Abstract
Aims
Many surgeons choose to perform total knee arthroplasty (TKA) surgery with the aid of a tourniquet. A tourniquet is a device that fits around the leg and restricts blood flow to the limb. There is a need to understand whether tourniquets are safe, and if they benefit, or harm, patients. The aim of this study was to determine the benefits and harms of tourniquet use in TKA surgery.
Methods
We searched MEDLINE, EMBASE, Cochrane Central Register of Controlled trials, and trial registries up to 26 March 2020. We included randomized controlled trials (RCTs), comparing TKA with a tourniquet versus without a tourniquet. Outcomes included: pain, function, serious adverse events (SAEs), blood loss, implant stability, duration of surgery, and length of hospital stay.
Results
We included 41 RCTs with 2,819 participants. SAEs were significantly more common in the tourniquet group (53/901 vs 26/898, tourniquet vs no tourniquet respectively) (risk ratio 1.73 (95% confidence interval (CI) 1.10 to 2.73). The mean pain score on the first postoperative day was 1.25 points higher (95% CI 0.32 to 2.19) in the tourniquet group. Overall blood loss did not differ between groups (mean difference 8.61 ml; 95% CI -83.76 to 100.97). The mean length of hospital stay was 0.34 days longer in the group that had surgery with a tourniquet (95% CI 0.03 to 0.64) and the mean duration of surgery was 3.7 minutes shorter (95% CI -5.53 to -1.87).
Conclusion
TKA with a tourniquet is associated with an increased risk of SAEs, pain, and a marginally longer hospital stay. The only finding in favour of tourniquet use was a shorter time in theatre. The results make it difficult to justify the routine use of a tourniquet in TKA surgery.
Cite this article: Bone Joint J 2021;103-B(5):830–839.
Keywords: Total knee arthroplasty, Tourniquet, Serious adverse events
Introduction
Over 106,000 total knee arthroplasties (TKAs) were performed in the UK in 2018.1,2 TKA is frequently undertaken with the aid of a tourniquet around the thigh.3 Over 90% of surgeons in the UK, USA, and in Europe routinely use tourniquets for TKA.4-6 A tourniquet is typically applied at high pressure around the leg for all or part of the procedure.
Tourniquets may help to create a bloodless field, facilitating easier surgery.4 The majority of knee arthroplasty components are cemented in situ to hold and stabilize them in the correct position on the bone.1 Some surgeons believe that using a tourniquet helps reduce bleeding and allows the cement to bond more effectively.4,7 Better cementing should reduce the chance of the knee arthroplasty loosening and failing, but there is no objective clinical evidence to support this. Effective cementing is achieved in hip and shoulder arthroplasty where the use of a tourniquet is not possible. In such surgery it is accepted that the absence of a tourniquet does not compromise the field of view, cause excessive intraoperative blood loss, or lead to long-term problems with implant survivorship.
A tourniquet can cause pain, both during and after surgery.8 In addition, a tourniquet causes both arterial and venous stasis within the lower leg. It is therefore possible that the use of a surgical tourniquet may increase the risk of postoperative venous thromboembolism (VTE). VTE is one of the most common complications after TKA surgery and a prominent cause of death. Research has found up to 1% develop symptomatic VTE and the in-hospital mortality has been reported as 7.1% in patients with a symptomatic VTE. This is substantially greater than when no VTE was identified (0.3%).9 Tourniquets may also cause wound and skin problems.4,10 Furthermore, it may be that after tourniquet deflation, systemic emboli formation contributes to the higher than expected incidence of postoperative cognitive deficit following TKA surgery.11
The continued use of tourniquets depends on the balance of harms versus benefits they confer to patients. The effects of using a tourniquet in TKA have previously been reported in systematic reviews, most recently in 2014.12-14 However, substantial additional data have become available that, when summarized, may have an important impact on clinical practice.15-23
This study aimed to review systematically the evidence to identify the benefits and the harms of surgery with a tourniquet compared to surgery without a tourniquet in patients undergoing knee arthroplasty.
Methods
This systematic review and meta-analysis is an abridged summary of a full Cochrane review.24 It was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines.25 The protocol was registered and published with the Cochrane database of systematic reviews.24 A search of OVID Medline (1946 to 26 March 2020), OVID Embase (1974 to 26 March 2020), and Cochrane Central Register of Controlled Trials via Cochrane Library was performed to identify RCTs. Details of the search strategy can be found in the Supplementary Material. Trial registries (clinicaltrial.gov and the WHO (World Health Organization International Clinical Trials Registry Platform (ICTRP)) was searched to identify further studies.
Eligibility criteria
We included RCTs and studies in which the allocation to intervention was quasi-randomized (e.g. by date of birth, hospital number). All other study types were excluded including non-randomized trials, cohort studies, and case series.
Population
We included anyone undergoing any type of knee arthroplasty (TKA, revision knee arthroplasty, and unicondylar knee arthroplasty) for any indication.
Intervention and comparators
We included studies comparing all types of tourniquet used for the full duration or part of the procedure. Comparator groups included: placebo or sham tourniquet (where a tourniquet is applied but not inflated); no tourniquet; and alternative measures to improve field of view or reduce intraoperative blood loss e.g. tranexamic acid. Studies that compared surgery with a tourniquet for the whole procedure versus surgery with a tourniquet for part of the procedure were excluded.
