Abstract
Rod cell oxidative stress is a major pathogenic factor in retinal disease, such as diabetic retinopathy (DR) and retinitis pigmentosa (RP). Personalized, non-destructive, and targeted treatment for these diseases remains elusive since current imaging methods cannot analytically measure treatment efficacy against rod cell compartment-specific oxidative stress in vivo. Over the last decade, novel MRI-based approaches that address this technology gap have been developed. This review summarizes progress in the development of MRI since 2006 that enables earlier evaluation of the impact of disease on rod cell compartment-specific function and the efficacy of anti-oxidant treatment than is currently possible with other methods. Most of the new assays of rod cell compartment-specific function are based on endogenous contrast mechanisms, and this is expected to facilitate their translation into patients with DR and RP, and other oxidative stress-based retinal diseases.
Keywords: Animal models, Calcium channels, Diabetes, Retinitis pigmentosa, MRI, Retinopathy, Subretinal space, Vision
1. Introduction
The most prevalent photoreceptor in the mammalian retina is the rod cell. Rod cells play an essential role in both vision and health of other cells in the retina (Berkowitz et al., 2014; Bissig et al., 2013; Cingolani et al., 2006; Curcio et al., 1996; Dong et al., 2006; Du et al., 2013; Kassen et al., 2009; Kolesnikov et al., 2010; Komeima et al., 2006; Rogers et al., 2007; Shen et al., 2005; Usui et al., 2009b). Rod cells have two major and highly compartmentalized functions related to vision: light detection in its posterior outer segments and synaptic terminal neurotransmitter release at its anterior pole; the extracellular space surrounding the outer segments [i.e., the subretinal space (SRS)] is also a key compartment for maintaining a healthy dark current and visual cycle (Adijanto et al., 2009; Cao et al., 1996; Li et al., 1994b).
Diabetic retinopathy (DR) and retinitis pigmentosa (RP) are two major diseases of the retina characterized by irreversible anatomical changes, deterioration of vision, and ultimately blindness (Campochiaro et al., 2015; Kern et al., 2010; Punzo et al., 2012). Current treatment options are either destructive and sub-optimal for DR (e.g., panretinal photocoagulation) or non-existent for RP (Campochiaro et al., 2015; Kern et al., 2010; Punzo et al., 2012). Rod cell-based oxidative stress has been implicated as a common pathogenic event underlying eventual histopathology in DR and RP, among other retinopathies (Fig. 1) (Cingolani et al., 2006; Dong et al., 2006; Du et al., 2013; Kassen et al., 2009; Komeima et al., 2006; Rogers et al., 2007; Shen et al., 2005; Usui et al., 2009b). A likely source of rod cell oxidative stress is the ellipsoid region of the inner segment which contain ~75% of retinal mitochondria (Johnson, Jr. et al., 2007; Medrano and Fox, 1995; Perkins et al., 2003). Notably, other abnormalities in, for example, DR, such as inflammation, appear as downstream consequences of oxidative stress (Du et al., 2013). In addition, the identification of rod cells as the main contributor to retinal oxidative stress complicates interpretation of systemic treatments that were previously thought to correct primarily endothelial cell oxidative stress.
Fig. 1.
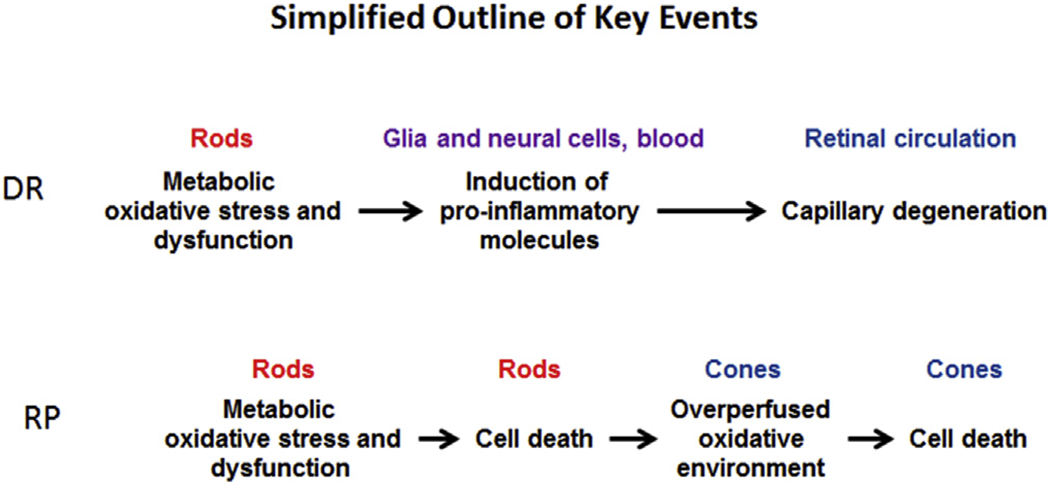
Simplified representation of key events in DR and RP over time; DR and RP time courses are not to scale and are not comparable. The key point here is that rod cell metabolic oxidative stress and dysfunction – either from a metabolic disturbance (DR) or genetic abnormality (RP) – are important early events.
These considerations provide a rationale for focusing on the evaluation of anti-oxidant therapy on rod cells in vivo. Much better outcomes in DR and RP can thus be expected if antioxidant therapy is started before gross changes are evident. However, testing the effectiveness of an antioxidant against rod oxidative stress has mostly relied on post-mortem studies and/or observing improvements in animal models following (often pleiotropic) anti-oxidant therapy (Berkowitz et al., 2007c, 2012a, 2009b; Campochiaro et al., 2015; Du et al., 2013, 2015; Fukuda et al., 2014; Galbinur et al., 2009; Jaliffa et al., 2009; Komeima et al., 2006; Rohrer et al., 2004; Sanz et al., 2007; Usui et al., 2009a; Yang et al., 2007; Zeng et al., 2014; Zheng et al., 2007). Such approaches i) are often unable to determine whether oxidative stress in rod cells per se has been corrected, and ii) are not useful for examining the same animal over time or for examining rod cell oxidative stress in patients. A need remains to non-invasively measure the efficacy of antioxidant therapy against early rod cell compartment-specific oxidative stress in diseases like DR and RP in order to personalize anti-oxidant therapy with regard to timing and dosing in vivo. In vulnerable neurons, including rod cells, one of the first consequences of pathogenic oxidative stress is cell dysfunction [e.g., (Du et al., 2013, 2015; Mao et al., 2014; Roddy et al., 2012; Wang and Michaelis, 2010)] (Fig. 1). Thus, we have focused this review on efforts on developing an optimized imaging platform that can non-invasively measure the effectiveness of antioxidant treatment in correcting rod cell compartment-specific pathophysiology before the appearance of other clinical biomarkers in disease.
1.1. Problems with current technology
Conventional non-invasive approaches measure either blood vessel/flow (optical coherence tomograph (OCT) angiography, fluorescein angiography), anatomy (fundus photography, OCT, adaptive optics), or a global function (ERG). Behavioral tests (e.g., optokinetic tracking) examine whole-animal visual performance (e.g., acuity and contrast sensitivity) usually under phototopic conditions. Thus, these common approaches do not measure the package of rod-specific functions together with retinal vascular and choroid functions that are specifically evaluated by MRI (see below).
At present, OCT is the gold-standard for evaluating anatomical changes in the laminar structure of the retina in vivo but provides little functional information especially about rod cells. The most common method for evaluating rod photoreceptor function in vivo is the electroretinogram (ERG), which measures an integrated panretinal signal. Several studies have used ERG used to evaluate anti-oxidant therapies on diabetes-induced rod dysfunction, however, ERG reports on the entire retina and thus provides no panretinal spatial resolution (Barile et al., 2005; Horio et al., 2004; Johnsen-Soriano et al., 2008; Midena et al., 1989; Samuels et al., 2012). Multi-focal ERG (mfERG) can distinguish electrical responses from different regions panretinally but suffers from extensive light scattering in small rodent eyes (Ball and Petry, 2000; Nusinowitz et al., 1999). In other words, electrophysiology is a relatively insensitive tool for evaluating focal dysfunction in common preclinical models. ERG measures an important but limited aspect of global rod function: movement of monovalent ions on a millisecond time scale after a flash of light in vivo. However, ERG does not report on changes in the flux of important divalent ions such as calcium. It is likely that this is why ERG has not been able to predict loss of visual performance in models of retinal degeneration and in aging in the absence of overt pathology (Bissig et al., 2013; McGill et al., 2012). ERG thus does not capture the increased calcium channel function that was found to be highly predictive of later declines in visual performance (Bissig et al., 2013). These considerations directly speak to the need for MRI methods that do quantitatively evaluate calcium channel function in vivo with high spatial resolution.
In addition, each approach is incomplete on its own (e.g., ERG provides function information without spatial resolution, OCT provides high spatial resolution but not information about rod function). Thus, the information available from each approach cannot be integrated into a coherent whole. For these reasons, the above methods have had limited success in evaluating the efficacy of antioxidant treatment, specifically on rod cells early in the course of disease, before the appearance of histopathology. The goal of this review is to highlight that MRI fills a non-invasive technology need not addressed by conventional technologies for i) a package of quantitative and non-invasive measurement of rod cell compartment-specific function together with retinal vessel and choroidal functions in vivo, ii) integrating retinal structure and rod function into a coherent picture and iii) early detection and evaluation of anti-oxidant treatment efficacy before the gross appearance of disease.
2. Previously learned lessons
Here, we briefly summarize three examples that illustrate how earlier and lower spatial resolution MRI studies of retinal disease and its treatment showed alternate views of retinal disease, and came to alternate conclusions that need to be evaluated alongside that of other studies (Berkowitz and Roberts, 2008, 2010; Trick and Berkowitz, 2005).
Example 1:
Tissue ablation surgery (e.g., panretinal photocoagulation) is the standard of care for proliferative and non-proliferative retinopathy. This surgery is commonly assumed to relieve retinal hypoxia at the border of vascular and avascular retina that is driving abnormal new vessel growth (i.e., retinal neovascularization). If this assumption is true, retinal hypoxia should precede the appearance of neovascularization. However, magnetic resonance studies did not find evidence to support a special early role of retinal hypoxia in proliferative retinopathy models, a conclusion in-line with that of other labs (Alder et al., 1991; Berkowitz, 2008; Diederen et al., 2006; Lau and Linsenmeier, 2014; Ola et al., 2006; Reeves et al., 2002; Wanek et al., 2014; Wright et al., 2011; Zhang et al., 2003).
In non-proliferative diabetic retinopathy models, the evidence to-date has failed to support a role for hypoxia at least before the appearance of histopathology (Alder et al., 1991; Diederen et al., 2006; Lau and Linsenmeier, 2014; Ola et al., 2006; Reeves et al., 2002; Wanek et al., 2014; Wright et al., 2011). To-date, only one study of three cats diabetic for 10 years has reported evidence for hypoxia together with vascular histopathology (Linsenmeier et al., 1998); it is not known if this is also true in more common animal models, such as the diabetic rat. Here, we present new data that begins to address this question. Pre-retinal oxygen tension was measured by a magnetic resonance method after 9–10 mo of diabetes, a time when vascular lesions are present in a non-proliferative diabetic rat model. In control and 9–10 mo streptozotocin-induced diabetic male Sprague Dawley rats, preretinal oxygen levels were measured with 19F NMR of a preretinal vitreous perfluorocarbon droplet during room air or 12% oxygen breathing in vivo (Arteel et al., 1998; Barber et al., 2005; Feit-Leichman et al., 2005; Handa et al., 1996; Kern et al., 2000; Kowluru et al., 2001; Zhang et al., 2003); the 12% condition was included to demonstrate the sensitivity of the method to hypoxia. Because of the small distances, a pre-retinal oxygen measurement reflects to some degree inner retinal oxygen levels but not as sensitively as an intraretinal oxygen measurement (Arteel et al., 1998; Barber et al., 2005; Feit-Leichman et al., 2005; Handa et al., 1996; Kern et al., 2000; Kowluru et al., 2001; Zhang et al., 2003). A level of hyperglycemia of 400–550 mg/dl was maintained for the duration of diabetes. Core temperature and blood gas parameters measured after the experiment and during either 12% oxygen breathing or room air were appropriate for each challenge (data not shown). In control rats, no significant difference (P > 0.05) was found between pre-retinal PO2 during room air in the 3 and 9–10 mo control groups (32 mm Hg ± 4 mm Hg, n = 6; 23 ± 2 mm Hg, n = 7, respectively; mean ± SEM). Pre-retinal vitreous oxygen tension during 12% oxygen inhalation (1 ± 4 mm Hg, n = 6) was significantly (P < 0.05) lower than that measured during room air breathing (~21%; 32 mm Hg ± 4 mm Hg, n = 6). Pre-retinal vitreous oxygen levels during room air breathing were not statistically different between 9 and 10 mo controls (23 ± 2 mm Hg, n = 10) and rats diabetic for 10–13 months (25 ± 8 mm Hg, n = 3) While these new data do not completely rule out the possibility of small areas of focal or subclinical hypoxia in retinas, it is hard to rationalize how such changes could play a major role in the development of a number of changes found in the retina during experimental diabetes, including upregulation of the potent mitogen vascular endothelial growth factor (VEGF) (Frank, 2004), and apoptosis of retinal cells (Bussink et al., 2003; Kern et al., 2000). These results, limited by measuring from a single location in the pre-retinal vitreous, do not support the presence of retinal hypoxia during the appearance of clinically relevant retinal vessel histopathology in the rat model. Overall, little evidence is available to support an early (i.e., pre-histopathologic) role of hypoxia in proliferative and non-proliferative retinopathy, although the role of hypoxia later in the disease remains unclear. More work is needed to identify early, non-hypoxia-based mechanisms in order to improve upon current treatment strategies.
Example 2:
It is a common belief that a leaky blood retinal barrier is the primary cause of diabetic retinal edema and subsequent vision loss. By taking advantage of MRI inherent sensitivity to water spin density and self-diffusion, together with MRI measurements of retinal thickness, it was established that diabetic male Sprague Dawley rats present with retinal edema from 2 to 9 mo of diabetes; 2 mo was the earliest time point examined by MRI and others find evidence for increased retinal thickness as early as 2 weeks of diabetes in the male Sprague Dawley rat (Berkowitz et al., 2012b; Clermont et al., 2011). However, there is less agreement as to when the blood retinal barrier (BRB) is disrupted. Studies find evidence for increased leakiness starting as early as 1 weeks of diabetes or as late as 8 mo of diabetes (Berkowitz et al., 2004; Xu et al., 2001). The reasons for the differences in the appearance of BRB damage in diabetic models are unclear. We note that many of the studies reporting earlier loss of BRB integrity involve tracers such as Evans blue and fluorescein that bind to albumin (Antonetti et al., 1998; Xu et al., 2001). In contrast, studies that report later BRB breakdown involve strictly passive tracers such as Gd-DTPA and sucrose (Berkowitz et al., 2004; Ennis and Betz, 1986). A potential unifying explanation is that the different tracers report on different types of BRB damage over time with earlier injury occurring to albumin-based paracellular transport mechanisms and later leakage due to loss of tight cell–cell junctions (Berkowitz et al., 2012b, 2004; Ennis and Betz, 1986; Grimes, 1985, 1988). In either case, a common misperception is that tracer accumulation equates to increased permeability. It is more accurate to view tracer accumulation as a function of tracer influx, the tracer plasma concentration integral over time, and tracer efflux (Berkowitz et al., 1992). Thus, if influx of a tracer did not change but tracer efflux decreased, one would measure net accumulation of the tracer. Indeed, new evidence supports the view that water efflux via Mueller cells, for example, is impaired by diabetes (Reichenbach et al., 2007). Growing evidence is expected to redirect efforts away from studies of water influx pathways as a cause of diabetic retinal edema to an improved appreciation of cellular mechanisms underlying impaired water removal.
Example 3:
In proliferative and non-proliferative disease, the most commonly detected abnormality across many labs and methods (including MRI) is retinal vessel dysfunction during, for example, a hyperoxic provocation (i.e., a stress test) (Kern et al., 2010; Lorenzi et al., 2010; Trick and Berkowitz, 2005). Importantly, treatments that correct retinal vessel dysfunction also correct later histopathology (Berkowitz et al., 2013; Trick and Berkowitz, 2005). Correction of impaired autoregulation early in the course of the disease as measured by MRI, acts as a prognostic treatment efficacy biomarker (Berkowitz et al., 2013; Berkowitz and Roberts, 2008, 2010; Trick and Berkowitz, 2005). From these results, evaluating retinal autoregulation using non-MRI methods that measure, for example, retinal vessel diameter, may be a useful approach in the clinical management of treatment efficacy in DR.
These three examples underscore MRI as a valuable discovery tool in the study of retinal pathophysiology and for gauging early treatment efficacy in vivo. However, in those early MRI studies, resolution was too low to interrogate rod cell function.
3. Why not use fMRI to study rod activity?
Common MRI methods for evaluating neuronal function involve approaches such as blood-oxygen level dependent (BOLD) or arterial spin labeling (ASL) approaches that capitalize on task-dependent hemodynamic changes to spotlight regions of the brain that are active (Detre and Wang, 2002). These methods are broadly used, in part, because they are reliable and do not involve injecting a contrast agent (Kim and Ogawa, 2012). BOLD, ASL, and related methods, have been modified for use to study the retina but at low resolution relative to the retinal thickness (Duong et al., 2002; La Garza et al., 2011; Li et al., 2008; Muir and Duong, 2011; Nair et al., 2011; Shih et al., 2011; 2012). Critically, BOLD and ASL only measure hemodynamically-dependent indices linked with neuronal activity and rod cells exist in an avascular environment. Thus, fMRI approaches are not expected to be particularly useful for specifically interrogating rod cell function.
