Abstract
Novelty triggers an increase in orienting behavior that is critical to evaluate the potential salience of unknown events. As novelty becomes familiar upon repeated encounters, this increase in response rapidly habituates as a form of behavioral adaptation underlying goal-directed behaviors. Many neurodevelopmental, psychiatric and neurodegenerative disorders are associated with abnormal responses to novelty and/or familiarity, although the neuronal circuits and cellular/molecular mechanisms underlying these natural behaviors in the healthy brain are largely unknown, as is the maladaptive processes that occur to induce impairment of novelty signaling in diseased brains. In rodents, the development of cutting-edge tools that allow for measurements of real time activity dynamics in selectively identified neuronal ensembles by gene expression signatures is beginning to provide advances in understanding the neural bases of the novelty response. Accumulating evidence indicate that midbrain circuits, the majority of which linked to dopamine transmission, promote exploratory assessments and guide approach/avoidance behaviors to different types of novelty via specific projection sites. The present review article focuses on midbrain circuit analysis relevant to novelty processing and habituation with familiarity.
Keywords: novelty, salience, neuronal circuits, midbrain, dopamine
1. Introduction
A major driving force for species survival and evolution is the ability to respond and adapt when faced with new changes in the environment. For any living organism, exposure to novelty (i.e. new environments/contexts, social experiences, food/ resources, sounds, odors, etc.) will naturally trigger an orienting response (Table 1) that will culminate with either an approach or avoidance behavior to the novel event (Fig. 1A). This increased response to novelty is necessary to evaluate the salience of unknown events, particularly the ones that could be potentially threatening, and provides a basic mechanism of survival. Nevertheless, upon evaluation, the heightened response to novelty rapidly habituates as novelty becomes familiar with repeated exposures (Fig. 1A). Altogether the novelty response and habituation (Table 1) leading to familiarity serve as fundamental processes underlying adaptation and prime goal-directed behaviors. Numerous neurodevelopmental (i.e. autism spectrum disorders1-3 / attention deficit hyperactivity disorder4,5), neuropsychiatric (i.e. schizophrenia6,7 / drug addiction8) and neurodegenerative disorders (Parkinson’s disease9) are frequently associated with abnormal response to novelty and/or disrupted habituation to familiarity10,11. Yet, the brain circuits and cellular/molecular mechanism underlying novelty and familiarity signaling in healthy brain has not been completely identified. In addition, how these circuits and mechanisms are disrupted in neuropathological conditions is largely unknown.
Table 1.
Key definitions related to the novelty response.
| Name | Definition |
|---|---|
| Novelty | An entity/stimulus or environment that an individual has not previously experienced in its lifetime. |
| Habituation | A form of associative learning in which a decrease in innate responses is elicited by repeated exposures to a stimulus or context. |
| Familiarity | A judgment or feeling of prior experience regardless of memory recollection. |
| Salience | The quality of a stimulus/context of being more prominent or noticeable as compared to the surroundings regardless of valence. *Novelty is a dimension of salience which drives an attentional bias and behavioral orienting. |
| Valence | An affective quality ascribed to the intrinsic attractiveness (positive valence) or aversiveness (negative valence) of a specific stimulus/context. *A novel stimulus may have an intrinsic positive valence, thus inducing an approach behavior, or a negative valence that promotes avoidance behavior. |
| Attention | The behavioral and cognitive process of selectively focusing on a specific aspect of information. *Novelty increases attention to elicit an appropriate behavioral response. |
| Orienting response | An individual’s immediate motor, behavioral and/or physiological response to novel stimuli or environmental changes. *Novelty triggers an orienting response by increasing attention and driving approach/avoidance behaviors. |
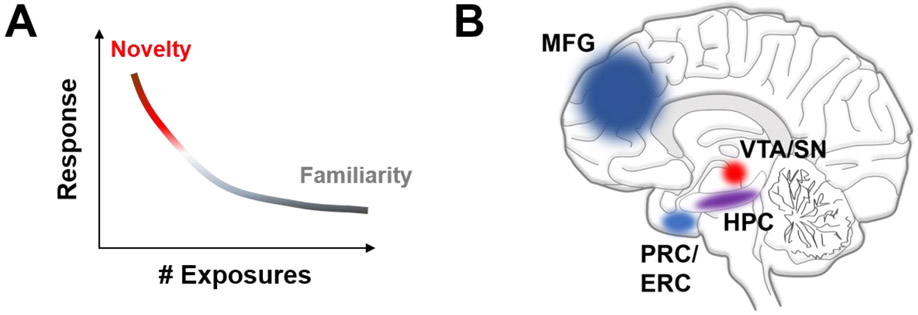
Figure 1. The novelty response and activated brain areas.
(A) Novel stimuli trigger an increase in behavioral response that rapidly decays as the stimulus becomes familiar with consecutive exposures. (B) Imaging studies in humans reveal that exposure to novelty increases the activity of MTL structures including the MFG (blue), PRC/ERC (light blue) or HPC (purple). Importantly, exposure to novelty raises the activity levels of midbrain DA centers within the VTA/SN (red) that are relevant for processing novelty-related salience. ERC, enthorinal cortex; HPC, hippocampus; MFG, middle frontal gyrus; PRC, perirhinal cortex; SN, substantia nigra; VTA, ventral tegmental area.
The response to novelty engages a wide neuronal network of many interconnected brain areas12. In humans, high-resolution functional imaging studies have determined that novel stimuli increase activity of medial temporal lobe (MTL) structures that include the hippocampus (HPC), the perirhinal, enthorinal and parahippocampal cortices, among other brain areas13,14 (Fig. 1B). For many decades, this network of interrelated structures has been deeply studied in the context of promoting novelty detection, memory encoding and retrieval15. Intriguingly, novel stimuli also raise the activity of midbrain areas such as dopamine (DA) neuron-rich systems in the substantia nigra (SN)/ventral tegmental area (VTA)16-19, the primary hub of reward-related processing (Fig. 1B). Novelty is considered another dimension of salience (Table 1) intrinsically linked to the value that is attributed to the different stimuli in the environment. Midbrain DA systems together with projections to the striatum are key in the evaluation of novelty signals and are both functionally connected to MTL structures such as the HPC, which conveys critical novelty-related information20. The role of midbrain areas in processing novelty and familiarity signaling has been a focus of interest over the last few years. In rodents, the development of cutting-edge tools that target specific cell types defined by genetic and projection profiles have allowed investigation of direct real-time activity dynamics and circuit elements encompassing the novelty response and habituation with familiarity. This review article provides an overview of novelty in terms of salience, and how specific midbrain systems, most of them linked to DA neurotransmission, guide behavioral responses to different novelty subtypes.
2. Types of novelty and stimulus salience
How do we define novelty? Novelty is inherently defined by a lack of previous experience and representation of a new event. However, there are many types of novelty that are based on the degree of prior knowledge with that new event. When an individual encounters novelty, each of the sensory features of the novel event are perceived, processed and integrated by thalamocortical circuits21. MTL structures rapidly process whether features of the novel stimulus have been previously encoded, as well as the mismatch between the novel event with regard to pre-existing information22-24. Novelty that somehow shares similar features with past experiences is referred to as “common/contextual novelty” and the features of the novel event are integrated to pre-existing representations15. In contrast, novelty with minimum prior experience is referred to as “absolute/distinct novelty” and requires the creation of new representations. Recent investigations suggest that these two types of novelty have different memory consolidation processes and involve divergent neuronal circuits25,26. Common novelty or events that can relate to prior knowledge selectively engage midbrain-hippocampal circuits and dopaminergic systems that promote memory storage via updating pre-existing neocortical networks26. This type of novelty is detected via the HPC which conveys the novelty signal to the dopaminergic midbrain to evaluate the salience of the new event15. In addition, the dopaminergic midbrain in turn sends a feedback DA signal to the HPC to trigger initial memory consolidation followed by an increased cortical memory consolidation, and therefore has a selective effect for the retention of the new event itself. Conversely, absolute novelty activates hindbrain-HPC systems, particularly DA signals from the locus coeruleus which boost initial memory consolidation in the HPC and enhance the retention of unrelated experiences, resulting in an overall effect on long-term memory consolidation26. Of note, absolute novelty can trigger a grace period for storing a global episodic memory trace and has a major repercussion on memory consolidation.
