Closely related species are typically assumed to demonstrate similar phenotypes driven by underlying conserved genotypes. When monitoring for the effect of antimicrobials on the types of species that may be selected for, this assumption may prove to be incorrect, and identification of additional genetic markers may be necessary.
KEYWORDS: antimicrobials, genomics, horizontal gene transfer, microbiology, phylogenetics
ABSTRACT
Perturbation of natural microbial communities by antimicrobials, such as triclosan, can result in selection for antibiotic tolerance, which is of particular concern when pathogens are present. Members of the genus Pseudomonas are found in many natural microbial communities and frequently demonstrate increased abundance following triclosan exposure. The pathogen and well-studied model organism Pseudomonas aeruginosa exhibits high triclosan tolerance; however, it is unknown if all Pseudomonas species share this trait or if there are susceptible strains. We characterized the triclosan tolerance phenotypes of diverse Pseudomonas isolates obtained from triclosan-exposed built environments and identified both tolerant and sensitive strains. High tolerance is associated with carriage of the enoyl-acyl carrier reductase (ENR) isozyme gene fabV, compared to the lesser protective effects of efflux or presence of ENRs. Given its unique importance, we examined fabV distribution throughout Pseudomonas species using large-scale phylogenomic analyses. We find fabV presence or absence is largely invariant at the species level but demonstrates multiple gain and loss events in its evolutionary history. We further provide evidence of its presence on mobile genetic elements. Our results demonstrate the surprising variability in triclosan tolerance in Pseudomonas and confirm fabV to be a useful indicator for high triclosan tolerance in Pseudomonas. These findings provide a framework for better monitoring of Pseudomonas in triclosan-exposed environments and interpreting effects on species and gene composition.
IMPORTANCE Closely related species are typically assumed to demonstrate similar phenotypes driven by underlying conserved genotypes. When monitoring for the effect of antimicrobials on the types of species that may be selected for, this assumption may prove to be incorrect, and identification of additional genetic markers may be necessary. We isolated several phylogenetically diverse members of Pseudomonas from indoor environments and tested their phenotypic tolerance toward the commonly used antimicrobial triclosan. Although Pseudomonas isolates are broadly regarded to be highly triclosan tolerant, we demonstrate the presence of both triclosan-tolerant and -susceptible strains, separated by a difference in tolerance of nearly 3 orders of magnitude. Bioinformatic and experimental investigation demonstrated that the presence of the gene fabV was associated with high tolerance. We demonstrate that fabV is not evenly distributed in all Pseudomonas species and that its presence could be a useful predictor of high triclosan tolerance suitable for antimicrobial monitoring efforts involving triclosan.
INTRODUCTION
There is concern that anthropogenic antimicrobials are profoundly impacting microbial communities in the form of selecting for undesirable traits like antimicrobial tolerance or favoring the survival of antimicrobial-tolerant pathogens (1–3). Developing the capacity to predict which taxa in a naturally occurring complex community will likely tolerate antimicrobial exposure requires a detailed understanding of antimicrobial phenotypes and genotypes beyond those of select model organisms.
The intensive use of the antimicrobial triclosan [5-chloro-2-(2,4-dichlorphenoxy)-phenol] has led to its widespread dissemination in both built and natural environments (4–8). Members of the cosmopolitan genus Pseudomonas are among those that interact most directly with environmental triclosan, affect human health (i.e., Pseudomonas aeruginosa), and are considered to be highly triclosan tolerant (5, 6, 8–13). Multiple studies have demonstrated genus-level increases in the total or relative abundance of Pseudomonas isolates present in environmental community extracts following exposure to triclosan (5, 7, 14, 15). However, it is unknown if triclosan exposure to a community containing diverse Pseudomonas species would select for all Pseudomonas species, a subset of species, or even variants of a single species, as Pseudomonas species have large and flexible pangenomes (16, 17). This is particularly important as selection could occur for pathogenic variants of P. aeruginosa or strains with unwanted metabolic capabilities (3, 18). There is some evidence that not all Pseudomonas species are highly tolerant, although susceptibility testing methods and taxonomic assignment are not consistent between studies (19, 20). Characterizing triclosan tolerance phenotypes in diverse Pseudomonas species could therefore help determine whether exposure to triclosan will result in selection for specific species, and if so, which.
Triclosan tolerance phenotypes and mechanisms in Pseudomonas have been principally examined in the well-studied model organism P. aeruginosa PAO1 (21–24). Triclosan’s sole known molecular targets are enoyl-acyl carrier reductase (ENR) isozymes, which catalyze the elongation step during fatty acid synthesis (23, 25, 26). P. aeruginosa PAO1 carries two of four known ENR isozymes, FabI and FabV, where FabI is highly sensitive to triclosan, while FabV is refractory and protective (23). P. aeruginosa PAO1 has additional protection via efflux of triclosan through resistance-nodulation-division (RND)-type multidrug efflux pumps (MDEP), including MexAB-OprM and TriABC-OpmH, that contribute various degrees of expression-dependent tolerance (21, 22, 24). Notably, the high MIC (≥128 mg/liter) of triclosan in P. aeruginosa PAO1 is attributed to the effect of FabV, with efflux playing a secondary role (23). Determining whether triclosan tolerance is linked to the presence or absence of these or other known factors in diverse Pseudomonas species could aid in determining whether triclosan-specific or multidrug tolerance genes would be selected for in triclosan-exposed communities.
In this study, we aimed to determine whether diverse Pseudomonas species demonstrated variability in triclosan tolerance, and if they did, what genetic factors drove the observed variation and whether they could be predictive of high tolerance. To this end, we characterized the triclosan tolerance phenotypes of phylogenetically diverse Pseudomonas isolates obtained from two environments commonly exposed to triclosan: indoor dust and hospital sink drains. Using a combined experimental and bioinformatic approach, we examined the phylogenetic context of triclosan tolerance variability and its association with various genetic factors. We establish fabV as a useful marker for inferring high triclosan tolerance in Pseudomonas and demonstrate evidence of its association with multiple mobile genetic elements. Our study broadly highlights the unexpected variation in antimicrobial tolerance in closely related species that differ from a few known type strains and the utility of gene-level identification rather than taxonomic assignment to predict tolerance phenotypes.
RESULTS
The built environment contains phylogenetically diverse Pseudomonas isolates from multiple established clades.
Thirty spatially distinct Pseudomonas isolates were isolated from two separate built environment source types, indoor dust (n = 25) and hospital sink drains (n = 5), assumed to come in contact with triclosan (Table 1; see supplemental text in the supplemental material) (5, 6, 27). Whole-genome sequencing (WGS) was performed on all study isolates to characterize study isolate diversity by phylogenetic analysis and whole-genome average nucleotide identity (ANI) to 160 known type strains. The WGS characteristics of the study isolates are listed in Table 2.
TABLE 1.
