Typhoid and paratyphoid fevers have a high incidence worldwide and coexist in many geographical areas, especially in low-middle-income countries (LMIC) in South and Southeast Asia. There is extensive consensus on the urgent need for better and affordable vaccines against systemic Salmonella infections.
KEYWORDS: OMV, GMMA, vaccine, Vi, Salmonella, enteric fever, typhoid fever, S. Paratyphi, S. Typhi
ABSTRACT
Typhoid and paratyphoid fevers have a high incidence worldwide and coexist in many geographical areas, especially in low-middle-income countries (LMIC) in South and Southeast Asia. There is extensive consensus on the urgent need for better and affordable vaccines against systemic Salmonella infections. Generalized modules for membrane antigens (GMMA), outer membrane exosomes shed by Salmonella bacteria genetically manipulated to increase blebbing, resemble the bacterial surface where protective antigens are displayed in their native environment. Here, we engineered S. Paratyphi A using the pDC5-viaB plasmid to generate GMMA displaying the heterologous S. Typhi Vi antigen together with the homologous O:2 O antigen. The presence of both Vi and O:2 was confirmed by flow cytometry on bacterial cells, and their amount was quantified on the resulting vesicles through a panel of analytical methods. When tested in mice, such GMMA induced a strong antibody response against both Vi and O:2, and these antibodies were functional in a serum bactericidal assay. Our approach yielded a bivalent vaccine candidate able to induce immune responses against different Salmonella serovars, which could benefit LMIC residents and travelers.
INTRODUCTION
Salmonella enterica serovars Typhi (S. Typhi) and Paratyphi (S. Paratyphi) subtypes A, B, and C cause enteric fevers, a major global health concern. S. Typhi (typhoid fever) causes an estimated 14.9 million cases annually and 116,800 associated deaths with postantimicrobial relapses in up to 10% of patients and chronic carriage in up to 6% of treated individuals (1); S. Paratyphi causes an estimated 3 million paratyphoid fever cases and approximately 19,000 deaths annually (1). These diseases coexist in many geographical areas, especially in low-middle-income countries (LMIC). S. Typhi incidence is high in South and Southeast Asia as well as Africa; an increasing incidence of S. Paratyphi A has been reported over the past 2 decades in different parts of Asia, including Nepal (2), Cambodia (3), and China (4).
Current treatments for S. enterica infections are hampered by the emergence of multidrug-resistant strains (5–8).
Vaccines are a powerful tool against systemic Salmonella infections. Several vaccines have been licensed for the prevention of typhoid fever; however, no vaccine is available against paratyphoid fever (7, 9). The licensed S. Typhi Ty21a live typhoid vaccine is safe but gives moderate protection after multiple dosing (10). Typhoid conjugate vaccines (TCV), in which S. Typhi Vi capsular polysaccharide is covalently linked to carrier proteins, offer several potential advantages over earlier generations of vaccines, especially enhanced immunogenicity and the ability to induce immune responses in infants (11). Similar strategies are currently being investigated for the development of a paratyphoid vaccine, including live attenuated (LAV) and nonliving vaccines (12). Some recent LAV candidates (e.g., the aroC-ssaV M01ZH09 and WT05 mutants) showed insufficient immunogenicity (13). LAV can cause lethal infections in immunocompromised hosts (14–17); therefore, conjugate vaccines would represent a much safer alternative. Recently, an O-antigen (OAg) glycoconjugate based on the immunodominant O:2 factor was proposed as a vaccine against S. Paratyphi A infections (18, 19).
A bivalent formulation would probably be the wiser choice to induce antibody responses that can potentially protect against both S. Typhi and S. Paratyphi A. Moreover, such a vaccine combination would increase the commercial attractiveness of the S. Paratyphi A component, especially considering the disproportionate incidence of the two diseases.
Recently, general modules for membrane antigens (GMMA) were proposed as an alternative delivery system for the OAg (20). GMMA are outer membrane vesicles (OMV) naturally shed by Gram-negative bacterium specifically engineered to increase blebbing and obtained through a simple and robust manufacturing process, possibly leading to affordable vaccines (21–23). GMMA contain mainly outer membrane proteins and lipopolysaccharides (LPS), together with luminal periplasmic proteins. GMMA are highly immunogenic and induce T-cell-dependent, boostable, isotype-switched, highly functional IgG profiles (24). This is crucial, given the importance of the quality of the antibody response in protection against salmonelloses (25, 26). Compared to traditional glycoconjugate vaccines, GMMA have the added value of combining multiple antigens in a single vaccine component, including polysaccharides and proteins possibly contributing to clinical protection. Indeed, GMMA from S. Typhimurium and S. Enteritidis are protective in animal models (24), and a Shigella sonnei GMMA-based vaccine was recently shown to be well tolerated and immunogenic in healthy adults and populations in areas where it is endemic (27–29).
In this study, we explored the possibility of inducing functional immune responses against S. Paratyphi A O:2 OAg and S. Typhi Vi polysaccharide antigen using GMMA from S. Paratyphi A as a delivery system.
RESULTS
Generation and characterization of OMV expressing Vi- and OAg-specific antigen.
