Abstract
Background and Aims
Lifestyle modification is the main treatment for nonalcoholic fatty liver disease (NAFLD), but remains challenging to implement. The aim of this pilot was to assess the acceptability and feasibility of a mobile-technology based lifestyle program for NAFLD patients.
Methods
We enrolled adult patients with NAFLD in a 6-month mobile-technology based program where participants received a FitBit with weekly tailored step count goals and nutritional assessments. Anthropometrics, hepatic and metabolic parameters, Fibroscan, physical function and activity, and health-related quality of life measures were obtained at enrollment and month 6. Semi-structured exit interviews were conducted to assess patient’s experience with the program.
Results
40 (63%) eligible patients were enrolled. Median age was 52.5 with 53% males, 93% whites, 43% with diabetes and median BMI 33.9. On baseline Fibroscan, 59% had F0-2 fibrosis and 70% had moderate-severe steatosis. 33 patients completed the study. Median percentage of days with valid FitBit data collection was 91. 4 patients increased and maintained, 19 maintained, and 8 increased but subsequently returned to baseline weekly step count. 59% of patients reported Fitbit was easy to use and 66% felt step count feedback motivated them to increase their activity. Roughly 50% of patients had reduction in weight, triglycerides and Fibroscan liver stiffness, and 75% had improvement in controlled attenuation parameter and physical function.
Conclusions
A 6-month mobile-technology based pilot lifestyle intervention was feasible and acceptable to NAFLD patients. The program promoted physical activity and was associated with improvement in clinical parameters in some patients.
Keywords: Exercise, Diet, NASH, Cirrhosis, Nutrition, Health behaviors, Obesity, Weight loss, EHealth, Steatosis
Introduction
The morbidity and mortality related to nonalcoholic fatty liver disease (NAFLD) continues to rise in the setting of the evolving obesity epidemic with global prevalence estimates of 25–30% [1]. Among high risk groups, prevalence rates are higher with estimates around 90% in patients undergoing bariatric surgery and 69% in patients with type II diabetes undergoing ultrasound [2, 3]. Patients with NAFLD can develop cirrhosis and hepatocellular carcinoma. Consequently, the clinical and public health significance of this burgeoning patient population cannot be understated. Presently there are no pharmacologic therapies specifically approved for NAFLD, though research is ongoing. The mainstay of therapy remains lifestyle interventions, improved nutrition and exercise, targeted to weight loss as this has been shown to improve histologic outcomes and health-related quality of life (HRQOL) [4].
In clinical practice it has remained challenging to help patients achieve and sustain weight loss. Structured lifestyle programs have been shown to have higher rates of adherence and success in achieving improvements in nutrition and increased physical activity [5]. There are several notable limitations to in-person structured lifestyle programs that limit accessibility and sustainability. This includes cost, distance to travel, and time constraints. Mobile technology-based interventions can circumvent many of these barriers and represent a promising mechanism to improve uptake and sustainability of lifestyle interventions. Simple to use electronic activity trackers can be used to provide patient specific feedback to promote and maintain increased physical activity levels [6]. When paired with concise structured nutritional assessments, these virtual programs represent potentially promising avenues to promote first line therapy for NAFLD.
The aim of this study was to develop and pilot test a low cost, scalable mobile technology-based lifestyle intervention program specifically designed for patients with NAFLD. We tested the feasibility and acceptability of recruiting NAFLD patients into a FitBit based intervention to encourage self-monitoring and provide real-time feedback for physical activity. We paired this with baseline and follow-up nutrition evaluation to promote overall healthy lifestyle behaviors. We hypothesized that NAFLD patients would find this intervention to be acceptable and that the design would be feasible to implement and maintain. Though not powered to assess impact on clinical outcomes, we explored its effects on metabolic and liver-related parameters.
Methods
Patient Population
We enrolled 40 adult patients with a diagnosis of NAFLD from our general hepatology outpatient clinic. To meet diagnostic criteria for NAFLD, a participant was required to have imaging (ultrasound, computed tomography, or Magnetic Resonance Imaging) demonstrating steatosis within the prior 24 months or a liver biopsy noting hepatic steatosis within the prior 36 months, with no or minimal weight loss since those tests. Patients with any other cause of liver disease (i.e., alcoholic, viral, autoimmune, genetic, cholestatic, etc.) were excluded from participation. Alcohol assessment was conducted based on chart review and participant self-report. Patients who reported drinking > 14 servings per week for males or > 7 servings per week in females were excluded. All participants were required to be able to participate in a walking program and nutritional interventions. Those with severe medical co-morbidities (i.e., severe cardiopulmonary disease, severe musculoskeletal disease, uncontrolled diabetes), hepatic decompensation or hepatocellular carcinoma were excluded. Individuals receiving medications that may cause hepatic steatosis or weight reduction, and those who had plans for bariatric procedures or enrollment in other structured lifestyle programs were also excluded. All eligible participants needed to have access to a computer or a smartphone with internet access.
