Graphical abstract
Keywords: Emulsion, Physical stability, Myofibrillar protein, High-intensity ultrasound, Low ionic strength
Highlights
-
•
The assembly behavior of myosin molecules limits the development of interfacial proteins.
-
•
High-intensity ultrasound (HIU) disrupted the filamentous myosin structure.
-
•
HIU improved the physical stability of myofibrillar protein (MPs) emulsion.
-
•
HIU pretreatment promoted the adsorption and rearrangement of MPs at the O/W interface.
-
•
HIU pretreatment enhanced the inter-droplet interactions.
Abstract
The specific molecular behavior of myofibrillar proteins (MPs) in low-salt media limits the development of muscle protein-based emulsions. This study aimed to evaluate the potential of high-intensity ultrasound (HIU; 150, 300, 450, and 600 W) to improve the physical stability of MP emulsion at low ionic strength and decipher the underlying mechanism. According to the physical stability analysis, HIU pretreatment, especially at 450 W power, significantly improved the physical stability of MP emulsions, as evidenced by the reduced particle size, enhanced inter-droplet interactions, and increased uniformity of the droplet size distribution (p < 0.05). The results of interfacial protein composition, Fourier transform infrared spectroscopy analysis, and microscopic morphology observation of the aqueous MP suspension suggested that HIU induced the depolymerization of filamentous myosin polymers and inhibited the subsequent self-assembly behavior. These effects may facilitate protein adsorption and molecular rearrangement at the oil–water interface, forming a complete interfacial layer and, thus, droplet stabilization. Confocal laser scanning microscopy observations further confirmed these results. In conclusion, these findings provide direct evidence for the role of HIU in improving the physical stability of MP emulsions at low ionic strength.
1. Introduction
An emulsion is a heterogeneous system consisting of two immiscible liquids (usually oil and water), one of which (the dispersed phase) is dispersed in another liquid (the continuous phase) in the form of small spherical droplets [1]. Conventional emulsions are generally categorized based on the arrangement of the two immiscible liquids as either water-in-oil or oil-in-water (O/W) types. The use of O/W emulsions as a delivery system for lipophilic bioactive substances has been widely employed in the food industry, particularly in foods such as beverages, ice cream, and sauces [2]. However, this system is thermodynamically unstable, tending toward phase separation over time through a series of physicochemical processes, such as flocculation, gravitational separation, Ostwald ripening, and creaming [3]. This challenge can often be overcome by adding surface-active substances (surfactants). There has been considerable interest in using natural proteins as surfactants to stabilize emulsions because of their amphiphilic properties that endow them with the ability to coat oil droplets while providing additional nutrients [4]. Most of the natural protein emulsifiers used in the food industry to date are plant- and milk-derived proteins, including soy, pea, flaxseed, casein, ovalbumin, and whey protein isolate [5], [6], [7].
Meat-derived proteins, particularly myofibrillar proteins (MPs), which account for approximately 50% of the total meat proteins, are usually regarded as “high quality” proteins because they contain all the essential amino acids (with a particularly high content of lysine) and are all highly digestible [8]. As early as the 1980s, Dickinson, et al. [9] reported that the surface activity of native MPs is almost the same as that of casein and much greater than that of gelatin. As a surface-active ingredient, however, MPs have not been fully utilized to the same extent as plant- or milk-derived proteins, and most of the existing studies on MP emulsification have focused on meat systems, such as frankfurter-type sausage [10]. Salt-soluble myosin is the major protein in MPs and responsible for a diverse range of functions in food systems. Myosin tends to pack and coil in low-salt media via electrostatic interactions in the tail (rod) region to form an insoluble filamentous polymer termed the myosin filament, thus rendering the MPs insoluble and unstable [11], [12]. This behavior makes it difficult for MP to be used effectively in low-ionic-strength media. Although MPs can be almost completely solubilized in relatively high-salt conditions (0.47–0.68 M NaCl), this is undesirable for a healthy diet [13]. Furthermore, high-salt is believed to be unfavorable to emulsion stability, partly due to charge shielding by the salt ions [14]. Hence, how to improve the physical stability of MP emulsion at low ionic strength is valuable for the development of low-salt emulsified meat products, and novel meat protein-based products such as protein beverages and fluid diets.
High-intensity ultrasound (HIU) is widely used in the food industry to inactivate microorganisms for prolonging the product shelf life and, to modify the functional properties of materials for enhancing the quality of various systems [15]. Recently, Li, et al. [16] reported that the stability of emulsion prepared by chicken myofibrillar protein could be effectively improved after HIU treatment. Chen et al. [17] reported a similar finding in cod myofibrillar protein, and they attributed this improvement to the physical modification of protein structure by HIU treatment. However, these studies have been conducted in the high salt media (0.6 M NaCl), while few studies have focus on the effect of HIU on the MP emulsion at low ionic strength. Moreover, our previous results showed that HIU treatment could dramatically improve the solubility of MPs in low-ionic-strength media [11]. For most proteins, the emulsifying properties are notably benefited by good solubility, but for MPs, the particular molecular behavior of myosin in low-ionic-strength media seems to lead to a unique interfacial property [5], [18], [19].
In this context, it would be valuable to understand the instability mechanism of the MP emulsion at low ionic strength and evaluate how HIU pretreatment of the proteins impacts its physical stability. These goals formed the basis of the present work. The physical stability, particle size and distribution, and zeta-potential of O/W emulsions prepared with an aqueous suspension of MPs pretreated by HIU at different output powers (150, 300, 450, and 600 W) were investigated. The interfacial protein was characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the structure and morphology of proteins in water were monitored by Fourier transform infrared (FTIR) spectroscopy and confocal laser scanning microscopy (CLSM), respectively, to enhance the understanding of the mechanism by which HIU induces the changes in the molecular behavior of proteins at the oil–water interface.
2. Materials and methods
2.1. Materials
Fresh pork meat (longissimus dorsi) was purchased within 24 h of slaughter from a local market (Harbin, Heilongjiang, China). After removing the non-muscle material (e.g., fat, skin, connective tissue), the pork longissimus muscle was stored in a −30 °C freezer and used within 1 week. Soybean oil was purchased from Jinlongyu Co., Ltd. (Beijing, China). All chemicals and reagents were at least of analytical grade.
2.2. Extraction of myofibrillar proteins (MPs)
The MPs were extracted according to a previous method [11]. In brief, the minced meat was homogenized with a 20 mM sodium phosphate buffer (pH 7.0, 25 mM NaCl, 5.0 mM EDTA), followed by centrifugation (10,000g, 15 min) at 4 °C. The resulting pellets were washed with 20 mM sodium phosphate buffer (pH 7.0, 0.1 M NaCl, 5.0 mM EDTA) three more times, and connective tissue and external fat were removed by filtration through four layers of coarse mesh gauze. Finally, the purified pellets were washed twice with 5.0 mM sodium phosphate buffer (1.0 mM EDTA, pH 7.0) to remove ionic. The extracted MPs were stored at 4 °C and used within 48 h of isolation.
2.3. High-intensity ultrasound (HIU) treatment
The HIU treatments were performed using a Scientz-II D ultrasound generator (Scientz Biotechnology Co., Ltd., Ningbo, China). The device was equipped with a 6.0-mm (diameter) titanium probe. The isolated MPs (10 mg/mL) were dispersed in 5.0 mM sodium phosphate (pH 7.0, 1.0 mM EDTA) and treated in a 50-mL beaker. The titanium probe was immersed in the liquid to a depth of 1.5 cm from the bottom, followed by treatment with ultrasound at 20 kHz at various output powers (0, 150, 300, 450 and 600 W) for 15 min (pulse durations of 2.0 s on and 3.0 s off). For maintaining the sample temperature below 20 °C, a rapid cooling system was implemented by circulating the ice water around the vessel during the ultrasound process.