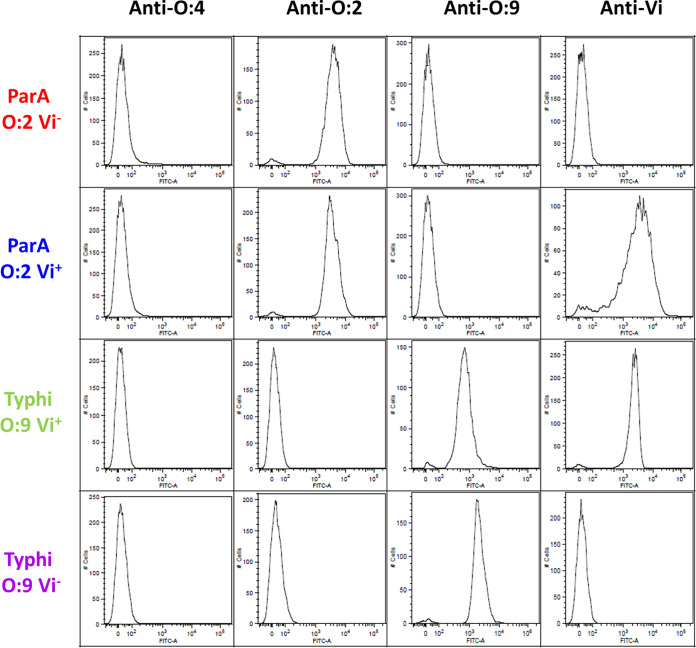
With the aim of engineering a S. Paratyphi A GMMA-producing strain that also would display the S. Typhi Vi antigen, S. Paratyphi A strain NVGH2041 (ParA O:2 Vi−), lacking the tolR gene for increased outer membrane blebbing, was transformed with pDC5-viaB; this is a plasmid that contains the entire viaB locus from S. Typhi and, therefore, all genes needed for Vi production and anchoring to the membrane (30). Simultaneous surface exposure of both Vi and O:2 on the bacterial surface of the resulting strain, indicated as ParA O:2 Vi+, was confirmed by flow cytometry using specific anti-O:2 and anti-Vi sera (Fig. 1). ParA O:2 Vi+ and O:2 Vi− were both recognized by the anti-O:2 serum, indicating that the presence of Vi does not hinder the binding of antibodies to OAg. GMMA were produced from ParA O:2 Vi+ and O:2 Vi− strains and were fully characterized through a panel of analytical methods. Both sets of GMMA had a similar size (average size of 72 and 83 nm in diameter; Table 1), as determined by dynamic light scattering (DLS), and a similar OAg/protein (wt/wt) ratio, as determined by high-performance anion exchange chromatography-pulsed amperometric detection (HPAEC-PAD). The amount of Vi (in micrograms) in ParA O:2 Vi+ GMMA was ∼10 times lower than the amount of OAg. To determine whether such a low Vi amount was due to heterologous expression of the viaB locus in S. Paratyphi A, S. Typhi BRD948 (Typhi O:9 Vi+) and its isogenic ΔtviB mutant (Typhi O:9 Vi−) were included as benchmarks in our analysis. Both S. Typhi strains display OAg containing the immunodominant O:9 factor, but only the native BRD948 is Vi+ due to the presence of the viaB locus in the chromosome. Similar to what is seen for ParA O:2 Vi+, surface exposure of both Vi and O:9 was detected on the bacterial surface of S. Typhi O:9 Vi+ (Fig. 1). Naturally released OMV were produced from S. Typhi strains and compared to GMMA obtained from S. Paratyphi A strains. OMV had more heterogeneous size than GMMA, with an average size of 81 and diameter of 133 nm and a higher polydispersion index (Table 1). The OAg/protein (wt/wt) ratio in S. Typhi O:9 Vi− OMV was similar to that measured in S. Paratyphi A GMMA, while Typhi O:9 Vi+ OMV showed a higher OAg/protein (wt/wt) ratio than the other preparations (Table 1). Importantly, the Vi/protein (wt/wt) ratio in Typhi O:9 Vi+ OMV was comparable to that of ParA O:2 Vi+ GMMA (Table 1).
FIG 1.
Display of polysaccharide antigens on the bacterial surface. Flow cytometry analysis of surface polysaccharides of ParA O:2 Vi−, ParA O:2 Vi+, S. Typhi O:9 Vi+, and S. Typhi O:9 Vi−. Flow cytometry was performed using rabbit anti-O:2, -O:9, and -Vi polyclonal serum, followed by Alexa Fluor 488-conjugated secondary antibodies. Bacteria stained with a rabbit anti-O:4 polyclonal serum were included as a negative control.
TABLE 1.
GMMA and OMV analytical characterization
| Antigen | Vi/OAga (wt/wt) ratio, % | Vi/proteina (wt/wt) ratio, % | OAg/proteina (wt/wt) ratio, % | Z avg diamb (nm) | Polydispersion indexb |
|---|---|---|---|---|---|
| ParA Vi+ GMMA | 9.6 | 4.2 | 43 | 83 | 0.14 |
| ParA Vi− GMMA | NA | NA | 47 | 72 | 0.13 |
| S. Typhi Vi+ OMV | 10.1 | 5.0 | 50 | 133 | 0.41 |
| S. Typhi Vi− OMV | NA | NA | 164 | 81 | 0.35 |
OAg content and Vi content were measured by HPAEC-PAD analysis and protein content by micro-BCA analysis, and reported ratios were calculated. NA, not applicable.
GMMA diameter and polydispersion index were calculated by DLS.