Outcomes
In this abridged review, we present data for the outcomes which were deemed to be medium quality or above. Results from outcomes which were graded as low and very low quality can be found in the full Cochrane review. We assessed the following outcomes: serious adverse events (SAEs; including deep vein thrombosis (DVT), pulmonary embolism (PE), infection, nerve damage, reoperation (excluding revision for implant failure), and mortality); pain (measured using mean pain score or mean change in pain score on a visual analogue scale (VAS), a numeric rating scale or other scale); function (measured with instruments such as Knee Society Score (KSS),26 Oxford Knee Score (OKS),27,28 or Hospital for Special Surgery (HSS) knee questionnaire;29 the planned MCID was 5.3 points in KSS for function);30 survival of implant (measured as risk of a revision); blood loss (measured with total blood loss, postoperative blood loss, and intraoperative blood loss); duration of surgry; length of hospital stay; and implant stability (using radiostereometric analysis (RSA) as a recognized surrogate marker of later implant failure).31,32
We grouped postoperative outcomes into days for the first week, and then up to three months; three to 12 months; greater than 12 months. Studies not reporting any of the outcomes listed were excluded.
Two review authors (IA, PW) independently screened titles and abstracts, assessed full texts of potentially eligible studies for inclusion, and independently assessed risk of bias for each study using the risk of bias tool in the Cochrane Handbook for systematic reviews and interventions.33 Disagreement was resolved following discussion with a senior author (MU).24
Statistical analysis
We used risk ratios (RRs) with 95% confidence intervals (CIs) to report categorical outcomes. We analyzed continuous data as mean differences (MDs) or as standardized mean differences (SMDs), depending on whether the same scale was used to measure an outcome, along with 95% CIs. We then translated the SMD back to a common scale by multiplying SMD by baseline standard deviation (SD) for the control group from the most representative study.34
For dichotomous outcomes, we calculated the absolute per cent change from the difference in risks between intervention and control groups using GRADEpro (GRADEpro 2015; McMaster University/Evidence Prime, Canada), and we expressed this as a percentage. For continuous outcomes, we calculated the absolute risk difference as improvement in the intervention group minus improvement in the control group, in the original units.
We calculated the relative per cent change for dichotomous data as the RR minus 1, expressed as a percentage. For continuous outcomes, we calculated the relative difference in change from baseline as the absolute benefit divided by the baseline mean of the control group.
For dichotomous outcomes, such as serious adverse events, we calculated the number needed to treat for an additional beneficial outcome (NNTB) from the control group event rate and the risk ratio, using the Visual Rx NNT calculator (Visual RX, UK).
We assessed statistical heterogeneity by visually inspecting the forest plot to assess for obvious differences in results between studies, and by using I² and chi-squared statistical tests.
If we were able to pool more than 10 trials, we decided to undertake formal statistical tests to investigate funnel plot asymmetry. For dichotomous data, we used a weighted linear regression based upon the odds ratio against its variance. In both cases, we considered a p-value below 0.05 as evidence that publication bias was present. We performed analyses using the “meta” R package (R Foundation for Statistical Computing, Austria).
We pooled outcomes of clinically and methodologically homogeneous studies, when meaningful, using a random‐effectsmodel. We performed analysis using Review Manager 5 (RevMan 2014, The Nordic Cochrane Centre, Denmark), and we produced forest plots for all analyses. Further details of the data extraction and the statistical analysis plan are detailed in the full Cochrane review.24
Results
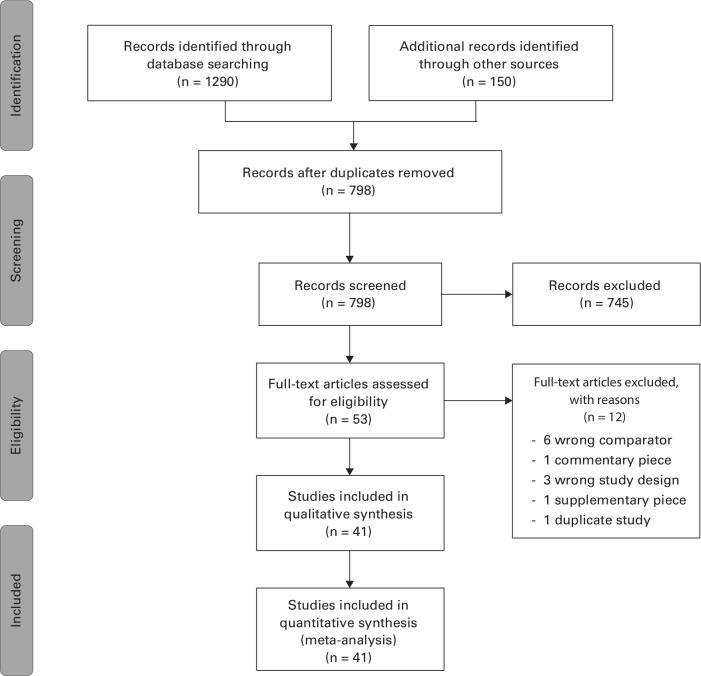
The search returned 1,290 citations through the databases and an additional 150 citations from trial registries. Following removal of duplicates, titles and abstracts were screened for eligibility and 53 full texts were assessed for inclusion. In total 41 RCTs met the inclusion criteria for this review. A PRISMA flow diagram of our search results can be seen in Figure 1.
Fig. 1.
Preferred Reporting Items for Systematic reviews and Meta-Analysis flow diagram demonstrating the results from the search and reasons for exclusion.
In total 2,819 participants were allocated to either surgery with a tourniquet (n = 1,461) or surgery without a tourniquet inflated (n = 1,466). In the tourniquet group, a tourniquet was used for the entire procedure in all studies. All trials included primary TKA only. In studies reporting sex, 1,777/2,721 (65%) were female. Where studies reported mean age, the mean age was 69.0 (SD 3.95) in the tourniquet group and 68.2 (SD 4.46) in the non-tourniquet group. Further details on the baseline characteristics can be seen in Supplementary Table i.
