Abstract
Hibernation is a unique evolutionary adaptation to conserve energy. During the pre-hibernation (i.e. fall) season, a progressive decline in core body temperature and further decrease in metabolism underlie a seasonal modulation in thermoregulation. The onset of hibernation requires marked changes in thermoregulatory attributes including adjustment in body temperature and tissue specific increases in thermogenic capacity. The hibernation season is characterized by a regulated suppression in thermogenesis allowing the onset of torpor interrupted by periodic activation of thermogenesis to sustain interbout arousals. Thyroid hormones are known to regulate both body temperature and metabolism, and for this reason, the hypothalamic-pituitary-thyroid axis and thyroid hormones have been investigated as modulators of thermogenesis in the phenomenon of hibernation, but the mechanisms remain poorly understood. In this review, we present an overview of what is known about the thermogenic roles of thyroid hormones in hibernating species across seasons and within the hibernating season (torpor-interbout arousal cycle). Overall, the hypothalamic-pituitary-thyroid axis and thyroid hormones play a role in the pre-hibernation season to enhance thermogenic capacity. During hibernation, thermogenesis is attenuated at the level of sympathetic premotor neurons within the raphe pallidus and by deiodinase expression in the hypothalamus. Further, as recent work highlights the direct effect of thyroid hormones within the central nervous system in activating thermogenesis, we speculate how similar mechanisms may occur in hibernating species to modulate thermogenesis across seasons and to sustain interbout arousals. However, further experiments are needed to elucidate the role of thyroid hormones in hibernation, moving towards the understanding that thyroid hormones metabolism, transport and availability within tissues may be the most telling indicator of thyroid status.
Keywords: HPT axis, CNS, Torpor, Tanycytes
1. Introduction
Plasticity in thermoregulatory and metabolic systems are key to hibernation. In small mammals, hibernation consists of prolonged intervals of controlled reductions in metabolic rate and body temperature (Tb), known as torpor, interrupted by short (<24 h) episodes of euthermia, which require intense bouts of non-shivering thermogenesis (Fig. 1). Hibernation studies have focused on elucidating the mechanisms driving changes in thermogenesis during hibernation including central nervous system (CNS) regulation of thermogenesis and modulation of tissue-specific thermogenic capacity. Thyroid hormones (TH) play key roles in regulating Tb and metabolism and have long been investigated as modulators of thermogenesis during hibernation, yet how this regulation occurs remains poorly understood. The purpose of this review is to summarize and discuss the role of TH in thermogenesis during hibernation in small mammals. We address how TH interact with brown adipose tissue (BAT) and the CNS to create a multifaceted system that regulates thermogenesis, and how this system is modulated during hibernation.
Fig. 1.
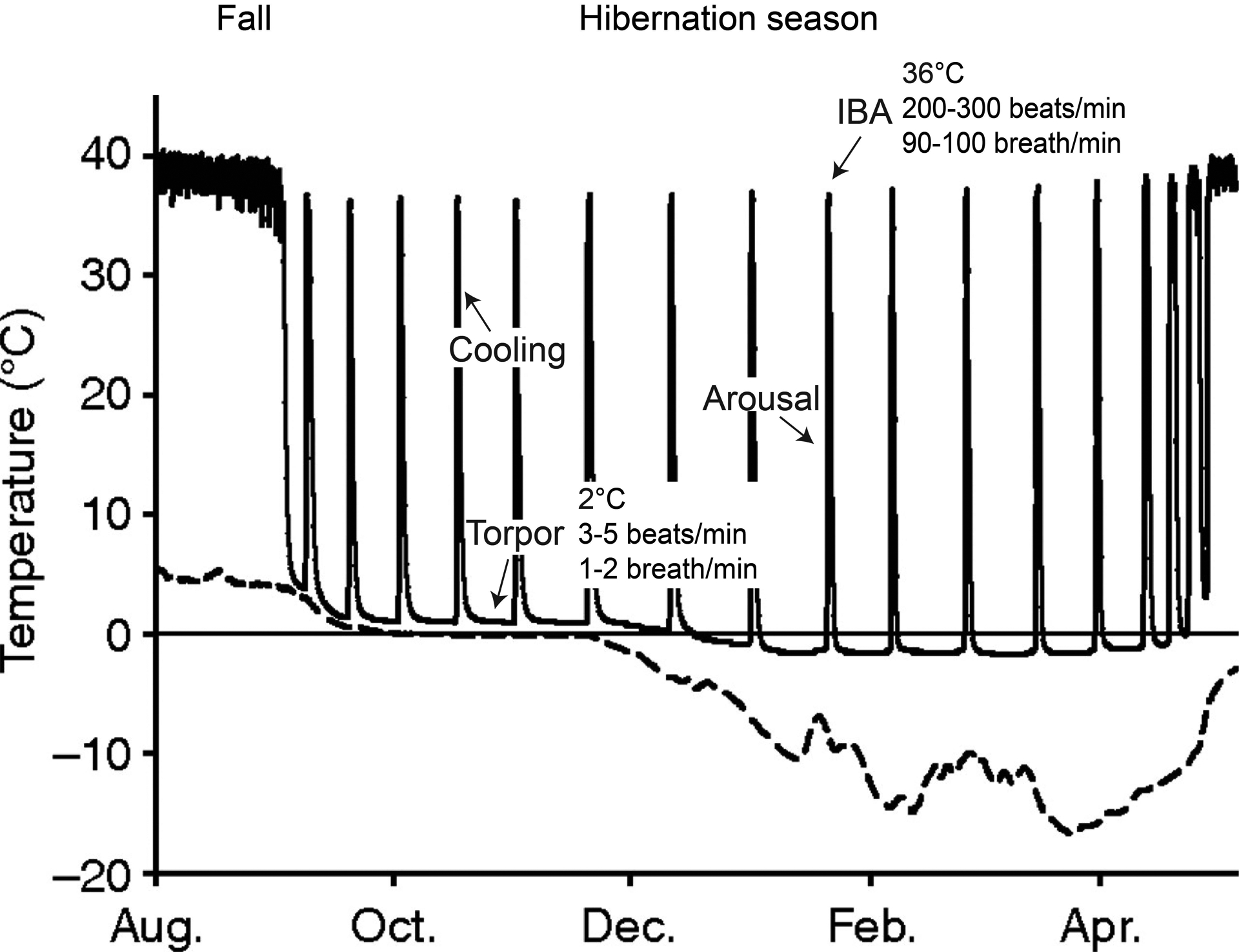
Seasonal onset of hibernation represented by seasonal changes in Tb in Arctic ground squirrel. A decrease in euthermic Tb indicates the pre-hibernation season (i.e. fall), which precedes the onset of hibernation. The hibernation season is not static but is comprised of extended periods of low metabolic rate and Tb (torpor bouts) interrupted by regular IBA. Animals actively suppress metabolism, which results in a decrease in Tb (and a further decrease in metabolism) during the cooling phase. During arousals, animals initially generate heat via non-shivering thermogenesis in BAT and this is supplemented by shivering once Tb attains ~16 °C and eventually metabolic rate and Tb are restored to euthermic levels. In the AGS, hibernacula temperatures can drop well below zero (dashed line) such that animals must also be thermogenic during torpor. The emergence from hibernation occurs in the spring and is associated with the reproductive period. The summer season follows. (Figure adapted from Williams et al., 2011 J. Exp. Biol. 214, 1300–1306).
