Abstract
Background
Irreversible electroporation (IRE) is a novel treatment for locally advanced pancreatic cancer (LAPC), but the predictive factors, based on cytokines and immunocytes of survival, are still lacking. This study aimed to establish a risk model based on cytokines and immunocytes for LAPC patients undergoing IRE treatment.
Patients and Methods
Peripheral blood samples were obtained from 31 LAPC patients and 8 healthy control subjects before IRE. The phenotypes of lymphocytes were analyzed by flow cytometry, and the cytokines were evaluated with Luminex microarray assay. Least absolute shrinkage and selection operator (LASSO) and Cox regression were applied to assess the prognostic factors for overall survival (OS) and progression-free survival (PFS). A receiver operating characteristic (ROC) curve and a concordance index (C-index) were used to compare the abilities to predict survival rates.
Results
The relationship between multiple cytokines and clinical factors was evaluated and their prognostic value was compared. The five best predictors for OS and PFS, including CA19-9, CD3+CD4+ T cells, CD3+CD8+ T cells, IL-17A, and TNF-α were selected and incorporated into a new immune panel. A risk model based on this immune panel was established and exhibited significantly higher values of C-indexes and AUC for OS and PFS prediction as compared with tumor marker score and TNM stage system.
Conclusion
We presented a risk model based on a microarray assay of cytokines and lymphocytes for LAPC patients after receiving IRE treatment for the first time. The established risk model showed relatively good performance in survival prediction and was able to facilitate tailed patient management in clinical practice.
Keywords: irreversible electroporation, locally advanced pancreatic cancer, cytokine, lymphocyte, prognosis
Introduction
Pancreatic cancer (PC) is the third leading cause of cancer mortality, and the survival rates of patients with PC have not improved significantly over the years.1 Surgery has provided the best chance for prolonged survival although only 20% of patients are eligible for surgical resection at diagnosis.2 PC can be clinically classified as resectable, borderline resectable, locally advanced, and metastatic ones according to the involvement of collateral blood vessels or the status of metastasis. Locally advanced pancreatic cancer (LAPC) represents almost 40% of all PCs3 and chemotherapy is the main treatment for LAPC in clinical practice.4 However, chemotherapy has been shown to have relatively low response rates and a limited increase in survival rates for LAPC patients.5 Also, the transformation rates from LAPC to resectable forms after chemotherapy were not high.6,7 Therefore, the unmet need for effective treatment has prompted researchers to develop novel therapies for LAPC. Considering more than 30% of LAPC patient’s deaths result from primary tumor progression,8,9 local destructive therapies are playing an increasingly important role in the treatment of LAPC.
Irreversible electroporation (IRE) is a novel, predominantly non-thermal ablative method that induces tumor cell death without destroying adjacent structures. Previous studies have consolidated the role of IRE in the treatment of LAPC, and found that IRE combined with chemotherapy resulted in significantly higher survival rates for LAPC patients compared with chemotherapy alone or chemoradiotherapy.10–13 Additionally, several studies have shown that the most common pattern of recurrence for LAPC patients after IRE was distant metastasis, which was also a major factor in decreased survival rates.14,15 The early detection or prediction of tumor progression is necessary for the choice of adjuvant intensified treatment. However, there are little data on the predictive factors of tumor progression and survival for LAPC patients undergoing IRE treatment. The regulatory evaluation of tumor response with imaging after IRE was only available two months after initial IRE therapy. Compared with radiological evaluation, circulating immune cells or cytokine markers may be the promising ones given their relationship with the tumor itself and the immunological response of the organism to treatment.
Using the large amount of data on immune cells and cytokines for LAPC patients who received IRE treatment, we aimed to evaluate the relationship between immune cells and cytokines and determine the prognostic value of these factors in terms of tumor progression and survival in LAPC patients undergoing IRE therapy.
Patients and Methods
Patients
Patients with LAPC who were initially treated with IRE from August 2015 to August 2017 at Sun Yat-sen University Cancer Center were retrospectively collected and another group of healthy people were analyzed as control in this study. The inclusion criteria were described as follows: 1) pathologically confirmed pancreatic adenocarcinoma and radiologically confirmed LAPC. All patients were classified as LAPC according to the criteria of NCCN Guidelines Version 1.2020;16 2) an Eastern Cooperative Oncology Group (ECOG) performance status score of 0, 1 or 2. The exclusions were as follows: 1) patients who had received other treatments, including surgical resection and RFA; 2) patients with metastatic implants; 3) patients with heart arrhythmia and a history of second primary malignant tumors; 4) patients who had missing information of circulating immune cells or cytokine markers, which were similar to those in our previous study.10,11 Approval of Institutional Review Board of Sun Yat-sen University Cancer Center (SYSUCC) was obtained. All procedures were in accordance with 1964 Helsinki Declaration. Written informed consent was obtained from patients prior to treatment.
Data Collection and Treatment Procedure
Clinical and pathological data were extracted from the medical record system of SYSUCC, including age, gender, tumor size, grade, site, neoadjuvant and adjuvant chemotherapy. The laboratory indexes, such as white blood cell (WBC) count, hemoglobin (HGB), platelet (PLT) count, serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glutamyl transpeptidase (GGT), albumin (ALB), total bilirubin (TBIL), indirect bilirubin (IBIL), C-reactive protein (CRP), carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA19-9) and carbohydrate antigen 125 (CA125). IRE treatment was performed with the NanoKnife equipment. The treatment procedure was the same as that described in our previous study.11 All IRE ablations were performed using an open technique by specialized pancreatic surgeons. All data were collected before IRE treatment. Overall survival (OS) and progression-free survival (PFS), which were defined as the duration from the date of diagnosis to death from all causes and tumor progression, respectively, or last follow-up, were regarded as the endpoints in this study. The follow-up date ended on April 30, 2020.
