Abstract
Objectives:
HPTN 075 enrolled men who have sex with men (MSM) and transgender women (TGW) in sub-Saharan Africa. Persons in HIV care or on antiretroviral treatment (ART) were not eligible to enroll. We evaluated antiretroviral (ARV) drug use, viral suppression, and drug resistance in this cohort over a 12-month follow-up period.
Methods:
Assessments included 64 participants with HIV (39 MSM, 24 TGW, and one gender not specified). ARV drugs were detected using a qualitative assay. Viral load (VL) and drug resistance testing were performed using commercial assays.
Results:
Over 12 months, the proportion of participants using ARV drugs increased from 28.1% to 59.4% and the proportion with VLs <400 copies/mL increased from 21.9% to 57.8%. The rate of ART failure (detection of drugs without viral suppression) was similar at screening and 12 months (12.0% and 11.1%, respectively) and was similar among MSM and TGW. Two participants developed HIV drug resistance during follow-up.
Conclusions:
Over 12 months, ARV drug use in the cohort more than doubled and viral suppression increased nearly threefold without a significant increase in ART failure or drug resistance. These results suggest that ART can be successfully scaled up for HIV prevention and treatment in this high-risk population.
Keywords: HIV, Antiretroviral drugs, HIV drug resistance, Viral suppression, Men who have sex with men, Transgender women, Sub-Saharan Africa
Introduction
Access to antiretroviral treatment (ART) continues to increase in sub-Saharan Africa (SSA), which reduces HIV transmission and HIV-related morbidity and mortality (Bor et al., 2013; Cohen et al., 2011; Granich et al., 2009). As in other regions, ART outcomes in SSA are impacted by ART adherence and HIV drug resistance (Gupta et al., 2012; Hogg et al., 2002; Lippman et al., 2020). Drug resistance has a greater impact on ART outcomes in regions of SSA and other resource-limited settings where drug resistance testing is not routinely used to guide HIV treatment (Crowell et al., 2020).
In SSA and elsewhere, men who have sex with men (MSM) and transgender women (TGW) have a disproportionately high HIV burden (Beyrer et al., 2012). In SSA, HIV prevalence among MSM and TGW has been reported at rates of 18%–55% (Crowell et al., 2020; Keshinro et al., 2016; Sandfort et al., 2015; Wirtz et al., 2017). Reported HIV incidence rates among MSM and TGW in SSA are also higher than those seen in the general population (6.8 cases per 100 person-years (PY) among MSM (Price et al., 2012) and 8.6 cases per 100 PY among MSM and TGW (Sanders et al., 2013) as compared to 0.6–3.5 cases per 100 PY in the general population (Braunstein et al., 2009)).
MSM and TGW suffer from stigmatization and criminalization in many regions of SSA, which negatively impacts access to HIV testing and treatment (Scheim et al., 2019; Schwartz et al., 2015; Stahlman et al., 2016). In studies from SSA, 0%–30% of MSM living with HIV were on ART (Charurat et al., 2015; Holland et al., 2015), as compared to 64%–67% of people living with HIV in the general population in Eastern and Southern Africa (UNAIDS – Fact sheet 2019). Rates of viral suppression are also low among MSM in Africa (estimated at 25% (Stannah et al., 2019)), as compared to MSM in Europe and Asia (63%–83% (Brown et al., 2018)). Information on HIV drug resistance among MSM in Africa is limited. In prior studies from Kenya and Nigeria, the rates of drug resistance among MSM who reported that they were ART-naive or initiating ART were 8.2% (Hassan et al., 2018) and 9.7% (Crowell et al., 2020), respectively. The study from Nigeria also detected drug resistance in 43% of ART-experienced MSM (Crowell et al., 2020). Further studies are needed to assess ART, viral suppression, and drug resistance in among MSM and TGW in Africa.
HIV Prevention Trials Network (HPTN) 075 was an observational cohort study that evaluated the feasibility of enrolling and retaining MSM and TGW at four study sites in three SSA countries (Kisumu, Kenya; Blantyre, Malawi; Cape Town and Soweto, South Africa), in preparation for future HIV prevention trials (Sandfort et al., 2020). The study enrolled both persons who were living with HIV and persons without HIV. Persons who reported that they had a previous positive HIV test were asked about their engagement in HIV care and treatment and were provided with information about the benefit of ART for HIV prevention; those who reported that they were on ART at follow-up visits received adherence counseling. In a previous study, retrospective antiretroviral (ARV) drug testing revealed that 63 (34.4%) of 183 persons with HIV who were screened for HPTN 075 were taking ARV drugs; 11 (17.5%) of those persons were not virally suppressed (Zhang et al., 2018). Many of those persons did not report being aware of their HIV status or that they were on ART at the screening visit. In this study, we analyzed ARV drug use, viral suppression, and HIV drug resistance at study screening and after 12 months of study engagement among MSM and TGW in HPTN 075.
