Abstract
Objective:
The menopausal transition is characterized by progressive changes in ovarian function and increasing circulating levels of gonadotropins, with some women having irregular menstrual cycles well before their final menstrual period. These observations indicate a progressive breakdown of the hypothalamic-pituitary-ovarian axis often associated with an increase in menopausal symptoms. Relationships between vasomotor symptoms (VMS) and depressed mood and sleep as well as a bidirectional association between VMS and depressed mood in mid-life women have been reported, but the endocrine foundations and hormone profiles associated with these symptoms have not been well described. Our objective was to determine the relationship between daily urinary hormone profiles and daily logs of affect and VMS during the early perimenopausal transition.
Study Design:
SWAN, the Study of Women’s Health Across the Nation, is a large, mutli-ethnic, multisite cohort study of 3302 women aged 42–52 at baseline, designed to examine predictors of health and disease in women as they traversed the menopause. Inclusion criteria were: an intact uterus and at least one ovary present, at least one menstrual period in the previous three months, no use of sex steroid hormones in the previous three months, and not pregnant or lactating. A subset (n=849) of women aged 43–53 years from all study sites in the first Daily Hormone Study collection were evaluated for this substudy.
Outcome Measures:
We measured daily VMS, and urinary hormones: follicle stimulating hormone (FSH), luteinizing hormone (LH), pregnanediol glucuronide (PdG) and estradiol (estrone conjugate, E1C).
Results:
A variable pattern of LH and negative LH feedback were the hormone patterns most strongly associated with increased VMS. In contrast, no hormone pattern was significantly related to negative mood.
Conclusion:
Fluctuations of LH associated with low progesterone production were associated with VMS but not negative mood, suggesting different endocrine patterns may be related to increased negative mood than to the occurrence of VMS.
Keywords: Menopausal Transition, Vasomotor symptoms, Mood, Luteinizing Hormone, progesterone
1.0. Introduction
The menopausal transition (MT) is characterized by changes in ovarian function and increasing circulating gonadotropin levels [1–4] with almost 90% of women experiencing regular menstrual cycles up to the last five years before their final menstrual period (FMP) and over 20% retaining normal ovarian function within one year prior to their FMP [5]. Some women have a form of irregular menstrual cycles well before their FMP, indicating a progressive breakdown of the hypothalamic-pituitary-ovarian (HPO) axis with increased symptoms. Relationships between vasomotor symptoms (VMS) and depressed mood and sleep [6, 7] as well as a bidirectional association between VMS and depressed mood in mid-life women [8] have been reported. While such symptoms may be mediated by similar endocrine foundations, the daily hormone profiles associated with these symptoms have not been described in midlife women.
Daily urinary hormone metabolites have been used to evaluate changes occurring in ovarian function in mid-life women whose initial observed intermenstrual interval did not meet the Kassam Algorithm criteria for ovulation [9, 10]. These were assumed to be anovulatory and have been investigated for their association with co-occurring VMS. That investigation concluded that a loss of estrogen negative feedback was the prominent contributing feature to VMS (10). Subsequent analyses revealed that VMS reporting was significantly greater during intermenstrual intervals of length 50+ days with low or medium pregnanediol glucuronide (PdG) levels than in intermenstrual intervals of shorter duration with high or medium PdG [11]. The most recent analyses [5] included the full cohort (N=510), followed participants for a longer time interval, and reported that irregular intermenstrual intervals increased as the FMP approached. These irregular intermenstrual intervals were characterized as having lowered PdG profiles compared to normal length, ovulatory menstrual cycles. Together, these findings suggested that a decline of estrogen and progesterone production was a principal component of irregular cycles. While these studies confirmed an increase in the occurrence of irregular intermenstrual intervals prior to the FMP, they did not explore the HPO feedback which might contribute to the breakdown of the HPO axis and, in turn, lead to increased irregular intermenstrual intervals and potential anovulation. Recent reports have indicated that central neural events downstream of the control of luteinizing hormone (LH) by kisspeptin are directly involved in VMS in both animal [12] and human [13] models.
Investigating early irregular intermenstrual intervals and their hormonal patterns may provide clues regarding their genesis and progression during the MT and assist in understanding of the endocrine basis of affective symptoms and VMS. We examined daily hormone metabolite excretion patterns that characterize the HPO axis during the early perimenopause to identify hormonal patterns associated with negative mood and VMS. We hypothesized the occurrence of negative mood and/or VMS is associated with specific alterations in the hormone patterns that characterize the functioning of the HPO axis in the early perimenopausal transition.
2.0. Methods
2.1. Participants
The Study of Women’s Health Across the Nation (SWAN) is a longitudinal, multi-racial/ethnic, multi-site, community-based study of menopause and aging among 3,302 pre- and early perimenopausal women who were aged 42–52 years at baseline. Each of seven sites recruited Caucasian women and a specified minority group (African Americans in Pittsburgh, Boston, Detroit, and Chicago; Japanese in Los Angeles; Chinese in the Oakland; and Hispanics in Newark). The Institutional Review Boards at all participating sites approved the study protocol, and all participants provided written, signed informed consent.
2.2. Data Collection
Design of the main cohort study has been described in detail previously [14]. SWAN baseline assessments consisted of interviewer- and self-administered study forms with detailed questions about medical, reproductive and menstrual history; lifestyle and psychosocial factors; and physical and psychological symptoms. Measurements of height and weight were obtained using standard protocols.
Data for the current report were collected as part of the Daily Hormone Study (DHS) begun at the first annual follow-up in a subset of SWAN women (n=849), aged 43–53 years, from all study sites. Inclusion criteria were: an intact uterus and at least one ovary present, at least one menstrual period in the previous three months, no use of sex steroid hormones in the previous three months, and not pregnant or lactating. Consistent with exclusions in prior analyses, 86 women with >20% missing data for daily symptoms or hormones were excluded, yielding 763 participants.