Study isolate and reference strain triclosan-related phenotypes and genotypes and phylogenomic characteristics
| Isolate ID | Count of homolog detected |
MIC (mg/liter)a |
% ANIb to closest type strain | Phylogenetic cladec | |||||
|---|---|---|---|---|---|---|---|---|---|
| FabV | FabI | MexB | MexK | TriB | TCS | TCS + PAβN | |||
| Dust | |||||||||
| 4A7 | 1 | 0 | 3 | 1 | 1 | >128 | 1 | 84.17 | P. putida |
| 6C6 | 1 | 0 | 3 | 1 | 1 | 128 | 8 | 84.3 | P. putida |
| 8A1 | 0 | 1 | 2 | 1 | 0 | 0.5 | ≤0.0625 | 83.18 | P. stutzeri |
| 10A6 | 0 | 1 | 3 | 1 | 1 | 0.5 | ≤0.0625 | 96.93 | P. oryzihabitans |
| 20A1 | 1 | 0 | 3 | 2 | 1 | >128 | 1 | 83.44 | P. putida |
| 31A8 | 1 | 0 | 3 | 2 | 1 | >128 | 2 | 99.36 | P. putida |
| 34A1 | 1 | 0 | 3 | 2 | 1 | >128 | 1 | 84.55 | P. putida |
| 39A1 | 0 | 1 | 3 | 1 | 1 | 0.5 | ≤0.0625 | 96.86 | P. oryzihabitans |
| 45C2 | 0 | 1 | 3 | 4 | 2 | 0.5 | ≤0.0625 | 88.11 | P. viridiflava |
| 56A10 | 1 | 0 | 3 | 2 | 1 | >128 | 2 | 99.32 | P. putida |
| 57B2 | 0 | 1 | 2 | 1 | 0 | 4 | ≤0.0625 | 89.53 | P. stutzeri |
| 62A4 | 0 | 1 | 2 | 1 | 0 | 0.5 | NDd | 83.21 | P. stutzeri |
| 66C3 | 0 | 1 | 4 | 1 | 1 | 0.5 | ND | 80.17 | P. putida |
| 69C1 | 0 | 1 | 2 | 1 | 0 | 1 | ≤0.0625 | 97.9 | P. stutzeri |
| 82B1 | 1 | 0 | 3 | 2 | 1 | >128 | 8 | 99.3 | P. putida |
| 88B1 | 1 | 0 | 3 | 2 | 1 | >128 | 2 | 84.55 | P. putida |
| 89C1 | 0 | 1 | 5 | 1 | 1 | 0.125 | ≤0.0625 | 98 | P. oryzihabitans |
| 95A6 | 0 | 1 | 3 | 1 | 0 | 0.125 | ≤0.0625 | 82.99 | P. stutzeri |
| 96A1 | 1 | 0 | 5 | 3 | 1 | >128 | 16 | 90.17 | P. fluorescens |
| 97C1 | 0 | 1 | 3 | 1 | 1 | 1 | ≤0.0625 | 98.37 | P. oryzihabitans |
| 99A1 | 0 | 1 | 2 | 1 | 0 | 1 | ND | 82.43 | P. stutzeri |
| 109A1 | 0 | 1 | 2 | 1 | 0 | 1 | ≤0.0625 | 97.58 | P. stutzeri |
| 114A4 | 1 | 0 | 3 | 3 | 1 | >128 | 16 | 91 | P. fluorescens |
| 115A1 | 0 | 1 | 2 | 1 | 0 | 2 | ≤0.0625 | 83.22 | P. stutzeri |
| 119A3 | 0 | 1 | 4 | 1 | 1 | 0.5 | ND | 80.14 | P. putida |
| Hospital sink | |||||||||
| HS1 | 1 | 0 | 4 | 3 | 1 | >128 | 32 | 90.13 | P. fluorescens |
| HS2 | 1 | 0 | 5 | 3 | 1 | >128 | >128 | 90.07 | P. fluorescens |
| HS3 | 1 | 1 | 4 | 3 | 1 | >128 | >128 | 98.73 | P. aeruginosa |
| HS4 | 1 | 1 | 4 | 2 | 1 | >128 | >128 | 99.35 | P. aeruginosa |
| HS5 | 1 | 0 | 4 | 2 | 1 | >128 | 32 | 99.67 | P. putida |
| Reference strain | |||||||||
| P. aeruginosa PAO1 | 1 | 1 | NAe | NA | NA | >128 | 16 | NA | P. aeruginosa |
| P. putida KT2440 | 1 | 0 | NA | NA | NA | >128 | 2 | NA | P. putida |
Shown are MICs for triclosan (TCS) alone or with 40 μg/ml phenylalanine-arginine-β-naphthylamide (PAβN).
ANI, average nucleotide identity.
Clades designated in Fig. 1.
ND, not determined. Because the inhibitor affected the growth control, the MIC was not calculated.
NA, not applicable.
TABLE 2.
WGS characteristics for all study isolates
| Isolate ID | Total length (bp) | Total no. of contigs | N50 | GC content | No. of ORFs | Coding density per Mb | Completeness (%)a | Contamination (%)a | Fold coverage | NCBI accession no. |
|---|---|---|---|---|---|---|---|---|---|---|
| 4A7 | 4,384,099 | 63 | 200,315 | 0.65 | 3,833 | 874.30 | 99.57 | 0.43 | 176.78 | GCA_013523165.1 |
| 6C6 | 4,766,710 | 114 | 97,492 | 0.64 | 4,252 | 892.02 | 99.25 | 0.48 | 81.15 | GCA_013523125.1 |
| 8A1 | 4,387,320 | 22 | 658,584 | 0.63 | 4,011 | 914.23 | 98.85 | 0.23 | 137.94 | GCA_013523115.1 |
| 10A6 | 5,011,469 | 74 | 143,141 | 0.66 | 4,582 | 914.30 | 98.28 | 0.51 | 106.98 | GCA_013523075.1 |
| 20A1 | 5,957,387 | 67 | 216,552 | 0.61 | 5,427 | 910.97 | 100 | 0.96 | 112.32 | GCA_013523055.1 |
| 31A8 | 4,933,281 | 64 | 140,977 | 0.62 | 4,472 | 906.50 | 100 | 0.14 | 150.84 | GCA_013523065.1 |
| 34A1 | 5,218,387 | 41 | 227,787 | 0.62 | 4,714 | 903.34 | 100 | 0.22 | 139.09 | GCA_013523035.1 |
| 39A1 | 5,149,393 | 73 | 212,731 | 0.66 | 4,756 | 923.60 | 98.28 | 0.59 | 125.36 | GCA_013523015.1 |
| 45C2 | 5,744,003 | 48 | 430,146 | 0.59 | 4,990 | 868.73 | 100 | 0.41 | 102.52 | GCA_013522975.1 |
| 56A10 | 5,310,052 | 73 | 162,085 | 0.61 | 4,863 | 915.81 | 100 | 0.91 | 125.39 | GCA_013522955.1 |
| 57B2 | 4,762,958 | 48 | 303,991 | 0.61 | 4,386 | 920.86 | 100 | 0.53 | 145.27 | GCA_013522965.1 |
| 62A4 | 4,274,196 | 38 | 216,335 | 0.63 | 3,939 | 921.58 | 99.17 | 0.18 | 156.81 | GCA_013522915.1 |
| 66C3 | 5,433,829 | 45 | 322,747 | 0.62 | 4,913 | 904.15 | 98.72 | 1.19 | 123.42 | GCA_013522925.1 |
| 69C1 | 4,420,499 | 19 | 530,907 | 0.64 | 4,134 | 935.19 | 99.89 | 0.08 | 121.17 | GCA_013522875.1 |
| 82B1 | 5,269,094 | 85 | 131,046 | 0.61 | 4,759 | 903.19 | 100 | 1.1 | 103.55 | GCA_013522865.1 |
| 88B1 | 5,221,008 | 57 | 191,778 | 0.62 | 4,737 | 907.30 | 100 | 0.22 | 121.01 | GCA_013522855.1 |
| 89C1 | 5,608,481 | 27 | 532,420 | 0.55 | 5,318 | 948.21 | 99.04 | 1.04 | 168.02 | GCA_013522795.1 |
| 95A6 | 4,993,631 | 108 | 114,700 | 0.62 | 4,672 | 935.59 | 98.53 | 3.42 | 150.17 | GCA_013522825.1 |
| 96A1 | 6,346,948 | 71 | 157,681 | 0.6 | 5,694 | 897.12 | 100 | 0.24 | 80.21 | GCA_013522805.1 |
| 97C1 | 5,024,421 | 37 | 364,598 | 0.66 | 4,597 | 914.93 | 98.6 | 0.61 | 143.27 | GCA_013522775.1 |
| 99A1 | 4,929,078 | 33 | 548,892 | 0.61 | 4,613 | 935.87 | 99.28 | 1.04 | 108.41 | GCA_013522755.1 |
| 109A1 | 5,134,915 | 194 | 194,981 | 0.63 | 4,812 | 937.11 | 99.89 | 2.52 | 113.41 | GCA_013522695.1 |
| 114A4 | 5,725,822 | 84 | 125,350 | 0.61 | 5,210 | 909.91 | 100 | 0.08 | 149.02 | GCA_013522705.1 |
| 115A1 | 4,824,973 | 29 | 381,757 | 0.63 | 4,430 | 918.14 | 98.96 | 0.65 | 124.02 | GCA_013522725.1 |
| 119A3 | 5,260,717 | 42 | 292,402 | 0.62 | 4,749 | 902.73 | 98.72 | 1.19 | 97.81 | GCA_013522655.1 |
| HS1 | 6,514,732 | 85 | 187,275 | 0.6 | 5,907 | 906.71 | 100 | 0.22 | 141.47 | GCA_013522665.1 |
| HS2 | 6,508,831 | 99 | 184,619 | 0.6 | 5,903 | 906.92 | 100 | 0.88 | 83.64 | GCA_013522615.1 |
| HS3 | 6,888,403 | 121 | 160,961 | 0.66 | 6,365 | 924.02 | 99.68 | 0.13 | 128.79 | GCA_013522595.1 |
| HS4 | 6,878,130 | 131 | 129,918 | 0.66 | 6,426 | 934.27 | 99.68 | 0.4 | 55.57 | GCA_013522575.1 |
| HS5 | 6,415,930 | 123 | 170,289 | 0.61 | 5,971 | 930.65 | 100 | 0.25 | 122.29 | GCA_013522605.1 |
CheckM estimate.
