The red-pigmented prodiginines are attracting increasing interest due to their broad biological activities. As with many secondary metabolites, the biosynthesis of prodiginines is regulated by both environmental and physiological factors.
KEYWORDS: stringent starvation protein, prodiginine biosynthesis, siderophore production, iron homeostasis, Pseudoalteromonas
ABSTRACT
Prodiginines are a family of red-pigmented secondary metabolites with multiple biological activities. The biosynthesis of prodiginines is affected by various physiological and environmental factors. Thus, prodiginine biosynthesis regulation is highly complex and multifaceted. Although the regulatory mechanism for prodiginine biosynthesis has been extensively studied in Serratia and Streptomyces species, little is known about that in the marine betaproteobacterium Pseudoalteromonas. In this study, we report that stringent starvation protein A (SspA), an RNA polymerase-associated regulatory protein, is required for the biosynthesis of prodiginine in Pseudoalteromonas sp. strain R3. The strain lacking sspA (ΔsspA) fails to produce prodiginine, which resulted from the downregulation of the prodiginine biosynthetic gene (pig) cluster. The effect of SspA on prodiginine biosynthesis is independent of histone-like nucleoid structuring protein (H-NS) and RpoS (σS). Further analysis demonstrates that the ΔsspA strain has a significant decrease in the transcription of the siderophore biosynthesis gene (pvd) cluster, leading to the inhibition of siderophore production and iron uptake. The ΔsspA strain regains the ability to synthesize prodiginine by cocultivation with siderophore producers or the addition of iron. Therefore, we conclude that SspA-regulated prodiginine biosynthesis is due to decreased siderophore levels and iron deficiency. We further show that the iron homeostasis master regulator Fur is also essential for pig transcription and prodiginine biosynthesis. Overall, our results suggest that SspA indirectly regulates the biosynthesis of prodiginine, which is mediated by the siderophore-dependent iron uptake pathway.
IMPORTANCE The red-pigmented prodiginines are attracting increasing interest due to their broad biological activities. As with many secondary metabolites, the biosynthesis of prodiginines is regulated by both environmental and physiological factors. At present, studies on the regulation of prodiginine biosynthesis are mainly restricted to Serratia and Streptomyces species. This work focused on the regulatory mechanism of prodiginine biosynthesis in Pseudoalteromonas sp. R3. We found that stringent starvation protein A (SspA) positively regulates prodiginine biosynthesis via affecting the siderophore-dependent iron uptake pathway. The connections among SspA, iron homeostasis, and prodiginine biosynthesis were investigated. These findings uncover a novel regulatory mechanism for prodigiosin biosynthesis.
INTRODUCTION
Prodiginines are a family of red-pigmented secondary metabolites produced by both Gram-positive and Gram-negative bacteria, such as Streptomyces spp., Serratia spp., and Pseudoalteromonas spp. (1, 2). These metabolites contain a common tripyrrole structure and can be classified into four groups (1). The first group consists of prodiginines with only a linear tripyrrole, including prodigiosin and undecylprodigiosin. The other three groups are cyclic derivatives of prodiginines: for example, cycloprodigiosin. In recent years, prodiginines have been attracting increasing interest due to their broad biological activities—mainly antibacterial (3), antimalarial (4), antifungal (5), anticancer (6), and immunosuppressive (7). Interestingly, recent studies in Streptomyces have revealed that prodiginines are also associated with programmed cell death (8, 9).
The biosynthesis of prodiginines has been well characterized in prodiginine-producing bacteria (1). In Serratia, the prodigiosin biosynthetic gene (pig) clusters contain at least 14 genes, pigA∼pigN. The pig clusters are arranged unidirectionally and transcribe as a polycistronic mRNA driven by a promoter upstream of pigA. The biosynthetic pathway of prodigiosin involves the biosynthesis of 4-methoxy-2,2′-bipyrrole-5-carbaldehyde (MBC) and 2-methyl-3-n-amyl-pyrrole (MAP). Once MBC and MAP are synthesized, the condensing enzyme PigC catalyzes them to form prodigiosin. The biosynthesis of undecylprodigiosin requires MBC and another monopyrrole, 2-undecylpyrrole (1). It was recently reported that the formation of cycloprodigiosin in Pseudoalteromonas rubra is derived from the cyclization of prodigiosin, which is catalyzed by an alkylglycerol monooxygenase-like enzyme (10).
The biosynthesis of prodiginines is affected by various environmental and physiological factors (1, 11). Several mechanisms underlying the regulation of prodiginine biosynthesis have been elucidated in Serratia and Streptomyces species. The acyl-homoserine lactone (AHL)-type quorum sensing (QS) system SmaI/R monitors bacterial population density and then controls the transcription of the pig cluster in Serratia sp. strain ATCC 39006 (12). Multiple two-component signal transduction systems (PigQ/W and RssB/A) and transcriptional regulators (PigP, MetR, RedD, and RedZ) regulate prodiginine production, although the signals sensed by most of these systems remain unknown (13–16).
The small nucleotides guanosine-tetraphosphate and guanosine-pentaphosphate (collectively referred to as ppGpp or alarmones) are also involved in the regulation of prodiginine biosynthesis (17, 18). In response to a variety of nutrient stresses (such as starvation for amino acid, carbon, nitrogen, phosphate, iron, or fatty acid), bacterial cells accumulate high concentrations of ppGpp to induce the stringent response (19, 20). ppGpp binds RNA polymerase (RNAP) and then changes transcription globally. Deletion of the ppGpp synthetase gene relA abolishes the production of undecylprodigiosin during nitrogen starvation in Streptomyces coelicolor A3(2), which is mediated by the pathway-specific regulator RedD (17). Similar to ppGpp, stringent starvation protein A (SspA), an RNAP-associated regulatory protein, is also induced during the stationary phase and under nutrient-limited conditions (21, 22). SspA plays an important role in stress response and is highly conserved among Gram-negative bacteria (23, 24). However, the involvement of SspA in the regulation of prodiginine biosynthesis has never been reported. Intriguingly, the starvation-induced expression of sspA requires a functional relA gene (21). In Francisella tularensis, ppGpp activates pathogenicity machinery via binding specifically to the SspA-containing heterodimer complex (25, 26). Therefore, it can be speculated that SspA may also be involved in the regulation of prodiginine biosynthesis, but direct evidence is still lacking.
Iron is an essential element for virtually all microorganisms. However, iron acquisition is difficult in aerobic environments since the majority of iron exists as an insoluble ferric (Fe3+) form (27). To overcome this problem, bacteria have evolved the most efficient mechanism for uptake of iron, which is dependent on siderophores (28). Siderophores are small organic molecules with extraordinarily high affinity for Fe3+ (29). They are synthesized in the cytoplasm and secreted into the environment, resulting in the formation of iron-siderophore complexes. Then these complexes are shuttled back into the cell to release iron. In addition to the siderophore-mediated iron uptake pathway, bacteria possess several other strategies of iron acquisition (28). Given that iron overload is highly toxic to bacterial cells, the intracellular iron concentrations must be tightly controlled. It is well described that the ferric uptake regulator Fur plays a critical role in the regulation of iron homeostasis (30, 31). Under iron-rich conditions, Fur interacts with its corepressor Fe2+ and represses the transcription of iron-responsive genes. Under iron-limited conditions, Fe2+ dissociates from the Fur-Fe2+ complex, leading to the derepression of the target genes. Recent studies have revealed that Fur can also function as an activator that affects many biological processes beyond iron metabolism (32, 33).
Pseudoalteromonas species are widely distributed in the marine environment and display a broad range of biological activities (34). Prodiginines are the most important biologically active metabolites produced by this genus (2). In contrast to Streptomyces and Serratia species, the regulatory mechanism for prodiginine biosynthesis in Pseudoalteromonas is still a mystery. Recently, we reported that SspA positively regulates biofilm formation and exopolysaccharide production in a red-pigmented bacterium, Pseudoalteromonas sp. strain R3 (35). Interestingly, the strain lacking sspA (ΔsspA) displayed white colonies on solid plates, suggesting that SspA is involved in the regulation of prodiginine biosynthesis. In this study, we present connections among SspA, iron homeostasis, and prodiginine biosynthesis in Pseudoalteromonas sp. R3. We show that SspA positively regulates the biosynthesis of prodiginine, which is dependent on the siderophore-mediated iron uptake pathway. Further analyses demonstrated that Fur is also required for prodiginine biosynthesis in Pseudoalteromonas sp. R3.
