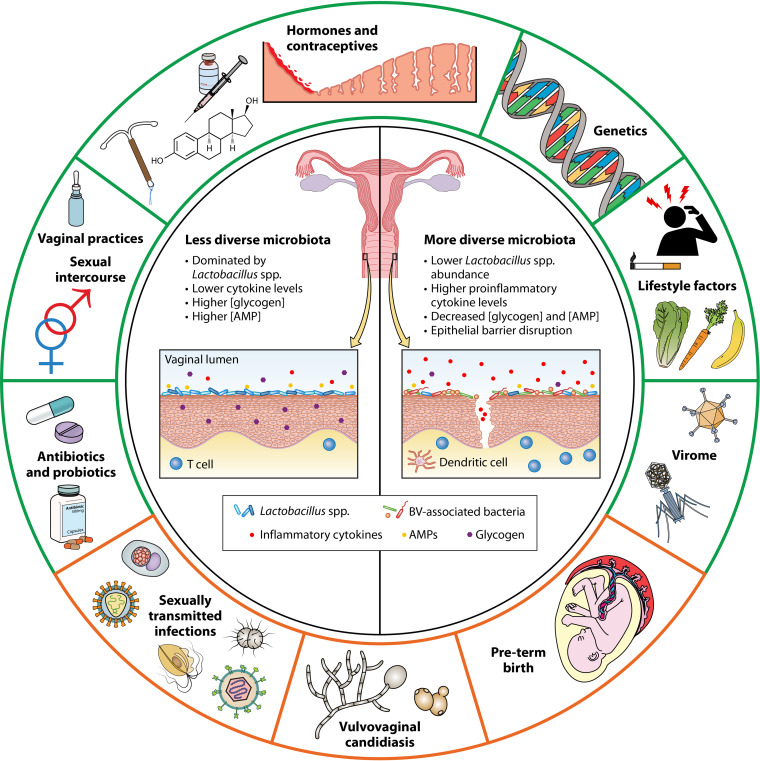
The female genital tract microbiota is part of a complex ecosystem influenced by several physiological, genetic, and behavioral factors. It is uniquely linked to a woman’s mucosal immunity and plays a critical role in the regulation of genital inflammation.
KEYWORDS: cytokines, inflammation, female genital tract, immune response, vaginal microbiota
ABSTRACT
The female genital tract microbiota is part of a complex ecosystem influenced by several physiological, genetic, and behavioral factors. It is uniquely linked to a woman’s mucosal immunity and plays a critical role in the regulation of genital inflammation. A vaginal microbiota characterized by a high abundance of lactobacilli and low overall bacterial diversity is associated with lower inflammation. On the other hand, a more diverse microbiota is linked to high mucosal inflammation levels, a compromised genital epithelial barrier, and an increased risk of sexually transmitted infections and other conditions. Several bacterial taxa such as Gardnerella spp., Prevotella spp., Sneathia spp., and Atopobium spp. are well known to have adverse effects; however, the definitive cause of this microbial dysbiosis is yet to be fully elucidated. The aim of this review is to discuss the multiple ways in which the microbiota influences the overall genital inflammatory milieu and to explore the causes and consequences of this inflammatory response. While there is abundant evidence linking a diverse genital microbiota to elevated inflammation, understanding the risk factors and mechanisms through which it affects genital health is essential. A robust appreciation of these factors is important for identifying effective prevention and treatment strategies.
INTRODUCTION
The human microbiota is an integral part of our immune system and plays an important role in the first line of defense in the female genital tract (FGT). Human immunity has evolved to live in symbiosis with beneficial bacteria to such an extent that our immune system recognizes commensal antigens in the body as self instead of foreign (1). Every woman has a unique genital microbiota, which can be broadly classified based on dominant bacterial taxa. These categories, generated using hierarchical clustering or nearest centroid classification of microbiota data, are defined as community state types (CSTs) or cervicotypes (2–4). While the distributions of dominant genital CSTs differ between studies, common clusters include a Lactobacillus iners-dominant CST, a Lactobacillus crispatus-dominant CST, and more diverse groupings, typically associated with the presence of bacterial vaginosis (BV), a clinical syndrome presenting with vaginal discharge and increased vaginal pH, characterized by a marked decrease in the abundance of Lactobacillus spp. and an overall increase in genital microbial diversity (5).
CHARACTERIZING THE FEMALE GENITAL TRACT MICROBIOTA
The vaginal microbiota is thought to play a crucial role in reproductive health, including the potential to protect against HIV and sexually transmitted infections (STIs), abnormal birth outcomes, and other pathogens (5–7). Its composition is dependent on several factors, including age, hormonal changes, and genital glycogen content (8), which, in the presence of the enzyme pullulanase or other amylases, can be used as an energy source for Lactobacillus spp., allowing increased colonization and the establishment of low-diversity CSTs (9–11). Typically, the cervicovaginal microbiota is characterized by a dominance of Lactobacillus species (12), a trait unique to humans (13), particularly L. crispatus, Lactobacillus jensenii, Lactobacillus gasseri, and L. iners (12, 14).
Although widely accepted, whether a low-diversity, Lactobacillus-dominated microbiota defines genital microbial health has been questioned in recent years. For example, a high relative abundance of Bifidobacterium breve can replace the production of lactic acid by lactobacilli (15). Furthermore, what is considered reproductively “optimal” has been associated with race in numerous analyses; however, race is a proxy for lived experiences that promote differences in biological and behavioral characteristics, including racism, and differences in microbiota should be attributed to these experiences rather than race itself. In a cohort of women living in North America, Lactobacillus species dominance was more common among white and Asian women, while Hispanic and black women had a more diverse microbiota, with a higher prevalence of communities not dominated by lactobacilli. This was seen in other studies where asymptomatic African American women were colonized with high proportions of L. iners, Gardnerella vaginalis, Sneathia spp., Prevotella spp., Atopobium spp., Mycoplasma hominis, Aerococcus spp., BV-associated bacterium 1 (BVAB1)/“Candidatus Lachnocurva vaginae” (16), BVAB2, and several other typically BV-associated anaerobes compared to white women, who were most likely colonized by L. crispatus, L. jensenii, L. gasseri, and Staphylococcus spp. (17). In studies of black women living in South Africa, the majority of women had a genital microbiota not defined by lactobacilli (2, 4, 18). Nonetheless, a low-diversity Lactobacillus-dominated vaginal environment (Fig. 1) is often associated with low inflammation, classically defined as having low levels of proinflammatory cytokines such as interleukin-1 (IL-1), IL-8, IL-16, tumor necrosis factor alpha(TNF-α), interferon gamma (IFN-γ), and monocyte chemoattractant protein 1 (MCP-1)/C-C motif chemokine ligand 2 (CCL2), among others, and high levels of anti-inflammatory/regulatory cytokines such as IL-1 receptor antagonist (IL-1RA) and IL-10 (19, 20). On the other hand, a higher-diversity microbiota is accompanied by higher inflammatory cytokine concentrations (2, 4, 21). The ratio of the BV-associated bacteria G. vaginalis and Atopobium vaginae to Lactobacillus spp. was associated with significantly elevated levels of IL-1α, IL-8, and IL-12(p70) and lower levels of IFN-γ-inducible protein 10 (IP-10) (22).
FIG 1.
Common causes and consequences of a diverse, inflammatory genital microbiota. The shift to a more diverse genital microbiota is characterized by a marked decline in the abundance of Lactobacillus spp. and lower concentrations of glycogen and antimicrobial peptides, including lactic acid. This is accompanied by high levels of proinflammatory cytokines and chemokines and epithelial barrier damage. Several factors can potentially influence this transition to a higher inflammatory state, including sexual/reproductive practices, the use of antibiotics or probiotics, the vaginal virome, genetics, and lifestyle-related factors such as the woman’s diet, stress, or smoking. This in turn leads to an increase in the risk of subsequent STI acquisition, vulvovaginal candidiasis infection, or preterm birth in some women.
Generally, genital inflammation is crucial for mounting an effective host immune response against bacterial pathogens and other STIs. This cytokine response is generated as part of protective immunity. For example, the initial spike in IL-1β concentrations in the presence of pathogens, followed by IL-8, is thought to play a role in activating the vaginal innate and adaptive immune response against BV-associated bacteria (23). At the mucosa, Toll-like receptors (TLRs) are able to bind and recognize a broad range of bacterial pathogen-associated molecular patterns (PAMPs), including lipopolysaccharide (LPS), peptidoglycans, flagellin, and bacterial DNA, triggering a signaling cascade that typically leads to the clearance of bacterial infections (24, 25). However, sustained cytokine production can be detrimental to the FGT, associated with damage to the epithelial barrier and higher T-cell infiltration to the genital mucosa (26). Chronic inflammation caused by vulvovaginitis can lead to serious long-term obstetric and gynecological complications, including tubal infertility and pelvic inflammatory diseases (27). Persistent inflammation can also increase a woman’s susceptibility to HIV (19).
This review focuses on the intrinsic relationship between a cisgender woman’s genital microbiota and its surrounding inflammatory milieu.
BACTERIAL VAGINOSIS
BV is by far the most common and well-researched genital condition and affects 20 to 70% of women (28–31). Despite antibiotic treatment, BV recurs in up to 50% of women within a year (32). The exact causes are multifactorial but result in increased colonization of the lower genital tract by pathogenic bacteria such as G. vaginalis, Prevotella spp., A. vaginae, Leptotrichia spp., Sneathia spp., Mobiluncus spp., and Megasphaera spp., among others, and lower abundances of Lactobacillus spp. (4, 33–35) (Table 1). There is limited information about specific bacteria that trigger incident BV, but it has been suggested that G. vaginalis, Prevotella bivia, A. vaginae, and Megasphaera type I play an important role in initiating BV episodes (36–38). BV can be diagnosed using Amsel criteria or Nugent scoring. Based on Amsel criteria, BV is defined as having three of the following four criteria: thin, white, yellow discharge; clue cells on wet-mount microscopy; pH >4.5; and fishy odor when adding a 10% potassium hydroxide solution to the wet mount (39). Nugent scoring, generally considered the gold standard for diagnosing BV, is a Gram stain scoring system that gives a score of 0 to 10 based on bacterial morphotype. A Nugent score of 7 to 10 indicates the presence of BV, while an intermediate genital microbiota is given a Nugent score of 4 to 6 (40). Women with highly diverse vaginal CSTs had an 18-fold-higher likelihood of being Nugent-defined BV positive (4). This microbial imbalance is often asymptomatic in women, with about 75% of women with Nugent-defined BV not experiencing any symptoms (41).
TABLE 1.
