Summary
The jugular-nodose ganglia contain the sensory peripheral neurons of the vagus nerve, linking visceral organs to the medulla oblongata. Accessing these ganglia in smaller animals without damaging the vascular and neural structures may be challenging, as ganglionic fibers imbed deeply into the carotid sheath, and vagal parasympathetic fibers cross through the interior of the ganglia. We describe a practical protocol for locating and accessing the mouse jugular-nodose ganglia in vivo, including instructions for intraganglionic injections and postperfusion dissection.
For complete details on the use and execution of this protocol, please refer to Han et al. (2018).
Subject areas: Metabolism, Microscopy, Neuroscience
Graphical abstract

Highlights
-
•
Practical approach to locate the mouse jugular-nodose ganglia
-
•
Detailed instructions on how to perform intraganglionic injections
-
•
Detailed description of ganglia-preserving postperfusion dissection
The jugular-nodose ganglia contain the sensory peripheral neurons of the vagus nerve, linking visceral organs to the medulla oblongata. Accessing these ganglia in smaller animals without damaging the vascular and neural structures may be challenging, as ganglionic fibers imbed deeply into the carotid sheath, and vagal parasympathetic fibers cross through the interior of the ganglia. We describe a practical protocol for locating and accessing the mouse jugular-nodose ganglia in vivo, including instructions for intraganglionic injections and postperfusion dissection.
Before you begin
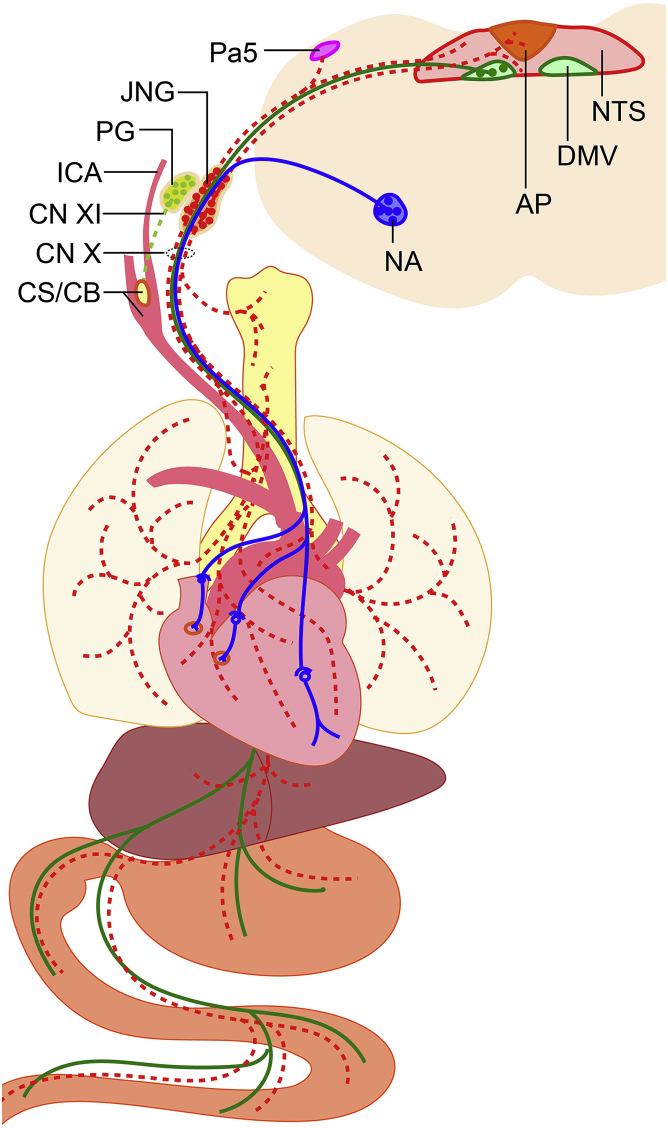
The protocol describes a straightforward procedure that allows addressing vagus-mediated physiological and behavioral phenomena. The protocol focuses on the jugular-nodose ganglia ("JNG") of the mouse. These ganglia, which are approximately 1 mm-wide in the mouse, host the vagal sensory neurons, embedded deeply at the dorsal medial corner of the carotid triangle, as shown in Figure 1. Unlike the guinea pig and other larger mammals, nodose and jugular ganglia in rodents are hardly distinct, merging into one jugular–nodose complex (Nassenstein et al., 2010, Mazzone and Undem, 2016). During development, the jugular neurons, which are situated in the rostral portion of the JNG (Mazzone and Undem, 2016) originally derive from neural crest cells (D'Amico-Martel and Noden, 1983) and express the critical signaling molecule Runx1/Wnt1 (Echelard et al., 1994). In contrast, the nodose neurons, which form the caudal portion of the jugular-nodose ganglia, derive during embryonic development from the epibranchial placodes (Baker and Bronner-Fraser, 2001) and express the paired-like homeodomain transcription factors Phox2a/Phox2b (Fode et al., 1998, Ma et al., 1998). Interestingly, several studies indicate that gut-innervating, nutrient-sensing nodose neurons express Cckar/Cckbr and Glp1r (Diepenbroek et al., 2017, Kupari et al., 2019). Jugular-nodose peripheral terminals innervate visceral organs, and their central terminals reach the medulla oblongata (i.e., nucleus tractus solitarius, area postrema, and paratrigeminal nucleus) (Han et al., 2018, McGovern et al., 2015, Altschuler et al., 1989, Kim et al., 2020).
