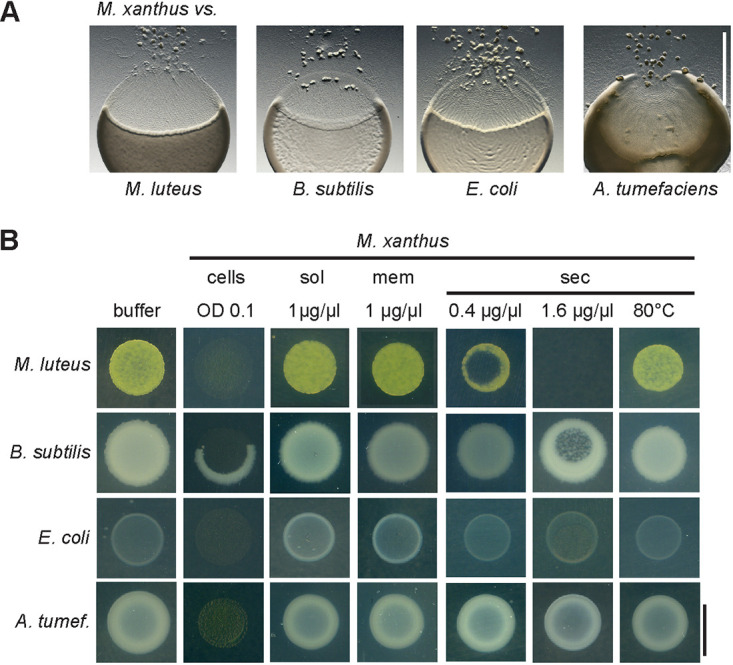
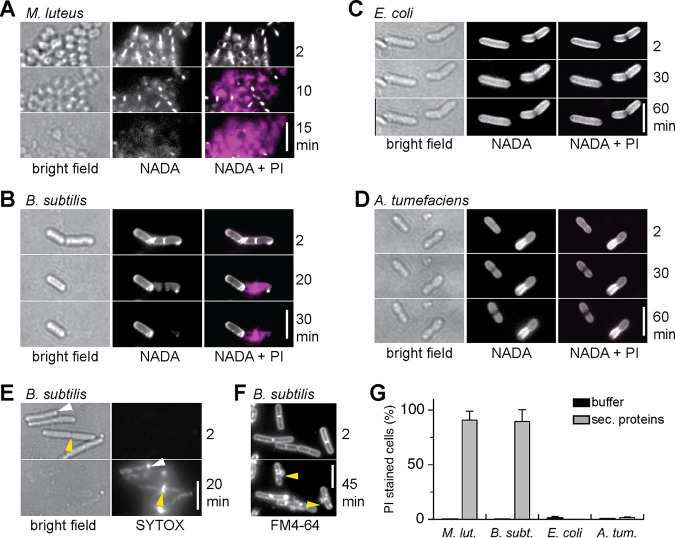
(A) Predation behavior of Myxococcus xanthus against Gram-positive species Micrococcus luteus and Bacillus subtilis and Gram-negative species Escherichia coli and Agrobacterium tumefaciens. M. xanthus was spotted next to prey bacteria on a CF minimal agar plate, incubated for 2 days, and documented with a stereo microscope. Scale bar, 5 mm. (B) Bacteriolytic activity of M. xanthus protein fractions. Cell suspensions of prey bacteria M. luteus, B. subtilis, E. coli, and A. tumefaciens adjusted to an OD600 of 0.08 were spotted on agar plates. Dried spots were overlaid with 2 µl of a sample of intact M. xanthus cells (OD600, 0.1), soluble proteins (sol), membrane proteins (mem), or secreted proteins (sec) at a protein concentration as indicated. Agar plates were incubated at 30°C overnight and visually screened for bacteriolytic activity. Results represent three biological replicates. Scale bar, 8 mm.
Next, we prepared different protein fractions of M. xanthus and compared their bacteriolytic activity against the four test strains to analyze how prey lysis might be mediated by isolated proteins. M. xanthus was cultivated in CYE medium, which contains Casitone as a nutrient and promotes vegetative growth. Cells were disrupted by French pressing, and the cleared cell extract was subjected to ultracentrifugation to separate soluble proteins (sol) from the membrane fraction (mem). To isolate secreted proteins (sec), we performed ammonium sulfate precipitation of M. xanthus culture supernatant. Samples of the isolated protein fractions were spotted directly onto live prey cells on agar plates at a concentration of 0.4 to 1.6 µg/µl, as indicated in Fig. 1B. To ensure maximum sensitivity, we used a diluted suspension of prey cells and incubated the plates overnight.
In contrast to intact M. xanthus cells, which lysed live cells of all of the tested prey bacteria, isolated soluble or membrane proteins did not cause detectable lysis of any of the test strains (Fig. 1B). In contrast, samples containing secreted proteins were active toward live cells of the Gram-positive species M. luteus and B. subtilis. Heating of the samples for 10 min at 80°C abolished the bacteriolytic effect. This indicates that protein activity is involved and excluded that the effect is due to high salt concentration after ammonium sulfate precipitation. The nonconcentrated culture supernatant did not cause prey lysis (data not shown). To achieve lysis of B. subtilis colonies, a 4-fold higher concentration of M. xanthus-secreted proteins was required. Notably, the Gram-negative test strains E. coli or A. tumefaciens were not affected by the secreted protein fraction (Fig. 1B).
These results show that bacteriolytic activity of isolated proteins against live cells can clearly be attributed to the M. xanthus secretome but not to putative surface-associated or membrane proteins. This supports a specific role of secreted lytic proteins in predation, in particular against Gram-positive prey.
Isolated secreted proteins kill and lyse Gram-positive but not Gram-negative prey species.
To confirm that the observations made on agar plates reflect lysis of prey bacteria and are not a consequence of growth inhibition, we used time-lapse microscopy and specific fluorescent dyes to resolve the bacteriolytic effects of M. xanthus-secreted proteins on live prey bacteria. To test for cell viability, we used the fluorescent nucleic acid stains propidium iodide (PI; red) and SYTOX (green), which bind to DNA only in dead cells with permeabilized membranes (36, 37). To visualize the peptidoglycan layer, we incorporated a green fluorescent d-amino acid (NADA-green) (38, 39), and to label the (outer) membrane, we used the lipophilic dye FM4-64 (red; Thermo Fisher) (37). M. xanthus-secreted proteins were added at a final concentration of 1 µg/µl to live bacteria stained with combinations of these fluorescent stains. Samples were placed on agarose-coated object slides and observed for 1 h.
Intact cells of the Gram-positive species M. luteus and B. subtilis were killed within 10 to 40 min after addition of M. xanthus-secreted proteins, as indicated by the appearance of PI fluorescence (Fig. 2A and B). Concomitant to cell death, cells visibly lost contrast in bright field, indicating cell lysis. This was confirmed by the loss of NADA fluorescence, which demonstrates degradation of the peptidoglycan layer (Fig. 2A and B). For B. subtilis, fluorescent-stained DNA visibly leaked from dead cells at the cell poles or near the division septa (Fig. 2E). Moreover, membrane staining revealed frequent membrane blebs near the division septa (Fig. 2F). DNA leakage from M. luteus cells was clearly detectable as well (Fig. 2A); however, the site of DNA leakage and membrane blebs could not be resolved due to the small size and clustering of coccoid M. luteus cells.
FIG 2.
Secreted proteins of M. xanthus lyse Gram-positive bacteria. Secreted proteins (1 µg/µl) isolated from M. xanthus culture supernatant were added to Gram-positive bacteria M. luteus (A) and B. subtilis (B) and to Gram-negative bacteria E. coli (C) and A. tumefaciens (D). The peptidoglycan layer was labeled with the fluorescent d-amino acid NADA-green (white). DNA staining with propidium iodide (PI) indicates dead cells (magenta). (E) SYTOX staining shows that DNA leaks from lysed B. subtilis cells predominantly from the cell pole (white triangle) or the septum area (yellow triangle). (F) Membrane blebs at the septum (yellow triangles) were frequently observed after treatment of B. subtilis with M. xanthus-secreted proteins. (G) Percentage of lysed cells 60 min after the addition of buffer (black) or M. xanthus-secreted proteins (gray). Data and standard deviations represent at least three biological replicates with >200 cells analyzed in total. See Table S3 in the supplemental material for statistical analysis. All scale bars represent 5 µm.
In total, 91% of M. luteus cells and 88% of B. subtilis cells were killed and lysed within 1 h after the addition of M. xanthus-secreted proteins, while no cells (0%) were killed in the buffer control (Fig. 2G). These results represent >200 cells analyzed per strain in three biological replicates.