Flow Cytometry and Cytokine Microarray Assay
All blood samples were collected using Na-heparin plasma tubes from patients one to three days before IRE. Peripheral blood mononuclear cells (PBMCs) were isolated from blood as previously described.17 The PBMCs were labeled with associated murine anti-human monoclonal antibodies. The CD3, CD3CD4, CD3CD8, CD4CD25, CD8CD25, and CD3CD16CD56 phenotype of lymphocytes were analyzed by flow cytometry (FC; CytoFLEX, Beckman Coulter, Brea, California, 92821, USA).
Blood was also obtained for cytokine analysis. Plasma samples cryopreserved at −80°C were thawed and centrifuged 1000g for 15 minutes at 4°C before transferring the cell and platelet-free supernatants. The cytokines were evaluated by the Luminex Bio-Plex system according to the manufacturer's instruction.
Statistical Analysis
The independent sample t-test, Mann–Whitney U-test and the chi-square test were used to compare the continuous and categorical variables, respectively. The survival differences in terms of OS and PFS were compared by the Log rank test and survival curves were analyzed using the Kaplan–Meier method. Multivariate logistic regression was conducted on the basis of clinicopathological variables and lymphocytes or cytokines selected by least absolute shrinkage and selection operator (LASSO) logistic regression model. The prediction algorithms were further validated using receiver operating characteristic (ROC) curves. Area under the ROC curve (AUC) and concordance index (C-index) of the multimarker algorithms were calculated and compared. Prognostic factors of survival, including OS and PFS, and the associated corresponding 95% confidence intervals (CIs) were determined. The correlative analyses were conducted using Pearson correlation coefficient and Mantel test. Statistical significance was considered when two-tailed P value < 0.05 was obtained. All statistical analyses were performed using R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristic
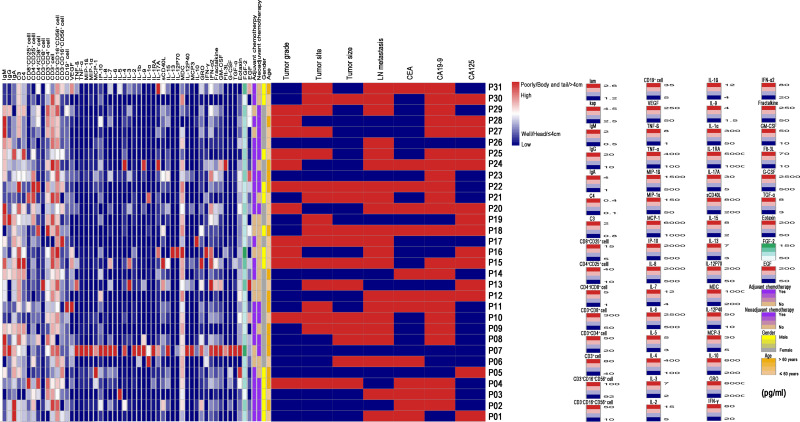
A total of 8 healthy control subjects and 31 LAPC patients who had received IRE treatment were included in this study. Four male and another four female healthy volunteers were included as the control group, with the median age of 59.0 years (range 43–75 years). In LAPC patients, there were 18 (58.1%) female patients and 13 (41.9%) male patients. The median age for all patients was 60.0 years (range 45–73 years). Most tumors were smaller than 4cm and located in the head of the pancreas. Sixteen patients (51.6%) had neoadjuvant chemotherapy while most of patients (71.0%) received adjuvant chemotherapy. The proportion of lymphocytes and the description of cytokines are summarized in Tables 1 and 2, respectively. The median tumor size was 36 mm (range 20–49 mm). A total of 21 patients (66.7%) were diagnosed with radiologic lymph node (LN) metastasis. There were 10 (32.3%) and 7 (22.6%) patients who had higher levels of CD3+CD4+ T cells and CD3+CD8+ T cells, respectively. As for CD3−CD16+CD56+ cells (natural killer cells, NK cells), 6 (19.4%) patients had decreased levels and 11 (35.5%) patients had elevated levels of cells. An overview of the distribution of all cytokines, lymphocytes and other clinicopathological variables is shown in Figure 1.
Table 1.