A multilocus sequence analysis (MLSA) maximum likelihood phylogeny was constructed from 34 single-copy genes found in all 30 study isolates, 160 Pseudomonas type strains, and a Cellvibrio japonicus outgroup (see Table S1 and supplemental data in the supplemental material). The resulting tree topology agreed well with those generated in prior phylogenetic and phylogenomic studies of Pseudomonas, based on the presence and preservation of the 13 previously described monophyletic clades with well-supported bootstrap values (Fig. 1) (28–30). All study isolates fell into six clades: P. putida, P. stutzeri, P. fluorescens, P. oryzihabitans, P. aeruginosa, and P. syringae. Eleven and eight study isolates were placed in clades P. putida and P. stutzeri, respectively, and were distributed throughout their member clade’s branches. The four study isolates found in the P. fluorescens clade, which we further divided into five subclades, were all placed within subclade 1. The remaining seven study isolates were placed in clades P. oryzihabitans, P. aeruginosa, and P. syringae.
FIG 1.
Maximum likelihood phylogeny of Pseudomonas type strains and new built environment isolates using 34 single-copy genes. The tree includes 160 Pseudomonas type strains and 30 study isolates. Phylogeny is rooted to C. japonicus. Bootstrap support values of <99 are annotated. The right-hand side displays clade assignments based on those from Hesse et al. (28). The P. fluorescens clade has five additional subclades designated by this study.
Thirteen study isolates demonstrated ≥95% ANI, a commonly accepted threshold for species delineation with a type strain (Table 1) (31). These were phylogenetically placed within clades P. putida, P. oryzihabitans, P. stutzeri, and P. aeruginosa. Two study isolates, HS3 and HS4, had ≥95% ANI with P. aeruginosa PAO1. The remaining 17 study isolates shared lower than 91% ANI with any type strain, but were phylogenetically placed within established clades. Only two study isolates, 119A3 and 66C3, were not embedded deeply within a clade. These were placed on the outermost branch of clade P. putida with type strains of P. coleopterorum and P. rhizosphaerae. Taken together, phylogenetic and ANI analyses demonstrate that despite occurrences of novel whole-genome diversity relative to established type strains, placement of all study isolates is consistent with established Pseudomonas phylogenies (28–30).
High- and low-triclosan-tolerance isolates were found among phylogenetically diverse Pseudomonas isolates.
The triclosan tolerance phenotypes of all 30 study isolates, along with reference controls P. aeruginosa PAO1 and P. putida KT2440, were characterized using broth microdilution. A strongly bimodal MIC distribution emerged, with 15 (50%) study isolates demonstrating a median MIC of 0.5 mg/liter and the other 15 a median MIC of 128 mg/liter (Fig. 2A and Table 1). These two triclosan tolerance phenotypes were subsequently termed the low-MIC group and high-MIC group isolates.
FIG 2.
Triclosan tolerance phenotypes of reference Pseudomonas strains, indoor dust, and hospital sink drain study isolates. (A) Triclosan MICs. (B) Triclosan relative growth inhibition curves. (C) Triclosan MIC with added 40 μg/ml PAβN. (D) Triclosan relative growth inhibition with added 40 μg/ml PAβN.
Low-MIC group study isolates had an invariably higher percentage of growth inhibition at all tested concentrations of triclosan compared to high-MIC group study isolates, highlighting their increased susceptibility. At 0.25 mg/liter, the lowest common tested triclosan concentration, the average percentage of growth inhibition of low-MIC isolates was 80.25% (minimum, 51.6%; maximum, 99.4%) compared to 2.33% inhibition (minimum, 0%; maximum, 7.65%) of high-MIC isolates (Fig. 2C). At 4 mg/liter, all low-MIC group study isolates had ≥95% growth inhibition, compared to high-MIC study isolates, with an average of 8.51% (minimum, 0%; maximum, 29.6%). While high-MIC group isolates generally began to demonstrate increases in percentage of growth inhibition starting at 4 to 8 mg/liter triclosan; only one study isolate, 6C6, ever reached ≥95% growth inhibition in the tested range. While there were minor within-group differences in MICs and percentages of growth inhibition, the dramatically larger differences between groups suggested a significant mechanistic underpinning warranting further examination.
The presence of fabV associates with high triclosan tolerance in phylogenetically diverse Pseudomonas isolates.
To identify genetic factors driving group-level differences in triclosan tolerance, all 30 isolates were searched for the presence of eight experimentally verified triclosan tolerance determinants, including ENRs and efflux pumps, similar to previous studies (see Tables S2 and S3 in the supplemental material) (32). A phylogeny-aware pangenome-wide association test demonstrated that carriage of a fabV homolog was 100% specific and sensitive to the 15 isolates demonstrating high triclosan tolerance (Fisher’s exact test, Bonferroni corrected P = 6.45 × 10−8) (Fig. 2A and C; see Table S4 in the supplemental material). Carriage of a P. aeruginosa triB homolog component belonging to the two-part membrane fusion TriABC-OpmH RND efflux system, known to have high triclosan affinity, was also found in all 15 high-MIC group isolates, as well as in 8 low-MIC group isolates (24). While not 100% specific, it nevertheless demonstrated statistical significance (Fisher’s exact test, Bonferroni-corrected P = 0.01) (24). All identified FabV enzymes had ≥58% amino acid identity and ≥98% coverage with P. aeruginosa PAO1 FabV and contained the typical YX8K motif. Importantly, no high-MIC isolates carried fabI containing any substitutions known to confer increased triclosan tolerance or additional fab variants (33). These results supported the hypothesis that the presence of fabV was the distinguishing characteristic between high- and low-MIC group study isolates, despite the potential contributions of other factors such as efflux (33).
Isolates with fabV demonstrate higher triclosan tolerance, even with efflux inhibition.
We next tested the impact of efflux inhibition on isolates with and without fabV. Microbroth susceptibility testing was conducted using triclosan and a constant concentration of 40 μg/ml phenylalanine-arginine-β-naphthylamide (PAβN), a widely used efflux pump inhibitor (34, 35). Four fabV-negative study isolates were excluded from MIC calculations because the concentration of PAβN used impacted growth at 0 mg/liter triclosan (Table 1). All study isolates demonstrated reduced triclosan tolerance in the presence of PaβN, as determined by MIC and/or percentage of growth inhibition (Fig. 2C and D). The effect of PAβN was more notable within the low-MIC group, where PAβN-treated study isolates had a median triclosan MIC of 0.0625 mg/liter compared to an untreated median triclosan MIC of 0.5 mg/ml. Comparatively, most, but not all, high-MIC group study isolates also demonstrated reductions in triclosan MIC. PAβN-treated high-MIC group study isolates had a median triclosan MIC of 8 mg/liter, compared to an untreated median triclosan MIC of 128 mg/ml. There is a possibility that PAβN treatment at this concentration resulted in not just efflux inhibition but also membrane permeabilization (36). However, even in the context of this unintended synergy, the protective effect of fabV carriage is still observed.
Efflux pump and fabI expression contribute to triclosan tolerance.
Functional screens using transposon mutagenesis were performed to identify additional or unknown positive or negative regulators of triclosan tolerance not identified by our bioinformatic screen. Transposon mutants from two high-MIC group study isolates, 56A10 and 96A1, belonging to clades P. putida and P. fluorescens, were screened for the absence of growth in 128 mg/liter triclosan. From a total of 10,000 transposon mutants generated, only three mutants (0.03%) were unable to grow at 128 mg/liter. Transposon mutants 56A10-A12, 56A10-H5, and 96A1-B4 were found to have triclosan MICs of 8 to 16 mg/liter, compared to wild-type MICs of ≥128 mg/liter (Table 3). All insertions for the three mutants were within an operon that is most homologous to P. aeruginosa PAO1 RND MDEP MexAB-OprM (see Fig. S1A in the supplemental material) (37). Because both 56A10 and 96A1 carry fabV as their sole ENR, no fabV interruption mutants were expected to be generated, as that would be lethal to the strain (23). Transposon mutant saturation amounted to ∼9% of potential dinucleotide insertions.
TABLE 3.
Transposon mutant genotype and corresponding triclosan phenotype
| Transposon mutant strain ID | Genotype | TCSa MIC (mg/liter) | Fold change |
|---|---|---|---|
| 56A10-H5 | mexA::Himar1 | 8–16 | 8–16b |
| 56A10-H5 | mexB::Himar1 | 8 | 16b |
| 96A1-B4 | oprM::Himar1 | 8 | 16b |
| 57B2-A1 | G7015_RS12405::Himar1 | 8 | 2c |
| 57B2-C3 | G7015_RS08705::Himar1 | 16 | 4c |
TCS, triclosan.
Fold decrease compared to the wild type.
Fold increase compared to the wild type.
