Abstract
Background:
There is evidence that the amygdala undergoes extensive development. The exact nature of this change remains less clear with evidence suggesting linear, curvilinear, and null effects. The aim of this study is the identification of a normative reference of left and right amygdala development by parceling variance into separate effects of age and longitudinal growth.
Methods:
Data come from the National Institutes of Health MRI Study of Normal Brain Development. Participants in this sample were 54% female and ranged in age from 5 to 18 years (mean = 11.37) at study entry.
Results:
As predicted, the age at initial scan moderated the slope of both left and right amygdala volumes demonstrating that the nature of longitudinal growth varies across age (i.e., steeper slopes observed among those first scanned at an early age). Follow up analysis showed that the positive longitudinal growth slope becomes non-significant at 13.1 years of age for the left amygdala, and 14.5 years for the right, suggesting growth of the left amygdala ‘peaks’ earlier than the right.
Conclusions:
Findings suggest that rapid increases in volumes at early ages decline as youth enter adolescence and may turn to minor declines in volume during late adolescence or early adulthood.
Keywords: amygdala, neural development, accelerated longitudinal design, multilevel modeling, normative development, cohort-sequential design
There appears to be clear evidence that the amygdala undergoes extensive morphological change across childhood and into early adulthood (1–5). Despite seminal research in this area, and recent advances using large-sample datasets, reports on the trajectory of amygdala development continue to be inconsistent, with researchers describing linear, curvilinear, and null effects in the left, right, and bilateral amygdalae (1–7). Moreover, longitudinal research examining change in the amygdala in relation to broader trends in physiological maturation remains limited.
A clear baseline reference for “normative” amygdala development may help increase consistency in studies investigating non-normative variation in individuals exposed to psychosocial stress (8–12) Amygdala volume is likely influenced by fluctuation in hormone release across puberty (13). Broadly, the timing of intertwining processes in child development (such as puberty) may be extraordinarily variable, both within and between individuals (14). Very few developmental landmarks occur within a tightly constrained time period across individuals, undermining the value of comparisons made across same-aged youth.
Cohort-sequential (CS; also known as accelerated longitudinal) design provides a compromise by simultaneously tracking “cohorts” of youth at varying ages over overlapping time periods. The true nature of change across age is then closely approximated by linking the age-overlapped data segments (15). Hoffman (16) cautions researchers using this approach that proper treatment of the data modeling change in ‘multiple dimensions of time’. Specifically, the passage of time may have different effects on the outcome depending on the point in time a participant entered the study. Current CS studies in developmental neuroscience have tended to consider the former – changes in the brain as participants age, but not the latter – the moderating influence of when a participant took part in the study (2,6). This ignores the fact that longitudinal growth trends in brain development (on the order of years) may be quite different in youth at six years of age versus age 12 or 16. For example, Albaugh et al. (6) used mixed-methods modeling to test for differences in amygdala volumes as an effect of age, as well as any moderating effect of sex or CBCL subscale score (specifically Aggression, Anxiety/Depression, and Attention Problems). A linear model of amygdala development across age showed superior fit over competing quadratic and cubic models, with volumes increasing consistently across age.
Yet, this finding of a linear trajectory of development conflicts with similar research suggesting that subcortical structures such as the amygdala may not grow at a constant pace across development (2). This inconsistency indeed suggests the need for analyses of CS data to incorporate both within and between-subject effects of development. To avoid assuming convergence, it is necessary to consider each type of developmental effect separately, as opposed to ‘smushing’ predictors with different meanings together. Hoffman (16) describes these as ‘alternative metrics’ of time. Briefly described, within subjects, data points vary along a metric of ‘study time’, or when they were collected relative to initial assessment. This can be incorporated into predictive models by adding an effect of change in time, age, or pubertal maturation since study entry. However, in CS studies, this initial assessment of each participant is conducted at varying points in ‘true’ time (e.g., chronological age, pubertal maturation). Therefore, the observations also vary between-subjects in terms of the part of developmental trajectory depicted. This variance can be incorporated into the model by adding the point of study entry or ‘cohort’ as a between-subjects predictor in the form of chronological age or pubertal development. Ultimately, this parceling of variance in neuroanatomical development into separate effects of age and time may enhance the precision and granularity of investigations into the growth of structures like the amygdala.
The aim of this study was to add to the literature by using the above modeling approach to identify a normative reference of left and right amygdala development and to examine lateral differences in development. Knowledge surrounding morphometric change in the amygdala largely stems from cross-sectional research, which cannot provide true evidence of developmental effects. Longitudinal research remains quite limited, and has tended to average amygdala volumes across hemispheres, despite cross-sectional evidence suggesting lateral differences (4,5). Thus, the current study will incorporate both within and between-subject effects of developmental time – specifically the time a participant spends in the study and their development at study entry, respectively. This approach avoids the age convergence assumption previously described and may better capture nuanced changes in the amygdala. Building on the work of Goddings et al. (2), “development” will be considered in terms of both chronological age and pubertal maturation. Exploratory analyses will examine sex difference. It was hypothesized A) that more dramatic growth will be evident early in development, followed by a plateau in early to mid-adolescence, and a possible decline in late adolescence or early adulthood, as identified in cross-sectional work (4); B) this trend will be evident in models that index development using chronological age, pubertal maturation, or a combination of both and C) right amygdalae will be larger on average and reach maximum volume earlier than left amygdalae (4).