Clinical Characteristics of Patients with LAPC Undergoing IRE Therapy
| Category Variable | Category | N (%) | Category Variable | Category | N (%) |
|---|---|---|---|---|---|
| Age (years) | ≤ 60 | 16 (51.6) | Tumor site | Head | 15 (48.4) |
| > 60 | 15 (48.4) | Body | 14 (45.2) | ||
| Gender | Female | 18 (58.1) | Tail | 2 (6.5) | |
| Male | 13 (41.9) | Tumor grade | Well/Moderate | 19 (61.3) | |
| Tumor size (cm) | ≤ 2 | 1 (3.2) | Poor | 12 (38.7) | |
| 2–4 | 18 (58.1) | LN metastasis | Absence | 10 (32.3) | |
| > 4 | 12 (38.7) | Presence | 21 (67.7) | ||
| Neoadjuvant chemotherapy | No | 15 (48.4) | CD19+ cell | Normal | 23 (74.2) |
| Yes | 16 (51.6) | Low | 5 (16.1) | ||
| Adjuvant chemotherapy | No | 9 (29.0) | High | 3 (9.7) | |
| Yes | 22 (71.0) | CD3+CD16+CD56+ cell | Normal | 30 (96.8) | |
| Progression within l year | No | 17 (54.8) | High | 1 (3.2) | |
| Yes | 14 (45.2) | CD3+CD4+ cell | Normal | 21 (67.7) | |
| Survival beyond 2 years | No | 13 (41.9) | High | 10 (32.3) | |
| Yes | 18 (58.1) | CD4+CD8+ cell | Normal | 9 (29.0) | |
| WBC (*109) | ≤ 10 | 27 (87.1) | Low | 15 (48.4) | |
| > 10 | 4 (12.9) | High | 7 (22.6) | ||
| HGB (g/L) | ≤ 120 | 8 (25.8) | CD8+CD25+ cell | Normal | 30 (96.8) |
| > 120 | 23 (74.2) | Low | 1 (3.2) | ||
| ALT (U/L) | ≤ 40 | 20 (64.5) | IgG | Low | 14 (45.2) |
| > 40 | 11 (35.5) | High | 17 (54.8) | ||
| AST1C | ≤ 40 | 8 (25.8) | IgA | Low | 12 (38.7) |
| > 40 | 23 (74.2) | High | 19 (61.3) | ||
| ALP (U/L) | ≤ 100 | 16 (51.6) | IgM | Low | 12 (38.7) |
| > 100 | 15 (48.4) | High | 19 (61.3) | ||
| ALB (g/L) | ≤ 40 | 13 (41.9) | CD3−CD16+CD56+ cell | Normal | 14 (45.2) |
| > 40 | 18 (58.1) | Low | 6 (19.4) | ||
| GGT (U/L) | ≤ 60 | 18 (58.1) | CD3+ cell | High | 11 (35.5) |
| > 60 | 13 (41.9) | Normal | 11 (35.5) | ||
| TBIL (umol/L) | ≤ 22.5 | 22 (71.0) | Low | 11 (35.5) | |
| > 22.5 | 9 (29.0) | CD3+CD8+ cell | High | 9 (29.0) | |
| IBIL (umol/L) | ≤ 15 | 26 (83.9) | Normal | 9 (29.0) | |
| > 15 | 5 (16.1) | Low | 15 (48.4) | ||
| CRP (ng/L) | ≤ 3 | 19 (61.3) | CD4+CD25+ cell | High | 7 (22.6) |
| > 3 | 12 (38.7) | Normal | 30 (96.8) | ||
| HBSAg | Absence | 28 (90.3) | High | 1 (3.2) | |
| Presence | 3 (9.7) | C3 | Low | 4 (12.9) | |
| CA125 (U/mL) | ≤ 35 | 19 (61.3) | High | 27 (87.1) | |
| > 35 | 12 (38.7) | C4 | Low | 15 (48.4) | |
| CA199 (U/mL) | ≤ 35 | 9 (29.0) | High | 16 (51.6) | |
| > 35 | 22 (71.0) | ||||
| CEA (ng/mL) | ≤ 5 | 18 (58.1) | |||
| > 5 | 13 (41.9) |
Abbreviations: MDC, macrophage-derived chemokine; sCD40L, soluble CD40 ligand; MIP, macrophage infectivity potentiator; TNF, tumor necrosis factor; VEGF, vascular epidermal growth factor; WBC, white blood cell count; PLT, platelet count; ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, glutamyl transpeptidase; ALB, albumin; TBIL, total bilirubin; IBIL, indirect bilirubin; CRP, C-reactive protein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19–9; CA125, carbohydrate antigen 125.
Table 2.
The Characteristics of Cytokines in Patients
| Continuous Variable | Median | Range | Variable | Median | Range |
|---|---|---|---|---|---|
| EGF | 51.12 | 355–231.54 | IL17A | 3.44 | 0.90–3365.00 |
| FGF2 | 79.70 | 17.28–189.91 | IL1RA | 31.13 | 0.90–5847.00 |
| Eotaxin | 90.08 | 18.46–241.16 | IL-1α | 6.64 | 4.04–315.71 |
| TGF-α | 2.53 | 2.53–8.30 | IL-9 | 1.25 | 1.25–4.23 |
| GCSF | 28.26 | 2.42–2874.00 | IL-1b | 2.83 | 2.83–13.35 |
| Flt3L | 2.23 | 2.23–74.09 | IL-2 | 1.53 | 0.75–19.97 |
| GMCSF | 7.14 | 1.96–51.83 | IL-3 | 1.54 | 1.54–7.11 |
| Fractalkine | 47.14 | 4.23–288.28 | IL-4 | 36.24 | 3.76–430.10 |
| IFN-α2 | 26.17 | 1.46–89.67 | IL-5 | 2.76 | 2.76–5.02 |
| IFN-γ | 6.18 | 1.88–88.55 | IL-6 | 13.34 | 3.05–2835.00 |
| GRO | 1384.0 | 7.37–8310.00 | IL-7 | 2.71 | 2.67–13.36 |
| IL-10 | 6.86 | 2.52–850.02 | IL-8 | 23.36 | 2.61–2020.00 |
| MCP3 | 3.62 | 3.62–33.06 | IP-10 | 550.90 | 163.69–2072.00 |
| IL-12P40 | 3.94 | 3.94–54.17 | MCP-1 | 343.64 | 127.87–6253.00 |
| MDC | 614.47 | 50.23–1001.00 | MIP-1α | 6.59 | 3.04–167.26 |
| IL-12P70 | 3.48 | 3.00–207.01 | MIP-1β | 54.50 | 13.61–1925.00 |
| IL-13 | 2.57 | 2.57–7.36 | TNF-α | 18.79 | 6.40–417.89 |
| IL-15 | 1.93 | 1.03–8.11 | TNF-β | 1.00 | 1.00–8.23 |
| sCD40L | 1597 | 230.34–8481.00 | VEGF | 47.82 | 3.72–298.02 |
Abbreviations: EGF, epidermal growth factor; FGF2, fibroblast growth factor-2; TGF-α, tumor growth factor-2; GCSF, granulocyte colony-stimulating factor; Flt3L, FMS like tyrosine kinase 3 ligand; GMCSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; GRO, growth regulates oncogene; IL, interleukin.