Data Collection
We enrolled individuals who met the eligibility criteria and provided written informed consent. At enrollment participants’ demographics including age, sex, race and formal education history were collected. The following data variables were obtained at time of enrollment: (1) medical comorbidities and use of medications to treat these comorbidities: diabetes, dyslipidemia, hypertension (HTN), coronary artery disease (CAD), history of cerebrovascular disease (CVA) or transient ischemic attack (TIA), depression, and thyroid disease; (2) vital signs and anthropometrics: blood pressure, height and weight, body mass index (BMI), waist and hip circumference; (3) laboratory studies (up to 6 months from time of enrollment): hepatic panel, lipid panel, and hemoglobin A1c; (4) Transient elastography (TE) (i.e., Fibroscan) including liver stiffness (kPa) and controlled attenuation parameter (CAP) measurements (abstracted from electronic medical record if collected within the past 12 months and participant did not have ≥ 5% weight loss since time of that TE-otherwise ordered at time of enrollment); (5) 6-min walk test (6MWT) to assess physical function [7]; (6) Patient-Reported Outcomes Measurement Information System (PROMIS) survey instruments to assess HRQOL [8]; (7) International Physical Activity Questionnaire (IPAQ) to assess baseline physical activity [9]; and (8) 24-h diet recall questionnaire.
The 6MWT is an inexpensive, efficient, easy to conduct and well-tolerated method to assess functional exercise capacity and thus was selected as an outcome measure in this pilot feasibility study over more rigorous, but comparatively more expensive and time-consuming measures of aerobic capacity like V02 peak fitness testing. PROMIS measures are scored on a T-score metric with a mean of 50 and a standard deviation (SD) of 10 according to data from the general US population. A higher PROMIS symptom score indicates high symptom burden, whereas a higher PROMIS function score indicates improved functioning. We included PROMIS short form measures of fatigue (8 items), physical function (6 items), ability to participate in social roles (6 items), satisfaction with social roles (6 items), and psychosocial illness impact (8 items). T-score meaningfully important differences (MIDs) ranges were used to assess PROMIS measures.
Lifestyle Intervention
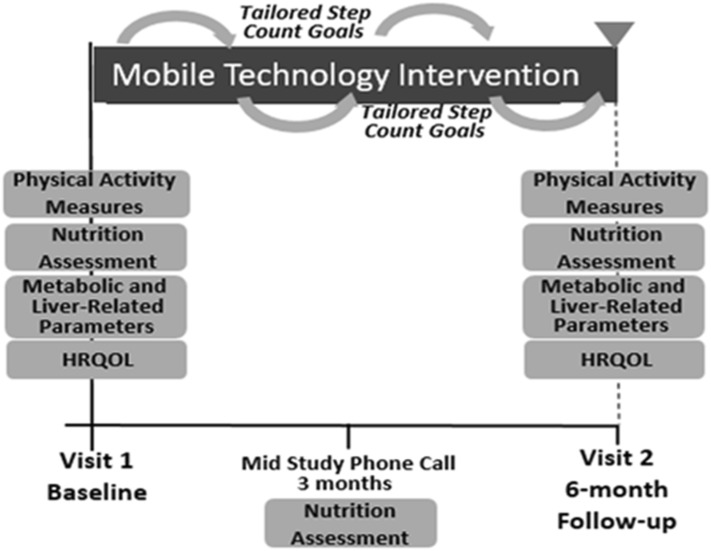
The mobile technology-based lifestyle intervention was 6-months in duration (Fig. 1). The design was based on a pre-existing study protocol that had been pilot-tested among individuals with cardiovascular disease and shown to be feasible and efficacious in that patient population.10 All participants received a Fitbit Zip at enrollment to track step counts. Participants were to wear their FitBit every day during waking hours for the entire study. To ensure accuracy of use and minimize potential barriers to navigating the Fitbit, researchers assisted patients with downloading the Fitbit software and creating a username for downloading step count data. If at any time during the study participants had questions regarding the use of the FitBit or problems uploading step count data, a research study staff member was readily available to assist via phone or email.
Fig. 1.
Mobile technology intervention design characteristics
The Fitbit wirelessly syncs data from the tracker to the Fitbit software or app. Users’ step count data was retrieved weekly for analysis. Study staff provided each of the participants with personalized feedback on physical activity with tailored step count goals (10% increase per week with maximum increase of 800 steps per week to a maximum of 10,000 steps per week) and motivational messages via email. This physical activity prescription was based on the United States Preventive Services Task Force recommendations and protocols used in the general obesity and cardiovascular disease literature [10, 11]. Patients with consecutive days without data recorded or with other signs of low Fitbit usage (days with minimal step counts) were contacted via email or phone to encourage increased use. This follow-up occurred no more frequently than once weekly. Weekly personalized feedback was provided to each participant for the first 3 months and transitioned to biweekly for the last 3 months of the study. A valid FitBit step count day was defined as any day with ≥ 300 steps recorded based on definitions and physical activity patterns used in prior behavioral health interventions using fitness activity trackers [12, 13].