Microbial and immunological changes associated with bacterial vaginosis
| Microbiological or immunological change(s) (reference[s]) |
|---|
| Increased microbial diversity |
| Transition to a Nugent score of 7–10; increased abundance of small Gram-variable rods or Gram-negative rods and curved Gram-variable rods (40, 211) |
| Diverse with low Lactobacillus abundance, typically characterized by decreased prevalence of CST I (L. crispatus dominant), CST II (L. gasseri), CST III (L. iners), and CST V (L. jensenii) and increased prevalence of CST IV (higher relative abundances of BVAB1, G. vaginalis, A. vaginae, and Prevotella spp., etc.) (2, 4, 212) |
| Soluble biomarkers |
| Increased vaginal pH (4, 213) |
| Lower l- and d-lactic acid concn (74) |
| Lower AMP (including α-defensins, HBD-2, and SLPI) concn (59) |
| Higher 12-hydroxyeicosatetraenoic acid (58) and SCFA (60, 61) levels |
| Polymicrobial biofilm formation by inflammatory bacteria such as G. vaginalis, A. vaginae, P. bivia, and/or F. nucleatum (113, 114, 119) |
| Changes in genital inflammation profile |
| Overall increase in cytokines and chemokines, with the exception of IP-10, MIG, GRO, CCL22, MIP-1α, and GM-CSF (2, 4, 42, 44) |
| Increased levels of MMPs (45–47) |
| Immune cells and humoral immunity |
| Phenotypic changes in APCs (2) |
| Maturation and activation of monocyte-derived DCs (CD40, CD83, and HLADR) (49) |
| Increased no. and activation of mucosal CD4+ T cells (CD69 and CCR5) (26, 57) |
| IgA response to G. vaginalis cytolysin (53) |
| Metaproteomic changes |
| Decrease in epithelial barrier integrity, cytoskeletal alterations, and cell membrane biological processes; reduced cell wall organization and peptidoglycan biosynthesis (26, 112, 214) |
| Host genetics |
| Polymorphisms in inflammatory cytokines (e.g., IL-1β, IL-10, IL-5, IL-6, and TNF-α) and Toll-like receptor (e.g., TLR-2, -4, and -7) genes (107–110) |
It is now well established that a BV-associated vaginal microbiota is linked to increased concentrations of several genital cytokines [proinflammatory markers such as IL-1α, IL-1β and TNF-α, TNF-β, IL-10, IL-8, IL-12(p70), IL-4, and FMS-related tyrosine kinase 3 ligand (FLT-3L)], while others are downregulated (chemokines such as IP-10/CXCL10, growth-regulated oncogene [GRO], macrophage-derived chemokine [MDC]/CCL22 and macrophage inflammatory protein 1α [MIP-1α]/CCL3, IL-7, and granulocyte-macrophage colony-stimulating factor [GM-CSF]) (2, 42) (Table 1). Genital inflammation is associated with high alpha (within-sample) bacterial diversity and low L. crispatus abundance (43). In a cohort of young South African women, higher abundances of Prevotella spp., Dialister spp., Parvimonas micra, and Sneathia sanguinegens and lower abundances of L. crispatus and Lactobacillus johnsonii-L. gasseri were predictive of high levels of inflammatory markers (4). Among sub-Saharan African women, higher concentrations of human β-defensin 2 (HBD-2) were predictive of BV by Nugent scores, and lower IL-1RA/IL-1β ratios were predictive of intermediate Nugent scores (44). The presence of BV-associated bacteria or LPS was also associated with increased host matrix metalloproteinases (MMPs) as part of a negative-feedback loop aimed at dampening inflammation (45, 46). Cytokines can be cleaved by MMPs; in the case of IL-8, cleavage of the C terminus by MMP8, upregulated during BV (47), led to a 10-fold-higher potency (48), creating a stronger inflammatory response to bacterial infection. BV-associated bacteria induced the production of IL-1α, IL-1β, and IL-8 when cocultured with vaginal epithelial cells (2). In vitro and in germfree mice, vaginal introduction of P. bivia increased IL-6 and IL-8 production and recruited and/or activated CD44+ CD4+ T cells to the genital mucosa compared to vaginal exposure to L. crispatus (21), suggesting a causative role of these microbiota members in inflammation and cellular activation.
Several of the cytokines upregulated with BV likely work in tandem to modulate mucosal immunity (Table 1). Cervicovaginal lavage fluids from women with BV induced the maturation and activation marker expression (CD40, CD83, and HLADR) of monocyte-derived dendritic cells (DCs) and the production of IL-12, IL-23, and p40 by these DCs (49). The maturation of DCs is brought about by the secretion of the growth factor GM-CSF and IL-4, a hallmark T-helper 2 (Th2) cytokine, while TNF-α and the combination of IL-1β and IFN-γ can cause DCs to produce the inflammatory cytokine IL-12 and the regulatory cytokine IL-10 (50). DCs are important for activating naive T cells and for the development of a Th immune response (51). This cytokine interplay could potentially play a role in cellular activation and recruitment in the presence of BV. The inflammatory cytokine IL-1β produced by activated macrophages leads to cellular proliferation, differentiation, and apoptosis (52). Similarly, Cauci et al. found that genital IL-1 concentrations, especially IL-1β, were almost 13-fold higher in women with BV and were essential in triggering an immunoglobulin A response to a hemolysin produced by G. vaginalis (53). Mitchell et al. showed that IL-1β concentrations were inversely associated with H2O2-producing Lactobacillus spp. in women with BV (54). Dysbiosis is associated with increased genital IL-1β concentrations and increased neutrophils or potentially other leukocytes (15, 55). It has also been hypothesized that microbially induced inhibition of TLR activation by certain pathogenic bacteria could lead to BV, including the downregulation of host heat shock protein production, which induces a proinflammatory response against pathogens (8, 56). Treatment of BV with metronidazole (an antibiotic that targets anaerobic bacteria and some protozoans) led to reductions in IL-1β, IL-8, and RANTES (regulated upon activation, normal T-cell expressed and secreted) as well as in the expression of the activation markers CD69 and CCR5 in mucosal CD4+ T cells (57).
In addition to influencing the secretion of cytokines, lactobacilli acidify the vagina and produce other metabolites and antimicrobial molecules that protect against pathogens. Genital metabolites can clearly delineate women with/without BV, with BV-positive women having higher levels of 12-hydroxyeicosatetraenoic acid, an inflammatory biomarker (58). Women with BV have significantly lower levels of antimicrobial peptides (AMPs), including α-defensins (produced by neutrophils), HBD-2 (produced by epithelial cells), and secretory leukocyte protease inhibitor (SLPI), in cervical mucus (59). After treatment, these AMP levels were comparable to those of women without BV, confirming that this was indeed due to BV. While Lactobacillus species are associated with low short-chain fatty acid (SCFA) levels, which can regulate host cytokine production, SCFAs are found at higher concentrations in women with BV, leading to the production of IL-8, IL-6, IL-1β, and TNF-α, some of them in a dose-dependent manner (60, 61).
CONTRIBUTORS TO VAGINAL MICROBIOTA DIVERSITY AND INFLAMMATION
Several physiological and behavioral factors may increase the risk of vaginal microbial dysbiosis and inflammation, including sexual intercourse, reproductive and contraceptive hormones, menstruation, use of antibiotics, vaginal insertion practices, and lifestyle habits (Fig. 1).
Sexual intercourse and exposure to semen.
Although the exact etiology of BV is unclear, higher total numbers of and new sexual partners put women at a higher risk of BV, regardless of partner gender. Condomless sex with a regular male partner after treatment for BV was associated with the presence of a diverse, inflammatory vaginal microbiota, with a higher prevalence of BV-associated bacteria, in particular Gardnerella, Atopobium, and Sneathia spp., and a decrease in Lactobacillus spp. (62). Semen is known to contain a complex community of microbes, dominated by Ralstonia spp., Lactobacillus spp., Corynebacterium spp., Staphylococcus spp., Prevotella spp., Finegoldia spp., Ureaplasma spp., Clostridiales spp., and several other bacterial taxa present during BV (63). The presence of spermatozoa in vaginal samples was a strong predictor of incident BV (64). A study involving heterosexual couples found Gardnerella biofilms on desquamated semen epithelial cells, suggesting that exposure to semen could be a cause of BV in women (65). Furthermore, a partner’s penile microbiota composition at the meatal opening and coronal sulcus was highly predictive of incident BV (sensitivity, specificity, and accuracy of ≥74.6%), with Parvimonas, L. iners, L. crispatus, Dialister, S. sanguinegens, and G. vaginalis being among the most predictive of incidence (66). In African women, reporting of recent unprotected sex was associated with decreased L. crispatus, Lactobacillus vaginalis, and other Lactobacillus species concentrations and increased G. vaginalis and L. iners concentrations (67), which would potentially lead to higher levels of genital inflammation. Among women who have sex with women (WSW), sexual contact with a partner with BV or with new partners was associated with an increased risk of BV (68) or a shift toward a more diverse vaginal CST dominated by G. vaginalis (69).
Menstrual cycle.
The vaginal microbiota can fluctuate rapidly throughout the menstrual cycle, with menses accompanied by decreased concentrations of L. jensenii and L. crispatus and increased concentrations of L. iners and G. vaginalis irrespective of the initial Nugent-defined BV status (36, 70, 71). Irregular menstrual cycles could render the vaginal microbiota more unstable and lead to a higher incidence of BV (70). Srinivasan et al. proposed that low estrogen levels during menses result in a decreased glycogen content in the vaginal epithelium, which in turn restricts the growth of Lactobacillus spp. that depend on glycogen as a critical nutrient. In contrast, the increase in G. vaginalis seen with menstruation could be due to menstrual blood providing an iron source supporting its growth (36).
Hormones and contraception.
Endogenous hormones can influence the vaginal microbiota, for example, at puberty, when hormonal changes influence shifts in the microbiota from one dominated by anaerobic bacteria to a more Lactobacillus species-dominated one (72). Estrogen increases vaginal epithelial thickness and glycogen availability (11). In turn, this state allows colonization by Lactobacillus spp. dependent on the metabolism of glycogen, which is converted to maltose, maltotriose, and α-dextrines, with lactic acid (a metabolite known to have antimicrobial and anti-inflammatory properties [73, 74]) being the by-product of this metabolic pathway (11, 75). This was seen in a cohort of young women where a higher-diversity, low-Lactobacillus inflammatory microbiota was associated with lower estrogen levels (4). This higher rate of colonization by lactobacilli has been associated with a lower prevalence of BV and lower cytokine levels. However, Gardnerella species is also capable of using products of glycogen metabolism by secreting its own α-glucosidase, thus potentially generating more nutrients for its colonization (76). Conversely, postmenopausal women experience a reduction in estrogen levels, leading to reduced colonization by Lactobacillus spp. and increased microbial diversity, although it does not necessarily lead to a higher prevalence of BV (77–79).
Observational data suggest that hormonal contraceptive use may affect the genital microbiota and immunity, although studies are flawed due to bias introduced by different condom use patterns in contracepting versus noncontracepting women or different behaviors in women who self-select various contraception methods. Some studies report a positive association between the use of various contraceptive methods and the prevalence of BV. Twelve months of intramuscular depot medroxyprogesterone acetate (DMPA-IM) use resulted in a lower proportion of women having Lactobacillus-dominant vaginal biomes (53% compared to 27% at baseline) (80). In women with BV, using DMPA-IM appeared to exacerbate inflammation, with elevated IL-8, MCP-1, and IP-10 concentrations (81). In randomized trials, Balle et al. found the combined contraceptive vaginal ring to be highly inflammatory compared to combined oral contraceptive pills (COCP) or injectable norethisterone enanthate (Net-EN) (82), and Brown et al. found that copper intrauterine device (copper-IUD) use led to increases in BV-associated bacteria and several inflammatory cytokines and chemokines compared to DMPA-IM or the levonorgestrel implant (B. P. Brown, R. F. Tanko, S. Z. Jaumdally, R. Bunjun, S. Dabee, A.-U. Happel, M. Onono, G. Nair, T. Palanee-Phillips, C. W. Scoville, K. Heller, D. D. Nyangahu, J. M. Baeten, S. E. Bosinger, A. Burgener, J.-A. S. Passmore, R. Heffron, and H. B. Jaspan, submitted for publication). Similarly, African women initiating copper-IUD experienced increases in Nugent scores and concentrations of BV-associated organisms by quantitative PCR (qPCR) (83, 84). Since menstruation has been linked to decreases in Lactobacillus numbers and increases in BV-associated organisms (36), it is possible that these effects are indirectly associated with amenorrhea and menorrhagia often experienced by women using DMPA-IM and copper-IUD, respectively.
In contrast to the studies described above, several studies have indicated that some hormonal contraceptives have a “protective” effect against BV (82, 85–89). Among black Kenyan women, consistent DMPA-IM use was accompanied by a significant decrease in G. vaginalis numbers and an overall decrease in bacterial loads, together with no change in Lactobacillus species numbers (90). In the randomized Evidence for Contraceptive Option and HIV Outcomes (ECHO) trial, DMPA-IM initiation was associated with an increase in Lactobacillus species relative abundance (Brown et al., submitted). A systematic review and meta-analysis of the effects of hormonal contraceptives showed that women using COCP or DMPA experienced BV less frequently (effect sizes of 0.68 for prevalent BV and 0.82 for incident BV), with similar results seen with estrogen-containing contraceptives in a randomized trial relative to no contraception (89, 91). Estradiol-progestin COCP users were more likely to be colonized by Lactobacillus spp. than women using condoms only but not DMPA-IM users in an observational study (92). Among South African young women randomized to various hormonal contraceptives, COCP initiation resulted in an increased relative abundance of L. iners (82). These findings could be due to a higher level of glycogen accumulation triggered by estrogen. Interestingly, in a recent study in women using COCP, transitioning from a normal to an intermediate microbiota (Nugent score of 4 to 6) was preceded by a general immunosuppressive profile with decreased IL-1β, MIP-3α, IL-6, vascular endothelial growth factor (VEGF), and BD-2 concentrations (44).