2.4. Emulsion preparation
Emulsions were prepared by slight modifications of the method described in our previous study [19]. Briefly, 5.0 g of soybean oil was mixed with 45 mL of aqueous MP suspension pretreated with different ultrasound powers (0, 150, 300, 450, and 600 W), then pre-emulsified using an Ultra-Turrax homogenizer (IKA T20 Basic, IKA-Werke GmbH and Co. KG, Staufen, Germany) at a speed of 10,000 rpm for 3 min. These coarse emulsions were further homogenized in a high-pressure homogenizer (ATS Engineer, Inc., Shanghai, China) at 60 MPa for two cycles, with a 5-min rest between cycles. The resulting fresh emulsions were used immediately for further experiments. In addition, to observe the storage stability of the MP emulsions, the particle size and appearance of all samples were recorded at the start and end of storage for 7 days at room temperature (approximately 22 °C). Sodium azide (3.0 mM in final emulsions) was added to all samples to prevent microbial growth during storage.
2.5. Physical stability analysis
Physical stability of emulsions prepared from MPs pretreated with different HIU powers was monitored using a LUMiSizer analytical centrifuge (LUM GmbH, Berlin, Germany) according to Liu et al. [20]. For this test, the LUMiSizer was set at 25 °C; rotational speed, 4000 rpm; scanning rate, once every 20 s for 6000 s; the number of scanning times, 300. During testing, the continuous centrifugal sedimentation accelerates instability processes, such as flocculation, aggregation, and creaming. Simultaneously, the parallel near-infrared (IR) light is passed through the entire sample cell, collecting the intensity of transmitted light as a function of time and position over the entire sample cell length. Data were shown as space- and time-related transmission profiles over the sample cell length. The curves of the integrated level of transmitted light plotted against time represent the “creaming rate” of the sample per scan during centrifugation. A higher creaming rate usually corresponds to lower physical stability [21].
2.6. Measurements of droplet size, size distribution, and zeta-potential of emulsions
Zeta-potential, as well as particle size, distribution, and polydispersity index (PDI), were monitored by static light scattering using a Mastersizer 2000 instrument (Malvern Instruments Ltd., Worcestershire, UK) and a zeta-potential analyzer (Zeta Plus, Malvern, UK), respectively, following a previously described procedure [19]. Before measurement, all emulsion samples were diluted 100-fold with 0.01 M phosphate buffer (pH 7.0) to avoid dynamic light scattering effects.
2.7. Characteristics of interfacial proteins
Adsorbed and non-adsorbed proteins within the emulsion systems (see Section 2.4) were separated by centrifugation (10,000g, 4 °C, 30 min), as reported by Li et al. [16]. After centrifugation, the serum phase was carefully collected using a long needle syringe to determine its non-adsorbed protein concentration by the Biuret reagent. The adsorbed MPs concentrations were then calculated as the difference between the protein content used for the original emulsion and that in the serum phase.
To identify the adsorbed MPs in the collected cream layer and the non-adsorbed MPs in the serum phase, SDS-PAGE was performed as previously described [11]. Before electrophoresis, the samples were diluted 5-fold with 5.0 mM sodium phosphate (pH 7.0, 1.0 mM EDTA), centrifuged (10,000g, 4 °C, 30 min) three times, and the recovered precipitate was carefully filtered through Whatman #1 filter paper to purify the protein. The protein was then re-dissolved in sample buffer (1 M Tris-HCl, 50% (v/v) glycerol, 20% (w/v) β-mercaptoethanol, 10% (w/v) SDS, and 1% (w/v) bromophenol blue) and heated at 100 °C for 3 min and cooled down prior to gel running. Then, 10 μL of each sample and markers were loaded on a 4–20% Bis-Tris gradient gel. The electrophoretic analysis was performed on a Bio-Rad Mini-PROTEAN II System Cell apparatus (Bio-Rad Laboratories Inc., Hercules, CA, USA) at a constant voltage of 120 V for 1 h. Gels were stained with Coomassie Brilliant Blue R-250 and decolorized with 10% (v/v) acetic acid and 5% (v/v) ethanol.
2.8. Fourier transform infrared spectroscopy analysis (FTIR) of myofibrillar proteins (MPs) in water
The dissociation of myofibrils into subunits of MPs was studied in water by recording the IR absorption data between 4000 and 500 cm−1 using a Nicolet 6700 spectrophotometer (Thermo Fisher Scientific, Madison, WI, USA) at room temperature (approximately 22 °C). Before determination, the sample suspensions were freeze-dried, mixed with potassium bromide in a certain proportion, and pelleted.
Accurate information on the changes in the α-helix of MPs was obtained by performing Fourier self-deconvolution of the amide-I bands using OMNIC 7.2 software (Thermo-Nicolet, Thermo Fisher Scientific). The α-helix contents of MPs were calculated by the method of Kong and Yu [22], using the integrated area of the peak characteristic of α-helix.
2.9. Confocal laser scanning microscopy (CLSM)
Micromorphology of the HIU-treated MP aqueous suspensions and the emulsion droplets coated by MPs treated with different HIU powers was observed under a Leica TCS SP5 confocal laser scanning microscope (Leica, Heidelberg, Germany) as described in our previous work with some modifications [1]. The protein and oil in MP emulsions (1.0 mL) were stained by mixing with 25 μL of Nile blue (0.1% in water) and 20 μL of Nile red, respectively, then incubated at room temperature for 30 min in the dark. Stained samples were placed on concave microscope slides with coverslips to observe the protein phase (633-nm helium–neon laser) and the oil phase (488 nm argon laser) using a 40 × HCPL APO/20 × oil-immersion objective.
2.10. Statistical analysis
All measurements were performed in triplicate, and data are displayed as mean ± standard deviation (SD). Significant differences between means (p < 0.05) were determined by one-way analysis of variance (ANOVA) with Tukey's multiple comparisons using the Statistix version 8.1 software package (Analytical Software, St Paul, MN, USA).
