Linezolid is one of the few antimicrobials available to treat severe infections due to drug-resistant Gram-positive bacteria; therefore, the emergence of linezolid-resistant enterococci carrying transferable resistance determinants is of great concern for public health. Linezolid resistance genes (cfr, optrA, and poxtA), often plasmid located, can be transmitted via horizontal gene transfer and have the potential to spread globally.
KEYWORDS: conjugative plasmid, Enterococcus faecalis, Enterococcus faecium, Enterococcus hirae, linezolid resistance, marine sediment, marine zooplankton, optrA, poxtA
ABSTRACT
Linezolid is a last-resort antibiotic for the treatment of severe infections caused by multidrug-resistant Gram-positive organisms; although linezolid resistance remains uncommon, the number of linezolid-resistant enterococci has increased in recent years due to worldwide spread of acquired resistance genes (cfr, optrA, and poxtA) in clinical, animal, and environmental settings. In this study, we investigated the occurrence of linezolid-resistant enterococci in marine samples from two coastal areas in Italy. Isolates grown on florfenicol-supplemented Slanetz-Bartley agar plates were investigated for their carriage of optrA, poxtA, and cfr genes; optrA was found in one Enterococcus faecalis isolate, poxtA was found in three Enterococcus faecium isolates and two Enterococcus hirae isolates, and cfr was not found. Two of the three poxtA-carrying E. faecium isolates and the two E. hirae isolates showed related pulsed-field gel electrophoresis (PFGE) profiles. Two E. faecium isolates belonged to the new sequence type 1710, which clustered in clonal complex 94, encompassing nosocomial strains. S1 PFGE/hybridization assays showed a double (chromosome and plasmid) location of poxtA and a plasmid location of optrA. Whole-genome sequencing revealed that poxtA was contained in a Tn6657-like element carried by two plasmids (pEfm-EF3 and pEh-GE2) of similar size, found in different species, and that poxtA was flanked by two copies of IS1216 in both plasmids. In mating experiments, all but one strain (E. faecalis EN3) were able to transfer the poxtA gene to E. faecium 64/3. The occurrence of linezolid resistance genes in enterococci from marine samples is of great concern and highlights the need to improve practices aimed at limiting the transmission of linezolid-resistant strains to humans from environmental reservoirs.
IMPORTANCE Linezolid is one of the few antimicrobials available to treat severe infections due to drug-resistant Gram-positive bacteria; therefore, the emergence of linezolid-resistant enterococci carrying transferable resistance determinants is of great concern for public health. Linezolid resistance genes (cfr, optrA, and poxtA), often plasmid located, can be transmitted via horizontal gene transfer and have the potential to spread globally. This study highlights the detection of enterococci carrying linezolid resistance genes from sediment and zooplankton samples from two coastal urban areas in Italy. The presence of clinically relevant resistant bacteria, such as linezolid-resistant enterococci, in marine environments could reflect their spillover from human and/or animal reservoirs and could indicate that coastal seawaters also might represent a source of these resistance genes.
INTRODUCTION
The anthropogenic release of antibiotics into the environment, due to their intensive use in human and veterinary medicine and in agriculture, has raised global public health concerns. The complex microbial communities of aquatic environments also include transient bacteria from different sources, such as hospital, domestic, and animal breeding effluents (1, 2). Antibiotic pollution imposes a selective pressure on bacterial populations, which can facilitate the development and spread of antibiotic resistances through horizontal gene transfer (HGT). The evidence for horizontal dissemination of antibiotic resistances between environmental bacteria and human pathogens demonstrates the importance of environmental resistomes.
Enterococci are members of gut microbiota of humans and animals. They are released in large amounts into the environment with feces and thus can be found in different niches, including soil, foods of animal origin, vegetables, and water. Enterococcus spp. have been well established as fecal indicators for routine monitoring of water quality, and this principle has been extended to foods (3). More recently, enterococci have been also proposed for monitoring of antibiotic resistance in food animals (4).
Although regarded as commensals, Enterococcus spp. are the leading causes of nosocomial infections worldwide (5). Acquired resistances are growing and considerably limit the therapeutic options; oxazolidinones are among the few available last-resort antibiotics recommended to treat severe infections caused by vancomycin-resistant enterococci and multidrug-resistant enterococci (6).
Oxazolidinones (linezolid and tedizolid) bind in the V domain of the 23S rRNA of the 50S ribosomal subunit and inhibit protein synthesis (7). Besides the mutations in 23S rRNA and/or in L3, L4, and L22 ribosomal proteins (6, 8), linezolid resistance can develop following acquisition of the resistance gene cfr and its variants, optrA, and poxtA. Cfr and Cfr-like methylases confer resistance to five classes of antimicrobial agents, including phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A (PhLOPSA phenotype), by posttranscriptional methylation of the 23S rRNA (9–13). The ATP-binding cassette (ABC)-F proteins OptrA and PoxtA lead to decreased susceptibility to phenicols, oxazolidinones (including tedizolid), and tetracyclines (PoxtA protein only) by a ribosomal protection mechanism (14–16). In enterococci, linezolid resistance genes are often carried by mobile genetic elements and are easily transferred between bacteria by HGT (14, 17–20).
Enterococci spread in many natural habitats and, besides the occurrence of linezolid-resistant enterococci in hospitals, their detection in other reservoirs is of special concern (21). The purpose of this study was to investigate the occurrence of linezolid resistance genes in enterococci isolated from marine samples collected in two coastal urban areas in Italy. To our knowledge, there have been no previous reports of linezolid resistance genes in enterococci from a marine environment.
RESULTS AND DISCUSSION
Detection of oxazolidinone resistance genes in florfenicol-resistant enterococci and antimicrobial susceptibility profiles.
Of 77 total samples (33 seawater samples, 33 sediment samples, and 11 zooplankton samples), only 10 sediment and 1 zooplankton samples from six sampling sites (Fig. 1) were positive for the presence of florfenicol-resistant enterococci. Thirty-five isolates were found to be positive for poxtA or optrA; however, only six different pulsotypes (one for each site) were detected by a SmaI pulsed-field gel electrophoresis (PFGE) assay. The six isolates (one Enterococcus faecalis isolate, three Enterococcus faecium isolates, and two Enterococcus hirae isolates) were then characterized (Table 1). The optrA gene was detected only in the E. faecalis isolate, poxtA was identified in the three E. faecium isolates and the two E. hirae isolates, and cfr was not found (Table 1).
