Trichinellosis is one of most neglected foodborne zoonoses worldwide. During Trichinella spiralis infection, the intestinal immune response is the first line of defense and plays a vital role in the host’s resistance.
KEYWORDS: P2X7R, NF-κB, NLPR3, IL-1β, macrophages, Trichinella spiralis
ABSTRACT
Trichinellosis is one of most neglected foodborne zoonoses worldwide. During Trichinella spiralis infection, the intestinal immune response is the first line of defense and plays a vital role in the host’s resistance. Previous studies indicate that purinergic P2X7 receptor (P2X7R) and pyrin domain-containing protein 3 (NLRP3) inflammasome are involved in the intestinal immune response in T. spiralis infection. However, the precise role of P2X7R and its effect on NLRP3 remains largely underdetermined. In this study, we aimed to investigate the role of P2X7R in the activation of NLRP3 in macrophages during the intestinal immune response against T. spiralis. We found that T. spiralis infection upregulated expression of P2X7R and activation of NLRP3 in macrophages in mice. In vivo, P2X7R deficiency resulted in increased intestinal adult and muscle larval burdens, along with decreased expression of NLRP3/interleukin-1β (IL-1β) in macrophages from the infected mice with T. spiralis. In In vitro experiments, P2X7R blockade inhibited activation of NLRP3/IL-1β via NF-κB and thus reduced the capacity of macrophages to kill newborn larvae of T. spiralis. These results indicate that P2X7R mediates the elimination of T. spiralis by activating the NF-κB/NLRP3/IL-1β pathway in macrophages. Our findings contribute to the understanding of the intestinal immune mechanism of T. spiralis infection.
INTRODUCTION
Trichinellosis, caused by the parasitic nematode Trichinella spiralis, is one of the most neglected foodborne zoonoses worldwide (1). Transmission to humans involves the consumption of raw or undercooked meat and its derivatives from infected animals (1, 2). Upon ingestion, larvae in infected meat are released and migrate to the small intestine, where they mature and then develop into adults (the enteral phase). After mating, adults release newborn larvae (NBL), and NBL migrate into striated muscle, where they encapsulate and form encapsulated larvae (the muscle phase). The intestinal mucosa is the first line of defense in the host’s resistance to T. spiralis infection (3). Therefore, the intestinal immune response of hosts plays a vital role in T. spiralis infection.
In the enteral phase of T. spiralis infection, adults and larvae feed on intestinal villi and invade intestinal tissue, which results in damage of intestinal tissue and cell death. Intestinal tissue damage and necrotic cells in infected hosts release a number of damage-associated molecular pattern molecules (DAMPs), including extracellular ATP. Extracellular ATP is sensed by the ATP-gated purinergic P2X receptors (P2XRs). Among them, the most involved receptor in infection and inflammation is P2X7 receptor (P2X7R) (4, 5). It has been demonstrated that many pathogens, such as Mycobacterium tuberculosis, Leishmania, Heligmosomoides bakeri, and Toxoplasma gondii, can evade the killing effects of infected hosts through interrupting the P2X7R pathway, which indicates that P2X7R acts as a trigger receptor of immune protection in infectious diseases (6–9). A previous study shows that both P2X7R expression and levels of interleukin-1β (IL-1β) increase in the jejunum of infected mice with T. spiralis, and IL-1β is one of the most important effector molecules to clear the parasite in infected hosts (10). However, the related immunological mechanism by which P2X7R mediates immune responses during T. spiralis infection has not been further elucidated.
Pyrin domain-containing protein 3 (NLRP3) inflammasome, a member of the Nod like-receptor (NLR) protein family, is widely expressed in macrophage and has the function of recognizing pathogens (10). There has been increasing research interest in the role of NLRP3 activation during parasitic infections (4, 11). It is reported that activated resident macrophages trigger an inflammatory response through NLRP3 inflammasome (12). Many macrophages accumulate in abdominal cavity during the enteral phase in murine trichinellosis, which indicates that macrophages serve as an important component in the intestinal immune response (13). Macrophages play an essential role in the control of some parasite infections, and P2X7R expression is upregulated in macrophages in models of chronic inflammatory diseases (9, 14). P2X7R in macrophages promotes the elimination of the intracellular parasite in vitro and plays an important role in regulation of NLRP3 inflammasome activation (12, 15). Protein NLRP3 and Pro-caspase-1 are two main components in peritoneal macrophages. NLRP3 is critical for innate immunity and for inducing the release of IL-1β (16). Nevertheless, there is little evidence of the effect of P2X7R on NLRP3 activation in the infection with the extracellular parasite T. spiralis.
In this study, we explored the effect of P2X7R on NLRP3 activation in macrophages and its protective mechanism during the intestinal immune response in murine T. spiralis infection. We found that T. spiralis infection upregulated expression of P2X7R and induced activation of NLRP3/IL-1β in macrophages in mice. P2X7R deficiency resulted in increased intestinal adult and muscle larval burdens, accompanied by depressed activation of NLRP3 in macrophages from the infected mice with T. spiralis. Moreover, P2X7R blockade inhibited activation of NLRP3/IL-1β via NF-κB and thus reduced the capacity of macrophages to kill NBL of T. spiralis in vitro.
RESULTS
P2X7R expression on macrophages was upregulated in the enteral phase of murine T. spiralis infection.
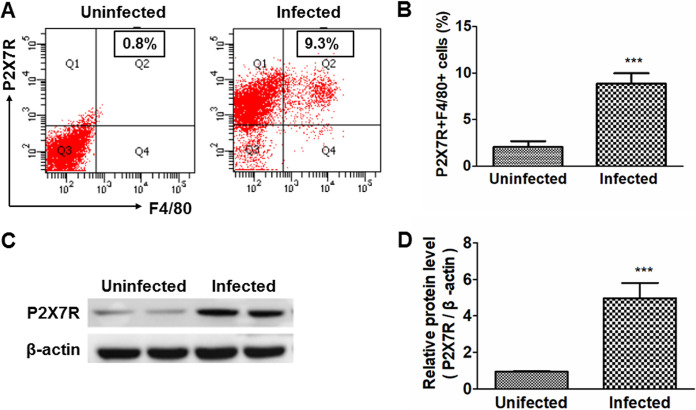
The effect of T. spiralis infection on P2X7R expression in mice was examined. T. spiralis-infected mice showed a significantly elevated percentage of P2X7R+F4/80 macrophages in mesenteric lymph nodes (MLN) (Fig. 1A and B) and higher P2X7R expression in peritoneal macrophages (Fig. 1C and D), indicating that T. spiralis infection induced increased expression of P2X7R on macrophages in murine trichinellosis.