RESULTS
Identification of the prodiginine biosynthetic gene cluster in Pseudoalteromonas sp. R3.
Pseudoalteromonas sp. R3 is capable of producing a red pigment, thus forming red colonies on a solid Zobell 2216E medium plate (36). BLASTp analysis demonstrated that the genome of R3 contains a putative prodiginine biosynthetic gene (pig) cluster, which is composed of 13 open reading frames: pigA∼M (Fig. 1A). The gene content and organization of the R3 pig cluster closely resemble those in other members of Pseudoalteromonas and Serratia sp. strain ATCC 39006. Interestingly, the Pseudoalteromonas pig clusters lack pigN and pigO at the 3′ end compared to the Serratia pig cluster. Except for PigL, the other 12 prodiginine biosynthesis proteins of strain R3 share higher than 80% sequence identity with their counterparts in P. rubra SCSIO 6842 (Table 1), which is a typical cycloprodigiosin-producing strain (37). To confirm the role of the R3 pig cluster in prodiginine biosynthesis, a mutant with a deletion of pigK, pigL, and pigM (ΔpigKLM) was constructed. In contrast to the wild-type (WT) strain, the ΔpigKLM strain failed to produce red pigment but displayed faint yellow colonies on solid medium (Fig. 1B). Therefore, it can be concluded that the red pigment produced by Pseudoalteromonas sp. R3 is a member of the prodiginine family, which is synthesized by the pig cluster.
FIG 1.
The prodiginine biosynthetic gene cluster in Pseudoalteromonas sp. R3. (A) Comparison of prodiginine biosynthetic gene clusters of Serratia sp. ATCC 39006, Pseudoalteromonas rubra SCSIO 6842, and Pseudoalteromonas sp. R3. (B) Growth of the wild-type (WT) and ΔpigKLM strains on solid Zobell 2216E plates.
TABLE 1.
Prodiginine biosynthetic genes in Pseudoalteromonas sp. R3
| Locus tag | Gene | Product | Identity (%) to: |
|
|---|---|---|---|---|
| Serratiaa | P. rubrab | |||
| ELR70_RS10930 | pigA | Acyl-CoA/acyl-ACP dehydrogenase | 68.93 | 94.01 |
| ELR70_RS10925 | pigB | NAD(P)-binding protein | 63.01 | 88.74 |
| ELR70_RS10920 | pigC | Phosphoenolpyruvate synthase | 72.62 | 95.80 |
| ELR70_RS10915 | pigD | Biosynthesis protein PigD | 74.82 | 94.82 |
| ELR70_RS10910 | pigE | Acetylornithine transaminase | 79.16 | 96.94 |
| ELR70_RS10905 | pigF | SAM-dependent methyltransferase | 78.47 | 97.35 |
| ELR70_RS10900 | pigG | Acyl carrier protein | 74.68 | 93.90 |
| ELR70_RS10895 | pigH | 7-Keto-8-aminopelargonate synthetase | 72.78 | 94.14 |
| ELR70_RS10890 | pigI | d-Alanine-poly(phosphoribitol) ligase | 60.91 | 86.75 |
| ELR70_RS10885 | pigJ | 3-Ketoacyl-ACP synthase | 59.59 | 81.00 |
| ELR70_RS10880 | pigK | RedY-like protein | 65.69 | 93.27 |
| ELR70_RS10875 | pigL | 4′-Phosphopantetheinyl transferase | 37.22 | 54.38 |
| ELR70_RS10870 | pigM | Nitroreductase family protein | 49.66 | 83.10 |
Serratia sp. ATCC 39006.
P. rubra SCSIO 6842.
Deletion of SspA abolishes prodiginine biosynthesis.
Recently, when we studied the involvement of SspA in biofilm formation in Pseudoalteromonas sp. R3, we found that the strain lacking sspA formed white colonies on solid plates (35). These results indicate that the ΔsspA strain cannot produce prodiginine. To further investigate the roles of SspA in prodiginine production, we performed genetic complementation with the multicopy plasmid pBBR1MCS-5, harboring a copy of sspA under the control of its native promoter. The complemented version of the ΔsspA strain (ΔsspAC) regained the ability to produce prodiginine, which exhibited red colonies on solid plates (Fig. 2A). In Escherichia coli, the sspA gene forms an operon with sspB (21). Similarly, the sspAB operon was also found in the genome of Pseudoalteromonas sp. R3. To test whether SspB is involved in prodiginine biosynthesis, a mutant strain lacking sspB (ΔsspB) was constructed. In contrast to the ΔsspA strain, the ΔsspB strain formed red colonies on solid plates, which is comparable to the wild type (see Fig. S1 in the supplemental material). These results suggest that deletion of sspA but not sspB abolishes the biosynthesis of prodiginine in Pseudoalteromonas sp. R3.
FIG 2.
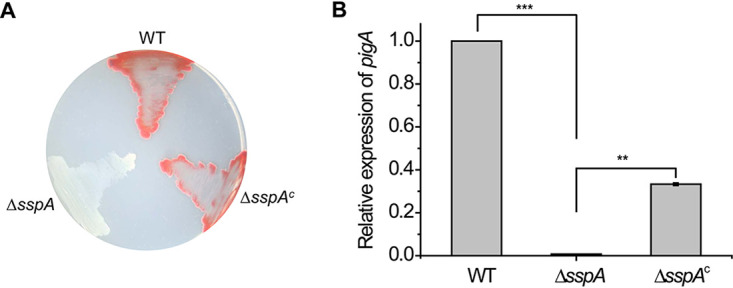
The sspA gene is required for the biosynthesis of prodiginine. (A) Growth of the WT, ΔsspA mutant, and ΔsspAC strains on solid plates. ΔsspAC represents the complemented version of the ΔsspA mutant. (B) Relative expression levels of pigA in indicated strains. The transcription level of the WT was defined as 1.0. Experiments were performed in biological triplicates, and error bars indicate the standard deviations. Asterisks indicate statistically significant differences compared to the WT: **, P < 0.01; ***, P < 0.001.
It has been shown that SspA negatively regulates histone-like nucleoid structuring protein (H-NS), which in turn downregulates the level of RpoS (σS) in E. coli (22). To determine whether H-NS and RpoS are involved in prodiginine biosynthesis in Pseudoalteromonas sp. R3, we constructed the hns and rpoS mutants. However, deletion of hns and rpoS did not affect the production of prodiginine (Fig. S1), suggesting that the regulation of prodiginine production by SspA is independent of H-NS and RpoS.
Next, we asked whether SspA regulates the transcription of the pig operon. To test this, we determined the transcription level of the pigA gene by quantitative real-time PCR (qRT-PCR). As shown by the results in Fig. 2B, deletion of sspA contributed to a dramatic decrease (∼140-fold) in pigA transcription compared to the wild type. Moreover, expression of the sspA gene in trans partially restored the transcription of pigA, which had an ∼47-fold increase relevant to the ΔsspA strain. Together, these results indicate that SspA is required for the regulation of prodiginine biosynthesis in Pseudoalteromonas sp. R3.
SspA positively regulates siderophore production.