Novelty can also be classified as contextual/spatial novelty or stimulus novelty23. Contextual/spatial novelty is associated with the detection of a mismatch in the arrangement of stimuli in a previously established context27, whereas stimulus novelty is associated with the novelty of the stimulus itself which has not been experienced before. In rodents, responses to stimulus novelty are typically assessed when an individual is presented to a new/unfamiliar conspecific or social stimulus28, new environments29, new objects30 or even new odors31. Each of this stimulus novelty is represented with a single or multiple complexity of sensory information that can trigger a novelty response of various intensities.
The novelty response to a new environment is commonly measured in terms of locomotor activity during the exploratory event (i.e. distance travelled or speed) when the animal is placed in an unfamiliar context. For social, inanimate object, or odor stimuli, the novelty response is measured as time the animal spends near the new stimuli, or time/number of active exploratory events of sniffing behavior when the animal is directing its nose in close proximity to the stimuli (less than 2 cm)28,32,33. To potentiate the novelty response due to stimulus novelty and avoid the novelty that originates from the context itself, stimulus novelty should be assessed in a previously experienced familiar context (i.e., the test animal should be habituated to the testing environment/cage).
As a novel stimulus become familiar upon repeated exposures, the level of exploratory behavior decreases. This process, known as habituation, represents a simple form of associative learning evolutionarily conserved across species34 and is the most elementary form of behavioral plasticity35. In mammals, habituation with repeated presentations can predict outcomes of higher cognitive functions and has been used to test the effectiveness of potential treatments that enhance cognitive capabilities36. The main goal of habituation processing is to act as a novelty filter, allowing an individual to remain focused on more relevant or unexpected stimuli10. If, following repeated episodes of habituation in a specific context, an individual is subsequently presented with a new stimulus in that same context, the stimulus novelty will trigger a rebounded investigation and increased levels of exploratory behavior37. Moreover, bearing in mind that novel stimuli are more salient than familiar stimuli, when given a choice between novel and familiar stimuli, test animals tend to exhibit a novelty preference (NP)38. Although responses of NP have been traditionally studied in terms of cognitive function and memory recognition or discrimination, how the salience of the novel stimulus influences the outcome of the NP response has not been thoroughly investigated. In rodents, a NP response is assessed when animals are exposed to a novel and a familiar stimulus of similar nature simultaneously and, the exploratory response towards each stimulus is measured over a time window ranging between 2 and 10 min39. For novel and familiar inanimate objects, it has been suggested that the memory recognition effect occurs within the first 1 or 2 min of the session38,40. Indeed, during the initial phase of testing NP scores tend to be higher since the novel object is newer at the beginning of the test but becomes more familiar with repeated exposures within the course of the session35,37.
Novelty increases alertness, attention, facilitates memory and is considered another dimension of salience that drives an orienting response41 (Table 1). Although in most scenarios this orienting response associates with an increase in exploratory behavior, this may be limited to certain types of novelty or contextual characteristics involved, which are based on the stimulus/context value. An increased exploratory behavior towards novelty is necessary to evaluate the overall salience of the unknown stimulus, enabling identification of new sources of potential reward but also, of sources that may be possibly threatening. From the perspective of stimulus salience assessment, novelty intrinsically generates an approach-avoidance conflict that is redefined as novelty becomes familiar and, therefore, becomes more predictable when this is evaluated upon multiple exposures24. According to this idea, a recent view for novelty exploration has been suggested as being a sequential process of learning and decision based on value and threat42, and, therefore DA neurotransmission would act as a primary signal in this novelty response (see below).
Are novel stimulus interactions more rewarding than familiar ones? In rodents, interactions with novel social conspecifics are both rewarding and reinforcing37,43-45, but those interactions with novel inanimate objects have been shown to be aversive46 or signal potential threat42. To investigate if interactions with novel social conspecifics are rewarding in mice, researchers have developed different versions of the conditioned place preference paradigm (CPP), which traditionally measures drug-induced reward/aversion by pairing a contextually distinct environment with the drug of interest and then determining if, after multiple training sessions, mice prefer or avoid that environment47. During the social novelty CPP test, mice learn to associate one compartment of the CPP apparatus with novel social interactions (instead of a drug); whereas, the other compartment is associated with interactions with a familiar or previously encountered social stimuli. Upon repeated conditionings, mice develop a preference for the compartment associated with the novel social stimulus, indicating that the novel social stimulus has a higher value than the familiar one37,45. However, given that conditioned response to familiar cues establish much slower during associative learning than those to novel cues, a behavioral term known as latent inhibition48, a preference for the compartment linked to novel social stimuli in the CPP paradigm, may be in part influenced by the novelty-induced facilitation of contextual associative learning.
3. Midbrain DA systems
Understanding DA function and its causal link with natural behaviors has been a focus of research for several decades. Neurons in the SN/VTA of the midbrain provide the majority of DA in the brain, and these neurons are primarily involved in decision-making49, goal-directed behaviors50, reinforcing learning51 and reward processing52-56. Overall, DA neurons are involved in computing emotionally salient stimuli of both positive and negative valence, and dysfunction of this system has been implicated in numerous neuropsychiatric disorders including substance abuse57,58, anxiety59 and depression60.
In humans, imaging studies have shown that the SN/VTA areas are activated by both actual reward and novelty, as well as by cues predicting their occurrence17-19. Interestingly, distinct DA midbrain regions seem to be preferentially activated by either reward or novelty suggesting the possibility of non-overlapping areas19. Midbrain DA neurons respond to novel or to unexpected rewards or reward-predicting cues by switching their firing rate from low-frequency firing to phasic burst firing at higher frequencies55, which significantly facilitates DA release and neurotransmission at post-synaptic sites. This increase in DA firing in response to novelty has been shown in several species and behavioral paradigms61-63, and rapidly habituates as the stimulus becomes familiar upon repeated exposures. In addition, early work in rats demonstrated that exposure to a novel environment raises the levels of DA in the nucleus accumbens (NAc)64,65, a phenomenon typically associated with reward. Despite all of the evidence indicating DA neurotransmission is strongly activated by novelty, the functional role of DA and the complete network underlying the response to novelty still remains elusive.
A broad theoretical framework and the canonical view of DA neuronal function is to compute reward prediction error (RPE) signals, the discrepancy between actual and predicted reward, to drive learning and predict future outcomes66. The goal of the RPE signal is to update values for future estimates and maximize choices with a positive result to reinforce those behaviors that may lead to positive outcomes. In the case of novelty, since a novel stimulus may predict outcomes of either positive or negative value, the larger response of DA neurons has been interpreted as a “novelty bonus”61. According to this idea, DA neurons respond to novelty as if novel stimuli are intrinsically rewarding or that they signal potential reward availability (ie., as positive RPE). Thus, in this scenario, increased DAergic neuronal activity and, presumably, increased DA release at downstream projection areas, act as a motivational signal to drive an exploratory behavior17. However, as mentioned above, encountering novelty is not necessarily associated with a rewarding experience and DA responses to novelty depend on the nature and the intrinsic value of the novel stimulus48.
3.1. The Ventral Tegmental Area
Over the last few years, modern neuroscience approaches have provided deeper insights into a functional role for DA signaling in reward/reinforcement processing and also in the novelty response. One such innovation is the use of fiber photometry optical recording paired with genetically encoded Ca2+ indicators or GCaMPs. Using this approach, bulk Ca2+ transients are measured as a proxy of neuronal activity and linked to a specific behavior. Pioneering work by the Deisseroth lab, utilized fiber photometry in combination with GCaMP expression in VTA DAergic neurons to demonstrate that, in behaving animals, activity of these neurons significantly increases during interaction with two types of novelty: when an animal encounters a novel social stimulus and when an animal encounters a novel inanimate object67 (Fig. 2A and 2B). In both cases, the strongest activation of VTA DAergic neurons occurs during the earlier bouts of interaction with the novel stimuli and then rapidly habituates with repeated exploratory events. Noticeably, the authors reported that whereas an increase in VTA DAergic neuronal activity predicts the onset of interaction to a novel social stimulus (i.e., an approach behavior), for a novel inanimate object, increased activity coincides with the termination of the exploratory event (i.e., withdrawal behavior)67. These results suggest that the temporal dynamics of VTA DAergic neuronal activity in response to novelty may be intrinsically associated with the overall salience of the novel stimulus. Indeed, rodents naturally prefer interactions with a novel social stimulus rather than a novel inanimate object when offered the choice, suggesting that these two types of novelty may be differently processed and may involve different networks of interconnected brain areas (Fig. 2). Moreover, a novel inanimate object may be perceived as an aversive stimulus in the first trials as a form of neophobia, and the release of DA which coincides with the termination of the exploratory event could reflect the reward associated with the avoidance of the object 42. Additional investigations have reported increased firing rate of VTA DA neurons in response to novel environments68,69 or to new odors48, as compared to familiar ones of similar nature.