FIG 1.
P2X7R 1 expression on macrophages was upregulated in the enteral phase of murine T. spiralis infection. Wild-type mice were administered orally with 300 larvae of T. spiralis in 0.2 ml of sterilized 0.9% saline. Uninfected control mice were administered orally with 0.2 ml of sterilized 0.9% saline. At 7 days postinfection (dpi), 10 mice from each group were sacrificed by cervical dislocation. (A) Proportions of P2X7R+F4/80+ cells in mesenteric lymph nodes (MLN) were detected by flow cytometry. (B) Ratios of P2X7R+F4/80+ cells to lymphocytes in MLN were calculated. (C and D) Expression of P2X7R on peritoneal macrophages was detected by Western blotting (C) and quantified (D). Data are expressed as means ± SEMs based on 10 mice in each group and from two independent combined experiments. Asterisks mark significant differences between different groups (***, P < 0.001).
NLRP3 in macrophages was activated in the enteral stage of murine T. spiralis infection.
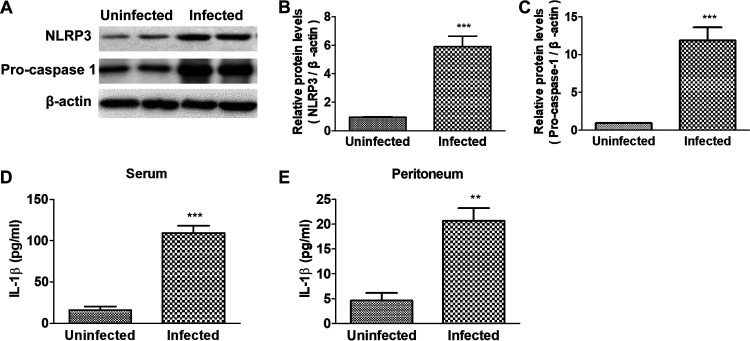
Protein NLRP3 and Pro-caspase-1 in peritoneal macrophages were detected, and concentrations of IL-1β in serum and peritoneal fluid were also investigated. As shown in Fig. 2A to C, the infected mice had higher protein levels of NLRP3 and Pro-caspase-1 in peritoneal macrophages than the uninfected mice. Concentrations of IL-1β in serum and peritoneum increased significantly in the infection group (Fig. 2D and E). Taken together, these results indicated that T. spiralis infection resulted in activation of NLRP3 in mice.
FIG 2.
NLRP3 in macrophages was activated in the enteral stage of murine T. spiralis infection. Wild-type mice were administered orally with 300 larvae of T. spiralis in 0.2 ml of sterilized 0.9% saline. Uninfected control mice were administered orally with 0.2 ml of sterilized saline. At 7 days postinfection (dpi), 10 mice from each group were sacrificed by cervical dislocation. (A to C) Expressions of NLRP3 and Pro-caspase-1 in peritoneal macrophages were detected by Western blotting (A) and quantified using the β-actin band for standardization (B and C). (D and E) Concentrations of IL-1β in mouse serum and peritoneal lavage fluids were detected by ELISA. Data are expressed as means ± SEMs based on 10 mice in each group and from two independent combined experiments. Asterisks mark significant differences between different groups (**, P < 0.01; ***, P < 0.001).
P2X7R deficiency increased the worm burdens in murine T. spiralis infection.
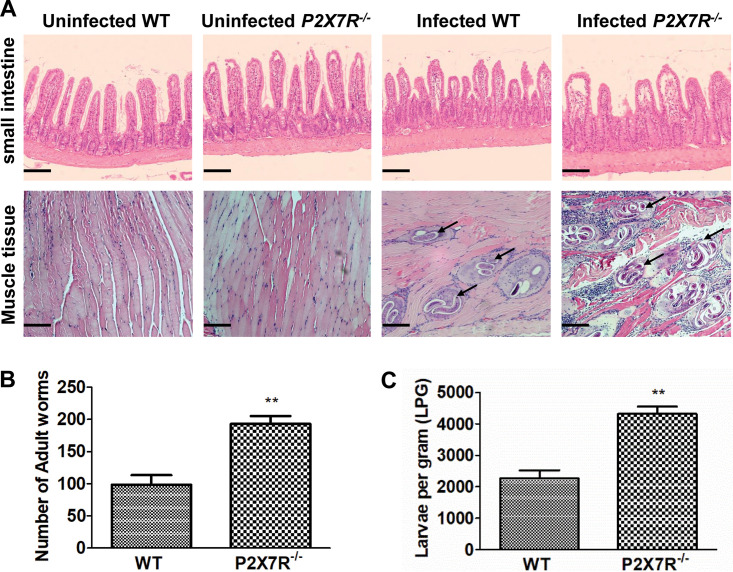
Since T. spiralis upregulated the expression of P2X7R, to verify the role of P2X7R in trichinellosis, we infected wild-type (WT) and P2X7R knockout (P2X7R−/−) mice with T. spiralis. Compared with the infected WT mice, the infected P2X7R−/− mice showed decreased activity, arched back, and towering hair (data not shown). P2X7R deficiency led to heavy worm burdens of intestinal adults (Fig. 3B) and muscle larvae in the infected mice (Fig. 3A and C). Furthermore, P2X7R deficiency aggravated pathological damage in the small intestine and muscle in the infected mice (Fig. 3A). Together, these data indicated that P2X7R deficiency increased the infection intensity and then exacerbated parasitological lesions in murine T. spiralis infection.
FIG 3.
