To understand how antibiotic resistance plasmids end up in human pathogens, it is crucial to learn how, where, and when they are transferred and maintained in members of bacterial communities such as the gut microbiome. To gain insight into the network of plasmid-mediated antibiotic resistance sharing in the gut microbiome, we investigated the transferability and maintenance of a multidrug resistance plasmid among the culturable bacteria of the zebrafish gut.
KEYWORDS: conjugation, gut microbiome, horizontal gene transfer, plasmid, plasmid network, plasmid persistence, zebrafish
ABSTRACT
By characterizing the trajectories of antibiotic resistance gene transfer in bacterial communities such as the gut microbiome, we will better understand the factors that influence this spread of resistance. Our aim was to investigate the host network of a multidrug resistance broad-host-range plasmid in the culturable gut microbiome of zebrafish. This was done through in vitro and in vivo conjugation experiments with Escherichia coli as the donor of the plasmid pB10::gfp. When this donor was mixed with the extracted gut microbiome, only transconjugants of Aeromonas veronii were detected. In separate matings between the same donor and four prominent isolates from the gut microbiome, the plasmid transferred to two of these four isolates, A. veronii and Plesiomonas shigelloides, but not to Shewanella putrefaciens and Vibrio mimicus. When these A. veronii and P. shigelloides transconjugants were the donors in matings with the same four isolates, the plasmid now also transferred from A. veronii to S. putrefaciens. P. shigelloides was unable to donate the plasmid, and V. mimicus was unable to acquire it. Finally, when the E. coli donor was added in vivo to zebrafish through their food, plasmid transfer was observed in the gut, but only to Achromobacter, a rare member of the gut microbiome. This work shows that the success of plasmid-mediated antibiotic resistance spread in a gut microbiome depends on the donor-recipient species combinations and therefore their spatial arrangement. It also suggests that rare gut microbiome members should not be ignored as potential reservoirs of multidrug resistance plasmids from food.
IMPORTANCE To understand how antibiotic resistance plasmids end up in human pathogens, it is crucial to learn how, where, and when they are transferred and maintained in members of bacterial communities such as the gut microbiome. To gain insight into the network of plasmid-mediated antibiotic resistance sharing in the gut microbiome, we investigated the transferability and maintenance of a multidrug resistance plasmid among the culturable bacteria of the zebrafish gut. We show that the success of plasmid-mediated antibiotic resistance spread in a gut microbiome can depend on which species are involved, as some are important nodes in the plasmid-host network and others are dead ends. Our findings also suggest that rare gut microbiome members should not be ignored as potential reservoirs of multidrug resistance plasmids from food.
INTRODUCTION
Today many bacterial pathogens responsible for nosocomial infections are resistant to most if not all available antibiotics (1, 2). In contrast to the late 1960s, when the U.S. Surgeon General stated that it is “time to close the book” on infectious diseases, we are now warned by authorities such as the WHO and NIAID about an emanating “postantibiotic” era (3). What was underestimated early on was the ability of bacteria to rapidly adapt to selective pressures such as those created by the prolific use of antibiotics. One important mechanism of bacterial adaptation to antibiotics is the acquisition of antibiotic resistance genes from other, even distantly related bacteria through horizontal gene transfer (HGT) (4). One of the main HGT mechanisms responsible for antibiotic resistance spread to pathogens is conjugation (5, 6). Conjugation requires cell-to-cell contact and allows transfer of plasmid DNA from a donor to a recipient cell. To slow down the spread of antibiotic resistance, we need to understand the bacterial reservoirs of the resistance genes and how and where pathogenic bacteria acquire these genes from these reservoirs by conjugation (7, 8).
One environment where antibiotic resistance genes are likely exchanged between resident and transient bacteria is the gastrointestinal tract of humans and animals. The gut is expected to be favorable to HGT for multiple reasons (9). It provides near-continuous nutrition to the gut microbiota and environmental conditions that allow for bacterial growth and high population densities that promote efficient conjugation. Plasmid transfer has been demonstrated in the mouse gastrointestinal tract (10, 11), in the guts of fleas and houseflies (12, 13), and even in the gut of zebrafish (14).
For quite some time, there has been evidence for plasmid transfer in the human gut (4, 15, 16). Moreover, a comparison of 1,183 human-associated bacteria and 1,052 bacteria from a broad range of environmental niches suggested that bacteria within the human gut microbiome may be 25-fold more likely to share genetic material than bacteria from other environments (17). Moreover, not only do plasmids carry accessory genes that encode antibiotic resistance, but they also encode various pathogenicity factors (see reference 18 for a comprehensive review) and genes that confer the ability to metabolize complex nutrients and degrade xenobiotic compounds. Due to their prevalence and potentially high rate of transfer in the gut, plasmids may provide functional redundancy to prevent the loss of key functions (18). However, such a functionally redundant network of mobile genetic elements could also lead to antibiotic resistance gene reservoirs that persist in the absence of antibiotic selection.
To understand how antibiotic resistance plasmids end up in human pathogens, it is crucial to learn how, where, and when they are transferred and maintained in members of bacterial communities such as the gut microbiome. One desirable model system for such studies are zebrafish (Danio rerio) because they have a well-defined and comparatively simple core microbiome and a digestive tract that is similar in organization and function to that of mammals. Therefore, they have been used to investigate host-microbe interactions, gut colonization, and differentiation (19–22), and recently also plasmid transfer (14). To gain insight into the network of plasmid-mediated antibiotic resistance sharing in the gut microbiome, we investigated the transferability and maintenance of the multidrug resistance (MDR) plasmid pB10::gfp among the culturable bacteria of the zebrafish gut microbiome, through both in vitro and in vivo studies. This plasmid belongs to the incompatibility group IncP-1, a well-known group of plasmids that are self-transmissible to a broad host range (BHR) of bacteria and likely to be involved in the spread of antibiotic resistance (23). In in vitro matings, the efficiency of plasmid transfer was found to depend on the combination of bacterial species acting as plasmid donors and recipients. In our in vivo study, only one particular species of the zebrafish gut microbiome effectively received and maintained the plasmid, even though it constituted a small fraction of that community. Given that conjugation requires cell contact, our findings suggest that the successful spread and persistence of a plasmid in a gut microbiome depend on the bacterial community composition and the spatial arrangement of its members. They also caution against using in vitro conjugation results to identify the likely reservoirs of MDR plasmids in a gut microbiome, as these can be rare community members.
RESULTS
Plasmid transfer to culturable bacteria from the zebrafish gut microbiome.
First we assessed the ability of plasmid pB10::gfp to transfer from an Escherichia coli donor to bacteria of the zebrafish gut microbiome. We performed these conjugation experiments on an agar surface using E. coli(pB10::gfp) as a plasmid donor to either (i) the entire microbiome isolated from the zebrafish intestinal tract or (ii) four numerically dominant species that had been isolated from these gut microbiomes.