Nutritional assessment included a 24-h diet recall at baseline and at month 3 by research study staff. Study staff were educated on how to perform accurate 24-h diet recall questionnaires and on evidence- based nutritional recommendations for patients with NAFLD based on tenets of the Mediterranean diet or carbohydrate-controlled diet for individuals with diabetes. During the phone assessment at month 3, results of baseline studies were reviewed and study staff provided overall feedback on physical activity and nutrition over the first 3-months of the study.
At month 6, participants had a second study visit. Anthropometrics, laboratory studies, TE, 6MWT and surveys were repeated. In addition, participants completed a concise qualitative exit interview by trained study staff to assess the patient’s experience with the program. Responses to interview questions were recorded by study staff and then transcribed. Transcripts were coded for thematic trends. Upon completion of the 6 month follow-up visit participants received a US $25 gift card as compensation for their time. Procedures of the study were approved by the University of Michigan Institutional Review Board.
Outcomes of Interest
The primary outcome of interest was feasibility and acceptability of the mobile technology based structured lifestyle intervention for patients with NAFLD. Feasibility and acceptability were defined using standardized parameters from the literature assessing exercise or physical activity interventions [14]. Feasibility was assessed based on dropout rates and completion rates for data collection (number of days with valid FitBit data, percentage of follow-up data elements completed). A feasible intervention required dropout rates ≤ 25% and completion rates for data collection of ≥ 80% [15]. Acceptability of the program was assessed based on number of eligible participants enrolled and qualitative exit interview feedback. An acceptable intervention required enrollment of > 50% of eligible subjects and subject feedback that overall reported participants were satisfied or very satisfied with the intervention and program design [16]. The secondary aim was to examine trends in changes in metabolic and liver-related clinical parameters, HRQOL, and physical activity patterns.
Statistical Analysis
To assess baseline characteristics and impact of the intervention on outcomes of interest we performed descriptive and bivariate analyses. Chi-square tests and Fisher exact tests were used for categorical variables and t-tests were used for continuous variables. Variables with distributions that deviated from normality were reported by median and interquartile range (Q1, Q3) and were compared using the Kruskal–Wallis test. P values ≤ 0.05 were considered statistically significant. All analyses were performed in STATA 14 (StataCorp, College Station, TX).
Results
Enrollment and Participant Baseline Characteristics
A total of 64 eligible patients were approached to successfully enroll the targeted total of 40 participants in the study, equaling to an enrollment rate of 62.5%. Among individuals who declined enrollment, the primary reason was time constraint to complete data collection that day (38%). The demographics of individuals who did compared to those who did not enroll in the study were similar (Table 1).
Table 1.
FitBit study enrollment
| Enrolled/approached: 40/64 | 62.5% | ||
| Reason for not enrolling: N = 24 | |||
| Time constraint | 9 (38%) | ||
| Not interested in study | 12 (50%) | ||
| No data | 3 (12%) | ||
| Variable | Not enrolled (N = 24) | Enrolled (N = 40) | P value |
|---|---|---|---|
| Age (median, IQR) | 53 (43–60) | 52.5 (43–61) | 0.82 |
| Sex (male N %) | 14 (70%) | 21 (52.5%) | 0.17 |
| Race (white N %) | 21 (87.5%) | 35 (87.5%) | 1.0 |
Baseline characteristics of participants are shown in Table 2. The median age of the cohort was 52.5 years with 53% males and 93% whites. Overall, 43% had diabetes, 38% had dyslipidemia and 55% were obese (median BMI 33.9). The majority of participants, 65%, had undergraduate or higher level of formal education. A total of 12 participants had a diagnosis of cirrhosis based on chart review of prior imaging results, ICD9-10 codes and outpatient hepatology provider assessments. On baseline Fibroscan, 59% had liver stiffness measurement scores < 10 kPa suggesting fibrosis stage 0–2 and 11 had scores > 14 suggesting cirrhosis, and 70% had CAP score > 260 dB/m suggesting moderate-severe steatosis. The median alanine aminotransferase (ALT) was 52 (IQR 38–68) U/L, Triglycerides (TG) 172.5 mg/dL (IQR 122–227) and HgA1c 5.8 (IQR 5.3–6.8).
Table 2.
