Abstract
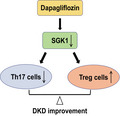
An imbalance between T helper 17 (Th17) and T regulatory (Treg) cell subsets contributes to the pathogenesis of diabetic kidney disease (DKD). However, the underlying regulatory mechanisms that cause this imbalance are unknown. Serum/glucocorticoid‐regulated kinase 1 (SGK1) has been suggested to affect Th17 polarization in a salt‐dependent manner, and sodium/glucose cotransporter 2 inhibitors (SGLT2i) have been demonstrated to regulate sodium‐mediated transportation in the renal tubules. This study aimed to evaluate the potential benefits of dapagliflozin (Dap) on DKD, as well as its influence on shifting renal T‐cell polarization and related cytokine secretion. We treated male db/db mice with Dap or voglibose (Vog) and measured blood and kidney levels of Th17 and Treg cells using flow cytometry. We found that Th17 cells were significantly increased, while Treg cells were significantly decreased in diabetic mice. Moreover, Dap suppressed the polarization of Th17/Treg cells by inhibiting SGK1 in diabetic kidneys, and this was accompanied by attenuation of albuminuria and tubulointerstitial fibrosis independent of glycemic control. Taken together, these results demonstrate that the imbalance of Th17/Treg cells plays an important role in the progression of DKD. Moreover, Dap protects against DKD by inhibiting SGK1 and reversing the T‐cell imbalance.
Keywords: diabetic kidney disease, inflammatory response, serum, glucocorticoid‐regulated kinase 1, sodium, glucose co‐transporter 2, Th17/Treg cell imbalance
An imbalance between Th17 and Treg cell subsets contributes to the pathogenesis of diabetic kidney disease. We found that Th17 cells were increased, while Treg cells were decreased in diabetic mice. Dapagliflozin suppressed the polarization of Th17/Treg cells by inhibiting SGK1 in diabetic kidneys, and this was accompanied by attenuation of albuminuria and tubulointerstitial fibrosis independent of glycemic control.
Abbreviations
- Dap
dapagliflozin
- DKD
diabetic kidney disease
- DM
diabetes mellitus
- ESRD
end‐stage renal disease
- IL
interleukin
- PMA
phorbol 12‐myristate 13‐acetate
- SGK1
serum/glucocorticoid‐regulated kinase 1
- SGLT2i
sodium/glucose cotransporter 2 inhibitors
- TGF‐β
tumor growth factor‐β
- Th17
T helper 17
- TNF‐α
tumor necrosis factor‐α
- Treg
T regulatory
- Vog
voglibose
Diabetic kidney disease (DKD), characterized by progressive albuminuria and reduced glomerular filtration, has become the leading cause of end‐stage renal disease (ESRD) worldwide [1]. With the prevalence of diabetes mellitus (DM) doubling in recent decades, DKD has accounted for about half of all ESRD etiologies [2]. The pathogenesis of DKD is multifactorial, and one identified pathogenic factor is an imbalance of T helper 17 (Th17) and T regulatory (Treg) cells. Early studies confirmed the accumulation of activated T cells and inflammatory factors in kidneys of DKD patients [3]. Further studies indicated that the accumulation of T cells in kidneys of DKD patients was characterized by an upregulation of pro‐inflammatory T cells (Th17 and Th1) and pro‐inflammatory cytokines [interleukin (IL)‐17, tumor necrosis factor‐α (TNF‐α), and IL‐6], and downregulated anti‐inflammatory T cells (Treg cells) and anti‐inflammatory cytokines [(IL‐10 and tumor growth factor‐β (TGF‐β)] [4, 5, 6, 7]. Serum/glucocorticoid‐regulated kinase 1 (SGK1) is a serine/threonine kinase that is constitutively expressed in various tissues [8]. Despite its regulatory efficacy on cellular Na+ transport [9], SGK1 can influence the development of Th17 cells via increasing cellular salinity. It has been confirmed that high salinity can induce SGK1 expression, while silencing SGK1 significantly can reduce the production of Th17 cells induced by high salt [10]. The underlying mechanism may be related to the activation of the SGK1/phosphorylated (p‐)Foxo1/IL‐23R pathway, and the stabilization and enhancement of Th17 cells [11]. However, whether SGK1 promotes the progression of DKD via stimulating an imbalance of Th17 and Treg cells remains unknown.
SGK1 is activated by excessive circulating glucose, which subsequently stimulates a number of ion channels, such as renal sodium/glucose cotransporter 2 (SGLT2) and epithelial sodium channel [12]. Sodium/glucose co‐transporters (SGLTs) belong to the solute carrier 5 family of glucose active transporters, which mainly exert the physiological transport of glucose via SGLT1 and SGLT2 subtypes [13, 14]. It has been found that the high‐carrying capacity, low‐affinity SGLT2 transporter is located almost exclusively in renal proximal tubular epithelial cells and is responsible for ~ 97% of the reabsorption of filtered glucose [15]; the remaining 3% is reabsorbed by SGLT1 under otherwise normal physiological conditions [16]. Glucose kinetic studies have demonstrated that SGLT2 inhibitors decrease the maximal carrying capacity of tubular glucose reabsorption, thereby increasing urinary glucose excretion and decreasing plasma glucose concentrations [17]. Clinically, SGLT2 inhibitors, a novel type of hypoglycemic agents, have been shown to confer antihypertensive, weight loss, uric acid lowering, cardioprotective, and renal protection properties, in addition to their hypoglycemic characteristics [18, 19, 20, 21, 22, 23]. Interestingly, a previous study showed that treatment with the SGLT2 inhibitor empagliflozin improved glycemic control and cardiac diastolic function and reduced expression of cardiac SGK1 in diabetic mice [12]. Based on the above findings, we aimed to evaluate the potential benefits of the SGLT2 inhibitor dapagliflozin (Dap) on DKD in diabetic mice, focusing on its influence on reversing the SGK1‐mediated Th17/Treg cell imbalance.