Ten thousand transposon mutants were generated from the fabV-negative, low-MIC group study isolate 57B2, which was embedded within clade P. stutzeri, resulting in 12 mutants (0.12%) with two distinct genetic interruptions. The first type, represented by 57B2-A1, had a transposon insertion upstream of an RND MDEP homologous to P. aeruginosa PAO1 MexJK. The second type, represented by 57B2-C3, had an insertion upstream of fabI (Fig. S1B). Triclosan MICs in both mutants ranged from 8 to 16 mg/liter, compared to the 4-mg/liter wild-type MIC. The increased triclosan MICs for both mutants were attributable to insertion of the transposon cassette’s outward-facing Ptac promoter in an orientation that could drive expression of a downstream target (supplemental material text and Fig. S1C). Transposon mutant saturation amounted to ∼18% of potential dinucleotide insertions.
Overall, both positive and negative screens returned known tolerance systems, namely, efflux ablation/overexpression and fabI overexpression (21). Therefore, no new genes or mechanisms that could confound the separation of MIC by fabV status were identified. However, it is possible that increasing transposon mutant saturation could identify additional mechanisms.
fabV demonstrates evidence of multiple independent acquisition events.
The demonstrated importance of FabV in contributing to high triclosan tolerance across different clades led us to ask how fabV was distributed throughout Pseudomonas strains. Homology searches for FabV were performed for all 160 Pseudomonas type strains and in the C. japonicus outgroup and mapped onto the existing phylogeny. Strikingly, fabV carriage was not monophyletic; instead, fabV appeared in multiple clusters and was loosely concordant with clade designation (Fig. 3i). Ancestral state reconstruction for fabV using a maximum likelihood model was performed to quantify the probabilities of its presence or absence. The resulting reconstruction broadly demonstrates <0.5 posterior probability for the presence of fabV at parent nodes leading to multiple fabV-carrying strains, providing support for four ancestral fabV gain events (Fig. 3i to iv) and one loss event in clade P. fluorescens subclade 2 (Fig. 3iv).
FIG 3.
Ancestral state reconstruction of fabV within the phylogeny of 160 Pseudomonas type strains and 30 study isolates. Only pie charts at nodes with log likelihoods for the presence/absence of fabV between 0.1 and 0.9 are displayed. Stars indicate hypothesized fabV gain events. Clade assignments are identical to those in Fig. 1. (i) fabV likelihood for the MLSA phylogeny. (ii) Inset of clades P. aeruginosa, P. oryzihabitans, and P. resinovorans. (iii) Inset of clade P. putida. (iv) Inset of clade P. fluorescens.
Despite the multiple gain and loss events, study isolates did not exhibit unusual fabV carriage given their phylogenetic placement: 13 of 15 study isolates absent for fabV were firmly embedded within clades that were exclusively absent for fabV, such as within clades P. stutzeri and P. oryzihabitans. The singular exception was fabV-negative study isolates 119A3 and 66C3, which grouped within clade P. putida, which contains mostly type strains carrying fabV. Importantly, 119A3 and 66C3 branch with other fabV-negative type strains of clade P. putida, suggesting a within-clade gain event.
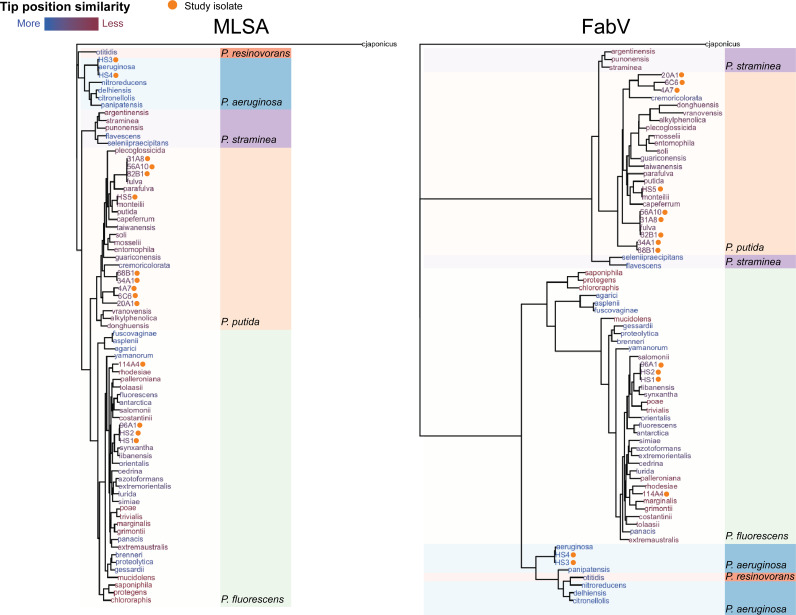
To further assess the potential shared (or dissimilar) origins of fabV, a pruned MLSA phylogeny containing all fabV-positive genomes (15 study isolates and 59 type strains) was compared to a fabV maximum likelihood phylogeny. The resulting FabV phylogeny was qualitatively and quantitatively distinct from that of the MLSA phylogeny (normalized Robinson-Foulds distance = 0.51, Kendall-Colijn distance = 120.5), and similarly supports at least three (and possibly four) distinct origins for the ancestral Pseudomonas fabV acquisitions (Fig. 4). Two gain events are observed at the bifurcation between P. fluorescens clade FabV and P. aeruginosa/P. resinovorans FabV. A distinct third (and potentially fourth) gain event is apparent in a separate branch containing FabV from clades P. straminea and P. putida.
FIG 4.
Species-gene tree comparison for Pseudomonas fabV homologs. The MLSA tree consists of all study isolates and type strains carrying fabV. The FabV tree is built from the FabV sequences identified in all study isolates and type strains. The coloring of species and isolates’ names at tree tips reflects the degree to which they differ in the two trees. Clade assignments are identical to those used in Fig. 1.
Taken together, ancestral state reconstruction and fabV phylogeny provide evidence that fabV carriage in Pseudomonas is characterized by multiple, potentially independent, gain events followed by vertical lineage transmission. Importantly, these results indicate that clade alone is not predictive of fabV.
Large-scale genome analysis shows fabV presence is largely invariant within Pseudomonas species groups.
We examined whether fabV was present in strains in single copies and whether highly similar strains (ANI of ≥95%) were likely to share similar fabV status. We greatly increased the scope of our genomic screening to 7,163 Pseudomonas genomes from NCBI. All genomes were assigned species groups based on a ≥95% ANI threshold match with one of the 160 Pseudomonas type strains (see Data Set S2 in the supplemental material). FabV BLAST searches for each strain showed that its fabV status was almost always identical to that of its representative type strain, with a significant odds ratio (OR) that a strain will carry fabV given its representative type strain is also carrying fabV (OR = 4.5 × 1015; 95% confidence interval [CI], 8.4 × 103 to 4.5 × 1015; P < 2.2 × 10−16) (see Fig. S3 in the supplemental material). In fact, there were only nine total instances (0.12%) in which a strain from a species group differed from the type strain’s fabV status. These differences were in the form of no fabV detected when expected (n = 2), fabV detected in multiple copies (n = 4), and fabV detected when no fabV was expected (n = 3). Examination of the NCBI BioSample metadata indicated that the seven strains with unusual fabV content relative to the type strain were each geographically and temporally distinct. Since their species membership was standardized to the highest type strain ANI, fabV discrepancies are not the results of taxonomic misassignments, but could be either contamination (biological or computational), incomplete or incorrect genome assemblies, or true de novo duplication or loss events that were distinct from the previously identified fabV gain/loss events.
Evidence of fabV on mobile genetic elements and within genomic islands.
We sought to identify whether fabV from the seven strains with unusual fabV copy number demonstrated evidence of chromosomal mobility. FabV sequences from all 4,914 fabV-positive strains were used to construct an expanded FabV phylogeny, which maintained a structure consistent with that that constructed from 59 type strains and 15 study isolates (Fig. 5A). Their overall similarity further supports that fabV has been gained independently in multiple clade-level lineages, followed by vertical dissemination. A conspicuous exception was a singular branch in clade P. fluorescens that contained seven fabV sequences belonging to seven previously identified strains with an unusual fabV copy number given their type strain (Fig. 5A).
FIG 5.
Identification of seven fabV genes with strong evidence of recent horizontal transfer. (A) Maximum likelihood tree of all FabV enzymes identified from 7,163 Pseudomonas genomes. Clade assignments are identical to those used in Fig. 1. Triangles indicate collapsed branches. The inset demonstrates the positioning of outlying fabV genes. Shown is the stylized Mauve output of seven whole-genome alignments with fabV genes of likely HGT origin. Locally colinear blocks between genomes are linked with identically colored vertical lines. Genes related to HGT are annotated or colored in. Each genome is identified by the ANI species group they were assigned, their NCBI strain name, and the RefSeq identifier. (B) The fabV gene region within genomes that carry two copies. The outlying fabV from panel A is visualized. (C) The fabV gene region within genomes whose species group did not typically carry fabV. The gene region is visualized over a 21,000-bp region.
