Bacteria living within fungal hyphae present an example of one of the most intimate relationships between fungi and bacteria. Even though there are several well-described examples of such partnerships, their prevalence within the fungal kingdom remains unknown.
KEYWORDS: BRE, Mortierella, Umbelopsis, bacterial-fungal interactions, endosymbionts
ABSTRACT
Mucoromycota representatives are known to harbor two types of endohyphal bacteria (EHB)—Burkholderia-related endobacteria (BRE) and Mycoplasma-related endobacteria (MRE). While both BRE and MRE occur in fungi representing all subphyla of Mucoromycota, their distribution is not well studied. Therefore, it is difficult to resolve the evolutionary history of these associations in favor of one of the following two alternative hypotheses explaining their origin: “early invasion” and “late invasion.” Our main goal was to fill this knowledge gap by surveying Mucoromycota fungi for the presence of EHB. We screened 196 fungal strains from 16 genera using a PCR-based approach to detect bacterial 16S rRNA genes, complemented with fluorescence in situ hybridization (FISH) imaging to confirm the presence of bacteria within the hyphae. We detected Burkholderiaceae in ca. 20% of fungal strains. Some of these bacteria clustered phylogenetically with previously described BRE clades, whereas others grouped with free-living Paraburkholderia. Importantly, the latter were detected in Umbelopsidales, which previously were not known to harbor endobacteria. Our results suggest that this group of EHB is recruited from the environment, supporting the late invasion scenario. This pattern complements the early invasion scenario apparent in the BRE clade of EHB.
IMPORTANCE Bacteria living within fungal hyphae present an example of one of the most intimate relationships between fungi and bacteria. Even though there are several well-described examples of such partnerships, their prevalence within the fungal kingdom remains unknown. Our study focused on early divergent terrestrial fungi in the phylum Mucoromycota. We found that ca. 20% of the strains tested harbored bacteria from the family Burkholderiaceae. Not only did we confirm the presence of bacteria from previously described endosymbiont clades, we also identified a new group of endohyphal Burkholderiaceae representing the genus Paraburkholderia. We established that more than half of the screened Umbelopsis strains were positive for bacteria from this new group. We also determined that, while previously described BRE codiverged with their fungal hosts, Paraburkholderia symbionts did not.
INTRODUCTION
Interactions between fungi and bacteria are common and widespread in the environment, as these organisms often occupy similar niches, and together, they are responsible for most decomposition processes in soil (1). However, not only can bacteria live in close proximity with the fungus, sometimes they are also present inside the fungal hyphae. Bacteria occupying this specific niche are referred to as endohyphal bacteria (EHB). EHB were first observed in spores of Endogone by Mosse in 1970 (2), although they were then described as “bacteria-like structures.”
Despite observing these bacteria-like structures in Endogone, the first formally described endobacterium associated with the Mucoromycota representative was “Candidatus Glomeribacter gigasporarum,” which was found to be living inside the hyphae of Gigasporales (3), and its prevalence seems to be limited to this order. “Candidatus Glomeribacter gigasporarum” belongs to Burkholderiaceae family and is considered one of the Burkholderia-related endobacteria (BRE). These bacteria are known to be vertically transmitted and were detected in both mycelium and spores (3). They help the fungus form a relationship with the plant by increasing ATP production and inducing detoxification of reactive oxygen forms (4). Another similar partnership involves Rhizopus microsporus (Mucoromycotina) and at least two species of BRE (Mycetohabitans rhizoxinica and Mycetohabitans endofungorum [Paraburkholderia rhizoxinica {synonym}] and Paraburkholderia endofungorum) (5, 6). In this relationship, bacteria were demonstrated to completely control asexual reproduction as well as partially control sexual reproduction of their host (7). More recently, a partnership between Mortierella elongata (Mortierellomycotina) and Mycoavidus cysteinexigens was discovered and described (8, 9). This partnership has been studied extensively, with both partners having their genomes assembled and annotated and series of physiological experiments carried out. It has been demonstrated that the bacterium relies on the fungal cysteine and that the host growth rate is higher in cured strains (9). There are also reports of endohyphal bacteria in Dikarya (10). In this group, single fungal hosts were shown to harbor few different bacterial endosymbionts from evolutionarily distant lineages (e.g., reference 11), which is not the case of Mucoromycota. Fungi from this phylum usually harbor one or two different lineages of bacteria, and these are quite uniform across the whole phylum, suggesting their evolutionary ancient relationship.
All of the above-mentioned interactions involving Mucoromycota representatives also involve bacteria from the Burkholderiaceae family (Burkholderiales, Betaproteobacteria, Proteobacteria), which are Gram-negative rod-shaped bacteria. Representatives of this clade are omnipresent in the environment, colonizing a wide range of ecological niches, from soil ecosystems to the human body; this family consists of obligately aerobic, facultatively anaerobic chemoorganotrophs as well as chemolithotrophs, both obligate and facultative (12). Recent phylogenomic studies of Burkholderia sensu lato led to the division of this genus into several new genera that seem to reflect the most prevalent lifestyle within the genus as well as phylogenetic grouping (13). The genus Paraburkholderia comprises environmental strains known to be sometimes beneficial to plants and Burkholderia sensu stricto comprises human and animal pathogens, while the newly established genus Mycetohabitans comprises two BRE isolated from Rhizopus microsporus (Mycetohabitans rhizoxinica and Mycetohabitans endofungorum) (14).
Another lineage of bacteria detected in the representatives of Glomeromycotina as well as in the Mortierella genus and Endogonales order is Mycoplasma-related (Mycoplasmataceae, Mollicutes, Tenericutes) endobacteria (MRE). Nauman et al. (15) identified MRE in Glomeromycotina, which was later confirmed by Naito et al. (16). Then, Desirò et al. (17) found MRE in Mortierella strains and concluded, after curing fungi of their endosymbionts, that these bacteria seem to be mild parasites. MRE were also detected in three out of four recently studied genomes of Endogonales (Mucoromycotina) representatives (18). Apart from being endosymbionts, bacteria from the family Mycoplasmataceae can lead a saprotrophic or parasitic lifestyle as well.
Together, these findings indicate that interactions between Mucoromycota fungi and bacteria are common and that they have been neglected for years (or there were no methods of studying them). Pawlowska et al. (19) suggested that it is inevitable to find new Mucoromycota-endosymbiont partnerships, and thus, looking for endosymbiotic bacteria as a part of primary research for each new species is recommended. In the same year, Takashima et al. (20) published a study in which they screened 238 strains of environmental Mortierella isolates originating from Japan using PCR and fluorescence in situ hybridization (FISH). They report that about 20% of the strains harbored BRE, which can be separated into three new subclades (called A, B, and C), but the authors were not able to draw conclusions about the factors driving these interactions. Moreover, they performed a FISH procedure confirming location of BRE inside the hyphae for five isolates. MRE seem to be less common within Mortierellomycotina, as Desirò et al. (17) report that only 12 out of 394 strains (ca. 3%) possess the bacteria in question.
Clearly, studying Mucoromycota-bacterial relationships can also help our understanding of the evolutionary history of interactions between fungi and bacteria. Mucoromycota are commonly described as early diverging, as they are one of the most ancient groups of land fungi (21). The Mucoromycota phylum comprises three subphyla: Glomeromycotina, Mortierellomycotina, and Mucoromycotina (22). The first one is rather uniform in the trophic mode of its representatives—almost all Glomeromycotina fungi are obligate endomycorrhizal partners of plants (with the exception of Geosiphon pyriformis, which forms a relationship with endosymbiotic cyanobacteria) (23). Mortierellomycotina are common and ubiquitous soil saprotrophs with a worldwide distribution (24), and are thought to form nonobligatory relationships with plant roots (25–27). Mucoromycotina is the most diverse subphylum in the phylum and encompasses the following three clearly distinct orders: Endogonales, Mucorales, and Umbelopsidales. Endogonales are mainly obligatory plant symbionts (28), while representatives of the other two orders are mostly common soil saprotrophs. However, many representatives of Mucorales are also isolated from spoiled fruits, vegetables, mushrooms, or bread (e.g., Rhizopus spp., Mucor spp., Choanephora cucurbitarum). There are also rare examples of Mucorales acting as opportunistic pathogens in immunocompromised patients (causing mucormycosis) (29).