Immunogenicity of GMMA/OMV in a preclinical murine model.
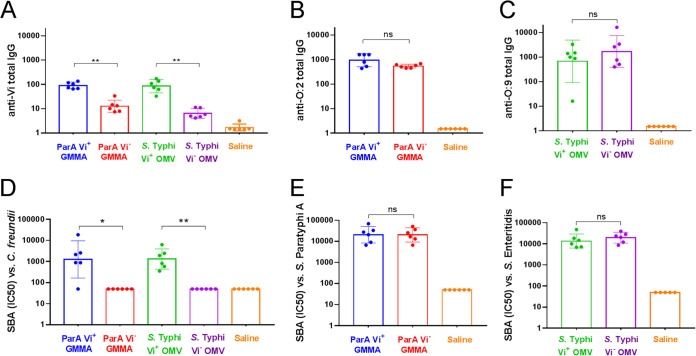
To test the possibility of inducing immune responses against both Vi and OAg with OMV/GMMA vaccine candidates, 4 groups of six C57BL/6 mice were immunized subcutaneously with vesicles prepared from ParA O:2 Vi+, ParA O:2 Vi−, Typhi O:9 Vi+, and Typhi O:9 Vi−. All animals received a booster vaccination on day 28, and sera were collected from individual animals on day 42. Each mouse received a dose equivalent to 0.5 μg of Vi antigen; this dose also resulted in the administration of similar amounts of OAg (Table 2). The immunogenicity of GMMA/OMV was assessed by measuring total IgG against Vi (Fig. 2A), O:2 (Fig. 2B), and O:9 (Fig. 2C). ParA O:2 Vi+ and ParA O:2 Vi− GMMA induced similar levels of anti-O:2 IgG, confirming that the display of Vi at the surface of the vesicles did not hinder the ability to induce anti-OAg IgG responses (Fig. 2B). Immunization with ParA O:2 Vi+ resulted in the induction of anti-Vi antibodies (Fig. 2A), showing that the Vi antigen was delivered in immunogenic form when using Vi+ vesicles. Interestingly, the anti-Vi response induced by the vesicles from S. Paratyphi A engineered to display Vi using episomal expression of the viaB locus was comparable to that of OMV from the naturally Vi+ serovar Typhi (Fig. 2A). This indicated that immune responses to Vi can be induced using vesicles produced from strains engineered for the heterologous display of Vi. Moreover, S. Typhi O:9 Vi+ and S. Typhi O:9 Vi− also induced similar levels of anti-O:9 IgG (Fig. 2C), once again confirming the lack of immune interference between Vi and OAg. Next, we tested the functional activity of resulting sera in a serum bactericidal assay (SBA) using bacterial strains displaying O:2, O:9, or Vi. Sera from mice immunized with ParA O:2 Vi+ and ParA O:2 Vi− GMMA showed similar bactericidal activity against the O:2-displaying S. Paratyphi A test strain (Fig. 2E). Thus, the display of Vi on the surface of S. Paratyphi A GMMA does not affect the ability to induce functional antibody responses capable of mediating bactericidal activity. We confirmed the ability of ParA O:2 Vi+ GMMA antisera to exert SBA against a C. freundii sensu lato (s.l.) strain, displaying the Vi antigen but not any other Salmonella-specific OAg determinants (Fig. 2D). As previously observed in enzyme-linked immunosorbent assay (ELISA), the functional activity of anti-Vi antibodies induced by ParA O:2 Vi+ GMMA was comparable to that induced by Typhi O:9 Vi+ OMV (Fig. 2D). This shows that vesicles from ParA O:2 Vi+ and Typhi O:9 Vi+, but not from their Vi− counterparts, can induce functional antibodies able to activate complement deposition and exert Vi-specific SBA. Finally, sera from mice immunized with Typhi O:9 Vi+ and Typhi O:9 Vi− OMV also showed similar bactericidal activity against the O:9-displaying S. Enteritidis test strain (Fig. 2F).
TABLE 2.
Mouse immunogenicity study: antigens and doses
| Group | Dose (μg) |
|||
|---|---|---|---|---|
| Protein | Vi | O:2 | O:9 | |
| ParA Vi+ GMMA | 12.0 | 0.5 | 5.2 | 0 |
| ParA Vi− GMMA | 11.0 | 0 | 5.2 | 0 |
| S. Typhi Vi+ OMV | 9.9 | 0.5 | 0 | 4.9 |
| S. Typhi Vi− OMV | 3.0 | 0 | 0 | 4.9 |
| Saline | 0 | 0 | 0 | 0 |
FIG 2.
Immunogenic (ELISA) and functional (SBA) assessment of vaccines. Total anti-O:2, anti-O9, and anti-Vi ELISA IgG (top) and SBA titers (IC50) against S. Paratyphi A (O:2-positive), S. Enteritidis (O:9-positive), and C. freundii s.l. (Vi-positive) strains (bottom) are shown. Unpaired, nonparametric t test (Mann-Whitney) was used to determine the statistically significant differences between groups (ns, not significant; *, P < 0.033; **, P < 0.002).
DISCUSSION
The possibility to deliver multiple antigens and to confer protection against multiple Salmonella serovars is becoming increasingly important in light of the awareness of the geographical coexistence of multiple Salmonella diseases, such as typhoid and paratyphoid fever.