1.1. Hibernation
Hibernation is an evolutionary strategy to conserve energy in part by attenuating thermogenesis with a subsequent decline in core Tb and further decrease in metabolism (Ruf and Geiser, 2015). The physiology and thermal biology of hibernation has perhaps been most extensively studied in the ground squirrels (GS), including 13-lined (Ictidomys tridecemlineatus), Richardson’s (Urocitellus richardsonii) and Arctic (Urocitellus parryii) ground squirrels. Within ground squirrels, often referred to as obligate hibernators, the onset of hibernation is regulated by an endogenous circannual rhythm which persists under constant environmental conditions (Pengelley et al., 1978; Frare et al., 2018). The onset of hibernation is characterized by a pre-hibernation phase (i.e. fall) in which thermogenesis, resting metabolic rate and energy expenditure decrease, reducing euthermic Tb by 2 °C (Russell et al., 2010; Sheriff et al., 2012, 2013; Frare et al., 2018) and shifting animals towards a positive energy balance. In addition, the volume of BAT increases and enhances the ability to produce heat (i.e. thermogenic capacity) (Abbotts and Wang, 1980; Ballinger et al., 2016; MacCannell et al., 2017). Thermogenic capacity is critical during the hibernation season to support regular interbout arousals (Ballinger and Andrews, 2018) characteristic of small mammalian hibernators. Ground squirrels do not consume food during their hibernation which lasts seven to eight months, but instead use stored fat to sustain their energetic demands (Williams et al., 2014).
The hibernation season is composed of repeated multi-day torpor bouts, interrupted by interbout arousal episodes known as interbout arousals (IBA) or interbout euthermia. A subsequent cooling phase precedes the next torpor bout (Fig. 1). Torpor bout length peaks during the middle of the hibernation season; torpor bouts are shorter early and late in hibernation. In mid-hibernation, a torpor bout in arctic ground squirrels (AGS) can last more than 21 consecutive days. Torpor involves a state of regulated hypometabolism during which animals suppress their metabolism and thermogenesis allowing their Tb to cool. Tb drops to a few degrees above ambient temperature (Ta) and can reach a minimum of −2.9 °C in free-living AGS (Barnes, 1989). During torpor, metabolic rate decreases to 1–2% of basal metabolic rate (Buck and Barnes, 2000), respiratory rate decreases to 1–2 breaths/min (personal observations), heart rate decreases to 3–5 beats/minute (Carey et al., 2003). Although Tb is very close to ambient temperature in most species of ground squirrels, AGS can increase their torpid metabolic rate as much as 36-fold as they generate heat to prevent themselves from freezing in sub-zero hibernacula (Richter et al., 2015), highlighting the metabolic plasticity of the species. The arousal or rewarming phase lasts on average 5 h in captive AGS (Karpovich et al., 2009) and, in other GS species, this has been shown to be initiated by BAT thermogenesis, accompanied by shivering once Tb reaches a minimum of 16 °C (Cannon and Nedergaard, 2004). During rewarming, metabolic rate increases to six times basal metabolic rate, and once Tb is restored to 35–36 °C, metabolic rate returns to just above basal levels (Tøien et al., 2001). After rewarming, respiratory rate levels off at 90–100 breath/minute and heart rate reaches 200–300 beats/min (Carey et al., 2003). These physiological parameters are maintained for about 15 h in the IBA, until the start of the next cooling phase (Karpovich et al., 2009). The onset of these prolonged bouts of torpor occurs through regulated suppression of thermogenesis and metabolism.
Much of our current understanding of the potential roles of thyroid hormones in hibernation and daily torpor, comes from the extensively-studied hibernating ground squirrels, as well as from Djungarian hamster (Phodopus sungorus), a species that shows spontaneous daily torpor (STD), but does not hibernate as defined by multi-day torpor. SDT is a hypometabolic state similar to hibernation (Ruf and Geiser, 2015) in which the degree of metabolic suppression and body temperature decrease is less dramatic and sustained for shorter intervals: metabolic rate decreases to just ~25% of basal metabolic rate (Cubuk et al., 2017), Tb decreases to a minimum of ~15 °C (Heldmaier and Steinlechner, 1981), and torpor bouts last less than 12 h. The winter expression of SDT in Djungarian hamsters is closer to hibernation than fasting-induced torpor, which Djungarian hamsters display in the summer season after food deprivation. Fasting-induced torpor, which is well described in mice, has been suggested to be regulated by a different mechanism (Cubuk et al., 2017), and is therefore not part of this review.
1.2. Thyroid hormones
TH secreted by the thyroid gland regulate thermogenesis, metabolism, and cognition. These roles for TH were recognized in the late 1800s when thyroidectomy became a common surgical intervention for thyroid disease. Physicians described thyroidectomized patients becoming cold, fat and mentally ill (Giddings, 1998; Sarkar et al., 2016).
1.2.1. HPT-axis
The hypothalamic-pituitary-thyroid (HPT)-axis regulates the production of TH which has been described in several reviews (Mullur et al., 2014; Ortiga-Carvalho et al., 2016) and we briefly summarize here. In the paraventricular nucleus of the hypothalamus (PVN), thyrotropin releasing hormone (TRH)-neurons project to the median eminence (ME), a key hypothalamic area linking the brain to the pituitary gland (i.e. hypophysis) through the hypophyseal portal system. TRH, released into the hypophyseal portal system reaches and stimulates thyrotroph cells in the pituitary gland, causing the release of thyroid stimulating hormone (TSH) into the circulatory system. TSH stimulates the thyroid gland triggering the secretion of the two primary TH, T3 (3,5,3′-triiodo-l-thyronine) and T4 (3,5,3′,5′-tetraiodo-l-thyronine), into the blood stream. The subsequent uptake of TH by target tissues is enzymatically regulated (see 1.2.2). T4 differs from T3 only by an additional iodine located at the 5′-position of the first thyroxine ring. Although T3 is considered the biologically active form of TH, a much larger proportion of T4 is secreted into the plasma compared to T3 (Abdalla and Bianco, 2014). However, T4 can be metabolized to T3 peripherally by deiodination and therefore acts as a reservoir, or prohormone, for T3. Uptake and conversion of TH in the hypothalamus and pituitary gland regulates the HPT-axis through a negative feedback mechanism; TH inhibits TRH release from TRH neurons and TSH release from thyrotroph cells (Ortiga-Carvalho et al., 2016).
1.2.2. TH availability
T3 availability is fundamental for TH action such as stimulating thermogenesis. In plasma, TH exist as bound and free TH. The majority of T3 and T4 is bound to and carried by proteins in the blood such as thyroxine-binding globulin (TBG), albumin, and transthyretin (TTR), leaving a small fraction of free TH (FTH) available for transport into target tissues (Yen, 2001). Circulating FTH enter tissues via trans-membrane protein transporters, including organic anion transporter (OAT1P1C), monocarboxylic acid transporter 8 (MCT8) and 10 (MCT10), large neutral amino acid transporters (LAT), and the sodium/taurocholate cotransporting polypeptide (SLC10A1), which vary in their specificity for the primary TH and their derivatives (Bernal et al., 2015). Within the target tissues, deiodinase enzymes convert TH to active or inactive forms. Deiodinase 1 (D1), primarily found in the plasma membrane of the thyroid, liver and kidneys, converts T4 to T3, which quickly equilibrates with plasma T3, thus largely contributing to T3 plasma levels (Gereben et al., 2008; Ortiga-Carvalho et al., 2016). Deiodinase 2 (D2), located in the endoplasmic reticulum in the CNS and BAT, is the primary generator of intracellular T3 through the deiodination of T4 (Gereben et al., 2008; Ortiga-Carvalho et al., 2016). Deiodinase 3 (D3), mainly expressed in the CNS, metabolizes T4 and T3 to metabolites: rT3 (3,3′5’–triiodo-L-thyronine) and T2 (3,3′-thyronine) (Ortiga-Carvalho et al., 2016). Thus, intracellular bioavailability, influenced by serum binding proteins, transporters and deiodinases, alters thyroid status in the periphery and in the CNS.
1.2.3. Thyroid hormone receptors
Thermogenic influence of TH is primarily through genomic effects; T3 binds to nuclear TH receptors (α1,β1,2) that together regulates transcription of specific genes by binding to the thyroid hormone response element (TRE) (Yen, 2001; Bernal et al., 2015). TH receptors also form heterodimers with other receptors such as retinoic X receptor, which enhances TH receptor binding to TRE, increases the range of TREs to which TH receptors can bind and consequently increases the number of target genes (Yen, 2001; Ortiga-Carvalho et al., 2016). Nonetheless, recent studies are investigating the nongenomic effects of TH (Incerpi et al., 2016). Even though the molecular mechanisms are not yet defined, it has been suggested that nongenomic modulation of thermogenesis and metabolism occur through a direct interaction between mitochondria and TH, in particular T2, previously thought to be an inactive TH (Incerpi et al., 2016). An alternative presumed nongenomic effect has been suggested to be mediate by 3-Iodothyronamine (T1AM), the last iodinated thyronamine produced by TH metabolism (Laurino et al., 2018). Interestingly, peripheral administration of T1AM promotes rapid hypothermia and hypometabolism in both mice and hamster (Braulke et al., 2008; Doyle Kristian et al., 2007; Scanlan et al., 2004), in contrast to TH effect on thermogenesis and metabolism. However, the T1AM mechanism of action is still unknown (Laurino et al., 2018).
