Within the last decade, we have learned that damaged mitochondria activate many of the same innate immune pathways that evolved to sense and respond to intracellular pathogens. These shared responses include cytosolic nucleic acid-sensing and type I interferon (IFN) expression, inflammasome activation that leads to pyroptosis, and selective autophagy (called mitophagy when mitochondria are the cargo).
KEYWORDS: LRRK2, Mycobacterium tuberculosis, cytosolic DNA sensing, innate immunity, macrophage, mtDNA, type I interferon
ABSTRACT
Within the last decade, we have learned that damaged mitochondria activate many of the same innate immune pathways that evolved to sense and respond to intracellular pathogens. These shared responses include cytosolic nucleic acid sensing and type I interferon (IFN) expression, inflammasome activation that leads to pyroptosis, and selective autophagy (called mitophagy when mitochondria are the cargo). Because mitochondria were once bacteria, parallels between how cells respond to mitochondrial and bacterial ligands are not altogether surprising. However, the potential for cross talk or synergy between bacterium- and mitochondrion-driven innate immune responses during infection remains poorly understood. This interplay is particularly striking, and intriguing, in the context of infection with the intracellular bacterial pathogen Mycobacterium tuberculosis (Mtb). Multiple studies point to a role for Mtb infection and/or specific Mtb virulence factors in disrupting the mitochondrial network in macrophages, leading to metabolic changes and triggering potent innate immune responses. Research from our laboratories and others argues that mutations in mitochondrial genes can exacerbate mycobacterial disease severity by hyperactivating innate responses or activating them at the wrong time. Indeed, growing evidence supports a model whereby different mitochondrial defects or mutations alter Mtb infection outcomes in distinct ways. By synthesizing the current literature in this minireview, we hope to gain insight into the molecular mechanisms driving, and consequences of, mitochondrion-dependent immune polarization so that we might better predict tuberculosis patient outcomes and develop host-directed therapeutics designed to correct these imbalances.
INTRODUCTION
Macrophages are exquisitely well-evolved to sense bacterial pathogens via a vast array of extracellular, endosomal, and cytosolic pattern recognition receptors and respond to threats via activation of antibacterial gene expression programs (1). In fact, macrophages even mount robust immune responses when they sense pieces of damaged mitochondria in their cytosol, despite the fact that mitochondria have not been free-living bacteria for over a billion years. Although the ability of mitochondrion-derived damage-associated molecular patterns (mito-DAMPs) to activate a variety of innate immune responses in macrophages and other cells is fairly well-appreciated today, we still know almost nothing about how mitochondrion-triggered innate immune responses alter a cell’s ability to sense and respond to an actual microbial threat.
Mounting evidence from a variety of disease models demonstrates that mitochondrial dysfunction and the release of mito-DAMPs can polarize innate immune responses and increase host susceptibility to certain pathogens (2–6). Furthermore, pathogens across kingdoms have been shown to interact with, and, in many cases, manipulate mitochondrial function to promote infection. Several recent reviews have tackled the vast microbe-mitochondrial interface literature and present valuable insights into unique and conserved strategies used by pathogens to manipulate mitochondrial metabolism and homeostasis (3, 5, 7, 8). Here, we will specifically focus on the surprising number of connections that have emerged between mitochondria and the important human pathogen Mycobacterium tuberculosis (Mtb). Mtb, the causative agent of tuberculosis, is an intracellular bacterial pathogen that survives and replicates within the harsh environment of a macrophage. Although long considered a strictly vacuolar pathogen, we now appreciate that Mtb permeabilizes or destabilizes its phagosome, accesses the macrophage cytosol, and triggers a variety of pathways downstream of cytosolic pattern recognition receptors. These same pathways, type I interferon expression, ubiquitin-mediated selective autophagy, and inflammasome activation/programed cell death, are activated by damaged mitochondria when they release ligands like mitochondrial DNA (mtDNA) into the cytosol (9–11). If macrophages sense and respond to Mtb and mitochondrial damage in the same ways, what happens when a cell senses both simultaneously? How are these signals integrated into a cohesive innate immune response? Is such a response inherently pro- or antibacterial? Finally, has a pathogen like Mtb evolved to disrupt the balance of these responses to promote its own survival within a host?
This review focuses on three seemingly distinct but likely intimately connected observations related to the mitochondrial-Mtb interface: (i) macrophages sense and respond to damaged mitochondria and Mtb by activating the same innate immune responses, (ii) Mtb can directly damage mitochondria in infected macrophages, and (iii) mutations in mitochondrion-associated genes alter host susceptibility to mycobacterial infection. By understanding how mitochondrion-triggered innate immune responses influence tuberculosis disease severity, we might one day develop host-directed therapeutics designed to correct imbalances in these responses and promote positive tuberculosis patient outcomes. Likewise, because mitochondrial mutations that confer susceptibility to mycobacterial infection are also associated with several chronic diseases, including Parkinson’s disease (PD) (12–15), Crohn’s disease (16–19), inflammatory bowel disease (20–22), and cancer, understanding how they alter mitochondrial function and dysregulate immune responses will help illuminate common molecular mechanisms driving these otherwise disparate diseases.
MITOCHONDRIAL DAMPs AND Mtb TRIGGER MANY OF THE SAME INNATE IMMUNE RESPONSES
Mitochondria are membrane-bound organelles that are involved in myriad biological processes. Unlike the bean-shaped organelles depicted in many textbooks, mitochondria actually form a highly dynamic, branched network that continually undergoes cycles of fission, fusion, transport, and degradation (23–25). The cycle of fission and fusion plays a critical role in maintaining cellular homeostasis, with mitochondrial fusion promoting energy production in proliferating cells via oxidative phosphorylation (26) and mitochondrial fission being required for clearance of damaged pieces of the network and programmed cell death via apoptosis (27, 28). While mitochondria are most well-known for their prominent role in generating energy in the form of ATP through oxidative phosphorylation, they also contribute to a number of other metabolic processes in the cell, including fatty acid metabolism and biosynthesis of macromolecules such as nucleotides, lipids, heme, and iron sulfur clusters (29). Beyond metabolic and catabolic roles, mitochondria are key players in regulation of the cell cycle, cell proliferation, cellular differentiation, and programmed cell death (30–32). In addition to these crucial roles of making energy, controlling cell proliferation, and regulating cell death, mitochondria are being increasingly appreciated as critical players in immunity, and there is growing evidence that the metabolic/bioenergetic state of an immune cell influences its ability to detect and respond to pathogens (33–35). To date, most of what we know about mitochondrion-driven innate immunity relates to the ability of mito-DAMPs (e.g., mtDNA, cardiolipin, formyl-methionine-labeled peptides, and cytochrome c) to engage pattern recognition receptors and trigger cytokine/chemokine production and/or cell death. Because the role of mito-DAMPs in eliciting immune responses has been reviewed extensively elsewhere (9, 36–38), we will focus here on mtDNA, the mito-DAMP that we currently know the most about during Mtb infection.
CYTOSOLIC DNA SENSING IS ACTIVATED BY mtDNA AND Mtb
The 16.6-kb mitochondrial genome is a double-stranded circular molecule that is rich in unmethylated CpG motifs and susceptible to oxidative stress (making it look more bacterial than eukaryotic) (39, 40). It is maintained independently of the eukaryotic genome by the mitochondrial DNA polymerase gamma (POLG). Organization of the mitochondrial genome is preserved by the mitochondrial transcription factor A (TFAM), which packs mtDNA into nucleosome-like protein-DNA complexes called nucleoids (41, 42). There are hundreds to thousands of copies of mtDNA present in every cell of the human body, with the exception of erythrocytes, which lack mitochondria.
Until somewhat recently, the role of the mitochondria in regulating immunity was mainly studied in the context of cell death and/or antiviral RNA sensing (for which mitochondria serve as an important signaling hub) (43–46). The first major study to implicate a mitochondrial ligand in directly eliciting inflammatory gene expression came from West et al., who used a Tfam+/− model to study the role of mtDNA in innate immune responses (11). They found that TFAM heterozygosity is sufficient to destabilize the mitochondrial genome and cause mtDNA leakage into the cytosol. They went on to demonstrate that in Tfam+/− cells, cytosolic mtDNA is recognized by the cytosolic DNA sensor cGAS (cyclic GMP-AMP synthase), which promotes chronic type I IFN expression and potentiates interferon-stimulated gene (ISG) expression upon cytosolic nucleic acid challenge. Thus, Tfam+/− mouse embryonic fibroblasts (MEFs) are resistant to herpes simplex virus 1 (HSV-1) and vesicular stomatitis virus (VSV) infection. A diagram of the canonical cGAS signaling cascade, whereby double-stranded DNA (dsDNA) sensing by cGAS generates cGAMP (cyclic GMP-AMP or 2′3′-cyclic GMP-AMP), which activates STING (stimulator of interferon genes), which recruits and activates TBK1 (Tank-binding kinase), which phosphorylates the transcription factor IRF3 (interferon regulatory factor 3), is shown in Fig. 1. This paradigm-shifting study from West et al. elevated the mitochondrion to an active player in triggering type I IFN expression via cytosolic DNA sensing. Subsequent studies have confirmed that several other types of mitochondrial damage can spill mtDNA into the cytosol and elicit type I IFN expression downstream of cGAS, including pore formation via the apoptosis proteins Bak/Bax (47–49) and gasdermins (50, 51). While cGAS is generally accepted as the major sensor of cytosolic mtDNA, mtDNA also has been shown to trigger type I IFN expression via toll-like receptor 9 (TLR9), although this phenomenon has mostly been observed in dendritic cells (Fig. 1) (9, 52).
FIG 1.
Both mtDNA and Mtb DNA engage cytosolic DNA surveillance pathways to activate type I interferon expression. Mtb infection can stimulate type I IFN expression by activating both cGAS, via Mtb dsDNA, and STING, via secretion of cyclic-di-AMP. To date, mtDNA has been shown to engage cGAS and TLR9. While TLR9 has been shown to play a role in determining Mtb infection outcomes in vivo, it remains unclear whether the endosomal receptor actually directly binds to Mtb-derived dsDNA during Mtb infection of macrophages (represented by the question mark). The figure was created with BioRender.
