Abstract
Background
Trastuzumab-dkst is a biosimilar of trastuzumab. The phase 3 HERITAGE trial demonstrated equivalent overall response rate (ORR) with trastuzumab-dkst or originator trastuzumab at 24 weeks in patients with HER2-positive metastatic breast cancer receiving chemotherapy. We now present the correlation of ORR with progression-free survival (PFS) for maintenance monotherapy with trastuzumab-dkst vs trastuzumab at 48 weeks of treatment, and the safety, tolerability, and immunogenicity.
Methods
HERITAGE is a multicenter, double-blind, randomized, parallel-group, phase 3 study. Patients were randomized 1:1 to receive trastuzumab-dkst or trastuzumab in combination with taxane followed by continued monotherapy until disease progression. The analysis included PFS at 48 weeks to support the primary efficacy endpoint of ORR and safety, tolerability, and immunogenicity of trastuzumab-dkst vs trastuzumab as maintenance monotherapy.
Results
Of 500 randomized patients, 342 entered the monotherapy phase; 214 patients received ≥48 weeks of treatment. There were no statistically significant differences between PFS, ORR, or interim overall survival at week 48 between trastuzumab-dkst and trastuzumab. Week 24 ORR was highly correlated with week 48 PFS (rb = 0.75). Cumulative treatment-emergent adverse events (TEAEs) and serious AEs were similar in both groups, with few grade ≥3 TEAEs. Immunogenicity was low and similar in both groups at 48 weeks.
Conclusion
The correlation between ORR and PFS supports the design of first-line metastatic trials assessing biosimilar trastuzumab. Overall, trastuzumab-dkst and trastuzumab were well tolerated with similar efficacy, including ORR and PFS, in combination with a taxane followed by monotherapy.
Keywords: Biosimilar, Combination therapy, Efficacy, Metastatic breast cancer, Monotherapy, Safety, Trastuzumab
Highlights
-
•
Trastuzumab-dkst is a biosimilar of trastuzumab.
-
•
Trastuzumab-dkst and trastuzumab had similar PFS, ORR, and OS at week 48.
-
•
Results support therapeutic equivalence between trastuzumab-dkst and trastuzumab.
-
•
Week 24 ORR was highly correlated with week 48 PFS.
-
•
ORR and PFS correlation supports ORR as a valid endpoint in clinical trials for MBC.
1. Introduction
Biologic agents, including monoclonal antibodies (mAbs), have improved outcomes for some cancers [1,2]. Despite significant therapeutic promise, biologics are structurally complex and often costly, limiting global access [[3], [4], [5], [6]]. Biosimilars to cancer drugs may lower healthcare costs and improve access. As patents for biologics expire, biosimilars provide a high-quality alternative [3].
Worldwide, breast cancer is the most common malignancy and the most frequent cause of cancer death in women [[7], [8], [9]]. About 25%–30% of breast cancers overexpress human epidermal growth factor receptor 2 (HER2) [10,11]. Trastuzumab is a humanized IgG1 mAb directed against HER2 [11]. With chemotherapy, trastuzumab has been shown to improve progression-free survival (PFS) and overall survival (OS) in HER2-positive metastatic breast cancer (MBC) and disease-free survival and OS in early-stage HER2-positive disease [[12], [13], [14], [15]]. Trastuzumab is approved for treatment of HER2-positive breast cancer and metastatic gastric or gastroesophageal junction adenocarcinoma [16].
Trastuzumab-dkst (Ogivri®; Viatris Inc, Zurich, Switzerland; formally MYL-1401O) is a trastuzumab biosimilar [17], with an amino acid sequence identical to that of trastuzumab (Herceptin®; Genentech, Inc, South San Francisco, CA). Similarity of trastuzumab-dkst to both United States– and European Union–sourced trastuzumab has been demonstrated in physicochemical, preclinical, and pharmacokinetic studies [18,19]. The phase 3 HERITAGE trial compared taxane-based chemotherapy with trastuzumab-dkst or trastuzumab as first-line therapy for MBC. The primary endpoint of overall response rate (ORR) at 24 weeks combined with taxanes has been reported [20] and was equivalent between groups, leading to regulatory approval of trastuzumab-dkst in the United States and Europe. After 24 weeks, patients with stable or responding disease continued mAb monotherapy until progression. We present safety, tolerability, immunogenicity, and correlation between ORR and PFS for trastuzumab-dkst or trastuzumab maintenance monotherapy after 48 weeks of therapy.
2. Patients and methods
This was a multicenter, double-blind, randomized, parallel-group, phase 3 study (NCT02472964) [21] in patients with HER2-positive MBC (Supplemental Figure) conducted in accordance with the International Council for Harmonisation Guidance for Industry E6 Good Clinical Practice, the Declaration of Helsinki, and applicable local regulatory requirements. All patients provided written informed consent before starting any study-related procedures. The full trial protocol and all other relevant study documentation were approved by the institutional review board or ethics committee at each study center before study initiation. Detailed methods, including eligibility criteria, study design, and treatment regimen details, have previously been described [20].
2.1. Eligibility
Patients were adults with histologically confirmed HER2-positive breast cancer having ≥1 measurable metastatic target lesion, Eastern Cooperative Oncology Group performance status of 0–2, and left ventricular ejection fraction (LVEF) within normal range [20]. Patients must not have received chemotherapy or HER2-targeted therapy within 1 year of diagnosis of metastatic disease.