Method
Participants
Data for this study comes from the National Institutes of Health MRI Study of Normal Brain Development (NIHPD), a longitudinal multi-method study undertaken to establish a public repository of anatomical neurological scans of typically developing youth (as described in BDCG & Evans, 2006). The project was exempted from review by the Institutional Review Board due to the use of de-identified data.
The original data package contained 1,058 MRI scans of 431 youth. Exclusion of left-handed and ambidextrous youth reduced this to 924 scans of 387 youth, while exclusion of scans that failed processing (most frequently due to motion artifacts) resulted in a final count of 637 scans of 330 youth (see Figure 1). Participants in the final sample were majority female (54%), ranged in age from 4.88 to 18.35 years (mean = 11.37, SE = 3.75) at study entry with a mean Tanner stage of 2.62 (1.37).
Figure 1.
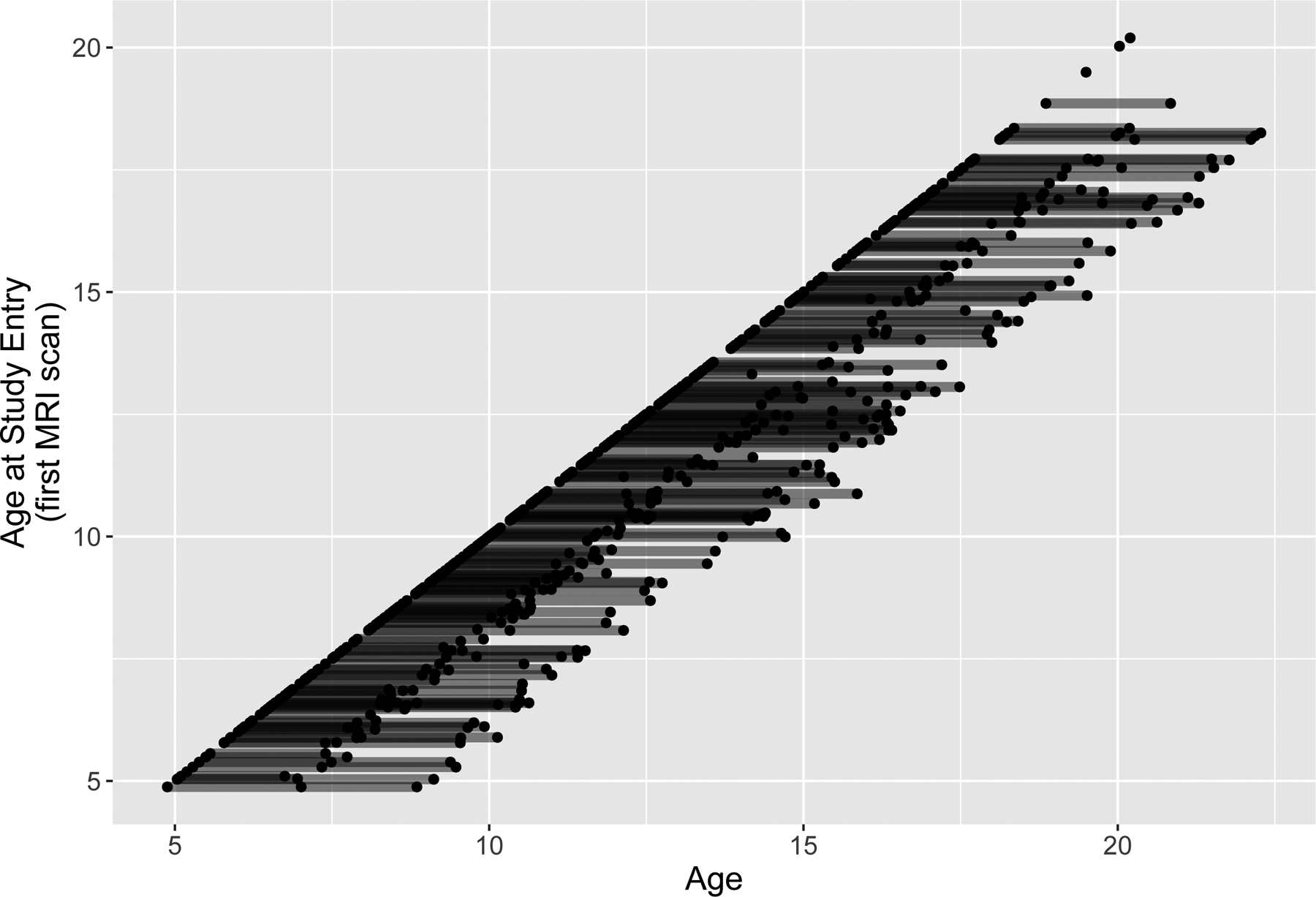
Accelerated longitudinal design of data collection in the National Institutes of Health MRI Study of Normal Brain Development. Participants (lines) are organized by age at study entry, with points denoting times of data collection. MRI, magnetic resonance imaging.
Measures
Pubertal progress was assessed using the Pubertal Development Scale (PDS; 18), a 10-item interview assessing progress in physical maturation (19). The PDS was administered by an on-site neurologist, and was omitted at their discretion when participants were age 10 or under. PDS scores were converted to a Tanner-stage metric using the methodology described by Shirtcliff, Dahl, and Pollak (19). Treatment of missingness in this and other variables of interest is described in supplemental materials.
MRI
Acquisition
A brief overview of scan acquisition procedures and parameters is provided below, as derived from the NIHPD MRI Manual, where a more complete description may be found (20). Multi-spectral magnetic resonance images were acquired at 1.5T. All MRI data were visually inspected at time of collection and scans were repeated in the event of substantial motion artifacts (20).