3. Results and discussion
3.1. Physical stability of emulsions
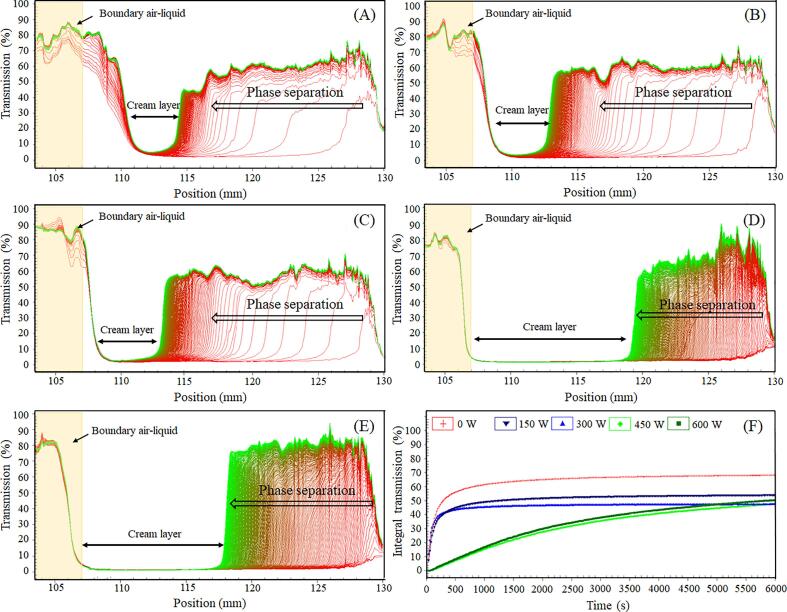
Fig. 1A–E shows the space- and time-resolved extinction profiles of MP emulsions during the analytical centrifugation. The liquid-air phase boundary (concave meniscus) shows a high transmission and is located left of the 107 mm from the center of rotation, while the low transmission to the right of 107 mm represents the cream layer. The greater the change in transmission of the overall profile with centrifugation, the less stable the emulsion [23]. As expected, the native MP (0 W) emulsion displayed a significant increase in transmission with centrifugation time (Fig. 1A), indicating its poor physical stability. In addition, it still showed a relatively higher transmission on the right side of the 107 mm than the other samples (Fig. 1A), which reveals an extensive phase separation, resulting in a cream layer of high transmission due to oil droplet accumulation in the upper region of the tube [24]. For obtaining high physically stable protein-based emulsions, the most important property of the amphiphilic proteins is their ability to form a stable amphipathic membrane at oil–water interfaces, with hydrophobic groups promptly mixed into the oil phase and the hydrophilic groups extending into the water phase [4]. Although the amphiphilic MPs possess the ability to form an interfacial membrane, the subsequent myosin assembly by rod–rod interactions would lead to an irreproducible interfacial behavior [18]. It could be argued then that the formation of an orderly self-assembled structure is the primary reason for the poor physical stability of MP emulsions.
Fig. 1.
The original transmission of the emulsions prepared by myofibrillar proteins (MPs) treated with different high-intensity ultrasound (HIU) powers at low ionic strength tested by LUMiSizer (A–E, MPs treated with HIU of 0, 150, 300, 450, 600 W, respectively). The integrated transmission-time plots (F) of emulsions prepared by MP treated with different HIU powers.
Although the HIU-treated MPs also produced emulsions that exhibited a typical phase separation process (the transmission profile migrating toward the left over time), the physical stability was improved compared with the native MP emulsion, as indicated by the change in the transmission profile (Fig. 1B–E). It is evident that the phase separation process of emulsions prepared with HIU-treated MPs differed from that of the native MP emulsion, speculating that HIU might improve the physical stability by altering the interfacial behavior of MPs, and this effect was power-dependent. Emulsions prepared from MPs pretreated by HIU at a power output of 450 W showed excellent physical stability among the investigated emulsion samples, as evidenced by the slower change in the transmission profile over time (Fig. 1D). These findings were further corroborated by the time-dependent integrated transmission profiles (Fig. 1F), which showed that the slope of profiles decreased when the HIU power increased, reflecting the improvement of the physical stability of the emulsion. It is plausible that the myosin filament assembled in water was destroyed and suppressed by HIU [11], [25], releasing free myosin monomers with an improved ability to form interfacial membranes and stabilize the oil droplets. Similar results have been reported by Ma et al. [26], who found that the high-pressure homogenization pretreatment could be effectively enhanced the emulsion stability of cod myofibrillar proteins, and attributed this effect to the physical modification of protein structure through the mechanical force provided by high-pressure homogenization. Notably, treatment with HIU at the power output of 600 W adversely influenced the physical stability of the emulsion, as reflected by the increase in transmittance (Fig. 1E). This result is probably related to the internal thermal effects of such high-power HIU treatments, which could lead to the aggregation of protein, resulting in an unstable interface behavior [27], [28].
3.2. Droplets characteristics of MP emulsion
3.2.1. Particle size and distribution
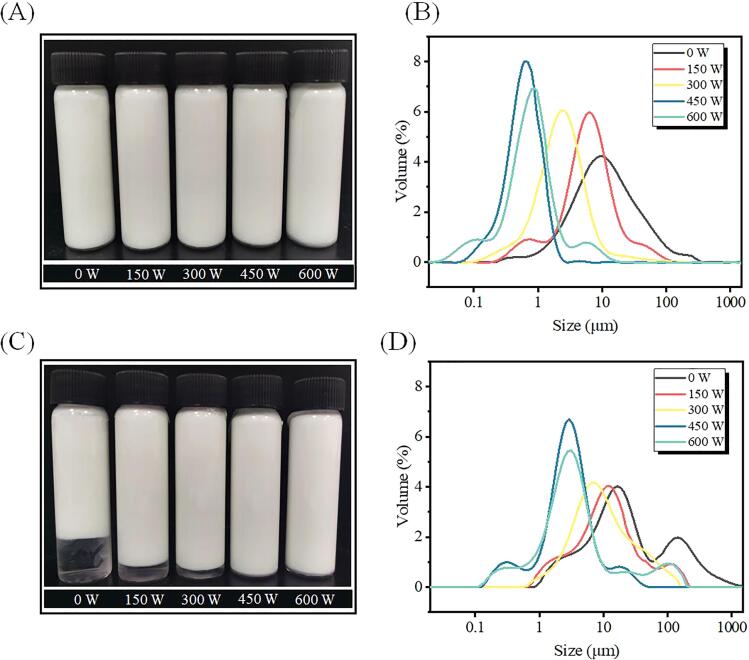
To further investigate the potential mechanism by which HIU pretreatment of the MPs enhanced the physical stability of the MP emulsions in low-salt media, the particle distribution, size (d4,3), and the PDI of the samples were analyzed before and after storage for 7 days. All the fresh emulsions showed a uniform white appearance (Fig. 2A). The fresh emulsion prepared from native MPs exhibited a wide particle size distribution (Fig. 2B) with relatively large particle mean diameters (Table 1). Applying HIU pretreatment shifted the peak of the particle distribution to the left but also decreased the particle size (d4,3) of the formed emulsions (Fig. 2B and Table 1), especially at the HIU power output of 450 W (p < 0.05), which resulted in the lowest PDI, indicating the highest particle homogeneity among all the samples tested (p < 0.05) (Table 1). These results illustrate that HIU treatment enhances the ability of MPs to form an emulsion. It is well known that the molecular size of a protein influences its emulsification properties. Generally, small proteins diffuse more readily than larger bulkier ones and usually show excellent emulsion-forming abilities [29]. It follows that during HIU treatment, the intense physical forces acting on the MPs cause the depolymerization of filamentous myosin polymer into smaller subunits or monomers, thereby promoting protein–oil interactions. A similar finding has emerged in HIU-treated chicken MPs in high-salt media (0.6 M NaCl). Although there are few myosin filaments in high-salt media, the same principle applies because most MPs still exist as macromolecules [16].
Fig. 2.
The appearance (A and C), and particle size distribution (B and D) of the emulsions prepared by myofibrillar proteins (MPs) treated with different high-intensity ultrasound (HIU) powers (0–600 W) at low ionic strength, before (A and B) and after (C and D) storage at approximately 22 °C for 7 day.
Table 1.
Effects of different high-intensity ultrasound (HIU) powers on the particle size (d4,3) and polydispersity index (PDI) of the emulsions prepared by HIU-treated myofibrillar proteins (MPs) at low ionic strength, before and after storage at approximately 22 °C for 7 day.
| HIU power (W) |
d4,3 (μm) |
PDI |
||
|---|---|---|---|---|
| day 0 | day 7 | day 0 | day 7 | |
| 0 | 29.07 ± 0.63a | 81.64 ± 1.23a | 2.21 ± 0.12a | 3.27 ± 0.09a |
| 150 | 12.90 ± 0.82b | 34.29 ± 1.78b | 1.71 ± 0.06b | 2.13 ± 0.17b |
| 300 | 7.80 ± 0.74c | 19.23 ± 0.73c | 1.14 ± 0.08c | 1.99 ± 0.10b |
| 450 | 0.97 ± 0.13e | 4.74 ± 0.95e | 0.61 ± 0.03d | 0.82 ± 0.16d |
| 600 | 2.07 ± 0.16d | 9.90 ± 1.37d | 0.99 ± 0.01c | 1.13 ± 0.09c |
a–e Indicate within the same column for the same index with different lowercase letters differ significantly (p < 0.05).