2.2. Study design
Patients were randomized 1:1 to receive taxane plus trastuzumab-dkst or trastuzumab using a centralized randomization procedure with stratification based on baseline covariates [20]. Taxanes included docetaxel or paclitaxel and were administered by physician’s choice at the study site. After 24 weeks of combination therapy, patients with stable or responding disease continued their assigned monotherapy until disease progression, unacceptable toxicity, or death. During monotherapy, patients with hormone receptor–positive disease could receive endocrine therapy.
2.3. Efficacy
After the first 24 weeks of combination therapy, tumor assessments were conducted every 12 weeks independent of delays in treatment administration and included imaging of the chest, abdomen, and pelvis, with bone scan as indicated.
2.4. Safety
The safety population included all patients who received ≥1 dose of trastuzumab-dkst or trastuzumab. Safety analyses used cumulative data through week 48 for all patients. A separate analysis was conducted using data from patients who received monotherapy only. Assessment of treatment-emergent adverse events (TEAEs) included type, incidence, severity (graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03), timing, seriousness, and relatedness. Laboratory abnormalities were also assessed.
2.5. Statistical analysis
The primary endpoint of ORR at week 24 was previously reported. The endpoint of efficacy and safety of trastuzumab-dkst and trastuzumab monotherapy at week 48 included a descriptive comparison of safety, tolerability, and immunogenicity, as well as evaluation of PFS, duration of response (DR), time to tumor progression (TTP), and interim OS. Final OS will occur during follow-up at 240 deaths or month 36 from the time of randomization of the last patient, whichever occurs first, and will be reported at study end.
Disease progression was defined according to Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) [22]. Patients were classified by response to therapy as having a complete response (CR), partial response (PR), or stable disease (SD). Patients who were intolerant to therapy or progressed during combination therapy were followed for safety and survival events.
Patient disposition, baseline characteristics, and treatment administration/compliance were descriptively summarized using SAS® software version 9.2 or later (SAS, Cary, NC). Analyses of secondary endpoints were not adjusted for multiplicity. Kaplan-Meier plots by treatment are presented, and a log-rank test of the 2 treatment groups unadjusted for any covariates was performed. The biserial correlation coefficient was used to assess associations between ORR at week 24 (responder or nonresponder) and PFS at week 48, irrespective of treatment assignment.
3. Results
3.1. Patient disposition and baseline characteristics
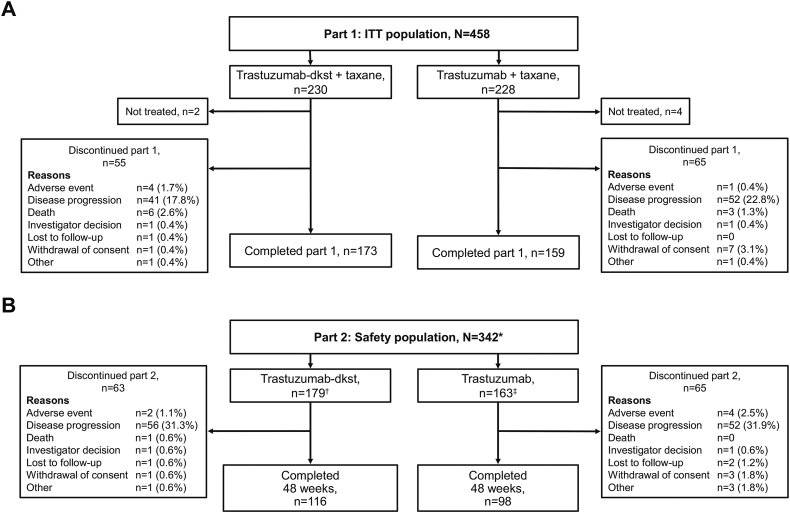
Of the 500 patients randomized, 493 received treatment (safety population). Of these, 458 were included in the intention-to-treat (ITT) efficacy analysis population (Fig. 1A), composed of patients who had not previously received first-line therapy. A total of 342 patients entered maintenance monotherapy (trastuzumab-dkst, n = 179; trastuzumab, n = 163; Fig. 1B). Overall, 214 patients completed 48 weeks of treatment (trastuzumab-dkst, n = 116; trastuzumab, n = 98). Baseline characteristics of patients enrolled to receive combination therapy were previously published [20]. Baseline characteristics of patients starting monotherapy were generally similar between groups (Table 1). Mean (SD) age was 54.1 (11.0) years, and the estrogen receptor– and progesterone receptor–negative population entering monotherapy was 55.3%.
Fig. 1.
Patient consort diagrams for the (A) ITT population and (B) safety population. ITT, intention-to-treat. ∗The safety population included 10 patients from the trastuzumab-dkst group and 12 patients from the trastuzumab group who were not considered part of the ITT population but were randomized for treatment in part 1 (combination therapy). †At the start of part 2 (monotherapy) and at the investigator’s discretion, 15 patients continued using taxane and later switched to monotherapy. ‡At the start of part 2 (monotherapy) and at the investigator’s discretion, 17 patients continued using taxane and later switched to monotherapy.
Table 1.
