Siglecs are sialic acid-binding immunoglobulin-like lectins that play an important role in tissue homeostasis, immune response, and pathogen infection. Bacterial sialidases act on natural ligands of Siglecs, interfering with the Siglec-mediated immune response. Glaesserella parasuis is a porcine bacterial pathogen that secretes sialidase.
KEYWORDS: Glaesserella parasuis, Glasser’s disease, sialidase, inflammatory response, Siglec-5
ABSTRACT
Siglecs are sialic acid-binding immunoglobulin-like lectins that play an important role in tissue homeostasis, immune response, and pathogen infection. Bacterial sialidases act on natural ligands of Siglecs, interfering with the Siglec-mediated immune response. Glaesserella parasuis is a porcine bacterial pathogen that secretes sialidase. However, little is known about the sialidase of G. parasuis and its impact on immune regulation. Here, we used wild-type G. parasuis, a sialidase-deficient mutant, and complementary strains to investigate the role of sialidase in porcine alveolar macrophage infection. Sialidase induced the release of proinflammatory cytokines, such as interleukin-1α (IL-1α), IL-6, and tumor necrosis factor alpha, from porcine alveolar macrophages. Moreover, sialidase desialylated the surface of porcine alveolar macrophages and altered the expression of Siglecs (the expression of Siglec-5 was reduced). Furthermore, sialidase led to a reduction in endogenous SH2 domain-containing protein tyrosine phosphatase (SHP-2) recruitment to Siglec-5 and simultaneously activated the inflammatory response via the mitogen-activated protein kinase and nuclear factor kappa light chain enhancer of activated B cell signaling pathways. This desialylation occurred before the release of proinflammatory cytokines, suggesting that the sialidase-induced inflammatory response was followed by reduced recruitment of SHP-2 to Siglec-5. Thus, this study is the first to demonstrate the role of sialidase in the inflammatory response of G. parasuis. This role resulted from the abrogation of negative regulation of Siglec-5 on proinflammatory cytokine release. This study helps to understand the molecular mechanism underlying the inflammatory response induced by sialidase secreted by G. parasuis and the acute inflammation caused by G. parasuis.
INTRODUCTION
Glaesserella parasuis (Haemophilus parasuis) is a Gram-negative commensal organism that colonizes the upper respiratory tract of conventional pigs. It can invade the body and cause Glasser’s disease, which is characterized by fibrinous polyserositis, arthritis, meningitis, acute pneumonia, and septicemia (1–3). Glasser’s disease is also a stress-associated disease that often occurs in weaned piglets of conventional herds (4). Viral diseases, such as swine flu and porcine reproductive and respiratory syndrome, exacerbate the severity of Glasser’s disease and result in mortality (5, 6). Glasser’s disease is prevalent in pig farms worldwide (7, 8). The isolation rate of G. parasuis in China is 9.7% in healthy pig herds, and it may go up to 22.6% in weaned pigs (7, 9). However, the key virulence factors of G. parasuis that are involved in the pathogenesis of acute inflammatory responses have yet to be elucidated.
Sialic acids are nine-carbon sugars exposed on the terminal monosaccharides of glycoproteins and glycolipids on the cell surface. These residues serve as a source for nutrition, decorate the cell surface to evade host immune recognition, and perturb natural self-recognition to enhance the inflammatory response (10–12). Sialidases, present in multiple pathogens, are thought to be virulence factors that facilitate colonization and pathogenicity. Bacterial sialidases cleave sialic acid at the end of the glycoconjugate to facilitate colonization. (10). Whether sialidase of G. parasuis acts on the sialic acid residues from host glycoconjugates and whether the subsequent effect unmasks the potential receptors required for the inflammatory response are two aspects that are largely unknown.
Siglecs are sialic acid-binding immunoglobulin-like lectins that remain anchored to the immune cell membranes and bind sialic acid residues to activate or inhibit the downstream signaling pathways (13, 14). Outside the cell, Siglecs comprise variable numbers of C2-set domains and an amino-terminal V-set immunoglobulin domain, which recognize the ligands containing sialylated glycans and mediate cell-cell and host-organism interactions during immunity and infection (15). Out of the eight Siglecs in swine, Siglec-2, -3, -5, and -10 have immune receptor tyrosine-based inhibition motifs (ITIMs) on the intracellular domain to transduce inhibitory signals. Siglec-14 and -15 have no intracellular domain, but they associate with the DNAX-activating protein of 12 kDa (DAP12) via the transmembrane region, containing an immune receptor tyrosine-based activation motif (ITAM) that can recruit phosphoinositide 3-kinase to activate downstream signals. Siglec-1 and -4 only mediate the internalization of pathogens and interaction between host cells (14, 15).
Since the local concentration of sialic acids on the surface of leukocytes is very high, sialic acid-binding sites of Siglecs are generally masked by glycan ligands via cis interactions (13). Bacterial sialidases can disrupt the cis interactions via desialylation of the glycan ligands (16). Almost all G. parasuis isolates express sialidase (NanH) and secrete it on or outside the bacterial cells (17–19). However, its specific role remains unclear. In this study, we investigated the function of sialidase secreted by G. parasuis in the infection of porcine alveolar macrophages using a sialidase-deficient mutant. Further, the mechanism of action for sialidase in promoting the release of inflammatory cytokines was also demonstrated.
RESULTS
Construction and identification of G. parasuis sialidase-deficient mutant.