Vaginal insertion practices.
Certain vaginal insertion practices, particularly vaginal douching, have been linked to a higher prevalence of BV (93, 94). Vaginal douching after menses increased the BV risk 5-fold, and women who had douched in the past week before sampling were twice as likely to have BV (95). In a study of women engaging in transactional sex, using a cloth to clean inside the vagina was associated with a hazard ratio (HR) of 1.58 for BV (86). In a meta-analysis, cleaning with soap intravaginally led to the development of BV or intermediate vaginal flora in women who had a normal vaginal microbiota at baseline (96).
Lifestyle-related factors: diet, stress, and smoking.
A woman’s diet appears to influence not only her gut microbiota but also her genital microbiota. Dietary fat intake has been associated with a heightened BV risk, while diets rich in or supplemented with folate, vitamin A, and calcium may be protective (97). Among pregnant adolescents, low vitamin D levels were associated with a higher prevalence of BV and higher vaginal TNF-α concentrations (98). “Naturally nutrient-rich” and glycemic load dietary scores were predictive of BV progression and persistence, which could be due to hyperglycemia-related oxidative stress, leading to impaired immune function, or via colonization from the rectum (99). In South African adolescents, body mass index positively correlated with an inflammation-associated vaginal microbiota (4), whereas Lokken et al. instead found a 20% lower risk of incident BV in obese than in normal-weight women (100).
Psychosocial stress can also lead to a higher prevalence and incidence of BV (101). Cortisol release during chronic stress can modulate estrogen production, leading to the inhibition of vaginal glycogen deposition and shifts to low-Lactobacillus states (102). Smoking has independently been associated with an increased risk of BV (103, 104), which the authors suggest could possibly occur through the exclusion of Lactobacillus spp. other than L. iners due to their lower stability in the FGT, facilitating the transition to BV. Smoking has been associated with low Lactobacillus abundance in the FGT, and smoking cessation led to a shift from a Lactobacillus-deficient CST to a Lactobacillus-dominated one (104).
Host genetics.
Although the effect of host genetics on gut microbiota composition has been well described (105, 106), evidence of a possible relationship between host genetics and the vaginal microbiota is scarcer (Table 1). Those factors that have been identified either modulate the risk of BV or modulate the inflammatory response to vaginal microbes (107–111). In a study of polymorphisms in inflammatory cytokines and TLR genes in pregnant women, Goepfert et al. found that women with BV were less likely to have polymorphisms at IL-1β exon 5 +3954, IL-10 −1082, and TLR4 399 loci, irrespective of race. In the same study, among black women, a polymorphism at the IL-6 −174 locus was associated with an increased risk of BV (107). Among HIV-negative women, the single nucleotide polymorphisms (SNPs) TLR7 rs5743737 and TLR7 rs1634323 were associated with a decreased BV risk, and TLR7 rs179012 was associated with an increased risk, while TLR2 rs3804099 was associated with a lower BV risk in women living with HIV (108). Polymorphisms in the TLR4 gene (896A>G polymorphism) led to a more subdued inflammatory response to LPS from G. vaginalis and other anaerobic Gram-negative rods among pregnant women (reduced production of IL-1 and IL-1RA), potentially allowing greater colonization of the vagina by these bacteria (109). A high relative abundance of Prevotella spp. was associated with increased vaginal cytokine levels and the activation of TLR and NF-κB pathways: Si et al. found a strong association between the polymorphism rs2069812 in the IL-5 gene and genital Prevotella melaninogenica abundance (110). A polymorphism in the TNF-α gene (TNFA-208G>A) led to higher vaginal fluid TNF-α concentrations in women with BV (111). These studies highlight the potential importance of host genetics and gene polymorphisms in modulating both the vaginal microbiota and the immune response to BV-associated bacteria in women.
INFLAMMATORY EFFECTS OF SPECIFIC BV-ASSOCIATED BACTERIA
There is a multitude of bacteria found in BV, yet studies in different populations have found various bacteria to be most inflammatory, likely due to their frequent cooccurrence. For example, even in the absence of symptomatic BV, Anahtar et al. found that higher relative abundances of Fusobacterium, Aerococcus, Sneathia, Gemella, Mobiluncus, and Prevotella were associated with the highest levels of inflammation in young South African women in the FRESH cohort, while Lennard et al. found higher relative abundances of BVAB1, S. sanguinegens, A. vaginae, BVAB2, G. vaginalis, Prevotella amnii, and Megasphaera, among others, in women with high inflammation levels as part of the WISH cohort (2, 4). Based on community functional inference (determined using phylogenetic information derived from 16S marker gene sequences, and a database of reference genomes, to predict functional potential), BVAB1 was found to be the strongest contributor to BV persistence and inflammation (4). Using metaproteomic techniques, Alisoltani et al. found that the molecular functions of bacterial cell wall, peptidoglycan, and cell membrane biological processes and cellular components were more predictive of genital inflammation than the presence of specific “nonoptimal” bacteria. Overall, however, higher levels of microbial proteins associated with G. vaginalis, Prevotella spp., Megasphaera spp., Sneathia amnii, and A. vaginae and lower levels of proteins of Lactobacillus origin were most strongly associated with high inflammation levels (112).
One of the characteristics of persistent BV is the formation of polymicrobial biofilms, thought to be initiated by G. vaginalis or A. vaginae (113, 114). These biofilms can provide bacteria with protection against antibiotics and antimicrobial compounds produced by Lactobacillus spp. (115, 116). In turn, BV-associated organisms, particularly G. vaginalis, secrete toxins such as vaginolysin and sialidase, which have cytolytic activity and induce host epithelial cells to produce cytokines such as IL-8 (117), thus creating a chronically highly inflamed milieu. It is also thought that colonization with G. vaginalis may initiate the transition of the vaginal microbiota to a more dysbiotic state because of its biofilm-forming ability, which allows symbiotic BV-associated bacteria like P. bivia, A. vaginae, or Fusobacterium nucleatum to adhere and colonize (118, 119).
A. vaginae is found in up to 55% of BV-positive women, as opposed to only 8% in Nugent-defined BV-negative women (120, 121), and higher concentrations are more likely to be found in women experiencing persistent BV (122). A. vaginae strongly induces the production of IL-6, IL-8, and HBD-4 in vitro via TLR2-to-NF-κB signaling, although the specific TLR2 agonist involved is yet to be defined (123). In a three-dimensional (3D) vaginal epithelial cell model, A. vaginae caused the production of molecules such as MIP-3α, HBD-2, IL-1β, IL-6, IL-8, and TNF-α, which have been shown to be associated with damage of epithelial barrier function (124).
Prevotella spp. can be found at low concentrations in the genital tract even in the absence of BV (125). However, a higher abundance of P. bivia is strongly associated with increased genital cytokine levels and inversely associated with Lactobacillus species abundance (2, 110). In in vitro cultures of vaginal epithelial cells, Prevotella induced high concentrations of IL-1α, IL-1β, IL-8, IL-6, MIP-2α, MIP-3α, MIP-2β, and RANTES compared to L. crispatus (2, 21, 124, 126). In a cohort of adult women from KwaZulu-Natal, South Africa, women with a higher relative abundance of P. bivia were 19 times more likely to have high genital tract inflammation levels and an increased risk for later HIV seroconversion. Here, LPS biosynthesis abundance was highly associated with inflammation (127). While other BV-associated bacteria also produce LPS (such as G. vaginalis) with differing inflammatory capabilities (128), P. bivia appears to contribute the highest LPS concentrations in vaginal secretions (129). In turn, LPS leads to the activation of the NF-κB pathway by binding to TLR4 and CD14 on genital epithelial cells, monocytes, and macrophages (24). Phenotypic and transcriptional analyses of antigen-presenting cells (APCs) from women with Prevotella-dominant vaginal communities showed an enrichment of genes related to NF-κB, TLR, nucleotide-binding oligomerization domain (NOD)-like receptor, and TNF-α signaling pathways (2).
Prevotella timonensis and Megasphaera elsdenii are associated with BV and, in vitro, induce a strong DC-mediated genital inflammatory response, including IL-1β, IL-6, IL-8, IL-12, and TNF-α, unlike L. crispatus, which induced neither Th differentiation nor a DC-mediated response (130). In a study of Kenyan women, the BV-associated bacteria Dialister micraerophilus, Eggerthella species type 1, M. hominis, Parvimonas species type 2, Gemella asaccharolytica, Sneathia spp., and Megasphaera spp., linked to an increased risk of HIV infection in this cohort, were associated with the upregulation of TNF-α and IL-1β (131).
Pathogenic bacteria can directly modulate inflammatory markers. As mentioned above, although BV is generally considered inflammatory, some chemokines, including IP-10, appear to be actively decreased in women with BV (132, 133). The mechanisms or bacteria mediating this effect are unclear. P. bivia can have a suppressive effect on IP-10 production by epithelial cells in vitro (134). Clinical strains of Finegoldia magna, a BV-associated pathobiont (135), express a serine proteinase, which degrades the chemokine monokine-induced gamma interferon (MIG) and suppresses its antimicrobial effect (136).
LACTOBACILLUS SPECIES ARE ASSOCIATED WITH LOW-INFLAMMATION STATES
Lactobacillus species contribute to host defenses by producing antimicrobial compounds such as lactic acid and bacteriocins. Lactic acid (both l- and d-isoforms), a key Lactobacillus species metabolite produced during anaerobic glucose metabolism, had a direct anti-inflammatory effect in a 3D human vaginal epithelial tissue model, where it triggered the production of the anti-inflammatory cytokine IL-1RA; suppressed the production of IL-6, IL-8, TNF-α, RANTES, and MIP-3α after stimulation by TLR2, -3, and -4 agonists; and inhibited the production of IL-6 and IL-8 upon exposure to seminal plasma (74). Lactobacilli can also physically protect the FGT in a nonspecific way by forming colonies on epithelial cells or by causing coaggregation between bacterial species and preventing the movement of pathogenic bacterial species (137). L. crispatus and L. iners are the two major Lactobacillus species colonizing the genital microbiota in women, although L. gasseri and L. jensenii are present at lower concentrations (138).
L. crispatus is known for its anti-inflammatory effect, and lower abundances of this species tend to be associated with higher inflammatory cytokine levels (124, 139). Compared to gut strains, genital L. crispatus have evolved to be more adapted to their local milieu, with a higher abundance of genes related to acid tolerance, redox reactions, pullulanase, and carbohydrate-binding molecules (140). In a vaginal epithelial cell culture system, L. crispatus as well as L. jensenii had a dampening effect on inflammation after TLR stimulation (139). L. crispatus and L. jensenii proteins in vaginal fluid were found to inhibit Escherichia coli growth (141, 142). L. crispatus, which is considered more beneficial, is more dependent on glycogen than L. iners (11, 143). Thus, circumstances where glycogen levels are low could potentially select for less optimal lactobacillus species, leading to lower lactic acid levels and higher pH.
While L. crispatus is rarely found in women with BV (144), L. iners can be present in the vaginas of women with and without BV (145, 146). L. iners has the smallest genome size of the vaginal lactobacilli (1.3-Mbp single chromosome) and has evolved to be highly specialized to the genital mucosa, with the majority of its genes encoding core metabolic proteins found among all lactobacilli (147). L. iners, the most commonly found bacterial species in the female genital tract (148), has been proposed as a “transitional” species that increases the likelihood of a shift to a more diverse microbiota than an L. crispatus-dominated microbiota (149). L. iners persists irrespective of BV status, suggesting that it is uniquely adapted to dynamic changes in the FGT (145). One possible reason is that L. iners possesses the gene for inerolysin, a pore-forming toxin that increases its adhesion capability and allows it to consistently take up nutrients from the host despite fluctuations in the genital milieu (150, 151). In addition, L. iners gene expression is increased up to 10% in women with BV, which included higher expression of a cytolysin, eight proteins for the CRISPR antibacteriophage defense system, mucin, and glycerol transport and metabolic enzymes (152). L. iners, found most often in African women with low-diversity microbiota and often associated with low inflammation (4), can also have an immune-dampening or probiotic effect, shown to be capable of disrupting G. vaginalis biofilms and reducing the risk of developing BV (153).