In the present study, we explored the possibility of producing a vesicle-based bivalent vaccine candidate against enteric fever based on GMMA delivering the Vi polysaccharide from S. Typhi and the somatic O-antigen from S. Paratyphi A. Different GMMA preparations were obtained at small scale and characterized, ensuring the reproducibility of the main analytical characteristics. However, additional work will be needed for the process scale-up, including the evaluation of lot-to-lot consistency. We show that an S. Paratyphi A GMMA engineered to display the Vi antigen from S. Typhi can induce both anti-Vi and anti-O:2 antibodies. This indicates that Vi does not render the underlying O-antigen inaccessible for recognition by the immune system. Furthermore, the immune responses induced by the O and Vi antigens are functional against both O:2+ and Vi+ target strains in a serum bactericidal assay, further supporting their potential for broad protective activity.
A typhoid-paratyphoid vaccine would be a great asset for LMIC and travelers, given that no paratyphoid vaccines are currently licensed. Glycoconjugates are a well-established bacterial vaccine approach and have been proposed as strategies against both S. Typhi and S. Paratyphi A (12, 20). More recently, GMMA have been proposed as an alternative delivery system for OAg (20) and are particularly attractive when multicomponent preparations are required and when impoverished communities are the vaccine target population. Compared to traditional glycoconjugates, GMMA show similar or better immunogenicity and a simpler manufacturing process (31), representing a promising alternative for the development of affordable multicomponent vaccines against Salmonella serovars (24).
S. Typhi OMV, naturally displaying the Vi antigen, were included in this study as internal controls and compared to S. Paratyphi A GMMA engineered to display the heterologous Vi polysaccharide. No differences were observed either in the amount of Vi found on the resulting vesicles or in the immunogenicity and functional activity of anti-Vi antibodies elicited upon immunization. Moreover, S. Typhi OMV could induce both anti-Vi and anti-O:9 antibodies, similar to what was observed with ParA O:2 Vi+ GMMA inducing both anti-Vi and anti-O:2 antibodies. These OMV therefore induced responses that would target both Vi+ S. Typhi (anti-Vi and anti-O:9 antibodies) and Vi− S. Typhi isolates, which occur in the field and are reported to be able to cause disease (32). Our previous work found that the Vi antigen is rapidly downregulated once the bacteria reach an intracellular location in the infected tissues, with the majority of the bacterial population becoming Vi− and no longer displaying a target for the immune response (33). Finally, these OMV would also target S. Enteritidis, which shares the O:9 antigen with S. Typhi.
In summary, our work shows that it is possible to deliver both O and Vi antigens using vesicle-based vaccine platforms, inducing strong and functional antibody responses against different polysaccharides. Moreover, the presence of protein antigens on Salmonella OMV/GMMA may represent an added value for GMMA vaccines compared to other polysaccharide-based formulations. In conclusion, bacterial outer membrane vesicles represent a flexible, affordable, and highly immunogenic platform for the development of multivalent Salmonella vaccines.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Salmonella Paratyphi A NVGH308 (displaying the O:2 OAg [34]) is the isolate that has been engineered with a ΔtolR mutation to increase outer membrane blebbing (i.e., GMMA production), resulting in strain NVGH2041. Serovar Paratyphi A does not naturally produce the Vi antigen; heterologous display of Vi in S. Paratyphi A ΔtolR strain NVGH2041 was obtained through episomal expression of the viaB locus using the pDC5-viaB plasmid, a gift from Andreas Baumler, University of California-Davis (30). These strains are referred to as ParA O:2 Vi− and ParA O:2 Vi+, respectively. Attenuated S. Typhi BRD948 (Ty2 ΔaroC ΔaroD ΔhtrA mutant naturally displaying the O:9 OAg and Vi antigen [35]) and Salmonella Typhi BRD948 ΔtviB (displaying O:9 but not Vi [36]) strains were used as S. Typhi OMV-producing strains (Typhi O:9 Vi+ and Typhi O:9 Vi−, respectively). All strains were grown at 30°C in liquid Luria-Bertani (LB) medium in rotary shakers for 16 h. For OMV/GMMA production, overnight cultures were diluted in HTMC medium (15 g/liter glycerol, 30 g/liter yeast extract, 0.5 g/liter MgSO4, 5 g/liter KH2PO4, 20 g/liter K2HPO4) to an optical density at 600 nm (OD600) of 0.3 and grown at 30°C for 8 h with a liquid-to-air volume ratio of 1:5. A supplement of a mixture of aromatic amino acids (Aro mix; 0.04 g/liter phenylalanine, 0.04 g/liter tryptophan, 0.01 g/liter para-aminobenzoic acid, and 0.01 g dihydrobenzoic acid) and 0.04 g/liter tyrosine was used for the S. Typhi strains (35). The ParA O:2 Vi+ strain was grown in the presence of 100 μg/ml ampicillin to retain plasmid pDC5-viaB expression.
Flow cytometry analysis.