In contrast to the Gram-positive species, Gram-negative E. coli and A. tumefaciens cells were not affected by secreted proteins from M. xanthus: cells retained their appearance and normal cell shape when observed in bright field and did not display PI fluorescence that would indicate cell death (Fig. 2C and D). One hour after the addition of M. xanthus-secreted proteins, 0% of E. coli and 1.7% of A. tumefaciens were visibly affected, compared to 1% of E. coli and 0.5% of A. tumefaciens in the buffer control (Fig. 2G; >200 cells analyzed in three biological replicates). Statistical analysis shows that the percentage of killed (PI stained) cells is significantly different for Gram-positive and Gram-negative species (P < 0.01; analysis of variance [ANOVA] and Tukey post hoc test; Table S3 in the supplemental material). Note that cells used for the quantitative analysis shown in Fig. 2G were not stained with NADA, as we found that its incorporation affected the viability of M. luteus and A. tumefaciens (data not shown).
The observations described here confirm that the secreted protein fraction from M. xanthus is sufficient for killing and lysing intact bacteria. Furthermore, they indicate degradation of the cell envelope by peptidoglycan-degrading enzymes. Moreover, these results emphasize the different susceptibility of the different prey species: M. xanthus-secreted proteins induced lysis of Gram-positive M. luteus and B. subtilis but not of Gram-negative E. coli and A. tumefaciens.
M. xanthus secretes LlpM, a family 19 glycoside hydrolase with lysozyme-like activity.
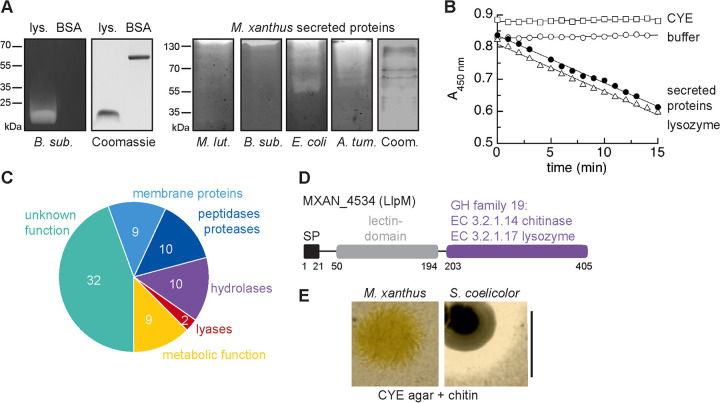
Enzymes with peptidoglycan-degrading activity had been previously isolated from M. xanthus culture supernatant and were shown to be effective against Gram-positive bacteria (27, 28). To further investigate the activity of these secreted proteins against different bacterial substrates, we performed in-gel zymography using polyacrylamide gels, which contained washed and autoclaved bacteria as a substrate. This approach allows to identify enzymes that lyse bacterial cell walls (40–42).
Lysozyme and bovine serum albumin (BSA) were used as positive and negative controls, respectively. Lysozyme, but not BSA, caused visible clearing of the bacterial substrate after renaturation, which can be seen by a localized loss of methylene blue staining (Fig. 3A). Under the seminative running conditions, the majority of secreted proteins of M. xanthus migrated only a small distance into the zymogram and the Coomassie-stained control gel. Sample retardation was most pronounced in zymograms containing autoclaved Gram-positive species, possibly due to their high content of peptidoglycan. Nevertheless, the samples caused clearing of all four bacterial substrates, M. luteus, B. subtilis, E. coli, and A. tumefaciens, which was reproducibly detected close to the top of the gel and indicates the presence of peptidoglycan-degrading enzymes in the samples (Fig. 3A).
FIG 3.
M. xanthus secretes proteins with peptidoglycan-degrading activity. (A) Zymogram analysis. One microgram of lysozyme, 1 µg BSA, or 5 µg of M. xanthus-secreted proteins was separated in gels containing autoclaved bacteria as a substrate as indicated. After electrophoresis under seminative conditions and renaturation, gels were stained with methylene blue and destained in H2O to reveal cleared zones that indicate bacteriolytic activity. Coomassie-stained gels were run under the same conditions as zymograms but lack substrate bacteria. (B) In a photometric assay, 10 µg/ml M. xanthus-secreted proteins (black circles) lyse lyophilized M. luteus cells at a similar rate as 0.5 µg/ml lysozyme (open triangles). Open squares, control with peptides precipitated from CYE growth medium (10 µg/ml); open circles, buffer control. Data represent average of three biological replicates for M. xanthus-secreted proteins and three technical replicates for controls. See also Fig. S4 in the supplemental material for a dose-response experiment. (C) A sample of M. xanthus-secreted proteins was analyzed by mass spectrometry. Seventy-two proteins were identified (Table S3) and belong to different functional groups as indicated. (D) MXAN_4534 (LlpM) is a candidate protein for peptidoglycan-degrading activity. It contains an N-terminal signal peptide (SP) for extracellular localization, a lectin domain, and a C-terminal domain with homology to glycoside hydrolases (GH) of family 19, which encompasses lysozyme and chitinase activities. Numbers indicate amino acid positions. (E) M. xanthus does not degrade chitin. M. xanthus or Streptomyces coelicolor, a chitin-degrading bacterium, was spotted onto agar plates containing rich growth medium and chitin. Clearing of turbid chitin indicates chitinolytic activity. Scale bar, 1 cm.
Using another assay to test for peptidoglycan degradation, we compared the activity of M. xanthus-secreted proteins to that of lysozyme in a photometric assay, using freeze-dried M. luteus cells as a substrate. Here, M. xanthus-secreted proteins at a concentration of 10 µg/ml caused a change in absorbance at 450 nm per minute (ΔA450/min) of 0.015 ± 0.003, which compares to a lysozyme concentration of 0.5 µg/ml (ΔA450/min of 0.015 ± 0.002) (Fig. 3B). A dose-response experiment confirmed that under these experimental conditions, the decrease in A450, i.e., lysis of M. luteus, was linearly dependent on increasing concentrations of M. xanthus-secreted proteins (Fig. S4). Negative controls with buffer only or with precipitated peptides from CYE medium did not induce a change in absorbance (Fig. 3B).
Putative peptidoglycan-degrading activity among M. xanthus secreted proteins has been reported before by Hart and Zahler (28) and Sudo and Dworkin (27); however, the identities of these enzymes remained unknown.
In order to identify proteins with bacteriolytic activity in the M. xanthus secretome, we used mass spectrometry to analyze proteins from gel regions that showed bacteriolytic activity in zymograms. This analysis resulted in 72 proteins (listed in Table S1), which we assessed bioinformatically for conserved functional domains, focusing on putative bacteriolytic candidates. The majority of the detected proteins (33) have unknown function, 18 of which are putative lipoproteins. Moreover, 9 membrane proteins were detected (Fig. 3C). Among the soluble proteins, candidates with annotated enzymatic function are predominant and include 10 proteases/peptidases, 10 hydrolases, 2 lyases, and 9 metabolic function (Fig. 3C). One candidate, protein MXAN_4534 (405 amino acids [aa]), displayed an annotated function directly related to the lysis of bacterial cells. Based on sequence homology, the C-terminal domain of protein MXAN_4534 belongs to the family 19 of glycoside hydrolases (from here on termed GH19), which encompasses chitinase (EC 3.2.1.14) and lysozyme-like (EC 3.2.1.17) activities (Fig. 3D). The N terminus of MXAN_4534 encompasses a lectin-like putative sugar-binding domain, as well as a signal peptide for secretion. The distribution of proteins with this domain organization is limited to several orders within the Actinobacteria and the orders Myxoccoccales and Bacillales (Fig. S2). MXAN_4534 was also detected in earlier studies among extracellular proteins of M. xanthus and is annotated as a class I chitinase (32, 34, 35). However, the activity of MXAN_4534 has not been characterized so far, and some members of the GH19 family are also known to hydrolyze peptidoglycan in addition to chitin (43–45). We tested for chitinolytic activity of M. xanthus on chitin-containing agar plates (or liquid cultures; data not shown). We detected no lysis of chitin by M. xanthus, although chitinolytic activity of Streptomyces coelicolor, which secretes GH19 enzyme ChiG (46), was clearly visible (Fig. 3E). This indicates that chitin is not, or is only to a very minor extent, enzymatically degraded by M. xanthus. The hydrolase MXAN_4534 might thus be used to degrade the peptidoglycan cell wall of bacterial prey. We therefore propose to rename MXAN_4534 to LlpM for lectin/lysozyme-like protein of M. xanthus.
LlpM displays bacteriolytic activity but is not required for predation.
To further characterize the enzymatic activity of LlpM, we heterologously expressed His6-tagged LlpM (LlpM-His) in E. coli BL21. Upon the induction of LlpM-His expression, the optical density of the growing E. coli culture decreased, suggesting cell lysis (Fig. S5). When E. coli cells expressing LlpM were examined microscopically, we observed cell debris as well as round-shaped cells, indicating the formation of spheroplasts due to degradation of the peptidoglycan layer (Fig. 4A). Based on sequence homology to other GH19 enzymes, we changed a putative catalytic glutamate residue, E260, to glutamine. Overexpression of LlpM (E260Q) no longer causes cell lysis, as reflected by rod-shaped E. coli cells (Fig. 4A), which indicates that enzymatic activity was lost. Next, we deleted the N-terminal signal peptide from residues 1 to 21 (Δ1-21), which should prevent the export into the periplasm. Indeed, overexpression of LlpM (Δ1-21) did not affect the cell shape of the E. coli host (Fig. 4A), presumably because this variant cannot reach the peptidoglycan layer in the periplasm.