1.2.4. TH stimulate thermogenesis by actions on brown adipose tissue
TH stimulates brown adipose tissue (BAT) thermogenesis through a genomic effect that synergizes with sympathetic nervous system (SNS) activation of BAT. BAT is a thermogenic tissue, characterized by a high density of mitochondria, blood vessels and sympathetic innervation (Jastroch et al., 2018). Mitochondrial uncoupling protein 1 (UCP1) confers to BAT the ability to produce heat. UCP1 allows the protons to return to the mitochondrial matrix, releasing the energy of the proton gradient as heat instead of synthetizing ATP (Nedergaard et al., 2001). SNS regulates BAT thermogenesis (Morrison and Madden, 2014) by releasing norepinephrine (NE) at sympathetic nerve terminals. NE activates the β3-type adrenergic receptors on the brown adipocytes, increasing intracellular cAMP levels, which activates PKA. PKA stimulates lipolysis, releasing free fatty acids (FFA), thus providing the substrates for the mitochondria respiratory chain, which dissipates energy as heat via UCP1 and increases thermogenesis (Nedergaard and Cannon, 2018). TH enhances adrenergic stimulation of BAT (de Jesus et al., 2001) by increasing expression of genes coding for proteins that enhance the response to β3 stimulation. The increase in cAMP, following β3 activation, also increases D2 activity and thus intracellular T3 levels. In this way, T3 amplifies cAMP response to adrenergic stimulation and increases UCP1 transcription in the brown adipocytes (Silva and Larsen, 1983; Silva and Matthews, 1988; de Jesus et al., 2001). A synergistic interaction between TH and SNS is fundamental for BAT thermogenesis.
TH may also stimulate BAT thermogenesis through central sites of action. In the ventromedial hypothalamus (VMH), T3 inhibits the activity of AMP-activated protein kinase (AMPK), activates the sympathetic premotor neurons located in the raphe pallidus (rPA) and drives BAT thermogenesis (López et al., 2010). Although the link between AMPK and SNS activation is not fully understood, UCP1 expression in BAT is known to be necessary for central-T3 activation of BAT (González-García and López, 2017). Interestingly, the central action of T3 also stimulates browning of white adipose tissue (Alvarez-Crespo et al., 2016).
We described the primary mechanisms how T3 increase BAT thermogenesis; interestingly, recent studies show that T2, considered an inactive metabolite, enhances BAT adrenergic tone; however the mechanisms are still not known (Cioffi et al., 2018).
1.2.5. Tanycytes may modulate TH effects on thermogenesis
Tanycytes are ependymal glial cells well characterized around the 3rd ventricle in the hypothalamus, and also found around the 4th ventricle and the central canal (Langlet et al., 2013), and are emerging as key players in energy metabolism and reproduction described in recent and comprehensive reviews (Prevot et al., 2018; Langlet, 2019). In general, tanycytes are characterized by long processes projecting to the medial basal hypothalamus (MBH) and the ME in spatial proximity with blood vessels and neurons (Prevot et al., 2018; Langlet, 2019; Rodríguez-Rodríguez et al., 2019). TH transporters MCT8 and OATP1c1 and deiodinases D2 and D3 are highly expressed in tanycytes (Bolborea and Dale, 2013; Langlet, 2019; Rodríguez-Rodríguez et al., 2019) and undergo marked seasonal changes in expression in seasonal species exhibiting SDT (Herwig et al., 2009; Petri et al., 2016). Hence, tanycytes regulate hypothalamic availability of T3. Modulation of T3 by tanycytes may contribute to the central T3-activation of BAT thermogenesis. Moreover, tanycytes regulate the HPT-axis through a local release of the TRH degrading enzyme Pyroglutamyl Peptidase II (PPII), and changes in endfeet morphology preventing TRH release into the portal system (Sánchez et al., 2009; Müller-Fielitz et al., 2017; Rodríguez-Rodríguez et al., 2019). Tanycytes, acting on the HPT-axis, regulate TH production, thus the TH effect on BAT thermogenesis.
2. Changes in the HPT-axis increase thermogenic capacity, which is regulated by SNS and tissue expression of deiodinases
An increase in thermogenic capacity during the hibernation season is necessary to sustain arousal. The increase in HPT axis activity during fall contributes to higher thermogenic capacity. A functional HPT-axis during hibernation may thus contribute to thermogenesis in arousal.
The hypothalamic TRH neurons maintain their neuronal activity across the seasons. TRH mRNA expression in whole hypothalamus is higher in the fall and winter compared to the spring in 13-lined GS (Schwartz et al., 2013). Euthermic AGS in summer, fall and IBA show a constant neuronal activation of TRH neurons in the PVN (Frare et al., 2018), however, the number of TRH-immunoreactive neurons (identified by TRH immunohistochemistry and counted manually), is higher in winter compared to summer AGS (Frare et al., 2019). The number of TRH positive neurons may increase because TRH expression increases reflecting an increase in synthetic/secretory activity; or, the increase may reflect neurogenesis of TRH secreting neurons. In either case, a winter increase in TRH-immunoreactive neurons is consistent with enhanced thermogenic capacity of the HPT-axis in winter.
The thyroid gland shows a basal secretory activity during the hibernation season, even in torpor, but significantly higher activity occurs in the pre-hibernation state. The thyroid gland is characterized by spherical colloids, containing T3 and T4 precursors and thyroglobulin, surrounded by secretory epithelial cells. A large spherical colloid and a squamous epithelium are associated with a “resting” gland; secretion is limited and thyroid activity is reduced. In contrast, a cuboidal to columnar epithelium and a small colloid are associated with a highly active gland. These distinct changes in thyroid morphology allow histological analyses to be used as a proxy for thyroid activity (Kališnik, 1972; Kot et al., 2013; Park et al., 2017). In one of the first papers investigating the thyroid gland in 13-lined GS, qualitative observations of thyroid histology did not highlight any seasonal change (Mann, 1916). However, a later study on the same species showed pronounced seasonal variation in thyroid activity during the year (Zalesky, 1935). High thyroid activity was also measured during the pre-hibernation phase in Richardson’s GS (Winston and Henderson, 1981) and AGS (Frare et al., 2018). In fall AGS, small colloids and columnar epithelium denote a high thyroid activity that settles into lower and summer-like morphology in mid-hibernation (both in torpor and IBA) (Frare et al., 2018). Another study in AGS reports large colloids in early hibernation (October), suggesting an initial resting of the gland and storage of TH (Nevretdinova et al., 1992). As the hibernation season progresses, thyroid activity increases, as the colloid’s volumes decrease (as seen in mid-hibernation), reaching maximal activity in May (Nevretdinova et al., 1992).
Iodine secretion is another indication of thyroid gland activity. Measurements of iodine secretion indicate TH is not secreted from the thyroid gland following direct cooling of the hypothalamus, between late summer (August) and mid-hibernation (December) in 13-lined GS (Hulbert and Hudson, 1976). Although thyroid secretion did not occur, direct hypothalamic cooling induced an immediate increase in Tb, which lead the author to conclude that the HPT-axis was unresponsive (Hulbert and Hudson, 1976). However, considering the recent findings of thermoregulatory pathways, these results might be better explained by an immediate sympathetic response to hypothalamic-cooling (Morrison, 2016), rather than a refractory HPT-axis. In fact, pharmacologically-induced cooling stimulates the HPT-axis in mid-hibernation, indicating a responsive HPT-axis (Frare et al., 2018), in contrast with the former hypothesis of a refractory HPT-axis during hibernation (Hulbert and Hudson, 1976). Further, thyroid secretory activity during the hibernation season is similar to the summer season, and the increase in secretion observed in the pre-hibernation season likely underlies the seasonal accumulation of BAT occurring in hibernators (Ballinger and Andrews, 2018).