P2X7R deficiency increased the worm burdens in murine T. spiralis infection. Wild-type and P2X7R–/– 26 mice were administered orally with 300 larvae of T. spiralis in 0.2 ml of sterilized 0.9% saline. Uninfected control mice were administered orally with 0.2 ml of sterilized 0.9% saline. At 7 days postinfection (dpi), 10 mice from each group were sacrificed by cervical dislocation, and the small intestines were obtained. (A) For the histopathological study, the sample sections were stained with hematoxylin and eosin (H&E) and observed under a microscope. Original magnification, ×100. Scale bars represent 100 μm. (B) Adults released from the small intestines into saline were collected and counted under a microscope. (C) At 42 dpi, 6 mice from each group were sacrificed, and T. spiralis muscle larvae were collected and counted. Arrows indicate larvae in muscle. Data are expressed as means ± SEMs from two independent combined experiments. Asterisks mark significant differences between different groups (**, P < 0.01).
P2X7R deficiency inhibited activation of NLRP3 in macrophages in the enteral stage of murine T. spiralis infection.
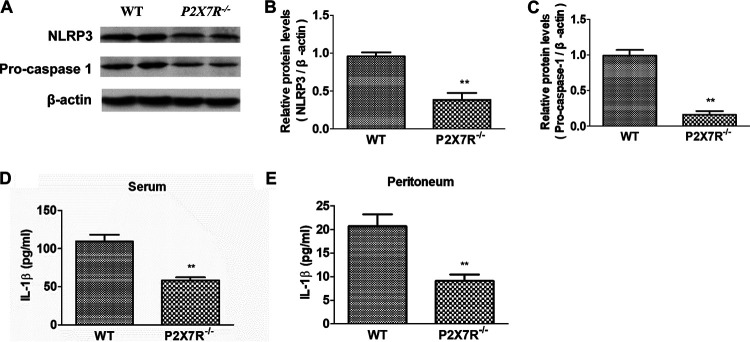
To investigate the effect of P2X7R on the activation of NLRP3 in murine trichinellosis, we compared the activated markers of NLRP3 in macrophages from the infected WT and P2X7R−/− mice with T. spiralis. As shown in Fig. 4A to C, P2X7R deficiency resulted in decreased expression levels of NLRP3 and Pro-capase-1 in peritoneal macrophages from the infected mice, and levels of IL-1β in serum and peritoneal fluid decreased in the infected P2X7R−/− mice (Fig. 4D and E). Collectively, P2X7R deficiency inhibited activation of NLRP3 in macrophages in murine trichinellosis.
FIG 4.
P2X7R deficiency inhibited activation of NLRP3 in macrophages in the enteral stage of murine T. spiralis infection. Wild-type and P2X7R–/–40 mice were administered orally with 300 larvae of T. spiralis in 0.2 ml of sterilized 0.9% saline. Uninfected control mice were administered orally with 0.2 ml of sterilized 0.9% saline. At 7 days postinfection (dpi), 10 mice from each group were sacrificed by cervical dislocation. (A to C) Expressions of NLRP3 and Pro-caspase-1 in peritoneal macrophages were detected by Western blot (A) and quantified using the β-actin band for standardization (B and C). (D and E) Concentrations of IL-1β in mouse serum and peritoneal lavage fluids were detected by ELISA. Data are expressed as means ± SEMs based on 10 mice in each group and from two independent combined experiments. Asterisks mark significant differences between different groups (**, P < 0.01).
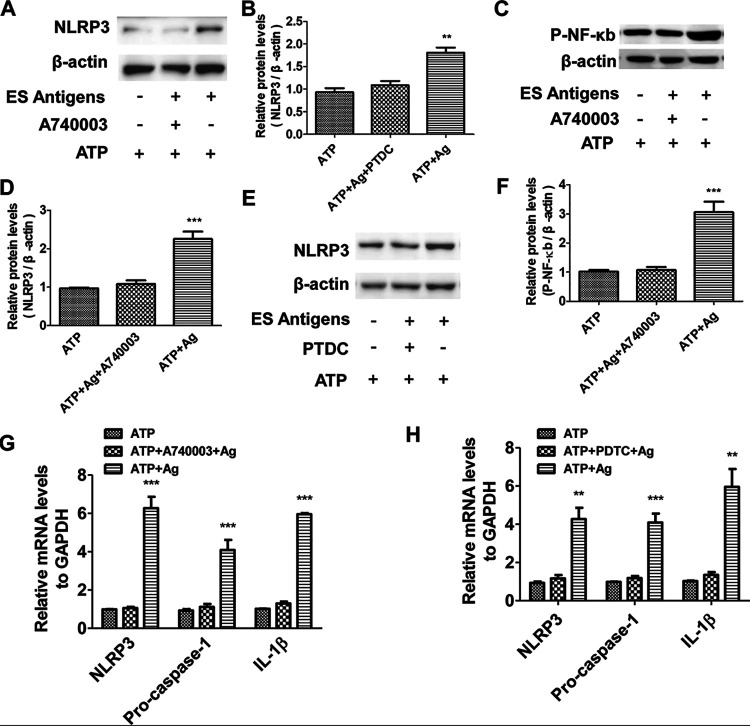
P2X7R blockade inhibited activation of NLRP3 in macrophages via NF-κB in vitro.
To confirm and explore the role of P2X7R and its detailed mechanism in the activation of NLRP3 in macrophages, we blocked P2X7R in monocyte-derived macrophages with a P2X7R antagonist (A740003) in vitro. Western blot analysis showed that the protein expressions of NLRP3 (Fig. 5A and B) and P-NF-κB (Fig. 5C and D) in P2X7R-blocked macrophages were downregulated after T. spiralis excretory-secretory (ES) antigens plus ATP treatment, along with decreased mRNA expression of NLRP3, Pro-caspase-1 and IL-1β (Fig. 5G). We inhibited the NF-κB signaling in macrophages with an NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC), and the protein expression of NLRP3 was suppressed after the inhibitor treatment (Fig. 5E and F), accompanied by decreased mRNA expression of NLRP3, Pro-caspase-1, and IL-1β (Fig. 5H). These data demonstrated that P2X7R blockade inhibited activation of NLRP3 in macrophages through the NF-κB pathway.
FIG 5.
