Abstract
The Opal multiplex technique is an established methodology for the detection of multiple biomarkers in one section. The protocol encompasses iterative single stainings and heating-mediated removal of the primary and secondary antibodies after each staining round, leaving untouched the Opal fluorophores which are deposited onto the antigen of interest. According to our experience, repetitive heating of skin sections often results in tissue damage, indicating an urgent need for milder alternatives to strip immunoglobulins. In this study, we demonstrate that considerable heating-related damage was found not only in skin but also in tissues of different origin, mostly characterized by low cell density. Importantly, the morphology remained fully intact when sections were repetitively exposed to β-mercaptoethanol-containing stripping buffer instead of multiple heating cycles. However, target epitopes appeared sensitive at a differential degree to multiple treatments with stripping buffer, as shown by loss in staining intensity, but in all cases, the staining intensity could be restored by increment of the primary antibody concentrations. Application of β-mercaptoethanol-containing stripping buffer instead of heating for antibody removal markedly improved the quality of the Opal multiplex technique, as a substantial higher number of differently colored cells could be visualized within a well-conserved morphological context:
Keywords: fluorescent antibody technique, immunohistochemistry, skin
Introduction
Formaldehyde reacts with end groups found in biological molecules, resulting in various hydroxymethyl adducts attached at the nitrogen, oxygen, sulfur, or carbon atoms in these end groups.1 This initial reaction is followed by a slowly proceeding process of crosslinking of these formaldehyde-modified end groups, thereby forming methylene bridges.1 The formalin-fixation induced adducts and crosslinks mask epitopes in tissue specimens and compromise antibody binding during subsequent immunohistochemical staining, leading to weak or false negative detection of certain proteins. A possible way to retrieve compromised antigenic sites in formalin-fixed paraffin-embedded (FFPE) specimens is treatment with heated antigen-retrieval buffer (e.g., citrate buffer pH 6 or Tris-EDTA [ethylenediaminetetraacetic acid] pH 9.0) to remove adducts and break crosslinks. The technique to unmask epitopes with heated buffer was discovered 25 years ago2 and is generally referred to as heat-induced epitope retrieval (HIER). The mechanism of unmasking epitopes is not fully understood but careful selection of the best HIER conditions (temperature, buffer, Ph, and exposure time) is critical for optimal antigen retrieval.1–3
Multiplex immunohistochemistry is a technique to simultaneously visualize multiple biomarkers in a single tissue section and is important, not only for clinical purposes (accurate diagnosis, evaluation of therapy choice, and prognosis), but also for the extension of our general understanding of the immunobiology of tissues, in particular, our insight in the phenotype, utilized signaling pathways, and cell cycle and activation state of cells in situ. Multicolor staining allows to detect coexpression of different molecules on individual cells, to simultaneously assess the distribution, abundancy, and heterogeneity of expression of various cell types in healthy versus diseased tissue, and to recognize spatial relationships between various cells. Numerous different strategies for multiple immunostaining of FFPE tissue have been described, generally comprising iterative immunostainings on the same section, including digitization of the stained specimen and subsequent removal of immunoglobulins (plus non-permanent dyes in some protocols) after each staining round.4–7
Despite the diversity in multiplexing staining methods, all protocols have in common that retrieval of epitopes in sections from FFPE tissue is an obligatory step after deparaffinization. In some protocols, HIER is not only applied for the antigen retrieval but also used to strip primary and secondary antibodies after each staining round to make a section ready for the next staining cycle.8,9 In this way, a specimen is exposed several times to HIER which may be detrimental to the tissue, that is, loss of epitopes and architecture and even (partial) detachment of tissue from the object glass.10,11 In skin sections, for example, the dermis often detaches upon multiple HIER exposures, while the epidermis is often delaminated. Application of stripping buffers has been suggested to avoid the issue of tissue destruction by HIER.4,5
The Opal method is a powerful multiplex immunofluorescence technique enabling to distinguish up to six markers by covalently depositing distinct Opal fluorophores onto the detected antigens, using HIER for antibody removal after each staining cycle. Despite the great potency of this method, it is less suitable for vulnerable tissue specimens or delicate antigenic determinants which are damaged by repetitive HIER treatments. This study was aimed to optimize the Opal workflow for HIER-sensitive FFPE human tissue sections, using β-mercaptoethanol-containing stripping buffer instead of HIER to strip immunoglobulins. This adjustment maintained the possibility to identify up to six biomarkers, while conserving the morphological context of the tissue and avoiding HIER-induced artifacts.