Materials and methods
Animals
Diabetic db/db mice and C57BLKS/J mice (8 weeks old) were purchased from Nanjing Institute of Biomedicine affiliated with Nanjing University (Nanjing, China). Animals were housed in a specific pathogen‐free room at 20–25 °C and were maintained on a continuous 12:12‐h light–dark cycle with free access to water and standard chow. After 2 weeks of adaptive housing, mice were randomly allocated into groups. Diabetic db/db mice were randomized to receive either normal saline as a vehicle control, Dap (1 mg·kg−1, Meilun, China, MB1917), or voglibose (Vog; 0.6 mg·kg−1, Meilun, MB2024) (n = 6 each group) [24]. The α‐glucosidase inhibitor Vog, used as a positive control, is also widely used to improve postprandial hyperglycemia without proven renoprotective effects [25]. Non‐diabetic C57BLKS/J mice that received normal saline served as controls (n = 6). All treatments and vehicle were administered to animals daily by gavage for 12 consecutive weeks. Body weights of all mice were measured once a week, blood glucose was measured every 4 weeks, and urine volumes were measured at 16 and 22 weeks after treatment. Blood was collected from the tail vein for blood glucose measurements. On the last day of week 22, animals were sacrificed, and renal tissues and blood were collected for further analyses. All experimental protocols were approved by the Ethics Committee of the Southern Medical University (No: NFYY‐2017‐39), and all animal studies were performed in accordance with the Institutional Animal Care Guidelines.
Immunohistochemistry
Mice were perfused with cold PBS, and kidney tissue samples were collected, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned into 3‐μm slices using a microtome (Leica RM 2235, Wetzlar, Germany). The tissues were then mounted onto glass slides and stained with PAS (Loogene, Beijing, China) and Masson chrome stain (Maiwei, Xiamen, China) as previously described [26]. For immunohistochemical analysis, the slides were stained with an anti‐SGK1 polyclonal antibody (1 : 1000; Abcam, Cambridge, UK, ab43606) or an anti‐SGLT2 polyclonal antibody (1 : 200; Proteintech, Chicago, IL, USA, 24654‐1‐AP), followed by an HRP‐conjugated secondary antibody (1 : 2000), as described previously [27]. All images were obtained using an Olympus microscope (Olympus, Tokyo, Japan).
Biochemical analysis
After a 5‐day acclimation period, mice were housed in metabolic chambers with free access to drinking water and food for 24 h to collect urine samples at the indicated durations. After centrifugation for 10 min at 3000 g, urinary albumin and creatinine levels were assayed using an ELISA Kit (Franke, Shanghai, China). The blood samples were placed into EP tubes for anticoagulation at 4 °C and then centrifuged for 10 min at 3000 g. Serum IL‐10 (Catalog Number: E0056m) and IL‐17 (Catalog Number: E0063m) concentrations were measured using an ELISA Kit (EIAab, Wuhan, China). The assays were performed according to the manufacturer's instructions, and absorbance was measured using the Bio‐Rad iMark Microplate Reader.
Flow cytometry analyses
Single‐cell suspensions were obtained following centrifugation of blood and kidney samples. In brief, 200 μL of whole blood was collected and incubated with 2 mL of erythrocyte lysates at 37 °C for 2–3 min. The samples were centrifuged at 250 g for 5 min and washed twice with PBS. Kidney tissues were cut into pieces and digested with 0.25% trypsin and 0.01% collagenase type II at 37 °C for 20 min. After centrifugation at 250 g for 5 min, solutions were filtered through a 200‐mesh sieve. The filtrates were centrifuged at 94 g. for 5 min and were incubated with 2 mL of erythrocyte lysates at 37 °C for 2–3 min. The samples were also centrifuged and washed for further use.
For analysis of intracellular cytokine production, kidney and blood mononuclear cells were stimulated with 150 ng·mL−1 phorbol 12‐myristate 13‐acetate (PMA) and 1 μg·mL−1 ionomycin in the presence of GolgiStop (BD Biosciences, San Diego, CA, USA) for 6 h. For Th17 cell analysis, the samples were digested and fixed with 100 μL of intracellular fixation and permeabilization buffer (eBioscience Catalog No. 88‐8824‐00, San Diego, CA, USA) for 20 min at room temperature in the dark and then washed with 2 mL permeabilization buffer twice. After centrifugation, the samples were resuspended in 100 µL permeabilization buffer and stained with a PE‐labeled anti‐mouse CD4 antibody (Catalog No. 12‐0041‐82; Invitrogen, Carlsbad, CA, USA) and a FITC‐labeled anti‐mouse IL‐17A antibody (Catalog No. A15377; Invitrogen) for 30 min in the dark before they were washed with 2 mL of permeabilization buffer. After addition of 500 μL staining buffer (eBioscience Catalog No. 00‐4222‐26), the cells were analyzed using the FACSCalibur cytometer (Becton Dickinson, New York, NY, USA). For analysis of Treg cells, the samples were digested before fixation and permeabilization, and were then stained with a PE‐labeled anti‐mouse CD4 antibody (Catalog No. 12‐0041‐82; Invitrogen) for 30 min at 4 °C. The samples were incubated with Foxp3 transcription factor staining buffer (eBioscience Catalog No. 00‐5523) for 30 min at 4 °C and a FITC‐labeled anti‐mouse FoxP3 antibody (Catalog No. A18662; Invitrogen) for 30 min in the dark. The CD4+Foxp3+ cells were considered Treg cells. The scatter patterns of lymphocytes are shown in Figs S1–S4.