Figure 2. Circuits underlying novelty responses to social and inanimate objects in rodents.
(A) Schematic representation of a sagittal view of a rodent brain showing midbrain circuits and interconnected areas that respond to novel and familiar social information. Novel social stimuli strongly activate VTA (red) DA systems that project to the NAc (orange), which is a hub integrating and consolidating socially-relevant information from the HPC (purple), and processing social reward via projections from the DR (gray). The LH (green) sends GABA inputs to the VTA that contribute to the social novelty response. The VTA also sends DA projections to the IPN (blue), which, via the habenulo (yellow) -interpeduncular axis, has an important role in resolving familiarity-based information. (B) Novel inanimate object stimuli activate midbrain DA systems in the VTA, but most importantly, in the SN lateral (light red) that projects to the TS (light orange). The VTA receives glutamate and GABA projections from the LH that also process salient information to novel inanimate objects. The habenulo-interpeduncular axis guides responses to familiar inanimate objects. Additional midbrain nuclei including the MnR (light gray) also play a role in response to novel objects. DR, dorsal raphe; HPC, hippocampus; IPN, interpeduncular nucleus; LH, lateral hypothalamus; MHb, medial habenula; MnR, median raphe; NAc, nucleus accumbens; SN, substantia nigra; TS, tail of the striatum; VTA, ventral tegmental area.
Despite the robust activation pattern of VTA DAergic neurons upon novel social and novel object stimuli interactions, direct manipulations of VTA DAergic neuronal activity either via optogenetic or chemogenetic tools have only been able to significantly affect behavioral responses to novel social stimuli but not to novel inanimate objects45,46,67. As Gunaydin et al previously discussed, one possibility behind these unsuccessful attempts might be related to the precise delivery timing of activity manipulations, which may be timescale relevant for social but not object novel stimuli investigations67. This could be clarified by using a more precise optogenetic manipulation such as the closed-loop stimulation system during novel encounters so that one may time-lock activation/inhibition of VTA DAergic neurons upon initial approach/contact with a novel stimulus or upon withdrawal. Another possibility might be that readouts of behavioral exploration in response to novel inanimate objects may trigger a much more complex approach-avoidance conflict than that observed with novel social interactions. Indeed, when presented to novel inanimate objects, mice perform “bouts” of investigation in which they initially approach the novel object and then quickly retreat42. This type of approach-avoidance conflict may represent an evaluation form for potential threat of the novel event and therefore, is intrinsically associated with the salience of novelty. In line with these results, there is also the possibility that distinctly wired relevant subpopulations of VTA neurons differentially respond to novelty based on stimulus salience (i.e. distinct responses depending on whether salience has a positive or negative valence), and only selective manipulations of specific VTA DA subgroups would elicit behavioral outcomes to different types of novelty.
A growing body of literature in the DA field suggests that DAergic neurons are highly diverse at the molecular level70, as well as in their activity71,72 and site-specific projection pattern73,74, resulting in a refined functional computational system75,76. DA neurons in the VTA are segregated into projection-specific site that innervate the striatum via the mesolimbic pathway, the prefrontal cortex (PFC) via the mesocortical pathway, the HPC or amydgala and extended areas, among others. Yet, even within the mesolimbic pathway, VTA DAergic neurons represent anatomically and functionally distinct populations that send axonal projections to different sub-compartments within the NAc, and those can shape opposing effects on motivational behaviors77-80. Considering that different brain areas are responsible for different types of novelty23, identifying the VTA microcircuits predictive of novelty responses should yield valuable insights toward understanding how the salience of novelty is evaluated to elicit an exploratory behavior. For novel social stimuli interactions, researchers have shown that only the VTA→NAc projection-specific component but not the VTA→PFC circuit is involved in the social novelty response67,81 (Fig. 2A). However, this VTA→NAc DA projection seems to be less responsive to novel inanimate object encounters, as opposed to the responses observed when directly recording VTA DAergic cell bodies67. The NAc represents a critical hub for the rewarding properties of social interactions44 and also for the storage of social memories82. Work from Okuyama et al. demonstrated that the NAc shell receives projections from the ventral CA1 region of the HPC, which are necessary for the memory of familiar social interactions but not for novel or familiar inanimate objects or context investigations82 (Fig. 2A), supporting the idea that different networks encode novelty of different salience. Midbrain DAergic neurons and striatal areas are both functionally connected to the MTL26 and, play dual roles in cognition/memory processing as well as salience evaluation that guides approach/avoidance behaviors. Moreover, divergent pathways from striatal and midbrain regions to the HPC are critical for processing familiar and novel stimuli information based on their expectancy, and are both associated with enhanced subsequent memory83. In rodents, tracing analysis have shown that VTA DAergic neurons send abundant axon projections that innervate the CA1 HPC68. Photostimulation of the DAergic VTA→CA1 circuit during exploration of novel environments is critical to enhance the reactivation of newly encoded hippocampal representations and therefore improve memory performance. If VTA DA signals contribute to novelty encoding, then attempts should be made to understand feedback mechanisms between the MTL and midbrain areas that would elucidate the novelty response and, in particular, the loss of stimulus salience with familiarity. Consistent with this idea, recent evidence has demonstrated a key role for midbrain DA signaling in responses to latent inhibition, when animals show learning rates significantly higher for novel stimuli as compared to familiar ones48. Interestingly, this work from Morrens et al. suggests that the behavioral adaptive strategy of latent inhibition is controlled by midbrain DA projections to cognitive centers, particularly the PFC.
Divergent VTA DA circuits are wired by functional projection-specific outputs as well as inputs. One of the most robust inputs to the VTA originates in the lateral hypothalamus (LH)84. Projections from the LH to the VTA have been identified as being glutamatergic, GABAergic or peptidergic in nature, though research suggests the GABAergic component may be more relevant for the novelty response85. Work from Nieh et al. showed that optogenetic stimulation of LH→VTA GABA projections increases interaction with novel social stimuli, and also promotes investigation of proximal salient objects, indicating the GABA LH→VTA pathway can drive an approach/avoidance behavior to novelty from both social and inanimate object stimuli (Fig. 2A and 2B). Interestingly, the glutamatergic component of the LH→VTA projection only affects novel object investigation but not novel social interaction, highlighting the functional specificity and network connectivity conveying salient information from inherently distinct types of novel stimuli. Neurons in the hypothalamus have been involved in social NP86,87 and, more recently, in object memory formation and in the establishment of appropriate responses to novel and familiar objects88. The extensive neuronal diversity and network connectivity of the hypothalamus sets this region as a key center for regulating novelty responses, via the VTA, and most probably many other structures, such as the HPC87. The LH sends abundant glutamatergic and GABAergic projections to the VTA and these inputs monosynaptically innervate VTA DAergic neurons, but also GABAergic neurons. Data suggest that the LH→VTA GABAergic component acts via disinhibition: Activation of the GABAergic projection from the LH elicits DA release in the NAc by suppressing the inhibition of VTA DA neurons by local VTA GABAergic neurons85. Similarly, the NAc sends abundant GABAergic inputs to the VTA, and electrophysiological and optogenetic studies indicate that these inputs also regulate VTA DAergic neurons through disinhibition via VTA GABAergic interneurons89-91. Accumulating data in the literature demonstrate that GABAergic neurons in the VTA play important roles in stress and reward processing92. Based on these previous findings, VTA GABAergic may be indirectly mediating novelty responses and may actually represent a fundamental component within the novelty network, although future research should be directed towards addressing this question.