Figure 1.
An overview of the distribution of all cytokines, lymphocytes and other clinicopathological variables in LAPC patients after IRE.
The Comparisons of Cytokines and Immune Cells
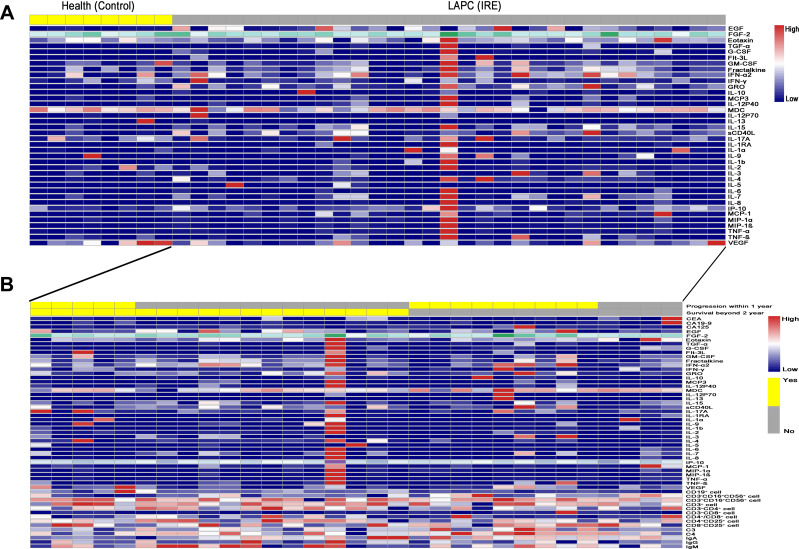
As shown in Figure 2A, compared with healthy control subjects, LAPC patients seemed to have significantly higher levels of EGF, GRO, IL-1RA, and MCP-1. Furthermore, the differences in the numbers of cytokines and immune cells in patients with different survival and tumor progression statuses were also compared. It was shown that elevated levels of Flt3l, GMCSF, MCP3, and IL-9 were associated with a decrease in the number of patients who were still alive two years after IRE treatment. In terms of tumor progression, patients with decreased levels of C4 and TNF-α or increased levels of MIP-1α and IL-17A were likely to have tumor progression within one year of receiving IRE treatment (Figure 2B).
Figure 2.
The comparisons of cytokines and immune cells in LAPC patients. (A) The comparisons of cytokines and immune cells between LAPC patients and healthy control. (B) The comparisons of cytokines and immune cells in LAPC patients with different survival or tumor progression statuses.
The Relationship Between Cytokines and Clinical Factors
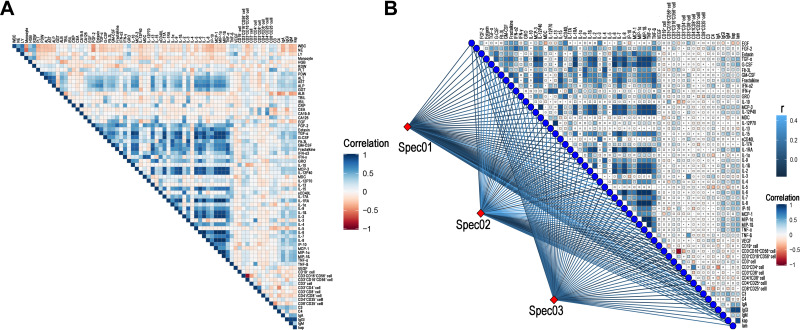
The correlation heatmap analysis of the relationship between cytokines and clinical factors is shown in Figure 3A. Positive relationships were observed among several cytokines, including EGF, TGF-ß, G-CSF, GM-CSF, fractalkine, GRO, IL-17A, IL-1RA, IL-9, IL-1ß, IL-2, MCP-1, MIP-1α, and MIP-1ß. Moreover, the level of CD3+CD4+ T cells and CD3−CD16+CD56+ cells were positively associated with FGF-2, Fil-3L, IFN-α2, IFN-γ, and IL-17A. Negative correlations were observed between CD3+CD8+ T cells and cytokines, including Flt-3L, MCP-3, IP-10, IL-1R CEA, and CA19-9. To further illustrate the correlations among these factors, factors of blood routine, biochemical routine, and tumor markers were classified as spec01, spec02, and spec03, respectively. The findings confirmed the positive relationship between tumor marker groups, including CEA and CA19-9, and ratios of CD3+CD4+ T cells, CD3+CD8+ T cells and CD3−CD16+CD56+ cells (Figure 3B). Positive correlations between IL-1R and TGF-α, G-CSF, MCP3, IL-12P40, respectively, were observed. Also, MIP-1α, MIP-ß and TNF-α were also positively associated with TGF-α, G-CSF, MCP3, IL-12P40, IL-1RA, IL-1ß, IL-2, IL-6, and IL-8, respectively.