FIG 4.
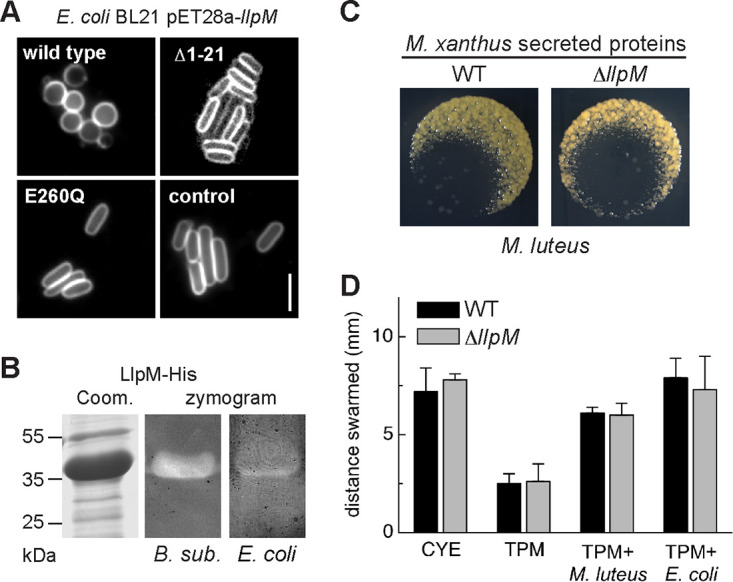
(A) LlpM has bacteriolytic activity. E. coli cells expressing LlpM-His form spheroplasts and lyse, which indicates peptidoglycan-degrading activity. No degradation is observed after deletion of the signal peptide (Δ1-21) or after mutation of a conserved catalytic glutamate residue (E260Q). Cell membranes were labeled with fluorescent dye FM4-64. Scale bar, 5 µm. See Fig. S5 in the supplemental material for corresponding growth curves. (B) Zymography of LlpM-His protein enriched by Ni-NTA chromatography confirms bacteriolytic activity using autoclaved B. subtilis or E. coli cells as a substrate. Zymograms were stained with methylene blue. (C) M. xanthus-secreted proteins lyse prey also in the absence of LlpM. Secreted proteins wild type and ΔllpM mutant were added to M. luteus cells spotted on agar plates and documented after incubation overnight at 30°C. Images represent three biological replicates. (D) M. xanthus wild type (black) and ΔllpM mutant (gray) were spotted to the center of agar plates that either contained nutrients (CYE), contained no nutrients (TPM), or were covered with a lawn of M. luteus or E. coli cells (TPM plus prey, as indicated). The distance swarmed within 3 days of incubation was measured at the swarm edge. Error bars represent the standard deviation of three biological replicates. The respective differences between wild type and ΔllpM mutant are statistically not significant (P > 0.1, Student's t test).
To purify LlpM, we overexpressed the full-length LlpM-His in the E. coli SHuffle strain, which supports the folding of periplasmic and extracellular proteins in the cytoplasm. In this strain, induction of LlpM expression does not induce lysis (data not shown), i.e., it seems to be retained in the cytoplasm. LlpM-His was enriched by affinity chromatography and showed activity in zymograms with autoclaved cells of B. subtilis or E. coli as a substrate (Fig. 4B). Taken together, these results show that LlpM is able to lyse the peptidoglycan layer of bacterial cell walls, similar to lysozyme.
To determine the putative role of LlpM in predation, we created a chromosomal deletion of gene llpM (MXAN_4534) and compared the bacteriolytic properties of the M. xanthus ΔllpM mutant strain to that of the wild type. First, we prepared secreted protein fractions from M. xanthus wild-type and ΔllpM strains and assayed their bacteriolytic activity against M. luteus on agar plates. Both protein extracts caused indistinguishable lysis of M. luteus (Fig. 4C; three biological replicates). Second, the M. xanthus wild type and ΔllpM were inoculated on different agar plates, which either contained Casitone as a nutrient (CYE), contained no nutrients (TPM), or were covered with a layer of M. luteus or E. coli cells as the only nutrient source (TPM plus prey, as indicated in Fig. 4D). On prey-covered TPM plates, swarm expansion of M. xanthus can serve as an indirect measure of predation performance since motility is coupled to the ability to access energy from prey biomass (10, 16, 25). We did not observe a significant difference (P > 0.1; Student's t test) in swarming between the M. xanthus wild type and ΔllpM mutant on either Gram-positive M. luteus or Gram-negative E. coli (Fig. 4D; three biological replicates). Control experiments on CYE plates demonstrate that motility is not affected by the ΔllpM mutation. Furthermore, both strains swarmed a significantly larger distance on nutrient-rich CYE agar compared to nutrient-free TPM agar (P < 0.005; Student's t test), demonstrating that motility under the experimental conditions is nutrient dependent. Prey lysis was also indistinguishable for both the M. xanthus wild type and ΔllpM mutant when observed on a population level in a qualitative assay (Fig. S6). This indicates that the overall predation performance of M. xanthus is not significantly affected by loss of LlpM (Fig. 4D).
In summary, our experiments show that LlpM fulfills the criteria for a predation factor: LlpM appears to be a secreted GH19 family protein, which is able to lyse bacteria in a lysozyme-like manner in vivo and in vitro. However, LlpM is not essential for prey cell lysis, highlighting the multifactorial bacteriolytic activity of the M. xanthus secretome. The role of the LlpM lectin domain remains to be investigated.
Activity of M. xanthus OMVs against different prey species.
The experiments described in Fig. 1 and 2 show that lysis of Gram-positive prey, like M. luteus or B. subtilis, can be achieved by isolated secreted proteins and independently of the secreting M. xanthus cells. However, Gram-negative preys E. coli and A. tumefaciens were not affected by this protein fraction. This indicates that additional mechanisms are important for specific prey cell killing. Previous studies have shown that outer membrane vesicles (OMVs) isolated from M. xanthus reduce the survival of E. coli, and have suggested a causative role in prey cell killing (30, 33). OMVs are secreted into the extracellular space or can remain attached to M. xanthus cells in long chains (30). We used ultracentrifugation to enrich OMVs from culture supernatant of vegetatively growing M. xanthus and verified the presence of OMVs by a protease protection assay (Fig. S7). To analyze their bacteriolytic properties, OMVs were spotted at a protein concentration of 0.1 µg/µl to diluted suspensions of M. luteus, B. subtilis, E. coli, and A. tumefaciens on agar plates, similar to the experiment shown in Fig. 1B. The OMV preparation did not affect overnight growth of Gram-positive M. luteus and B. subtilis (Fig. 5A; three biological replicates), and also for Gram-negative A. tumefaciens, we did not observe differences in colony formation between OMV treatment and buffer control. In contrast, treatment with OMVs abolished colony formation of Gram-negative E. coli. However, when we microscopically inspected E. coli cells after 3 h and 18 h of incubation with OMVs, we detected no signs of cell lysis (>150 cells analyzed in three biological replicates) (Fig. 5B). This indicates that the OMV preparation has a growth-inhibiting, but no lytic, effect on E. coli.
FIG 5.
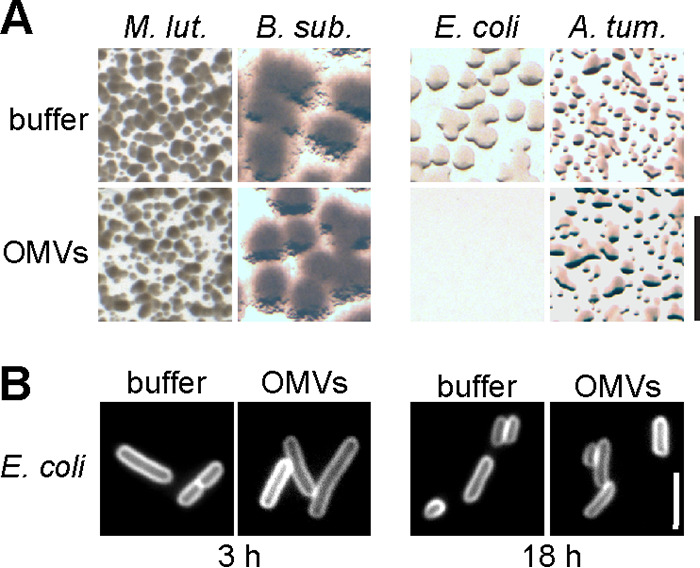
Activity of M. xanthus outer membrane vesicles (OMVs). (A) OMVs were enriched from cell-free supernatant of a M. xanthus culture by ultracentrifugation. Samples (0.1 µg/µl protein concentration) were added to prey bacteria spotted on agar plates as indicated, similar to Fig. 1B except that a 1:100 dilution of the prey cell suspension was used. After incubation overnight, bacterial spots were screened for bacterial growth within the area of sample application. Colony formation by E. coli was suppressed. Images represent three biological replicates. Scale bar, 1 mm. (B) E. coli cells incubated with M. xanthus OMVs for 3 h or 18 h do not alter their appearance compared to the buffer control. Shown are cells labeled with fluorescent membrane stain FM4-64. Images represent three biological replicates with >150 cells analyzed. Scale bar, 5 µm.