2.3. Procedures and measures
The protocol and specimen collection details of the DHS have been described in detail previously [15]. Beginning with the first day of menstrual cycle bleeding, participants collected a first morning urine specimen each day for the length of a menstrual cycle or 50 days, whichever came first. Women were provided with specimen collection kits and collected urine, filled two tubes with 5mL of urine and placed them in their freezer within two hours of collection. Participants also kept standardized daily bedtime logs of mood and vasomotor symptoms. When collections were completed, kits and logs were returned to the study site and kits were shipped frozen to the CLASS Laboratory at the University of Michigan for assay.
2.4. Hormone metabolite Measurement
Daily urinary metabolites for estradiol (estrone conjugate, E1C), pregnanediol glucuronide (PdG), immune-reactive LH, and follicle stimulating hormone (FSH) were measured using chemiluminescent assays as described previously [11, 15]. All hormone values were creatinine corrected.
2.5. Hormone patterns, Criteria for defining ELA and non-ELA Cycles
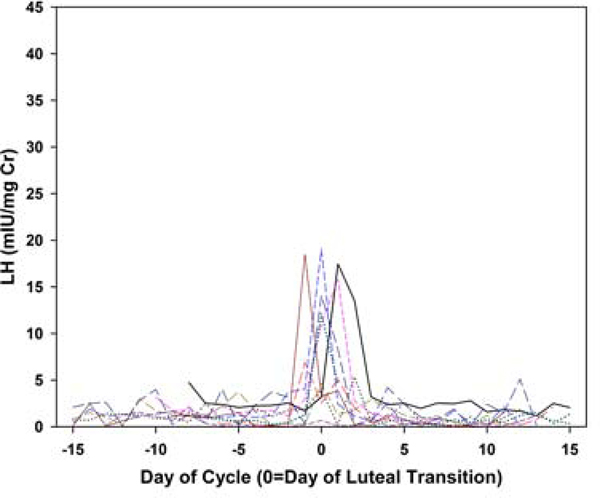
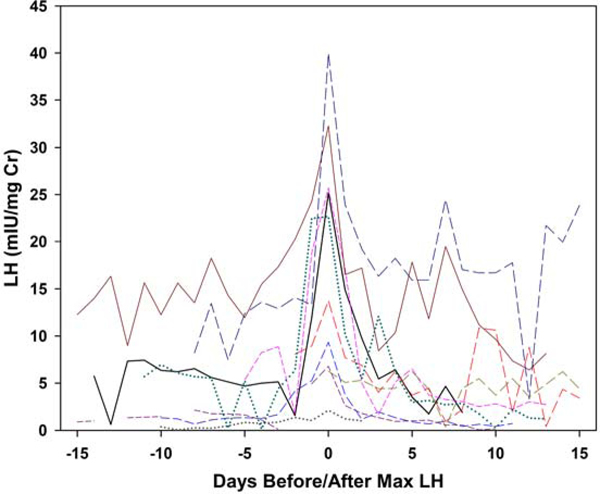
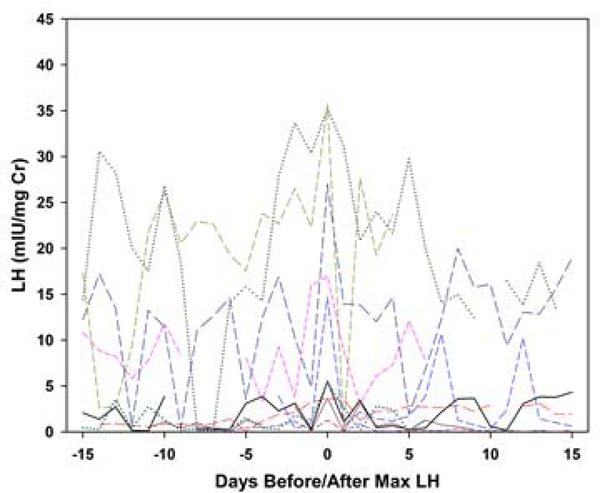
Each collection was categorized as having evidence of ovulatory luteal activity (ELA) or not (non-ELA), using the Kassam algorithm [9], which identifies robust ovulatory ovarian cycles based on the pattern of PdG. Four further classifications of the non-ELA collections were made based on three independent reviewers (BL, DM and NG). Generally accepted basic principles of the HPO axis, as four hormone events, were selected as representing the essential elements of ELA function. In all ELA, these events included: estrogen priming, estrogen-associated PdG increase, positive LH feedback, and negative LH suppression. These individual events are the primary mechanistic actions that are required to manifest normal, repeating ovulatory cycles, and all must be functional in a specific sequence and timeframe for the expression of regular menstrual cycles to occur [16]. The absence or defect in any one of these elements will likely affect ovarian function. Each element was evaluated by visual inspection by the same three independent reviewers with a combined 50 years of experience both assaying and evaluating human urinary hormone metabolites. Agreement between pairs of raters was 68% for LH suppression, 77% for LH surge and 87% for E1C associations. Collections were classified as assigned by at least two raters. Metabolite concentration and bleeding patterns were excluded from the four ELA criteria because levels of urinary metabolites do not always correspond to circulating levels of the parent hormone, and bleeding is generally considered a result rather than a cause of variations in ovarian function. In addition, two patterns of LH excretion patterns in non-ELA collections were visually identified (rater BL): 1) smooth increases and declines in LH metabolites; and 2) an oscillatory LH pattern. To illustrate, daily LH is plotted: versus days before/after day of luteal transition for a random sample of 10 ELA collections (Figure 1a); versus days before/after maximum LH for a random sample of 10 non-oscillatory non-ELA collections (Figure 1b); and versus days before/after maximum LH for a random sample of 10 oscillatory non-ELA collections (Figure 1c).In general, a single mid-segment peak in LH metabolites reflects the pre-ovulatory LH surge in ELA segments, a less well-defined mid-segment LH peak is observed in non-ELA segments with a smooth LH profile, and an absence of a single mid-segment peak in LH metabolites in any individual in collection intervals classified as oscillatory.