Scan processing protocol
All subsequent processing and analyses were performed by the first author, using the FreeSurfer image analysis suite (v6.0; surfer.nmr.mgh.harvard.edu) which performs automated segmentation and labeling of subcortical volumes. This release incorporates a newly developed ex vivo atlas of the amygdala and its sub-nuclei that provides enhanced precision over earlier iterations (21). An overview of the processing and segmentation pipeline is provided in supplemental materials (22,23). Output was then validated against original estimates performed using different software (MNI ANIMAL; 24,24).
Data Analysis
Proper treatment of CS data requires researchers to model both the within and between-subjects (cohort) effects of time (16). By design, participants in CS designs enter the study at varying ages, or stages of development. This between-subjects or “cohort” variance in age may significantly inform model predictions. Appropriately, separate variables were created for considering the effects of chronological time in the current analyses. At the within-subjects level, Time, was a continuous variable reflecting a participant’s time since first observation (i.e., time in study) and was computed by subtracting a participant’s date of birth from each scan date. A between-subjects variable, Age@1stScan referred to a participant’s age (in years) at study entry/first MRI scan, and was captured by subtracting date of birth from date of first scan. A second index of developmental progress was computed based on pubertal status. Similar within and between-subjects variables of “developmental time” were derived from the continuous Tanner scale scores (as described above). At the within-subjects level, the variable TSChange reflected the change in Tanner stage across time points. The between-subjects variable TS@1stScan referred to each participant’s Tanner stage score at study entry (initial MRI scan).
Modeling Normative Amygdala Development
Model development and effects testing was conducted using the stepped approach described by (16), who suggests a two-stage process of evaluating an incremental series of models adding fixed and random effects. In the initial stage, a series of unconditional longitudinal models (i.e., no between-subjects effects) were evaluated that tested fixed within-subject effects of variables capturing within-subject changes in development (chronological time: Time, pubertal development: TSChange). Such models are functionally equivalent to ordinary least squares linear regression. For each within-subjects variable, we next tested a random-effect, reflecting between-subject variability in the fixed effect on amygdala. Significance was determined by contrasting the deviance of models with and without a random effects term. A final unconditional model contained all fixed effects, and significant random effects. In a second stage, between-subjects predictors (e.g., Age@1stScan) were added to test their effect on the intercept. Cross-level interaction terms were then added to determine which between-subjects terms moderated the association between within-subjects terms and amygdala volume. Where necessary, significant interactions were decomposed using the Johnson-Neyman technique (25), which provides the range of values along a continuous moderator (e.g., Age@1stScan) for which the association between the predictor and outcome is significant (26).
This process was repeated, separately for left and right amygdala volumes, across three types of models that incorporated different metrics of time:
A Chronological Age model that predicted change in amygdala volumes according to the years since a participant’s entry into the study (Time), and the cohort-specific effect of chronological age at first scan (Age@1stScan).
A Pubertal Development model that predicted change in amygdala volumes according to the change in a participants’ pubertal status since study entry (TSChange) and the between-subjects effect of pubertal status at study entry (TS@1stScan).
A Combined model that incorporated both within-subjects predictors (Time and TSChange), and both cohort effects (Age@1stScan and TS@1stScan).
A broader review of mixed effects model analyses is provided in supplemental materials. Each model was first tested using the complete sample, then separately among boys and girls.
RESULTS
Analyses began by testing the Chronological Age model’s prediction of change in right amygdala volumes (see Table S1 for a listing of all mixed model formulas), where 83% of the variance in right amygdala volumes was between persons. A fixed linear effect of time (years) in study (Time) and its random variance across participants were both significant (17.30mm3/year, p < .001; −2ΔLL(1) = 6.92, p < .01; Table 2), indicating that right amygdala volumes increased on average over the course of the study, though this trend varied significantly across individuals. Age at first scan (Age@1stScan) was added next and exhibited a positive effect on the intercept, but a negative effect on the slope (change in amygdala volume over time since study entry). Each year of a participant’s Age@1stScan predicted a 14.12mm3 (p < .001) difference in right amygdala volumes, while attenuating the rate of growth by −5.81mm3/year (p < .001; see Table 2). Decomposition of this interaction using the J-N technique (Table 3) revealed that the slope of change in amygdala volumes during the study (Time) remained significant (i.e., p < .05) from 4.9 years (the age of the youngest participant in the sample) through 14.3 years (see Figure 2). No remarkable differences were observed across boys or girls (Tables S4, S5).
Table 2.
Change in Amygdala Volumes as a Function of Time and Age at Initial Scan
| Right Amygdala Volume (mm3) | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | |
| Fixed Effects Parameters | ||||||||
| Intercept | 1,635.98*** | 10.69 | 1,619.09*** | 11.03 | 1,457.41*** | 34.37 | 1,430.47*** | 34.96 |
| Time | 17.30*** | 2.54 | 19.31*** | 2.91 | 65.64*** | 8.52 | ||
| Age@1stscan | 14.12*** | 2.87 | 16.50*** | 2.92 | ||||
| Time × Age@1stScan | −4.13*** | 0.71 | ||||||
| Random Effects Parameters | ||||||||
| σ2 | 7,862.962 | 6,763.200 | 4,727.013 | 5,052.716 | ||||
| τ00 | 32,926.234 | 33,988.404 | 32,218.316 | 32,341.531 | ||||
| n | 330 | 330 | 330 | 330 | ||||
| Scans | 637 | 637 | 637 | 637 | ||||
| Left Amygdala Volume (mm3) | ||||||||
| B | SE | B | SE | B | SE | B | SE | |
| Fixed Effects Parameters | ||||||||
| Intercept | 1580.11*** | 9.81 | 1571.33*** | 10.12 | 1499.86*** | 32.06 | 1478.63*** | 32.60 |
| Time | 8.96*** | 2.38 | 9.76*** | 2.63 | 39.43*** | 8.34 | ||
| Age@1stscan | 6.25* | 2.68 | 8.10** | 2.73 | ||||
| Time × Age@1stScan | −2.61*** | 0.70 | ||||||
| Random Effects Parameters | ||||||||
| σ2 | 6302.988 | 5985.237 | 4888.177 | 4821.210 | ||||
| τ00 | 27944.557 | 28347.536 | 27642.634 | 27725.735 | ||||
| n | 330 | 330 | 330 | 330 | ||||
| Scans | 637 | 637 | 637 | 637 | ||||
Note. Time = Age at current scan – Age at first scan. Age@1stScan = chronological age at initial assessment/study entry.
