Introduction
Patients hospitalized with novel coronavirus disease 2019 (COVID-19) have increased risk of venous thromboembolism (VTE) [1, 2].Traditionally, diagnosis of VTE is radiographically confirmed with ultrasound or computed tomography. Due to the large number of patients with suspected VTE and the need to protect staff and conserve personal protective equipment, many hospitals were forced to develop guidelines to prioritize diagnostic imaging for the highest risk patients. This clinical reality led to circumstances in which empiric therapeutic anticoagulation may be considered without radiographic confirmation [3, 4].
Drawing on our clinical experiences, we hypothesized that many patients with COVID-19 were treated for VTE without a confirmatory diagnostic test, changing the certainty of the diagnosis, potentially misclassifying patients as having VTE who did not. This fact will cause important new challenges for researchers using national registries and databases to investigate VTE in patients with COVID-19. For example, many studies of VTE in patients with COVID-19 require VTE confirmation by duplex or CT scan [5, 6]. This study sought to determine the proportion of patients with COVID-19 empirically treated for VTE without diagnostic imaging.
Methods
We retrospectively identified patients hospitalized for COVID-19 at The Johns Hopkins Hospital from 3/1/2020 to 5/21/2020. We used our electronic health record to identify patients diagnosed with VTE and grouped them by (1) received standard treatment for VTE after imaging confirmation, or (2) received empiric treatment for VTE without diagnostic imaging. We collected patient demographics, clinical characteristics, and outcomes via a combination of automated database extraction and manual chart review. We defined major bleeding according to the International Society of Thrombosis and Hemostasis [7]. We compared outcomes between patients with and without VTE using Fisher’s exact or Chi-square tests, as appropriate. This study was approved by the Johns Hopkins Institutional Review Board.
Results
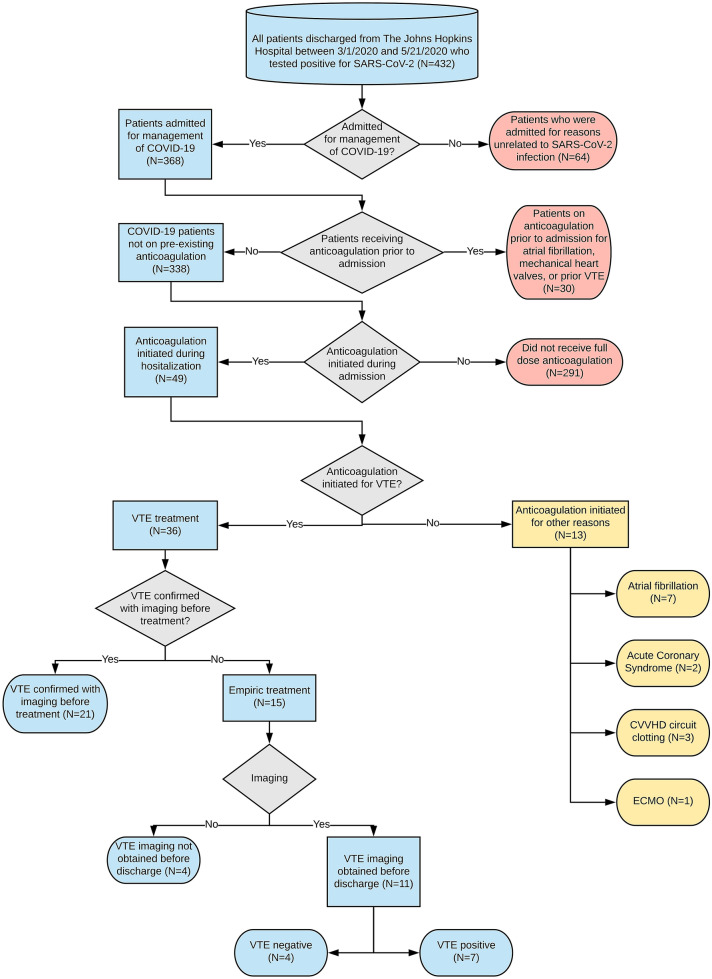
Of 338 COVID-19 patients not anticoagulated before admission, 36 (10.7%) received therapeutic anticoagulation for VTE. Twenty-one (58.3%) were initiated therapeutic anticoagulation after confirmation of VTE by imaging, and 15 (41.7%) were empirically treated before imaging. Of 15 patients treated empirically, 11 (73.3%) subsequently underwent diagnostic imaging (mean and median times between initiation of anticoagulation and confirmatory imaging was 5.5 days ± 4.5 days SD and 5 days (1–7 IQR), respectively. VTE was identified in 7 patients (63.6%, Fig. 1). The 4 patients without confirmed VTE were exposed to potential harm from anticoagulation, without any clinical benefit. In this group, the mean time between initiation of anticoagulation and imaging was 5 days (± 3.7 days SD). Bleeding complications occurred in 6 patients (16.7%), including 2 (n = 21, 9.5%) with confirmed VTE before anticoagulation and 4 (n = 15, 26.7%) empirically treated for VTE (Table 1).
