Abstract
Aim
Skin and soft tissue infections are classified into cellulitis and necrotizing fasciitis, which are difficult to distinguish. Necrotizing fasciitis has a poor prognosis and requires immediate intensive care. The diagnostic gold standard is to incise the lesion to determine whether necrosis has reached the fascia. We aimed to show that these infections can be differentiated using near‐infrared spectroscopy.
Methods
We describe two cases in an observational study about the utility of near‐infrared spectroscopy. Case 1 involved a 77‐year‐old man with a chief complaint of pain, redness, and swelling in the right lower leg for 1 week. Computed tomography of his legs showed no gas formation. Case 2 involved an 82‐year‐old man. He visited another hospital because of pain, redness, and swelling in the right thigh. Based on the X‐ray examination, necrotizing fasciitis was suspected, and he was transferred to our hospital.
Results
In Case 1, the regional oxygen saturation value was lower on the lesion side (41%) than on the healthy side (55%). We confirmed the depth of invasion by incision, leading to a diagnosis of necrotizing fasciitis. In Case 2, the thigh’s regional oxygen saturation was higher on the affected side (76%) than on the healthy side (61%). An incision was made for diagnosis, but the fascia was not necrotized. Thus, we diagnosed cellulitis and provided conservative treatment using antibiotics.
Conclusion
Near‐infrared spectroscopy can be utilized to measure tissue blood flow, and it could be useful as a non‐invasive diagnostic tool for skin and soft tissue infections.
Keywords: Cellulitis, near‐infrared spectroscopy, necrotizing fasciitis, skin, soft tissue infection
Currently, the diagnosis of necrotizing fasciitis is made by surgical incision. In this paper we report on a new diagnostic tool for Skin and soft tissue infections.
Introduction
Skin and soft tissue infections (SSTIs) are primarily classified based on the depth of the lesion. Cellulitis is where the lesion is relatively shallow from the skin to the soft tissues and can be improved by conservative treatment with proper antimicrobial agents. 1 A definite diagnosis is difficult because most cases do not present apparent symptoms early in the clinical course. The gold standard method for diagnosis is the confirmation of the injury depth and the necrotized area by well‐trained surgeons. 2 However, in the emergency department, quick and straightforward SSTI identification tools are required.
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score is an auxiliary diagnostic tool that scores the levels of hemoglobin (g/dL), serum creatinine (mg/dL), serum natrium (mEq/L), blood glucose (mg/dL), and C‐reactive protein (mg/dL). If the total score is 6 points or higher, necrotizing fasciitis is strongly suspected. 3 Although the LRINEC score is widely used, it has low sensitivity (68.2%) and specificity (84.8%). 4 Near‐infrared spectroscopy (NIRS) is a tool for measuring oxygen in living tissues. This method has been widely applied in the medical field since the 1990s. 5 It is widely used for monitoring cerebral tissue oxygenation during cardiac bypass surgery, 6 qualitatively assessing chest compressions during cardiopulmonary resuscitation, 7 and estimating neurological outcomes. 8 In dermatology, efforts to apply NIRS to blood flow evaluation during flap formation and prognosis of wound healing have been reported. 9 , 10 Previous studies have reported the use of NIRS for the diagnosis of necrotizing fasciitis. 11 We report two cases of SSTIs that were successfully differentiated as necrotizing fasciitis and cellulitis using NIRS comparing regional oxygen saturation (rSO2) between healthy and unhealthy skin.
Participants
A summary of the two cases is shown in Table 1. In both cases, rSO2 was measured using an INVOS oximeter (Medtronic, Minneapolis, MN, USA). Near‐infrared spectroscopy was measured at two sites, the affected area and the corresponding area on the healthy side. Measurements were taken only once at the initial treatment stage, immediately after the visit.
Table 1.