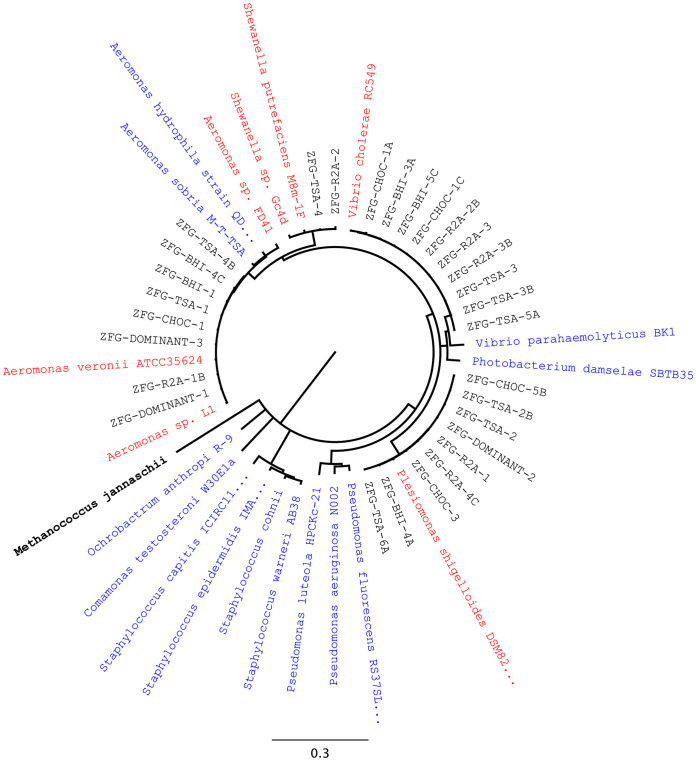
Conjugation experiments on R2A agar between E. coli AT1036(pB10::gfp) and the zebrafish gut microbiome (approximately 1 × 108 culturable bacteria) yielded pB10::gfp-containing microbiome members (called transconjugants) at a frequency of (1.5 ± 1.5) × 10−4 per donor. For simplicity, we often refer to this transconjugant/donor ratio from here on as the “transfer frequency,” which is reported as the average and standard deviation from three replicates. Based on analysis of their 16S rRNA gene sequences, these transconjugants all belonged to a single species, Aeromonas veronii. For the second in vitro method, we first isolated individual bacterial strains from the combined guts of four zebrafish, using different growth media. Based on differences in colony morphology, we isolated and purified 29 strains. Based on their 16S rRNA gene sequences, they belonged to the four gammaproteobacterial species Aeromonas veronii, Plesiomonas shigelloides, Shewanella putrefaciens, and Vibrio mimicus (Fig. 1). Conjugation experiments done using the E. coli(pB10::gfp) donor and these four species as recipients yielded transconjugants for A. veronii and P. shigelloides at frequencies of (3.4 ± 0.7) × 10−3 and (1.5 ± 0.0) × 10−4 per donor, respectively (Table 1). In contrast, transfer to V. mimicus and S. putrefaciens could not be detected (<1 × 10−8 transconjugants per donor). The plasmid was thus able to transfer from an E. coli donor to at least two of the four dominant culturable members of the zebrafish gut microbiome.
FIG 1.
All 29 unique zebrafish gut isolates grouped into four different genera based on their 16S rRNA gene sequences: Aeromonas, Plesiomonas, Vibrio, and Shewanella. ZFG, bacterial strains isolated from the zebrafish gut; DOMINANT, numerically dominant colony morphology types, originally identified on R2A agar, which successfully acquired the plasmid following conjugation with the donor E. coli; BHI, CHOC (chocolate), R2A, and TSA, media used to isolate the strains (see Materials and Methods). Red indicates culture collection strains most similar to isolates, and blue indicates zebrafish core microbiome reference strains (44). The tree was rooted using the archaean Methanococcus jannaschii.
TABLE 1.
Plasmid transfer frequencies in reciprocal matings demonstrate the importance of both donor and recipient identity in efficiency of plasmid transfer
| Recipient | Transfer frequency for donor of pB10::gfpa |
||
|---|---|---|---|
| E. coli AT1036 | A. veronii | P. shigelloides | |
| E. coli EC100 Nalr | (2.8 ± 1.4) × 10−2 | (8.8 ± 5.5) × 10−4 | ND |
| A. veronii Rifr | (3.4 ± 0.7) × 10−3 | (2.9 ± 0.2) × 10−1 | ND |
| P. shigelloides Rifr | (1.5 ± 0.0) × 10−4 | (2.2 ± 1.0) × 10−3 | ND |
| V. mimicus Rifr | ND | ND | ND |
| S. putrefaciens | ND | (4.2 ± 3.2) × 10−5 | ND |
Frequencies are expressed as transconjugants per donor after the matings (n = 3). ND, not detected: transconjugants were below the detection limit of 10−8.
Novel hosts may act as plasmid donor.
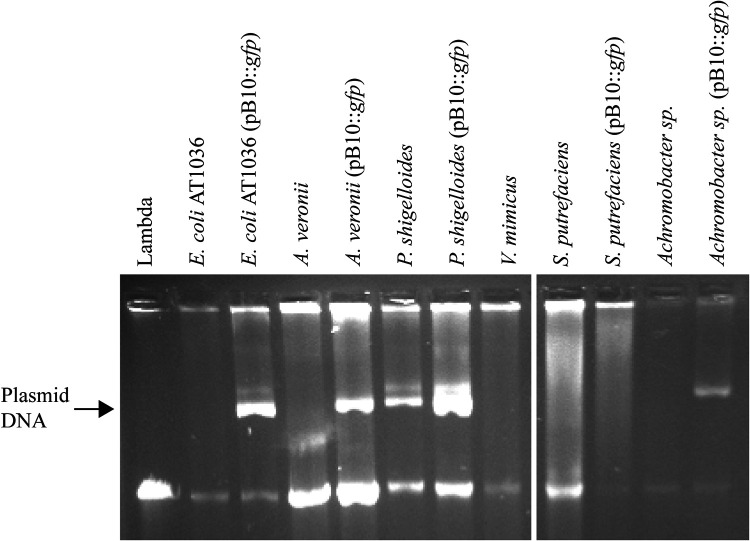
Since two culturable zebrafish gut microbiome members received the antibiotic resistance plasmid from E. coli, we determined if they could further spread it to other species and whether various donor-recipient combinations would affect the efficiency of plasmid transfer. To do this, the A. veronii and P. shigelloides transconjugants were each employed as donors in conjugation assays with five recipients: E. coli EC100 and rifampin-resistant (Rifr) mutants of each of the four gut isolates, A. veronii, P. shigelloides, S. putrefaciens, and V. mimicus. As shown in Table 1, the frequency of plasmid transfer to one particular recipient clearly depended on the identity of the donor, and the efficiency by which a donor transferred the plasmid depended on the recipient. Strikingly, though P. shigelloides was a good recipient, it was a bad plasmid donor as it was unable to transfer the plasmid to any of the five recipients. The lack of plasmid transfer from this host was probably not due to integration of plasmid pB10 in the chromosome, as a strong band of extrachromosomal plasmid DNA was visual on agarose gel, stronger than that of the endogenous plasmid of similar size in the strain without pB10 (Fig. 2). What is also clear from Table 1 is that A. veronii, but not E. coli, was able to transfer the plasmid to S. putrefaciens, albeit still at a low frequency, showing a clear donor effect. We were unable to use S. putrefaciens as a donor in these reciprocal transfer experiments since it was intrinsically resistant to both rifampin (Rif) and nalidixic acid (Nal), which precluded distinguishing between donors and transconjugants. Transfer to V. mimicus could not be detected at all. Since this could be due to the inability of the plasmid to either transfer to or replicate in this strain, we tried to introduce the plasmid through electroporation. As several attempts at this were also unsuccessful, we concluded that the plasmid cannot replicate in this host, making it impossible to use it as a plasmid donor. These results suggest that the trajectory of spread of a BHR MDR plasmid in a microbiome is determined by the identity of both the donor and recipient.
FIG 2.
Agarose gel electrophoresis of plasmid DNA extracted from plasmid-free and plasmid-containing strains.
The persistence of pB10::gfp in zebrafish gut bacteria was host dependent.
For a microbiome member to be an important reservoir of an MDR plasmid, it must not only acquire an incoming plasmid but also retain it sufficiently long in the absence of selection for plasmid-borne genes. To determine whether pB10::gfp was able to persist in our A. veronii, P. shigelloides, and S. putrefaciens isolates, we monitored the fraction of plasmid-containing cells in serially transferred populations in the absence of antibiotic selection for 10 days (100 generations). The plasmid was very persistent in P. shigelloides but much less so in the other two strains (Fig. 3). Thus, although A. veronii was both a rather good recipient and donor of the plasmid (Table 1), it was not good at maintaining the plasmid and may thus represent only a transient host. In contrast, P. shigelloides was very good at retaining the plasmid but unable to transfer it to other bacteria tested, suggesting a possible dead end for the plasmid in its transmission network. Finally, S. putrefaciens was very poor at both receiving and retaining the plasmid. These results show that persistence of the plasmid was variable in zebrafish gut bacteria and did not necessarily correlate with their host’s ability to receive or further transfer the plasmid.