P2X7R blockade inhibited activation of NLRP3 in macrophages via NF-κB in vitro. Human macrophage cell line U937 was cultured, and PMA (100 ng/ml) was added to stimulate U937 into adherent macrophage phenotype (PMA-U937). Then, cells were stimulated with 1 mM ATP for 30 min (the ATP group), following treatment with 10 μg/ml of T. spiralis ES antigens for 24 h (the ATP + ES antigen group). For the ATP + A740003 + ES antigen group, 10 μM A740003 (antagonist of P2X7R) was added before stimulation with ATP. Cells incubated with cell culture medium were used as the control. For the ATP + PTDC + ES antigen group, an NF-κB inhibitor, PTDC, was added before stimulation with ATP. (A to F) Protein expressions of NLRP3 and P-NF-κB in each group were detected by Western blotting (A, C, E) and quantified using the β-actin band for standardization (B, D, F). (G and H) mRNA expressions of NLRP3, Pro-caspase-1, IL-1β in each group were detected by qPCR. Each experiment had three replicates. Data are expressed as means ± SEMs from four independent combined experiments. Asterisks mark significant differences between different groups (**, P < 0.01, ***, P < 0.001).
P2X7R blockade inhibited the capacity of macrophages to kill NBL of T. spiralis in vitro.
To explore the effect of P2X7R on the capacity of macrophages to kill NBL, we cocultured the NBL with supernatants from cultured PMA-U937. We found that activated macrophages with ATP and T. spiralis ES antigen treatment killed more than 60% of NBL in vitro. However, blockade of P2X7R and inhibition of NF-κB resulted in a significant decrease of the killing capacity of macrophages (Fig. 6A and B). These results indicated that both P2X7R and the NF-κB pathway played a vital role in the parasiticidal capacity of macrophages.
FIG 6.
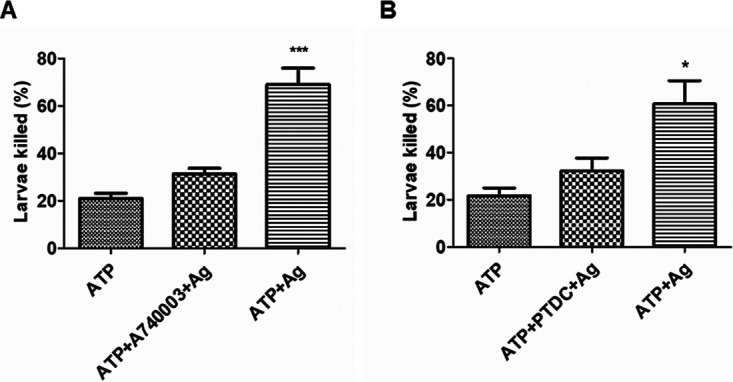
P2X7R blockade inhibited the capacity of macrophages to kill NBL of T. spiralis in vitro. Human macrophage cell line U937 was cultured, and PMA was added to stimulate U937 into the adherent macrophage phenotype (PMA-U937). Then, cells were stimulated with ATP for 30 min (the ATP group), followed by treatment with T. spiralis ES antigens for 24 h (the ATP + ES antigen group). For the ATP + A740003 + ES antigen group, A740003 (antagonist of P2X7R) was added before stimulation with ATP. Cells incubated with cell culture medium were used as the control. For the ATP + PTDC + ES antigen group, an NF-κB inhibitor, PTDC, was added before stimulation with ATP. Supernatants were collected from each group and cocultured with 100 newborn larvae. The death of larvae was determined under direct microscope by two independent observers in a blind fashion. (A and B) The percentages of death rate were calculated. Each experiment had three replicates. Data were expressed as means ± SEMs from four independent combined experiments. Asterisks mark significant differences between different groups (*, P < 0.05; ***, P < 0.001).
DISCUSSION
A number of studies have demonstrated that P2X7R has a central role in the inflammatory responses in some parasite infections, especially in the intestinal immune responses (9, 11, 17). The previous work shows that P2X7R expression is elevated in the small intestine of mice infected with T. spiralis (10), and P2X7R was proved to be located predominantly on macrophages in models of inflammatory diseases (18, 19). Our data showed that T. spiralis infection was associated with increased expression of P2X7R on macrophages, which indicates that P2X7R might play an important role in establishing an innate immune response against T. spiralis infection.
To verify the role of P2X7R in the elimination of T. spiralis in hosts, we compared the worm burdens of the infected P2X7R−/− mice and the infected WT control. P2X7R deficiency resulted in much higher worm burdens in the infected mice (evidenced by increased worm adults and muscle larvae numbers). Therefore, the pathological damage in the small intestine and muscle became deteriorated. Loss of function P2X7R has been linked to increased susceptibility to intracellular pathogens (20, 21). The macrophages infected with T. gondii and Leishmania amazonensis led to enhanced expression of P2X7R, which helps in the elimination of the parasites (22, 23). It has been reported that inhibition or deletion of P2X7R result in an increase in parasite load of T. gondii (14, 22). Our results, together with the previous studies, indicate that P2X7R participates in the elimination of both intracellular and extracellular parasites, although further studies are needed to investigate the detailed cellular and molecular mechanisms.
The inflammasome is a key line of immune defense against invading pathogens. Accumulating evidence indicates that NLRP3 activation is essential for the control of various parasitic infections (4, 11, 24). Our results showed that the T. spiralis-infected mice had higher expression of NLPR3 in peritoneal macrophages than the uninfected control, which is consistent with the previous studies (25, 26). Nevertheless, the cellular mechanism of NLRP3 activation in the parasite infection remains largely unknown. Many studies have identified that P2X7R is one of the most potent activators of the NLRP3 inflammasome (11, 27, 28). Our results showed that P2X7R deficiency inhibited NLRP3 activation in the infected mice, evidenced by decreased protein expressions of NLRP3 and Pro-caspase and lower concentrations of IL-1β in serum and peritoneum. In in vitro experiments, P2X7R blockade with the antagonist also led to consistent results. It has been demonstrated that under pathophysiological conditions caused by parasite infections, ATP released from dying cells enhances P2X7R activation to upregulate the NLRP3 inflammasome, subsequently promoting IL-1β secretion (11). The NLRP3 inflammasome is closely associated with P2X7R in T. gondii infection and thus inhibits parasite proliferation in small intestinal epithelial cells (9). P2X7R is one of the most potent activators of the NLRP3 inflammasome and, therefore, of mature IL-1β release (29). IL-1β is a crucial factor of host defense in response to infections and injuries (11, 29, 30). IL-1β production is strictly regulated at both the transcriptional and posttranslational levels through the activity of P2X7R-mediated NLRP3 inflammasome (9, 29). Many studies have reported that parasite infections produce significant amounts of IL-1β in different immune cells, and concentrations of IL-1β are closely associated with parasite elimination in hosts (9, 11, 21). The NLRP3 inflammasome is activated in response to Leishmania infection and is important for the restriction of parasite replication in vitro and in vivo (31). Our data showed that inhibition of the NLRP3/IL-1β pathway mediated by P2X7R decreased the capacity of macrophages to kill T. spiralis parasites both in vivo and in vitro. Our findings, consistent with previous studies, strongly suggest that P2X7R plays a pivotal part in facilitating IL-1β release for its important role in NLRP3 inflammasome activation and in mediating the resistance to parasites, and the mechanism needs to be further clarified.