Figure 1a.
Plots of daily LH metabolite excretion patterns for random samples of 10 participants per group. Ten individual ELA collections, LH versus days before/after day of luteal transition
Figure 1b.
Plots of daily LH metabolite excretion patterns for random samples of 10 participants per group. Ten non-ELA non-oscillatory LH collections, LH versus days before/after day of maximum LH
Figure 1c.
Plots of daily LH metabolite excretion patterns for random samples of 10 participants per group. 10 non-ELA oscillatory LH collections, LH versus days before/after day of maximum LH
2.6. Daily Mood and VMS Symptoms
Participants were asked to complete a daily diary at the end of each day of the urine collection. Questions included occurrence of hot flashes or night sweats in the prior 24 hours. Daily negative mood score was calculated as the mean of five dysphoric mood items – blue or down, mood swings, irritable, anxious, and feelings easily hurt – each of which was rated on a 4-point intensity scale: 1=not at all, 2=a little bit, 3=moderately, 4=a lot (Cronbach’s alpha=0.85). Based on the observed distribution, each day’s negative mood score was dichotomized as any negative mood (2+) versus none (<2). Each collection was categorized as no days with VMS, at or below the median of non-zero percent of days with VMS (median=16.7%), and above the median of days with VMS. Three categories were defined similarly for days with any negative mood (median=20.0%).
2.7. Covariates
Analyses adjusted for covariates, including race/ethnicity and geographic region (East, Midwest, West Coast). Variables from the corresponding SWAN parent visit interview and clinic visit data included: categorized measured body mass index (categorized as ≤25, 25–29.9, 30+ kg/m2); number of self-reported comorbidities (myocardial infarction/angina, diabetes, hypertension, high cholesterol, stroke, arthritis, over- or underactive thyroid, and osteoporosis) categorized as 0, 1, and 2+; age on the first day of the intermenstrual interval; use of antidepressant or anxiolytic medications; and early perimenopause (menstrual bleeding in the prior 3 months with increased cycle irregularity) versus premenopause (menstrual bleeding in the prior 3 months, no change in regularity).
2.8. Statistical Analyses
ELA and non-ELA collections were summarized and compared regarding participant characteristics, using chi-square and t-tests. Associations between hormone events and symptom frequency categories were estimated using chi-square tests and ordinal logistic regressions. Logistic regression analyses first compared ELA collections with all non-ELA collections. Subsequently, separately for each hormone event, ELAs were compared with non-ELAs with and without the hormone event, and the two non-ELA subgroups were compared to each other. Parallel analyses were run subdividing the non-ELA collections into those with all 5 elements of an intact HPO axis versus those with fewer than 5. The proportional odds assumption was met for all models.
3.0. Results
Of the 763 collections, 141 (18.5%) were classified as non-ELA and 622 (81.5%) as ELA. Non-ELA collections were more likely to occur during early perimenopause, at older ages, and to reflect a longer intermenstrual segment (Table 1). Almost all non-ELA collections contained one or more elements of an intact HPO axis. The presence of a two-fold or greater rise in E1C was identified in 90.1% of the non-ELA collections. A detectable E1C rise was followed by a three-fold rise above three previous base line in PdG in 85.1% of non-ELA collections, and was associated with a transient LH suppression in 85.8% of non-ELA collections.
Table 1:
Comparison of Non-ELA collections with and without evidence of luteal activity by Kassam criterion
| % (N) or Mean (Std Dev) | |||
|---|---|---|---|
| Characteristic | No evidence of luteal activity (non-ELA), N=141 | Evidence of luteal activity (ELA), n=622 | p-value |
| Menopause status: % (N) | <.0001 | ||
| Premenopausal | 13.5 (19) | 30.4 (189) | |
| Early perimenopausal | 86.5 (122) | 69.6 (433) | |
| Age (years): Mean (Std Dev) | 48.7 (2.5) | 47.0 (2.4) | <.0001 |
| Body mass index (kg/m2): % (N) | .2077 | ||
| <25 | 39.0 (55) | 47.1 (293) | |
| 25 – 29.9 | 29.1 (41) | 26.2 (163) | |
| 30+ | 31.9 (45) | 26.7 (166) | |
| Race/ethnicity: % (N) | .4472 | ||
| White | 28.4 (40) | 32.8 (204) | |
| Black | 27.0 (38) | 20.1 (125) | |
| Chinese | 17.0 (24) | 18.5 (115) | |
| Japanese | 19.2 (27) | 21.1 (131) | |
| Hispanic | 8.5 (12) | 7.6 (47) | |
| Antidepressant use: % (N) | 7.8 (11) | 10.5 (65) | .3430 |
| Anxiolytic use: % (N) | 5.0 (7) | 4.3 (27) | .8207 |
| Comorbidities: % (N) | .5000 | ||
| 0 | 50.4 (71) | 54.7 (340) | |
| 1 | 29.8 (42) | 29.3 (182) | |
| 2+ | 19.9 (28) | 16.1 (100) | |
| Collection length (days): % (N) | <.0001 | ||
| <21 | 11.4 (16) | 2.9 (18) | |
| 21 – 35 | 28.4 (40) | 90.7 (564) | |
| 36+ | 60.3 (85) | 6.4 (40) | |
| Reason for collection end: % (N) | <.0001 | ||
| Start of next menstrual flow | 55.3 (77) | 98.1 (610) | |
| 50 days elapsed | 44.7 (63) | 1.9 (12) | |
| Any days with vasomotor symptoms | 58.9 (83) | 45.2 (281) | .0033 |
| Any days with negative mood | 69.5 (98) | 73.5 (457) | .3393 |
| # HPO-axis elements: | -- | ||
| 0 | 0.7 (1) | 0.0 (0) | |
| 1 | 4.3 (6) | 0.0 (0) | |
| 2 | 2.8 (4) | 0.0 (0) | |
| 3 | 7.8 (11) | 0.0 (0) | |
| 4 | 42.6 (60) | 0.0 (0) | |
| 5 | 41.8 (59) | 100.0 (0) | |
| Estrogen priming: % (N) | 90.1 (127) | 100.0 (622) | -- |
| Estrogen-associated PdG increase: % (N) | 85.1 (120) | 100.0 (622) | -- |
| Negative LH feedback | 85.8 (121) | 100.0 (622) | -- |
| Positive LH feedback: % (N) | 94.3 (133) | 100.0 (622) | -- |
| Oscillatory LH: % (N) | 42.6 (60) | 0.0 (0) | -- |
An acute rise in LH metabolites following an E1C rise occurred in 94.3% of the non-ELA collections, indicating the positive feedback loop was generally operable. An oscillatory LH pattern occurred in 43% of the non-ELA segments. In contrast, all ELA collections exhibited a sustained suppression of LH for several consecutive days.