After storage for 7 days, all the emulsions showed obvious stratification, except for systems containing MPs pretreated by HIU at power outputs of 450 and 600 W, which still retained a uniform white appearance (Fig. 2C). Emulsions stabilized by native MPs exhibited an extensive increase in the particle size (Table 1) and a distinct bimodal distribution (Fig. 2D) compared to its fresh counterpart, highlighting its instability. The smaller particle size distribution peak was ascribed to the droplets in emulsion, whereas the subsequently larger one might be attributed to the contribution of the filamentous myosin polymer [30]. Interestingly, as the HIU power increased, the volume fraction of the large particle peak gradually decreased (Fig. 2D), accompanied by a significant reduction in the average particle size (Table 1). A similar trend was also observed in the result of PDI (Table 1). These observations corroborated the physical stability measurements and implied that the HIU treatment might improve the physical stability of MP emulsions by preventing the assembly of myosin filaments at the oil–water interface. In addition to this inhibitory effect on myosin filaments, the small oil droplets generated during homogenization also positively impact the stabilizing system. Ozturk and McClements [4] revealed that emulsions with smaller droplets usually demonstrate better stability to gravitational separation due to moving more slowly than larger droplets, consistent with findings from the present study. However, as mentioned above in Section 3.1, the over-processing effect of applying HIU at the power output of 600 W led to large particles both before and after storage and an increase in the d4,3 and PDI compared to HIU treatment at 450 W (Fig. 2 and Table 1). It was presumed that the excessively high HIU power increased hydrophobic associations [11], which would cause the flocculation of the interfacial protein, and affect the interface structure, resulting in large droplets with non-uniform droplet size distribution, resulting in larger and non-uniform distribution of droplets [19].
3.2.2. Zeta-potential
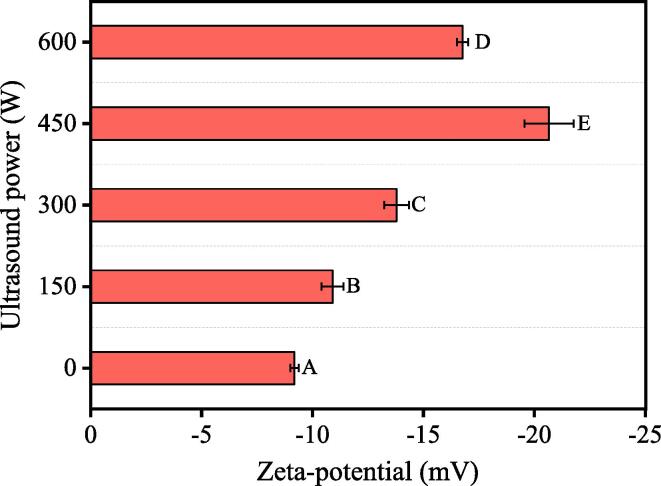
It is important to consider the droplet interactions when attempting to understand the effect of HIU pretreatment on MP emulsions at low ionic strength. In O/W emulsions, the electrostatic forces between droplets are the main determinant of the emulsion stability, and the zeta-potential is a direct indicator of the surface charge density of particles. As shown in Fig. 3, all the emulsions had droplets with negatively-charged surfaces, arising from the deprotonation of acidic amino acids contained in MPs at neutral pH [31]. The emulsion droplets stabilized by native MPs exhibited a relatively low net-negative charge (−9.18 mV), mainly attributed to the weak acidity caused by the closed protein structure [32]. As the HIU power increased, the net charge of the emulsion droplets showed a trend of initially increasing, followed by a reverse trend of decreasing value. The highest value (−20.66 mV) was observed in the emulsion prepared from MPs pretreated by HIU at a power output of 450 W (p < 0.05), consistent with the physical stability and particle size data (Figs. 1, 2 and Table 1).
Fig. 3.
The zeta-potential of the emulsions prepared by myofibrillar proteins (MPs) treated with different high-intensity ultrasound (HIU) powers (0–600 W) at low ionic strength. The letters A–E indicate the significant differences (p < 0.05) in the zeta potential, pertaining to the different HIU powers.
The increased zeta-potential can be explained by the HIU-induced physical degradation of filamentous polymers and unfolding of proteins, culminating in exposure of the internal polar sites [33]. Moreover, previous studies have shown that the increased charges could interfere with the rod–rod electrostatic interactions responsible for the assembly of myosin filaments [11], thereby, MPs acquire the ability to stabilize droplets. In addition, A higher zeta-potential generally signifies a strong electrostatic repulsion and a greater separation between adjacent droplets [19]. In this regard, the strong electrostatic repulsion between the emulsified oil droplets stabilized by MPs pretreated by HIU at a power output of 450 W would be expected to improve the resistance to unstable behavior caused by droplet collision.
Similar to the physical stability (Section 3.1) and particle size (Section 3.2.1) results, the zeta-potential also showed a reduction when the HIU was 600 W (Fig. 3). In our previous report, high power HIU treatment (600 W) of aqueous suspensions of MPs promoted intermolecular hydrophobic associations due to an excessive unfolding of the protein [11]. Extensive protein–protein interactions during protein aggregation would weaken the electrostatic repulsion between droplets because of the screening of charged groups, adversely affecting the physical stability of the emulsion. Likewise, Arredondo-Parada et al. [33] suggested that the ultrasound-induced decreases in the net charge of squid proteins could be correlated with the migration of hydrophobic apolar sites.