Production of IL-1β is a multistep process, including synthesis of pro-IL-1β, proteolytic cleavage to mature IL-1β, and release into the extracellular environment. The activation of the nuclear factor NF-κB induces the synthesis of pro-IL-1β in many inflammatory diseases (30). The seminal studies demonstrate that P2X7R is a potent stimulus for activation of the NF-κB pathway in microglia, osteoclasts, and osteoblasts (32–34). Previous studies found that NRLP3 is one of the downstream pathways of NF-κB (27), and we wondered whether the NF-κB pathway played a role in the effect of P2X7R on activation of NLRP3 in T. spiralis infection. It is reported that stimulation of monocyte-derived macrophages U937 in vitro may provide a useful model for further study of mechanisms of macrophage cytotoxicity and its activation in T. spiralis infection (35, 36). Thus, we investigated the role of NF-κB in P2X7R-mediated NLRP3 activation in U937 in response to T. spiralis ES antigens. The data showed that the expression of P-NF-κB in the treated macrophages decreased significantly after blockade of P2X7R. Furthermore, blockade of NF-κB inhibited both activation of NLRP3 and synthesis of IL-1β. As a result, the capacity of macrophages to kill T. spiralis larvae had a significant decrease after NF-κB blockade in vitro. Our results fit with the view that NF-κB plays an important role in mediation of P2X7R in the NLRP3/IL-1β pathway and then has an effect on the capacity of macrophages to kill parasites.
Taken together, our findings reveal the role of P2X7R in the induction of NLRP3 inflammasome activation in T. spiralis-induced macrophages. To the best of our knowledge, this is the first report that P2X7R plays an important role in the activation of NLRP3 during T. spiralis infection. P2X7R deficiency or blockade decreased the capacity of macrophages to kill the parasite by inhibiting NLRP3 activation in vivo and in vitro. Furthermore, our data showed that P2X7R mediated the parasiticidal activation of NLRP3 via NF-κB. These results may contribute to our better understanding of the intestinal immune mechanism of T. spiralis infection but also offer new insight into the identification of innate resistance during the enteral stage of trichinellosis.
MATERIALS AND METHODS
Mice and parasites.
P2X7R knockout (P2X7R−/−) mice in the C57BL/6 genetic background were purchased from The Jackson Laboratory (USA). Wild-type C57BL/6 mice were provided by the Hubei Province Center for Disease Control and Prevention (Wuhan, China). All mice were maintained in a standard specific-pathogen-free animal facility at Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). Our experiment was reviewed and approved by the Institutional Animal Care and Use Committee at Tongji Medical College (SCXK2016-0011). The life cycle of T. spiralis (ISS534) was maintained by serial passage in Kunming mice at 6-month intervals (37).
Experimental infection.
P2X7R−/− and WT mice, aged 6 to 8 weeks, were administered orally with 300 larvae of T. spiralis in 0.2 ml of sterilized 0.9% saline, following a previously published protocol (38). Uninfected control mice were administered orally with 0.2 ml of sterilized saline. At 7 days postinfection (dpi), 10 mice from each group were sacrificed by cervical dislocation. Blood was collected from the oculomotor sinus of mice under ether anesthesia. Small intestine, MLN, and peritoneal macrophages were collected for further analysis. At 42 dpi, 6 mice from each group were sacrificed; skinned and eviscerated mouse carcasses were collected for determination of skeletal muscle larvae.
Collection of intestinal adult worms.
To facilitate the recovery of adult worms from the intestine, the infected mice were not fed for 1 day prior to sacrifice. The small intestines were opened longitudinally and washed thrice in ice-cold 0.9% saline and then cut into 2-cm-long fragments with sharp scissors and cultured in normal saline at 37°C for 2.5 h. Then, adults released from the small intestine into saline were collected and counted under a microscope (39).
Determination of skeletal muscle larvae burden.
T. spiralis muscle larvae were collected and counted as previously described (40). In brief, the mice were sacrificed with euthanasia, and skinned and eviscerated mouse carcasses were cut into pieces and digested in 1% pepsin-hydrochloride digestion fluid for 1.5 h at 37°C. A magnetic spin bar was utilized to provide continual mixing during digestion, and then the entire digests from the beaker were poured through an 80 sieve (180-μm mesh) into a 2-liter separatory funnel. After settling for 30 min, larvae were collected following three additional washes and then resuspended in 0.9% saline. The larvae were counted under a microscope.
Preparation of excretory-secretory (ES) antigens of adults and larvae.
The ES antigens of adults and larvae were prepared as previously described (41, 42). Adults and larvae were pooled and cultured at 37°C in RPMI 1640 (Invitrogen) and 5% CO2, respectively, for 18 h. The supernatant containing ES antigens was collected after using an Amicon Ultra-3 centrifugal filter unit (Millipore, USA) at 4°C and centrifuged at 5,000 × g for 1 h. The protein concentration was detected with the bicinchoninic acid assay (BCA; Pierce, USA) according to the manufacturer’s protocol.
Isolation of peritoneal macrophages.