3.1. Associations of hormone events with symptom frequency
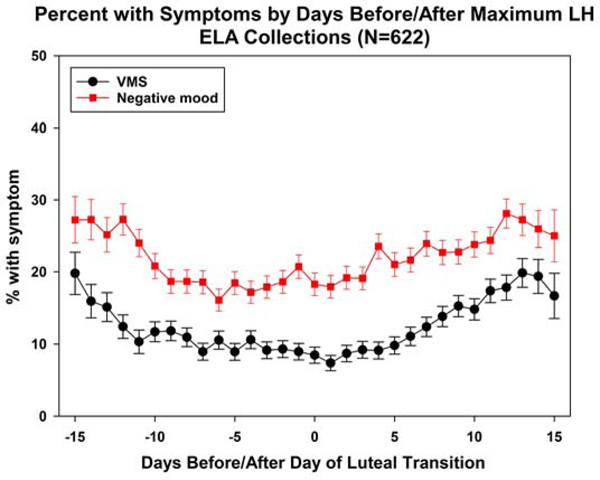
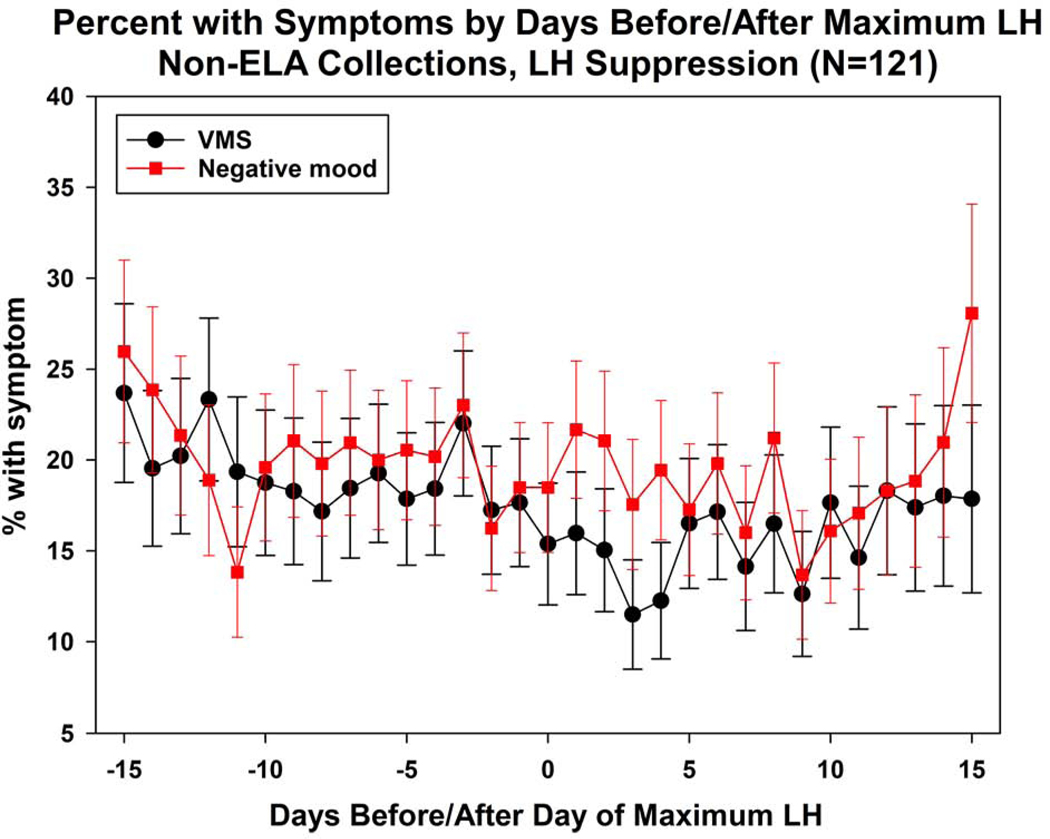
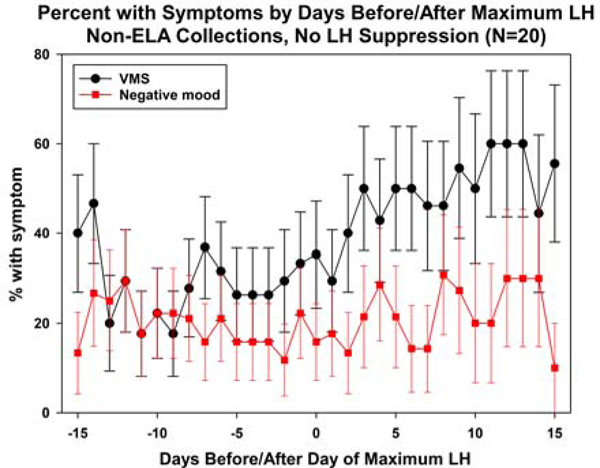
Compared with ELA collections, non-ELA collections had significantly more days with VMS (Table 2a). Over half of ELA collections had no VMS, compared with 41.1% of non-ELA collections. Subdividing non-ELA collections into those with 5 versus 0–4 elements of an intact HPO axis (hormone events), ELA collections and non-ELA collections had similar VMS frequency; in contrast, those with at least one element absent had significantly higher VMS frequency than the other two subgroups, before and after covariate adjustment. In turn, subdividing non-ELA collections by each of four ELA hormone events (positive LH feedback was not analyzed due to small cell counts), those without the event had the highest percentage of days with VMS, although pairwise differences of this group with ELA collections and non-ELA collections with the event tended not to be statistically significantly different due to small numbers for those without the event. The exception was negative LH feedback. Compared to ELA (Figure 2a) and non-ELA collections with LH negative feedback (Figure 2b), significantly more VMS occurred in non-ELA collections that lacked negative LH feedback (Figure 2c).