Risk of bias
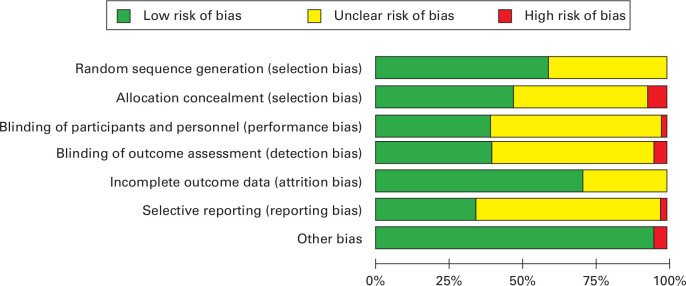
Three trials (including a total of 296 patients) met all methodological criteria for low risk of bias.15,16,35 The other trials had sources of bias including unclear risk of selection bias, performance bias, and detection bias, as blinding was not clearly stated in the methodology or protocol. A risk of bias summary is shown in Figure 2.
Fig. 2.
Risk of bias with judgements about each item presented as percentages across all included studies.
Serious adverse events
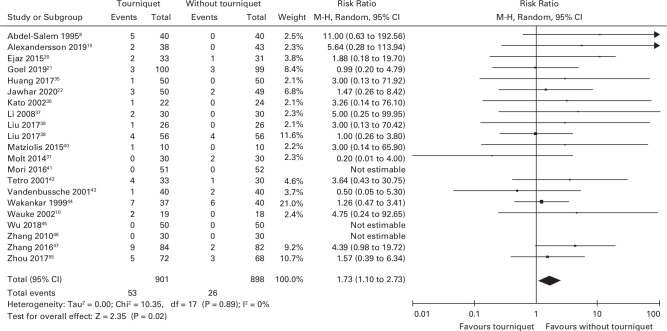
A total of 21 studies reported SAEs (n = 1,799).8,15,20-31,35–48-48 Of the 901 participants in the tourniquet group, 53 had a SAE, and 26 of the 898 participants in the no tourniquet group had a SAE. The risk of SAEs was greater in the tourniquet group compared to the no tourniquet group (RR 1.73 (95% CI 1.10 to 2.73); Figure 3). The number needed to harm (NNTH) was calculated as 48 (20 to 345) participants needed to have surgery with a tourniquet for one SAE to occur. Table I demonstrates the number of each SAE included in the analysis and the risk ratios.
Fig. 3.
Forest plot demonstrating the number of serious adverse events in the surgery with a tourniquet group compared to the surgery without a tourniquet group. CI, confidence interval. M-H, Mantel-Haenszel method.
Table I.
Types of serious adverse events and numbers within each group included in the meta-analysis.
| SAE | Tourniquet group, n (%) | Non-tourniquet group, n (%) | Risk ratio (95% CI) |
|---|---|---|---|
| DVT | 26/754 (3.4) | 11/745 (1.5) | 1.83 (0.92 to 3.65) |
| PE | 2/192 (0.52) | 0/224 (0) | 4.51 (0.49 to 41.81) |
| Infection | 19/427 (4.4) | 4/419 (0.95) | 2.17 (1.15 to 6.42) |
| Reoperation | 9/77 (11.7) | 5/80 (6.3) | 1.63 (0.61 to 4.34) |
| Mortality | 1/67 (1.5) | 3/70 (4.3) | 0.45 (0.07 to 3.01) |
CI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; SAE, serious adverse event.
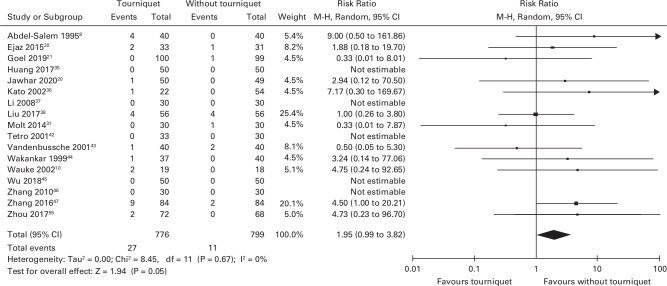
In total, 17 studies reported the number of symptomatic venous thromboembolic events (VTE; n = 1,575).8,20-31,35–37-42–48,42-48 There were 27 VTEs in 776 participants in the tourniquet group and 11 VTEs in 799 participants in the non-tourniquet group. Tourniquet use was associated with higher risk of VTE, of borderline statistical significance, compared to surgery without a tourniquet (RR 1.95 (95% CI 0.99 to 3.82); Figure 4). One study reported the number of postoperative asymptomatic DVTs (n = 103). However, these patients did not have any form of chemical thromboprophylaxis and all had routine ultrasounds. As this study was different from the others it was not included in the meta-analysis.41 This study did report significantly higher rate of DVT in the surgery with a tourniquet group (54.9%; 28 out of 51 patients) compared to surgery without a tourniquet (25%; 13 out of 52 patients).
Fig. 4.
Forest plot demonstrating the number of venous thromboembolic events in the surgery with a tourniquet group versus the surgery without a tourniquet group. CI, confidence interval. M-H, Mantel-Haenszel Method.