The patterns of presence and absence of BRE and MRE in Mucoromycota representatives enabled Bonfante and Desirò (30) to propose the following two different hypotheses on the evolution of bacterial-fungal interactions: early and late bacterial invasion. While these hypotheses apply to both types of EHB, in this paper, we focus on BRE. The early bacterial invasion hypothesis states that the common ancestor of all present BRE interacted with the common ancestor of extant Mucoromycota representatives. The diversity of BRE that we can observe today is thus a result of a codiversification of hosts and endosymbionts. The late bacterial invasion hypothesis states that there is at least some level of horizontal acquisition of BRE by representatives of different Mucoromycota lineages, which can also explain present day diversity of BRE. However, both scenarios are based on scarce data, especially considering the lack of information about EHB in the representatives of Mucoromycotina other than Endogonales and Rhizopus.
Therefore, the main goal of our study was to screen chosen fungal representatives of the Mucoromycota phylum for the presence of endohyphal bacteria and identify potential coevolution patterns in order to support or modify the current hypotheses on the evolution of bacterial-fungal partnerships within Mucoromycota. We included representatives of genera and orders underrepresented in endobacterial studies, such as Umbelopsis.
RESULTS
Among 196 strains belonging to 16 genera within the Mucoromycota phylum, 42 were demonstrated to be positive for bacteria from the Burkholderiaceae family, which constitutes 21% of all screened isolates (Table 1). As expected and previously reported (20), nearly 20% of Mortierella strains harbored bacteria from Burkholderiaceae. We also observed interactions between Umbelopsis and Burkholderiaceae. More than half (23 of 40) of screened strains of this genus tested positive for Burkholderiaceae. There was one strain (out of 15) of Mucor which seemed to have a relationship with Burkholderiaceae bacteria as well. As was expected, none of the screened arbuscular mycorrhizal fungi tested positive for the presence of bacteria from this group. However, we also identified Mycoplasma-related bacteria in Mortierella formicae, Diversispora sp., and two species of Glomus (data not shown). Some of the strains have had PCR bacterial product, but the identified bacteria belong to neither of the described groups. All of the data can be found in Table 1.
TABLE 1.
Prevalence of Burkholderiaceae in each screened genusa
| Subphylum | Order | Family | Genus | No. of strains tested | No. of strains with Burkholderiaceae |
|---|---|---|---|---|---|
| Glomeromycotina | Diversisporales | Diversisporaceae | Diversispora | 2 | 0 |
| Glomeromycotina | Glomerales | Glomeraceae | Glomus | 5 | 0 |
| Glomeromycotina | Glomerales | Claroideoglomeraceae | Claroideoglomus | 1 | 0 |
| Mortierellomycotina | Mortierellales | Mortierellaceae | Mortierella | 76 | 15 |
| Mucoromycotina | Mucorales | Chaetocladiaceae | Chaetocladium | 1 | 0 |
| Mucoromycotina | Mucorales | Cunninghamellaceae | Absidia | 2 | 0 |
| Mucoromycotina | Mucorales | Cunninghamellaceae | Cunninghamella | 3 | 0 |
| Mucoromycotina | Mucorales | Gilbertellaceae | Gilbertella | 1 | 0 |
| Mucoromycotina | Mucorales | Lichtheimiaceae | Lichtheimia | 2 | 0 |
| Mucoromycotina | Mucorales | Mucoraceae | Actinomucor | 3 | 0 |
| Mucoromycotina | Mucorales | Mucoraceae | Mucor | 15 | 1 |
| Mucoromycotina | Mucorales | Mucoraceae | Rhizopus | 42 | 3 |
| Mucoromycotina | Mucorales | Syncephalastraceae | Syncephalastrum | 1 | 0 |
| Mucoromycotina | Mucorales | Thamnidiaceae | Thamnidium | 1 | 0 |
| Mucoromycotina | Calcarisporiellales | Calcarisporiellaceae | Calcarisporiella | 1 | 0 |
| Mucoromycotina | Umbelopsidales | Umbelopsidaceae | Umbelopsis | 40 | 23 |
| Total | 196 | 42 |
The systematic classification follows the backbone proposed by Spatafora et al. (22).
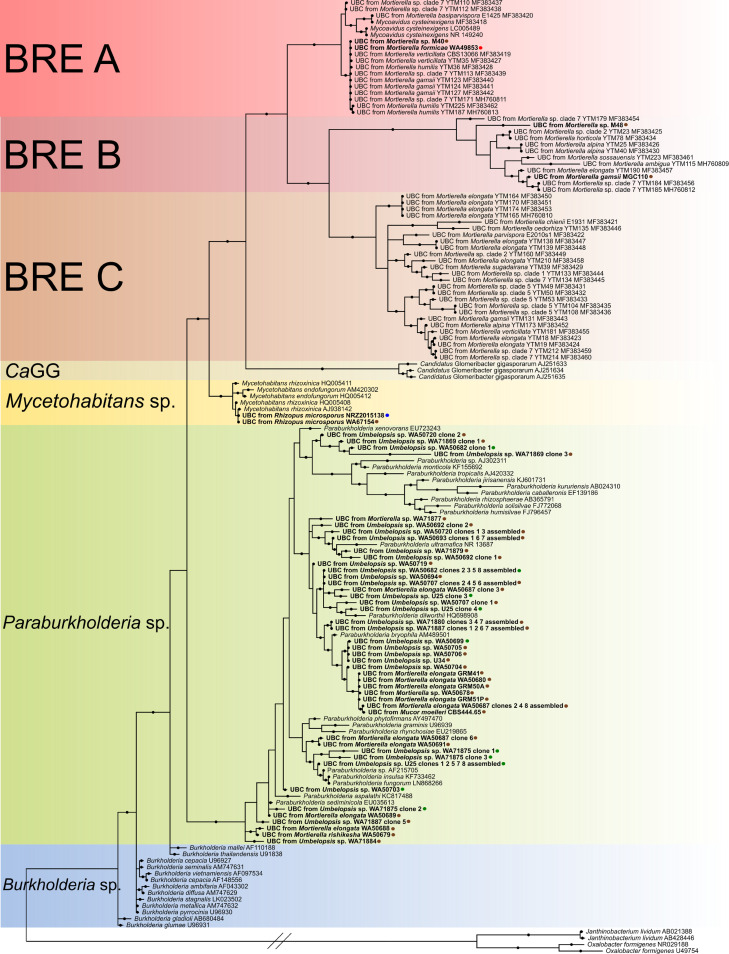
After selection of bacteria belonging to the Burkholderiaceae family, we reconstructed the phylogeny using 16S rRNA gene sequences of previously found Burkholderia-related endobacteria as well as free-living Burkholderiaceae. Our analysis showed that the identified Burkholderiaceae do not form a uniform group (Fig. 1). All obtained sequences split into the following two groups within Burkholderiaceae: one comprised strictly of endohyphal strains from Rhizopus microsporus, Mortierella spp., and arbuscular mycorrhizal fungi (Mycoavidus cysteinexigens [clades A, B, and C], M. rhizoxinica/M. endofungorum, and “Candidatus Glomeribacter gigasporarum” in Fig. 1; hereinafter referred to as BRE); and the other consisting mainly of free-living, environmental Paraburkholderia strains and endohyphal clones from this study (highlighted in green in Fig. 1). In the BRE clade, three main lineages can be distinguished, corresponding to Mycetohabitans spp., “Candidatus Glomeribacter gigasporarum,” and Mycoavidus cysteinexigens. Takashima et al. (20) further divided the Mycoavidus clade into three subclades, A, B, and C, and this pattern is also visible in our analysis. Two out of 15 endohyphal clones from our Mortierella spp. grouped within subclade A, and the same number grouped within subclade B (Fig. 1). None of our Burkholderiaceae sequences grouped within subclade C. Sequences obtained from Rhizopus microsporus all grouped within the Mycetohabitans spp. clade. The remaining 11 sequences of Burkholderiaceae from Mortierella strains, as well as all sequences from Umbelopsis spp. and one from Mucor moelleri, grouped with environmental sequences of Paraburkholderia sensu stricto (highlighted in green in Fig. 1).