To monitor the display of the O and Vi polysaccharide antigens on the surface of OMV/GMMA-producing strains, bacteria were grown for 16 h in liquid culture and analyzed by flow cytometry. Bacteria were pelleted at 4,000 × g for 5 min, washed with phosphate-buffered saline (PBS), and fixed using Cytofix fixation buffer (BD Biosciences) for 30 min. Fixed bacteria were then blocked with PBS containing 3% (wt/vol) bovine serum albumin (BSA) for 15 min and incubated for 1 h with rabbit polyclonal sera against O:2, O:9, or Vi (Denka Saiken), diluted 1:500 in PBS plus 1% (wt/vol) BSA. Rabbit polyclonal serum against O:4 (Denka Seiken) was used as a negative control. Samples were incubated with Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes) diluted 1:500 in PBS plus 1% BSA for 30 min. Flow cytometry analysis was performed using a FACSCanto II flow cytometer (BD Biosciences).
OMV/GMMA production.
OMV and GMMA were purified from the culture supernatant of each bacterial strain and characterized as previously described (23, 37). Bacteria were pelleted by centrifugation at 5,000 × g for 45 min. Cell-free supernatants were collected, filtered through 0.22-μm Stericup filters (Millipore), and ultracentrifuged at 175,000 × g for 2 h at 4°C using an SW32Ti rotor (Beckman Coulter). Pellets containing OMV/GMMA were resuspended in PBS, ultracentrifuged at 175,000 × g for 2 h, resuspended in PBS, filtered, and stored at 4°C until use.
Analytical characterization of GMMA/OMV.
GMMA/OMV were characterized in terms of antigen composition and size. A micro-bicinchoninic acid (BCA) kit (Thermo Scientific) was used for GMMA/OMV total protein quantification using BSA as a reference standard and by following the manufacturer’s instructions. The sugar monomers constituting the Vi and O polysaccharide repeating units were quantified through HPAEC-PAD, as previously described (38, 39). Particle size distribution of GMMA/OMV was evaluated by DLS, as previously reported (23, 40).
Animal experiments.
Female C57BL/6 mice were purchased from Envigo UK and used when over 6 weeks of age (mean weight 20 ± 3 g). The mice were housed in specific-pathogen-free containment facilities and were allowed water and food ad libitum. Six mice per group were vaccinated subcutaneously at days 0 and 28 with either GMMA or OMV diluted in saline and normalized to contain approximately 5 μg of OAg per dose and 0.5 μg of Vi per dose (in case of Vi-positive OMV/GMMA), as reported in Table 1. A separate control group of mice received saline. Individual sera were collected at day −1 (pooled sera) and at day 42 (individual sera). All animal experiments were performed in accordance with good animal practice as defined by the relevant international (Directive of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes, Brussels 543/5) and local (University of Cambridge) animal welfare guidelines. This research was regulated under the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012 following ethical review by the University of Cambridge Animal Welfare and Ethical Review Body (AWERB).
Assessment of anti-Vi and anti-OAg specific total IgG by ELISA.
Anti-OAg and anti-Vi antigen-specific IgG levels were measured 2 weeks after the second immunization (day 42) by ELISA as previously reported (41). Briefly, 96-well round-bottom MaxiSorp microtiter plates (Nunc, Roskilde, Denmark) were coated with 100 μl/well antigen overnight at 4°C. OAg purified from S. Paratyphi A (O:2) or S. Enteritidis (O:9) and Vi purified from C. freundii s.l. were used at 15 μg/ml and 2 μg/ml in carbonate or at 1 μg/ml in phosphate buffer, respectively (38, 42). Plates were blocked with PBS plus 5% fat-free milk (Sigma) for 2 h at room temperature (RT) and then washed 3 times with PBS plus 0.05% Tween 20 (PBS-T). Serum samples were diluted 1:100 and 1:4,000 in PBS-T supplemented with 0.1% BSA (diluent buffer), and both dilutions were assayed in triplicate. After incubation for 2 h at RT, plates were washed three times with PBS-T and incubated at 25°C for 1 h with anti-mouse goat IgG-alkaline phosphatase (Sigma), diluted 1:6,000, 1:8,800, and 1:2,600 (for Vi, O:2, or O:9, respectively) in diluent buffer. After washing three times with PBS-T, plates were developed by adding the alkaline phosphatase substrate (SIGMAFAST N2770; Sigma) and read at 405 nm and 490 nm using an ELx 800 reader (BioTek). ELISA units were expressed relative to a mouse antigen-specific antibody standard serum curve composed by 10 standard points and 2 blank wells (run in duplicate on each plate), with the best five-parameter fit determined by a modified Hill plot. One ELISA unit is defined as the reciprocal of the dilution of the standard serum that gives an absorbance value equal to 1 in this assay.
Assessment of serum bactericidal activity by SBA.
Individual mouse sera collected at day 42 were heat inactivated (HI) at 56°C for 30 min prior to being tested in a serum bactericidal assay based on luminescent readout against Salmonella Paratyphi A NVGH308, Salmonella Enteritidis CMCC3014, and Vi-positive Citrobacter freundii sensu lato strain 3056 (43, 44). SBA was performed in 96-well round-bottom sterile plates (Corning). Dilutions of HI test sera were incubated for 3 h in the presence of exogenous complement (baby rabbit complement [BRC]) and bacteria as previously described (43). Briefly, an adequate volume of reaction mixture containing the target bacterial cells (around 100,000 CFU/ml), BRC (50% for S. Enteritidis, 20% for S. Paratyphi A, and 5% for C. freundii s.l.), and buffer (PBS) was added to SBA plates containing HI serum dilutions and incubated for 3 h at 37°C. At the end of the incubation, the plates were centrifuged for 10 min at 4,000 × g, the supernatant was discarded to remove ATP derived from dead bacteria, and live bacterial pellets resuspended in PBS were transferred to a white round-bottom 96-well plate (Greiner) and mixed 1:1 (vol/vol) with BacTiter-Glo reagent (Promega). The reaction mixture was incubated for 5 min at RT in an orbital shaker, and the luminescence signal was measured using a luminometer (Viktor). A 4-parameter nonlinear regression was applied to raw luminescence for all the serum dilutions tested, as previously described (45). The SBA titer is reported as IC50, defined as serum dilutions giving 50% inhibition of the ATP level in the negative-control well. Titers below the minimum measurable level of luminescence were arbitrarily given an IC50 of 50, representing half of the first dilution of sera tested (i.e., 100). GraphPad Prism 7 software (GraphPad Software) was used for fitting and IC50 determination.