While it is assumed that novel stimuli increase the activity of DAergic neurons, the molecular signatures that drive a novelty responses and habituation with familiarity is poorly understood. In drosophila, the behavioral response to novel and familiar stimuli and particularly, the transition in neural activity upon familiarization, requires DA-mediated plasticity at synapses in the mushroom body93. In mice, Bariselli et al. demonstrated that exposure to novel stimuli, independent of their nature, induce specific forms of synaptic plasticity at glutamatergic synapses in the VTA. More precisely, novel stimuli increase the strength of excitatory inputs onto VTA DAergic neurons, measured by the insertion of GluA2-lacking AMPARs 24h after exposure46. This form of novelty-induced synaptic plasticity persists upon repeated exposures to social stimuli but not object stimuli, suggesting that it is necessary to sustain interactions with stimuli of higher salience for instance novel social stimuli vs. inanimate objects. Moreover, the non-canonical GluA2-lacking AMPA function can be induced by optogenetic stimulation of VTA DAergic neurons resulting in disruption of the habituation to novel social stimuli46.
3.2. The Substantia Nigra
The DA nigrostriatal pathway originating from the SN to the dorsal striatum has received abundant attention in the field of neurodegenerative disorders, given the functional loss of this pathway is strongly associated with Parkinson’s disease94,95 or Huntington’s disease96, among others neurodegenerative disorders. Anatomical and functional segregation of DA responses across the SN have also become a focus of interest for reward/aversive behavior97, as well as for the novelty response. Similar to VTA responses, DAergic neurons in the SN switch their firing rate from low-frequency spiking to a transient, high-frequency burst firing when mice are exposed to novel environments, and this switch requires intact K-ATP channel activity for novelty-dependent exploratory behavior98. More recently, a subpopulation of DAergic neurons in the lateral part of the SN (SNL) has been shown to respond to specific types of novelty, and this response is independently related to RPE signaling42,99. As compared to other midbrain DA neurons, those located in the SNL send specific projections innervating the tail of the striatum (TS) and have a unique set of inputs as demonstrated by viral-based neuronal tracing100. Fiber photometry experiments combined with GCaMP signaling have revealed that SNL→TS DA responses are highly activated by novel stimuli of different nature including new odors, tones or objects42,99 (Fig. 2B). Moreover, the TS DA response also encodes physical salience42 and is activated with some types of aversive stimuli such as an air puff or high intensity tones, suggesting the possibility that they may encode the threatening aspect of novelty. Indeed, the DA response in the TS is significantly larger when mice retract from a novel inanimate object but not a familiar one. This pattern of activity is similar to that previously reported for VTA DAergic neurons, where increased activity coincides with the termination of exploratory events only for novel objects but not novel social stimuli67. Taken together, these results confirm that divergent DA activity may signal the intrinsic salience of novel stimuli and maintain approach/avoidance behaviors based on their type of novelty101.
4. The Interpeduncular Nucleus
While accumulating evidence supports a functional role of increased DA system activity in response to novel stimuli, brain circuits and neuronal mechanisms leading to a cessation of behavioral responses when a novel stimulus transitions to familiar are poorly understood. Plasticity in the DA system may, in part, explain the habituation effect that is observed with repeated exposures of novel stimuli, and with the transition from novelty to familiarity46. However, a consensus body of work suggest that familiarity and novelty may involve non-overlapping brain regions102, and that a dual mechanism may lead to the interaction between separate familiarity and novelty signals15. Consistent with this idea, recent investigations indicate that the interpeduncular nucleus (IPN) of the midbrain may play a key role in familiarity signaling and the response of NP45.
The IPN is located ventral and medial to the VTA and is an integrative element of the habenulo-interpeduncular pathway103. The habenula (Hb) consists of a small, epithalamic structure evolutionarily conserved among vertebrates classically subdivided into the medial (MHb) and lateral (LHb) regions104-106. Neuronal cell types within the MHb and LHb have spatially defined transcriptional profiles with distinct electrophysiological properties that are engaged by different types of stimuli107,108. In a general view, the Hb connects the limbic forebrain with monoaminergic midbrain and hindbrain structures, integrating value-based, sensory and experience-dependent information to control motivational, emotional and cognitive processing109,110. Although the IPN receives abundant excitatory inputs from the MHb, other structures including the raphe, the lateral dorsal tegmental nucleus (LDTg) or the forebrain septal nuclei also innervate this nucleus111. In addition, the IPN sends output projections to regions involved in motivation and emotional behaviors including the raphe, tegmentum or pontine nucleus112,113, although the precise influence of these projections on behavior is largely unknown. The majority of IPN neurons express the glutamate decarboxylase (GAD) enzyme and presumably synthesize and release the neurotransmitter GABA, although glutamatergic and serotonergic neurons have also been identified within IPN sub-regions in rodents114. Despite being considered as an unpaired brain area, the IPN is a non-homologous structure that can be subdivided into multiple paired and unpaired subnuclei115. Whether physiological, anatomically and functionally relevant differences characterize each of these subdivisions requires further investigation.
The MHb-IPN pathway has been extensively studied in the context of drug addiction, particularly, this axis is a hub regulating the rewarding/aversive aspects of nicotine intake116-121 and the manifestation of nicotine withdrawal signs upon drug cessation122-125. Other investigations have linked the MHb-IPN axis with baseline anxiety126 or fear-related memories127,128, and with the control of emotional behavior in an experience-dependent manner115,129,130. Ablation of the MHb-IPN connection in mice via genetically-driven neurotoxicity results in a collection of behavioral abnormalities, among them, significant deficits in habituation upon repeated exposures to a new environment131. Noticeably, the absence of MHb-IPN function per se does not affect the initial response to a new environment but instead, it disrupts the process by which an animal habituates or familiarizes to novelty; the authors referred to this behavior as a maladaptation to new environments. Further research has demonstrated that the IPN may represent a neuroanatomical substrate for familiarity signaling and the expression of NP45. In particular, as mice habituate to novel stimuli upon repeated exposures, activity of IPN GABA neurons progressively increases. Both exposure to familiar social or inanimate object stimuli increases the activity of IPN GABAergic neurons as compared to exposure to novel stimuli with similar nature, measured with c-fos expression as a marker of neuronal activation. Silencing IPN GABAergic neurons with optogenetic tools induces exploratory activity of familiar stimuli as if it is novel; whereas, activating IPN GABAergic neurons reduces exploration of novel stimuli mimicking familiarity. Overall, these results indicate that the activity of these neurons is both necessary and sufficient for the expression of familiar responses and therefore a critical component of NP. Importantly, direct manipulations of IPN GABA tone has similar effects on encounters to stimuli of social and non-social nature (inanimate objects), implying a global response of the IPN for familiarity. In addition, functional circuit mapping has revealed that MHb cholinergic/glutamatergic afferents innervating the IPN are necessary and sufficient to drive a familiarity response (Fig. 2A and 2B).
The close proximity of the IPN with the VTA DA system suggests the possibility that these two midbrain areas may be synaptically connected. Indeed, several investigations have reported that the IPN is directly innervated by the DA system124: a subset of VTA DA neurons sends monosynaptic connections to the IPN, and this circuit is sufficient to trigger a social novelty response45. This mesointerpeduncular axis signals via D1 receptor expressing neurons in the IPN that are exclusively activated by novel but not familiar social stimuli interactions (Fig. 2A). With all the aforementioned inputs integrating novelty and familiarity signals in the IPN, it remains to be elucidated where IPN neurons convey information to guide responses of familiarity and NP. Reports have shown that IPN GABAergic neurons send inhibitory outputs to the LDTg, particularly to neurons in the LDTg that are monosynaptically connected to VTA DA neurons132. Through this IPN→LDTg circuit, the IPN controls aversion to nicotine, suggesting that the IPN can modulate VTA DA transmission via polysynaptic mechanisms (i.e. indirectly via LDTg→VTA pathways). Nevertheless, the precise IPN anatomical and functional synaptic connections involved in NP and reward-related responses are unclear.