Peritoneal macrophages from the uninfected and infected mice were collected after washing the peritoneal cavity with 3 ml sterile phosphate-buffered saline (PBS). Macrophages were isolated by adherence to plastic wells and confirmed with F4/80 immunostaining (10).
Flow cytometry.
Flow cytometry analysis was performed to assess expression levels of P2X7R in macrophages in MLN from mice. Briefly, live cells were isolated from MLN of T. spiralis-infected mice at 7 dpi. The cell surfaces were stained with anti-F4/80-FITC and anti-P2X7R-PE for 30 min at 4°C. All antibodies were purchased from eBioscience (San Diego, CA). Data were analyzed with FlowJo version 10.0.7 (Tree Star).
Histological analysis.
The small intestine (duodenum and jejunal segments) was obtained from the uninfected and infected mice at 7 dpi. All segments were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 h at 4°C. Then, samples were embedded in paraffin and cut into 5-μm sections. For the histopathological study, the samples were stained with hematoxylin and eosin (H&E).
Cell culture and in vitro treatment.
Human macrophage cell line U937 (Cell Bank, Chinese Academy of Sciences) was cultured with 5% CO2 for 24 h at 37°C in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) (Invitrogen). Phorbol myristate acetate (PMA) (100 ng/ml) was added to stimulate U937 into the adherent macrophage phenotype (PMA-U937). Then, cells were stimulated with 1 mM ATP for 30 min, followed by treatment with 10 μg/ml of T. spiralis ES antigens for 24 h. To evaluate the effect of P2X7R blockade on NLRP3 signals, 10 μM A740003 (antagonist of P2X7R; Sigma) was added before stimulation with ATP. Cells incubated with cell culture medium were used as the control. To determine the role of NF-κB in the activation of NLRP3 by P2X7R, an NF-κB inhibitor pyrrolidine dithiocarbamate (50 μM; Selleck Chemicals, USA) was added before stimulation with ATP. Supernatants and cultured cells were collected for further use.
Determination of parasiticidal capacity of macrophages in vitro.
To confirm the effect of P2X7R on killing T. spiralis larvae by activated macrophages in vitro, NBL were incubated with supernatants from cultured PMA-U937 with 5% CO2 for 24 h at 37°C, following the procedure described previously with some modifications (40, 43). Briefly, NBL were separated from female adult worms by filtration over nylon gauze and then washed thrice in sterilized saline. The number of NBL was adjusted to 1,000/ml with RPMI 1640 medium. For each well, 100 μl of larva suspension and 1 ml of supernatants from cultured PMA-U937 were added. The death of larvae was determined under direct microscope by two independent observers in a blind fashion with the following standard: worm body shown as “C” shape or completely straightened, no longer curled up, with a blurred interior. Each larva was observed for 30 s until the worm body no longer moved.
Western blot analysis.
Tissues and cells were lysed in RIPA lysis buffer (Beyotime Biotechnology, Nanjing, China) containing a cocktail of protease inhibitors (Roche, Basel, Switzerland). Samples were centrifuged at 12,000 × g for 15 min, and supernatants were collected for further analysis. The protein concentrations were detected with the BCA according to the manufacturer’s protocol. Western blot analysis was carried out according to the previous study (44). Briefly, samples (30 μg) were electrophoresed with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The protein was transferred to polyvinylidene difluoride (PVDF) membranes (0.45 μm; Merck) and then blocked with 5% milk in tris-buffered saline with Tween 20 (TBST). The following primary antibodies were used for the immune detection: rat anti-P2X7R (1:500, 148702; BioLegend), rabbit anti-NLRP3 (1:500, ab214185; Abcam), rabbit anti-Pro-caspase-1 (1:100, ab207802; Abcam), rabbit anti Phospho-NF-κB p65 (1:1,000, Cell Signaling Technology), and rabbit anti-β-actin (1:1,000; CST). Horseradish peroxidase (HRP)-conjugated anti-rat IgG antibodies (Ab) were used as the secondary Ab (1:1,000; Cell Signaling Technology) for P2X7R, whereas HRP-conjugated anti-rabbit IgG Ab (1:1,000, HAF005; R&D systems) were used as the secondary antibody for all the others. All the primary antibodies were incubated overnight at 4°C, while the secondary antibodies were incubated for 1 h at room temperature. After labeling with enhanced chemiluminescent substrate (ECL; Millipore), chemiluminescence signals of protein bands were detected using ImageJ software version 1.44 using the β-actin band for standardization.
Sample collection for cytokine analysis and ELISA.
Serum was collected from blood by centrifuging at 6, 000 × g for 5 min following coagulation of 0.5 ml whole blood. The peritoneal lavage was centrifuged at 800 × g for 5 min, and the supernatant was collected for enzyme-linked immunosorbent assay (ELISA) analysis. Concentrations of IL-1β in mouse serum and peritoneal lavage fluids were measured with ELISA kits (R&D Systems) according to the manufacturer’s instructions.
Quantitative real-time PCR.
Total RNA from tissues and cells was extracted with a TRIzol reagent kit (Invitrogen, Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. After being quantified using a Nanodrop 2000 instrument (Thermo Scientific, USA), RNA was reverse-transcribed to cDNA with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Thermo Fisher Scientific). Then, real-time PCR was performed using Power Sybr green PCR master mix (Applied Biosystems, USA) with gene-specific primers as listed in Table 1. The amplification reactions were carried out with an initial hold step (95°C for 5 min), followed by 40 cycles of a three-step PCR (95°C for 1 min, 60°C for 45 s, and 72°C for 30 s). Relative mRNA expression of the target gene was calculated in terms of the comparative cycling threshold (CT) normalized by GAPDH with the 2−ΔΔCt method.
TABLE 1.
Primer sequences of target mRNA
| Genes | Primers |
|
|---|---|---|
| Forward (5′ → 3′) | Reverse (5′ → 3′) | |
| NLRP3 | TTCTTTCTGTTTGCTGAGTTTTTG | TTCCTGGCATATCACAGTGG |
| Pro-caspase-1 | GAAGGACAAACCGAAGGTGA | TGGAAGAGCAGAAAGCGATA |
| IL-1β | AAACAGATGAAGTGCTCCTTCCAG | TGGAGAACACCACTTGTTGCTCCA |
| GAPDH | GTCAGTGGTGGACCTGACCT | AGGGGTCTACATGGCAACTG |
Statistical analysis.