Studies investigating the direct interaction between Lactobacillus spp. and BV-associated bacteria have mostly been in oversimplified in vitro systems lacking immune cells and mucus. In a CaSki epithelial cell culture model, a range of vaginal L. crispatus, L. jensenii, L. mucosae, and L. gasseri strains dampened the inflammatory response to G. vaginalis in a coculture model (154). However, lactobacilli isolated from women with a more diverse vaginal microbiota tended to independently induce higher concentrations of IL-6, IL-8, IL-1α, IL-1β, MIP-1α, and MIP-1β from CaSki cells than those from women with a Lactobacillus-dominant microbiota, and inflammation induction was inversely associated with their ability to adhere to epithelial cells (155). In coculture experiments, lactobacillus strains (particularly L. crispatus strains) inhibited the growth of Prevotella species, while only a few strains inhibited G. vaginalis growth. In addition, all the lactobacillus strains increased the production of IL-1RA and IL-10 by vaginal epithelial cell lines, which modulated the production of proinflammatory cytokines, including MIP-1α, MIP-1β, and IP-10, previously associated with a heightened risk of HIV acquisition (19, 156).
ANTIBIOTICS AND PROBIOTICS TO OPTIMIZE THE VAGINAL ECOSYSTEM
BV is currently treated with antibiotics that target anaerobic organisms (157–159). In U.S. and Kenyan cohorts, metronidazole has a profound short-term effect on the genital microbiota and BV prevalence, where bacteria such as G. vaginalis or other BV-associated bacteria become depleted within hours, to be replaced by less-antibiotic-sensitive lactobacilli such as L. iners posttreatment (160, 161). Clearance of BV was associated with significant decreases in concentrations of the proinflammatory cytokines IL-1α and IL-1β, although several chemokines were upregulated (including IP-10, MIG, MIP-3α, MCP-1, and MIP-1α), which could indicate that while treatment leads to a drastic reduction in BV-associated bacteria, it does not completely eradicate them (161). Unfortunately, these effects seem to be short-lived, with more than half of the women successfully treated presenting with recurrent BV at 12 weeks posttreatment and experiencing an increased prevalence of CST IV (G. vaginalis dominant) (A. Mtshali, S. Ngcapu, S. E. James, F. Osman, N. J. Garrett, C. Balle, J. Giandhari, K. Mngomezulu, G. Mzobe, T. de Oliveira, A. M. Rompalo, A. Mindel, S. S. Abdool Karim, J. A. S. Passmore, Q. Abdool Karim, H. B. Jaspan, and L. J. P. Liebenberg, submitted for publication).
Although findings are conflicting, studies have investigated the use of probiotic or biotherapeutic lactobacilli administered in conjunction with antibiotics to lower BV recurrence rates (162–165). A recent groundbreaking randomized controlled trial found that L. crispatus CTV-05, administered through vaginal application for 11 weeks following BV treatment with metronidazole gel, reduced the risk of BV recurrence by 34% (165). Certain lactobacilli, such as Lactobacillus reuteri RC-14 and L. rhamnosus GR-1, are able to dislodge G. vaginalis and A. vaginae and disrupt their biofilms through the production of bacteriocins or biosurfactant-like molecules and have been tested in clinical trials (166–170). The direct administration of Lactobacillus metabolites or prebiotics has also been explored: a study showed that the use of a combination of metronidazole and a vaginal gel containing lactic acid and glycogen was more effective in Lactobacillus colonization than metronidazole alone (171). In vitro studies have shown that lactic acid had an anti-inflammatory effect on cells in culture, upregulating the anti-inflammatory cytokine IL-1RA and inhibiting the production of IL-6, IL-8, TNF-α, RANTES, and MIP-3α when stimulated with TLR agonists (74).
CONSEQUENCES OF A HIGHLY DIVERSE MICROBIOTA AND INFLAMMATION
Having a highly inflammatory, diverse genital microbiota has been associated with an increased risk of genital infections, including several sexually transmitted infections (STIs), among others (Fig. 1).
Sexually transmitted infections.
Highly diverse microbiota and STIs have agonistic relationships in women. Having BV (Nugent score of 7 to 10) or an intermediate vaginal microbiota (Nugent score of 4 to 6) is associated with a higher rate of incident infections such as Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, or human papillomavirus (HPV), among others (7, 172–174), independent of sexual behavior. BV leads to chronic inflammation, which is associated with increased susceptibility to other genital infections, possibly via barrier disruption. Other possible mechanisms include the production of specific metabolites that enhance the growth or infectivity of pathogens (175). The fact that metronidazole treatment for BV leads to a significant decrease in the subsequent acquisition of STIs is further evidence of the key role of the genital microbiota in modulating STI risk (176, 177).
A recent systematic review linked vaginal microbiota with low Lactobacillus relative abundance to higher C. trachomatis incidence (7). Although the mechanism is unclear, commensal lactobacilli can inhibit the pathogenic effect of C. trachomatis, possibly via the activation of the innate immune response (124). In vitro, L. crispatus and its culture supernatant led to decreases in IL-6, IL-8, and TFN-α and an increase in IL-10 production by C. trachomatis-infected HeLa and J774 cells (178), while lactic acid potentially deactivated chlamydial elementary bodies, the infectious form of the pathogen, by inactivating their surface molecules, damaging their outer membrane, or disrupting chlamydial metabolic activity by hydrogen ions (179).
Higher susceptibility to N. gonorrhoeae is evident in women with Lactobacillus-deficient microbiota (180). In vitro and in a porcine vaginal mucosal model, several Lactobacillus spp. were directly able to inhibit N. gonorrhoeae adherence to epithelial cells, with clinical vaginal strains providing the most inhibition (181–184), while acidification due to lactic acid, particularly by L. crispatus, was able to inhibit N. gonorrhoeae growth in culture (185).
Women with BV were 3.5 times more likely to subsequently acquire M. genitalium than women with a normal vaginal microbiota in a dose-dependent manner, where the risk increased by 16% with each point increase in the Nugent score (186). The mechanisms for this have not been well explored.
T. vaginalis and BV commonly cooccur, and data suggest that BV precedes T. vaginalis infection (187, 188). This is possibly related to a low abundance of Lactobacillus spp. and a higher vaginal pH associated with BV, which favors the growth of T. vaginalis organisms (187).
Women with Nugent-defined BV are at a higher risk of HIV acquisition (189). Furthermore, in multiple African cohorts, HIV acquisition was associated with high-diversity microbiota and/or high relative abundances or absolute concentrations of specific microbes such as Prevotella, Sneathia, and other anaerobes (21, 190). Cellular immune responses and inflammation triggered in response to BV could provide more accessible target cells at the vaginal mucosa for HIV to infect, through their recruitment, activation, or barrier disruption (57). Alternatively, it is possible that the lower numbers of lactobacilli resulting from BV would lead to an increase in vaginal pH (191) associated with lower lactic acid levels (particularly l-lactic acid), which could in turn enable HIV infection (192, 193).
The absence of vaginal lactobacilli has also been associated with herpes simplex virus 2 (HSV-2) shedding (194, 195), while on the other hand, incident HSV-2 infections are associated with a 30% increased odds of having a subsequent BV episode (196), suggesting a reciprocal relationship between BV and HSV-2. The protective effect could be due to the adhesion of lactobacilli to epithelial cells (197) or the production of l-lactic acid, which has virucidal activity at low pH (164, 198).
Women with high-risk HPV are more likely to have a vaginal microbiota dominated by bacteria other than Lactobacillus spp., especially L. gasseri, which has been implicated in HPV clearance (199, 200). Having a high-diversity or an L. iners-dominated CST puts women at a higher risk of HPV acquisition or persistence, suggesting that a low-diversity, healthier CST could protect against HPV via the production of several antimicrobial factors, including lactic acid (7, 199).
Vulvovaginal candidiasis.
Vulvovaginal candidiasis (VVC), usually caused by Candida albicans, occurs more readily in a Lactobacillus-dominated microbiota, particularly with L. iners (201). Lower abundances of Megasphaera spp. and Mageeibacillus indolicus and higher abundances of Bifidobacterium bifidum, Aerococcus christensenii, L. mucosae, L. crispatus/L. helveticus, Streptococcus equinus/S. infantarius/S. lutetiensis, P. bivia, and Dialister propionicifaciens have been found in women with candidiasis (202). However, other studies point to a positive association between microbial dysbiosis and VVC instead. In a U.S. study, the C. albicans concentration was inversely associated with the Lactobacillus abundance and positively associated with the IL-8 concentrations (203). All of these studies, however, were associative, and we are not able to infer the directionality of the relationship between vaginal bacteria and candida. In vitro, however, lactobacilli can inhibit C. albicans growth through competition for adhesion sites and nutrients or the secretion of fungicidal compounds (204–206). Both cells and supernatants from L. crispatus and L. gasseri strains reduced the adhesion of Candida to HeLa cells, suggesting that Lactobacillus spp. produce compounds with fungistatic and fungicidal activities (207). Lactobacillus biosurfactants, lipopeptides that reduce pathogen growth and adhesion, showed antimicrobial activity against C. albicans, including via disrupting preformed biofilms (208) or competitive exclusion (209). Pretreating HeLa cells with L. crispatus induced the downregulation of TLR2/4 expression; increased IL-8, HBD-2, and HBD-3 concentrations; and decreased the adhesion and growth of C. albicans (205). The production of lactic acid by probiotic Lactobacillus spp. was also associated with reduced fungal growth (210).
CONCLUSION
The female genital microbiota and the consequent genital immune milieu are highly dynamic. While cytokines are an important part of the FGT immune system, the multiple physiological and behavioral factors associated with a diverse microbiota can lead to a cascade effect of overwhelming chronic inflammation, genital epithelial barrier damage, and increased risk of other infections. Research around this topic has increased significantly in recent years, and the role of certain key bacteria as well as community dynamics in modulating genital health is now well established. However, data describing the means through which particular taxa influence immunity in the FGT, and the ways in which specific cytokines interact with the genital milieu, are lacking. Understanding the mechanisms through which the host inflammatory response, triggered by shifts in the microbiota, can influence FGT health is essential for prevention and therapeutic strategies.