Baseline characteristics of enrolled patients
| Variable | Overall cohort N = 40 | Study completers N = 33 | Non-completers N = 7 | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) (median, IQR) | 52.5 (39.5–61) | 53 (43–61) | 44 (30–57) | 0.02 |
| Sex (% male) | 21 (52.5%) | 17 (51.5%) | 4 (57%) | 1.0 |
| Race (% white) | 37 (92.5%) | 30 (90.9%) | 7 (100%) | 1.0 |
| Clinical characteristics | ||||
| Cirrhosis | 12 (30%) | 9 (27.2%) | 3 (42%) | 0.41 |
| Diabetes | 17 (42.5%) | 14 (42.4%) | 3 (42%) | 0.41 |
| Hyperlipidemia or Hypertriglyceridemia | 15(37.5%) | 12(36.6%) | 3 (42%) | 0.41 |
| Hypertension | 17 (42.5%) | 14 (42.4%) | 3 (42%) | 0.41 |
| Depression | 9 (22.5%) | 6 (18.2%) | 3 (42%) | 0.41 |
| Education | ||||
| Elementary/junior high | 1 (2.5%) | 1 (3%) | 0 | 0.53 |
| High school | 13 (32.5%) | 9 (27%) | 4 (57%) | |
| Undergraduate | 17 (42.5%) | 15 (46%) | 2 (29%) | |
| Graduate or higher | 9 (22.5%) | 8 (24%) | 1 (14%) | |
| Physical exam (median, IQR) | ||||
| Waist circumference (inches) | 44 (40.8–48.8) | 42.5 (40.7–46) | 49.6 (41.5–51.5) | 0.001 |
| Truncal obesity | 33 (83%) | 26 (84%) | 7 (100%) | 0.56 |
| Weight (lb) | 209.5 (189.5–250) | 200 (186–236) | 270 (240–301) | 0.006 |
| BMI (kg/m2) | 33.9 (30.4–36.5) | 33.6 (29.6–35.3) | 37.7 (35.5–42.4) | 0.009 |
| Labs | ||||
| ALT (IU/L) | 52 (38–68) | 55 (40.5–70.5) | 40 (32–68) | 0.34 |
| LDL (mg/dL) | 120 (85–154) | 120 (80–134) | 120 (97–137) | 0.74 |
| HDL (mg/dL) | 43 (32.5–51.5) | 44 (39–55) | 34 (33–40) | 0.01 |
| TG (mg/dL) | 172.5 (122–227) | 148 (112–222) | 231 (180–462) | 0.03 |
| HgA1c | 5.8 (5.3–6.8) | 5.9 (5.3–6.7) | 5.7 (5.4–9) | 0.30 |
| Fibroscan | ||||
| Liver stiffness (kPa) | 7.15 (4.9–11.8) | 7 (4.8–10.3) | 9.6 (6.3–26.7) | 0.12 |
| Fibrosis stage | 0.54 | |||
| F0-2 | 22 (59%) | 19 (63%) | 4 (57%) | |
| F3 | 4 (11%) | 3 (10%) | 1 (14%) | |
| F4 | 11 (30%) | 8 (27%) | 2 (28%) | |
| CAP score | 329 (288–370) | 327.5 (278–356) | 362 (312–379) | 0.21 |
| Physical activity and function | ||||
| Moderate/vigorous intensity physical activity (median days/week)^ | 0 (0–2) | 0 (0–2) | 0 (0–2) | 1.0 |
| 6MWT distance (feet) | 1515 (1312–1800) | 1585 (1295–1822) | 1420 (1328–1536) | 0.30 |
| Post-test dyspnea (Borg scale: 0–10) | 2 (0–3) | 2 (0–3) | 1 (0.5–4) | 0.95 |
| HRQOL: T-score* (SD) | ||||
| Physical function | 49.9 (9.8) | |||
| Ability to participate in social roles | 56.2 (10.0) | |||
| Satisfaction with social roles | 50.2 (9.2) | |||
| Psychosocial illness impact | 48.9 (11.9) | |||
| Fatigue | 54.1 (10.2) | |||
Bolded values indicate result was significant with P value of 0.05 or less
BMI Body mass index, AST aspartate aminotransferase, ALT alanine aminotransferase, LDL low density lipoprotein, HDL high density lipoprotein, TG triglycerides, HgA1c hemoglobin A1c, 6MWT 6-min walk test, IQR interquartile range, SD standard deviation
*T-scores (mean = 50; SD = 10) are presented for each PROMIS domain. Higher scores for symptom domains (fatigue, psychosocial illness) indicate a higher symptom burden. Higher scores for functioning domains (physical function, ability to participate in social roles, satisfaction with social roles) indicate good functioning
^Measured using IPAQ
For physical activity, the median days per week of moderate or vigorous intensity physical activity reported on the IPAQ was 0 (IQR 0–2). For baseline physical function, the median distance walked in the 6MWT was 1515 feet (IQR 1311.5–1799.5; general population average is 1312–2296 feet) [17]. The associated Borg dyspnea scale was 2 (0–3), with higher numbers indicating more intense dyspnea. Regarding HRQOL, the baseline T-scores for physical function and satisfaction with social roles were similar to the general population means (49.9 and 50.2, respectively), whereas the ability to participate in social roles was slightly higher than population average (56.2) though fatigue symptom burden was also slightly higher (54.1).