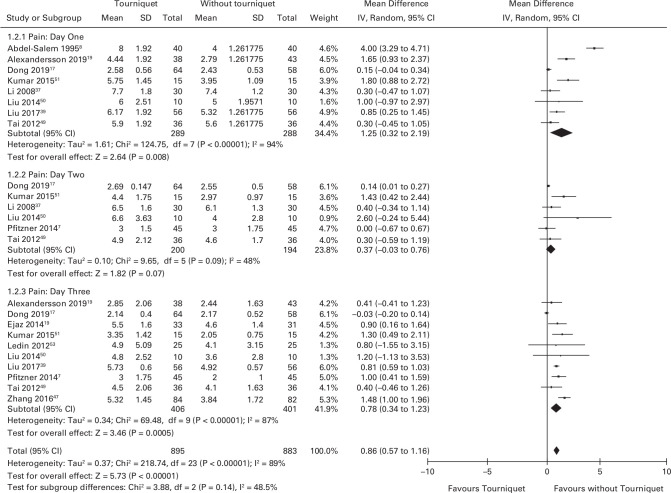
Pain
Eight studies (n = 577) reported pain using a VAS at day one (scale 0 to 10 with higher scores indicating more pain). The mean pain scores were 1.25 (95% CI 0.32 to 2.19) higher in the tourniquet group (Figure 5).8,15,17,37,39,49-51 Six studies (n = 394) reported pain using a VAS at day two.7,17,49-52 The mean pain scores were 0.37 (95% CI -0.03 to 0.76) higher in the tourniquet group. Ten studies (n = 807) reported pain at day three.7,15,17,19,39,47,49-53,53 The mean pain scores were 0.78 (95% CI 0.34 to 1.23) higher in the tourniquet group. Figure 5 demonstrates the pain scores in each group at day one, two, and three.
Fig. 5.
Forest plot demonstrating mean pain scores at day one, two and three. Pain scores were on a ten-point visual analogue scale (lower is better). CI, confidence interval; IV, inverse variance method; SD, standard deviation.
Function
Nine studies investigated the effect of tourniquet use on patient reported knee function scores. Four studies (n = 425) reported three-month scores.16,21,38,54 The standardized mean difference between the two groups was 0.64 lower in the tourniquet group (95% CI -1.52 to 0.52) compared to the group without a tourniquet. Five studies (n = 611) participants reported 12 month scores.8,21,35,38,55 The standardized mean difference was 0.06 lower (95% CI 0.22 to 0.10; I2 = 0%) in the group with a tourniquet.
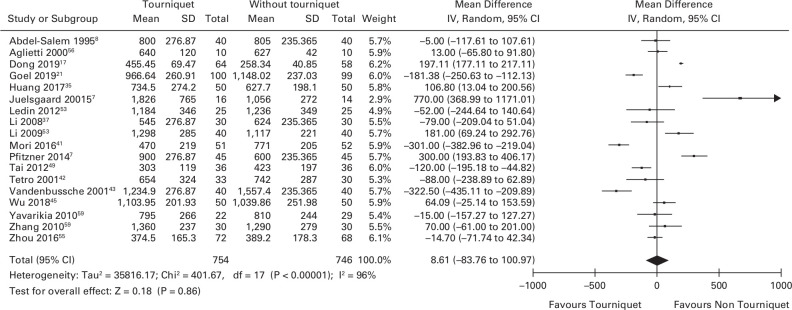
Blood loss
In total, 18 studies reported overall blood loss in the two treatment groups (n = 1,500).7,8,17,21,35,37,41-45,46,48,53,56–59-59 There was no difference in overall blood loss between patients who underwent knee arthroplasty surgery with and without a tourniquet. The mean difference was 8.61 ml (95% CI -83.76 to 100.97; Figure 6).
Fig. 6.
Forest plot demonstrating the overall blood loss in the surgery with a tourniquet group versus the surgery without a tourniquet group. CI, confidence interval; IV, inverse variance method; SD, standard deviation.
Duration of surgery
A total of 27 studies reported duration of surgery (n = 1,070).10,16,17,20,21,23,31,35-58–61,47,48,50,53,56,58-61 Surgery with a tourniquet was associated with a shorter length of surgery when compared to the group without a tourniquet. The mean reduction was 3.7 minutes (95% CI -5.53 to -1.87).
Length of hospital stay
Overall, 12 studies reported length of stay in patients undergoing knee arthroplasty surgery with and without a tourniquet (n = 995).8,31,35,42,43,45-53,60,53,60 Surgery with a tourniquet was associated with a longer hospital stay. The mean increase was 0.34 days (95% CI 0.03 to 0.64).
Implant stability
Two studies involving 130 patients assessed implant stability using radiostereometric analysis (RSA).18,31 There was no difference in implant maximum total point-motion between the two groups at eight weeks (mean difference (MD) -0.06 mm (95% CI -0.13 to 0.01)), 12 months (MD 0.05 mm (95% CI -0.09 to 0.18)), and 24 months (MD 0.06 mm (95% CI -0.08 to 0.19)).
GRADE assessment
The quality of evidence for all outcomes described in this abridged review were graded as moderate. These were downgraded by one level due to risk of bias. Many studies had unclear risk of allocation concealment and unclear risk of participant blinding. For further details on the Grading of Recommendations, Assessment, Development and Evaluation (GRADE; McMaster University/Evidence Prime, Canada) assessment please see ‘summary of findings table’ on the main Cochrane review.24
Publication bias
Publication bias was assessed using the aid of funnel plots for all major outcomes. Funnel plots were symmetrical for postoperative pain, function, and survival of the implant. Formal statistical tests were performed where more than ten trials were pooled (SAEs, blood loss, duration of surgery, and length of hospital stay). There were no statistically significant signs of publication bias for SAEs (p = 0.318), length of stay (p = 0.922), postoperative blood loss (p = 0.989) and overall blood loss (p = 0.178). There was evidence of publication bias for studies reporting intraoperative blood loss (p = 0.005) and duration of surgery (p = 0.014). Table II shows the results of publication bias testing.
Table II.