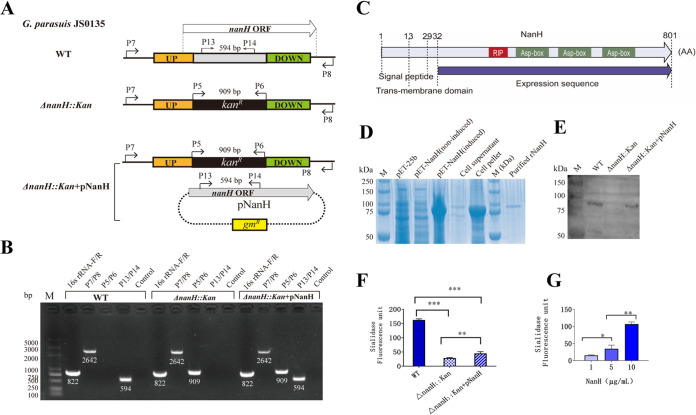
To investigate the role of sialidase secreted by G. parasuis in host immune cells, a sialidase-deficient mutant (ΔnanH::kan) of strain JS0135 and its complementary strain (ΔnanH::kan+pNanH) carrying plasmid pG3-nanH, expressing sialidase, were constructed and identified using PCR and four pairs of primers (Fig. 1A and B and Table 1; see also Table S1 in the supplemental material). Using the primers 16SrRNA-F/R, an 822-bp band was observed in the G. parasuis wild-type (WT), mutant, and complementary strains, indicating that these strains belong to G. parasuis species. Using the primers P7/P8, located outside the homologous arms, a 2,462-bp band appeared in ΔnanH::kan, ΔnanH::kan+pNanH, and WT samples, as the deleted region of nanH was similar in size to that of the kan cassette. A 909-bp band, indicative of the kan cassette, was detectable in ΔnanH::kan and ΔnanH::kan+pNanH strains, but not in the WT, using the primers P5/P6. A 594-bp fragment located in the inner region of the deleted nanH was obtained in the WT and ΔnanH::kan+pNanH but not in the ΔnanH::kan strain using the primers P13/14 (Fig. 1A and B). These results indicate that nanH was deleted via insertion of a kanamycin-resistant gene in the mutant, but the complementary strain containing the NanH expression plasmid was obtained. The recombinant sialidase NanH (rNanH) was expressed and purified to prepare a mouse anti-rNanH antibody for Western blotting. G. parasuis WT and complementary strains were confirmed to express sialidase, while the ΔnanH::kan strain did not express sialidase (Fig. 1C to E, Fig. S1). The results confirmed the successful construction of sialidase-deficient G. parasuis and the complementary strain.
FIG 1.
Identification of Glaesserella parasuis sialidase (NanH)-deficient mutant. (A) Schematic diagram showing wild-type (WT) G. parasuis, ΔnanH::kan mutant, and ΔnanH::kan+pNanH complementary strain. G. parasuis was identified using the primers 16SrRNA-F/R (822 bp). The primers P7/P8 were used to amplify nanH in the WT and the fragment including ΔnanH::kan in the ΔnanH::kan and ΔnanH::kan+pNanH strains (2,462 bp). The primers P5/P6 were used to amplify the kanamycin cassette in the ΔnanH::kan and ΔnanH::kan+pNanH strains (909 bp). The primers P13/P14 were used to amplify the inner fragment of nanH in WT and ΔnanH::kan+pNanH strains (594 bp). ORF, open reading frame. (B) PCR amplification without primers served as a PCR negative control. M, molecular size marker. (C) NanH consisted of a signal peptide (amino acids [aa] 1 to 29), transmembrane domain (aa 13 to 32), and functional domain (aa 33 to 801) according to the Signal P4.1 server. (D) Recombinant sialidase (rNanH) was expressed by plasmid pET-nanH after induction with isopropyl-β-d-1-thiogalactopyranoside. The vector pET-25b and noninduced plasmid pET-nanH did not express rNanH. rNanH was primarily included in the pellets but not in supernatants of the sonicated bacterial cells. The purified rNanH was obtained using the His tag and Ni Sepharose 6 Fast Flow. (E) Western blots using the mouse anti-sialidase antibodies revealed that WT and ΔnanH::kan+pNanH strains express NanH but the ΔnanH::kan strain does not. (E) Equivalent quantity of proteins from sonicated bacterial cells was used for sialidase activity detection. (F) The WT had the highest sialidase activity (P < 0.001), and the ΔnanH::kan+pNanH strain exhibited restoration of the activity to a level higher than the ΔnanH::kan strain (P < 0.01). (G) The sialidase activity of rNanH was dose dependent. For G. parasuis and rNanH, sialidase activity was detected in three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 1.
Strains and plasmids used in the study
| Strain or plasmid | Relevant characteristic or description | Source or reference |
|---|---|---|
| JS0135 | G. parasuis isolate, serotype 4 | China |
| ΔnanH::kan | JS0135 with sialidase deficient | This study |
| ΔnanH::kan + pNanH | JS0135 ΔnanH::kan carrying expression plasmid pNanH | This study |
| pK18mobsacB | Suicide vector, kanR | (32) |
| pK18-nanH | pK18mobsacB, ΔnanH::kanR | This study |
| pSHG3 | Shuttle vector, gmR | (31) |
| pNanH | pSHG3 carrying nanH gene | This study |
| pET-25b | Expression vector, ampR | Novagen |
| pET-NanH | pET-25b expressing sialidase, ampR | This study |
| p3XFlagCMV-14 | CMV promoter, 3× FLAG, ampR | Novagen |
| pSiglec-5-Flag | p3XFlagCMV-14 carrying Siglec-5 gene | This study |
Additionally, the G. parasuis WT exhibited higher sialidase activity than the ΔnanH::kan strain (P < 0.001); the ΔnanH::kan+pNanH strain restored the sialidase activity to a level that was higher than that of the ΔnanH::kan strain (P < 0.01) (Fig. 1G). Dose-dependent sialidase activity was also reported for rNanH (Fig. 1F).
Sialidase of G. parasuis increased the production of proinflammatory cytokines in 3D4/21 cells.