Magnetic bead cell sorting
Preparation of single‐cell suspension from kidney tissue: Minced kidney tissues were digested with 0.25% trypsin and 0.01% type II collagenase for 20 min at 37 °C, and enriched by centrifugation at 250 g for 5 min. The cell pellets were resuspended with RPMI 1640 media (C22400500BT; Gibco, Grand Island, NE, USA) containing 10% FCS (10091148; Gibco), 1 mm sodium pyruvate (S104174; Aladdin, Shanghai, China), 50 μm 2‐mercaptoethanol (BB‐92003; BestBio, Beijing, China), 2 mm glutamine, 25 mm HEPES, and 1 × nonessential amino acid (1140050; Gibco), and were passed through 200‐mesh screen. A total of 1 × 108 cells was used for cell sorting. Anti‐mouse CD4 and CD62 MicroBeads were used to select CD4+ CD62L+ naive T cells, as recommended by the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany; 130‐106‐643). The cells were gated on lymphocytes based on FSC‐SSC. The Th17 cells were collected from CD4+ CD62L+ cells after stimulation with recombinant TGF‐β (3 ng·mL−1; PeproTech, Rocky Hill, CT, USA), IL‐6 (300 IU·mL−1; PeproTech), and IL‐23 (300 IU·mL−1; PeproTech) for 90–108 min, at a cell density of 1 × 106 cells per mL. Approximately 5000 CD4+ T cells were acquired using cellquest software (Becton Dickinson, New York, NY, USA), and the mean fluorescence intensities of CD4 were determined using flow cytometry. The cells were gated on FSC‐SSC plots and were further plotted against CD4 and IL‐17. The CD4+ IL‐17+ cells were identified as Th17 cells. The Th17 cells were used for subsequent flow cytometry and western blot analyses.
Western blot analysis
Equal amounts of protein (20 μg) extracted from Th17 cells using RIPA (Biyuntian, Shanghai, China) were electrophoresed using 10% SDS/PAGE, transferred to Polyvinylidene fluoride membranes, blocked, and then incubated at 4 °C overnight with the following primary antibodies: SGK1 (1 : 500; Abcam, ab43606), p‐Foxo1 (1 : 500; Abclonal, Boston, MA, USA, AP0176), and IL‐23R (1 : 500; Abcam, ab175072). The blots were then incubated with an HRP‐conjugated secondary antibody (1 : 2500; Proteintech) at room temperature for 1 h and visualized using a Bio‐Rad Gel Doc EZ Imaging System (Bio‐Rad, Hercules, CA, USA) with enhanced chemiluminescence reagents (Millipore, Boston, MA, USA). All western blot analyses were performed at least three times, and the protein band densities were quantified using imagej software (NIH, National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
Continuous variables are presented as mean ± standard deviation (x ± SD). prism 5 (GraphPad software Inc., San Diego, CA, USA) and spss22.0 statistical software (IBM, International Business Machines Corporation, Armonk, USA) were used for statistical analysis. One‐way ANOVA with SNK test (each group has equal variance) or Dunnett's T3 test (equal variances not assumed) was used to assess statistical significance among the four groups. Comparisons of body weight and random blood glucose between groups were performed using repeated measurements (RM) followed by the SNK test or Dunnett's T3 test. A P‐value < 0.05 was considered statistically significant.
Results
Dapagliflozin reduces urinary albumin in DM mice without significantly affecting blood glucose and body weight
Body weight and random blood glucose were significantly higher in the DM, Dap, and Vog groups compared with the NC group. Body weight was not significantly different among mice in the Dap, Vog, and DM groups. Blood glucose was significantly lower in the Dap and Vog groups compared with the DM group (Fig. 1A,B). The ratio of urinary albumin/creatinine in the DM group was significantly higher compared with the NC group, but was significantly lower in the Dap group compared with the Vog and DM groups (Fig. 1C).
Fig. 1.
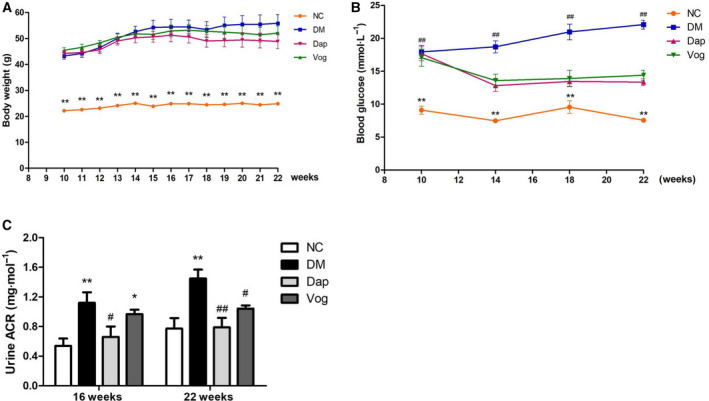
Dap treatment partly reversed random blood glucose and urinary albumin/creatinine ratio, but not body weight, in db/db mice. Mice were randomly allocated into the following groups: nondiabetic C57BLKS/J mice (n = 6); diabetic db/db mice treated with normal saline, Dap (1 mg·kg−1), or voglibose (Vog; 0.6 mg·kg−1) (n = 6 each group). (A) Body weight, (B) random blood glucose, (C) urinary albumin/creatinine ratio. Quantitative data are presented as mean ± SD. One‐way ANOVA with SNK test or Dunnett's T3 test was used for statistical analysis. *, P < 0.05 compared with the NC group, **, P < 0.01 compared with the NC group, #, P < 0.05 compared with the DM group, and ##, P < 0.01 compared with the DM group.