Summary of the characteristics, laboratory data on arrival, treatment, and outcome of two cases of skin and soft tissue infection
| Case 1 | Case 2 | |
|---|---|---|
| Age (years) | 77 | 82 |
| Sex | Male | Male |
| Medical history | AF, CKD | ALD |
| Glasgow Coma Scale (points) | 15 | 14 |
| Vital signs on arrival | ||
| Blood pressure (mmHg) | 102/55 | 126/57 |
| Heart rate (b.p.m.) | 95 | 97 |
| Respiratory rate (breaths/min) | 24 | 17 |
| SpO2 (%) | 98 (RA) | 97(O2 2L/min) |
| Body temperature (°C) | 36.6 | 37.1 |
| Gas formation on CT | (−) | (+) |
| Laboratory data | ||
| WBC/μL | 11,800 | 24,300 |
| Hemoglobin (g/dL) | 12.2 | 9.3 |
| Platelets (×104/μL) | 9.3 | 29.8 |
| Cre (mg/dL) | 2.18 | 2.00 |
| AST (U/L) | 89 | 25 |
| ALT (U/L) | 31 | 12 |
| T‐Bil (mg/dL) | 1.7 | 0.3 |
| CK (U/L) | 1,683 | 88 |
| Glu (mg/dL) | 163 | 149 |
| CRP (mg/dL) | 25.20 | 26.75 |
| LRINEC score (points) | 7 | 7 |
| rSO2 (lesion side) (%) | 41 | 76 |
| rSO2 (healthy side) (%) | 55 | 61 |
| Lesion / healthy ratio | 0.75 | 1.25 |
| Treatment | Abx debridement | Abx |
| Outcome | Survived | Survived |
Abbreviations: Abx, antibiotics; AF, atrial fibrillation; ALD, alcoholic liver disorder; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CKD, chronic renal failure; Cre, creatinine; CRP, C‐reactive protein; CT, computed tomography; Glu, glucose; LRINEC, Laboratory Risk Indicator for Necrotizing Fasciitis; rSO2, regional oxygen saturation; T‐Bil, total bilirubin; WBC, white blood cell count.
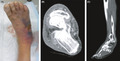
Case 1 was a 77‐year‐old man who had chronic atrial fibrillation and chronic renal failure as underlying diseases. He came to our hospital complaining of pain, redness, and swelling in his right lower leg, which persisted from the week before his visit. On arrival, he was alert and conscious. Initial vital signs and laboratory data are shown in Table 1. On physical examination, widespread redness, blistering, and skin erosion were observed from the right lower leg to the foot’s dorsum. When we punctured this lesion, serous effusion was aspirated (Fig. 1A). His LRINEC score was 7 points. Computed tomography (CT) of the leg showed no abscess and gas formation (Fig. 1B,C). His lower leg's rSO2 value was 41% on the affected side and 55% on the healthy side. The ratio between the affected and healthy sides was 0.75. The affected area was incised under local anesthesia in the emergency department, and necrotic tissue reaching the fascia was confirmed. He was diagnosed with necrotizing fasciitis. After emergency surgery, the mean blood pressure decreased to 50 mmHg; therefore, we developed an aggressive treatment plan consisting of catecholamines and antibiotics (2.4 billion units of penicillin and 1800 mg/day clindamycin) for 14 days.
Fig. 1.
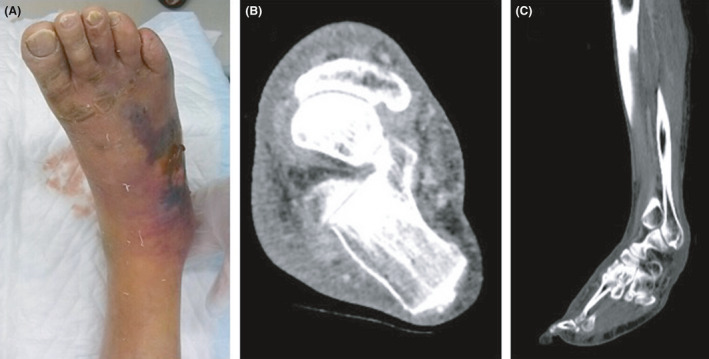
Skin and computed tomography findings in Case 1, a 77‐year‐old man with necrotizing fasciitis. A, An image of the affected area. The right lower limb was reddened, and blisters and purple color changes were found on the back of the foot. The dorsal foot artery was palpable. B, An axial image of the ankle joint. The findings were myomegaly and an increase in the concentration of fatty tissue in the subcutaneous tissue. C, No gas formation was observed in the sagittal image, and at first glance, it appeared to be cellulitis.
Streptococcus pyogenes was isolated from the wound culture. The patient’s general condition gradually stabilized after treatment initiation. On day 20, the wound was closed with skin grafting, and the patient began rehabilitation. On day 52, he was transferred to another hospital.