4. Breakthrough
In 2006, the first images of rat retina in vivo were published with a remarkable axial spatial resolution of 23.4 μm (Luan et al., 2006). At the time each retinal image collection required about 1 h. Over the years, with better MRI systems and acquisition schemes, images with this resolution can be collected in just a few minutes; similarly high resolution images can also be obtained in other common laboratory animals, for example in mice and zebrafish (in collaboration with Dr. Ryan Thummel, Wayne State University) (Fig. 2) (Chan et al., 2012; Chen et al., 2008, 2011; Duong, 2014; Wang et al., 2011).
Fig. 2.
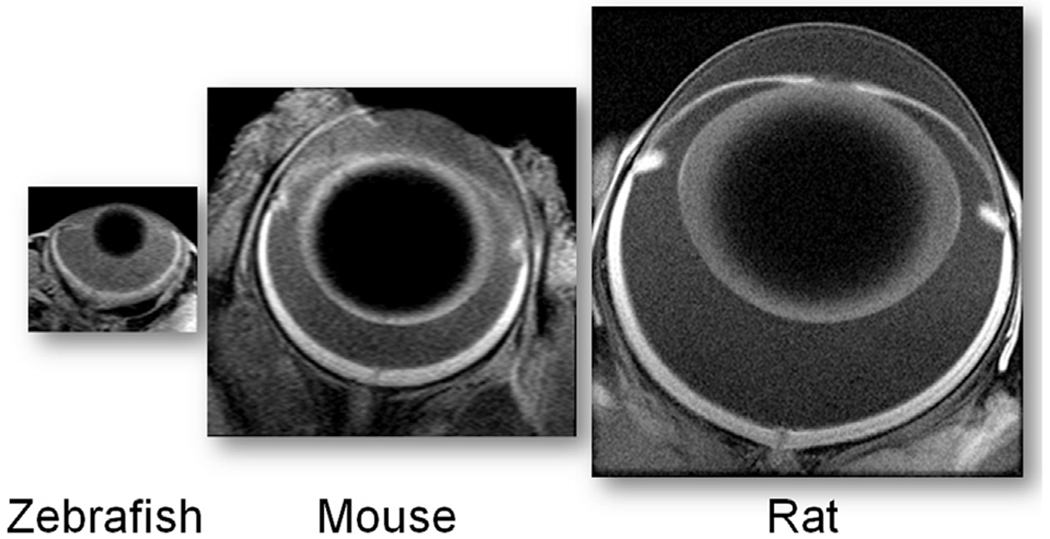
Representative MRI images (axial resolution 23.4 μm) of three common laboratory models acquired using established protocols (Berkowitz et al., 2014; Bissig et al., 2013); the images are proportionately sized based on ocular anatomy.
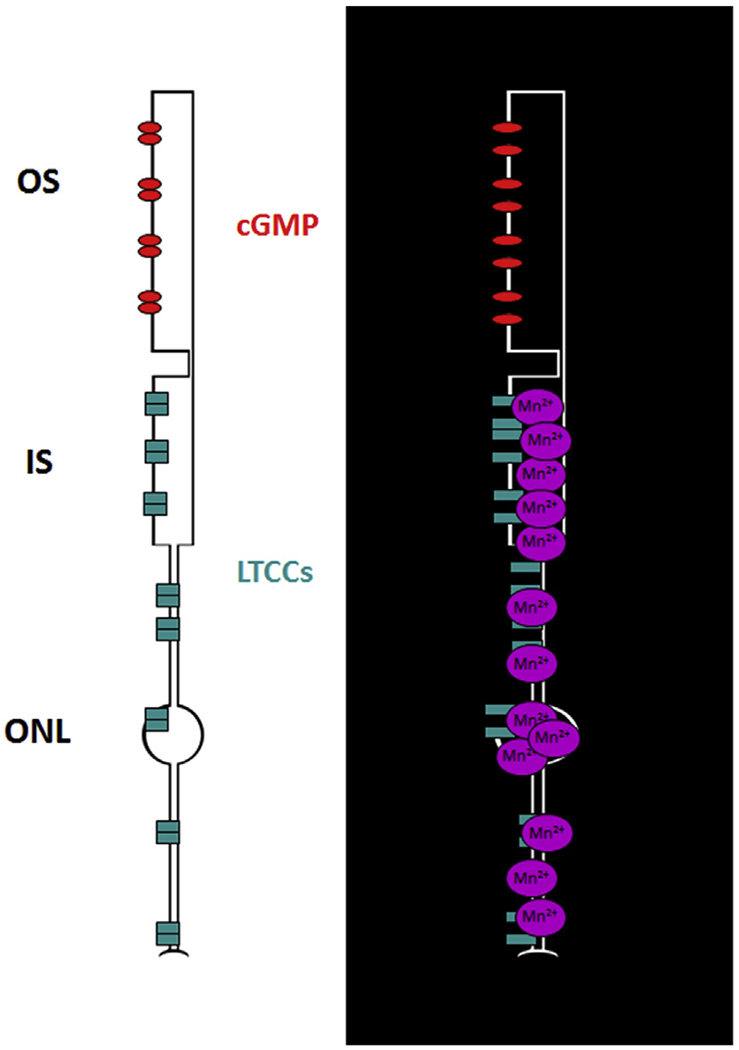
This 23.4 μm resolution is important because it allows for the collection of MRI data that can distinguish inner retina structure/function from that in the outer retina aided by the 200 μm thick retina having a well-defined laminar structure (Berkowitz et al., 2009a, 2010, 2012a, 2006, 2007d; Bissig and Berkowitz, 2011). The inner retina has a multi-laminar vasculature and a large number of different and overlapping cell types. These features can confound interpretation of MRI data from the inner retina. On the other hand, MRI of the outer retina is more readily interpreted because the outer retina is avascular and, in mice, ~97% of the cells are rods (Carter-Dawson et al., 1978). In addition, advantage of the distinct rod cell compartment-specific functions performed by rod nuclear and outer segment layers allows further spatial clarity (Fig. 3). These functions include i) activity-dependent voltage-gated L-type calcium channels (LTCCs) in the outer nuclear layer (ONL) and inner segments (IS) but not in the outer segments (OS), ii) light-dependent formation of tetrameric arrestin1 in the ONL and IS and its translocation as a monomer to the OS, and iii) light-evoked expansion of the extracellular space around the OS (i.e., the subretinal space, SRS) (Fig. 3) (Berkowitz et al., 2015a; Bissig and Berkowitz, 2012). Thus, MRI evaluates the same set of rod cells for compartment-specific function in vivo using different types of acquisitions (see below for details; summarized in Fig. 4) (Berkowitz et al., 2013, 2015a, 2015c; Bissig and Berkowitz, 2012). In addition, four “free” metrics are also obtained: retinal and choroidal thickness, and light-dependent expansion of the inner retinal circulation and choroid (Fig. 4). Further confidence is provided by the spatial agreement between transretinal MRI maps of function and OCT (Fig. 4).
Fig. 3.
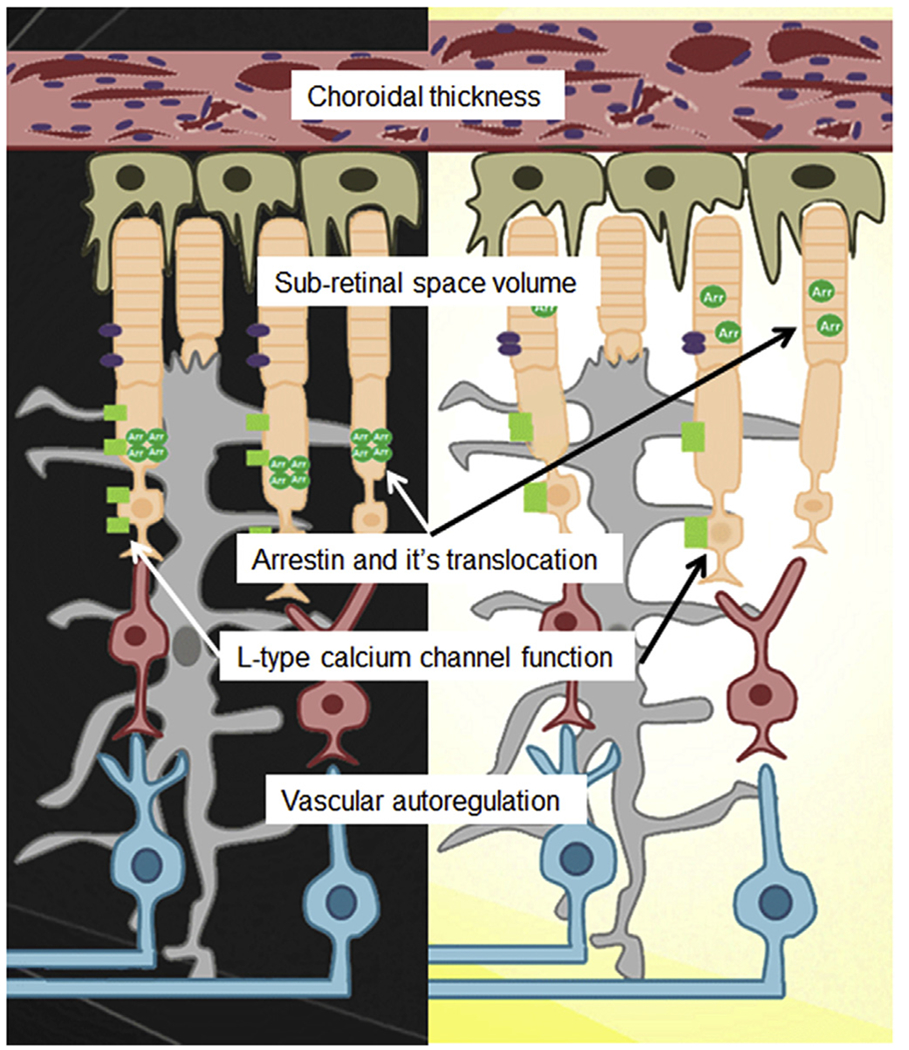
Simplified illustration of the rod cell functionome (white boxes in center) in dark (left) and light (right) as assessed by multi-functional MRI metrics. The array of essential information measured by MRI (shown in the white boxes) is unavailable from other imaging methods. Inner retinal circulatory plexi are not shown. Drawing courtesy of Jawan Gorgris.
Fig. 4.
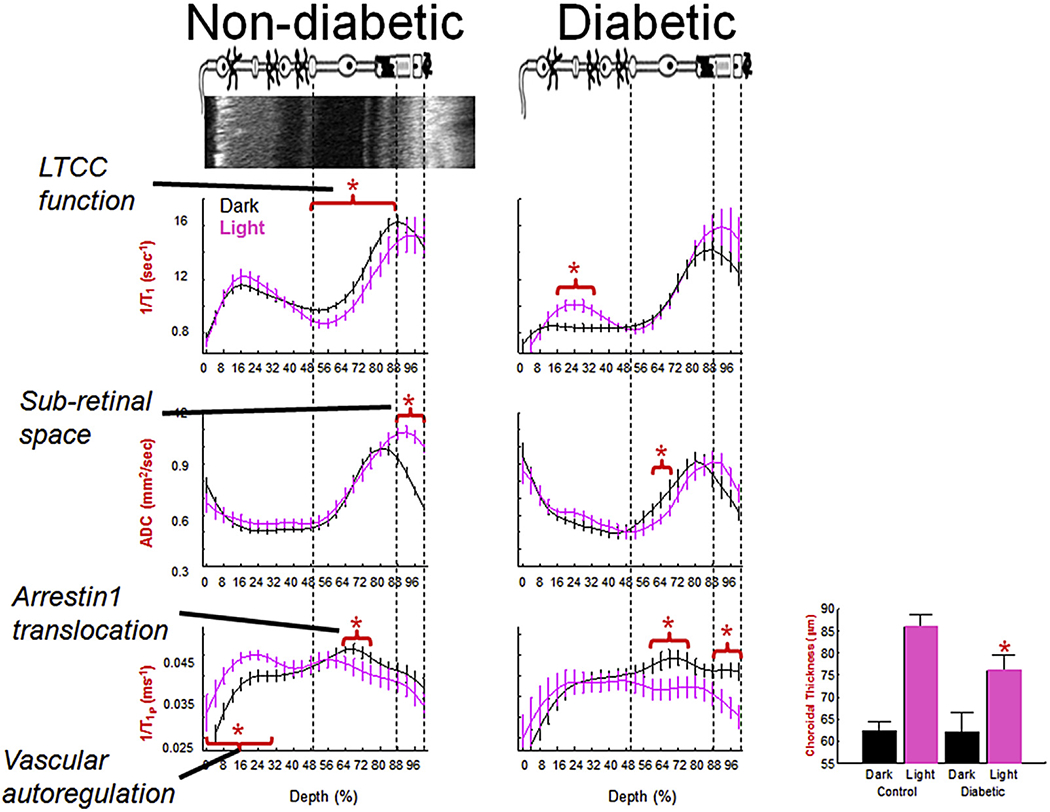
Summary of multi-functional MRI (graphs) spatially registered to OCT (top image, left). Rod functions in dark (black line) vs. light (pink line) in non-diabetic (left column) and diabetic (right column) mice. In summary, diabetes impairs light-evoked functions (except arrestin1 and its translocation) and these abnormalities were correctable with antioxidant treatments (Table 1). N’s for the dark and light data, respectively, are as follows: LTCC graphs (wt: 19 and 11, diabetic: 6 and 7); Sub-retina space graphs: (wt: 23 and 23, diabetic: 9 and 9); 1/T1ρ graphs (wt: 7 and 7, diabetic: 5 and 5). Light-evoked choroidal expansion is maintained in diabetics but to a significantly reduced degree (bargraph, paired dark/light data, n = 18 (wt) and 9 (diabetic)); an antioxidant did not correct this abnormality. *, P < 0.05. Data for each graph is obtained from central (+/− 0.4 − 1 mm from the optic nerve head) retina and shown as a function of distance from retina/non-retina borders, where 0% is the vitreous/retina border and 100% is the retina/choroid border. Above graphs: simplified schematic of retina and support circulations (Paques et al., 2003;Wangsa-Wirawan and Linsenmeier, 2003; Zhi et al., 2014). Red horizontal bracket and star above profiles indicates specific retinal regions with significant (P < 0.05) light-evoked differences. Based on OCT data (Fig. 4), vertical dashed lines approximate layer locations in retina.
In this review, we cover material from 2006 to the present, focusing on the use of MRI to simultaneously measure the above functions of rod cell compartments that are not effectively evaluated by other methods in vivo.
5. Rod nucleus/synapse/inner segment LTCCs
LTCCs are the major calcium entry path into rod cells (and other retinal neurons), and play an essential role in rod function (Chen et al., 2014; Krizaj, 2012; Molnar et al., 2012). For example, in the dark, rod cell membranes are depolarized resulting in the sustained opening of synaptic LTCCs (Fig. 5). Persistent opening of these LTCCs triggers continuous release of the neurotransmitter glutamate (Schmitz and Witkovsky, 1997). Thus, LTCCs bridge the two essential functions of the rod cell, light detection and signaling to the rest of the retina and brain; the role of LTCC in the other compartments of the rod are less clear (Xiao et al., 2007). Rod compartment-specific LTCC function in vivo can be evaluated using high resolution manganese-enhanced MRI (MEMRI).
Fig. 5.
Cartoon summarizing impact of rod cell membrane hyperpolarization in the light (left) and depolarization in the dark (right) on influx of manganese.
5.1. Brief history of MEMRI
In 1997, Alan Koretsky and his group demonstrated the potential value of using a non-toxic dose of the paramagnetic ion manganese as an intracellular MRI contrast agent whose accumulation occurred primarily in active neurons via voltage-gated LTCCs (Berkowitz et al., 2007b, 2009a, 2011, 2012a, 2013; Carlson et al., 1994; Cross et al., 2007; Drapeau and Nachshen, 1984; Jacob, 1990; Lin and Koretsky, 1997; Tofts et al., 2010). LTCCs play essential roles modulating neuronal activity including neurotransmitter release, and have a privileged role in activity-dependent gene expression via the transcription factor cAMP response element-binding protein (Mermelstein et al., 2000; Schmitz and Witkovsky, 1997). Manganese has three desirable properties. First, it enters cells over several hours and leaves slowly (days to weeks depending on the tissue) so interpretation is not confounded by efflux or time concerns (Tofts et al., 2010). Second, activity-dependent manganese entry into cells occurs primarily via LTCCs (Berkowitz et al., 2007b, 2009a, 2011, 2012a, 2013; Carlson et al., 1994; Cross et al., 2007; Drapeau and Nachshen, 1984). Importantly, based on the absence of toxicity effects at the present dose of manganese, there is little evidence to suggest that manganese blocks the calcium channels and prevents calcium entry (for more detail see section 9) (Berkowitz et al., 2013). Third, there is no endogenous agonist-releasable manganese store (Jacob, 1990), so the appearance of retained manganese unambiguously signals that its entry into the cytoplasm originated from outside the cell and not from an internal store (Jacob, 1990). For these reasons, MEMRI is a powerful discovery tool that is also useful for translating electrophysiology results obtained in isolated cells to preclinical models, and eventually, into the clinic (Tofts et al., 2010). Spatial agreement in activated regions between MEMRI and BOLD was noted in control animals (Duong et al., 2000). The Koretsky approach was, however, limited by the need to transiently breakdown the blood brain barrier in an anesthetized animal during a functional task. Breaking down the blood brain barrier even transiently is physiologically problematic. Nonetheless, it was clear from these early studies that MEMRI generates functional maps at much higher spatial resolution than other fMRI methods, as well as provides unique and direct interrogation of the extent of neuronal activation not measured by other fMRI methods.