M. xanthus cells kill Gram-positive and Gram-negative prey cells in a localized, cell contact-dependent manner.
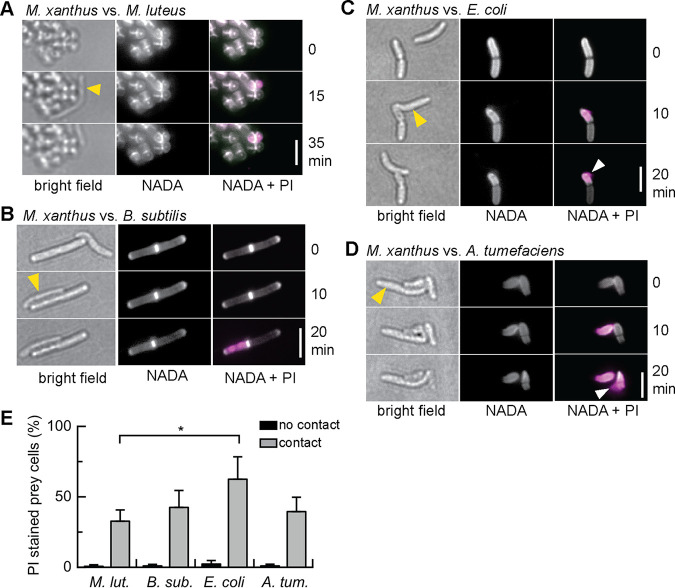
Secreted proteins and OMVs did not cause lysis of E. coli, but McBride and Zusman reported that individual M. xanthus cells are able to lyse E. coli cells in their immediate proximity (6). We thus followed predation on the level of individual cells using live-cell microscopy. To analyze how the killing mechanism used by individual M. xanthus cells might relate to secreted bacteriolytic proteins and OMVs, we compared the predation behavior of individual M. xanthus cells toward the prey organisms M. luteus, B. subtilis, E. coli, and A. tumefaciens. A mixture of growing M. xanthus cells and prey bacteria was observed on agarose pads for 1 h using light microscopy (Fig. 6). Individual M. xanthus cells approached prey cells and often remained in their immediate proximity for 10 to 30 min. In bright-field observation, the lysis of Gram-negative species E. coli and A. tumefaciens becomes apparent after cell contact with M. xanthus (black triangles), while Gram-positive species were not visibly affected (Fig. 6). To determine the viability of prey cells and analyze the effects on the prey cell envelope after contact with M. xanthus, we applied the fluorescent dyes PI and NADA to report cell death and stain the peptidoglycan layer, respectively, as described above for Fig. 2. We observed that individual cells of M. xanthus are able to induce cell death of all prey species in a cell contact-dependent manner, on a similar timescale, and with comparable efficiency (Fig. 7A to D): M. xanthus cells impaired the membrane integrity of prey cells in immediate proximity within 5 to 10 min of cell contact, as seen by PI staining.
FIG 6.
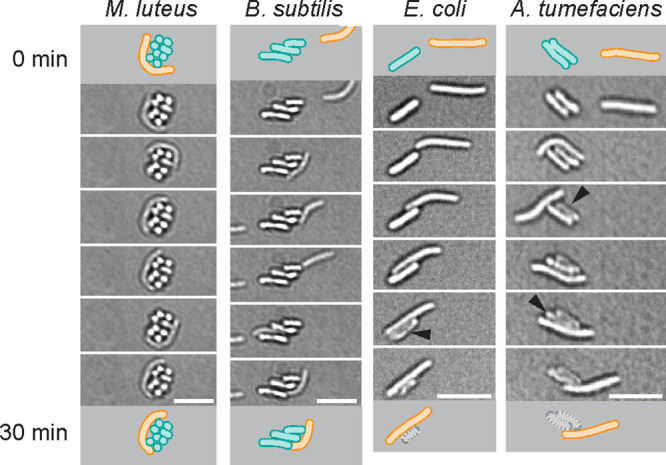
M. xanthus cells establish close contact to prey. Cell suspensions of M. xanthus were mixed with different prey species, as indicated, applied to agarose pads, and observed for 1 h in bright-field mode. Cartoons depict the position of a M. xanthus cell (orange) and prey cells (cyan) at the beginning and end of a 30-min interval. Gram-negative E. coli and A. tumefaciens visibly lyse after cell contact with M. xanthus, while Gram-positive M. luteus and B. subtilis appear intact. Scale bars, 5 µm.
FIG 7.
Prey killing by individual M. xanthus cells. Cell suspensions of M. xanthus were mixed with M. luteus (A), B. subtilis (B), E. coli (C), or A. tumefaciens (D) cells and observed for 1 h. NADA-green fluorescence (white) represents the peptidoglycan layer; PI fluorescence (magenta) represents the DNA of dead cells. Yellow arrows in bright-field image point to the M. xanthus cell. White arrows point to DNA leaking from Gram-negative prey. Scale bars, 5 µm. (E) Percentage of prey cells that show a PI signal after direct contact with a M. xanthus cell within 1 h (gray) or without contact with M. xanthus (black). Data and standard deviations represent three independent experiments and >200 cell contacts analyzed. *, P < 0.05; see Table S3 in the supplemental material for statistical analysis.
Notably, not every contact between M. xanthus and prey resulted in prey killing, but we observed that, on average, 32.7% of M. luteus, 42.5% of B. subtilis, 62.5% of E. coli, and 39.5% of A. tumefaciens cells, respectively, stained “dead” after contact with M. xanthus (Fig. 7E; >200 cell contacts analyzed per strain in at least three biological replicates). Statistical analysis showed that significantly more E. coli cells than M. luteus cells were killed by M. xanthus (P < 0.05); however, the observed differences between killing of E. coli, B. subtilis, and A. tumefaciens were not significant (Table S3). In parallel to our study, Zhang et al. reported that this “solitary” killing by M. xanthus causes E. coli cell lysis within minutes and with similar efficiency (47).
While the overall rate of prey killing was similar between the four prey species, the effects caused by contact with M. xanthus were still different for Gram-negative and Gram-positive prey. Gram-negative species E. coli and A. tumefaciens visibly deflated, and DNA leaked from cells (Fig. 7C and D, white arrows). In contrast, Gram-positive species M. luteus and B. subtilis retained their cell shape, and PI-labeled DNA remained within the cell (Fig. 7A and B). Notably, NADA staining revealed that the peptidoglycan layer was not entirely degraded after contact-dependent killing, both for Gram-negative and Gram-positive prey (Fig. 7A to D).
DISCUSSION
Although M. xanthus predation has been recognized long ago, the molecular mechanisms that mediate prey recognition, as well as prey killing and lysis, remain largely unidentified (4). Moreover, the broad prey spectrum indicates that M. xanthus must possess a versatile set of predation mechanisms to access nutrients from different bacterial species and fungi (2, 16, 17). So far, two secondary metabolites, unidentified secreted lytic proteins and OMVs, have been shown to contribute to the predation process (24, 25, 27, 28, 32, 33), and the cell contact-dependent killing of E. coli has been described (6, 47). In this study, we compared the bacteriolytic properties of different protein fractions of M. xanthus against M. luteus, B. subtilis, E. coli, and A. tumefaciens, aiming to identify active proteins and to infer possible mechanistic differences in the predation of Gram-positive and Gram-negative prey.
LlpM is a secreted bacteriolytic protein but not an essential predation factor.
We observed that a preparation of M. xanthus-secreted proteins induced lysis and peptidoglycan degradation of live Gram-positive bacteria (Fig. 1 and 2) and caused clearing of autoclaved Gram-positive, as well as Gram-negative, bacteria in zymograms (Fig. 3). These results are reminiscent of earlier studies that reported proteins with glucosaminidase and amidase activity enriched from M. xanthus culture supernatant (27, 28). In order to identify these enzymes, we performed mass spectrometry, and among the 72 M. xanthus-secreted proteins that we detected, we identified one protein with peptidoglycan hydrolase activity. MXAN_4534, which we termed LlpM for lectin/lysozyme-like protein ofMyxococcus, is a family 19 glycoside hydrolase with annotated lysozyme-like or chitinase activity (Fig. 3C and D). Our experiments with either bacteria or chitin as the substrate (Fig. 3E and Fig. 4) indicate that, in the case of LlpM, lysozyme-like activity is dominant over chitinase activity, in contrast to other GH19 enzymes where chitinase activity was described to be predominant (44, 45). However, when we tested whether an ΔllpM deletion affects the bacteriolytic activity of M. xanthus or the ability to swarm using prey bacteria as a nutrient source, we found no difference between the mutant and wild-type strains (Fig. 4C and D). Overall, this suggests that secreted LlpM might play a role in the degradation of prey biomass, but its function is apparently redundant to other secreted enzymes with lytic activity. The M. xanthus genome encodes 24 proteins (listed in Table S8 in the supplemental material) that are predicted to cleave peptidoglycan based on their homology to either lysozyme or N-acetylmuramoyl-l-alanine amidase; however, none, except for LlpM, were detected in our or previous M. xanthus secretome studies (35). Possibly, additional cell wall lytic enzymes are secreted by M. xanthus, which were not identified because their activity is not represented by their functional annotation. Indeed, we detected a high proportion of proteins with unknown function in our analysis (32 out of 72 proteins; Table S1). Moreover, M. xanthus secretes several proteases and peptidases that degrade proteins into amino acids, and it is likely that enzymes with different functions act synergistically in the degradation of bacterial biomass.