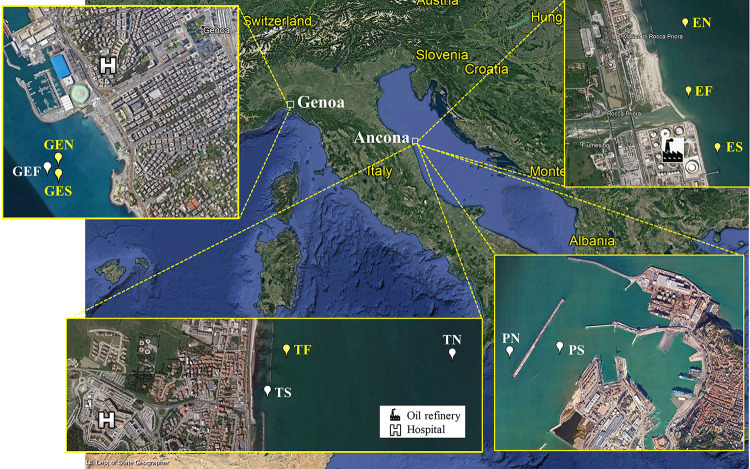
FIG 1.
Maps of two sampling areas located in the Ligurian Sea and the Adriatic Sea. The yellow pins indicate sites where florfenicol-resistant enterococci were isolated, and the white pins indicate sites where no florfenicol-resistant strains were recovered. Geographic coordinates and depth of sampling sites were as follows: GEN, 44°23′25.26″N, 8°56′40.56″E, 13.4 m; GES, 44°23′22.06″N, 8°56′44.59″E, 16.1 m; GEF, 44°23′21.48″N, 8°56′39.77″E, 16.2 m; EN, 43°38′51.06″N, 13°22′6.66″E, 4 m; EF, 43°38′41.16″N, 13°22′22.74″E, 3 m; ES, 43°38′37.20″N, 13°22′41.46″E, 3.4 m; TF, 43°36′45.96″N, 13°27′12.36″E, 3 m; PN, 43°37′21.30″N, 13°29′2.10″E, 8.8 m; PS, 43°37′22.78″N, 13°29′26.16″E, 7 m; TN, 43°37′17.94″N, 13°27′26.64″E, 7.6 m; TS, 43°36′40.02″N, 13°27′22.08″E, 2.4 m.
TABLE 1.
Linezolid resistance genes, antimicrobial susceptibility profiles, typing data, and gene locations
| Strain | Species | Sampling site | Sample | Oxazolidinone resistance genes |
MIC (mg/liter) of:a |
Typing results |
Estimated plasmid size(s) (kb) from S1 PFGE and hybridizationb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| optrA | cfr | poxtA | FFC | CHL | LZD | TZD | TE | VAN | SmaI PFGE | MLST | optrA | poxtA | ||||
| EN3 | E. faecalis | EN | Sediment | + | − | − | 128 | 128 | 4 | 4 | 128 | 1 | ST585 | 20, 140 | ||
| EF3 | E. faecium | EF | Sediment | − | − | + | 64 | 16 | 8 | 2 | 128 | 1 | C | ST1710 | 30, c | |
| ES2 | E. faecium | ES | Sediment | − | − | + | 32 | 16 | 8 | 2 | 128 | 1 | C1 | ST1710 | 15, 30, c | |
| TF3 | E. faecium | TF | Sediment | − | − | + | 64 | 32 | 2 | 2 | >128 | 0.5 | B | ST1711 | 30, 50, 80, c | |
| GE5 | E. hirae | GEN | Sediment | − | − | + | 64 | 64 | 4 | 2 | 128 | 0.5 | A1 | 25, 100 | ||
| GE2 | E. hirae | GES | Zooplankton | − | − | + | 64 | 64 | 8 | 3 | 128 | 0.5 | A | 25, 100 | ||
FFC, florfenicol; CHL, chloramphenicol; LZD, linezolid; TZD, tedizolid; TE, tetracycline; VAN, vancomycin. MIC resistance breakpoints (EUCAST or CLSI) were as follows: florfenicol, not applicable; chloramphenicol, resistant at ≥32mg/liter; linezolid, resistant at >4 mg/liter; tedizolid, susceptible at ≤0.5 mg/liter (only for E. faecalis); tetracycline, resistant at ≥16 mg/liter; vancomycin, resistant at >4 mg/liter.
c, chromosome.
The poxtA gene, which was first described in a methicillin-resistant Staphylococcus aureus (MRSA) strain from a patient with cystic fibrosis (15), was shortly thereafter reported in enterococci isolated from many different nonhuman sources, e.g., pigs and chickens (22–24) and retail meat and food-producing animals (25), as well as from air samples from a swine farm (26). Through a metagenomic approach, this gene was recently detected in livestock manure (27) and even in the microbiome of drinking water in environmental and clinical settings (28). The widespread dissemination of poxtA in nonhuman enterococci, mainly E. faecium strains, suggested that selection of this gene could occur in animal settings owing to extensive use of phenicols and doxycycline in veterinary medicine (29); poxtA-carrying strains could then reach bodies of water, including coastal waters, through manure contamination and runoff from husbandry and agricultural activities. In addition, poxtA has been increasingly reported in clinical isolates (30, 31), confirming its diffusion in human settings.
The six enterococcal isolates were all resistant to florfenicol (MIC range, 32 to 128 mg/liter), chloramphenicol (MIC range, 16 to 128 mg/liter), and tetracycline (MIC range, 128 to >128 mg/liter) and were either susceptible or resistant to linezolid (MIC range, 2 to 8 mg/liter) and tedizolid (MIC range, 2 to 4 mg/liter). All tested strains were susceptible to vancomycin (MIC range, 0.5 to 1 mg/liter) (Table 1).
Typing assays.