Methods
Study cohort
In HPTN 075, the enrollment of persons living with HIV was capped at 20/site. The study enrolled participants who were assigned male sex at birth. Persons who reported that they were in HIV care or treatment were not eligible to enroll. The study enrolled 401 participants, including 70 participants who tested positive for HIV infection. Participants were followed quarterly for 12 months. Study visits included interviews and physical exams. The analyses in this report included 64 participants with HIV who had samples available from the screening visit and the 12-month study visit (14 from Kisumu, 14 from Blantyre, 16 from Cape Town, and 20 from Soweto). The 64 participants included 39 participants who self-identified as male (MSM) and 24 participants who self-identified as transgender (TGW; 14 identified as transgender female, seven identified as female, and three who identified as a different gender type); one participant did not answer the question about gender identity. Six participants living with HIV were not included in the analyses because they had no 12-month visit.
Laboratory testing
Samples and data were collected in the main HPTN 075 study from June 2015 to July 2017. Retrospective testing for ARV drug use, HIV viral load (VL), and HIV genotyping was performed at the HPTN Laboratory Center (Johns Hopkins University, Baltimore, MD, USA). ARV drug testing was performed using a qualitative assay that detects drugs in five classes (nucleoside/nucleotide reverse transcriptase inhibitors [NRTIs], non-nucleoside reverse transcriptase inhibitors [NNRTIs], protease inhibitors [PIs], integrase inhibitors, and fusion inhibitors) (Zhang et al., 2017). Results from ARV testing at screening in HPTN 075 were reported previously (Sivay et al., 2020; Zhang et al., 2018). VL testing was performed by using the RealTime HIV-1 Viral Load Assay (Abbott Molecular, Des Plaines, IL) with a validated dilution protocol (lower limit of quantification: 400 copies/mL). Viral suppression was defined as having an HIV viral load HIV VL ≤ 400 copies/mL. HIV genotyping was performed for participants with VLs >400 copies/mL using the ViroSeq HIV-1 Genotyping System, v3.0 (Abbott Diagnostics, Des Plaines, IL).
Statistical analysis
The Chi-square test was used to compare the proportion of MSM vs. TGW who had ARV drugs detected or were virally suppressed. Logistic regression with an interaction term for gender and time was used to compare the increase in ARV drug use and viral suppression from baseline to 12 months in the two groups.
HIV sequences
HIV pol sequences generated with the ViroSeq system were submitted to GenBank (baseline: MG597250–MG597252, MG597256–MG597258, MT185161–MT185165, MT185167, MT185169, MT185171–MT185174, MT185176–MT185179, MT185181, MT185184–MT185186, MT185188, MT185190–MT185195, MT185213–MT185217, MT185220–MT185225, MT185227–MT185232, and MT185234; 12 months: MG597250–MG597252, MG597256–MG597258, MT185161–MT185165, MT185167, MT185169, MT185171–MT185174, MT185176–MT185179, MT185181, MT185184–MT185186, MT185188, MT185190–MT185195, MT185213–MT185217, MT185220–MT185225, MT185227–MT185232, MT185234, and.
Results
ARV drug use
ARV drugs were detected in 18 (28.1%) baseline samples and 38 (59.4%) 12-month samples (Figure 1). Most samples (47/56 [83.9%]) with ARV drugs detected had two NRTIs (tenofovir [TDF] with either lamivudine [3TC] or emtricitabine [FTC]) with one NNRTI (43 with efavirenz [EFV] and four with nevirapine [NVP]). No PIs were detected in baseline samples; two 12-month samples had a boosted PI (atazanavir/ritonavir [ATV/r]) detected with two NRTIs (TDF with 3TC or FTC). Seven samples had unexpected results: one baseline sample had NVP with 3TC only; four baseline samples and one 12-month sample had EFV alone; one baseline sample had two NNRTIs and two NRTIs detected (EFV, NVP, FTC, and TDF).
Figure 1. Proportion of participants with different study outcomes.