Figure 3.
The correlation heatmap analysis of the relationship among cytokines and clinical factors. (A) The association analysis among all cytokines and clinical factors. (B) The Mantel test for the association analysis among all cytokines and lymphocytes. The factors of blood routine, biochemical routine and tumor markers were classified as spec01, spec02 and spec03, respectively.
Risk Factors for OS and PFS
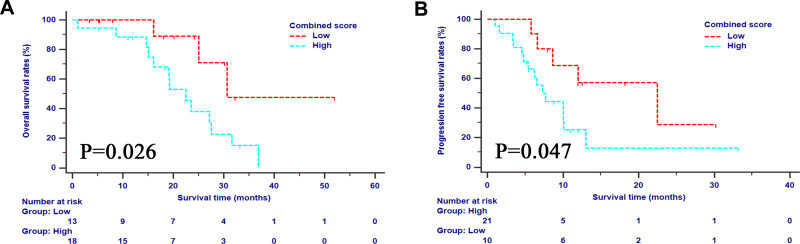
For the whole study cohort, the 1-, 2- and 3-OS rates were 92.9%, 56.3%, and 13.1%, respectively, while the 1- and 2-PFS rates were 35.9% and 19.1%, respectively. In order to investigate the prognostic factors of survival, high-dimensional microarray data were incorporated in the LASSO regression (Supplementary Figure 1). The five best predictors for OS and PFS, including CA19-9, CD3+CD4+ T cells, CD3+CD8+ T cells, IL-17A, and TNF-α, were selected and incorporated into a new immune panel. Higher values of CA19-9 or IL-17A, lower values of CD3+CD4+ T cells, CD3+CD8+ T cells, and TNF-α each contributed one point to the total score of the immune panel. The total scores ranged from 0 to 4. The scores of the immune panel were classified into different levels with the cutoff value of 2. Patients with higher scores on the immune panel had a significantly higher risk of decreased OS and PFS compared to those with lower scores (Figure 4). The immune panel, along with the clinicopathological indexes, was further analyzed with univariate and multivariate analyses. It was shown that the neoadjuvant chemotherapy (OS, HR=0.368, 95% CI 0.076–0.769, p=0.012; PFS, HR=0.183, 95% CI 0.055–0.610, p=0.006), tumor size (OS, HR=6.687, 95% CI 1.692–26.439, p=0.007; PFS, HR=1.345, 95% CI 1.077–3.794, p=0.046), tumor grade (OS, HR=2.914, 95% CI 1.921–9.218, p=0.049; PFS, HR=2.316, 95% CI 1.618–8.679, p=0.023), and immune panel (OS, HR=4.073, 95% CI 1.951–17.454, p=0.039; PFS, HR=4.819, 95% CI 1.225–18.959, p=0.024) were significant prognostic factors for both OS and PFS (Table 3).
Figure 4.
The survival analyses stratified by the selected immune panel in terms of OS (A) and PFS (B).
Abbreviations: OS, overall survival; PFS, progression-free survival.
Table 3.
Univariate and Multivariate Analyses of OS and PFS in LAPC Patients
| Characteristics | OS | PFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR | 95% CI | P | HR | 95% | P | HR | 95% | P | HR | 95% | P | |
| Age | 0.517 | 0.128–1.500 | 0.225 | NI | 0.798 | 0.329–1.936 | 0.618 | NI | ||||
| Gender | 2.005 | 0.738–5.447 | 0.172 | NI | 1.583 | 0.656–3.822 | 0.307 | NI | ||||
| Adjuvant chemotherapy | 1.036 | 0.291–3.687 | 0.957 | NI | 2.895 | 0.828–10.126 | 0.096 | NI | ||||
| Neoadjuvant chemotherapy | 0.201 | 0.061–0.663 | 0.008 | 0.368 | 0.076–0.769 | 0.012 | 0.296 | 0.111–0.783 | 0.015 | 0.183 | 0.055–0.610 | 0.006 |
| Tumor grade | 2.808 | 0.952–8.282 | 0.061 | 2.914 | 1.921–9.218 | 0.049 | 1.418 | 1.563–3.577 | 0.049 | 2.316 | 1.618–8.679 | 0.023 |
| Tumor site | 1.843 | 0.676–5.029 | 0.232 | NI | 1.799 | 0.727–4.451 | 0.204 | NI | ||||
| Tumor size | 5.103 | 1.558–16.716 | 0.007 | 6.687 | 1.692–26.439 | 0.007 | 2.557 | 1.033–6.329 | 0.042 | 1.345 | 1.077–3.794 | 0.046 |
| LN metastasis | 0.974 | 0.328–2.891 | 0.963 | NI | 1.264 | 0.521–3.068 | 0.604 | NI | ||||
| Immune panel | 3.570 | 1.015–12.561 | 0.047 | 4.073 | 1.951–17.454 | 0.039 | 3.212 | 1.026–10.053 | 0.045 | 4.819 | 1.225–18.959 | 0.024 |
Abbreviations: OS, overall survival; PFS, progression-free survival; NI, not included.
