Today, more than a billion people—one-sixth of the world’s population—are suffering from neglected tropical diseases. Human African trypanosomiasis, Chagas disease, and leishmaniasis are neglected tropical diseases caused by protozoan parasites belonging to the genera Trypanosoma and Leishmania.
KEYWORDS: metabolism, disease pathogenesis, kinetoplastids, neglected tropical diseases, host-parasite interactions, Trypanosoma cruzi, Chagas disease, Trypanosoma brucei, sleeping sickness, Leishmania, leishmaniasis, Trypanosoma
ABSTRACT
Today, more than a billion people—one-sixth of the world’s population—are suffering from neglected tropical diseases. Human African trypanosomiasis, Chagas disease, and leishmaniasis are neglected tropical diseases caused by protozoan parasites belonging to the genera Trypanosoma and Leishmania. About half a million people living in tropical and subtropical regions of the world are at risk of contracting one of these three infections. Kinetoplastids have complex life cycles with different morphologies and unique physiological requirements at each life cycle stage. This review covers the latest findings on metabolic pathways impacting disease pathogenesis of kinetoplastids within the mammalian host. Nutrient availability is a key factor shaping in vivo parasite metabolism; thus, kinetoplastids display significant metabolic flexibility. Proteomic and transcriptomic profiles show that intracellular trypanosomatids are able to switch to an energy-efficient metabolism within the mammalian host system. Host metabolic changes can also favor parasite persistence, and contribute to symptom development, in a location-specific fashion. Ultimately, targeted and untargeted metabolomics studies have been a valuable approach to elucidate the specific biochemical pathways affected by infection within the host, leading to translational drug development and diagnostic insights.
INTRODUCTION
The neglected tropical diseases (NTDs) are a group of infections that are common among the world’s poorest populations, affecting more than one billion people worldwide (1). NTDs remain major public health issues in parts of Asia, Africa, and South America, but they also affect populations in North America and Europe, due to population migration, climate change, or previously undetected endemicity (2). Due to lack of awareness and financial incentive for drug development, NTDs have remained largely underresearched. The trifecta of nutrition, health, and disease is complex, especially when factoring in parasites and their own metabolic needs. In addition, NTD-causing parasites have complex life cycles and modes of transmission (3). Understanding this plasticity on a metabolic level will deepen our understanding of parasitic diseases.
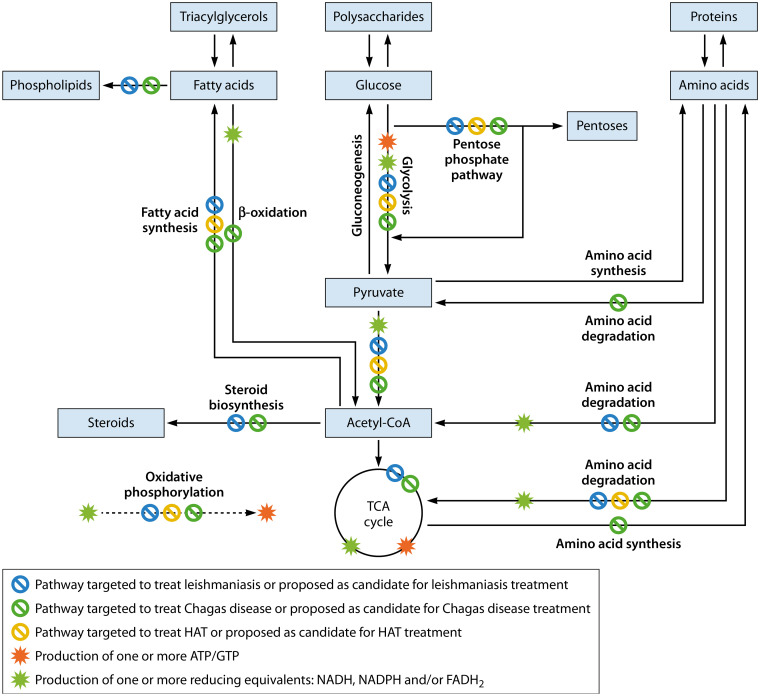
Metabolic pathways degrade macromolecules to produce energy (catabolic metabolic reactions), generate the building blocks of the cell (lipids, nucleotides, amino acids, and sugars; anabolic metabolic reactions), excrete waste products, and synthesize the small molecules involved in cell and system function and cell-cell communication, thus driving organismal phenotype (4, 5). Within a host system, parasites adapt their metabolism depending on their surroundings and nutrient needs. Parasites replicate and continue their life cycle, capitalizing on energy obtained from the host. Thus, parasites benefit from host cell metabolism, with (in some cases) reduced reliance on their own metabolic pathways. Parasites achieve this by influencing host cell gene expression and thus host metabolic pathways (6). Therefore, studying host-parasite interactions also means understanding the implications on the overall host metabolism (3), particularly with regard to energy-generating metabolic pathways. The major pathways for energy generation are the degradation of fatty acids, amino acids, and polysaccharide-derived monosaccharides, with the preferred monosaccharide usually glucose, when available. Glucose is degraded via glycolysis, leading to the production of pyruvate. Pyruvate can have multiple fates depending on oxygen availability. When oxygen is plentiful, it is converted to acetyl coenzyme A (acetyl-CoA), which proceeds through the citric acid cycle for full oxidation to CO2. The citric acid cycle is also called the tricarboxylic acid (TCA) cycle or the Krebs cycle. Glycolysis and the TCA cycle directly produce ATP and GTP. In addition, they produce reducing equivalents (FADH2 and NADH). These are regenerated to FAD and NAD+ through oxidative phosphorylation (mitochondrial respiration), which is also an ATP-producing process. Fatty acid catabolism (β-oxidation) and polysaccharide catabolism intersect at the level of acetyl-CoA. Amino acids are degraded to pyruvate, acetyl-CoA, or TCA cycle intermediates (Fig. 1) (5).
FIG 1.
Overall metabolic pathway integration and targets for intervention. Only metabolic pathways and inhibitors discussed in this review are shown. Adaptations unique to specific kinetoplastids and differences between mammalian and parasite metabolism are not displayed. Cofactors, additional reactants, conversion of oxaloacetate to pyruvate and pyruvate to oxaloacetate, energy cost, and urea and CO2 production are also not drawn. Targeting of parasite versus host metabolic pathways are not differentiated, because this is often hard to distinguish when nonselective inhibitors are used. Metabolites are boxed.
This review aims to compile literature on the metabolism of mammalian infection and discuss its impact on disease pathogenesis for the three different human kinetoplastid infections: Chagas disease (CD), human African trypanosomiasis (HAT), and various forms of leishmaniasis, caused by Trypanosoma cruzi, Trypanosoma brucei, and Leishmania, respectively. Studying the metabolic aspects of disease pathogenesis can uncover new mechanisms of infection and aid in developing drugs against these diseases.
LEISHMANIASES
Leishmaniases are caused by over 20 different species of Leishmania, which are transmitted by infected female phlebotomine sandflies. Leishmaniasis affects people in more than 100 countries in tropical and subtropical regions worldwide (7), with 12 to 15 million people infected (8). Leishmaniases have been called a disease complex rather than a single disease; their clinical manifestations are diverse but occur in three main categories—cutaneous, mucocutaneous, and visceral. Cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL) are the most common forms of the disease. CL manifests as ulcerative skin lesions, while symptoms of VL include fever, weight loss, and enlargement of the liver and spleen (7). VL can become fatal; risk of severe disease is heightened by coinfection with other pathogens such as HIV (9). Mucocutaneous leishmaniasis is a severely disfiguring form affecting the mucosal regions of the nose, mouth, and pharynx (10). Leishmania organisms have a two-stage life cycle in which they alternate between the extracellular promastigote stage in the sandfly vector and the intracellular amastigote stage in mammalian host macrophage phagolysosomes (7).
Pentavalent antimonial drug compounds remain a mainstay of leishmaniasis treatment (11). These drugs are highly toxic, and parasites are increasingly resistant to antimony in several regions of the world. Alternatives such as paromomycin and miltefosine come with the drawbacks of toxicity and increased drug resistance. Paromomycin in combination with sodium stibogluconate improves treatment success, but sodium stibogluconate also comes with the risk of cardiotoxicity (12). Amphotericin B in liposomal formulations is better tolerated than conventional amphotericin B formulations but can still cause adverse effects and requires parenteral administration (11). Miltefosine, though oral, is teratogenic and cannot be given to pregnant women. Treatment failure can be attributed to the host immune system and nutritional status of the host, parasite mechanisms of survival or drug resistance within the host, and extrinsic factors like access to medical treatment (13).
Approaching treatment failure by better understanding molecular-level changes occurring with infection could lead to more effective drug development (see below and “Avenues for Intervention”). In the early stages of Leishmania infection, catabolism of host-derived amino sugars (N-acetylglucosamine and glucosamine) is an essential source of carbon for the intracellular stage of Leishmania (14). Although a lack of hexose uptake cannot be compensated by amino acid catabolism in vivo (15), gluconeogenesis is also essential for parasite intramacrophage replication and in vivo virulence (16). Metabolomic analysis of isolated lysosomes has revealed enrichment of nucleosides, lower levels of specific amino acids (with others showing comparable concentrations), and equivalent levels of glucose and other sugars compared to whole cells (17). However, nutrient availability may be more limited within the Leishmania-housing phagolysosome, given that amastigotes engage in a stringent metabolic response, designed for sparing nutrient use. The parasites accomplish this by decreasing usage of glucose and amino acids while increasing catabolism efficiency, with minor increases in fatty acid β-oxidation and reduced biosynthesis of structural components such as proteins and lipids (18). This stringent response may account for the low growth rate observed for amastigotes in vivo (19).
In addition to this stringent metabolic state, Leishmania implements several additional mechanisms to compensate for this nutrient shortage, including increased nutrient transporter expression and modulation of host metabolism to favor parasite growth. Such mechanisms are essential given Leishmania auxotrophies, which include purines, a critical requirement for exogenous arginine, leucine, lysine, phenylalanine, tryptophan, and valine, with exogenous aspartate, glutamate, glutamine, and serine required to support growth and histidine, isoleucine, methionine, and threonine required to support protein synthesis (20–22). Leishmania must also scavenge biopterin, folic acid, riboflavin, and lipoic acid (20). Indeed, an atypical Leishmania donovani strain that causes cutaneous lesions instead of VL showed an increase in transporters, possibly representing an adaptation to the nutrient-limiting environment of the skin (23). In the macrophage-residing Leishmania, an arginine shortage in the phagolysosome environment is compensated by upregulating an arginine transporter. This upregulation highlights not only the importance of arginine to Leishmania survival but also that Leishmania monitors levels of arginine in the environment and induces an arginine-deprivation response (24, 25).
Host metabolic hijacking by the parasite has been demonstrated through metabolic alterations in infected macrophages. Macrophage glucose uptake and respiration can be controlled by Leishmania: L. infantum and L. major increase macrophage glycolysis early after infection (26–28). This requires live parasites (28). In contrast, L. major organisms downregulate the expression of their own glycolytic genes during the same period (27). At later time points in vitro, L. infantum, L. donovani, and L. mexicana promote macrophage oxidative phosphorylation via induction of 5′ AMP-activated protein kinase (AMPK) (26, 29); this has been postulated as a strategy to increase glucose availability for the parasite (30). However, a separate study using mouse bone marrow-derived macrophages infected with L. major showed no change in oxidative phosphorylation at 24 h postinfection (28). Analysis of monocyte-derived cells isolated from cutaneous leishmaniasis lesions revealed a decrease in cellular respiration, driven by local nitric oxide levels (31). These parasite-induced metabolic alterations may help reshape the immune response to favor reduced inflammatory cytokine production and thus parasite persistence (31).
