Antibiotic treatment failure of Staphylococcus aureus infections is very common. In addition to genetically encoded mechanisms of antibiotic resistance, numerous additional factors limit the efficacy of antibiotics in vivo.
KEYWORDS: Staphylococcus aureus, antibiotic tolerance, persisters
ABSTRACT
Antibiotic treatment failure of Staphylococcus aureus infections is very common. In addition to genetically encoded mechanisms of antibiotic resistance, numerous additional factors limit the efficacy of antibiotics in vivo. Identifying and removing the barriers to antibiotic efficacy are of major importance, as even if new antibiotics become available, they will likely face the same barriers to efficacy as their predecessors. One major obstacle to antibiotic efficacy is the proficiency of S. aureus to enter a physiological state that is incompatible with antibiotic killing. Multiple pathways leading to antibiotic tolerance and the formation of tolerant subpopulations called persister cells have been described for S. aureus. Additionally, S. aureus is a versatile pathogen that can infect numerous tissues and invade a variety of cell types, of which some are poorly penetrable to antibiotics. It is therefore unlikely that there will be a single solution to the problem of recalcitrant S. aureus infection. Instead, specific approaches may be required for targeting tolerant cells within different niches, be it through direct targeting of persister cells, sensitization of persisters to conventional antibiotics, improved penetration of antibiotics to particular niches, or any combination thereof. Here, we examine two well-described reservoirs of antibiotic-tolerant S. aureus, the biofilm and the macrophage, the barriers these environments present to antibiotic efficacy, and potential solutions to the problem.
INTRODUCTION
Antibiotics are arguably the most important class of medicine in modern health care. Despite their importance, their efficacy in the context of the infection environment remains poorly understood. Treatment failure is common and is becoming more widespread which imposes a heavy burden on global public health. Despite this threat, the FDA has not approved a new class of antibiotics since the lipopeptides in 2003 (1). The reasons for this dearth in drug discovery and development are complex, with economic factors playing a major role. New antibiotics are less profitable than drugs for chronic illnesses, such as diabetes or heart disease, which are frequently prescribed for a lifetime. Resistance to antibiotics, which render the drugs useless, spreads through societies and is fueled by use (2). This resistance has led to policies of antibiotic stewardship to limit resistance development to new agents, further limiting their use and the associated profits. Numerous efforts to solve this problem are ongoing, and there is reason for optimism. Nonetheless, antibiotic discovery and development remain a major challenge and emphasize the need to maximize the efficacy of our existing drugs to yield better patient outcomes and allow for shorter antibiotic-dosing regimens, which would also slow resistance development.
Staphylococcus aureus is the most common cause of nosocomial infection, is among the most common causes of community-associated bacterial infection, and is extremely difficult to treat with antibiotics (3). Antibiotic resistance (reviewed extensively elsewhere [4–6]), is a major problem in S. aureus; however, other factors also contribute significantly to poor antibiotic efficacy. Here, we will discuss treatment failure, focusing on the barriers to antibiotic penetration, the formation of antibiotic-tolerant S. aureus populations, and ways to overcome those barriers.
OBSTACLES
Biofilms.
A biofilm is a community of bacteria surrounded by an extracellular matrix that protects the bacteria from an array of assaults, including uptake by host phagocytic cells. Biofilms are also extremely difficult to kill with antibiotics. Up to 80% of all bacterial infections are estimated to be biofilm-associated (7), including many chronic infections frequently caused by S. aureus (endocarditis, osteomyelitis, chronic wound infection, and indwelling device infections). Free-floating or nonadhered biofilm aggregates are present in S. aureus knee joint infections and infections of the cystic fibrosis lung (8).
In the United States, chronic wounds affect approximately 2% of Americans, of which the most common are vascular ulcers, diabetic ulcers, and pressure ulcers (9). A total of 93.5% of chronic leg ulcers are infected with S. aureus (10). Bacterial biofilms are the leading cause of treatment failure in chronic wounds and usually require surgical intervention, such as debridement in conjunction with antibiotic therapy (11). With risk factors for chronic wounds, such as cardiovascular disease, diabetes, and obesity, rising globally, there is an urgent need for improved treatment strategies. It also represents an enormous financial health care burden, with Medicare cost estimates for chronic wound treatments now upward of $30 billion annually (11).
Surgical implant infections and osteomyelitis respond so poorly to antibiotics that therapy is routinely combined with surgical intervention (12, 13). Additionally, S. aureus can cause tissue abscesses comprising a dense S. aureus community encapsulated by fibrin and immune cells (14). The enclosed S. aureus communities share similarities with biofilms but have distinct genetic requirements (15). Encapsulation serves to contain the infection, but host defenses may fail to resolve the abscess (14) which likely contributes to the high infection relapse rates (20–50%) associated with skin and soft tissue infections (SSTIs) (16, 17) and chronic osteomyelitis (18). Antibiotic tolerance of S. aureus within abscess communities undoubtedly contributes to infection relapse, but the phenomenon has not been thoroughly studied (14).
A crucial criterion for antibiotic efficacy is that the drugs reach high enough concentrations at the site of infection to reduce pathogen burden or to halt pathogen replication so that the immune system can clear the infection. Antibiotics tend to be effective at micromolar levels, namely, 100 to 1,000 times higher than most host-targeted drugs (19). This greatly decreases the therapeutic window of antibiotics, increases the chance of toxicity, and compromises the clinical efficacy in any niche where antibiotic penetration is impeded. Biofilms act as a physical barrier and to some extent can impede or slow the penetration of glycopeptides, β-lactams, and phenicols (Table 1) (20–22). Previous studies have indicated that penetration of positively charged aminoglycosides, such as tobramycin, into biofilm is impeded by negatively charged components of the matrix, such as extracellular DNA and certain components of polysaccharides (23). Fluoroquinolones, macrolides, rifampicin, linezolid, and daptomycin penetrate well into biofilms (20, 21, 24–27).
TABLE 1.
Penetration of antibiotics into host cells and biofilms
| Antibiotic class | Antibiotic example(s) | Host cell penetrationa | Biofilm penetrationa |
|---|---|---|---|
| Macrolide | Azithromycin, telithromycin, erythromycin, clarithromycin | Efficient (59, 62, 145) | Efficient (146, 147) |
| Aminoglycoside | Gentamicin, tobramycin | Poor (26, 59) | Impeded/retarded penetration into biofilms (26, 148) |
| Oxazolidinone | Linezolid | Conflicting data on accumulation (59, 149) | Efficient (150) |
| β-lactam | Penicillin V, nafcillin, ampicillin, oxacillin | Poor penetration and low accumulation (53, 56–59, 61) | Impeded (21) |
| Glycopeptide | Vancomycin | Poor (59, 61, 62) | Penetration is retarded but can reach therapeutic levels within the biofilm (20, 151, 152) |
| Ansamycin | Rifampicin | Efficient (60–62) | Efficient (20, 27) |
| Quinolone | Ciprofloxacin, levofloxavin, moxifloxacin | Efficient (59, 61, 145) | Efficient (26, 153, 154) |
| Cephalosporin | Cefazolin, cefuroxime, cefotaxime | Poor (59, 61) | Impeded (21) |
| Tetracycline | Minocycline | Moderate (58, 59, 61) | Efficient (22, 155) |
| Lipopetide | Daptomycin | Poor (59) | Efficient (24, 25) |
| Lincosamide | Clindamycin | Temp dependent; moderate penetration at 37°C (58, 59) | Limited data on penetration (156) |
References are in parentheses.
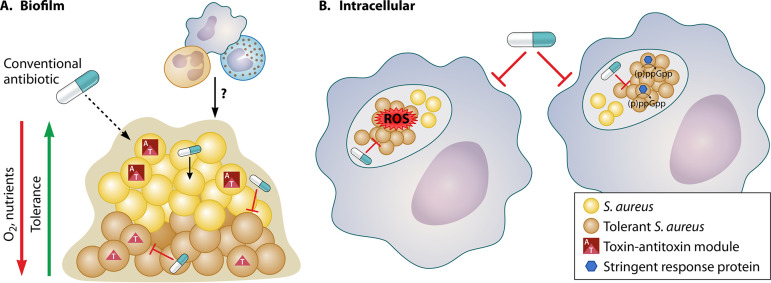
In addition to impeded penetration of some drugs, biofilms harbor high numbers of persister cells which are frequently implicated in treatment failure (28–33) (Fig. 1a). Persister cells are a subpopulation of antibiotic-tolerant cells that transiently survive antibiotic challenge. Antibiotic tolerance of biofilms has long been associated with metabolic quiescence due to poor nutrient and oxygen availability within the biofilm (26). Persister cell formation has been strongly associated with ATP depletion in S. aureus, so the presence of high numbers of persisters within the biofilm is not surprising (33–35). But, low ATP is not an essential requirement for antibiotic tolerance; direct inhibition of cellular processes, including cell wall synthesis or protein synthesis, can induce antibiotic tolerance while maintaining high ATP (34–36).