Based on thyroid secretory activity, we would expect high TH concentration in fall and constant TH concentration between summer and winter. Interestingly AGS do not show changes in TTH concentration in plasma across seasons (SI Table 1) (Frare et al., 2018; Williams et al., 2019). Only a few studies report FTH concentrations during hibernation. In AGS, FT3 and FT4 do not show any significant difference between summer, fall and IBA (Frare et al., 2018) (SI Table 2). Similarly in Richardson’s GS, FT4 concentration is the same in summer and IBA (Magnus and Henderson, 1988) (SI Table 2). Bound TH in the plasma maintain a stable pool of FTH to prevent hormonal fluctuations (Refetoff et al., 2000), and the stable plasma concentrations of TTH and FTH in hibernators may indicate normal physiological circulating level of TH across the year.
Experiments in which thyroidectomy was performed suggest a fundamental interaction between TH and thermogenesis, as shown by the different response to cold exposure between European hamsters thyroidectomized in different seasons (Canguilhem, 1970, 1972). Even if the procedure disrupted the occurrence of torpor, hamsters thyroidectomized in the fall were able to survive subsequent cold-exposure, while the hamsters thyroidectomized in the spring died of hypothermia (Canguilhem, 1970). These findings suggest a critical role of the thyroid and TH in providing thermogenic capacity before winter, which is consistent with the increase in thyroid activity during the fall (Winston and Henderson, 1981; Frare et al., 2018). In hyperthyroid mice, central T3 increases BAT volume and activity (Alvarez-Crespo et al., 2016; Weiner et al., 2016); similarly TH may drive the seasonal increase in BAT mass seen in hibernators (Ballinger and Andrews, 2018), which contributes to the increase in thermogenic capacity.
Interestingly, thyroidectomy performed at the end of the fall preparatory phase did not affect the hibernation pattern in Richardson GS (Henderson et al., 1981), or the metabolic rate in euthermic 13-lined GS (Hulbert and Hudson, 1976). The effects of TH on BAT and thermogenesis prior to thyroidectomy may have been sufficient for the GS to develop the thermogenic capacity needed for arousals from torpor. Moreover, the GS may rely mainly on sympathetic BAT activation as a compensatory thermogenic mechanism (Silva and Matthews, 1988).
TH and the SNS synergistically increase BAT thermogenesis (Silva and Matthews, 1988; Silva and Rabelo, 1997), and adrenergic activation of BAT enhances intracellular D2 activity. In hibernation, D2 activity in BAT is higher during torpor and arousal compared to euthermia (Liu et al., 2001), confirming higher thermogenic capacity in the hibernation season, and highlighting the promptness of BAT in sustaining arousal. Thus, HPT-axis and TH enhance thermogenic capacity, but SNS may be the ultimate gatekeeper of BAT thermogenesis in hibernation, allowing the onset of torpor and triggering arousal.
3. A downregulation of the HPT-axis contributes to maintaining torpor
A functional HPT-axis, with increased thermogenic capacity, sustains regular IBAs, but a “downregulation” allows torpor onset. Neuronal activity of TRH neurons in the PVN decreases during torpor compared to IBA in AGS (Frare et al., 2018), most likely reducing thermogenesis and Tb. TRH injections into the lateral ventricle (icv) or into the hippocampus reverse the torpid state by inducing thermogenesis in Syrian hamsters (Shintani et al., 2005; Tamura et al., 2005) and golden mantled GS (Stanton et al., 1980). A decrease in TRH neuronal activation and TRH receptor expression in the hypothalamus (Stanton et al., 1992) contributes to the downregulation of central TRH signaling within torpor.
Surprisingly, hibernation torpor is characterized by higher levels of plasma TTH compared to euthermia, which is counterintuitive as TH are known to increase thermogenesis. TT3 and TT4 concentration are higher in torpor compared to IBA in AGS (Nevretdinova and Shvareva, 1987; Frare et al., 2018) (SI Table 1), and in Richardson’s GS (Magnus and Henderson, 1988) (SI Table 3). Another study in Richardson’s GS report different patterns between TT3 and TT4 at different stages of the torpor-IBA cycle (Demeneix and Henderson, 1978a) (SI Table 3). Interestingly, one study in AGS showed a 50% increase in TT4, precursor for T3, during early arousal (Nevretdinova and Shvareva, 1987), supporting the proposed role of the HPT-axis in activating thermogenesis during arousal (Frare et al., 2018).
We speculate that increased plasma protein binding concomitant with an increase in TTH during torpor could be an alternative mechanism to prevent TH-mediated thermogenesis during torpor. Sustained basal secretory activity of the thyroid gland during torpor (Nevretdinova et al., 1992; Frare et al., 2018), and a decrease in TH clearance from plasma during torpor (Demeneix and Henderson, 1978b), suggest an accumulation of TH in plasma that reflects the increase in TTH concentration seen in torpor. During torpor, the amount of plasma proteins, such as albumin (Fig. 2), rise (Magnus and Henderson, 1988) likely increasing the amount of bound TH and thus TTH measured in blood. An increase in TH protein binding maintains FTH during torpor (Frare et al., 2018). However, when FTH is measured at low temperature, similar to torpor, FTH concentrations are lower than when FTH is measured at euthermic body temperature (Magnus and Henderson, 1988). These results suggest that during arousal bound TH is released with increasing temperature. Release of TH would increase the FTH fraction and FTH uptake by peripheral tissue such as BAT and stimulate BAT-thermogenesis.
Fig. 2.
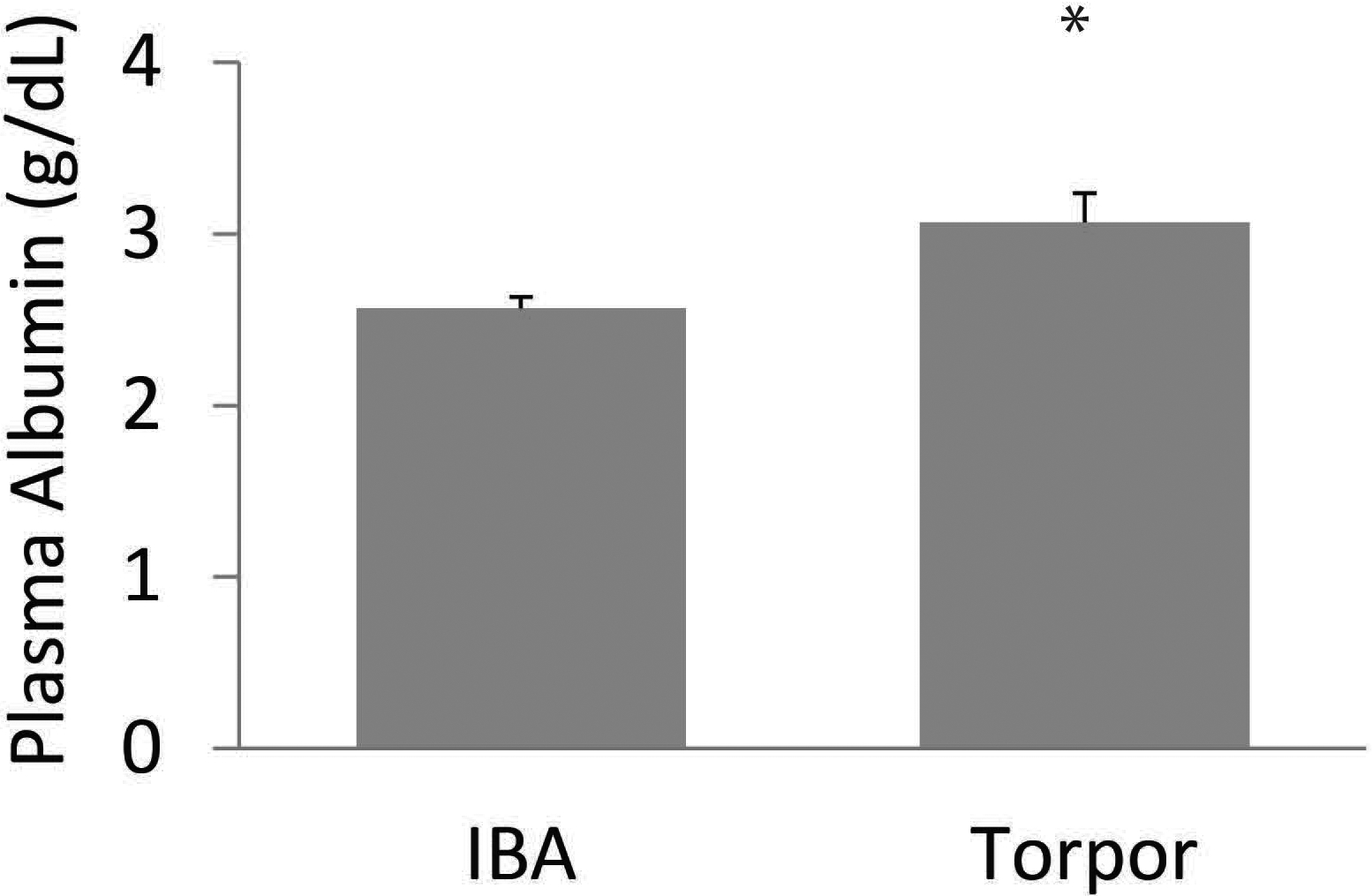
Albumin concentration in plasma in AGS. Albumin concentrations (g/dL) is significantly higher in torpor compared to interbout arousal (IBA) (*p < 0.05, t-test). Sample size n = 3–6.