p < .05.
p < .01.
p < .001.
σ2 = Random effects variance. τ00 = Between-subjects variance.
Table 3.
Johnson-Neyman Significance Regions for the Conditional Effect of Age at First Scan on Change in Amygdala Volumes across Time in Study
| Right Amygdala | ||||||
|---|---|---|---|---|---|---|
| Age at 1st Scan | Slope | SE | LCI | UCI | t | p |
| 20.00 | −17.55 | 6.44 | −30.17 | −4.93 | −2.73 | .007 |
| 19.00 | −13.41 | 5.81 | −24.79 | −2.02 | −2.31 | .022 |
| 18.00 | −9.26 | 5.19 | −19.45 | 0.92 | −1.78 | .075 |
| 17.00 | −5.12 | 4.60 | −14.14 | 3.90 | −1.11 | .267 |
| 16.00 | −0.98 | 4.04 | −8.90 | 6.94 | −0.24 | .809 |
| 15.00 | 3.16 | 3.53 | −3.75 | 10.08 | 0.90 | .370 |
| 14.00 | 7.31 | 3.08 | 1.26 | 13.35 | 2.37 | .018 |
| 13.00 | 11.45 | 2.74 | 6.08 | 16.83 | 4.17 | < .001 |
| 12.00 | 15.59 | 2.55 | 10.60 | 20.59 | 6.12 | < .001 |
| 11.00 | 19.74 | 2.53 | 14.78 | 24.70 | 7.80 | < .001 |
| 10.00 | 23.88 | 2.70 | 18.59 | 29.17 | 8.85 | < .001 |
| 9.00 | 28.02 | 3.02 | 22.11 | 33.94 | 9.29 | < .001 |
| 8.00 | 32.17 | 3.45 | 25.41 | 38.92 | 9.33 | < .001 |
| 7.00 | 36.31 | 3.95 | 28.56 | 44.05 | 9.19 | < .001 |
| 6.00 | 40.45 | 4.51 | 31.62 | 49.28 | 8.98 | < .001 |
| 5.00 | 44.60 | 5.09 | 34.61 | 54.58 | 8.76 | < .001 |
| Left Amygdala | ||||||
| Age at 1st Scan | Slope | SE | LCI | UCI | t | p |
| 20.00 | −12.92 | 6.28 | −25.24 | −0.60 | −2.06 | .041 |
| 19.00 | −10.33 | 5.67 | −21.44 | 0.79 | −1.82 | .069 |
| 18.00 | −7.74 | 5.07 | −17.68 | 2.20 | −1.53 | .128 |
| 17.00 | −5.15 | 4.49 | −13.95 | 3.66 | −1.15 | .253 |
| 16.00 | −2.55 | 3.95 | −10.29 | 5.18 | −0.65 | .518 |
| 15.00 | 0.04 | 3.44 | −6.72 | 6.79 | 0.01 | .992 |
| 14.00 | 2.63 | 3.01 | −3.27 | 8.53 | 0.87 | .383 |
| 13.00 | 5.22 | 2.68 | −0.03 | 10.47 | 1.95 | .052 |
| 12.00 | 7.81 | 2.49 | 2.93 | 12.68 | 3.14 | .002 |
| 11.00 | 10.40 | 2.47 | 5.56 | 15.24 | 4.21 | < .001 |
| 10.00 | 12.99 | 2.63 | 7.83 | 18.15 | 4.93 | < .001 |
| 9.00 | 15.58 | 2.95 | 9.81 | 21.36 | 5.29 | < .001 |
| 8.00 | 18.17 | 3.37 | 11.58 | 24.77 | 5.40 | < .001 |
| 7.00 | 20.76 | 3.86 | 13.20 | 28.33 | 5.38 | < .001 |
| 6.00 | 23.35 | 4.40 | 14.73 | 31.98 | 5.31 | < .001 |
| 5.00 | 25.95 | 4.97 | 16.20 | 35.69 | 5.22 | < .001 |
Note. LCI = 95% lower confidence interval. UCI = 95% upper confidence interval.
Figure 2.

Interactive effect of age at first scan and change in age (i.e., time in study) on change in right amygdala volumes.