Because Mtb resides within a membrane-bound compartment, termed the Mtb phagosome, following phagocytosis by macrophages, Mtb is traditionally categorized as a vacuolar pathogen. While visually, Mtb bacilli are mostly membrane-bound within macrophages, if one considers Mtb’s intracellular lifestyle in terms of what innate immune responses it elicits, the distinction between vacuolar and cytosolic pathogen becomes blurred. Indeed, early work from the Cox laboratory revealed that the transcriptional signature of Mtb-infected macrophages is almost identical to that of macrophages infected by Listeria monocytogenes (53), which famously completely breaks down its phagosome via secretion of a pore-forming toxin, listeriolysin O (LLO) (54, 55). More surprisingly, the transcriptional response elicited by both of these pathogens in many ways resembles the characteristic antiviral type I IFN response of a cytosolic DNA virus. Expression of this type I IFN signature during infection with both pathogens requires phagosomal permeabilization (via ESX-1 for Mtb and LLO for L. monocytogenes) and the STING/IRF3 axis (53) (Fig. 1). In the case of L. monocytogenes, work from the Portnoy laboratory showed that the major host determinant for type I IFN expression during L. monocytogenes infection is STING, as Listeria bacilli secrete c-di-AMP (cyclic di-3′,5′-AMP) via a bacterially encoded di-adenylate cyclase, effectively bypassing a cytosolic DNA sensor like cGAS (56, 57). During Mtb infection, ex vivo genetic studies suggest that the majority of type I IFN expression occurs downstream of cGAS, as the response is completely ablated in Mtb-infected Cgas−/− human and murine macrophage cell lines and primary murine macrophages (58–60). Curiously, in spite of this strong phenotype, loss of cGAS in mouse models of disease has a limited impact on type I IFN and Mtb in vivo infection outcomes (58, 60). More recent work found that STING is also dispensable for controlling Mtb infection in mice (61), despite earlier studies that identified and characterized a role for two Mtb adenylate cyclases, DisA and DacA, in stimulating type I IFN expression downstream of STING (62). These surprising observations suggest that type I IFN expression can be elicited downstream of one or more additional sensors in vivo or that the loss of these factors simultaneously impacts both pro- and antibacterial pathways such that phenotypes are obscured. Although the nature of these additional sensors remains elusive, several studies suggest TLR9, an endosomal sensor of CpG DNA, contributes to host resistance to Mtb (63–65), and RNA sensing pathways have been shown to contribute to type I IFN responses in vivo (66).
Even though we do not fully understand the contribution of specific host factors to eliciting type I IFN responses, the aforementioned studies and many others (67) strongly suggest that the ability to induce host type I interferon is a conserved intracellular bacterial adaptation shared by Mtb, L. monocytogenes, Legionella pneumophila, Chlamydia trachomatis, Brucella abortus, Coxiella burnetii, and Francisella tularensis (67). To date, most research has failed to define the nature of the cytosolic DNA ligand in cells infected with intracellular bacterial pathogens. We will address the potential relative contributions of bacterial (Mtb) and mitochondrial dsDNA to cytosolic DNA sensing in the next section.
MITOCHONDRIAL DAMPs AND Mtb PAMPS CAN MEDIATE IL-1β RELEASE AND TRIGGER PYROPTOSIS VIA ACTIVATION OF THE INFLAMMASOME
Inflammasomes are multisubunit, cytoplasmic protein complexes that consist of receptor/sensor molecules, the adaptor protein ASC, and the inflammatory cysteine protease caspase 1. Inflammasome assembly triggers caspase 1 activation, leading to the cleavage of pro-interleukin-1β (pro-IL-1β) and pro-IL-18 into mature, secreted forms of the cytokines (68, 69) and an inflammatory form of programmed cell death, called pyroptosis. Upon cellular stress or in the face of mitochondrial dysfunction, release of mito-DAMPs, including mitochondrial reactive oxygen species (ROS) (70), cardiolipin (71, 72), and extracellular ATP (73), can activate the NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome. In addition to triggering type I IFN expression through engaging cGAS, mtDNA also directly activates the NLRP3 and AIM2 (absent in melanoma) inflammasomes (Fig. 2) (74, 75). Because mtDNA can seemingly serve as a ligand for both cGAS and AIM2/NLRP3, an obvious unanswered question is whether mtDNA activates each of these cytosolic sensing pathways with similar efficiency and at the same time. One characteristic of mtDNA that could influence its ability to stimulate these two pathways independently is its oxidation state. Oxidized mtDNA has been shown to activate the NLR family pyrin domain-containing 3 (NLRP3) inflammasome (75) and has been shown to be a poor substrate of the cytosolic DNase TREX1 (three prime repair exonuclease 1) (76). Thus, oxidized mtDNA may persist longer in the cytosol, thereby affecting its ability to engage different PRRs. Likewise, the sequence, length, and subcellular localization of an mtDNA fragment may influence its ability to bind to/engage PRRs, although the effect of these variables has yet to be experimentally tested.
FIG 2.
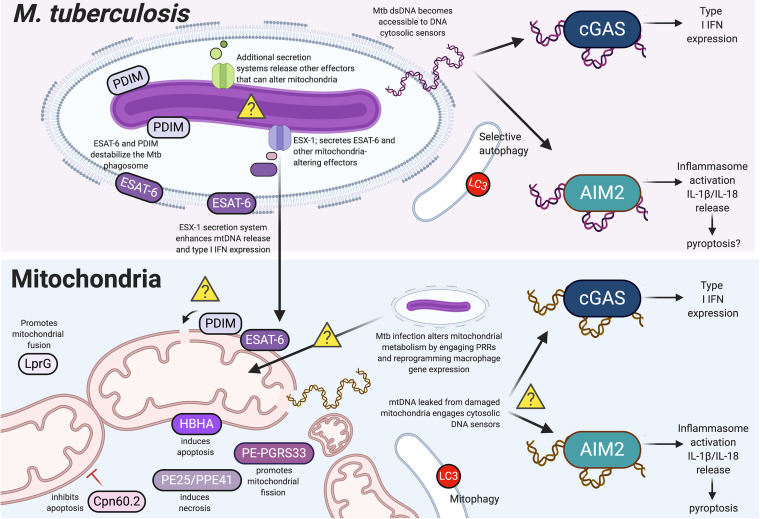
Cross talk between Mycobacterium tuberculosis and mitochondria can influence cytosolic sensing pathways. Schematic representation of molecular interplay between Mtb and mitochondria in infected cells. Yellow question marks indicate several phenomena whose mechanisms/consequences are unclear, namely, whether PDIM (phthiocerol dimycocerosates) and/or ESAT-6 (6-kDa early secretory antigenic target 6) directly alter the mitochondrial membrane, what secretion systems introduce mitochondrion-modulating Mtb effectors into host cells, how Mtb infection generally affects mitochondrial metabolism, and whether mtDNA engages with cGAS (cyclic GMP-AMP synthase) and AIM2 (absent in melanoma) simultaneously (and, if so, what the outcomes for an Mtb-infected cell are). Secreted Mtb virulence factors that have been shown to alter mitochondrial homeostasis are colored in shades of purple. The figure was created with BioRender.
The inflammasome also plays an important role in the immune response against Mtb, although certain aspects of the molecular mechanisms driving inflammasome activation and Mtb’s ability to manipulate this pathway within infected macrophages remain unclear. Activation of the inflammasome is certainly important during in vivo Mtb infection, as deletion of key inflammasome factors, including IL-18 (77), IL-1β (78), and IL-1 receptor (IL-1R) (78, 79), confer susceptibility to Mtb. Aim2−/− mice are also highly susceptible to Mtb infection and display impaired Th1 responses (80). In macrophages ex vivo, Mtb activates the AIM2 inflammasome in an ESX-1-dependent fashion (59), and a recently developed vaccine strain, BCGΔureC::hly, was also shown to stimulate the AIM2 inflammasome (81). BCGΔureC::hly is a bacille Calmette-Guérin (BCG) vaccine strain of Mtb, genetically engineered to express the L. monocytogenes LLO, which enables phagosomal permeabilization in what is otherwise an ESX-1 mutant (BCG lacks region of difference 1 [RD1], a region of the Mtb genome that encodes the ESX-1 secretion system). While AIM2 activation does appear to depend on the ESX-1 secretion system, we also know that AIM2 is not the only inflammasome induced by mycobacterial infection; significant levels of IL-1β are released from Aim2−/− bone marrow-derived macrophages, and nonvirulent strains of Mtb lacking RD1 can promote IL-1β release via the NLRP3 inflammasome (59, 82). Further complicating matters is a report that Mtb can inhibit AIM2 inflammasome activation in an ESX-1-dependent fashion, as evidenced by the fact that caspase-1 cleavage and IL-1β secretion are inhibited in Mtb-infected cells exposed to a secondary inflammasome trigger (82). Again, while these studies nicely demonstrate the involvement of the AIM2 and NLRP3 inflammasomes during Mtb infection of macrophages ex vivo, they only measure the final consequences and not the upstream drivers of inflammasome engagement. In all of these experiments, it is feasible that Mtb-derived ligands, mitochondrion-derived ligands, or both prime and/or trigger inflammasome activation. Indeed, the parallels between NLRP3 and AIM2 activation in response to Mtb infection and mitochondrial damage suggest an intriguing model whereby Mtb may mediate inflammasome activation through ESX-1-dependent perturbation of mitochondria and release of mtDNA/mito-DAMPs into the cytosol.
Mtb PERTURBS THE MITOCHONDRIAL NETWORK DURING MACROPHAGE INFECTION
Central to Mtb’s ability to modulate macrophage innate immune responses is its sec-independent type VII virulence-associated ESX-1 secretion system. One of the main consequences of ESX-1 secretion is destabilization/permeabilization of the Mtb phagosomal membrane, which allows for mixing of bacterial components from inside the phagosome with innate immune sensors in the host cytosol (Fig. 2). The prevailing paradigm asserts that permeabilization of the Mtb phagosome is achieved via cooperation between two major Mtb virulence factors, ESAT-6 (the major substrate of ESX-1) and surface glycolipids, called phthiocerol dimycocerosates (PDIMs) (83–87). ESX-1/ESAT-6 have been repeatedly implicated in destabilizing the Mtb phagosome (88–91). Although earlier work suggested that ESAT-6 formed pores in biological membranes, a bone fide pore-forming function for ESAT-6 has largely been dismissed as an artifact of residual detergent in ESAT-6 purified protein samples (92). Instead, work from the Ramakrishnan laboratory supports a model whereby ESX-1-dependent hemolysis requires direct contact between bacteria and membranes and ESX-1 secretion more accurately causes gross membrane disruption than discrete pores (92).
Like ESX-1, PDIM is required to maximize cytosolic access of both Mtb (83, 84) and M. marinum (a common experimental model for Mtb that can infect zebrafish) (85, 93). A recent set of biophysical experiments from Augenstreich et al. examined how various Mtb strains affected membrane polarity. They found that wild-type (WT) BCG elicits changes in membrane polarity of a lipid bilayer, while a PDIM mutant did not (86). Using hemolysis as a readout for membranolytic activity, the same group showed that BCG expressing ESX-1 but lacking PDIM are less efficient at potentiating membrane lysis compared to WT BCG or BCG expressing ESX-1. PDIM has also been shown to decrease plasma membrane polarity of THP-1 cells during BCG infection (94). Taken together, these experiments convincingly link ESX-1/ESAT-6 and PDIM to destabilization of the Mtb phagosome. However, we still know very little about how these virulence factors interfere with other lipid bilayers, like the mitochondrial membrane, in Mtb-infected cells.