Whole-genome alignments of the seven strains provide evidence of seven mobilizable fabV genes. Four strains, AK6U, 105819, HBP1, and Aramco J, have two copies of fabV. For each, one fabV copy is located within a near-identical 120,000-bp region and predicted to be a type IV integrative and conjugative transfer element (ICE) containing 23 predicted mobility-associated genes, including traI, traG, traU, and traC (Fig. 5B) (38). The other copy of fabV is located elsewhere in the genome, where all strains share similar surrounding gene content, including several metabolic genes, but no mobile elements (Fig. S3).
Three strains, P12, ZBG1, and 48C10, carry only a single copy of fabV. The regions surrounding these fabV genes do not demonstrate similar genetic content between each other (no shared locally colinear blocks beyond that attributed to fabV and an immediately adjacent site-specific recombinase). The genomic region surrounding these fabV genes was predicted to be a genomic island, which explains the lack of genetic synteny despite close phylogenetic proximity. Interestingly, these strains also share a large homologous region with the second fabV copy in the previous four strains, where the only difference is the absence of fabV, offering evidence of a loss of fabV (Fig. S3).
DISCUSSION
Here, we present evidence that phylogenetically distinct Pseudomonas species can exhibit a difference in triclosan tolerance of almost 3 orders of magnitude that is likely driven by carriage of fabV. While there are multiple additional mechanisms that can independently or synergistically increase triclosan tolerance, we confirm fabV to be a useful phylogeny-independent marker of high triclosan tolerance in Pseudomonas.
Both ENR and efflux pump homologs known to confer triclosan tolerance were detected in our 30 study isolates. While searches for all known ENRs FabI, FabV, FabK, and FabL were performed, only fabI and fabV genes were detected, and they are likely the predominant ENR genes of Pseudomonas. This was not unexpected as functional homologs of FabK and FabL in Pseudomonas have not been identified (23). Comparatively, a large diversity of RND-type efflux pump homologs of mexB and mexD were observed in all study isolates. Interestingly, the presence of the TriABC-OpmH membrane fusion gene, triB, whose expression is triclosan specific, was significantly associated with high triclosan tolerance, but the gene was also present in sensitive strains (24). Despite the multitude of potential triclosan tolerance factors, only the singular presence of fabV was associated with a difference in tolerance of 3 orders of magnitude between high- and low-MIC groups.
Differences in efflux capacity resulted in notable decreases in triclosan tolerance in both fabV- negative and -positive isolates. Percentage of growth inhibition was variable for fabV-negative study isolates, potentially due to differences in either efflux pump diversity or expression, which were further confirmed by the mexJK overexpression transposon mutant 57B2-C3. However, differences in fabI expression could also play a role, as shown by overexpression transposon mutant 57B2-A1. Treatment of fabV-positive study isolates with PAβN caused a uniform reduction in percentage of growth inhibition. Variations in reductions could be due to differences in efflux capability. The impact of efflux in fabV-positive isolates was further demonstrated in mexAB-oprM transposon mutant interruption strains 56A10-A12, 56A10-H5, and 96A1-B4, which resulted in an 8- to 16-fold decrease in triclosan tolerance. However, the strong explanatory effect of fabV suggests that it provides the majority of triclosan tolerance in Pseudomonas, with efflux contributing a lesser effect, similar to P. aeruginosa PAO1.
Our finding that tolerance in Pseudomonas can differ by several orders of magnitude carries specific suggestions for monitoring of Pseudomonas in natural and laboratory microbial communities exposed to triclosan. Principally, because increases in genus-level abundances may not correspond to all Pseudomonas species present in the community, using methods that offer species-level resolution, such as WGS or multilocus sequence typing, can offer better health- and study-relevant insights. For example, when monitoring a triclosan-exposed environment containing Pseudomonas, including P. aeruginosa, selection for fabV would carry implications for human health. In a different scenario, selection of high tolerance to the degree conferred by FabV and the detection of a Pseudomonas species that does not typically carry fabV could be particularly meaningful. We show that while an ANI of ≥95% to a type strain is strongly predictive of fabV status, there are instances where fabV was sometimes present but other times absent. fabV-negative species exhibiting high-level tolerance could indicate selection for one or a combination of factors, including increased efflux pump or fabI expression, acquisition of a tolerant ENR isozyme, a triclosan-metabolizing enzyme, or an unknown genetic factor. Of particular importance would be increased efflux capacity through increased expression or assortment of RND-type efflux pumps, as they can accommodate clinically relevant antibiotic substrates, inadvertently selecting for increased antibiotic tolerance (21). Alternatively, the presence of fabV in Pseudomonas species not known to typically carry fabV would suggest evidence of horizontal gene transfer (HGT), the mobility of which we have provided evidence for on a type IV ICE (39).
We identified both triclosan-tolerant and -sensitive Pseudomonas species in the built environment. However, we cannot determine whether tolerance was indeed selected for by triclosan. Given that environmental triclosan levels are typically in the pg/liter-to-ng/liter range, it is unclear whether the highly protective (≥128 mg/liter) effect of fabV is necessary for survival or whether lesser levels of tolerance conferred by efflux and/or other factors are sufficient (4, 40). A metagenomic analysis found fabV copy number to be higher in presumed triclosan-exposed environments compared to unexposed environments, suggesting that fabV-positive species are selected for (41). However, a retrospective study found non-fabV mechanisms may also be undergoing selection, while another study found the presence of horizontally transferred fabI (42, 43). Additionally, it is still unknown how fabV-carrying species react to low levels of triclosan, as the presence of fabV does not necessarily prohibit additional adaptations like increased efflux pump expression. It is likely that the utility of FabV levels of triclosan tolerance is dependent on the environment and the level and rate of triclosan exposure. It is also important to consider that ENRs and efflux pumps have additional metabolic roles that are also under selection (44, 45). Therefore, while further investigation is needed on multiple fronts, our study suggests that determining the fabV status of a Pseudomonas species in a triclosan-exposed microbial community is a useful first step in assessing potential selective effects.
More broadly, our findings highlight that supposedly conserved traits are not as well conserved as expected in even closely related species, highlighting the limitations of extrapolating knowledge from a few type species to whole genera common to amplicon sequencing. To better estimate the effect of antimicrobials on microbial communities, increased phenotypic and genotypic investigation of non-model, non-laboratory strains is necessary. Additionally, methods like WGS and metagenomic sequencing that provide species- and gene-level resolution can help to identify new and unexpected ways in which taxa may overcome antimicrobial challenges.
MATERIALS AND METHODS
Isolate collection, identification, and susceptibility testing.
Dust sample isolates originated from a culture isolate library obtained from a previous study (6). These were screened for Pseudomonas by patch plating on Pseudomonas isolation agar composed of Pseudomonas agar base (Thermo Fisher Scientific), 10 ml/liter glycerol, and CFC selective supplement (Thermo Fisher Scientific) containing 10 mg/liter cetrimide, 10 mg/liter fucidin, and 50 mg/liter cephalosporin (6). Isolates obtained from Rush University Medical Center sink drains were collected by first swabbing the inner rim of the sink drain and diluting in 1× phosphate-buffered saline (PBS). Colonies were picked and purity streaked onto tryptic soy agar (TSA) plates and further validated using Pseudomonas-specific primers (Table 4). Detailed methods for DNA extraction and PCR can be found in the supplemental text. Samples were submitted to Integrated DNA Technologies (IDT) for Sanger sequencing of the 16S universal primers (46, 47) (Table 4). The resulting sequences were then searched against the NCBI nr database using BLASTn. Triclosan MIC with and without 40 μg/ml PAβN was determined using microbroth susceptibility testing (see supplemental text for detailed methods).
TABLE 4.
PCR and qPCR primers used in this study
| Primer ID | Sequence | Purpose | Description | Reference |
|---|---|---|---|---|
| AGM_019 | GACGGGTGAGTAATGCCTA | PCR | 16S rRNA genes for Pseudomonas (PSP) | 73 |
| AGM_020 | CACTGGTGTTCCTTCCTATA | PCR | 16S rRNA genes for Pseudomonas (PSP) | 73 |
| 27F | AGAGTTTGATCMTGGCTCAG | PCR/Sanger sequencing | Universal 16S | 46 |
| 1319R | GACGGGCGGTGTGTRCA | PCR/Sanger sequencing | Universal 16S | 47 |
| AGM_023 | GGGCATACGGGAAGAAGTGA | PCR/Sanger sequencing | Gm3OUT | 74 |
| AGM_024 | GACTGCCCTGCTGCGTAACA | PCR/Sanger sequencing | Gm5OUT | 74 |
| AGM_067 | AGCTGGCGTTCGTTACCTGG | qPCR | 57b2_fabi | This study |
| AGM_068 | CATGGGCGTCTGCTTCTCGT | qPCR | 57b2_fabi | This study |
| AGM_069 | GGTCGTCTTCGTACCCTTGCT | qPCR | 57b2_mexb | This study |
| AGM_070 | TCGAGTAGGGATCGTGGTCGT | qPCR | 57b2_mexb | This study |
| AGM_071 | GTCCCTGCGCTGCAAAACTG | qPCR | 57b2_rpsl | This study |
| AGM_072 | TGGTTAGACGAACGCGGCAA | qPCR | 57b2_rpsl | This study |
Whole-genome sequencing, assembly, and annotation.