Performance of Prediction for OS and PFS
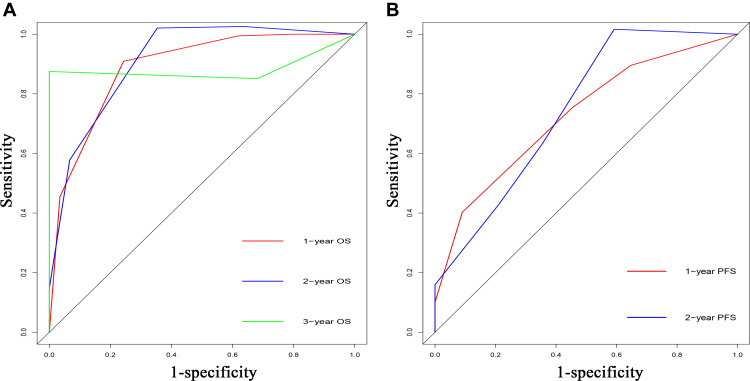
The predictive efficacy of significant risk factors for OS and PFS was further compared with the C-indexes and AUC. The C-indexes for OS and PFS were 0.826 (95% CI 0.751–0.901) and 0.724 (95% CI 0.627–0.821), respectively. The conventional tumor marker score, which was composed of CA19-9, CEA, and CA125, was used as a control. It was determined that the C-indexes of the tumor marker scores for OS and PFS were 0.489 (95% CI 0.333–0.645) and 0.478 (95% CI 0.349–0.607), respectively, which were both significantly lower than those of the established risk model (p<0.001). The ROC curves of the risk model for survival prediction are shown in Figure 5. The values of AUC for the risk model for 1-, 2-, and 3-OS prediction were 0.888, 0.911, and 0.883, respectively, while the values of the tumor marker score were 0.542, 0.542, and 0.582, respectively (p<0.050). Similarly, the risk model also had higher values of AUC than the tumor marker score for 1- and 2-PFS prediction (risk model vs tumor marker score, 1-year, 0.727 vs 0.532; 2-year, 0.745 vs 0.586, p<0.050).
Figure 5.
Comparisons of receiver operating characteristic (ROC) curves of the predictive system for predicting 1-, 2-, and 3-year OS (A) and 1- and 2-year PFS (B) for LAPC patients after IRE, respectively.
Discussion
In recent years, growing number of studies have focused on the potential of IRE for treatment and have shown that IRE is an effective method for treating LAPC.10,12,18 Although the associations between biomarkers and prognosis of LAPC patients undergoing IRE treatment have been explored before, the study only included limited dimensional of data.19 Thus, the identification of detailed molecular biomarkers is urgently needed to enhance the prediction of survival rates for LAPC patients after IRE. Considering that tumor staging or progression always incurs substantial metabolic costs by significantly altering the levels of cytokines or immunocytes, those altered molecular markers may be potential biomarkers for the survival prediction for LAPC patients undergoing IRE. To verify this hypothesis, we applied microarray assays of cytokines and immunocytes to baseline blood samples obtained from LAPC patients who had received IRE treatment, as well as to blood samples from healthy control subjects. In this study, an absolute risk model based on precision identification of multiple cytokines, lymphocytes, tumor markers and pathological characteristics was selected for LAPC patients who had received IRE for the first time. Our results underscored the improved performance of the risk model compared to conventional TNM stage or tumor markers in the context of prognosis stratification in LAPC patients undergoing IRE treatment.
In a previous study, we also found that IRE could significantly improve immune function and the elevation of CD8+ T cells indicated better survival rates for LAPC patients undergoing IRE.11,19 Based on these results, our recent work differentiates itself most significantly from previous work in the following ways: 1) it is specially designed for LAPC patients undergoing IRE treatment and 2) it expands on previous efforts by including broadly available cytokines and lymphocytes in multivariate survival models. Unsurprisingly, the risk model that was established based on the comprehensive evaluation of all of these parameters in this study outperforms the traditional TNM stage system or conventional tumor marker scores. To construct our multi-analyte panel, we selected CA19-9, CD3+CD4+ T cells, CD3+CD8+ T cells, IL-17A, and TNF-α. The tumor marker CA19-9 provided the routine indexes of tumor growth, which indicated the prognosis for LAPC patients.20,21 There was a closed relationship between CA19-9 and immune cell changes, indicating that these easily obtained clinical data might reflect the changes of immune cell. Moreover, CD3+CD4+ T cells and CD3+CD8+ T cells were two important immunocytes. In a study conducted by Scheffer,22 it was shown that IRE could activate CD4+ and CD8+ T cells. Apart from the activation, IRE was also shown to elevate the absolute numbers and proportions of CD8+ T cells, and the elevation of CD8+ T cells indicated a significantly better survival rate in LAPC patients undergoing IRE.19 Additionally, in this study, it was shown that CD3+CD8+ T cells were negatively correlated with CA19-9. Similar to results from other studies, lower levels of CA19-9 were correlated with a more immune-active microenvironment, which was reflected in the increased number of CD3+CD8+ T cells.23,24 As an important helper T cells, CD3+CD4+ T cells were also important for the inducement and enhancement of immune memory. The increased CD4+ T cells also contributed to enhanced adjuvanticity, which was essential for an immune response and the function of the abscopal effect induced by IRE.25
Similar to the findings of previous studies, IL-17A was correlated with decreased survival. A distribution of cytokines and clinicopathological variables has shown that patients with higher levels of IL-17A are more likely to have poorly differentiated and large tumors or LN metastasis. The regulation of tuft cells and stem cells of pancreatic cancer by IL-17A may contribute to tumor growth and progression.26 Conversely, TNF-α was essential for the lysis of tumor cells induced by cytotoxic T cells27 and played an important role in the polarization of macrophages into the M1-like subtype,28 which could contribute to the phagocytosis of tumor cells and antigen-presentation. Moreover, it was shown that TNF-α was positively associated with IL-1ß, IL-2, and IL-6, which were also pro-inflammatory cytokines from M1-subtype macrophages.28 In this study, the correlation analysis indicated that LAPC patients with higher levels of TNF-α were more likely to have tumor progression-free status for over one year and survival beyond two years after IRE treatment, illustrating that high TNF-α levels are an indication for better survival rates for LAPC patients.