Case 2 was that of an 82‐year‐old man with alcoholic liver disease. His chief complaint was pain, redness, and swelling in his right thigh 1 day before his initial evaluation at another hospital. His right knee X‐ray was significant for gas findings. He was transferred to our hospital with suspected necrotizing fasciitis. His consciousness levels were 14 points (E4V4M6) on the Glasgow Coma Scale on arrival. Initial vital signs and laboratory data are shown in Table 1. Widespread pain, redness, swelling, and feeling of warmth were noted from the right thigh to the knee; however, blisters were absent (Fig. 2A).
Fig. 2.
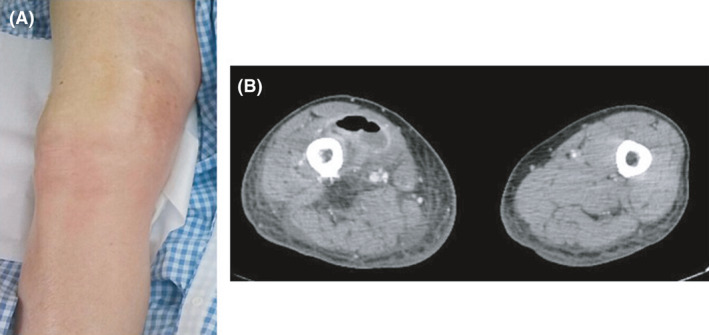
Skin and computed tomography findings in Case 2, an 82‐year‐old man with cellulitis. A, An image of the affected area. Although there was evidence of inflammation, automatic movement of the ankle was possible, and the dorsal foot artery was also palpable. B, The axial image showed swelling in the affected area compared with the healthy side. It showed subcutaneous abscess formation, gas production, and increased adipose tissue density.
His LRINEC score was 7 points. The CT showed fluid collection and gas formation inside the subcutaneous tissue from his right thigh to the knee joint (Fig. 2B).
His lower leg’s rSO2 value was 76% on the affected side and 61% on the healthy side. The ratio between the affected and healthy sides was 1.25. We incised the lesion to see the depth of damage to diagnose the lesion correctly. The fascia was not necrotized, and inflammation was confined in the subcutaneous tissue. Thus, we diagnosed him with subcutaneous abscess spreading from cellulitis and undertook incision and drainage without debridement. Catecholamines were not required during the course of treatment in this case. After admission, we selected conservative treatment with antibiotics (3 g meropenem and 1200 mg/day linezolid). A few days later, Escherichia coli was isolated from the wound culture; hence, we changed the antibiotics to 12 g/day sulbactam/ampicillin and continued the treatment for 23 days. The patient’s general condition did not deteriorate, and on day 41 he was transferred to a rehabilitation hospital.
Discussion
We suspected necrotizing fasciitis in both cases at the first visit and calculated the same LRINEC score of 7 points. Case 1 appeared to be cellulitis based on the CT image findings, and the diagnosis was difficult from physical and image findings. However, rSO2 was lower in the affected side in Case 1, where fascial necrosis was noted. In contrast, rSO2 was higher in the affected side in Case 2, which was diagnosed as cellulitis.
Near‐infrared spectroscopy measures blood flow and tissue oxygen status in the peripheral muscle tissue. 12 There are reports that these properties could be applied clinically for early recognition of lower limb ischemia caused by vascular occlusion and for the evaluation of tissue hypoperfusion in sepsis. 13 , 14 . In necrotizing fasciitis, the lower rSO2 could result from decreased blood flow caused by the necrosis. In contrast, rSO2 was higher in cellulitis, reflecting an increase in the blood flow associated with inflammation of soft tissues. Therefore, by comparing rSO2 of the affected and healthy sides, NIRS could be a useful diagnostic tool for non‐invasively and quickly distinguishing cellulitis and necrotizing fasciitis.
However, there are a few limitations in using NIRS for the differential diagnosis between necrotizing fasciitis and cellulitis. First, near‐infrared rays pass through various obstacles, such as subcutaneous tissues, bones, and blood, before reaching the receiver. The numerical value of rSO2 varies depending on the measurement site and individual. 15 Therefore, an absolute cut‐off value for rSO2 cannot be set, and the affected and healthy sides of the same site must be compared. This method is difficult to apply for lesions in the middle of the trunk (e.g., the perineum in Fournier’s gangrene) where no comparison target can be set. Second, if the affected area is the lower limb, the assessment might be difficult for cases with underlying diseases such as arteriosclerosis obliterans. In addition, the absolute value of NIRS could decrease in the presence of arrhythmia or circulatory insufficiency, and the numerical value should be evaluated carefully. 16
In conclusion, NIRS could be a non‐invasive quantitative diagnostic tool for differentiating SSTIs. We feel it is necessary to research this question at multiple facilities by prospective studies to verify its diagnostic sensitivity and specificity.