Fig. 1.
Flowchart for VTE data abstraction
Table 1.
Demographic and clinical characteristics of COVID-19 population at the johns hopkins hospital from March 1 to May 21, 2020
| Characteristics | COVID-19 patients without VTE | COVID-19 patients with VTE treated after imaging confirmatione | COVID-19 patients empirically treated for VTE | P valuea |
|---|---|---|---|---|
| Patients meeting criteria*, n | 302 | 21 | 15 | |
| Mean age (SD), years | 54.2 (18.0) | 53.6 (18.7) | 58.7 (14.9) | 0.63 |
| Female, n (%) | 140 (46.4) | 8 (38.1) | 7 (46.7) | 0.77 |
| Race, n (%) | ||||
| Black | 124 (41.1) | 11 (52.4) | 4 (26.6) | 0.15 |
| Caucasian | 71 (23.5) | 7 (33.3) | 3 (20.0) | |
| Other | 107 (34.4) | 3 (14.3) | 8 (53.3) | |
| Hispanic Ethnicity, n (%) | 81 (26.8) | 2 (9.5) | 5 (33.3) | 0.15 |
| Median body mass index (IQR), kg/m2 | 29.1 (24.6, 34.0) | 26.5 (24.9, 32.9) | 32.2 (25.6, 37.8) | 0.17 |
| Median length of stay (IQR), days | 6 (3, 11) | 16 (4, 22) | 12 (4, 24) | < 0.001 |
| Intubated, n (%) | 47 (15.6) | 8 (38.1) | 8 (53.3) | < 0.001 |
| Alive at discharge, n (%) | 276 (91.4) | 19 (90.5) | 10 (66.7) | 0.02 |
| Major bleedingb, n (%) | 2 (0.7) | 2 (9.5) | 4 (26.7) | < 0.001 |
| Fatal bleedingc | 1 (0.3) | 0 (0) | 0 (0) | 1.00 |
| Symptomatic bleeding in critical aread | 0 (0) | 1 (4.8) | 2 (13.3) | 0.001 |
| Bleeding causing fall in hgb > 2 g/dL | 1 (0.3) | 1 (4.8) | 2 (13.3) | 0.002 |
| Type of therapeutic anticoagulation, n (%) | ||||
| IV Heparin Infusion | 9 (3.0) | 13 (61.9) | 12 (80.0) | < 0.001 |
| LMWH | 3 (1.0) | 6 (28.6) | 3 (20.0) | |
| Other | 1 (0.3) | 2 (9.5) | 0 (0) | |
aP value obtained by Fisher’s exact, significance set at < 0.05
bMajor bleeding event as defined by ISTH (2005): (1) Fatal bleeding; (2) Symptomatic bleeding in a critical area or organ such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome; (3) Bleeding causing a fall in hemoglobin (hgb) level of 2 g/dL or more or leading to transfusion of two or more units of whole blood or red blood cells
cPatient anticoagulated for ECMO and died of bleeding complications while on central ECMO
dIncluded one patient with SAH after initiation of AC, one patient with retroperitoneal hematoma, and one patient with a thigh hematoma
eCT scan was less available than portable duplex ultrasound to patients in this study since many were too unstable to travel to CT. Therefore, VTE was not subdivided into PE and DVT as we did not feel we could rule out PE in patients with identified DVT and many clinicians suspecting PE used ultrasound to look for DVT instead as surrogate for PE
*Inclusion criteria: (1) Patients admitted to the hospital for management of COVID-19 who are not on preexisting anticoagulation
Discussion
Over 10% of patients hospitalized for COVID-19 treatment were treated for acute VTE during their hospitalization. Empiric anticoagulation for VTE was initiated before diagnostic imaging in over 40% of these patients. This dramatic practice shift from treating a VTE after diagnostic imaging to empiric anticoagulation adds substantial uncertainty and potential misclassification to the diagnosis of VTE. Once anticoagulation treatment begins, imaging is less useful. Even if a thrombus were present initially, it may not be detected after a few days of anticoagulation [4].