REFERENCES
- 1.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. 2013. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 497:258–262. 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, Ghebremichael MS, Nusbaum C, Huttenhower C, Virgin HW, Ndung’u T, Dong KL, Walker BD, Fichorova RN, Kwon DS. 2015. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42:965–976. 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, Koenig SSK, Fu L, Ma Z, Zhou X, Abdo Z, Forney LJ, Ravel J. 2012. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4:132ra52. 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennard K, Dabee S, Barnabas SL, Havyarimana E, Blakney A, Jaumdally SZ, Botha G, Mkhize NN, Bekker L-G, Lewis DA, Gray G, Mulder N, Passmore J-AS, Jaspan HB. 2018. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect Immun 86:e00410-17. 10.1128/IAI.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinnon LR, Achilles SL, Bradshaw CS, Burgener A, Crucitti T, Fredricks DN, Jaspan HB, Kaul R, Kaushic C, Klatt N, Kwon DS, Marrazzo JM, Masson L, McClelland RS, Ravel J, van de Wijgert JHHM, Vodstrcil LA, Tachedjian G. 2019. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retroviruses 35:219–228. 10.1089/AID.2018.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaambo E, Africa C, Chambuso R, Passmore J-AS. 2018. Vaginal microbiomes associated with aerobic vaginitis and bacterial vaginosis. Front Public Health 6:78. 10.3389/fpubh.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamarelle J, Thiébaut ACM, de Barbeyrac B, Bébéar C, Ravel J, Delarocque-Astagneau E. 2019. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: a systematic review and meta-analysis. Clin Microbiol Infect 25:35–47. 10.1016/j.cmi.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witkin SS, Linhares IM, Giraldo P. 2007. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol 21:347–354. 10.1016/j.bpobgyn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.van der Veer C, Hertzberger RY, Bruisten SM, Tytgat HLP, Swanenburg J, de Kat Angelino-Bart A, Schuren F, Molenaar D, Reid G, de Vries H, Kort R. 2019. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: implications for in vivo dominance of the vaginal microbiota. Microbiome 7:49. 10.1186/s40168-019-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, Spear WW, Landay A, Micci S, Lee B-H, Hamaker BR. 2014. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis 210:1019–1028. 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirmonsef P, Hotton AL, Gilbert D, Burgad D, Landay A, Weber KM, Cohen M, Ravel J, Spear GT. 2014. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS One 9:e102467. 10.1371/journal.pone.0102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrova MI, van den Broek M, Balzarini J, Vanderleyden J, Lebeer S. 2013. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol Rev 37:762–792. 10.1111/1574-6976.12029. [DOI] [PubMed] [Google Scholar]
- 13.Miller EA, Beasley DE, Dunn RR, Archie EA. 2016. Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front Microbiol 7:1936. 10.3389/fmicb.2016.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavlova SI, Kilic AO, Kilic SS, So J-S, Nader-Macias ME, Simoes JA, Tao L. 2002. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J Appl Microbiol 92:451–459. 10.1046/j.1365-2672.2002.01547.x. [DOI] [PubMed] [Google Scholar]
- 15.Campisciano G, Zanotta N, Licastro D, De Seta F, Comar M. 2018. In vivo microbiome and associated immune markers: new insights into the pathogenesis of vaginal dysbiosis. Sci Rep 8:2307. 10.1038/s41598-018-20649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm JB, France MT, Ma B, McComb E, Robinson CK, Mehta A, Tallon LJ, Brotman RM, Ravel J. 2020. Comparative metagenome-assembled genome analysis of “Candidatus Lachnocurva vaginae”, formerly known as bacterial vaginosis-associated bacterium-1 (BVAB1). Front Cell Infect Microbiol 10:117. 10.3389/fcimb.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, Strauss JF, The Vaginal Microbiome Consortium, Jefferson KK, Buck GA. 2014. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 160:2272–2282. 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balle C, Lennard K, Dabee S, Barnabas SL, Jaumdally SZ, Gasper MA, Maseko V, Mbulawa ZZA, Williamson A-L, Bekker L-G, Lewis DA, Passmore J-AS, Jaspan HB. 2018. Endocervical and vaginal microbiota in South African adolescents with asymptomatic Chlamydia trachomatis infection. Sci Rep 8:11109. 10.1038/s41598-018-29320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masson L, Passmore JS, Liebenberg LJ, Werner L, Baxter C, Arnold KB, Williamson C, Little F, Mansoor LE, Naranbhai V, Lauffenburger DA, Ronacher K, Walzl G, Garrett NJ, Williams BL, Couto-Rodriguez M, Hornig M, Lipkin WI, Grobler A, Abdool Karim Q, Abdool Karim SS. 2015. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 61:260–269. 10.1093/cid/civ298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdool Karim SS, Baxter C, Passmore JS, McKinnon LR, Williams BL. 2019. The genital tract and rectal microbiomes: their role in HIV susceptibility and prevention in women. J Int AIDS Soc 22:e25300. 10.1002/jia2.25300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, Padavattan N, Desai C, Droit L, Moodley A, Dong M, Chen Y, Ismail N, Ndung’u T, Ghebremichael MS, Wesemann DR, Mitchell C, Dong KL, Huttenhower C, Walker BD, Virgin HW, Kwon DS. 2017. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 46:29–37. 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, Mwaura M, Ndayisaba G, Delany-Moretlwe S, Buyze J, Vanham G, van de Wijgert JHHM. 2017. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep 7:11974. 10.1038/s41598-017-12198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cauci S. 2004. Vaginal immunity in bacterial vaginosis. Curr Infect Dis Rep 6:450–456. 10.1007/s11908-004-0064-8. [DOI] [PubMed] [Google Scholar]
- 24.Nasu K, Narahara H. 2010. Pattern recognition via the Toll-like receptor system in the human female genital tract. Mediators Inflamm 2010:976024. 10.1155/2010/976024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. 2005. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 206:306–335. 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 26.Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, Abou M, Westmacott GR, McCorrister S, Kwatampora J, Nyanga B, Kimani J, Masson L, Liebenberg LJ, Abdool Karim SS, Passmore J-AS, Lauffenburger DA, Kaul R, McKinnon LR. 2016. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 9:194–205. 10.1038/mi.2015.51. [DOI] [PubMed] [Google Scholar]
- 27.Sobel JD, Nyirjesy P. 2020. Vulvovaginitis, p 227–238. In Goldstein AT, Pukall CF, Goldstein I, Krapf JM, Goldstein SW, Goldstein G (ed), Female sexual pain disorders: evaluation and management, 2nd ed. John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- 28.Puran AC, Adler D, Wallace M, Bennie T, Phuti A, Abar B, Bekker L-G. 2014. Incidental findings of bacterial vaginosis and other infections in Papanicolaou smears of HIV-infected and HIV-uninfected adolescent females in South Africa. J AIDS HIV Res 6:172–176. [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson LF, Coetzee DJ, Dorrington RE. 2005. Sentinel surveillance of sexually transmitted infections in South Africa: a review. Sex Transm Infect 81:287–294. 10.1136/sti.2004.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenyon C, Colebunders R, Crucitti T. 2013. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 209:505–523. 10.1016/j.ajog.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Jespers V, Crucitti T, Menten J, Verhelst R, Mwaura M, Mandaliya K, Ndayisaba GF, Delany-Moretlwe S, Verstraelen H, Hardy L, Buvé A, van de Wijgert J, for the Vaginal Biomarkers Study Group . 2014. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in sub-Saharan Africa: a cross-sectional study. PLoS One 9:e109670. 10.1371/journal.pone.0109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. 2006. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 193:1478–1486. 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 33.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. 2007. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol 45:3270–3276. 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell C, Marrazzo J. 2014. Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol 71:555–563. 10.1111/aji.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datcu R, Gesink D, Mulvad G, Montgomery-Andersen R, Rink E, Koch A, Ahrens P, Jensen JS. 2013. Vaginal microbiome in women from Greenland assessed by microscopy and quantitative PCR. BMC Infect Dis 13:480. 10.1186/1471-2334-13-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, Marrazzo JM, Fredricks DN. 2010. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 5:e10197. 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert JA, John S, Sobel JD, Akins RA. 2013. Longitudinal analysis of vaginal microbiome dynamics in women with recurrent bacterial vaginosis: recognition of the conversion process. PLoS One 8:e82599. 10.1371/journal.pone.0082599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muzny CA, Blanchard E, Taylor CM, Aaron KJ, Talluri R, Griswold ME, Redden DT, Luo M, Welsh DA, Van Der Pol WJ, Lefkowitz EJ, Martin DH, Schwebke JR. 2018. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect Dis 218:966–978. 10.1093/infdis/jiy243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammadzadeh F, Dolatian M, Jorjani M, Alavi Majd H. 2014. Diagnostic value of Amsel’s clinical criteria for diagnosis of bacterial vaginosis. Glob J Health Sci 7:8–14. 10.5539/gjhs.v7n3p8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29:297–301. 10.1128/JCM.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klebanoff MA, Schwebke JR, Zhang J, Nansel TR, Yu K-F, Andrews WW. 2004. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol 104:267–272. 10.1097/01.AOG.0000134783.98382.b0. [DOI] [PubMed] [Google Scholar]
- 42.Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, Gamieldien H, Williamson C, Mckinnon LR, Walzl G, Abdool Karim Q, Abdool Karim SS, Passmore J-AS. 2014. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 90:580–588. 10.1136/sextrans-2014-051601. [DOI] [PubMed] [Google Scholar]
- 43.Shannon B, Gajer P, Yi TJ, Ma B, Humphrys MS, Thomas-Pavanel J, Chieza L, Janakiram P, Saunders M, Tharao W, Huibner S, Shahabi K, Ravel J, Kaul R. 2017. Distinct effects of the cervicovaginal microbiota and herpes simplex type 2 infection on female genital tract immunology. J Infect Dis 215:1366–1375. 10.1093/infdis/jix088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fichorova RN, Morrison CS, Chen P-L, Yamamoto HS, Govender Y, Junaid D, Ryan S, Kwok C, Chipato T, Salata RA, Doncel GF. 2020. Aberrant cervical innate immunity predicts onset of dysbiosis and sexually transmitted infections in women of reproductive age. PLoS One 15:e0224359. 10.1371/journal.pone.0224359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elkington PTG, O’Kane CM, Friedland JS. 2005. The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol 142:12–20. 10.1111/j.1365-2249.2005.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasioudis D, Linhares I, Ledger W, Witkin S. 2017. Bacterial vaginosis: a critical analysis of current knowledge. BJOG 124:61–69. 10.1111/1471-0528.14209. [DOI] [PubMed] [Google Scholar]
- 47.Zariffard MR, Anastos K, French AL, Munyazesa E, Cohen M, Landay AL, Spear GT. 2015. Cleavage/alteration of interleukin-8 by matrix metalloproteinase-9 in the female lower genital tract. PLoS One 10:e0116911. 10.1371/journal.pone.0116911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. 2000. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood 96:2673–2681. 10.1182/blood.V96.8.2673. [DOI] [PubMed] [Google Scholar]
- 49.St John EP, Martinson J, Simoes JA, Landay AL, Spear GT. 2007. Dendritic cell activation and maturation induced by mucosal fluid from women with bacterial vaginosis. Clin Immunol 125:95–102. 10.1016/j.clim.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakahara T, Urabe K, Fukagawa S, Uchi H, Inaba K, Furue M, Moroi Y. 2005. Engagement of human monocyte-derived dendritic cells into interleukin (IL)-12 producers by IL-1beta + interferon (IFN)-gamma. Clin Exp Immunol 139:476–482. 10.1111/j.1365-2249.2004.02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 52.Pulugulla SH, Adamik J, Grillini AN, Galson DL, Auron PE. 2016. Specific transcription factors distinctly regulate kinetics of IL-1B and TNF gene expression. J Immunol 196:189.14. [Google Scholar]
- 53.Cauci S, Driussi S, Guaschino S, Isola M, Quadrifoglio F. 2002. Correlation of local interleukin-1beta levels with specific IgA response against Gardnerella vaginalis cytolysin in women with bacterial vaginosis. Am J Reprod Immunol 47:257–264. 10.1034/j.1600-0897.2002.01096.x. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell C, Fredricks D, Agnew K, Hitti J. 2015. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Dis 42:358–363. 10.1097/OLQ.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cauci S, Guaschino S, De Aloysio D, Driussi S, De Santo D, Penacchioni P, Quadrifoglio F. 2003. Interrelationships of interleukin-8 with interleukin-1b and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod 9:53–58. 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 56.Campisi J, Leem TH, Fleshner M. 2003. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones 8:272–286. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rebbapragada A, Howe K, Wachihi C, Pettengell C, Sunderji S, Huibner S, Ball TB, Plummer FA, Jaoko W, Kaul R. 2008. Bacterial vaginosis in HIV-infected women induces reversible alterations in the cervical immune environment. J Acquir Immune Defic Syndr 49:520–522. 10.1097/QAI.0b013e318189a7ca. [DOI] [PubMed] [Google Scholar]
- 58.Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, Raftery D, Marrazzo JM, Fredricks DN. 2015. Metabolic signatures of bacterial vaginosis. mBio 6:e00204-15. 10.1128/mBio.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valore EV, Wiley DJ, Ganz T. 2006. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 74:5693–5702. 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mirmonsef P, Gilbert D, Zariffard MR, Hamaker BR, Kaur A, Landay AL, Spear GT. 2011. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol 65:190–195. 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL, Spear GT. 2012. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with Toll-like receptor ligands. Am J Reprod Immunol 67:391–400. 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratten L, Plummer E, Murray G, Danielewski J, Fairley C, Garland S, Hocking J, Tachedjian G, Chow EPF, Bradshaw C, Vodstrcil L. 23 July 2020. Sex is associated with the persistence of non‐optimal vaginal microbiota following treatment for bacterial vaginosis: a prospective cohort study. BJOG 10.1111/1471-0528.16430. [DOI] [PubMed]
- 63.Hou D, Zhou X, Zhong X, Settles ML, Herring J, Wang L, Abdo Z, Forney LJ, Xu C. 2013. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril 100:1261–1269.e3. 10.1016/j.fertnstert.2013.07.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallo MF, Warner L, King CC, Sobel JD, Klein RS, Cu-Uvin S, Rompalo AM, Jamieson DJ. 2011. Association between semen exposure and incident bacterial vaginosis. Infect Dis Obstet Gynecol 2011:842652. 10.1155/2011/842652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swidsinski A, Dörffel Y, Loening-Baucke V, Mendling W, Verstraelen H, Dieterle S, Schilling J. 2010. Desquamated epithelial cells covered with a polymicrobial biofilm typical for bacterial vaginosis are present in randomly selected cryopreserved donor semen. FEMS Immunol Med Microbiol 59:399–404. 10.1111/j.1574-695X.2010.00688.x. [DOI] [PubMed] [Google Scholar]