FIG 1.
Persisters and penetration in antibiotic treatment failure. Two primary challenges to antibiotic-mediated eradication of S. aureus infection are antibiotic-tolerant bacteria and poor antibiotic penetration to the infection site. Biofilms (A) and host cells (B) represent physical barriers to efficient penetration of antibiotics. However, even when antibiotics are able to penetrate these barriers, antibiotics may still fail due to the bacteria being in an antibiotic-tolerant state. Numerous mechanisms, including toxin-antitoxin modules, interactions with immune cells, and the stringent response, have been implicated in the formation of antibiotic-tolerant cells in different niches. Schematic credit: created with BioRender and modified by Patrick Lane, ScEYEnce Studios.
Elucidating mechanisms of persister formation has traditionally involved testing the capacity of genetic mutants to survive antibiotic challenge in vitro. Mutants of the agr quorum sensing system agrCA and agrD displayed increased persisters to levofloxacin in stationary phase in vitro. Conversely, an RNAIII mutation had decreased persisters under the same conditions (37). Phenol-soluble modulin (PSM) mutants formed fewer persisters to fluoroquinolones and gentamicin in vitro but had no impact on cell wall-acting antibiotics (37, 38). In addition, persisters under in vitro conditions were associated with reduced tricarboxylic acid (TCA) cycle activity (39, 40) and reduced membrane potential (39, 41). It is evident that a single pathway for antibiotic tolerance does not exist and not all pathways will lead to multidrug tolerance. We have yet to learn which of these mechanisms are relevant in the context of infection.
More recently, S. aureus antibiotic tolerance and persister formation have been examined in more physiologically relevant environments. A study by Ma et al. reported that there are mechanisms of persister formation that are specifically activated in the biofilm. Specifically, deleting the single type II toxin antitoxin (TA) module mazEF showed decreased survival to vancomycin in an in vitro biofilm model. This phenotype was confirmed in vivo with a mazEF mutant displaying fewer persisters to vancomycin in a deep-seated biofilm infection model in mice (42–44). Interestingly, the mazEF mutant was also associated with increased pathogenicity and mortality, and the authors concluded that the mutant was unable to transition from an acute infection into a chronic infection often associated with increased antibiotic tolerance. Importantly, we previously reported that a mutant devoid of three type II TA modules (mazEF, axe1/txe1, and axe2/txe2) had no contribution to persister formation under broth culture conditions (34). This major disparity in the contribution of TAs to persister formation under biofilm and planktonic conditions emphasizes the fact that numerous pathways to the persister state exist and their importance is dependent on environmental conditions. For this reason, stress response pathways leading to persister formation in biofilm-related infections are likely to be distinct from the pathways leading to persister formation in nonbiofilm-associated infections, such as bacteremia. It also highlights the salience of modeling physiologically relevant conditions in vitro.
Macrophages.
In S. aureus bacteremia, S. aureus is engulfed by macrophages within minutes of entering the bloodstream (45). Although macrophages exhibit an array of bactericidal assaults designed to kill invading pathogens, subpopulations of S. aureus reside, survive, and even proliferate within macrophages (45, 46). Infected macrophages may function as “Trojan horses,” shielding S. aureus from antibiotic and immune-mediated clearance, facilitating S. aureus dissemination to other tissues, and contributing to infection recurrence and secondary infections (45–48). Poor penetration of antibiotics into host cells (Table 1) on its own does not explain the ability of intracellular S. aureus to withstand antibiotic treatment, spurring recent efforts to elucidate a role for the host response in the formation of S. aureus persister cells (47–50).
S. aureus resides in multiple cell types, including macrophages, endothelial cells, epithelial cells, osteoblasts, osteoclasts, and neutrophils, and several classes of drugs fail to effectively penetrate host cells (51–53). Penetration of vancomycin, the front-line antibiotic prescribed for methicillin-resistant S. aureus (MRSA) bacteremia, is excluded from macrophages, likely due to its high molecular weight (1,449.2 g mol−1) and hydrophilicity (45, 54, 55). Although β-lactams may diffuse well into host cells, they do not accumulate well, presumably due to their acidity (56–58). Similarly, the highly polar aminoglycosides exhibit poor penetration into host cells (57, 59). Conversely, macrolides, rifampicin, and fluoroquinolones diffuse and accumulate well in host cells (Table 1) (60, 61). However, both macrolides and rifampicin exhibit poor killing efficacy intracellularly (62). In fact, only 5 out of 16 antibiotics (representing 7 antibiotic classes) tested in a comparative study caused a reduction in S. aureus burden following internalization by macrophages (62). Intracellular efficacy was consistently lower than extracellular efficacy for all antibiotics tested.
It appears that multiple pathways may contribute to antibiotic tolerance within host cells. We recently found that macrophage-derived reactive oxygen species (ROS) produced by activated macrophages during oxidative burst led to the formation of S. aureus antibiotic-tolerant persister cells. Exposure to ROS collapsed tricarboxylic acid (TCA) cycle activity, reducing ATP levels, and forcing S. aureus into a metabolic state that is incompatible with antibiotic killing (50). Furthermore, S. aureus recovered from mice deficient in the production of macrophage-derived ROS were more susceptible to antibiotic killing than S. aureus recovered from wild-type mice, supporting the in vitro findings (50).
Another study suggests that antibiotic pressure induces the formation of stable but reversible antibiotic-tolerant persister cells in a variety of host cell types, including unstimulated macrophages, keratinocytes, osteoblasts, epithelial cells, and monocytes, and that antibiotic-induced persister cells represent a major reservoir for secondary infection (48). Stringent response-deficient mutants of S. aureus formed fewer persisters in unstimulated macrophages (48). In addition, intracellular S. aureus persisters had induced expression of several stress response pathways, including the stringent response, cell wall stress, SOS, and heat shock responses that conferred multidrug tolerance (48). Clearly, drug penetration as well as multiple intracellular stress stimuli contribute to the recalcitrance of intracellular S. aureus populations to antibiotics (Figure 1b).
SOLUTIONS
Most conventional bactericidal antibiotics kill by corrupting ATP-dependent cellular processes, fluoroquinolones kill by preventing the religation step during DNA synthesis (63), aminoglycosides produce toxic peptides during translation (64), β-lactams induce a toxic futile cycle of cell wall synthesis and degradation (65), and glycopeptides prevent the cross-linking step of peptidoglycan synthesis (66). We now appreciate that any stress encountered during infection which slow or stop the processes targeted by antibiotics will likely lead to antibiotic tolerance. S. aureus can achieve antibiotic tolerance through multiple distinct mechanisms, as follows: activation of TA modules in biofilms (67), host-produced ROS in macrophages (50), nutrient limitation and activation of the stringent response during interaction with host cells (48), and likely numerous pathways that have yet to be identified. Therefore, we suggest that treatment failure should be studied in the context of a particular infection site, and strategies to overcome treatment failure may need to be niche specific. The logical but arduous task of improving antibiotic therapy of recalcitrant S. aureus infections is to first identify the persister reservoirs in vivo, determine how they formed, and strategize to eradicate them.
Antipersister drugs.
Antipersister antibiotics kill independently of the metabolic state of the cell and are more effective against biofilms than conventional antibiotics (68–70). Membrane-acting agents are attractive antipersister drugs as they exert rapid bactericidal activity, and in theory, they target persisters and growing cells indiscriminately. In addition, membrane-acting drugs often interact with multiple targets in the membrane which reduces the probability of resistance development (71). Daptomycin, a lipopeptide antibiotic, inserts into the cell membrane and disrupts fluid membrane microdomains. Daptomycin has potent activity against recalcitrant populations of S. aureus, including biofilms and persister cells (72), and in combination with linezolid, daptomycin is the leading therapy recommended to treat persistent MRSA bacteremia or vancomycin failure (72). However, despite its antipersister activity, its efficacy in vivo is limited by infection types; daptomycin is inactivated by surfactant and is therefore ineffective in the treatment of pneumonia or cystic fibrosis-associated infection (73). When prescribed, its efficacy against biofilm-associated infections is questionable, and treatment failure occurs in ∼20% cases (74–76). Tolerance to daptomycin is thought to arise from structural modifications to the membrane during infection which disrupt interaction with the drug (71, 74). In addition, mutants deficient in agr release phospholipid decoys which bind and inactivate daptomycin (77). This has major clinical relevance as agr mutants arise frequently in invasive S. aureus infections (78–80). This finding emphasizes that some pathways induce multidrug tolerance, while others may be specific to a single drug. Understanding the distinct mechanisms by which tolerance arises to individual drugs may lead to improved therapeutics. For instance, it was shown that a FASII inhibitor prevented phospholipid release from S. aureus cells leading to improved daptomycin killing (81).