FIG 1.
Bayesian phylogenetic tree of Burkholderiaceae-related endosymbionts based on partial 16S rRNA genes calculated as described in Materials and Methods. Branches marked with dots have posterior Bayesian probability higher than 0.8. Colors in the background indicate groups of sequences (from the top, three red clades of Burkholderia-related endosymbionts, A, B, and C; orange, “Candidatus Glomeribacter gigasporarum”; yellow, Mycetohabitans sp.; green, Paraburkholderia sp.; blue, Burkholderia sp.). UBC is an abbreviation for uncultured bacterial clone. Tips in bold are the ones obtained during this study. Colors of the dots next to the names indicate the source from which the hosts were obtained as follows: brown, soil; green, plant; blue, human with mucormycosis; and red, an ant. Two 16S rRNA gene sequences of Oxalobacter formigenes and two 16S rRNA gene sequences of Janthinobacterium lividum were used as an outgroup.
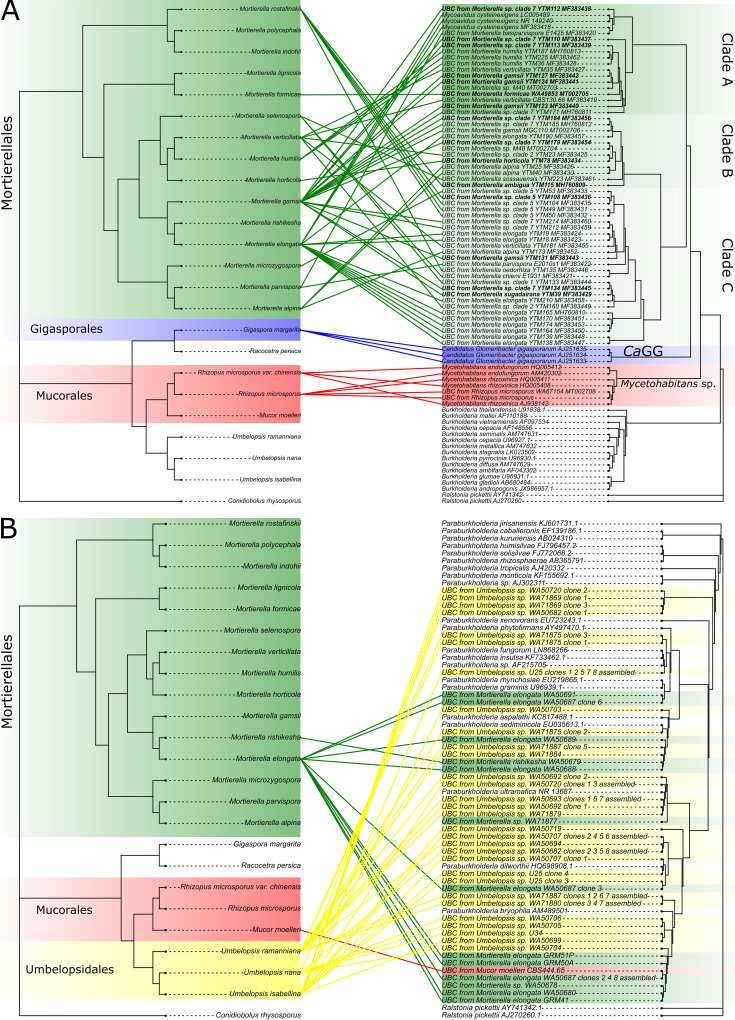
ParaFit analyses of bacteria and fungus phylogenies indicated an overall pattern of cospeciation in the BRE clade (ParaFitGlobal, P = 0.004), whereas it was not observed in the Paraburkholderia group (ParaFitGlobal, P = 1). Individual links tested with ParaFit can be found in Fig. 2.
FIG 2.
Tanglegrams of cophylogenetic relationships between fungal hosts (left) and their bacterial endosymbionts (right, BRE [A] and PRE [B]). All three trees were calculated using RAxML-NG as described in Materials and Methods. Color of highlight and links denote fungal orders and bacteria associated with its representatives. The links that were found to be statistically significant by ParaFit are denoted by bolding the bacterial tips. If the exact phylogenetic placement of the host was not known, the link was drawn to the closest species.
FISH confirmed the presence of endohyphal bacteria inside the living mycelia of Rhizopus microsporus (picture not shown), Umbelopsis sp. (Fig. 3B), and Mortierella elongata (picture not shown). For Mortierella elongata WA50687, three-dimensional visualization of hyphae with endohyphal bacteria was prepared (31). Although the identity of detected EHB was not confirmed by species-specific probe, it is highly likely that visualized bacteria are the ones detected by PCR, as usually only one strain of bacteria was identified in one fungal strain. Even in EHB-positive strains, bacterial cells were not present in all hyphae; for each strain, multiple empty hyphae were also observed (Fig. 3A).
FIG 3.
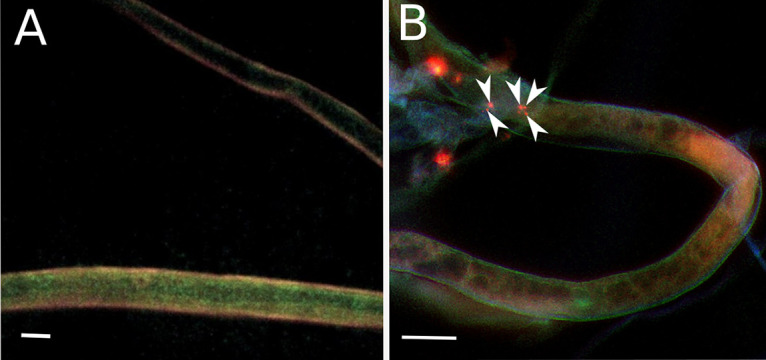
FISH visualization of endohyphal bacteria. Negative control (A) and in Umbelopsis sp. WA50699 (Umbelopsidales, Mucoromycotina) (B); bacterial cells within hyphae are indicated by arrowheads. Bar = 10 μm.
DISCUSSION
In this study, we aimed to expand knowledge about endohyphal bacteria living within hyphae of Mucoromycota representatives. To achieve this goal, we decided to use molecular methods for screening fungal isolates for the presence of bacteria and fluorescence microscopy for the confirmation of the endohyphal nature of the detected bacteria.
Currently, representatives of Mucoromycota are known to harbor endosymbionts from two distinct bacterial lineages—Burkholderia- and Mycoplasma-related ones (30)—in their hyphae, with the former probably being more widespread than the latter. In our search for EHB from previously undersampled lineages of this phylum, we detected bacteria that were previously undescribed. However, we also detected numerous endobacteria from already established clades of BRE and report similar percentage (20%) of BRE-harboring Mortierella as in the study conducted by Takashima et al. (20). All previously known lineages of BRE, including “Candidatus Glomeribacter gigasporarum” and Mycoavidus cysteinexigens, as well as a portion of BRE detected in Mortierella during this study, form a sister clade to Mycetohabitans. Notably, all endosymbionts of Umbelopsis spp. detected in our study grouped with environmental Paraburkholderia strains, and the endohyphal nature of this relationship is postulated.
At first glance, the prevalence of EHB in different lineages of Mucoromycota does not seem to be correlated with the phylogenetic position of the fungal host. Even though on a subphylum level, the colonization frequency is similar in Mortierellomycotina and Mucoromycotina (ca. 20% and ca. 24%, respectively); on an order level, the highest prevalence of EHB was observed in the representatives of Umbelopsidales (ca. 58%). The highest percentage of BRE-positive strains was observed among the strains isolated from soil (ca. 31%), and the lowest (apart from substrates represented by less than 5 strains) from clinical strains (ca. 4%). We want to elucidate, however, that the number of strains in our study does not allow us to draw conclusions about the impact of ecological niche on the prevalence of EHB and that the influence of fungal isolation substrate on prevalence and identity of EHB within Mucoromycota should be further investigated.