The enterococcal isolates belonged to three different SmaI PFGE types (A to C) and two subtypes (A1 and C1) (Table 1). E. faecium EF3 and ES2 were found to be closely related (type C and subtype C1, respectively), as were E. hirae GE5 (from marine sediment) and E. hirae GE2 (from zooplankton) (subtype A1 and type A, respectively). E. faecalis EN3 belonged to sequence type 585 (ST585), which has been associated with human enterococci (32–36). E. faecium EF3 and ES2 belonged to the same ST (ST1710), while E. faecium TF3 belonged to ST1711. Although neither ST has been described before, ST1710 clustered in clonal complex 94 (CC94), which encompasses human intestinal enterococci recovered from both community and hospitalized hosts (37). The proximity of our sampling sites to hospital and urban areas could suggest the spread in the environment of human strains carrying linezolid resistance genes.
Location of oxazolidinone resistance genes and detection of circular forms.
In E. faecalis EN3, the optrA gene was located on two plasmids of ∼20 kb and ∼140 kb; in the three poxtA-carrying E. faecium isolates, hybridization occurred on both the chromosome and plasmids. The poxtA gene was located on plasmids of different sizes, i.e., ∼30 kb in E. faecium EF3, ∼15 and ∼30 kb in E. faecium ES2, and ∼30, ∼50, and ∼80 kb in E. faecium TF3. In the closely related poxtA-carrying E. hirae GE5 and E. hirae GE2, only plasmid localization of the poxtA gene was detected. In both isolates, the poxtA probe hybridized on two plasmids of ∼25 and ∼100 kb (Table 1). Inverse PCR experiments and sequencing showed that no circular form of optrA genetic context was detectable. In contrast, minicircles were obtained from all poxtA genetic contexts.
Since optrA is located on plasmids of different sizes (∼20 kb and ∼140 kb) and whole-genome sequencing (WGS) revealed a single optrA genetic context with no evidences of circularization, it is reasonable to assume that recombination events between plasmids occurred (38). As regards poxtA, its location on plasmids of different sizes and even on the chromosome suggests intracellular mobility of the poxtA-carrying element due to insertion sequence (IS)-mediated recombination events.
Transferability of oxazolidinone resistance genes.
Five of six isolates successfully transferred the linezolid resistance genes in intraspecies and interspecies mating experiments, with frequencies ranging from 6.5 × 10−1 to 3 × 10−6 CFU per recipient. MICs and genotypes for both donors and selected transconjugants and transfer frequencies are indicated in Table 2. The higher frequencies were observed in intraspecies transfer of poxtA from E. faecium ES2 and E. faecium TF3 donors to the E. faecium 64/3 recipient. Conversely, E. hirae GE2 and GE5 successfully transferred poxtA to the E. faecium recipient. In E. faecium and E. hirae transconjugants, the poxtA gene was located on plasmids of ∼30 kb and ∼25 kb, respectively, and on the chromosome (Table 2). Despite several attempts, E. faecalis EN3 was not able to transfer the optrA gene to the E. faecium 64/3 recipient. The interspecies transfer of the resistance genes from E. hirae to E. faecium is worrisome since the former species is more common in animals for which phenicols and tetracyclines are widely used and thus it could be a reservoir of linezolid resistance genes for more pathogenic species such as E. faecium.
TABLE 2.
Florfenicol and linezolid MICs, resistance genotypes, and gene locations for relevant transconjugants
| Donor |
Recipient |
Transconjugant |
Estimated plasmid size(s) (kb) from S1 PFGE and hybridizationc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | MIC (mg/liter) of:a |
LZD resistance genotype | Strainb | Transfer frequency | MIC (mg/liter) of: |
LZD resistance genotype | |||
| FFC | LZD | FFC | LZD | ||||||
| E. faecalis EN3 | 128 | 4 | optrA | E. faecium 64/3 | NDd | ||||
| E. faecium EF3 | 64 | 8 | poxtA | E. faecium 64/3 | 5 × 10−5 | 64 | 4 | poxtA | 30, c |
| E. faecium ES2 | 32 | 8 | poxtA | E. faecium 64/3 | 6.5 × 10−1 | 64 | 4 | poxtA | 30, c |
| E. faecium TF3 | 64 | 2 | poxtA | E. faecium 64/3 | 1.1 × 10−1 | 32 | 2 | poxtA | 30, c |
| E. hirae GE5 | 64 | 4 | poxtA | E. faecium 64/3 | 7.5 × 10−5 | 64 | 4 | poxtA | 25, c |
| E. hirae GE2 | 64 | 8 | poxtA | E. faecium 64/3 | 3 × 10−6 | 64 | 4 | poxtA | 25, c |
FFC, florfenicol; LZD, linezolid.
The florfenicol and linezolid MICs for E. faecium 64/3 were 4 mg/liter and 1 mg/liter, respectively.
c, chromosome.
ND, not detectable.
WGS analysis.
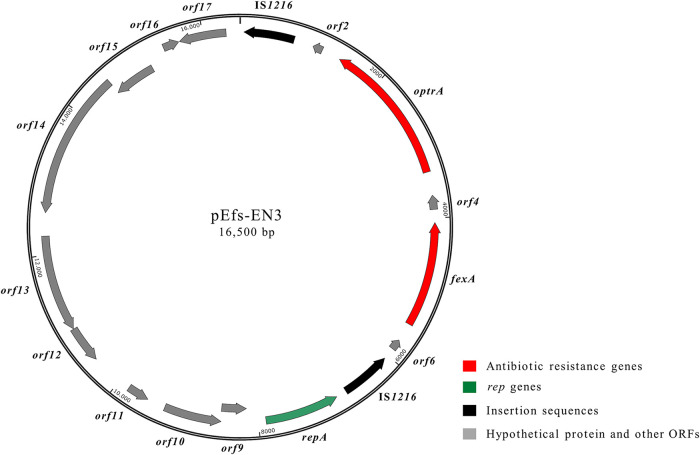
All six test strains were subjected to WGS analysis. The maps of the plasmids are shown in Fig. 2 to 4. Bioinformatic analysis of the draft genome of E. faecalis EN3, coupled with PCR mapping and Sanger sequencing experiments, revealed that the optrA gene was part of a 16,500-bp plasmid named pEfs-EN3 (G+C content, 33.0%) (GenBank accession number MT683614), according to the nomenclature of optrA variants reported by Morroni et al. (39). E. faecalis EN3 showed the optrA DP variant, which has been described in different E. faecalis clones from humans and pigs (40). The optrA genetic environment (6,810 bp), bounded by two IS1216 elements arranged in the same orientation, also contained the fexA gene located 687 bp upstream of optrA (Fig. 2). A similar organization was previously described in plasmids of E. faecalis isolates from dogs in China (41). The repA, parA, and prgN genes (orf8, orf10, and orf11, respectively) responsible for plasmid replication and partitioning were also detected. The plasmid pEfs-EN3 belonged to the RepA_N family and showed a rep9 type, both of which are typical features of E. faecalis sex pheromone-responsive plasmids (42). Interestingly, pheromone-responsive conjugative optrA-carrying plasmids have been identified in E. faecalis strains of swine origin (43).