Demonstrating the probability of publication bias for outcomes which included more than 10 studies.
| Outcome | Bias estimate (SE) | p-value* |
|---|---|---|
| Serious adverse events | 0.567 (0.552) | 0.318 |
| Pain | 3.875 (2.168) | 0.097 |
| Intraoperative blood loss | -8.732 (2.596) | 0.005 |
| Overall blood loss | 5.585 (3.968) | 0.178 |
| Postoperative blood loss | -0.049 (3.420) | 0.989 |
| Transfusion rate | 0.47 (0.63) | 0.468 |
| Length of stay | 0.219 (2.182) | 0.922 |
| Duration of surgery | -2.947 (1.113) | 0.014 |
For continuous data, (pain, blood loss, length of stay, and duration of surgery) we tested asymmetry by using a weighted linear regression of the standardised mean against its standard error. For dichotomous data (serious adverse events and transfusion), we used a weighted linear regression based upon the odds ratio against its variance. In both cases, we considered a p-value below 0.05 as evidence that publication bias was present.
SE, standard error.
Discussion
This review of 41 RCTs is the largest of its kind to date and involves 2,819 participants. The findings demonstrate that tourniquet use is associated with increased risk of SAEs, postoperative pain, and longer hospital stay. The only finding in favour of tourniquets was a shorter time in theatre. The perceived benefit of tourniquet use is improved cementing and long-term survival; however, based on evidence from two included studies we found no difference in implant micromotion up to two years postoperatively (as a surrogate marker of longer-term implant survival). It is important to note that only three of the included studies in this review had a low risk of bias, with the remainder having some type of methodological bias. We found no good quality evidence to quantify the direct impact of tourniquet use on implant survival. Further registry-based studies or high-quality trials may answer this question.
The increase in the risk of SAEs (DVT, PE, infection, reoperation, and mortality) related to surgery (RR 1.73 (95% CI 1.10 to 2.73) and NNTH is 48 (95% CI 20 to 345)), which we found are likely to be highly clinically relevant. If our findings are representative, a change in practice to performing surgery without a tourniquet could approximately halve the risk of VTE. In 2018, 106,000 TKAs were performed in the UK.1,2 Based on estimates showing that over 90% of UK surgeons use a tourniquet3 and a NNTH of 48, a change in practice could potentially prevent around 2,000 SAEs per year in the UK alone. To put these findings further into context a Cochrane review reported that the effect of using antiembolic stockings to prevent postoperative DVT gave an odds ratio of 0.47 (95% CI 0.32 to 0.68).62
We acknowledge that the estimates of SAEs are based on a large number of trials with a low number of participants and events which can cause problems with precision estimates. However, in the absence of large multicentre trials or registry data, meta-analysis of multiple small trials may be the only way to obtain reliable evidence of an effect in rare but serious outcomes such as this.
The point estimate for mean difference in pain on the first postoperative day is (mean 1.25 higher on a VAS scale zero to 10 (95% CI 0.32 to 2.19)) when a tourniquet is used is above the published minimum clinically important difference (MCID) of 1.0.63 However, the confidence intervals for our estimate include a difference below the MCID threshold. Therefore, it is possible that the higher pain levels associated with a tourniquet may not be clinically relevant. Further data in the future will help to improve the precision of this estimate. It is important to also recognize that there is controversy and challenges in establishing an MCID for pain.64 As expected, the differences in pain were less on subsequent postoperative days as the effects of the intervention are reduced, and these levels were below the MCID. However, this may still be relevant as it is well established that an early rise in postoperative pain increases the risk of persistent chronic pain.65,66 Chronic pain remains a problem in a substantial portion of patients having TKA.65
There was no evidence of a difference in overall blood loss, function, or implant stability. Surgery with a tourniquet was associated with an increased length of hospital stay (mean increase 0.34 days (95% CI 0.03 to 0.64)) which may be clinically relevant to patients, surgeons, and healthcare providers. Over a year based on 106,000 TKAs performed per year, this would equate to 36,040 excess bed days. The cost of one excess bed day is £346, which could be extrapolated to an excess annual cost of £12,469,840 due to the use of a tourniquet.67 TKA with a tourniquet was associated with a reduced duration of surgery (-3.7 minutes (95% CI -5.53 to -1.87)). This equates to 6,537 hours less when a tourniquet is used compared to no tourniquet over one year. The cost of operating theatre time has been shown to cost £1,200 per hour.68 By using a tourniquet, this could save the NHS £7,844,000 per year in terms of operating time. When combining both economic outcomes, very crudely, the use of a tourniquet could potentially cost the NHS £4,625,840 per year.
There have been four previous systematic reviews between 2010 and 2014.12-14 Our findings are consistent with but add substantially to the most recent review in 2014 by Zhang et al.4 They reported on 13 RCTs involving 689 participants and showed no significant difference in overall blood loss but an increased risk of thrombotic events (RR 5.0 (95% CI 1.31 to 19.10)) and non-thrombotic complications (RR 2.03 (95% CI 1.12 to 3.67) in the surgery with a tourniquet group. Since completing this search, a recent RCT has been published which has similar findings to our review, including higher postoperative pain scores in the tourniquet group and reduced duration of surgery. This study also found that surgery with a tourniquet was associated with significantly lower patient reported knee function and range of motion at three weeks.69 If further trials are needed, they should focus on evaluating the risks of systemic emboli on cognitive function, and health-related quality of life. Ideally, prospective registry type data may facilitate more precision in estimating implant survival. Further research into the impact of tourniquet use in revision knee arthroplasty should also be considered in line with a recently established research priorities.70
Using a tourniquet during knee arthroplasty surgery is a practice that has largely been unchallenged until recently, with a focus on the benefits, but very little on the potential harms. The evidence presented from our data synthesis shows substantial risks and no major advantage to patients, which questions the routine use of a tourniquet in TKA.
Take home message
- The results demonstrate that the use of a tourniquet in total knee arthroplasty surgery is associated with increased risks to the patient, including serious adverse effects and increased levels of postoperative pain.
- There is no evidence to suggest any major advantage to the patient in the use of a tourniquet for these procedures.
- These findings suggest that the risks of tourniquet use should be strongly considered prior to their use.