Method
Patient Material
Human abdominal skin was obtained as anonymized discarded tissue from corrective plastic surgery of the abdomen (n=4). Biopsies from the skin were fixed in formalin and embedded in paraffin according to standard procedures. FFPE whole tissue sections from lung (n=1), tonsil (n=1), kidney (n=1), melanoma (n=6), and colon (n=1) were kindly provided by the Department of Pathology at the Amsterdam University Medical Centers, Amsterdam, the Netherlands. In addition, an FFPE tissue microarray (TMA) including human tissues from the female reproductive system (n=7), digestive system (n=20), endocrine system (n=3), lymphoid tissues (n=6), kidney (n=3), lung (n=2), skin (n=2), and muscle (n=1) was also provided by the Department of Pathology. The TMA consisted of triplicate 0.6 mm cores. The institutional Medical Ethics Review Committee granted a waiver for the anonymous use of human leftover FFPE material of diagnostic procedures. The study was carried out in agreement with the Dutch law (Medical Research Involving Human Subjects Act) and following the Declaration of Helsinki principles. According to the Dutch law, researchers are allowed to use anonymous human tissue without patient consent. Tissue sections of 4 µm thickness were used for immunohistochemical and immunofluorescence stainings.
Antibodies
Primary antibodies used for both immunohistochemical and immunofluorescence stainings included mouse anti-human Melan-A (clone A103, Dako/Agilent, Santa Clara, CA), mouse anti-human pan cytokeratin (clone C11, Abcam, Cambridge, UK), mouse anti-human CD8 (clone C8/144B, Dako/Agilent), mouse anti-human CD45 (mix of clones 2B11 and PD7/26, Dako/Agilent), rabbit anti-human CD3 (polyclonal, Dako/Agilent), and mouse anti-human mast cell tryptase (clone AA1, Dako/Agilent).
HIER
FFPE tissue sections were deparaffinized in xylene and rehydrated by serial passage through graded concentrations of ethanol. Endogenous peroxidase in tissues was blocked with 0.3% H2O2/methanol for 10 min. Next, HIER was performed for 5 min at 95C in Tris-EDTA pH 9.0 buffer using the Lab Vision PT Module (Thermo Fisher Scientific, Waltham, MA). In some experiments, Citrate pH 6.0 buffer was used instead of Tris-EDTA. Multiple HIER treatments (up to six times) in Tris-EDTA pH 9.0 buffer was performed to mimic repetitive antibody stripping to study possible heating-induced damage. Thereafter, sections were stained with hematoxylin (Klinipath/VWR International, Amsterdam, The Netherlands) and eosin (Merck Millipore, Burlington, MA) and mounted for review. Images were acquired on a Leica DM microscope using Leica software (Leica Biosystems, Wetzlar, Germany).
Immunohistochemistry
After standard HIER in Tris-EDTA pH 9.0 buffer, sections were washed in cold running tap water, then 3 times in Tris-buffered saline–0.05% Tween20 (TBST) and blocked with Superblock (Scytek Laboratories, Logan, UT) for 10 min, before incubation with a primary antibody for 60 min. Antibodies were diluted in Normal Antibody Diluent (Immunologic/VWR International, Radnor, PA). Next, tissue sections were washed and incubated for 30 min with horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit polymer (Immunologic, Duiven, The Netherlands). Epitope–antibody binding was visualized using NovaRED as chromogen (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin (Klinipath/VWR International) and mounted in glycerol-gelatin (Sigma Aldrich, Saint Louis, MO). Images were acquired on a Leica DM microscope using Leica software (Leica Biosystems). To make sections ready for the next staining round, coverslips were removed after warming up the mounting medium in demi water at 50C, followed by washing the section in cold running tap water and demi water. Finally, antibodies and dye were stripped with β-mercaptoethanol-containing stripping buffer pH 7.5 (2% SDS/Tris-HCl, 0.7% β-mercaptoethanol) for 30 min at 50C. Sections were washed in cold running tap water and TBST, before being incubated with the next primary antibody. Last, sections were stained with hematoxylin (Klinipath/VWR International) and mounted for review.