Table 2a.
Symptom Frequency vs Hormone patterns: VMS Frequency vs Hormone Patterns
| % (N) in VMS frequency category | OR (95% CI), non-ELA subgroup vs. ELA | OR (95% CI), 1st non-ELA subgroup vs. 2nd non-ELA subgroup | |||||
|---|---|---|---|---|---|---|---|
| No days | ≤ median (=16.7%) | >median | Unadjusted | Covariateadjusted(a) | Unadjusted | Covariateadjusted(a) | |
| ELA(b) | 54.8 (341) | 24.1 (150) | 21.1 (131) | Reference | Reference | -- | -- |
| Non-ELA(c) | |||||||
| All | 41.1 (58) | 23.4 (33) | 35.5 (50) | 1.87 (1.33, 2.63) | 1.57 (1.09, 2.26) | -- | -- |
| # HPO-axis elements: | |||||||
| 5 of 5 | 57.6 (34) | 22.0 (13) | 20.3 (12) | 0.91 (0.54, 1.53) | 0.82 (0.48, 1.41) | 0.29 (0.15, 0.56) | 0.33 (0.17, 0.64) |
| 0 – 4 of 5 | 29.3 (24) | 24.4 (20) | 46.3 (38) | 3.10 (2.01, 4.78) | 2.48 (1.57, 3.91) | Reference | Reference |
| Estrogen priming: | |||||||
| Yes | 41.7 (53) | 23.6 (30) | 34.7 (44) | 1.81 (1.27, 2.58) | 1.53 (1.05, 2.24) | 0.73 (0.26, 2.02) | 0.81 (0.29, 2.28) |
| No | 35.7 (5) | 21.4 (3) | 42.9 (6) | 2.48 (0.93, 6.62) | 1.90 (0.70, 5.18) | Reference | Reference |
| Estrogen-associated PdG(d) rise: | |||||||
| Yes | 41.7 (50) | 22.5 (27) | 35.8 (43) | 1.86 (1.29, 2.67) | 1.59 (1.08, 2.34) | 0.97 (0.41, 2.29) | 1.07 (0.45, 2.57) |
| No | 38.1 (8) | 28.6 (6) | 33.3 (7) | 1.91 (0.86, 4.27) | 1.48 (0.65, 3.38) | Reference | Reference |
| LH(e) suppression: | |||||||
| Yes | 45.5 (55) | 20.7 (25) | 33.9 (41) | 1.63 (1.13, 2.35) | 1.37 (0.93, 2.01) | 0.43 (0.18, 1.04) | 0.43 (0.17, 1.07) |
| No | 15.0 (3) | 40.0 (8) | 45.0 (9) | 3.82 (1.64, 8.88) | 3.21 (1.34, 7.67) | Reference | Reference |
| Oscillatory LH: | |||||||
| No | 51.8 (42) | 22.2 (18) | 25.9 (21) | 1.18 (0.76, 1.84) | 1.04 (0.66, 1.64) | 0.35 (0.18, 0.48) | 0.37 (0.19, 0.71) |
| Yes | 26.7 (16) | 25.0 (15) | 48.3 (29) | 3.42 (2.07, 5.64) | 2.80 (1.65, 4.76) | Reference | Reference |
Covariates include: race/ethnicity, geographic region (east, midwest, west coast), menopause status (early peri- versus premenopausal), categorized BMI (≤25, 25–29.9, 30+), number of comorbidities (0, 1, 2+), antidepressant use, anxiolytic use, age,
No evidence of luteal activity,
Evidence of luteal activity,
Pregnanediol glucuronide,
Luteinizing Hormone
Statistically significant pairwise differences (p<.05) are presented in bold
Figure 2a.
Plots of mean daily negative mood and VMS in ELA collections, with mean VMS and Negative mood.
Figure 2b.
Plots of mean daily negative mood and VMS in Non-ELA with LH suppression.
Figure 2c.
Plots of mean daily negative mood and VMS in non-ELA with no LH suppression.
Non-ELA collections retaining an element of an intact HPO axis, tended to have significantly more days with VMS than ELA collections, with odds ratios ranging from 1.63, 95% confidence intervals (1.13, 2.35) for VMS associated with negative LH feedback to 1.86 (1.29, 2.67) for VMS with an estrogen-associated rise. In short, for each criterion, the non-ELA collections with an event had an intermediate VMS frequency compared with ELA and non-ELA collections without an event. In all comparisons, covariate adjustment had minimal impact.
In contrast to VMS frequency, associations of ELA hormone events with frequency of negative mood were much smaller, and none was statistically significant before or after adjustment for covariates (Table 2b).
Table 2b.