4. TH availability in the CNS may be regulated by tanycytes and could affect HPT-axis and thermogenesis
As mentioned above, tanycytes regulate the HPT-axis through local release of PPII, and changes in endfeet morphology preventing TRH release into the portal system (Sánchez et al., 2009; Müller-Fielitz et al., 2017). We do not know how PPII and tanycytes endfeet morphology changes across seasons or during hibernation. We speculate that an increase in peripheral FT3 during arousal could inhibit TRH neurons and increase PPII expression, further suppressing TRH release into the portal system, thus promoting the onset of torpor. Further investigations are needed to assess any detailed structural changes in tanycytes’ morphology regulating neurohormonal release of TRH and to measure changes in PPII.
Hypothalamic deiodinases, which are mainly located in tanycytes, may regulate SDT (Murphy et al., 2012). The onset of SDT is associated with a decrease in the hypothalamic mRNA expression of D2, and an increase in D3 mRNA and MCT8 (Herwig et al., 2009; Petri et al., 2016), suggesting a larger MCT8 uptake of TH by tanycytes and a consequent deactivation via D3 (Herwig et al., 2009; Bank et al., 2015). Lower hypothalamic T3 gates STD onset (Bank et al., 2017). Similar studies are needed in ground squirrels to understand the role of hypothalamic T3 in hibernation.
We know that central T3 regulates thermogenesis through the VMH-rPA pathway activating BAT thermogenesis as seen in mice (López et al., 2010), and α-tanycytes may be a likely source of T3. These α-tanycytes express deiodinases and TH transporters. Furthermore, α-tanycyte processes extend into the VMH (Prevot et al., 2018). Hypothalamic D2 mRNA expression increases in normothermia compared to torpor in Djungarian hamster (Bank et al., 2015). Thus, an increase in hypothalamic T3 availability promotes thermogenesis via the VMH-rPA pathway. Based on studies in SDT, we expect that a decrease in hypothalamic T3 availability contributes to the seasonal decrease in cFos expression in the premotor neurons in the rPA that we have observed in euthermic AGS during the hibernation season (Frare et al., 2018). Hypothalamic D2 expression increases in 13-lined GS in the spring (Schwartz and Andrews, 2013), and under long photoperiod in Djungarian hamster (Herwig et al., 2009; Petri et al., 2016) and in Syrian hamster (Revel et al., 2006; Yasuo et al., 2007) suggesting an increase in hypothalamic T3. The increase in T3 most likely affects the reproductive phase, but it may also increase the winter euthermic Tb by 1–2 °C and contribute to the increase in euthermic Tb seen at the end of the hibernation season and maintained throughout the summer.
Hypothalamic effects of TH on thermogenesis, metabolism and energy balance is independent of plasma levels. We suggest tanycytes as a regulator of TH availability. Different tanycytes populations regulate T3 availability in defined hypothalamic nuclei, controlling specific functions such as TRH release into the portal vessel of the pituitary and the T3-mediated VMH-rPA thermogenesis. Seasons may drive tanycytes modulation of T3 availability to regulate the HPT-axis and thermogenesis accordingly, consistent with the role of these ependymal cells in regulating seasonal phenotypes.
5. Methodological issues in measuring FTH
Results from studies examining stability of the physiological pool of FTH in the plasma of hibernating animals have been inconsistent and we speculate differences among studies may reflect methodological issues with measurements.
Within Richardson’s ground squirrel, for example, Demeneix and Henderson (1978b) found higher FT4 in torpor compared to euthermia, whereas Magnus and Henderson (1988) observed the opposite pattern (SI Table 2, underlined values). In both studies, equilibrium dialysis (ED) lasting 20–24 h was used to separate the unbound TH to measure the percent fraction of FTH. The percent fraction of FTH multiplied by TTH (measured by RIA) resulted in the actual FTH concentration. ED was performed at two different temperatures 6 °C and 37 °C, chosen to represent torpor Tb and euthermic Tb respectively.
The studies show conflicting results in the percent FT4 (%FT4) measurements performed at the same assay temperature 37 °C and 6 °C following the same protocol, except for a lower specific activity for the [125I]T4 used. Therefore, the actual concentration of FT4 was different comparing values at the same assay temperature (SI Table 2). As the ED assay temperatures reflected the animal Tb, the authors draw their conclusion using FT4 concentration at the specific Tb: FT4 obtained at 37 °C assay group was indicative of euthermia and FT4 obtained at 6 °C indicative of torpor. The early study showed a higher FT4 in torpor compared to euthermia (Demeneix and Henderson, 1978b), while the opposite trend was reported in the later one (Magnus and Henderson, 1988). Differences were associated with different affinity in [125I]T4, possible contamination and the difference ED length (20 versus 24 h).
The different techniques used and the difference within the same methods, show the need to use an accurate and reliable method in measuring FTH. In particular, the temperature during ED and separation methods may affect the fraction of bound and free TH as the binding to plasma proteins is temperature dependent.
In AGS, FTH have been measured using LC-MS/MS and RIA. LC-MS/MS required an initial separation between the free and bound TH fractions as in ED, on the contrary RIA measures FTH directly from plasma (comprised of free and bound TH). Before LC-MS/MS quantification, FTH were separated via ultrafiltration for 40 min at 37 °C (Frare et al., 2018). The separation temperature may have been critical and prevented a detection of torpor-state differences in FTH. However, FTH concentrations were similar in summer and fall between the RIA and the LC-MS/MS, suggesting that ultrafiltration does not significantly alter FTH concentration (SI Table 2). ED did not provide a direct measurement of FTH, instead the ultrafiltrate was immediately used in the LC-MS/MS to directly quantify FTH, providing a more reliable measurement. Due to the limited amount of studies, the inconsistent results between species (AGS and Richardson’s GS) and within species (Richardson’s GS), we cannot yet evaluate the role of FTH in hibernation. A consistent and reliable method is needed to standardize results between studies and laboratories, keeping in mind that changes in TTH concentrations do not always reflect changes in FTH concentration, and that the month of sampling could affect the TH values, as well as the time of the day (Demeneix and Henderson, 1978a). Moreover, in light of the current knowledge of the role of TH in the CNS and the importance of TH transporters and deiodinases, we should rely less on plasma data as an indicator of thyroid status because it may not fully reflect availability of TH and TH metabolites within the CNS.