FIG 3.
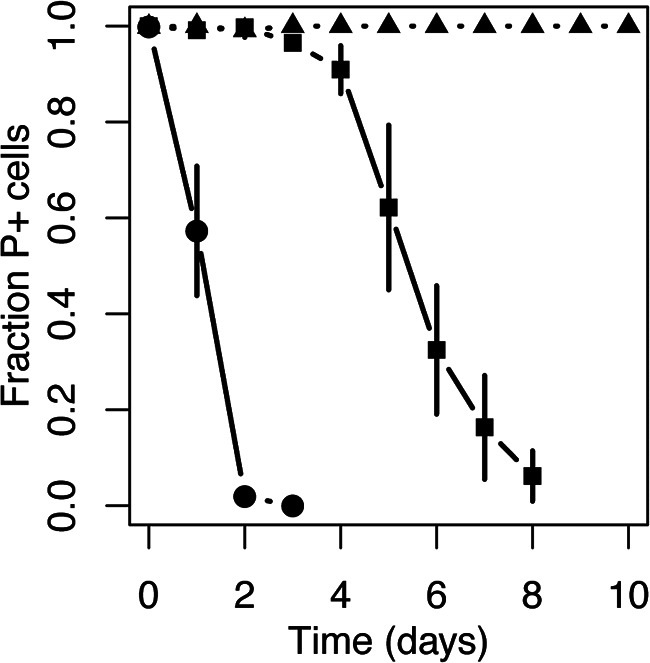
The persistence of plasmid pB10::gfp in zebrafish gut isolates over 100 generations of growth in the absence of antibiotic selection varied greatly from high (P. shigelloides [solid triangles]), to moderate (A. veronii [solid squares]), to poor (S. putrefaciens [solid circles]). Each experiment consisted of three replicate populations, and the error bars represent the standard deviation.
Plasmid transfer in vivo.
Next, we examined if the plasmid could transfer to bacteria in the gut of zebrafish exposed to tetracycline (Tc), one of the antibiotics for which the plasmid encodes resistance and which is frequently used in aquaculture (24). Briefly, with 32 fish divided over eight separate tanks, half of the fish were fed twice daily with food premixed with the plasmid donor (treated: tanks A to D), while the other half received food premixed with an isogenic plasmid-free strain (untreated: tanks E to H). After analyzing the gut microbiomes of the eight fish populations harvested on the 23rd day, 23 green fluorescent colonies were observed on transconjugant-selective agar plates at an average frequency of (1.2 ± 0.8) per 106 culturable gut bacteria from the zebrafish in three of the four treated tanks. In contrast, no green fluorescent strains were detected in the guts of the untreated groups. No fluorescent E. coli AT1036(pB10::gfp) donors were detected on any of the donor-permissive agar plates, verifying that this E. coli strain was incapable of establishing itself in the zebrafish gut. All 23 fluorescent colonies looked identical, and based on comparisons of an ∼700-bp fragment of their 16S rRNA gene sequences, they all belonged to the genus Achromobacter. Their closest relatives were the Achromobacter spp. A. denitrificans, A. insolitus, and A. xylosoxidans, with >99% sequence identity across the V1 to V4 region. Thus, while other means of HGT cannot be ruled out, the 23 green fluorescent colonies likely represent transconjugants that arose from a minimum of three independent plasmid transfer events (at least once for each of the three tanks). Furthermore, since plasmid donors were no longer fed to the fish during the last 2 days before harvesting the microbiomes, these transconjugants were present in the zebrafish gut for at least 2 days posttreatment.
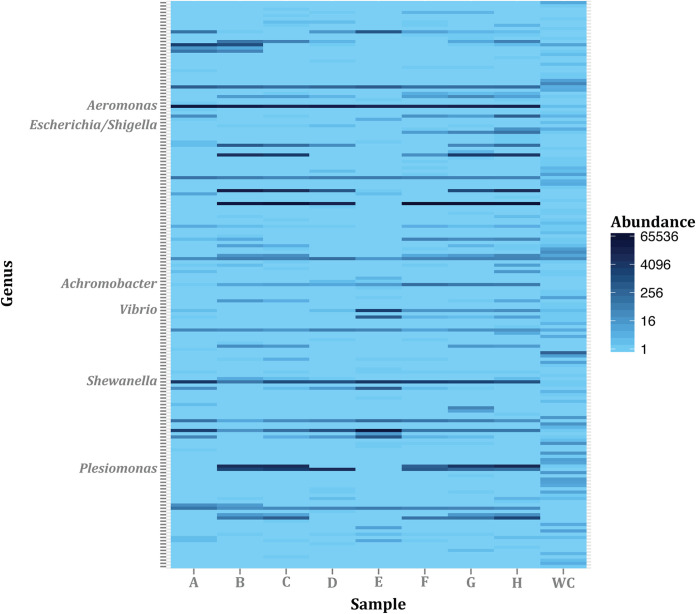
Since Achromobacter is generally described as a common waterborne organism (25) and has not previously been referenced as a resident of the zebrafish gut microbiome, we determined if Achromobacter was indeed present within the guts of our zebrafish populations. This was done by constructing and sequencing 16S rRNA amplicon libraries using genomic DNA (gDNA) isolated from the gut microbiome samples. The DNA sequence analysis showed that Achromobacter was present at a low frequency in the gut microbiome of both the treated and untreated populations [(7.9 ± 4.2) × 10−3% and (8.2 ± 5.6) × 10−2%, respectively] (Fig. 4). In comparison, Aeromonas represented 40.3% ± 27.1% and 16.2% ± 5.0% of the gut microbiomes of treated and untreated populations, respectively. Despite being the most numerically dominant genus in these guts and a good plasmid recipient in vitro (see the section “Plasmid transfer to culturable bacteria from the zebrafish gut microbiome”), no transconjugants of this genus were identified at the end of this in vivo experiment. Our findings suggest that factors other than species abundance determine the in vivo trajectories of plasmid transfer and establishment in a gut microbiome.
FIG 4.
Achromobacter was present at low abundance among the many genera in the gut microbiota of the treated zebrafish populations [(7.9 ± 4.2) × 10−3% in samples A to D] and untreated populations [(8.2 ± 5.6) × 10−2% in samples E to H], and not detectable in the water control (WC). Only the relevant cultured genera or operational taxonomic units (OTUs) are indicated. The relative abundance corresponds to the number of paired-end reads for each taxa normalized to the total number of paired-end reads per sample. A complete list of all the OTUs can be found in Table S1.
Transferability and persistence of pB10::gfp in Achromobacter.
To determine if a high frequency of transfer from E. coli to Achromobacter could in part explain why Achromobacter was the only species of in vivo transconjugants detected, we determined the transfer frequency in vitro. On an agar surface, plasmid pB10::gfp transferred from E. coli AT1036 to Achromobacter at a frequency of (4.3 ± 2.0) × 10−2 transconjugants donor−1. When comparing this to plasmid transfer under the same conditions from the same E. coli strain to other species used in this study (Table 1), this frequency was on average 13 times higher than transfer to A. veronii, 287 times higher than that to P. shigelloides, and similar to the frequency of transfer between two isogenic E. coli strains. Thus, even though Achromobacter was present at only 0.008% of the gut microbiome, its high proficiency as a recipient for pB10::gfp likely allowed it to acquire the plasmid within the zebrafish gut.
For gut bacteria to become new reservoirs of horizontally acquired MDR plasmids, they not only need to receive the plasmid, they should also be able retain it under conditions of low or no antibiotic pressure and be good plasmid donors. Therefore, we also measured the persistence of pB10::gfp in Achromobacter in serial batch culture and its frequency of transfer to E coli as an example recipient strain. After approximately 100 generations of growth in the absence of antibiotic selection for plasmid maintenance, about 80% of the Achromobacter population still retained the plasmid (Fig. 5). Since some bacterial strains have shown no loss of plasmid pB10::gfp in this time frame and others a much more rapid loss (26), this Achromobacter sp. strain seems to be quite a good host for this plasmid. Moreover, this strain was also capable of transferring the plasmid to E. coli EC100 at a high frequency of (3.0 ± 1.8) × 10−2 transconjugants donor−1, suggesting it is a capable plasmid donor and thus a likely reservoir. A combination of a high plasmid transfer frequency and good plasmid persistence likely explained how this plasmid-host pair formed in the gut and remained there for at least 2 days since the last E. coli donor cells were added.