5. The raphe
Midbrain neurons in the median raphe (MnR) and dorsal raphe (DR) are the predominant sources of serotonin (5-HT) synthesis in the brain133. These neurons send extensive axonal projections that innervate forebrain areas, including the prefrontal and entorhinal/perirhinal cortices and the HPC134-138, where the release of 5-HT contributes to emotional and cognitive function. For many decades, disruption of 5-HT neurotransmission has been linked to psychiatric disorders especially depression and anxiety139,140. More recently, a growing body of literature suggest the possibility that the 5-HT system has a direct effect encoding reward-related signals44,141,142. Using real-time measurements of DR 5-HT neuronal activity with fiber photometry, recent investigations have shown that these neurons are highly activated by rewarding stimuli including sucrose, food or sex, but not by aversive stimuli such as a foot-shock142. Importantly, DR 5-HT neurons also respond to novelty and the magnitude of their activation depends on the nature and intrinsic salience of the novel stimuli. For instance, novel social stimuli robustly activate DR 5-HT neurons, whereas investigation of novel inanimate objects are associated with brief Ca2+ transients of smaller magnitude142,143 (Fig. 2A). Optogenetic manipulations of DR 5-HT neuronal activity showed that these neurons are necessary and sufficient to trigger investigation of novel social stimuli but not novel inanimate objects143. Similar effects are seen using chemogenetic inhibition of these neurons, which does not affect NP response for inanimate objects144. Instead, chemogenetic inhibition of 5-HT neurons in the MnR raphe impair a novelty response to inanimate objects (Fig. 2B), suggesting the MnR and DR 5-HT nuclei may differentially process novel stimuli depending on their nature and/or salience. Interestingly, DR 5-HT neurons send abundant axon projections to the NAc where they convey social novelty responses. Indeed, optogenetic stimulation of 5-HT terminal activity in the NAc recapitulates the behavioral phenotypes of DR 5-HT somatic manipulations143 (Fig. 2A).
DR neurons are noticeably heterogeneous in terms of anatomical, electrophysiological and neurotransmitter signaling that may reflect their functional specialization guiding behavior138. A sparse subpopulation of DA neurons in the DR has been shown to play a unique role in sociability and aversion-related behaviors145,146. These DR DAergic neurons increase their activity when mice investigate novel social stimuli and to a lesser extent with novel object stimuli interactions67; they share similar activity pattern as described for VTA DAergic neurons. Increases in DR DAergic neural activity are mainly observed during initial contact with a novel social stimulus, and significantly decay upon consecutive encounters. In addition, optogenetic activation of these DR DAergic neurons is sufficient to promote investigation towards novel social individuals but not novel objects145, again, as previously described for VTA DAergic neurons. However, stimulation of the DR neurons intrinsically drives aversion, whereas stimulation of VTA DAergic neurons is known to be rewarding147. The divergence of these two DAergic neuronal populations in reward/aversion responses but their equal effects on the social novelty response has been attributed to opposite motivational drives in sociability: While VTA DAergic neurons are assumed to represent the substrate for social reward, DR DAergic neurons putatively drive seeking for social contact as an attempt to mitigate social loneliness145. Thus, DR DAergic neuron activation is significantly stronger if mice undergo acute social isolation and their photoinhibition only affects interaction with novel social stimuli in socially isolated mice, but not group-housed animals145. Overall, these results indicate that DR DAergic neurons guide investigation of novel social stimuli when there is a specific motivational drive for sociability in an effort to relieve an aversive behavioral state, and point towards the idea that specific neuronal responses may have significant effects in processing novelty information according to the basal internal states of an individual.
6. Perspective/Outstanding questions for future directions
Novelty represents another dimension of salience that increases alertness, attention and drives behavioral orienting. As such, novelty engages midbrain DA systems that contribute to assorted approach/avoidance conflicts with a behavioral outcome that depends on the overall salience of the novel event. Accumulating evidence points towards specific components of the DA system responding to different types of novelty, but how subsets of DA neurons and their associated microcircuits integrate salience-specific novelty information requires further research. For social stimuli interactions, a major circuit comprising VTA DAergic neurons and DA release in the NAc triggers the novelty response. The NAc integrates VTA DA signals but also 5-HT signals from DR neurons, acting as a hub for socially salient information. Of note, the systems governing this novelty response are fine-tuned by the special needs for survival, and under socially restricted circumstances, additional DA neurons that reside in the DR drive the novel social response. For inanimate object interactions, SNL DA and projection-specific sites to the TS play a significant role in the response to novelty. These overall findings suggest that divergent brain regions may be responsible for different types of novelty responses based on stimulus salience and that they are susceptible to internal demands.
Intriguingly, in most scenarios, DA responses only increase with the first novelty encounter thus posing a series of outstanding questions. First, what are the synaptic plasticity/mechanisms of DA responses that occur upon consecutive exposures to novelty that lead to the transition to familiarity? Persistent changes in glutamatergic inputs onto DAergic neurons might underlie responses to social novelty but not to inanimate objects. Second, what additional neuronal circuits and networks respond to familiarity and facilitate the habituation of DA responses? Recent data implicate the MHb→IPN axis as one circuit-level component critical for familiarity signaling, but additional networks are likely involved. Importantly, how are these midbrain circuits and DA responses to novelty integrated into MTL structures to identify and guide appropriate responses to novelty vs. familiarity? Moreover, how are these circuits and DA responses to novelty shaped by lifespan experience, assuming that absolute novelty dominates at early stages of life and then decays with age and experience? Finally, how is novelty-induced exploratory behavior modulated by the basal internal state of an individual and the special needs for survival (food/social availability, etc), and which clusters of neurons may be involved in each specific situation?
An interesting aspect of novelty is the fact that the brain is hardwired to seek out stimuli that are new. Novelty seeking (NS) in humans and animals is defined as an increased exploration of novel stimuli and/or environments and the willingness to take risk to obtain them. From an evolutionary perspective, seeking new foods, social encounters, or environments opens up opportunities for adaptation and increased chances of survival. In modern society, NS is a driving force for creativity and innovation, leading to novel ideas and technologies, and ultimately improving the advancement of human wellbeing and progress. Within the last few years, increased attention has been paid to this motivational aspect of novelty exploration and how they are driven by phasic activation of midbrain DA systems, although the complete brain network and mechanisms underlying these processes remain largely unknown. High levels of NS and excessive novelty value can lead to maladaptive learning and decision-making, as seen in several neurological disorders such as drug addiction, schizophrenia or attention deficit hyperactivity disorder. Elucidating the precise neuronal circuits and cellular/molecular mechanisms that govern NS will not only help to clarify the evolutionarily conserved driving forces behind the processing of novelty and familiarity, but also, may provide invaluable insights towards the development of novel strategies for the diagnosis and/or treatment of a wide range of neuropathological conditions with abnormal NS response.
Highlights.