3.3. Identification of interfacial proteins
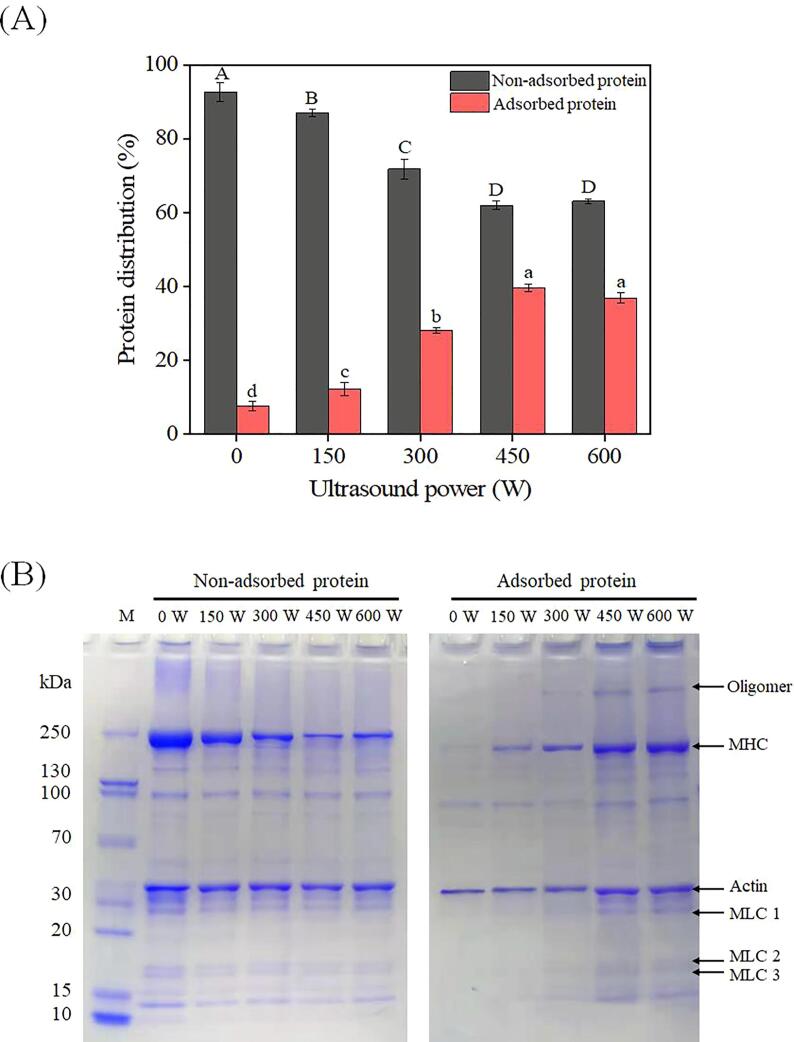
To further understand the interfacial behavior of the HIU-treated MPs at low ionic strength, the levels of interfacial and non-adsorbed proteins in emulsions were monitored. As shown in Fig. 4A, the interfacial protein levels of the emulsions stabilized by HIU-treated MPs were significantly higher than that of the control (p < 0.05) and progressively increased to a maximum of 39.74% with the increase in HIU power. On the contrary, the concentration of non-adsorbed proteins in the water phase decreased gradually (p < 0.05). It has long been argued that a stable interfacial layer is a prerequisite for the formation of a stable emulsion, as it can lower the interfacial tension and simultaneously provide additional resistance between adjacent droplets [34]. In other words, the interfacial proteins play a critical role in the physical stability of the emulsion. As mentioned in Section 1, the assembly of myosin filaments in low-salt media might make it difficult for MPs to remain adsorbed at the oil–water interface. However, HIU treatment might undermine and suppress such polymer formation, strengthening MP adsorption ability (Fig. 4A). To verify this, our immediate interest was in identifying the protein profiles of the non-adsorbed and adsorbed MPs.
Fig. 4.
Percentages of non-adsorbed proteins (in the aqueous layer) and adsorbed proteins (in the cream layer) of the emulsions prepared by myofibrillar proteins (MPs) treated with different high-intensity ultrasound (HIU) powers (0–600 W) at low ionic strength (A), and the SDS-PAGE pattern of the non-adsorbed and adsorbed proteins of emulsions (B). The letters A–D and a–d indicate the significant differences (p < 0.05) in the non-adsorbed proteins and adsorbed proteins, pertaining to the different HIU powers. M: mark; MHC: myosin heavy chain; MLC: myosin light chain.
As shown in Fig. 4B, all the non-adsorbed proteins exhibited banding patterns typical of MPs, with the major bands, including the myosin heavy chain (MHC), actin, and myosin light chain (MLC), almost identical to previous findings [11], [25]. A relatively low level of protein was detected in the cream layer of the control, attributed to its poor adsorption capability. Some actin remained in the cream layer, consistent with previous reports [16], [35]. Unlike myosin, actin generally exists as spherical monomers with good functional properties at low ionic strength [36]. Elsewhere, Ma et al. [35] also concluded that actin is a major component of the interfacial membrane of emulsions stabilized by cod myofibrillar proteins in low-salt conditions. However, actin adsorption alone may be insufficient to form a stable emulsion system. The present results showed a drastic change in the electrophoretic pattern of MPs both in the water phase and at the oil–water interface following HIU pretreatment, particularly in the MHC (Fig. 4B). Moreover, As the HIU power (0–600 W) increased, the subunit levels of MPs, particularly MHC, gradually decreased in the water phase and increased in the cream layer, consistent with the above results of the adsorbed and non-adsorbed protein levels (Fig. 4A). Albeit less conspicuous, the MLC bands also increased with increasing HIU power, despite remaining faint even at high HIU output power (Fig. 4B). It is known that MHC comprises an important part of the myosin rod, whereas the essential light chain (MLC1) and the regulatory light chain (MLC2) wrap around myosin alpha-helical neck region constitutes a light chain region of myosin and, provides mechanical support [37], [38]. Accordingly, it is not surprising that the expression level of myosin-derived bands increased in the cream layer as long as the myosin filaments were destroyed by HIU pretreatment. These results also illustrate that the myosin molecules undergo molecular rearrangement and orientation at the oil–water interface without the structural limitations of myosin filaments.
It is noteworthy that after HIU treatment, a high-molecular-weight oligomer was seen at the top of the SDS-PAGE gel of the adsorbed proteins (Fig. 4B). This phenomenon reinforces a previous study that the HIU treatment of the aqueous MP suspension could induce the formation of soluble oligomers [11]. During HIU treatment, the continuous mechanical shock and cavitation would evoke the splitting of water molecules, generating highly reactive free radicals [39]. This process might result in a certain extent of protein oxidation, which is characterized by disulfide bond-mediated intermolecular covalent crosslinking. Previous nano-liquid chromatography-electrospray ionization-tandem mass spectrometry analysis has shown that such intermolecular crosslinking mostly occurs in MHC sites [11], which indirectly confirmed the depolymerization of myosin filaments. The present results also showed that the soluble oligomers had an excellent adsorption capacity (Fig. 4B). This is expected to facilitate repulsion between droplets, as the covalent oligomers can provide more steric hindrance, thereby improving the physical stability of the emulsion [30], [35].
3.4. Transformation of protein structure induced by HIU
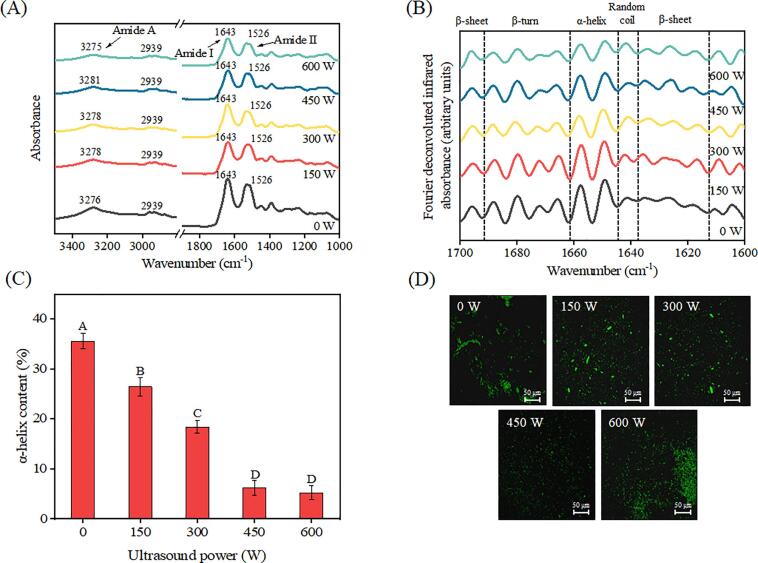
To provide further evidence supporting the role of HIU in the disruption of filamentous myosin polymers, structure information on the HIU-treated MPs in water was obtained by FTIR spectroscopy. The FTIR spectra (Fig. 5A) display the amide A (3200–3400 cm−1) region reflecting the intermolecular H-bonded N–H and O–H stretching vibrations, and the amide I (1643 cm−1) and amide II (1526 cm−1) regions assigned to C O stretching and N–H bending vibrations, respectively [40], [41]. It can be seen that the HIU process had only minor effects on the entire FTIR spectra of MPs, suggesting that no remarkable change occurred in the protein polypeptide backbone. However, the peak position of amide A, shown in Fig. 5A, exhibits a slight and gradual blue shift (from 3276 to 3281 cm−1) with increasing HIU power (from 0 to 450 W), indicative of altered intra-/intermolecular interactions. A reasonable explanation for this observation is that during HIU, the intense physical forces (cavitation and mechanical shock) induced the dissociation of myofibrils by destroying the non-covalent interactions (hydrogen or hydrophobic bond) involving N–H or O–H [11]. As a result, the N–H or O–H bond shortens, and the electron density increases, in turn, leading to a shift in the absorption peak toward a higher frequency in the IR spectrum of protein [42]. In contrast, increasing the HIU power up to 600 W promoted intermolecular hydrophobic interactions causing a slight red-shift in the absorption peak of amide A (Fig. 5A) consistent with the previous result [11], [43].