L. amazonensis macrophage infection in vitro increases alanine, proline, arginine, valine, isoleucine or leucine, ornithine, hypoxanthine, glutamic acid, and histidine, as well as phosphocholine (32). In macrophages infected with L. donovani, glutamine consumption and host glutamine metabolism were increased (33). This concurs with findings of decreased glutamine in experimental CL lesions (34). Infected macrophages also upregulate arginine and proline metabolism while downregulating steroid and fatty acid biosynthesis (27), though this contrasts with observations of increased cholesterol uptake, total fatty acids, and lipid droplet formation in other studies (28, 35). Overall, given the parasite energetic needs and amino acid and purine auxotrophies (20, 21), these adaptations likely favor parasite proliferation. In addition, Leishmania affects host lipid metabolism, including increasing lysophosphocholine (LPC), total phospholipids, triacylglycerol, diacylglycerol, and monoacylglycerol, as well as specific phosphocholines, phosphoethanolamines, hydroxyoctadecadienoic acid metabolites, and hydroxyeicosatetraenoic acid metabolites, in vitro (35–38) and in vivo (34). The role of these adaptations in pathogenesis is still unknown but warrants investigation. Given that miltefosine was initially developed to target host phospholipid metabolism for cancer treatment (39), it may restore these infection-induced host phospholipid alterations in addition to directly killing Leishmania, although this needs to be demonstrated experimentally.
Strikingly, some of these metabolic alterations are distal to the site of leishmaniasis lesions or even systemic. For example, serum triglyceride levels were significantly higher in VL patients (40), and a study of Indian VL patients showed that an increase in splenic parasite load came with a decrease in serum cholesterol (41). While systemic metabolic alterations are not unexpected in the case of VL, they have also been reported in CL. Tissue metabolomic analysis showed that specific phosphocholines were increased in the skin beyond the site of the lesion (34). Diffuse cutaneous leishmaniasis was associated with increased plasma cadaverine compared to other CL forms. Ornithine was also lower in plasma of mucocutaneous and localized cutaneous leishmaniasis patients than in healthy controls (42).
Parasite metabolites can also affect immune responses; for example, lipids from L. braziliensis amastigotes stimulate nitric oxide production (43). Increased LPC levels worsen dendritic cell infection with L. major by decreasing inducible nitric oxide synthase (iNOS) expression (44). Examples of other provirulence Leishmania metabolic pathways include mannan metabolism, required for adaptation to variable carbohydrate availability (45), and ether glycerolipid production (46).
Overall, the adaptability of Leishmania within its host is a testament to the underlying mechanisms an intracellular parasite uses to survive in different organs of the host. Considerable effort has unveiled metabolic adaptations in vitro; exciting advances are beginning to unravel whether these adaptations also occur in situ and in vivo. New technologies such as single-cell metabolomics and sequencing may enable better characterization of these changes at early or disease resolution time points when parasite burden is low.
CHAGAS DISEASE
CD is caused by the protozoan parasite T. cruzi, transmitted by triatomine kissing bugs. It is endemic to 21 Latin American countries, with 6 to 7 million infected individuals worldwide (47). Due to population mobility, its incidence has been increasing in the United States, Canada, and Europe (48). Infection occurs in two stages, acute and chronic. During the acute stage, symptoms include fever, enlarged lymph nodes, and abdominal or chest pain. After 8 to 12 weeks, parasite levels become undetectable by microscopy, and the infected individual enters the chronic stage. Individuals with chronic T. cruzi infection who lack disease symptoms have the intermediate form of CD. Symptomatic chronic CD is characterized by cardiac and/or gastrointestinal dysfunction, including cardiac apical aneurysms, megaesophagus, and megacolon (47).
Within the mammalian host, T. cruzi alternates between an intracellular amastigote stage in host cell cytoplasm and an extracellular trypomastigote stage, across multiple organs and tissues (47). In mouse chronic CD models, parasite persistence is predominantly in the large intestine, stomach, and gut mesenteric tissue, with transient colonization at multiple other sites (49). This pleiotropism may be facilitated by T. cruzi metabolic flexibility, whereby amastigotes can use glucose, amino acids, and fatty acids as their main carbon sources (50–53). Upon interaction with the extracellular matrix, trypomastigotes reduce glycolytic activity (54). This was also observed at the mRNA level early following trypomastigote invasion of fibroblasts, with reemergence of glycolysis-associated transcripts 24 h after invasion (52) and amastigote uptake of glucose from host pools (50). Intracellular amastigotes also upregulate TCA cycle, oxidative phosphorylation, and β-oxidation-associated transcripts and amino acid permeases compared to trypomastigotes (52). This is supported by increased fatty acid oxidation proteins in amastigotes compared to trypomastigotes (51) and adaptations in T. cruzi growth rates depending on exogenous nutrient availability (55), further supporting metabolic flexibility.
Deciphering the effect of infection on host metabolism can ultimately lead to a better understanding of how intracellular T. cruzi organisms proliferate within the mammalian host. Genes related to mitochondrial function, such as oxidative phosphorylation pathway genes, were downregulated in chronic CD murine models (56). An increased abundance of swollen mitochondria in infected cardiac tissue further indicates that mitochondrial dysfunction may relate to disease severity in chagasic cardiomyopathy (56). Indeed, a general increase in oxidative stress is associated with disease progression in human CD and oxidative stress is linked to damaged mitochondrial function in chagasic patients (57, 58). Untargeted metabolomics studies of acute experimental infection with T. cruzi strain Y parasites also demonstrated oxidative stress in heart tissue and a decline in TCA cycle intermediates such as fumarate, further supporting decreased oxidative metabolism in cardiac tissue (59). This was also observed in vitro in two separate studies of macrophage infection and in monocytes isolated from CD patients, was enhanced by gamma interferon (IFN-γ), and was associated with increased production of nitric oxide and reactive oxygen species. These are antiparasitic but also contribute to tissue damage and impair CD8+ T cell function (29, 60, 61). This contrasts with in vitro reports of increased mitochondrial respiration in infected fibroblasts (50), suggesting host cell-type-specific effects or differences between in vitro and in vivo settings.
Increased glucose uptake and glycolysis were observed in infected cardiac tissue (59), in vitro in macrophages (60) and fibroblasts and myoblasts (50), and in monocytes isolated from CD patients (61). However, a separate study did not observe increased glycolysis in infected macrophages at 18 h postinfection (29). This discrepancy may be due to the infecting parasite strain, since two of these conflicting studies (29, 60) used the same 18-h postinfection time point, and two studies used human cells (29, 61). Activation of glycolysis promoted parasite clearance in activated macrophages, whereas restriction of glucose availability or replacement of glucose with fructose prevented reactive oxygen and nitrogen species production, with no effects on cytokine production (60). This was only partially confirmed in monocytes isolated from CD patients: similar to in vitro murine macrophage infection (60), inhibition of glycolysis reduced nitric oxide production (61). However, glycolysis inhibition also reduced interleukin 1β (IL-1β) secretion (61). Oxidant production was also dependent on the pentose phosphate pathway (60). This observation contrasts with reports for fibroblasts, in which inhibition of glycolysis blocked T. cruzi proliferation. The latter reliance on glycolysis was on both host and parasite levels (50, 55).
Differences between these studies suggest a host cell-type-specific relationship between host glycolysis and T. cruzi proliferation. This may be related to the presence of other alternative nutrients or higher reliance on alternative pathways for energy metabolism. Indeed, cells that were defective in mitochondrial fatty acid oxidation were also less competent to support the growth of T. cruzi amastigotes (62). The amino acids tryptophan, isoleucine, leucine, and valine were increased in infected heart tissue, while purine nucleotides were depleted by infection in heart tissue (59). T. cruzi, like Leishmania, is auxotrophic for tryptophan, leucine, isoleucine, valine, and purines (21). Parasites also require exogenous glutamine for growth (55). Kynurenine, glycerophosphocholine, and long-chain fatty acids were also increased with infection in heart tissue (59). T. cruzi can scavenge and incorporate host lipids (63).
However, T. cruzi shows specific tropism even within target organs. In the heart, parasite load was highest at the heart base, which may indicate a preference for a specific cardiac metabolic environment (64). Untargeted metabolomics revealed distinct chemical signatures between the apex and base of the heart. Host nucleosides were elevated at the apex of the heart, specifically, AMP, which regulates AMPK (64). AMPK inhibition in vitro promotes intracellular parasite growth (62). In chronic T. cruzi strain Sylvio X10/4 infection, significant overall metabolic perturbations in the heart were observed only in the apical region, providing one explanatory mechanism for the localized apical damage observed in human CD. Infection had position-specific impact on specific metabolite families such as acylcarnitines (65). Host metabolism was also affected by T. cruzi infection in the gastrointestinal (GI) tract in an experimental mouse model, in a spatially resolved fashion. For example, total and long-chain acylcarnitines were significantly elevated in the small intestine in acute CD. Kynurenine was elevated by infection in the stomach and large intestine in the acute stage. The largest metabolic perturbations were observed in the chronic stage in the esophagus and large intestine, sites of gastrointestinal CD, suggesting a metabolic cause of disease tropism in this context (66). Thus, T. cruzi is able to infect and cause host metabolic alterations in a variety of tissue locations.
Overall, T. cruzi depends on nutrient scavenging and on metabolism adaptations for in vivo proliferation. Infection also induces adaptive and maladaptive host metabolic adaptations. Thus, there is considerable scope to build on this knowledge to develop new CD treatments (see “Avenues for Intervention” below).
HUMAN AFRICAN TRYPANOSOMIASIS
HAT occurs in sub-Saharan Africa and is transmitted through a tsetse fly vector. The two clinical forms of HAT are the slow-progressing form caused by Trypanosoma brucei gambiense in central and western Africa and the fast-progressing form caused by T. brucei rhodesiense in parts of eastern and southern Africa (67, 68). Approximately 70 million people live in regions where they are at risk of contracting HAT (69). Unlike T. cruzi and Leishmania, T. brucei is extracellular at all stages (67). Initial symptoms are nonspecific and include fever, headaches, and joint pain (hemolymphatic stage). Eventually, the parasite crosses the blood-brain barrier, leading to psychiatric and behavioral disturbances, ataxia, seizures, coma, and ultimately death (70).
Bloodstream form T. brucei lives in glucose-rich environments and thus depends on glucose for its ATP production (71) and anabolic processes (65, 72). Indeed, RNA interference (RNAi) data showed a greater reliance of bloodstream form T. brucei on glycolysis, whereas procyclics (insect stage) were more dependent on fatty acid metabolism (73) and proline catabolism, though glucose remains the preferred energy source (74). The observed change in glycolysis products in urine and plasma during experimental T. brucei brucei mouse infection (75) could reflect parasite glycolysis or host adaptation to energy needs caused by infection. If reflecting host metabolism, this may lead to competition between host and parasite for glucose. Bloodstream forms of T. brucei do not significantly rely on a full TCA cycle (72). Beyond glucose, anabolic processes in bloodstream T. brucei use glutamine to produce glutamate, 2-oxoglutarate, and some succinate. Extracellular cysteine is used by the parasite to produce glutathione, trypanothione, and coenzyme A (76). Extracellular acetate feeds into parasite fatty acid biosynthesis (77).