In 2005, Dan Turnbull and his group reported that there was not a need to breakdown the blood barrier to encode function as long as the functional task lasted for several hours after systemic injection of manganese (Yu et al., 2005). The Turnbull approach offered another major advantage: functional encoding outside of the magnet while the animal is awake and freely moving. Only after the prolonged functional task is presented for a fixed period of time is the animal anesthetized and imaged to measure how much manganese accumulated. This represented an opportunity for a new approach to functional retinal imaging: combining Turnbulls’ prolonged encoding of functional information with very high resolution images of the retina. MEMRI has been called the imaging modality of choice for non-invasively studying retinal ion homeostasis (Ramos de Carvalho et al., 2014). Much of the basic operational aspect of the MEMRI has been reviewed previously (Berkowitz et al., 2013).
Below, the sensitivity and physiologic accuracy of MEMRI for studying rod cell stimulation is demonstrated by comparing the extent of manganese accumulation in different rod compartments to known modulators of rod function, such as light-evoked changes in cGMP-gated channels, the visual cycle, and horizontal cell (HC) inhibitory signaling (Berkowitz et al., 2009a, 2006).
5.2. cGMP and the visual cycle
In the dark, rod cell membranes are persistently depolarized by open cGMP channels resulting in prolonged opening of the associated LTCCs. In line with this, MEMRI measures a greater uptake of manganese in rod nuclear and inner segment layers in dark vs. light; further, the uptake was linearly and inversely proportional to the log of light intensity used for adaptation, similar to that reported between background light adaptation and extracellular current in isolated rod photoreceptors (Fig. 6) (Berkowitz et al., 2009a, 2006). Genetic inhibition of just rod phototransduction in rod transducin knockout mice (GNAT1−/−) prevents both cGMP opening and the differential uptake of manganese in rod nuclear and inner segment layers between dark and light adapted mice (Berkowitz et al., 2014). Further, visual cycle impairment at the level of RPE65 reduces 11-cis-retinal production, leading to a buildup of unbound opsin with closure of cGMP channels and LTCCs (Berkowitz et al., 2009a). Consistent with this expectation, pharmacologic suppression of RPE65 resulted in a decrease in the amount of manganese taken up in the outer retina relative to that in dark-adapted mice without such visual cycle manipulation (Berkowitz et al., 2009a). These data were used to support MEMRI’s sensitivity to impaired visual cycle.
Fig. 6.
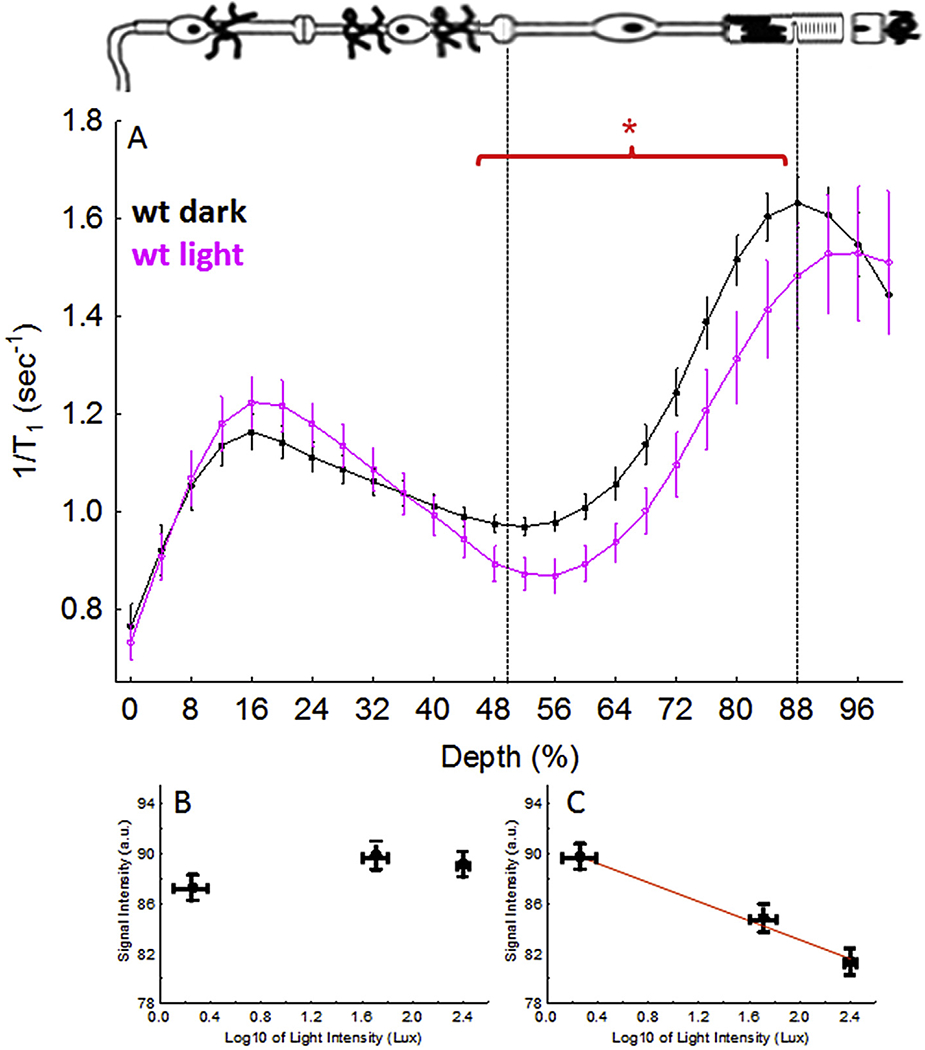
Functional LTCC topography as measured by MEMRI in dark and light adaptation. (A) Data for C57Bl/6 mice (wt); graph and graphing conventions are as in Fig. 4. B) and C) illustrate how manganese uptake in inner retina (0–50% depth) or outer retina (50–100% depth), as measured using T1 weighted MRI, response to changes in light intensity, respectively, in male albino control Sprague—Dawley rats after exposure to the following light intensities: 1.8 ± 0.7 (n = 10, mean ± SEM), 51.3 ± 11.7 (n = 5), and 250.2 ± 19.3 (n = 6) lux. (Berkowitz et al., 2009a). In C), the red line indicates a significant (P < 0.05) linear correlation. *, P < 0.05.
Considerably lower than normal uptake of manganese was measured transretinally in 6 week old rd12 mice, which have a loss-of-function mutation in RPE65; notably, this decrease was correctable with systemic 11-cis-retinal treatment (Berkowitz et al., 2009a; Pang et al., 2005). However, more recent research showed that rd12 mice undergo a significant loss of cones by 5 weeks of age, potentially explaining the impact of this mutation across the whole retina (Fig. 7); in addition, 11-cis-retinal can exert anti-oxidant effects separate from the visual cycle, thus providing an alternative benefit, as measured by MEMRI (manuscript in press) (Pang et al., 2010).
Fig. 7.
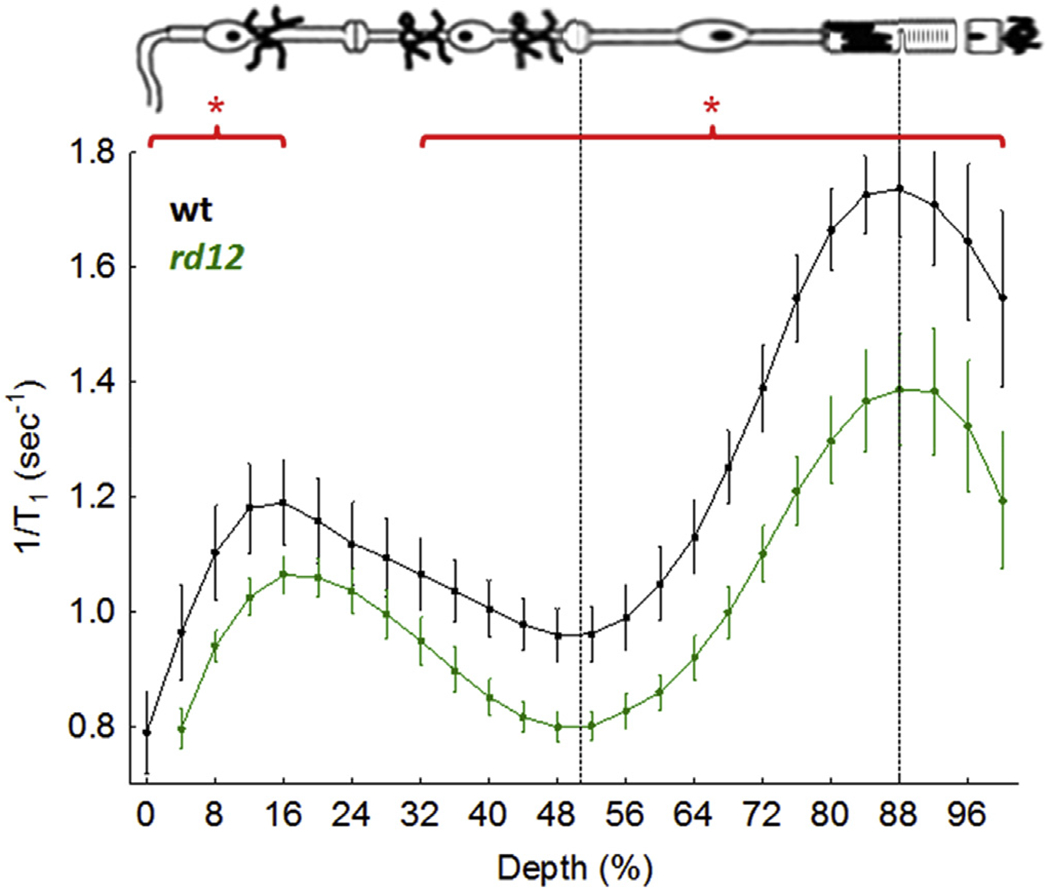
Functional LTCC topography as measured by MEMRI in dark adapted wt mice (n = 6) or 6 week old rd12 mice (n = 7); graphing conventions are as in Fig. 4. *, P < 0.05.
It is worth noting while different groups agree that the outer retina of rats accumulates more manganese in the dark than light adapted conditions, with this uptake sensitive to diabetes (Berkowitz et al., 2009a, 2006; De La Garza et al., 2012; Muir et al., 2015), there is an apparent discrepancy regarding uptake manganese uptake in the inner retina with dark and light. For example data illustrated in Fig. 4 finds no differences in the extent of manganese uptake in the inner retina between dark and light; however Duong et al. reported higher MEMRI activity in light compared with dark adaption (De La Garza et al., 2012; Muir et al., 2015). There are several possible explanations for the different results in the inner retina measured by the two groups. One is that we administer manganese to awake and freely moving animals, whereas Duong et al. injects anesthetized animals that are then allowed to wake up during dark or light adaptation; this experimental design raises the possibility of a residual anesthesia effect on retinal manganese uptake. Perhaps the most likely difference is that one group generally performs its studies in the absence of patching, and the other patches one eye to obtain dark adapted and light adapted retinae in the same animal. In an earlier paper, a significant light-dependent Mn2+ uptake in the inner retina was noted, perhaps involving a pathway from the patched eye through the brain to the unpatched eye (Bissig and Berkowitz, 2011). These considerations do not support a discrepancy between groups, but do highlight the sensitivity of MEMRI outcomes to the experimental design.
These results establish the sensitivity and accuracy of MEMRI to light detection as it impacts rod cell LTCCs.
5.3. Inhibitory signaling and rod cells
In 2007, sodium iodate (24 h post injection of 30 mg/kg, IP), an insult that can cause RPE damage, leading to blood retinal barrier breakdown, photoreceptor degeneration, retinal dysfunction, and loss of visual behavior was observed to also produce a greater than normal uptake of manganese in rods (and cells of the inner retina) (Berkowitz et al., 2007d). Notably, the supernormal uptake was not linked with iodate-induced opening of the blood retinal barrier (Berkowitz et al., 2007d). The interpretation of these results was not clear but it has been suggested that iodate-induced damage to the photoreceptors produces a loss of glutamatergic signaling to horizontal cells (HC) which in turn impaired HC inhibitory signaling back onto the rods (Schubert et al., 2006). Normally, HC depolarization in the dark following glutamatergic signaling results in closure of LTCCs on rods (Babai and Thoreson, 2009; Liu et al., 2013). The purpose of HC inhibitory signaling in bright light is most commonly understood in terms of i) controlling conegenerated synaptic gain and ii) lateral inhibition onto cone cells forming the basis of the center-surround organization of vision (Thoreson and Mangel, 2012; Yang and Wu, 1991). In dim light, HC inhibitory signaling onto rod cells has also been documented and suggested to fulfill these same two purposes (Babai and Thoreson, 2009; Mansergh et al., 2005; Morgans et al., 2001; Schmitz and Witkovsky, 1997). In total darkness, HC inhibitory signaling may act to throttle down the extent of LTCC opening to regulate how much calcium enters into rod (and possibly cells of the inner retina). It was beyond the scope of this review to investigate the exact mechanism(s) by which HCs communicate with photoreceptors, a currently active area of research by other labs, or with inner retina (Kamermans et al., 2001; Thoreson and Mangel, 2012; Yang and Wu, 1991).
When HC inhibitory signaling to rod cells is impaired, the extent of LTCC opening is expected to be greater than normal, allowing more calcium and manganese into rod cells (and possibly cells of the inner retina). HC inhibitory signaling is involved in the processing of light information by i) control of photoreceptor-generated synaptic gain and ii) lateral inhibition onto photoreceptor cells forming the basis of the center-surround organization of vision (Thoreson and Mangel, 2012; Yang and Wu, 1991). HC inhibitory signaling may also occur to the inner nuclear layer but that has been less studied (Thoreson and Mangel, 2012; Yang and Wu, 1991).
Thus, a recent study investigated whether loss of glutamatergic signaling to HC leads to impaired HC inhibitory signaling back onto the rods in the dark using MEMRI and mice null for proteins known to regulate glutamatergic-evoked HC inhibitory signaling to photoreceptors (i.e., Cav1.4 and arrestin-1) in the light (Babai and Thoreson, 2009; Berkowitz et al., 2015b; Mansergh et al., 2005; Morgans et al., 2001; Schmitz and Witkovsky, 1997). The data from the Cav1.4 experiments are summarized in Fig. 8 and supports the idea that impaired photoreceptor glutamateric signaling to the HC (as would also be expected following sodium iodate treatment) results in loss of HC inhibitory signaling back to the photoreceptors leading to supernormal uptake of manganese in both rods and cells of the inner retina (Berkowitz et al., 2015b). The above data provide the first application of MEMRI to study inhibitory signaling in vivo.
Fig. 8.
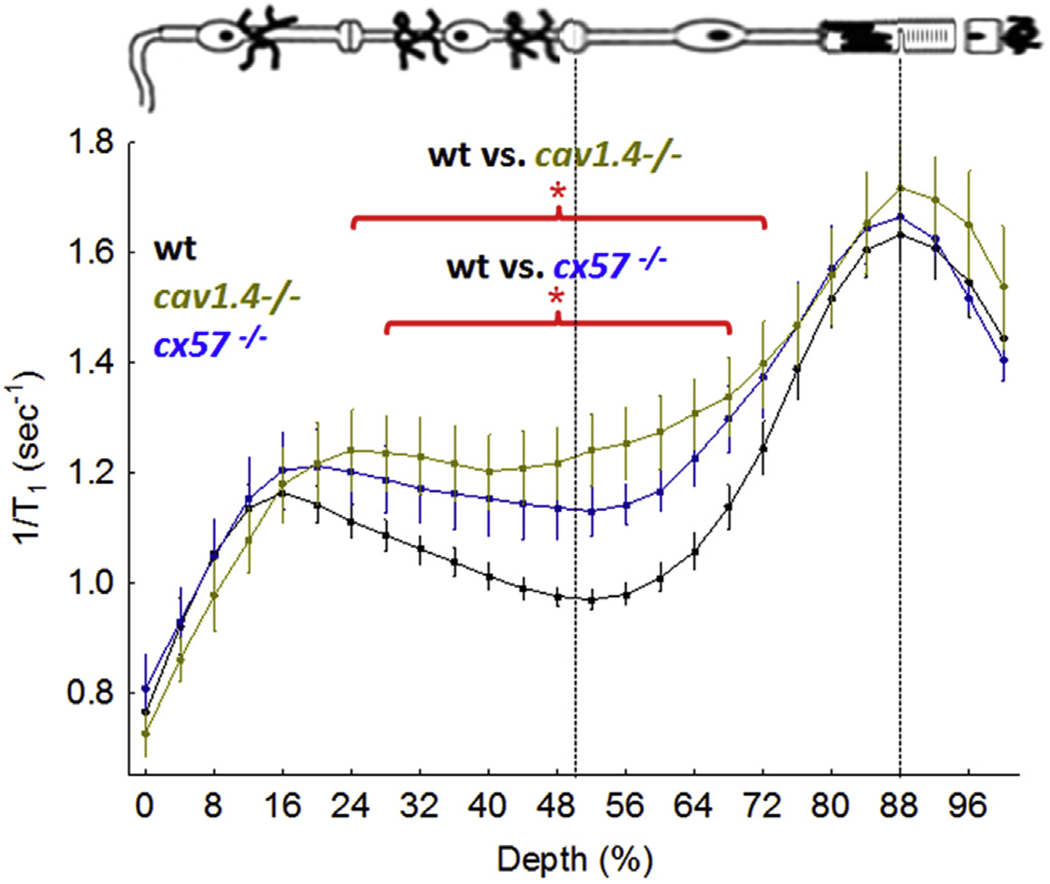
Functional LTCC topography as measured by MEMRI in dark adapted wt mice (closed black circles, n = 19), Cav1.4−/− mice (closed gold circle, n = 5), and cx57−/− mice (closed blue circle, n = 5). Graphing conventions are as in Fig. 4 (Berkowitz et al., 2015b). *, P < 0.05.