Statistical analysis.
Unpaired, nonparametric t test (Mann-Whitney) was used to determine the statistically significant differences between groups, using GraphPad Prism 7 software (GraphPad Software).
ACKNOWLEDGMENTS
This work was funded by a grant from GCRF Networks in Vaccine Research and Development, which was cofunded by the MRC, and BBSRC, which was awarded jointly to P. Mastroeni of the University of Cambridge as the lead applicant and Francesca Micoli (GSK) as a coapplicant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This study was undertaken at the request of and sponsored by a GlaxoSmithKline Biologicals SA in-kind contribution. GSK Vaccines Institute for Global Health (Srl) (GVGH) is an affiliate of GlaxoSmithKline Biologicals SA.
We thank Andreas Baumler, UC Davis, for discussions and for providing pDC5-viaB plasmid.
Conceived and designed the experiments: G.G., A.S., O.R., F. Micoli, and P.M. Performed the experiments: G.G., R.A., V.A., F. Mancini, M.G.A., P.K., P.M., and D.P. Analyzed the data: G.G., R.A., V.A., F. Mancini, M.G.A., P.K., F.N., A.S., O.R., F. Micoli, and P.M. Contributed to the writing of the manuscript: G.G., O.R., F. Micoli, and P.M. All authors had full access to the data and approved the final manuscript.
G.G., R.A., V.A., F. Mancini, M.G.A., F.N., O.R., and F. Micoli are employees of the GSK group of companies. A.S. was employed by the GSK group of companies at the time of the study, owns GSK shares, and is listed as an inventor on patents owned by the GSK group of companies. This does not alter the authors’ adherence to all journal policies on data and material sharing.
REFERENCES
- 1.GBD 2017 Typhoid and Paratyphoid Collaborators. 2019. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 19:369–381. doi: 10.1016/S1473-3099(18)30685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zellweger RM, Basnyat B, Shrestha P, Prajapati KG, Dongol S, Sharma PK, Koirala S, Darton TC, Dolecek C, Thompson CN, Thwaites GE, Baker SG, Karkey A. 2017. A 23-year retrospective investigation of Salmonella Typhi and Salmonella Paratyphi isolated in a tertiary Kathmandu hospital. PLoS Negl Trop Dis 11:e0006051. doi: 10.1371/journal.pntd.0006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuijpers LMF, Phe T, Veng CH, Lim K, Ieng S, Kham C, Fawal N, Fabre L, Le Hello S, Vlieghe E, Weill FX, Jacobs J, Peetermans WE. 2017. The clinical and microbiological characteristics of enteric fever in Cambodia, 2008–2015. PLoS Negl Trop Dis 11:e0005964. doi: 10.1371/journal.pntd.0005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arndt MB, Mosites EM, Tian M, Forouzanfar MH, Mokhdad AH, Meller M, Ochiai RL, Walson JL. 2014. Estimating the burden of paratyphoid A in Asia and Africa. PLoS Negl Trop Dis 8:e2925. doi: 10.1371/journal.pntd.0002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hohmann EL. 2001. Nontyphoidal salmonellosis. Clin Infect Dis 32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 6.Mirza SH, Beeching NJ, Hart CA. 1996. Multi-drug resistant typhoid: a global problem. J Med Microbiol 44:317–319. doi: 10.1099/00222615-44-5-317. [DOI] [PubMed] [Google Scholar]
- 7.Kariuki S, Gordon MA, Feasey N, Parry CM. 2015. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 33(Suppl 3):C21–C29. doi: 10.1016/j.vaccine.2015.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1. [Google Scholar]
- 9.Baliban SM, Lu YJ, Malley R. 2020. Overview of the nontyphoidal and paratyphoidal Salmonella vaccine pipeline: current status and future prospects. Clin Infect Dis 71:S151–S154. doi: 10.1093/cid/ciaa514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest BD, LaBrooy JT, Beyer L, Dearlove CE, Shearman DJ. 1991. The human humoral immune response to Salmonella typhi Ty21a. J Infect Dis 163:336–345. doi: 10.1093/infdis/163.2.336. [DOI] [PubMed] [Google Scholar]
- 11.Micoli F, Bjarnarson SP, Arcuri M, Aradottir Pind AA, Magnusdottir GJ, Necchi F, Di Benedetto R, Carducci M, Schiavo F, Giannelli C, Pisoni I, Martin LB, Del Giudice G, MacLennan CA, Rappuoli R, Jonsdottir I, Saul A. 2020. Short Vi-polysaccharide abrogates T-independent immune response and hyporesponsiveness elicited by long Vi-CRM197 conjugate vaccine. Proc Natl Acad Sci U S A 117:24443–24449. doi: 10.1073/pnas.2005857117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLennan CA, Martin LB, Micoli F. 2014. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vaccin Immunother 10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR, Everest P, Wu T, Pickard D, Holden DW, Dougan G, Griffin GE, House D, Santangelo JD, Khan SA, Shea JE, Feldman RG, Lewis DJ. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun 70:3457–3467. doi: 10.1128/iai.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant AJ, Oshota O, Chaudhuri RR, Mayho M, Peters SE, Clare S, Maskell DJ, Mastroeni P. 2016. Genes required for the fitness of Salmonella enterica serovar Typhimurium during infection of immunodeficient gp91-/- phox mice. Infect Immun 84:989–997. doi: 10.1128/IAI.01423-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess J, Ladel C, Miko D, Kaufmann SH. 1996. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol 156:3321–3326. [PubMed] [Google Scholar]