To explore the underlying mechanism for SspA-regulated prodiginine biosynthesis, RNA sequencing (RNA-seq) experiments were performed. We compared the transcriptomes of the wild-type and ΔsspA strains at the stationary phase. In total, 791 (16.3%) and 782 (16.1%) of the 4,866 open reading frames (ORFs) were significantly up- and downregulated (>2-fold), respectively, in the ΔsspA strain. Therefore, SspA is a global regulator that modulates the expression of various genes in the stationary phase in Pseudoalteromonas sp. R3, which is in agreement with that observed in E. coli (21, 22). It is worth noting that the downregulated gene list contained a gene cluster (ELR70_RS23345∼ELR70_RS23450) involved in siderophore pyoverdine biosynthesis and uptake (pvd cluster) (Table 2). Among them, ELR70_RS23385 (now designated pvdA) had the largest fold change (∼22.5-fold) between the wild-type and ΔsspA strains. This gene encodes an l-ornithine N5-oxygenase with 49% identity and 67% similarity to Pseudomonas aeruginosa PvdA, which catalyzes a key step of the pyoverdine biosynthetic pathway (38). Consistently, the qRT-PCR analysis demonstrated that the transcription level of pvdA was significantly decreased in the ΔsspA strain, which could be partially restored by the expression of sspA in trans (Fig. 3A).
TABLE 2.
Siderophore biosynthetic genes in Pseudoalteromonas sp. R3a
| Locus tag | Gene | Predicted protein function | Log2 ΔsspA/WT expression ratio |
|---|---|---|---|
| ELR70_RS23345 | pvdE | Cyclic peptide export ABC transporter | −2.00 |
| ELR70_RS23350 | Siderophore-iron reductase | −3.36 | |
| ELR70_RS23355 | Isochorismate synthase | −3.63 | |
| ELR70_RS23360 | (2,3-Dihydroxybenzoyl) adenylate synthase | −2.60 | |
| ELR70_RS23365 | Isochorismatase family protein | −4.04 | |
| ELR70_RS23370 | pvdF | Ferric siderophore synthetase subunit F | −3.83 |
| ELR70_RS23375 | 2,3-Dihydro-2,3-dihydroxybenzoate dehydrogenase | −2.94 | |
| ELR70_RS23385 | pvdA | Lysine N6-hydroxylase/l-ornithine N5-oxygenase | −4.49 |
| ELR70_RS23395 | Nonribosomal peptide synthetase | −2.98 | |
| ELR70_RS23400 | pvdL | Nonribosomal peptide synthetase | −3.13 |
| ELR70_RS23405 | pvdI | Nonribosomal peptide synthetase | −4.09 |
| ELR70_RS23410 | Nonribosomal peptide synthetase | −3.83 | |
| ELR70_RS23415 | TonB-dependent receptor | −2.11 | |
| ELR70_RS23430 | N5-Hydroxyornithine transformylase PvdF | −1.42 | |
| ELR70_RS23435 | Type I polyketide synthase | −2.75 | |
| ELR70_RS23440 | Nonribosomal peptide synthetase | −2.54 | |
| ELR70_RS23450 | Penicillin acylase family protein | −2.13 |
In each case, expression of the gene in the ΔsspA mutant was downregulated versus expression in the WT.
FIG 3.
Deletion of sspA inhibits the production of siderophores. (A) Relative expression levels of pvdA in the WT, ΔsspA mutant, and ΔsspAC strains. ΔsspAC represents the complemented version of ΔsspA. The transcription level of the WT was defined as 1.0. Experiments were performed in biological triplicates, and error bars indicate the standard deviations. Asterisks indicate statistically significant differences compared to the WT. (B) Siderophore production of indicated strains on the O-CAS plates. NC represents negative control. Asterisks indicate statistically significant differences compared to the WT: **, P < 0.01; ***, P < 0.001.
We next determined siderophore production using the overlaid chrome azurol S (O-CAS) assay (39). As shown by the results in Fig. 3B, the Pseudoalteromonas sp. R3 wild-type strain produced an orange halo surrounding the bacterial colony, which is an indicator of the presence of siderophores. However, the diameter of the orange halo zone was reduced in the ΔsspA strain, which could be fully recovered in the complemented version of the ΔsspA strain. Therefore, siderophore production is severely compromised in the strain lacking sspA.
SspA-regulated prodiginine biosynthesis is relevant to siderophore production.
To further explore the relationship between siderophore production and prodiginine synthesis in Pseudoalteromonas sp. R3, the pvdA gene was deleted from the genome. In the O-CAS assay, the ΔpvdA strain also failed to produce an orange halo surrounding the bacterial colony, reinforcing the fact that PvdA is essential for siderophore production (Fig. 4A). Deletion of pvdA had no impact on cell growth. However, the biosynthesis of prodiginine was retarded in the ΔpvdA strain (Fig. 4B). After incubation for 24 h, the mutant formed faint red colonies on a solid medium, while the wild type formed bright red colonies. These results indicate that siderophore production is relevant to prodiginine biosynthesis.
FIG 4.

Siderophore production is involved in the biosynthesis of prodiginine. (A) Siderophore production of the WT and ΔsspA strains on the O-CAS plates. (B) Growth of the WT and ΔsspA strains on solid Zobell 2216E plates at different times. (C) Cross-feeding bioassay for detection of prodiginine biosynthesis. The Pseudoalteromonas sp. R3 strains, S. oneidensis MR-1, or Pseudoalteromonas sp. T1lg65 and E. coli DH5α were streaked on the top, while the ΔsspA strain was streaked on the bottom in parallel. The cross-feeding plates were cultured at 30°C for 48 h and then photographed.
It is well documented that siderophore pyoverdines are public goods that can be shared among neighboring cells (40–42). If the reason prodiginine biosynthesis was abolished in the ΔsspA strain is due to decreased siderophore levels, the mutant should regain the ability to synthesize prodiginine by cocultivation with siderophore producers. To test this, the cross-feeding bioassay was performed on 2216E plates (Fig. 4C). Indeed, cocultivation with the wild-type or the ΔsspAC strain changed the colony color of the ΔsspA strain from white to red. In contrast, the ΔsspA strain remained white when cocultivated with the ΔpvdA strain and the ΔsspA strain per se. Interestingly, the colony color of the ΔsspA strain was also changed by Pseudoalteromonas sp. strain T1lg65 and Shewanella oneidensis MR-1 (which belong to Alteromonadales), but not Escherichia coli DH5α. These results suggest that the Pseudoalteromonas ΔsspA strain can obtain siderophores from other bacteria in the surroundings, thereby enabling the bacterium to synthesize prodiginines.
The addition of Fe restores prodiginine biosynthesis in the ΔsspA strain.
Thus far, our data suggest that the absence of SspA inhibits siderophore production, thus preventing the biosynthesis of prodiginine. Given that the principal role of siderophore is to chelate iron from the surroundings, we hypothesized that deletion of ΔsspA may affect the uptake of iron. To test this, the levels of extracellular iron and intracellular iron were measured by atomic absorption spectroscopy (AAS) (Fig. 5A). After incubation in an iron-rich medium (containing 40 mg/liter FeCl3) for 24 h, the contents of extracellular and intracellular iron in the wild type were 4.6 and 121.4 mg/liter, respectively. However, deletion of sspA resulted in an ∼5.4-fold increase in extracellular iron and an ∼27.5-fold decrease in intracellular iron. Moreover, complementation of sspA partially restored the extracellular and intracellular levels of iron compared to the wild type (Fig. 5A). These results verify that iron uptake is compromised in the strain lacking sspA, leading to the deficiency of iron.
FIG 5.
Iron deficiency is accountable for the phenotype of the ΔsspA strain. (A) Extracellular iron and intracellular iron in the WT, ΔsspA mutant, and the complemented version (ΔsspAC) of the ΔsspA mutant. (B) Prodiginine biosynthesis of the ΔsspA strain in the presence of FeSO4 and FeCl3. (C) Effects of iron on the relative expression of the pigA gene in the ΔsspA strain. The transcription level of the WT was defined as 1.0. (D) Effects of iron on prodiginine production in the wild type. Cultures of the WT strain grown in Zobell 2216E were pelleted for prodiginine production without or with 0.4 and 0.8 mM FeCl3. The optical density at 535 nm (OD535) was read for the detection of prodiginine. Meanwhile, the number of CFU was determined for each culture. The relative prodiginine concentration was expressed as OD535/log CFU. In panels A, C, and D, experiments were performed in biological triplicates, and error bars indicate the standard deviations. Asterisks indicate statistically significant differences compared to the WT: **, P < 0.01; ***, P < 0.001.