A recent study in zebrafish suggested the controversial hypothesis that HC-specific connexin 57 (Cx57) regulates inhibitory feedback to photoreceptors (Ciolofan et al., 2007; Dedek et al., 2008; Hombach et al., 2004; Kamermans et al., 2001; Klaassen et al., 2011; O’Brien et al., 2012a,b; Pandarinath et al., 2010; Shelley et al., 2006). Connexins, transmembrane proteins that form channels that make up hexameric hemichannels between apposed cells, provide a communication path between neurons and contribute to information processing. The availability of connexin57 knockout mice (cx57−/−; a kind gift from Dr. Sheila Nirenberg, Weill Medical College of Cornell University) provided an opportunity to test this hypothesis with MEMRI. Here, we present new data that are consistent with the results in zebrafish in that cx57−/−mice demonstrate a greater than normal uptake in their rod cells (Fig. 8). In addition, a greater than normal uptake in the inner retina was noted in the cx57−/− mice, providing provisional evidence for HC inhibitory “feed forward” signaling (Thoreson and Mangel, 2012; Yang and Wu, 1991). Together, this supernormal and symmetric uptake phenotype at least raises the possibility for a role for cx57-modulated HC inhibitory signaling in the dark adapted awake and freely moving mouse in vivo.
Another gap junction protein at the photoreceptor synapse, but not known to be involved in HC inhibitory signaling, is the neuronal gap junction connexin 36 (Cx36), which is also expressed in cones, particularly at the pedicle and cone-to-rod contacts, but not in rods per se; Cx36 is also expressed in retinal amacrine and ganglion cells (Feigenspan et al., 2004; Güldenagel et al., 2001; O’Brien et al., 2012a, b; Schubert et al., 2005). Functionally, Cx36 is thought to contribute to regulation of cone-rod interactions and rod synaptic transmission based on post-mortem examination (Asteriti et al., 2014; Güldenagel et al., 2001). Cx36 is expressed at the outer plexiform layer in a laminar and non-interacting arrangement with cx57 (Ciolofan et al., 2007; Deans and Paul, 2001; O’Brien et al., 2012a, b). Here, new data are presented of dark adapted cx36−/− mice (a kind gift from Dr. Marla Feller) demonstrating focal regions of subnormal uptake, consistent with a role for Cx36 in the inner and outer segments, and in the inner plexiform and ganglion cells layers (Fig. 9). These MEMRI data support independent contributions from cx57 and cx36 in the dark adapted mouse in vivo (Ciolofan et al., 2007).
Fig. 9.
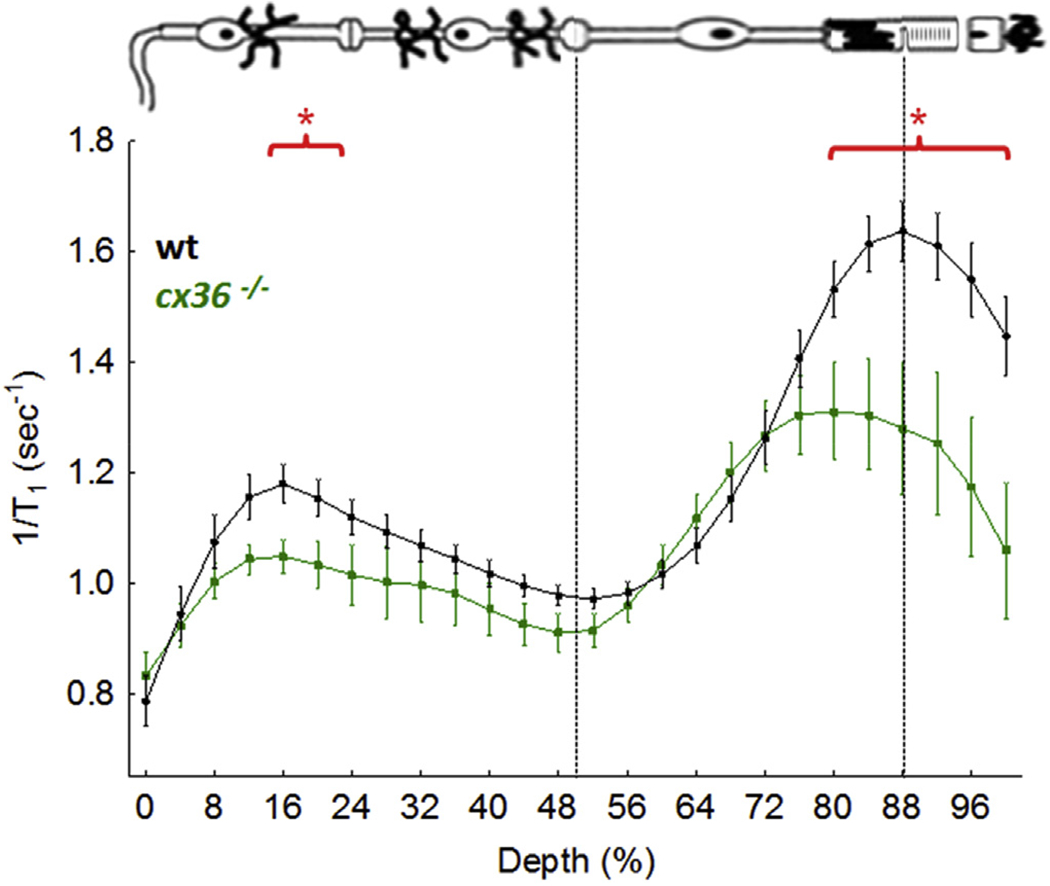
Functional LTCC topography as measured by MEMRI in dark adapted wt mice (n = 19) or cx36−/− mice (n = 5); graphing conventions are as in Fig. 4. *, P < 0.05.
Together, the data establish MEMRI as the first objectively useful method for evaluating light detection and processing as it impacts rod cell LTCCs in vivo.
5.4. MEMRI and Cav1.* subtypes
The retina does not contain a single type of LTCC but instead three LTCC subtypes, Cav1.2, 1.3, and 1.4 channels, each with different roles based on their different biophysical properties. Arguably the most important subtype is the Cav1.4 channel located only at the photoreceptor synapse. Cav1.4 channels play an essential role in vision since Cav1.4−/− mice are blind and mutations in the CACNA1F gene, which encode Cav1.4 LTCC α1 subunits, are linked with loss of vision in Åland Island Eye Disease, cone-rod dystrophy, X-linked retinal disorder, night blindness-associated transient tonic down-gaze, and incomplete congenital stationary night blindness (Baumann et al., 2004; Burtscher et al., 2014; Busquet et al., 2010; Koschak et al., 2003; Mansergh et al., 2005; Morgans et al., 2001; Strom et al., 1998; Xiao et al., 2007). In contrast, the functional role of Cav1.3 channels is less clear, in part because i) current antibodies for identifying these channels and used for immunological localization are non-specific and ii) a selective antagonist has not yet been established (Zou et al., 2012). Cav1.3 channels have been documented in cells of the inner retina and in retinal pigment epithelium cells based on electrophysiological evidence, and their mRNA exists in rod cells and cells of the inner retina (Habermann et al., 2003; Morgans et al., 2005; Rosenthal et al., 2001; Xiao et al., 2007; Xu et al., 2002; Zou et al., 2012). However, Cav1.3−/− mice do not exhibit an impaired visual phenotype as measured by electrophysiology and water maze testing (McKinney and Murphy, 2006; Wu et al., 2007). The remaining major LTCC subtype, Cav1.2, is expressed in the inner plexiform and ganglion layers, but not rod cells in young mice, although in older mice Cav1.2 LTCCs appear to be functional in the outer retina in vivo (Berkowitz et al., 2014; Busquet et al., 2010).
To separately interrogate LTCC Cav1.* subtypes, mice were pretreated (i.e., before the injection of manganese) with D-cis-diltiazem (DIL) at a dose that appeared to antagonizes only Cav1.2 channels in vivo (Berkowitz et al., 2014). DIL IC50’s, generated in different labs using expressed channels in cells, suggest some subtype specificity [i.e., IC50 ~45 μM (Cav1.2) vs. ~326 μM (Cav1.3) and ~92 μM (Cav1.4)] (Baumann et al., 2004; Berkowitz et al., 2014; Bissig et al., 2013; Glossmann et al., 1983; Tarabova et al., 2007). However, the exact DIL IC50’s for the LTCC subtypes in the retina in vivo are not known (Berkowitz et al., 2014; Bissig et al., 2013). Nonetheless, spatial agreement was noted between where Cav1.2 is located on immunohistochemistry (inner retina) and DIL-induced focal suppression of manganese uptake (inner retina) (Baumann et al., 2004; Berkowitz et al., 2014; Bissig et al., 2013; Glossmann et al., 1983; Tarabova et al., 2007). Cav1.3, an order of magnitude less sensitive than Cav1.2 channels to DIL based on the above IC50’s, is thought to be more widely expressed than just inner retina and evidence for DIL-related suppression in other parts of the retina of wildtype (wt) mice was noted (Xiao et al., 2007). Together, these data in vivo are consistent with the notion that Cav1.3 channels are less responsive to DIL treatment than Cav1.2 channels (Lipscombe et al., 2004; Xu and Lipscombe, 2001). If DIL substantially antagonized Cav1.4 channels, one expects a reduction in HC inhibitory signaling to the rod cells (see above) and thus a greater than normal uptake of manganese. A supernormal uptake in rod cells was not seen in the mice treated with DIL (Berkowitz et al., 2014). Collectively, these considerations support the use of DIL for unraveling the contribution of LTCC Cav1.* subtypes in vivo using MEMRI (Bissig et al., 2013). Thus, MEMRI alone is not able to separate the functional contributions from cav1.* channels. However, MEMRI with and without DIL treatment, together with the highly localized Cav1.4 at the photoreceptor synapse, laminar-structure of the retina, and high spatial resolution of MRI has been useful in unraveling the various subtype contributions in vivo.
MEMRI with and without DIL studies have raised the possibility that the number and/or spatial location of LTCC subtypes varies within the retina in, for example, age and disease; a similar phenomenon has been documented in the brain (Jurkovicova-Tarabova et al., 2012; Sinnegger-Brauns et al., 2009). For example, we find evidence that manganese uptake in rod cells and other cells of the retina steadily increases with age (Fig. 10). MEMRI with and without DIL experiments suggest that this was due to increased Cav1.3 expression that was predictive of visual performance declines in healthy aging (Bissig et al., 2013); exactly how increased Cav1.3 leads to vision loss remains unclear. Also, in Cav1.3−/− mice and in 10 mo old mice, DIL suppressed uptake in the outer plexiform layer and outer nuclear layer, suggesting compensatory up regulation of retinal Cav1.2 channels not present in wt or in younger mice, respectively; knocking out specific proteins regulating photoreceptor synapse activity also modified the array of Cav1.2 channels in the retina as measured by MEMRI with and without DIL (Jurkovicova-Tarabova et al., 2012). Intriguingly, discordant reports of the efficacy of the Cav1.2-specific antagonist DIL in RP models may be, at least in part, a result of this plasticity of the Cav1.2 subtype (Barabas et al., 2010; Berkowitz et al., 2011, 2014; Bissig et al., 2013).
Fig. 10.
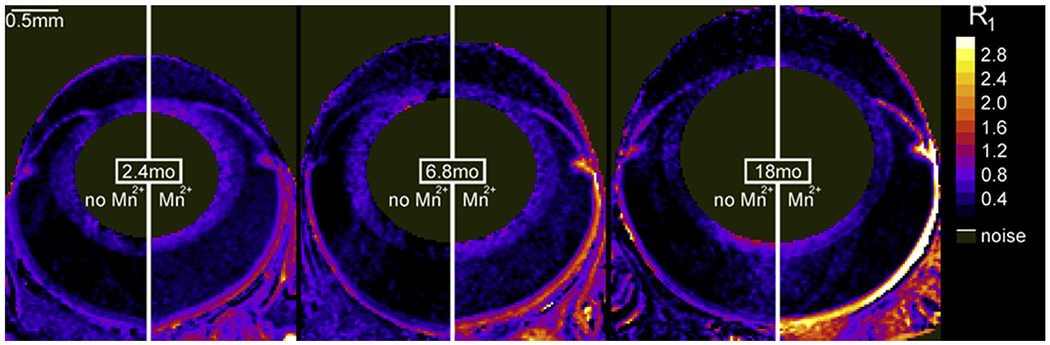
Representative maps of tissue R1 (in units of s−1) from baseline (no Mn2+, left half of each composite image) and Mn2+-injected long Evans rats (right half of each composite image) (Bissig et al., 2013). In Mn2+-injected rats, R1 — which is linearly related to tissue Mn2+ concentration (Chuang et al., 2009) — increases with age; this change was not linked with blood–retinal barrier breakdown (Bissig et al., 2013). In addition, the no-manganese baseline R1s were stable over time (Bissig et al., 2013).
Mapping the number and spatial location of retinal LTCC subtypes in vivo using MEMRI with and without DIL will likely help guide/optimize calcium channel therapy in aging and in disease (Fox et al., 2003).
5.5. Other photosensitive cells
Rod cells are not the only photosensitive cells that can be studied by MEMRI. For example, MEMRI readily detects light-stimulated changes in intrinsically photosensitive retinal ganglion cells in postnatal day 7 (P7) mice (when ipRGC content is high and photoreceptors are not functional), and in inner retinal cells genetically modified to express channelrhodopsin in rd1 mice (optogenetics) (Berkowitz et al., 2010; Ivanova et al., 2010). In both cases, MEMRI outcomes matched the known physiology of these channels which function in an opposite manner to that of rod cells by having greater opening in the light than in the dark. It remains unclear at present whether the manganese entered the cells directly via melanopsin or channelrhodopsin channels, or indirectly via opening of neighboring LTCCs on the plasmalemmal membrane. Work is currently on-going to determine if MEMRI is also useful to study cone photoreceptors using zebrafish and coneonly mice (Klaassen et al., 2011; Samardzija et al., 2014).
6. Non-MEMRI assays of rod cell function
Disease or aging will alter a variety of rod functions and not just rod LTCCs. Thus, two additional MRI indices of rod cell function—measured without administration of exogenous contrast agents — have been developed to complement MEMRI and to facilitate translation of MRI of retinal function into humans. These methods are i) apparent diffusion coefficient (ADC) MRI, which measures light-evoked expansion of both SRS [controlled in part by the photoreceptors and in part by the retinal pigment epithelium (RPE)] and of the choroid (Berkowitz et al., 2015a; Bissig and Berkowitz, 2012), and ii) measurement of the spin-lattice relaxation rate in the rotating-frame (1/T1ρ) which evaluates light-stimulated rod arrestin and its translocation, together with retinal vessel expansion (Berkowitz et al., 2015c). As summarized in Fig. 4, in diabetes, for example, abnormal rod function was measured on MEMRI, as well as on ADC MRI and in the light-evoked responses of the retinal vessels and choroid; anti-oxidant treatment corrects most of these defects (except choroidal expansion), supporting a role of oxidative stress in rod cell dysfunction (Table 1). Details about these additional methods are presented below. These results support triangulating the rod cell dysfunctionome using a variety of complementary functional MRI indices.
Table 1.
Anti-oxidant approaches so far examined that correct early diabetes-induced MRI retinal abnormalities.
| • α-lipoic acid corrects • Impaired autoregulation (IOVS, 2006 Sep; 47(9):4077-82) • Subnormal rod manganese uptake (IOVS, 2007 Oct; 48(10):4753-8) • Impaired subretinal space expansion (IOVS, 2015 Jan 8; 56(1):606-15) • Cu/Zn SOD overexpression corrects • Subnormal rod manganese uptake (IOVS, 2009; 50(5):2351-8) • Peroxisome-targeted catalase corrects • Subnormal rod manganese uptake (IOVS, 2015; 56(5):3095-102) • Retinaldehydes correct • Subnormal rod manganese uptake (Mol Vis. 2012; 18:372-6) • Impaired subretinal space expansion (in press, 2015) |
6.1. ADC MRI
ADC MRI provides an index of water self-diffusion in a particular diffusion-weighted magnetic field gradient direction. ADC can be thought of as a barrier index: the more barriers water hits as it diffuses, the lower the water mobility, and thus the lower the ADC measured. In the retina, advantage is taken of this property and the fact that the subretinal space (SRS) expands several fold in light compared to that in dark (Adijanto et al., 2009; Chen et al., 2008, 2011; Li et al., 1994a; Nair et al., 2010). In-line with this physiology, ADC MRI measures a greater axial ADC in the SRS in the light than in dark (Bissig and Berkowitz, 2012); the same extent of light-stimulated SRS expansion is measured independently of the presence of manganese (Fig. 11). The sensitivity of ADC MRI to rod function was confirmed by the lack of an increase in the SRS ADC in the absence of rod phototransduction in GNAT1−/− mice (Fig. 11) (Bissig and Berkowitz, 2012). Here, we present new data, based on literature studies of SRS hydration physiology (Wolfensberger et al., 1999), that predict that the light-evoked increase in ADC in the far posterior retina would be absent if the SRS was acidotic. Pretreatment with a carbonic anhydrase inhibitor acetazolamide, which targets the retinal pigment epithelium (RPE) and induces retinal acidosis, prevented the light-evoked increase in SRS ADC (Fig. 11) (Yamamoto and Steinberg, 1992). These studies support interpretation of ADC MRI of light-dependent SRS expansion as a measure of a combination of rod and RPE function.