Membrane-acting agents can also potentiate aminoglycosides (70, 82). Aminoglycosides are a broad-spectrum antibiotic that require proton motive force (PMF) for uptake into the cell. Following uptake, aminoglycosides bind to the 30S ribosomal subunit promoting tRNA mismatching which leads to the production of toxic peptides and cell death (64). This class of antibiotics is very effective against respiring cells which maintain a PMF at sufficient levels to facilitate drug uptake. However, aminoglycosides have limited utility against tolerant populations that maintain a PMF below the threshold required for drug uptake, such as anaerobically growing cells, small colony variants, and metabolically indolent cells within a biofilm (36, 70, 83). Pairing membrane-acting agents with aminoglycosides can increase drug uptake and sensitize recalcitrant S. aureus populations. Importantly, research has repeatedly shown that once the penetration barrier is overcome, aminoglycosides are highly bactericidal, even against persister cells, suggesting protein synthesis is sufficiently active in persisters for its corruption to lead to cell death (70, 84, 85). Rhamnolipids, biosurfactants produced by Pseudomonas aeruginosa, permeabilize the S. aureus membrane to allow PMF-independent diffusion of tobramycin into the cell (70, 86). This combination of tobramycin and rhamnolipids rapidly sterilized anaerobically growing cells and small colony variants and increased efficacy against biofilms by ∼1,000-fold. In another study, synthetic retinoids which are analogues of vitamin A that target lipid bilayers showed impressive activity alone and in combination with gentamicin in a mouse model of chronic MRSA (70, 82). Despite the promise of membrane-acting agents, identifying novel agents that specifically interact with bacterial membranes and exhibit low toxicity against host cells is challenging.
Another avenue of interest is the use of antimicrobial peptides (AMPs) to treat recalcitrant S. aureus infections. AMPs are a diverse class of naturally occurring defense peptides produced by all organisms, with activity against bacteria, viruses, and fungi (87, 88). Cationic AMPs usually consist of 10 to 50 amino acids which form positively charged, hydrophobic structures that are soluble in water, with very little secondary structure. With a high ratio of hydrophobic amino acids and an overall positive charge, AMPs selectively bind and disrupt the negatively charged bacterial cell membranes or cell walls, which can cause lysis and cell death (89). Due to their mechanism of action, it is unsurprising that AMPs have shown efficacy against biofilms; derivatives of LL37 eradicated MRSA biofilms on thermally wounded human skin equivalent models (90), and several studies have demonstrated efficacy against S. aureus infections in vivo (91–93). Synthetic cationic peptide nanoparticles containing a hydrophobic cholesterol core form micelles in the S. aureus membrane. When administered intraperitoneally in rabbits, these nanoparticles were able to cross the blood brain barrier and suppress S. aureus growth in a meningitis model (94).
AMPs can also be used to potentiate conventional antibiotics. The human β-defensins and CAP18 produced by epithelial cells synergize with β-lactams and improve killing of S. aureus in vitro (95). The frog-produced Citropin 1.1 displayed antibiofilm efficacy alone and improved the activity of rifampicin and minocycline in a central venous catheter rat model of S. aureus infection (96). In addition to their bactericidal activity, AMPs also recruit immune cells to the site of infection and modulate cytokine production (97). The role of AMPs in immune homeostasis as well as high production costs will likely hinder their therapeutic development (98). In addition, resistance to AMPs is conferred by any mutation that reduces the negative charge of the membrane, thus decreasing drug interaction (99). This is thought to be a general strategy used by gut bacteria to tolerate host-produced AMPs but also presents an insight into the mechanism that resistance could occur if AMPs were to be developed as therapeutics.
Another potent antipersister drug class is the acyldepsipeptides (ADEPs). ADEPs bind to the ClpP peptidase in S. aureus. Under normal conditions, ClpP degrades proteins in conjunction with the ClpC and ClpX unfoldases in an ATP-dependent manner. ADEPs cause the catalytic core of ClpP to expand, resulting in the formation of a dysregulated protease that digests bacterial proteins in an ATP-independent manner (69). Development of ADEPs was abandoned by Bayer in the 2000s due to the high frequency of resistance; mutants of the nonessential clpP arise at a frequency of 10−6. However, clpP mutants display decreased virulence and increased susceptibility to other antibiotics (69, 100). Indeed, ADEP combined with rifampicin led to impressive in vivo activity against biofilms in a murine deep-seated S. aureus infection (69). Recently, modifications to the ADEP structure has led to improved biological activity and metabolic stability (101, 102). ADEPs also have activity against Bacillus, Streptococcus, and Enterococcus bacteria, and they currently remain in preclinical development (100, 103).
Despite the promise of antipersister drugs, eradication of the population in vivo remains arduous, and antipersister activity alone may be insufficient to eradicate some recalcitrant infections. This is evidenced in a mouse catheter model where, following 7 days of treatment, daptomycin cleared less than 10% of S. aureus biofilms (104). In this scenario, it is possible that other factors such as drug penetration or membrane composition were impeding therapy. Additionally, the capacity of many antipersister drugs to pass into host cells and target intracellular persisters has yet to be explored.
Improving antibiotic penetration.
Evidence of in vivo reservoirs of S. aureus poorly accessed by antibiotics has fueled several innovative strategies for improving drug delivery to tackle this problem.
Ultrasound.
Ultrasound technology, traditionally used in medical imaging, uses sound waves that travel through soft tissues and fluids. When combined with intravenously administered microbubbles, ultrasound applications can be expanded to improve drug delivery. Upon exposure to an ultrasound wave, gas-filled microbubbles will oscillate; at low pressures, bubbles continuously expand and contract; and at higher pressures, the bubbles violently collapse. Ultrasound pressures can be exploited to create fluid movements around the bubble to induce shear stress to nearby biological structures, such as biofilms (105). A disadvantage of microbubbles is their short half-life following injection and their relatively large size (1 to 4 μm in diameter) (106), which may hamper penetration into biofilms. Maintaining microbubbles in the more stable liquid phase (nanodroplets) is hypothesized to improve penetration into biofilms due to their smaller size (0.1 to 0.4 μm in diameter) (107). Upon ultrasound activation, nanodroplets expand into microbubbles, creating shear stress from inside the biofilm (108). This experimental treatment strategy has successfully improved antibiotic efficacy against S. aureus biofilms in vitro (107, 108), improved transdermal drug delivery (109), and transiently permeabilized the blood brain barrier (110); in theory, this method could be expanded to improve drug penetration to any niche that is vascularized. In addition, it has the potential for rapid clinical translation, as ultrasound is already used routinely in wound debridement, some nanodroplet formulations are variants of FDA-approved ultrasound contrast agents that are already in clinical practice (111), and the low-pressure ultrasound settings required can be achieved with existing ultrasound hardware at pressures below the FDA set limits for diagnostic imaging (107).
Liposomes and nanoparticles.
Technological advances in nanoscience have yielded promising results in drug delivery using nanoparticles. Research has been dominated in the area of cancer therapeutics, but nanoparticle-bound-antibiotics is a growing research area. Pharmacokinetic data assess the length of time specific doses of antibiotics remain in the body. Dosing regimens desired to achieve clinical efficacy are decided based on pharmacokinetics, toxicity, and in vitro susceptibility data. For most infections, antibiotics must penetrate out of the blood and into host tissues to achieve efficacy. This is dependent on many factors, including initial concentration in the serum, blood flow to the tissue, penetration barriers such as host membranes, as well as effects of disease on these factors (112). Encapsulating drugs in liposomes, artificial spherical nanoparticle phospholipid vesicles, can drastically affect the pharmacokinetic properties and is a promising strategy for improving drug delivery. Drugs encapsulated in phospholipid, a component of the host membrane, interact with host cells and other biological structures, such as bacterial biofilms, to facilitate drug delivery. The aliphatic composition of phospholipids facilitates incorporation of hydrophilic drugs into the hydrophilic aqueous space or hydrophobic drugs into the hydrophobic membrane (112). An advantage to this strategy is improving the pharmacokinetics of antibiotics and potentially decreasing toxicity. An obstacle of liposomes is their osmotic fragility which can improved by incorporating polyethylene glycol into the outer membrane. This has been shown to improve blood circulation times (113, 114).