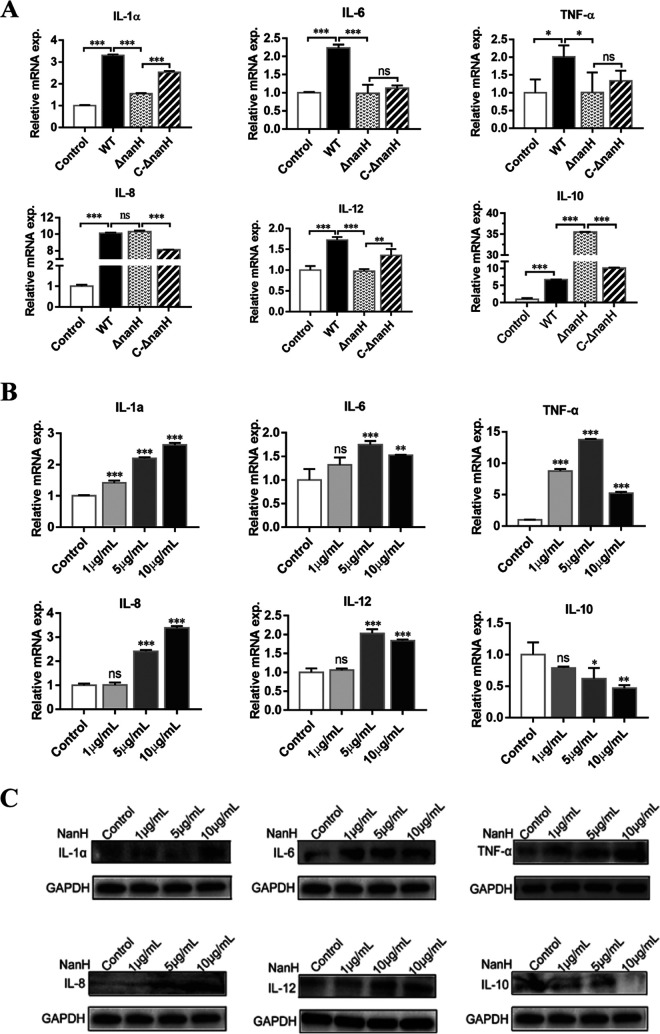
To understand the impact of sialidase secreted by G. parasuis on the inflammatory response, WT, ΔnanH::kan, and ΔnanH::kan+pNanH strains were used to infect 3D4/21 cells for 12 h, and the mRNAs of inflammatory cytokines were detected by real-time quantitative reverse transcription-PCR (qRT-PCR). The WT enhanced the mRNAs of proinflammatory cytokines, including interleukin-1α (IL-1α), IL-6, and tumor necrosis factor alpha (TNF-α), in 3D4/21 cells (P < 0.05). While the mRNA levels of IL-1α, IL-6, and TNF-α (P < 0.05) were reduced in the ΔnanH::kan strain, the ΔnanH::kan+pNanH strain restored their mRNA levels close to that induced by the WT. IL-8 increased significantly in all the three strains but decreased in cells infected with the ΔnanH::kan strain. The IL-8 level was not restored in the ΔnanH::kan+pNanH strain. This could be because entire bacterial cells extensively exchange IL-8, with sialidase having a relatively small impact on it. IL-12, known to enhance the adaptive immunity via cytotoxic activity of the macrophages, also displayed a decline in the ΔnanH::kan strain (P < 0.001) and improvement in the ΔnanH::kan+pNanH strain (P < 0.01). The anti-inflammatory cytokine IL-10 increased in all three strains; the ΔnanH::kan strain exhibited the highest mRNA level (P < 0.001). The results indicate that sialidase expression in WT G. parasuis can inhibit IL-10 production and promote inflammatory responses (Fig. 2A).
FIG 2.
Inflammatory cytokines induced by sialidase of Glaesserella parasuis. (A) The 3D4/21 cells were infected with wild-type (WT), sialidase-deficient (ΔnanH::kan), and complementary (ΔnanH::kan+pNanH) strains of G. parasuis at a multiplicity of infection of 10 for 12 h. Thereafter, the mRNA levels of IL-1α, IL-6, tumor necrosis factor alpha (TNF-α), IL-8, IL-12, and IL-10 in 3D4/21 cells were detected. Asterisks indicate comparisons between the control cells without infection and cells infected with the WT, WT and ΔnanH::kan, and ΔnanH::kan and ΔnanH::kan+pNanH strains. (B) For cells treated with recombinant sialidase (rNanH) for 3 h, mRNA levels of IL-1α, IL-6, IL-8, TNF-α, IL-12, and IL-10 were measured. Asterisks indicate comparisons between the control cells without treatment and the cells treated with rNanH (1 μg, 5 μg, and 10 μg). exp., expression. (C) Using Western blotting, inflammatory cytokine production was probed following treatment of the cells with rNanH for 3 h (C). All real-time quantitative reverse transcription PCRs were conducted independently thrice, and statistical analysis was performed using one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significance. Western blotting was repeated twice.
A bacterial lipooligosaccharide-induced inflammatory response mediated via Toll-like receptor 4 (TLR4) was also reported in G. parasuis (20, 21). Therefore, inflammatory cytokine levels of 3D4/21 cells were further tested following treatment of the cells with rNanH for 3 h. Cells treated with rNanH (5 μg/ml and 10 μg/ml) exhibited high levels of mRNA encoding proinflammatory cytokines IL-1α, IL-6, TNF-α, IL-8, and IL-12 (P < 0.01). However, IL-10 was reduced in cells treated with rNanH at concentrations of 5 μg/ml and 10 μg/ml (P < 0.05) (Fig. 2B). Western blotting was used to detect the cytokines following treatment with rNanH for 3 h. Increased release of IL-1α, IL-6, TNF-α, IL-8, and IL-12 and reduced secretion of IL-10 were confirmed from the qRT-PCR results (Fig. 2C). The results indicate that sialidase of G. parasuis enhances the production of the proinflammatory cytokines (IL-1α, IL-6, IL-8, and TNF-α) and inhibits that of anti-inflammatory cytokine (IL-10) in porcine alveolar macrophage 3D4/21 cells, thereby contributing to the inflammatory response induced by G. parasuis.
Sialidase of G. parasuis confers cell surface desialylation and negative regulation of Siglec-5 and Siglec-10.