- 16.Muotiala A. 1992. Anti-IFN-gamma-treated mice—a model for testing safety of live Salmonella vaccines. Vaccine 10:243–246. doi: 10.1016/0264-410x(92)90159-h. [DOI] [PubMed] [Google Scholar]
- 17.Sinha K, Mastroeni P, Harrison J, de Hormaeche RD, Hormaeche CE. 1997. Salmonella typhimurium aroA, htrA, and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect Immun 65:1566–1569. doi: 10.1128/IAI.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micoli F, Rondini S, Gavini M, Lanzilao L, Medaglini D, Saul A, Martin LB. 2012. O:2-CRM(197) conjugates against Salmonella Paratyphi A. PLoS One 7:e47039. doi: 10.1371/journal.pone.0047039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravenscroft N, Cescutti P, Gavini M, Stefanetti G, MacLennan CA, Martin LB, Micoli F. 2015. Structural analysis of the O-acetylated O-polysaccharide isolated from Salmonella paratyphi A and used for vaccine preparation. Carbohydr Res 404:108–116. doi: 10.1016/j.carres.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Berti F, Micoli F. 2020. Improving efficacy of glycoconjugate vaccines: from chemical conjugates to next generation constructs. Curr Opin Immunol 65:42–49. doi: 10.1016/j.coi.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, Rossi O, Martin LB, Auerbach J, Di Cioccio V, Saul A. 2015. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS One 10:e0134478. doi: 10.1371/journal.pone.0134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi O, Pesce I, Giannelli C, Aprea S, Caboni M, Citiulo F, Valentini S, Ferlenghi I, MacLennan CA, D'Oro U, Saul A, Gerke C. 2014. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: relative activation of TLR4 and TLR2 pathways in different mutants. J Biol Chem 289:24922–24935. doi: 10.1074/jbc.M114.566570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Benedetto G, Alfini R, Cescutti P, Caboni M, Lanzilao L, Necchi F, Saul A, MacLennan CA, Rondini S, Micoli F. 2017. Characterization of O-antigen delivered by generalized modules for membrane antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 35:419–426. doi: 10.1016/j.vaccine.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 24.Micoli F, Rondini S, Alfini R, Lanzilao L, Necchi F, Negrea A, Rossi O, Brandt C, Clare S, Mastroeni P, Rappuoli R, Saul A, MacLennan CA. 2018. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc Natl Acad Sci U S A 115:10428–10433. doi: 10.1073/pnas.1807655115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goh YS, Armour KL, Clark MR, Grant AJ, Mastroeni P. 2016. IgG subclasses targeting the flagella of Salmonella enterica serovar Typhimurium can mediate phagocytosis and bacterial killing. J Vaccines Vaccin 7:322. doi: 10.4172/2157-7560.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Dominguez-Medina C, Cumley NJ, Heath JN, Essex SJ, Bobat S, Schager A, Goodall M, Kracker S, Buckley CD, May RC, Kingsley RA, MacLennan CA, Lopez-Macias C, Cunningham AF, Toellner KM. 2017. IgG1 is required for optimal protection after immunization with the purified porin OmpD from Salmonella Typhimurium. J Immunol 199:4103–4109. doi: 10.4049/jimmunol.1700952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Launay O, Lewis DJM, Anemona A, Loulergue P, Leahy J, Scire AS, Maugard A, Marchetti E, Zancan S, Huo Z, Rondini S, Marhaba R, Finco O, Martin LB, Auerbach J, Cohen D, Saul A, Gerke C, Podda A. 2017. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine 22:164–172. doi: 10.1016/j.ebiom.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obiero CW, Ndiaye AGW, Scire AS, Kaunyangi BM, Marchetti E, Gone AM, Schutte LD, Riccucci D, Auerbach J, Saul A, Martin LB, Bejon P, Njuguna P, Podda A. 2017. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front Immunol 8:1884. doi: 10.3389/fimmu.2017.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launay O, Ndiaye AGW, Conti V, Loulergue P, Scire AS, Landre AM, Ferruzzi P, Nedjaai N, Schutte LD, Auerbach J, Marchetti E, Saul A, Martin LB, Podda A. 2019. Booster vaccination with GVGH Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: results from a phase 1 clinical trial. Front Immunol 10:335. doi: 10.3389/fimmu.2019.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffatellu M, Santos RL, Chessa D, Wilson RP, Winter SE, Rossetti CA, Lawhon SD, Chu H, Lau T, Bevins CL, Adams LG, Baumler AJ. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect Immun 75:4342–4350. doi: 10.1128/IAI.