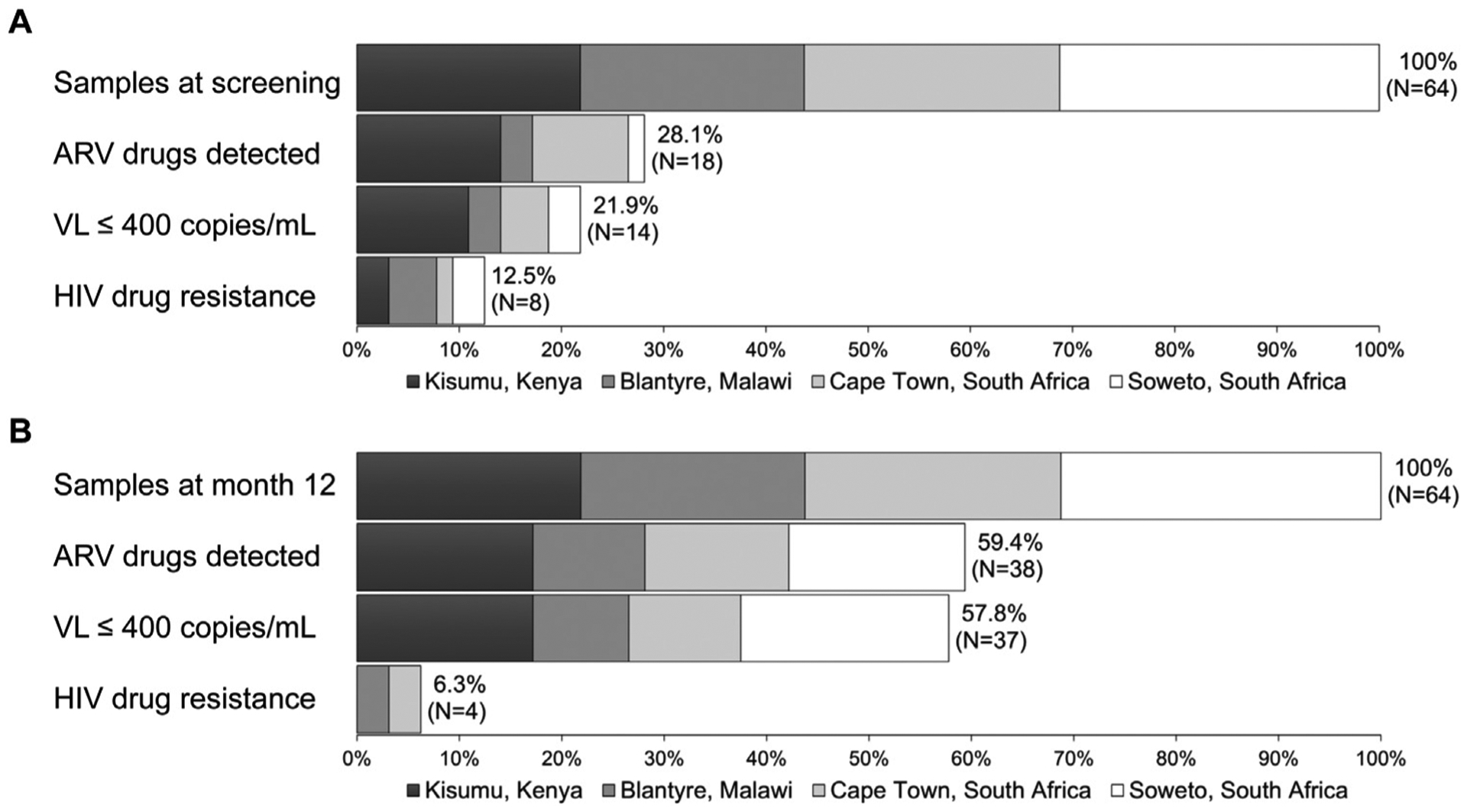
The panels show data for the 64 participants at screening (Panel A) and at month 12 (Panel B). HIV drug resistance testing was only performed for participants with viral loads >400 copies/mL. The proportion of participants with drug resistance in each group is shown for all participants; participants who were virally suppressed were classified as having no drug resistance. Abbreviations: ARV, antiretroviral; VL, viral load; and mL, milliliter.