In the left amygdala, 83% of variance was attributable to between-person differences. Fixed linear and random effects variances of time in study (Time) were both significant (8.97mm3/year, p < .001; −2ΔLL(1) = 5.63, p < .05; Table 2), suggesting a positive linear increase in left amygdala volumes on average, as well as substantial variation in the trend across participants. Participant age at study entry (Age@1stScan) predicted the intercept of left amygdala volumes, with an increase of 6.25mm3/year of age. Age@1stScan further moderated the slope of left amygdala volume growth. Each year of chronological age at study entry (Age@1stScan) predicted a −2.61mm3/year decrease in the rate of left amygdala growth (p < .001; see Table 2). Application of the J-N technique revealed that the slope became non-significant at age 13.0 years (Table 3; see Figure 3). In sex-specific analyses, Age@1stScan affected the intercept for boys but not girls, while the reverse was true for the interaction between Time and Age@1stScan (Tables S6, S7).
Figure 3.
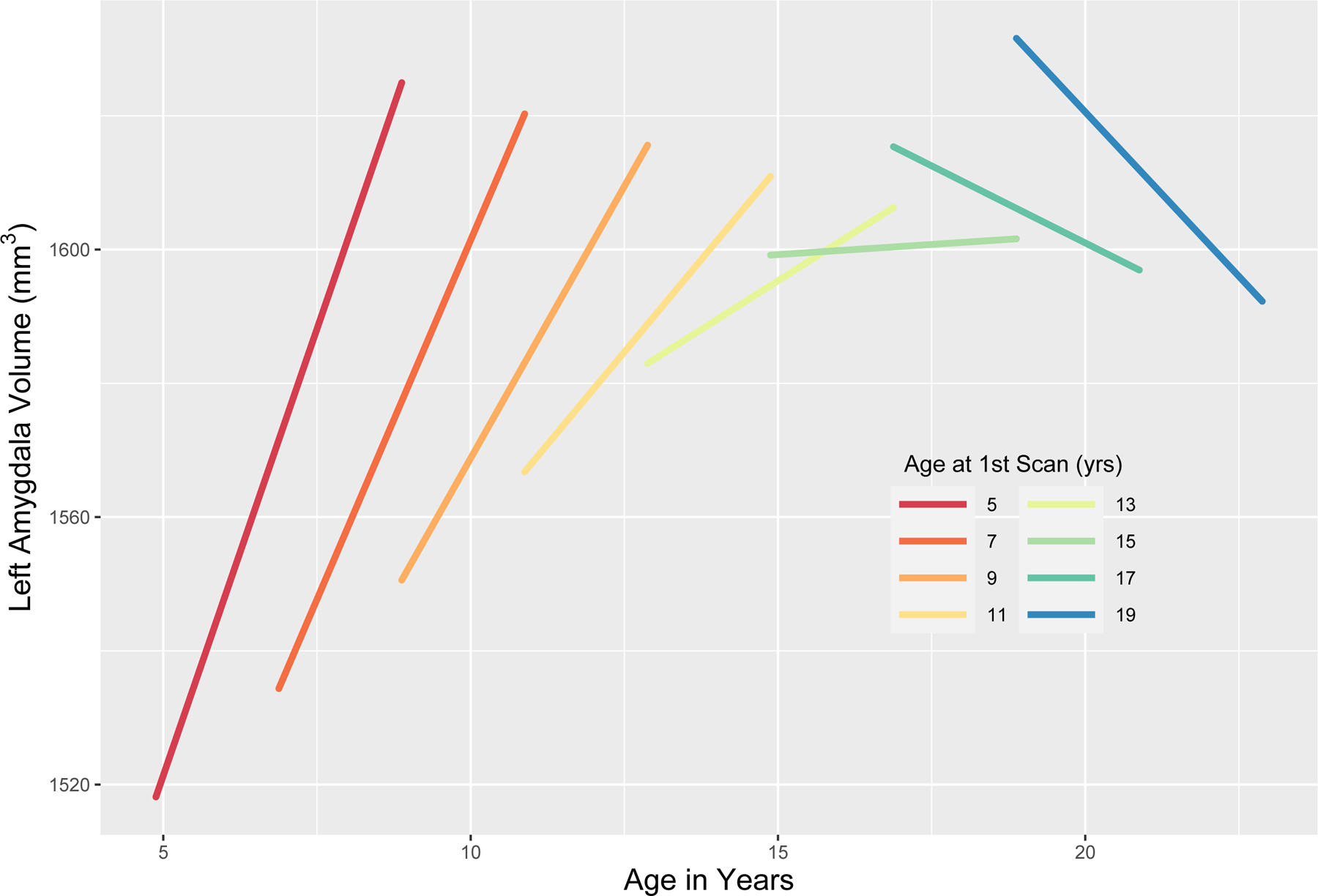
Interactive effect of age at first scan and change in age (i.e., time in study) on change in left amygdala volumes.
Analyses next examined growth along a pubertal development time metric, beginning with the right amygdala (see Table 4), and using the same approach. The fixed effect of pubertal change (TSChange) was significant (p < .05), with right amygdala volumes growing by 34.89mm3 with each Tanner stage. The corresponding random effect was non-significant, suggesting that this trend was largely stable across participants. Tanner stage at study entry (TS@1stScan) predicted the model intercept, in that initial right amygdalae volumes were 17.26mm3 (p < .05) greater for each Tanner stage reached by study entry (Table 4), though did not meaningfully influence the slope. In sex-specific analyses, the effect of TSChange on the intercept was significant for girls, though not boys, while the reverse was true for the between-subjects effect of TS@1stScan (Table S8).
Table 4.