Fig. 5.
The Fourier transform infrared (FTIR) spectra (A) and Fourier deconvoluted Amide I (B) (1700–1600 cm−1) of myofibrillar proteins (MPs) treated with different high-intensity ultrasound (HIU) powers at low ionic strength, and the α-helix structure content (C) estimated from deconvoluted FTIR spectra. The confocal laser scanning microscopy (CLSM) micrographs (D) of HIU-treated MP aqueous suspensions. The letters A–D indicate the significant differences (p < 0.05) in the α-helix structure content, pertaining to the different HIU powers.
The absorption of the amide I band is ascribed to C O stretching vibrations derived from α-helical structures and water vibration and is often associated with the change in protein conformation. The corresponding deconvoluted spectrum is shown in Fig. 5B. Bands near 1692–1662, 1662–1645, and 1645–1638 cm−1 correspond to β-turn, α-helix, and random coil structures, respectively, and the 1700–1692 and 1638–1615 cm−1 are related to β-sheet components [41]. After HIU treatment, noticeable reductions in the absorption of β-turn components and increases in the absorption of β-sheets and random coils were observed (Fig. 5B). These changes are consistent with the reorganization of MPs from an ordered rigid structure to a disordered flexible structure; β-turns are commonly associated with a highly ordered protein structure, whereas the β-sheet and random coil structures have been described as typical of flexible and open structures [44]. Furthermore, the increased β-sheet region is related to the increase in protein–protein interactions between exposed hydrophobic regions, promoting the formation of intermolecular β-sheet structures [42].
The α-helix is an important structure for MPs, especially in low salt media, because it is the predominant form of the myosin rod responsible for assembling myosin filaments [11], [45]. To assess the effect of HIU treatment on the myosin rods in the water more accurately, a quantitative estimation of the α-helical structure was conducted. As shown in Fig. 5C, the exposure of MPs to intense physical forces caused a gradual HIU power-dependent decrease in α-helical content, similar to previous reports [11], [30]. Due to its intrinsic hydrophobic character, the myosin tail has been regarded as the dominant unit for interfacial adsorption in emulsion systems [9]. However, because of its unique periodic charge distribution, it tends to assemble into filaments through intermolecular electrostatic interactions, hindering its development at the oil–water interface [46]. The fact that the α-helical content decreased following HIU treatment highlights the HIU-induced disintegration of the helical structures, disrupting the electrostatic balance between rods, concomitantly suppressing the assembly of filamentous myosin polymers. Microscopic observations obtained by CLSM of the aqueous MP suspensions treated with different HIU powers provided more direct evidence (Fig. 5D). Prior to the HIU treatment, several complete fibrous structures with a relatively large particle size were observed in the native MPs, consistent with atomic force microscopy observations that MPs exist mainly as filamentous polymers in low-ionic-strength media [11]. With an increase in the HIU power, the filamentous polymers were gradually transformed into small units and dispersed uniformly in water; meanwhile, a trend of hydrophobic associations at 600 W was also apparent (Fig. 5D). These findings further confirmed both the existence of filamentous polymers in low-ionic-strength media and the efficiency of HIU in destroying and suppressing them. Combined with the above results of emulsion stability, we proposed that this aspect may be a major mechanism of HIU-induced improvement of MP emulsion stability at low ionic strength.
3.5. Microscopic morphology of droplets in emulsions
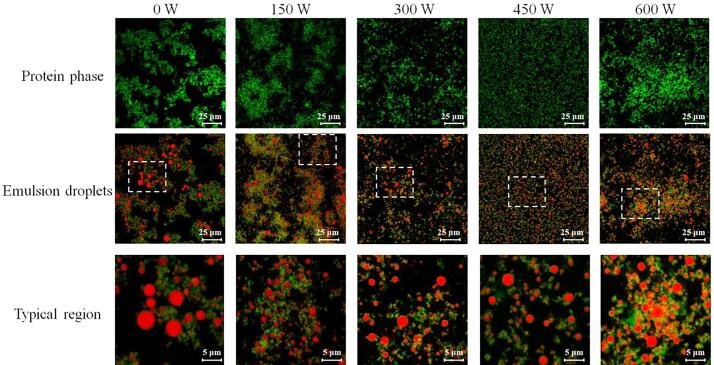
In addition to the microscopic morphology of the HIU-treated MP aqueous suspensions, CLSM was also applied to visualize the actual distribution of oil droplets and proteins in the MP emulsions. As shown in Fig. 6, the oil droplets (red) in emulsion coated by native MPs (green) showed a larger size than the other samples, and a obviously aggregating trend of being close to each other, again implicating the emulsion instability. Such aggregation tendency appears to be driven by the assembly of the interfacial protein. This was most apparent in the protein phase, in which filamentous structures, albeit loose and not very complete, can be observed (Fig. 6). Moreover, in the typical region shown in Fig. 6, only a small amount of protein can be seen coating the oil droplets of the native MP emulsion, suggesting the separation of proteins from the oil–water interface. It could be that most of the separated interfacial proteins are involved in the assembly of myosin filaments. After HIU treatment, the self-assembled fibrous aggregates gradually dissipated with increasing HIU power and were replaced with several small units released into the continuous phase or uniformly localized at the oil droplets, with more marked changes at the HIU power of 450 W (Fig. 6). This observation further suggested that the HIU pretreatment disrupts and inhibits protein self-assembly behavior, promoting the dispersion of droplets and improving the physical stability of the emulsion. By contrast, when the MPs were pretreated by HIU at the highest power output examined (600 W), an apparent aggregation trend of the emulsion droplets occurred, perhaps because of exposure of hydrophobic sites, which are otherwise buried within the interior of the native protein and the MPs treated at the relatively lower power levels.
Fig. 6.
Confocal laser scanning microscopy (CLSM) micrographs of the emulsions prepared by myofibrillar proteins (MPs) treated with different high-intensity ultrasound (HIU) powers (0–600 W) at low ionic strength. To better monitor the protein assembly behavior, the protein phase was observed separately, and typical regions were selected to observe the coating of interfacial proteins.