Figure 1.
Schematic overview of the vagus nerve system
Schematic representation of nodose-jugular ganglia visceral and central terminations, as shown on a cross-sectional view of the medulla oblongata and vagal nuclei. Vagal visceral efferent neurons, originating from the Nucleus Ambiguus (NA) and the Dorsal Motor Nucleus of the Vagus (DMV), provide parasympathetic tone to visceral organs, including the heart and the gastrointestinal tract (blue trace). In mice, the Jugular and Nodose ganglia merge to form the Jugular-Nodose ganglia (JNG). JNG sensory pseudomonopolar neurons monitor visceral perception and synapse onto the Paratrigeminal nucleus (Pa5), the Nucleus of the Tractus Solitarius (NTS), and the Area Postrema (AP) in the medulla oblongata. The Petrosal ganglia (PG), consisting of sensory neurons partially innervating the craniofacial and cervical tissues such as the Carotid Sinus and Body (CS/CB), in some cases merge with JNG to form a JNG/PG complex.
We first describe how to safely perform intra-ganglionic injections (Part I). We use as an example the delivery of CCK-conjugated saporin (CCK-SAP), a neurotoxin that can be used to specifically ablate gut-innervating JNG neurons (Han et al., 2018). However, the same protocol can be equally used for viral vectors (e.g., AAVs/HSV/PRV/Rabies) infection. This part of the protocol requires the following:
A laboratory system for isoflurane anesthesia administration to rodents.
A stereomicroscope mounted on a fixed platform.
A microinjection system.
Parasympathetic efferents, arising from medullary nucleus ambiguus and dorsal motor nucleus of vagus (DMV), partially branch into the heart, lung, and respiratory tract (Kalia and Sullivan, 1982). Inappropriate chemical or mechanical irritation to these fibers will inevitably trigger a parasympathetic overtone, including cardiac arrests due to sinoatrial node inhibition (Vaseghi and Shivkumar, 2008). The following suggestions might help with increasing the survival rate: (1) Preoperative administration of atropine, (2) Close monitoring of respiratory and cardiac functions, with immediate pauses to the procedures upon any signs of cardiorespiratory irregularity, (3) Maintain the surgically exposed area hydrated (moist), (4) minimize the duration of the surgery, (5) if bilateral JNG manipulation is necessary, consider scheduling the contralateral surgeries at least one week apart from each other to reduce complications. Many experts (Lee et al., 2020, McGovern et al., 2012, McGovern et al., 2015, Mazzone and Undem, 2016) have successfully performed bilateral JNG injections on the same surgery day. Thus, the outer needle diameter, and the volume, infusion rate, and toxicity of injective will impact the decision for staged surgery. (6) Finally, the JNG is deeply situated at the skull base immediately adjacent to the jugular canal exit. The major vagus nerve descending branch enters the carotid sheath and run alongside the carotid artery and internal jugular vein down to the neck, chest, and abdomen. Therefore, any incautious penetration of the carotid sheath may lead to lethal arterial or venous bleeding. Accessing the ganglia without affecting the surrounding nervous and vascular tissue is critical for post-surgical recovery and proper behavioral assessment. A sharply focused surgical field under the stereomicroscope and blunt surgical instruments are critical to improving the surgical outcome.
The second part of the protocol involves post-perfusion JNG dissection (Part II). This protocol requires:
A rodent transcardiac perfusion system.
A stereomicroscope dissection setup.
JNG is located outside of, but in close proximity to, the skull. Dissecting the vagus nerve root by applying scissors to nerves outside the skull will inevitably lead to a fragmented ganglion. Besides, transcardiac perfusion blurs the visualizable histological differences between the carotid artery and the vagus nerve. Our protocol provides a novel approach to achieve integral JNG dissection by accessing the interior of the skull.
Preparation of the surgery setup
Timing: 30 min
-
1.
Sterilize surgical tools (Figure 2A) and supplies: glass syringes, metal needles, retractors, fine scissors, a spinal cord hook, ultra-fine forceps, scalpel, wooden cotton swabs, drapes, gauze pads.
-
2.
Prepare sterile 0.9% saline solution, ophthalmic ointment, depilatory ointment, povidone-iodine prep pads, buprenorphine, carprofen, and atropine.