Intervention Uptake
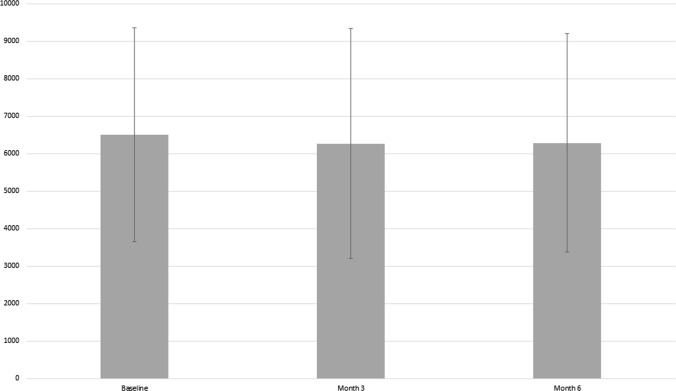
Overall, 36 participants were still in the program at month 3 and 33 participants (83%) completed the entire 6-month program. Baseline characteristics of participants who did versus those who did not complete the study were similar aside from higher baseline weight, BMI, waist circumference and TG and lower HDL in the non-completers (Table 2). Among individuals who did not complete the study, 3 were lost to follow-up and 4 reported that they were either no longer interested in participating or did not have the time to participate any longer. Median percentage of days with valid FitBit data collection was 91% for the cohort. The median weekly step count was 6088 (IQR 4848–7966) at the initiation of the study. At the end of the program, the median weekly step count was 5336 (IQR 3757–8156). When analyzed as means with standard deviation (SD), at baseline the mean daily step count was 6499 (SD 2848), at month 3 was 6269 (SD 3064) and at month 6 6282 (SD 2910) (Fig. 2). F. Of the 33 participants who completed the study, 4 increased and maintained, 19 maintained, and 8 increased but subsequently returned to baseline weekly step count, while step count data were missing for 2 participants.
Fig. 2.
Mean daily step count with standard deviation across the study period
Secondary Outcomes
The pre- and post-intervention changes in clinical variables for the 33 participants who completed the intervention are shown in Table 3. There were statistically significant improvements in HDL, LDL, TG and HgA1c. Among completers there was also a statistically significant improvement in physical function as assessed by the 6MWT distance. Overall neither the change in step count nor changes in HRQOL measures were statistically different between baseline and end of the intervention. Roughly 50% patients had reduction in weight, and ALT. Approximately 2/3 of participants who completed the study had a reduction in TG and an increase in 6MWT. Lastly, 42.4% of participants showed reduction in both Fibroscan liver stiffness and CAP scores (Table 4). The relationship between patterns in change in step count and improvement in clinical parameters is also demonstrated in Table 4.
Table 3.
Pre–post intervention data for 33 program completers
| Variable | Baseline | 6-Month | Median (IQR) Δ Pre–Post | P-value |
|---|---|---|---|---|
| Waist circumference (in) | 42.5 (40.7–46) | 43.7 (41–47) | 0.5 (− 0.75, 1.9) | 0.04 |
| Weight (lb) | 200 (186–236) | 207 (185.1–229.4) | 0.5 (− 5, 7) | 0.56 |
| BMI | 33.6 (29.6–35.3) | 32.3 (29.7–35.9) | − 0.3 (− 0.78, 1.0) | 1.0 |
| SBP mmHg | 132(126–142) | 131 (120–136) | 3 (− 3, 10) | 0.16 |
| ALT (U/L) | 55 (40.5–70.5) | 44 (29–75.5) | − 2.5 (− 14, 5) | 0.31 |
| HDL (mg/dL) | 44 (39–55) | 49.5 (39–56) | 4 (− 1, 6) | < 0.01 |
| LDL (mg/dL) | 120 (80–134) | 100.5 (69–116.5) | − 7 (− 18, 1) | < 0.01 |
| TG (mg/dL) | 148 (122–227) | 137.5 (96.5–193.5) | − 22 (− 53, 11) | 0.03 |
| HgA1c | 5.9 (5.3–6.7) | 5.8 (5.4–6.6) | 0.1 (− 0.1, 0.2) | < 0.01 |
| Fibroscan kPA | 7 (4.8–10.3) | 7.6 (5.7–9.5) | 0 (− 2.3, 1.1) | 1.00 |
| Fibroscan F Score | − 1 (− 1, 1) | 0.67 | ||
| F0-2 | 19 (63%) | 21 (63.6%) | ||
| F3 | 3 (10%) | 7 (21.2%) | ||
| F4 | 8 (27%) | 5 (15.2%) | ||
| Fibroscan CAP (dB/m) | 327.5 (278–356) | 305 (254–354) | − 10.5 (− 54, 35) | 0.19 |
| 6MWT distance (feet) | 1585 (1295–1822) | 1725 (1495–1870) | 59.2 (− 19, 175) | < 0.01 |
| Post-test dyspnea (Borg scale: 0–10) | 2 (0–3) | 1 (0.5–2) | − 1 (− 2, 1) | 0.10 |
| Step count^ | 6088 (4848–7966) | 5336.90 (3757–8156) | − 203 (− 2038, 789) | 0.52 |
| HRQOL# T-score (SD) | ||||
| Physical function | 50.6(8.2) | 50.9 (8.2) | 0.3 | |
| Ability to participate in social roles | 55.9 (9.5) | 56.5 (8.7) | 0.6 | |
| Satisfaction with social roles | 51.5 (11.8) | 53.4 (10.1) | 1.9 | |
| Psychosocial illness impact | 48.1 (9.1) | 47.9 (8.5) | -0.2 | |
| Fatigue | 52.2(9.4) | 50.7 (8.9) | -1.5 | |
Bolded values indicate result was significant with P value of 0.05 or less
^General population median daily step count = 5117
#Negative change in symptoms (fatigue, satisfaction, psychosocial illness impact) indicate reduced symptom burden from baseline to end of intervention. Positive change in health and functioning indicate improved health/functioning from baseline to end of intervention. Results presented as T-scores (mean = 50; SD = 10); higher PROMIS T-scores reflect a greater level of the construct measured. To interpret changes in PROMIS scores, minimally important differences (MID) are used. MID for physical function 1.9–2; MID social roles and psychosocial impact 2.3–3.4; MID fatigue 3–5
Table 4.