Next, we tested whether prodiginine synthesis in the ΔsspA strain could be reversed by exogenous addition of iron. Indeed, the ΔsspA strain changed the colony color from white to red when supplemented with either Fe2+ or Fe3+. The impact of Fe2+ on colony color was more evident than that of Fe3+, as shown in Fig. 5B. In comparison, the colony color of the ΔsspA strain was not affected by other divalent cations, including Ca2+, Mg2+, Zn2+, Mn2+, and Cu2+ (see Fig. S2 in the supplemental material). As we expected, the addition of Fe3+ significantly increased the transcription of pigA in the ΔsspA strain (∼2.6-fold versus wild type; ∼11.5-fold versus ΔsspA mutant) (Fig. 5C).
The effect of iron on the wild type of Pseudoalteromonas sp. R3 was also investigated. The wild type exhibited ∼2.9- and ∼8.0-fold increases in prodiginine production when supplemented with 0.4 mM and 0.8 mM FeCl3, respectively (Fig. 5D). Consistently, the transcription of pigA was elevated ∼2.6-fold in the wild type when cultured with FeCl3 (see Fig. S3 in the supplemental material). Collectively, these data demonstrate that iron strongly promotes prodiginine biosynthesis in Pseudoalteromonas sp. R3.
Fur is required for pigA transcription and prodiginine biosynthesis.
To explore how iron regulates prodiginine biosynthesis in Pseudoalteromonas sp. R3, we focused our attention on the ferric uptake regulator Fur, which plays a critical role in iron homeostasis in many bacteria such as E. coli (28, 33). The transcription of fur in Pseudoalteromonas sp. R3 was increased ∼1.6-fold under the iron-limited condition (supplemented with 0.2 mM iron chelator 2,2′-dipyridyl) and decreased ∼4.4-fold under the iron-rich condition (supplemented with 0.4 mM FeCl3) (see Fig. S4 in the supplemental material). Additionally, our RNA-seq data demonstrated that the transcription of fur was significantly enhanced (∼2.4-fold) in the iron-deficient ΔsspA strain. Similar results were obtained from qRT-PCR analysis (Fig. S4). These data suggest that Fur is also responsive to iron levels in Pseudoalteromonas sp. R3.
We next constructed a strain lacking fur (Δfur). The mutant displayed yellow colonies (Fig. 6A) that were similar to those of the ΔpigKLM strain, as shown above (Fig. 1B). Complementation of fur fully recovered the colony color of the Δfur strain (Fig. 6A). Consistently, the Δfur strain had a significant decrease (∼3.4-fold) in pigA transcription compared to the wild type, which can be partially restored in the complemented strain (Fig. 6B). However, the colony color of the Δfur strain was barely affected by the addition of either Fe2+ or Fe3+ (Fig. 6C), which is in contrast to the ΔsspA strain. Interestingly, the Δfur strain changed color from yellow to white, which resembles the ΔsspA strain when supplemented with 2,2′-dipyridyl (Fig. 6D). Together, these results demonstrate that Fur is required for prodiginine production in Pseudoalteromonas sp. R3.
FIG 6.
Fur is required for the biosynthesis of prodiginine. (A) Growth of the WT, Δfur mutant, and ΔfurC strains on solid plates. ΔfurC represents the complemented version of Δfur. (B) Relative expression of the pigA gene in indicated strains. The transcription level of the WT was defined as 1.0. Experiments were performed in biological triplicates, and error bars indicate the standard deviations. Asterisks indicate statistically significant differences compared to the WT. (C) Prodiginine production of the Δfur strain in the presence of FeSO4 and FeCl3. (D) Culture colors of the Δfur strain. Cultures (OD600 of ∼0.6) of the Δfur strain grown in Zobell 2216E were pelleted for photography without or with 0.2 mM iron chelator 2,2′-dipyridyl. Asterisks indicate statistically significant differences compared to the WT: **, P < 0.01; ***, P < 0.001.
DISCUSSION
Prodiginines are a family of bacterial secondary metabolites that are influenced by both environmental and physiological factors. The regulation of prodiginine biosynthesis has been extensively studied in Serratia and Streptomyces species (1). In contrast, little is known about the regulatory mechanism in the marine betaproteobacterium Pseudoalteromonas (2). In this study, we showed that SspA, an RNA polymerase (RNAP)-associated regulatory protein, is required for the biosynthesis of prodiginine in Pseudoalteromonas sp. R3.
In Pseudoalteromonas sp. R3, SspA positively regulates the transcription of the prodiginine biosynthetic gene (pig) cluster. However, the regulation by SspA is indirect. On the basis of the findings of this study, a model of SspA-regulated prodiginine biosynthesis was proposed (Fig. 7). SspA positively regulates the expression of the siderophore biosynthetic gene (pvd) cluster. Loss of SspA results in the inhibition of siderophore production, which prevents siderophore-dependent iron uptake, thereby leading to iron deficiency and then repressing the transcription of the pig cluster. In addition to iron, Fur is also essential for pig transcription and prodiginine biosynthesis. Overall, the effect of SspA on prodiginine biosynthesis by SspA is mediated by the siderophore-dependent iron uptake pathway.
FIG 7.
Model for SspA-regulated prodiginine biosynthesis in Pseudoalteromonas sp. R3. The regulation of prodiginine biosynthesis by SspA is indirect and mediated by the siderophore-dependent iron uptake pathway. SspA positively regulates the transcription of the siderophore biosynthetic gene (pvd) cluster through an unknown mechanism. In the absence of SspA, the production of siderophore is inhibited, resulting in iron deficiency and making the strain incapable of prodiginine biosynthesis. The iron homeostasis master regulator Fur is also essential for pig transcription and prodiginine biosynthesis. It is likely that Fur senses and interacts with Fe2+ to form Fur-Fe2+ complexes, which bind to the promoter region of pigA, thus activating the transcription of the pig cluster. For clarity, the production, secretion, and uptake of siderophores are simplified in the model.
SspA plays an important role in stress response during the stationary phase and under nutrient starvation conditions (22). As an RNA polymerase-associated protein, SspA regulates gene expression through modulating the transcription activity of RNAP holoenzyme (24). It is notable that the regulon of SspA displays a striking overlap with that of the global regulator H-NS and RpoS (22). In E. coli, SspA positively regulates the transcription of genes involved in acid resistance by downregulation of H-NS, which in turn negatively regulates RpoS (22, 43, 44). Similarly, the regulation of virulence gene expression by SspA in enterohemorrhagic E. coli is also mediated by the cellular level of H-NS (45). Interestingly, our recent studies demonstrated that H-NS is involved in SspA-regulated quorum sensing but not biofilm formation in Pseudoalteromonas (35, 46). In this study, we showed that the regulation of prodiginine production by SspA in Pseudoalteromonas sp. R3 is independent of H-NS and RpoS. Therefore, the role of SspA in certain bacterial physiological processes—at least prodiginine biosynthesis—is distinct from those of H-NS and RpoS, although the three proteins are all global regulators involved in stress response. In addition to RpoS, several studies demonstrated that the effect of SspA on RNAP activities is mediated by other sigma factors, such as σ70 and σE (35, 47, 48). This conclusion is supported by the structural information that SspA specifically interacts with the σ70-RNAP holoenzyme (24). Under stress conditions, SspA inhibits σ70-dependent gene expression via suppressing promoter escape. The involvement of these sigma factors in SspA-regulated prodiginine biosynthesis needs to be further investigated.