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raso MM, Gasperini G, Alfini R, Schiavo F, Aruta MG, Carducci M, Forgione MC, Martini S, Cescutti P, Necchi F, Micoli F. 2020. GMMA and glycoconjugate approaches compared in mice for the development of a vaccine against Shigella flexneri serotype 6. Vaccines 8:160. doi: 10.3390/vaccines8020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hornick RB, Greisman SE, Woodward TE, DuPont HL, Dawkins AT, Snyder MJ. 1970. Typhoid fever: pathogenesis and immunologic control. N Engl J Med 283:686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- 33.Janis C, Grant AJ, McKinley TJ, Morgan FJ, John VF, Houghton J, Kingsley RA, Dougan G, Mastroeni P. 2011. In vivo regulation of the Vi antigen in Salmonella and induction of immune responses with an in vivo-inducible promoter. Infect Immun 79:2481–2488. doi: 10.1128/IAI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullagh D, Dobinson HC, Darton T, Campbell D, Jones C, Snape M, Stevens Z, Plested E, Voysey M, Kerridge S, Martin LB, Angus B, Pollard AJ. 2015. Understanding paratyphoid infection: study protocol for the development of a human model of Salmonella enterica serovar Paratyphi A challenge in healthy adult volunteers. BMJ Open 5:e007481. doi: 10.1136/bmjopen-2014-007481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop A, House D, Perkins T, Baker S, Kingsley RA, Dougan G. 2008. Interaction of Salmonella enterica serovar Typhi with cultured epithelial cells: roles of surface structures in adhesion and invasion. Microbiology 154:1914–1926. doi: 10.1099/mic.0.2008/016998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart PJ, O'Shaughnessy CM, Siggins MK, Bobat S, Kingsley RA, Goulding DA, Crump JA, Reyburn H, Micoli F, Dougan G, Cunningham AF, MacLennan CA. 2016. Differential killing of Salmonella enterica serovar Typhi by antibodies targeting Vi and lipopolysaccharide O:9 antigen. PLoS One 11:e0145945. doi: 10.1371/journal.pone.0145945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi O, Caboni M, Negrea A, Necchi F, Alfini R, Micoli F, Saul A, MacLennan CA, Rondini S, Gerke C. 2016. Toll-like receptor activation by generalized modules for membrane antigens from lipid A mutants of Salmonella enterica serovars Typhimurium and Enteritidis. Clin Vaccine Immunol 23:304–314. doi: 10.1128/CVI.00023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micoli F, Rondini S, Gavini M, Pisoni I, Lanzilao L, Colucci AM, Giannelli C, Pippi F, Sollai L, Pinto V, Berti F, MacLennan CA, Martin LB, Saul A. 2013. A scalable method for O-antigen purification applied to various Salmonella serovars. Anal Biochem 434:136–145. doi: 10.1016/j.ab.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannelli C, Raso MM, Palmieri E, De Felice A, Pippi F, Micoli F. 2020. Development of a specific and sensitive HPAEC-PAD method for quantification of Vi polysaccharide applicable to other polysaccharides containing amino uronic acids. Anal Chem 92:6304–6311. doi: 10.1021/acs.analchem.9b05107. [DOI] [PubMed] [Google Scholar]
- 40.De Benedetto G, Cescutti P, Giannelli C, Rizzo R, Micoli F. 2017. Multiple techniques for size determination of generalized modules for membrane antigens from Salmonella typhimurium and Salmonella enteritidis. ACS Omega 2:8282–8289. doi: 10.1021/acsomega.7b01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rondini S, Micoli F, Lanzilao L, Hale C, Saul AJ, Martin LB. 2011. Evaluation of the immunogenicity and biological activity of the Citrobacter freundii Vi-CRM197 conjugate as a vaccine for Salmonella enterica serovar Typhi. Clin Vaccine Immunol 18:460–468. doi: 10.1128/CVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Micoli F, Rondini S, Pisoni I, Giannelli C, Di Cioccio V, Costantino P, Saul A, Martin LB. 2012. Production of a conjugate vaccine for Salmonella enterica serovar Typhi from Citrobacter Vi. Vaccine 30:853–861. doi: 10.1016/j.vaccine.2011.11.108. [DOI] [PubMed] [Google Scholar]
- 43.Necchi F, Saul A, Rondini S. 2017. Development of a high-throughput method to evaluate serum bactericidal activity using bacterial ATP measurement as survival readout. PLoS One 12:e0172163. doi: 10.1371/journal.pone.0172163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Necchi F, Saul A, Rondini S. 2018. Setup of luminescence-based serum bactericidal assay against Salmonella Paratyphi A. J Immunol Methods 461:117–121. doi: 10.1016/j.jim.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Rossi O, Molesti E, Saul A, Giannelli C, Micoli F, Necchi F. 2020. Intra-laboratory evaluation of luminescence based high-throughput serum bactericidal assay (L-SBA) to determine bactericidal activity of human sera against Shigella. High Throughput 9:14. doi: 10.3390/ht9020014. [DOI] [PMC free article] [PubMed] [Google Scholar]