Baseline characteristics of patients entering combination and monotherapy phases: ITT population.
|
Patient characteristic |
Combination therapy phase |
Monotherapy phase |
||
|---|---|---|---|---|
| Trastuzumab-dkst + taxane (N = 230) | Trastuzumab + taxane (N = 228) | Trastuzumab-dkst (N = 179) | Trastuzumab (N = 163) | |
| Age, y | ||||
| Mean (SD) | 54.3 (11.0) | 52.9 (11.2) | 55.1 (10.4) | 53.1 (11.6) |
| Median (range) | 55.0 (26–79) | 54.0 (26–82) | 56.0 (31–79) | 54.0 (26–81) |
| Age category, n (%) | ||||
| <50 y | 74 (32.2) | 86 (37.7) | 51 (28.5) | 60 (36.8) |
| ≥50 y | 156 (67.8) | 142 (62.3) | 128 (71.5) | 103 (63.2) |
| Race, n (%) | ||||
| Asian | 70 (30.4) | 72 (31.6) | 51 (28.5) | 48 (29.4) |
| Black/African American | 1 (0.4) | 2 (0.9) | 1 (0.6) | 0 |
| White | 159 (69.1) | 154 (67.5) | 127 (70.9) | 115 (70.6) |
| Tumor endocrine status, n (%) | ||||
| ER/PgR negative | 128 (55.7) | 127 (55.7) | 101 (56.4) | 88 (54.0) |
| ER or PgR positive | 102 (44.3) | 101 (44.3) | 78 (43.6) | 75 (46.0) |
| Tumor progression into metastatic phase, n (%) | ||||
| <2 y | 146 (63.5) | 153 (67.1) | 120 (67.0) | 115 (70.6) |
| ≥2 y | 75 (32.6) | 71 (31.1) | 54 (30.2) | 45 (27.6) |
| Missing | 9 (3.9) | 4 (1.8) | 5 (2.8) | 3 (1.8) |
| Previous treatment (adjuvant) | ||||
| Trastuzumab | 22 (9.6) | 16 (7.0) | 12 (6.7) | 10 (6.1) |
| Taxane | 46 (20.0) | 42 (18.4) | 29 (16.2) | 28 (17.2) |
| Assigned taxane, n (%) | ||||
| Docetaxel | 193 (83.9) | 192 (84.2) | 152 (84.9) | 144 (88.3) |
| Paclitaxel | 35 (15.2) | 32 (14.0) | 27 (15.1) | 19 (11.7) |
| No treatment | 2 (0.9) | 4 (1.8) | – | – |
| Presence of visceral metastases, n (%) | ||||
| Yes | 172 (74.8) | 185 (81.1) | 128 (71.5) | 131 (80.4) |
| No | 58 (25.2) | 43 (18.9) | 51 (28.5) | 32 (19.6) |
| No. of metastatic sites, n (%) | ||||
| 1 | 58 (25.2) | 61 (26.8) | 50 (27.9) | 51 (31.3) |
| 2 | 87 (37.8) | 67 (29.4) | 65 (36.3) | 48 (29.4) |
| 3 | 44 (19.1) | 57 (25.0) | 35 (19.6) | 42 (25.8) |
| ≥4 | 41 (17.8) | 43 (18.9) | 29 (16.2) | 22 (13.5) |
| CNS first site of metastasis, n (%) | ||||
| Yes | 1 (0.4) | 2 (0.9) | 1 (0.6) | 1 (0.6) |
| No | 229 (99.6) | 226 (99.1) | 178 (99.4) | 162 (99.4) |
CNS, central nervous system; ER, estrogen receptor; ITT, intention-to-treat; PgR, progesterone receptor; SD, standard deviation.
3.2. Efficacy
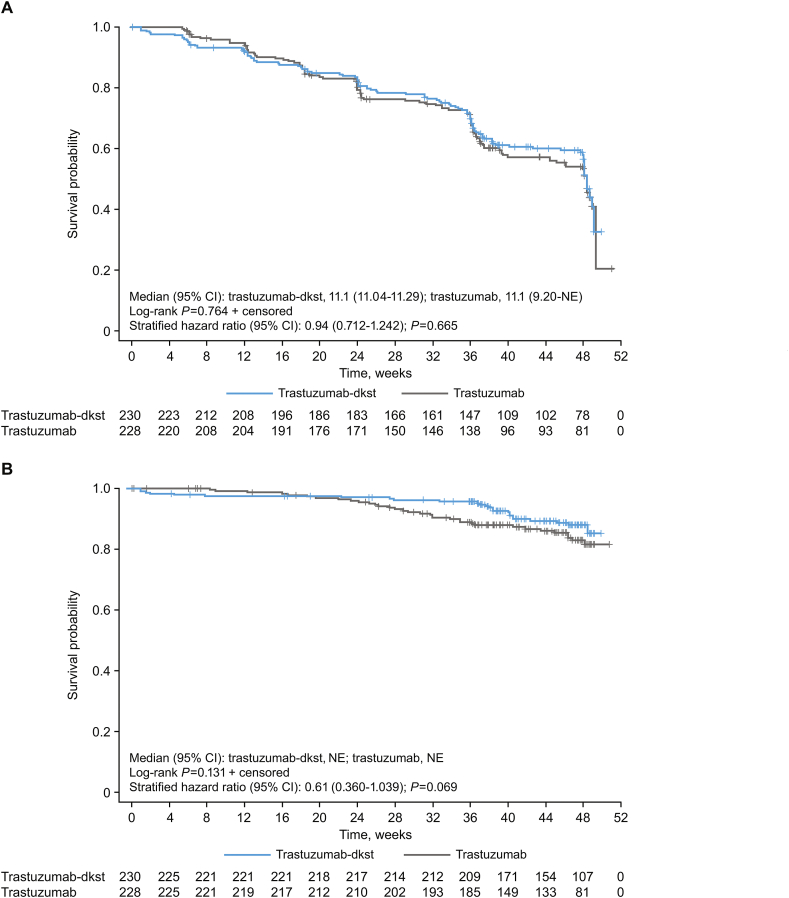
Week 48 TTP, PFS, and OS endpoints were previously reported [20]. Median DR through week 48 was similar with trastuzumab-dkst (9.7 months; 95% CI, 7.4–9.9) and trastuzumab (9.7 months; 95% CI, 7.7–9.9) in the ITT population, with no statistically significant differences (log-rank test, P = 0.790). At week 48, age, race, previous adjuvant/neoadjuvant chemotherapy/HER2-targeted treatment, and region were potential covariates to influence the hazard ratio for TTP and included in the final model. According to the final model at week 48, age (≥50 vs < 50 years) influenced TTP (hazard ratio, 0.69; P = 0.013). Because of the small number of patients with tumor progression, the data were of limited clinical relevance. The 95% CI of the TTP ratio (trastuzumab-dkst to trastuzumab) included “1” for all subgroups at week 48 (ie, no relevant subgroup