Fig. 11.
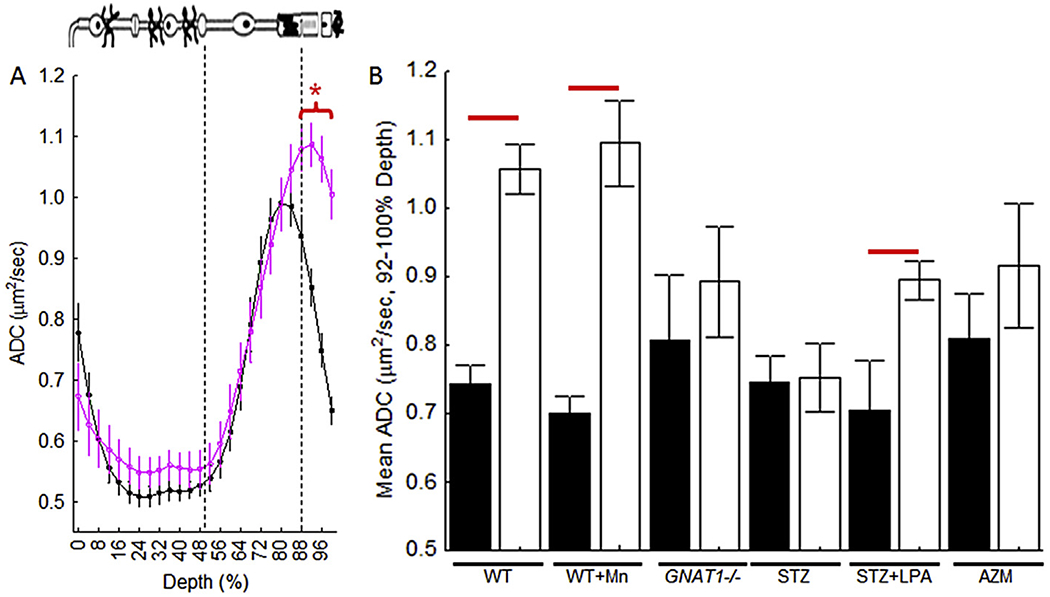
Light detection using ADC MRI. A) Summary of central retinal ADC with retinal depth during dark (closed symbols, n = 23) and light (open symbols, n = 23) in untreated mice (WT). Approximate location of retinal layers is indicated (dotted blue lines and insert cartoon). Profiles are spatially normalized to retinal thickness (0% = vitreous/retina border, 100% = vitreous/choroid border). Red bracket/horizontal line, P < 0.05. B) Summary of paired data (filled = dark, open = light) of WT (n = 23), MnCl2-treated mice (WT + Mn, n = 6), GNAT1−/− mice (n = 6), untreated + vehicle treated diabetic mice (STZ, n = 9), diabetic mice treated acutely with the anti-oxidant a-lipoic acid (STZ + LPA, n = 8), on-diabetic acetazolamide-treated mice (AZM, n = 8) (Berkowitz et al., 2015a).
The contribution of oxidative stress to SRS hydration physiology has not been previously investigated. As mentioned above, diabetes produced an impairment in the light-evoked SRS/ADC increases (Fig. 4) that was corrected by treatment with a potent antioxidant, α-lipoic acid (LPA) (Table 1) (Berkowitz et al., 2007c). These data support ADC MRI for monitoring the impact of an oxidative stress environment on rod/RPE function.
ADC MRI also provides information about the thickness of the major blood supply to the rod cells, the choroid. This is important because techniques that simultaneously image co-localized photoreceptor structure/function and choroidal structure/function in vivo have not been available. Most previous MRI studies measure choroidal thickness in vivo using an intravascular contrast agent (Berkowitz et al., 2007a; Shih et al., 2013). Instead, ADC MRI nulls the choroidal blood signal (essentially very fast moving water) in the axial direction allowing a measurement of a “retina”-only thickness (Bissig and Berkowitz, 2012; Chen et al., 2008). Subtracting the “retina”-only thickness from the “retina/choroid” thickness, calculated from non-diffusion weighted images, yields the choroidal thickness. Agreement was found between diffusion weighted choroid thickness and those in the literature thus supporting the accuracy of this approach in C57BL/6J mice (Fig. 4) (Barber et al., 2005; Muir and Duong, 2011). As an additional accuracy check, light-adapted Sprague–Dawley rats agreement was noted between ADC MRI measures of choroidal thickness (63 ± 8 μm, n = 5, mean ± SEM) and a previous estimate (66 ± 8 μm) obtained using an exogenous vascular contrast agent (Berkowitz et al., 2007d).
The choroid has an extensive autonomic innervation that controls its volume in response to changes in light, among other factors (Fitzgerald et al., 1992, 1996; Fuchsjäger-Mayrl et al., 2001; Fuchsjäger-Mayrl et al., 2003; Garhofer et al., 2002; Longo et al., 2000). Indeed, significant light-evoked expansion of choroidal thickness in mice was observed using ADC MRI (Berkowitz et al., 2015a). To assess sensitivity, it was noted that light-adapted choroidal thickness and its regulation are reduced with diabetes and age as has been reported in other studies using other methods (Dallinger et al., 1998; Fitzgerald et al., 2001; Ko et al., 2013). This also occurs in mice; the diabetes results are presented in Fig. 4. In the light, the choroidal thickness in 2 mo C57Bl/6J mice (86 ± 3 μm, n = 18) was significantly (P < 0.05) greater than that in 10 mo C57Bl/6J mice (66 ± 5 μm, n = 4); dark values did not change with age (62 ± 2 μm vs. 59 ± 7 μm, respectively). ADC MRI thus provides assays of photoreceptor/RPE function together with choroidal thickness and its light-evoked expansion that can be combined with MEMRI.
6.2. 1/T1ρ MRI
1/T1ρ, the spin-lattice relaxation rate constant in the rotating frame, describes the decay of the transverse magnetization out of a spin-lock radio-frequency field. 1/T1ρ is particularly sensitive to changes in (i) macromolecular content in avascular tissues as well as to (ii) vascular volume in well perfused tissue like the brain (Hulvershorn et al., 2005; Jin and Kim, 2013; Kettunen et al., 2002; Li et al., 2007; Magnotta et al., 2012; Regatte et al., 2003). 1/T1ρ has been used clinically (Borthakur et al., 2004; Haris et al., 2015; Hulvershorn et al., 2005). Because of its sensitivity to large molecules, it was hypothesized that the difference between avascular outer nuclear/inner segment tetrameric rod Arrestin 1 (Arr1) in the dark – and its reduction via translocation upon light exposure – would be detectable as a change in 1/T1ρ in the outer retina (Gurevich et al., 2011; Huang et al., 2010). Tetrameric Arr1 plays an important role in regulating rod synapse function (Gurevich et al., 2011; Huang et al., 2010). Exposure to bright light produces a dramatic reduction in tetrameric Arr1 concentration in these compartments with translocation of the monomer form of Arr1 to the outer segments; only Arr1 monomer binds rhodopsin to stop phototransduction (Hanson et al., 2007; Philp et al., 1987). Importantly, this translocation of Arr1 into the outer segments does not occur unless visual cycle regeneration of rhodopsin via the visual cycle is normal (Mendez et al., 2003). As shown in Fig. 4, 1/T1ρ transretinal profiles clearly demonstrate a greater 1/T1ρ in the dark than in the light (P < 0.05). The opposite pattern was noted in the inner retina (i.e., <50% depth into the retina) (P < 0.05); these results were independent of the presence of manganese (Berkowitz et al., 2015c). Confirmation that this change in 1/T1ρ in the rod cells was provided by the fact that the arrestin 1 signal at 64–72% was absent in Arr1−/− null mice (kind gift from Dr. Jeannie Chen, USC) and greater than normal with Arr1 overexpression (data not shown) (Berkowitz et al., 2015c).
1/T1ρ in blood is known to be much shorter than in tissue (Hulvershorn et al., 2005; Jin and Kim, 2013; Kettunen et al., 2002; Magnotta et al., 2012). This allowed an evaluation of light-dependent changes in the inner retina vascular volume based on changes in 1/T1ρ (Fig. 4) (Hulvershorn et al., 2005; Jin and Kim, 2013; Kettunen et al., 2002; Magnotta et al., 2012); the light-evoked change in inner retinal blood volume was confirmed using MRI and the blood-pool contrast agent monocrystalline iron oxide nanocolloid (MION) (Shih et al., 2011). Supporting this finding, our lab finds no photoregulation of 1/T1ρ in the inner retina in (i) guinea pigs (which are without an inner retinal circulation, data not shown) and (ii) diabetic mice (Fig. 4). The data in the diabetic mice are particularly relevant because diabetes is known to impair retinal vascular autoregulation (Kern et al., 2010). Since the retinal circulation provides ~10% of the oxygen required by the photoreceptors in the dark to support the great energy needed for continuous ion channel opening (41–44), evaluating the photoregulatory changes in inner retina blood volume is likely useful as an indirect probe of functional changes in photoreceptor oxygen consumption.
Using a combination of MRI approaches, rod compartment-specific LTCC subtypes can be studied together with light-stimulated changes in SRS, tetrameric Arr1, choroidal thickness, and retinal vessel autoregulation in vivo. To ensure spatial accuracy in vivo, high resolution MRI assays of rod function can be aligned against optical coherence tomography (OCT) images.
7. MRI and oxidative stress
Oxidative stress is well known to impair neuronal function, including LTCC function, suggesting the hypothesis that MRI indices are sensitive to the presence of oxidative stress (Downs and Helms, 2013; Fusi et al., 2001; Guerra et al., 1996; Guzman et al., 2010; Kourie, 1998; Shirotani et al., 2001; Yang et al., 2013). All of the published MRI data to-date are consistent with this hypothesis. For example, in 2007, it was observed that ouabain, a specific inhibitor of Na+/K+-ATPase activity that increases neuronal oxidative stress, significantly reduced uptake of manganese in rod cells as measured by MEMRI (Riegel et al., 2010). This lower-than-normal pathophenotype was also found early in many diseases with a prominent oxidative stress etiology, including models of DR, RP, glaucoma, and ischemia/reperfusion (Campochiaro et al., 2015; Du et al., 2013; Fukuda et al., 2014; Handa, 2012; Inman et al., 2013; Osborne et al., 2004; Roddy et al., 2012); the ADC MRI and 1/T1ρ MRI-measurement of autoregulation to retinal oxidative stress data were presented above (Berkowitz et al., 2007b, 2011, 2013, 2008b; Calkins et al., 2008). Intriguingly, in a rat model of retinopathy of prematurity, a model not associated with oxidative stress, a greater than normal uptake in rod cells and cells of the inner retina was observed; these results are not consistent with oxidative stress but instead suggest a loss of HC inhibitory signaling (Berkowitz et al., 2007b, 2011). To be clear, simply observing multiple aspects of dysfunction linked with oxidative stress is not sufficient to use functional MRI indices as a tool for the unambiguous detection of oxidative stress per se since subnormal uptake may arise for reasons unrelated to oxidative stress. However, and most importantly, a range of treatments known to reduce retinal oxidative stress also correct the MEMRI defects in DR (Table 1); in DR, antioxidant treatment also corrected the early defect measured by ADC MRI (Berkowitz et al., 2015a).
Together, these data suggest that MRI is a powerful approach for objectively evaluating antioxidant treatment efficacy in a rod cell compartment-specific manner before the appearance of other clinical biomarkers in disease in vivo.
8. New perspectives
Aging:
Although many retinal diseases occur against a background of aging, the influence of the aging process on disease progress remains vastly underexplored. Aging alone causes declines in vision even in the absence of disease and available biomarkers have been insufficient in explaining the physiology behind these declines (Fitzgerald et al., 2001; Gresh et al., 2003; Grunwald et al., 1993, 1998; Kolesnikov et al., 2010; Lehmann et al., 2012; Spear, 1993). While the extent of vision loss with age in healthy patients is modest, its occurrence alone is a significant predictor of degrading day-to-day quality of life, deleterious aging outcomes (e.g., hip fracture), and reduced survival (Swindell et al., 2010). Thus, an increasingly older population raises an urgent and continuing need to understand the origins of age-related physiologic vision loss and how this impacts disease-related vision loss (Bissig et al., 2013). Until recently, biomarkers of retinal physiology have been insufficient for understanding how the “healthy” retina ages (Spear, 1993). In particular, the role of retinal LTCCs and their subtypes in age-related vision loss has been virtually unexplored. A strong predictive link between age-related escalating increases in photoreceptor Cav1.3 LTCC activity as measured by MEMRI and progressive declines in contrast sensitivity has been observed (Bissig et al., 2013). In support of this hypothesis, healthy C57Bl/6J mice have relatively stable photopic vision until after 18 mo of age (data not shown) (Bissig et al., 2013; Kolesnikov et al., 2010; Lehmann et al., 2012; Spear, 1993) and non-escalating retinal LTCC function, as measured by MEMRI, with light and dark adaptation before 18 months of age. Together, these data have uncovered a previously unsuspected target in age-related impaired sight loss: early and progressive increases in retina LTCC function; intriguingly, this calcium hypothesis of aging is reminiscent of a similar hypothesis first identified in CA1 region of hippocampus and now linked with cognitive declines (Bissig and Berkowitz, 2014). As noted elsewhere, these aging changes may help explain some of the later time course features in DR as measured by MEMRI (Berkowitz et al., 2013).
9. Addressing concerns about MEMRI and MRI
A considerable body of work over the past decade has demonstrated that MRI has the spatial resolution and sensitivity to be a reliable and physiologically accurate technology for analytically measuring rod cell biology non-invasively in a range of animal models. Here, we address some of the major concerns.
MEMRI requires injection of manganese, an essential metal at low levels (e.g., manganese-superoxide dismutase) but a neurotoxin at high levels of exposure. For MEMRI to be useful it is critical to identify doses that are non-toxic and that allow for adequate detection of manganese with good contrast-to-noise on MRI examination. At the doses used in studies of rodent retina [44 (rat) – 66 (mouse) mg/kg, IP], no evidence was observed that manganese altered retinal health based on lack of changes in 1) light adaptation, 2) synaptic release at the rod synapse, light-evoked expansion of the 4) subretinal space, 5) choroid, and 6) retinal vessels, 7) arrestin 1 and its light-dependent translocation, 8) blood retinal barrier integrity, 9) intraocular pressure, 10) electroretinogram parameters (ERG) and 11) histology (Berkowitz et al., 2006, 2007a, 2007b, 2015a, 2015b, 2015c). Here, new data are presented that neither 12) visual acuity nor 13) contrast sensitivity were altered 4 h post injection of manganese (66 mg/kg MnCl2 treatment in dark adapted C57Bl6 mice IP), as measured by optokinetic tracking (P > 0.05) (non-treated (n = 5) acuity 0.389 ± 0.004 cycles/degree and contrast sensitivity 16.9 ± 1.2 unitless parameter; treated (n = 5) acuity 0.394 ± 0.005 c/d and contrast sensitivity 19.2 ± 1.7 unitless parameter); also, no changes from baseline values were observed at 24 h and 1 week post MnCl2 treatment (data not shown). In addition, we evaluated 14) rod-dominated retinal superoxide production with a lucigenin assay and confirmed similar (P > 0.05) levels in untreated mice (n = 3,100.0 ± 10.9% of controls after normalizing for protein (mean ± SEM)) and mice 4 h post treatment with manganese (66 mg/kg, IP; n = 3,102.6 ± 18.7% of controls after normalizing for protein). Collectively, these 14 different indices strongly suggest a lack of toxic effects of manganese at the singly acute systemic doses of MnCl2 used in retinal studies.
A related concern is whether or not MEMRI can be safely applied in humans. To this end, note that the Mn2+ injectate TeslaScan is a manganese-based contrast agent previously approved over a decade ago as safe and effective for use in humans by the Food and Drug Administration (FDA) (Karlsson et al., 2015; Tofts et al., 2010; Torres et al., 1997). Teslascan was originally investigated as a blood pool contrast agent to identify the tumor burden in the liver. Teslascan has shown to lack adverse events, even in patients with cancer and patients that needed cardiac reperfusion (Karlsson et al., 2015). Teslascan is converted in vivo into the lipid soluble Mn dipyridoxyl ethyldiamine (Karlsson et al., 2015). In addition to not showing toxicity, Teslascan and its metabolite act as anti-oxidants because they are superoxide dismutase mimetics, and have ironbinding abilities in vitro (Karlsson et al., 2015). Preclinical and early clinical studies confirm their beneficial protection against oxidative stress in different tissues (Karlsson et al., 2015). To determine if the low concentration of chelated manganese in Teslascan would be useful in functional studies, it was observed that the Mn2+ in Teslascan is taken up by the rat retina in a stimulus-dependent manner (Berkowitz et al., 2013; Tofts et al., 2010). Together, these considerations have motivated on-going human MEMRI studies using Teslascan to detect accumulation of Mn2+ with activation in retinal and brain neurons. Note, however, that the Teslascan used in this clinical study was prepared in-house because there are currently no commercial sources of Teslascan due to a low market earning (Karlsson et al., 2015). Thus, while feasible and safe in patients, wide application of MEMRI likely awaits improved availability of Teslascan.