-
3.
Sterilize the surgical table, stereomicroscope, ring light illuminator, microinjection pump surface disinfected with alcohol (70% ethanol).
-
4.
Turn on the heating pad, set temperature to 37°C.
-
5.
Cover the pre-heated surgical platform with a sterile drape.
-
6.
Confirm isoflurane level in the anesthesia apparatus.
-
7.
Prepare a clean cage for housing the mouse after surgery..
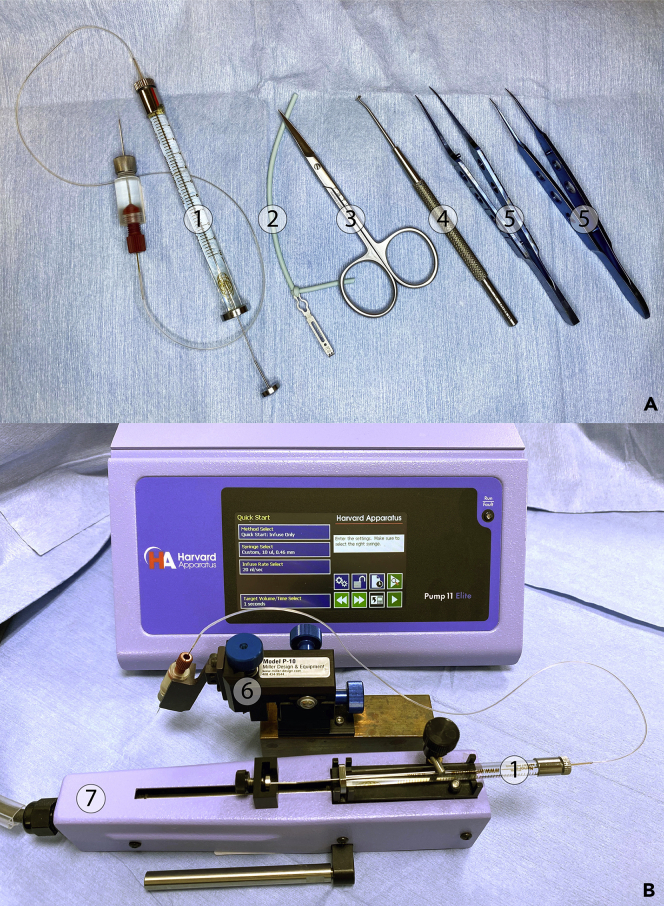
Figure 2.
Microinjection system of JNG virus infection/chemical delivery
(A) The surgical toolset consists of the assembled NanoFil sub-microliter injection system ①, a retractor attached to an elastomer ②, fine scissors ③, a spinal cord hook ④, and ultra-fine forceps ⑤.
(B) Mount the NanoFil sub-microliter syringe ① onto the Nanomite remote infusion pump ⑦, then clamp the injection holder with the micropositioner ⑥.
Preparation of the NanoFil sub-microliter injection system
Timing: 20 min
-
8.Front-load the microsyringe
-
a.Unfreeze the CCK-SAP [IT-31, ATS] and keep it on ice after centrifugation.
-
b.Withdraw the CCK-SAP solution with a 10 μL 33G Neuros Syringe [65460, Hamilton].
-
c.Remove the locking cap and silicone gasket from the 10 μL NanoFil microsyringe [NANOFIL, WPI].
-
d.To front-fill the NanoFil microsyringe:
-
i.Keep the NanoFil plunger to the lowest volume.
-
ii.Insert the Neuros Syringe needle into the NanoFil microsyringe cavity. Ensure that the Neuros Syringe needle tip contacts the NanoFil plunger. This is critical for degassing the NanoFil microsyringe.
-
iii.Slowly fill 2 μL of the CCK-SAP solution into NanoFil microsyringe. Then pull the NanoFil plunger backwards while pushing Neuros Syringe onwards until the desired amount is reached.
-
i.
-
a.
CRITICAL: Residual air bubbles in the NanoFil system will negatively affect the ability to inject precise volumes at the appropriate rate.
CRITICAL: The required needle filling amount is = 3 μL (the total dead volume of Nanofil needle, holder, and tubing) + 0.5 μL (each JNG) × sides × animals.
-
9.Close the NanoFil injecting system (Figure 2A).
-
a.Secure the 36 G beveled NanoFil needle [NF36BV-2, WPI] to the NanoFil injection holder [NFINHLD, WPI], which must be closely attached to one end of the SilFlex tubing [SILFLEX-2, WPI].
-
b.Insert the other end of the SilFlex tubing into the NanoFil syringe through the locking cap and the silicone gasket. Tightly screw the locking cap and slowly push the NanoFil plunger to degas the whole NanoFil injecting system.
-
a.