Dapagliflozin improves renal pathological changes and inhibits SGLT2 and SGK1 expression
PAS and Masson staining showed that kidney fibrosis was increased in the DM group, as evidenced by a thickened glomerular basement membrane, widened mesangial matrix, and an obvious fibrotic renal tubulointerstitium. Treatment with Dap alleviated the renal fibrosis, as evidenced by attenuated thickness of the renal basement membrane and the width of mesangial matrix. We did not observe any significant difference in renal fibrosis between the DM and Vog groups (Fig. 2A‐D). Furthermore, immunohistochemical analyses showed that SGLT2 protein expression and SGK1 protein expression were significantly higher in the kidneys of the DM group compared with the NC group, but significantly lower in the Dap group compared with the DM group. In addition, SGLT2 expression was not significantly different between the Vog and DM groups, while SGK1 expression was reduced in the Vog group compared with the DM group (Fig. 2E–H).
Fig. 2.
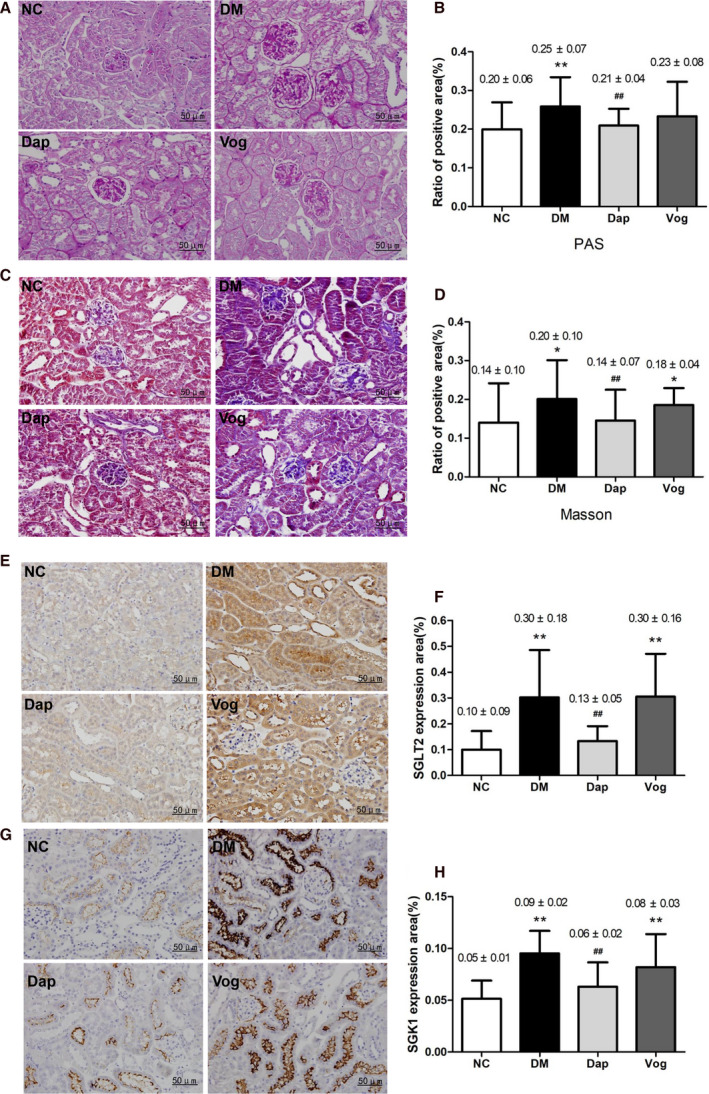
Dap treatment partly reversed renal fibrosis and inhibited SGLT2 and SGK1 expression in db/db mice. We did not observe any significant difference in renal fibrosis between the DM and Vog groups Mice were randomly allocated into the following groups: nondiabetic C57BLKS/J mice (n = 6); diabetic db/db mice treated with normal saline, Dap (1 mg·kg−1), or voglibose (Vog; 0.6 mg·kg−1) (n = 6 each group). Kidney tissue samples were fixed in 4% paraformaldehyde and stained with PAS and Masson. For immunohistochemical analysis, the slides were stained with anti‐SGK1 or anti‐SGLT2 polyclonal antibodies, followed by HRP‐conjugated secondary antibody. (A) Representative images of PAS staining and (B) semi‐quantitative analysis; (C) representative images of Masson staining and (D) semi‐quantitative analysis; (E) representative images of immunohistochemical staining for renal SGLT2 and (F) semi‐quantitative analysis; (G) representative images of immunohistochemical staining for renal SGK1 and (H) semi‐quantitative analysis. Error bar = 50 μm. Quantitative data are presented as mean ± SD. One‐way ANOVA with SNK test or Dunnett's T3 test was used for statistical analysis. *, P < 0.05 compared with the NC group, **, P < 0.01 compared with the NC group, #, P < 0.05 compared with the DM group, and ##, P < 0.01 compared with the DM group.
Dapagliflozin improves the Th17/Treg cell imbalance in peripheral blood and renal tissues of DM mice
Compared to the NC group, the number of Th17 cells was significantly increased in both blood and kidneys from the DM group, while the number of Treg cells was decreased and the ratio of Th17/Treg cells was significantly increased. Further analysis revealed that the increased serum IL‐17 and decreased serum IL‐10 levels were positively correlated with DM status, which we did not observe in mice from the NC group. Treatment with Dap reversed the changes in the serum inflammatory cytokines and the imbalance of Th17/Treg cells, while treatment with Vog did not significantly affect the serum inflammatory cytokines and T‐cell subsets (Fig. 3A–J).