Paraburkholderia spp. seem to be plant associated (usually being isolated from rhizosphere), and some strains can potentially have a positive impact on plant health (13, 14). As more than one-half of the screened strains of Umbelopsis spp. tested positive for Paraburkholderia sp. and one-third of them were isolated from the plant material, we hypothesize that the relationship between fungus and bacteria could be beneficial for plants, especially since it is postulated that the fungal role in relationships with endohyphal bacteria is providing a safe environment for them. However, data are still scarce, and physiological experiments using cleared and infected isogenic isolates, similar to those performed by Uehling et al. (9), as well as comparative transcriptomics experiments of such strains (32) and sampling more strains from different locations around the world, are needed to assess the actual impact of Umbelopsis on plants as well as to examine how this impact changes with the presence of endobacteria.
The ancient origin (350 to 400 million years ago [MYA]) of BRE (Mycoavidus-Glomeribacter-Mycetohabitans lineage) in Mucoromycota was postulated by Mondo et al. (7) and Uehling et al. (9). Their results would thus support the early BRE invasion hypothesis in Mucoromycota as proposed by Bonfante and Desirò (30). In our study, the coevolution of EHB and fungal hosts may be observed on the order level in the BRE clade (i.e., Mortierellales, Mycoavidus sensu lato; Gigasporales, “Candidatus Glomeribacter sp.”; Mucorales, Mycetohabitans spp.). We also prove that there is a significant coevolutionary pattern between Mucoromycota and BRE sensu stricto, which suggests that the common ancestor of this clade interacted with an ancestor of Mucoromycota and they coevolved from this moment onwards. Conversely, our results support the late invasion hypothesis for bacteria identified as Paraburkholderia spp. It seems that symbionts from this genus may be recruited from the environment when it is advantageous for partners and form more transient relationships with Mucoromycota than BRE. Our hypothesis is in agreement with the final conclusion of Bonfante and Desirò (30). They hypothesize that soil, with its living components, has acted as a facilitator in transferring free-living bacteria inside fungi. We also speculate that the event of interaction between ancestors of Mucoromycota and BRE may have enabled fungi to interact with different types of bacteria. That would explain why Paraburkholderia representatives, closely related to BRE, were found in closely related Mortierella and Umbelopsis. As the current state of knowledge is largely incomplete, further studies are required to fully understand the nature of initiating and maintaining relationships between fungi and bacteria as well as their evolutionary origin.
In conclusion, screening of 196 fungal strains of Mucoromycota revealed EHB from the Burkholderiaceae family in ca. 20% of them. Some of the detected bacteria could be assigned to previously described endosymbiotic clades (Mycoavidus sensu lato, Mycetohabitans spp.), but others clustered with free-living Paraburkholderia. Most importantly, this study allowed for identification of potentially endohyphal bacteria in Umbelopsis spp. belonging to Paraburkholderia spp. The hypotheses regarding the time of invasion of EHB in Mucoromycota could not be resolved with certainty. However, we lean toward the early invasion hypothesis for BRE and the late invasion hypothesis for Paraburkholderia spp.
MATERIALS AND METHODS
Fungal strains collection and identification.
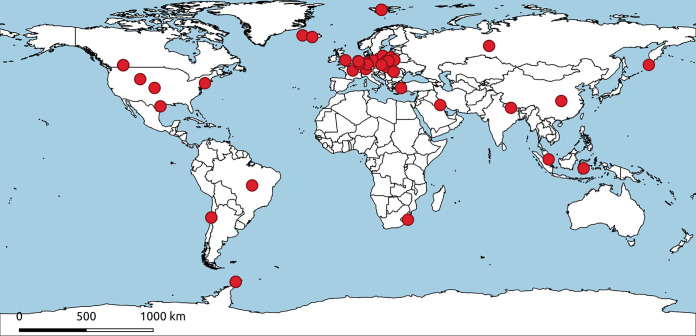
Between 2015 and 2019, we collected soil from Europe, Antarctica, and the Arctic. Mucoromycotina and Mortierellomycotina representatives were isolated from soil using the Warcup method on water agar (WA) plates (33). Emerging hyphae were subsequently transferred to new malt extract agar (MEA) plates in order to obtain pure colonies of each strain. Since culturing obligate biotrophs is difficult, Glomeromycotina spores were suspended in water and used for further analysis. Additionally, 32 strains from the Westerdijk Fungal Biodiversity Institute culture collection, 39 strains from the Nationales Referenzzentrum (NRZ) für Mykobakterien culture collection, and 8 strains from the Jagiellonian University collection were also used in this study. A detailed list of all of the strains used and sampling sites is presented in Table 2 and Table S1 in the supplemental material and is visualized in Fig. 4. The map of sampling sites was prepared using qGIS 3.4 Madeira (34).
TABLE 2.
Fungal strains used in the study
| Strain ID | Phylogenetic placement | Place of origin |
|---|---|---|
| Act | Actinomucor elegans | Poland |
| AG | Absidia glauca | Poland |
| BEG11 | Glomus geosporum | Poland |
| BEG12 | Glomus mosseae | Poland |
| BEG144 | Glomus intraradices | Poland |
| BEG23 | Glomus claroideum | Poland |
| CBS 101040 | Lichtheimia corymbifera | France |
| CBS 102.35 | Absidia fusca | Germany |
| CBS 103.35 | Lichtheimia ramosa | NDa |
| CBS 117697 | Actinomucor kuwaitensis | Kuwait |
| CBS 120585 | Mucor indicus | France |
| CBS 120811 | Syncephalastrum racemosum | ND |
| CBS 123972 | Mucor hiemalis | Germany |
| CBS 123973 | Mucor circinelloides | Germany |
| CBS 142.35 | Mucor circinatus | Brazil |
| CBS 156.74 | Chaetocladium brefeldii | The Netherlands |
| CBS 167.53 | Cunninghamella elegans | Canada |
| CBS 185.68 | Mucor jensenii | Russia |
| CBS 185.77 | Mucor amphibiorum | USA |
| CBS 190.32 | Gilbertella persicaria | USA |
| CBS 222.81 | Mucor racemosus | The Netherlands |
| CBS 226.32 | Mucor plumbeus | Canada |
| CBS 236.35 | Mucor lusitanicus | Germany |
| CBS 242.35 | Mucor hiemalis | Germany |
| CBS 243.67 | Mucor jensenii | South Africa |
| CBS 308.87 | Rhizopus microsporus | USA |
| CBS 318.78 | Cunninghamella elegans | Turkey |
| CBS 338.72 | Actinomucor elegans | Nepal |
| CBS 366.70 | Mucor circinelloides | The Netherlands |
| CBS 372.95 | Cunninghamella bertholletiae | China |
| CBS 411.52 | Thamnidium elegans | Poland |
| CBS 422.71 | Mucor indicus | Indonesia |
| CBS 444.65 | Mucor moelleri | USA |
| CBS 515.94 | Rhizopus arrhizus | Singapore |
| CBS 969.68 | Mucor circinelloides | Russia |
| CBS279.70 | Calcarisporiella thermophila | England |
| H2C1 | Mortierella elongata | Iceland |
| H2C2 | Mortierella elongata | Iceland |
| J6 | Glomus claroideum | Poland |
| M21 | Mortierella sp. | Canada |
| M19 | Mortierella sp. | Canada |
| M23 | Mortierella sp. | Canada |
| M25 | Mortierella alpina | Romania |
| M26 | Mortierella sp. | Canada |
| M4 | Mortierella parvispora | Poland |
| M40 | Mortierella sp. | Canada |
| M44 | Mortierella sp. | Canada |
| M48 | Mortierella sp. | Canada |