FIG 2.
Schematic representation of the optrA-carrying pEfs-EN3 plasmid (16,500 bp) from E. faecalis EN3 (accession number MT683614). Arrows indicate the positions and directions of transcription of the different genes.
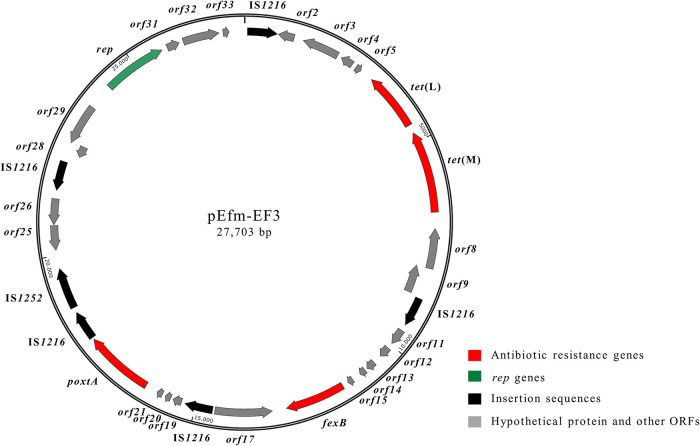
FIG 3.
Schematic representation of the poxtA-carrying pEfm-EF3 plasmid (27,703 bp) from E. faecium EF3 (accession number MT683615). Arrows indicate the positions and directions of transcription of the different genes.
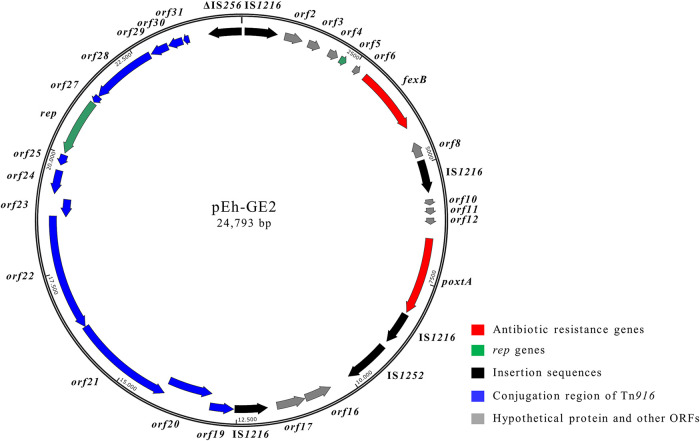
FIG 4.
Schematic representation of the poxtA-carrying pEh-GE2 plasmid (24,793 bp) from E. hirae GE2 (accession number MT683616). Arrows indicate the positions and directions of transcription of the different genes.
Since the hybridization assays suggested that a poxtA-carrying plasmid of ∼30 kb was shared by the E. faecium isolates, we decided to proceed with its assembly. In E. faecium EF3, the poxtA gene was located on a 27,703-bp plasmid designated pEfm-EF3 (G+C content, 35.0%) (GenBank accession number MT683615). The genetic context of poxtA (4,003 bp), flanked by two IS1216 elements in the same orientation, was in turn inserted in a Tn6657-like transposon also containing fexB, as originally described in the MRSA strain AOUC-0915 (GenBank accession number MH746818) (20). Upstream of the Tn6657-like transposon, a tetracycline resistance region containing tet(L) and tet(M) genes arranged in tandem was found; downstream of the Tn6657-like transposon, four genes (orf28 to orf31) involved in plasmid partitioning and replication were detected (Fig. 3). pEfm-EF3 exhibited 99% DNA identity (coverage, 100%) with regions of pC25-1 and pC27-2, two broad-host-range Inc18 plasmids from a CC17 E. faecium strain of pig origin from China (GenBank accession numbers MH784601 and MH784602, respectively) (44).
In E. faecium ES2 and E. faecium TF3, poxtA-carrying plasmids identical to pEfm-EF3 were found. It is noteworthy that the three E. faecium isolates were collected from different sampling sites (Table 1). Furthermore, the closely related E. faecium EF3 and E. faecium ES2 belonged to ST1710, while E. faecium TF3 was assigned to ST1711, suggesting spread of pEfm-EF3 by HGT may occur among isolates with different backgrounds.
WGS analysis of E. hirae GE2 revealed that the poxtA gene was located on a 24,793-bp plasmid named pEh-GE2 (G+C content, 38.0%) (GenBank accession number MT683616). BLASTN analysis revealed that in pEh-GE2 two regions exhibited high levels of DNA identity with different genetic elements. The 12.8-kb region containing the poxtA genetic context (orf1 to orf18) showed a high level of DNA identity (99%) with a Tn6657-like transposon (20). As observed in pEfm-EN3, the poxtA genetic context was bracketed by IS1216 elements in the same orientation (Fig. 4). The pEh-GE2 region spanning orf19 to orf31 (14.7 kb) and carrying the Tn916 conjugation region (including the rep gene) showed 99% DNA identity with plasmid 3 of E. faecium E4457 (GenBank accession number LR135260) (Fig. 4).