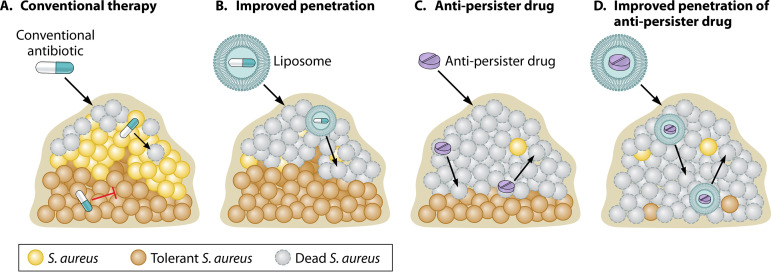
Encapsulating front-line anti-Mycobacterium tuberculosis drugs (isoniazid, pyrazinamide, and rifampicin) in liposomes significantly increased their therapeutic index (115). Similar strategies have been applied to S. aureus; flucloxacillin, rifampicin, and vancomycin encapsulated in liposomes all improved killing of S. aureus cells internalized in macrophages and embedded in biofilms (45, 55). It is interesting to consider the potential of encapsulating antipersister drugs in liposomes as a dual approach for improving antibiotic efficacy against biofilms (Fig. 2).
FIG 2.
A combined approach for improving antibiotic efficacy against S. aureus biofilms. (A) Biofilms display remarkable tolerance to antibiotics. Susceptible cells at the biofilm periphery die (dead S. aureus), while less metabolically active cells within the biofilm are tolerant to conventional antibiotics (tolerant S. aureus). Failure to eradicate the biofilm leads to relapse in infection following removal of the antibiotic. (B) Encapsulating drugs in liposomes will improve penetration of some conventional antibiotics that do not penetrate well through the biofilm matrix. This strategy is limited, as it does not improve killing of antibiotic-tolerant persister cells (tolerant S. aureus) and leads to relapse. (C) Targeting biofilms with antibiotics which kill persister cells (antipersister drug) improves efficacy, but if drug penetration is impeded into the biofilm, some cells will remain viable following drug treatment and lead to relapse. (D) Encapsulating antipersister drugs in liposomes improves penetration of drugs that are active against persister cells. Combining strategies to improve antibiotic efficacy against biofilms will improve the chances of eradicating all cells within a biofilm and prevent relapse of infection following removal of the antibiotic. Schematic credit: created with BioRender and modified by Patrick Lane, ScEYEnce Studios.
Other types of nanoparticles, such as polymeric nanoparticles, also serve as excellent drug carriers. Polymeric nanoparticles are polymer-based nanoparticles which can protect drugs from degradation, improve pharmacokinetics, and achieve localized high concentrations of drug at the site of infection. Formulations can be modified to tailor drug release and maximize the amount of time the drug is maintained above the MIC (116, 117). This strategy is proposed to increase the therapeutic potential of the drug while minimizing the chance of resistance development.
Antibody-based antibiotic therapy.
The ability of intracellular S. aureus to evade both antibiotic and immune-mediated killing, as well as seed secondary infections, suggests that sensitizing intracellular populations to antibiotics is a crucial strategy for improving efficacy. Antibody-antibiotic conjugate (AAC) therapy has proven more effective than antibiotic treatment alone in eradicating intracellular S. aureus (118). AAC is composed of an anti-S. aureus antibody linked to a rifamycin antibiotic using a protease-sensitive linker (118). S. aureus is opsonized by the anti-S. aureus antibody and phagocytosed by immune cells. Upon internalization, the linker is cleaved by host cell proteases, releasing the antibiotic in close proximity to and, importantly, in the same compartment as the bacteria (118). The AAC therapy was shown to effectively kill in vitro persister cells, internalized S. aureus, and, in conjunction with vancomycin treatment, prevented internalized S. aureus from causing secondary infection in mice (118). The superior potency, efficacy, and specificity of the AAC therapy, particularly in combination with vancomycin treatment, render AAC therapy a viable option for solving the following two major problems associated with treatment failure: antibiotic-tolerant bacteria and poor antibiotic penetration into host cells. Despite the promise of this approach, high production costs may hinder the translation of this therapy to the clinic (119).
Metabolic priming.
As persister cells are frequently in a state of metabolic quiescence, coercing them out of that state by providing metabolites is an established means of sensitizing persisters to antibiotics. Allison et al. found that addition of carbon metabolites sensitized Escherichia coli and S. aureus to aminoglycoside antibiotics (84). Interestingly, metabolites that stimulated the beginning steps of glycolysis (i.e., glucose, mannitol, and fructose) induced the most rapid and efficacious killing of persisters, while metabolites that stimulated the end of glycolysis, as well as the pentose phosphate and the Entner-Doudoroff pathways, resulted in less potentiation (84). Supplementing these metabolites increased PMF, facilitating aminoglycoside uptake without requiring resumption of growth (84). This treatment strategy was effective under both aerobic and anaerobic conditions, against S. aureus and E. coli biofilms, and in a murine chronic catheter-associated urinary tract infection model (84). Subsequently, a similar phenomenon was described for P. aeruginosa where addition of TCA cycle intermediates similarly stimulated respiration and increased PMF, sensitizing P. aeruginosa to aminoglycoside antibiotics (85). Stalling or shunting the TCA cycle was associated with increased tolerance (85). In S. aureus, decreased TCA cycle activity was shown to contribute to rifampicin tolerance following ROS exposure (50). Upon addition of excess glucose, rifampicin susceptibility was restored, presumably by circumventing the TCA cycle generating ATP through substrate-level phosphorylation (50). However, the feasibility of metabolic priming to sensitize S. aureus persisters in patients remains to be seen. It is likely that the specific limiting nutrient(s) would need to be identified for a given niche of interest, and it is therefore unlikely that a single molecule will serve as a universal persister resuscitation signal. Additionally, the nutrients available for resuscitating persisters are limited, as any nutrient easily metabolized by host cells would be difficult to deliver at sufficiently high levels to the pathogen populations.
Immunomodulation.
In addition to directly targeting the bacteria, it may be possible to target host pathways that induce antibiotic tolerance during infection. High levels of ROS are associated with an M1-like proinflammatory macrophage response (50, 120–122). Thus, in the case of ROS-dependent induction of antibiotic tolerance, modulation of the immune response toward an anti-inflammatory M2-like response where ROS production is decreased (120, 122, 123) may improve antibiotic efficacy. Indeed, the use of antioxidants was shown to increase antibiotic efficacy in a murine bacteremia model of S. aureus infection (50). Additionally, corticosteroids, such as dexamethasone, are commonly used in combination with antibiotics to treat bacterial infections (124, 125). The effects of corticosteroids are largely immunosuppressive, although effects on innate immune cells, such as macrophages and monocytes, are complex (126–130). In an S. aureus arthritis model, coadministration of dexamethasone and antibiotics significantly decreased disease severity and resulted in rapid resolution of infection compared with antibiotic treatment alone (131). Other studies using human macrophages have found that treatment of macrophages with dexamethasone increases phagocytosis, as well as killing of S. aureus (132).
In contrast, chronic and recurrent S. aureus biofilms effectively evade macrophage-mediated killing by skewing the macrophage response to an alternatively activated M2-like state (133–136). Treatment of S. aureus tissue and catheter-associated biofilms with either EP67, a C5α receptor agonist, or classically activated M1-like macrophages decreased biofilm bacterial burden compared with antibiotic treatment alone (135). However, the effect(s) of classically or alternatively activated macrophages in combination with antibiotic treatment outcome in biofilm infections is unclear.
Despite the promise of targeting the host to increase antibiotic efficacy, immunomodulation carries with it its own set of risks and challenges. The initial proinflammatory immune response is critical for controlling initial S. aureus burden, thus bringing into question when an immunosuppressive treatment should be administered (50, 137, 138). In addition, the benefits of activating or suppressing the immune response may be niche and infection type dependent. In the case of corticosteroids, treatment outcome may be adversely affected by patient age and comorbidities (139, 140). Similarly, stimulating an immune response may lead to tissue damage and cytotoxicity associated with systemic inflammation (138). Despite potential adverse effects, immunomodulation remains an exciting and promising strategy for combating antibiotic treatment failure, but more research is required to explore its promise and limitations.
SUMMARY
Research in E. coli and more recently in S. aureus has underscored that there is not a singular mechanism of persister formation, and consequently, there is not a single “cure” for the problem. Therefore, understanding the nature of treatment failure to each drug for each indication is crucial for improving therapy. Although the examination of multidrug tolerance in broth culture has led to many important foundational discoveries, we need to now consider the barriers to the efficacy of clinically relevant antibiotics during infection. We must determine where S. aureus persisters are located and how they form in relevant murine models and eventually in patients. Murine models are a powerful resource which have afforded us immeasurable insight into the nature of S. aureus infections, but they have important limitations (141); some receptors of key S. aureus toxins present in humans are absent in mice (142), and neutrophils from mice do not express defensins, the potent antimicrobial peptide expressed by human neutrophils (143). The examination of antibiotic efficacy using human primary cells and the recently developed humanized mouse lines must be encouraged in order to bridge the gap from mouse relevance to human relevance (144).