FIG 5.
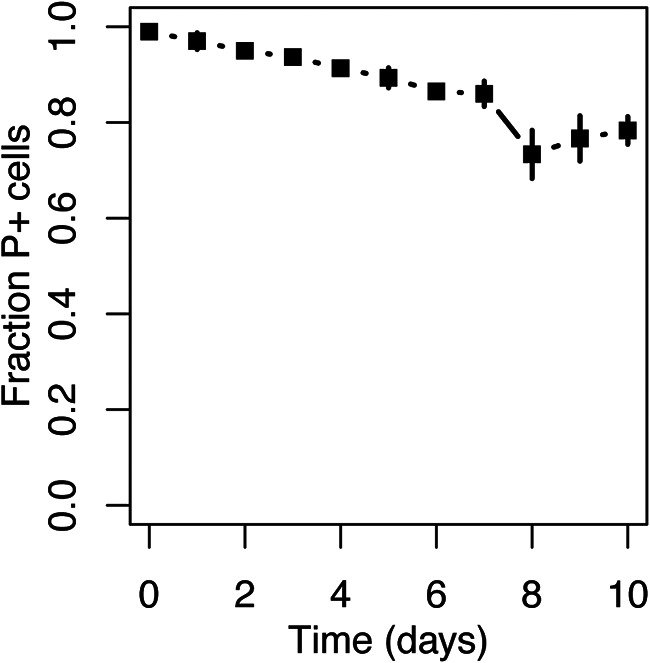
Plasmid pB10::gfp displays good persistence in Achromobacter. The experiment consisted of three replicate populations, and the error bars represent the standard deviation.
DISCUSSION
If we want to slow down the alarmingly rapid spread of resistance to critically important antibiotics, we need to better understand the plasmid transfer networks that facilitate this spread. Conjugation of self-transmissible plasmids is likely a major pathway for horizontal gene transfer among bacteria, and so-called “epidemic” plasmids play a critical role in global resistance spread, in particular among multidrug-resistant Enterobacteriaceae (5, 6). Using zebrafish as a model system, we showed here that an MDR plasmid introduced with the fish food can transfer and establish itself in one of the quite rare gut microbiome members, creating a new reservoir of mobile resistance genes. Our in vitro data also suggest that key features of such successful reservoir bacteria are not only a high plasmid transfer efficiency, which itself depends on the combination of donor and recipient bacteria in the transfer network, but also the ability to maintain an MDR plasmid in the absence of antibiotics. The latter can be due to a low segregational loss rate, low plasmid fitness cost, or high plasmid reinfection rate, each of which would benefit the plasmid-host pair in vivo both in the absence or in the presence of antibiotics.
The gut microbiome of zebrafish appears to be relatively conserved, with a small group of core genera that are present in most sampled zebrafish (27), including those in this study (see Table S1 in the supplemental material). Of the most abundant bacterial genera in our zebrafish microbiomes, Aeromonas, Shewanella, Vibrio, and Plesiomonas, the first three are known to be part of this core microbiome (27). Furthermore, most of the bacteria present in the gut belonged to the Gram-negative phylum Proteobacteria. This group of bacteria actively participates in HGT (28) and is within the host range of several MDR plasmid families, including the IncP-1 plasmids such as our model plasmid pB10 (29). The phylum Proteobacteria also contains several human pathogens listed by the WHO in 2017 as being of priority 1 (“critical”) (30). The gut microbiome of zebrafish is thus an ideal model system for research pertaining to the transfer and maintenance of MDR plasmids in microbial communities.
We clearly showed that the success of plasmid transfer between bacterial isolates from the gut microbiome depends on the identity of both the donor and recipient. Some hosts were good recipients but poor donors, or vice versa, while others were both good donors and recipients. This is in line with a previous study from our group showing that the donor species defined the host range of pB10 within a wastewater activated sludge community (31). It is also consistent with the conjugation studies by Dionisio et al. (32) and Benz et al. (33). Dionisio et al. (32) showed a significant difference among enterobacterial species and even E. coli strains in the ability to donate the F-type plasmid R1 and postulated that the best donor strains can act as “amplifiers” in a community and thereby facilitate the spread of antibiotic resistance. Using extended-spectrum β-lactamase (ESBL)-producing IncF and IncI plasmids and associated clinical E. coli and Salmonella enterica hosts, Benz et al. (33) further demonstrated that the transfer network of these plasmids correlated in vitro and in vivo. In this particular study with narrow-host-range (NHR) plasmids and associated hosts, transferability was largely governed by the completeness of the plasmid-encoded transfer system—the presence of coresident plasmids in the donor or plasmids belonging to the same incompatibility group in the recipient. While the limitations are more difficult to predict for BHR plasmids due to the large genetic diversity between potential hosts, we confirm here that some strains can be important nodes in the plasmid transfer network, while others may be dead ends. To slow down the spread of antibiotic resistance, it is important to identify these critical nodes in microbiomes as well as the molecular mechanisms underlying their proficiency as a plasmid donor or recipient.
One striking finding of our study was the inability of P. shigelloides to transfer the plasmid to any of the five different species tested, in spite of it being a rather good recipient when combined with each donor. While we cannot exclude that this strain may transfer this plasmid under different conditions, it could well be an example of a dead-end species within a plasmid transfer network. This poor conjugation proficiency could be due to inhibition of conjugation by another resident plasmid. Figure 2 shows that it was the only host showing a second plasmid DNA band on an agarose gel after plasmid extraction. Negative regulatory effects of coresident plasmids on the conjugative transfer of a specific plasmid have been long known (34). This is referred to as “fertility inhibition” and has previously been demonstrated for IncP-1 plasmids like pB10 (35). Dionisio et al. (32) also provided some evidence that plasmid-encoded fertility inhibition systems like FinOP on plasmid R1 were involved in the variable ability to serve as a donor, likely due to interaction with native plasmids of these strains. This phenomenon has also been more recently described for several plasmid combinations (36), but for many, the molecular mechanisms remain elusive. Whatever the mechanism here, given that the combination of donor and recipient hosts determines successful plasmid transfer, the transferability of a plasmid within a gut microbiome likely depends on who neighbors whom. Our findings suggest the gene transfer network might not only be determined by the composition of a bacterial community but also by the spatial arrangement of its members.
Transfer of a plasmid to a given host does not necessarily translate to successful establishment in that host (37, 38). If the replication or partitioning systems do not function optimally, or if the cost of the plasmid is high, the plasmid will fail to persist in that population (39). This was shown here by the inability of the plasmid to persist in A. veronii. In contrast, the plasmid was highly persistent in P. shigelloides and persisted quite well in Achromobacter. Thus, even in the absence of antibiotics, gastrointestinal bacteria that efficiently acquire and retain an MDR plasmid could ensure the persistence of that plasmid and its resistance genes within the gut microbiome. Importantly, the efficiency by which a host receives a plasmid does not necessarily correlate with its ability to subsequently retain it. This serves as a reminder that plasmid transfer from a donor, establishment in a recipient, and its subsequent persistence are distinctly unique processes that all contribute to the success of plasmids in any microbiome.