| M54 | Mortierella gamsii | Romania |
| MGC110 | Mortierella gamsii | Poland |
| MGC142 | Mortierella zychae | Poland |
| N1131 | Mortierella alpina | The Arctic |
| N1231A | Mortierella minutissima | The Arctic |
| N1525 | Mortierella hyalina | The Arctic |
| N2121 | Mortierella minutissima | The Arctic |
| N2131 | Mortierella hyalina | The Arctic |
| N3532 | Mortierella minutissima | The Arctic |
| N4235 | Mortierella minutissima | The Arctic |
| N4323B | Mortierella gamsii | The Arctic |
| N4332 | Mortierella minutissima | The Arctic |
| N4421 | Mortierella minutissima | The Arctic |
| N4422 | Mortierella minutissima | The Arctic |
| N5321 | Mortierella alpina | The Arctic |
| N5431B | Mortierella alpina | The Arctic |
| N5531 | Mortierella alpina | The Arctic |
| N6431 | Mortierella alpina | The Arctic |
| NRZ-2015-138 | Rhizopus microsporus | Germany |
| NRZ-2015-182 | Rhizopus arrhizus | Germany |
| NRZ-2015-216 | Rhizopus microsporus | Germany |
| NRZ-2016-056 | Rhizopus arrhizus | Germany |
| NRZ-2016-117 | Rhizopus microsporus | Germany |
| NRZ-2016-214 | Rhizopus microsporus | Germany |
| NRZ-2016-230 | Rhizopus arrhizus | Germany |
| NRZ-2016-254 | Rhizopus arrhizus | Germany |
| NRZ-2016-325 | Rhizopus arrhizus | Germany |
| NRZ-2016-328 | Rhizopus arrhizus | Germany |
| NRZ-2017-035 | Rhizopus microsporus | Germany |
| NRZ-2017-167 | Rhizopus microsporus | Germany |
| NRZ-2017-218 | Rhizopus microsporus | Germany |
| NRZ-2017-239 | Rhizopus microsporus | Germany |
| NRZ-2017-267 | Rhizopus microsporus | Germany |
| NRZ-2017-370 | Rhizopus microsporus | Germany |
| NRZ-2017-401 | Rhizopus arrhizus | Germany |
| NRZ-2017-426 | Rhizopus arrhizus | Germany |
| NRZ-2017-431 | Rhizopus arrhizus | Germany |
| NRZ-2018-015 | Rhizopus microsporus | Germany |
| NRZ-2018-028 | Rhizopus microsporus | Germany |
| NRZ-2018-083 | Rhizopus arrhizus | Germany |
| NRZ-2018-084 | Rhizopus arrhizus | Germany |
| NRZ-2018-111 | Rhizopus arrhizus | Germany |
| NRZ-2018-169 | Rhizopus microsporus | Germany |
| NRZ-2018-178 | Rhizopus microsporus | Germany |
| NRZ-2018-330 | Rhizopus stolonifer | Germany |
| NRZ-2018-357 | Rhizopus microsporus | Germany |
| NRZ-2018-385 | Rhizopus arrhizus | Germany |
| NRZ-2018-414 | Rhizopus microsporus | Germany |
| NRZ-2018-419 | Rhizopus arrhizus | Germany |
| NRZ-2018-423 | Rhizopus microsporus | Germany |
| NRZ-2018-463 | Rhizopus arrhizus | Germany |
| NRZ-2018-475 | Rhizopus arrhizus | Germany |
| NRZ-2018-476 | Rhizopus arrhizus | Germany |
| NRZ-2018-478 | Rhizopus arrhizus | Germany |
| NRZ-2018-560 | Rhizopus arrhizus | Germany |
| NRZ-2018-581 | Rhizopus microsporus | Germany |
| NRZ-2018-591 | Rhizopus arrhizus | Germany |
| S1433 | Mortierella polygonia | Antarctica |
| S1bC | Mortierella elongata | Iceland |
| S1bD | Mortierella elongata | Iceland |
| S2bC | Mortierella elongata | Iceland |
| S3123A | Mortierella alpina | Antarctica |
| S3323 | Mortierella alpina | Antarctica |
| S3421 | Mortierella alpina | Antarctica |
| MGC163b | Umbelopsis sp. | Poland |
| MGC164 | Umbelopsis sp. | Poland |
| U25 | Umbelopsis sp. | Poland |
| U34 | Umbelopsis angularis | Poland |
| U41 | Umbelopsis angularis | Poland |
| U810 | Umbelopsis angularis | Poland |
| WA18942 | Mortierella sp. | Poland |
| WA18944 | Mortierella calciphila | Poland |
| WA49853 | Mortierella formicae | Poland |
| WA50677 | Mortierella verticillata | Poland |
| WA50678 | Mortierella sp. | Poland |
| WA50678 | Mortierella elongata | Poland |
| GRM41 | Mortierella elongata | Poland |
| WA50679 | Mortierella rishikesha | Poland |
| WA50680 | Mortierella elongata | Poland |
| WA50680 | Mortierella elongata | Poland |
| WA50681 | Mortierella elongata | Poland |
| WA50682 | Umbelopsis ramanniana sensu lato | Poland |
| WA50683 | Mortierella zychae | Poland |
| WA50684 | Mortierella zychae | Poland |
| WA50685 | Mortierella elongata | Poland |
| WA50687 | Mortierella elongata | Poland |
| WA50688 | Mortierella elongata | Poland |
| WA50689 | Mortierella elongata | Poland |
| WA50691 | Mortierella elongata | Poland |
| WA50692 | Umbelopsis ramanniana sensu lato | Poland |
| WA50693 | Umbelopsis ramanniana sensu lato | Poland |
| WA50694 | Umbelopsis ramanniana sensu lato | Poland |
| WA50697 | Umbelopsis ramanniana sensu lato | Poland |
| WA50698 | Umbelopsis ramanniana sensu lato | Poland |
| WA50699 | Umbelopsis ramanniana sensu lato | Poland |
| WA50700 | Umbelopsis ramanniana sensu lato | Poland |
| WA50701 | Umbelopsis ramanniana sensu lato | Poland |
| WA50702 | Umbelopsis ramanniana sensu lato | Poland |
| WA50703 | Umbelopsis isabellina | Poland |
| WA50704 | Umbelopsis ramanniana sensu lato | Poland |
| WA50705 | Umbelopsis ramanniana sensu lato | Poland |
| WA50706 | Umbelopsis ramanniana sensu lato | Poland |
| WA50707 | Umbelopsis ramanniana | Poland |
| WA50719 | Umbelopsis ramanniana sensu lato | Poland |
| WA50720 | Umbelopsis ramanniana sensu lato | Poland |
| WA51536 | Umbelopsis vinacea | Poland |
| WA67140 | Mortierella gamsii | Romania |
| WA67141 | Mortierella alpina | Romania |
| WA67145 | Mortierella zychae | Poland |
| WA67154 | Rhizopus microsporus | Poland |
| WA67162 | Mortierella gamsii | Romania |
| WA67163 | Mortierella hyalina | Poland |
| WA67166 | Mortierella sp. | Poland |
| WA67171 | Mortierella alpina | Poland |
| WA67176 | Mortierella sp. | Chile |
| WA67179 | Mortierella gemmifera | Chile |
| WA67203 | Mortierella zychae | Poland |
| WA67204 | Mortierella zychae | Poland |
| WA67205 | Mortierella sp. | Poland |
| WA67206 | Mortierella zychae | Poland |
| WA67211 | Mortierella zychae | Poland |
| WA67219 | Mortierella sp. | Chile |
| WA71869 | Umbelopsis ramanniana sensu lato | Poland |
| WA71874 | Umbelopsis sp. | Poland |
| WA71875 | Umbelopsis isabellina | Poland |
| WA71876 | Umbelopsis ramanniana sensu lato | Poland |
| WA71877 | Mortierella sp. | Poland |
| WA71878 | Umbelopsis ramanniana | Poland |
| WA71879 | Umbelopsis ramanniana | Poland |
| WA71880 | Umbelopsis ramanniana | Poland |
| WA71881 | Umbelopsis ramanniana | Poland |
| WA71883 | Umbelopsis ramanniana | Poland |
| WA71884 | Umbelopsis ramanniana | Poland |
| WA71885 | Umbelopsis ramanniana | Poland |
| WA71887 | Umbelopsis ramanniana | Poland |
| WA71891 | Umbelopsis angularis | Poland |
| WA71892 | Umbelopsis vinacea | Poland |
| WA74572 | Umbelopsis sp. | Poland |
| WA74573 | Umbelopsis sp. | Poland |
| XWhI | Mortierella bainieri | The Arctic |
| XWhII | Mortierella minutissima | The Arctic |
| Zl7 | Claroideoglomus claroideum | Poland |
| ZR16 | Diversispora sp. | Poland |
| ZR16 Se-3 | Diversispora sp. | Poland |
| GMR42 | Mortierella elongata | Poland |
| GRM50A | Mortierella elongata | Poland |
| GRM51prim | Mortierella elongata | Poland |
| GRM51A | Mortierella elongata | Poland |
ND, no data.