differences were observed). At week 48, 128 (55.7%) trastuzumab-dkst patients and 126 (55.3%) trastuzumab patients had not experienced disease progression. According to the log-rank test, time-to-event curves for both groups were also not significantly different at week 48 (P = 0.842). Median time for PFS by Kaplan-Meier estimates was 11.1 months in both groups (Fig. 2A). Through week 48, 205 (89.1%) trastuzumab-dkst patients survived compared with 194 (85.1%) trastuzumab patients, and survival curves for both groups were not significantly different (P = 0.131; median not reached for Kaplan-Meier estimates for OS because of the relatively small number of patients in the ITT population who died before week 48; Fig. 2B).
Fig. 2.
(A) Kaplan-Meier plot of progression-free survival at week 48 based on investigator assessment in the ITT population. (B) Kaplan-Meier plot of overall survival at week 48 in the ITT population. ITT, intention-to-treat; NE, not estimable. Numbers at risk are displayed at the bottom of the figure. The stratified hazard was done by assigned taxane, tumor progression, and tumor endocrine status.
Additional responders were documented during the monotherapy phase. At week 24, CR was observed in 1.3% and 0% of patients receiving trastuzumab-dkst and trastuzumab, respectively; at week 48, this increased to 1.7% and 0.4%, respectively, as 1 additional patient in each group demonstrated CR. At week 24, PR was observed in 68.3% and 64.0% of patients receiving trastuzumab-dkst and trastuzumab, respectively. An additional 5 patients receiving trastuzumab demonstrated PR during monotherapy, increasing the percentage of patients with PR to 66.2%. Confirmed ORR at week 48 for trastuzumab-dkst and trastuzumab was 70.0% and 66.7%, respectively.
The ORR at week 24 correlated with PFS in responders and nonresponders at week 48 (Fig. 3A). Association of ORR at week 24 and PFS for the total sample at week 48 was strong (rb = 0.75, biserial correlation coefficient for study population; Fig. 3B).
Fig. 3.
ORR at week 24 was predictive of PFS at week 48. (A) ORR at week 24 with PFS at week 48 in responders and nonresponders. (B) ORR at week 24 and Kaplan-Meier plot of PFS probability. ORR, overall response rate; PFS, progression-free survival.
3.3. Safety and tolerability
Results from patients receiving combination therapy were previously reported [20]. Cumulative overall TEAE rate was 57.3% (trastuzumab-dkst, 54.7%; trastuzumab, 60.1%) during the monotherapy phase (Table 2). Of the 5015 total TEAEs reported, only 513 started during monotherapy. The most frequently reported TEAEs during monotherapy were headache (7.3%), anemia (5.0%), and alopecia (3.2%). More patients receiving trastuzumab monotherapy discontinued treatment because of TEAEs compared with those receiving trastuzumab-dkst monotherapy (4.9% [n = 8] vs 1.7% [n = 3]). Four patients discontinued treatment because of treatment-related TEAEs (trastuzumab-dkst, 1.2% [n = 3]; trastuzumab, 0.4% [n = 1]).
Table 2.
Summary of TEAEs (occurring in ≥10% of patients in any group) and SAEs (occurring in ≥2% of patients in any group) in combination therapy (24 weeks) and monotherapy (48 weeks) phases: Safety population.