Multiplex Immunofluorescence
Multiplex immunofluorescence staining was performed with the Opal 7-color fluorescence immunohistochemistry kit (Akoya Biosciences, Marlborough, MA), according to the manufacturer’s protocol, except for the repetitive heat-mediated antibody-stripping treatments. In short, after deparaffinization, rehydration, and blocking endogenous peroxidase, HIER was performed for 5 min. at 95C in Tris-EDTA pH 9.0 buffer in a Lab Vision PT Module (Thermo Fisher Scientific). Sections were washed in TBST and blocked with blocking/antibody diluent for 10 min, before being incubated with primary antibody for 60 min. Then, sections were incubated with polymer HRP Ms + Rb for 10 min, followed by incubation with an Opal fluorophore (Opal480, Opal520, Opal570, Opal620, Opal690, or Opal780) for 10 min. Bound primary and secondary antibodies were then eluted with HIER treatment (as aforementioned) or with β-mercaptoethanol-containing stripping buffer (defined as above) for 30 min in a water bath at 50C. After washing in cold running tap water, demi water, and TBST, the process of staining and antibody removal was repeated using a different Opal fluorophore. Finally, after staining with the sixth Opal fluorophore, tissue specimens were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min and mounted in ProLong Diamond Antifade Mountant (ThermoFisher Scientific).
Imaging
Vectra Polaris Automated Quantitative Pathology Imaging System (Akoya Biosciences) was used for multispectral imaging at 20× magnification. Thereafter, whole slide images were loaded into InForm image analysis software (Akoya Bioscience).
Results
Repetitive Heating Is Deleterious to the Morphology of Tissues With Low Cell Density
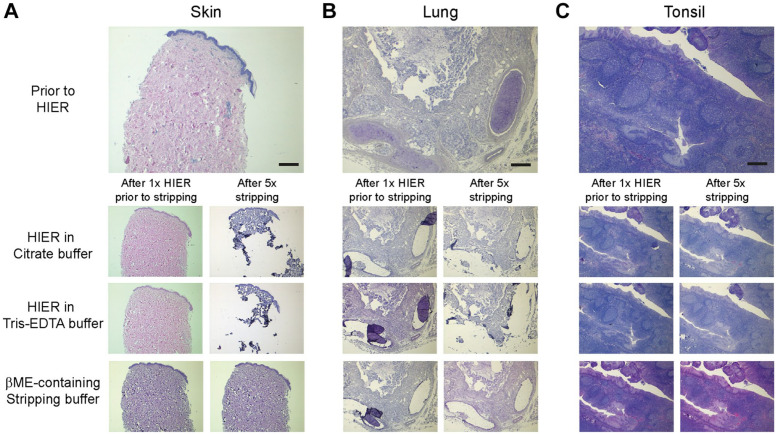
In some multiplex immunohistochemistry protocols for detection of multiple markers within a single tissue section, such as the Opal method, HIER is used to remove antibodies after each staining step. Repetitive high-temperature heating of tissue sections may lead to tissue damage. We investigated in tissues of different origin the effect of multiple HIER treatments on morphology, using Tris-EDTA pH 9.0 or Citrate pH 6.0 as retrieval buffer. We found that all FFPE whole tissue sections survived the obligatory initial HIER treatment, which is needed to enable binding of primary antibodies (Fig. 1, Supplementary Fig. 1A–C). However, additional HIER treatments led to considerable tissue damage (such as partial detachment and delamination) in sections from skin, lung, and kidney, whereas cell-dense tissues, such as tonsil, melanoma, and colon remained fully intact, even after six treatments (Fig. 1, Supplementary Fig. 1A–C). As compared with the whole tissue sections, the small-sized (only 0.6 mm in diameter) circular tissue sections in the TMA appeared to be even more vulnerable for repetitive HIER exposures, regardless of the cell density. As expected, heating-related damage was found in all human skin (2/2), lung (2/2), and kidney (3/3) sections in the TMA, but in addition, significant tissue damage was observed in 51% of the cell-dense tissues (19/37) as well (Supplementary Fig. 1D). In most cases, parts of the tissue section had detached from the glass or, if not completely detached, the tissue was folded.
Figure 1.