Each experiment was performed at least twice with 6 to 10 mice or samples per group. All results were presented as means ± standard error of the means (SEMs) of two independent experiments. Statistical significance was determined using an unpaired Student’s t test or one-way analysis of variance (ANOVA) (GraphPad Prism version 7.0 software). The significant difference levels are indicated by *, P < 0.05; **, P < 0.01, and ***, P < 0.001.
ACKNOWLEDGMENTS
This work was funded by grants from the National Nature Science Foundation of China (grant 81000739 and 81772220 to Jiahui Lei) and the Fundamental Research Funds for the Central Universities (grant HUST 2016YXMS199 to Jiahui Lei).
REFERENCES
- 1.Robertson LJ. 2018. Parasites in food: from a neglected position to an emerging issue. Adv Food Nutr Res 86:71–113. 10.1016/bs.afnr.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rostami A, Gamble HR, Dupouy-Camet J, Khazan H, Bruschi F. 2017. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol 64:65–71. 10.1016/j.fm.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang XZ, Sun XY, Bai Y, Song YY, Hu CX, Li X, Cui J, Wang ZQ. 2020. Protective immunity in mice vaccinated with a novel elastase-1 significantly decreases Trichinella spiralis fecundity and infection. Vet Res 51:43. 10.1186/s13567-020-00767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. 2017. The P2X7 receptor in infection and inflammation. Immunity 47:15–31. 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. 1996. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738. 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 6.Matty MA, Knudsen DR, Walton EM, Beerman RW, Cronan MR, Pyle CJ, Hernandez RE, Tobin DM. 2019. Potentiation of P2RX7 as a host-directed strategy for control of mycobacterial infection. Elife 8:e39123. 10.7554/eLife.39123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaves MM, Sinflorio DA, Thorstenberg ML, Martins MDA, Moreira-Souza ACA, Rangel TP, Silva CLM, Bellio M, Canetti C, Coutinho-Silva R. 2019. Non-canonical NLRP3 inflammasome activation and IL-1β signaling are necessary to L. amazonensis control mediated by P2X7 receptor and leukotriene B4. PLoS Pathog 15:e1007887. 10.1371/journal.ppat.1007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun R, Urban JF Jr, Notari L, Vanuytsel T, Madden KB, Bohl JA, Ramalingam TR, Wynn TA, Zhao A, Shea-Donohue T. 2016. Interleukin-13 receptor α1-dependent responses in the intestine are critical to parasite clearance. Infect Immun 84:1032–1044. 10.1128/IAI.00990-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan JH, Huang R, Wang Z, Huang S, Choi IW, Zhou Y, Lee YH, Chu JQ. 2018. P2X7 receptor mediates NLRP3-dependent IL-1β secretion and parasite proliferation in Toxoplasma gondii-infected human small intestinal epithelial cells. Parasit Vectors 11:1. 10.1186/s13071-017-2573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating C, Pelegrin P, Martínez CM, Grundy D. 2011. P2X7 receptor-dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. J Immunol 187:1467–1474. 10.4049/jimmunol.1100423. [DOI] [PubMed] [Google Scholar]
- 11.Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. 2018. The P2X7 receptor: a main player in inflammation. Biochem Pharmacol 151:234–244. 10.1016/j.bcp.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Callaway JB, Ting JP. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687. 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding J, Bai X, Wang XL, Wang YF, Shi HN, Rosenthal B, Boireau P, Wu XP, Liu MY, Liu XL. 2016. Developmental profile of select immune cells in mice infected with Trichinella spiralis during the intestinal phase. Vet Parasitol 231:77–82. 10.1016/j.vetpar.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Corrêa G, Marques da Silva C, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R. 2010. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect 12:497–504. 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Müller T, Vieira RP, Grimm M, Dürk T, Cicko S, Zeiser R, Jakob T, Martin SF, Blumenthal B, Sorichter S, Ferrari D, Di Virgillio F, Idzko M. 2011. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol 44:456–464. 10.1165/rcmb.2010-0129OC. [DOI] [PubMed] [Google Scholar]
- 16.Lu F, Lan Z, Xin Z, He C, Guo Z, Xia X, Hu T. 2020. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol 235:3207–3221. 10.1002/jcp.29268. [DOI] [PubMed] [Google Scholar]
- 17.Huang SW, Walker C, Pennock J, Else K, Muller W, Daniels MJ, Pellegrini C, Brough D, Lopez-Castejon G, Cruickshank SM. 2017. P2X7 receptor-dependent tuning of gut epithelial responses to infection. Immunol Cell Biol 95:178–188. 10.1038/icb.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang M, Cui BW, Wu YL, Zhang Y, Shang Y, Liu J, Yang HX, Qiao CY, Zhan ZY, Ye H, Jin Q, Nan JX, Lian LH. 2020. P2X7R orchestrates the progression of murine hepatic fibrosis by making a feedback loop from macrophage to hepatic stellate cells. Toxicol Lett 333:22–32. 10.1016/j.toxlet.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Xu SL, Lin Y, Liu W, Zhu XZ, Liu D, Tong ML, Liu LL, Lin LR. 2020. The P2X7 receptor mediates NLRP3-dependent IL-1β secretion and promotes phagocytosis in the macrophage response to Treponema pallidum. Int Immunopharmacol 82:106344. 10.1016/j.intimp.2020.106344. [DOI] [PubMed] [Google Scholar]