Alternatives: Other techniques such as glass micropipettes systems with gas pressure, or mineral oil backfilling, are also viable options. Typically, glass micropipettes with minimal diameters of around 20–50 μm can inject small volumes and cause little disruption because of the small tip size. In the current protocol, we selected the stainless steel 36G beveled NanoFil needle [NF36BV-2, WPI], given its robustness and moderate outer diameter (120 μm).
Preparation of Nanomite microinjection system
Timing: 5 min
-
10.To program the Nanomite syringe pump [Pump 11 Elite, Harvard Apparatus] (Figure 2B):
-
a.Method Selection: "Quick Start: Infuse Only";
-
b.Syringe Selection: "Custom, 10 μL, 0.46 mm";
-
c.Infuse Rate Selection: "2 nL/s";
-
d.Target Volume/Time Selection: “250 s” or “500 nL”.
-
a.
-
11.
Mount the NanoFil microsyringe onto the remote infuse unit.
-
12.
Clamp the NanoFil injection holder to the micromanipulator [P-10, Miller Design].
-
13.
Gently press the "▹▹" button until liquid flows out of the NanoFil needle.
CRITICAL: The 36G beveled NanoFil needle [NF36BV-2, WPI] gets clogged easily. Therefore, only fill well-filtered solutions to the syringe, and keep immersing the needle tip into saline to prevent crystallization.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| CCK-SAP | Advanced Targeting Systems | SKU: IT-31 |
| RIMADYL® (Carprofen) 50 mg/mL | Pfizer Animal Health | NADA # 141-199 |
| Buprenex Injection Ampule 0.3 mg/mL | Henry Schein Medical | 1181317 | Diatron US - 258 |
| Atropine Sulfate Injection SDV 0.4 mg/mL | Henry Schein Medical | 2580069 | American Regent Inc - 00517040125 |
| Artificial Tears Lubricating Ophthalmic Ointment Sterile | Henry Schein Medical | 1296576 | Geri-Care Pharmaceuticals - Q187-08 |
| Nair Hair Remover Aloe Vera/Lanolin Lotion | Henry Schein Medical | 1248837 | Church Dwight - 2709004 |
| Isoflurane Liquid Inhalation | Henry Schein Medical | 1182097 | Piramal Critical Care(RXElite) - 66794001725 |
| Sterile Saline Solutions | Medline Industries | PCS1650 |
| Povidone-Iodine PVP Prep Pads | Medline Industries | MDS093917 |
| Sterile Alcohol Prep Pads | Medline Industries | MDS090670 |
| Paraformaldehyde Solution, 4% in PBS | Thermo Scientific | J19943K2 |
| PBS Tablets, Phosphate Buffered Saline | Fisher Bioreagents | BP2944100 |
| Krebs-Henseleit Buffer Modified | Sigma-Aldrich | K3753-10L |
| Heparin sodium salt | Sigma-Aldrich | H3149-250KU |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL6J | The Jackson Laboratory | Stock# 000664 |
| Other | ||
| 10 μL, Neuros Syringe, Model 1701 RN, 33 gauge | Hamilton | 65460-06 |
| Sub-Microliter Injection System | World Precision Instruments | 10 μL (NANOFIL) |
| Nanofil Application Kits | World Precision Instruments | Beveled (IO-KIT) |
| 36 gauge Beveled NanoFil needle | World Precision Instruments | NF36BV-2 |
| Scalpel Surgical #10 Sterile Disposable | Henry Schein Medical | 1126189 | Henry Schein Inc. — 4319 |
| Sterile 100% Cotton Woven Gauze Sponges | Medline Industries | NON21420 |
| Disposable Drapes | Medline Industries | NON21001 |
| Monomid Nylon Suture | CP Medical | CPQ1666B |
| Magnetic Fixator Retraction System | Fine Science Tools | 18200-10 |
| Fine Scissors - Sharp | Fine Science Tools | 14060-09 |
| Spinal Cord Hook | Fine Science Tools | 10162-12 |
| Forceps | Fine Science Tools | 11911-11 |
| Vannas Spring Scissors | Fine Science Tools | 15071-08 |
| Bone Cutting Forceps | Fine Science Tools | Dumont #15a |
| Remote Infuse/Withdraw Pump 11 Elite Nanomite Programmable Syringe Pump | Harvard Apparatus | 70-4507 |
| Micromanipulator | Miller Design | P-10 |
| Heated Pad | Harvard Apparatus | 34-1446 |
| Precision Stereo Zoom Binocular Microscope (IV) on Boom Stand | World Precision Instruments | PZMIV-BS |
| Isoflurane Vaporizer | VetEquip | 911103 |
| Induction Chamber | VetEquip | 941443 |
Step-by-step method details
Part I. JNG virus infection/chemical delivery
Timing: 1 h
Timing: 30 min for preoperative preparation
Timing: 30 min for JNG intraganglionic injection
-
1.Preoperative preparation. Preoperative drug administration, anesthesia induction, and surgical site preparation.