In Pseudoalteromonas sp. R3, the inhibition of siderophore-dependent iron uptake is accountable for the defective prodiginine biosynthesis of the ΔsspA strain. The cross-feeding assays showed that Pseudoalteromonas sp. T1lg65 and S. oneidensis MR-1 but not E. coli DH5α were able to restore prodiginine biosynthesis of the ΔsspA strain. S. oneidensis MR-1 produces three siderophores, putrebactin, avaroferrin, and bisucaberin (49, 50), while E. coli DH5α produces enterobactin and aerobactin (51). Although the siderophores produced by Pseudoalteromonas sp. R3 have not been identified yet, it is reasonable that they are similar in structure to those accepted from other sources. As such, Pseudoalteromonas strains can recognize siderophores produced by other bacteria for prodiginine biosynthesis. Interestingly, being blocked in siderophore production (ΔpvdA) significantly impairs prodiginine biosynthesis, but the mutant still synthesizes prodiginine (Fig. 4B). It is likely that alternative iron uptake systems, such as the Feo system, are activated in the absence of siderophores. A similar phenomenon has been observed in E. coli (52).
SspA positively regulates the siderophore-dependent iron uptake system, suggesting that SspA is involved in the regulation of iron homeostasis. Consistently, previous reports revealed that iron limitation triggers the ppGpp-dependent stringent response, which in turn increases the expression of several iron uptake systems, including siderophores (52, 53). Our results demonstrated that iron homeostasis affects prodiginine biosynthesis. The essential role of iron in prodigiosin biosynthesis was revealed several decades ago, but the underlying reason remains unknown (54, 55). In this study, we present evidence that Fur, the master regulator of iron homeostasis, plays a critical role in prodiginine biosynthesis. Like many other bacteria, Pseudoalteromonas Fur senses and interacts with Fe2+, resulting in the formation of Fur-Fe2+ complexes (Fig. 7). It can be speculated that the complexes bind to the promoter region of pigA, thus activating the transcription of the pig cluster. Efforts to confirm this hypothesis are under way.
Except for the red-pigmented prodiginine, Pseudoalteromonas sp. R3 can also produce an unknown yellow-pigmented compound since the ΔpigKLM strain displays yellow colonies on solid medium. The ΔsspA strain forms white colonies, indicating that the biosynthesis of both compounds is under the control of SspA. The Δfur strain also forms yellow colonies, which can be changed to white by the addition of an iron chelator (Fig. 6). Thus, Fur is required for the biosynthesis of prodiginine but not the yellow-pigmented compound, while iron is essential for the production of both compounds. At present, the yellow-pigmented compound in Pseudoalteromonas sp. R3 has not yet been characterized. Two types of yellow pigments, tambjamine and alterochromides, have been reported to be produced by multiple species of Pseudoalteromonas bacteria (56–59). Tambjamine shares the MBC moiety and its biosynthetic pathway with prodiginines (2). Our results showed that the transcription of pigA, which is involved in MBC biosynthesis, is remarkably reduced in the Δfur strain. Because the Δfur strain displays yellow colonies, it is impossible that Pseudoalteromonas sp. R3 produces the yellow pigment tambjamine. The alterochromide lipopeptides are synthesized by a gene cluster containing nonribosomal peptide synthetases (60). Intriguingly, the genome of Pseudoalteromonas sp. R3 contains a putative biosynthetic gene cluster for alterochromides. Our transcriptome data showed that several genes in this cluster (ELR70_RS10160∼ELR70_RS10190) were downregulated in the ΔsspA strain. Hence, it is very likely that the yellow pigment in Pseudoalteromonas R3 is a member of alterochromides. We are working to identify this yellow pigment.
SspA-regulated prodiginine biosynthesis may be ecologically advantageous to bacterial cells. Under conditions of nutrient starvation, the expression of sspA is induced. SspA positively controls the siderophore-dependent iron uptake pathway, thus facilitating iron uptake and prodiginine production. On the one hand, prodiginines have a wide range of biological activities (2), enabling the producers to outcompete valuable resources with other microorganisms. On the other hand, prodiginines are important for inducing cell death (8, 9), and overload with iron is highly toxic to bacterial cells since it efficiently catalyzes the Fenton reaction (61). It is likely that iron and prodiginines are coregulated to initiate cell death of a portion of the population, which is beneficial for the survivors.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All bacterial strains and plasmids used in this study are shown in Table 3. The Pseudoalteromonas strains were grown in Zobell 2216E medium at 30°C (36). The E. coli and S. oneidensis strains were grown in lysogeny broth (LB) at 37 and 30°C, respectively. Solid media contained 1.5% (wt/vol) agar. When needed, the medium was supplemented with chemicals at the following concentrations: ampicillin (Amp), 100 μg/ml; gentamicin (Gm), 50 μg/ml; kanamycin (Km), 50 μg/ml; and 2,6-diaminopimelic acid (DAP), 0.3 mmol/liter.
TABLE 3.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| WM3064 | Donor strain for conjugation; ΔdapA | W. Metcalf, UIUC |
| DH5α | Host strain for routine cloning | Lab stock |
| Pseudoalteromonas | ||
| R3 | Wild type | 36 |
| ΔsspA mutant | Mutant of strain R3 with disrupted gene sspA | 35 |
| ΔsspB mutant | Mutant of strain R3 with disrupted gene sspB | This study |
| ΔpigKLM mutant | Mutant of strain R3 with disrupted genes pigK, pigL, and pigM | This study |
| ΔpvdA mutant | Mutant of strain R3 with disrupted gene pvdA | This study |
| Δfur mutant | Mutant of strain R3 with disrupted gene fur | This study |
| Δhns mutant | Mutant of strain R3 with disrupted gene hns | This study |
| ΔrpoS mutant | Mutant of strain R3 with disrupted gene rpoS | This study |
| T1lg65 | Yellow-pigmented isolate | 67 |
| S. oneidensis MR-1 | Putrebactin-producing bacterium | 49 |
| Plasmids | ||
| pHGM01 | Apr Gmr Cmr; suicide vector | 62 |
| pBBR1MCS-5 | Gmr; delivery vector for gene complementation | 63 |
| pBBR1MCS-5-fur | Complement vector carrying fur | This study |
| pBBR1MCS-5-sspA | Complement vector carrying sspA | This study |
Construction of in-frame deletion mutants and complementation.
The gene deletion mutants of Pseudoalteromonas sp. R3 were constructed by attB-based mutagenesis as described previously (36, 62). First, two fragments flanking the target gene were amplified by PCR and then were joined by the fusion PCR method. Second, the fused fragments were introduced into the suicide plasmid pHGM01 using Gateway BP clonase II enzyme mix (Invitrogen) and transformed into E. coli WM3064 (DAP auxotroph). Third, the resulting plasmids were transferred into Pseudoalteromonas sp. R3 via conjugation. Integration of the mutagenesis constructs into the chromosome was selected by resistance to gentamicin and verified by PCR. Finally, the correct transconjugant was grown in Zobell 2216E broth overnight and then plated onto the Zobell 2216E plate supplemented with 2% sucrose. Gentamicin-sensitive and sucrose-resistant colonies were screened by PCR for deletion of the targeted genes. The deletion mutations were verified by sequencing.
The broad-host-range plasmid pBBR1MCS-5 was used for genetic complementation of the Pseudoalteromonas mutants (63). A fragment containing the gene of interest and its native promoter was amplified by PCR and then cloned into pBBR1MCS-5. After verification by sequencing, the resulting recombinant plasmids were then transferred into the corresponding mutant via conjugation. The primers used in this study are listed in Table 4.
TABLE 4.