Viral suppression
Among the 64 participants, 14 (21.9%) were virally suppressed at screening and 37 (57.8%) were virally suppressed at 12 months (Figure 1). Among those who were virally suppressed, 12/14 (85.7%) had at least one ARV drug detected at screening and 35/37 (94.6%) had at least one ARV drug detected at 12 months. One participant was virally suppressed at both visits in the absence of ARV drugs; this participant was classified as a viremic controller. Among the 50 participants who were not virally suppressed at screening, six (12.0%) had at least one ARV drug detected (one with EFV/3TC/TDF, one with NVP/3TC, and four with EFV alone). Among the 27 participants who were not virally suppressed at 12 months, three (11.1%) had at least one drug detected (two with EFV/FTC/TDF and one with EFV/3TC/TDF).
HIV drug resistance
HIV drug resistance testing was performed for samples with VLs >400 copies/mL. Ten (15.6%) of the 64 participants had one or more major drug resistance mutations detected (8/50 [16%] at screening and 4/27 [14.8%] at 12 months; Figure 1). Table 1 shows results from ARV drug testing and HIV drug resistance testing for these ten participants.
Table 1.
Results from antiretroviral drug testing and HIV drug resistance testing for participants who had HIV drug resistance at study entry (screening) or at the end of study follow-up (12 months).
| Start of study | End of study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Viral Load | NRTIs detected | NNRTIs detected | Major NRTI mutations | Major NNRTI mutations | Viral Load | NRTIs detected | NNRTIs detected | PIs detected | Major NRTI mutations | Major NNRTI mutations |
| 1 | 18,900 | 3TC | NVP | M184V | K103N | <400 | 3TC, TDF | - | ATV/r | N/A | N/A |
| 2 | 480 | 3TC, TDF | EFV | - | K103N | <400 | 3TC, TDF | EFV | - | N/A | N/A |
| 3 | 34,100 | - | - | - | K103N | <400 | 3TC, TDF | EFV | - | N/A | N/A |
| 4 | 1,180 | - | - | - | K103N | <400 | FTC, TDF | EFV | - | N/A | N/A |
| 5 | 8,440 | - | - | - | K103N | <400 | FTC, TDF | EFV | - | N/A | N/A |
| 6 | 43,200 | - | - | - | Y188C | 34,720 | - | - | - | - | - |
| 7 | 195,990 | - | - | - | K103N, G190A | 4,770 | - | - | - | - | K103N |
| 8 | 17,520 | - | EFV | M184I | K103N, V106M | 1,280 | FTC, TDF | EFV | - | L74V, M184V | K103N, V106M |
| 9 | 430 | - | EFV | - | - | 4,510 | FTC, TDF | EFV | - | K65R | K103N, Y188C |
| 10 | 48,100 | - | - | - | - | 21,910 | 3TC, TDF | EFV | - | K65R | K103N, V106M |
The table shows results of antiretroviral drug testing and HIV drug resistance testing. Testing was performed at the start of the study (screening visit) and at the end of the study (12-month visit). Results are shown only for participants who had HIV drug resistance mutations detected at one or both visits. HIV viral load values are shown as copies/mL. HIV drug resistance testing was not performed at 12 months for five virally suppressed participants (N/A).
Abbreviations: NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate; FTC, emtricitabine; NVP, nevirapine; EFV, efavirenz; ATZ, atazanavir; and r, ritonavir.
Among the eight participants with drug resistance at screening (cases 1–8), six had NNRTI resistance only and two had multiclass resistance (NNRTI plus NRTI resistance). Among the four participants with drug resistance at 12 months (cases 7–10), one had NNRTI resistance only and three had multiclass resistance (NNRTI plus NRTI resistance). None of the participants had PI resistance.
Among the eight participants who had drug resistance at screening, five were virally suppressed at 12 months (cases 1–5); at 12 months, four were on a regimen of EFV and TDF with either 3TC or FTC; and one was on a regimen with a boosted PI (ATV/r) with TDF and 3TC. The remaining three participants were still viremic at the end of the study (cases 6–8). ARV drugs were detected in only one of those cases at 12 months (EFV/TDF/FTC, case 8). In the two participants not on ART (cases 6 and 7), one or more of the resistance mutations present at screening was no longer detected; the participant who was still on ART (case 8) acquired an additional NRTI mutation during the study. Two additional participants had no resistance at screening (cases 9 and 10), but had multiclass resistance at the end of study. In both cases, the participants had NNRTI resistance (K103N with Y188C or V106M) and NRTI resistance (K65R, which confers resistance to TDF and other drugs). Of note, five of the eight participants who had drug resistance at screening did not have ARV drugs detected (Table 1, cases 3–7); this corresponds to an overall resistance rate of 7.8% among the 64 participants included in the study. In all five cases, the mutations detected conferred resistance to NNRTIs (three had K103N alone, one had Y188C alone, and one had K103N plus G190A). Three of the five participants were on ART and were virally suppressed at 12 months; NNRTI resistance was still detected in one of the remaining two participants at 12 months (Table 1, case 7).