- 20.Miller CM, Zakrzewski AM, Robinson DP, Fuller SJ, Walker RA, Ikin RJ, Bao SJ, Grigg ME, Wiley JS, Smith NC. 2015. Lack of a functioning P2X7 receptor leads to increased susceptibility to toxoplasmic ileitis. PLoS One 10:e0129048. 10.1371/journal.pone.0129048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller CM, Zakrzewski AM, Ikin RJ, Boulter NR, Katrib M, Lees MP, Fuller SJ, Wiley JS, Smith NC. 2011. Dysregulation of the inflammatory response to the parasite, Toxoplasma gondii, in P2X7 receptor-deficient mice. Int J Parasitol 41:301–308. 10.1016/j.ijpara.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, Mui EJ, Witola WH, Coyne JJ, Hargrave AC, Jamieson SE, Blackwell JM, Wiley JS, Smith NC. 2010. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol 184:7040–7046. 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaves SP, Torres-Santos EC, Marques C, Figliuolo VR, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B. 2009. Modulation of P2X(7) purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect 11:842–849. 10.1016/j.micinf.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Celias DP, Motrán CC, Cervi L. 2020. Helminths turning on the NLRP3 inflammasome: pros and cons. Trends Parasitol 36:87–90. 10.1016/j.pt.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Jin X, Yang Y, Ding J, Liu X, Shi H, Luo X, Jia W, Cai X, Vallee I, Boireau P, Bai X, Liu M. 2020. Nod-like receptor pyrin domain containing 3 plays a key role in the development of Th2 cell-mediated host defenses against Trichinella spiralis infection. Vet Parasitol 109159. 10.1016/j.vetpar.2020.109159. [DOI] [PubMed] [Google Scholar]
- 26.Jin X, Bai X, Yang Y, Ding J, Shi H, Fu B, Boireau P, Liu M, Liu X. 2020. NLRP3 played a role in Trichinella spiralis-triggered Th2 and regulatory T cells response. Vet Res 51:107. 10.1186/s13567-020-00829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutterwala FS, Haasken S, Cassel SL. 2014. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci 1319:82–95. 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacheco PAF, Faria RX. 2021. The potential involvement of P2X7 receptor in COVID-19 pathogenesis: a new therapeutic target? Scand J Immunol 93:e12960. 10.1111/sji.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F. 2017. The P2X7 receptor-interleukin-1 liaison. Front Pharmacol 8:123. 10.3389/fphar.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh T, Otsuka A, Contassot E, French LE. 2015. The inflammasome and IL-1β: implications for the treatment of inflammatory diseases. Immunotherapy 7:243–254. 10.2217/imt.14.106. [DOI] [PubMed] [Google Scholar]
- 31.Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva ALN, Mineo TWP, Gutierrez FRS, Bellio M, Bortoluci KR, Flavell RA, Bozza MT, Silva JS, Zamboni DS. 2013. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat Med 19:909–915. 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 32.Thawkar BS, Kaur G. 2019. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J Neuroimmunol 326:62–74. 10.1016/j.jneuroim.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Di Virgilio F, Adinolfi E. 2017. Extracellular purines, purinergic receptors and tumor growth. Oncogene 36:293–303. 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genetos DC, Karin NJ, Geist DJ, Donahue HJ, Duncan RL. 2011. Purinergic signaling is required for fluid shear stress-induced NF-κB translocation in osteoblasts. Exp Cell Res 317:737–744. 10.1016/j.yexcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larrick JW, Fischer DG, Anderson SJ, Koren HS. 1980. Characterization of a human macrophage-like cell line stimulated in vitro: a model of macrophage functions. J Immunol 125:6–12. [PubMed] [Google Scholar]
- 36.Piaggi S, Salvetti A, Gomez-Morales MA, Pinto B, Bruschi F. 2020. Glutathione-S-transferase omega 1 and nurse cell formation during experimental Trichinella infection. Vet Parasitol 109114. 10.1016/j.vetpar.2020.109114. [DOI] [PubMed] [Google Scholar]
- 37.Guan F, Hou X, Nie G, Xiao Y, Zhang Q, Liu WQ, Li YL, Lei JH. 2013. Effect of Trichinella spiralis infection on the immune response to HBV vaccine in a mouse model. Foodborne Pathog Dis 10:882–887. 10.1089/fpd.2013.1545. [DOI] [PubMed] [Google Scholar]
- 38.Jiang P, Wang ZQ, Cui J, Zhang X. 2012. Comparison of artificial digestion and Baermann's methods for detection of Trichinella spiralis pre-encapsulated larvae in muscles with low-level infections. Foodborne Pathog Dis 9:27–31. 10.1089/fpd.2011.0985. [DOI] [PubMed] [Google Scholar]
- 39.Pozio E, Marucci G, Casulli A, Sacchi L, Mukaratirwa S, Foggin CM, Rosa GL. 2004. Trichinella papuae and Trichinella zimbabwensis induce infection in experimentally infected varans, caimans, pythons and turtles. Parasitology 128:333–342. 10.1017/s0031182003004542. [DOI] [PubMed] [Google Scholar]
- 40.Buys J, Wever R, van Stigt R, Ruitenberg EJ. 1981. The killing of newborn larvae of Trichinella spiralis by eosinophil peroxidase in vitro. Eur J Immunol 11:843–845. 10.1002/eji.1830111018. [DOI] [PubMed] [Google Scholar]
- 41.Liu RD, Qi X, Sun GG, Jiang P, Zhang X, Wang LA, Liu XL, Wang ZQ, Cui J. 2016. Proteomic analysis of Trichinella spiralis adult worm excretory-secretory proteins recognized by early infection sera. Vet Parasitol 231:43–46. 10.1016/j.vetpar.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Wang ZQ, Liu RD, Sun GG, Song YY, Jiang P, Zhang X, Cui J. 2017. Proteomic analysis of Trichinella spiralis adult worm excretory-secretory proteins recognized by sera of patients with early trichinellosis. Front Microbiol 31:986. 10.3389/fmicb.2017.00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi X, Yue X, Han Y, Jiang P, Yang F, Lei JJ, Liu RD, Zhang X, Wang ZQ, Cui J. 2018. Characterization of two Trichinella spiralis adult-specific DNase II and their capacity to induce protective immunity. Front Microbiol 9:2504. 10.3389/fmicb.2018.02504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Liu RD, Bai SJ, Hao HN, Yue WW, Xu YXY, Long SR, Cui J, Wang ZQ. 2020. Molecular characterization of a Trichinella spiralis aspartic protease and its facilitation role in larval invasion of host intestinal epithelial cells. PLoS Negl Trop Dis 14:e0008269. 10.1371/journal.pntd.0008269. [DOI] [PMC free article] [PubMed] [Google Scholar]