Change in Amygdala Volumes as a Function of Pubertal Development and Tanner Stage at Initial Scan
| Right Amygdala Volume (mm3) | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | |
| Fixed Effects Parameters | ||||||||
| Intercept | 1635.98*** | 10.69 | 1636.84*** | 11.95 | 1592.97*** | 25.18 | 1591.72*** | 25.27 |
| TSChange | 34.89*** | 6.25 | 35.72*** | 6.26 | 43.50** | 14.27 | ||
| TS@1stScan | 17.26 | 8.74 | 17.93* | 8.81 | ||||
| TSChange × TS@1stScan | −4.63 | 7.64 | ||||||
| Random Effects Parameters | ||||||||
| σ2 | 7862.962 | 6997.066 | 6996.983 | 6970.463 | ||||
| τ00 | 32926.234 | 33702.795 | 33171.360 | 33184.714 | ||||
| n | 330 | 274 | 274 | 274 | ||||
| Scans | 637 | 513 | 513 | 513 | ||||
| Left Amygdala Volume (mm3) | ||||||||
| B | SE | B | SE | B | SE | B | SE | |
| Fixed Effects Parameters | ||||||||
| Intercept | 1580.11*** | 9.81 | 1579.65*** | 11.13 | 1573.30*** | 23.40 | 1572.80*** | 23.47 |
| TSChange | 16.63** | 5.78 | 19.96** | 7.24 | 24.28 | 16.32 | ||
| TS@1stScan | 2.37 | 8.14 | 2.64 | 8.19 | ||||
| TSChange × TS@1stScan | −2.35 | 7.94 | ||||||
| Random Effects Parameters | ||||||||
| σ2 | 6302.988 | 5956.590 | 5042.971 | 5042.055 | ||||
| τ00 | 27944.557 | 29257.289 | 29280.836 | 29293.357 | ||||
| n | 330 | 274 | 274 | 274 | ||||
| Scans | 637 | 513 | 513 | 513 | ||||
Note. TSChange = Tanner stage at current scan – Tanner stage at first scan. TS@1stScan = pubertal development (Tanner stage) at initial assessment/study entry.
p < .05.
p < .01.
p < .001.
σ2 = Random effects variance. τ00 = Between-subjects variance.
Analyses next examined change in the left amygdala as a function of pubertal development (TSChange; Table 4). On average, left amygdala volumes increased 16.63mm3 (p < .01) with each Tanner stage, with meaningful variation across individuals (i.e., a significant random effect; −2ΔLL(1) = 8.37, p < .01). Pubertal development at study entry (TS@1stScan) was added to an unconditional model that retained the fixed (but not random) effect, though did not significantly affect either the intercept or slope (Table 4). No terms reached significance in modeling specific to boys or girls (Table S9).
A Combined Model was considered next and tested the effects of chronological age and pubertal development in a single model predicting amygdala volumes (see mixed model formulas in Table 1). Results from these analyses may be found in supplemental materials.
Table 1.
Formulae of Mixed Models Predicting Amygdala Volumes
| Chronological Age | Right/Left Amygdalati = π0i + π1i*(Timeti) + eti |
| π0i = β00 + β01*(Age@1stScani) + r0i | |
| π1i = β10 + β11*(Age@1stScani) | |
| Pubertal Development | Right/Left Amygdalati = π0i + π1i*(TSChangeti) + eti |
| π0i = β00 + β01*(TS@1stScani) + r0i | |
| π1i = β10 + β11*(TS@1stScani) | |
| Combined | Right/Left Amygdalati = π0i + π1i*(Timeti) + π2i*(TSChangeti) + π3i*(TSChangeti* Timeti) + eti |
| π0i = β00 + β01*(Age@1stScani) + β02*(TS@1stScani) + r0i | |
| π1i = β10 + β11*(Age@1stScani) + β12*(TS@1stScani) | |
| π2i = β20 + β21*(Age@1stScani) + β22*(TS@1stScani) | |
| π3i = β30 + β31*(Age@1stScani) + β32*(TS@1stScani) |
DISCUSSION
Though collective knowledge about the fully formed amygdala is nearly 200 years old (27), neuroscientists are just now beginning to examine its development. The current study builds on previous work exploring the amygdala’s growth (1,2,6), while making important empirical and methodological advances. Foremost, the findings provide a well-founded reference for amygdala growth in typically developing youth, which might be used as a baseline for assessing the severity of deviation in non-typical or disordered youth. Further, results reveal a previously unidentified difference in the growth of the left and right amygdalae, which may open avenues for future investigations. Moreover, the analyses provide a template for treating data from CS design studies, where researchers must take care to consider multiple effects of ‘time’. The results demonstrate how the selection of an index of ‘developmental’, be it puberty or age, can impact results.
As anticipated based on extant cross-sectional research, positive growth in right and left amygdala volumes was observed. Notably, however, the trajectory of change appeared to differ across the left and right sides of the brain. For each year of participation in the study, right amygdala volumes increased 19.04mm3 on average, while a similar, but more gradual increase was seen in the left amygdala, which grew at less than half the rate (9.92mm3/year). As anticipated, the age at which children were initially scanned moderated the slope of both left and right amygdala volumes, indicating that the nature of growth varies across development. Specifically, steeper slopes were observed among youth who entered the study at an early age (i.e., < 10 yrs.). This is depicted visually in Figure 2, which shows the linear slopes of right and left amygdala volumes across different ages of study entry. Dramatic gains in volume at ages 5 and 6 become more moderate as youth enter early adolescence. Decomposition of the interaction between age at first scan and time in study using the JN technique found that the slope becomes non-significant at 13.1 years of age for the left amygdala, and 14.5 years for the right. Thus, the volumetric growth of the left amygdala ‘peaks’ earlier than the right.