This discrepancy between practice and existing research on VTE in the setting of COVID-19 is alarming. This misclassification may underestimate the true incidence of VTE events in patients with COVID-19. Moreover, exclusion of patients from COVID-19/VTE research who are empirically anticoagulated before confirmatory VTE testing and ultimately test negative for VTE, may lead authors to underestimate bleeding complications associated with anticoagulation [8]. As expected, patients who were empirically treated for VTE were more likely to be critically ill at the time of initiation of anticoagulation. More than half of patients who were empirically treated were intubated at the time of treatment, compared to 38% of patients who were treated after diagnostic imaging.
One recent study suggested a more liberal use of therapeutic anticoagulation based on its association with increased in-hospital survival for patients with COVID-19 [9]. However, therapeutic anticoagulation is not without risks. We found over 25% of those treated empirically experienced major bleeding.
While this study was limited by small sample size from a single center, these data are consistent with reports from other institutions during the pandemic and underscore the potential harm of empiric anticoagulation. Furthermore, our experience emphasizes the importance of obtaining diagnostic imaging whenever possible. Imaging should be prioritized for patients with a high pre-test probability for VTE and for those with high risk of bleeding on anticoagulation [10].
Conclusions
We have submitted this small, single center retrospective study, not to provide clinical guidance on the complex issue of empiric anticoagulation for suspected VTE in the setting of COVID-19, but rather, to highlight the role of empiric anticoagulation for suspected (but not confirmed) VTE in patients with COVID-19.
A significant proportion (40%) of patients with COVID19 at our institution were treated empirically for VTE without imaging confirmation, which illustrates a significant change in practice with important implications for quality improvement, research, and regulatory affairs. VTE empirically treated without confirmatory testing should be clearly, but differentially, reported in databases and registries to facilitate research and quality improvement efforts, particularly in the COVID-19 era.
Acknowledgements
Mr. Lau, Ms. Varasteh Kia, Drs. Owodunni and Haut report research funding from The Patient-Centered Outcomes Research Institute (PCORI). Mr. Lau, Ms. Varasteh Kia, Drs. Owodunni, Streiff, and Haut report research funding from the Agency for Healthcare Research and Quality (AHRQ). Mr. Lau, Drs. Streiff and Haut report research funding from the NIH/NHLBI. Mr. Lau reports research funding from the Institute for Excellence in Education and The Johns Hopkins University Catalyst Award. Dr. Streiff reports research funding from Boehringer-Ingelheim, Janssen, Portola and Roche and consulted for Bristol Myers Squibb, Dispersol, Janssen, Pfizer and Portola and has given expert witness testimony in various medical malpractice cases. Mr. Lau, Ms. Varasteh Kia, and Dr. Haut report research funding from the DOD/Army Medical Research Acquisition Activity. Dr. Haut reports research funding from the Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF). Dr. Haut reports royalties from Lippincott, Williams, Wilkins for a book, "Avoiding Common ICU Errors," and was a paid speaker for the Vizient Hospital Improvement Innovation Network (HIIN) VTE Prevention Acceleration Network.
Author contributions
All authors listed have contributed sufficiently to the project to be included as authors and those who are qualified to be authors are listed in the author byline.
Funding
This work was not supported by any contract or grant.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel L. Eisenson, Email: daniel@jhmi.edu
Oluwafemi P. Owodunni, Email: oowodun1@jhmi.edu
Brandyn D. Lau, Email: blau2@jhmi.edu
Mujan Varasteh Kia, Email: mvarast1@jhmi.edu.
Peggy S. Kraus, Email: pkraus2@jhmi.edu
Christine G. Holzmueller, Email: cholzmu1@jhmi.edu
Dauryne L. Shaffer, Email: dshaffe1@jhmi.edu
Michael B. Streiff, Email: mstreif@jhmi.edu
Elliott R. Haut, Email: ehaut1@jhmi.edu
References
- 1.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients With COVID-19 in a New York city health system. JAMA. 2020 doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piazza G, Morrow DA. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. JAMA. 2020;324(24):2548. doi: 10.1001/jama.2020.23422. [DOI] [PubMed] [Google Scholar]
- 3.Trigonis RA, Holt DB, Yuan R, et al. Incidence of venous thromboembolism in critically Ill coronavirus disease 2019 patients receiving prophylactic anticoagulation. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02138-z5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients With COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients: Definitions of major bleeding in clinical studies. J Thromb Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 8.Florecki KL, Owodunni OP, VarastehKia M, et al. What does venous thromboembolism mean in the national surgical quality improvement program? J Surg Res. 2020;251:94–99. doi: 10.1016/j.jss.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients With COVID-19. J Am Coll Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obi AT, Barnes GD, Wakefield TW, et al. Practical diagnosis and treatment of suspected venous thromboembolism during COVID-19 pandemic. J Vasc Surg Venous Lymphat Disord. 2020;8(4):526–534. doi: 10.1016/j.jvsv.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]