Cell-associated and cell-independent mechanisms differentially contribute to predation of Gram-positive and Gram-negative prey bacteria.
To further dissect the contribution of cell-associated versus cell-independent mechanisms to predation, we compared the bacteriolytic properties of M. xanthus intact cells as well as of different isolated protein fractions.
Based on the analysis of four species (M. luteus, B. subtilis, E. coli, and A. tumefaciens), our observations reveal mechanistic differences in the killing and degradation of Gram-positive and Gram-negative prey bacteria. Using live-cell microscopy, we find that individual cells of M. xanthus induce cell death to both Gram-negative and Gram-positive test strains within minutes in a cell contact-dependent manner (Fig. 7). The peptidoglycan layer remains intact overall during cell contact-dependent killing, which may indicate a localized penetration of the cell envelope. For Gram-negative prey, this results in leakage of DNA (and the cytoplasm) from the cell, which provides direct access to cytoplasmic proteins and their amino acids as nutrients.
In contrast, Gram-positive prey with a thick peptidoglycan layer retain their cell shape, and DNA does not leak from cells upon contact with an individual M. xanthus cell. Here, additional secreted lytic proteins appear to be required to break open the cell envelope, which might only be provided by a quorum of M. xanthus cells at sufficient concentration. The potential advantages of large cell groups secreting degradative enzymes for predation have been discussed repeatedly (2, 5, 18–20), based on cell density-dependent growth rates that have been observed for M. xanthus under certain conditions (48). Taking our analysis of four prey species into account, we would expect that a putative effect of M. xanthus cell density is more pronounced while preying on Gram-positive bacteria compared to Gram negative. However, we recognize that for a general understanding of putative group-specific predation mechanisms, additional prey species will need to be tested.
Although M. xanthus-secreted lytic proteins have the capacity to lyse autoclaved Gram-positive and Gram-negative bacteria in vitro (Fig. 3A), they have no effect on live Gram-negative bacteria (Fig. 1 and 2), presumably because they cannot overcome the outer membrane barrier. Potentially, lytic factors packed into OMVs (30, 31, 33, 49), which then fuse with the outer membrane of a target cell and release their contents, could mediate the killing and lysis of Gram-negative prey. Indeed, several putative lytic proteins, such as LlpM as well as antibiotics, have been detected in OMVs (32, 34). We found that treatment with M. xanthus OMVs impairs growth of E. coli growing on agar plates (Fig. 5A), in agreement with previous reports (30, 33). However, we did not observe that incubation with OMVs induced the lysis of intact bacteria (Fig. 5B), which may indicate a growth-inhibiting, rather than lytic activity, of our OMV preparation. It has been shown that starving cells produce higher levels of OMVs (33). Our experiments were performed under conditions that promote vegetative growth but still show very efficient prey killing by intact M. xanthus and by secreted proteins. Nevertheless, it is possible that the contribution of OMVs to predation is more pronounced under starvation conditions.
It has been reported that individual M. xanthus cells mediate the cell contact-dependent killing of E. coli (6, 47), and we find that this is true for additional Gram-negative, as well as for Gram-positive, prey species (Fig. 6 and 7): only prey cells in immediate proximity to an M. xanthus cell are killed, while nearby cells remain intact. In principle, this effect could be mediated by OMVs if they were secreted into the surrounding extracellular space and stay local (49). Based on our experiments, we currently presume that OMVs are not the main causative agent of cell contact-dependent prey killing. We did not detect OMV-induced, cell-independent lysis of E. coli, and isolated OMVs did not affect survival of A. tumefaciens, B. subtilis, or M. luteus (Fig. 5), whereas individual M. xanthus cells killed these prey species on the same timescale and with comparable efficiency as E. coli (Fig. 7). Instead, we hypothesize that an additional cell-associated mechanism exists to target predation factors onto or across the cell envelope of prey. The delivery of protein effectors into eukaryotic or prokaryotic cells is, in many bacteria, mediated by protein secretion systems of types II, III, IV, and VI (50, 51). Besides components for type II/III systems, the M. xanthus genome encodes a type VI secretion system (52). The investigation of these secretion systems and the identification of their substrates might provide valuable information on M. xanthus predation.
While our observations highlight the important contribution of individual M. xanthus cells to prey cell killing, they do not reveal whether Gram-negative and Gram-positive cells are killed by the same cell contact-dependent mechanism or whether independent systems exist. The identification of predation effectors and subsequent mutant analysis will be instrumental in addressing this question. Finally, additional characteristics of prey bacteria, for example, biofilm formation, cell differentiation, or antibiotic production, are certainly important factors that further modulate the predation behavior of M. xanthus. Integrating the observations made for individual cells in laboratory settings into the investigation of reciprocal predator-prey relationships in the environment will be a challenging but critical aspect in future studies on bacterial predation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial strains used in this study are listed in Table 1. M. xanthus strains were grown in CYE medium (10 mM MOPS, pH 7.6, 1% Casitone, 0.5% yeast extract, and 8 mM MgSO4) or CF minimal medium (10 mM morpholinepropanesulfonic acid [MOPS], pH 7.6, 8 mM MgSO4, 1 mM KH2PO4, 0.015% Casitone, 0.2% sodium citrate, 0.1% sodium pyruvate, 0.02% ammonium sulfate) at 30°C or 32°C, and 200 rpm. A. tumefaciens, E. coli, B. subtilis, and M. luteus strains were cultivated in LB medium at 30°C or 37°C. S. coelicolor was grown in ISP2 medium (22 mM glucose, 1% malt extract, and 0.4% yeast extract) at 30°C and 200 rpm. 1.5% agar was added to liquid media for the preparation of agar plates. We added 100 µg/ml kanamycin where appropriate. To test bacterial growth on chitin, colloidal chitin was prepared from practical-grade shrimp shells according to a previously published protocol (53), autoclaved, and included into liquid media or agar plates at a concentration of 2% (wt/vol).
TABLE 1.
Bacterial strains used in this study
| Organism | Strain | Reference no. or source |
|---|---|---|
| M. xanthus DK1622 | Wild type | 58 |
| M. xanthus CKB64 | ΔllpM (MXAN_4534) deletion mutant | This study |
| E. coli DH5a | For plasmid construction | Thermo Fisher |
| E. coli BL21(DE3) | For heterologous protein expression | New England Biolabs |
| E. coli SHuffle | For heterologous protein expression | New England Biolabs |
| Agrobacterium tumefaciens C58 | 59 | |
| Bacillus subtilis 168 | Bacillus Genetic Stock Center | |
| Micrococcus luteus DSM 20030 | DSMZ (German Collection of Microorganisms and Cell Cultures) | |
| Streptomyces coelicolor DSM 40233 | DSMZ (German Collection of Microorganisms and Cell Cultures) |
Preparation of M. xanthus protein fractions.
M. xanthus strains were inoculated to 15 ml CYE medium and incubated at 30°C and 200 rpm. The next day, cultures were diluted 1:100 to 50 to 400 ml CYE medium and incubated for 2 days, resulting in an optical density at 600 nm (OD600) of 1.3 to 1.8. To enrich membrane proteins, cells of 50 ml culture were harvested by centrifugation, washed with phosphate-buffered saline (PBS) buffer, and subjected to two passages of French pressing. Cell debris was removed by centrifugation 2 times for 30 min at 13,000 rpm and 4°C. The cleared cell extract was then subjected to ultracentrifugation for 1 h at 38,500 rpm (Ti70.1 rotor; Beckman Coulter), and the resulting membrane pellet was resuspended in 200 µl PBS buffer.
For preparation of secreted proteins, cell-free culture supernatants were obtained by centrifugation of 200 ml culture 2 times for 15 min at 8,000 rpm and sequential filtration using 0.45-µm and 0.22-µm pore size filters. Ammonium sulfate was slowly added to supernatants to a saturation of 40%, and the solution was stirred at 4°C for 1 h to allow proteins to precipitate. Secreted proteins were pelleted by centrifugation at 13,000 rpm for 20 min and 4°C and dissolved in approximately 400 µl PBS buffer for further analysis. OMVs were pelleted from cell-free supernatant of 400 ml CYE culture by sequential ultracentrifugation for 1 h at 38,500 rpm, 4°C (Ti70.1 rotor). OMV pellets were resuspended in 200 µl PBS buffer.