| Combination therapy phase only |
Monotherapy phase only |
|||
|---|---|---|---|---|
| Adverse events (preferred term), n (%) | Trastuzumab-dkst + taxane (N = 247) | Trastuzumab + taxane (N = 246) | Trastuzumab-dkst (N = 179) | Trastuzumab (N = 163) |
| Patients with ≥1 TEAE | 239 (96.8) | 233 (94.7) | 98 (54.7) | 98 (60.1) |
| CTCAE preferred terma | ||||
| Alopecia | 142 (57.9) | 135 (54.9) | 6 (3.4) | 5 (3.1) |
| Neutropenia | 142 (57.5) | 131 (53.3) | 2 (1.1) | 4 (2.5) |
| Diarrhea | 51 (20.6) | 51 (20.7) | 6 (3.4) | 0 |
| Asthenia | 54 (21.9) | 40 (16.3) | 5 (2.8) | 3 (1.8) |
| Leukopenia | 42 (17.0) | 51 (20.7) | 1 (0.6) | 2 (1.2) |
| Nausea | 49 (19.8) | 34 (13.8) | 4 (2.2) | 4 (2.5) |
| Anemia | 40 (16.2) | 40 (16.3) | 5 (2.8) | 12 (7.4) |
| Peripheral edema | 35 (14.2) | 28 (11.4) | 1 (0.6) | 3 (1.8) |
| Arthralgia | 30 (12.1) | 11 (4.5) | 5 (2.8) | 2 (1.2) |
| Peripheral sensory neuropathy | 29 (11.7) | 34 (13.8) | 4 (2.2) | 2 (1.2) |
| Fatigue | 28 (11.3) | 33 (13.4) | 3 (1.7) | 6 (3.7) |
| Peripheral neuropathy | 28 (11.3) | 28 (11.4) | 3 (1.7) | 7 (4.3) |
| Vomiting | 26 (10.5) | 19 (7.7) | 3 (1.7) | 5 (3.1) |
| Pyrexia | 21 (8.5) | 30 (12.2) | 4 (2.2) | 2 (1.2) |
| SAEs | ||||
| Patients with ≥1 SAE | 94 (38.1) | 89 (36.2) | 5 (2.8) | 4 (2.5) |
| CTCAE preferred terma | ||||
| Neutropenia | 68 (27.5) | 62 (25.2) | 0 | 0 |
| Febrile neutropenia | 11 (4.5) | 10 (4.1) | 0 | 0 |
| Leukopenia | 4 (1.6) | 12 (4.9) | 0 | 0 |
| Pneumonia | 4 (1.6) | 5 (2.0) | 2 (1.1) | 0 |
CTCAE, Common Terminology Criteria for Adverse Events; SAE, severe adverse event; TEAE, treatment-emergent adverse event.
Coded using Medical Dictionary for Regulatory Activities, version 18.0.
A total of 330 serious AEs (SAEs) were reported in 188 patients through week 48. During monotherapy, 9 SAEs were reported (trastuzumab-dkst, 2.8% [n = 5]; trastuzumab, 2.5% [n = 4]; Table 2).
From study initiation, 65 deaths occurred (trastuzumab-dkst, 9.7% [n = 24]; trastuzumab, 15.9% [n = 39]); only 7 (2.8%) trastuzumab-dkst patients and 5 (2.0%) trastuzumab patients died within 28 days of their last dose, indicating that most patients did not die on treatment. Through week 48, 10 patients experienced fatal TEAEs. During combination therapy, 8 fatal TEAEs occurred (trastuzumab-dkst, 50.0% [n = 4]; trastuzumab, 50.0% [n = 4]). During monotherapy, 2 patients receiving trastuzumab-dkst each experienced 1 fatal TEAE (dyspnea and unconfirmed carditis, respectively) that were not considered related to study drug.
3.4. TEAEs of special interest
Using the standardized Medical Dictionary for Regulatory Activities (MedDRA) query (SMQ) for hypersensitivity (narrow) with the additional preferred term (PT) of infusion-related reaction (IRR), 106 events were identified in 53 (21.5%) patients receiving trastuzumab-dkst and 105 events in 56 (22.8%) patients receiving trastuzumab through week 48, the majority of which occurred during combination therapy. Identified events that occurred in >1% of patients receiving trastuzumab-dkst were rash (8.9% vs 10.2% for trastuzumab), IRR (6.9% vs 4.9%), hypersensitivity (2.0% vs 2.8%), allergic cough (1.2% vs 0.4%), dermatitis (1.2% vs 0.4%), and rash pruritic (1.2% vs 0.4%). Treatment-related IRRs were reported in 9 (3.6%) patients receiving trastuzumab-dkst and 6 (2.4%) receiving trastuzumab through week 48. Three (1.2%) patients in each group reported a grade ≥3 hypersensitivity event, and 1 (0.4%) in each group experienced a grade ≥3 IRR. Three (1.2%) patients in each group experienced a serious hypersensitivity event or IRR (trastuzumab-dkst, 1 drug hypersensitivity event and 2 anaphylactic reactions; trastuzumab, 1 IRR and 2 hypersensitivity events). Grade 3 anaphylactic reactions were reported in 2 (0.8%) patients receiving trastuzumab-dkst, one treatment related and the other related to concomitant medications (piperacillin/tazobactam). Both events resolved. No anaphylactic events were reported with trastuzumab.
During maintenance monotherapy, 1 (0.4%) patient receiving trastuzumab experienced an IRR compared with none receiving trastuzumab-dkst. The SMQ hypersensitivity (narrow) identified 3 (1.7%) patients receiving trastuzumab-dkst and 4 (2.5%) receiving trastuzumab who experienced a potential hypersensitivity event. No hypersensitivity or IRR events were serious, grade 3, or resulted in interruption or permanent treatment discontinuation during monotherapy. Through week 48, all IRRs resolved the same day as onset with interruption of the infusion and/or conservative treatment.