Fig. 3.
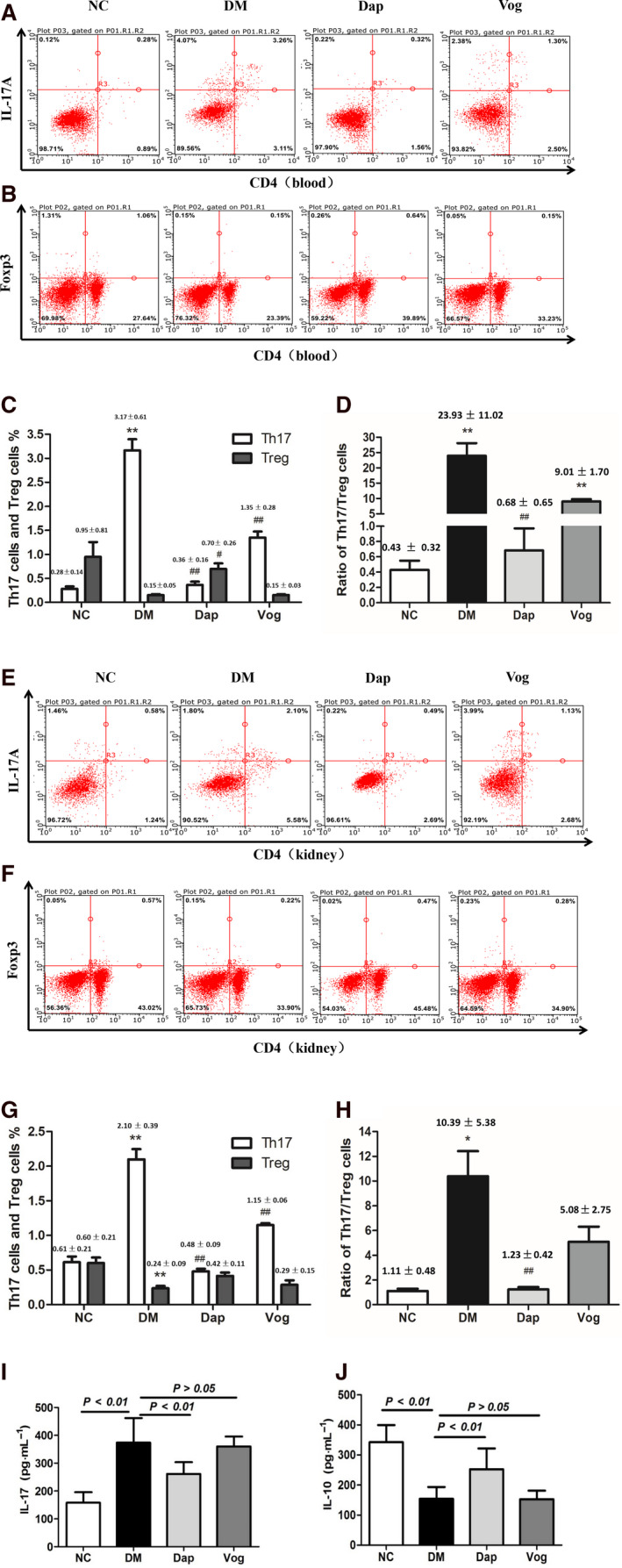
Dap treatment changed the subpopulations of Th17 and Treg cells in db/db mice (n = 6) after 12 weeks of intervention. Single‐cell suspensions were obtained following centrifugation of kidney and blood samples, followed by stimulation with 150 ng·mL−1 PMA and 1 μg·mL−1 ionomycin in the presence of GolgiStop for 6 h. For Th17 cell analysis, the samples were stained with a PE‐labeled anti‐mouse CD4 antibody and then were incubated with a FITC‐labeled anti‐mouse IL‐17A antibody. For analysis of Treg cells, the antibodies used included a PE‐labeled anti‐mouse CD4 antibody and a FITC‐labeled anti‐mouse FoxP3 antibody. (A) Flow cytometry measurements for blood Th17 cells; (B) flow cytometry measurements for blood Treg cells; (C) quantitative analyses for blood Th17 and Treg cells; (D) quantitative analyses for the blood Th17/Treg cell ratio; (E) flow cytometry measurements for kidney Th17 cells; (F) flow cytometry measurements for kidney Treg cells; (G) quantitative analyses for kidney Th17 and Treg cells; (H) quantitative analyses for the kidney Th17/Treg cell ratio; (I) serum levels of IL‐17 in each group; and (J) serum levels of IL‐10 in each group. Quantitative data are presented as mean ± SD. One‐way ANOVA with SNK test or Dunnett's T3 test was used for statistical analysis. *, P < 0.05 compared with the NC group, **, P < 0.01 compared with the NC group, #, P < 0.05 compared with the DM group, and ##, P < 0.01 compared with the DM group.
Dapagliflozin inhibits SGK1, p‐Foxo1, and IL‐23R expression in Th17 cells from DM kidneys
To further clarify the effect of SGK1 on Th17 cell differentiation, Th17 cells were isolated from renal tissues and measured using western blot analysis. The results showed that the levels of SGK1 pathway‐related proteins, including p‐Foxo1 and IL‐23R, were significantly increased in the DM group. Treatment with Dap inhibited SGK1, p‐Foxo1, and IL‐23R protein expression in the kidneys from the DM mice, while treatment with Vog did not significantly affect the expression of these proteins (Fig. 4A–D).
Fig. 4.