FIG 4.
World map showing locations from which fungal strains were obtained (projection EPSG:4326-WGS84). Each red dot represents one strain. If the exact isolation location is known (location name or coordinates), the dot is placed as accurately as possible. If not, the dot is placed in the geographic center of the country.
DNA extraction, amplification, and sequencing.
Whole genomic DNA was extracted using ExtractMe genomic DNA kit (Blirt S.A., Gdańsk, Poland) according to the manufacturer’s protocol. An internal transcribed spacer (ITS) rRNA gene fragment was amplified using a 20-μl PCR mixture, which consisted of 10 μl of 2× TaqNova-RED PCR master mix (Blirt S.A., Gdańsk, Poland), 1.5 μl each of ITS1f and ITS4 primers in 10 pmol μl−1 concentration (35), up to 7 μl of template DNA (depending on the template’s concentration), and distilled water up to 20 μl. PCR was performed as follows: 4 min in 95°C for initial denaturation, 35 cycles of 30 s in 95°C, 30 s in 54°C, 1 min in 72°C for annealing, and 10 min in 72°C for final elongation.
PCR amplicons were visualized by 1% agarose gel electrophoresis and purified using ExtractMe genomic clean up kit (Blirt S.A., Gdańsk, Poland) and used as a template for Sanger sequencing with the ABI PRISM BigDye Terminator cycle sequencing ready reaction kit 3.1 (Applied Biosystems, Warrington, UK) with the same primers as those used in PCR. Sequencing was outsourced to Genomed (Genomed S.A., Warszawa, Poland). A BLASTn search (36) against the UNITE database (https://unite.ut.ee) (37) was performed for the obtained ITS fragments in order to assess taxonomic placement of each strain. Fungal sequence data generated for this study are available in GenBank under accession numbers MT009408 to MT009438 and MT009444 to MT009481.
Detection of endofungal bacteria.
DNA isolates from each strain were then used as a template for PCR targeting bacterial 16S rRNA genes. PCR was performed in a 25-μl volume consisting of 2.5 μl of 10× DreamTaq green buffer (Thermo Fisher Scientific, Waltham, MA, USA), 0.5 μl of deoxynucleoside triphosphates (dNTPs) mix (Thermo Fisher Scientific, Waltham, MA, USA), 0.5 μl of each of two universal bacterial primers, i.e., 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) in 10 pmol μl−1 concentration, 0.1 μl Taq DNA polymerase (Qiagen, Hilden, Germany), 1 μl of template DNA, and 19.9 μl of distilled water. For difficult templates (resulting in a small amount of expected product), PCR was repeated using Taq PCR core kit (Qiagen, Hilden, Germany) using the same template as before. Reaction was performed in a 50-μl volume consisting of 5 μl of 10× CoralLoad PCR buffer, 10 μl of 5× Q-solution, 1 μl of dNTPs mix (Thermo Fisher Scientific, Waltham, MA, USA), 2.5 μl of each primer, 0.25 μl of Taq DNA polymerase, at least 3 μl of template DNA (depending on concentration), and distilled water up to 50 μl. PCR was performed as follows: 3 min in 94°C for initial denaturation, 35 cycles of 30 s in 94°C, 30 s in 53°C, 1 min in 72°C for annealing, and 10 min in 72°C for final elongation. Presence of the PCR product was confirmed on 1% agarose gel and then purified and cloned.
Cloning of 16S rRNA gene PCR products.
Purified 16S rRNA genes were then cloned on pGEM-T Easy vector using pGem-T Easy vector systems kit (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocol and transformed into Dh5-alpha-competent E. coli cells (MCLAB, San Francisco, CA, USA). Transformed Dh5-alpha cells were plated on LB agar medium (Sigma-Aldrich, Saint Louis, MO, USA) supplemented with 200 μl of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal), 200 μl of isopropyl-β-d-thiogalactopyranoside (IPTG), and 200 μl of ampicillin per 100 ml of medium. Transformants of each strain were plated twice, and successfully cloned colonies were chosen from each plate for further investigation.
Subsequently, direct PCR was performed for each colony in a 20-μl volume consisting of 10 μl 2× TaqNova-RED PCR master mix (Blirt S.A., Gdańsk, Poland), 1.5 μl (each) of M13F and M13R primers, 7 μl of distilled water, and a small amount of material from the bacterial colony. PCR was performed as follows: 3 min in 95°C for initial denaturation, 35 cycles of 30 s in 95°C, 30 s in 55°C, 1 min in 72°C for annealing, and 5 min in 72°C for a final elongation. Presence of the PCR product was then confirmed on 1% agarose gel and purified as described previously. Five DNA clones from each fungal strain were used as a template for Sanger sequencing and sequenced as described previously. Bacterial sequence data generated for this study are available in GenBank under accession numbers MT002691 to MT002716, MW055707 to MW055867, and MW080027 to MW080031 (BRE) and MT031989 to MT032002 (MRE).
Phylogenetic analyses of detected endobacteria.
Forward and reverse reads of 16S rRNA gene sequences obtained in the previous step were assembled using Geneious Prime 2019.2 (Geneious, Auckland, New Zealand). Sequences belonging to the Burkholderiaceae family were selected using BLASTn searches (36) against the NCBI database (38). Only these sequences were used for further analysis. Then, if the sequences assembled from clone reads from one fungal strain were similar in at least 98%, a consensus sequence from them was created using cons 6.6.0.0 from the EMBOSS package (39). If not, the sequences were used separately. We then combined obtained sequences with publicly available 16S rRNA gene sequences of previously detected BRE and free-living Burkholderiaceae, aligned them together using MAFFT (v.7.271; –auto) (40), and trimmed them using trimAl (v.1.2rev59; -automated1) (41). Trimmed alignment was then visually inspected and used for calculating a phylogenetic tree. ModelTest-NG was used to check which evolutionary model of substitutions should be used (TIM3 + I + G4), and RAxML-NG (v.0.8.0) was subsequently used for finding the best tree and calculating 1,000-bootstrap replicates (42).
The same alignment was used for finding the best Bayesian tree using MrBayes (43, 44) with the best fit model of nucleotide evolution (generalized time reversible [GTR] and inverted gamma-distributed rate variation). Metropolis-coupled Markov chain Monte Carlo (MCMC) chains were run for 500,000 generations, with trees sampled every 100 generations, and an initial burn-in threshold was set to 1,250 trees; from the remaining trees, the consensus phylogram was computed using the 50% majority rule.
To establish whether endohyphal bacteria coevolved with their fungal hosts, we used the global fits method. First, sequences of small and large ribosomal subunits for each fungal host were obtained from NCBI (accession numbers used for the tree can be found in Table S2 in the supplemental material), aligned separately using MAFFT, trimmed using trimAl, and, after separately checking for the best evolutionary model using ModelTest-NG, concatenated. Then, the fungal tree was calculated using RAxML-NG with the same settings as for the bacterial tree described above. We also calculated two separate phylogenetic trees for bacteria, one for Paraburkholderia sequences and one for Burkholderia sensu lato, using the same software and settings as before. Afterward, the global hypothesis of coevolution between fungal hosts and harbored bacteria was tested for both groups using the ParaFit function from ape v.5.3 R package (45) with 999 permutations to implement a global test as well as individual links. The interaction was considered to be significant if the ParaFitGlobal P value was <0.05. Individual links between hosts and bacteria were visualized on tanglegrams (Fig. 2) created using phytools v.0.6.99 R package (46).