The zebrafish gut microbiome members that were detected as new hosts of our BHR MDR plasmid differed between the in vitro and in vivo conjugation experiments. In in vitro matings between E. coli and the extracted zebrafish gut microbiome, the only species of transconjugants detected (A. veronii) was numerically dominant in this microbiome, based on both 16S rRNA gene amplicon sequencing (Fig. 4) and plate counts (Fig. 1). In matings between pure cultures, it acquired the plasmid at a moderately high frequency compared to other tested species (Table 1). Consistent with this, Fu et al. (14) recently demonstrated that Aeromonas species represented the dominant fraction of the zebrafish gut microbiome based on a cultivation-independent method. They also showed that members of this genus were common among the transconjugants of the IncP-1α plasmid they introduced in in vivo experiments. In contrast to the findings of Fu et al. (14) and of our in vitro experiment, the only transconjugant we detected in our in vivo experiment was Achromobacter, a minority member of the zebrafish gut microbiome (Fig. 4). Its low proportion in the gut community explains why it was not detected on any of the agar media nor as a transconjugant in the in vitro mating with extracted gut microbiome, where the roughly 5,000-fold more dominant A. veronii apparently crowded the transconjugant plates. Achromobacter was also present in the gut of the zebrafish used by Fu et al. (14), varying in abundance along the fore-, mid-, and hindgut, but it was not shown to acquire their resistance plasmid. There could be several possible reasons for this discrepancy, none of which are mutually exclusive: (i) there might be differences in plasmid donor strains and the way they were administered to the fish, (ii) Achromobacter may not be a favorable host for the IncP-1α plasmids used by Fu et al. as there is no complete overlap between the host ranges of IncP-1α and -β plasmids (pB10 in our study) (40), (iii) the Achromobacter. strains in these two studies may have been distinctly different, and plasmid host range is known to vary greatly between and even within species (26), and (iv) our cultivation-dependent technique had a better detection limit, allowing us to identify transconjugants present at low abundance.
Achromobacter was the best recipient in in vitro conjugation experiments between E. coli(pB10) and pure cultures of recipients. The plasmid was also rather persistent in this host—more so than in A. veronii. High plasmid transfer frequencies and plasmid persistence can satisfy the “existence conditions” for plasmids in bacterial populations, as any plasmid loss can possibly be overcome by reinfection of the plasmid from neighboring cells (39, 41). Using in vitro conjugation experiments to identify which microbiome members play an important role in the spread of a particular plasmid may thus be misleading, as these results may be determined by relative abundance and plasmid transfer frequency but not by plasmid persistence and in vivo conditions. Our message is therefore in contrast with that of Benz et al. (33), as they concluded that their results from in vitro transfer of NHR plasmids were a good predictor of the plasmids in vivo spread. We caution that the transfer network of a broad-host-range plasmid like ours may be less predictable from in vitro studies given the much broader diversity of hosts the plasmid can transfer to and replicate in compared to an NHR plasmid. Moreover, our findings uniquely emphasize that even rather rare species in a gut microbiome may become important reservoirs of antibiotic resistance if their ability to acquire resistance plasmids from bacteria introduced with food is high.
The detection of only one species in the zebrafish gut that received our plasmid in our in vivo experiments does not by any means imply that it was the only member that received the plasmid. It has been demonstrated that the host range of bacteria to which a plasmid can transfer exceeds the range in which they can replicate (42, 43). Therefore, some members like A. veronii here may have received the plasmid in the zebrafish gut, passed it on to others, and subsequently lost it. Other members were likely not culturable (14, 27, 44). Thus, the frequency and range of plasmid transfer are likely underestimated. We can also not exclude that conjugative transfer of the plasmid from the E. coli donor to Achromobacter took place in the water environment prior to Achromobacter establishing in the gut. Irrespective of the route, Achromobacter transconjugants were present in the zebrafish gut for at least 2 days post-donor treatment and are thus likely an important link in this plasmid transfer network.
While a full understanding of the plasmid transfer network in a gut microbiome will require cultivation-independent monitoring of introduced and indigenous plasmids with methods such as proximity ligation (Hi-C) (45, 46), several important messages can be drawn from this cultivation-dependent study: (i) successful transfer of the plasmid is very much dependent on the combination of donor and recipient, and therefore an MDR plasmid network in a non-well-mixed system like the gut is in part defined by both the composition and spatial organization of the microbiome, (ii) caution should be taken when drawing conclusions about the range of MDR plasmid spread from in vitro data, as Achromobacter would have been missed here without the in vivo experiment, and (iii) in spite of their low relative abundance, rare gut microbiome members could be important reservoirs of MDR plasmids introduced through food.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
Our model plasmid was the BHR MDR plasmid of the IncP-1β family, pB10::gfp. This plasmid was previously constructed by inserting mini-Tn5-PA1-04/03::gfp, a Tn5 derivative transposon encoding green fluorescent protein (GFP), in the 68.3-kb plasmid pB10 (47). This plasmid is self-transmissible and codes for resistance to tetracycline, amoxicillin, sulfonamide, streptomycin, ionic mercury, and kanamycin (Km [the latter encoded on the mini-Tn5]). The antibiotics kanamycin (50 μg ml−1) and tetracycline (10 μg ml−1) were used to select for the plasmid.
Table 2 specifies the relevant characteristics of each bacterial strain used in the study. As a donor strain, we used E. coli AT1036, an auxotrophic mutant that cannot grow without diaminopimelic acid (DAP) used to supplement the growth medium. Omission of DAP from the transconjugant selective plates thus allows for easy “counterselection” against this host. E. coli EC100 was used as a recipient due to its inherent resistance to nalidixic acid (Nal), thereby allowing for selective growth of this host. Both E. coli strains were cultured on Luria-Bertani (LB) or tryptic soy (TS) medium at 30°C, and DAP or Nal was added to a final concentration of 100 μg ml−1 when required, respectively. Achromomacter, Aeromonas veronii, Plesiomonas shigelloides, Shewanella putrefaciencs, and Vibrio mimicus were cultured in TS at 26°C. To obtain Rif-resistant (Rifr) mutants of the latter four strains, they were serially subcultured in TS supplemented with increasing concentrations of rifampin: from 20 to 200 μg ml−1.
TABLE 2.
Bacterial strains used in this study
| Bacterial strain | Genotype and relevant characteristic(s) | Source |
|---|---|---|
| E. coli | ||
| AT1036 | dapD4 Δ(gpt-proA)62 lacY1 glnX44(AS) λ rfbC1 mgl51 lysA27::Mu rpsL20 (Strr) xylA5 mtl-1 thiE1 | Coli Genetic Stock Center |
| EC100 Nalr | Nalr mutant of E. coli EC100 [F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ− rpsL (Strr) nupG] | Epicentre |
| Achromobacter(pB10::gfp) | Zebrafish gut isolate containing plasmid pB10::gfp | This study |
| Achromobacter sp. strain | Plasmid-free derivative of Achromobacter(pB10::gfp) | This study |
| A. veronii sp. strain | Zebrafish gut isolate | This study |
| A. veronii(pB10::gfp) | Zebrafish gut isolate containing plasmid pB10::gfp | This study |
| A. veronii Rifr | Rifr mutant of A. veronii | This study |
| A. veronii(pB10::gfp) Rifr | Rifr mutant of A. veronii containing pB10::gfp | This study |
| P. shigelloides sp. strain | Zebrafish gut isolate | This study |
| P. shigelloides Rifr | Rifr mutant of P. shigelloides | This study |
| P. shigelloides(pB10::gfp) Rifr | Rifr mutant of P. shigelloides containing pB10::gfp | This study |
| S. putrefaciens sp. strain | Zebrafish gut isolate | This study |
| S. putrefaciens(pB10::gfp) | S. putrefaciens containing pB10::gfp | This study |
| V. mimicus sp. strain | Zebrafish gut isolate | This study |
| V. mimicus Rifr | Rifr mutant of V. mimicus | This study |
General DNA manipulation techniques.
Plasmid DNA was isolated and purified using a PureYield plasmid miniprep system (Promega), and gel electrophoresis and plasmid electroporation were carried out using standard techniques (48). Genomic DNA (gDNA) was isolated using a Genelute bacterial gDNA kit (Sigma-Aldrich). All PCR experiments, except those described below in the section “Zebrafish gut microbiome diversity,” were performed using Taq Master Mix (NEB) as per the manufacturer’s instructions. The reaction parameters included an initial denaturation step of 10 min at 94°C, followed by 30 cycles of denaturation (30 s at 94°C), a variable annealing step dependent upon the average primer annealing temperature, and an elongation step at 72°C, with the extension time depending on the amplicon size.