Based on this immune-based panel and other independent prognostic factors, a risk model was established that showed a higher prognostic efficiency in survival prediction compared with tumor marker scores. It was shown that the immune-based panel contributed to the strongest predictive power, compared with other independent prognostic factors. Additionally, significantly different prognoses were stratified by the established risk score for LAPC patients, who were classified as the same stage according to TNM stage system, illustrating that the established risk model had a significantly better ability for prognostic stratification. IRE is an inflammation-inducing treatment, which has been shown to not only destroy tumor cells, but to also cause a release of tumor-associated neo-antigens, and stimulate the cellular and humoral immune in the local and systemic environment.19,25,29 The changes of immune indexes and immune responses were the main characteristics that were compared after IRE treatment and after surgery.30 Considering tumor marker scores, which only reflected the tumor burden itself, and the TNM stage, which were generally designed based on pathological factors only, the inclusion of cytokines and immunocytes from a large microarray assay, along with clinicopathological features, ensured better OS and PFS prediction of the established risk model.
It is important to note that precise prediction of progression is essential for individual treatment. An important advantage of this study was the utilization of a microarray assay specially designed for IRE treatment to determine the immune panel. The comparisons of values from the C-index and the AUC showed the powerful efficacy of the combination of clinicopathological characteristics and immune features in survival prediction. Using this model, clinicians could perform a specific survival prediction of LAPC patients undergoing IRE treatment and specialize the adjuvant treatment for those with a high risk of decreased survival, which fits the current trend of personalized medicine.
This study had several limitations. Truly, as the first study of predictive system based on high-dimensional microarray data for LAPC patients undergoing IRE, a major limitation is the relatively small sample size. The lack of external validation and specificity to IRE due to the absence of data from patients after IRE was another limitation. Therefore, it is necessary to evaluate the changes of these variables after IRE in the further study. A longer follow-up period and an external validation based on larger cohorts are also needed. Finally, the proportion of neoadjuvant chemotherapy in patients was only 51.6% in this study. Given the neoadjuvant chemotherapy emerged as a standard care of LAPC patients in the recent years, studies based on more patients after neoadjuvant chemotherapy were needed to validate the results of this study.
Conclusion
In summary, we evaluated the microarray assay of cytokines and lymphocytes in LAPC patients undergoing IRE and developed a risk score for OS and PFS prediction for the first time. The risk score showed great efficacy in survival prediction and might facilitate highly tailed patient management in clinical practice in LAPC patients after IRE.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (81171890; 81672390), the Major National Scientific Research Projects of China (NO. 2013CB910304), and Guangdong Basic and Applied Basic Research Foundation (2020A1515110954).
Data Sharing Statement
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (http://www researchdata.org.cn), with the approval number as RDDA2020001533.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Kommalapati A, Tella SH, Goyal G, Ma WW, Mahipal A. Contemporary management of localized resectable pancreatic cancer. Cancers. 2018;10(1):24. doi: 10.3390/cancers10010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14(4):1320–1326. doi: 10.1245/s10434-006-9249-8 [DOI] [PubMed] [Google Scholar]
- 4.Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1751–1756. doi: 10.1245/s10434-009-0413-9 [DOI] [PubMed] [Google Scholar]
- 5.Kang H, Jo JH, Lee HS, et al. Comparison of efficacy and safety between standard-dose and modified-dose FOLFIRINOX as a first-line treatment of pancreatic cancer. World J Gastrointest Oncol. 2018;10(11):421–430. doi: 10.4251/wjgo.v10.i11.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barenboim A, Lahat G, Geva R, et al. Neoadjuvant FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer: an intention to treat analysis. Eur J Surg Oncol. 2018;44(10):1619–1623. doi: 10.1016/j.ejso.2018.07.057 [DOI] [PubMed] [Google Scholar]
- 7.Xie DR, Yang Q, Chen DL, et al. Gemcitabine-based cytotoxic doublets chemotherapy for advanced pancreatic cancer: updated subgroup meta-analyses of overall survival. Jpn J Clin Oncol. 2010;40(5):432–441. doi: 10.1093/jjco/hyp198 [DOI] [PubMed] [Google Scholar]