Primers used in this study
| Primer | Primer sequence |
|---|---|
| In-frame deletion | |
| pigKLM-5O | GGGGACAAGTTTGTACAAAAAAGCAGGCTTTGTCCAGCAGCATAGTGTT |
| pigKLM-5I | AATAATGCGCTGCAAGGGCCAGCGTACTGGTTCAACATGA |
| pigKLM-3O | GGGGACCACTTTGTACAAGAAAGCTGGGTCTGCAAACTAAATCCGACGC |
| pigKLM-3I | GGCCCTTGCAGCGCATTATTCGAATGCTTTGACCGAATCC |
| sspB-5O | GGGGACAAGTTTGTACAAAAAAGCAGGCTGAATGATCGAGCAGCCAGTC |
| sspB-5I | GGTCCGGGTTCGCTATCTAT CGAATCGGATGGTGACAGAGC |
| sspB-3O | GGGGACCACTTTGTACAAGAAAGCTGGGTTGTCAAAAGAGCCCCACGTAT |
| sspB-3I | ATAGATAGCGAACCCGGACCTGTCGGTTCAACAGCTCAACTC |
| hns-5O | GGGGACAAGTTTGTACAAAAAAGCAGGCTTTGCTTTAAGCCTGCGGAAT |
| hns-5I | GGTCCGGGTTCGCTATCTATGCATTCGTTGGTGTTAACTTAGAA |
| hns-3O | GGGGACCACTTTGTACAAGAAAGCTGGGTCTGAACCTGAGCGTGTCTGT |
| hns-3I | ATAGATAGCGAACCCGGACCGCGCTTTATTCAAATCACTGTAAC |
| rpoS-5O | GGGGACAAGTTTGTACAAAAAAGCAGGCTGCGTAAGATGGCGTTACTCAG |
| rpoS-5I | GGTCCGGGTTCGCTATCTATTACAACATGAGGGCTTGAGCA |
| rpoS-3O | GGGGACCACTTTGTACAAGAAAGCTGGGTATAAGGTACGATGGCGCTGG |
| rpoS-3I | ATAGATAGCGAACCCGGACCTTGGGCTCCTCGACTGAAAC |
| fur-5O | GGGGACAAGTTTGTACAAAAAAGCAGGCTAGTGGGCCTGGACTTAAACG |
| fur-5I | GTAGATCACCGCTAACGCAGCAGCGACGTAGAAGCGTGT |
| fur-3O | GGGGACCACTTTGTACAAGAAAGCTGGGTGGTACTACGGTGAGGCACAG |
| fur-3I | CTGCGTTAGCGGTGATCTACGTTGTGATCAGTCATTAATC |
| pvdA-5O | GGGGACAAGTTTGTACAAAAAAGCAGGCTACCCCACGACCATATAAGCG |
| pvdA-5I | GTAGATCACCGCTAACGCAGGGCCAAACCCGATCCCAAT |
| pvdA-3O | GGGGACCACTTTGTACAAGAAAGCTGGGTGTCGCGCAGCATATTGAGTT |
| pvdA-3I | CTGCGTTAGCGGTGATCTACGAGCAAACCCATGGCATCAG |
| qRT-PCR | |
| fur-RT-F | TCGGTCTTGCAACGGTTTAC |
| fur-RT-R | TCTTCCTGGCGCGTTTCAAT |
| pvdA-RT-F | GCGTACGTCTGACCAAAAACC |
| pvdA-RT-R | AAATCTCAGCACCACTTTGCC |
| pigA-RT-F | TGTCTTTGTTCCCGAGTCGC |
| pigA-RT-R | TGCCAAATTGCTTACGTTCCT |
| 16S-RT-F | CTGGAACTGAGACACGGTCC |
| 16S-RT-R | GGAGTTAGCCGGTGCTTCTT |
| Complementation | |
| sspA-CF | CCAAGCTTGGAGTAGAAGAGCACGGCGTAAA |
| sspA-CR | CGGAATTCCGACATTCAGCACAATCTGCCC |
| fur-CF | CCGGAATTCTGAGCTATATCAGGCATTGA |
| fur-CR | CGCGGATCCGCGGACTCTTCAAATTGCGT |
RNA extraction, sequencing, and analysis.
The Pseudoalteromonas wild-type (WT) and ΔsspA mutant strains were grown overnight to the stationary phase. Total RNA was extracted using the RNAiso Plus kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The RNA concentration was determined using the NanoDrop ND-1000 spectrophotometer (NanoDrop Instruments, Wilmington, DE, USA). The removal of rRNA, library construction, RNA sequencing, and transcriptome analysis were performed by Zhejiang Tianke High Technology Development Co., Ltd. (Hangzhou, China), as described previously (64). RNA sequencing was performed using the Illumina HiSeq 2500 platform (Illumina, San Diego, CA, USA). The unique reads were mapped to the genome of Pseudoalteromonas sp. R3 (accession no. CP034835.1 and CP034834.1). The count of FPKM (fragments per kilobase per million fragments mapped) of each gene was calculated to compare gene expression levels between the WT and sspA mutant.
Quantitative real-time PCR.
The transcription levels of pigA, pvdA, and fur were determined by qRT-PCR analysis as described previously. The Pseudoalteromonas strains were grown overnight to the stationary phase, and then the total RNA was extracted as mentioned above. The cDNA was synthesized using HiScript IIQ Select RT SuperMix (Vazyme, Nanjing, China). The qRT-PCR analysis was performed using the CFX Connect real-time PCR detection system (Bio-Rad, USA) as described previously (36). The cycle threshold (CT) values for each gene were normalized against the CT values of the 16S rRNA gene. The primers used for qRT-PCR analysis are listed in Table 4.
Cross-feeding bioassays.
Cross-feeding bioassays were used to assess whether prodiginine production was restored in the ΔsspA strain when cocultivated with other strains. All studies were performed on Zobell 2216E plates unless otherwise stated. Each tested strain (including Pseudoalteromonas strains, S. oneidensis MR-1, and Escherichia coli DH5α) was streaked next to the ΔsspA strain, and then the plates were incubated at 30°C for 48 h.
Prodiginine production assay.
Pseudoalteromonas sp. R3 was inoculated in 100 ml of Zobell 2216E broth without or with FeCl3 (final concentration of 0.4 or 0.8 mM) and incubated for 24 h at 30°C. One milliliter of culture was harvested by centrifugation, and then prodiginines were extracted from cell pellets with acidified ethanol as described previously (65). Prodiginines display a maximum absorbance at ∼535 nm in acidic ethanol; thus, the optical density at 535 nm (OD535) was read using a Sunrise microplate reader (Tecan, Mannedorf, Switzerland). Meanwhile, the number of CFU was determined for each culture. The relative prodiginine concentration was expressed as OD535/log CFU.
Siderophore assays.
The production and secretion of siderophore were determined by the overlaid chrome azurol S (O-CAS) assay as described previously (39). Each tested strain was grown in Zobell 2216E broth to the mid-log phase (OD600 of ∼0.6), and then 10 μl of each culture was dropped onto Zobell 2216E plates. After incubation for 48 h at 30°C, the plates were overlaid with 30 ml CAS medium. Siderophore production was observed 2 h later.
Quantification of intracellular and extracellular iron.
Atomic absorption spectroscopy (AAS) was used to measure the intracellular and extracellular levels of iron. The Pseudoalteromonas strains were grown in an iron-rich medium (Zobell 2216E supplemented with 40 mg/liter FeCl3) at 30°C for 24 h. After centrifugation and filtration, the cell-free culture supernatants were used to determine the extracellular levels of iron. The cell pellets were washed with 5 ml deionized water, sonicated, and centrifuged at 12,000 rpm for 5 min. The cell lysates were used to qualify the intracellular levels of iron. Iron concentrations were determined by flame-atomic absorption spectroscopy using a Perkin Elmer Analyst AA-800 (Singapore) as described previously (66).
Other analyses.
Comparative sequence analysis and alignment were performed using BLASTp research in NCBI. Student's t test was used to compare statistical differences between the groups of experiment data. Experimental values are presented as the mean ± standard error of the mean (SEM).
Data availability.