Sub analysis of patients with clinical improvements
| Sub-analysis of those with improvements* | Median change (IQR) | Change in step count | |||
|---|---|---|---|---|---|
| Decreased | Maintained | Increased | |||
| Weight loss (lb) | 15^ (45.5%) | − 7 (− 12.9, − 2) | 2 | 9 | 2 |
| ≥ 5% weight loss | 6 (18.2%) | ||||
| Reduction in ALT (U/L) | 17^ (51.5%) | − 30 (− 78, − 11) | 2 | 12 | 2 |
| Reduction to < 35 | 10 (33.3%) | ||||
| Reduction in TG (mg/dL) | 21 (63.6%) | − 2.5 (− 6.5, − 0.3) | 4 | 14 | 3 |
| Reduction to < 150 | 9 (31%) | ||||
| Reduction in liver stiffness (kPa) | 14^ (42.4%) | − 12 (− 26, − 6) | 2 | 10 | 1 |
| ≥ 10% Reduction in kPa | 7 (30.4%) | ||||
| Reduction in CAP (dB/m) | 14^ (42.4%) | − 46 (− 85, − 20) | 3 | 13 | 2 |
| ≥ 10% Reduction in CAP | 7 (50%) | ||||
| Increase in 6MWT (feet) | 23^ (69.6%) | 140 (35–255) | 4 | 14 | 3 |
| 6MWT > population mean | 5 (17.9%) | ||||
Bolded values indicate result was significant with P value of 0.05 or less
*Top row for each variable represents number of participants with any improvement for that variable. Second row for each variable represents number of participants with more marked improvement
^2 patients missing 6-month step count data for weight loss and 6MWT; 1 patient had missing 6-month step count data for reduction in ALT, kPa and CAP
Qualitative Outcomes
Participant responses to the semi-structured exit interviews focused on feasibility and acceptability of the program as well as facilitators and barriers to behavior change are shown in Table 5. Overall, the majority of participants (59%) reported the Fitbit was easy to use and 66% felt step count feedback motivated them to increase their activity. Bad weather was the predominant reported barrier to increased physical activity, whereas objective step count data was the main facilitator.
Table 5.
Semi-structured program exit interviews to assess program feasibility, acceptability and facilitators and barriers to behavior change
| What did you hope you would gain from the program? | Receive objective activity goals (21) |
| Be more active/healthier lifestyle (20) | |
| Help with research (8) | |
| Feedback about mobile technology application and study design | FitBit and app easy to use and liked using (19) |
| Had difficulty syncing FitBit (12) | |
| Prefer wrist based FitBit (5) | |
| Forgot to wear FitBit (8) | |
| Thoughts about step count feedback | Helped motivate physical activity (21) |
| Motivation waned over time (5) | |
| Did not change activity based on feedback (4) | |
| What things supported physical activity? | Objective step count numbers (14) |
| Physical activity of household members (6) | |
| What barriers did you have to physical activity? | Weather (12) |
| Work (7) | |
| Musculoskeletal pain/limitations (6) | |
| Family needs (5) | |
| Motivation to change (4) | |
| Illness (3) | |
| Vacation/social events (3) | |
| Other comments | Desired more assistance with improving diet (3) |
Parentheses indicate number of participants with that response
Discussion
Successful implementation of first line therapy for NAFLD—lifestyle changes, remains a significant challenge for patients and providers. Mobile technology-based structured lifestyle interventions have been tested in the overall obesity population and among patients with diabetes and cardiovascular diseases, but they have not been extensively trialed among patients with NAFLD. In this study, we tested a 6-month mobile technology-based structured lifestyle intervention for patients with NAFLD. Our findings demonstrate that this type of program is acceptable to NAFLD patients, with two-thirds of patients approached about the study electing to enroll. Furthermore, this intervention was feasible to implement and maintain with high levels of adherence with wearing the FitBit over a 6-month period and overall positive experiences as demonstrated by exit interview feedback.
A major gap in our current knowledge relates to the reasons for low uptake and factors impacting response to lifestyle changes in NAFLD patients. We hypothesize that a significant contributing factor stems from the lack of evidenced based guidance for designing and implementing lifestyle programs in real-world settings for patients with NAFLD. This gap is a result of a lack of data on effectiveness of lifestyle programs in the real-world and patient preference to guide the development of programs tailored to the individual patient to ensure maximum effectiveness. Data from the obesity literature indicates that programs that remove logistical barriers (time constraints, cost) and increase the interactions/follow-up with real-time feedback have a higher likelihood for real-world effectiveness [18]. Mobile technology-based lifestyle programs encompass many of these features and have been proven to be effective for weight loss among cohorts with similar phenotypes such as diabetes [19]. In our study, lack of interest and time constraint to complete data collection for enrollment were the primary reasons for not participating. The reasons for lack of interest were not further evaluated in this study, but are the subject of our separate dedicated qualitative studies focused on evaluating motivation to change health behaviors among patients with NAFLD. The limitations in terms of time constraint to complete data collection are a reflection of our study design in this pilot where patients were approached during their routinely scheduled clinic visit. As a result, potential participants were not alerted ahead of time about the study and had not planned to stay in clinic for longer duration. We have revised our subsequent study protocols to alert potential participants about the opportunity to enroll in studies so they can adjust their anticipated visit times accordingly. In response to the COVID-19 pandemic, we have also modified many of our studies to allow remote consent and to adapt some study procedures so they can be done remotely.