Author contributions
I. Ahmed: Designed the study, Performed the search, Screened abstracts and full texts, Extracted and analyzed the data, Produced and revised the final manuscript.
A. Chawla: Performed the search, Screened the abstracts and full texts, Produced and revised the final manuscript.
M. Underwood: Conceptualized the study, Acted as senior author to resolve disagreements during the search, Produced and revised the final manuscript.
A. J. Price: Produced and revised the final manuscript.
A. Metcalfe: Produced and revised the final manuscript.
C. E. Hutchinson: Produced and revised final manuscript.
J. Warwick: Produced and revised the final manuscript.
K. Seers: Produced and revised the final manuscript.
H. Parsons: Data analysis, Produced and revised the final manuscript.
P. D. H. Wall: Conceived of the study, Performed the search, Screened the abstracts and full texts, Extracted and analyzed the data, Produced and revised the final manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.The review forms part of a larger project (SAFE-TKR Study) and is independent-research-funded by the National Institute for Health Research (NIHR) under a Post-Doctoral Fellowship Award (PDF-2015-08-108). The study is sponsored by the University of Warwick. The study funder and sponsor had no role in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit for publication. The researchers are independent and the views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Data sharing:
Data may be made available upon reasonable request.
Acknowledgements
We would like to acknowledge the Cochrane Musculoskeletal group who provided continuous advice and guidance to ensure the work was carried out the highest of standards. The Musculoskeletal group provided guidance throughout all stages of this review from conception through to completion of the full Cochrane review.
We would like to acknowledge the following members of the Safety and Feasibility Evaluation of Knee Replacement Surgery (SAFE-TKR) Study Group: Ms Bushra Rahman, Ms Jaclyn Brown, Mr James Smith, Mrs Christine Goulden, Mrs Jan Dixon, Dr Nele Demeyere and Professor J. M. Wilkinson.
We would also like to acknowledge the contribution of Andrew Sprowson, who died unexpectedly on 13 March 2015. Andrew was one of the main collaborators on this project and had made a significant contribution to the study design and in securing research funding. Andrew was an academic orthopaedic surgeon who was dedicated to improving evidence-based care in his field. He was an exceptionally enthusiastic researcher and surgeon and is greatly missed by his academic and clinical colleagues.
The full Cochrane review can be found via the following reference: Ahmed I, Chawla A, Underwood M, Price AJ, Metcalfe A, Hutchinson C, Warwick J, Seers K, Parsons H, Wall PD. Tourniquet use for knee replacement surgery. Cochrane Database Syst Rev. 2020 Dec 8;12:CD012874.
Ethical review statement
Patient members were involved at all stages of this review. Three patient members were part of the Safety and Feasibility Evaluation of Knee Replacement Surgery (SAFE-TKR) Study patient advisory group: Mrs Jan Dixon, Mrs Christine Goulden and Mr James Smith. Prior to starting the review, the aims and outcomes of interest were discussed with the group. The PPI group agreed that the outcomes studied were of substantial clinical importance. The results have also been discussed with this group and a plain language summary was produced. This summary is freely available as part of the main Cochrane review.
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
This article was primary edited by A. Wood and first proof edited by G. Scott.
Follow Warwick CTU @WarwickCTU
Follow R&D at UHCW @UHCW_RandD
Supplementary material
Results of search strategy and baseline characteristics of the included studies.
References
- 1.No authors listed . 16th Annual Report. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 2019. https://reports.njrcentre.org.uk/portals/0/pdfdownloads/njr%2016th%20annual%20report%202019.pdf (date last accessed 18 February 2021).
- 2.No authors listed . Scottish Arthroplasty Project Annual Report. Scottish arthroplasty project. 2019. https://www.arthro.scot.nhs.uk/Reports/Main.html?1
- 3.Gibbs V, Price A, Wall PDH, et al. Surgical tourniquet use in total knee replacement surgery: a survery of BASK members. Knee. 2016;23(4). [Google Scholar]
- 4.Zhang W, Li N, Chen S, Tan Y, Al-Aidaros M, Chen L. The effects of a tourniquet used in total knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2014;9(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.No authors listed . 1st Annual Report. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 2004. https://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/NJR_AR_1.pdf (date last accessed 18 February 2021).
- 6.No authors listed . Annual report. Swedish Knee Arthroplasty Register. 2012. http://myknee.se/pdf/117_SKAR_2012_Engl_1.0.pdf (date last accessed 18 February 2021).