All isolate genomic DNA extracts were prepared to a minimum concentration of 2 ng/μl and a minimum mass of 60 ng, for a target coverage of 1.5 Gbp (see supplemental text for details on genomic DNA extraction and quantification). Genomic DNA was shipped to the Broad Institute for additional DNA quantification using a fluorescent dye-based method followed by paired-end short-read (2 × 150 bp) sequencing using a Nextera XT DNA Library kit (Illumina) with an Illumina HiSeq system. The resulting sequencing read BAM files were converted to fastq format with SAMtools v1.6 (48). Sequencing adapters were trimmed, and low-quality reads (quality score of <30 [<Q30]) were removed using fastp 0.20.0 (49). High-quality read pairs were merged with BBMap v38.69 and assembled using SPAdes v3.13.0 (50, 51). Assembly quality statistics were obtained using QUAST v5.0.2, and estimates of assembly completeness and contamination were acquired with CheckM v1.07 (52, 53). Open reading frames (ORFs) were predicted with Prodigal v2.6.3 (54).
Transposon mutant generation.
Conjugation was performed via biparental mating of the recipient isolate and donor E. coli SM10(ƛpir) carrying the Himar1C9 delivery vector pBT20 (55). Isolates and SM10(ƛpir) cells were streaked onto tryptic soy agar (TSA) plates and incubated at 30°C for 20 to 24 h. Three colonies per strain were picked and grown in 5 ml tryptic soy broth (TSB) for 20 to 24 h at 30°C and 170 rpm. For biparental mating, a 1:1 volume of isolate and SM10(ƛpir) cultures were obtained, plated onto TSA, and incubated at 37°C. Subsequent mating spots were picked with cotton tip swabs and resuspended in phosphate-buffered saline (PBS). Fifty microliters was plated onto SM10(ƛpir)-containing auxotrophic minimal medium M63 agar selection plates containing 30 μg/ml gentamicin (Alfa Aesar) and incubated for 24 to 48 h at 37°C. M63 agar contained the following: 1× M63 salts (2 g/liter ammonium sulfate [Millipore Sigma], 2 g/liter, 13.6 g/liter potassium phosphate monobasic [Millipore Sigma], 0.5 mg/liter ferrous ammonium sulfate heptahydrate [Millipore Sigma], pH 7), 15 g/liter bacteriological agar (Millipore Sigma), 20 mM sodium succinate (Alfa Aesar), 20 mM l-glutamic acid (Millipore Sigma), 20 mM magnesium chloride (Millipore Sigma), and 10 mM ferrous ammonium sulfate heptahydrate.
For negative selection, individual transposon mutant colonies were picked onto 96-well plates containing TSB and grown for 20 to 24 h at 30°C and 170 rpm. Next, a sterile replicator pin was used to transfer contents from each well onto a TSA selection plate containing 128 mg/liter triclosan and incubated for 24 to 48 h at 37°C. Transferred contents that did not exhibit growth were traced back to the 96-well plate to confirm growth in the original well. If the original well had growth, contents from the well were spotted onto a TSA plate containing 30 μg/ml gentamicin and grown for 20 to 24 h at 37°C. For positive selection, mating spots resuspended in PBS were plated onto M63 agar plates containing 30 μg/ml gentamicin and either 0.5, 1, 2, 4, 8, or 16 mg/liter triclosan. Insertions were identified using inverse PCR (supplemental text).
Transposon saturation was calculated as number of colonies per strain/number of TA dinucleotides in the genome.
Bioinformatic screen for triclosan tolerance factors.
BLAST v2.7.1+ searches were performed against a database of functionally verified homologs (Table S2) (56). All hits were filtered for ≥40% amino acid identity and an alignment length of ≥80%. FabI and FabV hit sequences were confirmed for the distinguishing presence of their respective YX6K and YX8K catalytic motifs using JalView v2.11 (26, 33, 57). OrthoFinder v2.4.1 was used to cluster hits into orthologous groups and inputted in Scoary v1.16.6 to test for orthogroups associated with ≥128 mg/liter tolerance (58, 59).
MLSA phylogenetic analysis and pairwise ANI calculations.
Assemblies of 160 Pseudomonas type strains and one Cellvibrio japonicus type strain were downloaded from NCBI on 4 January 2020 and had ORFs identified using Prodigal (Data Set S2) (54). All type strains and study isolates underwent homology searching with BLAST for 34 single-copy genes used by Hesse et al. (28) by using ≥40% amino acid identity and ≥80% coverage thresholds and filtering for the highest bit score (Table S3). Nucleotide sequences were aligned individually with MAFFT v7.313 using the L-INS-I option and trimmed with TrimAl v1.4.rev15 using the “automated1” parameter. Individual gene alignments were concatenated, and a maximum likelihood phylogenetic tree was built using RAxML v8.2.12 using the -m GTRGAMMA substitution model and 100 bootstraps (60). The resulting tree was rooted to the C. japonicus outgroup with the ape R package and visualized using the ggtree R package v2.2.1 (61, 62). Pairwise ANI calculations for study isolates and type strains was performed using fastANI v1.3 (31). Clade assignments were based on those from Hesse et al. (28).
Ancestral state reconstruction and FabV phylogeny tree comparison.
Ancestral state reconstruction for FabV was performed using the “ace” function from the ape R package v5.4 using the “ML” method and “ARD” model (63). Visualizations were made using ggtree (62). All FabV amino acid sequences found in all type strains and study isolates were aligned and trimmed with MAFFT and TrimAl as described previously. A maximum likelihood phylogeny was constructed using RAxML with the PROTGAMMAAUTO substitution model and 100 bootstraps (60). The normalized Robinson-Foulds distance was calculated using the RF.dist function, with normalize and rooted options set to TRUE, from the phangorn R package v2.5.5 (64). The Kendall-Colijn distance was calculated using the treeDif function from the treespace R package v1.1.3.2 (65). Topological differences were visualized using the plotTreeDif function from the treespace R package.
Large-scale Pseudomonas genome FabV analysis.
A total of 9,620 assemblies matching the “Pseudomonas” genus tag were downloaded from the NCBI RefSeq database on 27 March 2020. Quality filtering was performed using QUAST and CheckM as described above. Assemblies were filtered for ≤300 contigs, ≥10 kb at N50, ≥90% completeness, ≤10% contamination, and ≥50% overall quality (defined as completeness − 5× contamination), retaining 8,742 assemblies (52, 53, 66). FastANI was used for pairwise ANI calculations between all 160 type strains (31). Type strains with ≥95% pairwise ANI were grouped (designated with the suffix “_group”). Afterward, the ANI between each type strain and the remaining assemblies was calculated. Species group assignments were based on the highest match and ≥95% ANI. This process resulted in 7,163 genomes used in the rest of the analyses (Data Set S2). FabV homology searching, alignment, trimming, and maximum likelihood tree construction were performed as previously described. The resulting tree was visualized using ggtree, with branches collapsed using the “max” parameter (62). The odds ratio for fabV presence versus absence given type strain status was obtained via a one-tailed Fisher’s exact test using the fisher.test function in R.
HGT prediction.
GenBank format assemblies from genomes with outlying fabV composition were downloaded from the NCBI RefSeq database and aligned and visualized with Mauve v2.4.0 using the progressiveMauve option (67, 68). Locally colinear blocks are defined by progressiveMauve. The IslandViewer FTP site was used for genomic island prediction (69). The IceFinder web portal was used for ICE region identification (70, 71).
Data availability.
All analyzed bacterial genomes are publicly available on the NCBI website and downloaded from the RefSeq database (68). Study isolate genome assemblies are available under BioProject accession no. PRJNA606080. Supplemental data and analytical scripts are available at https://github.com/hartmann-lab/fabv_paper under the MIT license (72). All strains are available upon request (erica.hartmann@northwestern.edu).