Testing for bacteriolytic activity on agar plates.
Prey species M. luteus, B. subtilis, E. coli, and A. tumefaciens were grown in LB medium overnight. Cells were washed twice with PBS buffer, and the optical density was adjusted to an OD600 of 0.08. Ten microliters of cell suspensions were spotted on agar plates with 0.5× CTT medium (0.5% Casitone, 10 mM Tris-HCl, pH 7.6, 1 mM KH2PO4, and 8 mM MgSO4) (54). Dried spots were overlaid with 2 µl of a sample of M. xanthus cells or protein fractions, and plates were incubated overnight at 30°C and documented with a flatbed scanner. For analysis of M. xanthus OMVs, the cell suspensions were used in 1:100 dilution, and plates were documented using a stereomicroscope.
Zymography.
To screen for bacteriolytic activity of secreted proteins, we adapted the following zymography protocol from reference 55. Twelve percent acrylamide gels and running buffer contained only 0.02% SDS, and autoclaved bacterial cells were included into the separating gel. We used a final concentration of 40 mg/ml for cells of A. tumefaciens, E. coli, and M. luteus, but reduced the amount to 10 mg/ml for cells of B. subtilis, which showed intense staining and was difficult to destain at a higher concentration. Protein samples were prepared with sample buffer without reducing agents (62.5 mM Tris-HCl, pH 7.6, 4% SDS, 25% glycerol, and 0.01% bromophenol blue) and were not boiled before loading. After electrophoresis, gels were rinsed in double-distilled water (ddH2O) and transferred to renaturation buffer (20 mM sodium phosphate, pH 7, 10 mM MgCl2, and 0.1% Triton X-100). Gels were incubated overnight at 30°C while renaturation buffer was exchanged once. After rinsing in ddH2O, gels were stained in 0.1% methylene blue and 0.01% KOH for 3 h at room temperature and destained in ddH2O until clearing zones were visible.
Photometric assay for bacteriolytic activity.
Lyophilized cells of M. luteus (Sigma; catalog no. M3770) were resuspended in PBS buffer to an A450 of 0.8. Half a microgram of hen egg-white lysozyme (Sigma; catalog no. L6876), 10 µg of secreted proteins prepared from M. xanthus culture supernatant (see above), or 10 µg peptides of CYE medium alone was added to 1 ml of this cell suspension. A450 was recorded every 1 min for 15 min, and ΔA450/min was calculated by linear regression.
Mass spectrometry analysis.
Samples of secreted proteins were separated in parallel on a zymogram (see above) and on an identical polyacrylamide gel without bacterial substrate. The zymogram was used as an orientation for the migration of proteins with bacteriolytic activity, and gel spots were manually excised from the Coomassie-stained polyacrylamide gel. Gel spots were rinsed 2 times for 15 min at 37°C with washing solution (20 mM ammonium bicarbonate and 30% acetonitrile), reduced with 10 mM dithiothreitol (DTT) (45 min, 60°C), and alkylated with 50 mM iodoacetamide (30 min, 25°C). Proteins were treated with trypsin overnight at 37°C and eluted from gel spots with 0.1% trifluoroacetic acid (TFA). Proteins were identified using a Synapt G2-S high-definition mass spectrometer equipped with a lock spray source for electrospray ionization and a time of flight (TOF) detector (Waters, USA) according to a previously published protocol (56).
Strain construction.
Plasmids and oligonucleotides used in this study are listed in Tables 2 and 3, respectively. For the heterologous overexpression of MXAN_4534 (LlpM) protein in E. coli strains, the coding sequence was amplified from M. xanthus DK1622 chromosomal DNA using oligonucleotides BP32 and BP44 (wild-type protein) or BP32 and BP42 (variant Δ1-21), and the fragments were cloned to NcoI and EcoRI sites of pET28a. The point mutation E260Q was introduced by quick-change PCR using oligonucleotides BP77 and BP78. The resulting plasmids were used to transform E. coli strains DH5α, BL21, or SHuffle. To delete the llpM gene from the M. xanthus chromosome, 1,000-bp regions upstream and downstream the coding region were amplified using primers P1-4534 and P2-4534 or P3-4534 and P4-4534, respectively, fused by PCR, and cloned to the XbaI and BamHI sites of pBJ114 (57). The resulting plasmid pCKB58 was used to transform M. xanthus DK1622 wild type. Transformants were selected in two steps for Kans and Galr, and deletion of the llpM gene was confirmed by PCR.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Source or reference no. |
|---|---|---|
| pET28a | For protein expression, Kmr | Novagene |
| pCKB42 | pET28a-llpM | This study |
| pCKB22 | pET28a-llpM (Δ1-21) | This study |
| pCKB80 | pET28a-llpM (E260Q) | This study |
| pBJ114 | galK Kmr | 57 |
| pCKB58 | Flanking regions of MXAN_4534 (llpM) in pBJ114; for markerless deletion of ORFa | This study |
ORF, open reading frame.
TABLE 3.
Oligonucleotides used in this study
| Oligonucleotide | Sequencea |
|---|---|
| BP44 | 5′-CGCCATGGCCGGCAAGTTCGTGTCACGC-3′ |
| BP32 | 5′-GCGAATTCTCGCAGCCCAGGTTGCCGCC-3′ |
| BP42 | 5′-CGCCATGGCCCTGGGAGGCTGTGGC-3′ |
| BP77 | 5′-TTCGCCAACACGGCCCATCAGACGGGCAACTACGTGTAC-3′ |
| BP78 | 5′-GTACACGTAGTTGCCCGTCTGATGGGCCGTGTTGGCGAA-3′ |
| BP81 | 5′-GGCAACTACGTGTACGTGGAGCAGATCAATCGCGGTGAC-3′ |
| BP82 | 5′-GTCACCGCGATTGATCTGCTCCACGTACACGTAGTTGCC-3′ |
| P1-4534 | 5′-GCGGATCCCTGCTCATCGACCTGGCGAC-3′ |
| P2-4534 | 5′-GCCAGGGCTCACGCCGAGCATCAGCAGGGCCAGACTGCGTGA-3′ |
| P3-4534 | 5′-ATGCTCGGCGTGAGCCCTGGC-3′ |
| P4-4534 | 5′-GCTCTAGAGTGTTCTTCGCGGATTGCGCC-3′ |
Restrictions sites are underlined.
Overexpression and purification of LlpM.
E. coli BL21 containing pCKB42, pCKB22, or pCKB80 was grown in LB medium at 37°C and 200 rpm. Protein expression was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) when an OD600 of 0.7 was reached. Samples for microscopy were collected 3 h after induction and stained with FM4-64 to determine cell shape (see below). LlpM-His was overexpressed in E. coli strain SHuffle (New England Biolabs) containing plasmid pCKB42 at 30°C and 200 rpm for 12 h and enriched from cleared soluble protein extract using a nickel-nitrilotriacetic acid (Ni-NTA) column (1 ml HiTrap Sepharose; GE Healthcare) and buffer containing 50 mM Na-phosphate, pH 7.6, 150 mM NaCl, and 20 to 500 mM imidazole.
Lawn predation assay.
To compare predation efficiency of M. xanthus strains, we tested swarming on buffered agar plates containing a lawn of prey bacteria as the only nutrient source (10, 16). M. xanthus strains were grown for 2 days in 15 ml CYE medium at 30°C, and prey bacteria were incubated in 15 ml LB medium at 30°C overnight. Bacteria were harvested by centrifugation, and the cell pellet was washed twice with TPM buffer (10 mM Tris-HCl, pH 7.6, 8 mM MgSO4, and 1 mM KH2PO4). The density of M. xanthus strains was adjusted to an OD600 of 6, and that of prey bacteria was adjusted to an OD600 of 12. We plated 0.5 ml of each prey cell suspension to a TPM agar plate (TPM buffer plus 1.5% agar), which was incubated at room temperature until the surface had dried. Ten microliters of a M. xanthus cell suspension were spotted in the center of a prey-covered TPM plate. CYE and TPM plates without prey were used as control. Plates were incubated for 3 days at 32°C, and the swarm radius of M. xanthus was determined as the mean of 4 measurements.
Light microscopy.
To observe the effect of M. xanthus-secreted proteins, prey bacteria were cultivated overnight in LB medium at 30°C. One milliliter culture was harvested by centrifugation, and the cell pellet was washed and resuspended in 1 ml PBS buffer. Proteins precipitated from cell-free M. xanthus culture supernatant (see above) were added to a 10-µl aliquot of cell suspension at a final concentration of 1 µg/µl. To analyze the effect of M. xanthus OMVs on E. coli, 5 µl of OMV preparation (0.5 µg) was added to 5 µl of E. coli cell suspension and incubated at 30°C. Samples were spotted to object slides covered with agarose pads containing CF minimal medium, covered with a cover glass, and documented with a 100× objective starting 2 min after the addition of secreted proteins or OMVs.