Outcomes by study site and gender identity
The frequency of ARV drug use at screening varied by study site (64.3% in Kenya, as compared to 37.5% in Cape Town, 14.3% in Malawi, and 5.0% in Soweto; Figure 1; Table 2). A similar trend was seen for viral suppression at screening (50.0% in Kenya as compared to 18.8% in Cape Town, 14.3% in Malawi, and 10.0% in Soweto). The frequency of ARV drug use and viral suppression increased over the 12-month follow-up period at all four sites (Figure 1 and Table 2). At 12 months, the frequency of ARV drug use ranged from 50.0% to 78.6% and the frequency of viral suppression ranged from 42.9% to 78.6%. The greatest increases over time were seen in Soweto, which had the lowest rate of ARV drug use and viral suppression at the start of the study; at this site, the rate of ARV drug use increased 11-fold (from 5.0% to 55.0%) and the rate of viral suppression 6.5-fold (from 10.0% to 65.0%).
Table 2.
Frequency of antiretroviral drug use and viral suppression at screening and at 12 months by study site
| Kisumu, Kenya (N = 14) | Blantyre, Malawi (N = 14) | Cape Town, South Africa (N = 16) | Soweto, South Africa (N = 20) | |
|---|---|---|---|---|
| ARV drug use | ||||
| ARV drug use at screening | 9/14 (64.3%) | 2/14 (14.3%) | 6/16 (37.5%) | 1/20 (5.0%) |
| ARV drug use at 12 months | 11/14 (78.6%) | 7/14 (50.0%) | 9/16 (56.3%) | 11/20 (55.0%) |
| Increase in ARV drug use | 1.2-fold | 3.5-fold | 1.5-fold | 11-fold |
| Viral suppression | ||||
| Virally suppressed at screening | 7/14 (50.0%) | 2/14 (14.3%) | 3/16 (18.8%) | 2/20 (10.0%) |
| Virally suppressed at 12 months | 11/14 (78.6%) | 6/14 (42.9%) | 7/16 (43.8%) | 13/20 (65.0%) |
| Increase in viral suppression | 1.6-fold | 3.0-fold | 2.3-fold | 6.5-fold |
The table shows the frequency of ARV drug use and viral suppression at screening and 12 months at each of the four study sites. The fold change increase in ARV drug use and viral suppression during the 12-month follow-up period is also shown.
Abbreviations: ARV, antiretroviral.
ARV drug use and viral suppression were also analyzed among MSM and TGW (Table 3). The increases in ARV drug use and viral suppression over the 12-month follow-up period were higher among participants who identified as male. However, these differences were not statistically significant.
Table 3.
Frequency of antiretroviral drug use and viral suppression at screening and at 12 months by gender identity.
| TGW (N = 24) | MSM (N = 39) | P-value | |
|---|---|---|---|
| ARV drug use | |||
| ARV drug use at screening | 8/24 (33.3%) | 10/39 (25.6%) | 0.511a |
| ARV drug use at 12 months | 15/24 (62.5%) | 23/39 (59.0%) | 0.781a |
| Increase in ARV drug use | 1.9-fold | 2.3-fold | 0.774b |
| Viral suppression | |||
| Virally suppressed at screening | 6/24 (25.0%) | 8/39 (20.5%) | 0.677a |
| Virally suppressed at 12 months | 15/24 (62.5%) | 22/39 (56.4%) | 0.634a |
| Increase in viral suppression | 2.5-fold | 2.8-fold | 0.997b |
The table shows the frequency of ARV drug use and viral suppression at screening and 12 months by gender identity. The fold change increase in ARV drug use and viral suppression during the 12-month follow-up period is also shown.
Abbreviations: TGW, transgender women; MSM, men who have sex with men; and ARV, antiretroviral.
Chi-Square p-value.
P-value from logistic regression.
Discussion
During 12 months of participation in the HPTN 075 study, the frequency of ARV drug use more than doubled (from 28.1% to 59.4%), and the rate of viral suppression increased nearly threefold (from 21.9% to 57.8%) without a significant increase in the frequency of treatment failure or drug resistance. Among those with ARV drugs detected, 33.3% had VLs ≥400 copies/mL at study entry as compared to only 7.9% at the end of the study, which indicates improved ART adherence.