Incidence of treatment-associated pulmonary toxicity was low for the 48-week study, with dyspnea (7.1%), pneumonia (3.4%), and pneumonitis (1.2%) being reported most frequently. With trastuzumab-dkst, 41 pulmonary toxicity events occurred vs 43 events with trastuzumab. During monotherapy, 9 pulmonary toxicity events occurred: dyspnea (trastuzumab-dkst, n = 4; trastuzumab, n = 1), pneumonia (trastuzumab-dkst, n = 2; trastuzumab, n = 0), pneumonitis (trastuzumab-dkst, n = 0; trastuzumab, n = 1), and pulmonary fibrosis (trastuzumab-dkst, n = 1; trastuzumab, n = 0).
Incidence of cardiac events was low for the 48-week study and comparable in both groups (trastuzumab-dkst, n = 29; trastuzumab, n = 18). In total, 17 cardiac events occurred during maintenance monotherapy. The most common cardiac event was decreased ejection fraction, which occurred in 10 patients (trastuzumab-dkst, 3.4% [n = 6]; trastuzumab, 2.5% [n = 4]). One event each (0.4%) of cardiac failure, cardiotoxicity, carditis, and LV dysfunction occurred with trastuzumab-dkst, and 1 event each (0.4%) of cardiomyopathy and congestive cardiomyopathy occurred with trastuzumab. The LVEF values were similar between groups through week 48. Eighteen patients had LVEF <50% at least once during the study (trastuzumab-dkst, 4.0% [n = 10]; trastuzumab, 3.3% [n = 8]; P = 0.637). Most patients who had a 50% drop in LVEF had previously received anthracyclines and had conditions potentially associated with a second cardiac event. Sixteen patients (trastuzumab-dkst, 4.0% [n = 10]; trastuzumab, 2.4% [n = 6]) who had LVEF <50% at least once postbaseline recovered to >50% during the study.
3.5. Immunogenicity
Before treatment, 14 of 237 patients (5.9%) receiving trastuzumab-dkst and 22 of 240 (9.2%) receiving trastuzumab were positive for antidrug antibodies (ADA). Antibodies were transient with low titers, and incidence of neutralizing antibodies (NAb) was low and similar between groups. Overall, ADA rate postbaseline was 3.9% with trastuzumab-dkst and 4.4% with trastuzumab (Table 3). Treatment-induced ADA rate with trastuzumab-dkst and trastuzumab was 1.7% and 1.8%, respectively. Between weeks 24 and 48, 1 new patient receiving trastuzumab-dkst was ADA positive. There were no differences between groups regarding immunogenicity.
Table 3.
| n, % | Trastuzumab-dkst (N = 247) | Trastuzumab (N = 246) |
|---|---|---|
| Overall ADA rate | 9 (3.9) | 10 (4.4) |
| Overall NAb rate | 1 (0.4) | 3 (1.3) |
|
n, % |
Trastuzumab-dkst (N = 233) |
Trastuzumab (N = 224) |
| Treatment-induced ADA rate | 4 (1.7) | 4 (1.8) |
| Treatment-induced NAb rate | 1 (0.4) | 2 (0.9) |
ADA, antidrug antibodies; NAb, neutralizing antibodies.
Percentages were based on the number of patients in the safety population with non-missing postbaseline samples available in each group and include 228 patients in the trastuzumab-dkst group and 227 patients in the trastuzumab group.
Percentages were based on the number of patients in the safety population with available ADA postbaseline results and include 229 patients in the trastuzumab-dkst group and 227 patients in the trastuzumab group. Postbaseline includes only on-treatment samples until week 48 and excludes end-of-treatment/end-of-study samples.
4. Discussion
Trastuzumab combined with taxane has resulted in improved ORR in MBC and improved pathologic complete response in early breast cancer, indicating similar sensitivity in both HER2-positive disease settings. Week 48 results for DR, TTP, PFS, and OS were similar between the trastuzumab-dkst and trastuzumab groups (ITT population), with no statistically significant differences. All secondary efficacy analyses supported the conclusion of therapeutic equivalence. Further post hoc analysis suggests that the ORR of the responder and nonresponder subgroups provides a good prediction of prolonged PFS and that the behavior is similar in both groups. There was a strong positive correlation between ORR at week 24 and PFS at week 48 in the HERITAGE trial.
Trastuzumab-dkst and trastuzumab were well tolerated both in combination with taxane and as monotherapy. Incidence of TEAEs was similar between groups, and the most common TEAEs were alopecia, neutropenia, and diarrhea. Of the 5015 TEAEs that occurred through week 48, 513 were during monotherapy, suggesting that concomitant taxane therapy may have significantly contributed to toxicity.
The study population that experienced LV dysfunction had predisposing factors for cardiotoxicity, including prior anthracycline use, chest wall radiotherapy, diabetes, and hypertension. Changes in LVEF observed in this study are consistent with trastuzumab literature [23,24]. Incidence of IRRs was lower in this study (trastuzumab-dkst, 21.5%; trastuzumab, 22.8%) compared with the 40% of patients receiving trastuzumab as estimated by the EU-trastuzumab summary of product characteristics [25]. Of the 2 anaphylactic events that occurred with trastuzumab-dkst, both resolved, and 1 was considered related to treatment. Incidence of SAEs was similar between groups receiving monotherapy, and no new safety findings were observed. One death in each group was considered possibly related to treatment (both respiratory failure). Incidence of ADA against trastuzumab-dkst and trastuzumab was low and consistent with literature [26].