However, there is also metabolic flexibility in T. brucei. Bloodstream forms are able to not only survive but also proliferate in a medium containing glycerol instead of glucose (71), with glycerol serving as a source for gluconeogenesis in vitro (71, 78). Abundant glycerol could be the reason why parasites associate with adipose tissue, though this has yet to be directly demonstrated. Indirect evidence is provided by elevation of glycolytic/gluconeogenic enzymes in adipose tissue resident parasites (79). A better understanding of glycerol metabolism could open new avenues for developing better drug targets (71). Additional adaptations to adipose tissue tropism compared to bloodstream tropism include increased expression of enzymes involved in the TCA cycle, purine salvage, the pentose phosphate pathway, and sterol and lipid metabolism, including β-oxidation. Parasites isolated from adipose tissue were confirmed ex vivo to be capable of metabolizing fatty acids (79). Additional underexplored temporal metabolic flexibility may also occur, with in vitro studies showing strong circadian regulation of metabolic genes in cultured bloodstream T. brucei forms (80).
Given that T. brucei is extracellular (67), the impact on host metabolism will be more indirect than during Leishmania or T. cruzi infection. Host lipids are highly affected by HAT. A global untargeted metabolic profiling of plasma from rhodesiense trypanosomiasis in Uganda showed significant differences in host lipid profiles with infection. HAT patients had lower levels of phosphatidylcholines (PCs) with a high number of saturated bonds compared to the control samples. The concentration of PCs with two or fewer unsaturated bonds was increased with infection (81). Urine and plasma chemical compositions of mice infected with T. brucei brucei, a Trypanosoma brucei subspecies that causes nonhuman infection, showed enhanced lipid oxidation with infection (75). The study also showed depleted levels of PC with infection but did not assess degree of saturation (75). There was an increase in the choline concentration in plasma samples, suggesting a breakdown of PCs in the presence of infection (75, 81). Since PCs are among the metabolites most affected by HAT infection in both patient samples and in vivo mouse studies, future studies can aim to study the role of PCs in the disease pathogenesis of HAT.
Changes in host amino acid metabolism also occur due to HAT. In the plasma sample study from rhodesiense trypanosomiasis in Uganda, higher concentrations of phenylalanine and lower concentrations of other amino acids, like histidine and alanine, occurred with infection (81). Central nervous system (CNS)-stage disease was associated with an increase in 5-hydroxytryptophan and kynurenine and decreased tryptophan in the CNS compared to the case for the hemolymphatic stage (82). A study in voles infected with T. brucei gambiense also showed significant perturbations in phenylalanine metabolism, with, however, a decrease in phenylalanine levels in the chronic stage (45- to 49-day infection period) (83). Hypoaminoacidemia was also observed in the plasma of voles infected with T. brucei gambiense for 21 days. The aromatic amino acids tryptophan and tyrosine were significantly reduced and may play a role in the neuropsychiatric syndromes associated with HAT (84); these results concur with human CNS studies (82). However, in contrast to the study in patients, the concentration of phenylalanine, also an aromatic amino acid, was unchanged with infection (84). This discrepancy between tyrosine and phenylalanine levels could be attributed to the rate of tyrosine breakdown being considerably greater than the dietary intake of tyrosine as well as the rate at which phenylalanine can be converted to tyrosine (83). Discrepancies between studies could be attributed to the various metabolic differences produced by the two different types of strains used for infection, to the duration of infection, or to the mammalian system studied. T. brucei also produces the amino acid metabolites indolepyruvate, hydroxyphenylpyruvate, and phenylpyruvate, which inhibit production of the proinflammatory cytokine IL-1β, via downmodulation of macrophage glycolytic metabolism. This has been postulated to attenuate HAT symptoms and favor host survival long enough to ensure parasite transmission (85, 86). These metabolites also decreased inducible nitric oxide synthase expression and IL-6 induction, likely favoring parasite persistence (86). Additional investigation of immunometabolism in HAT is warranted.
Ultimately, T. brucei is able to proliferate and survive within the host system due to its metabolic flexibility. Additionally, infection causes perturbation in overall host lipid and amino acid metabolism.
AVENUES FOR DIAGNOSIS AND PATIENT MONITORING
There is a need for better ways to diagnose leishmaniasis, CD, and sleeping sickness and monitor disease progression and treatment efficacy. Several metabolomics studies have identified metabolites, primarily host derived, that can serve as biomarkers to address these needs. For example, serum samples from an experimental model of CD showed significant perturbations in host amino acid metabolism with infection. The essential amino acids isoleucine, leucine, phenylalanine, tryptophan, and valine were decreased with infection, as was glutamine. Acylcarnitines were also affected by infection (87). Long-chain fatty acids were decreased by infection in plasma, while p-cresol sulfate, allantoin, and kynurenine were elevated (59). A machine learning model built on the cardiac metabolome could predict acute experimental T. cruzi infection outcome (64); such an approach can serve as a framework to identify disease severity-associated and prognostic circulatory metabolite biomarkers.
The ability to differentiate between the two stages of HAT infection ultimately dictates the course of treatment to be chosen. A metabolomic analysis of cerebrospinal fluid (CSF) and plasma from patients in Angola revealed several biomarkers that were different based on disease stage. Stage 2 HAT could be distinguished from stage 1 by an increase in neopterin and hydroxytryptophan levels, a slight increase in kynurenine, and a decrease in tryptophan in the CSF samples. In plasma, an increase in aminododecanoic acid and decrease in ornithine are both indicators of stage 2 HAT (82). A second study observed amino acid depletion in the CSF in HAT stage 2 (88). Indolepyruvate produced by the bloodstream form of T. brucei could also be an important indicator of parasite presence and disease state (85).
In the context of leishmaniasis, several studies described above reported differences in selected serum or plasma metabolites compared to uninfected controls (40–42). The serum metabolome can also predict CL treatment success, with elevated pretreatment taurine, allantoin, N-acetylglutamine, and pyruvate predictive of successful pentavalent antimonial treatment (89).
One cautionary note is that these biomarkers, while useful to monitor disease progression or treatment, may not directly reflect the specific changes occurring at the sites of disease. For example, long-chain fatty acids showed the opposite direction of change in the heart and the plasma during acute T. cruzi infection (59), highlighting the importance of studying metabolism right at the site of disease progression to understand pathogenesis. A further limitation is that these metabolic changes are predominantly host derived and often not specific to the kinetoplastid disease of concern. Thus, they may be useful only in combination with other diagnostic methods and of limited utility to determine the presence of an infection. Their strength instead may lie in predicting disease severity (64) or treatment response (89) once infection status has been established.
AVENUES FOR INTERVENTION
Drug development commonly targets the pathogen but can also address pathogen or immune response-induced collateral damage (protolerance mechanism [90]). Though pan-kinetoplastid treatment strategies would be desirable to decrease drug development costs, given the differences in target organs, parasite localization, and timeline of disease progression, treatments of these different diseases will likely require very different pharmacokinetic properties and have different acceptable adverse-effect profiles. Thus, individual optimization of these treatment avenues will likely be necessary for each kinetoplastid disease.
The current HAT treatment, eflornithine, inhibits parasite polyamine metabolism (91). Likewise, miltefosine, in clinical use for leishmaniasis, targets parasite lipid metabolism (92) and allopurinol, used exclusively in veterinary leishmaniasis, inhibits purine salvage (93). Azole family compounds, which failed in clinical trials for CD, targeted parasite ergosterol metabolism (94, 95). By identifying additional metabolic pathways affected by infection, the next generation of drug development can target pathways essential to parasite survival or disease pathogenesis. As described below, there are multiple preclinical drug development studies targeting metabolism in kinetoplastid infection.
For example, T. cruzi, T. brucei, and Leishmania are sensitive to fatty acid biosynthesis inhibitors (19, 96, 97), and inhibitors of glycolysis and respiration kill all three kinetoplastids (98, 99). These need not be nonspecific: compound GNF7686 selectively targets T. cruzi mitochondrial respiration with more than a 100-fold selectivity window compared to host cells (100). Some inhibitors of T. brucei hexokinase also show low inhibition of host hexokinase (101). Kinetoplastid segregation of initial steps of glycolysis to glycosome organelles (unlike for mammals) also provides an opportunity for the development of selective interventions. For example, interfering with glycosomal enzyme localization impairs ATP production and cures HAT in mouse models. These compounds also showed acceptable efficacy against intracellular T. cruzi amastigotes in vitro, though in vivo activity was not tested (102). Alternatively, research with T. brucei suggests that even nonspecific inhibitors (such as those targeting glycolysis, with potential off-target effects on host glycolysis) may be sufficiently safe as long as the inhibitors have a greater impact on flux in the parasite than in the host (103). Some of these treatments also target host metabolism or the intersection between host and parasite metabolism. For example, chronic-stage CD treatment with resveratrol activates the AMPK pathway, reduces cardiac oxidative stress and parasite load, and improves cardiac function (104). Polyamine production requires metabolism of arginine to ornithine by arginase enzymes. In parallel, arginine is a substrate for host nitric oxide synthase, which produces antiparasitic nitric oxide. The host and parasite both express arginases, with L. major arginase expression and L. infantum lipids promoting host arginase activity. Arginase inhibition slows cutaneous leishmaniasis lesion progression in BALB/c mice by restricting availability of polyamines without affecting nitric oxide or cytokine production (38, 105, 106), though knocking out arginase in C57BL/6 mice had no impact on cutaneous leishmaniasis lesion size or parasite load (107). In contrast, inhibition of arginase increased nitric oxide production and T. brucei killing in vitro (108). Arginase may also contribute to CD progression (109), and arginine supplementation reduces T. cruzi load and improves disease severity in vitro and in vivo by increasing nitric oxide production (110).
Indeed, given the key role of metabolism in shaping immune responses (111), several studies have investigated the impact of metabolism modulators on immune responses with the goal of developing new treatments for kinetoplastid infection. For example, 3-hydroxykynurenine is a tryptophan metabolite produced by macrophages and dendritic cells, among others, with immunomodulatory properties. Mice infected with T. cruzi and treated with 3-hydroxykynurenine in the acute disease stage showed reduction in chronic-stage electrocardiogram alterations, inflammation and fibrosis in the heart, and reduced parasite load (112). In contrast, inhibiting the enzyme producing these metabolites, indoleamine 2,3 dioxygenase (IDO), significantly reduced cutaneous leishmaniasis lesion size and parasite load (113). Glutaminase inhibition impaired antiparasitic immune responses during L. donovani infection, with reduced IFN-γ and increased IL-10 production, supporting the development of strategies that promote glutamine metabolism as new treatments for visceral leishmaniasis (33). Knocking out AMPK in myeloid cells improved L. infantum clearance by promoting increased proinflammatory nitric oxide synthase expression and reduced arginase expression (M1 macrophage phenotype) (26). Reducing cholesterol levels via simvastatin treatment improved cutaneous leishmaniasis lesion size via enhanced macrophage antioxidant production (114).
Metabolic modulation can also induce disease tolerance: improved disease severity with no changes in parasite burden. For example, acylcarnitine metabolism was perturbed during acute and chronic CD, and carnitine supplementation improved acute-stage survival in vivo, including cardiac function, but without affecting parasite load (66). Metformin, an AMPK modulator that also has AMPK-independent effects (115), improved cardiac function in experimental CD, with no significant effects on parasite load (104).