Novelty triggers an orienting response to evaluate the salience of the new event
Dopamine midbrain circuits guide approach/avoidance behaviors to novelty
Divergent midbrain circuits drive the novelty responses based on stimulus salience
Acknowledgments
This article was prepared by invitation to S.M. for the special issue “Novelty and memory” in the Neurobiology of Learning and Memory. A.R.T is supported by the National Institute on Drug Abuse award number DA041482 and DA047678. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- 1.Tam FI, King JA, Geisler D, et al. Altered behavioral and amygdala habituation in high-functioning adults with autism spectrum disorder: An fMRI study. Sci Rep. Published online 2017. doi: 10.1038/s41598-017-14097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, Jin P. Dysfunction of Habituation Learning: A Novel Pathogenic Paradigm of Intellectual Disability and Autism Spectrum Disorder. Biol Psychiatry. Published online 2019. doi: 10.1016/j.biopsych.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 3.McDiarmid TA, Belmadani M, Liang J, et al. Systematic phenomics analysis of autism-associated genes reveals parallel networks underlying reversible impairments in habituation. Proc Natl Acad Sci U S A. Published online 2020. doi: 10.1073/pnas.1912049116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tistarelli N, Fagnani C, Troianiello M, Stazi MA, Adriani W. The nature and nurture of ADHD and its comorbidities: A narrative review on twin studies. Neurosci Biobehav Rev. Published online 2020. doi: 10.1016/j.neubiorev.2019.12.017 [DOI] [PubMed] [Google Scholar]
- 5.Ceceli AO, Esposito G, Tricomi E. Habit Expression and Disruption as a Function of Attention-Deficit/Hyperactivity Disorder Symptomology. Front Psychol. Published online 2019. doi: 10.3389/fpsyg.2019.01997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinelli C, Rigoli F, Averbeck B, Shergill SS. The value of novelty in schizophrenia. Schizophr Res. Published online 2018. doi: 10.1016/j.schres.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modinos G, Allen P, Zugman A, et al. Neural Circuitry of Novelty Salience Processing in Psychosis Risk: Association with Clinical Outcome. Schizophr Bull. Published online 2020. doi: 10.1093/schbul/sbz089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW. In search of predictive endophenotypes in addiction: Insights from preclinical research. Genes, Brain Behav. Published online 2016. doi: 10.1111/gbb.12265 [DOI] [PubMed] [Google Scholar]
- 9.Schomaker J, Berendse HW, Foncke EMJ, et al. Novelty processing and memory formation in Parkinson’s disease. Neuropsychologia. Published online 2014. doi: 10.1016/j.neuropsychologia.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 10.Schmid S, Wilson DA, Rankin CH. Habituation mechanisms and their importance for cognitive function. Front Integr Neurosci. Published online 2015. doi: 10.3389/fnint.2014.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDiarmid TA, Bernardos AC, Rankin CH. Habituation is altered in neuropsychiatric disorders—A comprehensive review with recommendations for experimental design and analysis. Neurosci Biobehav Rev. Published online 2017. doi: 10.1016/j.neubiorev.2017.05.028 [DOI] [PubMed] [Google Scholar]
- 12.Knight RT. Contribution of human hippocampal region to novelty detection. Nature. Published online 1996. doi: 10.1038/383256a0 [DOI] [PubMed] [Google Scholar]
- 13.Kafkas A, Montaldi D. Two separate, but interacting, neural systems for familiarity and novelty detection: A dual-route mechanism. Hippocampus. Published online 2014. doi: 10.1002/hipo.22241 [DOI] [PubMed] [Google Scholar]
- 14.Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. Published online 2006. doi: 10.1016/j.neuron.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 15.Kafkas A, Montaldi D. How do memory systems detect and respond to novelty? Neurosci Lett. Published online 2018. doi: 10.1016/j.neulet.2018.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunzeck N, Doeller CF, Dolan RJ, Duzel E. Contextual interaction between novelty and reward processing within the mesolimbic system. Hum Brain Mapp. Published online 2012. doi: 10.1002/hbm.21288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunzeck N, Düzel E. Absolute Coding of Stimulus Novelty in the Human Substantia Nigra/VTA. Neuron. Published online 2006. doi: 10.1016/j.neuron.2006.06.021 [DOI] [PubMed] [Google Scholar]
- 18.Bunzeck N, Thiel C. Neurochemical modulation of repetition suppression and novelty signals in the human brain. Cortex. Published online 2016. doi: 10.1016/j.cortex.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 19.Krebs RM, Heipertz D, Schuetze H, Duzel E. Novelty increases the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: Evidence from high-resolution fMRI. Neuroimage. Published online 2011. doi: 10.1016/j.neuroimage.2011.06.038 [DOI] [PubMed] [Google Scholar]
- 20.Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. Published online 2005. doi: 10.1016/j.neuron.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Nourski KV, Steinschneider M, Rhone AE, Kawasaki H, Howard MA, Banks MI. Auditory predictive coding across awareness states under anesthesia: An intracranial electrophysiology study. J Neurosci. Published online 2018. doi: 10.1523/JNEUROSCI.0967-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rainer G, Ranganath C. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. Published online 2003. [DOI] [PubMed] [Google Scholar]
- 23.Schomaker J, Meeter M. Short- and long-lasting consequences of novelty, deviance and surprise on brain and cognition. Neurosci Biobehav Rev. Published online 2015. doi: 10.1016/j.neubiorev.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 24.Schomaker J, Meeter M. Predicting the unknown: Novelty processing depends on expectations. Brain Res. Published online 2018. doi: 10.1016/j.brainres.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 25.Fernández G, Morris RGM. Memory, Novelty and Prior Knowledge. Trends Neurosci. Published online 2018. doi: 10.1016/j.tins.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 26.Duszkiewicz AJ, McNamara CG, Takeuchi T, Genzel L. Novelty and Dopaminergic Modulation of Memory Persistence: A Tale of Two Systems. Trends Neurosci. Published online 2019. doi: 10.1016/j.tins.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. Published online 2003. doi: 10.1038/nn1049 [DOI] [PubMed] [Google Scholar]
- 28.Fernández M, Mollinedo-Gajate I, Peñagarikano O. Neural Circuits for Social Cognition: Implications for Autism. Neuroscience. Published online 2018. doi: 10.1016/j.neuroscience.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 29.Thompson SM, Berkowitz LE, Clark BJ. Behavioral and neural subsystems of rodent exploration. Learn Motiv. Published online 2018. doi: 10.1016/j.lmot.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaser R, Heyser C. Spontaneous object recognition: A promising approach to the comparative study of memory. Front Behav Neurosci. Published online 2015. doi: 10.3389/fnbeh.2015.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez-Andrade G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory. J Reprod Dev. Published online 2005. doi: 10.1262/jrd.17031 [DOI] [PubMed] [Google Scholar]
- 32.Linster C, Kelsch W. A computational model of oxytocin modulation of olfactory recognition memory. eNeuro. Published online 2019. doi: 10.1523/ENEURO.0201-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molas S, Gener T, Güell J, et al. Hippocampal changes produced by overexpression of the human CHRNA5/A3/B4 gene cluster may underlie cognitive deficits rescued by nicotine in transgenic mice. Acta Neuropathol Commun. 2014;2(1). doi: 10.1186/s40478-014-0147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy JS, Thorpe WH. Learning and Instinct in Animals. J Anim Ecol. Published online 1964. doi: 10.2307/2644 [DOI] [Google Scholar]
- 35.Rankin CH, Abrams T, Barry RJ, et al. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. Published online 2009. doi: 10.1016/j.nlm.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grayson B, Leger M, Piercy C, Adamson L, Harte M, Neill JC. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav Brain Res. Published online 2015. doi: 10.1016/j.bbr.2014.10.025 [DOI] [PubMed] [Google Scholar]
- 37.Bariselli S, Contestabile A, Tzanoulinou S, Musardo S, Bellone C. SHANK3 Downregulation in the Ventral Tegmental Area Accelerates the Extinction of Contextual Associations Induced by Juvenile Non-familiar Conspecific Interaction. Front Mol Neurosci. Published online 2018. doi: 10.3389/fnmol.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. Published online 2010. doi: 10.1101/lm.1650110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antunes M, Biala G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn Process. Published online 2012. doi: 10.1007/s10339-011-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ennaceur A One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav Brain Res. Published online 2010. doi: 10.1016/j.bbr.2009.12.036 [DOI] [PubMed] [Google Scholar]
- 41.Ernst D, Becker S, Horstmann G. Novelty competes with saliency for attention. Vision Res. Published online 2020. doi: 10.1016/j.visres.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 42.Menegas W, Akiti K, Amo R, Uchida N, Watabe-Uchida M. Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat Neurosci. Published online 2018. doi: 10.1038/s41593-018-0222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hung LW, Neuner S, Polepalli JS, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science (80- ). Published online 2017. doi: 10.1126/science.aan4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. Published online 2013. doi: 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molas S, Zhao-Shea R, Liu L, Degroot SR, Gardner PD, Tapper AR. A circuit-based mechanism underlying familiarity signaling and the preference for novelty. Nat Neurosci. 2017;20(9). doi: 10.1038/nn.4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bariselli S, Hörnberg H, Prévost-Solié C, et al. Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat Commun. Published online 2018. doi: 10.1038/s41467-018-05382-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. In: Neuroscience and Biobehavioral Reviews. ; 2003. doi: 10.1016/S0149-7634(03)00018-6 [DOI] [PubMed] [Google Scholar]