-
a.Weigh the animal.
-
b.Administer preoperative analgesia: Subcutaneous administration (s.c.) of 0.05 mg/Kg Buprenorphine and anticholinergics: 0.1 mg/Kg Atropine (s.c.), 15 mins before starting surgery.
-
c.Place the animal inside an induction chamber with 3% isoflurane for 1 min. Then maintain the isoflurane to 1.5% after transferring the animal to the surgical platform.
-
d.Apply ophthalmic ointment to both eyes.
-
e.Use tape to fix the animal on a supine position and spread the hair-removal cream from chin to thorax. Five minutes later, wipe the cream and hair away using saline pads.
-
f.Disinfect the surgical area with povidone-iodine prep pads and wipe the area with 75% ethanol pads, three times.
-
a.
Note: Fresh dilute Buprenorphine stock solution (0.3 mg/mL) with 0.9% saline into working solution (0.015 mg/mL). Storage of Buprenorphine dilutions is forbidden.
Note: Dilute Atropine stock solution (0.4 mg/mL) with 0.9% saline into working solution (0.02 mg/mL). Atropine dilutions can be kept for six month at room temperature.
CRITICAL: 6–8 weeks old, 20–25 g males and females mice are optimal for JNG surgery. Excessive adipose tissue surrounding the carotid sheath in an overweight animal will obstruct the surgical vision and possibly lead to an imprecise approach followed by hemorrhage.
-
2.
JNG intraganglionic injection.
This part involves retracting the submandibular glands, and then the sternocleidomastoid and omohyoid muscles, to expose the carotid sheath. Second, the vagus nerve should be detached from the carotid artery until the JNG becomes accessible. Then 500 nL of CCK-SAP, at 2 nL/s, can be delivered with the Nanofil Sub-Microliter Injection System mounted on the programmable syringe pump. In detail:-
a.To expose the JNG (Figure 3 and Methods video S1).
-
i.Cut a midline incision ① using a scalpel to expose the submandibular glands ② (Figure 3A).
-
ii.Bilaterally retract the submandibular glands ② outwards to expose the trachea (Figure 3B).
-
iii.Laterally retract the sternocleidomastoid muscle ③ (Figure 3C).
- iv.
- v.
-
vi.Open the carotid sheath rostrally until fully exposing the vagus root and superior laryngeal branch ⑥ (Figure 3H).
-
vii.Carefully remove the connective tissue surrounding the vagus root ⑥ rostral to the superior laryngeal branch to expose the superior cervical ganglion ⑦ (Figure 3I).
-
viii.Cautiously tear off the brown adipose tissue ⑧, which is located on the ventral side of the superior cervical ganglion ⑦, to uncover the roots of the hypoglossal nerve ⑨ and the vagus nerve ⑥ (Figures 3J and 3K).
-
ix.Pull the superior cervical ganglion ⑦ rostrally to reveal the hypoglossal nerve ⑨ and the vagus nerve ⑥ (Figures 3L and 3M).
-
x.Detach the hypoglossal nerve ⑨ away from the vagus nerve ⑥ using a spinal cord hook (Figure 3N) to gain a clear view of the JNG swellings ⑩, which are located immediately adjacent to the base of the skull where the vagus nerve exits ⑥ (Figure 3O).
 CRITICAL: Immerse all surgical tools into saline before contacting any carotid arteries or cranial nerves. Frequently drop saline into the surgical area to maintain it hydrated.
CRITICAL: Immerse all surgical tools into saline before contacting any carotid arteries or cranial nerves. Frequently drop saline into the surgical area to maintain it hydrated.
Methods video S1. JNG intraganglionic injection, related to part I, step 2Download video file (20.5MB, flv) -
i.
-
b.Gently lower and insert the 36G beveled NanoFil needle [WPI, NF36BV-2] into the JNG swellings ⑩ with the micropositioner [Miller Design, P-10]. Then, infuse 200 nL CCK-SAP at 2 nL/s (Figure 3P).
-
c.Close the skin with a sterile suture and apply povidone-iodine.
-
d.Transfer the animal into a clean cage warmed at 37°C.
-
e.Apply post-operative analgesia, carprofen, 5 mg/Kg (s.c.) twice/day for three consecutive days.
-
a.
Note: Dilute Carprofen stock solution (50 mg/mL) with 0.9% saline into working solution (0.5 mg/mL). Keep Carprofen stock solution under refrigeration 2°C–8°C. Diluted Carprofen should be stored refrigerated and disposed of after 30 days.
Pause point: Suspend the vagal surgery procedure immediately upon any signs of cardiorespiratory irregularity.
CRITICAL: For post-operative days 1 through 7, mice (6–8 weeks old, 20–25 g) typically recover from surgery satisfactorily. The animals reaching 95% of pre-operative body weight or over, and behaving normally in their home cages without displaying any signs of pain or distress, are ready for behavioral testing and further analyses.