We must continue drug discovery and development efforts with a focus on efficacy against persister cells. We must develop improved delivery methods to allow antibiotics to reach recalcitrant populations. Admittedly, many of the solutions proposed here are costly and may currently suffer from commercial viability issues. However, there is a critical need to improve antibiotic therapies; Medicare is burdened with costs of over $30 billion annually for chronic wounds alone; and risk factors for chronic bacterial infections, such as obesity and diabetes, are increasing at an alarming rate (11). Perhaps government investment in these novel therapies will be less expensive in the long run as they promise to improve killing of problematic subpopulations of S. aureus and get us closer to the ultimate goal—efficient and reliable eradication of S. aureus infection.
Biographies

Sarah E. Rowe, Ph.D. is a research assistant professor in the Department of Microbiology and Immunology at the University of North Carolina at Chapel Hill. She received her Ph.D. from University College Dublin in 2010 where she studied mechanisms of biofilm formation in Staphylococcus aureus and Staphylococcus epidermidis in the laboratory of James P. O’Gara. Her postdoctoral fellowship was with Kim Lewis at Northeastern University where she studied antibiotic tolerance in Escherichia coli and S. aureus. In 2015, she joined Synlogic Therapeutics in Cambridge, MA, as a scientist to engineer synthetic probiotics for the treatment of rare diseases. In 2016, she joined the Conlon lab at the University of North Carolina at Chapel Hill as a research assistant professor. Here, she is focused on various aspects of antibiotic treatment failure of staphylococcal infection and ways to solve this problem.

Jenna E. Beam is a Ph.D. candidate in the Department of Microbiology and Immunology at the University of North Carolina at Chapel Hill. She received her BS in microbiology from The University of Texas at Austin in 2017. She is currently under the mentorship of Brian Conlon studying how interactions between S. aureus and macrophages impact antibiotic treatment outcome.

Brian P. Conlon, Ph.D. is an assistant professor in the Department of Microbiology and Immunology at the University of North Carolina at Chapel Hill. He received a BS in microbiology from the National University of Ireland, Galway in 2006. He received his Ph.D. from University College Dublin in 2010 under the mentorship of Jim O’Gara, examining biofilm formation in staphylococci. He then went to Northeastern University in 2011 to study persister cell formation in S. aureus under the mentorship of Kim Lewis. It was here he developed an interest in the contribution of the host infection environment to the efficacy of antibiotics. He began his independent lab at University of North Carolina at Chapel Hill in 2016, where he has been examining how bacterial interspecies interactions and host-pathogen interactions can alter the physiological state of a pathogen and contribute to antibiotic tolerance and treatment failure.
REFERENCES
- 1.Fair RJ, Tor Y. 2014. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem 6:25–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett JG, Gilbert DN, Spellberg B. 2013. Seven ways to preserve the miracle of antibiotics. Clin Infect Dis 56:1445–1450. 10.1093/cid/cit070. [DOI] [PubMed] [Google Scholar]
- 3.Richter K, Van den Driessche F, Coenye T. 2017. Innovative approaches to treat Staphylococcus aureus biofilm-related infections. Essays Biochem 61:61–70. 10.1042/EBC20160056. [DOI] [PubMed] [Google Scholar]
- 4.Foster TJ. 2017. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol Rev 41:430–449. 10.1093/femsre/fux007. [DOI] [PubMed] [Google Scholar]
- 5.McGuinness WA, Malachowa N, DeLeo FR. 2017. Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med 90:269–281. [PMC free article] [PubMed] [Google Scholar]
- 6.Vestergaard M, Frees D, Ingmer H. 2019. Antibiotic resistance and the MRSA problem. Microbiol Spectr 7:10.1128/microbiolspec.GPP3-0057-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reffuveille F, Josse J, Vallé Q, Mongaret C, Gangloff SC. 2017. Staphylococcus aureus biofilms and their impact on the medical field. The rise of virulence and antibiotic resistance in Staphylococcus aureus. InTechOpen, London, UK. [Google Scholar]
- 8.Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. 2015. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis 211:641–650. 10.1093/infdis/jiu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. 2009. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17:763–771. 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA. 2006. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3:225–231. 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negut I, Grumezescu V, Grumezescu AM. 2018. Treatment strategies for infected wounds. Molecules 23:2392. 10.3390/molecules23092392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan S, Stevens NT, Humphreys H, O'Gara JP, O'Neill E. 2015. Current and future approaches to the prevention and treatment of staphylococcal medical device-related infections. Curr Pharm Des 21:100–113. 10.2174/1381612820666140905123900. [DOI] [PubMed] [Google Scholar]
- 13.Urish KL, Cassat JE. 2020. Staphylococcus aureus osteomyelitis: bone, bugs, and surgery. Infect Immun 88:e00932-19. 10.1128/IAI.00932-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofstee MI, Riool M, Terjajevs I, Thompson K, Stoddart MJ, Richards RG, Zaat SAJ, Moriarty TF. 2020. 3-Dimensional in vitro Staphylococcus aureus abscess communities display antibiotic tolerance and protection from neutrophil clearance. Infect Immun 88:e00293-20. 10.1128/IAI.00293-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. 2009. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J 23:3393–3404. 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery CP, David MZ, Daum RS. 2015. Host factors that contribute to recurrent staphylococcal skin infection. Curr Opin Infect Dis 28:253–258. 10.1097/QCO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sreeramoju P, Porbandarwalla NS, Arango J, Latham K, Dent DL, Stewart RM, Patterson JE. 2011. Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am J Surg 201:216–220. 10.1016/j.amjsurg.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Conterno LO, Turchi MD. 2013. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev 6:CD004439. 10.1002/14651858.CD004439.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis K. 2017. New approaches to antimicrobial discovery. Biochem Pharmacol 134:87–98. 10.1016/j.bcp.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Dunne WM, Jr, Mason EO, Jr, Kaplan SL. 1993. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother 37:2522–2526. 10.1128/aac.37.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh R, Ray P, Das A, Sharma M. 2010. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother 65:1955–1958. 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Sahore S, Kaur P, Rani A, Ray P. 2016. Penetration barrier contributes to bacterial biofilm-associated resistance against only select antibiotics, and exhibits genus-, strain- and antibiotic-specific differences. Pathog Dis 74:ftw056. 10.1093/femspd/ftw056. [DOI] [PubMed] [Google Scholar]
- 23.Goltermann L, Tolker-Nielsen T. 2017. Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrob Agents Chemother 61:e02696-16. 10.1128/AAC.02696-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudjemaa R, Briandet R, Revest M, Jacqueline C, Caillon J, Fontaine-Aupart MP, Steenkeste K. 2016. New insight into daptomycin bioavailability and localization in Staphylococcus aureus biofilms by dynamic fluorescence imaging. Antimicrob Agents Chemother 60:4983–4990. 10.1128/AAC.00735-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart PS, Davison WM, Steenbergen JN. 2009. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother 53:3505–3507. 10.1128/AAC.01728-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters MC, III, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323. 10.1128/aac.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Z, Stewart PS. 2002. Penetration of rifampin through Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 46:900–903. 10.1128/aac.46.3.900-903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crabbé A, Jensen PO, Bjarnsholt T, Coenye T. 2019. Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol 27:850–863. 10.1016/j.tim.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Lewis K. 2001. Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007. 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mah TF, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39. 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 31.Sønderholm M, Bjarnsholt T, Alhede M, Kolpen M, Jensen PO, Kuhl M, Kragh KN. 2017. The consequences of being in an infectious biofilm: microenvironmental conditions governing antibiotic tolerance. Int J Mol Sci 18:2688. 10.3390/ijms18122688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 33.Waters EM, Rowe SE, O'Gara JP, Conlon BP. 2016. Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells? PLoS Pathog 12:e1006012. 10.1371/journal.ppat.1006012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, Clair G, Adkins JN, Cheung AL, Lewis K. 2016. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol 1:16051. 10.1038/nmicrobiol.2016.51. [DOI] [PubMed] [Google Scholar]