General zebrafish husbandry and gut bacterium isolation.
Zebrafish (Scientific Hatcheries, Inc.) of unknown genetic background were maintained in a recirculating system (Aquaneering) at 28.5°C and fed twice daily with soy protein concentrate-based pellet food unless otherwise stated. Procedures involving animals were approved by the University of Idaho Institutional Animal Care and Use Committee. Prior to harvesting of the gut bacteria, the fish were starved for 24 h and individually anesthetized with 170 mg/liter MS222 (tricaine methanosulfonate, pH ∼7.0 [Argent Laboratories]). Each fish was aseptically dissected in a petri dish, using a surgical blade. The entire fore-, mid-, and hind gut were placed into sterile 2-ml microcentrifuge tubes and placed on ice. Disposable plastic inoculation loops were used to grind the guts and squeeze out the bacteria against the round bottom of the microcentrifuge tube. To suspend the bacteria, 2 ml of PBS (pH 7.4, 4°C) was added, and the samples were vortexed vigorously for 1 min. To separate the bacterial suspension and the gut lining, the samples were centrifuged at 800 rpm for 1 min, and the bacterium-containing supernatant was transferred to a sterile 2-ml microcentrifuge tube. To collect the bacterial cells, the bacterial suspensions were centrifuged at 8,000 rpm for 4 min and the pellet suspended in 2 ml PBS (pH 7.4, 4°C). This procedure was repeated twice to wash the bacterial cells.
16S rRNA identity of clonal isolates.
Individual bacterial colonies from the combined guts of four zebrafish were obtained by spreading 100 μl of a 10-fold dilution series onto brain heart infusion (BHI), Reasoner's 2A (R2A), TS, and chocolate agar and incubated at 26°C for 48 h. All media contained cycloheximide (100 μg ml−1) to inhibit fungal growth. To obtain clonal isolates, a total of 29 colonies with unique morphologies were streaked onto their respective isolation media and TS agar (TSA). Single colonies were inoculated into 5 ml TS broth and grown overnight at 26°C, and 1 ml of each culture was used for gDNA purification. One nanogram of gDNA was used as the template for 16S rRNA gene amplification using the 27f (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492 (5′-TACGGYTACCTTGTTACGACTT-3′) 16S rRNA primers described by Roeselers et al. (27). The ∼1.6-kb PCR products were sequenced across the first ∼700 bp by Sanger sequencing at Elim Biopharmaceuticals, Inc. (Hayward, CA), using the 27f primer. The identity of clonal isolates was determined by comparing their partial 16S rRNA gene sequences, which span the V1 to V4 regions, to known sequences using SeqMatch at the Ribosomal Database Project (https://rdp.cme.msu.edu/). The RDP classifier does not provide identification beyond the genus level. Therefore, further phylogenetic analysis was performed by globally aligning the unknown sequences with best hits from the RDP using a cost matrix of 65% similarity and with gap open and extension penalties of 12 and 3, respectively. The alignment was used to construct a neighbor-joining tree using a Tamura-Nei genetic distance model and bootstrap of 100. Both tasks were executed using the Alignment and Tree Builder functions within the Geneious R10 software package. The identity of isolates used in reciprocal conjugation experiments was further confirmed by comparing the sequence generated using both the 27f and 1492 primers to reference sequences from the RDP. Species-level classifications were made if the sequences shared >98% identity to a particular reference sequence.
In vitro plasmid transfer.
The original plasmid donor in the quantitative conjugation assays with zebrafish gut isolates as recipients was E. coli AT1036(pB10::gfp), a DAP auxotroph. After these conjugation assays, the resulting transconjugants served as donors in subsequent matings with Rifr mutants of these isolates, as well as with Nalr E. coli EC100.
To investigate plasmid transfer from the E. coli donor to the zebrafish gut microbial community in vitro, the recipient community was prepared in 1 ml PBS from the combined guts of four zebrafish as described in the section “General zebrafish husbandry and gut bacterium isolation,” and the conjugation assay was carried out on R2A. For all other in vitro conjugation experiments, the donors and recipients were prepared from cultures that were grown overnight at 30°C for E. coli and 26°C for zebrafish gut isolates, and the matings were carried out on TSA.
Cells from 1 ml of each culture were collected by centrifugation at 8,000 × g for 2 min and resuspended in 100 μl PBS. The donor and recipient cultures were mixed in equal parts, applied as spots onto agar plates, and incubated for 16 h at 26°C. The medium did not contain any antibiotics, nor DAP, the lack of which prevents E. coli AT1036 from proliferating. An equal volume of donor and recipient was also applied as spots onto separate agar plates. The cells were scraped from the plate using a sterile inoculation loop and resuspended in 1.0 ml PBS buffer, and a dilution series was spread onto differentially selective agar media to enumerate the donors (50 μg ml−1 Km, 10 μg ml−1 Tc, and 100 μg ml−1 DAP), recipients (200 μg ml−1 Rif or Nal) and transconjugants (200 μg ml−1 Rif or Nal, 50 μg ml−1 Km, and 10 μg ml−1 Tc). Colonies were counted after 2 to 3 days of incubation at 26°C. Green fluorescent transconjugants were also verified by excitation at 488 nm, and donor, recipient, and transconjugant identities were determined by comparing their 16S rRNA gene sequences to known sequences within the RDP database, as described above.
Plasmid persistence assays.
The ability of pB10::gfp to persist in a host was determined by monitoring the fraction of plasmid-containing cells in a population over 10 days, as described previously (49). Briefly, precultures were grown overnight in the presence of kanamycin and tetracycline, and on each subsequent day, 4.9 μl of culture was transferred into 5 ml of fresh medium without antibiotics and incubated in a shaking incubator for 24 h, yielding approximately 10 generations per day. Cultures were spread daily onto nonselective TSA such that approximately 100 to 400 colonies were obtained per sample. The fraction of plasmid-containing colonies within each sampled population was determined by counting the fluorescent and nonfluorescent colonies during exposure to a 488-nm light source.
In vivo plasmid transfer.
Thirty-two zebrafish of mixed sex were randomly divided into eight groups of four. Each group was put into one of eight tanks (width, 10 cm; height, 15 cm; depth, 25 cm) containing 2 liters of filtered water. The eight tanks were divided into two treatment groups in which the fish were fed dry food treated with E. coli AT1036 that either contained plasmid pB10::gfp (treated tanks: A to D) or was plasmid free (untreated tanks: E to H). The water was replaced daily with freshwater containing 20 μg ml−1 tetracycline, and the fish were fed twice daily with ∼35 mg food per tank. The food was prepared every 7 days by suspending the E. coli AT1036 cells with or without plasmid in soybean oil and mixing it with the soy protein-based pellet food (Tetramin) such that the final concentration was ∼1 × 106 CFU mg−1 dry food (the CFU count dropped slightly to ∼5 × 105 CFU mg−1 during storage over a 9-day period). Thus, each 2-liter tank received approximately 3 × 107 CFU daily. The fish were fed with the E. coli-containing food for 20 days. During the last 2 days, they were fed with untreated food to minimize E. coli donor cells in the gut at the time of harvest. The presence of the donor at that time could confound the transconjugant counts as it may result in plasmid transfer on agar plates rather than in the zebrafish gut.
On the 23rd day, all fish were euthanized, and their gut material was harvested aseptically by dissection as described in the section “General zebrafish husbandry and gut bacterium isolation.” The gut content from four fish per tank was then pooled and suspended in PBS using the pestle and mortar technique, and bacterial counts were determined by plating a 10-fold dilution series onto different selective TS agar media as follows. Total culturable CFU were quantified on TSA lacking all antibiotics, except cycloheximide (100 μg ml−1). Donor bacteria were quantified based on fluorescence on TSA supplemented with the pB10::gfp-selective antibiotics tetracycline (10 μg ml−1), kanamycin (50 μg ml−1), and streptomycin (50 μg ml−1), as well as with 100 μM DAP to support the growth of the auxotrophic E. coli AT1036 host. Transconjugant bacteria were enumerated on TSA plates supplemented with the same plasmid-selective antibiotics but no DAP (thus counterselecting the donor). The transconjugant bacteria were distinguishable from the intrinsically resistant gut microbiota based on the fluorescent phenotype encoded by pB10::gfp. It should be noted that such transconjugant enumerations will always be an underestimation, due not only to limited culturability of all gut bacteria but also because not all bacteria can properly express and fold the fluorescent protein (50).