Can the high resolution functional images similar to those described in animals models herein be obtained in patients? Spatially the problem is simply defined: given a 250 μm thick retina, one needs 50 μm or less resolution to collect enough information to confidently separate inner from outer retina. By taking advantage of the good coil filling factor and non-isotropic sampling (e.g., thick slices), fine resolution images, approaching those in rodents, but in humans without sedation is possible using a cued-blinking procedure (Beenakker et al., 2013, 2015; Berkowitz et al., 2001; Berkowitz et al., 2013; Lindner et al., 2014; Sirin et al., 2015; Zhang et al., 2011). For example, we recently published human images collected at 58 μm axial resolution (Berkowitz et al., 2013). The possibility of using MEMRI in patients was discussed above. ADC MRI requires no exogenous contrast agent and is likely to be useful in human studies. Clinical application of 1/T1ρ MRI of brain tissue in patients is already in place and FDA limitations for specific applied radiation (SAR) is possible with available acquisition schemes (Borthakur et al., 2004; Haris et al., 2015). Thus, while challenges undoubtedly remain in making high resolution functional retina images in patients routine, the results to-date clearly highlight likely progress in this area.
Will MRI be useful as a screening tool in patients? Not likely because MRI throughput is relatively low, and MRI facilities are located outside of vision labs and Ophthalmology clinics. On the other hand, these are not limitations if MRI is used as a discovery tool in, for example, animal studies, Phase 2 clinical trials or in patients with difficult to manage disease. Most major hospitals have clinically accessible MRI centers, and the recent availability of relatively low cost bench top preclinical MRI systems raises the possibility that more labs will be able to routinely benefit from MRI in their laboratories.
The expense of MRI is also considered to be problematic. However, it is important to consider not just the cost but the cost-benefit ratio. For example, the equipment used for non-MRI methods is considerably less expensive than a MRI machine; most major research centers, however, have small animal MRI available as core facilities. The benefit of existing non-MRI methods, as discussed above, is less clear because they provide incomplete and incompatible assays that do not measure many essential aspects of retinal function; thus, current methods have a low cost but also a low benefit. To see why the low cost/low benefit approach is problematic, we note that it is standard practice to wait until the appearance of gross histopathology to implement laser therapy; this is clearly costly to the retina and patient. Applying existing tools to expensive genetically modified mouse models and genetic therapies is also very costly because these popular approaches have not shown good predictive value for future clinical success (e.g., (McGill et al., 2012)). On the other hand, MRI provides a unique discovery engine that measures a wealth of retinal and rod compartment-specific functions in the same eye at the same location at the same time, that are unavailable with other technologies, resulting in much higher benefits for directed, personalized, and targeted therapy.
10. Summary and future directions
It is currently a major challenge to translate findings in animal models into the clinic in part because of vast differences in methodology and available indices. The best bench-to-bedside (and back) bridge is likely to be the development and application of imaging biomarkers that inform about disease progression and treatment efficacy in both preclinical and clinical situations. It has become clear that current optical and electrophysiology methods do not provide such a bridge in many situations. This problem can perhaps be best appreciated early in the course of retinopathies (i.e., in the absence of gross anatomy changes), a critical time point when drug intervention is most likely to be successful at reducing vision loss. In other words, before the appearance of gross changes there is currently little to guide the management of retinal disease and to answer essential questions such as what is the optimal time/dose/route/schedule for therapeutic intervention? Is a treatment reaching its target? How well do current experimental models mimic the clinical condition?
This review highlights MRI as a promising tool for addressing the above problems. High resolution MRI stands alone in its ability to analytically measure rod cell compartment-specific function in vivo. MRI is thus a modern phenotype discovery tool that can complement genotyping assays. The availability of this optimized imaging technology enables earlier evaluation of disease progression and better management of disease-preventing anti-oxidant treatment than is currently possible. Most of the newer assays of rod cell functions are based on endogenous contrast mechanisms, and this is expected to facilitate their translation into patients with DR and RP, and other oxidative-stress-based retinal diseases.
MRI studies uniquely allow for in vivo testing of ex vivo-derived hypothesis and this has already motivated new lines of scientific inquiry into, for example, the problem of vision loss in aging and in DR. In addition, MRI provides new and useful biomarker of therapeutic efficacy against threats to sight. As MRI studies of rod photoreceptors become more common used by other labs, better testing of mechanistic hypotheses and early evaluation of treatment efficacy in vivo is expected.
Acknowledgments
We gratefully acknowledge on-going and very helpful input from the following colleagues: Drs. Tim Kern, Rod Braun, and Jena Steinle. Financial support for these studies from the following sources is also acknowledged: Juvenile Diabetes Research Foundation (BAB), Mouse Metabolic and Phenotyping Centers Pilot and Feasibility Program (BAB), National Eye Institute (BAB, DB), Beckman Foundation (BAB), Wayne State University Bridge Funding (BAB), Research to Blindness (KEI).
Abbreviations:
- ADC
apparent diffusion coefficient; Arr1, Arrestin 1
- ASL
arterial spin labeling
- BRB
blood retinal barrier
- BOLD
blood-oxygen level dependent
- Cx36
connexin 36
- Cx57
connexin 57
- DIL
D-cis-diltiazem
- DR
diabetic retinopathy
- ERG
electroretinogram
- HC
horizontal cell
- IS
inner segments
- MEMRI
manganese-enhanced MRI
- OCT
optical coherence tomography
- ONL
outer nuclear layer
- RPE
retinal pigment epithelium
- RP
retinitis pigmentosa
- 1/T1r
spin-lattice relaxation rate in the rotating-frame
- SRS
subretinal space
- LTCCs
voltage-gated L-type calcium channels
- wt
wildtype.
References
- Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS, 2009. CO2-induced ion and fluid transport in human retinal pigment epithelium. J. General Physiol 133, 603–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder VA, Yu DY, Cringle SJ, Su EN, 1991. Changes in vitreal oxygen tension distribution in the streptozotocin diabetic rat. Diabetologia 34, 469–476. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW, 1998. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes 47, 1953–1959. [DOI] [PubMed] [Google Scholar]
- Arteel GE, Thurman RG, Raleigh JA, 1998. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur. J. Biochem 253, 743–750. [DOI] [PubMed] [Google Scholar]
- Asteriti S, Gargini C, Cangiano L, 2014. Mouse rods signal through gap junctions with cones. Elife 3, e01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babai N, Thoreson WB, 2009. Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J. Physiol 587, 2353–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SL, Petry HM, 2000. Noninvasive assessment of retinal function in rats using multifocal electroretinography. Investig. Ophthalmol. Vis. Sci 41, 610–617. [PubMed] [Google Scholar]
- Barabas P, Cutler PC, Krizaj D, 2010. Do calcium channel blockers rescue dying photoreceptors in the Pde6b ( rd1 ) mouse? Adv. Exp. Med. Biol 664, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CEN, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK, 2005. The Ins2Akita mouse as a model of early retinal complications in diabetes. Investig. Ophthalmol. Vis. Sci 46, 2210–2218. [DOI] [PubMed] [Google Scholar]
- Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM, 2005. The RAGE axis in early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci 46, 2916–2924. [DOI] [PubMed] [Google Scholar]
- Baumann L, Gerstner A, Zong X, Biel M, Wahl-Schott C, 2004. Functional characterization of the L-type Ca2+ channel Cav1.4 from mouse retina. Investig. Ophthalmol. Vis. Sci 45, 708–713. [DOI] [PubMed] [Google Scholar]
- Beenakker JWM, van Rijn GA, Luyten GPM, Webb AG, 2013. High-resolution MRI of uveal melanoma using a microcoil phased array at 7 T. NMR Biomed. 26, 1864–1869. [DOI] [PubMed] [Google Scholar]
- Beenakker JW, Shamonin DP, Webb AG, Luyten GPM, Stoel BC, 2015. Automated retinal topographic maps measured with magnetic resonance imagingretinal topographic maps measured with MRI. Investig. Ophthalmol. Vis. Sci 56, 1033–1039. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Bissig D, Bergman D, Bercea E, Kasturi VK, Roberts R, 2011. Intraretinal calcium channels and retinal morbidity in experimental retinopathy of prematurity. Mol. Vis 17, 2516–2526. [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Bissig D, Dutczak O, Corbett S, North R, Roberts R, 2013. MRI biomarkers for evaluation of treatment efficacy in preclinical diabetic retinopathy. Expert Opin. Med. Diagn 7, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Bissig D, Patel P, Bhatia A, Roberts R, 2012a. Acute systemic 11-cis-retinal intervention improves abnormal outer retinal ion channel closure in diabetic mice. Mol. Vis 18, 372–376. [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Bissig D, Ye Y, Valsadia P, Kern TS, Roberts R, 2012b. Evidence for diffuse central retinal edema in vivo in diabetic male Sprague Dawley rats. In: Evidence for Diffuse Central Retinal Edema in Vivo in Diabetic Male Sprague Dawley Rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Gorgis J, Patel A, Baameur F, Gurevich VV, Craft CM, Kefalov VJ, Roberts R, 2015c. Feb. Development of an MRI biomarker sensitive to tetrameric visual arrestin 1 and its reduction via light-evoked translocation in vivo. FASEB J. 29 (2), 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Grady EM, Khetarpal N, Patel A, Roberts R, 2015a. Oxidative stress and light-evoked responses of the posterior segment in a mouse model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci 56, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, McDonald C, Ito Y, Tofts PS, Latif Z, Gross J, 2001. Measuring the human retinal oxygenation response to a hyperoxic challenge using MRI: eliminating blinking artifacts and demonstrating proof of concept. Magn. Reson. Med 46, 412–416. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Murphy GG, Craft CM, Surmeier DJ, Roberts R, 2015b. Genetic dissection of horizontal cell inhibitory signaling in mice in complete darkness in vivo. Investig. Ophthalmol. Vis. Sci 56, 3132–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, 2008. Prognostic MRI biomarkers of treatment efficacy for retinopathy. NMR Biomed. 21, 957–967. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, Bissig D, 2010. Light-dependant intraretinal ion regulation by melanopsin in young awake and free moving mice evaluated with manganese-enhanced MRI. Mol. Vis 16, 1776–1780. [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, Luan H, Bissig D, Bui BV, Gradianu M, Calkins DJ, Vingrys AJ, 2007a. Manganese-enhanced MRI studies of alterations of intraretinal ion demand in models of ocular injury. Investig. Ophthalmol. Vis. Sci 48, 3796–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, Oleske DA, Chang M, Schafer S, Bissig D, Gradianu M, 2009a. Quantitative mapping of ion channel regulation by visual cycle activity in rodent photoreceptors in vivo. Investig. Ophthalmol. Vis. Sci 50, 1880–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, Penn JS, Gradianu M, 2007b. High-resolution manganese-enhanced MRI of experimental retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci 48, 4733–4740. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, Stemmler A, Luan H, Gradianu M, 2007c. Impaired apparent ion demand in experimental diabetic retinopathy: correction by lipoic acid. Investig. Ophthalmol. Vis. Sci 48, 4753–4758. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Tofts PS, Sen HA, Ando N, de Juan E Jr., 1992. Accurate and precise measurement of blood-retinal barrier breakdown using dynamic Gd-DTPA MRI. Investig. Ophthalmol. Vis. Sci 33, 3500–3506. [PubMed] [Google Scholar]
- Berkowitz B, Roberts R, 2010. Evidence for a critical role of panretinal pathophysiology in experimental ROP. Doc. Ophthalmol 120, 13–24. [DOI] [PubMed] [Google Scholar]
- Berkowitz B, 2008. Hypoxia and retinal neovascularization. In: Penn JS. (Ed.), Retinal and Choroidal Angiogenesis. Springer, Netherlands, pp. 151–168. [Google Scholar]
- Berkowitz BA, Gradianu M, Bissig D, Kern TS, Roberts R, 2009b. Retinal ion regulation in a mouse model of diabetic retinopathy: natural history and the effect of Cu/Zn superoxide dismutase overexpression. Investig. Ophthalmol. Vis. Sci 50, 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Gradianu M, Schafer S, Jin Y, Porchia A, Iezzi R, Roberts R, 2008. Ionic dysregulatory phenotyping of pathologic retinal thinning with manganese-enhanced MRI. Investig. Ophthalmol. Vis. Sci 49, 3178–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Grady EM, Roberts R, 2014. Confirming a prediction of the calcium hypothesis of photoreceptor aging in mice. Neurobiol. Aging 35, 1883–1891. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, Goebel DJ, Luan H, 2006. Noninvasive and Simultaneous imaging of layer-specific retinal functional adaptation by manganese-enhanced MRI. Investig. Ophthalmol. Vis. Sci 47, 2668–2674. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, Luan H, Bissig D, Bui BV, Gradianu M, Calkins DJ, Vingrys AJ, 2007d. Manganese-enhanced MRI studies of alterations of intraretinal ion demand in models of ocular injury. Investig. Ophthalmol. Vis. Sci 48, 3796–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Roberts R, Luan H, Peysakhov J, Mao X, Thomas KA, 2004. Dynamic contrast-enhanced MRI measurements of passive permeability through blood retinal barrier in diabetic rats. Investig. Ophthalmol. Vis. Sci 45, 2391–2398. [DOI] [PubMed] [Google Scholar]
- Bissig D, Berkowitz BA, 2012. Light-dependent changes in outer retinal water diffusion in rats in vivo. Mol. Vis 18, 2561–2xxx. [PMC free article] [PubMed] [Google Scholar]
- Bissig D, Berkowitz BA, 2014. Testing the calcium hypothesis of aging in the rat hippocampus in vivo using manganese-enhanced MRI. Neurobiol. Aging 35, 1453–1458. [DOI] [PubMed] [Google Scholar]
- Bissig D, Berkowitz BA, 2011. Same-session functional assessment of rat retina and brain with manganese-enhanced MRI. NeuroImage 58, 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig D, Goebel D, Berkowitz BA, 2013. Diminished vision in healthy aging is associated with increased retinal L-type voltage gated calcium channel ion influx. PLoS One 8, e56340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A, Wheaton AJ, Gougoutas AJ, Akella SVS, Regatte RR, Charagundla SR, Reddy R, 2004. In vivo measurement of T1-ρ dispersion in the human brain at 1.5 tesla. J. Magn. Reson. Imaging 19, 403–409. [DOI] [PubMed] [Google Scholar]
- Burtscher V, Schicker K, Novikova E, Pohn B, Stockner T, Kugler C, Singh A, Zeitz C, Lancelot ME, Audo I, Leroy BP, Freissmuth M, Herzig S, Matthes J, Koschak A, 2014. Spectrum of Cav1.4 dysfunction in congenital stationary night blindness type 2. Biochim. Biophys. Acta 1838, 2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquet P, Khoi Nguyen N, Schmid E, Tanimoto N, Seeliger MW, Ben-Yosef T, Mizuno F, Akopian A, Striessnig J, Singewald N, 2010. CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int. J. Neuropsychopharmacol 13, 499–513. [DOI] [PubMed] [Google Scholar]
- Bussink J, Kaanders JH, Van Der Kogel AJ, 2003. Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother. Oncol 67, 3–15. [DOI] [PubMed] [Google Scholar]
- Calkins D, Horner PJ, Roberts R, Gradianu M, Berkowitz BA, 2008. Manganese-enhanced MRI of the DBA/2J mouse model of hereditary glaucoma. Investig. Ophthalmol. Vis. Sci 49, 5083–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA, Strauss RW, Lu L, Hafiz G, Wolfson Y, Shah SM, Sophie R, Mir TA, Scholl HP, 2015. Sep 1. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid. Redox Signal 23 (7), 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Govardovskii V, Li JD, Steinberg RH, 1996. Systemic hypoxia dehydrates the space surrounding photoreceptors in the cat retina. Investig. Ophthalmol. Vis. Sci 37, 586–596. [PubMed] [Google Scholar]
- Carlson RO, Masco D, Brooker G, Spiegel S, 1994. Endogenous ganglioside GM1 modulates L-type calcium channel activity in N18 neuroblastoma cells. J. Neurosci 14, 2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, Lavail MM, Sidman RL, 1978. Differential effect of the rd mutation on rods and cones in the mouse retina. Investig. Ophthalmol. Vis. Sci 17, 489–498. [PubMed] [Google Scholar]