However, this finding contradicts the current hypotheses, which anticipated faster development of the right amygdala. Weems (10) synthesis of the literature reveals that the right amygdala may be more susceptible to effects of stress exposure. Notably, the typically developing participants in the current sample were unlikely to have endured such extreme forms of stress. Still, even normative youth undergo some stressful experiences during development; therefore, it would seem reasonable to assume that the right amygdala would exhibit slightly earlier growth in a normative sample. Conversely, however, this assumes that in the pristine brain of an individual whose development was completely stress-free, we should expect the right and left amygdala to grow and change in a symmetrical fashion. On its face, this assumption seems untenable, given our extensive knowledge about functional and structural lateral differences in the brain (28). Moreover, based on model intercept values in the results of the current study, right amygdala volumes are generally larger than left, a finding consistent with a wealth of evidence from adult studies (29). Presumably, this may stem from prolonged development of the right amygdala, therefore a later ‘peak’ in volume as shown in the findings.
It is notable that differences in amygdala development across boys and girls were generally unremarkable, though with some caveats. Both sexes demonstrated significant increases in right amygdala volume across their time in study, though this effect was only observed when youth were younger than 13. In the left amygdala, the effect of age at assessment on time in study was significant only for girls, though the magnitude of the effect was quite similar in boys. For both sexes, meaningful change in left amygdala volumes was only detected prior to age 12. This contrasts with Hu et al. (3) who also modeled sex differences in lateral amygdala volumes, though in cross-sectional data. There the authors found that girls reached peak amygdala volume (both hemispheres), while boys amygdala volumes continued to increase throughout adolescence. Differences in these findings may be attributable to our choice to model multiple dimensions of time, or our use of longitudinal data. Regarding puberty, we noted that while lateral volumes increased with in-study pubertal development, there was no significant effect of pubertal status at study entry for boys or girls. We are aware of no similar research modeling lateral amygdala development against puberty in such a wide age-range, therefore these findings will need replication in future work.
Though this study brings volumetric and developmental differences in the right and left amygdala into focus, the mechanism underlying this effect remains unclear. While scholars often characterize it as a unified structure, the amygdala is actually comprised of multiple subregions containing highly differentiated cells (30). Functional neuroimaging reveals that the cells in these substructures may show specialized responses to aspects of emotionally salient stimuli (31). Lateral differences in cellular composition (i.e., sub-amygdalar volumes) might account for lateral differences in responsive activation to stimuli (e.g., 32). Moreover, and relevant to the current study, such differences might also underlie the variation in size and growth seen across the left and right amygdalae, though investigation of this possibility will require substructure segmentation – an important next step for amygdala research. In vivo research into the substructure of the human amygdala is only recently emerging from expanded work with high-resolution scans (e.g., 7T), multimodal approaches, and advanced segmentation techniques (21,33,34)
Though the findings reveal meaningful, dynamic change in amygdala volumes across development, it is important to consider that these results were only observed in models where development was indexed according to age in years. In contrast, corresponding analyses that predicted change in amygdala volumes according to puberty (or a combination of age and puberty), failed to show any change in the nature of amygdala growth as children developed. This difference in results might be a product of the way this study and many others assess pubertal development. The Tanner stage system translates the appearance of several outward pubertal changes onto a graded five-level scale of development (35,36). However, a discrete, five-point scale may not provide the sensitivity necessary to index subtle changes in neuroanatomical development. Moreover, variance in Tanner stages does not appear until the start of adrenarche, which occurs at approximately 6 to 8 years of age in girls, and a year later in boys (35). Results in the current study show that by this point, the amygdala has already undergone dramatic change, which would not be indexed by a Tanner stage metric. Similarly, declining amygdala volumes in late adolescence/early adulthood, when most youth have reached Tanner stage 5, cannot be captured using this system.
We believe these analyses provide a template for treating data from CS design studies, where researchers must take care to consider multiple effects of ‘time’. Commonly, researchers using CS data have modeled change in outcomes as a function of change in within-subject variables, such as chronological age or pubertal development. However, in doing, so investigators implicitly assume negligible influence of between-subjects differences in age at assessment. Ultimately, this presumes that the effect of the passage of time on an outcome variable (e.g., amygdala volume) is invariant (i.e., linear) across development. Yet, rarely, if ever are developmental trajectories truly linear across the lifespan. More typically, the waxing and waning of environmental and biological influences is likely to produce curves and bends in developmental trends. This work proposes a remedy for future research with CS designs that incorporates influence of both within and between-subjects effects using mixed effects model analyses.