Zebrafish gut microbiome diversity.
After sampling the bacterial cell suspensions from the in vivo plasmid transfer experiment for enumeration by plate counting, the remainder of the bacterial cells were collected by centrifugation at 8,000 × g and stored at −20°C. To account for the bacterial diversity in the water and food, a water control was constructed as follows. Approximately 20 mg of food was mixed with 20 μl of soybean oil and suspended in 2 ml of the system water.
The gDNA was extracted from the zebrafish gut microbiome and water control samples using the two-step enzymatic and bead-beating lysis method described by Yuan et al. (51). Briefly, the frozen cells were thawed on ice and suspended in 900 μl Tris-EDTA buffer (TE; pH 8.0). Fifty microliters of lysozyme (10 mg/ml; Sigma-Aldrich), 6 μl mutanolysin (25 kU/ml; Sigma-Aldrich), and 3 μl lysostaphin (4,000 U/ml; Sigma-Aldrich) were added to 250 μl cell suspension and incubated for 1 h at 37°C. Thereafter, 600 mg of 0.1-mm-diameter zirconia-silica beads (BioSpec) were added to the lysate, and the microbial cells were mechanically disrupted with a Mini-BeadBeater-96 (BioSpec) at 2,100 rpm for 1 min. The gDNA was purified from the lysate using a QIAamp DNA minikit (Qiagen).
Sequence libraries of the V1 to V3 region of the 16S rRNA genes from each of the samples were constructed in accordance with the “Dual barcoded two-step PCR” procedure from Illumina (52). Briefly, using the universal 16S rRNA primers 27F and 534R, the V1 to V3 region of 16S rRNA genes was amplified from 2 ng of DNA in 50-μl reaction mixtures containing the following components: 1× standard Taq reaction buffer (NEB), 3 mM MgCl2 (NEB), 0.24 mg ml−1 bovine serum albumin (BSA; Fermentas), 200 μM deoxynucleoside triphosphates (dNTPs; Fermentas), 50 nM each of the 27f and 534r primer mixtures, and 0.025 U μl−1 Taq DNA polymerase (NEB). The cycling parameters were 95°C for 2 min, followed by 20 cycles of 95°C for 1 min, 51°C for 1 min, and 68°C for 1 min, followed by 68°C for 10 min and a hold step at 10°C. The PCR products were purified using a QIAquick PCR purification kit and visualized on a 1% agarose gel. To attach the barcodes, the PCR products were diluted 15-fold in PCR-grade water, and 1 μl of each was transferred into 20 μl PCR mixture containing 1× standard Taq reaction buffer, 4.5 mM MgCl2, 0.24 mg ml−1 BSA, 75 nM the barcoded primer, 200 μM dNTPs, and 0.05 U μl−1 Taq DNA polymerase. The cycling parameters were 95°C for 1 min, followed by 10 cycles of 95°C for 30 s, 60°C for 30 s, and 68°C for 1 min, followed by 68°C for 5 min and a hold step at 10°C. The PCR products were once again purified using a QIAquick PCR purification kit and visualized on a 1% agarose gel. The purified, barcoded amplicon libraries were quantified, pooled equimolar, and prepared for sequencing on a MiSeq DNA sequencer (Illumina) by the Genomics Resources Core Facility at the Institute for Bioinformatics and Evolutionary Studies (Moscow, ID) according to their standard operating procedures.
Raw unclipped DNA sequence reads from the Illumina platform were cleaned, assigned, and filtered by the Genomics Resources Core Facility using custom scripts, after which the sequence reads were assigned to bacterial taxa using the Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences (53) at the Ribosomal Database Project (https://rdp.cme.msu.edu/). The operational taxonomic unit (OTU) table was interrogated and visualized using R 3.3.0.
Data availability.
The 16S RNA gene sequences have been deposited in the Sequence Read Archive at NCBI under accession no. PRJNA601447.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded in part by National Institute of Allergy and Infectious Diseases grant R01 AI084918 from the National Institutes of Health (NIH). D.P. was supported by a summer fellowship from the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the NIH (grant no. P20GM103408). We are also grateful to the IBEST Genomics Research Core for help with DNA sequencing, supported by an Institutional Development Award (IDeA) from the NIGMS of the NIH under grant no. P30 GM103324.
We also thank Matt Singer for dissecting the zebrafish from the in vivo experiment.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Kåhrström CT. 2013. Entering a post-antibiotic era? Nat Rev Microbiol 11:146. 10.1038/nrmicro2983. [DOI] [Google Scholar]
- 2.World Health Organization. 2018. Global Antimicrobial Resistance Surveillance System (GLASS) report: early implementation 2017–2018. World Health Organization, Geneva, Switzerland.
- 3.World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland.
- 4.Broaders E, Cormac GM, Marchesi G, Marchesi JR. 2013. Mobile genetic elements of the human gastrointestinal tract. Gut Microbes 4:271–280. 10.4161/gmic.24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carattoli A. 2013. Plasmids and the spread of resistance. Int J Med Microbiol 303:298–304. 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Mathers AJ, Peirano G, Pitout JDD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson DI, Hughes D. 2012. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist Updat 15:162–172. 10.1016/j.drup.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Piddock L. 2017. Understanding drug resistance will improve the treatment of bacterial infections. Nat Rev Microbiol 15:639–640. 10.1038/nrmicro.2017.121. [DOI] [PubMed] [Google Scholar]
- 9.Aminov RI. 2011. Horizontal gene exchange in environmental microbiota. Front Microbiol 2:158. 10.3389/fmicb.2011.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licht T, Christensen B, Krogfelt K, Molin S. 1999. Plasmid transfer in the animal intestine and other dynamic bacterial populations: the role of community structure and environment. Microbiology 145:2615–2622. 10.1099/00221287-145-9-2615. [DOI] [PubMed] [Google Scholar]
- 11.García-Quintanilla M, Ramos-Morales F, Casadesús J. 2008. Conjugal transfer of the Salmonella enterica virulence plasmid in the mouse intestine. J Bacteriol 190:1922–1927. 10.1128/JB.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinnebusch BJ, Rosso ML, Schwan TG, Carniel E. 2002. High-frequency conjugative transfer of antibiotic resistance genes to Yersinia pestis in the flea midgut. Mol Microbiol 46:349–354. 10.1046/j.1365-2958.2002.03159.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda A, Usui M, Okubo T, Tamura Y. 2016. Horizontal transfer of plasmid-mediated cephalosporin resistance genes in the intestine of houseflies (Musca domestica). Microb Drug Resist 22:336–341. 10.1089/mdr.2015.0125. [DOI] [PubMed] [Google Scholar]
- 14.Fu J, Yang D, Jin M, Liu W, Zhao X, Li C, Zhao T, Li C, Zhao T, Wang J, Gao Z, Shen Z, Qiu Z, Li J. 2017. Aquatic animals promote antibiotic resistance gene dissemination in water via conjugation: role of different regions within the zebra fish intestinal tract, and impact on fish intestinal microbiota. Mol Ecol 26:5318–5333. 10.1111/mec.14255. [DOI] [PubMed] [Google Scholar]
- 15.Balis E, Vatopoulos AC, Kanelopoulou M, Mainas E, Hatzoudis G, Kontogianni V, Malamou-Lada H, Kitsou-Kiriakopoulou S, Kalapothaki V. 1996. Indications of in vivo transfer of an epidemic R plasmid from Salmonella enteritidis to Escherichia coli of the normal human gut flora. J Clin Microbiol 34:977–979. 10.1128/JCM.34.4.977-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datta N, Richards H, Datta C. 1981. Salmonella typhi in vivo acquires resistance to both chloramphenicol and co-trimoxazole. Lancet 317:1181–1183. 10.1016/S0140-6736(81)92350-3. [DOI] [PubMed] [Google Scholar]
- 17.Smillie CS, Smith MB, Friedman J, Cordero OX, David LA, Alm EJ. 2011. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480:241–244. 10.1038/nature10571. [DOI] [PubMed] [Google Scholar]