The RNA sequencing data have been deposited in the NCBI database under SRA accession no. SRR13125106.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (31670114) and Zhejiang Provincial Natural Science Foundation of China (LY20C010002).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Williamson NR, Fineran PC, Leeper FJ, Salmond GPC. 2006. The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol 4:887–899. doi: 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]
- 2.Sakai-Kawada FE, Ip CG, Hagiwara KA, Awaya JD. 2019. Biosynthesis and bioactivity of prodiginine analogs in marine bacteria, Pseudoalteromonas: a mini review. Front Microbiol 10:1715. doi: 10.3389/fmicb.2019.01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alihosseini F, Ju KS, Lango J, Hammock BD, Sun G. 2008. Antibacterial colorants: characterization of prodiginines and their applications on textile materials. Biotechnol Prog 24:742–747. doi: 10.1021/bp070481r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papireddy K, Smilkstein M, Kelly JX, Shweta Salem SM, Alhamadsheh M, Haynes SW, Challis GL, Reynolds KA. 2011. Antimalarial activity of natural and synthetic prodiginines. J Med Chem 54:5296–5306. doi: 10.1021/jm200543y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You Z, Zhang S, Liu X, Zhang J, Wang Y, Peng Y, Wu W. 2019. Insights into the anti-infective properties of prodiginines. Appl Microbiol Biotechnol 103:2873–2887. doi: 10.1007/s00253-019-09641-1. [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Tomás R, Viñas M. 2010. New insights on the antitumoral properties of prodiginines. Curr Med Chem 17:2222–2231. doi: 10.2174/092986710791331103. [DOI] [PubMed] [Google Scholar]
- 7.Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP. 2007. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol 2:605–618. doi: 10.2217/17460913.2.6.605. [DOI] [PubMed] [Google Scholar]
- 8.Tenconi E, Traxler MF, Hoebreck C, van Wezel GP, Rigali S. 2018. Production of prodiginines is part of a programmed cell death process in Streptomyces coelicolor. Front Microbiol 9:1742. doi: 10.3389/fmicb.2018.01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenconi E, Traxler M, Tellatin D, van Wezel GP, Rigali S. 2020. Prodiginines postpone the onset of sporulation in Streptomyces coelicolor. Antibiotics 9:847. doi: 10.3390/antibiotics9120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rond T, Stow P, Eigl I, Johnson RE, Chan LJG, Goyal G, Baidoo EEK, Hillson NJ, Petzold CJ, Sarpong R, Keasling JD. 2017. Oxidative cyclization of prodigiosin by an alkylglycerol monooxygenase-like enzyme. Nat Chem Biol 13:1155–1159. doi: 10.1038/nchembio.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yip CH, Yarkoni O, Ajioka J, Wan KL, Nathan S. 2019. Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl Microbiol Biotechnol 103:1667–1680. doi: 10.1007/s00253-018-09611-z. [DOI] [PubMed] [Google Scholar]
- 12.Thomson NR, Crow MA, Mcgowan SJ, Cox A, Salmond GPC. 2000. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol Microbiol 36:539–556. doi: 10.1046/j.1365-2958.2000.01872.x. [DOI] [PubMed] [Google Scholar]
- 13.Guthrie EP, Flaxman CS, White J, Hodgson DA, Bibb MJ, Chater KF. 1998. A response-regulator-like activator of antibiotic synthesis from Streptomyces coelicolor A3(2) with an amino-terminal domain that lacks a phosphorylation pocket. Microbiology 144:727–738. doi: 10.1099/00221287-144-3-727. [DOI] [PubMed] [Google Scholar]
- 14.Fineran PC, Slater H, Everson L, Hughes K, Salmond GPC. 2005. Biosynthesis of tripyrrole and β‐lactam secondary metabolites in Serratia: integration of quorum sensing with multiple new regulatory components in the control of prodigiosin and carbapenem antibiotic production. Mol Microbiol 56:1495–1517. doi: 10.1111/j.1365-2958.2005.04660.x. [DOI] [PubMed] [Google Scholar]
- 15.Horng YT, Chang KC, Liu YN, Lai HC, Soo PC. 2010. The RssB/RssA two-component system regulates biosynthesis of the tripyrrole antibiotic, prodigiosin, in Serratia marcescens. Int J Med Microbiol 300:304–312. doi: 10.1016/j.ijmm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Pan X, Sun C, Tang M, You J, Osire T, Zhao Y, Xu M, Zhang X, Shao M, Yang S, Yang T, Rao Z. 2019. LysR-type transcriptional regulator MetR controls prodigiosin production, methionine biosynthesis, cell motility, H2O2 tolerance, heat tolerance, and exopolysaccharide synthesis in Serratia marcescens. Appl Environ Microbiol 86:e02241-19. doi: 10.1128/AEM.02241-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraburtty R, Bibb M. 1997. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J Bacteriol 179:5854–5861. doi: 10.1128/jb.179.18.5854-5861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takano E, Gramajo HC, Strauch E, Andres N, White J, Bibb MJ. 1992. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2). Mol Microbiol 6:2797–2804. doi: 10.1111/j.1365-2958.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 19.Dalebroux ZD, Swanson MS. 2012. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol 10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- 20.Steinchen W, Zegarra V, Bange G. 2020. (p)ppGpp: magic modulators of bacterial physiology and metabolism. Front Microbiol 11:2072. doi: 10.3389/fmicb.2020.02072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams MD, Ouyang TX, Flickinger MC. 1994. Starvation-induced expression of SspA and SspB: the effects of a null mutation in sspA on Escherichia coli protein synthesis and survival during growth and prolonged starvation. Mol Microbiol 11:1029–1043. doi: 10.1111/j.1365-2958.1994.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 22.Hansen AM, Qiu Y, Yeh N, Blattner FR, Durfee T, Jin DJ. 2005. SspA is required for acid resistance in stationary phase by downregulation of H-NS in Escherichia coli. Mol Microbiol 56:719–734. doi: 10.1111/j.1365-2958.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- 23.Hansen AM, Gu Y, Li M, Andrykovitch M, Waugh DS, Jin DJ, Ji X. 2005. Structural basis for the function of stringent starvation protein A as a transcription factor. J Biol Chem 280:17380–17391. doi: 10.1074/jbc.M501444200. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Shi J, He D, Tong B, Zhang C, Wen A, Zhang Y, Feng Y, Lin W. 2020. Structural basis for transcription inhibition by E. coli SspA. Nucleic Acids Res 48:9931–9942. doi: 10.1093/nar/gkaa672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faron M, Fletcher JR, Rasmussen JA, Long ME, Allen LA, Jones BD. 2013. The Francisella tularensis migR, trmE, and cphA genes contribute to F. tularensis pathogenicity island gene regulation and intracellular growth by modulation of the stress alarmone ppGpp. Infect Immun 81:2800–2811. doi: 10.1128/IAI.00073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuthbert BJ, Ross W, Rohlfing AE, Dove SL, Gourse RL, Brennan RG, Schumacher MA. 2017. Dissection of the molecular circuitry controlling virulence in Francisella tularensis. Genes Dev 31:1549–1560. doi: 10.1101/gad.303701.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melton ED, Swanner ED, Behrens S, Schmidt C, Kappler A. 2014. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat Rev Microbiol 12:797–808. doi: 10.1038/nrmicro3347. [DOI] [PubMed] [Google Scholar]
- 28.Andrews SC, Robinson AK, Rodriguez-Quinones F. 2003. Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 29.Hider RC, Kong X. 2010. Chemistry and biology of siderophores. Nat Prod Rep 27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 30.Troxell B, Hassan HM. 2013. Transcriptional regulation by ferric uptake regulator (Fur) in pathogenic bacteria. Front Cell Infect Microbiol 3:59. doi: 10.3389/fcimb.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fillat MF. 2014. The FUR (ferric uptake regulator) superfamily: diversity and versatility of key transcriptional regulators. Arch Biochem Biophys 546:41–52. doi: 10.1016/j.abb.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Yu C, Genco CA. 2012. Fur-mediated global regulatory circuits in pathogenic Neisseria species. J Bacteriol 194:6372–6381. doi: 10.1128/JB.00262-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo SW, Kim D, Latif H, O'Brien EJ, Szubin R, Palsson BO. 2014. Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nat Commun 5:4910. doi: 10.1038/ncomms5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmström C, Kjelleberg S. 1999. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol Lett 30:285–293. doi: 10.1016/S0168-6496(99)00063-X. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Zhang J, Ding M, Wu S, Li S, Zhang M, Yin J, Qiu M. 2020. SspA positively controls exopolysaccharides production and biofilm formation by up-regulating the algU expression in Pseudoalteromonas sp. R3. Biochem Biophys Res Commun 533:988–994. doi: 10.1016/j.bbrc.2020.09.118. [DOI] [PubMed] [Google Scholar]