- 8.Loehrer PJ, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern cooperative oncology group trial. J Clin Oncol. 2011;29(31):4105–4112. doi: 10.1200/JCO.2011.34.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–1853. doi: 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 10.He C, Huang X, Zhang Y, Cai Z, Lin X, Li S. Comparison of survival between irreversible electroporation followed by chemotherapy and chemotherapy alone for locally advanced pancreatic cancer. Front Oncol. 2020;10:6. doi: 10.3389/fonc.2020.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He C, Wang J, Zhang Y, Lin X, Li S. Irreversible electroporation after induction chemotherapy versus chemotherapy alone for patients with locally advanced pancreatic cancer: a propensity score matching analysis. Pancreatology. 2020;20(3):477–484. doi: 10.1016/j.pan.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 12.He C, Wang J, Sun S, et al. Irreversible electroporation versus radiotherapy after induction chemotherapy on survival in patients with locally advanced pancreatic cancer: a propensity score analysis. BMC Cancer. 2019;19(1):394. doi: 10.1186/s12885-019-5607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruarus AH, Vroomen L, Geboers B, et al. Percutaneous irreversible electroporation in locally advanced and recurrent pancreatic cancer (PANFIRE-2): a multicenter, prospective, single-arm, phase II study. Radiology. 2020;294(1):212–220. doi: 10.1148/radiol.2019191109 [DOI] [PubMed] [Google Scholar]
- 14.Holland MM, Bhutiani N, Kruse EJ, et al. A prospective, multi-institution assessment of irreversible electroporation for treatment of locally advanced pancreatic adenocarcinoma: initial outcomes from the AHPBA pancreatic registry. HPB. 2019;21(8):1024–1031. doi: 10.1016/j.hpb.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Martin RC, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262(3):486–494. doi: 10.1097/SLA.0000000000001441 [DOI] [PubMed] [Google Scholar]
- 16.NCCN clinical practice guidelines in oncology. Pancreatic adenocarcinoma version 1, 2020; 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed April22, 2021.
- 17.Huang X, He C, Lin G, et al. Induced CD10 expression during monocyte-to-macrophage differentiation identifies a unique subset of macrophages in pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun. 2020;524(4):1064–1071. doi: 10.1016/j.bbrc.2020.02.042 [DOI] [PubMed] [Google Scholar]
- 18.He C, Wang J, Zhang Y, Cai Z, Lin X, Li S. Comparison of combination therapies in the management of locally advanced pancreatic cancer: induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation. Cancer Med. 2020;9(13):4699–4710. doi: 10.1002/cam4.3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He C, Wang J, Sun S, Zhang Y, Li S. Immunomodulatory effect after irreversible electroporation in patients with locally advanced pancreatic cancer. J Oncol. 2019;2019:9346017. doi: 10.1155/2019/9346017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen ZT, Zhou H, Li AM, et al. Clinical outcomes and prognostic factors of stereotactic body radiation therapy combined with gemcitabine plus capecitabine for locally advanced unresectable pancreatic cancer. J Cancer Res Clin Oncol. 2020;146(2):417–428. doi: 10.1007/s00432-019-03066-z [DOI] [PubMed] [Google Scholar]
- 21.Gemenetzis G, Groot VP, Blair AB, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 2019;270(2):340–347. doi: 10.1097/SLA.0000000000002753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheffer HJ, Stam AGM, Geboers B, et al. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation. Oncoimmunology. 2019;8(11):1652532. doi: 10.1080/2162402X.2019.1652532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang D, Horner A, Brehm E, et al. Early serum tumor marker dynamics predict progression-free and overall survival in single PD-1/PD-L1 inhibitor treated advanced NSCLC-A retrospective cohort study. Lung Cancer. 2019;134:59–65. doi: 10.1016/j.lungcan.2019.05.033 [DOI] [PubMed] [Google Scholar]
- 24.Hwang HK, Kim HI, Kim SH, et al. Prognostic impact of the tumor-infiltrating regulatory T-cell (Foxp3(+))/activated cytotoxic T lymphocyte (granzyme B(+)) ratio on resected left-sided pancreatic cancer. Oncol Lett. 2016;12(6):4477–4484. doi: 10.3892/ol.2016.5252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He C, Huang X, Zhang Y, Lin X, Li S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin Transl Med. 2020;10:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Zoltan M, Riquelme E, et al. Immune cell production of interleukin 17 induces stem cell features of pancreatic intraepithelial neoplasia cells. Gastroenterology. 2018;155(1):210–223.e213. doi: 10.1053/j.gastro.2018.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parriott G, Deal K, Crean S, Richardson E, Nylen E, Barber A. T-cells expressing a chimeric-PD1-Dap10-CD3zeta receptor reduce tumour burden in multiple murine syngeneic models of solid cancer. Immunology. 2020;160(3):280–294. doi: 10.1111/imm.13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Huang Q, Hu X, et al. Evaluating the osteoimmunomodulatory properties of micro-arc oxidized titanium surface at two different biological stages using an optimized in vitro cell culture strategy. Mater Sci Eng C. 2020;110:110722. doi: 10.1016/j.msec.2020.110722 [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Wen X, Tian L, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. 2019;10(1):899. doi: 10.1038/s41467-019-08782-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandit H, Hong YK, Li Y, et al. Evaluating the regulatory immunomodulation effect of irreversible electroporation (IRE) in pancreatic adenocarcinoma. Ann Surg Oncol. 2019;26(3):800–806. doi: 10.1245/s10434-018-07144-3 [DOI] [PubMed] [Google Scholar]