- 48.Morrens J, Aydin Ç, Janse van Rensburg A, Esquivelzeta Rabell J, Haesler S. Cue-Evoked Dopamine Promotes Conditioned Responding during Learning. Neuron. Published online 2020. doi: 10.1016/j.neuron.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 49.Floresco SB, West AR, Ash B, Moorel H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. Published online 2003. doi: 10.1038/nn1103 [DOI] [PubMed] [Google Scholar]
- 50.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. Published online 2007. doi: 10.1016/j.tins.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 51.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. Published online 1988. doi: 10.1073/pnas.85.14.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev. Published online 1998. doi: 10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- 53.Tsai HC, Zhang F, Adamantidis A, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science (80- ). Published online 2009. doi: 10.1126/science.1168878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang CY, Esber GR, Marrero-Garcia Y, Yau HJ, Bonci A, Schoenbaum G. Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat Neurosci. Published online 2015. doi: 10.1038/nn.4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science (80- ). Published online 1997. doi: 10.1126/science.275.5306.1593 [DOI] [PubMed] [Google Scholar]
- 56.Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. Published online 1985. [PubMed] [Google Scholar]
- 57.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. Published online 2016. doi: 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lüscher C The Emergence of a Circuit Model for Addiction. Annu Rev Neurosci. Published online 2016. doi: 10.1146/annurev-neuro-070815-013920 [DOI] [PubMed] [Google Scholar]
- 59.Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. Published online 2015. doi: 10.1038/nn.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nestler EJ, Carlezon WA. The Mesolimbic Dopamine Reward Circuit in Depression. Biol Psychiatry. Published online 2006. doi: 10.1016/j.biopsych.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 61.Kakade S, Dayan P. Dopamine: Generalization and bonuses. Neural Networks. Published online 2002. doi: 10.1016/S0893-6080(02)00048-5 [DOI] [PubMed] [Google Scholar]
- 62.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. Published online 1992. doi: 10.1152/jn.1992.67.1.145 [DOI] [PubMed] [Google Scholar]
- 63.Schultz W Dopamine reward prediction-error signalling: A two-component response. Nat Rev Neurosci. Published online 2016. doi: 10.1038/nrn.2015.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. Published online 1996. doi: 10.1016/S0306-4522(96)00382-X [DOI] [PubMed] [Google Scholar]
- 65.Legault M, Wise RA. Novelty-evoked elevations of nucleus accumbens dopamine: Dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur J Neurosci. Published online 2001. doi: 10.1046/j.0953-816X.2000.01448.x [DOI] [PubMed] [Google Scholar]
- 66.Berke JD. What does dopamine mean? Nat Neurosci. Published online 2018. doi: 10.1038/s41593-018-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunaydin LA, Grosenick L, Finkelstein JC, et al. Natural neural projection dynamics underlying social behavior. Cell. Published online 2014. doi: 10.1016/j.cell.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. Published online 2014. doi: 10.1038/nn.3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeuchi T, Duszkiewicz AJ, Sonneborn A, et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. Published online 2016. doi: 10.1038/nature19325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique Properties of Mesoprefrontal Neurons within a Dual Mesocorticolimbic Dopamine System. Neuron. Published online 2008. doi: 10.1016/j.neuron.2008.01.022 [DOI] [PubMed] [Google Scholar]
- 71.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. Published online 2012. doi: 10.1038/nature10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. Published online 2009. doi: 10.1038/nature08028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lammel S, Lim BK, Ran C, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. Published online 2012. doi: 10.1038/nature11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thibeault KC, Kutlu MG, Sanders C, Calipari ES. Cell-type and projection-specific dopaminergic encoding of aversive stimuli in addiction. Brain Res. Published online 2019. doi: 10.1016/j.brainres.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verharen JP, Zhu Y, Lammel S. Aversion hot spots in the dopamine system. Curr Opin Neurobiol. Published online 2020. doi: 10.1016/j.conb.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morales M, Margolis EB. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. Published online 2017. doi: 10.1038/nrn.2016.165 [DOI] [PubMed] [Google Scholar]
- 77.Berridge KC, Kringelbach ML. Pleasure Systems in the Brain. Neuron. Published online 2015. doi: 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salamone JD, Correa M. The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron. Published online 2012. doi: 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dreyer JK, Vander Weele CM, Lovic V, Aragona BJ. Functionally distinct dopamine signals in nucleus accumbens core and shell in the freely moving rat. J Neurosci. Published online 2016. doi: 10.1523/JNEUROSCI.2326-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Jong JW, Afjei SA, Pollak Dorocic I, et al. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron. Published online 2019. doi: 10.1016/j.neuron.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gunaydin LA, Deisseroth K. Dopaminergic dynamics contributing to social behavior. Cold Spring Harb Symp Quant Biol. Published online 2014. doi: 10.1101/sqb.2014.79.024711 [DOI] [PubMed] [Google Scholar]
- 82.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science (80- ). Published online 2016. doi: 10.1126/science.aaf7003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kafkas A, Montaldi D. Striatal and midbrain connectivity with the hippocampus selectively boosts memory for contextual novelty. Hippocampus. Published online 2015. doi: 10.1002/hipo.22434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-Brain Mapping of Direct Inputs to Midbrain Dopamine Neurons. Neuron. Published online 2012. doi: 10.1016/j.neuron.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 85.Nieh EH, Vander Weele CM, Matthews GA, et al. Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron. Published online 2016. doi: 10.1016/j.neuron.2016.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soden ME, Miller SM, Burgeno LM, Phillips PEM, Hnasko TS, Zweifel LS. Genetic Isolation of Hypothalamic Neurons that Regulate Context-Specific Male Social Behavior. Cell Rep. Published online 2016. doi: 10.1016/j.celrep.2016.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang L, Zou B, Xiong X, et al. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. Ann Intern Med. Published online 2013. doi: 10.1523/JNEUROSCI.3200-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kosse C, Burdakov D. Natural hypothalamic circuit dynamics underlying object memorization. Nat Commun. Published online 2019. doi: 10.1038/s41467-019-10484-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bocklisch C, Pascoli V, Wong JCY, et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science (80- ). Published online 2013. doi: 10.1126/science.1237059 [DOI] [PubMed] [Google Scholar]
- 90.Yang H, de Jong JW, Tak YE, Peck J, Bateup HS, Lammel S. Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations. Neuron. Published online 2018. doi: 10.1016/j.neuron.2017.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. Published online 2011. doi: 10.1523/JNEUROSCI.1504-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouarab C, Thompson B, Polter AM. VTA GABA Neurons at the Interface of Stress and Reward. Front Neural Circuits. Published online 2019. doi: 10.3389/fncir.2019.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hattori D, Aso Y, Swartz KJ, Rubin GM, Abbott LF, Axel R. Representations of Novelty and Familiarity in a Mushroom Body Compartment. Cell. Published online 2017. doi: 10.1016/j.cell.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Masato A, Plotegher N, Boassa D, Bubacco L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol Neurodegener. Published online 2019. doi: 10.1186/s13024-019-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mallet, Delgado, Chazalon, Miguelez, Baufreton. Cellular and Synaptic Dysfunctions in Parkinson’s Disease: Stepping out of the Striatum. Cells. Published online 2019. doi: 10.3390/cells8091005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koch ET, Raymond LA. Dysfunctional striatal dopamine signaling in Huntington’s disease. J Neurosci Res. Published online 2019. doi: 10.1002/jnr.24495 [DOI] [PubMed] [Google Scholar]