Importantly, overall retention of participants in this 6-month program was excellent, with 33 of 40 enrolled participants completing the entire program and 36 remaining in the program at 3 months. Of note, those who did not complete the study did have worse baseline metabolic profiles, indicating that perhaps these patients needed most help to keep them engaged in the program. Among the 33 participants who completed the program, the median percentage of days with valid FitBit data collection was 91%. Our rates of adherence using the fitness activity tracker are higher than those reported in other physical activity intervention trials including the recent STRIVE trial of a home-based exercise program among individuals with cirrhosis [20]. This is likely related to variations in definition for adherence, study design features for follow-up among participants with patterns indicating low adherence, and patient population enrolled. We applied standardized definitions for valid FitBit data days and modeled our intervention protocol after similar studies among patients with metabolic disease. Prior studies have shown that mobile technology devices can help enhance the intervention’s acceptability and perceived value of the program through real-time feedback and goal setting [21]. This was supported by the findings from our qualitative exit interviews whereby the majority (64%) of participants reported seeing their daily step count was motivating. While the overall average step count over the duration of the intervention did not improve for all participants, 69% of completers were able to maintain or increase their step counts over the duration of the study. In future studies we will need to investigate mechanisms to maintain participant’s engagement in the program over time.
We did not have sufficient power to analyze the impact of the intervention on clinical and HRQOL outcomes in this pilot study. There were however overall positive trends in metabolic, liver-related and physical function outcomes. At month 6, there were improvements in lipid panels (HDL, LDL, TG) as well as HgA1c. Among completers there was also a statistically significant improvement in the 6MWT distance. Interestingly, despite the positive qualitative program feedback, we did not see significant improvements in HRQOL measures. Notably, roughly half of participants had a reduction in weight and ALT, two-thirds had improvement in TG and 6MWT and 42% and reduction in liver stiffness and CAP measurements. Among those with these improvements, the majority maintained their step count over the duration of the study. This confirms findings of prior studies that demonstrated improvements in hepatic steatosis and fibrosis can occur with improved nutrition and increased physical activity even if the targeted 5 or 10% reduction in body weight is not achieved [22].
There are several limitations to note for this study. First, as a pilot study we only aimed to enroll a total of 40 patients from a single tertiary center, which limited our ability to speak to scalability to a larger and more diverse population, particularly since our participants were predominantly white. Second, inherent to pilot studies, we did not have power to assess impact of the program on clinical outcomes of interest. As a pilot feasibility study, due to cost limitations and time constraint, we chose less rigorous outcome measures such as the 6MWT over V02peak fitness testing and transient elastography over MR elastography with proton density fat fraction. We plan to incorporate these more rigorous measures in the next phase of our lifestyle intervention where the focus is on impact on clinical outcomes rather than feasibility and acceptability of the program design [23]. Third, participants in the program may be more motivated to change their behavior than other patients and our results may represent best case scenario. Finally, our intervention program was basic and better results might have been obtained by incorporating structured nutritional counseling, and motivational behavioral interventions. For this pilot feasibility trial, due to cost limitations and time constraints, the nutritional assessment and feedback were performed by trained research study staff. In our subsequent larger scale study that was designed based on results gleaned from this study, with the support of grant funding we were able to have a registered dietician perform the nutritional assessments and counseling which may enhance the impact of our intervention.
In conclusion, in this pilot study of a 6-month mobile technology-based lifestyle intervention specifically designed for patients with NAFLD, we demonstrated feasibility and acceptability of the program with an overall positive program participation experience. Adherence with wearing the FitBit was good and roughly half of the participants increased and maintained higher step counts till the end of the program. In future work we will incorporate a stronger focus on nutrition assessment and interventions as 80–90% of weight loss is associated with dietary changes [24, 25]. We will also incorporate participant-identified preferences to enhance motivation to change health behaviors. Though pharmacological therapies are likely necessary for many patients with NAFLD, particularly those who have advanced fibrosis, effective, scalable lifestyle programs are essential to decrease morbidity and mortality of NAFLD due to its high global prevalence.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- HTN
Hypertension
- CAD
Coronary artery disease
- CVA
Cerebrovascular disease
- TIA
Transient ischemic attack
- BMI
Body mass index
- TE
Transient elastography
- CAP
Controlled attenuation parameter
- 6MWT
6-minute walk test
- PROMIS
Patient-reported outcomes measurement information system
- HRQOL
Health related quality of life
- IPAQ
International physical activity questionnaire
- SD
Standard deviation
- MIDs
Meaningfully important differences
Funding
MAT was supported by the American Association for the Study of Liver Diseases (AASLD) Clinical Translational and Outcomes Research Award.