- 35.Shan Y, Brown Gandt A, Rowe SE, Deisinger JP, Conlon BP, Lewis K. 2017. ATP-dependent persister formation in Escherichia coli. mBio 8:e02267-16. 10.1128/mBio.02267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobritz MA, Belenky P, Porter CB, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ. 2015. Antibiotic efficacy is linked to bacterial cellular respiration. Proc Natl Acad Sci U S A 112:8173–8180. 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu T, Wang XY, Cui P, Zhang YM, Zhang WH, Zhang Y. 2017. The Agr quorum sensing system represses persister formation through regulation of phenol soluble modulins in Staphylococcus aureus. Front Microbiol 8:2189. 10.3389/fmicb.2017.02189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bojer MS, Lindemose S, Vestergaard M, Ingmer H. 2018. Quorum sensing-regulated phenol-soluble modulins limit persister cell populations in Staphylococcus aureus. Front Microbiol 9:255. 10.3389/fmicb.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Bojer MS, George SE, Wang Z, Jensen PR, Wolz C, Ingmer H. 2018. Inactivation of TCA cycle enhances Staphylococcus aureus persister cell formation in stationary phase. Sci Rep 8:10849. 10.1038/s41598-018-29123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zalis EA, Nuxoll AS, Manuse S, Clair G, Radlinski LC, Conlon BP, Adkins J, Lewis K. 2019. Stochastic variation in expression of the tricarboxylic acid cycle produces persister cells. mBio 10:e01930-19. 10.1128/mBio.01930-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nabb DL, Song S, Kluthe KE, Daubert TA, Luedtke BE, Nuxoll AS. 2019. Polymicrobial interactions induce multidrug tolerance in Staphylococcus aureus through energy depletion. Front Microbiol 10:2803. 10.3389/fmicb.2019.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwan BW, Valenta JA, Benedik MJ, Wood TK. 2013. Arrested protein synthesis increases persister-like cell formation. Antimicrob Agents Chemother 57:1468–1473. 10.1128/AAC.02135-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pontes MH, Groisman EA. 2019. Slow growth determines nonheritable antibiotic resistance in Salmonella enterica. Sci Signal 12:eaax3938. 10.1126/scisignal.aax3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pontes MH, Groisman EA. 2020. A physiological basis for nonheritable antibiotic resistance. mBio 11:e00817-20. 10.1128/mBio.00817-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surewaard BG, Deniset JF, Zemp FJ, Amrein M, Otto M, Conly J, Omri A, Yates RM, Kubes P. 2016. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 213:1141–1151. 10.1084/jem.20160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jorch SK, Surewaard BG, Hossain M, Peiseler M, Deppermann C, Deng J, Bogoslowski A, van der Wal F, Omri A, Hickey MJ, Kubes P. 2019. Peritoneal GATA6+ macrophages function as a portal for Staphylococcus aureus dissemination. J Clin Invest 129:4643–4656. 10.1172/JCI127286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krauss JL, Roper PM, Ballard A, Shih CC, Fitzpatrick JAJ, Cassat JE, Ng PY, Pavlos NJ, Veis DJ. 2019. Staphylococcus aureus infects osteoclasts and replicates intracellularly. mBio 10:e02447-19. 10.1128/mBio.02447-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peyrusson F, Varet H, Nguyen TK, Legendre R, Sismeiro O, Coppee JY, Wolz C, Tenson T, Van Bambeke F. 2020. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat Commun 11:2200. 10.1038/s41467-020-15966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peyrusson F, Tulkens PM, Van Bambeke F. 2018. Cellular pharmacokinetics and intracellular activity of gepotidacin against Staphylococcus aureus isolates with different resistance phenotypes in models of cultured phagocytic cells. Antimicrob Agents Chemother 62:e02245-17. 10.1128/AAC.02245-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowe SE, Wagner NJ, Li L, Beam JE, Wilkinson AD, Radlinski LC, Zhang Q, Miao EA, Conlon BP. 2020. Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat Microbiol 5:282–290. 10.1038/s41564-019-0627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fraunholz M, Sinha B. 2012. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol 2:43. 10.3389/fcimb.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol 164:3713–3722. 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 53.Roesler J, Hockertz S, Vogt B, Lohmann-Matthes ML. 1991. Staphylococci surviving intracellularly in phagocytes from patients suffering from chronic granulomatous disease are killed in vitro by antibiotics encapsulated in liposomes. J Clin Invest 88:1224–1229. 10.1172/JCI115425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barcia-Macay M, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Evaluation of the extracellular and intracellular activities (human THP-1 macrophages) of telavancin versus vancomycin against methicillin-susceptible, methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. J Antimicrob Chemother 58:1177–1184. 10.1093/jac/dkl424. [DOI] [PubMed] [Google Scholar]
- 55.Scriboni AB, Couto VM, Ribeiro LNM, Freires IA, Groppo FC, de Paula E, Franz-Montan M, Cogo-Müller K. 2019. Fusogenic liposomes increase the antimicrobial activity of vancomycin against Staphylococcus aureus biofilm. Front Pharmacol 10:1401. 10.3389/fphar.2019.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abed N, Couvreur P. 2014. Nanocarriers for antibiotics: a promising solution to treat intracellular bacterial infections. Int J Antimicrob Agents 43:485–496. 10.1016/j.ijantimicag.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Renard C, Vanderhaeghe HJ, Claes PJ, Zenebergh A, Tulkens PM. 1987. Influence of conversion of penicillin G into a basic derivative on its accumulation and subcellular localization in cultured macrophages. Antimicrob Agents Chemother 31:410–416. 10.1128/aac.31.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tulkens PM. 1991. Intracellular distribution and activity of antibiotics. Eur J Clin Microbiol Infect Dis 10:100–106. 10.1007/BF01964420. [DOI] [PubMed] [Google Scholar]
- 59.Bongers S, Hellebrekers P, Leenen LPH, Koenderman L, Hietbrink F. 2019. Intracellular penetration and effects of antibiotics on Staphylococcus aureus inside human neutrophils: a comprehensive review. Antibiotics (Basel) 8:54. 10.3390/antibiotics8020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Acocella G, Carlone NA, Cuffini AM, Cavallo G. 1985. The penetration of rifampicin, pyrazinamide, and pyrazinoic acid into mouse macrophages. Am Rev Respir Dis 132:1268–1273. 10.1164/arrd.1985.132.6.1268. [DOI] [PubMed] [Google Scholar]
- 61.Darouiche RO, Hamill RJ. 1994. Antibiotic penetration of and bactericidal activity within endothelial cells. Antimicrob Agents Chemother 38:1059–1064. 10.1128/aac.38.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother 50:841–851. 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malik M, Zhao X, Drlica K. 2006. Lethal fragmentation of bacterial chromosomes mediated by DNA gyrase and quinolones. Mol Microbiol 61:810–825. 10.1111/j.1365-2958.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 64.Davis BD. 1987. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev 51:341–350. 10.1128/MR.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho H, Uehara T, Bernhardt TG. 2014. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 159:1300–1311. 10.1016/j.cell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Healy VL, Lessard IA, Roper DI, Knox JR, Walsh CT. 2000. Vancomycin resistance in enterococci: reprogramming of the d-ala-d-Ala ligases in bacterial peptidoglycan biosynthesis. Chem Biol 7:R109–119. 10.1016/s1074-5521(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 67.Ma D, Mandell JB, Donegan NP, Cheung AL, Ma W, Rothenberger S, Shanks RMQ, Richardson AR, Urish KL. 2019. The toxin-antitoxin MazEF drives Staphylococcus aureus biofilm formation, antibiotic tolerance, and chronic infection. mBio 10:e01658-19. 10.1128/mBio.01658-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bauer J, Siala W, Tulkens PM, Van Bambeke F. 2013. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob Agents Chemother 57:2726–2737. 10.1128/AAC.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conlon BP, Nakayasu ES, Fleck LE, LaFleur MD, Isabella VM, Coleman K, Leonard SN, Smith RD, Adkins JN, Lewis K. 2013. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 503:365–370. 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radlinski LC, Brzozowski SE, Wilkinson R, Huang A, Eswara R, Conlon P, Brian P. 2019. Chemical induction of aminoglycoside uptake overcomes antibiotic tolerance and resistance in Staphylococcus aureus. Cell Chem Biol 26:1355–1364.e4. 10.1016/j.chembiol.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurdle JG, O'Neill AJ, Chopra I, Lee RE. 2011. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62–75. 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 73.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 191:2149–2152. 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 74.Humphries RM, Pollett S, Sakoulas G. 2013. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev 26:759–780. 10.1128/CMR.00030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seaton RA, Menichetti F, Dalekos G, Beiras-Fernandez A, Nacinovich F, Pathan R, Hamed K. 2015. Evaluation of effectiveness and safety of high-dose daptomycin: results from patients included in the European Cubicin((R)) Outcomes Registry and Experience. Adv Ther 32:1192–1205. 10.1007/s12325-015-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stefani S, Campanile F, Santagati M, Mezzatesta ML, Cafiso V, Pacini G. 2015. Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: a review of the available evidence. Int J Antimicrob Agents 46:278–289. 10.1016/j.ijantimicag.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Pader V, Hakim S, Painter KL, Wigneshweraraj S, Clarke TB, Edwards AM. 2016. Staphylococcus aureus inactivates daptomycin by releasing membrane phospholipids. Nat Microbiol 2:16194. 10.1038/nmicrobiol.2016.194. [DOI] [PubMed] [Google Scholar]