Our prior study demonstrated that a high proportion of people with HIV, who were screened for participation in HPTN 075 did not report being aware of their HIV status (Fogel et al., 2019). ARV drug testing revealed that many of those individuals were on ART, which would have excluded them from study participation. This is consistent with our findings from other cohorts and settings that show that study participants often do not disclose that they are on ART (Fogel et al., 2013; Fogel et al., 2019; Marzinke et al., 2014). In this study, 28.1% of the persons tested at screening were using ARV drugs; in all but six of these cases, the pattern of ARV drugs detected was consistent with ART (five had only one or two ARV drugs detected and one had four ARV drugs detected). These persons did not report to being in HIV care or on ART to study staff. This highlights the importance of using an objective biomedical measure to assess ARV drug use. The frequency of ARV drug use among all 183 persons with HIV who were screened for participation in HPTN 075 (34.4% (Zhang et al., 2018)) and among the 64 enrolled participants in this study (28.1%) is consistent with a prior study of ART among MSM in Nigeria. In that study, which included both MSM and TGW, 31% of persons reported that they were on ART (Charurat et al., 2015). In a study of MSM from seven major cities in Cameroon that used several measures to assess ART, the frequency of ART ranged from 0% to 25% (Holland et al., 2015).
The rate of viral suppression among the 183 persons with HIV who were screened for HPTN 075 (32.2%, data not shown) was higher than the rate of 25% previously reported among MSM in SSA (Stannah et al., 2019). The rate was lower at screening among those included in this study (21.9%), which likely reflects exclusion from the enrollment of those who reported that they were in HIV care or treatment. The rate of viral suppression increased to 57.8% over 12 months of follow-up, which approaches the rate of 63–83% observed among MSM in Europe and Asia (Brown et al., 2018). In a few cases, participants who were taking ARV drugs were not virally suppressed; however, the frequency did not change significantly over the course of the study (12.0% at baseline and 11.1% at failure). Differences were observed between the four sites in the prevalence of ARV use and viral suppression at the start and end of the study, with the highest rates observed at the site in Kenya. These differences may have reflected differences in access to HIV care or other factors. All four sites referred participants with HIV to local clinics for ART, and HIV care at all sites included VL monitoring for those on ART. Availability of drug resistance testing varied among the sites, which may have impacted ART outcomes. The site in Soweto was the only site to offer HIV care on location, which may have contributed to Soweto having the greatest increase in ARV drug use and viral suppression during the course of the study.
HIV drug resistance was detected in samples from eight (12.5%) of the 64 participants at screening. In five of these cases, NNRTI resistance mutations were detected in the absence of ARV drugs; none of the five participants reported prior positive HIV test or prior ART. Some of these cases could represent transmitted drug resistance. Other studies of African MSM have reported rates of transmitted drug resistance of 8.2% (Kenya (Hassan et al., 2018)) and 9.7% (Nigeria (Crowell et al., 2020)). However, it is also possible that one or more of the five participants in our cohort had prior exposure to an NNRTI for reasons other than ART (e.g., recreational EFV use (Gatch et al., 2013; Rough et al., 2014)). In three of the five cases, the participants started ART during the study and were virally suppressed at 12 months.
Uptake of ART in this observational study demonstrates that obstacles such as stigma and criminalization of same-sex activities do not prohibit the use of ART in this high-risk population. In HPTN 075, stigma reduction and staff training on cultural sensitivities, along with the promotion of ART for prevention and adherence counseling for those who reported that they were on ART, may have contributed to this positive outcome. The low rates of ART failure and emergence of drug resistance during the course of the HPTN 075 study provide further hope for the successful scale-up of ART uptake among MSM and TGW in SSA. Study sites in HPTN 075 provided referral for ART and other support services, but did not provide ART or peer navigation to treatment services. Additional interventions would likely further increase the proportion of MSM and TGW who are virally suppressed on ART, helping to meet 95-95-95 targets for ending the HIV/AIDS epidemic by 2030 (Granich et al., 2018).