Symptom Frequency vs Hormone patterns: Mood Frequency vs Hormone Patterns
| % (N) in negative mood frequency category | OR (95% CI), non-ELA subgroup vs. ELA | OR (95% CI), 1st non-ELA subgroup vs. 2nd non-ELA subgroup | |||||
|---|---|---|---|---|---|---|---|
| No days | ≤ median (=20.0%) | >median | Unadjusted | Covariate-adjusted(a) | Unadjusted | Covariate-adjusted(a) | |
| ELA | 26.5 (165) | 37.0 (230) | 36.5 (227) | Reference | Reference | -- | -- |
| Non-ELA: | |||||||
| All | 30.5 (43) | 35.5 (50) | 34.0 (48) | 1.16 (0.83, 1.62) | 1.11 (0.77, 1.58) | -- | -- |
| # HPO-axis elements: | |||||||
| 5 of 5 | 39.9 (23) | 30.5 (18) | 30.5 (18) | 0.65 (0.39, 1.06) | 0.72 (0.43, 1.20) | 0.62 (0.33, 1.14) | 0.68 (0.36, 1.27) |
| 0 – 4 of 5 | 24.4 (20) | 39.0 (32) | 36.6 (30) | 1.05 (0.69, 1.60) | 1.06 (0.68, 1.67) | Reference | Reference |
| Estrogen priming: | |||||||
| Yes | 29.9 (38) | 36.2 (46) | 33.9 (43) | 0.87 (0.61, 1.24) | 0.93 (0.64, 1.34) | 1.10 (0.40, 3.02) | 1.28 (0.45, 3.63) |
| No | 35.7 (5) | 28.6 (4) | 35.7 (5) | 0.79 (0.30, 2.10) | 0.72 (0.26, 1.96) | Reference | Reference |
| Estrogenassociated PdG rise: | |||||||
| Yes | 30.8 (37) | 36.7 (44) | 32.5 (39) | 0.83 (0.58, 1.18) | 0.89 (0.61, 1.30) | 0.73 (0.31, 1.72) | 0.90 (0.38, 2.17) |
| No | 28.6 (6) | 28.6 (6) | 42.9 (9) | 1.13 (0.51, 2.52) | 0.99 (0.43, 2.26) | Reference | Reference |
| LH suppression: | |||||||
| Yes | 30.6 (37) | 34.7 (42) | 34.7 (42) | 0.88 (0.61, 1.25) | 0.92 (0.63, 1.35) | 1.10 (0.46, 2.63) | 1.17 (0.48, 2.84) |
| No | 30.0 (6) | 40.0 (8) | 30.0 (6) | 0.79 (0.35, 1.80) | 0.79 (0.34, 1.84) | Reference | Reference |
| Oscillatory LH: | |||||||
| No | 35.8 (29) | 30.9 (25) | 33.3 (27) | 0.75 (0.49, 1.15) | 0.79 (0.51, 1.23) | 0.73 (0.39, 1.34) | 0.73 (0.30, 1.37) |
| Yes | 23.3 (14) | 41.7 (25) | 35.0 (21) | 1.03 (0.63, 1.68) | 1.09 (0.65, 1.83) | Reference | Reference |
Covariates include: race/ethnicity, geographic region (east, midwest, west coast), menopause status (early peri- versus premenopausal), categorized BMI (≤25, 25–29.9, 30+), number of comorbidities (0, 1, 2+), antidepressant use, anxiolytic use, age
4.0. Discussion
The present results confirmed that a significant decline in estrogen production and/or excretion was not an early or major contributor to the early breakdown of the fully operational HPO axis. Apparent adequate estrogen but decreased progesterone and distinct non-ELA patterns of LH excretion were the most frequently observed features in non-ELA collections. Changes in the excretion patterns of ovarian and pituitary hormones during the early stages of the menopausal transition were examined for their associations with increases in VMS and negative mood. The difference in VMS frequency for collections with and without estrogen priming was smaller than the difference in VMS frequency for oscillatory and non-oscillatory LH.
Our definition that all ELA segments include a robust E1C peak and a compressed LH surge followed by a sustained rise in PdG, was central to the comparisons between ELA and non-ELA collections. Gonadotropin profiles in ELA segments are consistently predictable being largely constrained by ovarian steroid feedback loops [17, 18]. In contrast, the patterns of gonadotropin excretion are quite variable in non-ELA segments, particularly for LH, which was induced to surge and then suppressed within the same collection. Despite modest deficiencies in ovarian control of LH, the major feedback loops of the HPO axis remained largely present, albeit temporally abnormal in most non-ELA segments. While the ability of modestly increased PdG, when associated with a preceding E1C rise, was usually related to LH surges in non-ELA collections, the sustained negative feedback on LH associated with PdG rises in ELA collections was most often not observed in non-ELA collections. The daily excretion profile of LH metabolites in ELA collections was relatively uniform, with a single mid-collection peak. In contrast, the daily whole segment LH excretion profile for representative non-ELA segments with a smooth LH excretion were less uniform than ELA profiles, although a mid-segment peak often occurred. The LH excretion profile for representative non-ELA segments that exhibited variable LH had no obvious uniformity with LH surges as a predominant feature.
LH variability and negative LH feedback were the events most strongly associated with increased VMS, although VMS frequency was significantly associated with all hormone patterns. In contrast, none of the hormone patterns was related to a significant increase in negative mood. In the whole non-ELA collection patterns with a smooth LH profile (not oscillatory), frequency of negative mood and VMS were similar to those in ELA collections and in collections with no observed LH suppression, VMS appeared to increase. These comparisons suggest that the same endocrine mechanisms are not involved in the genesis of negative mood as found for VMS.
The increased odds ratio for non-ELA collections with an E1C peak preceding a rise in PdG suggests that a more complete development and progression of the ovarian follicular complex may be as important as the subsequent inadequacy of progesterone to elicit LH variability and VMS. The lowered estrogen feedback is consistent with earlier studies with VMS [10] and the additional lowering of estrogen production required for increased negative mood.