All of the trees and tanglegrams were edited using FigTree (47), iTOL (48), and Inkscape (49) software.
Visualization of endobacteria.
The strains from different orders of Mucoromycota (namely, Mortierellales, Umbelopsidales, and Mucorales; strains Mortierella elongata WA50687, Umbelopsis sp. WA50699, Rhizopus microsporus WA67154) putatively harboring EHB were chosen for visualization procedure. Small (0.5 cm2) fragments of fungal cultures were taken from 2% MEA plates, washed in 1× phosphate-buffered saline (PBS) three times, and fixed in 10% formalin (no additional permeabilization was implemented). Samples were then centrifuged at 4,500 rpm for 8 min and incubated for 3 h at 4°C. Subsequently, samples were centrifuged with 4,500 rpm for 8 min, after which supernatant was replaced with autoclaved Milli-Q water. This last procedure was repeated twice, and after the last round of centrifuging, biomass was suspended in 1× PBS (pH 7.4). All samples were stored at −20°C until further analyses were performed.
Fluorescence in situ hybridization (FISH) analyses were performed according to Nielsen et al. (50) with the following modifications. The procedure was performed in suspension instead of slides. At least 2 μg of each fungal colony was suspended in 40 μl of hybridization buffer and incubated at 46°C overnight. Then, samples were centrifuged, and hybridization buffer was replaced by washing buffer. After 15 min of washing at 48°C, washing buffer was discarded, and samples were resuspended in cold distilled water (dH2O). Finally, samples were transferred to wells on slides in proper aliquot to obtain a thin hyphal layer, facilitating microscopic observation (volumes between 5 and 40 μl were tested). Fungal biomass after FISH procedure without addition of probe was used as the negative control. The negative control was needed to assess autofluorescence.
The fungal hyphae were recognized by bright-field microscopy; then, the endohyphal bacteria were stained by FISH universal bacterial probe EUB338 labeled with Cy3 and observed under a microscope with a proper set of filters (excitation, 552 nm; emission, 565 nm). Detailed probe information is available in probeBase (51). The EHB were visualized using an Olympus IX81F– ZDC2 confocal microscope and Andor iQ software, objectives CLARA100×/60×/40×.
Data availability.
Sequences produced in the study can be found in the NCBI database under GenBank accession numbers MT031989 to MT032002 (MRE), MT009408 to MT009438 and MT009444 to MT009481 (fungal ITS), MT002691 to MT002716, MW055707 to MW055867, and MW080027 to MW080031 (BRE).
Supplementary Material
ACKNOWLEDGMENTS
We want to thank Michał Gorczak, Łukasz Istel, Grzegorz Ostrowski, Lidia Serewa, Igor Siedlecki, Grit Walther, and Szymon Zubek for providing Mucoromycota strains. We also acknowledge Bartosz Kiersztyn for his assistance with FISH, and University of Chemistry and Technology, Prague for access to the fluorescent microscopy facility. We want to thank Gregory Bonito and Anna Muszewska for valuable comments on this manuscript. We also want to acknowledge Kacper Maciszewski and Kaley Scott for the text revisions. Finally, we thank the anonymous reviewers whose comments contributed to the improvement of the manuscript.
The research was supported by the National Science Centre, Poland, under grants 2017/25/B/NZ8/00473 (PI: Julia Pawłowska) and 2016/23/B/NZ8/00897 (PI: Marta Wrzosek). Aleksandra Miłobędzka, the researcher working on this project, was supported by the European Structural and Investment 671 Funds, OP RDE-funded project “ChemJets” (number CZ.02.2.69/0.0/0.0/16_027/0008351).
A.O. and J.P. conceived the idea of the study. K.D., A.B., A.G., and B.S. performed molecular laboratory work. A.M. performed FISH experiments. A.O. performed phylogenetic and statistical analysis. A.O. and J.P. wrote the manuscript and prepared figures. A.M. and M.W. edited the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Baldrian P, Voříšková J, Dobiášová P, Merhautová V, Lisá L, Valášková V. 2011. Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant Soil 338:111–125. doi: 10.1007/s11104-010-0324-3. [DOI] [Google Scholar]
- 2.Mosse B. 1970. Honey-coloured, sessile Endogone spores. Archiv Mikrobiol 74:146–159. doi: 10.1007/BF00446902. [DOI] [Google Scholar]
- 3.Bianciotto V, Lumini E, Bonfante P, Vandamme P. 2003. “Candidatus Glomeribacter gigasporarum” gen. nov., sp. nov., an endosymbiont of arbuscular mycorrhizal fungi. Int J Syst Evol Microbiol 53:121–124. doi: 10.1099/ijs.0.02382-0. [DOI] [PubMed] [Google Scholar]
- 4.Salvioli A, Ghignone S, Novero M, Navazio L, Venice F, Bagnaresi P, Bonfante P. 2016. Symbiosis with an endobacterium increases the fitness of a mycorrhizal fungus, raising its bioenergetic potential. ISME J 10:130–144. doi: 10.1038/ismej.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partida-Martinez LP, Hertweck C. 2005. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 437:884–888. doi: 10.1038/nature03997. [DOI] [PubMed] [Google Scholar]
- 6.Partida-Martinez LP, Groth I, Schmitt I, Richter W, Roth M, Hertweck C. 2007. Burkholderia rhizoxinica sp. nov. and Burkholderia endofungorum sp. nov., bacterial endosymbionts of the plant-pathogenic fungus Rhizopus microsporus. Int J Syst Evol Microbiol 57:2583–2590. doi: 10.1099/ijs.0.64660-0. [DOI] [PubMed] [Google Scholar]
- 7.Mondo SJ, Lastovetsky OA, Gaspar ML, Schwardt NH, Barber CC, Riley R, Sun H, Grigoriev IV, Pawlowska TE. 2017. Bacterial endosymbionts influence host sexuality and reveal reproductive genes of early divergent fungi. Nat Commun 8:1843. doi: 10.1038/s41467-017-02052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato Y, Narisawa K, Tsuruta K, Umezu M, Nishizawa T, Tanaka K, Yamaguchi K, Komatsuzaki M, Ohta H. 2010. Detection of betaproteobacteria inside the mycelium of the fungus Mortierella elongata. Microbes Environ 25:321–324. doi: 10.1264/jsme2.me10134. [DOI] [PubMed] [Google Scholar]
- 9.Uehling J, Gryganskyi A, Hameed K, Tschaplinski T, Misztal PK, Wu S, Desirò A, Vande Pol N, Du Z, Zienkiewicz A, Zienkiewicz K, Morin E, Tisserant E, Splivallo R, Hainaut M, Henrissat B, Ohm R, Kuo A, Yan J, Lipzen A, Nolan M, LaButti K, Barry K, Goldstein AH, Labbé J, Schadt C, Tuskan G, Grigoriev I, Martin F, Vilgalys R, Bonito G. 2017. Comparative genomics of Mortierella elongata and its bacterial endosymbiont Mycoavidus cysteinexigens. Environ Microbiol 19:2964–2983. doi: 10.1111/1462-2920.13669. [DOI] [PubMed] [Google Scholar]
- 10.Deveau A, Bonito G, Uehling J, Paoletti M, Becker M, Bindschedler S, Hacquard S, Hervé V, Labbé J, Lastovetsky OA, Mieszkin S, Millet LJ, Vajna B, Junier P, Bonfante P, Krom BP, Olsson S, van Elsas JD, Wick LY. 2018. Bacterial–fungal interactions: ecology, mechanisms and challenges. FEMS Microbiol Rev 42:335–352. doi: 10.1093/femsre/fuy008. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman MT, Arnold AE. 2010. Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl Environ Microbiol 76:4063–4075. doi: 10.1128/AEM.02928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coenye T. 2014. The family Burkholderiaceae, p 759–776. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes. Springer, Berlin, Germany. [Google Scholar]