A few limitations of the study should be noted. The rate of viral suppression in this study may be overestimated as some persons with VLs <400 copies/mL may have had levels of HIV RNA detected with more sensitive methods. In this study, the ARV drugs detected in most cases included one NNRTI and two NRTIs, which is consistent with ART regimens offered regionally at the time the study was performed. Failure to detect two NRTIs in some cases may have reflected suboptimal ART adherence, and the detection of EFV alone could represent recreational EFV use (Gatch et al., 2013; Rough et al., 2014). The proportion of those on ART may also have been higher than reported, because ARV drugs may not have been detected in those with intermittent ARV drug use. Finally, this study did not evaluate demographic, behavioral, and clinical factors associated with ART uptake and viral suppression in HPTN 075. Those analyses will be described in a separate report and will help inform public health programs and intervention studies using ART for HIV prevention and treatment in MSM and TGW in SSA.
Acknowledgments
The authors thank the HPTN 075 study team and participants for the provision of samples and data used in this study. We also thank the laboratory staff who helped with sample management and testing.
Funding
This work was supported by the grants from the Division of AIDS Research of the U.S. National Institutes of Mental Health (NIMH)and the Division of AIDS of the U.S. National Institute of Allergy and Infectious Diseases (NIAID) [Nos. UM1-AI068613 (Eshleman), UM1-AI068617 (Donnell), and UM1-AI068619 (Cohen/El-Sadr)]. Dr. Sandfort received additional support from a NIMH center grant [No. P30-MH43520 (Remien)].
Footnotes
Competing interests
None of the authors have a financial or personal relationship with other people or organizations that could inappropriately influence (bias) their work, with the following exceptions: Susan Eshleman has collaborated on research studies with investigators from Abbott Diagnostics and Abbott Diagnostics has provided reagents for collaborative research studies.
Ethics statement
Approval for the HPTN 075 study was obtained from the institutional review boards (IRBs) at the study sites. Informed consent was obtained from all participants at study screening and study enrollment. Written consent was obtained at three study sites; at one site, oral consent was obtained to avoid inadvertent disclosure, at the direction of the local IRB.
References
- Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012;380(9839):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013;339 (6122):961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein SL, van de Wijgert JH, Nash D. HIV incidence in sub-Saharan Africa: a review of available data with implications for surveillance and prevention planning. AIDS Rev 2009;11(3):140–56. [PubMed] [Google Scholar]
- Brown AE, Attawell K, Hales D, Rice BD, Pharris A, Supervie V, et al. Monitoring the HIV continuum of care in key populations across Europe and Central Asia. HIV Med 2018; [DOI] [PubMed] [Google Scholar]
- Charurat ME, Emmanuel B, Akolo C, Keshinro B, Nowak RG, Kennedy S, et al. Uptake of treatment as prevention for HIV and continuum of care among HIV-positive men who have sex with men in Nigeria. J Acquir Immune Defic Syndr 2015;68 (Suppl 2):S114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell TA, Kijak GH, Sanders-Buell E, O’Sullivan AM, Kokogho A, Parker ZF, et al. Transmitted, pre-treatment and acquired antiretroviral drug resistance among men who have sex with men and transgender women living with HIV in Nigeria. Antivir Ther 2020;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel JM, Wang L, Parsons TL, Ou SS, Piwowar-Manning E, Chen Y, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV Prevention Trials Network 052). J Infect Dis 2013;208(10):1624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel JM, Zhang Y, Palumbo PJ, Guo X, Clarke W, Breaud A, et al. Use of antiretroviral drug testing to assess the accuracy of self-reported data from HIV-infected people who inject drugs. AIDS Behav 2019;23(8):2101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Kozlenkov A, Huang RQ, Yang W, Nguyen JD, Gonzalez-Maeso J, et al. The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacology 2013;38(12):2373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granich R, Gupta S, Wollmers M, Ruffner M, Williams B. Modeling the HIV Epidemic: Why the 95-95-95 Target and ART Effectiveness Parameters Matter. Int J Virol AIDS 2018;5(041). [Google Scholar]
- Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009;373(9657):48–57. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012;380(9849):1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AS, Esbjornsson J, Wahome E, Thiong’o A, Makau GN, Price MA, et al. HIV-1 subtype diversity, transmission networks and transmitted drug resistance amongst acute and early infected MSM populations from Coastal Kenya. PLoS One 2018;13(12)e0206177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O’Shaughnessy MV, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS 2002;16(7):1051–8. [DOI] [PubMed] [Google Scholar]