Drug development can also build on metabolic models, to predict pathways that are essential to the parasite and thus can serve as good targets for antiparasitics. For example, a proteomic network analysis showed reactions involved in carbohydrate metabolism and amino acid metabolism as essential to T. cruzi (116). A genome-scale metabolic T. cruzi model predicted lethality for drugs targeting pairs of enzymes in the citric acid cycle and gluconeogenesis/glycolysis pathways, in the citric acid cycle and glutamate metabolism, or in glutamate metabolism and oxidative phosphorylation (117). A similar approach in Leishmania predicted threonine biosynthesis as a novel target (118). Genes involved in Leishmania purine metabolism, methionine metabolism, steroid metabolism, vitamin B6 metabolism, oxidative phosphorylation, and the pentose phosphate pathway were also predicted to be lethal using a computational, constraint-based L. donovani metabolic model (119). Kinetic modeling in T. brucei predicts glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and glucose transport as drug targets in this parasite (103). Translation of these in silico findings was demonstrated in T. cruzi, where machine learning based on T. cruzi metabolism and prior drug screening hits successfully predicted compounds that then demonstrated in vitro and in vivo antiparasitic activity (120).
Metabolic modulators do not have to be used alone; instead, they could be combined with existing drugs to facilitate dose reduction, improve safety profiles, or address tissue damage not currently restored by antiparasitics. For example, combining glutamine with miltefosine for the treatment of VL showed improved reduction in parasite load in the spleen (33). Supplementing meglumine antimoniate with allantoin improved Leishmania clearance in vitro (89). Given that carnitine supplementation improved cardiac function, but without affecting parasite load in acute CD (66), a path forward for clinical implementation will likely require combination with existing antiparasitics. Eflornithine is usually used in combination with nifurtimox for sleeping sickness (121).
Host-parasite metabolomics can also be used to determine drug mechanism of action and whether new drugs proceed via pathways similar to those of previously characterized agents. For example, this approach revealed that the experimental compounds S205, S448, and S1000 have a mechanism of action in CD distinct from that of benznidazole and nifurtimox (122). A similar approach was applied to Leishmania (123). This approach can also identify candidate mechanisms of action, for example, by demonstrating that sertraline, a candidate for drug repurposing for leishmaniasis, induces multitarget metabolic dysfunction in the parasite (124). Similar studies used metabolomics to demonstrate that AN5568 acts on S-adenosyl-l-methionine metabolism in T. brucei and causes redox dysfunction (125) and that miltefosine affects Leishmania sphingolipid and sterol metabolism (126). However, one of the barriers that still remains is the high level of metabolite commonalities occurring within the host and parasite and being able to differentiate between these common metabolites for the treated condition (122).
Metabolomics can also help in understanding drug treatment failure or success. For example, metabolomics of miltefosine-resistant Leishmania demonstrated resistance-associated changes in phosphatidylcholines (92). Susceptibility to an antiparasitic depends upon the metabolic environment of the parasites and thus the sites of in vivo infection. An in vitro study showed that in the presence of glutamine, T. cruzi amastigotes were more susceptible to the effect of ketoconazole (127). Interestingly, glutamine levels are lower in the large intestine than other gastrointestinal sites (66), which matches sites of reduced parasite clearance during posaconazole treatment (128). Thus, controlling the host metabolic environment may be a possible intervention to increase susceptibility to existing drugs. T. cruzi can enter a dormant drug-resistant state (129), although the associated metabolic changes are still unknown. Studying these will require highly sensitive techniques, such as single-cell transcriptomics or metabolomics. Treatment failure can also result from differential tissue damage, leading to altered drug distribution. This has been understudied in the context of infection. Indeed, pharmacokinetic studies are usually performed in uninfected animals (for an example, see reference 130). However, differential metabolism and tissue distribution of the animal anesthetic ketamine were observed in an untargeted metabolomics study of T. cruzi-infected hearts (131) and VL lowered liver and spleen amphotericin B levels (132).
Beyond drug treatment, nutrition also impacts disease severity. Host malnutrition is linked to increased death rate in T. cruzi-infected mice (133). In the acute stage of CD, a high-fat diet reduced parasitemia, parasite load in the heart, and ultimately mortality (134). In contrast, a murine model of the chronic stage of CD showed that a high-fat diet increased oxidative mitochondrial stress, contributing ultimately to cardiac dysfunction and cardiomyopathy (135). An atherogenic diet is also believed to offer some protection against L. donovani infection (136).
CONCLUSIONS AND FUTURE DIRECTIONS
Intracellular pathogens depend on the host to fulfill their carbon as well as other nutrient requirements, and extracellular parasites such as T. brucei nevertheless must scavenge host nutrients. To meet these needs, and in response to infection, kinetoplastids cause several host metabolic alterations. Greater commonality was observed when comparing metabolic adaptations in leishmaniasis and CD than for HAT, CD, and leishmaniasis together. Both Leishmania and T. cruzi initially downregulate parasite glycolysis upon infection (27, 54), whereas T. brucei relies on glycolysis during mammalian bloodstream infection (73). Likewise, both Leishmania and T. cruzi upregulate parasite β-oxidation during infection, though this increase is small in Leishmania (18, 52). T. brucei from adipose tissue was also able to consume fatty acids (79). In terms of host metabolic alterations during infection, greater similarities were likewise observed when comparing Leishmania and T. cruzi infections: for example, both downregulate host glycolysis, at least at early time points (26–28). Other common trends include changes in host oxidative phosphorylation and AMPK modulation as well as changes in phosphocholine and amino acid abundances. However, the direction of changes in these metabolic pathways are often unique to each kinetoplastid infection. For example, in vitro Leishmania infection was primarily associated with increased host oxidative phosphorylation (26, 29), while most studies of T. cruzi infection indicate decreased host oxidative phosphorylation (29, 60, 61). AMPK activity helped Leishmania proliferation (26), while it hindered the growth of T. cruzi (62, 104). An increase in phosphocholines was observed in Leishmania and T. cruzi infection, while a depletion of overall phosphocholines was observed for T. brucei, with just specific phosphocholines being increased (27, 43, 61). The bloodstream form of T. brucei metabolizes amino acids (76). This impact of infection on amino acid metabolism is common across kinetoplastids, but with different directionality depending on the species: the aromatic amino acids tryptophan and tyrosine decreased with T. brucei infection (84). In contrast, the levels of several amino acids were increased by T. cruzi infection in cardiac tissue in mouse models (59) and by Leishmania infection in macrophages (32), though acute T. cruzi infection was associated with depleted tryptophan in the large intestine (66).
A further limitation is the diversity of systems in which these metabolic changes have been evaluated, hampering cross-study comparisons. Although T. cruzi colonizes a broad range of tissues in vivo (137), few in vitro studies consider multiple cell types in parallel, and separate in vitro studies have shown conflicting impacts on cellular glycolysis and oxidative phosphorylation (26, 50, 60). Few studies use multiple Leishmania species or Leishmania, T. cruzi, or T. brucei strains in parallel. Likewise, while differences in parasite metabolism between vector stages and mammalian stages are unsurprising, there are also large differences between in vitro and in vivo pathogen metabolism. For example, doubling time for lesional L. mexicana is over three times slower than when infecting macrophages in vitro, reflected in 5-times-slower fatty acid turnover in the lesion (19). Likewise, in vitro settings lack the complex signals derived from multiple intercellular interactions that develop in vivo. For example, unlike observations in vitro (26), in vivo L. major infection was associated with decreased host cell respiration, induced by local immune response-derived nitric oxide (31). Thus, in vitro findings should only be extrapolated with caution and should always be validated in vivo. Reliance on animal models is unavoidable to enable the invasive tissue collection necessary to assess host and parasite metabolism at sites of disease and to tightly control infection duration, diet, comorbidities, medication usage, etc. Nevertheless, the relevance of each animal model to the disease being represented should always be considered. Concordance between multiple animal models (especially across species, but also across infection durations and diets) strengthens translatability of findings to humans. Nevertheless, sufficient overlap is expected. Indeed, rats and humans share all but eight metabolic enzymes, and only a minority of metabolic subsystems include species-specific reactions (138).
Demonstrating which of these metabolic changes are associated with pathology, which are instead protective and help the host compensate for the stress of infection, and which are merely “passenger” metabolic changes is still in its infancy. Thus, studies of infection metabolism benefit from being coupled with interventional investigations from a fundamental as well as a drug development perspective. This is particularly true for phospholipid alterations, whose biological significance has been understudied compared to changes in central energy metabolism (except with regard to miltefosine treatment in leishmaniasis). It will also be important to begin differentiating between “keystone” metabolic adaptations and the possibility that infection instead induces a multiplicity of small-scale metabolic alterations that cumulatively affect infection outcome. The latter may be missed by data analysis techniques that rely exclusively on univariate fold change analyses. Furthermore, fold change in metabolomics data is not always a direct representation of the exact fold change in the underlying metabolome, even though directionality of change is usually conserved (139). Similarly, fold change at the transcriptome level is not necessarily the same as fold change at the functional level (140).
Another common trend is parasite metabolic flexibility. Leishmania organisms adapt their metabolism to nutrient-restrictive conditions in vivo (18), while T. cruzi organisms are able to reduce the dependence on glycolysis upon exposure to the extracellular matrix (54). Metabolic flexibility in T. brucei allowed the parasites to proliferate in the presence of glycerol rather than glucose in vitro (71). However, many of these studies have been performed in vitro or have not considered metabolic adaptations to different tissue sites and cellular environments. In addition, there is considerable variability between individual parasite cells (129), although this has not yet been studied at the metabolic level. Future studies should aim to use single-cell approaches like single-cell Raman or single-cell metabolomics, which could provide more insight on the metabolic fingerprint of a single cell (141, 142).
Many studies of the metabolism of infection have only relied on a single or a few time points, and usually a single parasite strain. As costs decrease for omics (especially for liquid chromatography-mass spectrometry-based metabolomics), more time-resolved investigations should be pursued, enabling the determination of disease trajectories on a metabolic level. These advances will enable assessment of metabolic resilience and metabolic disease tolerance, aspects that we are now discovering play key roles in infection outcomes in kinetoplastid diseases and beyond (66). Likewise, usage of multiple parasite strains (and host strains or species in the case of experimental disease models) is essential to enable generalizations on the impact of infection on metabolism and to facilitate translatability to patients. Strain-specific impacts are to be expected, but strain-independent effects should be prioritized for drug and biomarker development, given that the infecting parasite strain is usually unknown in a clinical setting.
The majority of host-kinetoplastid metabolism studies have considered only mammalian and parasite metabolism. However, from a physiological standpoint the human microbiome has often been considered an organ by itself (143). In the GI tract of an in vivo CD model, different tissue types had various metabolic and microbiome alterations with infection (66). In CL, skin dysbiosis is not restricted to the site of the skin lesions and a global change in the skin microbiota is observed (144), although microbiome metabolic profiling in this context has not been investigated. Therefore, there is still considerable scope to define how microbiota metabolism affects kinetoplastid disease progression.