An early report that links LH secretion to VMS was one that employed the relatively new LH immunoassays in postmenopausal women, before kisspeptin was discovered [19]. The oscillations observed in this report may be predictive of events that follow with aging, including increased sensitivity to less dynamic LH pulses in the postmenopause. While estrogen production in the early MT is relatively normal, estrogen production in women appears to decline years later [20]. In aged rodents, a decline in the positive estrogen feedback is associated with a general hypothalamic circadian disruption [21], and an attenuation of the midcycle LH surge has been attributed to reduced estradiol responsiveness of kisspeptin neurons [22]. A loss of positive estrogen feedback, which was not observed in this study of pre- and early perimenopausal women, has been reported in older perimenopausal women and may represent a progression of the failure of the ovary to entrain LH [23]. The association of changes in LH secretion with estrogen priming has been well-established [24]. However, in both much older men and women, when steroid production has declined, regular LH pulses dissipate and may represent a reflection of conventional aging [25] and a reduction in VMS. However, these minute-to-minute changes in LH and FSH secretion associated with aging bear no resemblance to the variable LH patterns associated with VMS. Thus, these variable patterns of LH are unlikely to represent a consequence of the hypothalamic-pituitary changes that occur later with general aging.
The variable LH profiles with surges explains not only why increasing circulating LH is not a reliable and early indicator of menopausal stage but may also explain the variable expression of related symptoms both within and between women. Women who retain regular menstrual cycles through most of the MT will experience only the short-lived mid-cycle LH surges. In contrast, women with low progesterone production and who experience LH surges and variability may experience more symptoms.
The near omnipresence of E1C peaks in non-ELA collections argues against possible ovarian quiescence as a single mechanism leading to VMS. The E1C peaks in many non-ELA collections were likely quantitatively less than those in Kassam-adjudicated ELA collections but were associated with both positive and negative feedback loops, indicating adequate estrogen feedback. In contrast, the lower concentrations of PdG rises observed here support a general decline in progesterone production [5] and may represent the major component of the early HPO axis breakdown. PdG rose more than threefold above baseline for three consecutive days in 85% of the non-ELA collections evaluated; yet many of these segments failed the Kassam algorithm to qualify as ovulatory luteal phases [9]. These lower than expected elevations of PdG usually occurred within ten days of a two-fold or greater rise in E1C, suggesting general synchrony between follicular maturation and luteinization of mature follicles. The temporal proximity of these events suggests the overall integrity of the ovarian follicular function in which the estrogen-producing complex of theca and granulosa cells matured sufficiently to be capable of first synthesizing androgens, then producing estrogens, and ultimately being converted to luteinized cells capable of producing progesterone, at a rate somewhat less than in association with a normal corpus luteum. However, the failure of a concomitant sustained suppression of LH associated with the low PdG profiles suggests that the synchronization of subsequent recruitment of follicles, follicular maturation, and an optimal ovulatory process that leads to appropriate luteinization and luteal support is the leading event in the progression of a general HPO axis breakdown. Thus, control of the feedback mechanisms that coordinates and limits the mid-cycle LH surge to insure complete luteinization of mature granulosa cells may be one of the first components of the HPO axis to fail. The timing and completeness of events are critical for normal repetitive ovarian cycles to occur. While prominent LH surges were usually present, these were not regular in occurrence but were scattered throughout individual non-ELA collections indicating the decline in HPO axis synchrony.
The variability in elevations of LH provide a partial explanation for increased adrenal androgens and insulin dysregulation in mid-aged women by acting through extra-gonadal LH receptors in the adrenal cortex [26, 27] and pancreatic beta cells [28, 29]. These LH profiles are also consistent with a central mechanism involving kisspeptin secretion, which has been linked to the elicitation of VMS in the rodent model [12] and in humans [13]. Taken together, these observation link changes in the HPO axis to VMS associated with the MT, and future studies of this type may provide insight on both the mechanism(s) and progressive severity of VMS and other symptoms.
The major strength of this study was the application of daily hormone metabolite patterns in a relatively large multi-racial/ethnic group of midlife women to characterize individual qualities of the HPO axis. A limitation of this study was the subjective nature of self-reporting of symptoms and the use of urinary metabolites rather than circulating parent hormones. A possible limitation in negative mood assessment was that a range of values was based on a relatively healthy population and inclusion of women with more extreme mood/depression would have expanded the range. We also note that sample sizes in the non-ELA group without each hormonal feature of interest tended to be small -- as participants were in the early stage of the menopausal transition – which limited our power to detect differences from the other non- ELA group and the ELA group. This issue is expected to dissipate in future analyses of subsequent collections, when participants are farther along in the menopausal transition. It will also be interesting to further explore past premenstrual VMS symptoms, and whether women report an increase in psychological symptoms [30].
5.0. Conclusion
These results provide evidence of potential a direct causal pathway between variable patterns of LH excretion and VMS but not negative mood and may provide future new management and therapy strategies, particularly in cohorts that are more advanced in the MT.
Highlights.
We measured daily vasomotor symptoms, and urinary hormone levels: follicle stimulating hormone (FSH), luteinizing hormone (LH), pregnanediol glucuronide (PdG) and estradiol (estrone conjugate, E1C).
A variable pattern of LH and negative LH feedback were the hormone patterns most strongly associated with increased vasomotor symptoms.
No hormone pattern was significantly related to negative mood.
Acknowledgements
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Funding
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495).
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Ethical approval
The Institutional Review Boards at all participating sites approved the study protocol, and all participants provided written, signed informed consent.
ethics
The authors declare that informed consent was obtained for experimentation with human subjects.
The privacy rights of human subjects have always been observed.
Provenance and peer review
This article was not commissioned and was externally peer reviewed.
Research data (data sharing and collaboration)
There are no linked research data sets for this paper. Data will be made available on request.