Trastuzumab-dkst is indicated as a single agent or in combination with taxanes for the treatment of HER2-positive metastatic breast cancer, as well as for the treatment of early-stage HER2-positive disease [27]. In the metastatic first-line setting, current treatment includes pertuzumab [28]. Extrapolation, which has been practiced with biosimilars across indications and settings when sufficient scientific justification and totality of evidence support such use under the guidelines of the European Medicines Agency, US Food and Drug Administration (FDA), and World Health Organization [3], may enable the use of trastuzumab-dkst in combination with pertuzumab as well as with other agents that have been combined with trastuzumab in clinical practice.
Limitations of the HERITAGE trial are consistent with other biosimilar clinical development programs, including use of a short-term primary efficacy endpoint to initially assess similarity between trastuzumab-dkst and trastuzumab. Assessment of ORR at 24 weeks was chosen as the primary endpoint as a short-term measure of clinical activity and safety related to the use of trastuzumab-dkst as first-line therapy for MBC. The present results bolster these previously reported findings, with similarity between trastuzumab-dkst and trastuzumab (administered as maintenance monotherapy after combination therapy with taxane) demonstrated across numerous endpoints, including DR, TTP, and PFS. Longer-term assessment of OS and safety will be necessary to continue to evaluate trastuzumab-dkst in patients with HER2-positive MBC.
5. Conclusions
A positive correlation was observed between ORR and PFS in this study, potentially supporting the use of ORR as a valid endpoint in clinical trials for MBC. These data further support the FDA-approved trastuzumab-dkst as a biosimilar to trastuzumab and provide another treatment option for patients with HER2-positive MBC, as well as other trastuzumab indications, based on biosimilar extrapolation. Overall, no significant differences were observed between trastuzumab-dkst and trastuzumab in clinical activity, and both products were well tolerated with no new significant safety issues.
Declaration of competing interest
HS Rugo has received travel, accommodations, and expenses from Amgen, Merck, Viatris Inc, Pfizer, and Puma Biotechnology and research funding (provided to the Regents of the University of California) from Eisai, Genentech/Roche, Lilly, Macrogenics, Merck, Novartis, OBI Pharma, Daiichi, Immunomedics, and Pfizer. EJ Pennella was a paid employee of Mylan Inc (now Viatris Inc) during the time of the study and may hold stock with the company. U Gopalakrishnan, HF Koch, and A Barve are paid employees of Viatris Inc and may hold stock with the company. M Hernandez-Bronchud has served as a consultant/advisory board member for Viatris Inc. S Loganathan, S Deodhar, and A Marwah are paid employees of Biocon Research Ltd and may hold stock with the company. C Akewanlop has received travel, accommodations, and expenses from Amgen, AstraZeneca, Roche, and Bristol-Myers Squibb. CF Waller is a consultant/advisory board member for Viatris Inc. J Herson, A Manikhas, JD Parra, MLT Abesamis-Tiambeng, I Vynnychenko, I Bondarenko, V Sriuranpong, S Roy, and EP Yanez Ruiz have nothing to disclose.
Acknowledgments
The authors would like to acknowledge Gopichand Mamillapalli for his contributions to this manuscript. Editorial assistance was provided under the direction of the authors by MedThink SciCom, with support from Viatris Inc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.03.009.
Contributor Information
Hope S. Rugo, Email: Hope.Rugo@ucsf.edu.
Eduardo J. Pennella, Email: eduardo.pennella@gmail.com.
Unmesh Gopalakrishnan, Email: Unmesh.G@viatris.com.
Miguel Hernandez-Bronchud, Email: mhbronchud@gmail.com.
Jay Herson, Email: jay.herson@earthlink.net.
Hans Friedrich Koch, Email: hans-friedrich.koch@viatris.com.
Subramanian Loganathan, Email: subramanian.l101@biocon.com.
Sarika Deodhar, Email: Sarika.Deodhar@biocon.com.
Ashwani Marwah, Email: Ashwani.Marwah@biocon.com.
Alexey Manikhas, Email: alexeyman72@mail.ru.
Igor Bondarenko, Email: oncology@dsma.dp.ua.
Joseph D. Parra, Email: joeydparra@mac.com.
Maria Luisa T. Abesamis-Tiambeng, Email: matiambengmd@yahoo.com.
Charuwan Akewanlop, Email: charuwan.ake@mahidol.ac.th.
Ihor Vynnychenko, Email: vynnych@gmail.com.
Virote Sriuranpong, Email: vsmdcu40@gmail.com.
Sirshendu Roy, Email: sirshendu_roy_ms@yahoo.com.
Eduardo Patricio Yanez Ruiz, Email: eduardo.yanez@icos.cl.
Abhijit Barve, Email: Abhijit.Barve@viatris.com.
Cornelius F. Waller, Email: cornelius.waller@uniklinik-freiburg.de.
Funding
This work was supported by Viatris Inc, Canonsburg, PA, and Biocon Limited, Bangalore, India.
Role of the funding source
The study sponsors had a role in the design of the study; the collection, analysis, and interpretation of the data; and the writing of the manuscript. All authors agreed to submit the manuscript for publication.