The novel finding of lateral differences across development encourages subsequent research investigating the sub-structural makeup of the left and right amygdala. It may be that specific regions of the amygdala drive the effect, potentially due to respective involvement in specific functions of affective processing – such as orientation to threatening stimuli (32). Indeed, this reflects a broader trend in human neuroscience towards investigation at the substructural, cellular, and subcellular levels. Despite increasing support for a decline in amygdala volumes during early adulthood (4), there is presently a paucity of longitudinal research examining changes in the amygdala during this period. Our results suggest the need for future investigations that might better elucidate the amygdala’s changes during this period, and explore potential mechanisms. Finally, results demonstrate the importance of considering variability in timing and tempo of neurodevelopment across multiple metrics of development.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Russell is supported by a grant from the National Institute of Mental Health (T32-MH018931). Dr Weems is supported by grants from the National Institute of Justice (2019-R2-CX-0013), Department of Justice Office on Violence Against Women (2017-SI-AX-0004), US Environmental Protection Agency (84004001), Youth Policy Institute of Iowa, and contracts with the state of Iowa (Child Support Training BOC-18-003 and Service Training FOSU-20-001& 21-001; prime sponsor for both HHS-US Department of Health & Human Services).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. (1996): Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol 366: 223–230. [DOI] [PubMed] [Google Scholar]
- 2.Goddings A-L, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore S-J (2014): The influence of puberty on subcortical brain development. NeuroImage 88: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu S, Pruessner JC, Coupé P, Collins DL (2013): Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. NeuroImage 74: 276–287. [DOI] [PubMed] [Google Scholar]
- 4.Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H (2012): Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PloS One 7: e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S (2014): Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage 96: 67–72. [DOI] [PubMed] [Google Scholar]
- 6.Albaugh MD, Nguyen T-V, Ducharme S, Collins DL, Botteron KN, D’Alberto N, et al. (2017): Age-related volumetric change of limbic structures and subclinical anxious/depressed symptomatology in typically developing children and adolescents. Biol Psychol 124: 133–140. [DOI] [PubMed] [Google Scholar]
- 7.Goddings A-L, Mills K, Clasen L, Giedd J, Viner R, Blakemore S-J (2014): Longitudinal MRI to assess effect of puberty on subcortical brain development: An observational study. The Lancet 383: S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. (2015): Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry 77: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. (2010): Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci 13: 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weems CF (2017): Severe stress and the development of the amygdala in youth: A theory and its statistical implications. Dev Rev 46: 44–53. [Google Scholar]
- 11.Weems CF, Klabunde M, Russell JD, Reiss AL, Carrión VG (2015): Post-traumatic stress and age variation in amygdala volumes among youth exposed to trauma. Soc Cogn Affect Neurosci 10: 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weems CF, Scott BG, Russell JD, Reiss AL, Carrión VG (2013): Developmental variation in amygdala volumes among children with posttraumatic stress. Dev Neuropsychol 38: 481–495. [DOI] [PubMed] [Google Scholar]
- 13.Scherf KS, Smyth JM, Delgado MR (2013): The amygdala: An agent of change in adolescent neural networks. Horm Behav 64: 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehl M, Hooker K, Sliwinski MJ (2015): A brief historical overview of intraindividual variability research across the lifespan. In: Diehl M, Hooker K, Sliwinski MJ, editors. Handbook of Intraindividual Variability across the Life Span. New York, NY: Routledge, pp 3–15. [Google Scholar]
- 15.Miyazaki Y, Raudenbush SW (2000): Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychol Methods 5: 44–63. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman L (2015): Longitudinal Analysis: Modeling within-Person Fluctuation and Change. New York, NY: Routledge. [Google Scholar]
- 17.Brain Development Cooperative Group, Evans AC (2006): The NIH MRI study of normal brain development. NeuroImage 30: 184–202. [DOI] [PubMed] [Google Scholar]
- 18.Petersen AC, Crockett L, Richards M, Boxer A (1988): A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 17: 117–133. [DOI] [PubMed] [Google Scholar]
- 19.Shirtcliff EA, Dahl RE, Pollak SD (2009): Pubertal development: Correspondence between hormonal and physical development. Child Dev 80: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NIHPD (2006, November): The MRI Study of Normal Brain Development: MRI procedure manual. Retrieved from https://pediatricmri.nih.gov/nihpd/info/Documents/Protocol_Nov06.pdf
- 21.Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, et al. (2017): High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. NeuroImage 155: 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischl B, Salat DH, Busa E, Albert MS, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 23.Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012): Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 61: 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- 25.Johnson PO, Neyman J (1936): Tests of certain linear hypotheses and their application to some educational problems. Stat Res Mem 1: 57–93. [Google Scholar]
- 26.Preacher KJ, Curran PJ, Bauer DJ (2006): Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat 31: 437–448. [Google Scholar]
- 27.Burdach KF (1819): Vom Baue Und Leben Des Gehrins, vol. 1. Leipzig: Dyk’schen Buchhandlung. [Google Scholar]
- 28.Ocklenburg S, Güntürkün O (2018): The Lateralized Brain: The Neuroscience and Evolution of Hemispheric Asymmetries. London: Academic Press. [Google Scholar]
- 29.Guadalupe T, Mathias SR, vanErp TGM, Whelan CD, Zwiers MP, Abe Y, et al. (2017): Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging Behav 11: 1497–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson LW, Petrovich GD (1998): What is the amygdala? Trends Neurosci 21: 323–331. [DOI] [PubMed] [Google Scholar]
- 31.Balderston NL, Schultz DH, Hopkins L, Helmstetter FJ (2015): Functionally distinct amygdala subregions identified using DTI and high-resolution fMRI. Soc Cogn Affect Neurosci 10: 1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M (2001): Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4: 437–441. [DOI] [PubMed] [Google Scholar]
- 33.Crone EA, Elzinga BM (2015): Changing brains: how longitudinal functional magnetic resonance imaging studies can inform us about cognitive and social-affective growth trajectories. Wiley Interdiscip Rev Cogn Sci 6: 53–63. [DOI] [PubMed] [Google Scholar]
- 34.De Martino F, Yacoub E, Kemper V, Moerel M, Uludağ K, De Weerd P, et al. (2018): The impact of ultra-high field MRI on cognitive and computational neuroimaging. NeuroImage 168: 366–382. [DOI] [PubMed] [Google Scholar]
- 35.Dorn LD, Biro FM (2011): Puberty and its measurement: A decade in review. J Res Adolesc 21: 180–195. [Google Scholar]
- 36.Tanner JM (1962): Growth at Adolescence. Springfield, IL: Thomas. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