Although targeting metabolism is complex, multiple successes demonstrate that this is possible (see “Avenues for Intervention” above). Several of these successes have been with pleiotropic multitarget agents (e.g., carnitine [66], metformin [104], and resveratrol [104] in CD). One intriguing possibility is that these interventions were successful perhaps exactly because of this multiplicity of effects, enabling restoration of the multiple infection-induced metabolic changes. Such a possibility warrants further investigation as we seek to build on these insights into metabolism of infection to defeat kinetoplastid diseases. There is also still significant scope for exploring immunometabolism modulators to treat these diseases.
Overall, there have been major advances in our understanding of metabolism during kinetoplastid infection. Further advances will be facilitated by new data analysis methods and new technologies, including increased spatial analysis in situ in infected tissues and single-cell analyses. These insights will lead to new ways to monitor and treat these infections.
ACKNOWLEDGMENTS
Research on kinetoplastids in the McCall laboratory is supported by NIH award number 1R21AI148886 and PhRMA foundation award number 45188.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Biographies

Adwaita R. Parab is a master’s candidate at the University of Oklahoma, where she has been studying host-parasite and host-microbiome interactions in the context of metabolism. She is passionate about discovering mechanisms of disease pathogenesis and has worked on multiple projects to study the role of the host immune response in Trypanosoma cruzi infection and the discovery of host metabolic pathways affected by cutaneous leishmaniasis.

Laura-Isobel McCall is an Assistant Professor at the University of Oklahoma who has been working on kinetoplastids since 2007. She obtained her B.Sc. and Ph.D. in microbiology and immunology from McGill University, where both her undergraduate honors thesis and Ph.D. thesis focused on Leishmania tropism, under the supervision of Dr. Greg Matlashewski. She pursued postdoctoral training with Dr. James McKerrow at the University of California San Francisco and San Diego, where she studied Leishmania, T. cruzi, and T. brucei. Her laboratory at the University of Oklahoma focuses on using three-dimensional spatial mapping (chemical cartography) of host-pathogen-microbiome chemical interactions as a unique way to guide drug development and biomarker discovery.
REFERENCES
- 1.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. 2007. Control of neglected tropical diseases. N Engl J Med 357:1018–1027. 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 2.Lidani KCF, Andrade FA, Bavia L, Damasceno FS, Beltrame MH, Messias-Reason IJ, Sandri TL. 2019. Chagas disease: from discovery to a worldwide health problem. Front Public Health 7:166. 10.3389/fpubh.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuzarte-Luís V, Mota MM. 2018. Parasite sensing of host nutrients and environmental cues. Cell Host Microbe 23:749–758. 10.1016/j.chom.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Patti GJ, Yanes O, Siuzdak G. 2012. Innovation: metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol 13:263–269. 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voet D, Voet JG, Pratt CW. 2016. Fundamentals of biochemistry: life at the molecular level, 5th ed. Life at the molecular level. Wiley Global Education, New York, NY. [Google Scholar]
- 6.Xu T, Ping J, Yu Y, Yu F, Yu Y, Hao P, Li X. 2010. Revealing parasite influence in metabolic pathways in Apicomplexa infected patients. BMC Bioinformatics 11(Suppl 11):S13. 10.1186/1471-2105-11-S11-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burza S, Croft SL, Boelaert M. 2018. Leishmaniasis. Lancet 392:951–970. 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 8.Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. 2017. Leishmaniasis: a review. F1000Res 6:750. 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karunaweera ND, Ferreira MU. 2018. Leishmaniasis: current challenges and prospects for elimination with special focus on the South Asian region. Parasitology 145:425–429. 10.1017/S0031182018000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soong L, Henard CA, Melby PC. 2012. Immunopathogenesis of non-healing American cutaneous leishmaniasis and progressive visceral leishmaniasis. Semin Immunopathol 34:735–751. 10.1007/s00281-012-0350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, Carvalho E, Ephros M, Jeronimo S, Magill A. 2017. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg 96:24–45. 10.4269/ajtmh.16-84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvar J, Croft S, Olliaro P. 2006. Chemotherapy in the treatment and control of leishmaniasis. Adv Parasitol 61:223–274. 10.1016/S0065-308X(05)61006-8. [DOI] [PubMed] [Google Scholar]
- 13.Ponte-Sucre A, Gamarro F, Dujardin J-C, Barrett MP, López-Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B. 2017. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11:e0006052. 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naderer T, Heng J, McConville MJ. 2010. Evidence that intracellular stages of Leishmania major utilize amino sugars as a major carbon source. PLoS Pathog 6:e1001245. 10.1371/journal.ppat.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders EC, Naderer T, Chambers J, Landfear SM, McConville MJ. 2018. Leishmania mexicana can utilize amino acids as major carbon sources in macrophages but not in animal models. Mol Microbiol 108:143–158. 10.1111/mmi.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naderer T, Ellis MA, Sernee MF, De Souza DP, Curtis J, Handman E, McConville MJ. 2006. Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase. Proc Natl Acad Sci U S A 103:5502–5507. 10.1073/pnas.0509196103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Remaileh M, Wyant GA, Kim C, Laqtom NN, Abbasi M, Chan SH, Freinkman E, Sabatini DM. 2017. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358:807–813. 10.1126/science.aan6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders EC, Ng WW, Kloehn J, Chambers JM, Ng M, McConville MJ. 2014. Induction of a stringent metabolic response in intracellular stages of Leishmania mexicana leads to increased dependence on mitochondrial metabolism. PLoS Pathog 10:e1003888. 10.1371/journal.ppat.1003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloehn J, Saunders EC, O’Callaghan S, Dagley MJ, McConville MJ. 2015. Characterization of metabolically quiescent Leishmania parasites in murine lesions using heavy water labeling. PLoS Pathog 11:e1004683. 10.1371/journal.ppat.1004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayak A, Akpunarlieva S, Barrett M, Burchmore R. 2018. A defined medium for Leishmania culture allows definition of essential amino acids. Exp Parasitol 185:39–52. 10.1016/j.exppara.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Opperdoes FR, Butenko A, Flegontov P, Yurchenko V, Lukeš J. 2016. Comparative metabolism of free-living Bodo saltans and parasitic trypanosomatids. J Eukaryot Microbiol 63:657–678. 10.1111/jeu.12315. [DOI] [PubMed] [Google Scholar]
- 22.Boitz JM, Ullman B. 2006. A conditional mutant deficient in hypoxanthine-guanine phosphoribosyltransferase and xanthine phosphoribosyltransferase validates the purine salvage pathway of Leishmania donovani. J Biol Chem 281:16084–16089. 10.1074/jbc.M600188200. [DOI] [PubMed] [Google Scholar]
- 23.McCall L-I, Zhang W-W, Dejgaard K, Atayde VD, Mazur A, Ranasinghe S, Liu J, Olivier M, Nilsson T, Matlashewski G. 2015. Adaptation of Leishmania donovani to cutaneous and visceral environments: in vivo selection and proteomic analysis. J Proteome Res 14:1033–1059. 10.1021/pr5010604. [DOI] [PubMed] [Google Scholar]
- 24.Goldman-Pinkovich A, Balno C, Strasser R, Zeituni-Molad M, Bendelak K, Rentsch D, Ephros M, Wiese M, Jardim A, Myler PJ, Zilberstein D. 2016. An arginine deprivation response pathway is induced in Leishmania during macrophage invasion. PLoS Pathog 12:e1005494. 10.1371/journal.ppat.1005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman-Pinkovich A, Kannan S, Nitzan-Koren R, Puri M, Pawar H, Bar-Avraham Y, McDonald J, Sur A, Zhang W-W, Matlashewski G, Madhubala R, Michaeli S, Myler PJ, Zilberstein D. 2020. Sensing host arginine is essential for parasites’ intracellular development. mBio 11:e02023-20. 10.1128/mBio.02023-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreira D, Rodrigues V, Abengozar M, Rivas L, Rial E, Laforge M, Li X, Foretz M, Viollet B, Estaquier J, da Silva AC, Silvestre R. 2015. Leishmania infantum modulates host macrophage mitochondrial metabolism by hijacking the SIRT1-AMPK axis. PLoS Pathog 11:e1004684. 10.1371/journal.ppat.1004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillon LAL, Suresh R, Okrah K, Corrada Bravo H, Mosser DM, El-Sayed NM. 2015. Simultaneous transcriptional profiling of Leishmania major and its murine macrophage host cell reveals insights into host-pathogen interactions. BMC Genomics 16:1108. 10.1186/s12864-015-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabhi I, Rabhi S, Ben-Othman R, Rasche A, Daskalaki A, Trentin B, Piquemal D, Regnault B, Descoteaux A, Guizani-Tabbane L, Sysco Consortium . 2012. Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view. PLoS Negl Trop Dis 6:e1763. 10.1371/journal.pntd.0001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ty MC, Loke P, Alberola J, Rodriguez A, Rodriguez-Cortes A. 2019. Immuno-metabolic profile of human macrophages after Leishmania and Trypanosoma cruzi infection. PLoS One 14:e0225588. 10.1371/journal.pone.0225588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogdan C. 2020. Macrophages as host, effector and immunoregulatory cells in leishmaniasis: impact of tissue micro-environment and metabolism. Cytokine: X 2:100041. 10.1016/j.cytox.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postat J, Olekhnovitch R, Lemaître F, Bousso P. 2018. A metabolism-based quorum sensing mechanism contributes to termination of inflammatory responses. Immunity 49:654–665.e5. 10.1016/j.immuni.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Muxel SM, Mamani-Huanca M, Aoki JI, Zampieri RA, Floeter-Winter LM, López-Gonzálvez Á, Barbas C. 2019. Metabolomic profile of BALB/c macrophages infected with Leishmania amazonensis: deciphering L-arginine metabolism. Int J Mol Sci 20:6248. 10.3390/ijms20246248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira C, Mesquita I, Barbosa AM, Osório NS, Torrado E, Beauparlant C-J, Droit A, Cunha C, Carvalho A, Saha B, Estaquier J, Silvestre R. 2020. Glutamine supplementation improves the efficacy of miltefosine treatment for visceral leishmaniasis. PLoS Negl Trop Dis 14:e0008125. 10.1371/journal.pntd.0008125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parab AR, Thomas D, Lostracco-Johnson S, de Siqueira-Neto JL, McKerrow J, Dorrestein PC, McCall L-I. 2020. Metabolite profiling of experimental cutaneous leishmaniasis lesions demonstrates significant perturbations in tissue phospholipids. bioRxiv 2020.05.13.094649.