- 78.Fischer J, Lee JC, Peters G, Kahl BC. 2014. Acapsular clinical Staphylococcus aureus isolates lack agr function. Clin Microbiol Infect 20:O414–O417. 10.1111/1469-0691.12429. [DOI] [PubMed] [Google Scholar]
- 79.Fowler VG, Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. 2004. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 190:1140–1149. 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- 80.Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. 2008. agr function in clinical Staphylococcus aureus isolates. Microbiology (Reading) 154:2265–2274. 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pee CJE, Pader V, Ledger EVK, Edwards AM. 2019. A FASII inhibitor prevents Staphylococcal evasion of daptomycin by inhibiting phospholipid decoy production. Antimicrob Agents Chemother 63:e02105-18. 10.1128/AAC.02105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim W, Zhu W, Hendricks GL, Van Tyne D, Steele AD, Keohane CE, Fricke N, Conery AL, Shen S, Pan W, Lee K, Rajamuthiah R, Fuchs BB, Vlahovska PM, Wuest WM, Gilmore MS, Gao H, Ausubel FM, Mylonakis E. 2018. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 556:103–107. 10.1038/nature26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lechner S, Lewis K, Bertram R. 2012. Staphylococcus aureus persisters tolerant to bactericidal antibiotics. J Mol Microbiol Biotechnol 22:235–244. 10.1159/000342449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meylan S, Porter CBM, Yang JH, Belenky P, Gutierrez A, Lobritz MA, Park J, Kim SH, Moskowitz SM, Collins JJ. 2017. Carbon sources tune antibiotic susceptibility in Pseudomonas aeruginosa via tricarboxylic acid cycle control. Cell Chem Biol 24:195–206. 10.1016/j.chembiol.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Radlinski L, Rowe SE, Kartchner LB, Maile R, Cairns BA, Vitko NP, Gode CJ, Lachiewicz AM, Wolfgang MC, Conlon BP. 2017. Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus. PLoS Biol 15:e2003981. 10.1371/journal.pbio.2003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ageitos JM, Sánchez-Pérez A, Calo-Mata P, Villa TG. 2017. Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol 133:117–138. 10.1016/j.bcp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 88.Reddy KV, Yedery RD, Aranha C. 2004. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24:536–547. 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Zhang LJ, Gallo RL. 2016. Antimicrobial peptides. Curr Biol 26:R14–R19. 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 90.Haisma EM, de Breij A, Chan H, van Dissel JT, Drijfhout JW, Hiemstra PS, El Ghalbzouri A, Nibbering PH. 2014. LL-37-derived peptides eradicate multidrug-resistant Staphylococcus aureus from thermally wounded human skin equivalents. Antimicrob Agents Chemother 58:4411–4419. 10.1128/AAC.02554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao G, Lange D, Hilpert K, Kindrachuk J, Zou Y, Cheng JT, Kazemzadeh-Narbat M, Yu K, Wang R, Straus SK, Brooks DE, Chew BH, Hancock RE, Kizhakkedathu JN. 2011. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 32:3899–3909. 10.1016/j.biomaterials.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 92.Kwakman PH, Te Velde AA, Vandenbroucke-Grauls CM, van Deventer SJ, Zaat SA. 2006. Treatment and prevention of Staphylococcus epidermidis experimental biomaterial-associated infection by bactericidal peptide 2. Antimicrob Agents Chemother 50:3977–3983. 10.1128/AAC.00575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Menousek J, Mishra B, Hanke ML, Heim CE, Kielian T, Wang G. 2012. Database screening and in vivo efficacy of antimicrobial peptides against methicillin-resistant Staphylococcus aureus USA300. Int J Antimicrob Agents 39:402–406. 10.1016/j.ijantimicag.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu L, Xu K, Wang H, Tan PK, Fan W, Venkatraman SS, Li L, Yang YY. 2009. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat Nanotechnol 4:457–463. 10.1038/nnano.2009.153. [DOI] [PubMed] [Google Scholar]
- 95.Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, Yamazaki K, Sayama K, Taubman MA, Kurihara H, Hashimoto K, Sugai M. 2003. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun 71:3730–3739. 10.1128/iai.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cirioni O, Giacometti A, Ghiselli R, Kamysz W, Orlando F, Mocchegiani F, Silvestri C, Licci A, Chiodi L, Lukasiak J, Saba V, Scalise G. 2006. Citropin 1.1-treated central venous catheters improve the efficacy of hydrophobic antibiotics in the treatment of experimental staphylococcal catheter-related infection. Peptides 27:1210–1216. 10.1016/j.peptides.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 97.Mansour SC, Pena OM, Hancock RE. 2014. Host defense peptides: front-line immunomodulators. Trends Immunol 35:443–450. 10.1016/j.it.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Batoni G, Maisetta G, Esin S. 2016. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim Biophys Acta 1858:1044–1060. 10.1016/j.bbamem.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 99.Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, Goodman AL. 2015. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347:170–175. 10.1126/science.1260580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brotz-Oesterhelt H, Beyer D, Kroll HP, Endermann R, Ladel C, Schroeder W, Hinzen B, Raddatz S, Paulsen H, Henninger K, Bandow JE, Sahl HG, Labischinski H. 2005. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat Med 11:1082–1087. 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]
- 101.Carney DW, Schmitz KR, Truong JV, Sauer RT, Sello JK. 2014. Restriction of the conformational dynamics of the cyclic acyldepsipeptide antibiotics improves their antibacterial activity. J Am Chem Soc 136:1922–1929. 10.1021/ja410385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Griffith EC, Zhao Y, Singh AP, Conlon BP, Tangallapally R, Shadrick WR, Liu J, Wallace MJ, Yang L, Elmore JM, Li Y, Zheng Z, Miller DJ, Cheramie MN, Lee RB, LaFleur MD, Lewis K, Lee RE. 2019. Ureadepsipeptides as ClpP activators. ACS Infect Dis 5:1915–1925. 10.1021/acsinfecdis.9b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown Gandt A, Griffith EC, Lister IM, Billings LL, Han A, Tangallapally R, Zhao Y, Singh AP, Lee RE, LaFleur MD. 2018. In vivo and in vitro effects of a ClpP-activating antibiotic against vancomycin-resistant enterococci. Antimicrob Agents Chemother 62:e00424-18. 10.1128/AAC.00424-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weiss EC, Zielinska A, Beenken KE, Spencer HJ, Daily SJ, Smeltzer MS. 2009. Impact of sarA on daptomycin susceptibility of Staphylococcus aureus biofilms in vivo. Antimicrob Agents Chemother 53:4096–4102. 10.1128/AAC.00484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu J, Zhang N, Jr, Li L, Zhang N, Sr., Ma Y, Zhao C, Wu Q, Li Y, He N, Wang X. 2018. The synergistic bactericidal effect of vancomycin on UTMD treated biofilm involves damage to bacterial cells and enhancement of metabolic activities. Sci Rep 8:192. 10.1038/s41598-017-18496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tzu-Yin W, Wilson KE, Machtaler S, Willmann JK. 2013. Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications. Curr Pharm Biotechnol 14:743–752. 10.2174/1389201014666131226114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Durham PG, Sidders AE, Dayton PA, Conlon BP, Papadopoulou V, Rowe SE. 2020. Harnessing ultrasound-stimulated phase change contrast agents to improve antibiotic efficacy against methicillin-resistant Staphylococcus aureus biofilms. bioRxiv 10.1101/2020.06.01.127340. [DOI] [PMC free article] [PubMed]
- 108.Guo H, Wang Z, Du Q, Li P, Wang Z, Wang A. 2017. Stimulated phase-shift acoustic nanodroplets enhance vancomycin efficacy against methicillin-resistant Staphylococcus aureus biofilms. IJN 2017:4679–4690. 10.2147/IJN.S134525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oberli MA, Schoellhammer CM, Langer R, Blankschtein D. 2014. Ultrasound-enhanced transdermal delivery: recent advances and future challenges. Ther Deliv 5:843–857. 10.4155/tde.14.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Konofagou EE, Tung YS, Choi J, Deffieux T, Baseri B, Vlachos F. 2012. Ultrasound-induced blood-brain barrier opening. Curr Pharm Biotechnol 13:1332–1345. 10.2174/138920112800624364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fix SM, Koppolu BP, Novell A, Hopkins J, Kierski TM, Zaharoff DA, Dayton PA, Papadopoulou V. 2019. Ultrasound-stimulated phase-change contrast agents for transepithelial delivery of macromolecules, toward gastrointestinal drug delivery. Ultrasound Med Biol 45:1762–1776. 10.1016/j.ultrasmedbio.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kohno S, Tomono K, Maesaki S. 1998. Drug delivery systems for infection: liposome-lncorporating antimicrobial drugs. J Infect Chemother 4:159–173. 10.1007/BF02490162. [DOI] [Google Scholar]