Disclosures
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: N/A.
Acknowledgements
The authors would like to thank Editage for the English language review.
Funding information
No funding information provided.
References
- 1. Napolitano LM. Severe soft tissue infections. Infect. Dis. Clin. North Am. 2009; 23: 571–91. [DOI] [PubMed] [Google Scholar]
- 2. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J. Bone Joint Surg. Am. 2003; 85: 1454–60. [PubMed] [Google Scholar]
- 3. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit. Care Med. 2004; 32: 1535–41. [DOI] [PubMed] [Google Scholar]
- 4. Fernando SM, Tran A, Cheng W et al. Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC score: a systematic review and meta‐analysis. Ann. Surg. 2019; 269: 58–65. [DOI] [PubMed] [Google Scholar]
- 5. Takakura H. Near‐infrared spectroscopy: NIRS. Equilibrium Res. 2015; 74: 552–6.[Japanese]. [Google Scholar]
- 6. Rogers CA, Stoica S, Ellis L, Stokes EA, Wordsworth S, Dabner L. Randomized trial of near‐infrared spectroscopy for personalized optimization of cerebral tissue oxygenation during cardiac surgery. Br. J. Anaesth. 2017; 119: 384–93. [DOI] [PubMed] [Google Scholar]
- 7. Schewe JC, Thudium MO, Kappler J et al. Monitoring of cerebral oxygen saturation during resuscitation in out‐of‐hospital cardiac arrest: a feasibility study in a physician staffed emergency medical system. Scand. J. Trauma Resusc. Emerg. Med. 2014; 22: 58. Published 2014 Oct 5. 10.1186/s13049-014-0058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koyama Y, Inoue Y, Hisago S et al. Improving the neurological prognosis following OHCA using real‐time evaluation of cerebral tissue oxygenation. Am. J. Emerg. Med. 2018; 36: e5–7. [DOI] [PubMed] [Google Scholar]
- 9. Hill WF, Webb C, Monument M, McKinnon G, Hayward V, Temple‐Oberle C. Intraoperative near‐infrared spectroscopy correlates with skin flap necrosis: a prospective cohort study. Plast. Reconstr. Surg. Glob. Open. 2020; 8: e2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jayachandran M, Rodriguez S, Solis E, Lei J, Godavarty A. Critical review of noninvasive optical technologies for wound imaging. Adv. Wound Care (New Rochelle). 2016; 5: 349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang T‐L, Hung C‐R. Role of tissue oxygen saturation monitoring in diagnosing necrotizing fasciitis of the lower limbs. Ann. Emerg. Med. 2004; 44: 222–8. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe T, Matsushita M, Nishikimi N, Sakurai T, Komori K, Nimura Y. An assessment of ischemic limbs using near‐infrared spectroscopy in patients with atherosclerotic occlusive disease. Jpn. J. Vasc. Surg. 2000; 9: 701–6. [Google Scholar]
- 13. Green MS, Sehgal S, Tariq R. Near‐infrared spectroscopy: the new must have tool in the intensive care unit? Semin. Cardiothorac. Vasc. Anesth. 2016; 20: 213–24. [DOI] [PubMed] [Google Scholar]
- 14. Patton‐Rivera K, Beck J, Fung K et al. Using near‐infrared reflectance spectroscopy (NIRS) to assess distal‐limb perfusion on venoarterial (V‐A) extracorporeal membrane oxygenation (ECMO) patients with femoral cannulation. Perfusion. 2018; 33: 618–23. [DOI] [PubMed] [Google Scholar]
- 15. Yoshitani K, Kawaguchi M, Miura N et al. Effects of hemoglobin concentration, skull thickness, and the area of the cerebrospinal fluid layer on near‐infrared spectroscopy measurements. Anesthesiology 2007; 106: 458–62. [DOI] [PubMed] [Google Scholar]
- 16. Vranken NPA, Lindelauf AAMA, Simons AP, et al. Cerebral and limb tissue oxygenation during peripheral venoarterial extracoporeal life support. J. Intensive Care Med. 2020; 35: 179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