To date, most of what we know about how PDIM and ESX-1 secretion are linked to mitochondrial damage and innate immune regulation is correlative. As described above, work from the Cox laboratory was among the first to demonstrate that type I IFN expression during Mtb infection requires ESX-1 secretion (53, 95), and our subsequent studies identified cGAS as the major cytosolic DNA sensor driving type I IFN expression in response to Mtb-derived dsDNA (which we found bound to cGAS in Mtb-infected cells) (60). A recent transposon sequencing (Tn-seq) experiment designed to identify Mtb mutants defective in intracellular growth reported high correlation between mutants in ESX-1 and mutants in PDIM and showed that production and transport of PDIM is required for type I IFN expression during Mtb infection of macrophages (96). These data support a model whereby permeabilization of the Mtb phagosome allows for release of bacterial dsDNA into the cytosol, where it can engage with cGAS and elicit cytosolic innate immune sensing. However, due to growing interest in the involvement of cytosolic DNA of mitochondrial and/or nuclear origin in eliciting type I IFN expression during Mtb infection, studies have begun to challenge this model. Notably, Wiens and Ernst infected macrophages with three phylogenetically distinct strains of the Mtb complex and found that the level of type I IFN response correlates with a strain’s ability to induce mitochondrial stress (as measured by mitoSOX, a fluorogenic dye used to measure mitochondrial superoxide in live cells) and induce cytosolic mtDNA accumulation (97). Likewise, using a collection of transposon mutants in ESX-1 genes in M. marinum, Lienard et al. were able to uncouple M. marinum phagosomal access from type I IFN expression (98). Their work shows that in M. marinum-infected bone marrow-derived macrophages (BMDMs), permeabilization of the phagosome is not sufficient to induce type I IFN production and that mtDNA, but not M. marinum dsDNA, accumulates in the cytosol in an ESX-1-dependent fashion. These authors conclude that an unidentified ESX-1-dependent function or secreted factor (not ESAT-6) is required to release host mtDNA into the cytosol. Critically, while both of these reports attempt to dismiss the contribution of Mtb dsDNA to driving type I IFN expression, it is possible that the amount of cytosolic Mtb dsDNA changes dynamically over the course of infection (both studies examine a single time point: 24 h postinfection). The same caveat could apply to cytosolic mtDNA levels, which one can imagine will increase over the course of infection as mitochondrial damage accumulates. Thus, it is possible that activation of type I IFN is initiated at very early time points after phagocytosis via bacteria-derived dsDNA and later is augmented and/or sustained through Mtb-dependent release of mtDNA. Experiments designed to alter the mtDNA content of cells and/or identify and characterize Mtb mutants that lack the ability to induce type I IFN altogether or fail to release mito-DAMPs will help tease apart the contribution of each dsDNA source to the type I IFN response during Mtb infection.
Beyond directly or indirectly affecting the release of mito-DAMPs, Mtb, ESAT-6, and ESX-1 secretion have also been implicated in generally altering mitochondrial function. A very recent paper from Yabaji et al. demonstrates that the expression of ESAT-6 upregulates superoxide dismutase (SOD2), an enzyme that detoxifies free radical by-products of mitochondrial respiration. Upregulated SOD2 activity, in turn, inhibits the acidification of the Mtb phagosome (99). Although the molecular mechanisms linking ESAT-6 to increased SOD2 activity remain elusive, loss of SOD2 by short interfering RNA knockdown is sufficient to limit BCG survival in J774.A.1 macrophages, supporting the idea that mitochondrial superoxide contributes to Mtb killing. Thus, ESAT-6 may facilitate mycobacterial survival by upregulating radical detoxifying enzymes in the mitochondria. Along the same lines, work from Cumming et al. demonstrated that a dramatic reprogramming of cellular respiration occurs in Mtb-infected cells (100). They show that a decrease in maximal respiration and a decrease in basal respiratory capacity are dependent on an intact ESX-1 secretion system (wild-type Mtb induced these changes while the BCG vaccine strain did not). Overall, these changes correspond to a quiescent energy phenotype, with bioenergetic metabolism further decelerating with increasing multiplicity of infection (MOI). These changes in mitochondrial respiration corresponded to changes in the dependence of mitochondria on different energy sources, whereby Mtb-infected macrophages are more dependent on exogenous fatty acids than are uninfected cells. While several outstanding questions remain, namely, how Mtb both up- and downregulates glycolysis at different time points during infection and how these changes benefit the pathogen, this research provides an important framework for understanding how intracellular bacterial pathogens reprogram mitochondrial metabolism.
Another very recent study suggests that one way Mtb infection restricts macrophage glycolysis is via induction of miR-21, which targets the phosphofructokinase muscle (PFK-M) isoform, an enzyme that controls a critical rate-limiting step of glycolysis (101). It will be interesting to determine whether these changes can be ascribed to the direct action of Mtb virulence factors versus the activation of various innate immune pathways, which themselves have been shown to reprogram macrophage energetics (102). Some potential candidates for mitochondrial metabolism-modulating Mtb effectors are LprG and PE-PGRS33 (Fig. 2). Treatment of monocyte-derived macrophages with recombinant LprG induces mitochondrial fission and decreases the basal respiratory rate, while addition of PE-PGRS33 induces mitochondrial fusion (103). Although both of these factors have predicted mitochondrial targeting sequences and PE-PGRS33 colocalizes with the mitochondrial network in transfected cells (104), the precise mechanisms for how they interfere with mitochondrial network dynamics and/or how they get from Mtb to the mitochondria have yet to be explored. Likewise, a recent large-scale protein-protein interaction study of Mtb effector and host proteins (105) identified several Mtb secreted proteins that had high-confidence interactions with mitochondrial proteins (e.g., Rv2469c, PE25, and Rv2941), although follow-up analyses on whether these factors modulate mitochondrial biology are still needed. As additional insights are made into the mechanisms through which Mtb effectors impact mitochondrial homeostasis, it will be interesting and important to compare and contrast with the strategies employed by myriad other pathogens across kingdoms.
Lastly, there are several mycobacterial proteins shown to regulate macrophage cell death via interacting with the mitochondrion. For example, heparin-binding hemagglutinin (HBHA), a major secreted antigen during Mtb infection, localizes to mitochondria and can induce macrophage apoptosis via caspase activation. HBHA treatment of RAW 264.7 macrophages causes Bax translocation to the mitochondria, cytochrome c release, and decreased mitochondrial membrane potential, suggesting a role for HBHA in triggering programmed cell death (106). An opposite, antiapoptotic function has been ascribed to a mycobacterial chaperone protein, Cpn60.2 (GroEL2), which was shown to interact with and inhibit mortalin, a mitochondrial protein and host mediator of apoptosis (107). Another Mtb-secreted complex, PE25/PPE41, can induce necrosis but not apoptosis in RAW 264.7 macrophages (108) (Fig. 2). While these cell death studies are somewhat limited by the use of the RAW 264.7 macrophage cell line, which does not encode an active inflammasome, these findings demonstrate that Mtb has evolved multiple ways to modulate cell death via interacting with mitochondrial proteins, consistent with the pathogen’s need to control how and when an infected macrophage dies.
MITOCHONDRIAL MUTATIONS CONFER SUSCEPTIBILITY TO MYCOBACTERIAL INFECTION
Additional insights into mitochondria/Mtb cross talk come from genome-wide association studies (GWAS) that repeatedly correlate human single-nucleotide polymorphisms (SNPs) in mitochondrial genes with susceptibility to mycobacterial infection. Because GWAS are generally more successful in genetically similar human populations, we know the most about mycobacterial susceptibility loci from experiments conducted with patients infected with Mycobacterium leprae (Mlep) the causative agent of leprosy, which persists in certain isolated, rural communities. Mlep and Mtb are highly related pathogens (both encode an analogous virulence-associated ESX-1 secretion system), and the severity of both leprosy and tuberculosis is highly correlated with type I interferon responses in vivo (109, 110). Numerous susceptibility loci have been associated with exacerbated leprosy in human patients, most of them in bone fide immune sensors and signaling molecules (e.g., NOD2, IL-6, RIPK2, TNFSF15, SOCS1, IL-12B, IL-18RAP/IL-18R1, and IL-23R) (111–119). However, a curious number of leprosy-associated mutations appear in mitochondrion-related genes, again pointing to a role for this organelle in regulating immune outcomes during mycobacterial infection.
One of the most studied genes associated with leprosy susceptibility and other inflammatory disorders, like Crohn’s disease, is LRRK2 (17, 19, 111, 115, 120–123), which encodes a massive, multifunctional protein with both GTPase and kinase activity (124) (Fig. 3). Mutations in LRRK2 are the largest genetic contributor to familial PD (12, 125–127), and the best-characterized LRRK2 mutation is LRRK2G2019S, a gain-of-function allele with overactive kinase activity. While the LRRK2G2019S SNP has not been specifically identified in GWAS to confer leprosy or tuberculosis susceptibility, an SNP in a nearby amino acid also in the kinase domain (M2397T) has been linked to both leprosy and Crohn’s disease (121). Because of LRRK2’s strong links to PD, the vast majority of mechanistic studies have investigated its function, and the function of the LRRK2G2019S allele, in neurons and other cells of the central nervous system (CNS). Through that research, we have learned that LRRK2 contributes to mitochondrial homeostasis, lysosomal acidification, and autophagy/mitophagy (128–131). However, in light of its numerous genetic links to mycobacterial infection and chronic inflammatory disorders, it has become clear that LRRK2 function is not relevant solely in the brain.
FIG 3.
Mutations in functionally diverse mitochondrial proteins confer susceptibility to mycobacterial disease. Shown is a representation of proteins associated with leprosy/tuberculosis susceptibility via either GWAS or direct experimental investigation. From left to right, proteins are Presenilins-associated rhomboid-like protein (PARL), Parkinson’s disease 2 (PARK2), PTEN-induced kinase 1 (PINK1), leucine-rich repeat kinase 2 (LRRK2), mitofusin 2 (MFN2), optic atrophy 1 (OPA1), fatty acid metabolism-immunity nexus (FAMIN), mitochondrial transcription factor A (TFAM), and mitochondrial DNA polymerase gamma (POLG). The figure was created with BioRender.