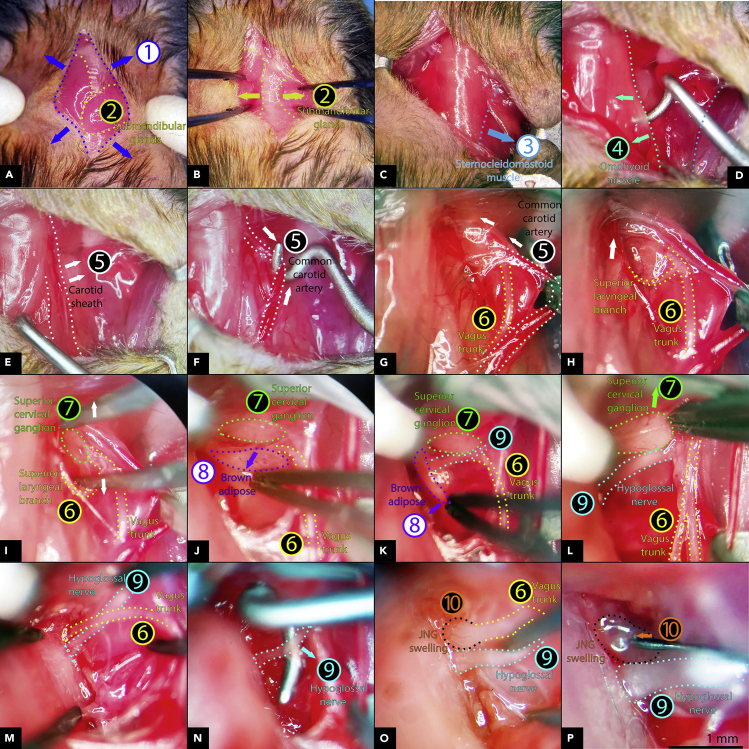
Figure 3.
Intraganglionic CCK-SAP delivery to JNG
(A) Midline incision ① to expose the submandibular glands ②.
(B) Expose of the trachea by retracting the submandibular glands ② sideways.
(C–E) Retract the sternocleidomastoid muscle laterally ③(C) and the omohyoid muscle medially ④ (D) to expose the carotid sheath ⑤(E).
(F) Penetrate the carotid sheath with blunt a spinal cord hook and separate the common carotid artery (⑤ dotted lines) from the carotid.
(G and H) Slide the spinal hook along the common carotid artery toward the skull base and tear the rostral carotid sheath and connective tissue to uncover the vagus trunk and superior laryngeal branch ⑥.
(I–K) Carefully shred down the brown adipose tissue ⑧ to gain access to the superior cervical ganglion ⑦ and the root of the hypoglossal nerve ⑨.
(L and M) Push the superior cervical ganglion ⑦ rostrally to expose the hypoglossal nerve roots ⑨ and the vagus nerve ⑥.
(N) Separate and pull the hypoglossal nerve ⑨ caudally away from the vagus nerve root with a spinal cord hook.
(O) Clean view of the JNG swelling ⑩ rostral to the hypoglossal nerve ⑨ and immediately adjacent to the cranium.
(P) Insert NanoFil needle tip medially into JNG ⑩, Scale bar, 1 mm.
Part II. Post-perfusion JNG dissection
Timing: 3 work days
-
3.Transcardiac perfusion.
-
a.Induce and maintain the animal into deep anesthesia with 3% isoflurane.
-
b.Transcardially perfuse 50 mL of a saline + heparin (10U/L) solution, followed by 50 mL ice-cold 4% PFA dissolved in PBS. Dissect the skull and cervical tissues.
-
c.After 5-h post perfusion fixation in PFA, thoroughly wash the tissue with PBS. Rinse the tissue with sufficient PBS for 3 h, three times.
-
a.
-
4.JNG dissection.
-
a.Remove excess skin and adipose tissue (Figure 4A).
- b.
-
c.Discriminate the light-colored jugular foramen ④ from the hypoglossal canal ③. Note that the vagus and hypoglossal nerves pass through the jugular foramen and the hypoglossal canal, respectively, after exiting the medulla oblongata (Figure 4C).
-
d.Split the occipital bone ① along the midline, and then remove the temporal bone ② away from the occipital bone ① (Figures 4C–4E).
-
e.Expose and dissect the vagus nerve and JNG ⑤ using Vannas spring scissors [FST, 15071-08] (Figures 4F–4I).
-
a.