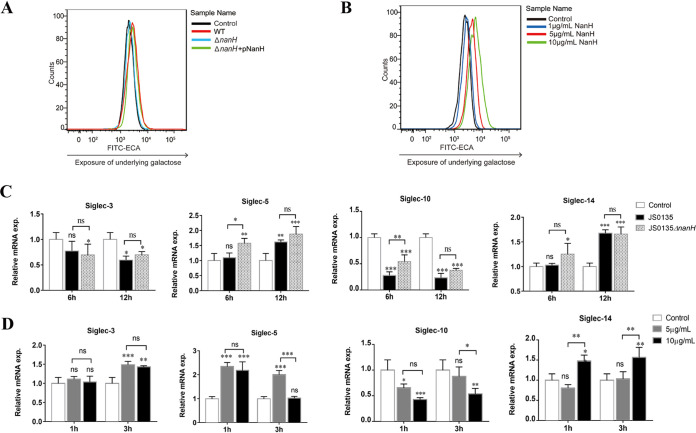
One of the multiple functions of bacterial sialidase in infection and pathogenicity is cell surface desialylation (10, 16). Therefore, the desialylation of porcine alveolar macrophage 3D4/21 cells by sialidase of G. parasuis was investigated in this study. Complying with our expectations, 3D4/21 cells infected with the WT bound the agglutinin lectin of Erythrina cristagalli, indicating that the terminal sialic acids of 3D4/21 cell surface were removed and the underlying Galβ1-4GlcNAcβ1 was exposed. Cells infected with the ΔnanH::kan strain showed no binding, while those infected with the complementary strain restored binding activity to a certain extent (Fig. 3A). Additionally, the 3D4/21 cells treated with rNanH bound the agglutinin lectin of E. cristagalli in a dose-dependent manner (Fig. 3B). The results indicate that sialidase of G. parasuis causes surface desialylation of 3D4/21 cells in vitro.
FIG 3.
Desialylation and altered Siglec expression in 3D4/21 cells upon the action of sialidase of Glaesserella parasuis. (A) The desialylation of 3D4/21 cells was detected using a flow cytometer based on exposure to underlying galactose. Cells infected with wild-type (WT) G. parasuis at a multiplicity of infection of 10 for 6 h showed more galactose exposure than the mutant (ΔnanH::kan) and complementary (ΔnanH::kan+pNanH) strains. (B) Cells treated with recombinant sialidase (rNanH) for 3 h showed desialylation in a dose-dependent manner. (C) The mRNAs for Siglec-3, -5, -10, and -14 in 3D4/21 cells infected with WT and ΔnanH::kan strains were detected using real-time quantitative reverse transcription PCR (qRT-PCR). Asterisks above each pillar indicate the comparisons between control cells and cells infected with WT and ΔnanH::kan strains. Additional asterisks indicate the comparison between WT and ΔnanH::kan strains. (D) The mRNAs for Siglec-3, -14, -5, and -10 were detected in 3D4/21 cells following rNanH treatment. Asterisks above each pillar indicate comparisons between the control cells and cells treated with 5 μg/ml and 10 μg/ml rNanH. Additional asterisk indicates the comparison between 5 μg/ml and 10 μg/ml of rNanH. All qRT-PCRs were performed in three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significance.
Certain Siglecs appear on different immune cells, and multiple Siglecs can be expressed on the same cell type (14). Microenvironmental triggers influence the loss and gain in Siglec expression (14). In pigs, Siglecs are exposed on the surface of porcine alveolar macrophages, and whether G. parasuis affects Siglec expression is not clear. Therefore, mRNAs encoding Siglec-2, -3, -5, -10, -14, and -15 were detected following infection of 3D4/21 cells with G. parasuis, as they regulate the immune response via inhibitory and activating motifs. Siglec-1 and -4, with no regulatory effects, were not detected. Consequently, Siglec-2 and -15 were undetectable in 3D4/21 cells. Siglec-3 and Siglec-14 showed no significant differences in cells infected with the WT and ΔnanH::kan strains (P > 0.05). Increases in Siglec-5 and decreases in Siglec-10 were observed after infection with the WT and ΔnanH::kan strains. However, cells infected with the ΔnanH::kan strain exhibited higher mRNA levels for Siglec-5 and Siglec-10 than those with the WT at 6 h postinfection (P < 0.05). The results suggest that sialidase negatively regulates Siglec-5 and Siglec-10 expression (Fig. 3C).
Lipopolysaccharide stimulation may also induce changes in Siglec expression (24). Therefore, 3D4/21 cells were treated with rNanH. No change was observed for mRNA encoding Siglec-3 in cells treated with rNanH at a concentration of 5 μg/ml and 10 μg/ml. However, mRNA encoding Siglec-14 increased upon treatment of the cells with 10 μg/ml rNanH compared to treatment with 5 μg/ml rNanH (P < 0.01). In accordance with the results for bacterial infection, Siglec-5 increased and Siglec-10 decreased upon treatment of the cells with rNanH. Cells treated with 10 μg/ml rNanH showed lower levels of Siglec-5 and Siglec-10 than those treated with 5 μg/ml rNanH at 3 h posttreatment (P < 0.001 and P < 0.05). The observations indicate that sialidase of G. parasuis negatively regulates the expression of Siglec-5 and Siglec-10 (Fig. 3D). The present results not only indicate G. parasuis infection and rNanH treatment as exogenous stimuli that alter Siglec-5 and Siglec-10 expression in 3D4/21 cells but also confirm that sialidase of G. parasuis negatively regulates Siglec-5 and Siglec-10.
Sialidase of G. parasuis abrogated the negative regulatory effect of Siglec-5 on the signaling pathway for inflammatory response.