How might LRRK2 and mutations in LRRK2 alter peripheral inflammatory responses and confer susceptibility to infection? First, there are several established links between LRRK2 and the autophagy pathway, a conserved process that clears both damaged mitochondria and cytosolically exposed pathogens via a lysosome-dependent process. As autophagy is a dynamic process involving transport of vesicles, a number of Rab proteins (small GTPases that play key roles in endosomal vesicle transport) are directly involved in various stages of autophagy. A recent proteomics study identified RAB10 and several related Rab family members as substrates of LRRK2 (132), and a follow-up study found that RAB10 is recruited to depolarized mitochondria in a process that is dependent on the mitophagy factors PINK1 and Parkin (129) (which are also associated with susceptibility to bacterial infection; see below). These data directly link LRRK2 kinase activity to mitophagy and suggest that mutations in LRRK2 may impact the ability of macrophages to target Mtb and/or mitochondria for turnover in autophagosomes, resulting in the accumulation of cytosolically exposed immune ligands and dysregulated innate immune responses.
Second, LRRK2 has been implicated in regulation of endosomal dynamics and phagosomal maturation. Hartlova et al. showed that LRRK2 is required for recruitment of class III phosphatidylinositol 3-kinase (PI3K) and the Beclin-1 binding protein Rubicon to phagosomes and that loss of LRRK2 promotes acidification of Mtb-containing phagosomes and limits Mtb replication in macrophages. Notably, these changes are seemingly independent of selective autophagy, as LC3 recruitment to Mtb phagosomes is unaltered by loss of LRRK2 or by the presence of the Lrrk2G2019S mutation. In vivo, they report that loss of LRRK2 restricts Mtb replication in the lungs at early time points and correlates with increased inflammatory cytokine expression (133). Subsequent work, also from the Gutierrez laboratory, showed that following pathogen-induced or sterile endomembrane damage, LRRK2 recruits Rab8A and components of the ESCRT (endosomal sorting complexes required for transport) repair complex to damaged endolysosomes, which accumulate in the absence of LRRK2 and Rab8A (134). Because the intracellular niches of both Mtb and Mlep need to be precisely regulated by both the host and pathogen, it makes sense that even slight defects in phagosomal maturation could impact the ability of these bacterial pathogens to survive and replicate. It will be interesting for future studies to determine how LRRK2 phenotypes in macrophages ex vivo translate to an in vivo model, i.e., does LRRK2’s ability to control maturation of the Mtb phagosome inside macrophages explain the importance of the protein in regulating mycobacterial disease severity? One could begin these admittedly challenging experiments by determining whether macrophages are the major cells driving Lrrk2−/− and Lrrk2 mutant phenotypes in vivo.
Third, the loss of LRRK2 can induce mitochondrial damage that leads to mito-DAMP release. Our laboratory recently found that loss of LRRK2 in human and mouse macrophages causes mitochondrial instability such that mtDNA accumulates in the cytosol, resulting in chronic cGAS stimulation and elevated basal type I interferon expression in resting macrophages (135). Consequently, these reprogrammed Lrrk2−/− macrophages produce a blunted type I IFN response following Mtb infection. We found that mitochondrial dysfunction and mito-DAMP release are driven by a combination of two major stresses endured by Lrrk2−/− cells: (i) hyperphosphorylation of the mitochondrial fission protein DRP1 and increased mitochondrial fragmentation and (ii) oxidative stress resulting from low levels of purine metabolites. These findings are reminiscent of those from an earlier study that connected LRRK2 mutations to altered mitochondrial respiratory capacity and mtDNA damage in the rat primary midbrain (136), suggesting that chronic type I IFN expression is an underappreciated contributor to LRRK2-associated PD phenotypes in the CNS.
Moving forward, it will be critically important to translate these findings in Lrrk2−/− macrophages to cells harboring actual human SNPs in LRRK2. Intriguingly, in parts of the world, 3 to 5% of the population are carriers of the LRRK2G2019S mutation, suggesting that there is a selective advantage to harboring this allele (137, 138). One could not predict such selective pressure to be driven by associated with PD, since the disease afflicts individuals who are well beyond reproductive age, and the mutation is certainly not advantageous. To address this evolutionary curiosity, recent studies have used several infection models to probe the function of LRRK2 in immunity and pathogenesis. Shutinoski et al. found that in a sepsis model, where adult mice were intravenously injected with the gut pathogen Salmonella enterica serovar Typhimurium, that mice carrying the Lrrk2G2019S allele better control infection, with reduced bacterial growth and increased animal survival times postinoculation (139). They also induced encephalitis by infecting newborn pups intranasally with reovirus and found that although mice harboring LRRK2G2019S have lower viral titers, pups harboring the mutation experience significantly increased mortality (139). Together, this important study suggests that Lrrk2G2019S mice are more prone to inflammation, which could protect against or promote disease, depending on the route of infection or type of pathogen.
Common variants of mitophagy-related factors, like PARK2 (Parkinson’s disease 2), PARL (Presenilins-associated rhomboid-like protein), and PINK1 (PTEN-induced kinase 1) (Fig. 3), also increase leprosy risk. These three proteins in particular are critical for mitochondrial turnover via mitophagy. Briefly discussed earlier, mitophagy is a conserved mechanism that tags damaged mitochondria with the ubiquitin “eat me” signal for subsequent destruction in autophagolysosomes. Mitophagy is triggered when mitochondrial depolarization stabilizes the kinase PINK1 on the mitochondrial outer membrane, where it can recruit the E3-ubiquitin ligase Parkin (PARK2). Parkin-mediated ubiquitination of mitochondrial substrates, including BCL2 (B cell lymphoma 2), mitofusins, and VDAC (voltage-dependent anion channel), provides the “eat me” signal that recruits autophagy adaptors that link the tagged/damaged mitochondria to autophagosomes (140–142). Mitophagy is highly analogous to xenophagy, whereby cytosolically exposed pathogens are recognized, tagged with ubiquitin, and targeted to autophagolysosomes (143, 144). In fact, one study found that in the absence of PARK2, significantly fewer Mtb organisms are ubiquitinated and targeted to LC3+ autophagosomes (145). Remarkably, Park2−/− mice are incredibly susceptible to Mtb infection (145). Because only about 30% of Mtb cells in any infected macrophage are ever tagged with ubiquitin and targeted to selective autophagy, this strong in vivo phenotype is actually at odds with the idea that Parkin simply restricts Mtb replication inside macrophages. While we can speculate that Park2−/− mice succumb to Mtb infection because they fail to degrade damaged mitochondria, leading to mito-DAMP accumulation and hyperinduction of type I IFN and/or inflammasome activation, such a mechanism has yet to be directly tested.
SNPs in several proteins required to maintain proper mitochondrial network structure also show up in leprosy GWAS. OPA1 (optic atrophy 1) is a mitochondrial inner membrane dynamin-like GTPase required for maintaining normal mitochondrial morphology (Fig. 3). Researchers identified two OPA1 variants that showed positive association with increased risk for lepromatous (i.e., more severe) leprosy. One allele (rs414237) correlated with lower OPA1 mRNA expression (146), suggesting that the susceptibility phenotype is attributable to the loss of OPA1. The mitochondrial fusion regulator mitofusin 2 (MFN2) has been implicated in controlling Mtb’s ability to survive and replicate inside macrophages ex vivo and in a mouse model of infection, consistent with GWAS that identified human SNPs in MFN2 associated with susceptibility to tuberculosis in a population of Han Chinese (147) (Fig. 3). While siRNA knockdown of Mfn2 in primary BMDMs restricts Mtb replication (148), Mfn2fl/fl-Cre-LysM mice are susceptible to infection with both Mtb and Listeria monocytogenes, and expression of ESAT-6 has been shown to promote interaction between MFN2 and the NLRP3 inflammasome, leading to IL-1β secretion (149). While discrepancies between ex vivo and in vivo Mfn2−/− Mtb infection phenotypes need to be resolved and the precise nature of the mitochondrial damage driving these phenotypes remains unclear, these data highlight the diverse contributions that a mitochondrial outer membrane protein and fusion regulator can have on Mtb replication and downstream inflammatory responses.
Mutations in factors required to replicate and maintain the mitochondrial genome are also associated with mycobacterial susceptibility. Mutations in TFAM, the mitochondrial transcription factor A, and POLG, the mitochondrial DNA polymerase, are associated with susceptibility to leprosy (150) (Fig. 3). In another study, knockdown of TFAM was shown to reduce Mycobacterium bovis-induced IFN-β production in BMDMs and J774A.1 macrophages (151), although these results are somewhat at odds with previously published findings whereby loss of TFAM increases cytosolic mtDNA and IFN-β expression in MEFs and BMDMs (11). It will be interesting to see whether human SNPs in TFAM result in destabilization of the mitochondrial genome and/or altered type I IFN expression in leprosy patients.
Lastly, very recent work discovered a role for FAMIN (encoded by C13orf31 or LACC1) in regulating immunometabolism through controlling the bioenergetic state of macrophages. Loss of FAMIN decreases mitochondrial ROS production and impairs the ability of macrophages to control both BCG and Salmonella intracellular replication. Intriguingly, macrophages express the highest levels of C13orf31 mRNA of any cell type (152), hinting at a connection between C13orf31 SNPs and susceptibility to macrophage-dwelling bacteria like Mtb. Indeed, a missense mutation in C13orf31 contributes to leprosy risk (153).
Together, these GWAS and related studies strongly argue that humans and/or mouse models harboring mitochondrial mutations have altered ability to control Mtb infection. But altered how? Do certain mutations protect against Mtb while others exacerbate disease? Do some mutations increase local inflammation such that Mtb replication is controlled (as is the case in Lrrk2−/− mice), or does dysregulated release of mito-DAMPs create hyperinflammation that exacerbates disease (as we predict is the case of Park2−/− mice)? A concerted comprehensive approach is needed to experimentally define how mitochondrial mutations affect release and accumulation of mito-DAMPs in vivo and the subsequent effects of these DAMPs on activating type I IFN expression, selective autophagy, and the inflammasome/pyroptosis. Only then might one be able to link certain mitochondrial phenotypes with tuberculosis patient outcomes, as in the example, high circulating mtDNA → increased type I IFN expression → acute tuberculosis disease.