Declaration
Conflict of interest
The authors have no conflicts of interests to disclose.
Footnotes
An editorial commenting on this article is available at 10.1007/s10620-021-07018-x.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of nonalcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Tapper EB, Lai M. Weight loss results in significant improvements in quality of life for patients with nonalcoholic fatty liver disease: a prospective cohort study. Hepatology. 2016;63:1184–1189. doi: 10.1002/hep.28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konerman MA, Walden P, Jackson E, Lok AS, Rubenfire M. Impact of a structured lifestyle program on patients with metabolic syndrome complicated by nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;00:1–12. doi: 10.1111/apt.15063. [DOI] [PubMed] [Google Scholar]
- 6.Kumar AM, Lyden AM, Carlozzi NE, Sen A, Richardson CR, Jackson EA. The physical activity daily (PAD) trial: the rationale and design of a randomized controlled trial evaluating an internet walking program to improve maximal walking distance among patients with peripheral arterial disease. Contemp Clin Trials. 2018;67:23–30. doi: 10.1016/j.cct.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VanWagner LB, Uttal S, Lapin B, et al. Use of six-minute walk test to measure functional capacity after liver transplantation. Phys Ther. 2016;96:1456–1467. doi: 10.2522/ptj.20150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Cocker KA, De Bourdeaudhuij IM, Cardon GM. What do pedometer counts represent? A comparison between pedometer data and data from four different questionnaires. Public Health Nutr. 2009;12:74–81. doi: 10.1017/s1368980008001973. [DOI] [PubMed] [Google Scholar]
- 10.Roberts LM, Jaeger BC, Baptista LC, et al. Wearable technology to reduce sedentary behavior and CVD risk in older adults: a pilot randomized clinical trial. Clin Interv. Aging. 2019;14:1817–1828. doi: 10.2147/CIA.S222655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griauzde DH, Kullgren JT, Liestenfeltz B, Richardson C, Heisler M. A mobile phone-based program to promote healthy behaviors among adults with prediabetes: study protocol for a pilot randomized controlled trial. Pilot Feasibility Stud. 2018;4:48. doi: 10.1186/s40814-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block VJ, Lizée A, Crabtree-Hartman E, et al. Continuous daily assessment of multiple sclerosis disability using remote step count monitoring. J Neurol. 2017;264:316–326. doi: 10.1007/s00415-016-8334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dlugonski D, Pilutti LA, Sandroff BM, Suh Y, Balantrapu S, Motl RW. Steps per day among persons with multiple sclerosis: variation by demographic, clinical, and device characteristics. Arch Phys Med Rehabil. 2013;94:1534–1539. doi: 10.1016/j.apmr.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 14.El-Kotob R, Giangregorio LM. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud. 2018;4:137–137. doi: 10.1186/s40814-018-0326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Román E, Torrades MT, Nadal MJ, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59:1966–1975. doi: 10.1007/s10620-014-3086-6. [DOI] [PubMed] [Google Scholar]
- 16.Connolly B, Thompson A, Douiri A, Moxham J, Hart N. Exercise-based rehabilitation after hospital discharge for survivors of critical illness with intensive care unit-acquired weakness: a pilot feasibility trial. J Crit Care. 2015;30:589–598. doi: 10.1016/j.jcrc.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chetta A, Zanini A, Pisi G, et al. Reference values for the 6-min walk test in healthy subjects 20–50 years old. Respir Med. 2006;100:1573–1578. doi: 10.1016/j.rmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Joseph MS, Tincopa MA, Walden P, Jackson EA, Conte ML, Rubenfire M. The impact of structured exercise programs on metabolic syndrome and its components: a systematic review. Diabetes Metab Syndr Obes. 2019;12:2395–2404. doi: 10.2147/dmso.s211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khokhar B, Jones J, Ronksley PE, Armstrong MJ, Caird J, Rabi D. Effectiveness of mobile electronic devices in weight loss among overweight and obese populations: a systematic review and meta-analysis. BMC Obes. 2014;1:22. doi: 10.1186/s40608-014-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai JC, Dodge JL, Kappus MR, et al. A multicenter pilot randomized clinical trial of a home-based exercise program for patients with cirrhosis: the strength training intervention (STRIVE) Am College Gastroenterol. 2020 doi: 10.14309/ajg.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Med Internet Res. 2014;16:e192. doi: 10.2196/jmir.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ok D-P, Ko K, Bae JY. Exercise without dietary changes alleviates nonalcoholic fatty liver disease without weight loss benefits. Lipids Health Disease. 2018;17:207–207. doi: 10.1186/s12944-018-0852-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31. doi: 10.1186/1471-2466-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westerterp KR. Physical activity as determinant of daily energy expenditure. Physiol Behav. 2008;93:1039–1043. doi: 10.1016/j.physbeh.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]