- 66.Mehta SD, Zhao D, Green SJ, Agingu W, Otieno F, Bhaumik R, Bhaumik D, Bailey RC. 2020. The microbiome composition of a man’s penis predicts incident bacterial vaginosis in his female sex partner with high accuracy. Front Cell Infect Microbiol 10:433. 10.3389/fcimb.2020.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jespers V, van de Wijgert JH, Cools P, Verhelst R, Verstraelen H, Delany-Moretlwe S, Mwaura M, Ndayisaba GF, Mandaliya K, Menten J, Hardy L, Crucitti T, for the Vaginal Biomarkers Study Group . 2015. The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. BMC Infect Dis 15:115. 10.1186/s12879-015-0825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forcey DS, Vodstrcil LA, Hocking JS, Fairley CK, Law M, McNair RP, Bradshaw CS. 2015. Factors associated with bacterial vaginosis among women who have sex with women: a systematic review. PLoS One 10:e0141905. 10.1371/journal.pone.0141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plummer EL, Vodstrcil LA, Fairley CK, Tabrizi SN, Garland SM, Law MG, Hocking JS, Fethers KA, Bulach DM, Murray GL, Bradshaw CS. 2019. Sexual practices have a significant impact on the vaginal microbiota of women who have sex with women. Sci Rep 9:19749. 10.1038/s41598-019-55929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eschenbach DA, Thwin SS, Patton DL, Hooton TM, Stapleton AE, Agnew K, Winter C, Meier A, Stamm WE. 2000. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis 30:901–907. 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 71.Lopes dos Santos Santiago G, Cools P, Verstraelen H, Trog M, Missine G, El Aila N, Verhelst R, Tency I, Claeys G, Temmerman M, Vaneechoutte M. 2011. Longitudinal study of the dynamics of vaginal microflora during two consecutive menstrual cycles. PLoS One 6:e28180. 10.1371/journal.pone.0028180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alvarez-Olmos MI, Barousse MM, Rajan L, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL. 2004. Vaginal lactobacilli in adolescents. Sex Transm Dis 31:393–400. 10.1097/01.olq.0000130454.83883.e9. [DOI] [PubMed] [Google Scholar]
- 73.O’Hanlon DE, Moench TR, Cone RA. 2013. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8:e80074. 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hearps AC, Tyssen D, Srbinovski D, Bayigga L, Diaz DJD, Aldunate M, Cone RA, Gugasyan R, Anderson DJ, Tachedjian G. 2017. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol 10:1480–1490. 10.1038/mi.2017.27. [DOI] [PubMed] [Google Scholar]
- 75.Cruickshank R, Sharman A. 1934. The biology of the vagina in the human subject. BJOG 41:208–226. 10.1111/j.1471-0528.1934.tb08759.x. [DOI] [Google Scholar]
- 76.Bhandari P, Tingley JP, Abbott DW, Hill JE. 2020. Characterization of an α-glucosidase enzyme conserved in Gardnerella spp. isolated from the human vaginal microbiome. bioRxiv. 10.1101/2020.05.11.086124. [DOI] [PMC free article] [PubMed]
- 77.Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, Viscidi RP, Burke AE, Ravel J, Gravitt PE. 2014. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 21:450–458. 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cauci S, Driussi S, De Santo D, Penacchioni P, Iannicelli T, Lanzafame P, De Seta F, Quadrifoglio F, de Aloysio D, Guaschino S. 2002. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol 40:2147–2152. 10.1128/jcm.40.6.2147-2152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffmann JN, You HM, Hedberg EC, Jordan JA, McClintock MK. 2014. Prevalence of bacterial vaginosis and Candida among postmenopausal women in the United States. J Gerontol B Psychol Sci Soc Sci 69:S205–S214. 10.1093/geronb/gbu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitchell CM, McLemore L, Westerberg K, Astronomo R, Smythe K, Gardella C, Mack M, Magaret A, Patton D, Agnew K, McElrath MJ, Hladik F, Eschenbach D. 2014. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis 210:651–655. 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Molatlhegi RP, Liebenberg LJ, Leslie A, Noel-Romas L, Mabhula A, Mchunu N, Perner M, Birse K, Ngcapu S, Adamson JH, Govender K, Garrett NJ, Samsunder N, Burgener AD, Abdool Karim SS, Abdool Karim Q, Passmore J-AS, McKinnon LR. 2020. Plasma concentration of injectable contraceptive correlates with reduced cervicovaginal growth factor expression in South African women. Mucosal Immunol 13:449–459. 10.1038/s41385-019-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balle C, Konstantinus IN, Jaumdally SZ, Havyarimana E, Lennard K, Esra R, Barnabas SL, Happel A-U, Moodie Z, Gill K, Pidwell T, Karaoz U, Brodie E, Maseko V, Gamieldien H, Bosinger SE, Myer L, Bekker L-G, Passmore J-AS, Jaspan HB. 2020. Hormonal contraception alters vaginal microbiota and cytokines in South African adolescents in a randomized trial. Nat Commun 11:5578. 10.1038/s41467-020-19382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Achilles SL, Austin MN, Meyn LA, Mhlanga F, Chirenje ZM, Hillier SL. 2018. Impact of contraceptive initiation on vaginal microbiota. Am J Obstet Gynecol 218:622.e1–622.e10. 10.1016/j.ajog.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peebles K, Kiweewa FM, Palanee-Phillips T, Chappell C, Singh D, Bunge KE, Naidoo L, Makanani B, Jeenarain N, Reynolds D, Hillier SL, Brown ER, Baeten JM, Balkus JE, MTN-020/ASPIRE Study Team . 6 June 2020. Elevated risk of bacterial vaginosis among users of the copper intrauterine device: a prospective longitudinal cohort study. Clin Infect Dis 10.1093/cid/ciaa703. [DOI] [PMC free article] [PubMed]
- 85.Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. 2007. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis 34:954–959. [PubMed] [Google Scholar]
- 86.McClelland RS, Richardson BA, Graham SM, Masese LN, Gitau R, Lavreys L, Mandaliya K, Jaoko W, Baeten JM, Ndinya-Achola JO. 2008. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sex Transm Dis 35:617–623. 10.1097/OLQ.0b013e31816907fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pettifor A, Delany S, Kleinschmidt I, Miller WC, Atashili J, Rees H. 2009. Use of injectable progestin contraception and risk of STI among South African women. Contraception 80:555–560. 10.1016/j.contraception.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van de Wijgert JHHM, Verwijs MC, Turner AN, Morrison CS. 2013. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS 27:2141–2153. 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 89.Vodstrcil LA, Hocking JS, Law M, Walker S, Tabrizi SN, Fairley CK, Bradshaw CS. 2013. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PLoS One 8:e73055. 10.1371/journal.pone.0073055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roxby AC, Fredricks DN, Odem-Davis K, Ásbjörnsdóttir K, Masese L, Fiedler TL, De Rosa S, Jaoko W, Kiarie JN, Overbaugh J, McClelland RS. 2016. Changes in vaginal microbiota and immune mediators in HIV-1-seronegative Kenyan women initiating depot medroxyprogesterone acetate. J Acquir Immune Defic Syndr 71:359–366. 10.1097/QAI.0000000000000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, De Guingand D, Morton AN, Fairley CK. 2013. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis 56:777–786. 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- 92.Brooks JP, Edwards DJ, Blithe DL, Fettweis JM, Serrano MG, Sheth NU, Strauss JF, Buck GA, Jefferson KK. 2017. Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception 95:405–413. 10.1016/j.contraception.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ness RB, Soper DE, Holley RL, Peipert J, Randall H, Sweet RL, Sondheimer SJ, Hendrix SL, Hillier SL, Amortegui A, Trucco G, Bass DC, PID Evaluation and Clinical Health (PEACH) Study Investigators . 2001. Douching and endometritis: results from the PID Evaluation and Clinical Health (PEACH) study. Sex Transm Dis 28:240–245. 10.1097/00007435-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Klebanoff MA, Nansel TR, Brotman RM, Zhang J, Yu K-F, Schwebke JR, Andrews WW. 2010. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis 37:94–99. 10.1097/OLQ.0b013e3181bc063c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwebke JR, Desmond RA, Oh MK. 2004. Predictors of bacterial vaginosis in adolescent women who douche. Sex Transm Dis 31:433–436. 10.1097/01.olq.0000129948.91055.9f. [DOI] [PubMed] [Google Scholar]
- 96.Low N, Chersich MF, Schmidlin K, Egger M, Francis SC, van de Wijgert JHHM, Hayes RJ, Baeten JM, Brown J, Delany-Moretlwe S, Kaul R, McGrath N, Morrison C, Myer L, Temmerman M, van der Straten A, Watson-Jones D, Zwahlen M, Hilber A. 2011. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 8:e1000416. 10.1371/journal.pmed.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu K, Goldenberg RL, Klebanoff MA. 2007. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr 137:2128–2133. 10.1093/jn/137.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akoh CC, Pressman EK, Cooper E, Queenan RA, Pillittere J, O’Brien KO. 2018. Low vitamin D is associated with infections and proinflammatory cytokines during pregnancy. Reprod Sci 25:414–423. 10.1177/1933719117715124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thoma ME, Klebanoff MA, Rovner AJ, Nansel TR, Neggers Y, Andrews WW, Schwebke JR. 2011. Bacterial vaginosis is associated with variation in dietary indices. J Nutr 141:1698–1704. 10.3945/jn.111.140541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lokken EM, Richardson BA, Kinuthia J, Mwinyikai K, Abdalla A, Jaoko W, Mandaliya K, Shafi J, Scott McClelland R. 2019. A prospective cohort study of the association between body mass index and incident bacterial vaginosis. Sex Transm Dis 46:31–36. 10.1097/OLQ.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nansel TR, Riggs MA, Yu K-F, Andrews WW, Schwebke JR, Klebanoff MA. 2006. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol 194:381–386. 10.1016/j.ajog.2005.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Amabebe E, Anumba DOC. 2018. Psychosocial stress, cortisol levels, and maintenance of vaginal health. Front Endocrinol (Lausanne) 9:568. 10.3389/fendo.2018.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. 2008. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis 35:78–83. 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 104.Brotman RM, He X, Gajer P, Fadrosh D, Sharma E, Mongodin EF, Ravel J, Glover ED, Rath JM. 2014. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect Dis 14:471. 10.1186/1471-2334-14-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dąbrowska K, Witkiewicz W. 2016. Correlations of host genetics and gut microbiome composition. Front Microbiol 7:1357. 10.3389/fmicb.2016.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kurilshikov A, Wijmenga C, Fu J, Zhernakova A. 2017. Host genetics and gut microbiome: challenges and perspectives. Trends Immunol 38:633–647. 10.1016/j.it.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 107.Goepfert AR, Varner M, Ward K, Macpherson C, Klebanoff M, Goldenberg RL, Mercer B, Meis P, Iams J, Moawad A, Carey JC, Leveno K, Wapner R, Caritis SN, Miodovnik M, Sorokin Y, O’Sullivan MJ, Van Dorsten JP, Langer O. 2005. Differences in inflammatory cytokine and Toll-like receptor genes and bacterial vaginosis in pregnancy. Am J Obstet Gynecol 193:1478–1485. 10.1016/j.ajog.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 108.Mackelprang RD, Scoville CW, Cohen CR, Ondondo RO, Bigham AW, Celum C, Campbell MS, Essex M, Wald A, Kiarie J, Ronald A, Gray G, Lingappa JR, Partners in Prevention HSV/HIV Transmission Study Team . 2015. Toll-like receptor gene variants and bacterial vaginosis among HIV-1 infected and uninfected African women. Genes Immun 16:362–365. 10.1038/gene.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Genc MR, Vardhana S, Delaney ML, Onderdonk A, Tuomala R, Norwitz E, Witkin SS. 2004. Relationship between a Toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant women. Eur J Obstet Gynecol Reprod Biol 116:152–156. 10.1016/j.ejogrb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 110.Si J, You HJ, Yu J, Sung J, Ko G. 2017. Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell Host Microbe 21:97–105. 10.1016/j.chom.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 111.Genç MR, Vardhana S, Delaney ML, Witkin SS, Onderdonk AB, MAP Study Group . 2007. TNFA-308G>A polymorphism influences the TNF-α response to altered vaginal flora. Eur J Obstet Gynecol Reprod Biol 134:188–191. 10.1016/j.ejogrb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 112.Alisoltani A, Manhanzva MT, Potgieter M,. et al. 2020. Microbial function and genital inflammation in young South African women at high risk of HIV infection. Microbiome 8:165. 10.1186/s40168-020-00932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hardy L, Jespers V, Dahchour N, Mwambarangwe L, Musengamana V, Vaneechoutte M, Crucitti T. 2015. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLoS One 10:e0136658. 10.1371/journal.pone.0136658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, Hale LP, Lochs H. 2005. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106:1013–1023. 10.1097/01.AOG.0000183594.45524.d2. [DOI] [PubMed] [Google Scholar]
- 115.Patterson JL, Girerd PH, Karjane NW, Jefferson KK. 2007. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol 197:170.e1–170.e7. 10.1016/j.ajog.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. 2002. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res 66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 117.Gelber SE, Aguilar JL, Lewis KLT, Ratner AJ. 2008. Functional and phylogenetic characterization of vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol 190:3896–3903. 10.1128/JB.01965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Machado A, Jefferson KK, Cerca N. 2013. Interactions between Lactobacillus crispatus and bacterial vaginosis (BV)-associated bacterial species in initial attachment and biofilm formation. Int J Mol Sci 14:12004–12012. 10.3390/ijms140612004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castro J, Machado D, Cerca N. 2019. Unveiling the role of Gardnerella vaginalis in polymicrobial bacterial vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J 13:1306–1317. 10.1038/s41396-018-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferris MJ, Masztal A, Martin DH. 2004. Use of species-directed 16S rRNA gene PCR primers for detection of Atopobium vaginae in patients with bacterial vaginosis. J Clin Microbiol 42:5892–5894. 10.1128/JCM.42.12.5892-5894.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burton JP, Devillard E, Cadieux PA, Hammond J-A, Reid G. 2004. Detection of Atopobium vaginae in postmenopausal women by cultivation-independent methods warrants further investigation. J Clin Microbiol 42:1829–1831. 10.1128/jcm.42.4.1829-1831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferris MJ, Norori J, Zozaya-Hinchliffe M, Martin DH. 2007. Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. J Clin Microbiol 45:1016–1018. 10.1128/JCM.02085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Libby EK, Pascal KE, Mordechai E, Adelson ME, Trama JP. 2008. Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect 10:439–446. 10.1016/j.micinf.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 124.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. 2014. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis 209:1989–1999. 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- 125.Larsen JM. 2017. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 151:363–374. 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fichorova RN, Lai JJ, Schwartz JL, Weiner DH, Mauck CK, Callahan MM. 2011. Baseline variation and associations between subject characteristics and five cytokine biomarkers of vaginal safety among healthy non-pregnant women in microbicide trials. Cytokine 55:134–140. 10.1016/j.cyto.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 127.Passmore J-AS, Williams BL. 2016. Role of vaginal microbiota in genital inflammation and enhancing HIV acquisition in women, abstr TUSS0604. Abstr 21st Int AIDS Conf, Durban, South Africa.