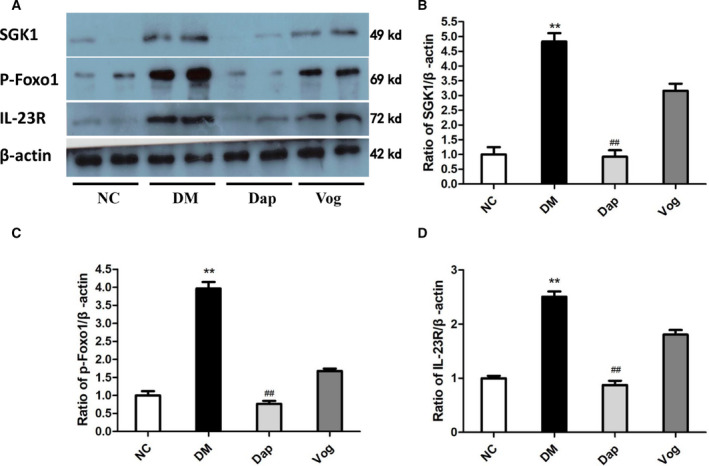
Dap treatment reduced the protein levels of SGK1, p‐Foxo1, and IL‐23R in kidney Th17 cells of db/db mice. Mice were randomly allocated into the following groups: Th17 cells were isolated from the kidneys in nondiabetic C57BLKS/J mice (NC), and diabetic db/db mice treated with normal saline (DM), Dap (1 mg·kg−1), or voglibose (Vog; 0.6 mg·kg−1) (n = 6 for each group). The Th17 cells were collected from CD4+ CD62L+ naive T cells after stimulation with recombinant TGF‐β (3 ng·mL−1), IL‐6 (300 IU·mL−1), and IL‐23 (300 IU·mL−1). Total cell proteins were extracted from Th17 cells for western blot analyses. Equal amounts of protein (20 μg) extracted from Th17 cells were incubated with the following primary antibodies: SGK1, p‐Foxo1, and IL‐23R. (A) representative images for SGK1, p‐Foxo1, and IL‐23R expression; (B) semi‐quantitative analysis for SGK1 expression; (C) semi‐quantitative analysis for p‐Foxo1 expression; (D) semi‐quantitative analysis for IL‐23R expression. Quantitative data are presented as mean ± SD. One‐way ANOVA with SNK test or Dunnett's T3 test was used for statistical analysis. **, P < 0.01 compared with the NC group; ##, P < 0.01 compared with the DM group.
Discussion
In China, about 20–40% of DM patients have DKD [28], which has been attributed to metabolic–hemodynamic changes, oxidative stress, inflammatory responses, and the renin–angiotensin system [29]. The current study evaluated the potential benefits of the SGLT2 inhibitor Dap on DKD in a diabetic mouse model, focusing primarily on the ability of Dap to influence the Th17/Treg cell imbalance.
We confirmed that db/db mice gradually develop DKD, as evidenced by our histological analyses, as well as by increases in body weight, blood glucose levels, and the ratio of urinary protein/creatinine during DM progression. Increasing evidence indicates that T‐cell‐induced inflammatory responses play a vital role in the pathogenesis of DKD in DM [30] because T cells accumulate in the renal juxtaglomerular apparatus [3]. Furthermore, an imbalance of pro‐inflammatory T cells (Th17 and Th1) and anti‐inflammatory T cells (Treg cells) [4] was reported in a previous study. Results of our study confirmed these findings, showing that the Th17 cells in the blood and kidneys were significantly increased, while Treg cells were significantly decreased in diabetic mice. We also found increases in serum IL‐17 and decreases in serum IL‐10 levels. These results suggest that an imbalance of T cells and related changes in inflammatory factors are important determinants of DKD pathogenesis.
Th17 cells are a subset of CD4+ helper T cells, which are widely believed to induce inflammation in the pathogenesis of autoimmune diseases by producing cytokines such as IL‐17, TNF‐α, and IL‐6. Th17 cells counteract Treg cells, which function by maintaining immune homeostasis and tolerance by secreting anti‐inflammatory cytokines IL‐10 and TGF‐β [31]. Our findings are consistent with previous clinical studies that showed that the Th17/Treg cell ratio was significantly higher in type 2 DM (T2DM) patients with DKD compared to T2DM patients without DKD or healthy controls [5]. Another in vivo study showed that the mycophenolate mofetil improved pathological kidney changes in DM patients, which were accompanied by reduced numbers of Th17 cells in the kidney [6]. Taken together, these findings confirm the previous hypothesis that a Th17/Treg cell imbalance plays a key role in the development and progression of DKD [7].
We also found that circulating IL‐17 levels were higher, IL‐10 levels were lower, and there was an imbalance of Th17/Treg cells in DM mice compared with controls. These findings suggest that a Th17/Treg cell imbalance accelerates the development of DKD via inducing inflammatory responses. Previous studies indicated that SGK1 regulates the Th17/Treg cell balance via influencing cell salinity [10]. Indeed, Wu et al. [11] confirmed that high‐salt treatment induced SGK1 expression, and exposure of IL‐23R‐deficient T cells to IL‐23 reduced SGK1 expression. In contrast, SGK1 deficiency does not prevent Na+‐mediated Th17 differentiation in an IL‐23R‐dependent manner [11]. Furthermore, IL‐23R and IL‐17A expression in CD4+ memory T cells lacking Foxo1 increased compared with wild‐type cells. IL‐23R could also be trans‐activated by the RORγt promoter, an important IL‐17 transcription regulator, which can be inhibited by Foxo1. Wu et al. [11] also indicated that activation of SGK1 can sequentially inactivate Foxo1 via phosphorylation, subsequently leading to the activation of RORγt by relieving the inhibition of RORγt, and promoting IL‐23R expression, ultimately resulting in Th17 differentiation [11]. Results of our study indicate that the activation of the SGK1, p‐Foxo1, and IL‐23R pathways is associated with a Th17/Treg imbalance, suggesting that SGK1 regulates the pathogenesis of DKD via influencing the Th17/Treg cell ratio. Given that there are few reports on the influence of SGK1 on other immune cells in the kidney, the influence of Dap on various immune cells during DKD development warrants further research.