TARGETING MITOCHONDRIAL FUNCTION THERAPEUTICALLY TO IMPROVE TUBERCULOSIS PATIENT OUTCOMES
Furthering our understanding of how mitochondrial dysfunction alters immune responses during infection introduces a variety of new opportunities for therapeutic intervention designed to target mitochondrial function (154). Abundant evidence suggests that modulation of mitochondria could alter Mtb infection outcomes and/or the efficacy of current treatment plans. One recent study that examined human blood RNA signatures over the course of tuberculosis treatment found that the strongest predictor of treatment failure is downregulation of mitochondrial gene expression in pretreatment patients (155). Gene expression studies in Mlep-infected macrophage cell lines and from patient nerve biopsies both revealed a global downregulation of mitochondrial gene expression associated with leprosy (156). These studies suggest that patients with compromised mitochondrial function experience more severe mycobacterial disease, but again, the molecular changes driving these outcomes are completely unknown.
Several studies have already tested mitochondria-modulating drugs during Mtb infection of cells ex vivo. Experiments designed to understand the role of cholesterol in modulating macrophage function found that treatment of THP-1 cells with a small molecule called M1 rescued mitochondrial membrane potential and restored the ability of cholesterol-treated cells to control Mtb replication (157). Likewise, treatment of Mtb-infected THP-1 cells with an inhibitor of the mitochondrial pyruvate carrier (resulting in a decrease of acetyl-coenzyme A) dramatically limited Mtb replication over a 48-h time course (158). While we are far from mechanistically understanding how such treatments alter the ability of Mtb to survive and replicate in macrophages, these types of experiments should help motivate additional studies on how we might target mitochondria to skew innate and adaptive immune responses to Mtb. Lastly, it is worth considering the consequences of tuberculosis antibiotic regimens on mitochondrial health and homeostasis. Most current tuberculosis treatment plans span between 6 and 9 months, providing ample opportunity for unintended side effects related to mitochondrial function. Rifampin, for example, has long been known to inhibit mitochondrial RNA polymerase and promote cytochrome c release (159). Pyrazinamide and isoniazid, two other first-line drugs, increase ROS production in ex vivo experiments (159). If tuberculosis treatment alters mitochondrial function in a way that promotes Mtb pathogenesis, we may be inadvertently interfering with the ability of patients’ own immune cells to control infection and/or inflammation. As pathogens like Mtb continue to acquire resistance to many first-line antibiotics, there is a growing need to understand the intricacies of the host response to infection so that we can develop host-directed interventions, including strategies that modulate mitochondrial homeostasis, to improve patient outcomes.
REFERENCES
- 1.Janeway CA, Jr, Medzhitov R. 2002. Innate immune recognition. Annu Rev Immunol 20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Khan S, Raj D, Jaiswal K, Lahiri A. 2020. Modulation of host mitochondrial dynamics during bacterial infection. Mitochondrion 53:140–149. doi: 10.1016/j.mito.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Tiku V, Tan MW, Dikic I. 2020. Mitochondrial functions in infection and immunity. Trends Cell Biol 30:263–275. doi: 10.1016/j.tcb.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faas MM, de Vos P. 2020. Mitochondrial function in immune cells in health and disease. Biochim Biophys Acta Mol Basis Dis 1866:165845. doi: 10.1016/j.bbadis.2020.165845. [DOI] [PubMed] [Google Scholar]
- 5.Ramond E, Jamet A, Coureuil M, Charbit A. 2019. Pivotal role of mitochondria in macrophage response to bacterial pathogens. Front Immunol 10:2461. doi: 10.3389/fimmu.2019.02461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spier A, Stavru F, Cossart P. 7 March 2019. Interaction between intracellular bacterial pathogens and host cell mitochondria. Microbiol Spectr doi: 10.1128/microbiolspec.BAI-0016-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand SK, Tikoo SK. 2013. Viruses as modulators of mitochondrial functions. Adv Virol 2013:738794. doi: 10.1155/2013/738794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan M, Syed GH, Kim SJ, Siddiqui A. 2015. Mitochondrial dynamics and viral infections: A close nexus. Biochim Biophys Acta 1853:2822–2833. doi: 10.1016/j.bbamcr.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley JS, Tait SW. 2020. Mitochondrial DNA in inflammation and immunity. EMBO Rep 21:e49799. doi: 10.15252/embr.201949799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhanwani R, Takahashi M, Sharma S. 2018. Cytosolic sensing of immuno-stimulatory DNA, the enemy within. Curr Opin Immunol 50:82–87. doi: 10.1016/j.coi.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. 2015. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessi DR, Sammler E. 2018. LRRK2 kinase in Parkinson's disease. Science 360:36–37. doi: 10.1126/science.aar5683. [DOI] [PubMed] [Google Scholar]
- 13.Anand VS, Braithwaite SP. 2009. LRRK2 in Parkinson's disease: biochemical functions. FEBS J 276:6428–6435. doi: 10.1111/j.1742-4658.2009.07341.x. [DOI] [PubMed] [Google Scholar]
- 14.Braithwaite SP. 2009. LRRK2 in Parkinson's disease: building an understanding of disease etiology. FEBS J 276:6427. doi: 10.1111/j.1742-4658.2009.07340.x. [DOI] [PubMed] [Google Scholar]
- 15.Brice A. 2005. Genetics of Parkinson's disease: LRRK2 on the rise. Brain 128:2760–2762. doi: 10.1093/brain/awh676. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Luo Z, Ward C, Ibanez DP, Liu H, Zhong X, Sharma NK, Qin B, Fan W, Wang D. 2020. Generation of two LRRK2 homozygous knockout human induced pluripotent stem cell lines using CRISPR/Cas9. Stem Cell Res 45:101804. doi: 10.1016/j.scr.2020.101804. [DOI] [PubMed] [Google Scholar]
- 17.Ikezu T, Koro L, Wolozin B, Farraye FA, Strongosky AJ, Wszolek ZK. 2020. Crohn's and Parkinson's disease-associated LRRK2 mutations alter type II interferon responses in human CD14(+) blood monocytes ex vivo. J Neuroimmune Pharmacol 15:794–800. doi: 10.1007/s11481-020-09909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu TC, Naito T, Liu Z, VanDussen KL, Haritunians T, Li D, Endo K, Kawai Y, Nagasaki M, Kinouchi Y, McGovern DP, Shimosegawa T, Kakuta Y, Stappenbeck TS. 2017. LRRK2 but not ATG16L1 is associated with Paneth cell defect in Japanese Crohn's disease patients. JCI Insight 2:e91917. doi: 10.1172/jci.insight.91917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridler C. 2018. Parkinson disease: LRRK2 variants linked to PD and Crohn's disease. Nat Rev Neurol 14:126. doi: 10.1038/nrneurol.2018.10. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Lenardo MJ. 2012. The role of LRRK2 in inflammatory bowel disease. Cell Res 22:1092–1094. doi: 10.1038/cr.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ. 2011. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol 12:1063–1070. doi: 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vora P, McGovern DP. 2012. LRRK2 as a negative regulator of NFAT: implications for the pathogenesis of inflammatory bowel disease. Expert Rev Clin Immunol 8:227–229. doi: 10.1586/eci.12.11. [DOI] [PubMed] [Google Scholar]
- 23.Busch KB, Kowald A, Spelbrink JN. 2014. Quality matters: how does mitochondrial network dynamics and quality control impact on mtDNA integrity? Philos Trans R Soc Lond B Biol Sci 369:20130442. doi: 10.1098/rstb.2013.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lackner LL. 2014. Shaping the dynamic mitochondrial network. BMC Biol 12:35. doi: 10.1186/1741-7007-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie LL, Shi F, Tan Z, Li Y, Bode AM, Cao Y. 2018. Mitochondrial network structure homeostasis and cell death. Cancer Sci 109:3686–3694. doi: 10.1111/cas.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao CH, Wang R, Wang Y, Kung CP, Weber JD, Patti GJ. 2019. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. Elife 8:e41351. doi: 10.7554/eLife.41351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youle RJ, Karbowski M. 2005. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 28.Ma K, Chen G, Li W, Kepp O, Zhu Y, Chen Q. 2020. Mitophagy, mitochondrial homeostasis, and cell fate. Front Cell Dev Biol 8:467. doi: 10.3389/fcell.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spinelli JB, Haigis MC. 2018. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol 20:745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. 2012. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 16:1150–1180. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bock FJ, Tait SWG. 2020. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 32.Tait SW, Green DR. 2013. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol 5:a008706. doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta MM, Weinberg SE, Chandel NS. 2017. Mitochondrial control of immunity: beyond ATP. Nat Rev Immunol 17:608–620. doi: 10.1038/nri.2017.66. [DOI] [PubMed] [Google Scholar]
- 34.Liu PS, Ho PC. 2018. Mitochondria: a master regulator in macrophage and T cell immunity. Mitochondrion 41:45–50. doi: 10.1016/j.mito.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Geltink RIK, Kyle RL, Pearce EL. 2018. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol 36:461–488. doi: 10.1146/annurev-immunol-042617-053019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley JS, Tait SW. 2019. Mitochondria and pathogen immunity: from killer to firestarter. EMBO J 38:e102325. doi: 10.15252/embj.2019102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West AP. 2017. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 391:54–63. doi: 10.1016/j.tox.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 38.West AP, Shadel GS, Ghosh S. 2011. Mitochondria in innate immune responses. Nat Rev Immunol 11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youle RJ. 2019. Mitochondria-striking a balance between host and endosymbiont. Science 365:eaaw9855. doi: 10.1126/science.aaw9855. [DOI] [PubMed] [Google Scholar]