- 128.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen A-M, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lähdesmäki H, Huttenhower C, Gevers D, Cullen TW, Knip M, DIABIMMUNE Study Group, Xavier RJ. 2016. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165:842–853. 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aroutcheva A, Ling Z, Faro S. 2008. Prevotella bivia as a source of lipopolysaccharide in the vagina. Anaerobe 14:256–260. 10.1016/j.anaerobe.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Teijlingen NH, Helgers LC, Zijlstra-Willems EM, van Hamme JL, Ribeiro CMS, Strijbis K, Geijtenbeek TBH. 2020. Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells. J Reprod Immunol 138:103085. 10.1016/j.jri.2020.103085. [DOI] [PubMed] [Google Scholar]
- 131.Sabo MC, Lehman DA, Wang B, Richardson BA, Srinivasan S, Osborn L, Matemo D, Kinuthia J, Fiedler TL, Munch MM, Drake AL, Fredricks DN, Overbaugh J, John-Stewart G, McClelland RS, Graham SM. 2020. Associations between vaginal bacteria implicated in HIV acquisition risk and proinflammatory cytokines and chemokines. Sex Transm Infect 96:3–9. 10.1136/sextrans-2018-053949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Masson L, Arnold KB, Little F, Mlisana K, Lewis DA, Mkhize N, Gamieldien H, Ngcapu S, Johnson L, Lauffenburger DA, Abdool Karim Q, Abdool Karim SS, Passmore J-AS. 2016. Inflammatory cytokine biomarkers to identify women with asymptomatic sexually transmitted infections and bacterial vaginosis who are at high risk of HIV infection. Sex Transm Infect 92:186–193. 10.1136/sextrans-2015-052072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kyongo JK, Crucitti T, Menten J, Hardy L, Cools P, Michiels J, Delany-Moretlwe S, Mwaura M, Ndayisaba G, Joseph S, Fichorova R, van de Wijgert J, Vanham G, Ariën KK, Jespers V. 2015. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in sub-Saharan African women, with relevance to HIV risk and prevention. Clin Vaccine Immunol 22:526–538. 10.1128/CVI.00762-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, Nibert ML, Singh BN, Onderdonk AB. 2013. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 89:460–466. 10.1136/sextrans-2013-051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shipitsyna E, Roos A, Datcu R, Hallén A, Fredlund H, Jensen JS, Engstrand L, Unemo M. 2013. Composition of the vaginal microbiota in women of reproductive age—sensitive and specific molecular diagnosis of bacterial vaginosis is possible? PLoS One 8:e60670. 10.1371/journal.pone.0060670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karlsson C, Eliasson M, Olin AI, Mörgelin M, Karlsson A, Malmsten M, Egesten A, Frick IM. 2009. SufA of the opportunistic pathogen Finegoldia magna modulates actions of the antibacterial chemokine MIG/CXCL9, promoting bacterial survival during epithelial inflammation. J Biol Chem 284:29499–29508. 10.1074/jbc.M109.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nunn KL, Wang Y-Y, Harit D, Humphrys MS, Ma B, Cone R, Ravel J, Lai SK. 2015. Enhanced trapping of HIV-1 by human cervicovaginal mucus is associated with Lactobacillus crispatus-dominant microbiota. mBio 6:e01084-15. 10.1128/mBio.01084-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108:4680–4687. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rose WA, McGowin CL, Spagnuolo RA, Eaves-Pyles TD, Popov VL, Pyles RB. 2012. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One 7:e32728. 10.1371/journal.pone.0032728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Q, Zhang L, Ross P, Zhao J, Zhang H, Chen W. 2020. Comparative genomics of Lactobacillus crispatus from the gut and vagina reveals genetic diversity and lifestyle adaptation. Genes (Basel) 11:360. 10.3390/genes11040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kalyoussef S, Nieves E, Dinerman E, Carpenter C, Shankar V, Oh J, Burd B, Angeletti RH, Buckheit KW, Fredricks DN, Madan RP, Keller MJ, Herold BC. 2012. Lactobacillus proteins are associated with the bactericidal activity against E. coli of female genital tract secretions. PLoS One 7:e49506. 10.1371/journal.pone.0049506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mildner M, Stichenwirth M, Abtin A, Eckhart L, Sam C, Gläser R, Schröder J-M, Gmeiner R, Mlitz V, Pammer J, Geusau A, Tschachler E. 2010. Psoriasin (S100A7) is a major Escherichia coli-cidal factor of the female genital tract. Mucosal Immunol 3:602–609. 10.1038/mi.2010.37. [DOI] [PubMed] [Google Scholar]
- 143.Onderdonk AB, Delaney ML, Fichorova N. 2016. The human microbiome during bacterial vaginosis. Clin Microbiol Rev 29:223–238. 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. 2009. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 9:116. 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tamrakar R, Yamada T, Furuta I, Cho K, Morikawa M, Yamada H, Sakuragi N, Minakami H. 2007. Association between Lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infect Dis 7:128. 10.1186/1471-2334-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fredricks DN, Fiedler TL, Marrazzo JM. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353:1899–1911. 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 147.Macklaim JM, Gloor GB, Anukam KC, Cribby S, Reid G. 2011. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc Natl Acad Sci U S A 108:4688–4695. 10.1073/pnas.1000086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, Foster JA, Forney LJ. 2007. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 1:121–133. 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 149.Petrova MI, Reid G, Vaneechoutte M, Lebeer S. 2017. Lactobacillus iners: friend or foe? Trends Microbiol 25:182–191. 10.1016/j.tim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 150.Kwak W, Han Y-H, Seol D, Kim H, Ahn H, Jeong M, Kang J, Kim H, Kim TH. 2020. Complete genome of Lactobacillus iners KY using Flongle provides insight into the genetic background of optimal adaption to vaginal econiche. Front Microbiol 11:1048. 10.3389/fmicb.2020.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rampersaud R, Planet PJ, Randis TM, Kulkarni R, Aguilar JL, Lehrer RI, Ratner AJ. 2011. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J Bacteriol 193:1034–1041. 10.1128/JB.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Macklaim JM, Fernandes AD, Di Bella JM, Hammond J-A, Reid G, Gloor GB. 2013. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 1:12. 10.1186/2049-2618-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saunders S, Bocking A, Challis J, Reid G. 2007. Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surf B Biointerfaces 55:138–142. 10.1016/j.colsurfb.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 154.Chetwin E, Manhanzva MT, Abrahams AG, Froissart R, Gamieldien H, Jaspan H, Jaumdally SZ, Barnabas SL, Dabee S, Happel A-U, Bowers D, Davids L, Passmore J-AS, Masson L. 2019. Antimicrobial and inflammatory properties of South African clinical Lactobacillus isolates and vaginal probiotics. Sci Rep 9:1917. 10.1038/s41598-018-38253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manhanzva MT, Abrahams AG, Gamieldien H, Froissart R, Jaspan H, Jaumdally SZ, Barnabas SL, Dabee S, Bekker LG, Gray G, Passmore J-AS, Masson L. 2020. Inflammatory and antimicrobial properties differ between vaginal Lactobacillus isolates from South African women with non-optimal versus optimal microbiota. Sci Rep 10:6196. 10.1038/s41598-020-62184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Happel AU, Kullin B, Gamieldien H, Wentzel N, Zauchenberger CZ, Jaspan HB, Dabee S, Barnabas SL, Jaumdally SZ, Dietrich J, Gray G, Bekker LG, Froissart R, Passmore JAS. 2020. Exploring potential of vaginal Lactobacillus isolates from South African women for enhancing treatment for bacterial vaginosis. PLoS Pathog 16:e1008559. 10.1371/journal.ppat.1008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Centers for Disease Control and Prevention. 2015. Bacterial vaginosis—2015 STD treatment guidelines. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 158.Bradshaw CS, Sobel JD. 2016. Current treatment of bacterial vaginosis—limitations and need for innovation. J Infect Dis 214:S14–S20. 10.1093/infdis/jiw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Laghi L, Picone G, Cruciani F, Brigidi P, Calanni F, Donders G, Capozzi F, Vitali B. 2014. Rifaximin modulates the vaginal microbiome and metabolome in women affected by bacterial vaginosis. Antimicrob Agents Chemother 58:3411–3420. 10.1128/AAC.02469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mayer BT, Srinivasan S, Fiedler TL, Marrazzo JM, Fredricks DN, Schiffer JT. 2015. Rapid and profound shifts in the vaginal microbiota following antibiotic treatment for bacterial vaginosis. J Infect Dis 212:793–802. 10.1093/infdis/jiv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Joag V, Obila O, Gajer P, Scott MC, Dizzell S, Humphrys M, Shahabi K, Huibner S, Shannon B, Tharao W, Mureithi M, Oyugi J, Kimani J, Kaushic C, Ravel J, Anzala O, Kaul R. 2019. Impact of standard bacterial vaginosis treatment on the genital microbiota, immune milieu, and ex vivo human immunodeficiency virus susceptibility. Clin Infect Dis 68:1675–1683. 10.1093/cid/ciy762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Larsson P-G, Stray-Pedersen B, Ryttig KR, Larsen S. 2008. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health 8:3. 10.1186/1472-6874-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heczko PB, Tomusiak A, Adamski P, Jakimiuk AJ, Stefański G, Mikołajczyk-Cichońska A, Suda-Szczurek M, Strus M. 2015. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: a randomised, double-blind, placebo-controlled trial. BMC Womens Health 15:115. 10.1186/s12905-015-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. 2017. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol 168:782–792. 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 165.Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, Green L, Miller S, Powell J, Parks T, Hemmerling A. 2020. Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. N Engl J Med 382:1906–1915. 10.1056/NEJMoa1915254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McMillan A, Dell M, Zellar MP, Cribby S, Martz S, Hong E, Fu J, Abbas A, Dang T, Miller W, Reid G. 2011. Disruption of urogenital biofilms by lactobacilli. Colloids Surf B Biointerfaces 86:58–64. 10.1016/j.colsurfb.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 167.Reid G, Heinemann C, Velraeds M, van der Mei HC, Busscher HJ. 1999. Biosurfactants produced by Lactobacillus. Methods Enzymol 310:426–433. 10.1016/s0076-6879(99)10033-8. [DOI] [PubMed] [Google Scholar]