6. Conclusion
The seasonal modulation of thermoregulation during hibernation resides not only in the plasma concentration of TH, but within the different components of the HPT-axis, and target tissue such as BAT and the hypothalamus. A key perspective that emerges is the role of TH in increasing thermogenic capacity during the hibernation season, most likely through synergism between TH and the SNS in activating BAT-thermogenesis (Fig. 3). The increased HPT-axis response to pharmacologically-induced cooling (Frare et al., 2018) highlights a responsive HPT-axis and the increase in thermogenic capacity during hibernation. This interpretation is supported by a seasonal increase in TRH neuronal activation (Frare et al., 2019) and TRH mRNA expression (Schwartz et al., 2013) in the hypothalamus and higher D2 activity in BAT (Liu et al., 1998). In addition, the increase in TT4 in early arousal (Nevretdinova and Shvareva, 1987), is consistent with the role of the HPT-axis and TH as thermogenic components in the arousal process.
Fig. 3.
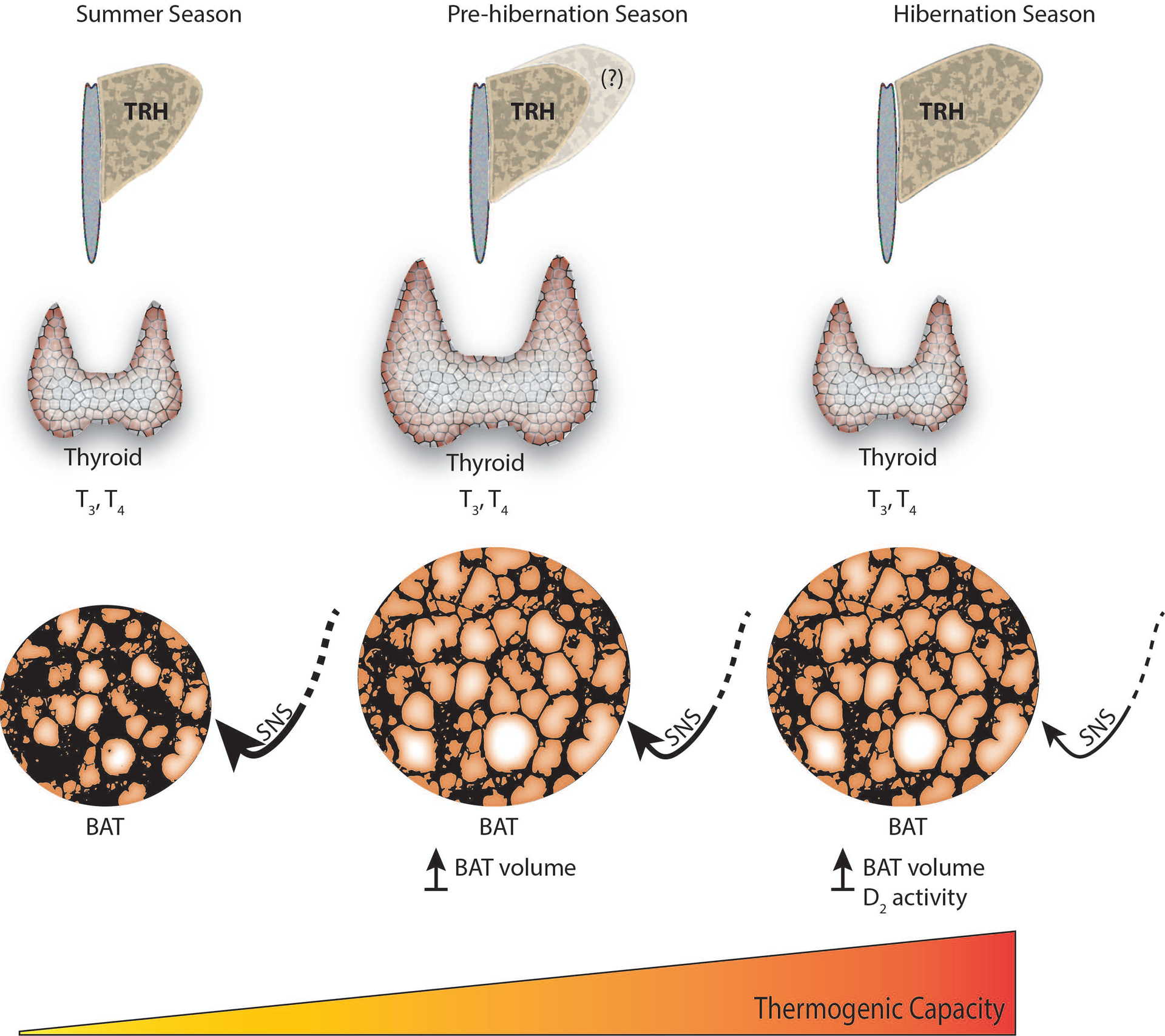
Changes in thyroid status leading to a seasonal increase in thermogenic capacity occurs with a gradual decrease in sympathetic tone.
In addition, we illustrated how the circulating levels of TH as in indicator of TH function is not a reliable indication of HPT activation, and how published results are inconsistent even within the same species. We highlight the need for an accurate technique to measure FTH to complement TTH analyses. We described how the effect of TH relates to tissue concentration of TH, and to deiodinases activity highlighting how measurements of mRNA expression and protein levels of deiodinases may be more indicative of the thyroid status.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by grants from the National Science Foundation to C.T.W. (IOS-1806216) and K.L.D. (IOS-1258179); and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers 2P20GM103395 and P20GM130443. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of competing interest
C.F. and C.T.W have no competing interest to declare. KLD has a financial interest in Be Cool Pharmaceutics.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mce.2020.111054.