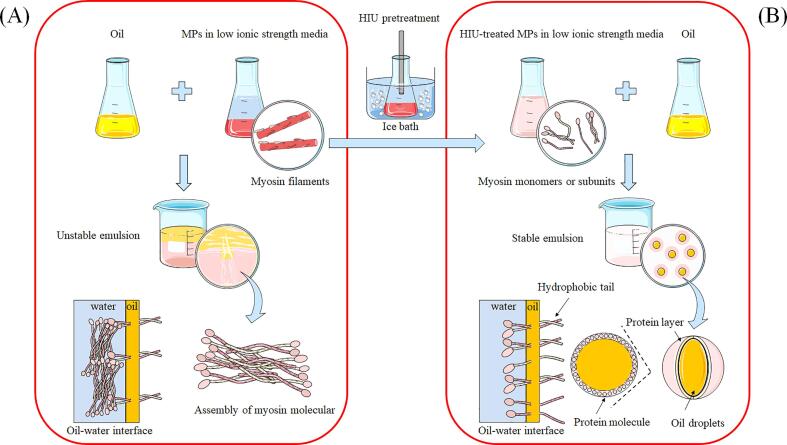
Several studies have highlighted the importance of the interfacial membrane for the stability of protein-based emulsions, providing a physical barrier and preventing droplet coalescence [47], [48]. However, the interfacial membrane is dynamic in nature and its performance mainly depends on the molecular properties of the adsorbed protein [5]. For myosin in MPs, its bipolar termini tend to self-associate by electrostatic interactions, forming highly ordered filamentous structures [49]. Such specific self-assembly behavior is not only a local but also a global behavior in MP emulsion systems (Figs. 6 and 7A). This renders the myosin incompetent regarding permanence at the oil–water interface following emulsification, resulting in emulsion instability (Fig. 1A). The present findings suggest that the HIU pretreatment can destroy and suppress the formation of filamentous polymers by unwinding the double-stranded helix of the myosin rod. The resulting myosin monomer or subunit is evenly dispersed in the continuous phase, thereby orienting its hydrophobic tail toward the oil droplets and its hydrophilic moieties toward the water, without self-assembly limitations (Figs. 5 and 7B). This coated interfacial protein layer provides additional forces, such as electrostatic repulsion and steric hindrance, keeping the emulsions stable over time.
Fig. 7.
Proposed mechanism to improve the physical stability of the emulsion prepared by high-intensity ultrasound (HIU)-treated myofibrillar proteins (MPs): The emulsion prepared by native MPs (A) and by HIU-treated MPs (B).
4. Conclusions
This study demonstrated that the physical stability of MP emulsions in low-ionic-strength media could be effectively improved by HIU pretreatment. This improvement may be attributed to the disintegration of the filamentous myosin polymer caused by the mechanical force produced during the ultrasound treatment and the subsequent inhibition of self-assembly behavior, thus promoting protein orientation and molecular rearrangement at the oil–water interface. The formation of a complete and dense interfacial film enhances the inter-droplet interactions (i.e., electrostatic repulsion and steric hindrance), which play a vital role in the subsequent stability of the emulsion. The physical stability of the emulsions prepared with HIU-pretreated MPs at low ionic strength is dependent on the HIU power, and the highest physical stability corresponded to the MPs treated at 450 W, which produced the smallest droplet sizes, the most homogeneous particle size distribution, the highest zeta-potential, and near-complete interfacial layer structure. Overall, the HIU pretreatment represents a promising approach for physical modulation to improve the physical stability of MP emulsions at low ionic strength.
CRediT authorship contribution statement
Haotian Liu: Conceptualization, Formal analysis, Investigation, Writing - original draft, Visualization. Jingnan Zhang: Investigation, Software. Hui Wang: Formal analysis, Investigation, Visualization. Qian Chen: Writing - review & editing, Supervision, Project administration. Baohua Kong: Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by the Major Science and Technology Projects of Heilongjiang Province (2020ZX07B02, 2019ZX07B03).
Contributor Information
Qian Chen, Email: chenqianego7@126.com.
Baohua Kong, Email: kongbh63@hotmail.com.
References
- 1.Liu H., Li Y., Diao X., Kong B., Liu Q. Effect of porcine bone protein hydrolysates on the emulsifying and oxidative stability of oil-in-water emulsions. Colloid Surf. A. 2018;538:757–764. [Google Scholar]
- 2.McClements D.J. Nanoparticle- and microparticle-based delivery systems: encapsulation, protection and release of active compounds. J. Alloy. Compd. 2014;261:54–61. [Google Scholar]
- 3.McClements D.J., Li Y. Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components. Adv. Colloid Interfaces. 2010;159:213–228. doi: 10.1016/j.cis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Ozturk B., McClements D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016;7:1–6. [Google Scholar]
- 5.Lam R.S.H., Nickerson M.T. Food proteins: a review on their emulsifying properties using a structure–function approach. Food Chem. 2013;141:975–984. doi: 10.1016/j.foodchem.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Bengoechea C., Romero A., Aguilar J.M., Cordobés F., Guerrero A. Temperature and pH as factors influencing droplet size distribution and linear viscoelasticity of O/W emulsions stabilised by soy and gluten proteins. Food Hydrocolloid. 2010;24:783–791. [Google Scholar]
- 7.Karaca A.C., Low N., Nickerson M. Emulsifying properties of canola and flaxseed protein isolates produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011;44:2991–2998. [Google Scholar]
- 8.Bohrer B.M. Review: Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017;65:103–112. [Google Scholar]
- 9.Dickinson E., Murray B.S., Stainsby G., Brock C.J. Behaviour of adsorbed myosin at the oil-water interface. Int. J. Biol. Macromol. 1987;9:302–304. [Google Scholar]
- 10.Herrero A.M., Carmona P., Pintado T., Jiménez-Colmenero F., Ruíz-Capillas C. Olive oil-in-water emulsions stabilized with caseinate: elucidation of protein–lipid interactions by infrared spectroscopy. Food Hydrocolloid. 2011;25:12–18. [Google Scholar]
- 11.Liu H., Zhang H., Liu Q., Chen Q., Kong B. Solubilization and stable dispersion of myofibrillar proteins in water through the destruction and inhibition of the assembly of filaments using high-intensity ultrasound. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105160. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Xu X., Liu D., Zhou G., Han M., Wang P. Rheological behavior, conformational changes and interactions of water-soluble myofibrillar protein during heating. Food Hydrocolloid. 2018;77:524–533. [Google Scholar]
- 13.Krishnamurthy G., Chang H.-S., Hultin H.O., Feng Y., Srinivasan S., Kelleher S.D. Solubility of chicken breast muscle proteins in solutions of low ionic strength. J. Agric. Food Chem. 1996;44:408–415. [Google Scholar]
- 14.Li Y., Liu H., Liu Q., Kong B., Diao X. Effects of zein hydrolysates coupled with sage (salvia officinalis) extract on the emulsifying and oxidative stability of myofibrillar protein prepared oil-in-water emulsions. Food Hydrocolloid. 2019;87:149–157. [Google Scholar]
- 15.Xie Y., Wang J., Wang Y., Wu D., Liang D., Ye H., Cai Z., Ma M., Geng F. Effects of high-intensity ultrasonic (HIU) treatment on the functional properties and assemblage structure of egg yolk. Ultrason. Sonochem. 2020;60 doi: 10.1016/j.ultsonch.2019.104767. [DOI] [PubMed] [Google Scholar]
- 16.Li K., Fu L., Zhao Y.-Y., Xue S.-W., Wang P., Xu X.-L., Bai Y.-H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocolloid. 2020;98 [Google Scholar]
- 17.Chen J., Zhang X., Xue S., Xu X. Effects of ultrasound frequency mode on myofibrillar protein structure and emulsifying properties. Int. J. Biol. Macromol. 2020;163:1768–1779. doi: 10.1016/j.ijbiomac.2020.09.114. [DOI] [PubMed] [Google Scholar]
- 18.Zorba Ö. The effects of the amount of emulsified oil on the emulsion stability and viscosity of myofibrillar proteins. Food Hydrocolloid. 2006;20:698–702. [Google Scholar]