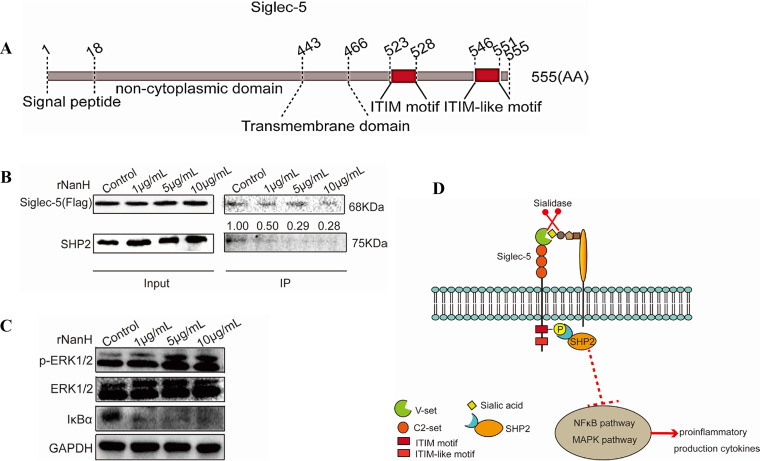
Porcine Siglec-5 consists of ITIM and ITIM-like motifs (Fig. 4A, Fig. S2), which antagonize the kinase-dependent activation cascades via the recruitment of SHP-2 (23). To elucidate the impact of sialidase on the Siglec-5 signaling pathway, recruitment of SHP-2 to Siglec-5 in 3D4/21 cells was examined. The 3D4/21 cells expressing Siglec-5 were treated with rNanH, and Siglec-5 was immunoprecipitated and probed for coimmunoprecipitation with SHP-2. A baseline level of endogenous SHP-2 was associated with Siglec-5 in the untreated 3D4/21 cells. An obvious decrease in SHP-2 recruitment was observed in cells treated with rNanH at a concentration of 5 μg/ml and 10 μg/ml compared to those treated with 1 μg/ml rNanH (Fig. 4B). The results indicate that sialidase of G. parasuis reduces the recruitment of endogenous SHP-2 to Siglec-5 and activates the downstream signal activation.
FIG 4.
Sialidase of Glaesserella parasuis abrogated the Siglec-5-mediated inhibitory signal and activated the proinflammatory signals in 3D4/21 cells. (A) Porcine Siglec-5 comprises a signal peptide (aa 1 to 18), a noncytoplasmic domain (aa 19 to 443), a transmembrane domain (aa 444 to 466), and a cytoplasmic domain (aa 447 to 555) containing an immunoreceptor tyrosine-based inhibition motif (ITIM) (aa 523 to 528) and ITIM-like motif (aa 546 to 551). (B) Siglec-5-Flag expressed by 3D4/21 cells pretreated with recombinant sialidase was coimmunoprecipitated with Flag antibody-conjugated beads and analyzed via Western blotting using anti-Flag and anti-SH2 domain-containing protein tyrosine phosphatase (SHP-2) antibodies. Reduced recruitment of SHP-2 occurred in a dose-dependent manner. (C) The 3D4/21 cells pretreated with rNanH were used for immunoblot analysis. Increased extracellular signal-regulated kinase 1/2 phosphorylation and IκBα degradation occurred in a dose-dependent manner. (D) Schematic diagram showing the activation of mitogen-activated protein kinase and nuclear factor kappa light chain enhancer of activated B cell signaling pathways via the abolishment of the Siglec-5-mediated inhibitory signal by sialidase of G. parasuis.
Bacterial infections characteristically activate the pattern recognition receptors, such as TLR4, to initiate the mitogen-activated protein kinase (MAPK) and nuclear factor kappa light chain enhancer of activated B cells (NF-κB) signaling pathways, which then induce an inflammatory response. Proinflammatory cytokine production was enhanced by sialidase of G. parasuis (Fig. 2). Thus, we hypothesize that sialidase activates the MAPK and NF-κB pathways. As expected, 3D4/21 cells treated with rNanH showed increased phosphorylation of extracellular signal-regulated kinase1/2 (ERK1/2) in a dose-dependent manner and increased IκB degradation in response to different concentrations of rNanH (Fig. 4C). The results indicate that sialidase of G. parasuis induces an inflammatory response via activation of the MAPK and NF-κB signaling pathways.
Taken together, the present results indicate that sialidase of G. parasuis not only reduces the expression of Siglec-5 but also diminishes the recruitment of SHP2 to Siglec-5 in 3D4/2 cells, contributing to proinflammatory cytokine release via activation of the MAPK and NF-κB signaling pathways (Fig. 4D).
DISCUSSION
Siglecs regulate the host immune responses via recognition of the self-glycans. However, increasing numbers of studies have shown that pathogens interact with Siglecs to influence the outcome of infection, which further contributes to their survival and pathogenicity within the host (24). The present study reported that sialidase of G. parasuis promotes proinflammatory cytokine production, induces surface desialylation of 3D4/21 cells, and alters Siglec expression following infection of the cells with the WT and a sialidase-deficient mutant of G. parasuis. The inflammatory response induced by sialidase was associated with reduced recruitment of SHP-2 to Siglec-5, enhanced ERK phosphorylation, and accelerated IκB degradation.
In resting immune cells, inhibitory Siglecs bind to the cis-sialoglycan, which then recruits SHP1/2 to limit the inflammatory reactions (14). A decrease in the sialic acid level indicates the elimination of the Siglec ligands and activation of downstream signals; however, natural ligands of most Siglecs are unknown (13). Siglec-5 contains ITIM and ITIM-like motifs, which recruit SHP-2, associated with negative regulation of proinflammatory cytokine release (14). Sialidase of G. parasuis causes desialylation of the 3D4/21 cell surface, negatively regulating the expression of Siglec-5 and diminishing recruitment of SHP-2 to Siglec-5 (Fig. 3A and 4B). Once the native inhibitory effect of Siglec-5 on the inflammation response is abrogated, activation of the downstream signaling pathway occurs. Additionally, desialylation and Siglec-5 expression decrease at 6 h postinfection with G. parasuis (Fig. 3B). Proinflammatory cytokine production primarily occurred 12 h postinfection (Fig. 2A). Thus, it was hypothesized that the inflammatory response induced by sialidase was derived from the reduced expression of Siglec-5 and diminished recruitment of SHP-2 to Siglec-5 following disruption of the cis ligands via desialylation (Fig. 4D).