Cell-poor tissue is sensitive to repetitive high-temperature heating, while morphology is well conserved after multiple treatments with stripping buffer. On top is shown, hematoxylin and eosin staining of human skin (A), lung (B), and tonsil (C) tissue before HIER. At the bottom is shown treatment of skin (A), lung (B), and tonsil (C) tissue after one treatment (left) and five subsequent treatments (right) with HIER in citrate buffer, HIER in Tris-EDTA buffer, or β-mercaptoethanol-containing stripping buffer. Bars equal 1 mm. Abbreviation: HIER, heat-induced epitope retrieval; EDTA, ethylenediaminetetraacetic acid; βME, β-mercaptoethanol.
As stripping buffer can be used as an alternative way to remove antibodies, we questioned what effect this treatment would have on the tissue morphology. To investigate this, tissue sections were subjected to the obligatory single HIER treatment first, followed by repetitive exposures to β-mercaptoethanol-containing stripping buffer. We found that all types of tissue maintained their morphology with stripping buffer, even after five rounds of treatment (Fig. 1, Supplementary Fig. 1). In the small specimens in the TMA, we observed that the tissue morphology was maintained in the majority (88%) of tissues after multiple rounds of β-mercaptoethanol-containing stripping buffer, as compared with the tissue in the multiple-heating treated TMA sample (Supplementary Fig. 1D), hence underlining that exposure to stripping buffer is a relatively mild treatment, even for vulnerable tissues such as those present in TMA.
These data indicate that application of stripping buffer is preferred over HIER for repetitive antibody removal in multiplex immunohistochemistry, in particular for cell-poor tissues such as skin, lung, and kidney, to retain tissue spatial and morphological context.
Sensitivity of Epitopes to the Stripping Buffer
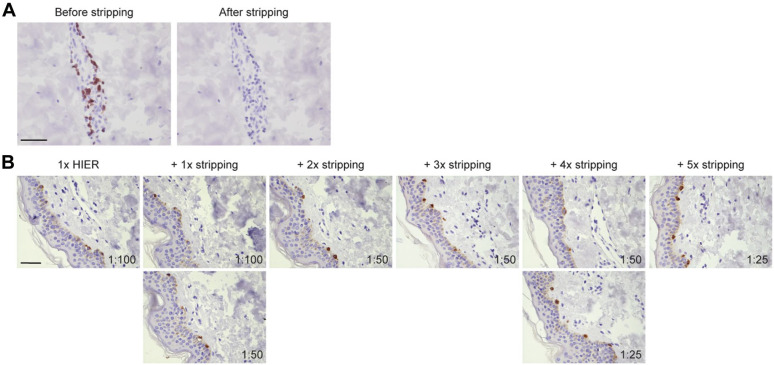
We showed that stripping buffer does not affect tissue morphology and could be a better way to remove antibodies, compared with HIER. As complete removal of primary and secondary antibodies in between the staining steps is essential in multiplex immunostaining to avoid crossreactivity, this prompted us to demonstrate the efficacy of β-mercaptoethanol-containing stripping buffer to successfully removing all immunoglobulins. To this end, we exposed a CD3-stained human skin section (Fig. 2A, left) to the stripping buffer, and subsequently, the same section was incubated with poly-HRP secondary antibody and NovaRED for visualization (Fig. 2A, right). Lack of staining indicated that originally bound primary and secondary antibodies (and also the non-permanent NovaRED chromogen) had been successfully erased by the stripping buffer (Fig. 2A). This control to check whether complete antibody removal occurred was included for all antibodies in all our experiments.
Figure 2.
Sensitivity of epitopes for repetitive treatment with stripping buffer. (A) CD3-stained human skin tissue (left) was subjected to one round of treatment with β-mercaptoethanol-containing stripping buffer followed by incubation with poly-HRP anti-rabbit and NovaRED visualization (right). Lack of staining after treatment with staining buffer indicated successful removal of primary and secondary antibodies. (B) Melan-A staining on human skin tissue after one round of HIER treatment with Tris-EDTA buffer, followed by indicated rounds of treatment with stripping buffer. Antibody dilutions are indicated. Bars equal 100 µm. Abbreviation: HIER, heat-induced epitope retrieval; EDTA, ethylenediaminetetraacetic acid.