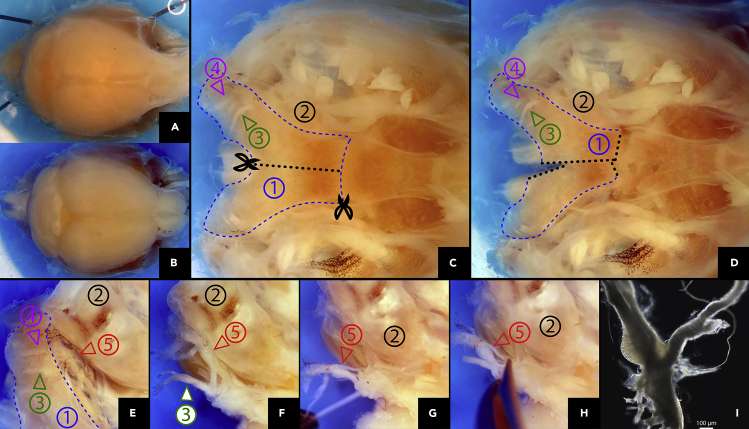
Figure 4.
Post-perfusion JNG dissection
(A and B) Truncate the cervix between the first and second cervical spinal cord segments (A) and cut the skullcap (B).
(C) Remove the brain and spinal cord, expose the skull's upper surface, locate the occipital bone ①, the temporal bone ②, the hypoglossal canal ③, and the jugular foramen ④.
(D) Cut the occipital bone ① along the midline (black dotted line).
(E) Push the occipital bone ① away from the temporal bone ② to expose the vagus nerve and the JNG ⑤. (F-H), Isolate the vagus nerve and JNG ⑤ using Vannas spring scissors. (I), Bright-field image of dissected JNG, scale bar, 100 μm.
Expected outcomes
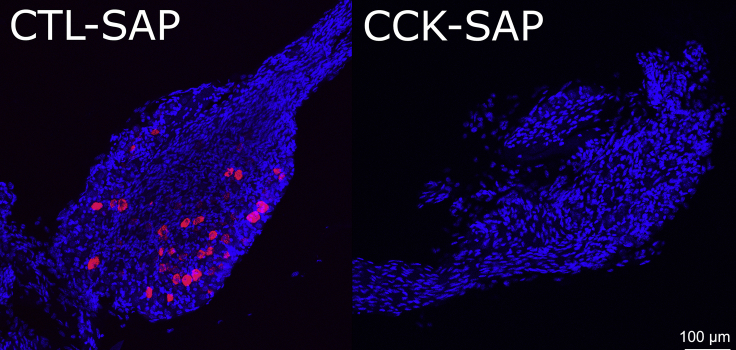
The CCK-SAP intraganglionic injections can efficiently eliminate ganglionic CCK receptor-positive neurons from JNG within 48 h. Animals will be able to receive the contralateral injection one week after the first unilateral injection. Animals can perform behavior tasks one week after the second unilateral CCK-SAP administration, and be insensitive to CCK-induced satiety. Molecular analysis reveals over 95% apoptosis of CCK receptor-positive cells, as shown in Figure 5.
Figure 5.
Validation of intra-JNG surgical approach based on CCKAR gene expression
Efficiency of CCK-SAP-induced CCK receptor cell apoptosis in JNG as shown by RNAScope detection. Left, CCKRA (red) expression in control animal injected with unconjugated saporin in JNG; Right, complete CCKRA cell elimination in animal injected with CCK-SAP in JNG, scale bar, 100 μm. Note the procedure preserves ganglia.
Limitations
While the nodose ganglia are located outside the skull, the jugular ganglia are located deep within the jugular foramina. This is a site where getting access to may be considerably difficult. So, injections primarily targeting the jugular ganglia are not ideal for this protocol. We do not recommend pulling the vagus nerve away from the skull, which will inevitably trigger parasympathetic overtone, including cardiac arrest due to sinoatrial node inhibition.
Troubleshooting
Problems 1–4 are related to “part I, step 2. JNG intraganglionic injection”
Problem 1
In overweight animals, excessive adipose tissue surrounding the carotid sheath is likely to obstruct the surgical field, leading to potential mistargeting that may result in hemorrhage.
Potential solution
6–8 weeks old, 20–25 g mice of either sex are optimal for JNG surgery. Make sure the tips of all surgical tools are blunt. Employ a stereomicroscope and sufficient illumination to obtain a sharp surgical view.
Problem 2
Long term exposure and dehydration of the surgical area can irritate the vagus nerve and carotid body, which may lead to sudden heart malfunction.
Potential solution
Preoperative administration of atropine. Immerse all surgical tools into saline and apply saline using cotton tips to moisture the vagus nerve and arteries. Minimize the duration of the surgery. Immediately pause the procedure upon observing any signs of cardiorespiratory irregularity. If bilateral JNG manipulation is necessary, consider scheduling the staged surgery for contralateral side one week apart from each other to reduce complications.
Problem 3
Especially for surgeons unexperienced in this procedure, mechanical pressure on the vagus nerve crossing through the JNG may lead to cardiovascular/respiratory failure.
Potential solution
Select sharp needle tips having outer diameters less than 50 μm; alternatively, utilize glass pipettes, and reduce infusion rates.