Results of bacterial infection and rNanH treatment confirmed that sialidase of G. parasuis negatively regulates Siglec-5 (Fig. 3C and D). On the other hand, Siglec-14 was positively regulated in response to treatment with rNanH (Fig. 3D). Previous studies have shown that Siglec-5 is an inhibitory receptor, while Siglec-14 associates with the activation adapter, DAP12, in humans. Although Siglec-5 and Siglec-14 have similar ligand binding domains, they induce signaling pathways in opposite directions for a balanced immune response (25, 26). Hence, we speculate that Siglec-5 and Siglec-14 are paired receptors and mediate opposite signaling pathways in G. parasuis infection. However, further investigation is warranted.
In addition, the study confirmed that the NanH-dependent inflammatory response was mediated via the activation of MAPK and NF-κB signaling pathways, which remains associated with desialylation by sialidase of G. parasuis (Fig. 4C). Earlier it was reported that sialidase of Clostridium perfringens activates the ERK1/2 signaling pathway and increases the level of phosphorylated ERK1/2 (27) via desialylation of the monocytes. However, there exists no evidence to support that the desialylation of Siglecs directly activates the NF-κB signaling pathway. Sialidase (Neu1) secreted in humans contributes to the TLR4-mediated inflammatory response by negatively regulating the interaction of TLR4 and Siglec-E. In G. parasuis, TLR1, -2, -4, and -6 were required for NF-κB and MAPK activation (28, 29). Therefore, it is anticipated that sialidase of G. parasuis activates the NF-κB and MAPK signaling pathways by disrupting the interaction between TLR4 and Siglec-5 on the cell surface via desialylation of either or both proteins. The abrogation of Siglec-5 inhibitory signal and elimination of its potential repressive effects on the TLR4 signaling pathway can further aggravate the inflammatory response, supporting the importance of sialidase in systemic infection and bacteremia induced by G. parasuis. Therefore, further research is also required to explore the molecular mechanism underlying the inflammation response regulated by interaction of TLR4 and Siglec-5 during G. parasuis infection.
While no changes in Siglec-3 expression were reported, Siglec-5 and Siglec-10 expression increased and decreased, respectively, in porcine alveolar macrophages of 3D4/21 cells infected with G. parasuis in vitro (Fig. 2A). Further, Siglec-3 expression increased in porcine mononuclear cells isolated from pigs with septicemia caused by G. parasuis, but Siglec-5 and Siglec-10 expression exhibited no significant change (2). It is likely that different cell types have diverse patterns of Siglec expression for immune cells (14).
In conclusion, to the best of our knowledge, this study is the first to provide evidence to support the hypothesis that the unmasking of Siglec-5 from cis ligands increases the inflammatory responses in porcine alveolar macrophages. The Siglec-sialic acid interaction plays a vital role in tissue homeostasis and the inflammatory response (14). Microbial sialidase may act as an additional factor that breaks the balance between these factors. Therefore, unraveling the action of sialidase of G. parasuis on Siglec-sialic acid interaction would indeed help in understanding their involvement in the pathogenesis of Glasser’s disease and other infectious diseases caused by pathogens expressing sialidase.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
G. parasuis JS0135 is a clinical isolate of serotype 4 from China and shows medium virulence in swine. The WT and its sialidase-deficient and complementary strains were grown on tryptic soy broth or tryptic soy agar (TSB or TSA; Difco, Detroit, MI, USA) supplemented with 10 μg/ml NAD and 5% fetal bovine serum. The Escherichia coli strains DH5α and Rosetta (DE3) were grown on Luria-Bertani (LB; Difco, BD Biosciences, San Diego, CA, USA) broth or LB agar. When required, the media were supplemented with kanamycin (50 μg/ml), gentamicin (20 μg/ml), or ampicillin (100 μg/ml).
Construction of NanH-deficient mutant and rNanH expression.
Plasmids pK18-nanH and pNanH were constructed (Table 1; see also Table S1 in the supplemental material) and transformed using the primers listed in Table S1, as reported previously (30). The suicidal plasmid pK18-nanH was introduced into G. parasuis via natural transformation to obtain the ΔnanH::kan mutant. The shuttle plasmid pNanH harboring the genR gene and nanH operon was introduced into the ΔnanH::kan mutant via electroporation to construct the complementary strain (30). The sialidase-encoding gene, nanH, was amplified from the WT strain using primers P9/10 and cloned into the E. coli expression vector pET-25b (Table S1; MT625975). E. coli Rosetta (DE3) was transformed with pET-NanH to express recombinant sialidase (Table 1, Fig. S1). The rNanH protein was purified using Ni-Sepharose 6 Fast Flow by His tag (GE Healthcare Biosciences, Pittsburgh, PA, USA). The concentration of purified rNanH was measured using a bicinchoninic acid (BCA) kit (Bioshap, Hefei, China).
Sialidase activity.
WT, mutant, and complementary strains of G. parasuis were grown in TSA for 16 h. Cultures were then collected, washed with phosphate-buffered saline (PBS), and resuspended in PBS. The bacterial suspensions were sonicated and centrifuged at 12,000 rpm for 5 min. The supernatant was collected and the concentration measured using a BCA kit adjusted to 500 μg/ml. Sialidase activity for a 10-μl sample (500 μg/ml) was measured using a sialidase activity detection kit according to the manufacturers’ protocol (Beyotime, Shanghai, China). The sialidase activity of rNanH-treated samples was measured using the same method.
Cell culture and G. parasuis infection in vitro.
Porcine alveolar macrophages (3D4/21) were cultured in an incubator with 5% carbon dioxide. Cells were maintained in RPMI 1640 complete medium containing 100 U/ml penicillin G, 100 mg/ml streptomycin, 4.5 g/liter d-glucose, 1.5 g/liter sodium bicarbonate, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 10 mM HEPES buffer (Gibco, USA), and 10% heat-inactivated fetal bovine serum (Gemini, USA). WT, mutant, and complementary strains of G. parasuis were grown on TSA supplemented with the required antibiotics. The bacterial cultures were then collected, washed twice with PBS, and resuspended in RPMI 1640 complete medium at a multiplicity of infection (MOI) of 10.