WGS analysis of E. hirae GE5 revealed a poxtA-carrying plasmid with complete synteny to pEh-GE2, despite the two strains coming from different sampling sites and samples (sediment and zooplankton, respectively) (Table 1). Interestingly, the poxtA-carrying plasmids of E. hirae and E. faecium isolates shared only the Tn6657-like region (coverage, 55%; DNA identity, 99%), suggesting the widespread dissemination of this element in enterococci. The pEh-GE2 belonged to the Rep_trans family, which includes small plasmids largely spread among enterococcal populations (42). Hybridization analysis also showed the presence of an optrA-carrying plasmid (∼140 kb) in E. faecalis EN3 and a poxtA plasmid (∼100 kb) in E. hirae GE2 and GE5 isolates that were not assembled. WGS analysis also ruled out the presence of cfr(B), cfr(C), cfr(D), and cfr(E) genes. No mutations were detected in the genes encoding the 23S rRNA or ribosomal proteins.
Conclusions.
The emergence of linezolid-resistant enterococci due to transferable resistance determinants is a matter of concern worldwide. To the best of our knowledge, there have been no previous reports of the detection of enterococci carrying linezolid resistance genes in marine sediment and zooplankton. The evidence that the coastal seawaters also could serve as a reservoir of oxazolidinone resistance genes is of great concern for public health. Further surveillance and control efforts are needed to counteract the spread of linezolid-resistant bacteria in human and animal settings to prevent the formation of environmental reservoirs of resistance genes that are transmissible to humans via different routes, including bathing, aquaculture, and seafood consumption.
MATERIALS AND METHODS
Sampling sites, sample processing, and bacterial isolation.
Sampling activities were carried out at 11 sites in two areas located on the western (Ligurian Sea) and eastern (Adriatic Sea) coasts of Italy, in a framework of a research project aimed at the detection of antibiotic-resistant bacteria from the marine environment (N. Cedraro, S. Simoni, C. Vignaroli, L. Vezzulli, and F. Biavasco, unpublished results). Sampling sites located in the Ligurian Sea (n = 3) were in front of the harbor and the hospital of Genoa city (GEN, GES, and GEF), whereas sampling sites in the Adriatic Sea (n = 8) were in front of an urban area close to the river Esino estuary and to an oil refinery (ESN, ESS, and ESF) and in front of the hospital (TN, TS, and TF) and the harbor of Ancona city (PN and PS) (Fig. 1). At each site, seven samples (seawater, n = 3; sediment, n = 3; zooplankton, n = 1) were collected in July 2019.
All 77 samples were incubated overnight at 37°C in azide broth (Oxoid, Basingstoke, UK) for the selective enrichment of enterococci. Sediment samples (5 g) were immediately added to the enrichment broth, whereas seawater and zooplankton samples were processed as follows. Seawater samples (400 ml) were filtered through 0.22-μm filter membranes (Merk Life Science, Milan, Italy), and the filters were incubated in 30 ml azide broth. Zooplanktonic organisms (50-ml aliquots) were collected by dragging the water horizontally (∼1-m depth) with a 200-μm-mesh plankton net. Aliquots (50 ml) of the collected material were centrifuged for 10 min at 15,000 × g; pellets were resuspended in 5 ml artificial sterile seawater and added to 40 ml of azide broth. Each enrichment culture (100 μl) was spread on Slanetz-Bartley agar plates supplemented with florfenicol (10 mg/liter) for the selection of resistant enterococcal isolates. From each selective agar plate, eight presumptive resistant enterococcal colonies were randomly picked for further analysis.
Genotypic and phenotypic characterization.
Selected florfenicol-resistant enterococci were screened by PCR for the presence of cfr, optrA, and poxtA genes using primer pairs described previously (22). The PCR products were subjected to Sanger sequencing.
Isolates carrying linezolid resistance genes were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Vitek-MS; bioMérieux) and tested for their susceptibility to florfenicol, chloramphenicol, linezolid, tetracycline, and vancomycin (Sigma-Aldrich, St. Louis, MO) by standard broth microdilution assays and to tedizolid using Etest strips (Liofilchem, Roseto degli Abruzzi, Italy). Susceptibility tests were interpreted according to clinical EUCAST (v10.0) (https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf; https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/QC/v_11.0_EUCAST_QC_tables_routine_and_extended_QC_pdf.pdf) or CLSI (51) breakpoints. E. faecalis ATCC 29212 was used as a quality control (EUCAST quality control tables v10.0) (http://www.eucast.org).
SmaI PFGE, S1 PFGE, Southern blotting, and hybridization assays.
Typing was performed by SmaI PFGE as described previously (45). Genomic DNA embedded in agarose gel plugs was digested with S1 nuclease (Thermo Fisher Scientific, Milan, Italy), and chromosomes and plasmids separated by PFGE as described previously (46). After S1 PFGE, total DNA was blotted onto positively charged nylon membranes (Ambion-Celbio, Milan, Italy) and hybridized with biotin-labeled cfr, optrA, and poxtA DNA probes as described elsewhere (47).
Detection of circular forms.
To investigate the excision of genetic contexts carrying linezolid resistance genes, PCR assays were performed using outward-directed primer pairs targeting the linezolid resistance genes, i.e., (i) poxtAdiv-FW (GACGAGCCGACCAACCACCT) and poxtAdiv-RV (TTCAGGCGGACAAAAATCCAA) and (ii) optrAdiv-FW (GAAAAATAACACAGTAAAAGGC) and optrAdiv-RV (TTTTTCCACATCCATTTCTACC). Briefly, 5 μl of genomic DNA was added in a final volume of 25 μl of master mix containing 0.2 μM each primer, 500 mM deoxynucleoside triphosphate (dNTP) mix, 7 mM MgCl2, and 2 U Dream Taq DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA). PCR conditions were as follows: 94°C for 3 min; 30 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 5 min; and 72°C for 5 min. PCR was performed in a GeneAmp PCR system 9700 thermal cycler (Applied Biosystems). PCR products were resolved by electrophoresis on a 1.0% agarose gel. The cfr- and poxtA-carrying S. aureus AOUC-0915 (48) and the cfr- and optrA-carrying E. faecium E35048 (49) isolates were used as positive controls in PCR experiments.
Conjugation experiments.
Conjugal transfer was performed on a membrane filter as described previously (47). In mating experiments, all isolates carrying linezolid resistance genes were used as donors and Enterococcus faecium 64/3 was used as a recipient (50). Transconjugants were selected on brain heart infusion agar (Oxoid) containing florfenicol (10 mg/liter), fusidic acid (25 mg/liter), and rifampin (25 mg/liter), and grown colonies were tested for the presence of linezolid resistance genes by PCR and for their susceptibility to florfenicol and linezolid.