- 7.Pfitzner T, von Roth P, Voerkelius N, et al. Influence of the tourniquet on tibial cement mantle thickness in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):96–101. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77-B(2):250–253. [PubMed] [Google Scholar]
- 9.Shahi A, Bradbury TL, Guild GN, Saleh UH, Ghanem E, Oliashirazi A. What are the incidence and risk factors of in-hospital mortality after venous thromboembolism events in total hip and knee arthroplasty patients? Arthroplast Today. 2018;4(3):343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wauke K, Nagashima M, Kato N, Ogawa R, Yoshino S. Comparative study between thromboembolism and total knee arthroplasty with or without tourniquet in rheumatoid arthritis patients. Arch Orthop Trauma Surg. 2002;122(8):442–446. [DOI] [PubMed] [Google Scholar]
- 11.Deo H, West G, Butcher C, Lewis P. The prevalence of cognitive dysfunction after conventional and computer-assisted total knee replacement. Knee. 2011;18(2):117–120. [DOI] [PubMed] [Google Scholar]
- 12.Alcelik I, Pollock RD, Sukeik M, Bettany-Saltikov J, Armstrong PM, Fismer P. A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27(3):331–340. [DOI] [PubMed] [Google Scholar]
- 13.Smith TO, Hing CB. Is a tourniquet beneficial in total knee replacement surgery? A meta-analysis and systematic review. Knee. 2010;17(2):141–147. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Yin L, Chen Z-Y, et al. The effect of tourniquet use in total knee arthroplasty: grading the evidence through an updated meta-analysis of randomized, controlled trials. Eur J Orthop Surg Traumatol. 2014;24(6):973–986. [DOI] [PubMed] [Google Scholar]
- 15.Alexandersson M, Wang EY, Eriksson S. A small difference in recovery between total knee arthroplasty with and without tourniquet use the first 3 months after surgery: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc. 2019;27(4):1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayik O, Demirel M, Birisik F, et al. The effects of tourniquet application in total knee arthroplasty on the recovery of thigh muscle strength and clinical outcomes. J Knee Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 17.Dong J, Min S, He K-H, Peng L-H, Cao J, Ran W. Effects of the nontourniquet combined with controlled hypotension technique on pain and long-term prognosis in elderly patients after total knee arthroplasty: a randomized controlled study. J Anesth. 2019;33(5):587–593. [DOI] [PubMed] [Google Scholar]
- 18.Ejaz A, Laursen AC, Jakobsen T, et al. Absence of a tourniquet does not affect fixation of Cemented TKA: a randomized RSA study of 70 patients. J Arthroplasty. 2015;30(12):2128–2132. [DOI] [PubMed] [Google Scholar]
- 19.Ejaz A, Laursen AC, Kappel A, et al. Faster recovery without the use of a tourniquet in total knee arthroplasty. Acta Orthop. 2014;85(4):422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ejaz A, Laursen AC, Kappel A, et al. Tourniquet induced ischemia and changes in metabolism during TKA: a randomized study using microdialysis. BMC Musculoskelet Disord. 2015;16(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel R, Rondon AJ, Sydnor K, et al. Tourniquet use does not affect functional outcomes or pain after total knee arthroplasty: a prospective, double-blinded, randomized controlled trial. J Bone Joint Surg Am. 2019;101-A(20):1821–1828. [DOI] [PubMed] [Google Scholar]
- 22.Jawhar A, Skeirek D, Stetzelberger V, Kollowa K, Obertacke U. No effect of tourniquet in primary total knee arthroplasty on muscle strength, functional outcome, patient satisfaction and health status: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1045–1054. [DOI] [PubMed] [Google Scholar]
- 23.Jawhar A, Hermanns S, Ponelies N, Obertacke U, Roehl H. Tourniquet-Induced ischaemia during total knee arthroplasty results in higher proteolytic activities within vastus medialis cells: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3313–3321. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed I, Chawla A, Underwood M, et al. Tourniquet use for knee replacement surgery. Cochrane Database Syst Rev. 2017;77(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 27.Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80-B(1):63–69. [DOI] [PubMed] [Google Scholar]
- 28.Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89-B(8):1010–1014. [DOI] [PubMed] [Google Scholar]
- 29.Windsor RE, Insall JN, Warren RF. The Hospital for Special Surgery Knee Ligament Rating Form. Am J Knee Surg. 1988;1:140–145. [Google Scholar]
- 30.Lee WC, Kwan YH, Chong HC, Yeo SJ. The minimal clinically important difference for knee Society clinical rating system after total knee arthroplasty for primary osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2017;25(11):3354–3359. [DOI] [PubMed] [Google Scholar]
- 31.Molt M, Harsten A, Toksvig-Larsen S. The effect of tourniquet use on fixation quality in cemented total knee arthroplasty a prospective randomized clinical controlled RSA trial. Knee. 2014;21(2):396–401. [DOI] [PubMed] [Google Scholar]
- 32.Pijls BG, Plevier JWM, Nelissen RGHH. Rsa migration of total knee replacements. Acta Orthop. 2018;89(3):320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Green S. Cochrane Handbook for systematic reviews of interventions. 2011. http://www.cochrane-handbook.org.march/
- 34.Higgins JPT, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Welch VA. eds. Cochrane Handbook for systematic reviews of interventions, 2020. [Google Scholar]
- 35.Huang Z, Xie X, Li L, et al. Intravenous and topical tranexamic acid alone are superior to tourniquet use for primary total knee arthroplasty: a prospective, randomized controlled trial. J Bone Joint Surg Am. 2017;99-A(24):2053–2061. [DOI] [PubMed] [Google Scholar]
- 36.Kato N, Nakanishi K, Yoshino S, Ogawa R. Abnormal echogenic findings detected by transesophageal echocardiography and cardiorespiratory impairment during total knee arthroplasty with tourniquet. Anesthesiology. 2002;97(5):1123–1128. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Qian Q-rong, Wu H-shan, et al. The use of a pneumatic tourniquet in total knee arthroplasty: a prospective, randomized study. Zhonghua Wai Ke Za Zhi. 2008;46(14):1054–1057. Article in Chinese [PubMed] [Google Scholar]