Cytokine detection.
The 3D4/21 cells were infected with WT G. parasuis, the ΔnanH::kan mutant, and the complementary mutant at an MOI of 10. Cytokines were detected 12 h postinfection using qRT-PCR with the primers listed in Table S2. For cells treated with rNanH, cytokines were detected after 3 h from treatment. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an endogenous control, and qRT-PCR was independently repeated three times. After 3D4/21 cells were treated for 3 h with rNanH at 1 μg/ml, 5 μg/ml, and 10 μg/ml, cytokine secretion was probed twice using Western blotting. Amicon ultracentrifugal ultrafiltration tubes (Millipore, Burlington, MA, USA) were used to collect the supernatants of 3D4/21 cells for Western blotting with antibodies against multiple cytokines (ABclonal, China).
Flow cytometry analysis of cell surface desialylation.
Cell surface desialylation was analyzed according to the method reported for Streptococcus pneumoniae (16). Briefly, 3D4/21 cells were infected with WT G. parasuis, the ΔnanH::kan mutant, and the complementary strain at an MOI of 10 for 6 h. The cell monolayer was washed twice with PBS and stained with fluorescein isothiocyanate-conjugated E. cristagalli agglutinin lectin (Vector Laboratories, Burlingame, CA) for 45 min at 4°C. Cells were digested with trypsin, washed with PBS, and subjected to BD FACSVerse flow cytometry to analyze the exposed Galβ1-4GlcNAcβ1 units. Results were analyzed using FlowJo 7.0 software. The desialylation of 3D4/21 cells by rNanH was conducted by following the same method. Detection was performed at 3 h poststimulation by rNanH.
Siglec detection.
The 3D4/21 cells were infected with G. parasuis WT and mutant strains at an MOI of 10. Infected cells were collected at 6 h and 12 h postinfection, and the mRNAs for Siglec-2, -3, -5, -10, -14, and -15 were detected using qRT-PCR performed with the primers listed in Table S2. The 3D4/21 cells were next treated with 5 μg/ml and 10 μg/ml rNanH for 1 h and 3 h. Thereafter, mRNAs for Siglec-5 and Siglec-10 were detected. Porcine GAPDH was amplified as an endogenous control, and qRT-PCR was independently repeated three times.
Immunoprecipitation and signal pathway detection.
The expression plasmid pSiglec-5-Flag was constructed (Table 1 and Fig. S2) and transfected into 3D4/21 cells using jetPRIME transfection (PolyPlus, France). At 48 h posttransfection, the cell line expressing Siglec-5-Flag was treated with rNanH (1 μg/ml, 5 μg/ml, and 10 μg/ml) for 3 h. Cells were then lysed for 15 min in 0.2 ml of ice-cold lysis buffer (Beyotime, China) containing a protease inhibitor cocktail (EDTA-free) (MCE, USA). Siglec-5 was immunoprecipitated with mouse anti-Flag antibody (ABclonal, China) at 4°C overnight. The precipitated proteins were probed with mouse anti-Flag and rabbit anti-SHP-2 primary antibodies using Western blotting. This was followed by probing with horseradish peroxidase-conjugated secondary antibodies (ABclonal, China) and enhanced chemiluminescence reagents (Bio-Rad). For signal pathway detection, cell lysates of 3D4/21 cells treated with rNanH (1 μg/ml, 5 μg/ml, and 10 μg/ml) for 3 h were subjected to Western blotting with rabbit anti-p-ERK1/2 and ERK1/2, rabbit anti-IκB, and anti-GAPDH antibodies (CST, Boston, MA).
Statistical analysis.
GraphPad Prism, version 7.0, was used for statistical analysis. A P value of <0.05 was considered statistically significant. One-way analysis of variance (ANOVA) was used to analyze the sialidase activity and cytokine production, while two-way ANOVA was used to compare Siglec expression.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Key Research and Development Program of China (no. 2016YFD0500702) and Major Science and Technology Projects of Hubei Province (no. 66). There is no bearing on founding data deposited with CrossRef.