However, we cannot exclude that multiple rounds of stripping buffer may be detrimental to any target epitope and hamper epitope detection in later staining cycles. To test this, we performed immunohistochemical staining on adjacent sections that have been subjected to the obligatory single HIER treatment followed by a differential number of treatments with stripping buffer. We observed for all antibodies in our panel that exposure to repetitive stripping cycles resulted in loss in staining intensity at a different rate and already starting after the first stripping cycle (Fig. 2B). In all cases, the staining intensity could be restored by using a higher concentration of antibody (Fig. 2B). The required increase of antibody concentration to regain optimal staining did vary among antibodies, indicating that epitopes have differential sensitivity to multiple rounds of stripping buffer. In general, after the fifth stripping round, the antibodies were applied four to six times more concentrated to maintain the staining quality. Based on the sensitivity of the different epitopes for the stripping buffer, we determined the optimal sequence of epitope detection in multiplex staining (Supplementary Table 1).
Multiplex Immunofluorescence Staining
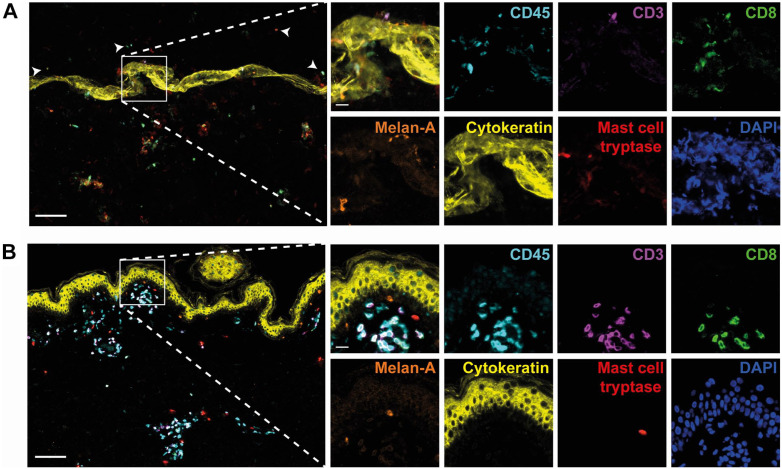
We demonstrated that antibody removal by β-mercaptoethanol-containing stripping buffer is superior over HIER treatment in case of tissues with low cell density, and in addition, we assessed the sensitivity of our target epitopes to this stripping buffer, providing a vital clue about the optimal order of epitope detection by our panel of primary antibodies. Next, we implemented the application of stripping buffer in the Opal method and aimed to study if our adjustment would lead to improved multiplex immunostaining of cell-poor tissue, using healthy human skin as typical representative. Opal fluorophores were paired to antibodies based on brightness of the fluorophores, antigen abundance, colocalization of markers, and skin-tissue autofluorescence. Preliminary tests showed that covalently deposited Opal fluorophores were not removed by the stripping buffer (data not shown). Direct comparison of the standard Opal protocol (including repetitive HIER) with our adjusted protocol (stripping buffer instead of HIER) revealed that application of stripping buffer markedly improved the quality of the Opal immunofluorescence staining technique, as a substantial higher number of differently colored cells could be visualized (Fig. 3A vs. 3B), while maintaining the spatial and morphological context. Concomitantly, cells detached during repetitive HIER treatment and subsequently randomly stuck to the slide, while this did not occur with our adjusted protocol. As a result of the damage caused by HIER treatment, cells could not be imaged in focus. Fluorophore intensity was unaffected by the stripping buffer. Signal intensity and exposure times were comparable between both protocols, when stained with similar antibody concentrations. Collectively, our data suggest that application of stripping buffer instead of HIER for antibody removal in the Opal protocol result in improved quality of this septuple immunofluorescence staining technique.
Figure 3.
Application of stripping buffer instead of HIER results in improved quality of the septuple immunofluorescence staining technique. Opal multiplex immunofluorescence staining of human skin using HIER with Tris-EDTA buffer (A) or β-mercaptoethanol-containing stripping buffer (B) for antibody removal. Representative immunofluorescence images showing CD45 (in cyan), CD3 (in purple), CD8 (in green), Melan-A (in orange), cytokeratin (in yellow), mast cell tryptase (in red) and DAPI (in blue). Arrow heads indicate cells that detached during HIER treatment and subsequently randomly stuck to the slide. Bars equal 100 µm. Abbreviation: HIER, heat-induced epitope retrieval; EDTA, ethylenediaminetetraacetic acid; DAPI, 4′,6-diamidino-2-phenylindole.