- 36.Yu Z, Yu D, Mao Y, Zhang M, Ding M, Zhang J, Wu S, Qiu J, Yin J. 2019. Identification and characterization of a LuxI/R-type quorum sensing system in Pseudoalteromonas. Res Microbiol 170:243–255. doi: 10.1016/j.resmic.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Wang P, Zeng Z, Cai X, Wang G, Wang X. 2016. Complete genome sequence of Pseudoalteromonas rubra SCSIO 6842, harboring a putative conjugative plasmid pMBL6842. J Biotechnol 224:66–67. doi: 10.1016/j.jbiotec.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Visca P, Ciervo A, Orsi N. 1994. Cloning and nucleotide sequence of the pvdA gene encoding the pyoverdin biosynthetic enzyme l-ornithine N5-oxygenase in Pseudomonas aeruginosa. J Bacteriol 176:1128–1140. doi: 10.1128/jb.176.4.1128-1140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez-Miranda S, Cabirol N, George-Téllez R, Zamudio-Rivera LS, Fernández FJ. 2007. O-CAS, a fast and universal method for siderophore detection. J Microbiol Methods 70:127–131. doi: 10.1016/j.mimet.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Kümmerli R, Brown SP. 2010. Molecular and regulatory properties of a public good shape the evolution of cooperation. Proc Natl Acad Sci U S A 107:18921–18926. doi: 10.1073/pnas.1011154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kümmerli R, Santorelli LA, Granato ET, Dumas Z, Dobay A, Griffin AS, West SA. 2015. Co-evolutionary dynamics between public good producers and cheats in the bacterium Pseudomonas aeruginosa. J Evol Biol 28:2264–2274. doi: 10.1111/jeb.12751. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien S, Luján AM, Paterson S, Cant MA, Buckling A. 2017. Adaptation to public goods cheats in Pseudomonas aeruginosa. Proc R Soc B 284:20171089. doi: 10.1098/rspb.2017.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Gottesman S. 2006. Modes of regulation of RpoS by H-NS. J Bacteriol 188:7022–7025. doi: 10.1128/JB.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen AM, Jin DJ. 2012. SspA up-regulates gene expression of the LEE pathogenicity island by decreasing H-NS levels in enterohemorrhagic Escherichia coli. BMC Microbiol 12:231. doi: 10.1186/1471-2180-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Li S, Zhao Y, Wang Y, Zhang W, Wu S, Zhang J, Hu Z, Ding M, Meng Q, Yin J, Yu Z. 2021. Stringent starvation protein A and LuxI/LuxR-type quorum sensing system constitute a mutual positive regulation loop in Pseudoalteromonas. Biochem Biophys Res Commun 534:885–890. doi: 10.1016/j.bbrc.2020.10.082. [DOI] [PubMed] [Google Scholar]
- 47.Hansen AM, Lehnherr H, Wang X, Mobley V, Jin DJ. 2003. Escherichia coli SspA is a transcription activator for bacteriophage P1 late genes. Mol Microbiol 48:1621–1631. doi: 10.1046/j.1365-2958.2003.03533.x. [DOI] [PubMed] [Google Scholar]
- 48.Yin Y, Withers TR, Wang X, Yu HD. 2013. Evidence for sigma factor competition in the regulation of alginate production by Pseudomonas aeruginosa. PLoS One 8:e72329. doi: 10.1371/journal.pone.0072329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, Li S, Wang S, Dong Z, Gao H. 2018. Complex iron uptake by the putrebactin-mediated and Feo systems in Shewanella oneidensis. Appl Environ Microbiol 84:e01752-18. doi: 10.1128/AEM.01752-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Liang H, Liu L, Jiang X, Wu S, Gao H. 2020. Biosynthesis of diverse siderophores in Shewanella oneidensis: a result of promiscuous enzymes. Appl Environ Microbiol 86:e00030-20. doi: 10.1128/AEM.00030-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raymond K, Dertz E, Kim S. 2003. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A 100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinella D, Albrecht C, Cashel M, D'Ari R. 2005. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol Microbiol 56:958–970. doi: 10.1111/j.1365-2958.2005.04601.x. [DOI] [PubMed] [Google Scholar]
- 53.Miethke M, Westers H, Blom EJ, Kuipers OP, Marahiel MA. 2006. Iron starvation triggers the stringent response and induces amino acid biosynthesis for bacillibactin production in Bacillus subtilis. J Bacteriol 188:8655–8657. doi: 10.1128/JB.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silverman MP, Munoz EF. 1973. Effect of iron and salt on prodigiosin synthesis in Serratia marcescens. J Bacteriol 114:999–1006. doi: 10.1128/JB.114.3.999-1006.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen GR, Reichelt JL, Gray PP. 1983. Influence of environmental factors and medium composition on Vibrio gazogenes growth and prodigiosin production. Appl Environ Microbiol 45:1727–1732. doi: 10.1128/AEM.45.6.1727-1732.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franks A, Haywood P, Holmström C, Egan S, Kjelleberg S, Kumar N. 2005. Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata. Molecules 10:1286–1291. doi: 10.3390/10101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burke C, Thomas T, Egan S, Kjelleberg S. 2007. The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Environ Microbiol 9:814–818. doi: 10.1111/j.1462-2920.2006.01177.x. [DOI] [PubMed] [Google Scholar]
- 58.Sobolevskaya MP, Smetanina OF, Speitling M, Shevchenko LS, Dmitrenok PS, Laatsch H, Kuznetsova TA, Ivanova EP, Elyakov GB. 2005. Controlling production of brominated cyclic depsipeptides by Pseudoalteromonas maricaloris KMM 636T. Lett Appl Microbiol 40:243–248. doi: 10.1111/j.1472-765X.2005.01635.x. [DOI] [PubMed] [Google Scholar]
- 59.Speitling M, Smetanina OF, Kuznetsova TA, Laatsch H. 2007. Bromoalterochromides A and A′, unprecedented chromopeptides from a marine Pseudoalteromonas maricaloris strain KMM 636T. J Antibiot (Tokyo) 60:36–42. doi: 10.1038/ja.2007.5. [DOI] [PubMed] [Google Scholar]
- 60.Ross AC, Gulland LE, Dorrestein PC, Moore BS. 2015. Targeted capture and heterologous expression of the Pseudoalteromonas alterochromide gene cluster in Escherichia coli represents a promising natural product exploratory platform. ACS Synth Biol 4:414–420. doi: 10.1021/sb500280q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin M, Jiang Y, Sun L, Yin J, Fu H, Wu G, Gao H. 2013. Unique organizational and functional features of the cytochrome c maturation system in Shewanella oneidensis. PLoS One 8:e75610. doi: 10.1371/journal.pone.0075610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 64.Hua X, Chen Q, Li X, Yu Y. 2014. Global transcriptional response of Acinetobacter baumannii to a subinhibitory concentration of tigecycline. Int J Antimicrob Agents 44:337–344. doi: 10.1016/j.ijantimicag.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 65.Slater H, Crow M, Everson L, Salmond GP. 2003. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol Microbiol 47:303–320. doi: 10.1046/j.1365-2958.2003.03295.x. [DOI] [PubMed] [Google Scholar]
- 66.Hoepken HH, Korten T, Robinson SR, Dringen R. 2004. Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J Neurochem 88:1194–1202. doi: 10.1046/j.1471-4159.2003.02236.x. [DOI] [PubMed] [Google Scholar]
- 67.Yu Z, Ding Y, Yin J, Yu D, Zhang J, Zhang M, Ding M, Zhong W, Qiu J, Li J. 2018. Dissemination of genetic acquisition/loss provides a variety of quorum sensing regulatory properties in Pseudoalteromonas. Int J Mol Sci 19:3636. doi: 10.3390/ijms19113636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data have been deposited in the NCBI database under SRA accession no. SRR13125106.