REFERENCES
- 1.Oliveira S, Pijoan C. 2004. Haemophilus parasuis: new trends on diagnosis, epidemiology and control. Vet Microbiol 99:1–12. doi: 10.1016/j.vetmic.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Álvarez-Estrada Á, Rodríguez-Ferri EF, Martínez-Martínez S, Álvarez B, Fernández-Caballero T, Domínguez J, Gutiérrez-Martín CB. 2019. TLR2, Siglec-3 and CD163 expressions on porcine peripheral blood monocytes are increased during sepsis caused by Haemophilus parasuis. Comp Immunol Microbiol Infect Dis 64:31–39. doi: 10.1016/j.cimid.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Amano H, Shibata M, Takahashi K, Sasaki Y. 1997. Effects on endotoxin pathogenicity in pigs with acute septicemia of Haemophilus parasuis infection. J Vet Med Sci 59:451–455. doi: 10.1292/jvms.59.451. [DOI] [PubMed] [Google Scholar]
- 4.MacInnes JI, Desrosiers R. 1999. Agents of the “suis-ide diseases” of swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Can J Vet Res 63:83–89. [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang N, Liu H, Wang P, Huang J, Han H, Wang Q. 2019. Illumina MiSeq sequencing investigation of microbiota in bronchoalveolar lavage fluid and cecum of the swine infected with PRRSV. Curr Microbiol 76:222–230. doi: 10.1007/s00284-018-1613-y. [DOI] [PubMed] [Google Scholar]
- 6.Pomorska-Mól M, Dors A, Kwit K, Czyżewska-Dors E, Pejsak Z. 2017. Coinfection modulates inflammatory responses, clinical outcome and pathogen load of H1N1 swine influenza virus and Haemophilus parasuis infections in pigs. BMC Vet Res 13:376. doi: 10.1186/s12917-017-1298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B, Ku X, Yu X, Sun Q, Wu H, Chen F, Zhang X, Guo L, Tang X, He Q. 2019. Prevalence and antimicrobial susceptibilities of bacterial pathogens in Chinese pig farms from 2013 to 2017. Sci Rep 9:9908. doi: 10.1038/s41598-019-45482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunaga F, Tsuchiaka S, Kishimoto M, Aoki H, Kakinoki M, Kure K, Okumura H, Okumura M, Okumura A, Nagai M, Omatsu T, Mizutani T. 2020. Development of a one-run real-time PCR detection system for pathogens associated with porcine respiratory diseases. J Vet Med Sci 82:217–223. doi: 10.1292/jvms.19-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P, Zhang C, Aragon V, Zhou X, Zou M, Wu C, Shen Z. 2019. Investigation of Haemophilus parasuis from healthy pigs in China. Vet Microbiol 231:40–44. doi: 10.1016/j.vetmic.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Varki A, Gagneux P. 2012. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci 1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vimr E, Lichtensteiger C, Steenbergen S. 2000. Sialic acid metabolism’s dual function in Haemophilus influenzae. Mol Microbiol 36:1113–1123. doi: 10.1046/j.1365-2958.2000.01925.x. [DOI] [PubMed] [Google Scholar]
- 12.Vimr ER. 2013. Unified theory of bacterial sialometabolism: how and why bacteria metabolize host sialic acids. ISRN Microbiol 2013:816713. doi: 10.1155/2013/816713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crocker PR, Paulson JC, Varki A. 2007. Siglecs and their roles in the immune system. Nat Rev Immunol 7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 14.Lübbers J, Rodríguez E, van Kooyk Y. 2018. Modulation of immune tolerance via Siglec-sialic acid interactions. Front Immunol 9:2807. doi: 10.3389/fimmu.2018.02807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CH, Yeh YC, Yang KD. 2019. Functions and therapeutic targets of Siglec-mediated infections, inflammations and cancers. J Formos Med Assoc 120:5–24. doi: 10.1016/j.jfma.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Chang YC, Uchiyama S, Varki A, Nizet V. 2012. Leukocyte inflammatory responses provoked by pneumococcal sialidase. mBio 3:e00220-11. doi: 10.1128/mBio.00220-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Moliner V, Soler-Llorens P, Moleres J, Garmendia J, Aragon V. 2012. Distribution of genes involved in sialic acid utilization in strains of Haemophilus parasuis. Microbiology 158:2117–2124. doi: 10.1099/mic.0.056994-0. [DOI] [PubMed] [Google Scholar]
- 18.Bregón-Villahoz M, Gutiérrez-Martín CB, Álvarez-Estrada Á, Rodríguez-Ferri EF, Frandoloso R, Martínez-Martínez S. 2017. Molecular study of an outer fragment of Haemophilus parasuis neuraminidase and utility with diagnostic and immunogen purposes. Res Vet Sci 115:463–469. doi: 10.1016/j.rvsc.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Lichtensteiger CA, Vimr ER. 1997. Neuraminidase (sialidase) activity of Haemophilus parasuis. FEMS Microbiol Lett 152:269–274. doi: 10.1111/j.1574-6968.1997.tb10438.x. [DOI] [PubMed] [Google Scholar]
- 20.Bouchet B, Vanier G, Jacques M, Gottschalk M. 2008. Interactions of Haemophilus parasuis and its LOS with porcine brain microvascular endothelial cells. Vet Res 39:42. doi: 10.1051/vetres:2008019. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Z, Zhang B, He H, Chen X, Ren Y, Yue H, Tang C. 2017. lgtF effects of Haemophilus parasuis LOS induced inflammation through regulation of NF-kappaB and MAPKs signaling pathways. Microb Pathog 110:380–384. doi: 10.1016/j.micpath.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Lock K, Zhang J, Lu J, Lee SH, Crocker PR. 2004. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology 209:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Macauley MS, Crocker PR, Paulson JC. 2014. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol 14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angata T. 2018. Possible influences of endogenous and exogenous ligands on the evolution of human Siglecs. Front Immunol 9:2885. doi: 10.3389/fimmu.2018.02885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. 2006. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J 20:1964–1973. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 26.Ali SR, Fong JJ, Carlin AF, Busch TD, Linden R, Angata T, Areschoug T, Parast M, Varki N, Murray J, Nizet V, Varki A. 2014. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med 211:1231–1242. doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamatos NM, Curreli S, Zella D, Cross AS. 2004. Desialylation of glycoconjugates on the surface of monocytes activates the extracellular signal-related kinases ERK 1/2 and results in enhanced production of specific cytokines. J Leukoc Biol 75:307–313. doi: 10.1189/jlb.0503241. [DOI] [PubMed] [Google Scholar]
- 28.Chen GY, Brown NK, Wu W, Khedri Z, Yu H, Chen X, van de Vlekkert D, D'Azzo A, Zheng P, Liu Y. 2014. Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. Elife 3:e04066. doi: 10.7554/eLife.04066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Liu T, Langford P, Hua K, Zhou S, Zhai Y, Xiao H, Luo R, Bi D, Jin H, Zhou R. 2015. Haemophilus parasuis induces activation of NF-κB and MAP kinase signaling pathways mediated by toll-like receptors. Mol Immunol 65:360–366. doi: 10.1016/j.molimm.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Cai X, Qi Y, Liu Y, Cao Q, Wang X, Chen H, Xu X. 2018. Improvement in the efficiency of natural transformation of Haemophilus parasuis by shuttle-plasmid methylation. Plasmid 98:8–14. doi: 10.1016/j.plasmid.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Wang X, Cao Q, Feng F, Xu X, Cai X. 2016. ClpP participates in stress tolerance and negatively regulates biofilm formation in Haemophilus parasuis. Vet Microbiol 182:141–149. [DOI] [PubMed] [Google Scholar]
- 32.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.