- 40.Boguszewska K, Szewczuk M, Kaźmierczak-Barańska J, Karwowski BT. 2020. The similarities between human mitochondria and bacteria in the context of structure, genome, and base excision repair system. Molecules 25:2857. doi: 10.3390/molecules25122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrido N, Griparic L, Jokitalo E, Wartiovaara J, van der Bliek AM, Spelbrink JN. 2003. Composition and dynamics of human mitochondrial nucleoids. Mol Biol Cell 14:1583–1596. doi: 10.1091/mbc.e02-07-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman BA, Durisic N, Mativetsky JM, Costantino S, Hancock MA, Grutter P, Shoubridge EA. 2007. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol Biol Cell 18:3225–3236. doi: 10.1091/mbc.e07-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills EL, Kelly B, O'Neill LAJ. 2017. Mitochondria are the powerhouses of immunity. Nat Immunol 18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 44.Vazquez C, Horner SM. 2015. MAVS coordination of antiviral innate immunity. J Virol 89:6974–6977. doi: 10.1128/JVI.01918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banoth B, Cassel SL. 2018. Mitochondria in innate immune signaling. Transl Res 202:52–68. doi: 10.1016/j.trsl.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutta S, Das N, Mukherjee P. 2020. Picking up a fight: fine tuning mitochondrial innate immune defenses against RNA viruses. Front Microbiol 11:1990. doi: 10.3389/fmicb.2020.01990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, Geoghegan ND, Chappaz S, Davidson S, San Chin H, Lane RM, Dramicanin M, Saunders TL, Sugiana C, Lessene R, Osellame LD, Chew TL, Dewson G, Lazarou M, Ramm G, Lessene G, Ryan MT, Rogers KL, van Delft MF, Kile BT. 2018. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359:eaao6047. doi: 10.1126/science.aao6047. [DOI] [PubMed] [Google Scholar]
- 48.Rongvaux A, Jackson R, Harman CC, Li T, West AP, de Zoete MR, Wu Y, Yordy B, Lakhani SA, Kuan CY, Taniguchi T, Shadel GS, Chen ZJ, Iwasaki A, Flavell RA. 2014. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, Huang DC, Kile BT. 2014. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang LS, Hong Z, Wu W, Xiong S, Zhong M, Gao X, Rehman J, Malik AB. 2020. mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity 52:475–486. doi: 10.1016/j.immuni.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerur N, Fukuda S, Banerjee D, Kim Y, Fu D, Apicella I, Varshney A, Yasuma R, Fowler BJ, Baghdasaryan E, Marion KM, Huang X, Yasuma T, Hirano Y, Serbulea V, Ambati M, Ambati VL, Kajiwara Y, Ambati K, Hirahara S, Bastos-Carvalho A, Ogura Y, Terasaki H, Oshika T, Kim KB, Hinton DR, Leitinger N, Cambier JC, Buxbaum JD, Kenney MC, Jazwinski SM, Nagai H, Hara I, West AP, Fitzgerald KA, Sadda SR, Gelfand BD, Ambati J. 2018. cGAS drives noncanonical-inflammasome activation in age-related macular degeneration. Nat Med 24:50–61. doi: 10.1038/nm.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Julian MW, Shao G, Bao S, Knoell DL, Papenfuss TL, VanGundy ZC, Crouser ED. 2012. Mitochondrial transcription factor A serves as a danger signal by augmenting plasmacytoid dendritic cell responses to DNA. J Immunol 189:433–443. doi: 10.4049/jimmunol.1101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. 2012. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bielecki J, Youngman P, Connelly P, Portnoy DA. 1990. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 55.Tilney LG, Portnoy DA. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol 109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. 2011. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun 79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodward JJ, Iavarone AT, Portnoy DA. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins AC, Cai H, Li T, Franco LH, Li XD, Nair VR, Scharn CR, Stamm CE, Levine B, Chen ZJ, Shiloh MU. 2015. Cyclic GMP-AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe 17:820–828. doi: 10.1016/j.chom.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, Schmid-Burgk JL, Schmidt T, Hornung V, Cole ST, Ablasser A. 2015. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe 17:799–810. doi: 10.1016/j.chom.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. 2015. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17:811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamashiro LH, Wilson SC, Morrison HM, Karalis V, Chung JJ, Chen KJ, Bateup HS, Szpara ML, Lee AY, Cox JS, Vance RE. 2020. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat Commun 11:3382. doi: 10.1038/s41467-020-17156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, Bishai WR. 2015. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med 21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schurz H, Daya M, Moller M, Hoal EG, Salie M. 2015. TLR1, 2, 4, 6 and 9 variants associated with tuberculosis susceptibility: a systematic review and meta-analysis. PLoS One 10:e0139711. doi: 10.1371/journal.pone.0139711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mortaz E, Adcock IM, Tabarsi P, Masjedi MR, Mansouri D, Velayati AA, Casanova JL, Barnes PJ. 2015. Interaction of pattern recognition receptors with Mycobacterium tuberculosis. J Clin Immunol 35:1–10. doi: 10.1007/s10875-014-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bharti D, Kumar A, Mahla RS, Kumar S, Ingle H, Shankar H, Joshi B, Raut AA, Kumar H. 2014. The role of TLR9 polymorphism in susceptibility to pulmonary tuberculosis. Immunogenetics 66:675–681. doi: 10.1007/s00251-014-0806-1. [DOI] [PubMed] [Google Scholar]
- 66.Cheng Y, Schorey JS. 2018. Mycobacterium tuberculosis-induced IFN-beta production requires cytosolic DNA and RNA sensing pathways. J Exp Med 215:2919–2935. doi: 10.1084/jem.20180508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patrick KL, Bell SL, Watson RO. 2016. For better or worse: cytosolic DNA sensing during intracellular bacterial infection induces potent innate immune responses. J Mol Biol 428:3372–3386. doi: 10.1016/j.jmb.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 68.Vanaja SK, Rathinam VA, Fitzgerald KA. 2015. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol 25:308–315. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bryant C, Fitzgerald KA. 2009. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol 19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Zhou R, Yazdi AS, Menu P, Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 71.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS. 2013. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. 2009. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A 106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 74.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. 2011. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. 2012. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, Tuting T, Hartmann G, Barchet W. 2013. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun 67:2585–2589. doi: 10.1128/IAI.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. 2010. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol 184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Juffermans NP, Florquin S, Camoglio L, Verbon A, Kolk AH, Speelman P, van Deventer SJ, van Der Poll T. 2000. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J Infect Dis 182:902–908. doi: 10.1086/315771. [DOI] [PubMed] [Google Scholar]
- 80.Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M, Takeda K. 2012. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol 24:637–644. doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- 81.Saiga H, Nieuwenhuizen N, Gengenbacher M, Koehler AB, Schuerer S, Moura-Alves P, Wagner I, Mollenkopf HJ, Dorhoi A, Kaufmann SH. 2015. The Recombinant BCG DeltaureC::hly Vaccine Targets the AIM2 Inflammasome to Induce Autophagy and Inflammation. J Infect Dis 211:1831–1841. doi: 10.1093/infdis/jiu675. [DOI] [PubMed] [Google Scholar]
- 82.Shah S, Bohsali A, Ahlbrand SE, Srinivasan L, Rathinam VA, Vogel SN, Fitzgerald KA, Sutterwala FS, Briken V. 2013. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-beta and AIM2 inflammasome-dependent IL-1beta production via its ESX-1 secretion system. J Immunol 191:3514–3518. doi: 10.4049/jimmunol.1301331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quigley J, Hughitt VK, Velikovsky CA, Mariuzza RA, El-Sayed NM, Briken V. 2017. The cell wall lipid pdim contributes to phagosomal escape and host cell exit of Mycobacterium tuberculosis. mBio 8:e00148-17. doi: 10.1128/mBio.00148-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lerner TR, Queval CJ, Fearns A, Repnik U, Griffiths G, Gutierrez MG. 2018. Phthiocerol dimycocerosates promote access to the cytosol and intracellular burden of Mycobacterium tuberculosis in lymphatic endothelial cells. BMC Biol 16:1. doi: 10.1186/s12915-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osman MM, Pagan AJ, Shanahan JK, Ramakrishnan L. 2020. Mycobacterium marinum phthiocerol dimycocerosates enhance macrophage phagosomal permeabilization and membrane damage. PLoS One 15:e0233252. doi: 10.1371/journal.pone.0233252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Augenstreich J, Haanappel E, Sayes F, Simeone R, Guillet V, Mazeres S, Chalut C, Mourey L, Brosch R, Guilhot C, Astarie-Dequeker C. 2020. Phthiocerol dimycocerosates from Mycobacterium tuberculosis increase the membrane activity of bacterial effectors and host receptors. Front Cell Infect Microbiol 10:420. doi: 10.3389/fcimb.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Augenstreich J, Arbues A, Simeone R, Haanappel E, Wegener A, Sayes F, Le Chevalier F, Chalut C, Malaga W, Guilhot C, Brosch R, Astarie-Dequeker C. 15 February 2017. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol doi: 10.1111/cmi.12726. [DOI] [PubMed] [Google Scholar]