References
- Abbotts B, Wang LCH, 1980. Seasonal thermogenic capacity in a hibernator, Spermophilus richardsonii. J. Comp. Physiol 140, 235–240. [Google Scholar]
- Abdalla SM, Bianco AC, 2014. Defending plasma T3 is a biological priority. Clin. Endocrinol 81, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Crespo M, Csikasz RI, Martínez-Sánchez N, Díeguez C, Cannon B, Nedergaard J, López M, 2016. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol. Metabol 5, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger MA, Andrews MT, 2018. Nature’s fat-burning machine: brown adipose tissue in a hibernating mammal. J. Exp. Biol 221, jeb162586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger MA, Hess C, Napolitano MW, Bjork JA, Andrews MT, 2016. Seasonal changes in brown adipose tissue mitochondria in a mammalian hibernator: from gene expression to function. Am. J. Physiol. Regul. Integr. Comp. Physiol 311, R325–R336. [DOI] [PubMed] [Google Scholar]
- Bank JHH, Kemmling J, Rijntjes E, Wirth EK, Herwig A, 2015. Thyroid hormone status affects expression of daily torpor and gene transcription in Djungarian hamsters (Phodopus sungorus). Horm. Behav 75, 120–129. [DOI] [PubMed] [Google Scholar]
- Bank JHH, Cubuk C, Wilson D, Rijntjes E, Kemmling J, Markovsky H, Barrett P, Herwig A, 2017. Gene expression analysis and microdialysis suggest hypothalamic triiodothyronine (T3) gates daily torpor in Djungarian hamsters (Phodopus sungorus). J. Comp. Physiol. B 187, 857–868. [DOI] [PubMed] [Google Scholar]
- Barnes BM, 1989. Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator. Science 244, 1593–1595. [DOI] [PubMed] [Google Scholar]
- Bernal J, Guadaño-Ferraz A, Morte B, 2015. Thyroid hormone transporters—functions and clinical implications. Nat. Rev. Endocrinol 11, 406–417. [DOI] [PubMed] [Google Scholar]
- Bolborea M, Dale N, 2013. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 36, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke LJ, Klingenspor M, DeBarber A, Tobias SC, Grandy DK, Scanlan TS, Heldmaier G, 2008. 3-Iodothyronamine: a novel hormone controlling the balance between glucose and lipid utilisation. J. Comp. Physiol. B 178, 167–177. [DOI] [PubMed] [Google Scholar]
- Buck CL, Barnes BM, 2000. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am. J. Physiol. Regul. Integr. Comp. Physiol 279, R255–R262. [DOI] [PubMed] [Google Scholar]
- Canguilhem B, 1970. [Effects of radiothyroidectomy and to thyroid hormone injections on the beginning of hibernation of the European hamster (Cricetus cricetus)]. C. R. Seances Soc. Biol. Fil 164, 1366–1369. [PubMed] [Google Scholar]
- Canguilhem B, 1972. [Role of thyroxine in the initiation of hibernation and resistance to cold in European hamsters (Cricetus cricetus) thyroidectomized in spring]. C. R. Seances Soc. Biol. Fil 166, 688–692. [PubMed] [Google Scholar]
- Cannon B, Nedergaard J, 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL, 2003. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev 83, 1153–1181. [DOI] [PubMed] [Google Scholar]
- Cioffi F, Gentile A, Silvestri E, Goglia F, Lombardi A, 2018. Effect of iodothyronines on thermogenesis: focus on Brown adipose tissue. Front. Endocrinol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubuk C, Markowsky H, Herwig A, 2017. Hypothalamic control systems show differential gene expression during spontaneous daily torpor and fasting-induced torpor in the Djungarian hamster (Phodopus sungorus). PLoS One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC, 2001. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J. Clin. Invest 108, 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeneix BA, Henderson NE, 1978a. Serum T4 and T3 in active and torpid ground squirrels, Spermophilus richardsoni. Gen. Comp. Endocrinol 35, 77–85. [DOI] [PubMed] [Google Scholar]
- Demeneix BA, Henderson NE, 1978b. Thyroxine metabolism in active and torpid ground squirrels, Spermophilus richardsoni. Gen. Comp. Endocrinol 35, 86–92. [DOI] [PubMed] [Google Scholar]
- Doyle Kristian P, Suchland Katherine L, Ciesielski Thomas MP, Lessov Nikola S, Grandy David K, Scanlan Thomas S, Stenzel-Poore Mary P, 2007. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke 38, 2569–2576. [DOI] [PubMed] [Google Scholar]
- Frare C, Jenkins M, Soldin S, Drew K, 2018. The raphe pallidus and the hypothalamic-pituitary-thyroid axis gate seasonal changes in thermoregulation in the hibernating Arctic Ground Squirrel (Urociltellus parryii). Front. Physiol 9, 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frare C, Jenkins ME, McClure KM, Drew KL, 2019. Seasonal decrease in thermogenesis and increase in vasoconstriction explain seasonal response to 6 N-cyclohexyladenosine-induced hibernation in the Arctic Ground Squirrel (Urocitellus parryii). J. Neurochem 151, 316–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC, 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev 29, 898–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings AE, 1998. The history of thyroidectomy. J. R. Soc. Med 91, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-García I, López M, 2017. UCP1 and T3: a key “(un)couple” in energy balance. Temperature 4, 18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldmaier G, Steinlechner S, 1981. Seasonal pattern and energetics of short daily torpor in the Djungarian hamster, Phodopus sungorus. Oecologia 48, 265–270. [DOI] [PubMed] [Google Scholar]
- Henderson NE, Demeneix BA, McGraw KI, Meronek RP, 1981. Hibernation in thyroidectomized ground squirrels, Spermophilus richardsoni. Gen. Comp. Endocrinol 43, 543–548. [DOI] [PubMed] [Google Scholar]
- Herwig A, Wilson D, Logie TJ, Boelen A, Morgan PJ, Mercer JG, Barrett P, 2009. Photoperiod and acute energy deficits interact on components of the thyroid hormone system in hypothalamic tanycytes of the Siberian hamster. Am. J. Physiol. Regul. Integr. Comp. Physiol 296, R1307–R1315. [DOI] [PubMed] [Google Scholar]
- Hulbert A, Hudson J, 1976. Thyroid function in a hibernator, Spermophilus tridecemlineatus. Am. J. Physiol. Leg. Content 230, 1211–1216. [DOI] [PubMed] [Google Scholar]
- Incerpi S, Davis PJ, Pedersen JZ, Lanni A, 2016. Nongenomic actions of thyroid hormones. In: Belfiore A, LeRoith D (Eds.), Principles of Endocrinology and Hormone Action. Springer International Publishing; ), Cham, pp. 1–26. [Google Scholar]
- Jastroch M, Oelkrug R, Keipert S, 2018. Insights into brown adipose tissue evolution and function from non-model organisms. J. Exp. Biol 221, jeb169425. [DOI] [PubMed] [Google Scholar]
- Kališnik M, 1972. A histometric thyroid gland activation index (preliminary report). J. Microsc 95, 345–348. [DOI] [PubMed] [Google Scholar]
- Karpovich SA, Toien O, Buck CL, Barnes BM, 2009. Energetics of arousal episodes in hibernating arctic ground squirrels. J. Comp. Physiol. B 179, 691–700. [DOI] [PubMed] [Google Scholar]
- Kot Brian Chin Wing, et al. , 2013. Stereology of thethyroid gland in indo-pacific bottlenose dolphin (Tursiops aduncus) in comparison with human (Homo sapiens): Quantitative and functional implications. PloS One 8 (5), e62060.. 10.1371/journal.pone.0062060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet F, 2019. Tanycyte gene expression dynamics in the regulation of energy homeostasis. Front. Endocrinol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B, 2013. Tanycyte-like cells form a blood–cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J. Comp. Neurol 521, 3389–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurino A, Landucci E, Raimondi L, 2018. Central effects of 3-iodothyronamine reveal a novel role for mitochondrial monoamine oxidases. Front. Endocrinol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lin Q, Li Q, Huang C, Sun R, 1998. Uncoupling protein mRNA, mitochondrial GTP-binding, and T4 5′-deiodinase activity of brown adipose tissue in Daurian ground squirrel during hibernation and arousal. Comp. Biochem. Physiol. Mol. Integr. Physiol 120, 745–752. [DOI] [PubMed] [Google Scholar]
- Liu X, Li Q, Lin Q, Sun R, 2001. Uncoupling protein 1 mRNA, mitochondrial GTP-binding, and T4 5′-deiodinase of brown adipose tissue in euthermic Daurian ground squirrel during cold exposure. Comp. Biochem. Physiol. Mol. Integr. Physiol 128, 827–835. [DOI] [PubMed] [Google Scholar]
- López M, Varela L, Vázquez MJ, Rodríguez-Cuenca S, González CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, et al. , 2010. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med 16, 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCannell A, Sinclair K, Friesen-Waldner L, McKenzie CA, Staples JF, 2017. Water–fat MRI in a hibernator reveals seasonal growth of white and brown adipose tissue without cold exposure. J. Comp. Physiol. B 187, 759–767. [DOI] [PubMed] [Google Scholar]
- Magnus TH, Henderson NE, 1988. Thyroid hormone resistance in hibernating ground squirrels, Spermophilus richardsoni. I. Increased binding of triiodo-L-thyronine and L-thyroxine by serum proteins. Gen. Comp. Endocrinol 69, 352–360. [DOI] [PubMed] [Google Scholar]