- 168.Boris S, Barbés C. 2000. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2:543–546. 10.1016/s1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 169.Yang S, Reid G, Challis JRG, Gloor GB, Asztalos E, Money D, Seney S, Bocking AD. 2020. Effect of oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the vaginal microbiota, cytokines and chemokines in pregnant women. Nutrients 12:368. 10.3390/nu12020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yefet E, Colodner R, Strauss M, Gam Ze Letova Y, Nachum Z. 2020. A randomized controlled open label crossover trial to study vaginal colonization of orally administered Lactobacillus reuteri RC-14 and rhamnosus GR-1 in pregnant women at high risk for preterm labor. Nutrients 12:1141. 10.3390/nu12041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Decena DCD, Co JT, Manalastas RM, Palaypayon EP, Padolina CS, Sison JM, Dancel LA, Lelis MA. 2006. Metronidazole with Lactacyd vaginal gel in bacterial vaginosis. J Obstet Gynaecol Res 32:243–251. 10.1111/j.1447-0756.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 172.Abbai NS, Reddy T, Ramjee G. 2016. Prevalent bacterial vaginosis infection—a risk factor for incident sexually transmitted infections in women in Durban, South Africa. Int J STD AIDS 27:1283–1288. 10.1177/0956462415616038. [DOI] [PubMed] [Google Scholar]
- 173.Balkus JE, Richardson BA, Rabe L, Taha TE, Mgodi N, Kasaro MP, Ramjee G, Hoffman IF, Karim SSA. 2014. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV-1 negative women. Sex Transm Dis 41:123–128. 10.1097/OLQ.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bautista CT, Wurapa EK, Sateren WB, Morris SM, Hollingsworth BP, Sanchez JL. 2017. Association of bacterial vaginosis with chlamydia and gonorrhea among women in the U.S. Army. Am J Prev Med 52:632–639. 10.1016/j.amepre.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 175.Ziklo N, Huston WM, Hocking JS, Timms P. 2016. Chlamydia trachomatis genital tract infections: when host immune response and the microbiome collide. Trends Microbiol 24:750–765. 10.1016/j.tim.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 176.Schwebke JR, Desmond R. 2007. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol 196:517.e1–517.e6. 10.1016/j.ajog.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Balkus JE, Manhart LE, Lee J, Anzala O, Kimani J, Schwebke J, Shafi J, Rivers C, Kabare E, Scott McClelland R. 2016. Periodic presumptive treatment for vaginal infections may reduce the incidence of sexually transmitted bacterial infections. J Infect Dis 213:1932–1937. 10.1093/infdis/jiw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rizzo A, Fiorentino M, Buommino E, Donnarumma G, Losacco A, Bevilacqua N. 2015. Lactobacillus crispatus mediates anti-inflammatory cytokine interleukin-10 induction in response to Chlamydia trachomatis infection in vitro. Int J Med Microbiol 305:815–827. 10.1016/j.ijmm.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 179.Gong Z, Luna Y, Yu P, Fan H. 2014. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS One 9:e107758. 10.1371/journal.pone.0107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zeng J, Yang R, He W, Zhong X, Liu W, Zhu H, Zhang X, Luo Q. 2019. Modulation effect of vaginal mucosal microflora and susceptibility to Neisseria gonorrhoeae infections: a systematic review and meta-analysis. Arch Gynecol Obstet 300:261–267. 10.1007/s00404-019-05200-1. [DOI] [PubMed] [Google Scholar]
- 181.Spurbeck RR, Arvidson CG. 2010. Lactobacillus jensenii surface-associated proteins inhibit Neisseria gonorrhoeae adherence to epithelial cells. Infect Immun 78:3103–3111. 10.1128/IAI.01200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vielfort K, Sjölinder H, Roos S, Jonsson H, Aro H. 2008. Adherence of clinically isolated lactobacilli to human cervical cells in competition with Neisseria gonorrhoeae. Microbes Infect 10:1325–1334. 10.1016/j.micinf.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 183.Breshears LM, Edwards VL, Ravel J, Peterson ML. 2015. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol 15:276. 10.1186/s12866-015-0608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Foschi C, Salvo M, Cevenini R, Parolin C, Vitali B, Marangoni A. 2017. Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: an in vitro study. Front Cell Infect Microbiol 7:502. 10.3389/fcimb.2017.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Graver MA, Wade JJ. 2011. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob 10:8. 10.1186/1476-0711-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lokken EM, Balkus JE, Kiarie J, Hughes JP, Jaoko W, Totten PA, McClelland RS, Manhart LE. 2017. Association of recent bacterial vaginosis with acquisition of Mycoplasma genitalium. Am J Epidemiol 186:194–201. 10.1093/aje/kwx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brotman RM. 2011. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest 121:4610–4617. 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Brotman RM, Bradford LL, Conrad M, Gajer P, Ault K, Peralta L, Forney LJ, Carlton JM, Abdo Z, Ravel J. 2012. Association between Trichomonas vaginalis and vaginal bacterial community among reproductive-age women. Sex Transm Dis 39:807–812. 10.1097/OLQ.0b013e3182631c79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. 2008. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22:1493–1501. 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McClelland RS, Lingappa JR, Srinivasan S, Kinuthia J, John-Stewart GC, Jaoko W, Richardson BA, Yuhas K, Fiedler TL, Mandaliya KN, Munch MM, Mugo NR, Cohen CR, Baeten JM, Celum C, Overbaugh J, Fredricks DN. 2018. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis 18:554–564. 10.1016/S1473-3099(18)30058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mirmonsef P, Krass L, Landay A, Spear GT. 2012. The role of bacterial vaginosis and trichomonas in HIV transmission across the female genital tract. Curr HIV Res 10:202–210. 10.2174/157016212800618165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Aldunate M, Tyssen D, Latham C, Ramsland P, Perlmutter P, Moench T, Cone R, Tachedjian G. 2014. Vaginal concentrations of lactic acid potently inactivate HIV-1 compared to short chain fatty acids present during bacterial vaginosis. AIDS Res Hum Retroviruses 30:A228. 10.1089/aid.2014.5499.abstract. [DOI] [Google Scholar]
- 193.O’Hanlon DE, Moench TR, Cone RA. 2011. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis 11:200. 10.1186/1471-2334-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. 2005. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis 40:1422–1428. 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 195.Borgdorff H, Tsivtsivadze E, Verhelst R, Marzorati M, Jurriaans S, Ndayisaba GF, Schuren FH, van de Wijgert JH. 2014. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J 8:1781–1793. 10.1038/ismej.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Masese L, Baeten JM, Richardson BA, Bukusi E, John-Stewart G, Jaoko W, Shafi J, Kiarie J, McClelland RS. 2014. Incident herpes simplex virus type 2 infection increases the risk of subsequent episodes of bacterial vaginosis. J Infect Dis 209:1023–1027. 10.1093/infdis/jit634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Conti C, Malacrino C, Mastromarino P. 2009. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol 60:19–26. [PubMed] [Google Scholar]
- 198.Isaacs CE, Xu W. 2013. Theaflavin-3,3′-digallate and lactic acid combinations reduce herpes simplex virus infectivity. Antimicrob Agents Chemother 57:3806–3814. 10.1128/AAC.00659-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, Gravitt PE. 2014. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis 210:1723–1733. 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shannon B, Yi TJ, Perusini S, Gajer P, Ma B, Humphrys MS, Thomas-Pavanel J, Chieza L, Janakiram P, Saunders M, Tharao W, Huibner S, Shahabi K, Ravel J, Rebbapragada A, Kaul R. 2017. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol 10:1310–1319. 10.1038/mi.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.van de Wijgert JHHM, Morrison CS, Cornelisse PGA, Munjoma M, Moncada J, Awio P, Wang J, Van der Pol B, Chipato T, Salata RA, Padian NS. 2008. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 48:203–210. 10.1097/QAI.0b013e3181743936. [DOI] [PubMed] [Google Scholar]
- 202.Eastment MC, Balkus JE, Richardson BA, Srinivasan S, Kimani J, Anzala O, Schwebke J, Fiedler TL, Fredricks DN, McClelland RS. 29 July 2020. Association between vaginal bacterial microbiota and vaginal yeast colonization. J Infect Dis 10.1093/infdis/jiaa459. [DOI] [PMC free article] [PubMed]
- 203.Spear GT, Zariffard MR, Cohen MH, Sha BE. 2008. Vaginal IL-8 levels are positively associated with Candida albicans and inversely with lactobacilli in HIV-infected women. J Reprod Immunol 78:76–79. 10.1016/j.jri.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Donnarumma G, Molinaro A, Cimini D, De Castro C, Valli V, De Gregorio V, De Rosa M, Schiraldi C. 2014. Lactobacillus crispatus L1: high cell density cultivation and exopolysaccharide structure characterization to highlight potentially beneficial effects against vaginal pathogens. BMC Microbiol 14:137. 10.1186/1471-2180-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rizzo A, Losacco A, Carratelli CR. 2013. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human β-defensins 2 and 3. Immunol Lett 156:102–109. 10.1016/j.imlet.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 206.Boris S, Suárez JE, Vázquez F, Barbés C. 1998. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun 66:1985–1989. 10.1128/IAI.66.5.1985-1989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Parolin C, Marangoni A, Laghi L, Foschi C, Ñahui Palomino RA, Calonghi N, Cevenini R, Vitali B. 2015. Isolation of vaginal lactobacilli and characterization of anti-Candida activity. PLoS One 10:e0131220. 10.1371/journal.pone.0131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morais IMC, Cordeiro AL, Teixeira GS, Domingues VS, Nardi RMD, Monteiro AS, Alves RJ, Siqueira EP, Santos VL. 2017. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microb Cell Fact 16:155. 10.1186/s12934-017-0769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.De Gregorio PR, Parolin C, Abruzzo A, Luppi B, Protti M, Mercolini L, Silva JA, Giordani B, Marangoni A, Nader-Macías MEF, Vitali B. 2020. Biosurfactant from vaginal Lactobacillus crispatus BC1 as a promising agent to interfere with Candida adhesion. Microb Cell Fact 19:133. 10.1186/s12934-020-01390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Köhler GA, Assefa S, Reid G. 2012. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gynecol 2012:636474. 10.1155/2012/636474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. 2005. Utility of Amsel criteria, Nugent score, and quantitative PCR for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol 43:4607–4612. 10.1128/JCM.43.9.4607-4612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.France M, Bing M, Gajer P, Brown S, Humphrys MS, Holm JB, Brotman RM, Ravel J. 2020. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8:166. 10.1186/s40168-020-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.O’Hanlon DE, Come RA, Moench TR. 2019. Vaginal pH measured in vivo: lactobacilli determine pH and lactic acid concentration. BMC Microbiol 19:13. 10.1186/s12866-019-1388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, Novak RM, McCorrister S, McKinnon LR, Cohen CR, Mackelprang R, Lingappa J, Lauffenburger DA, Klatt NR, Burgener AD. 2016. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog 12:e1005889. 10.1371/journal.ppat.1005889. [DOI] [PMC free article] [PubMed] [Google Scholar]