- 35.Mesquita I, Ferreira C, Moreira D, Kluck GEG, Barbosa AM, Torrado E, Dinis-Oliveira RJ, Gonçalves LG, Beauparlant C-J, Droit A, Berod L, Sparwasser T, Bodhale N, Saha B, Rodrigues F, Cunha C, Carvalho A, Castro AG, Estaquier J, Silvestre R. 2020. The absence of HIF-1α increases susceptibility to Leishmania donovani infection via activation of BNIP3/mTOR/SREBP-1c axis. Cell Rep 30:4052–4064.e7. 10.1016/j.celrep.2020.02.098. [DOI] [PubMed] [Google Scholar]
- 36.Henriques C, Atella GC, Bonilha VL, de Souza W. 2003. Biochemical analysis of proteins and lipids found in parasitophorous vacuoles containing Leishmania amazonensis. Parasitol Res 89:123–133. 10.1007/s00436-002-0728-y. [DOI] [PubMed] [Google Scholar]
- 37.Negrão F, Abánades DR, Jaeeger CF, Rocha DFO, Belaz KRA, Giorgio S, Eberlin MN, Angolini CFF. 2017. Lipidomic alterations of in vitro macrophage infection by L. infantum and L. amazonensis. Mol Biosyst 13:2401–2406. 10.1039/c7mb00381a. [DOI] [PubMed] [Google Scholar]
- 38.Paloque L, Perez-Berezo T, Abot A, Dalloux-Chioccioli J, Bourgeade-Delmas S, Le Faouder P, Pujo J, Teste M-A, François J-M, Schebb NH, Mainka M, Rolland C, Blanpied C, Dietrich G, Bertrand-Michel J, Deraison C, Valentin A, Cenac N. 2019. Polyunsaturated fatty acid metabolites: biosynthesis in Leishmania and role in parasite/host interaction. J Lipid Res 60:636–647. 10.1194/jlr.M091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ. 2012. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 40.Lal CS, Verma RB, Verma N, Siddiqui NA, Rabidas VN, Pandey K, Singh D, Kumar S, Paswan RK, Kumari A, Sinha P, Das P. 2016. Hypertriglyceridemia: a possible diagnostic marker of disease severity in visceral leishmaniasis. Infection 44:39–45. 10.1007/s15010-015-0811-9. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh J, Lal CS, Pandey K, Das VNR, Das P, Roychoudhury K, Roy S. 2011. Human visceral leishmaniasis: decrease in serum cholesterol as a function of splenic parasite load. Ann Trop Med Parasitol 105:267–271. 10.1179/136485911X12899838683566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malta-Santos H, França-Costa J, Macedo A, Queiroz ATL, Fukutani KF, Muxel SM, Khouri R, Van Weyenbergh J, Boaventura V, Barral A, Costa JM, Floh EIS, Andrade BB, Floeter-Winter LM, Borges VM. 2020. Differential expression of polyamine biosynthetic pathways in skin lesions and in plasma reveals distinct profiles in diffuse cutaneous leishmaniasis. Sci Rep 10:10543. 10.1038/s41598-020-67432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carfagna IE, Penas FN, Bott E, Lammel EM, Goren NB, Belaunzarán ML, Gimenez G. 2020. Involvement of lipids from Leishmania braziliensis promastigotes and amastigotes in macrophage activation. Mol Immunol 125:104–114. 10.1016/j.molimm.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Tounsi N, Meghari S, Moser M, Djerdjouri B. 2015. Lysophosphatidylcholine exacerbates Leishmania major-dendritic cell infection through interleukin-10 and a burst in arginase1 and indoleamine 2,3-dioxygenase activities. Int Immunopharmacol 25:1–9. 10.1016/j.intimp.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Sernee MF, Ralton JE, Nero TL, Sobala LF, Kloehn J, Vieira-Lara MA, Cobbold SA, Stanton L, Pires DEV, Hanssen E, Males A, Ward T, Bastidas LM, van der Peet PL, Parker MW, Ascher DB, Williams SJ, Davies GJ, McConville MJ. 2019. A family of dual-activity glycosyltransferase-phosphorylases mediates mannogen turnover and virulence in Leishmania parasites. Cell Host Microbe 26:385–399.e9. 10.1016/j.chom.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Zufferey R, Ben Mamoun C. 2006. Leishmania major expresses a single dihydroxyacetone phosphate acyltransferase localized in the glycosome, important for rapid growth and survival at high cell density and essential for virulence. J Biol Chem 281:7952–7959. 10.1074/jbc.M512911200. [DOI] [PubMed] [Google Scholar]
- 47.Rassi A, Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 48.Bern C, Kjos S, Yabsley MJ, Montgomery SP. 2011. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev 24:655–681. 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis MD, Francisco AF, Taylor MC, Jayawardhana S, Kelly JM. 2016. Host and parasite genetics shape a link between Trypanosoma cruzi infection dynamics and chronic cardiomyopathy. Cell Microbiol 18:1429–1443. 10.1111/cmi.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah-Simpson S, Lentini G, Dumoulin PC, Burleigh BA. 2017. Modulation of host central carbon metabolism and in situ glucose uptake by intracellular Trypanosoma cruzi amastigotes. PLoS Pathog 13:e1006747. 10.1371/journal.ppat.1006747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atwood JA, 3rd, Weatherly DB, Minning TA, Bundy B, Cavola C, Opperdoes FR, Orlando R, Tarleton RL. 2005. The Trypanosoma cruzi proteome. Science 309:473–476. 10.1126/science.1110289. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Shah-Simpson S, Okrah K, Belew AT, Choi J, Caradonna KL, Padmanabhan P, Ndegwa DM, Temanni MR, Corrada Bravo H, El-Sayed NM, Burleigh BA. 2016. Transcriptome remodeling in Trypanosoma cruzi and human cells during intracellular infection. PLoS Pathog 12:e1005511. 10.1371/journal.ppat.1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engel JC, de Cazzulo BMF, Stoppani AOM, Cannata JJB, Cazzulo JJ. 1987. Aerobic glucose fermentation by Trypanosoma cruzi axenic culture amastigote-like forms during growth and differentiation to epimastigotes. Mol Biochem Parasitol 26:1–10. 10.1016/0166-6851(87)90123-X. [DOI] [PubMed] [Google Scholar]
- 54.Mattos EC, Canuto G, Manchola NC, Magalhães RDM, Crozier TWM, Lamont DJ, Tavares MFM, Colli W, Ferguson MAJ, Alves MJM. 2019. Reprogramming of Trypanosoma cruzi metabolism triggered by parasite interaction with the host cell extracellular matrix. PLoS Negl Trop Dis 13:e0007103. 10.1371/journal.pntd.0007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumoulin PC, Burleigh BA. 2018. Stress-induced proliferation and cell cycle plasticity of intracellular amastigotes. mBio 9:e00673-18. 10.1128/mBio.00673-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garg N, Popov VL, Papaconstantinou J. 2003. Profiling gene transcription reveals a deficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts: implications in chagasic myocarditis development. Biochim Biophys Acta 1638:106–120. 10.1016/s0925-4439(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 57.de Oliveira TB, Pedrosa RC, Filho DW. 2007. Oxidative stress in chronic cardiopathy associated with Chagas disease. Int J Cardiol 116:357–363. 10.1016/j.ijcard.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 58.Wen J-J, Yachelini PC, Sembaj A, Manzur RE, Garg NJ. 2006. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med 41:270–276. 10.1016/j.freeradbiomed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Gironès N, Carbajosa S, Guerrero NA, Poveda C, Chillón-Marinas C, Fresno M. 2014. Global metabolomic profiling of acute myocarditis caused by Trypanosoma cruzi infection. PLoS Negl Trop Dis 8:e3337. 10.1371/journal.pntd.0003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koo S-J, Szczesny B, Wan X, Putluri N, Garg NJ. 2018. Pentose phosphate shunt modulates reactive oxygen species and nitric oxide production controlling in macrophages. Front Immunol 9:202. 10.3389/fimmu.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanmarco LM, Eberhardt N, Bergero G, Quebrada Palacio LP, Adami PM, Visconti LM, Minguez ÁR, Hernández-Vasquez Y, Carrera Silva EA, Morelli L, Postan M, Aoki MP. 2019. Monocyte glycolysis determines CD8+ T cell functionality in human Chagas disease. JCI Insight 4:e123490. 10.1172/jci.insight.123490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caradonna KL, Engel JC, Jacobi D, Lee C-H, Burleigh BA. 2013. Host metabolism regulates intracellular growth of Trypanosoma cruzi. Cell Host Microbe 13:108–117. 10.1016/j.chom.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gazos-Lopes F, Martin JL, Dumoulin PC, Burleigh BA. 2017. Host triacylglycerols shape the lipidome of intracellular trypanosomes and modulate their growth. PLoS Pathog 13:e1006800. 10.1371/journal.ppat.1006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCall L-I, Morton JT, Bernatchez JA, de Siqueira-Neto JL, Knight R, Dorrestein PC, McKerrow JH. 2017. Mass spectrometry-based chemical cartography of a cardiac parasitic infection. Anal Chem 89:10414–10421. 10.1021/acs.analchem.7b02423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dean DA, Gautham Siqueira-Neto JL, McKerrow JH, Dorrestein PC, McCall L-I. 2020. Spatial metabolomics identifies localized chemical changes in heart tissue during chronic cardiac Chagas disease. bioRxiv 2020.06.29.178038. [DOI] [PMC free article] [PubMed]
- 66.Hossain E, Khanam S, Dean DA, Wu C, Lostracco-Johnson S, Thomas D, Kane SS, Parab AR, Flores K, Katemauswa M, Gosmanov C, Hayes SE, Zhang Y, Li D, Woelfel-Monsivais C, Sankaranarayanan K, McCall L-I. 2020. Mapping of host-parasite-microbiome interactions reveals metabolic determinants of tropism and tolerance in Chagas disease. Sci Adv 6:eaaz2015. 10.1126/sciadv.aaz2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Büscher P, Cecchi G, Jamonneau V, Priotto G. 2017. Human African trypanosomiasis. Lancet 390:2397–2409. 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- 68.Aksoy S, Buscher P, Lehane M, Solano P, Van Den Abbeele J. 2017. Human African trypanosomiasis control: achievements and challenges. PLoS Negl Trop Dis 11:e0005454. 10.1371/journal.pntd.0005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franco J, Simarro P, Diarra A, Jannin J. 2014. Epidemiology of human African trypanosomiasis. Clin Epidemiol 6:257–275. 10.2147/CLEP.S39728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kennedy PGE, Rodgers J. 2019. Clinical and neuropathogenetic aspects of human African trypanosomiasis. Front Immunol 10:39. 10.3389/fimmu.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pineda E, Thonnus M, Mazet M, Mourier A, Cahoreau E, Kulyk H, Dupuy J-W, Biran M, Masante C, Allmann S, Rivière L, Rotureau B, Portais J-C, Bringaud F. 2018. Glycerol supports growth of the Trypanosoma brucei bloodstream forms in the absence of glucose: analysis of metabolic adaptations on glycerol-rich conditions. PLoS Pathog 14:e1007412. 10.1371/journal.ppat.1007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Creek DJ, Mazet M, Achcar F, Anderson J, Kim D-H, Kamour R, Morand P, Millerioux Y, Biran M, Kerkhoven EJ, Chokkathukalam A, Weidt SK, Burgess KEV, Breitling R, Watson DG, Bringaud F, Barrett MP. 2015. Probing the metabolic network in bloodstream-form Trypanosoma brucei using untargeted metabolomics with stable isotope labelled glucose. PLoS Pathog 11:e1004689. 