SGK1 can stimulate SGLT2 activation, and Dap has been recognized as a hypoglycemic agent that inhibits SGLT2 in renal tubular epithelial cells [32]. Interestingly, SGLT2 resorbs Na+ in addition to glucose in urine, potentially increasing salinity, which may induce the imbalance of Th17/Treg cells and lead to DKD pathogenesis. Accordingly, Dap may confer renal protection by inhibiting SGLT2‐mediated high salinity and the imbalance of Th17/Treg cells in DM mice. This was confirmed by our findings that Dap treatment decreased renal SGK1 protein expression, reduced circulating IL‐17 levels, increased circulating IL‐10 levels, and significantly attenuated the pathological kidney changes in DM mice, all of which were not observed in mice treated with Vog. Vog reduces blood glucose levels by inhibiting intestinal sugar absorption, which differs from the glucose‐lowering mechanism of Dap. Thus, Dap may reverse the imbalance of Th17/Treg cells by inhibiting Na+ resorption.
These results indicate that Dap confers protection in DM patients, primarily via its favorable influence on Th17/Treg cells and inflammation, rather than via its hypoglycemic effects. These mechanisms may be important for understanding the renal protective efficacy of Dap in DM patients.
In conclusion, we found that increased expression of SGLT2 could result in an imbalance of Th17/Treg cells in the kidney via activation of the SGK1/p‐Foxo1/IL‐23R pathway and that this underlies DKD pathogenesis. Accordingly, Dap treatment may confer renal protection in DM patients via reversing the SGK1‐mediated Th17/Treg cell imbalance.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
DW designed the study, performed the experiments, and prepared the manuscript. ZZ and ZS performed the experiments and prepared the manuscript, researched data, and assisted with revising of the manuscript. YY and SL proposed the hypothesis, assisted with performing the experiments, and analyzed the data. YX is the guarantor of this work and supervised the entire project, researched the data, and wrote the manuscript.
Supporting information
Fig. S1. The scatter plots of Th17 lymphocytes from kidney tissues of mice from each group are shown inside the circles. NC: non‐diabetic C57BLKS/J mice; DM: diabetic db/db mice treated with normal saline; Dap: diabetic db/db mice treated with dapagliflozin; Vog: diabetic db/db mice treated with voglibose.
Fig. S2. The scatter plots of Treg lymphocytes from kidney tissues of mice from each group are shown inside the circles. NC: non‐diabetic C57BLKS/J mice; DM: diabetic db/db mice treated with normal saline; Dap: diabetic db/db mice treated with dapagliflozin; Vog: diabetic db/db mice treated with voglibose.
Fig. S3. The scatter plots of Th17 lymphocytes from peripheral blood of mice from each group are shown inside the circles. NC: non‐diabetic C57BLKS/J mice; DM: diabetic db/db mice treated with normal saline; Dap: diabetic db/db mice treated with dapagliflozin; Vog: diabetic db/db mice treated with voglibose.
Fig. S4. The scatter plots of Treg lymphocytes from peripheral blood of mice from each group are shown inside the circles. NC: non‐diabetic C57BLKS/J mice; DM: diabetic db/db mice treated with normal saline; Dap: diabetic db/db mice treated with dapagliflozin; Vog: diabetic db/db mice treated with voglibose.
Acknowledgements
This research was supported by grants from the Dean Foundation of Nanfang Hospital (2016C025) and the National Natural Science Foundation of China (81900759).