Supplementary Material
ACKNOWLEDGMENTS
We thank Mary Hayden for assistance in obtaining Rush University Medical Center sink study isolates, Samuel Miller for providing pBT20, Alan Hauser for providing strains SM10(λpir) and PAO1, Rolf Halden for providing strain KT2440, and Alberto Lopez for assistance with setting up transposon mutagenesis.
This work was supported by the Alfred P. Sloan Foundation (G-2017-9807 and G-2016-7201), the Centers for Disease Control and Prevention (75D30118C02915), the Northwestern University Biotechnology Training Program, the Northwestern University Office of Undergraduate Research (758SUMMER1915505, 762ACADYR1915064, and 758SUMMER1915367), and the Searle Leadership Fund. The conclusions, opinions, and other statements in this publication are the authors’ and not necessarily those of the sponsoring institution.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Wright GD. 2010. Antibiotic resistance in the environment: a link to the clinic? Curr Opin Microbiol 13:589–594. doi: 10.1016/j.mib.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano CA, Rybak MJ. 2015. Efficacy of triclosan as an antimicrobial hand soap and its potential impact on antimicrobial resistance: a focused review. Pharmacother J Hum Pharmacol Drug Ther 35:328–336. doi: 10.1002/phar.1553. [DOI] [PubMed] [Google Scholar]
- 3.Lanini S, D'Arezzo S, Puro V, Martini L, Imperi F, Piselli P, Montanaro M, Paoletti S, Visca P, Ippolito G. 2011. Molecular epidemiology of a Pseudomonas aeruginosa hospital outbreak driven by a contaminated disinfectant-soap dispenser. PLoS One 6:e17064. doi: 10.1371/journal.pone.0017064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhillon GS, Kaur S, Pulicharla R, Brar SK, Cledón M, Verma M, Surampalli RY. 2015. Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health 12:5657–5684. doi: 10.3390/ijerph120505657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBain AJ, Bartolo RG, Catrenich CE, Charbonneau D, Ledder RG, Price BB, Gilbert P. 2003. Exposure of sink drain microcosms to triclosan: population dynamics and antimicrobial susceptibility. Appl Environ Microbiol 69:5433–5442. doi: 10.1128/aem.69.9.5433-5442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahimipour AK, Ben Mamaar S, McFarland AG, Blaustein RA, Chen J, Glawe AJ, Kline J, Green JL, Halden RU, Van Den Wymelenberg K, Huttenhower C, Hartmann EM. 2018. Antimicrobial chemicals associate with microbial function and antibiotic resistance indoors. mSystems 3:e00200-18. doi: 10.1128/mSystems.00200-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke A, Azulai D, Dueker ME, Vos M, Perron GG. 2019. Triclosan alters microbial communities in freshwater microcosms. Water 11:961. doi: 10.3390/w11050961. [DOI] [Google Scholar]
- 8.Welsch TT, Gillock ET. 2011. Triclosan-resistant bacteria isolated from feedlot and residential soils. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:436–440. doi: 10.1080/10934529.2011.549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribas F, Perramon J, Terradillos A, Frias J, Lucena F. 2000. The Pseudomonas group as an indicator of potential regrowth in water distribution systems. J Appl Microbiol 88:704–710. doi: 10.1046/j.1365-2672.2000.01021.x. [DOI] [PubMed] [Google Scholar]
- 10.Kittinger C, Lipp M, Baumert R, Folli B, Koraimann G, Toplitsch D, Liebmann A, Grisold AJ, Farnleitner AH, Kirschner A, Zarfel G. 2016. Antibiotic resistance patterns of Pseudomonas spp. isolated from the River Danube. Front Microbiol 7. doi: 10.3389/fmicb.2016.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y, Kajii S, Nishiyama M, Iguchi A. 2013. Susceptibility of Pseudomonas aeruginosa isolates collected from river water in Japan to antipseudomonal agents. Sci Total Environ 450–451:148–154. doi: 10.1016/j.scitotenv.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Remold SK, Purdy-Gibson ME, France MT, Hundley TC. 2015. Pseudomonas putida and Pseudomonas fluorescens species group recovery from human homes varies seasonally and by environment. PLoS One 10:e0127704. doi: 10.1371/journal.pone.0127704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaulke CA, Barton CL, Proffitt S, Tanguay RL, Sharpton TJ. 2016. Triclosan exposure is associated with rapid restructuring of the microbiome in adult zebrafish. PLoS One 11:e0154632. doi: 10.1371/journal.pone.0154632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svenningsen H, Henriksen T, Priemé A, Johnsen AR. 2011. Triclosan affects the microbial community in simulated sewage-drain-field soil and slows down xenobiotic degradation. Environ Pollut 159:1599–1605. doi: 10.1016/j.envpol.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 15.Ma L, Xie Y, Han Z, Giesy JP, Zhang X. 2017. Responses of earthworms and microbial communities in their guts to triclosan. Chemosphere 168:1194–1202. doi: 10.1016/j.chemosphere.2016.10.079. [DOI] [PubMed] [Google Scholar]
- 16.Freschi L, Vincent AT, Jeukens J, Emond-Rheault J-G, Kukavica-Ibrulj I, Dupont M-J, Charette SJ, Boyle B, Levesque RC. 2019. The Pseudomonas aeruginosa pan-genome provides new insights on its population structure, horizontal gene transfer, and pathogenicity. Genome Biol Evol 11:109–120. doi: 10.1093/gbe/evy259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun S-R, Wassenaar TM, Nookaew I, Hauser L, Wanchai V, Land M, Timm CM, Lu T-YS, Schadt CW, Doktycz MJ, Pelletier DA, Ussery DW. 2016. Diversity of Pseudomonas genomes, including Populus-associated isolates, as revealed by comparative genome analysis. Appl Environ Microbiol 82:375–383. doi: 10.1128/AEM.02612-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meade MJ, Waddell RL, Callahan TM. 2001. Soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp. denitrificans inactivate triclosan in liquid and solid substrates. FEMS Microbiol Lett 204:45–48. doi: 10.1111/j.1574-6968.2001.tb10860.x. [DOI] [PubMed] [Google Scholar]
- 19.Aiello AE, Marshall B, Levy SB, Della-Latta P, Larson E. 2004. Relationship between triclosan and susceptibilities of bacteria isolated from hands in the community. Antimicrob Agents Chemother 48:2973–2979. doi: 10.1128/AAC.48.8.2973-2979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tattawasart U, Maillard J-Y, Furr JR, Russell AD. 1999. Comparative responses of Pseudomonas stutzeri and Pseudomonas aeruginosa to antibacterial agents. J Appl Microbiol 87:323–331. doi: 10.1046/j.1365-2672.1999.00811.x. [DOI] [PubMed] [Google Scholar]
- 21.Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkhoff-Schweizer RR, Schweizer HP. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob Agents Chemother 45:428–432. doi: 10.1128/AAC.45.2.428-432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuanchuen R, Karkhoff-Schweizer RR, Schweizer HP. 2003. High-level triclosan resistance in Pseudomonas aeruginosa is solely a result of efflux. Am J Infect Control 31:124–127. doi: 10.1067/mic.2003.11. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Lin J, Ma J, Cronan JE, Wang H. 2010. Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan-resistant enoyl-acyl carrier protein reductase. Antimicrob Agents Chemother 54:689–698. doi: 10.1128/AAC.01152-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mima T, Joshi S, Gomez-Escalada M, Schweizer HP. 2007. Identification and characterization of TriABC-OpmH, a triclosan efflux pump of Pseudomonas aeruginosa requiring two membrane fusion proteins. J Bacteriol 189:7600–7609. doi: 10.1128/JB.00850-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem 274:11110–11114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 26.Massengo-Tiassé RP, Cronan JE. 2008. Vibrio cholerae FabV defines a new class of enoyl-acyl carrier protein reductase. J Biol Chem 283:1308–1316. doi: 10.1074/jbc.M708171200. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Hartmann EM, Kline J, Van Den Wymelenberg K, Halden RU. 2018. Assessment of human exposure to triclocarban, triclosan and five parabens in U.S. indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry. J Hazard Mater 360:623–630. doi: 10.1016/j.jhazmat.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Hesse C, Schulz F, Bull CT, Shaffer BT, Yan Q, Shapiro N, Hassan KA, Varghese N, Elbourne LDH, Paulsen IT, Kyrpides N, Woyke T, Loper JE. 2018. Genome-based evolutionary history of Pseudomonas spp. Environ Microbiol 20:2142–2159. doi: 10.1111/1462-2920.14130. [DOI] [PubMed] [Google Scholar]