SmaI PFGE was carried out and patterns were analyzed to confirm the genetic background of transconjugants. Conjugation frequencies were expressed as the ratio of the cell number (CFU per milliliter) of the transconjugant to that of the recipient.
WGS and sequence analysis.
Genomic DNA was extracted using a commercial kit (Sigma-Aldrich). Next-generation sequencing was carried out using the Illumina MiSeq platform (MicrobesNG, Birmingham, UK) by using a 2 × 250-bp paired-end approach. De novo assembly was performed with SPAdes v3.11.1 (http://cab.spbu.ru/software/spades), and open reading frames (ORFs) (minimum length, 50 amino acids) were annotated with the RAST server (http://rast.nmpdr.org) and ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder). The quality of the final contigs was improved with the Burrows-Wheeler Aligner. The gaps between the plasmid contigs were closed by PCR mapping using primers targeting unique DNA regions and Sanger sequencing of the resulting amplicons, after purification with a GenElute PCR cleanup kit (Sigma-Aldrich).
The presence of mutations in genes encoding all copies of the 23S rRNA and ribosomal proteins L3 and L4 were investigated by WGS analysis, comparing the sequences to those from linezolid-susceptible E. faecalis ATCC 29212 (GenBank accession number ALOD01000000). The nucleotide sequences were compared with sequences in the GenBank database using BLASTn (http://blast.ncbi.nlm.nih.gov/blast). The ST was determined through the Center for Genomic Epidemiology (https://cge.cbs.dtu.dk/services/MLST) and the multilocus sequence typing (MLST) database (https://pubmlst.org/general.shtml).
Data availability.
The whole genomes of six isolates are available under the BioProject accession number PRJNA679166. The sequence of plasmids characterized in this study were submitted to GenBank and assigned accession numbers MT683614, MT683615, and MT683616.
ACKNOWLEDGMENTS
This work was supported by PRIN 2017 grant 20177J5Y3P from MIUR-Italy and by Progetto Strategico di Ateneo 2017–Polytechnic University of Marche (In the Hunt of New Antibiotics: Active Compounds from Both Chemical Synthesis and Natural Sources).
REFERENCES
- 1.Grenni P, Ancona V, Barra Caracciolo A. 2018. Ecological effects of antibiotics on natural ecosystems: a review. Microchem J 136:25–39. 10.1016/j.microc.2017.02.006. [DOI] [Google Scholar]
- 2.Karkman A, Do TT, Walsh F, Virta MPJ. 2018. Antibiotic-resistance genes in wastewater. Trends Microbiol 26:220–228. 10.1016/j.tim.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Jay JM. 2005. Indicators of food microbial quality and safety, p 473–495. In Jay JM, Loessner MJ, Golden DA (ed), Modern food microbiology, 7th ed. Springer Science and Business Media, New York, NY. [Google Scholar]
- 4.European Food Safety Authority. 2008. Report from the Task Force on Zoonoses Data Collection including guidance for harmonized monitoring and reporting of antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. from food animals. EFSA J 141:1–44. 10.2903/j.efsa.2008.141r. [DOI] [Google Scholar]
- 5.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10:266–278. 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender J, Cattoir V, Hegstad K, Sadowy E, Coque TM, Westh H, Hammerum AM, Schaffer K, Burns K, Murchan S, Novais C, Freitas AR, Peixe L, Del Grosso M, Pantosti A, Werner G. 2018. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: towards a common nomenclature. Drug Resist Updat 40:25–39. 10.1016/j.drup.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DN, Schluenzen F, Harms JM, Starosta AL, Connell SR, Fucini P. 2008. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc Natl Acad Sci U S A 105:13339–133344. 10.1073/pnas.0804276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long KS, Vester B. 2012. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother 56:603–612. 10.1128/AAC.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. 2006. The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob Agents Chemother 50:2500–2505. 10.1128/AAC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande LM, Ashcraft DS, Kahn HP, Pankey G, Jones RN, Farrell DJ, Mendes RE. 2015. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 59:6256–6261. 10.1128/AAC.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y, Dai L, Sahin O, Wu Z, Liu M, Zhang Q. 2017. Emergence of a plasmid-borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter. J Antimicrob Chemother 72:1581–1588. 10.1093/jac/dkx023. [DOI] [PubMed] [Google Scholar]
- 12.Pang S, Boan P, Lee T, Gangatharan S, Tan SJ, Daley D, Lee YT, Coombs GW. 2020. Linezolid-resistant ST872 Enterococcus faecium harbouring optrA and cfr(D) oxazolidinone resistance genes. Int J Antimicrob Agents 55:105831. 10.1016/j.ijantimicag.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Stojković V, Ulate MF, Hidalgo-Villeda F, Aguilar E, Monge-Cascante E, Pizarro-Guajardo M, Tsai K, Tzoc E, Camorlinga M, Paredes-Sabja D, Quesada-Gómez C, Fujimori DG, Rodríguez C. 2019. cfr(B), cfr(C), and a new cfr-like gene, cfr(E), in Clostridium difficile strains recovered across Latin America. Antimicrob Agents Chemother 64:e01074-19. 10.1128/AAC.01074-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, Wang D, Wang Z, Shen Y, Li Y, Feßler AT, Wu C, Yu H, Deng X, Xia X, Shen J. 2015. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190. 10.1093/jac/dkv116. [DOI] [PubMed] [Google Scholar]