To observe predation behavior on the single-cell level, 1 ml of growing cultures of M. xanthus or prey bacteria were harvested by centrifugation, and the cell pellets were resuspended in PBS buffer. M. xanthus and prey cells were mixed at a ratio of approximately 1:10, applied to object slides with agarose pads, and covered with a cover glass. Slides were incubated for 20 min prior to observation to allow M. xanthus cells to attach to the surface and initiate gliding motility. Cell behavior was documented for 60 min with a 60× objective.
Lipophilic dye FM4-64 was added at a final concentration of 0.2 ng/ml to stain cell membranes. The nucleic acid stains SYTOX green (Thermo Fisher) and propidium iodide (PI; Merck) were used at final concentrations of 5 µM and 30 µM, respectively. For staining of peptidoglycan with the fluorescent d-amino acid NADA-green (Tocris Bioscience), prey bacteria were grown to early exponential phase in LB medium, and NADA was added to a final concentration of 0.5 mM for E. coli and B. subtilis. For M. luteus and A. tumefaciens, 0.25 mM was used. Cells were grown for 3 to 4 h, harvested by centrifugation, washed two times with PBS buffer to remove excess NADA stain, and used for microscopy.
Light microscopy was carried out on an Olympus BX51 equipped with a 100× objective, charge-coupled-device (CCD) camera (Retiga 3; QImaging) and LED light source (Sola 365; Lumencor). To detect red fluorescence of FM4-46 or PI, a filter set for 530 to 550 nm excitation and 575 to 625 nm emission was used. Green NADA or SYTOX fluorescence was detected with a filter set for 450 to 490 nm excitation and 500 to 550 nm emission.
Supplementary Material
ACKNOWLEDGMENTS
Mass spectrometry was kindly performed by Sina Schäkermann using infrastructure financed by the State of North Rhine-Westfalia (Großgeräte der Länder, granted to Julia Bandow [RUB]). We thank Meriyem Aktas and Bernd Masepohl for discussions. We gratefully acknowledge Franz Narberhaus (RUB) for critical reading of the manuscript and for ongoing support of our research.
This work was funded by a MERCUR start-up grant from the Mercator-Foundation (An-2016-0033) and a research grant by the Deutsche Forschungsgemeinschaft (Ka 3361/3-1) to C.K.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Mercier R, Mignot T. 2016. Regulations governing the multicellular lifestyle of Myxococcus xanthus. Curr Opin Microbiol 34:104–110. doi: 10.1016/j.mib.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz-Dorado J, Marcos-Torres FJ, García-Bravo E, Moraleda-Muñoz A, Pérez J. 2016. Myxobacteria: moving, killing, feeding, and surviving together. Front Microbiol 7:781. doi: 10.3389/fmicb.2016.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jurkevitch E. 2007. Predatory prokaryotes - biology, ecology and evolution. Springer, Heidelberg, Germany. [Google Scholar]
- 4.Thiery S, Kaimer C. 2020. The predation strategy of Myxococcus xanthus. Front Microbiol 11:2. doi: 10.3389/fmicb.2020.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez J, Moraleda-Muñoz A, Marcos-Torres FJ, Muñoz-Dorado J. 2016. Bacterial predation: 75 years and counting! Environ Microbiol 18:766–779. doi: 10.1111/1462-2920.13171. [DOI] [PubMed] [Google Scholar]
- 6.McBride MJ, Zusman DR. 1996. Behavioral analysis of single cells of Myxococcus xanthus in response to prey cells of Escherichia coli. FEMS Microbiol Lett 137:227–231. doi: 10.1111/j.1574-6968.1996.tb08110.x. [DOI] [PubMed] [Google Scholar]
- 7.Berleman JE, Scott J, Chumley T, Kirby JR. 2008. Predataxis behavior in Myxococcus xanthus. Proc Natl Acad Sci U S A 105:17127–17132. doi: 10.1073/pnas.0804387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berleman JE, Chumley T, Cheung P, Kirby JR. 2006. Rippling is a predatory behavior in Myxococcus xanthus. J Bacteriol 188:5888–5895. doi: 10.1128/JB.00559-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Vaksman Z, Litwin DB, Shi P, Kaplan HB, Igoshin OA. 2012. The mechanistic basis of Myxococcus xanthus rippling behavior and its physiological role during predation. PLoS Comput Biol 8:e1002715. doi: 10.1371/journal.pcbi.1002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham VD, Shebelut CW, Diodati ME, Bull CT, Singer M. 2005. Mutations affecting predation ability of the soil bacterium Myxococcus xanthus. Microbiology (Reading) 151:1865–1874. doi: 10.1099/mic.0.27824-0. [DOI] [PubMed] [Google Scholar]
- 11.Müller S, Strack SN, Ryan SE, Kearns DB, Kirby JR. 2015. Predation by Myxococcus xanthus induces Bacillus subtilis to form spore-filled megastructures. Appl Environ Microbiol 81:203–210. doi: 10.1128/AEM.02448-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laloux G. 2019. Shedding light on the on the cell biology of the predatory bacterium Bdellovibrio bacteriovorus. Front Microbiol 10:3136. doi: 10.3389/fmicb.2019.03136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sockett RE. 2009. Predatory lifestyle of Bdellovibrio bacteriovorus. Annu Rev Microbiol 63:523–539. doi: 10.1146/annurev.micro.091208.073346. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg E, Varon M. 1984. Antibiotics and lytic enzymes, p 109–125. In Rosenberg E (ed), Myxobacteria - development and cell interactions. Springer Verlag, New York, NY. [Google Scholar]
- 15.Müller S, Strack SN, Hoefler BC, Straight PD, Kearns DB, Kirby JR. 2014. Bacillaene and sporulation protect Bacillus subtilis from predation by Myxococcus xanthus. Appl Environ Microbiol 80:5603–5610. doi: 10.1128/AEM.01621-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan AD, MacLean RC, Hillesland KL, Velicer GJ. 2010. Comparative analysis of Myxococcus predation on soil bacteria. Appl Environ Microbiol 76:6920–6927. doi: 10.1128/AEM.00414-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendes-Soares H, Velicer GJ. 2013. Decomposing predation: testing for parameters that correlate with predatory performance by a social bacterium. Microb Ecol 65:415–423. doi: 10.1007/s00248-012-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keane R, Berleman J. 2016. The predatory life cycle of Myxococcus xanthus. Microbiology (Reading) 162:1–11. doi: 10.1099/mic.0.000208. [DOI] [PubMed] [Google Scholar]
- 19.Marshall RC, Whitworth DE. 2019. Is “wolf-pack” predation by antimicrobial bacteria cooperative? Cell behaviour and predatory mechanisms indicate profound selfishness, even when working alongside kin. Bioessays 41:e1800247. doi: 10.1002/bies.201800247. [DOI] [PubMed] [Google Scholar]
- 20.Berleman JE, Kirby JR. 2009. Deciphering the hunting strategy of a bacterial wolfpack. FEMS Microbiol Rev 33:942–957. doi: 10.1111/j.1574-6976.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman BS, Nierman WC, Kaiser D, Slater SC, Durkin AS, Eisen JA, Eisen J, Ronning CM, Barbazuk WB, Blanchard M, Field C, Halling C, Hinkle G, Iartchuk O, Kim HS, Mackenzie C, Madupu R, Miller N, Shvartsbeyn A, Sullivan SA, Vaudin M, Wiegand R, Kaplan HB. 2006. Evolution of sensory complexity recorded in a myxobacterial genome. Proc Natl Acad Sci U S A 103:15200–15205. doi: 10.1073/pnas.0607335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korp J, Vela Gurovic MS, Nett M. 2016. Antibiotics from predatory bacteria. Beilstein J Org Chem 12:594–607. doi: 10.3762/bjoc.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Findlay BL. 2016. The chemical ecology of predatory soil bacteria. ACS Chem Biol 11:1502–1510. doi: 10.1021/acschembio.6b00176. [DOI] [PubMed] [Google Scholar]
- 24.Müller S, Strack SN, Ryan SE, Shawgo M, Walling A, Harris S, Chambers C, Boddicker J, Kirby JR. 2016. Identification of functions affecting predator-prey interactions between Myxococcus xanthus and Bacillus subtilis. J Bacteriol 198:3335–3344. doi: 10.1128/JB.00575-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y, Wei X, Ebright R, Wall D. 2011. Antibiotic production by myxobacteria plays a role in predation. J Bacteriol 193:4626–4633. doi: 10.1128/JB.05052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao Y, Gerth K, Müller R, Wall D. 2012. Myxobacterium-produced antibiotic TA (myxovirescin) inhibits type II signal peptidase. Antimicrob Agents Chemother 56:2014–2021. doi: 10.1128/AAC.06148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudo S, Dworkin M. 1972. Bacteriolytic enzymes produced by Myxococcus xanthus. J Bacteriol 110:236–245. doi: 10.1128/JB.110.1.236-245.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart BA, Zahler SA. 1966. Lytic enzyme produced by Myxococcus xanthus. J Bacteriol 92:1632–1637. doi: 10.1128/JB.92.6.1632-1637.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haskå G. 1981. Activity of bacteriolytic enzymes adsorbed to clays. Microb Ecol 7:331–341. doi: 10.1007/BF02341428. [DOI] [PubMed] [Google Scholar]