10.1371/journal.ppat.1004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alsford S, Turner DJ, Obado SO, Sanchez-Flores A, Glover L, Berriman M, Hertz-Fowler C, Horn D. 2011. High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome Res 21:915–924. 10.1101/gr.115089.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamour N, Rivière L, Coustou V, Coombs GH, Barrett MP, Bringaud F. 2005. Proline metabolism in procyclic Trypanosoma brucei is down-regulated in the presence of glucose. J Biol Chem 280:11902–11910. 10.1074/jbc.M414274200. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Utzinger J, Saric J, Li JV, Burckhardt J, Dirnhofer S, Nicholson JK, Singer BH, Brun R, Holmes E. 2008. Global metabolic responses of mice to Trypanosoma brucei brucei infection. Proc Natl Acad Sci U S A 105:6127–6132. 10.1073/pnas.0801777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnston K, Kim D-H, Kerkhoven EJ, Burchmore R, Barrett MP, Achcar F. 2019. Mapping the metabolism of five amino acids in bloodstream form Trypanosoma brucei using U-13C-labelled substrates and LC–MS. Biosci Rep 39:BSR20181601. 10.1042/BSR20181601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazet M, Morand P, Biran M, Bouyssou G, Courtois P, Daulouède S, Millerioux Y, Franconi J-M, Vincendeau P, Moreau P, Bringaud F. 2013. Revisiting the central metabolism of the bloodstream forms of Trypanosoma brucei: production of acetate in the mitochondrion is essential for parasite viability. PLoS Negl Trop Dis 7:e2587. 10.1371/journal.pntd.0002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kovářová J, Nagar R, Faria J, Ferguson MAJ, Barrett MP, Horn D. 2018. Gluconeogenesis using glycerol as a substrate in bloodstream-form Trypanosoma brucei. PLoS Pathog 14:e1007475. 10.1371/journal.ppat.1007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trindade S, Rijo-Ferreira F, Carvalho T, Pinto-Neves D, Guegan F, Aresta-Branco F, Bento F, Young SA, Pinto A, Van Den Abbeele J, Ribeiro RM, Dias S, Smith TK, Figueiredo LM. 2016. Trypanosoma brucei parasites occupy and functionally adapt to the adipose tissue in mice. Cell Host Microbe 19:837–848. 10.1016/j.chom.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rijo-Ferreira F, Pinto-Neves D, Barbosa-Morais NL, Takahashi JS, Figueiredo LM. 2017. Trypanosoma brucei metabolism is under circadian control. Nat Microbiol 2:17032. 10.1038/nmicrobiol.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamour SD, Gomez-Romero M, Vorkas PA, Alibu VP, Saric J, Holmes E, Sternberg JM. 2015. Discovery of infection associated metabolic markers in human African trypanosomiasis. PLoS Negl Trop Dis 9:e0004200. 10.1371/journal.pntd.0004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vincent IM, Daly R, Courtioux B, Cattanach AM, Biéler S, Ndung'u JM, Bisser S, Barrett MP. 2016. Metabolomics identifies multiple candidate biomarkers to diagnose and stage human African trypanosomiasis. PLoS Negl Trop Dis 10:e0005140. 10.1371/journal.pntd.0005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seed JR, Hall JE, Sechelski J. 1982. Phenylalanine metabolism in Microtus montanus chronically infected with Trypanosoma brucei gambiense. Comp Biochem Physiol B 71:209–215. 10.1016/0305-0491(82)90242-5. [DOI] [PubMed] [Google Scholar]
- 84.Newport GR, Page CR, Ashman PU, Stibbs HH, Seed JR. 1977. Alteration of free serum amino acids in voles infected with Trypanosoma brucei gambiense. J Parasitol 63:15–24. 10.2307/3280098. [DOI] [PubMed] [Google Scholar]
- 85.McGettrick AF, Corcoran SE, Barry PJG, McFarland J, Crès C, Curtis AM, Franklin E, Corr SC, Mok KH, Cummins EP, Taylor CT, O’Neill LAJ, Nolan DP. 2016. Trypanosoma brucei metabolite indolepyruvate decreases HIF-1α and glycolysis in macrophages as a mechanism of innate immune evasion. Proc Natl Acad Sci U S A 113:E7778–E7787. 10.1073/pnas.1608221113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Campbell NK, Williams DG, Fitzgerald HK, Barry PJ, Cunningham CC, Nolan DP, Dunne A. 2019. Trypanosoma brucei secreted aromatic ketoacids activate the Nrf2/HO-1 pathway and suppress pro-inflammatory responses in primary murine glia and macrophages. Front Immunol 10:2137. 10.3389/fimmu.2019.02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lizardo K, Ayyappan JP, Ganapathi U, Dutra WO, Qiu Y, Weiss LM, Nagajyothi JF. 2019. Diet alters serum metabolomic profiling in the mouse model of chronic chagas cardiomyopathy. Dis Markers 2019:4956016. 10.1155/2019/4956016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McLuskey K, Wandy J, Vincent I, van der Hooft JJJ, Rogers S, Burgess K, Daly R. 2020. Decomposing metabolite set activity levels with PALS. bioRxiv 2020.06.07.138974. [DOI] [PMC free article] [PubMed]
- 89.Vargas DA, Prieto MD, Martínez-Valencia AJ, Cossio A, Burgess KEV, Burchmore RJS, Gómez MA. 2019. Pharmacometabolomics of meglumine antimoniate in patients with cutaneous leishmaniasis. Front Pharmacol 10:657. 10.3389/fphar.2019.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCarville JL, Ayres JS. 2018. Disease tolerance: concept and mechanisms. Curr Opin Immunol 50:88–93. 10.1016/j.coi.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bacchi CJ, Garofalo J, Mockenhaupt D, McCann PP, Diekema KA, Pegg AE, Nathan HC, Mullaney EA, Chunosoff L, Sjoerdsma A, Hutner SH. 1983. In vivo effects of α-dl-Difluoromethylornithine on the metabolism and morphology of Trypanosoma brucei brucei. Mol Biochem Parasitol 7:209–225. 10.1016/0166-6851(83)90022-1. [DOI] [PubMed] [Google Scholar]
- 92.Shaw CD, Lonchamp J, Downing T, Imamura H, Freeman TM, Cotton JA, Sanders M, Blackburn G, Dujardin JC, Rijal S, Khanal B, Illingworth CJR, Coombs GH, Carter KC. 2016. In vitro selection of miltefosine resistance in promastigotes of Leishmania donovani from Nepal: genomic and metabolomic characterization. Mol Microbiol 99:1134–1148. 10.1111/mmi.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yasur-Landau D, Jaffe CL, David L, Baneth G. 2016. Allopurinol resistance in Leishmania infantum from dogs with disease relapse. PLoS Negl Trop Dis 10:e0004341. 10.1371/journal.pntd.0004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morillo CA, Waskin H, Sosa-Estani S, del Carmen Bangher M, Cuneo C, Milesi R, Mallagray M, Apt W, Beloscar J, Gascon J, Molina I, Echeverria LE, Colombo H, Perez-Molina JA, Wyss F, Meeks B, Bonilla LR, Gao P, Wei B, McCarthy M, Yusuf S, STOP-CHAGAS Investigators . 2017. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: the STOP-CHAGAS trial. J Am Coll Cardiol 69:939–947. 10.1016/j.jacc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 95.Molina I, Gómez I Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sánchez-Montalvá A, Vidal X, Pahissa A. 2014. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med 370:1899–1908. 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 96.Morita YS, Paul KS, Englund PT. 2000. Specialized fatty acid synthesis in African trypanosomes: myristate for GPI anchors. Science 288:140–143. 10.1126/science.288.5463.140. [DOI] [PubMed] [Google Scholar]
- 97.D’Avila H, Freire-de-Lima CG, Roque NR, Teixeira L, Barja-Fidalgo C, Silva AR, Melo RCN, Dosreis GA, Castro-Faria-Neto HC, Bozza PT. 2011. Host cell lipid bodies triggered by Trypanosoma cruzi infection and enhanced by the uptake of apoptotic cells are associated with prostaglandin E2 generation and increased parasite growth. J Infect Dis 204:951–961. 10.1093/infdis/jir432. [DOI] [PubMed] [Google Scholar]
- 98.Martínez-Flórez A, Galizzi M, Izquierdo L, Bustamante JM, Rodriguez A, Rodriguez F, Rodríguez-Cortés A, Alberola J. 2020. Repurposing bioenergetic modulators against protozoan parasites responsible for tropical diseases. Int J Parasitol Drugs Drug Resist 14:17–27. 10.1016/j.ijpddr.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carvalho L, Luque-Ortega JR, Manzano JI, Castanys S, Rivas L, Gamarro F. 2010. Tafenoquine, an antiplasmodial 8-aminoquinoline, targets Leishmania respiratory complex III and induces apoptosis. Antimicrob Agents Chemother 54:5344–5351. 10.1128/AAC.00790-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khare S, Roach SL, Barnes SW, Hoepfner D, Walker JR, Chatterjee AK, Neitz RJ, Arkin MR, McNamara CW, Ballard J, Lai Y, Fu Y, Molteni V, Yeh V, McKerrow JH, Glynne RJ, Supek F. 2015. Utilizing chemical genomics to identify cytochrome b as a novel drug target for Chagas disease. PLoS Pathog 11:e1005058. 10.1371/journal.ppat.1005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharlow ER, Lyda TA, Dodson HC, Mustata G, Morris MT, Leimgruber SS, Lee K-H, Kashiwada Y, Close D, Lazo JS, Morris JC. 2010. A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity. PLoS Negl Trop Dis 4:e659. 10.1371/journal.pntd.0000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dawidowski M, Emmanouilidis L, Kalel VC, Tripsianes K, Schorpp K, Hadian K, Kaiser M, Mäser P, Kolonko M, Tanghe S, Rodriguez A, Schliebs W, Erdmann R, Sattler M, Popowicz GM. 2017. Inhibitors of PEX14 disrupt protein import into glycosomes and kill parasites. Science 355:1416–1420. 10.1126/science.aal1807. [DOI] [PubMed] [Google Scholar]
- 103.Haanstra JR, Gerding A, Dolga AM, Sorgdrager FJH, Buist-Homan M, Du Toit F, Faber KN, Holzhütter H-G, Szöör B, Matthews KR, Snoep JL, Westerhoff HV, Bakker BM. 2017. Targeting pathogen metabolism without collateral damage to the host. Sci Rep 7:40406. 10.1038/srep40406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vilar-Pereira G, Carneiro VC, Mata-Santos H, Vicentino ARR, Ramos IP, Giarola NLL, Feijó DF, Meyer-Fernandes JR, Paula-Neto HA, Medei E, Bozza MT, Lannes-Vieira J, Paiva CN. 2016. Resveratrol reverses functional Chagas heart disease in mice. PLoS Pathog 12:e1005947. 10.1371/journal.ppat.1005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kropf P, Fuentes JM, Fähnrich E, Arpa L, Herath S, Weber V, Soler G, Celada A, Modolell M, Müller I. 2005. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J 19:1000–1002. 10.1096/fj.04-3416fje. [DOI] [PubMed] [Google Scholar]
- 106.Muleme HM, Reguera RM, Berard A, Azinwi R, Jia P, Okwor IB, Beverley S, Uzonna JE. 2009. Infection with arginase-deficient Leishmania major reveals a parasite number-dependent and cytokine-independent regulation of host cellular arginase activity and disease pathogenesis. J Immunol 183:8068–8076. 10.4049/jimmunol.0803979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paduch K, Debus A, Rai B, Schleicher U, Bogdan C. 2019. Resolution of cutaneous leishmaniasis and persistence of Leishmania major in the absence of arginase 1. J Immunol 202:1453–1464. 10.4049/jimmunol.1801249. [DOI] [PubMed] [Google Scholar]