- Holland CE, Papworth E, Billong SC, Tamoufe U, LeBreton M, Kamla A, et al. Antiretroviral treatment coverage for men who have sex with men and female sex workers living with HIV in Cameroon. J Acquir Immune Defic Syndr 2015;68 (Suppl 2):S232–40. [DOI] [PubMed] [Google Scholar]
- Keshinro B, Crowell TA, Nowak RG, Adebajo S, Peel S, Gaydos CA, et al. High prevalence of HIV, chlamydia and gonorrhoea among men who have sex with men and transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc 2016;19(1):21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman SA, Mooney AC, Puren A, Hunt G, Grignon JS, Prach LM, et al. The role of drug resistance in poor viral suppression in rural South Africa: findings from a population-based study. BMC Infect Dis 2020;20(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzinke MA, Clarke W, Wang L, Cummings V, Liu TY, Piwowar-Manning E, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis 2014;58(1):117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MA, Rida W, Mwangome M, Mutua G, Middelkoop K, Roux S, et al. Identifying at-risk populations in Kenya and South Africa: HIV incidence in cohorts of men who report sex with men, sex workers, and youth. J Acquir Immune Defic Syndr 2012;59(2):185–93. [DOI] [PubMed] [Google Scholar]
- Rough K, Dietrich J, Essien T, Grelotti DJ, Bansberg DR, Gray G, et al. Whoonga and the abuse and diversion of antiretrovirals in Soweto, South Africa. AIDS Behav 2014;18(7):1378–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders EJ, Okuku HS, Smith AD, Mwangome M, Wahome E, Fegan G, et al. High HIV-1 incidence, correlates of HIV-1 acquisition, and high viral loads following seroconversion among MSM. AIDS 2013;27(3):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandfort TGM, Hamilton EL, Marais A, Guo X, Sugarman J, Chen YC, et al. The feasibility of recruiting and retaining men who have sex with men and transgender women in a multinational prospective HIV prevention research cohort study in sub-Saharan Africa (HPTN 075). J Int AIDS Soc 2020;22(S6): e25600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandfort TG, Lane T, Dolezal C, Reddy V. Gender expression and risk of HIV infection among black South African men who have sex with men. AIDS Behav 2015;19 (12):2270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheim A, Lyons C, Ezouatchi R, Liestman B, Drame F, Diouf D, et al. Sexual behavior stigma and depression among transgender women and cisgender men who have sex with men in Cote d’Ivoire. Ann Epidemiol 2019;33: 79–83 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SR, Nowak RG, Orazulike I, Keshinro B, Ake J, Kennedy S, et al. The immediate eff ect of the Same-Sex Marriage Prohibition Act on stigma, discrimination, and engagement on HIV prevention and treatment services in men who have sex with men in Nigeria: analysis of prospective data from the TRUST cohort. Lancet HIV 2015;2(7):e299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivay M, Palumbo PJ, Zhang Y, Cummings V, Guo X, Hamilton EL, et al. HIV drug resistance, phylogenetic analysis, and superinfection among men who have sex with men and transgender women in sub-Saharan Africa: HPTN 075. 2020. [DOI] [PMC free article] [PubMed]
- Stahlman S, Liestman B, Ketende S, Kouanda S, Ky-Zerbo O, Lougue M, et al. Characterizing the HIV risks and potential pathways to HIV infection among transgender women in Cote d’Ivoire, Togo and Burkina Faso. J Int AIDS Soc 2016;19(3 Suppl 2):20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannah J, Dale E, Elmes J, Staunton R, Beyrer C, Mitchell KM, et al. HIV testing and engagement with the HIV treatment cascade among men who have sex with men in Africa: a systematic review and meta-analysis. Lancet HIV 2019;6(11) e769–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS – Fact Sheet 2019. Available from: https://www.unaids.org/en/resources/fact-sheet.
- Wirtz AL, Trapence G, Kamba D, Gama V, Chalera R, Jumbe V, et al. Geographical disparities in HIV prevalence and care among men who have sex with men in Malawi: results from a multisite cross-sectional survey. Lancet HIV 2017;4(6) e260–e9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Clarke W, Marzinke MA, Piwowar-Manning E, Beauchamp G, Breaud A, et al. Evaluation of a multidrug assay for monitoring Adherence to a regimen for HIV preexposure prophylaxis in a clinical study, HIV Prevention Trials Network 073. Antimicrob Agents Chemother 2017;61(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fogel JM, Guo X, Clarke W, Breaud A, Cummings V, et al. Antiretroviral drug use and HIV drug resistance among MSM and transgender women in sub-Saharan Africa. AIDS 2018;32(10):1301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