Data availability statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical approval
This study was conducted in accordance with the International Council for Harmonisation Guidance for Industry E6 Good Clinical Practice, the Declaration of Helsinki, and applicable local regulatory requirements. All patients provided written informed consent before starting any study-related procedures. The full trial protocol and all other relevant study documentation were approved by the institutional review board or ethics committee at each study center before study initiation.
Suggested running head
Safety and efficacy of trastuzumab-dkst.
Prior presentation
These data have previously been presented in part at the European Society for Medical Oncology 2016 Congress; October 7–11, 2016; Copenhagen, Denmark, and at the American Society of Clinical Oncology Annual Meeting; June 1–5, 2018; Chicago, IL.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sharkey R.M., Goldenberg D.M. Targeted therapy of cancer: new prospects for antibodies and immunoconjugates. CA Cancer J Clin. 2006;56:226–243. doi: 10.3322/canjclin.56.4.226. [DOI] [PubMed] [Google Scholar]
- 2.Bellati F., Napoletano C., Gasparri M.L., Visconti V., Zizzari I.G., Ruscito I. Monoclonal antibodies in gynecological cancer: a critical point of view. Clin Dev Immunol. 2011;2011:890758. doi: 10.1155/2011/890758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rugo H.S., Linton K.M., Cervi P., Rosenberg J.A., Jacobs I. A clinician’s guide to biosimilars in oncology. Canc Treat Rev. 2016;46:73–79. doi: 10.1016/j.ctrv.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Baer Ii W.H., Maini A., Jacobs I. Barriers to the access and use of rituximab in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: a physician survey. Pharmaceuticals (Basel) 2014;7:530–544. doi: 10.3390/ph7050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammers P., Criscitiello C., Curigliano G., Jacobs I. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: a physician survey in the United States and emerging markets. Pharmaceuticals (Basel) 2014;7:943–953. doi: 10.3390/ph7090943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCamish M., Woollett G. Worldwide experience with biosimilar development. mAbs. 2011;3:209–217. doi: 10.4161/mabs.3.2.15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Key T.J., Verkasalo P.K., Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 8.Beslija S., Bonneterre J., Burstein H.J., Cocquyt V., Gnant M., Heinemann V. For the Central European Cooperative Oncology Group (CECOG). Third consensus on medical treatment of metastatic breast cancer. Ann Oncol. 2009;20:1771–1785. doi: 10.1093/annonc/mdp261. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization, International Agency for Research on Cancer . 2012. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012.http://globocan.iarc.fr/Pages/DataSource_and_methods.aspx accessed 1 November 2017. [Google Scholar]
- 10.Iqbal N., Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenbeis A.M., Grau S.J. Monoclonal antibodies and Fc fragments for treating solid tumors. Biologics. 2012;6:13–20. doi: 10.2147/BTT.S19955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez E.A., Romond E.H., Suman V.J., Jeong J.-H., Sledge G., Geyer C.E., Jr. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart M.J., Procter M., Leyland-Jones B., Goldhirsch A., Untch M., Smith I. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 14.Slamon D., Eiermann W., Robert N., Pienkowski T., Martin M., Press M. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 16.Herceptin [package insert] Genentech, Inc; South San Francisco, CA: 2018. [Google Scholar]
- 17.Us Food and Drug Administration FDA approves first biosimilar for the treatment of certain breast and stomach cancers. 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm587378.htm accessed 1 December 2017.
- 18.Waller C.F., Vutikullird A., Lawrence T.E., Shaw A., Liu M.S., Baczkowski M. A pharmacokinetics phase 1 bioequivalence study of the trastuzumab biosimilar MYL-1401O vs. EU-trastuzumab and US-trastuzumab. Br J Clin Pharmacol. 2018;84:2336–2343. doi: 10.1111/bcp.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chtioui H., Vallotton L., Audran R., Dao K., Rothuizen L.E., Winterfeld U. 2015. A bioequivalence study for Hercules, a biosimilar trastuzumab candidate in development. Poster presented at Pharmacology. [London, UK] [Google Scholar]
- 20.Rugo H.S., Barve A., Waller C.F., Hernandez-Bronchud M., Herson J., Yuan J. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: a randomized clinical trial. JAMA. 2017;317:37–47. doi: 10.1001/jama.2016.18305. [DOI] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov . Met. Br. Ca. (HERiTAge); 2018. Study of efficacy and safety of MYL-1401O + taxane vs Herceptin© + taxane for 1st line.https://clinicaltrials.gov/ct2/show/NCT02472964 accessed 22 October 2019. [Google Scholar]
- 22.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Canc. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Baselga J., Cortés J., Kim S.-B., Im S.-A., Hegg R., Im Y.-H. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez E.A., Barrios C., Eiermann W., Toi M., Im Y.-H., Conte P. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35:141–148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herceptin [summary of product characteristics]. Grenzach-Wyhlen. Roche Pharma AG; Germany: 2010. [Google Scholar]
- 26.Ismael G., Hegg R., Muehlbauer S., Heinzmann D., Lum B., Kim S.-B. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 27.Ogivri [package insert] Mylan GmbH; Zurich, Switzerland: 2017. [Google Scholar]
- 28.Sapna F., Athwal P.S.S., Kumar M., Randhawa S., Kahlon S. Therapeutic strategies for human epidermal receptor-2 positive metastatic breast cancer: a literature review. Cureus. 2020;12 doi: 10.7759/cureus.9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.