- 88.de Jonge MI, Pehau-Arnaudet G, Fretz MM, Romain F, Bottai D, Brodin P, Honore N, Marchal G, Jiskoot W, England P, Cole ST, Brosch R. 2007. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol 189:6028–6034. doi: 10.1128/JB.00469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ray S, Vazquez Reyes S, Xiao C, Sun J. 2019. Effects of membrane lipid composition on Mycobacterium tuberculosis EsxA membrane insertion: a dual play of fluidity and charge. Tuberculosis 118:101854. doi: 10.1016/j.tube.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Leon J, Jiang G, Ma Y, Rubin E, Fortune S, Sun J. 2012. Mycobacterium tuberculosis ESAT-6 exhibits a unique membrane-interacting activity that is not found in its ortholog from non-pathogenic Mycobacterium smegmatis. J Biol Chem 287:44184–44191. doi: 10.1074/jbc.M112.420869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Q, Wang D, Jiang G, Liu W, Deng Q, Li X, Qian W, Ouellet H, Sun J. 2016. EsxA membrane-permeabilizing activity plays a key role in mycobacterial cytosolic translocation and virulence: effects of single-residue mutations at glutamine 5. Sci Rep 6:32618. doi: 10.1038/srep32618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conrad WH, Osman MM, Shanahan JK, Chu F, Takaki KK, Cameron J, Hopkinson-Woolley D, Brosch R, Ramakrishnan L. 2017. Mycobacterial ESX-1 secretion system mediates host cell lysis through bacterium contact-dependent gross membrane disruptions. Proc Natl Acad Sci U S A 114:1371–1376. doi: 10.1073/pnas.1620133114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu J, Tran V, Li M, Huang X, Niu C, Wang D, Zhu J, Wang J, Gao Q, Liu J. 2012. Both phthiocerol dimycocerosates and phenolic glycolipids are required for virulence of Mycobacterium marinum. Infect Immun 80:1381–1389. doi: 10.1128/IAI.06370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Astarie-Dequeker C, Le Guyader L, Malaga W, Seaphanh FK, Chalut C, Lopez A, Guilhot C. 2009. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog 5:e1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watson RO, Manzanillo PS, Cox JS. 2012. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barczak AK, Avraham R, Singh S, Luo SS, Zhang WR, Bray MA, Hinman AE, Thompson M, Nietupski RM, Golas A, Montgomery P, Fitzgerald M, Smith RS, White DW, Tischler AD, Carpenter AE, Hung DT. 2017. Systematic, multiparametric analysis of Mycobacterium tuberculosis intracellular infection offers insight into coordinated virulence. PLoS Pathog 13:e1006363. doi: 10.1371/journal.ppat.1006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wiens KE, Ernst JD. 2016. The mechanism for type I interferon induction by Mycobacterium tuberculosis is bacterial strain-dependent. PLoS Pathog 12:e1005809. doi: 10.1371/journal.ppat.1005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lienard J, Nobs E, Lovins V, Movert E, Valfridsson C, Carlsson F. 2020. The Mycobacterium marinum ESX-1 system mediates phagosomal permeabilization and type I interferon production via separable mechanisms. Proc Natl Acad Sci U S A 117:1160–1166. doi: 10.1073/pnas.1911646117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yabaji SM, Dhamija E, Mishra AK, Srivastava KK. 2020. ESAT-6 regulates autophagous response through SOD-2 and as a result induces intracellular survival of Mycobacterium bovis BCG. Biochim Biophys Acta Proteins Proteom 1868:140470. doi: 10.1016/j.bbapap.2020.140470. [DOI] [PubMed] [Google Scholar]
- 100.Cumming BM, Addicott KW, Adamson JH, Steyn AJ. 2018. Mycobacterium tuberculosis induces decelerated bioenergetic metabolism in human macrophages. Elife 7:e39169. doi: 10.7554/eLife.39169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hackett EE, Charles-Messance H, O'Leary SM, Gleeson LE, Munoz-Wolf N, Case S, Wedderburn A, Johnston DGW, Williams MA, Smyth A, Ouimet M, Moore KJ, Lavelle EC, Corr SC, Gordon SV, Keane J, Sheedy FJ. 2020. Mycobacterium tuberculosis limits host glycolysis and il-1beta by restriction of PFK-M via microRNA-21. Cell Rep 30:124–136. doi: 10.1016/j.celrep.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sancho D, Enamorado M, Garaude J. 2017. Innate immune function of mitochondrial metabolism. Front Immunol 8:527. doi: 10.3389/fimmu.2017.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aguilar-Lopez BA, Correa F, Moreno-Altamirano MMB, Espitia C, Hernandez-Longoria R, Oliva-Ramirez J, Padierna-Olivos J, Sanchez-Garcia FJ. 2019. LprG and PE_PGRS33 Mycobacterium tuberculosis virulence factors induce differential mitochondrial dynamics in macrophages. Scand J Immunol 89:e12728. doi: 10.1111/sji.12728. [DOI] [PubMed] [Google Scholar]
- 104.Cadieux N, Parra M, Cohen H, Maric D, Morris SL, Brennan MJ. 2011. Induction of cell death after localization to the host cell mitochondria by the Mycobacterium tuberculosis PE_PGRS33 protein. Microbiology 157:793–804. doi: 10.1099/mic.0.041996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Penn BH, Netter Z, Johnson JR, Von Dollen J, Jang GM, Johnson T, Ohol YM, Maher C, Bell SL, Geiger K, Golovkine G, Du X, Choi A, Parry T, Mohapatra BC, Storck MD, Band H, Chen C, Jager S, Shales M, Portnoy DA, Hernandez R, Coscoy L, Cox JS, Krogan NJ. 2018. An Mtb-human protein-protein interaction map identifies a switch between host antiviral and antibacterial responses. Mol Cell 71:637–648. doi: 10.1016/j.molcel.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sohn H, Kim JS, Shin SJ, Kim K, Won CJ, Kim WS, Min KN, Choi HG, Lee JC, Park JK, Kim HJ. 2011. Targeting of Mycobacterium tuberculosis heparin-binding hemagglutinin to mitochondria in macrophages. PLoS Pathog 7:e1002435. doi: 10.1371/journal.ppat.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joseph S, Yuen A, Singh V, Hmama Z. 2017. Mycobacterium tuberculosis Cpn60.2 (GroEL2) blocks macrophage apoptosis via interaction with mitochondrial mortalin. Biol Open 6:481–488. doi: 10.1242/bio.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tundup S, Mohareer K, Hasnain SE. 2014. Mycobacterium tuberculosis PE25/PPE41 protein complex induces necrosis in macrophages: Role in virulence and disease reactivation? FEBS Open Biol 4:822–828. doi: 10.1016/j.fob.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, Komisopoulou E, Kelly-Scumpia K, Chun R, Iyer SS, Sarno EN, Rea TH, Hewison M, Adams JS, Popper SJ, Relman DA, Stenger S, Bloom BR, Cheng G, Modlin RL. 2013. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science 339:1448–1453. doi: 10.1126/science.1233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Toledo Pinto TG, Batista-Silva LR, Medeiros RCA, Lara FA, Moraes MO. 2018. Type I interferons, autophagy and host metabolism in leprosy. Front Immunol 9:806. doi: 10.3389/fimmu.2018.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marcinek P, Jha AN, Shinde V, Sundaramoorthy A, Rajkumar R, Suryadevara NC, Neela SK, van Tong H, Balachander V, Valluri VL, Thangaraj K, Velavan TP. 2013. LRRK2 and RIPK2 variants in the NOD 2-mediated signaling pathway are associated with susceptibility to Mycobacterium leprae in Indian populations. PLoS One 8:e73103. doi: 10.1371/journal.pone.0073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sales-Marques C, Salomao H, Fava VM, Alvarado-Arnez LE, Amaral EP, Cardoso CC, Dias-Batista IM, da Silva WL, Medeiros P, da Cunha Lopes Virmond M, Lana FC, Pacheco AG, Moraes MO, Mira MT, Pereira Latini AC. 2014. NOD2 and CCDC122-LACC1 genes are associated with leprosy susceptibility in Brazilians. Hum Genet 133:1525–1532. doi: 10.1007/s00439-014-1502-9. [DOI] [PubMed] [Google Scholar]
- 113.Xiong JH, Mao C, Sha XW, Jin Z, Wang H, Liu YY, Ning Y. 2016. Association between genetic variants in NOD2, C13orf31, and CCDC122 genes and leprosy among the Chinese Yi population. Int J Dermatol 55:65–69. doi: 10.1111/ijd.12981. [DOI] [PubMed] [Google Scholar]
- 114.Zhang F, Liu H, Chen S, Low H, Sun L, Cui Y, Chu T, Li Y, Fu X, Yu Y, Yu G, Shi B, Tian H, Liu D, Yu X, Li J, Lu N, Bao F, Yuan C, Liu J, Liu H, Zhang L, Sun Y, Chen M, Yang Q, Yang H, Yang R, Zhang L, Wang Q, Liu H, Zuo F, Zhang H, Khor CC, Hibberd ML, Yang S, Liu J, Zhang X. 2011. Identification of two new loci at IL23R and RAB32 that influence susceptibility to leprosy. Nat Genet 43:1247–1251. doi: 10.1038/ng.973. [DOI] [PubMed] [Google Scholar]
- 115.Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, Cui Y, Yan XX, Yang HT, Yang RD, Chu TS, Zhang C, Zhang L, Han JW, Yu GQ, Quan C, Yu YX, Zhang Z, Shi BQ, Zhang LH, Cheng H, Wang CY, Lin Y, Zheng HF, Fu XA, Zuo XB, Wang Q, Long H, Sun YP, Cheng YL, Tian HQ, Zhou FS, Liu HX, Lu WS, He SM, Du WL, Shen M, Jin QY, Wang Y, Low HQ, Erwin T, Yang NH, Li JY, Zhao X, Jiao YL, Mao LG, Yin G, Jiang ZX, Wang XD, Yu JP. 2009. Genomewide association study of leprosy. N Engl J Med 361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 116.Ali S, Srivastava AK, Chopra R, Aggarwal S, Garg VK, Bhattacharya SN, Bamezai RN. 2013. IL12B SNPs and copy number variation in IL23R gene associated with susceptibility to leprosy. J Med Genet 50:34–42. doi: 10.1136/jmedgenet-2012-101214. [DOI] [PubMed] [Google Scholar]
- 117.Liu H, Irwanto A, Tian H, Fu X, Yu Y, Yu G, Low H, Chu T, Li Y, Shi B, Chen M, Sun Y, Yuan C, Lu N, You J, Bao F, Li J, Liu J, Liu H, Liu D, Yu X, Zhang L, Yang Q, Wang N, Niu G, Ma S, Zhou Y, Wang C, Chen S, Zhang X, Liu J, Zhang F. 2012. Identification of IL18RAP/IL18R1 and IL12B as leprosy risk genes demonstrates shared pathogenesis between inflammation and infectious diseases. Am J Hum Genet 91:935–941. doi: 10.1016/j.ajhg.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu H, Irwanto A, Fu X, Yu G, Yu Y, Sun Y, Wang C, Wang Z, Okada Y, Low H, Li Y, Liany H, Chen M, Bao F, Li J, You J, Zhang Q, Liu J, Chu T, Andiappan AK, Wang N, Niu G, Liu D, Yu X, Zhang L, Tian H, Zhou G, Rotzschke O, Chen S, Zhang X, Liu J, Zhang F. 2015. Discovery of six new susceptibility loci and analysis of pleiotropic effects in leprosy. Nat Genet 47:267–271. doi: 10.1038/ng.3212. [DOI] [PubMed] [Google Scholar]
- 119.Sales-Marques C, Cardoso CC, Alvarado-Arnez LE, Illaramendi X, Sales AM, Hacker MA, Barbosa MGM, Nery J, Pinheiro RO, Sarno EN, Pacheco AG, Moraes MO. 2017. Genetic polymorphisms of the IL6 and NOD2 genes are risk factors for inflammatory reactions in leprosy. PLoS Negl Trop Dis 11:e0005754. doi: 10.1371/journal.pntd.0005754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cardoso CC, Pereira AC, de Sales Marques C, Moraes MO. 2011. Leprosy susceptibility: genetic variations regulate innate and adaptive immunity, and disease outcome. Future Microbiol 6:533–549. doi: 10.2217/fmb.11.39. [DOI] [PubMed] [Google Scholar]
- 121.Fava VM, Manry J, Cobat A, Orlova M, Van Thuc N, Ba NN, Thai VH, Abel L, Alcais A, Schurr E, Canadian Lrrk2 in Inflammation Team . 2016. A missense LRRK2 variant is a risk factor for excessive inflammatory responses in leprosy. PLoS Negl Trop Dis 10:e0004412. doi: 10.1371/journal.pntd.0004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang D, Xu L, Lv L, Su LY, Fan Y, Zhang DF, Bi R, Yu D, Zhang W, Li XA, Li YY, Yao YG. 2015. Association of the LRRK2 genetic polymorphisms with leprosy in Han Chinese from Southwest China. Genes Immun 16:112–119. doi: 10.1038/gene.2014.72. [DOI] [PubMed] [Google Scholar]