- 19.Liu H., Han G., Zhang H., Liu Q., Kong B. Improving the physical and oxidative stability of emulsions based on the interfacial electrostatic effects between porcine bone protein hydrolysates and porcine bone protein hydrolysate-rutin conjugates. Food Hydrocolloid. 2019;94:418–427. [Google Scholar]
- 20.Liu F., Wang D., Xu H., Sun C., Gao Y. Physicochemical properties of β-carotene emulsions stabilized by chlorogenic acid–lactoferrin–glucose/polydextrose conjugates. Food Chem. 2016;196:338–346. doi: 10.1016/j.foodchem.2015.09.047. [DOI] [PubMed] [Google Scholar]
- 21.Dammak I., Sobral P.J.d.A. Effect of different biopolymers on the stability of hesperidin-encapsulating O/W emulsions. J. Food Eng. 2018;237:33–43. [Google Scholar]
- 22.Kong J., Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 23.Xin L., Xu W., Jiawei L., Duoxia X., Yanping C., Baoguo S. The effect of unadsorbed proteins on the physiochemical properties of the heteroaggregates of oppositely charged lactoferrin coated lutein droplets and whey protein isolate coated DHA droplets. Food Funct. 2018;9:3956–3964. doi: 10.1039/c8fo00371h. [DOI] [PubMed] [Google Scholar]
- 24.Dammak I., do Amaral Sobral P.J. Investigation into the physicochemical stability and rheological properties of rutin emulsions stabilized by chitosan and lecithin. J. Food Eng. 2018;229:12–20. [Google Scholar]
- 25.Sun Q., Chen Q., Xia X., Kong B., Diao X. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrason. Sonochem. 2019;54:311–320. doi: 10.1016/j.ultsonch.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Ma W., Wang J., Wu D., Xu X., Wu C., Du M. Physicochemical properties and oil/water interfacial adsorption behavior of cod proteins as affected by high-pressure homogenization. Food Hydrocolloid. 2020;100 [Google Scholar]
- 27.Zou Y., Xu P., Wu H., Zhang M., Sun Z., Sun C., Wang D., Cao J., Xu W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int. J. Biol. Macromol. 2018;113:640–647. doi: 10.1016/j.ijbiomac.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 28.Hu H., Wu J., Li-Chan E.C.Y., Zhu L., Zhang F., Xu X., Fan G., Wang L., Huang X., Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocolloid. 2013;30:647–655. [Google Scholar]
- 29.Gravel A., Doyen A. The use of edible insect proteins in food: challenges and issues related to their functional properties. Innov. Food Sci. Emerg. 2020;59 [Google Scholar]
- 30.Chen X., Xu X., Han M., Zhou G., Chen C., Li P. Conformational changes induced by high-pressure homogenization inhibit myosin filament formation in low ionic strength solutions. Food Res. Int. 2016;85:1–9. doi: 10.1016/j.foodres.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Saricaoglu F.T., Gul O., Tural S., Turhan S. Potential application of high pressure homogenization (HPH) for improving functional and rheological properties of mechanically deboned chicken meat (MDCM) proteins. J. Food Eng. 2017;215:161–171. [Google Scholar]
- 32.Chen X., Xiong Y.L., Xu X. High-pressure homogenization combined with sulfhydryl blockage by hydrogen peroxide enhance the thermal stability of chicken breast myofibrillar protein aqueous solution. Food Chem. 2019;285:31–38. doi: 10.1016/j.foodchem.2019.01.131. [DOI] [PubMed] [Google Scholar]
- 33.Arredondo-Parada I., Torres-Arreola W., Suárez-Jiménez G.M., Ramírez-Suárez J.C., Juárez-Onofre J.E., Rodríguez-Félix F., Marquez-Rios E. Effect of ultrasound on physicochemical and foaming properties of a protein concentrate from giant squid (Dosidicus gigas) mantle. LWT – Food Sci. Technol. 2020;121 [Google Scholar]
- 34.Piorkowski D.T., McClements D.J. Beverage emulsions: recent developments in formulation, production, and applications. Food Hydrocolloid. 2014;42:5–41. [Google Scholar]
- 35.Ma W., Wang J., Xu X., Qin L., Wu C., Du M. Ultrasound treatment improved the physicochemical characteristics of cod protein and enhanced the stability of oil-in-water emulsion. Food Res. Int. 2019;121:247–256. doi: 10.1016/j.foodres.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Galluzzo S.J., Regenstein J.M. Role of chicken breast muscle proteins in meat emulsion formation: myosin, actin and synthetic actomyosin. J. Food Sci. 1978;43:1761–1765. [Google Scholar]
- 37.Craig R., Woodhead J.L. Structure and function of myosin filaments. Curr. Opin. Struct. Biol. 2006;16:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Lowey S., Trybus K.M. Common structural motifs for the regulation of divergent class II myosins. J. Biol. Chem. 2010;285:16403–16407. doi: 10.1074/jbc.R109.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang D.-C., Gao X.-Q., Ge Q.-F., Zhou G.-H., Zhang W.-G. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason. Sonochem. 2017;38:317–325. doi: 10.1016/j.ultsonch.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Chen X., Zhou R., Xu X., Zhou G., Liu D. Structural modification by high-pressure homogenization for improved functional properties of freeze-dried myofibrillar proteins powder. Food Res. Int. 2017;100:193–200. doi: 10.1016/j.foodres.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Pinilla C.M.B., Brandelli A., López-Caballero M.E., Montero P., Gómez-Guillén M.d.C. Structural features of myofibrillar fish protein interacting with phosphatidylcholine liposomes. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109687. [DOI] [PubMed] [Google Scholar]
- 42.Harnkarnsujarit N., Kawai K., Suzuki T. Effects of freezing temperature and water activity on microstructure, color, and protein conformation of freeze-dried bluefin tuna (Thunnus orientalis) Food Bioprocess Technol. 2015;8:916–925. [Google Scholar]
- 43.Wang Y.-Y., Tayyab Rashid M., Yan J.-K., Ma H. Effect of multi-frequency ultrasound thawing on the structure and rheological properties of myofibrillar proteins from small yellow croaker. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siddique M.A.B., Maresca P., Pataro G., Ferrari G. Effect of pulsed light treatment on structural and functional properties of whey protein isolate. Food Res. Int. 2016;87:189–196. doi: 10.1016/j.foodres.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Thompson R.C., Buvoli M., Buvoli A., Leinwand L.A. Myosin filament assembly requires a cluster of four positive residues located in the rod domain. FEBS Lett. 2012;586:3008–3012. doi: 10.1016/j.febslet.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L., Cai R., Wang P., Xu X., Zhou G., Sun J. Manipulating interfacial behavior and emulsifying properties of myosin through alkali-heat treatment. Food Hydrocolloid. 2018;85:69–74. [Google Scholar]
- 47.Nishinari K., Fang Y., Guo S., Phillips G.O. Soy proteins: a review on composition, aggregation and emulsification. Food Hydrocolloid. 2014;39:301–318. [Google Scholar]
- 48.Tokle T., McClements D.J. Physicochemical properties of lactoferrin stabilized oil-in-water emulsions: effects of pH, salt and heating. Food Hydrocolloid. 2011;25:976–982. [Google Scholar]
- 49.Kramer R.M., Shende V.R., Motl N., Pace C.N., Scholtz J.M. Toward a molecular understanding of protein solubility: increased negative surface charge correlates with increased solubility. Biophys. J. 2012;102:1907–1915. doi: 10.1016/j.bpj.2012.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]