Discussion
Techniques to perform multiplex immunofluorescence staining in a single tissue section take advantage of the possibility to remove bound primary and secondary antibodies after each completed staining round leaving the color on the target epitope untouched, hence enabling the performance of multiple cycles of staining and immunoglobulin stripping. In this study, we investigated the suitability of repetitive HIER exposures to perform antibody stripping—as used in the standard Opal multiplex immunofluorescence method—and found that this treatment is disastrous for sections with low cell density, such as sections from skin, lung, and kidney, resulting in detachment of a large part of the tissue. In contrast, cell-dense specimens could resist iterative high-temperature heating. We have no explanation for this differential effect of HIER on cell-poor versus cell-rich tissues.
Release of primary and secondary antibodies from immunohistological stained sections can be achieved by stripping buffers.4,5 To solve the problem of HIER-induced damage during antibody stripping in sections with low cell density, we tested the repetitive use of β-mercaptoethanol-containing stripping buffer and found that this treatment maintained the tissue morphology quite well. However, multiple exposures of tissue sections to stripping buffer is not without harm, as we found that the staining intensity decreased after each treatment with this buffer, suggesting that the stripping buffer had a detrimental effect on the epitopes. This loss of staining intensity could be overcome by using higher concentrations of antibody. The observation that stripping-buffer exposure can cause reduction in staining intensity in subsequent staining is in line with our earlier study.7 Cattoretti and colleagues refer this loss of detection to as re-masking of the epitopes, and suggested that this problem can be solved by adding disaccharides throughout the process, and avoiding glycerol/gelatin mounting media, which negatively affect reproducibility of epitope detection.12–14
Nowadays, many laboratories prefer the use of autostainers over manual staining. Currently, these are mostly applied for single immunohistochemical stainings using 3,3’-diaminobenzidine (DAB) chromogen. As it is possible to equip these (semi-)automated instruments with devices needed for epitope retrieval steps, for example, HIER, it should not be a problem to customize autostainers for the use of β-mercaptoethanol-containing stripping buffer as antibody-removal application, which is simply a matter of adjusting time, temperature, and type of buffer, provided good extraction due to the toxicity of β-mercaptoethanol. Concomitantly, predesigned Opal multiplex panels are optimized already for use on autostainers. Therefore, we believe our current multiplex immunofluorescence protocol can be implemented in an automated platform. Nevertheless, it would still be necessary to perform appropriate assay development steps manually, for example, antibody optimization, antibody-Opal fluorophore pairings, and so on, which is also needed for the conventional Opal workflow.
In conclusion, we propose to use β-mercaptoethanol-containing stripping buffer, instead of HIER, to erase antibodies in the Opal protocol for simultaneous detection of multiple biomarkers. This adaptation avoids heat-induced tissue damage, in particular, in vulnerable tissue sections with low cell density, thereby enabling the identification of multiple biomarkers, while retaining spatial and morphological context.
Supplemental Material
Supplemental material, sj-pdf-1-jhc-10.1369_00221554211007793 for Improvement of Opal Multiplex Immunofluorescence Workflow for Human Tissue Sections by Marcella Willemsen, Gabrielle Krebbers, Marcel W. Bekkenk, Marcel B.M. Teunissen and Rosalie M. Luiten in Journal of Histochemistry & Cytochemistry
Acknowledgments
We would like to thank Dr. Elena de Miguel for sharing her advanced expertise on multispectral imaging at the Department of Molecular Cell Biology & Immunology at the Amsterdam University Medical Centers, location Vumc, Amsterdam, the Netherlands.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MW and GK performed experiments, microscopy, and data analysis, and drafted the manuscript; MWB and RML participated in manuscript revision; MBMT critically revised the work and edited the manuscript. All authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by TRANSCAN JTC 2014 through the Dutch Cancer Society under Grant Number 7800.
Contributor Information
Marcella Willemsen, Department of Dermatology, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Netherlands Institute for Pigment Disorders, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands.