- 15.Antonelli A, D'Andrea MM, Brenciani A, Galeotti CL, Morroni G, Pollini S, Varaldo PE, Rossolini GM. 2018. Characterization of poxtA, a novel phenicol-oxazolidinone-tetracycline resistance gene from an MRSA of clinical origin. J Antimicrob Chemother 73:1763–1769. 10.1093/jac/dky088. [DOI] [PubMed] [Google Scholar]
- 16.Sharkey LKR, O’Neill AJ. 2018. Antibiotic resistance ABC-F proteins: bringing target protection into the limelight. ACS Infect Dis 4:239–246. 10.1021/acsinfecdis.7b00251. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Wang Y, Schwarz S. 2013. Presence and dissemination of the multiresistance gene cfr in Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 68:1697–1706. 10.1093/jac/dkt092. [DOI] [PubMed] [Google Scholar]
- 18.Lazaris A, Coleman DC, Kearns AM, Pichon B, Kinnevey PM, Earls MR, Boyle B, O'Connell B, Brennan GI, Shore AC. 2017. Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecium (VRE) from a hospital outbreak: co-location of cfr and optrA in VRE. J Antimicrob Chemother 72:3252–3257. 10.1093/jac/dkx292. [DOI] [PubMed] [Google Scholar]
- 19.Morroni G, Brenciani A, Antonelli A, D'Andrea MM, Di Pilato V, Fioriti S, Mingoia M, Vignaroli C, Cirioni O, Biavasco F, Varaldo PE, Rossolini GM, Giovanetti E. 2018. Characterization of a multiresistance plasmid carrying the optrA and cfr resistance genes from an Enterococcus faecium clinical isolate. Front Microbiol 9:2189. 10.3389/fmicb.2018.02189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Andrea MM, Antonelli A, Brenciani A, Di Pilato V, Morroni G, Pollini S, Fioriti S, Giovanetti E, Rossolini GM. 2019. Characterization of Tn6349, a novel mosaic transposon carrying poxtA, cfr and other resistance determinants, inserted in the chromosome of an ST5-MRSA-II strain of clinical origin. J Antimicrob Chemother 74:2870–2875. 10.1093/jac/dkz278. [DOI] [PubMed] [Google Scholar]
- 21.Hölzel CS, Tetens JL, Schwaiger K. 2018. Unraveling the role of vegetables in spreading antimicrobial-resistant bacteria: a need for quantitative risk assessment. Foodborne Pathog Dis 15:671–688. 10.1089/fpd.2018.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenciani A, Fioriti S, Morroni G, Cucco L, Morelli A, Pezzotti G, Paniccià M, Antonelli A, Magistrali CF, Rossolini GM, Giovanetti E. 2019. Detection in Italy of a porcine Enterococcus faecium isolate carrying the novel phenicol-oxazolidinone-tetracycline resistance gene poxtA. J Antimicrob Chemother 74:817–818. 10.1093/jac/dky505. [DOI] [PubMed] [Google Scholar]
- 23.Lei CW, Kang ZZ, Wu SK, Chen YP, Kong LH, Wang HN. 2019. Detection of the phenicol-oxazolidinone-tetracycline resistance gene poxtA in Enterococcus faecium and Enterococcus faecalis of food-producing animal origin in China. J Antimicrob Chemother 74:2459–2461. 10.1093/jac/dkz198. [DOI] [PubMed] [Google Scholar]
- 24.Kang ZZ, Lei CW, Yao TG, Zhang Y, Wang YL, Ye XL, Wang XC, Gao YF, Wang HN. 2019. Whole-genome sequencing of Enterococcus hirae CQP3-9, a strain carrying the phenicol-oxazolidinone-tetracycline resistance gene poxtA of swine origin in China. J Glob Antimicrob Resist 18:71–73. 10.1016/j.jgar.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Elghaieb H, Freitas AR, Abbassi MS, Novais C, Zouari M, Hassen A, Peixe L. 2019. Dispersal of linezolid-resistant enterococci carrying poxtA or optrA in retail meat and food-producing animals from Tunisia. J Antimicrob Chemother 74:2865–2869. 10.1093/jac/dkz263. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Ripa L, Feßler AT, Hanke D, Sanz S, Olarte C, Eichhorn I, Schwarz S, Torres C. 2020. Detection of poxtA- and optrA-carrying E. faecium isolates in air samples of a Spanish swine farm. J Glob Antimicrob Resist 22:28–31. 10.1016/j.jgar.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Li X, Fu Y, Chen Y, Wang Y, Ye D, Wang C, Hu X, Zhou L, Du J, Shen J, Xia X. 2020. Association of florfenicol residues with the abundance of oxazolidinone resistance genes in livestock manures. J Hazard Mater 399:123059. 10.1016/j.jhazmat.2020.123059. [DOI] [PubMed] [Google Scholar]
- 28.Dias MF, da Rocha Fernandes G, de Paiva MC, de Matos Salim AC, Bueno Santos A, Amaral Nascimento AM. 2020. Exploring the resistome, virulome and microbiome of drinking water in environmental and clinical settings. Water Res 174:115630. 10.1016/j.watres.2020.115630. [DOI] [PubMed] [Google Scholar]
- 29.Torres C, Alonso CA, Ruiz-Ripa L, León-Sampedro R, Del Campo R, Coque TM. 2018. Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol Spectr 6:ARBA-0032-2018. 10.1128/microbiolspec.ARBA-0032-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papagiannitsis CC, Tsilipounidaki K, Malli E, Petinaki E. 2019. Detection in Greece of a clinical Enterococcus faecium isolate carrying the novel oxazolidinone resistance gene poxtA. J Antimicrob Chemother 74:2461–2462. 10.1093/jac/dkz155. [DOI] [PubMed] [Google Scholar]
- 31.Egan SA, Shore AC, O'Connell B, Brennan GI, Coleman DC. 2020. Linezolid resistance in Enterococcus faecium and Enterococcus faecalis from hospitalized patients in Ireland: high prevalence of the MDR genes optrA and poxtA in isolates with diverse genetic backgrounds. J Antimicrob Chemother 75:1704–1711. 10.1093/jac/dkaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshpande LM, Castanheira M, Flamm RK, Mendes RE. 2018. Evolving oxazolidinone resistance mechanisms in a worldwide collection of enterococcal clinical isolates: results from the SENTRY Antimicrobial Surveillance Program. J Antimicrob Chemother 73:2314–2322. 10.1093/jac/dky188. [DOI] [PubMed] [Google Scholar]