- 123.Derkinderen P, Neunlist M. 2018. Crohn's and Parkinson disease: is LRRK2 lurking around the corner? Nat Rev Gastroenterol Hepatol 15:330–331. doi: 10.1038/s41575-018-0006-9. [DOI] [PubMed] [Google Scholar]
- 124.Blanca Ramirez M, Lara Ordonez AJ, Fdez E, Hilfiker S. 2017. LRRK2: from kinase to GTPase to microtubules and back. Biochem Soc Trans 45:141–146. doi: 10.1042/BST20160333. [DOI] [PubMed] [Google Scholar]
- 125.Berwick DC, Harvey K. 2011. LRRK2 signaling pathways: the key to unlocking neurodegeneration? Trends Cell Biol 21:257–265. doi: 10.1016/j.tcb.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 126.Blanca Ramirez M, Madero-Perez J, Rivero-Rios P, Martinez-Salvador M, Lara Ordonez AJ, Fernandez B, Fdez E, Hilfiker S. 2017. LRRK2 and Parkinson's disease: from lack of structure to gain of function. Curr Protein Pept Sci 18:677–686. doi: 10.2174/1389203717666160311121748. [DOI] [PubMed] [Google Scholar]
- 127.Bonifati V. 2006. Parkinson's disease: the LRRK2-G2019S mutation: opening a novel era in Parkinson's disease genetics. Eur J Hum Genet 14:1061–1062. doi: 10.1038/sj.ejhg.5201695. [DOI] [PubMed] [Google Scholar]
- 128.Singh A, Zhi L, Zhang H. 2019. LRRK2 and mitochondria: Recent advances and current views. Brain Res 1702:96–104. doi: 10.1016/j.brainres.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wauters F, Cornelissen T, Imberechts D, Martin S, Koentjoro B, Sue C, Vangheluwe P, Vandenberghe W. 2020. LRRK2 mutations impair depolarization-induced mitophagy through inhibition of mitochondrial accumulation of RAB10. Autophagy 16:203–222. doi: 10.1080/15548627.2019.1603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roosen DA, Cookson MR. 2016. LRRK2 at the interface of autophagosomes, endosomes and lysosomes. Mol Neurodegener 11:73. doi: 10.1186/s13024-016-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Manzoni C. 2012. LRRK2 and autophagy: a common pathway for disease. Biochem Soc Trans 40:1147–1151. doi: 10.1042/BST20120126. [DOI] [PubMed] [Google Scholar]
- 132.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, Mann M. 2016. Phosphoproteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5:e12813. doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hartlova A, Herbst S, Peltier J, Rodgers A, Bilkei-Gorzo O, Fearns A, Dill BD, Lee H, Flynn R, Cowley SA, Davies P, Lewis PA, Ganley IG, Martinez J, Alessi DR, Reith AD, Trost M, Gutierrez MG. 2018. LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J 37:e98694. doi: 10.15252/embj.201798694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herbst S, Campbell P, Harvey J, Bernard EM, Papayannopoulos V, Wood NW, Morris HR, Gutierrez MG. 2020. LRRK2 activation controls the repair of damaged endomembranes in macrophages. EMBO J 39:e104494. doi: 10.15252/embj.2020104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weindel CG, Bell SL, Vail KJ, West KO, Patrick KL, Watson RO. 2020. LRRK2 maintains mitochondrial homeostasis and regulates innate immune responses to Mycobacterium tuberculosis. Elife 9:e51071. doi: 10.7554/eLife.51071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Howlett EH, Jensen N, Belmonte F, Zafar F, Hu X, Kluss J, Schule B, Kaufman BA, Greenamyre JT, Sanders LH. 2017. LRRK2 G2019S-induced mitochondrial DNA damage is LRRK2 kinase dependent and inhibition restores mtDNA integrity in Parkinson's disease. Hum Mol Genet 26:4340–4351. doi: 10.1093/hmg/ddx320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Berg D, Schweitzer KJ, Leitner P, Zimprich A, Lichtner P, Belcredi P, Brussel T, Schulte C, Maass S, Nagele T, Wszolek ZK, Gasser T. 2005. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson's disease. Brain 128:3000–3011. doi: 10.1093/brain/awh666. [DOI] [PubMed] [Google Scholar]
- 138.Schulte C, Gasser T. 2011. Genetic basis of Parkinson's disease: inheritance, penetrance, and expression. Appl Clin Genet 4:67–80. doi: 10.2147/TACG.S11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shutinoski B, Hakimi M, Harmsen IE, Lunn M, Rocha J, Lengacher N, Zhou YY, Khan J, Nguyen A, Hake-Volling Q, El-Kodsi D, Li J, Alikashani A, Beauchamp C, Majithia J, Coombs K, Shimshek D, Marcogliese PC, Park DS, Rioux JD, Philpott DJ, Woulfe JM, Hayley S, Sad S, Tomlinson JJ, Brown EG, Schlossmacher MG. 2019. Lrrk2 alleles modulate inflammation during microbial infection of mice in a sex-dependent manner. Sci Transl Med 11:eaas9292. doi: 10.1126/scitranslmed.aas9292. [DOI] [PubMed] [Google Scholar]
- 140.Durcan TM, Fon EA. 2015. The three 'P's of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev 29:989–999. doi: 10.1101/gad.262758.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Escobar-Henriques M, Joaquim M. 2019. Mitofusins: disease gatekeepers and hubs in mitochondrial quality control by E3 ligases. Front Physiol 10:517. doi: 10.3389/fphys.2019.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Narendra DP, Youle RJ. 2011. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal 14:1929–1938. doi: 10.1089/ars.2010.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sharma V, Verma S, Seranova E, Sarkar S, Kumar D. 2018. Selective autophagy and xenophagy in infection and disease. Front Cell Dev Biol 6:147. doi: 10.3389/fcell.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patrick KL, Bell SL, Weindel CG, Watson RO. 2019. Exploring the "multiple-hit hypothesis" of neurodegenerative disease: bacterial infection comes up to bat. Front Cell Infect Microbiol 9:138. doi: 10.3389/fcimb.2019.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, Cox JS. 2013. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 501:512–516. doi: 10.1038/nature12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiang YL, Zhang DF, Wang D, Li YY, Yao YG. 2015. Common variants of OPA1 conferring genetic susceptibility to leprosy in Han Chinese from southwest China. J Dermatol Sci 80:133–141. doi: 10.1016/j.jdermsci.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 147.Qi H, Zhang YB, Sun L, Chen C, Xu B, Xu F, Liu JW, Liu JC, Chen C, Jiao WW, Shen C, Xiao J, Li JQ, Guo YJ, Wang YH, Li QJ, Yin QQ, Li YJ, Wang T, Wang XY, Gu ML, Yu J, Shen AD. 2017. Discovery of susceptibility loci associated with tuberculosis in Han Chinese. Hum Mol Genet 26:4752–4763. doi: 10.1093/hmg/ddx365. [DOI] [PubMed] [Google Scholar]
- 148.Lee J, Choi JA, Cho SN, Son SH, Song CH. 2019. Mitofusin 2-deficiency suppresses Mycobacterium tuberculosis survival in macrophages. Cells 8:1355. doi: 10.3390/cells8111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tur J, Pereira-Lopes S, Vico T, Marin EA, Munoz JP, Hernandez-Alvarez M, Cardona PJ, Zorzano A, Lloberas J, Celada A. 2020. Mitofusin 2 in macrophages links mitochondrial ROS production, cytokine release, phagocytosis, autophagy, and bactericidal activity. Cell Rep 32:108079. doi: 10.1016/j.celrep.2020.108079. [DOI] [PubMed] [Google Scholar]
- 150.Wang D, Li GD, Fan Y, Zhang DF, Bi R, Yu XF, Long H, Li YY, Yao YG. 2017. The mtDNA replication-related genes TFAM and POLG are associated with leprosy in Han Chinese from southwest China. J Dermatol Sci 88:349–356. doi: 10.1016/j.jdermsci.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 151.Song Y, Hussain T, Wang J, Liao Y, Yue R, Sabir N, Zhao D, Zhou X. 2020. Mitochondrial transcription factor A regulates Mycobacterium bovis-induced IFN-beta production by modulating mitochondrial DNA replication in macrophages. J Infect Dis 221:438–448. doi: 10.1093/infdis/jiz461. [DOI] [PubMed] [Google Scholar]
- 152.Cader MZ, Boroviak K, Zhang Q, Assadi G, Kempster SL, Sewell GW, Saveljeva S, Ashcroft JW, Clare S, Mukhopadhyay S, Brown KP, Tschurtschenthaler M, Raine T, Doe B, Chilvers ER, Griffin JL, Kaneider NC, Floto RA, D'Amato M, Bradley A, Wakelam MJ, Dougan G, Kaser A. 2016. C13orf31 (FAMIN) is a central regulator of immunometabolic function. Nat Immunol 17:1046–1056. doi: 10.1038/ni.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang D, Fan Y, Malhi M, Bi R, Wu Y, Xu M, Yu XF, Long H, Li YY, Zhang DF, Yao YG. 2018. Missense variants in HIF1A and LACC1 contribute to leprosy risk in Han Chinese. Am J Hum Genet 102:794–805. doi: 10.1016/j.ajhg.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sheedy FJ, Divangahi M. 2021. Targeting immunometabolism in host defense against Mycobacterium tuberculosis. Immunology 162:145–159. doi: 10.1111/imm.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thompson EG, Du Y, Malherbe ST, Shankar S, Braun J, Valvo J, Ronacher K, Tromp G, Tabb DL, Alland D, Shenai S, Via LE, Warwick J, Aderem A, Scriba TJ, Winter J, Walzl G, Zak DE, Catalysis TB-Biomarker Consortium . 2017. Host blood RNA signatures predict the outcome of tuberculosis treatment. Tuberculosis 107:48–58. doi: 10.1016/j.tube.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guerreiro LT, Robottom-Ferreira AB, Ribeiro-Alves M, Toledo-Pinto TG, Rosa Brito T, Rosa PS, Sandoval FG, Jardim MR, Antunes SG, Shannon EJ, Sarno EN, Pessolani MC, Williams DL, Moraes MO. 2013. Gene expression profiling specifies chemokine, mitochondrial and lipid metabolism signatures in leprosy. PLoS One 8:e64748. doi: 10.1371/journal.pone.0064748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Asalla S, Mohareer K, Banerjee S. 2017. Small molecule mediated restoration of mitochondrial function augments anti-mycobacterial activity of human macrophages subjected to cholesterol induced asymptomatic dyslipidemia. Front Cell Infect Microbiol 7:439. doi: 10.3389/fcimb.2017.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mehrotra P, Rao KVS, Chatterjee S. 2017. A mathematical model predicting host mitochondrial pyruvate transporter activity to be a critical regulator of Mycobacterium tuberculosis pathogenicity. Biosystems 155:1–9. doi: 10.1016/j.biosystems.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 159.Cahill C, Phelan JJ, Keane J. 2020. Understanding and exploiting the effect of tuberculosis antimicrobials on host mitochondrial function and bioenergetics. Front Cell Infect Microbiol 10:493. doi: 10.3389/fcimb.2020.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]