- 108.Gobert AP, Daulouede S, Lepoivre M, Boucher JL, Bouteille B, Buguet A, Cespuglio R, Veyret B, Vincendeau P. 2000. l-Arginine availability modulates local nitric oxide production and parasite killing in experimental trypanosomiasis. Infect Immun 68:4653–4657. 10.1128/iai.68.8.4653-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dar MA, Hölscher C. 2018. Arginase-1 is responsible for IL-13-mediated susceptibility to Trypanosoma cruzi infection. Front Immunol 9:2790. 10.3389/fimmu.2018.02790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carbajosa S, Rodríguez-Angulo HO, Gea S, Chillón-Marinas C, Poveda C, Maza MC, Colombet D, Fresno M, Gironès N. 2018. L-arginine supplementation reduces mortality and improves disease outcome in mice infected with Trypanosoma cruzi. PLoS Negl Trop Dis 12:e0006179. 10.1371/journal.pntd.0006179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O’Neill LAJ, Kishton RJ, Rathmell J. 2016. A guide to immunometabolism for immunologists. Nat Rev Immunol 16:553–565. 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Knubel CP, Martínez FF, Acosta Rodríguez EV, Altamirano A, Rivarola HW, Diaz Luján C, Fretes RE, Cervi L, Motrán CC. 2011. 3-Hydroxy kynurenine treatment controls T. cruzi replication and the inflammatory pathology preventing the clinical symptoms of chronic Chagas disease. PLoS One 6:e26550. 10.1371/journal.pone.0026550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Makala LHC, Baban B, Lemos H, El-Awady AR, Chandler PR, Hou D-Y, Munn DH, Mellor AL. 2011. Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J Infect Dis 203:715–725. 10.1093/infdis/jiq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parihar SP, Hartley M-A, Hurdayal R, Guler R, Brombacher F. 2016. Topical simvastatin as host-directed therapy against severity of cutaneous leishmaniasis in mice. Sci Rep 6:33458. 10.1038/srep33458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rena G, Grahame Hardie D, Pearson ER. 2017. The mechanisms of action of metformin. Diabetologia 60:1577–1585. 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roberts SB, Robichaux JL, Chavali AK, Manque PA, Lee V, Lara AM, Papin JA, Buck GA. 2009. Proteomic and network analysis characterize stage-specific metabolism in Trypanosoma cruzi. BMC Syst Biol 3:52. 10.1186/1752-0509-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shiratsubaki IS, Fang X, Souza ROO, Palsson BO, Silber AM, Siqueira-Neto JL. 2020. Genome-scale metabolic models highlight stage-specific differences in essential metabolic pathways in Trypanosoma cruzi. PLoS Negl Trop Dis 14:e0008728. 10.1371/journal.pntd.0008728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meshram RJ, Goundge MB, Kolte BS, Gacche RN. 2019. An in silico approach in identification of drug targets in Leishmania: a subtractive genomic and metabolic simulation analysis. Parasitol Int 69:59–70. 10.1016/j.parint.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Sharma M, Shaikh N, Yadav S, Singh S, Garg P. 2017. A systematic reconstruction and constraint-based analysis of Leishmania donovani metabolic network: identification of potential antileishmanial drug targets. Mol Biosyst 13:955–969. 10.1039/c6mb00823b. [DOI] [PubMed] [Google Scholar]
- 120.Ekins S, de Siqueira-Neto JL, McCall L-I, Sarker M, Yadav M, Ponder EL, Kallel EA, Kellar D, Chen S, Arkin M, Bunin BA, McKerrow JH, Talcott C. 2015. Machine learning models and pathway genome data base for Trypanosoma cruzi drug discovery. PLoS Negl Trop Dis 9:e0003878. 10.1371/journal.pntd.0003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kansiime F, Adibaku S, Wamboga C, Idi F, Kato CD, Yamuah L, Vaillant M, Kioy D, Olliaro P, Matovu E. 2018. A multicentre, randomised, non-inferiority clinical trial comparing a nifurtimox-eflornithine combination to standard eflornithine monotherapy for late stage Trypanosoma brucei gambiense human African trypanosomiasis in Uganda. Parasit Vectors 11:105. 10.1186/s13071-018-2634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hennig K, Abi-Ghanem J, Bunescu A, Meniche X, Biliaut E, Ouattara AD, Lewis MD, Kelly JM, Braillard S, Courtemanche G, Chatelain E, Béquet F. 2019. Metabolomics, lipidomics and proteomics profiling of myoblasts infected with Trypanosoma cruzi after treatment with different drugs against Chagas disease. Metabolomics 15:117. 10.1007/s11306-019-1583-5. [DOI] [PubMed] [Google Scholar]
- 123.Armitage EG, Godzien J, Peña I, López-Gonzálvez Á, Angulo S, Gradillas A, Alonso-Herranz V, Martín J, Fiandor JM, Barrett MP, Gabarro R, Barbas C. 2018. Metabolic clustering analysis as a strategy for compound selection in the drug discovery pipeline for leishmaniasis. ACS Chem Biol 13:1361–1369. 10.1021/acschembio.8b00204. [DOI] [PubMed] [Google Scholar]
- 124.Lima ML, Abengózar MA, Nácher-Vázquez M, Martínez-Alcázar MP, Barbas C, Tempone AG, López-Gonzálvez Á, Rivas L. 2018. Molecular basis of the leishmanicidal activity of the antidepressant sertraline as a drug repurposing candidate. Antimicrob Agents Chemother 62:e01928-18. 10.1128/AAC.01928-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Steketee PC, Vincent IM, Achcar F, Giordani F, Kim D-H, Creek DJ, Freund Y, Jacobs R, Rattigan K, Horn D, Field MC, MacLeod A, Barrett MP. 2018. Benzoxaborole treatment perturbs S-adenosyl-L-methionine metabolism in Trypanosoma brucei. PLoS Negl Trop Dis 12:e0006450. 10.1371/journal.pntd.0006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Armitage EG, Alqaisi AQI, Godzien J, Peña I, Mbekeani AJ, Alonso-Herranz V, López-Gonzálvez Á, Martín J, Gabarro R, Denny PW, Barrett MP, Barbas C. 2018. Complex interplay between sphingolipid and sterol metabolism revealed by perturbations to the Leishmania metabolome caused by miltefosine. Antimicrob Agents Chemother 62:e02095-17. 10.1128/AAC.02095-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dumoulin PC, Vollrath J, Tomko SS, Wang JX, Burleigh B. 2020. Glutamine metabolism modulates azole susceptibility in Trypanosoma cruzi amastigotes. Elife 9:e60226. 10.7554/eLife.60226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Francisco AF, Lewis MD, Jayawardhana S, Taylor MC, Chatelain E, Kelly JM. 2015. Limited ability of posaconazole to cure both acute and chronic Trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrob Agents Chemother 59:4653–4661. 10.1128/AAC.00520-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sánchez-Valdéz FJ, Padilla A, Wang W, Orr D, Tarleton RL. 2018. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. Elife 7:e34039. 10.7554/eLife.34039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perin L, Pinto L, Balthazar Nardotto GH, da Silva Fonseca K, Oliveira Paiva B, Fernanda Rodrigues Bastos Mendes T, Molina I, Correa-Oliveira R, Melo de Abreu Vieira P, Martins Carneiro C. 2020. Population pharmacokinetics and biodistribution of benznidazole in mice. J Antimicrob Chemother 75:2213–2221. 10.1093/jac/dkaa130. [DOI] [PubMed] [Google Scholar]
- 131.Hoffman K, Liu Z, Hossain E, Bottazzi ME, Hotez P, Jones K, McCall L-I. 2020. Alterations to the cardiac metabolome induced by chronic T. cruzi infection relate to the degree of cardiac pathology. bioRxiv 2020.09.17.300608. [DOI] [PMC free article] [PubMed]
- 132.Gershkovich P, Wasan EK, Sivak O, Li R, Zhu X, Werbovetz KA, Tidwell RR, Clement JG, Thornton SJ, Wasan KM. 2010. Visceral leishmaniasis affects liver and spleen concentrations of amphotericin B following administration to mice. J Antimicrob Chemother 65:535–537. 10.1093/jac/dkp465. [DOI] [PubMed] [Google Scholar]
- 133.Carlomagno MA, Riarte A, Moreno M, Segura EL. 1987. Effects of calorie-restriction on the course of Trypanosoma cruzi infection. Nutr Res 7:1031–1040. 10.1016/S0271-5317(87)80174-4. [DOI] [Google Scholar]
- 134.Nagajyothi F, Weiss LM, Zhao D, Koba W, Jelicks LA, Cui M-H, Factor SM, Scherer PE, Tanowitz HB. 2014. High fat diet modulates Trypanosoma cruzi infection associated myocarditis. PLoS Negl Trop Dis 8:e3118. 10.1371/journal.pntd.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lizardo K, Ayyappan JP, Cui M-H, Balasubramanya R, Jelicks LA, Nagajyothi JF. 2019. High fat diet aggravates cardiomyopathy in murine chronic Chagas disease. Microbes Infect 21:63–71. 10.1016/j.micinf.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ghosh J, Das S, Guha R, Ghosh D, Naskar K, Das A, Roy S. 2012. Hyperlipidemia offers protection against Leishmania donovani infection: role of membrane cholesterol. J Lipid Res 53:2560–2572. 10.1194/jlr.M026914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lewis MD, Fortes Francisco A, Taylor MC, Burrell-Saward H, McLatchie AP, Miles MA, Kelly JM. 2014. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol 16:1285–1300. 10.1111/cmi.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blais EM, Rawls KD, Dougherty BV, Li ZI, Kolling GL, Ye P, Wallqvist A, Papin JA. 2017. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nat Commun 8:14250. 10.1038/ncomms14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu H, Xing S, Nierves L, Lange PF, Huan T. 2020. Fold-change compression: an unexplored but correctable quantitative bias caused by nonlinear electrospray ionization responses in untargeted metabolomics. Anal Chem 92:7011–7019. 10.1021/acs.analchem.0c00246. [DOI] [PubMed] [Google Scholar]
- 140.Wang D, Eraslan B, Wieland T, Hallström B, Hopf T, Zolg DP, Zecha J, Asplund A, Li L-H, Meng C, Frejno M, Schmidt T, Schnatbaum K, Wilhelm M, Ponten F, Uhlen M, Gagneur J, Hahne H, Kuster B. 2019. A deep proteome and transcriptome abundance atlas of 29 healthy human tissues. Mol Syst Biol 15:e8503. 10.15252/msb.20188503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li M, Xu J, Romero-Gonzalez M, Banwart SA, Huang WE. 2012. Single cell Raman spectroscopy for cell sorting and imaging. Curr Opin Biotechnol 23:56–63. 10.1016/j.copbio.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 142.Pan N, Rao W, Kothapalli NR, Liu R, Burgett AWG, Yang Z. 2014. The single-probe: a miniaturized multifunctional device for single cell mass spectrometry analysis. Anal Chem 86:9376–9380. 10.1021/ac5029038. [DOI] [PubMed] [Google Scholar]
- 143.Baquero F, Nombela C. 2012. The microbiome as a human organ. Clin Microbiol Infect 18(Suppl 4):2–4. 10.1111/j.1469-0691.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 144.Gimblet C, Meisel JS, Loesche MA, Cole SD, Horwinski J, Novais FO, Misic AM, Bradley CW, Beiting DP, Rankin SC, Carvalho LP, Carvalho EM, Scott P, Grice EA. 2017. Cutaneous leishmaniasis induces a transmissible dysbiotic skin microbiota that promotes skin inflammation. Cell Host Microbe 22:13–24.e4. 10.1016/j.chom.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]