- 33.Elghaieb H, Tedim AP, Abbassi MS, Novais C, Duarte B, Hassen A, Peixe L, Freitas AR. 2020. From farm to fork: identical clones and Tn6674-like elements in linezolid-resistant Enterococcus faecalis from food-producing animals and retail meat. J Antimicrob Chemother 75:30–35. 10.1093/jac/dkz419. [DOI] [PubMed] [Google Scholar]
- 34.He T, Shen Y, Schwarz S, Cai J, Lv Y, Li J, Feßler AT, Zhang R, Wu C, Shen J, Wang Y. 2016. Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J Antimicrob Chemother 71:1466–1473. 10.1093/jac/dkw016. [DOI] [PubMed] [Google Scholar]
- 35.Càmara J, Camoez M, Tubau F, Pujol M, Ayats J, Ardanuy C, Domínguez MÁ. 2019. Detection of the novel optrA gene among linezolid-resistant enterococci in Barcelona, Spain. Microb Drug Resist 25:87–93. 10.1089/mdr.2018.0028. [DOI] [PubMed] [Google Scholar]
- 36.Zhou W, Gao S, Xu H, Zhang Z, Chen F, Shen H, Zhang C. 2019. Distribution of the optrA gene in Enterococcus isolates at a tertiary care hospital in China. J Glob Antimicrob Resist 17:180–186. 10.1016/j.jgar.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Freitas AR, Novais C, Ruiz-Garbajosa P, Coque TM, Peixe L. 2009. Dispersion of multidrug-resistant Enterococcus faecium isolates belonging to major clonal complexes in different Portuguese settings. Appl Environ Microbiol 75:4904–4908. 10.1128/AEM.02945-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Sante L, Morroni G, Brenciani A, Vignaroli C, Antonelli A, D'Andrea MM, Di Cesare A, Giovanetti E, Varaldo PE, Rossolini GM, Biavasco F. 2017. pHTβ-promoted mobilization of non-conjugative resistance plasmids from Enterococcus faecium to Enterococcus faecalis. J Antimicrob Chemother 72:2447–2453. 10.1093/jac/dkx197. [DOI] [PubMed] [Google Scholar]
- 39.Morroni G, Brenciani A, Simoni S, Vignaroli C, Mingoia M, Giovanetti E. 2017. Commentary: nationwide surveillance of novel oxazolidinone resistance gene optrA in Enterococcus isolates in China from 2004 to 2014. Front Microbiol 8:1631. 10.3389/fmicb.2017.01631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freitas AR, Tedim AP, Novais C, Lanza VF, Peixe L. 2020. Comparative genomics of global optrA-carrying Enterococcus faecalis uncovers a common chromosomal hotspot for optrA acquisition within a diversity of core and accessory genomes. Microb Genom 6:e000350. 10.1099/mgen.0.000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y, Fan R, Wang Y, Lei L, Feßler AT, Wang Z, Wu C, Schwarz S, Wang Y. 2019. Analysis of combined resistance to oxazolidinones and phenicols among bacteria from dogs fed with raw meat/vegetables and the respective food items. Sci Rep 9:15500. 10.1038/s41598-019-51918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clewell DB, Weaver KE, Dunny GM, Coque TM, Francia MV, Hayes F. 2014. Extrachromosomal and mobile elements in enterococci: transmission, maintenance, and epidemiology, p 309–320. In Gilmore MS, Clewell DB, Ike Y, Shankar N (ed), Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, MA. [PubMed] [Google Scholar]
- 43.Shang Y, Li D, Shan X, Schwarz S, Zhang SM, Chen YX, Ouyang W, Du XD. 2019. Analysis of two pheromone-responsive conjugative multiresistance plasmids carrying the novel mobile optrA locus from Enterococcus faecalis. Infect Drug Resist 12:2355–2362. 10.2147/IDR.S206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Wang M, Gao Y, Chen L, Wang L. 2019. Emergence of plasmid-mediated oxazolidinone resistance gene poxtA from CC17 Enterococcus faecium of pig origin. J Antimicrob Chemother 74:2524–2530. 10.1093/jac/dkz250. [DOI] [PubMed] [Google Scholar]
- 45.Ripa S, Zampaloni C, Vitali LA, Giovanetti E, Montanari MP, Prenna M, Varaldo PE. 2001. SmaI macrorestriction analysis of Italian isolates of erythromycin-resistant Streptococcus pyogenes and correlations with macrolide-resistance phenotypes. Microb Drug Resist 7:65–71. 10.1089/107662901750152828. [DOI] [PubMed] [Google Scholar]
- 46.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 47.Brenciani A, Morroni G, Pollini S, Tiberi E, Mingoia M, Varaldo PE, Rossolini GM, Giovanetti E. 2016. Characterization of novel conjugative multiresistance plasmids carrying cfr from linezolid-resistant Staphylococcus epidermidis clinical isolates from Italy. J Antimicrob Chemother 71:307–313. 10.1093/jac/dkv341. [DOI] [PubMed] [Google Scholar]
- 48.Antonelli A, D'Andrea MM, Galano A, Borchi B, Brenciani A, Vaggelli G, Cavallo A, Bartoloni A, Giovanetti E, Rossolini GM. 2016. Linezolid-resistant cfr-positive MRSA, Italy. J Antimicrob Chemother 71:2349–2351. 10.1093/jac/dkw108. [DOI] [PubMed] [Google Scholar]
- 49.Brenciani A, Morroni G, Vincenzi C, Manso E, Mingoia M, Giovanetti E, Varaldo PE. 2016. Detection in Italy of two clinical Enterococcus faecium isolates carrying both the oxazolidinone and phenicol resistance gene optrA and a silent multiresistance gene cfr. J Antimicrob Chemother 71:1118–1119. 10.1093/jac/dkv438. [DOI] [PubMed] [Google Scholar]
- 50.Werner G, Klare I, Witte W. 1997. Arrangement of the vanA gene cluster in enterococci of different ecological origin. FEMS Microbiol Lett 155:55–61. 10.1111/j.1574-6968.1997.tb12685.x. [DOI] [PubMed] [Google Scholar]
- 51.Clinical and Laboratory Standards Institute (CLSI) . 2020. Performance standards for antimicrobial susceptibility testing. CLSI M100. CLSI, Wayne, PA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The whole genomes of six isolates are available under the BioProject accession number PRJNA679166. The sequence of plasmids characterized in this study were submitted to GenBank and assigned accession numbers MT683614, MT683615, and MT683616.