- Mann FC, 1916. The ductless glands and hibernation. Am. J. Physiol. Leg. Content 41, 173–188. [Google Scholar]
- Morrison SF, 2016. Central Control of Body Temperature, F1000Research, p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, 2014. Central nervous system regulation of Brown adipose tissue. Comp. Physiol 4, 1677–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Fielitz H, Stahr M, Bernau M, Richter M, Abele S, Krajka V, Benzin A, Wenzel J, Kalies K, Mittag J, et al. , 2017. Tanycytes control the hormonal output of the hypothalamic-pituitary-thyroid axis. Nat. Commun 8, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullur R, Liu Y-Y, Brent GA, 2014. Thyroid hormone regulation of metabolism. Physiol. Rev 94, 355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Jethwa PH, Warner A, Barrett P, Nilaweera KN, Brameld JM, Ebling FJP, 2012. Effects of manipulating hypothalamic triiodothyronine concentrations on seasonal body weight and torpor cycles in siberian hamsters. Endocrinology 153, 101–112. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B, 2018. Chapter 9 - Brown adipose tissue as a heat-producing thermoeffector. In: Romanovsky AA (Ed.), Handbook of Clinical Neurology. Elsevier, pp. 137–152. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B, 2001. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta Bioenerg 1504, 82–106. [DOI] [PubMed] [Google Scholar]
- Nevretdinova ZG, Shvareva NV, 1987. [Thyroid hormone levels in the peripheral blood of the ground squirrel Citellus parryi during winter hibernation]. Zh. Evol. Biokhim. Fiziol 23, 42–47. [PubMed] [Google Scholar]
- Nevretdinova Z, Solovenchuk L, Lapinski A, 1992. Some aspects of lipid metabolism and thyroid function in arctic ground squirrel, Citellus parryi during hibernation. Arctic Med. Res 51, 196–204. [PubMed] [Google Scholar]
- Ortiga-Carvalho Tania, et al. , 2016. Hypothalamus-Pituitary-Thyroid Axis. Comprehensive Physiology 6 (3), 1387–1428. 10.1002/cphy.c150027. [DOI] [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE, 2016. Hypothalamus-pituitary-thyroid Axis. In: Comprehensive Physiology. American Cancer Society, pp. 1387–1428. [DOI] [PubMed] [Google Scholar]
- Park Gi Cheol, et al. , 2017. TSH-independent release of thyroid hormones through cold exposure in aging rats. Oncotarget 8 (52), 89431–89438. 10.18632/oncotarget.19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelley ET, Aloia RC, Barnes BM, 1978. Circannual rhythmicity in the hibernating ground squirrel Citellus lateralis under constant light and hyperthermic ambient temperature. Comp. Biochem. Physiol. A Physiol 61, 599–603. [Google Scholar]
- Petri I, Diedrich V, Wilson D, Fernández-Calleja J, Herwig A, Steinlechner S, Barrett P, 2016. Orchestration of gene expression across the seasons: hypothalamic gene expression in natural photoperiod throughout the year in the Siberian hamster. Sci. Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot V, Dehouck B, Sharif A, Ciofi P, Giacobini P, Clasadonte J, 2018. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev 39, 333–368. [DOI] [PubMed] [Google Scholar]
- Refetoff S, 2000. Thyroid hormone serum transport proteins. In: Endotext, Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, et al. (Eds.). MDText.com, Inc., South Dartmouth (MA. [Google Scholar]
- Revel FG, Saboureau M, Pévet P, Mikkelsen JD, Simonneaux V, 2006. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology 147, 4680–4687. [DOI] [PubMed] [Google Scholar]
- Richter MM, Williams CT, Lee TN, Tøien Ø, Florant GL, Barnes BM, Buck CL, 2015. Thermogenic capacity at subzero temperatures: how low can a hibernator go? Physiol. Biochem. Zool 88, 81–89. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez A, Lazcano I, Sánchez-Jaramillo E, Uribe RM, Jaimes-Hoy L, Joseph-Bravo P, Charli J-L, 2019. Tanycytes and the control of thyrotropin-releasing hormone flux into portal capillaries. Front. Endocrinol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf T, Geiser F, 2015. Daily torpor and hibernation in birds and mammals. Biol. Rev 90, 891–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RL, O’Neill PH, Epperson LE, Martin SL, 2010. Extensive use of torpor in 13-lined ground squirrels in the fall prior to cold exposure. J. Comp. Physiol 180, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez E, Vargas MA, Singru PS, Pascual I, Romero F, Fekete C, Charli J-L, Lechan RM, 2009. Tanycyte Pyroglutamyl Peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid Axis through glial-axonal associations in the median eminence. Endocrinology 150, 2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Banerjee S, Sarkar R, Sikder B, 2016. A review on the history of ‘thyroid surgery. Indian J. Surg 78, 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, et al. , 2004. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med 10, 638–642. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Andrews MT, 2013. Circannual transitions in gene expression: lessons from seasonal adaptations. Curr. Top. Dev. Biol 105, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Hampton M, Andrews MT, 2013. Seasonal and regional differences in gene expression in the brain of a hibernating mammal. PLoS One 8, e58427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Williams CT, Kenagy GJ, Buck CL, Barnes BM, 2012. Thermoregulatory changes anticipate hibernation onset by 45 days: data from free-living arctic ground squirrels. J. Comp. Physiol. B 182, 841–847. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Fridinger RW, Tøien Ø, Barnes BM, Buck CL, 2013. Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol. Biochem. Zool 86, 515–527. [DOI] [PubMed] [Google Scholar]
- Shintani M, Tamura Y, Monden M, Shiomi H, 2005. Thyrotropin-releasing hormone induced thermogenesis in Syrian hamsters: site of action and receptor subtype. Brain Res. 1039, 22–29. [DOI] [PubMed] [Google Scholar]
- Silva JE, Larsen PR, 1983. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305, 712–713. [DOI] [PubMed] [Google Scholar]
- Silva JE, Matthews PS, 1988. Full expression of uncoupling protein gene requires the concurrence of norepinephrine and triiodothyronine. Mol. Endocrinol 2, 706–713. [DOI] [PubMed] [Google Scholar]
- Silva JE, Rabelo R, 1997. Regulation of the uncoupling protein gene expression. Eur. J. Endocrinol 136, 251–264. [DOI] [PubMed] [Google Scholar]
- Stanton TL, Winokur A, Beckman AL, 1980. Reversal of natural CNS depression by TRH action in the hippocampus. Brain Res. 181, 470–475. [DOI] [PubMed] [Google Scholar]
- Stanton TL, Caine SB, Winokur A, 1992. Seasonal and state-dependent changes in brain TRH receptors in hibernating ground squirrels. Brain Res. Bull 28, 877–886. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Shintani M, Nakamura A, Monden M, Shiomi H, 2005. Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res. 1045, 88–96. [DOI] [PubMed] [Google Scholar]
- Tøien Ø, Drew KL, Chao ML, Rice ME, 2001. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol 281, R572–R583. [DOI] [PubMed] [Google Scholar]
- Weiner J, Kranz M, Kloting N, Kunath A, Steinhoff K, Rijntjes E, Köhrle J, Zeisig V, Hankir M, Gebhardt C, et al. , 2016. Thyroid hormone status defines brown adipose tissue activity and browning of white adipose tissues in mice. Sci. Rep 6, 38124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CT, Goropashnaya AV, Buck CL, Fedorov VB, Kohl F, Lee TN, Barnes BM, 2011. Hibernating above the permafrost: effects of ambient temperature and season on expression of metabolic genes in liver and brown adipose tissue of arctic ground squirrels. J. Exp. Biol 214, 1300–1306. [DOI] [PubMed] [Google Scholar]
- Williams CT, Barnes BM, Kenagy GJ, Buck CL, 2014. Phenology of hibernation and reproduction in ground squirrels: integration of environmental cues with endogenous programming. J. Zool 292, 112–124. [Google Scholar]
- Williams C, Chmura H, Zhang V, Dillon D, Wilsterman K, Barnes BM, Buck CL, 2019. Environmental heterogeneity affects seasonal variation in thyroid hormone physiology of free-living arctic ground squirrels. Can. J. Zool 97 (9), 783–790. 10.1139/cjz-2018-0302. [DOI] [Google Scholar]
- Winston BW, Henderson NE, 1981. Seasonal changes in morphology of the thyroid gland of a hibernator, Spermophilus richardsoni. Can. J. Zool 59, 1022–1031. [Google Scholar]
- Yasuo S, Yoshimura T, Ebihara S, Korf H-W, 2007. Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology 148, 4385–4392. [DOI] [PubMed] [Google Scholar]
- Yen PM, 2001. Physiological and molecular basis of thyroid hormone action. Physiol. Rev 81, 1097–1142. [DOI] [PubMed] [Google Scholar]
- Zalesky M, 1935. A study of the seasonal changes in the thyroid gland of the thirteen-lined ground squirrel (citellus tridecemlineatus), with particular reference to its sexual cycle. Anat. Rec 62, 109–137. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