- 97.Collins AL, Saunders BT. Heterogeneity in striatal dopamine circuits: Form and function in dynamic reward seeking. J Neurosci Res. Published online 2020. doi: 10.1002/jnr.24587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schiemann J, Schlaudraff F, Klose V, et al. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci. Published online 2012. doi: 10.1038/nn.3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Menegas W, Babayan BM, Uchida N, Watabe-Uchida M. Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. Elife. Published online 2017. doi: 10.7554/eLife.21886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Menegas W, Bergan JF, Ogawa SK, et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. Elife. Published online 2015. doi: 10.7554/eLife.10032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Watabe-Uchida M, Uchida N. Multiple dopamine systems: Weal and woe of dopamine. Cold Spring Harb Symp Quant Biol. Published online 2018. doi: 10.1101/sqb.2018.83.037648 [DOI] [PubMed] [Google Scholar]
- 102.Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. In: Neuropharmacology. ; 1998. doi: 10.1016/S0028-3908(98)00030-6 [DOI] [PubMed] [Google Scholar]
- 103.Morley BJ. The interpeduncular nucleus. Int Rev Neurobiol. Published online 1986. doi: 10.1016/S0074-7742(08)60108-7 [DOI] [PubMed] [Google Scholar]
- 104.Sutherland RJ. The dorsal diencephalic conduction system: A review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. Published online 1982. doi: 10.1016/0149-7634(82)90003-3 [DOI] [PubMed] [Google Scholar]
- 105.Aizawa H, Amo R, Okamoto H. Phylogeny and ontogeny of the habenular structure. Front Neurosci. Published online 2011. doi: 10.3389/fnins.2011.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin C, Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. Published online 2009. doi: 10.1016/j.neuroscience.2009.03.085 [DOI] [PubMed] [Google Scholar]
- 107.Hashikawa Y, Hashikawa K, Rossi MA, et al. Transcriptional and Spatial Resolution of Cell Types in the Mammalian Habenula. Neuron. Published online 2020. doi: 10.1016/j.neuron.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wallace ML, Huang KW, Hochbaum D, Hyun M, Radeljic G, Sabatini BL. Anatomical and single-cell transcriptional profiling of the murine habenular complex. Elife. Published online 2020. doi: 10.7554/eLife.51271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. Published online 2020. doi: 10.1038/s41583-020-0292-4 [DOI] [PubMed] [Google Scholar]
- 110.Fakhoury M The habenula in psychiatric disorders: More than three decades of translational investigation. Neurosci Biobehav Rev. Published online 2017. doi: 10.1016/j.neubiorev.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 111.Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. Published online 1990. doi: 10.1016/0361-9230(90)90072-8 [DOI] [PubMed] [Google Scholar]
- 112.Quina LA, Harris J, Zeng H, Turner EE. Specific connections of the interpeduncular subnuclei reveal distinct components of the habenulopeduncular pathway. J Comp Neurol. Published online 2017. doi: 10.1002/cne.24221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bueno D, Lima LB, Souza R, et al. Connections of the laterodorsal tegmental nucleus with the habenular-interpeduncular-raphe system. J Comp Neurol. Published online 2019. doi: 10.1002/cne.24729 [DOI] [PubMed] [Google Scholar]
- 114.Hemmendinger LM, Moore RY. Interpeduncular nucleus organization in the rat: Cytoarchitecture and histochemical analysis. Brain Res Bull. Published online 1984. doi: 10.1016/0361-9230(84)90018-2 [DOI] [PubMed] [Google Scholar]
- 115.Molas S, DeGroot SR, Zhao-Shea R, Tapper AR. Anxiety and Nicotine Dependence: Emerging Role of the Habenulo-Interpeduncular Axis. Trends Pharmacol Sci. 2017;38(2). doi: 10.1016/j.tips.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frahm S, Ślimak MA, Ferrarese L, et al. Aversion to Nicotine Is Regulated by the Balanced Activity of β4 and α5 Nicotinic Receptor Subunits in the Medial Habenula. Neuron. Published online 2011. doi: 10.1016/j.neuron.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 117.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. Published online 2011. doi: 10.1038/nature09797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duncan A, Heyer MP, Ishikawa M, et al. Habenular TCF7L2 links nicotine addiction to diabetes. Nature. Published online 2019. doi: 10.1038/s41586-019-1653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ables JL, Antolin-Fontes B, Ibañez-Tallon I. Habenular synapses and nicotine. In: Neuroscience of Nicotine: Mechanisms and Treatment. ; 2019. doi: 10.1016/B978-0-12-813035-3.00010-1 [DOI] [Google Scholar]
- 120.Antolin-Fontes B, Li K, Ables JL, et al. The habenular G-protein–coupled receptor 151 regulates synaptic plasticity and nicotine intake. Proc Natl Acad Sci U S A. Published online 2020. doi: 10.1073/pnas.1916132117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ables JL, Görlich A, Antolin-Fontes B, et al. Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc Natl Acad Sci U S A. Published online 2017. doi: 10.1073/pnas.1717506114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Antolin-Fontes B, Ables JL, Görlich A, Ibañez-Tallon I. The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology. Published online 2015. doi: 10.1016/j.neuropharm.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Görlich A, Antolin-Fontes B, Ables JL, et al. Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. Proc Natl Acad Sci U S A. Published online 2013. doi: 10.1073/pnas.1313103110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao-Shea R, Degroot SR, Liu L, et al. Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun. Published online 2015. doi: 10.1038/ncomms7770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Casserly AP, Tsuji J, Zhao-Shea R, et al. Integrated miRNA-/mRNA-Seq of the Habenulo-Interpeduncular Circuit During Acute Nicotine Withdrawal. Sci Rep. 2020;10(1). doi: 10.1038/s41598-020-57907-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pang X, Liu L, Ngolab J, et al. Habenula cholinergic neurons regulate anxiety during nicotine withdrawal via nicotinic acetylcholine receptors. Neuropharmacology. Published online 2016. doi: 10.1016/j.neuropharm.2016.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soria-Gómez E, Busquets-Garcia A, Hu F, et al. Habenular CB1 Receptors Control the Expression of Aversive Memories. Neuron. Published online 2015. doi: 10.1016/j.neuron.2015.08.035 [DOI] [PubMed] [Google Scholar]
- 128.Zhang J, Tan L, Ren Y, et al. Presynaptic Excitation via GABAB Receptors in Habenula Cholinergic Neurons Regulates Fear Memory Expression. Cell. Published online 2016. doi: 10.1016/j.cell.2016.06.026 [DOI] [PubMed] [Google Scholar]
- 129.Agetsuma M, Aizawa H, Aoki T, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. Published online 2010. doi: 10.1038/nn.2654 [DOI] [PubMed] [Google Scholar]
- 130.Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron. Published online 2013. doi: 10.1016/j.neuron.2013.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kobayashi Y, Sano Y, Vannoni E, et al. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front Behav Neurosci. Published online 2013. doi: 10.3389/fnbeh.2013.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wolfman SL, Gill DF, Bogdanic F, et al. Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nat Commun. Published online 2018. doi: 10.1038/s41467-018-04654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. Published online 1992. doi: 10.1152/physrev.1992.72.1.165 [DOI] [PubMed] [Google Scholar]
- 134.Vertes RP. A PHA - L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. Published online 1991. doi: 10.1002/cne.903130409 [DOI] [PubMed] [Google Scholar]
- 135.Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. Published online 1986. doi: 10.1002/cne.902430307 [DOI] [PubMed] [Google Scholar]
- 136.Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-Cell bodies and terminals. Neuroscience. Published online 1981. doi: 10.1016/0306-4522(81)90146-9 [DOI] [PubMed] [Google Scholar]
- 137.Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct Funct. Published online 2016. doi: 10.1007/s00429-014-0924-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ren J, Isakova A, Friedmann D, et al. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife. Published online 2019. doi: 10.7554/eLife.49424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou W, Jin Y, Meng Q, et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci. Published online 2019. doi: 10.1038/s41593-019-0468-2 [DOI] [PubMed] [Google Scholar]
- 140.Mann JJ. Role of the serotonergic system in the pathogenesis of major depressionand suicidal behavior. Neuropsychopharmacology. Published online 1999. doi: 10.1016/S0893-133X(99)00040-8 [DOI] [PubMed] [Google Scholar]
- 141.Liu Z, Zhou J, Li Y, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. Published online 2014. doi: 10.1016/j.neuron.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Y, Zhong W, Wang D, et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun. Published online 2016. doi: 10.1038/ncomms10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walsh JJ, Christoffel DJ, Heifets BD, et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature. Published online 2018. doi: 10.1038/s41586-018-0416-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fernandez SP, Muzerelle A, Scotto-Lomassese S, et al. Constitutive and Acquired Serotonin Deficiency Alters Memory and Hippocampal Synaptic Plasticity. Neuropsychopharmacology. Published online 2017. doi: 10.1038/npp.2016.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matthews GA, Nieh EH, Vander Weele CM, et al. Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell. Published online 2016. doi: 10.1016/j.cell.2015.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matthews GA, Tye KM. Neural mechanisms of social homeostasis. Ann N Y Acad Sci. Published online 2019. doi: 10.1111/nyas.14016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Witten IB, Steinberg EE, Lee SY, et al. Recombinase-driver rat lines: Tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. Published online 2011. doi: 10.1016/j.neuron.2011.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]