- 29.Gomila M, Peña A, Mulet M, Lalucat J, García-Valdés E. 2015. Phylogenomics and systematics in Pseudomonas. Front Microbiol 6. doi: 10.3389/fmicb.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomila M, Busquets A, Mulet M, García-Valdés E, Lalucat J. 2017. Clarification of taxonomic status within the Pseudomonas syringae species group based on a phylogenomic analysis. Front Microbiol 8:2422. doi: 10.3389/fmicb.2017.02422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. 2018. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan R, Roy N, Choi K, Lee S-W. 2018. Distribution of triclosan-resistant genes in major pathogenic microorganisms revealed by metagenome and genome-wide analysis. PLoS One 13:e0192277. doi: 10.1371/journal.pone.0192277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao J, Rock CO. 2016. Resistance mechanisms and the future of bacterial enoyl-acyl carrier protein reductase (FabI) antibiotics. Cold Spring Harb Perspect Med 6:a027045. doi: 10.1101/cshperspect.a027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrer-Espada R, Shahrour H, Pitts B, Stewart PS, Sánchez-Gómez S, Martínez-de-Tejada G. 2019. A permeability-increasing drug synergizes with bacterial efflux pump inhibitors and restores susceptibility to antibiotics in multi-drug resistant Pseudomonas aeruginosa strains. Sci Rep 9:3452. doi: 10.1038/s41598-019-39659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonnet P, Izard D, Mullié C. 2012. Prevalence of efflux-mediated ciprofloxacin and levofloxacin resistance in recent clinical isolates of Pseudomonas aeruginosa and its reversal by the efflux pump inhibitors 1–(1-naphthylmethyl)-piperazine and phenylalanine-arginine-β-naphthylamide. Int J Antimicrob Agents 39:77–80. doi: 10.1016/j.ijantimicag.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Lamers RP, Cavallari JF, Burrows LL. 2013. The efflux inhibitor phenylalanine-arginine beta-naphthylamide (PAβN) permeabilizes the outer membrane of Gram-negative bacteria. PLoS One 8:e60666. doi: 10.1371/journal.pone.0060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJC, Yoo HS, Zhang C, Zhang Y, Sobral BW. 2014. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res 42:D581–D591. doi: 10.1093/nar/gkt1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christie PJ, Whitaker N, González-Rivera C. 2014. Mechanism and structure of the bacterial type IV secretion systems. Biochim Biophys Acta 1843:1578–1591. doi: 10.1016/j.bbamcr.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan R, Lee MH, Joo H-J, Jung Y-H, Ahmad S, Choi J-H, Lee S-W. 2015. Triclosan resistance in a bacterial fish pathogen, Aeromonas salmonicida subsp. salmonicida, is mediated by an enoyl reductase, FabV. J Microbiol Biotechnol 25:511–520. doi: 10.4014/jmb.1407.07021. [DOI] [PubMed] [Google Scholar]
- 40.Li M, He Y, Sun J, Li J, Bai J, Zhang C. 2019. Chronic exposure to an environmentally relevant triclosan concentration induces persistent triclosan resistance but reversible antibiotic tolerance in Escherichia coli. Environ Sci Technol 53:3277–3286. doi: 10.1021/acs.est.8b06763. [DOI] [PubMed] [Google Scholar]
- 41.Khan R, Kong HG, Jung Y-H, Choi J, Baek K-Y, Hwang EC, Lee S-W. 2016. Triclosan resistome from metagenome reveals diverse enoyl acyl carrier protein reductases and selective enrichment of triclosan resistance genes. Sci Rep 6:32322. doi: 10.1038/srep32322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciusa ML, Furi L, Knight D, Decorosi F, Fondi M, Raggi C, Coelho JR, Aragones L, Moce L, Visa P, Freitas AT, Baldassarri L, Fani R, Viti C, Orefici G, Martinez JL, Morrissey I, Oggioni MR, BIOHYPO Consortium. 2012. A novel resistance mechanism to triclosan that suggests horizontal gene transfer and demonstrates a potential selective pressure for reduced biocide susceptibility in clinical strains of Staphylococcus aureus. Int J Antimicrob Agents 40:210–220. doi: 10.1016/j.ijantimicag.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Skovgaard S, Nielsen LN, Larsen MH, Skov RL, Ingmer H, Westh H. 2013. Staphylococcus epidermidis isolated in 1965 are more susceptible to triclosan than current isolates. PLoS One 8:e62197. doi: 10.1371/journal.pone.0062197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heath RJ, Su N, Murphy CK, Rock CO. 2000. The enoyl-[acyl-carrier-protein] reductases FabI and FabL from Bacillus subtilis. J Biol Chem 275:40128–40133. doi: 10.1074/jbc.M005611200. [DOI] [PubMed] [Google Scholar]
- 45.Li X-Z, Plésiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ludwig W. 2007. Nucleic acid techniques in bacterial systematics and identification. Int J Food Microbiol 120:225–236. doi: 10.1016/j.ijfoodmicro.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Turner S, Pryer KM, Miao VPW, Palmer JD. 1999. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46:327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bushnell B. 2014. BBMap: a fast, accurate, splice-aware aligner. Report no. LBNL-7065E. Lawrence Berkeley National Lab, Berkeley, CA. https://www.osti.gov/biblio/1241166-bbmap-fast-accurate-splice-aware-aligner. Accessed 5 February 2020. [Google Scholar]
- 51.Nurk S, Bankevich A, Antipov D, Gurevich A, Korobeynikov A, Lapidus A, Prijibelsky A, Pyshkin A, Sirotkin A, Sirotkin Y, Stepanauskas R, McLean J, Lasken R, Clingenpeel SR, Woyke T, Tesler G, Alekseyev MA, Pevzner PA. 2013. Assembling genomes and mini-metagenomes from highly chimeric reads, p 158–170. In Deng M, Jiang R, Sun F, Zhang X (ed), Research in computational molecular biology. Lecture notes in computer science. Springer, Berlin, Germany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gurevich A, Saveliev V, Vyahhi N, Tesler G. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kulasekara HD, Ventre I, Kulasekara BR, Lazdunski A, Filloux A, Lory S. 2005. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol Microbiol 55:368–380. doi: 10.1111/j.1365-2958.2004.04402.x. [DOI] [PubMed] [Google Scholar]
- 56.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol 20:238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brynildsrud O, Bohlin J, Scheffer L, Eldholm V. 2016. Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol 17:238. doi: 10.1186/s13059-016-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 62.Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. 2017. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 63.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 64.Schliep KP. 2011. phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jombart T, Kendall M, Almagro‐Garcia J, Colijn C. 2017. treespace: statistical exploration of landscapes of phylogenetic trees. Mol Ecol Resour 17:1385–1392. doi: 10.1111/1755-0998.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parks DH, Rinke C, Chuvochina M, Chaumeil P-A, Woodcroft BJ, Evans PN, Hugenholtz P, Tyson GW. 2017. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol 2:1533–1542. doi: 10.1038/s41564-017-0012-7. [DOI] [PubMed] [Google Scholar]
- 67.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.NCBI. 2020. RefSeq: NCBI Reference Sequence Database. https://www.ncbi.nlm.nih.gov/refseq/. Accessed 17 July 2020.
- 69.Bertelli C, Laird MR, Williams KP, Lau BY, Hoad G, Winsor GL, Brinkman FSL, Simon Fraser University Research Computing Group. 2017. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res 45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu M, Li X, Xie Y, Bi D, Sun J, Li J, Tai C, Deng Z, Ou H-Y. 2019. ICEberg 2.0: an updated database of bacterial integrative and conjugative elements. Nucleic Acids Res 47:D660–D665. doi: 10.1093/nar/gky1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Microbial Bioinformatics Group. 2020. ICEfinder. https://db-mml.sjtu.edu.cn/ICEfinder/ICEfinder.html. Accessed 17 July 2020.
- 72.McFarland AG, Bertucci HK, Littman XE, Shen J, Huttenhower C, Hartmann EM. 2020. hartmann-lab/fabv_paper. Triclosan tolerance is driven by a conserved mechanism in diverse Pseudomonas species. GitHub https://github.com/hartmann-lab/fabv_paper. Accessed 22 July 2020. [DOI] [PMC free article] [PubMed]
- 73.Spilker T, Coenye T, Vandamme P, LiPuma JJ. 2004. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol 42:2074–2079. doi: 10.1128/jcm.42.5.2074-2079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Withers TR, Yin Y, Yu HD. 2014. Identification of novel genes associated with alginate production in Pseudomonas aeruginosa using mini-himar1 mariner transposon-mediated mutagenesis. J Vis Exp 85:e51346. doi: 10.3791/51346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analyzed bacterial genomes are publicly available on the NCBI website and downloaded from the RefSeq database (68). Study isolate genome assemblies are available under BioProject accession no. PRJNA606080. Supplemental data and analytical scripts are available at https://github.com/hartmann-lab/fabv_paper under the MIT license (72). All strains are available upon request (erica.hartmann@northwestern.edu).