Dan Wang, Zikun Zhang, Zekun Si contributed equally to this work
Data Accessibility
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Forbes JM and Cooper ME (2013) Mechanisms of diabetic complications. Physiol Rev 93, 137–188. [DOI] [PubMed] [Google Scholar]
- 2. Kowalski A, Krikorian A and Lerma EV (2015) Diabetes and chronic kidney disease. Dis Mon 61, 378–386. [DOI] [PubMed] [Google Scholar]
- 3. Galkina E and Ley K (2006) Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 17, 368–377. [DOI] [PubMed] [Google Scholar]
- 4. Jagannathan‐Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM and Nikolajczyk BS (2011) Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol 186, 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abouzeid S and Sherif N (2015) Role of alteration in Treg/Th17 cells' balance in nephropathic patients with Type 2 diabetes mellitus. Electron Physician 7, 1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim SM, Lee SH, Lee A, Kim DJ, Kim YG, Kim SY, Jeong KH, Lee TW, Ihm CG, Lim SJ et al. (2015) Targeting T helper 17 by mycophenolate mofetil attenuates diabetic nephropathy progression. Transl Res 166, 375–383. [DOI] [PubMed] [Google Scholar]
- 7. Zeng C, Shi X, Zhang B, Liu H, Zhang L, Ding W and Zhao Y (2012) The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med 90, 175–186. [DOI] [PubMed] [Google Scholar]
- 8. Lang F, Bohmer C, Palmada M, Seebohm G, Strutz‐Seebohm N and Vallon V (2006) (Patho)physiological significance of the serum‐ and glucocorticoid‐inducible kinase isoforms. Physiol Rev 86, 1151–1178. [DOI] [PubMed] [Google Scholar]
- 9. Liu J, Ma S, Mu J, Chen T, Xu Q, Dun W, Tian J and Zhang M (2017) Integration of white matter network is associated with interindividual differences in psychologically mediated placebo response in migraine patients. Hum Brain Mapp 38, 5250–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN and Hafler DA (2013) Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A and Kuchroo VK (2013) Induction of pathogenic TH17 cells by inducible salt‐sensing kinase SGK1. Nature 496, 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Barron B, Mayoux E, Rector RS, Whaley‐Connell A et al. (2017) Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol 16, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wright EM (2013) Glucose transport families SLC5 and SLC50. Mol Aspects Med 34, 183–196. [DOI] [PubMed] [Google Scholar]
- 14. Ferrannini E (2017) Sodium‐glucose co‐transporters and their inhibition: clinical physiology. Cell Metab 26, 27–38. [DOI] [PubMed] [Google Scholar]
- 15. Heerspink HJ, Perkins BA, Fitchett DH, Husain M and Cherney DZ (2016) Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134, 752–772. [DOI] [PubMed] [Google Scholar]
- 16. Liu JJ, Lee T and DeFronzo RA (2012) Why do SGLT2 inhibitors inhibit only 30–50% of renal glucose reabsorption in humans? Diabetes 61, 2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, Farrell K, Rothenberg P and Henry RR (2013) Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo‐controlled study. Diabetes Care 36, 2154–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kohan DE, Fioretto P, Tang W and List JF (2014) Long‐term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 85, 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yale JF, Bakris G, Cariou B, Nieto J, David‐Neto E, Yue D, Wajs E, Figueroa K, Jiang J, Law G et al. (2014) Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 16, 1016–1027.24965700 [Google Scholar]
- 20. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC and EMPA‐REG RENAL trial investigators (2014) Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol 2, 369–384. [DOI] [PubMed] [Google Scholar]
- 21. Fioretto P, Stefansson BV, Johnsson E, Cain VA and Sjostrom CD (2016) Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia 59, 2036–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B et al. (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375, 323–334. [DOI] [PubMed] [Google Scholar]
- 23. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA et al. (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380, 347–357. [DOI] [PubMed] [Google Scholar]
- 24. Shin SJ, Chung S, Kim SJ, Lee EM, Yoo YH, Kim JW, Ahn YB, Kim ES, Moon SD, Kim MJ et al. (2016) Effect of sodium‐glucose co‐transporter 2 inhibitor, dapagliflozin, on renal renin‐angiotensin system in an animal model of type 2 diabetes. PLoS One 11, e0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaku K (2014) Efficacy of voglibose in type 2 diabetes. Expert Opin Pharmacother 15, 1181–1190. [DOI] [PubMed] [Google Scholar]
- 26. Wang D, Guan MP, Zheng ZJ, Li WQ, Lyv FP, Pang RY and Xue YM (2015) Transcription factor Egr1 is involved in high glucose‐induced proliferation and fibrosis in rat glomerular mesangial cells. Cell Physiol Biochem 36, 2093–2107. [DOI] [PubMed] [Google Scholar]
- 27. Zeng Y, Li C, Guan M, Zheng Z, Li J, Xu W, Wang L, He F and Xue Y (2014) The DPP‐4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein‐E‐knockout mice via AMPK‐ and MAPK‐dependent mechanisms. Cardiovasc Diabetol 13, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang H, Luo W, Sun Y, Qiao Y, Zhang L, Zhao Z and Lv S (2016) Wnt/beta‐catenin signaling mediated‐UCH‐L1 expression in podocytes of diabetic nephropathy. Int J Mol Sci 17, 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duran‐Salgado MB and Rubio‐Guerra AF (2014) Diabetic nephropathy and inflammation. World J Diabetes 5, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kornete M, Mason ES and Piccirillo CA (2013) Immune regulation in T1D and T2D: prospective role of Foxp3+ Treg cells in disease pathogenesis and treatment. Front Endocrinol 4, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang C, Xiao C, Wang P, Xu W, Zhang A, Li Q and Xu X (2014) The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Hum Immunol 75, 289–296. [DOI] [PubMed] [Google Scholar]
- 32. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G and Brown J (2005) Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non‐insulin‐dependent diabetes. Diabetes 54, 3427–3434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The scatter plots of Th17 lymphocytes from kidney tissues of mice from each group are shown inside the circles. NC: non‐diabetic C57BLKS/J mice; DM: diabetic db/db mice treated with normal saline; Dap: diabetic db/db mice treated with dapagliflozin; Vog: diabetic db/db mice treated with voglibose.
Fig. S2. The scatter plots of Treg lymphocytes from kidney tissues of mice from each group are shown inside the circles. NC: non‐diabetic C57BLKS/J mice; DM: diabetic db/db mice treated with normal saline; Dap: diabetic db/db mice treated with dapagliflozin; Vog: diabetic db/db mice treated with voglibose.
Fig. S3. The scatter plots of Th17 lymphocytes from peripheral blood of mice from each group are shown inside the circles. NC: non‐diabetic C57BLKS/J mice; DM: diabetic db/db mice treated with normal saline; Dap: diabetic db/db mice treated with dapagliflozin; Vog: diabetic db/db mice treated with voglibose.
Fig. S4. The scatter plots of Treg lymphocytes from peripheral blood of mice from each group are shown inside the circles. NC: non‐diabetic C57BLKS/J mice; DM: diabetic db/db mice treated with normal saline; Dap: diabetic db/db mice treated with dapagliflozin; Vog: diabetic db/db mice treated with voglibose.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.