- 38.Liu P-L, Li D-Q, Zhang Y-K, et al. Effects of unilateral tourniquet used in patients undergoing simultaneous bilateral total knee arthroplasty. Orthop Surg. 2017;9(2):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu P, DQ L, Zhang YK, et al. Influence of tourniquet on wound healing in total knee arthroplasty: a randomized and paired clinical trial. Int J Clin Exp Med. 2017;10(2):3653–3660. [Google Scholar]
- 40.Matziolis G, Drahn T, Schröder JH, Krocker D, Tuischer J, Perka C. Endothelin-1 is secreted after total knee arthroplasty regardless of the use of a tourniquet. J Orthop Res. 2005;23(2):392–396. [DOI] [PubMed] [Google Scholar]
- 41.Mori N, Kimura S, Onodera T, et al. Use of a pneumatic tourniquet in total knee arthroplasty increases the risk of distal deep vein thrombosis: a prospective, randomized study. Knee. 2016;23(5):887–889. [DOI] [PubMed] [Google Scholar]
- 42.Tetro AM, Rudan JF. The effects of a pneumatic tourniquet on blood loss in total knee arthroplasty. Can J Surg. 2001;44(1):33–38. [PMC free article] [PubMed] [Google Scholar]
- 43.Vandenbussche E, Duranthon L-D, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 1999;81-B(1):30–33. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Lu X, Ma Y, et al. Efficacy and safety of limb position on blood loss and range of motion after total knee arthroplasty without tourniquet: a randomized clinical trial. Int J Surg. 2018;60:182–187. [DOI] [PubMed] [Google Scholar]
- 46.Zhang F-J, Xiao Y, Liu Y-B, Tian X, Gao Z-G. Clinical effects of applying a tourniquet in total knee arthroplasty on blood loss. Chin Med J. 2010;123(21):3030–3033. [PubMed] [Google Scholar]
- 47.Zhang Q, Dong J, Gong K, et al. Effects of tourniquet use on perioperative outcome in total knee arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016;30(4):421–425. Article in Chinese [PubMed] [Google Scholar]
- 48.Liu D-H, G-T M, Gong Y, Liu J-S, Zhou W. Relationship between pneumatic tourniuqet application in total knee arthroplasty and hypercoagulability. Journal of Clinical Rehabilitative Tissue Engineering Research. 2011;15(9):1541–1543. [Google Scholar]
- 49.Tai T-W, Chang C-W, Lai K-A, Lin C-J, Yang C-Y. Effects of tourniquet use on blood loss and soft-tissue damage in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94-A(24):2209–2215. [DOI] [PubMed] [Google Scholar]
- 50.Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar N, Yadav C, Singh S, et al. Evaluation of pain in bilateral total knee replacement with and without tourniquet; a prospective randomized control trial. J Clin Orthop Trauma. 2015;6(2):85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B, Qian Q, Wu H, et al. The use of a pneumatic tourniquet in total knee arthroplasty: a prospective, randomised study. Chinese Journal of Surgery. 2008;46(14):1054–1057. [PubMed] [Google Scholar]
- 53.Ledin H, Aspenberg P, Good L. Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion. Acta Orthop. 2012;83(5):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozkunt O, Sariyilmaz K, Gemalmaz HC, Dikici F. The effect of tourniquet usage on cement penetration in total knee arthroplasty: a prospective randomized study of 3 methods. Medicine. 2018;97(4):e9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou K, Ling T, Wang H, et al. Influence of tourniquet use in primary total knee arthroplasty with drainage: a prospective randomised controlled trial. J Orthop Surg Res. 2017;12(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aglietti P, Baldini A, Vena LM, Abbate R, Fedi S, Falciani M. Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res. 2000;371:169–177. [DOI] [PubMed] [Google Scholar]
- 57.Juelsgaard P, Larsen UT, Sørensen JV, Madsen F, Søballe K. Hypotensive epidural anesthesia in total knee replacement without tourniquet: reduced blood loss and transfusion. Reg Anesth Pain Med. 2001;26(2):105–110. [DOI] [PubMed] [Google Scholar]
- 58.Li B, Wen Y, Wu H, et al. The effect of tourniquet use on hidden blood loss in total knee arthroplasty. Int Orthop. 2009;33(5):1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yavarikia A, Amjad GG, Davoudpour K. The influence of tourniquet use and timing of its release on blood loss in total knee arthroplasty. Pak J Biol Sci. 2010;13(5):249–252. [DOI] [PubMed] [Google Scholar]
- 60.Harsten A, Bandholm T, Kehlet H, Toksvig-Larsen S. Tourniquet versus no tourniquet on knee-extension strength early after fast-track total knee arthroplasty; a randomized controlled trial. Knee. 2015;22(2):126–130. [DOI] [PubMed] [Google Scholar]
- 61.Kiss H, Raffl M, Neumann D, Hutter J, Dorn U. Epinephrine-augmented hypotensive epidural anesthesia replaces tourniquet use in total knee replacement. Clin Orthop Relat Res. 2005;436:184–189. [DOI] [PubMed] [Google Scholar]
- 62.Sachdeva A, Dalton M, Amaragiri SV, et al. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. 2010;7:Cd001484. [DOI] [PubMed] [Google Scholar]
- 63.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. [DOI] [PubMed] [Google Scholar]
- 64.Olsen MF, Bjerre E, Hansen MD, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017;15(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wylde V, Beswick AD, Dennis J, Gooberman-Hill R. Post-Operative patient-related risk factors for chronic pain after total knee replacement: a systematic review. BMJ Open. 2017;7(11):e018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wylde V, Lenguerrand E, Gooberman-Hill R, et al. Effect of local anaesthetic infiltration on chronic postsurgical pain after total hip and knee replacement: the apex randomised controlled trials. Pain. 2015;156(6):1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.NHS Improvement . Reference costs 2017/18: highlights, analysis and introduction to the data. London, UK: NHS Improvement, 2018. [Google Scholar]
- 68.Fletcher D, Edwards D, Tolchard S, Baker R, Berstock J. Improving theatre turnaround time. BMJ Qual Improv Rep. 2017;6(1):u219831.w8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao H-Y, Yeersheng R, Kang X-W, et al. The effect of tourniquet uses on total blood loss, early function, and pain after primary total knee arthroplasty: a prospective, randomized controlled trial. Bone Joint Res. 2020;9(6):322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathews JA, Kalson NS, Tarrant PM, Toms AD, Revision Knee Replacement Priority Setting Partnership steering group . Top ten research priorities for problematic knee arthroplasty. Bone Joint J. 2020;102-B(9):1176–1182. [DOI] [PubMed] [Google Scholar]