- 113.Mei L, Fu L, Shi K, Zhang Q, Liu Y, Tang J, Gao H, Zhang Z, He Q. 2014. Increased tumor targeted delivery using a multistage liposome system functionalized with RGD, TAT and cleavable PEG. Int J Pharm 468:26–38. 10.1016/j.ijpharm.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 114.Pattni BS, Chupin VV, Torchilin VP. 2015. New developments in liposomal drug delivery. Chem Rev 115:10938–10966. 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 115.Justo OR, Moraes AM. 2003. Incorporation of antibiotics in liposomes designed for tuberculosis therapy by inhalation. Drug Deliv 10:201–207. 10.1080/713840401. [DOI] [PubMed] [Google Scholar]
- 116.Labruere R, Sona AJ, Turos E. 2019. Anti-methicillin-resistant Staphylococcus aureus nanoantibiotics. Front Pharmacol 10:1121. 10.3389/fphar.2019.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lakshminarayanan R, Ye E, Young DJ, Li Z, Loh XJ. 2018. Recent advances in the development of antimicrobial nanoparticles for combating resistant pathogens. Adv Healthc Mater 7:e1701400. 10.1002/adhm.201701400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Morisaki JH, Kim J, Park S, Darwish M, Lee BC, Hernandez H, Loyet KM, Lupardus P, Fong R, Yan D, Chalouni C, Luis E, Khalfin Y, Plise E, Cheong J, Lyssikatos JP, Strandh M, Koefoed K, Andersen PS, Flygare JA, Wah Tan M, Brown EJ, Mariathasan S. 2015. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 527:323–328. 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 119.Mariathasan S, Tan M-W. 2017. Antibody-antibiotic conjugates: a novel therapeutic platform against bacterial infections. Trends Mol Med 23:135–149. 10.1016/j.molmed.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 120.Griess B, Mir S, Datta K, Teoh-Fitzgerald M. 2020. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med 147:48–60. 10.1016/j.freeradbiomed.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee KY. 2019. M1 and M2 polarization of macrophages: a mini-review. Med Biol Sci Eng 2:1–5. 10.30579/mbse.2019.2.1.1. [DOI] [Google Scholar]
- 122.Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. 2016. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev 2016:2795090. 10.1155/2016/2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thurlow LR, Joshi GS, Richardson AR. 2018. Peroxisome proliferator-activated receptor gamma is essential for the resolution of Staphylococcus aureus skin infections. Cell Host Microbe 24:261–270.e264. 10.1016/j.chom.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 124.Aberdein J, Singer M. 2006. Clinical review: a systematic review of corticosteroid use in infections. Crit Care 10:203. 10.1186/cc3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Y, Li H, Sun H, Gong L, Guo L, Shi Y, Cai C, Gu H, Song Z, Yang L, Tong Y, Wei C, Zou Q, Zeng H. 2016. A novel nitro-dexamethasone inhibits agr system activity and improves therapeutic effects in MRSA sepsis models without antibiotics. Sci Rep 6:20307. 10.1038/srep20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Busillo JM, Azzam KM, Cidlowski JA. 2011. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem 286:38703–38713. 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cain DW, Cidlowski JA. 2017. Immune regulation by glucocorticoids. Nat Rev Immunol 17:233–247. 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ehrchen JM, Roth J, Barczyk-Kahlert K. 2019. More than suppression: glucocorticoid action on monocytes and macrophages. Front Immunol 10:2028. 10.3389/fimmu.2019.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim H-Y, Müller N, Herold MJ, van den Brandt J, Reichardt HM. 2007. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology 122:47–53. 10.1111/j.1365-2567.2007.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakamura Y, Murai T, Ogawa Y. 1996. Effect of in vitro and in vivo administration of dexamethasone on rat macrophage functions: comparison between alveolar and peritoneal macrophages. Eur Respir J 9:301–306. 10.1183/09031936.96.09020301. [DOI] [PubMed] [Google Scholar]
- 131.Sakiniene E, Bremell T, Tarkowski A. 1996. Addition of corticosteroids to antibiotic treatment ameliorates the course of experimental Staphylococcus aureus arthritis. Arthritis Rheum 39:1596–1605. 10.1002/art.1780390921. [DOI] [PubMed] [Google Scholar]
- 132.van der Goes A, Hoekstra K, van den Berg TK, Dijkstra CD. 2000. Dexamethasone promotes phagocytosis and bacterial killing by human monocytes/macrophages in vitro. J Leukoc Biol 67:801–807. 10.1002/jlb.67.6.801. [DOI] [PubMed] [Google Scholar]
- 133.Alboslemy T, Yu B, Rogers T, Kim M-H. 2019. Staphylococcus aureus biofilm-conditioned medium impairs macrophage-mediated antibiofilm immune response by upregulating KLF2 expression. Infect Immun 87:e00643-18. 10.1128/IAI.00643-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS. 2011. Protective role of IL-1beta against post-arthroplasty Staphylococcus aureus infection. J Orthop Res 29:1621–1626. 10.1002/jor.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. 2013. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J Immunol 190:2159–2168. 10.4049/jimmunol.1202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benoit M, Desnues B, Mege JL. 2008. Macrophage polarization in bacterial infections. J Immunol 181:3733–3739. 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 138.Fura JM, Sarkar S, Pidgeon SE, Pires MM. 2017. Combatting bacterial pathogens with immunomodulation and infection tolerance strategies. Curr Top Med Chem 17:290–304. 10.2174/1568026616666160829160707. [DOI] [PubMed] [Google Scholar]
- 139.Drozdowicz LB, Bostwick JM. 2014. Psychiatric adverse effects of pediatric corticosteroid use. Mayo Clin Proc 89:817–834. 10.1016/j.mayocp.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 140.Yasir M, Goyal A, Bansal P, Sonthalia S. 2020. Corticosteroid adverse effects. In StatPearls. StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- 141.Mestas J, Hughes CC. 2004. Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738. 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 142.Spaan AN, van Strijp JAG, Torres VJ. 2017. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 15:435–447. 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Risso A. 2000. Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J Leukoc Biol 68:785–792. [PubMed] [Google Scholar]
- 144.Cheung C, Gonzalez FJ. 2008. Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J Pharmacol Exp Ther 327:288–299. 10.1124/jpet.108.141242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kirst HA, Sides GD. 1989. New directions for macrolide antibiotics: pharmacokinetics and clinical efficacy. Antimicrob Agents Chemother 33:1419–1422. 10.1128/aac.33.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parra-Ruiz J, Vidaillac C, Rybak MJ. 2012. Macrolides and staphylococcal biofilms. Rev Esp Quimioter 25:10–16. [PubMed] [Google Scholar]
- 147.Yasuda H, Ajiki Y, Koga T, Kawada H, Yokota T. 1993. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob Agents Chemother 37:1749–1755. 10.1128/aac.37.9.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chiang WC, Nilsson M, Jensen PO, Hoiby N, Nielsen TE, Givskov M, Tolker-Nielsen T. 2013. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 57:2352–2361. 10.1128/AAC.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pascual A, Ballesta S, Garcia I, Perea EJ. 2002. Uptake and intracellular activity of linezolid in human phagocytes and nonphagocytic cells. Antimicrob Agents Chemother 46:4013–4015. 10.1128/aac.46.12.4013-4015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fernandez-Barat L, Motos A, Panigada M, Alvarez-Lerma F, Vina L, Lopez-Aladid R, Ceccato A, Bassi GL, Nicolau DP, Lopez Y, Munoz L, Guerrero L, Soy D, Israel T, Castro P, Torres A. 2019. Comparative efficacy of linezolid and vancomycin for endotracheal tube MRSA biofilms from ICU patients. Crit Care 23:251. 10.1186/s13054-019-2523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Darouiche RO, Dhir A, Miller AJ, Landon GC, Raad II, Musher DM. 1994. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J Infect Dis 170:720–723. 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 152.Jefferson KK, Goldmann DA, Pier GB. 2005. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob Agents Chemother 49:2467–2473. 10.1128/AAC.49.6.2467-2473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Anderl JN, Franklin MJ, Stewart PS. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 44:1818–1824. 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ishida H, Ishida Y, Kurosaka Y, Otani T, Sato K, Kobayashi H. 1998. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob Agents Chemother 42:1641–1645. 10.1128/AAC.42.7.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stone G, Wood P, Dixon L, Keyhan M, Matin A. 2002. Tetracycline rapidly reaches all the constituent cells of uropathogenic Escherichia coli biofilms. Antimicrob Agents Chemother 46:2458–2461. 10.1128/aac.46.8.2458-2461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Souli M, Giamarellou H. 1998. Effects of slime produced by clinical isolates of coagulase-negative staphylococci on activities of various antimicrobial agents. Antimicrob Agents Chemother 42:939–941. 10.1128/AAC.42.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]