- 30.Remis JP, Wei D, Gorur A, Zemla M, Haraga J, Allen S, Witkowska HE, Costerton JW, Berleman JE, Auer M. 2014. Bacterial social networks: structure and composition of Myxococcus xanthus outer membrane vesicle chains. Environ Microbiol 16:598–610. doi: 10.1111/1462-2920.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palsdottir H, Remis JP, Schaudinn C, O'Toole E, Lux R, Shi W, McDonald KL, Costerton JW, Auer M. 2009. Three-dimensional macromolecular organization of cryofixed Myxococcus xanthus biofilms as revealed by electron microscopic tomography. J Bacteriol 191:2077–2082. doi: 10.1128/JB.01333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berleman JE, Allen S, Danielewicz MA, Remis JP, Gorur A, Cunha J, Hadi MZ, Zusman DR, Northen TR, Witkowska HE, Auer M. 2014. The lethal cargo of Myxococcus xanthus outer membrane vesicles. Front Microbiol 5:474. doi: 10.3389/fmicb.2014.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans AG, Davey HM, Cookson A, Currinn H, Cooke-Fox G, Stanczyk PJ, Whitworth DE. 2012. Predatory activity of Myxococcus xanthus outer-membrane vesicles and properties of their hydrolase cargo. Microbiology (Reading) 158:2742–2752. doi: 10.1099/mic.0.060343-0. [DOI] [PubMed] [Google Scholar]
- 34.Kahnt J, Aguiluz K, Koch J, Treuner-Lange A, Konovalova A, Huntley S, Hoppert M, Søgaard-Andersen L, Hedderich R. 2010. Profiling the outer membrane proteome during growth and development of the social bacterium Myxococcus xanthus by selective biotinylation and analyses of outer membrane vesicles. J Proteome Res 9:5197–5208. doi: 10.1021/pr1004983. [DOI] [PubMed] [Google Scholar]
- 35.Whitworth DE, Slade SE, Mironas A. 2015. Composition of distinct sub-proteomes in Myxococcus xanthus: metabolic cost and amino acid availability. Amino Acids 47:2521–2531. doi: 10.1007/s00726-015-2042-x. [DOI] [PubMed] [Google Scholar]
- 36.Roth BL, Poot M, Yue ST, Millard PJ. 1997. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol 63:2421–2431. doi: 10.1128/AEM.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luther A, Urfer M, Zahn M, Müller M, Wang SY, Mondal M, Vitale A, Hartmann JB, Sharpe T, Monte FL, Kocherla H, Cline E, Pessi G, Rath P, Modaresi SM, Chiquet P, Stiegeler S, Verbree C, Remus T, Schmitt M, Kolopp C, Westwood MA, Desjonquères N, Brabet E, Hell S, LePoupon K, Vermeulen A, Jaisson R, Rithié V, Upert G, Lederer A, Zbinden P, Wach A, Moehle K, Zerbe K, Locher HH, Bernardini F, Dale GE, Eberl L, Wollscheid B, Hiller S, Robinson JA, Obrecht D. 2019. Chimeric peptidomimetic antibiotics against Gram-negative bacteria. Nature 576:452–458. doi: 10.1038/s41586-019-1665-6. [DOI] [PubMed] [Google Scholar]
- 38.Kuru E, Tekkam S, Hall E, Brun YV, Van Nieuwenhze MS. 2015. Synthesis of fluorescent D-amino acids and their use for probing peptidoglycan synthesis and bacterial growth in situ. Nat Protoc 10:33–52. doi: 10.1038/nprot.2014.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuru E, Lambert C, Rittichier J, Till R, Ducret A, Derouaux A, Gray J, Biboy J, Vollmer W, Van Nieuwenhze M, Brun YV, Sockett RE. 2017. Fluorescent D-amino-acids reveal bi-cellular cell wall modifications important for Bdellovibrio bacteriovorus predation. Nat Microbiol 2:1648–1657. doi: 10.1038/s41564-017-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lou Q, Zhu T, Hu J, Ben H, Yang J, Yu F, Liu J, Wu Y, Fischer A, Francois P, Schrenzel J, Qu D. 2011. Role of the SaeRS two-component regulatory system in Staphylococcus epidermidis autolysis and biofilm formation. BMC Microbiol 11:146. doi: 10.1186/1471-2180-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valence F, Lortal S. 1995. Zymogram and preliminary characterization of Lactobacillus helveticus autolysins. Appl Environ Microbiol 61:3391–3399. doi: 10.1128/AEM.61.9.3391-3399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lepeuple AS, Van Gemert E, Chapot-Chartier MP. 1998. Analysis of the bacteriolytic enzymes of the autolytic Lactococcus lactis subsp. cremoris strain AM2 by renaturing polyacrylamide gel electrophoresis: identification of a prophage-encoded enzyme. Appl Environ Microbiol 64:4142–4148. doi: 10.1128/AEM.64.11.4142-4148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wohlkönig A, Huet J, Looze Y, Wintjens R. 2010. Structural relationships in the lysozyme superfamily: significant evidence for glycoside hydrolase signature motifs. PLoS One 5:e15388. doi: 10.1371/journal.pone.0015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunner F, Stintzi A, Fritig B, Legrand M. 1998. Substrate specificities of tobacco chitinases. Plant J 14:225–234. doi: 10.1046/j.1365-313X.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang SL, Chang WT. 1997. Purification and characterization of two bifunctional chitinases/lysozymes extracellularly produced by Pseudomonas aeruginosa K-187 in a shrimp and crab shell powder medium. Appl Environ Microbiol 63:380–386. doi: 10.1128/AEM.63.2.380-386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoell IA, Dalhus B, Heggset EB, Aspmo SI, Eijsink VG. 2006. Crystal structure and enzymatic properties of a bacterial family 19 chitinase reveal differences from plant enzymes. FEBS J 273:4889–4900. doi: 10.1111/j.1742-4658.2006.05487.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Wang Y, Lu H, Liu Q, Wang C, Hu W, Zhao K. 2019. Dynamics of solitary predation by Myxococcus xanthus on Escherichia coli observed at the single-cell level. Appl Environ Microbiol 86:e02286-19. doi: 10.1128/AEM.02286-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg E, Keller KH, Dworkin M. 1977. Cell density-dependent growth of Myxococcus xanthus on casein. J Bacteriol 129:770–777. doi: 10.1128/JB.129.2.770-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitworth DE. 2011. Myxobacterial vesicles death at a distance? Adv Appl Microbiol 75:1–31. doi: 10.1016/B978-0-12-387046-9.00001-3. [DOI] [PubMed] [Google Scholar]
- 50.Cianciotto NP, White RC. 2017. Expanding role of type II secretion in bacterial pathogenesis and beyond. Infect Immun 85:e00014-17. doi: 10.1128/IAI.00014-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galán JE, Waksman G. 2018. Protein-injection machines in bacteria. Cell 172:1306–1318. doi: 10.1016/j.cell.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konovalova A, Petters T, Søgaard-Andersen L. 2010. Extracellular biology of Myxococcus xanthus. FEMS Microbiol Rev 34:89–106. doi: 10.1111/j.1574-6976.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- 53.Hsu SC, Lockwood JL. 1975. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol 29:422–426. doi: 10.1128/AM.29.3.422-426.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hodgkin J, Kaiser D. 1977. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A 74:2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernadsky G, Beveridge TJ, Clarke AJ. 1994. Analysis of the sodium dodecyl sulfate-stable peptidoglycan autolysins of select gram-negative pathogens by using renaturing polyacrylamide gel electrophoresis. J Bacteriol 176:5225–5232. doi: 10.1128/JB.176.17.5225-5232.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wenzel M, Patra M, Senges CH, Ott I, Stepanek JJ, Pinto A, Prochnow P, Vuong C, Langklotz S, Metzler-Nolte N, Bandow JE. 2013. Analysis of the mechanism of action of potent antibacterial hetero-tri-organometallic compounds: a structurally new class of antibiotics. ACS Chem Biol 8:1442–1450. doi: 10.1021/cb4000844. [DOI] [PubMed] [Google Scholar]
- 57.Julien B, Kaiser AD, Garza A. 2000. Spatial control of cell differentiation in Myxococcus xanthus. Proc Natl Acad Sci U S A 97:9098–9103. doi: 10.1073/pnas.97.16.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaiser D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A 76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Larebeke N, Engler G, Holsters M, Van den Elsacker S, Zaenen I, Schilperoort R, Schell J. 1974. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature 252:169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.