- 18.Ogilvie LA, Firouzmand S, Jones BV. 2012. Evolutionary, ecological and biotechnological perspectives on plasmids resident in the human gut mobile metagenome. Bioeng Bugs 3:13–31. 10.4161/bbug.3.1.17883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns AR, Stephens WZ, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJM. 2016. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J 10:655–664. 10.1038/ismej.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan CC, Love DR. 2012. Molecular characterization of bacterial community structure along the intestinal tract of zebrafish (Danio rerio): a pilot study. ISRN Microbiol 2012:590385. 10.5402/2012/590385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo P, Iturria I, Mohedano ML, Caggianiello G, Rainieri S, Fiocco D, Angel Pardo M, López P, Spano G. 2015. Zebrafish gut colonization by mCherry-labelled lactic acid bacteria. Appl Microbiol Biotechnol 99:3479–3490. 10.1007/s00253-014-6351-x. [DOI] [PubMed] [Google Scholar]
- 22.Stephens WZ, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJM. 2016. The composition of the zebrafish intestinal microbial community varies across development. ISME J 10:644–654. 10.1038/ismej.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popowska M, Krawczyk-Balska A. 2013. Broad-host-range IncP-1 plasmids and their resistance potential. Front Microbiol 4:44. 10.3389/fmicb.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuševljak N, Dutil L, Rajić A, Uhland FC, McClure C, St‐Hilaire S, Reid‐Smith RJ, McEwen SA. 2013. Antimicrobial use and resistance in aquaculture: findings of a globally administered survey of aquaculture‐allied professionals. Zoonoses Public Health 60:426–436. 10.1111/zph.12017. [DOI] [PubMed] [Google Scholar]
- 25.Garrity GM, Brenner DJ, Krieg NR, Staley JT (ed). 2005. Bergey's manual of systematic bacteriology, vol 2. The Proteobacteria, part C: the Alpha-, Beta-, Delta-, and Epsilonproteobacteria. Springer, New York, NY. [Google Scholar]
- 26.De Gelder L, Ponciano JM, Joyce P, Top EM. 2007. Stability of a promiscuous plasmid in different hosts: no guarantee for a long-term relationship. Microbiology (Reading) 153:452–463. 10.1099/mic.0.2006/001784-0. [DOI] [PubMed] [Google Scholar]
- 27.Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. 2011. Evidence for a core gut microbiota in the zebrafish. ISME J 5:1595–1608. 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloesges T, Popa O, Martin W, Dagan T. 2011. Networks of gene sharing among 329 proteobacterial genomes reveal differences in lateral gene transfer frequency at different phylogenetic depths. Mol Biol Evol 28:1057–1074. 10.1093/molbev/msq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H, Yano H, Brown CJ, Top EM. 2010. Predicting plasmid promiscuity based on genomic signature. J Bacteriol 192:6045–6055. 10.1128/JB.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland.
- 31.De Gelder L, Vandecasteele FPJ, Brown CJ, Forney LJ, Top EM. 2005. Plasmid donor affects host range of promiscuous IncP-1β plasmid pB10 in an activated-sludge microbial community. Appl Environ Microbiol 71:5309–5317. 10.1128/AEM.71.9.5309-5317.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dionisio F, Matic I, Radman M, Rodrigues OR, Taddei F. 2002. Plasmids spread very fast in heterogeneous bacterial communities. Genetics 162:1525–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benz F, Huisman JS, Bakkeren E, Herter JA, Stadler T, Ackermann M, Diard M, Egli A, Hall AR, Hardt W, Bonhoeffer S. 2020. Plasmid- and strain-specific factors drive variation in ESBL-plasmid spread in vitro and in vivo. ISME J 10.1038/s41396-020-00819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta N, Hedges RW, Shaw EJ, Sykes RB, Richmond MH. 1971. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol 108:1244–1249. 10.1128/JB.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santini JM, Stanisich VA. 1998. Both the fipA gene of pKM101 and the pifC gene of F inhibit conjugal transfer of RP1 by an effect on traG. J Bacteriol 180:4093–4101. 10.1128/JB.180.16.4093-4101.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gama JA, Zilhão R, Dionisio F. 2017. Conjugation efficiency depends on intra and intercellular interactions between distinct plasmids: plasmids promote the immigration of other plasmids but repress co-colonizing plasmids. Plasmid 93:6–16. 10.1016/j.plasmid.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Bingle LE, Thomas CM. 2001. Regulatory circuits for plasmid survival. Curr Opin Microbiol 4:194–200. 10.1016/s1369-5274(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 38.Adamczyk M, Jagura-Burdzy G. 2003. Spread and survival of promiscuous IncP-1 plasmids. Acta Biochim Pol 50:425–453. 10.18388/abp.2003_3696. [DOI] [PubMed] [Google Scholar]
- 39.Ponciano JM, De Gelder L, Top EM, Joyce P. 2007. The population biology of bacterial plasmids: a hidden Markov model approach. Genetics 176:957–968. 10.1534/genetics.106.061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norberg P, Bergström M, Jethava V, Dubhashi D, Hermansson M. 2011. The IncP-1 plasmid backbone adapts to different host bacterial species and evolves through homologous recombination. Nat Commun 2:268. 10.1038/ncomms1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart FM, Levin BR. 1977. The population biology of bacterial plasmids: a priori conditions for the existence of conjugationally transmitted factors. Genetics 87:209–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musovic S, Oregaard G, Kroer N, Sørensen SJ. 2006. Cultivation-independent examination of horizontal transfer and host range of an IncP-1 plasmid among Gram-positive and Gram-negative bacteria indigenous to the barley rhizosphere. Appl Environ Microbiol 72:6687–6692. 10.1128/AEM.00013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waters VL. 1999. Conjugative transfer in the dissemination of beta-lactam and aminoglycoside resistance. Front Biosci 4:D433–D456. 10.2741/waters. [DOI] [PubMed] [Google Scholar]
- 44.Cantas L, Roger J, Sørby T, Aleström P, Sørum H. 2012. Culturable gut microbiota diversity in zebrafish. Zebrafish 9:26–37. 10.1089/zeb.2011.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marbouty M, Baudry L, Cournac A, Koszul R. 2017. Scaffolding bacterial genomes and probing host-virus interactions in gut microbiome by proximity ligation (chromosome capture) assay. Sci Adv 3:e1602105. 10.1126/sciadv.1602105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stalder T, Press MO, Sullivan S, Liachko I, Top EM. 2019. Linking the resistome and plasmidome to the microbiome. ISME J 13:2437–2446. 10.1038/s41396-019-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Meervenne E, Van Coillie E, Kerckhof FM, Devlieghere F, Herman L, De Gelder LS, Top EM, Boon N. 2012. Strain-specific transfer of antibiotic resistance from an environmental plasmid to foodborne pathogens. Biomed Res Int 2012:834598. 10.1155/2012/834598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 49.Loftie-Eaton W, Yano H, Burleigh S, Simmons RS, Hughes JM, Rogers LM, Hunter SS, Settles ML, Forney LJ, Ponciano JM, Top EM. 2016. Evolutionary paths that expand plasmid host-range: implications for spread of antibiotic resistance. Mol Biol Evol 33:885–897. 10.1093/molbev/msv339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cormack BP, Valdivia RH, Falkow S. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38. 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 51.Yuan S, Cohen DB, Ravel J, Abdo Z, Forney LJ. 2012. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS One 7:e33865. 10.1371/journal.pone.0033865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Illumina. 2013. 16S metagenomic sequencing library preparation. Illumina, San Diego, CA.
- 53.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S RNA gene sequences have been deposited in the Sequence Read Archive at NCBI under accession no. PRJNA601447.