Problem 4
The glossopharyngeal nerves insert into the base of the skull, and enter the jugular foramina along with the vagus nerve. The petrosal ganglia, which is composed of sensory neurons innervating the posterior third of the tongue and the carotid sinus/body, reside in the jugular foramina. In some cases, the petrosal ganglion merges with the jugular ganglion to form a petrosal-jugular-nodose complex. Injecting into the nodose ganglia chemicals that can be absorbed by neuronal fibers, such as lipophilic dyes, may contaminate the petrosal ganglia.
Potential solution
The “Nodose” part of the “petrosal-jugular-nodose complex” resides outside the skull, and thus can be clearly distinguished from the glossopharyngeal nerve. Pulling the glossopharyngeal nerve with a blunt hook away from the vagus nerve during the JNG injection can minimize chemical diffusion into the glossopharyngeal nerve. Reduce the infusion rate and maintain the needle tip within the JNG for a short period of time (e.g., 5 minutes) after stopping the infusion. This can eliminate the leakage into the glossopharyngeal nerve. It is advisable to try multiple infusate concentrations and volumes to assure that the injections are restricted to the nodose ganglion proper.
Problem 5 (related to “part II. post-perfusion JNG dissection”)
Single cell RNA sequence and RNAscope protocol require fresh tissue dissection without PFA fixation.
Potential solution
The procedure can be modified for fresh tissue dissection:
Perfuse transcardiacally the animal using an ice-cold Krebs-Henseleit buffer solution and transect and immerse the head immediately into the same ice-cold Krebs-Henseleit buffer solution. Then, dissect the JNG as described in the protocol.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Wenfei Han, MD, PhD Wenfei.Han@mssm.edu
Materials availability
This study did not generate new unique reagents.
Data and code availability
The published article includes all [datasets/code] generated or analyzed during this study.
Acknowledgments
This work was supported by the US National Institutes of Health grants R01DC014859 and by the Food Allergy Science Initiative (FASI).
Author contributions
W.H. and I.E.d.A. conceived the study. W.H. performed JNG injections and dissections. W.H. and I.E.d.A. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100474.
References
- Altschuler S.M., Bao X.M., Bieger D., Hopkins D.A., Miselis R.R. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J. Comp. Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Baker C.V., Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev. Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A., Noden D.M. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am. J. Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Diepenbroek C., Quinn D., Stephens R., Zollinger B., Anderson S., Pan A., De Lartigue G. Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;313 doi: 10.1152/ajpgi.00095.2017. G342–g352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard Y., Vassileva G., McMahon A.P. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- Fode C., Gradwohl G., Morin X., Dierich A., Lemeur M., Goridis C., Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Han W., Tellez L.A., Perkins M.H., Perez I.O., Qu T., Ferreira J., Ferreira T.L., Quinn D., Liu Z.W., Gao X.B. A neural circuit for gut-induced reward. Cell. 2018;175:665–678.e23. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M., Sullivan J.M. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Hadley S.H., Maddison M., Patil M., Cha B., Kollarik M., Taylor-Clark T.E. Mapping of sensory nerve subsets within the vagal ganglia and the brainstem using reporter mice for Pirt, TRPV1, 5-HT3, and Tac1 expression. eNeuro. 2020;7 doi: 10.1523/ENEURO.0494-19.2020. ENEURO.0494-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari J., Häring M., Agirre E., Castelo-Branco G., Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019;27:2508–2523.e4. doi: 10.1016/j.celrep.2019.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Krieger J.P., Vergara M., Quinn D., McDougle M., De Araujo A., Darling R., Zollinger B., Anderson S., Pan A. Blunted vagal cocaine- and amphetamine-regulated transcript promotes hyperphagia and weight gain. Cell Rep. 2020;30:2028–2039.e4. doi: 10.1016/j.celrep.2020.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Chen Z., Del Barco Barrantes I., De La Pompa J.L., Anderson D.J. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Mazzone S.B., Undem B.J. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 2016;96:975–1024. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern A.E., Davis-Poynter N., Farrell M.J., Mazzone S.B. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience. 2012;207:148–166. doi: 10.1016/j.neuroscience.2012.01.029. [DOI] [PubMed] [Google Scholar]
- McGovern A.E., Driessen A.K., Simmons D.G., Powell J., Davis-Poynter N., Farrell M.J., Mazzone S.B. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J. Neurosci. 2015;35:7041–7055. doi: 10.1523/JNEUROSCI.5128-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C., Taylor-Clark T.E., Myers A.C., Ru F., Nandigama R., Bettner W., Undem B.J. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J. Physiol. 2010;588:4769–4783. doi: 10.1113/jphysiol.2010.195339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi M., Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog. Cardiovasc. Dis. 2008;50:404–419. doi: 10.1016/j.pcad.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all [datasets/code] generated or analyzed during this study.