- 13.Sawana A, Adeolu M, Gupta RS. 2014. Molecular signatures and phylogenomic analysis of the genus Burkholderia: proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front Genet 5:429. doi: 10.3389/fgene.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrada-de los Santos P, Palmer M, Chávez-Ramírez B, Beukes C, Steenkamp E, Briscoe L, Khan N, Maluk M, Lafos M, Humm E, Arrabit M, Crook M, Gross E, Simon M, dos Reis Junior F, Whitman W, Shapiro N, Poole P, Hirsch A, Venter S, James E. 2018. Whole genome analyses suggests that Burkholderia sensu lato contains two additional novel genera (Mycetohabitans gen. nov., and Trinickia gen. nov.): implications for the evolution of diazotrophy and nodulation in the Burkholderiaceae. Genes 9:389. doi: 10.3390/genes9080389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naumann M, Schüssler A, Bonfante P. 2010. The obligate endobacteria of arbuscular mycorrhizal fungi are ancient heritable components related to the Mollicutes. ISME J 4:862–871. doi: 10.1038/ismej.2010.21. [DOI] [PubMed] [Google Scholar]
- 16.Naito M, Desirò A, González JB, Tao G, Morton JB, Bonfante P, Pawlowska TE. 2017. ‘Candidatus Moeniiplasma glomeromycotorum’, an endobacterium of arbuscular mycorrhizal fungi. Int J Syst Evol Microbiol 67:1177–1184. doi: 10.1099/ijsem.0.001785. [DOI] [PubMed] [Google Scholar]
- 17.Desirò A, Hao Z, Liber JA, Benucci GMN, Lowry D, Roberson R, Bonito G. 2018. Mycoplasma-related endobacteria within Mortierellomycotina fungi: diversity, distribution and functional insights into their lifestyle. ISME J 12:1743–1757. doi: 10.1038/s41396-018-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Y, Desirò A, Na H, Sandor L, Lipzen A, Clum A, Barry K, Grigoriev IV, Martin FM, Stajich JE, Smith ME, Bonito G, Spatafora JW. 2019. Phylogenomics of Endogonaceae and evolution of mycorrhizas within Mucoromycota. New Phytol 222:511–525. doi: 10.1111/nph.15613. [DOI] [PubMed] [Google Scholar]
- 19.Pawlowska TE, Gaspar ML, Lastovetsky OA, Mondo SJ, Real-Ramirez I, Shakya E, Bonfante P. 2018. Biology of fungi and their bacterial endosymbionts. Ann Rev Phytopathol 56: 289–309. doi: 10.1146/annurev-phyto-080417-045914. [DOI] [PubMed] [Google Scholar]
- 20.Takashima Y, Seto K, Degawa Y, Guo Y, Nishizawa T, Ohta H, Narisawa K. 2018. Prevalence and intra-family phylogenetic divergence of Burkholderiaceae-related endobacteria associated with species of Mortierella. Microbes Environ 33:417–427. doi: 10.1264/jsme2.ME18081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonfante P, Venice F. 2020. Mucoromycota: going to the roots of plant-interacting fungi. Fungal Biol Rev 34:100–113. doi: 10.1016/j.fbr.2019.12.003. [DOI] [Google Scholar]
- 22.Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY, O'Donnell K, Roberson RW, Taylor TN, Uehling J, Vilgalys R, White MM, Stajich JE. 2016. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108:1028–1046. doi: 10.3852/16-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluge M. 2002. A fungus eats a cyanobacterium: the story of the Geosiphon pyriformis endocyanosis. Biol Environ Proc R Ir Acad 102:11–14. doi: 10.3318/BIOE.2002.102.1.11. [DOI] [Google Scholar]
- 24.Tedersoo L, Bahram M, Polme S, Koljalg U, Yorou NS, Wijesundera R, Ruiz LV, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Rosas M, Riit T, Ratkowsky D, Pritsch K, Poldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, Partel K, Otsing E, Nouhra E, Njouonkou AL, Nilsson RH, Morgado LN, Mayor J, May TW, Majuakim L, Lodge DJ, Lee SS, Larsson K-H, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo L-D, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, De Kesel A, et al. 2014. Global diversity and geography of soil fungi. Science 346:1256688. doi: 10.1126/science.1256688. [DOI] [PubMed] [Google Scholar]
- 25.Bonito G, Reynolds H, Robeson MS, Nelson J, Hodkinson BP, Tuskan G, Schadt CW, Vilgalys R. 2014. Plant host and soil origin influence fungal and bacterial assemblages in the roots of woody plants. Mol Ecol 23:3356–3370. doi: 10.1111/mec.12821. [DOI] [PubMed] [Google Scholar]
- 26.Keim J, Mishra B, Sharma R, Ploch S, Thines M. 2014. Root-associated fungi of Arabidopsis thaliana and Microthlaspi perfoliatum. Fungal Divers 66:99–111. doi: 10.1007/s13225-014-0289-2. [DOI] [Google Scholar]
- 27.Toju H, Tanabe AS, Sato H. 2018. Network hubs in root-associated fungal metacommunities. Microbiome 6:116. doi: 10.1186/s40168-018-0497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desirò A, Rimington WR, Jacob A, Pol NV, Smith ME, Trappe JM, Bidartondo MI, Bonito G. 2017. Multigene phylogeny of Endogonales, an early diverging lineage of fungi associated with plants. IMA Fungus 8:245–257. doi: 10.5598/imafungus.2017.08.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 30.Bonfante P, Desirò A. 2017. Who lives in a fungus? The diversity, origins and functions of fungal endobacteria living in Mucoromycota. ISME J 11:1727–1735. doi: 10.1038/ismej.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okrasińska A, Bokus A, Duk K, Gęsiorska A, Sokołowska B, Miłobędzka A, Wrzosek M, Pawłowska J. 3 November 2020. 3D visualization of bacteria within the hyphae of Mortierella elongata WA50687 using FISH. YouTube video, 00:06, posted by alisosiemnascie. https://youtu.be/DU81FZs-Bqk.
- 32.Lastovetsky OA, Krasnovsky LD, Qin X, Gaspar ML, Gryganskyi AP, Huntemann M, Clum A, Pillay M, Palaniappan K, Varghese N, Mikhailova N, Stamatis D, Reddy TBK, Daum C, Shapiro N, Ivanova N, Kyrpides N, Woyke T, Pawlowska TE. 2020. Molecular dialogues between early divergent fungi and bacteria in an antagonism versus a mutualism. mBio 11:e02088-20. doi: 10.1128/mBio.02088-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warcup JH. 1950. The soil-plate method for isolation of fungi from soil. Nature 166:117–118. doi: 10.1038/166117b0. [DOI] [PubMed] [Google Scholar]
- 34.qGIS Development Team. 2018. qGIS geographic information system. Open Source Geospatial Foundation, Beaverton, OR. [Google Scholar]
- 35.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 36.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glöckner FO, Tedersoo L, Saar I, Kõljalg U, Abarenkov K. 2019. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 47:D259–D264. doi: 10.1093/nar/gky1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NCBI Resource Coordinators. 2017. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 45:D12–D17. doi: 10.1093/nar/gkw1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice P, Longden I, Bleasby A. 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 40.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. 2019. RAxML-NG: a fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35:4453–4455. doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 44.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 45.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 46.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 47.Rambaut A. 2012. FigTree. http://tree.bio.ed.ac.uk/software/figtree/.
- 48.Letunic I, Bork P. 2019. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrington B. 2020. Inkscape. https://inkscape.org/.
- 50.Nielsen PH, Daims H, Lemmer H. 2009. FISH handbook for biological wastewater treatment. IWA Publishing, London, UK. [Google Scholar]
- 51.Loy A, Horn M, Wagner M. 2003. probeBase: an online resource for rRNA-targeted oligonucleotide probes. Nucleic Acids Res 31:514–516. doi: 10.1093/nar/gkg016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences produced in the study can be found in the NCBI database under GenBank accession numbers MT031989 to MT032002 (MRE), MT009408 to MT009438 and MT009444 to MT009481 (fungal ITS), MT002691 to MT002716, MW055707 to MW055867, and MW080027 to MW080031 (BRE).