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Soules MR, et al. , Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric, 2001. 4(4): p. 267–72. [PubMed] [Google Scholar]
- [2].Soules MR, et al. , Stages of Reproductive Aging Workshop (STRAW). J Womens Health Gend Based Med, 2001. 10(9): p. 843–8. [DOI] [PubMed] [Google Scholar]
- [3].Soules MR, et al. , Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril, 2001. 76(5): p. 874–8. [DOI] [PubMed] [Google Scholar]
- [4].Soules MR, et al. , Executive summary: Stages of Reproductive Aging Workshop (STRAW) Park City, Utah, July, 2001. Menopause, 2001. 8(6): p. 402–7. [DOI] [PubMed] [Google Scholar]
- [5].Santoro N, et al. , Menstrual Cycle Hormone Changes in Women Traversing Menopause: Study of Women’s Health Across the Nation. J Clin Endocrinol Metab, 2017. 102(7): p. 2218–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chung HF, et al. , The role of sleep difficulties in the vasomotor menopausal symptoms and depressed mood relationships: an international pooled analysis of eight studies in the InterLACE consortium. Psychol Med, 2018. 48(15): p. 2550–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Campbell IG, et al. , Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep, 2011. 34(11): p. 1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Natari RB, et al. , The bidirectional relationship between vasomotor symptoms and depression across the menopausal transition: a systematic review of longitudinal studies. Menopause, 2018. 25(1): p. 109–120. [DOI] [PubMed] [Google Scholar]
- [9].Kassam A, et al. , Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect, 1996. 104(4): p. 408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Skurnick JH, et al. , Longitudinal changes in hypothalamic and ovarian function in perimenopausal women with anovulatory cycles: relationship with vasomotor symptoms. Fertil Steril, 2009. 91(4): p. 1127–34. [DOI] [PubMed] [Google Scholar]
- [11].Gold EB, et al. , Relation of daily urinary hormone patterns to vasomotor symptoms in a racially/ethnically diverse sample of midlife women: study of women’s health across the nation. Reprod Sci, 2007. 14(8): p. 786–97. [DOI] [PubMed] [Google Scholar]
- [12].Padilla SL, et al. , A Neural Circuit Underlying the Generation of Hot Flushes. Cell Rep, 2018. 24(2): p. 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Szeliga A, et al. , The role of kisspeptin/neurokinin B/dynorphin neurons in pathomechanism of vasomotor symptoms in postmenopausal women: from physiology to potential therapeutic applications. Gynecol Endocrinol, 2018. 34(11): p. 913–919. [DOI] [PubMed] [Google Scholar]
- [14].Sowers MF, The menopause transition and the aging process: a population perspective. Aging (Milano), 2000. 12(2): p. 85–92. [DOI] [PubMed] [Google Scholar]
- [15].Santoro N, et al. , Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab, 2003. 284(3): p. E521–30. [DOI] [PubMed] [Google Scholar]
- [16].Messinis IE, Messini CI, and Dafopoulos K, Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online, 2014. 28(6): p. 714–22. [DOI] [PubMed] [Google Scholar]
- [17].Yen SS, et al. , The operating characteristics of the hypothalamic-pituitary system during the menstrual cycle and observations of biological action of somatostatin. Recent Prog Horm Res, 1975. 31: p. 321–63. [DOI] [PubMed] [Google Scholar]
- [18].Lasley BL, Wang CF, and Yen SS, The effects of estrogen and progesterone on the functional capacity of the gonadotrophs. J Clin Endocrinol Metab, 1975. 41(5): p. 820–6. [DOI] [PubMed] [Google Scholar]
- [19].Tataryn IV, et al. , LH, FSH and skin temperaure during the menopausal hot flash. J Clin Endocrinol Metab, 1979. 49(1): p. 152–4. [DOI] [PubMed] [Google Scholar]
- [20].Santoro N, et al. , Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab, 2008. 93(5): p. 1711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wise PM, Krajnak KM, and Kashon ML, Menopause: the aging of multiple pacemakers. Science, 1996. 273(5271): p. 67–70. [DOI] [PubMed] [Google Scholar]
- [22].Lederman MA, et al. , Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology, 2010. 58(1): p. 314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Weiss G, et al. , Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA, 2004. 292(24): p. 2991–6. [DOI] [PubMed] [Google Scholar]
- [24].Chang SP, et al. , Differences in the ratio of bioactive to immunoreactive serum luteinizing hormone during vasomotor flushes and hormonal therapy in postmenopausal women. J Clin Endocrinol Metab, 1984. 58(5): p. 925–9. [DOI] [PubMed] [Google Scholar]
- [25].Pincus SM, et al. , Effects of age on the irregularity of LH and FSH serum concentrations in women and men. Am J Physiol, 1997. 273(5): p. E989–95. [DOI] [PubMed] [Google Scholar]
- [26].Pabon JE, et al. , Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J Clin Endocrinol Metab, 1996. 81(6): p. 2397–400. [DOI] [PubMed] [Google Scholar]
- [27].Lasley B, et al. , Identification of Immunoreactive Luteinizing Hormone Receptors in the Adrenal Cortex of the Female Rhesus Macaque. Reprod Sci, 2016. 23(4): p. 524–30. [DOI] [PubMed] [Google Scholar]
- [28].Parkash J, Lei Z, and Rao CV, The Presence of Human Chorionic Gonadotropin/Luteinizing Hormone Receptors in Pancreatic beta-Cells. Reprod Sci, 2015. 22(8): p. 1000–7. [DOI] [PubMed] [Google Scholar]
- [29].Rao CV, Involvement of Luteinizing Hormone in Alzheimer Disease Development in Elderly Women. Reprod Sci, 2017. 24(3): p. 355–368. [DOI] [PubMed] [Google Scholar]
- [30].Mishra GD and Kuh D, Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ, 2012. 344: p. e402 [DOI] [PMC free article] [PubMed] [Google Scholar]