Gabrielle Krebbers, Department of Dermatology, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Netherlands Institute for Pigment Disorders, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands.
Marcel W. Bekkenk, Department of Dermatology, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands Netherlands Institute for Pigment Disorders, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands.
Marcel B.M. Teunissen, Department of Dermatology, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands.
Rosalie M. Luiten, Department of Dermatology, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Netherlands Institute for Pigment Disorders, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Infection & Immunity Institute, Amsterdam University Medical Centers, location AMC, University of Amsterdam, Amsterdam, The Netherlands.
Literature Cited
- 1. Dapson RW. Macromolecular changes caused by formalin fixation and antigen retrieval. Biotech Histochem. 2007;82(3):133–40. doi: 10.1080/10520290701567916. [DOI] [PubMed] [Google Scholar]
- 2. Shi S-R, Gu J, Kalra KL, Chen T, Cote RJ, Taylor CR. Antigen retrieval technique: a novel approach to immunohistochemistry on routinely processed tissue sections. Cell Vision. 1995;2:6–22. [Google Scholar]
- 3. van der Loos CM. A focus on fixation. Biotech Histochem. 2007;82(3):141–54. doi: 10.1080/10520290701375302. [DOI] [PubMed] [Google Scholar]
- 4. Pirici D, Mogoanta L, Kumar-Singh S, Pirici I, Margaritescu C, Simionescu C, Stanescu R. Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. J Histochem Cytochem. 2009;57(6):567–75. doi: 10.1369/jhc.2009.953240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glass G, Papin JA, Mandell JW. SIMPLE: a sequential immunoperoxidase labeling and erasing method. J Histochem Cytochem. 2009;57(10):899–905. doi: 10.1369/jhc.2009.953612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 7. de Boer OJ, Krebbers G, Mackaaij C, Florquin S, de Rie MA, van der Wal AC, Teunissen MBM. Comparison of two different immunohistochemical quadruple staining approaches to identify innate lymphoid cells in formalin-fixed paraffin-embedded human tissue. J Histochem Cytochem. 2020;68(2):127–38. doi: 10.1369/0022155419897257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tornehave D, Hougaard DM, Larsson L. Microwaving for double indirect immunofluorescence with primary antibodies from the same species and for staining of mouse tissues with mouse monoclonal antibodies. Histochem Cell Biol. 2000;113(1):19–23. doi: 10.1007/s004180050002. [DOI] [PubMed] [Google Scholar]
- 9. Toth ZE, Mezey E. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem. 2007;55(6):545–54. doi: 10.1369/jhc.6A7134.2007. [DOI] [PubMed] [Google Scholar]
- 10. Vinod KR, Jones D, Udupa V. A simple and effective heat induced antigen retrieval method. MethodsX. 2016;3:315–9. doi: 10.1016/j.mex.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eckhard AH, O’Malley JT, Nadol JB, Jr, Adams JC. Mechanical compression of coverslipped tissue sections during heat-induced antigen retrieval prevents section detachment and preserves tissue morphology. J Histochem Cytochem. 2019;67(6):441–52. doi: 10.1369/0022155419826940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gendusa R, Scalia CR, Buscone S, Cattoretti G. Elution of high-AFFINITY (>10-9 KD) antibodies from tissue sections: clues to the molecular mechanism and use in sequential immunostaining. J Histochem Cytochem. 2014;62(7):519–31. doi: 10.1369/0022155414536732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boi G, Scalia CR, Gendusa R, Ronchi S, Cattoretti G. Disaccharides protect antigens from drying-induced damage in routinely processed tissue sections. J Histochem Cytochem. 2016;64(1):18–31. doi: 10.1369/0022155415616162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolognesi MM, Manzoni M, Scalia CR, Zannella S, Bosisio FM, Faretta M, Cattoretti G. Multiplex staining by sequential immunostaining and antibody removal on routine tissue sections. J Histochem Cytochem. 2017;65(8):431–44. doi: 10.1369/0022155417719419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jhc-10.1369_00221554211007793 for Improvement of Opal Multiplex Immunofluorescence Workflow for Human Tissue Sections by Marcella Willemsen, Gabrielle Krebbers, Marcel W. Bekkenk, Marcel B.M. Teunissen and Rosalie M. Luiten in Journal of Histochemistry & Cytochemistry