- Chan KC, Cheng JS, Fan S, Zhou IY, Yang J, Wu EX, 2012. In vivo evaluation of retinal and callosal projections in early postnatal development and plasticity using manganese-enhanced MRI and diffusion tensor imaging. NeuroImage 59, 2274–2283. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Q, Zhang H, Yang X, Wang J, Berkowitz BA, Wickline SA, Song SK, 2008. In vivo quantification of T1, T2, and apparent diffusion coefficient in the mouse retina at 11.74T. Magn. Reson. Med 59, 731–738. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Q, Chen S, Wickline SA, Song SK, 2011. In vivo diffusion tensor MRI of the mouse retina: a noninvasive visualization of tissue organization. NMR Biomed. 24, 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Krizaj D, Thoreson WB, 2014. Intracellular calcium stores drive slow non-ribbon vesicle release from rod photoreceptors. Front. Cell. Neurosci 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KH, Koretsky AP, Sotak CH, 2009. Temporal changes in the T1 and T2 relaxation rates (DeltaR1 and DeltaR2) in the rat brain are consistent with the tissue-clearance rates of elemental manganese. Magn. Reson. Med 61, 1528–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani C, Rogers B, Lu L, Kachi S, Shen J, Campochiaro PA, 2006. Retinal degeneration from oxidative damage. Free Radic. Biol. Med 40, 660–669. [DOI] [PubMed] [Google Scholar]
- Ciolofan C, Lynn BD, Wellershaus K, Willecke K, Nagy JI, 2007. Spatial relationships of connexin36, connexin57 and zonula occludens-1 in the outer plexiform layer of mouse retina. Neuroscience 148, 473–488. [DOI] [PubMed] [Google Scholar]
- Clermont A, Chilcote TJ, Kita T, Liu J, Riva P, Sinha S, Feener EP, 2011. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes 60, 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DJ, Flexman JA, Anzai Y, Sasaki T, Treuting PM, Maravilla KR, Minoshima S, 2007. In vivo manganese MR imaging of calcium influx in spontaneous rat pituitary adenoma. AJNR Am. J. Neuroradiol 28, 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, Millican CL, 1996. Photoreceptor loss in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci 37, 1236–1249. [PubMed] [Google Scholar]
- Dallinger S, Findl O, Strenn K, Eichler HG, Wolzt M, Schmetterer L, 1998. Age dependence of choroidal blood flow. J. Am. Geriatr. Soc 46, 484–487. [DOI] [PubMed] [Google Scholar]
- Deans MR, Paul DL, 2001. Mouse horizontal cells do not express connexin26 or connexin36. Cell Commun. Adhes 8, 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Pandarinath C, Alam NM, Wellershaus K, Schubert T, Willecke K, Prusky GT, Weiler R, Nirenberg S, 2008. Ganglion cell adaptability: does the coupling of horizontal cells play a role? PLoS One 3, e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Wang J, 2002. Technical aspects and utility of fMRI using BOLD and ASL. Clin. Neurophysiol 113, 621–634. [DOI] [PubMed] [Google Scholar]
- De La Garza BH, Li G, Shih YY, Duong TQ, 2012. Layer-specific manganese-enhanced MRI of the retina in light and dark adaptation. Investig. Ophthalmol. Vis. Sci 53, 4352–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen RM, Starnes CA, Berkowitz BA, Winkler BS, 2006. Reexamining the hyperglycemic pseudohypoxia hypothesis of diabetic oculopathy. Investig. Ophthalmol. Vis. Sci 47, 2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A, Shen J, Krause M, Akiyama H, Hackett SF, Lai H, Campochiaro PA, 2006. Superoxide dismutase 1 protects retinal cells from oxidative damage. J. Cell Physiol 208, 516–526. [DOI] [PubMed] [Google Scholar]
- Downs CA, Helms MN, 2013. Regulation of ion transport by oxidants. Am. J. Physiol. Lung Cell Mol. Physiol 305, L595–L603. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Nachshen DA, 1984. Manganese fluxes and manganese-dependent neurotransmitter release in presynaptic nerve endings isolated from rat brain. J. Physiol 348, 493–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Cramer M, Lee CA, Tang J, Muthusamy A, Antonetti DA, Jin H, Palczewski K, Kern TS, 2015. Adrenergic and serotonin receptors affect retinal superoxide generation in diabetic mice: relationship to capillary degeneration and permeability. FASEB J. 29, 2194–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Veenstra A, Palczewski K, Kern TS, 2013. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. PNAS 110, 16586–16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ, Ngan SC, Ugurbil K, Kim SG, 2002. Functional magnetic resonance imaging of the retina. Investig. Ophthalmol. Vis. Sci 43, 1176–1181. [PMC free article] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG, 2000. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn. Reson. Med 43, 383–392. [DOI] [PubMed] [Google Scholar]
- Duong TQ, 2014. Magnetic resonance imaging of the retina: from mice to men. Magn. Reson. Med 71, 1526–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis SR, Betz AL, 1986. Sucrose permeability of the blood-retinal and blood-brain barriers. Effects of diabetes, hypertonicity, and iodate. Investig. Ophthalmol. Vis. Sci 27, 1095–1102. [PubMed] [Google Scholar]
- Feigenspan A, Janssen-Bienhold U, Hormuzdi S, Monyer H, Degen J, Sohl G, Willecke K, Ammermuller J, Weiler R, 2004. Expression of connexin36 in cone pedicles and OFF-cone bipolar cells of the mouse retina. J. Neurosci 24, 3325–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit-Leichman RA, Kinouchi R, Takeda M, Fan Z, Mohr S, Kern TS, Chen DF, 2005. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Investig. Ophthalmol. Vis. Sci 46, 4281–4287. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ME, Caldwell RB, Reiner A, 1992. Vasoactive intestinal polypeptide-containing nerve fibers are increased in abundance in the choroid of dystrophic RCS rats. Curr. Eye Res 11, 501–515. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ME, Gamlin PD, Zagvazdin Y, Reiner A, 1996. Central neural circuits for the light-mediated reflexive control of choroidal blood flow in the pigeon eye: a laser Doppler study. Vis. Neurosci 13, 655–669. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MEC, Tolley E, Frase S, Zagvazdin Y, Miller RF, Hodos W, Reiner A, 2001. Functional and morphological assessment of age-related changes in the choroid and outer retina in pigeons. Vis. Neuro 18, 299–317. [DOI] [PubMed] [Google Scholar]
- Fox DA, Poblenz AT, He L, Harris JB, Medrano CJ, 2003. Pharmacological strategies to block rod photoreceptor apoptosis caused by calcium overload: a mechanistic target-site approach to neuroprotection. Eur. J. Ophthalmol 13 (Suppl. 3), S44–S56. [DOI] [PubMed] [Google Scholar]
- Frank RN, 2004. Diabetic retinopathy. N. Engl. J. Med 350, 48–58. [DOI] [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, Malec M, Amoako-Mensah T, Kolodjaschna J, Schmetterer L, 2003. Changes in choroidal blood flow during light/dark transitions are not altered by atropine or propranolol in healthy subjects. Vis. Res 43, 2185–2190. [DOI] [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, Polska E, Malec M, Schmetterer L, 2001. Unilateral light-dark transitions affect choroidal blood flow in both eyes. Vis. Res 41, 2919–2924. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Ohneda O, Oshika T, 2014. Oxidative stress retards vascular development before neural degeneration occurs in retinal degeneration rd1 mice. Graefes Arch. Clin. Exp. Ophthalmol 252, 411–416. [DOI] [PubMed] [Google Scholar]
- Fusi F, Saponara S, Gagov H, Sgaragli G, 2001. 2,5-Di-t-butyl-1,4-benzohydroquinone (BHQ) inhibits vascular L-type Ca(2+) channel via superoxide anion generation. Br. J. Pharmacol 133, 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldenagel M, Ammermüller J, Feigenspan A, Teubner B, Degen J, Söhl G, Willecke K, Weiler R, 2001. Visual transmission deficits in mice with targeted disruption of the Gap Junction gene connexin36. J. Neurosci 21, 6036–6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbinur T, Obolensky A, Berenshtein E, Vinokur V, Chowers I, Chevion M, Banin E, 2009. Effect of para-aminobenzoic acid on the course of retinal degeneration in the rd10 mouse. J. Ocul. Pharmacol. Ther 25, 475–482. [DOI] [PubMed] [Google Scholar]
- Garhofer G, Huemer KH, Zawinka C, Schmetterer L, Dorner GT, 2002. Influence of diffuse luminance flicker on choroidal and optic nerve head blood flow. Curr. Eye Res 24, 109–113. [DOI] [PubMed] [Google Scholar]
- Glossmann H, Linn T, Rombusch M, Ferry DR, 1983. Temperature-dependent regulation of d-cis-[3H]diltiazem binding to Ca2+ channels by 1,4-dihydropyridine channel agonists and antagonists. FEBS Lett. 160, 226–232. [DOI] [PubMed] [Google Scholar]
- Gresh J, Goletz PW, Crouch RK, Rohrer B, 2003. Structure-function analysis of rods and cones in juvenile, adult, and aged C57bl/6 and Balb/c mice. Vis. Neurosci 20, 211–220. [DOI] [PubMed] [Google Scholar]
- Grimes PA, 1985. Fluorescein distribution in retinas of normal and diabetic rats. Exp. Eye Res 41, 227–238. [DOI] [PubMed] [Google Scholar]
- Grimes PA, 1988. Carboxyfluorescein distribution in ocular tissues of normal and diabetic rats. Curr. Eye Res 7, 981–988. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Hariprasad SM, DuPont J, 1998. Effect of aging on foveolar choroidal circulation. Arch. Ophthalmol 116, 150–154. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Piltz J, Patel N, Bose S, Riva CE, 1993. Effect of aging on retinal macular microcirculation: a blue field simulation study. Investig. Ophthalmol. Vis. Sci 34, 3609–3613. [PubMed] [Google Scholar]
- Guerra L, Cerbai E, Gessi S, Borea PA, Mugelli A, 1996. The effect of oxygen free radicals on calcium current and dihydropyridine binding sites in guinea-pig ventricular myocytes. Br. J. Pharmacol 118, 1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Hanson SM, Song X, Vishnivetskiy SA, Gurevich EV, 2011. The functional cycle of visual arrestins in photoreceptor cells. Prog. Retin. Eye Res 30, 405–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ, 2010. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468, 696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann CJ, O’Brien BJ, Wässle H, Protti DA, 2003. AII amacrine cells express L-type calcium channels at their output synapses. J. Neurosci 23, 6904–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa JT, Berkowitz BA, Wilson CA, Ando N, Sen HA, Jaffe GJ, 1996. Hypoxia precedes the development of experimental preretinal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol 234, 43–46. [DOI] [PubMed] [Google Scholar]
- Handa JT, 2012. How does the macula protect itself from oxidative stress? Mol. Asp. Med 33, 418–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Vishnivetskiy SA, Hubbell WL, Gurevich VV, 2007. Opposing effects of inositol hexakisphosphate on rod arrestin and arrestin2 self-association. Biochemistry 47, 1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haris M, Yadav SK, Rizwan A, Singh A, Cai K, Kaura D, Wang E, Davatzikos C, Trojanowski JQ, Melhem ER, Marincola FM, Borthakur A, 2015. T1rho MRI and CSF biomarkers in diagnosis of Alzheimer’s disease. NeuroImage Clin. 7, 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach S, Janssen-Bienhold U, Söhl G, Schubert T, Büssow H, Ott T, Weiler R, Willecke K, 2004. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur. J. Neurosci 19, 2633–2640. [DOI] [PubMed] [Google Scholar]
- Horio N, Clermont AC, Abiko A, Abiko T, Shoelson BD, Bursell SE, Feener EP, 2004. Angiotensin AT(1) receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia 47, 113–123. [DOI] [PubMed] [Google Scholar]
- Huang SP, Brown BM, Craft CM, 2010. Visual arrestin 1 acts As a modulator for N-ethylmaleimide-sensitive factor in the photoreceptor synapse. J. Neurosci 30, 9381–9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn J, Borthakur A, Bloy L, Gualtieri EE, Reddy R, Leigh JS, Elliott MA, 2005. T1-ρ contrast in functional magnetic resonance imaging. Magn. Reson. Med 54, 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman DM, Lambert WS, Calkins DJ, Horner PJ, 2013. alpha-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS One 8, e65389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Roberts R, Bissig D, Pan ZH, Berkowitz BA, 2010. Retinal channelrhodopsin-2-mediated activity in vivo evaluated with manganese-enhanced magnetic resonance imaging. Mol. Vis 16, 1059–106. [PMC free article] [PubMed] [Google Scholar]
- Jacob R, 1990. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J. Physiol 421, 55–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaliffa C, Ameqrane I, Dansault A, Leemput J, Vieira V, Lacassagne E, Provost A, Bigot K, Masson C, Menasche M, Abitbol M, 2009. Sirt1 involvement in rd10 mouse retinal degeneration. Investig. Ophthalmol. Vis. Sci 50, 3562–3572. [DOI] [PubMed] [Google Scholar]
- Jin T, Kim SG, 2013. Characterization of non-hemodynamic functional signal measured by spin-lock fMRI. NeuroImage 78, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen-Soriano S, Garcia-Pous M, Arnal E, Sancho-Tello M, Garcia-Delpech S, Miranda M, Bosch-Morell F, Diaz-Llopis M, Navea A, Romero FJ, 2008. Early lipoic acid intake protects retina of diabetic mice. Free Radic. Res 42, 613–617. [DOI] [PubMed] [Google Scholar]
- Johnson JE Jr., Perkins GA, Giddabasappa A, Chaney S, Xiao W, White AD, Brown JM, Waggoner J, Ellisman MH, Fox DA, 2007. Spatiotemporal regulation of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. Mol. Vis 13, 887–919. [PMC free article] [PubMed] [Google Scholar]
- Jurkovicova-Tarabova B, Griesemer D, Pirone A, Sinnegger-Brauns MJ, Striessnig J, Friauf E, 2012. Repertoire of high voltage-activated Ca2+ channels in the lateral superior olive: functional analysis in wild-type, Ca(v)1.3(−/−), and Ca(v)1.2DHP(−/−) mice. J. Neurophysiol 108, 365–379. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R, 2001. Hemichannel-mediated inhibition in the outer retina. Science 292, 1178–1180. [DOI] [PubMed] [Google Scholar]
- Karlsson JE, El-Saadi W, Ali M, Puskar W, Skogvard P, Engvall JE, Andersson RG, Maret E, Jynge P, 2015. Mangafodipir as a cardioprotective adjunct to reperfusion therapy: a feasibility study in patients with ST-segment elevation myocardial infarction. Eur. Heart J. — Cardiovasc. Pharmacother 1, 39–45. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA, Hyde DR, 2008. CNTF induces photoreceptor neuroprotection and Mnller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp. Eye Res 88, 1051–1064. [DOI] [PubMed] [Google Scholar]
- Kern TS, Tang J, Berkowitz BA, 2010. Validation of structural and functional lesions of diabetic retinopathy in mice. Mol. Vis 16, 2121–2131. [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Tang J, Mizutani M, Kowluru RA, Nagaraj RH, Romeo G, Podesta F, Lorenzi M, 2000. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Investig. Ophthalmol. Vis. Sci 41, 3972–3978. [PubMed] [Google Scholar]
- Kettunen MI, Gröhn OHJ, Silvennoinen MJ, Penttonen M, Kauppinen RA, 2001. Effects of intracellular pH, blood, and tissue oxygen tension on T1-ü relaxation in rat brain. Magn. Reson. Med 48, 470–477. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ogawa S, 2012. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow. Metab 32, 1188–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T, Klooster J, Claassen Y, Shields CR, Ten Eikelder HMM, Janssen-Bienhold U, Zoidl G, McMahon DG, Kamermans M, 2011. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol 9, e1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko A, Cao S, Pakzad-Vaezi K, Brasher PM, Merkur AB, Albiani DA, Kirker AW, Cui J, Matsubara J, Forooghian F, 2013. Optical coherence tomography-based correlation between choroidal thickness and drusen load in dry age-related macular degeneration. Retina 33, 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Fan J, Crouch RK, Kefalov VJ, 2010. Age-related deterioration of rod vision in mice. J. Neurosci 30, 11222–11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L, Campochiaro PA, 2006. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. PNAS 103, 11300–11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Walter D, Hoda JC, Heinzle T, Grabner M, Striessnig J, 2001. Cav1.4alpha1 subunits can form slowly Inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent Inactivation. J. Neurosci 23, 6041–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourie JI, 1998. Interaction of reactive oxygen species with ion transport mechanisms. Am. J. Physiol 275, C1–C24. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Tang J, Kern TS, 2001. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 50, 1938–1942. [DOI] [PubMed] [Google Scholar]
- Krizaj D, 2012. Calcium stores in vertebrate photoreceptors. Adv. Exp. Med. Biol 740, 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Garza BHD, Muir ER, Li G, Shih YY, Duong TQ, 2011. Blood oxygenation level-dependent (BOLD) functional MRI of visual stimulation in the rat retina at 11.7 T. NMR Biomed. 24, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JC, Linsenmeier RA, 2014. Increased intraretinal PO2 in short-term diabetic rats. Diabetes 63, 4338–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K, Schmidt KF, Löwel S, 2012. Vision and visual plasticity in ageing mice. Restor. Neurol. Neurosci 30, 161–178. [DOI] [PubMed] [Google Scholar]
- Li JD, Gallemore RP, Dmitriev A, Steinberg RH, 1994a. Light-dependent hydration of the space surrounding photoreceptors in chick retina. Investig. Ophthalmol. Vis. Sci 35, 2700–2711. [PubMed] [Google Scholar]
- Li JD, Govardovskii VI, Steinberg RH, 1994b. Light-dependent hydration of the space surrounding photoreceptors in the cat retina. Vis. Neurosci 11, 743–752. [DOI] [PubMed] [Google Scholar]
- Li X, jamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S, 2007. In vivo T1-ρ and T2 mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Osteoarthr. Cartil 15, 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cheng H, Duong TQ, 2008. Blood-flow magnetic resonance imaging of the retina. NeuroImage 39, 1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Koretsky AP, 1997. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magn. Reson. Med 38, 378–388. [DOI] [PubMed] [Google Scholar]
- Lindner T, Langner S, Graessl A, Rieger J, Schwerter M, Muhle M, Lysiak D, Kraus O, Wuerfel J, Guthoff RF, Falke K, Hadlich S, Krueger PC, Hosten N, Niendorf T, Stachs O, 2014. High spatial resolution in-ávivo magnetic resonance imaging of the human eye, orbit, nervus opticus and optic nerve sheath at 7.0 Tesla. Exp. Eye Res 125, 89–94. [DOI] [PubMed] [Google Scholar]
- Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL, McLeod DS, Lutty GA, 1998. Retinal hypoxia in long-term diabetic cats. Investig. Ophthalmol. Vis. Sci 39, 1647–1657. [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W, 2004. L-type calcium channels: the low down. J. Neurophysiol 92, 2633–2641. [DOI] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC, Barnes S, 2013. Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J. Physiol 591, 3309–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo A, Geiser M, Riva CE, 2000. Subfoveal choroidal blood flow in response to light-dark exposure. Investig. Ophthalmol. Vis. Sci 41, 2678–2683. [PubMed] [Google Scholar]
- Lorenzi M, Feke GT, Pitler L, Berisha F, Kolodjaschna J, McMeel JW, 2010. Defective myogenic response to posture change in retinal vessels of wellcontrolled type 1 diabetic patients with no retinopathy. Investig. Ophthalmol. Vis. Sci 51, 6770–6775. [DOI] [PubMed] [Google Scholar]
- Luan H, Roberts R, Sniegowski M, Goebel DJ, Berkowitz BA, 2006. Retinal thickness and subnormal retinal oxygenation response in experimental diabetic retinopathy. Investig. Ophthalmol. Vis. Sci 47, 320–328. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Heo HY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA, 2012. Detecting activity-evoked pH changes in human brain. PNAS 109, 8270–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT, 2005. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum. Mol. Genet 14, 3035–3046. [DOI] [PubMed] [Google Scholar]
- Mao H, Seo SJ, Biswal MR, Li H, Conners M, Nandyala A, Jones K, Le YZ, Lewin AS, 2014. Mitochondrial oxidative stress in the retinal pigment epithelium leads to localized retinal degeneration. Investig. Ophthalmol. Vis. Sci 55, 4613–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TJ, Prusky GT, Douglas RM, Yasumura D, Matthes MT, Lowe RJ, Duncan JL, Yang H, Ahern K, Daniello KM, Silver B, LaVail MM, 2012. Discordant anatomical, electrophysiological, and visual behavioral profiles of retinal degeneration in rat models of retinal degenerative disease. Investig. Ophthalmol. Vis. Sci 53, 6232–6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Murphy GG, 2006. The L-type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn. Mem 13, 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano CJ, Fox DA, 1995. Oxygen consumption in the rat outer and inner retina: light- and pharmacologically-induced inhibition. Exp. Eye Res 61, 273–284. [DOI] [PubMed] [Google Scholar]
- Mendez A, Lem J, Simon M, Chen J, 2003. Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J. Neurosci 23, 3124–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Bito H, Deisseroth K, Tsien RW, 2000. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J. Neurosci 20, 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midena E, Segato T, Radin S, di Giorgio G, Meneghini F, Piermarocchi S, Belloni AS, 1989. Studies on the retina of the diabetic db/db mouse. I. Endothelial cell-pericyte ratio. Ophthalmic Res. 21, 106–111. [DOI] [PubMed] [Google Scholar]
- Molnar T, Barabas P, Birnbaumer L, Punzo C, Kefalov V, Krizaj D, 2012. Store-operated channels regulate intracellular calcium in mammalian rods. J. Physiol 590, 3465–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR, 2005. Photoreceptor calcium channels: insight from night blindness. Vis. Neurosci 22, 561–568. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Gaughwin P, Maleszka R, 2001. Expression of the alpha1F calcium channel subunit by photoreceptors in the rat retina. Mol. Vis 7, 202–209. [PubMed] [Google Scholar]
- Muir ER, Duong TQ, 2011. MRI of retinal and choroidal blood flow with laminar resolution. NMR Biomed. 24, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir ER, Chandra SB, De La Garza BH, Velagapudi C, Abboud HE, Duong TQ, 2015. Layer-specific manganese-enhanced MRI of the diabetic rat retina in light and dark adaptation at 11.7 tesla light and dark adapted MRI of the diabetic retina. Investig. Ophthalmol. Vis. Sci 56, 4006–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair G, Tanaka Y, Kim M, Olson DE, Thule PM, Pardue MT, Duong TQ, 2011. MRI reveals differential regulation of retinal and choroidal blood volumes in rat retina. NeuroImage 54, 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair G, Shen Q, Duong TQ, 2010. Relaxation time constants and apparent diffusion coefficients of rat retina at 7 Tesla. Int. J. Imaging Syst. Technol 20, 126.-. [Google Scholar]
- Nusinowitz S, Ridder WH, Heckenlively JR, 1999. Rod multifocal electroretinograms in mice. Investig. Ophthalmol. Vis. Sci 40, 2848–2858. [PubMed] [Google Scholar]
- O’Brien JJ, Chen X, Macleish PR, O’Brien J, Massey SC, 2012a. Photoreceptor coupling mediated by connexin36 in the primate retina. J. Neurosci 32, 4675–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola MS, Berkich DA, Xu Y, King MT, Gardner TW, Simpson I, LaNoue KF, 2005. Analysis of glucose metabolism in diabetic rat retinas. Am. J. Physiol. Endocrinol. Metab 290, E1057–E1067. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J, 2004. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res 23, 91–147. [DOI] [PubMed] [Google Scholar]
- O’Brien JJ, Chen X, MacLeish PR, O’Brien J, Massey SC, 2012b. Photoreceptor coupling mediated by connexin 36 in the primate retina. J. Neurosci 32, 4675–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandarinath C, Bomash I, Victor JD, Prusky GT, Tschetter WW, Nirenberg S, 2008. A novel mechanism for switching a neural system from one state to another. Front. Comput. Neurosci 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J, Noorwez SM, Malhotra R, McDowell JH, Kaushal S, Hauswirth WW, Nusinowitz S, Thompson DA, Heckenlively JR, 2005. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol. Vis 11, 152–162. [PubMed] [Google Scholar]
- Pang J, Boye SE, Lei B, Boye SL, Everhart D, Ryals R, Umino Y, Rohrer B, Alexander J, Li J, Dai X, Li Q, Chang B, Barlow R, Hauswirth WW, 2010. Self-complementary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Ther. 17, 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques M, Tadayoni R, Sercombe R, Laurent P, Genevois O, Gaudric A, Vicaut E, 2003. Structural and hemodynamic analysis of the mouse retinal microcirculation. Investig. Ophthalmol. Vis. Sci 44, 4960–4967. [DOI] [PubMed] [Google Scholar]
- Perkins GA, Ellisman MH, Fox DA, 2003. Three-dimensional analysis of mouse rod and cone mitochondrial cristae architecture: bioenergetic and functional implications. Mol. Vis 9, 60–73. [PubMed] [Google Scholar]
- Philp NJ, Chang W, Long K, 1987. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 225, 127–132. [DOI] [PubMed] [Google Scholar]
- Punzo C, Xiong W, Cepko CL, 2012. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J. Biol. Chem 287, 1642–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos de Carvalho JE, Verbraak FD, Aalders MC, van Noorden CJ, Schlingemann RO, 2014. Recent advances in ophthalmic molecular imaging. Surv. Ophthalmol 59, 393–413. [DOI] [PubMed] [Google Scholar]
- Reeves D, Lee M, Hatala D, Kern TS, 2002. Nitroimidazole derivative as a histologic marker for retinal ischemia. ARVO Meet. Abstr 43, 785. [Google Scholar]
- Regatte RR, Akella SVS, Borthakur A, Reddy R, 2003. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J. Magn. Reson. Imaging 17, 114–121. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A, 2005. Muller cells as players in retinal degeneration and edema. Graefes Arch. Clin. Exp. Ophthalmol 245, 627–636. [DOI] [PubMed] [Google Scholar]
- Riegel RE, Valvassori SS, Moretti M, Ferreira CL, Steckert AV, de SB, Dal-Pizzol F, Quevedo J, 2010. Intracerebroventricular ouabain administration induces oxidative stress in the rat brain. Int. J. Dev. Neurosci 28, 233–237. [DOI] [PubMed] [Google Scholar]
- Roddy GW, Rosa RH Jr., Oh JY, Ylostalo JH, Bartosh TJ Jr., Choi H, Lee RH, Yasumura D, Ahern K, Nielsen G, Matthes MT, Lavail MM, Prockop DJ, 2012. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol. Ther 20, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BS, Symons RCA, Komeima K, Shen J, Xiao W, Swaim ME, Gong YY, Kachi S, Campochiaro PA, 2007. Differential sensitivity of cones to iron-mediated oxidative damage. Investig. Ophthalmol. Vis. Sci 48, 438–445. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Pinto FR, Hulse KE, Lohr HR, Zhang L, Almeida JS, 2004. Multidestructive pathways triggered in photoreceptor cell death of the rd mouse as determined through gene expression profiling. J. Biol. Chem 279, 41903–41910. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Thieme H, Strauss O, 2001. Fibroblast growth factor receptor 2 (FGFR2) in brain neurons and retinal pigment epithelial cells act via stimulation of neuroendocrine L-type channels (Ca(v)1.3). FASEB J. 15, 970–977. [DOI] [PubMed] [Google Scholar]
- Samardzija M, Caprara C, Heynen SR, Willcox DeParis S, Meneau I, Traber G, Agca C, von Lintig J, Grimm C, 2014. A mouse model for studying cone photoreceptor pathologies. Investig. Ophthalmol. Vis. Sci 55, 5304–5313. [DOI] [PubMed] [Google Scholar]
- Samuels IS, Lee CA, Petrash JM, Peachey NS, Kern TS, 2012. Exclusion of aldose reductase as a mediator of ERG deficits in a mouse model of diabetic eye disease. Vis. Neurosci 29, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz MM, Johnson LE, Ahuja S, Ekstrom PA, Romero J, van VT, 2007. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience 145, 1120–1129. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Witkovsky P, 1997. Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neuroscience 78, 1209–1216. [DOI] [PubMed] [Google Scholar]
- Schubert T, Degen J, Willecke K, Hormuzdi SG, Monyer H, Weiler R, 2005. Connexin36 mediates gap junctional coupling of alpha-ganglion cells in mouse retina. J. Comp. Neurol 485, 191–201. [DOI] [PubMed] [Google Scholar]
- Schubert T, Weiler R, Feigenspan A, 2006. Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J. Neurophysiol 96, 1278–1292. [DOI] [PubMed] [Google Scholar]
- Shelley J, Dedek K, Schubert T, Feigenspan A, Schultz K, Hombach S, Willecke K, Weiler R, 2006. Horizontal cell receptive fields are reduced in connexin57-deficient mice. Eur. J. Neurosci 23, 3176–3186. [DOI] [PubMed] [Google Scholar]
- Shen J, Yang X, Dong A, Petters RM, Peng YW, Wong F, Campochiaro PA, 2005. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell Physiol 203, 457–464. [DOI] [PubMed] [Google Scholar]
- Shih YY, De La Garza BH, Muir ER, Rogers WE, Harrison JM, Kiel JW, Duong TQ, 2011. Lamina-specific functional MRI of retinal and choroidal responses to visual stimuli. Investig. Ophthalmol. Vis. Sci 52, 5303–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Muir ER, Li G, De La Garza BH, Duong TQ, 2012. High-resolution 3D MR microangiography of the rat ocular circulation. Radiology 264, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YY, Wang L, De La Garza BH, Li G, Cull G, Kiel JW, Duong TQ, 2013. Quantitative retinal and choroidal blood flow during light, dark adaptation and flicker light stimulation in rats using fluorescent microspheres. Curr. Eye Res 38, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotani K, Katsura M, Higo A, Takesue M, Mohri Y, Shuto K, Tarumi C, Ohkuma S, 2001. Suppression of Ca2+ influx through L-type voltage-dependent calcium channels by hydroxyl radical in mouse cerebral cortical neurons. Brain Res. Mol. Brain Res 92, 12–18. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J, 2009. Expression and 1,4-dihydropyridine-Binding properties of brain L-type calcium channel isoforms. Mol. Pharmacol 75, 407–414. [DOI] [PubMed] [Google Scholar]
- Sirin S, Schlamann M, Metz K, Bornfeld N, Schweiger B, Holdt M, Temming P, Schuendeln M, Goericke S, 2015. High-resolution MRI using orbit surface coils for the evaluation of metastatic risk factors in 143 children with retinoblastoma. Neuroradiology. 1–10. [DOI] [PubMed] [Google Scholar]
- Spear PD, 1993. Neural bases of visual deficits during aging. Vis. Res 33, 2589–2609. [DOI] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, pfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BHF, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A, 1998. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat. Genet 19, 260–263. [DOI] [PubMed] [Google Scholar]
- Swindell W, Ensrud K, Cawthon P, Cauley J, Cummings S, Miller R, Study Of Osteoporotic Fractures Research Group, 2010. Indicators of “Healthy Aging” in older women (65-69 years of age). A data-mining approach based on prediction of long-term survival. BMC Geriatr. 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabova B, Lacinova L, Engel J, 2007. Effects of phenylalkylamines and benzo thiazepines on Cav1.3-mediated Ca2+ currents in neonatal mouse inner hair cells. Eur. J. Pharmacol 573, 39–48. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC, 2012. Lateral interactions in the outer retina. Prog. Retin. Eye Res 31, 407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofts PS, Porchia A, Jin Y, Roberts R, Berkowitz BA, 2010. Toward clinical application of manganese-enhanced MRI of retinal function. Brain Res. Bull 81, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CG, Lundby B, Sterud AT, McGill S, Gordon PB, Bjerknes HS, 1997. MnDPDP for MR imaging of the liver: results from the European phase III studies. Acta Radiol. 38, 631 —637. [DOI] [PubMed] [Google Scholar]
- Trick GL, Berkowitz BA, 2005. Retinal oxygenation response and retinopathy. Prog. Retin. Eye Res 24, 259–274. [DOI] [PubMed] [Google Scholar]
- Usui S, Komeima K, Lee SY, Jo YJ, Ueno S, Rogers BS, Wu Z, Shen J, Lu L, Oveson BC, Rabinovitch PS, Campochiaro PA, 2009a. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol. Ther 17, 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui S, Oveson BC, Lee SY, Jo YJ, Yoshida T, Miki A, Miki K, Iwase T, Lu L, Campochiaro PA, 2009b. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem 110, 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanek J, Teng PY, Blair NP, Shahidi M, 2014. Inner retinal oxygen delivery and metabolism in streptozotocin diabetic rats. Investig. Ophthalmol. Vis. Sci 55, 1588–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Song SK, Zhang H, Berkowitz BA, Chen S, Wickline SA, Chen J, 2008. Photoreceptor degeneration changes magnetic resonance imaging features in a mouse model of retinitis pigmentosa. Magn. Reson. Med 65, 1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK, 2010. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA, 2003. Retinal oxygen: fundamental and clinical aspects. Arch. Ophthalmol 121, 547–557. [DOI] [PubMed] [Google Scholar]
- Wolfensberger TJ, Dmitriev AV, Govardovskii VI, 1999. Inhibition of membrane bound carbonic anhydrase decreases subretinal pH and volume. Doc Ophthalmol. 97, 261–271. [DOI] [PubMed] [Google Scholar]
- Wright WS, McElhatten RM, Harris NR, 2011. Increase in retinal hypoxia inducible factor-2alpha, but not hypoxia, early in the progression of diabetes in the rat. Exp. Eye Res 93, 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Marmorstein AD, Striessnig J, Peachey NS, 2007. Voltage-dependent calcium channel CaV1.3 subunits regulate the light peak of the electroretinogram. J. Neurophysiol 97, 3731–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Chen X, Steele EC Jr., 2007. Abundant L-type calcium channel Ca(v)1.3 (alpha1D) subunit mRNA is detected in rod photoreceptors of the mouse retina via in situ hybridization. Mol. Vis 13, 764–771. [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Zhao JW, Yang XL, 2002. Expression of voltage-dependent calcium channel subunits in the rat retina. Neurosci. Lett 329, 297–300. [DOI] [PubMed] [Google Scholar]
- Xu Q, Qaum T, Adamis AP, 2001. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Investig. Ophthalmol. Vis. Sci 42, 789–794. [PubMed] [Google Scholar]
- Xu W, Lipscombe D, 2001. Neuronal CaV1.3alpha1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci 21, 5944–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto F, Steinberg RH, 1992. Effects of intravenous acetazolamide on retinal pH in the cat. Exp. Eye Res 54, 711–718. [DOI] [PubMed] [Google Scholar]
- Yang LP, Wu LM, Guo XJ, Tso MO, 2007. Activation of endoplasmic reticulum stress in degenerating photoreceptors of the rd1 mouse. Investig. Ophthalmol. Vis. Sci 48, 5191–5198. [DOI] [PubMed] [Google Scholar]
- Yang L, Xu J, Minobe E, Yu L, Feng R, Kameyama A, Yazawa K, Kameyama M, 2013. Mechanisms underlying the modulation of L-type Ca2+ channel by hydrogen peroxide in guinea pig ventricular myocytes. J. Physiol. Sci 63, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XL, Wu SM, 1991. Feedforward lateral inhibition in retinal bipolar cells: input-output relation of the horizontal cell-depolarizing bipolar cell synapse. Proc. Natl. Acad. Sci. U. S. A 88, 3310–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wadghiri YZ, Sanes DH, Turnbull DH, 2005. In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat. Neurosci 8, 961 —968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Ding M, Chen XX, Lu Q, 2014. Microglial NADPH oxidase activation mediates rod cell death in the retinal degeneration in rd mice. Neuroscience 275, 54–61. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ito Y, Berlin E, Roberts R, Berkowitz BA, 2003. Role of hypoxia during normal retinal vessel development and in experimental retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci 44, 3119–3123. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nateras OSE, Peng Q, Kuranov RV, Harrison JM, Milner TE, Duong TQ, 2011. Lamina-specific anatomic magnetic resonance imaging of the human retina. Investig. Ophthalmol. Vis. Sci 52, 7232–7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Kern TS, Ball S, Berkowitz BA, 2007. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 50, 1987–1996. [DOI] [PubMed] [Google Scholar]
- Zhi Z, Chao JR, Wietecha T, Hudkins KL, Alpers CE, Wang RK, 2014. Noninvasive imaging of retinal morphology and microvasculature in obese mice using optical coherence tomography and optical microangiography. Investig. Ophthalmol. Vis. Sci 55, 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Lee A, Yang J, 2012. The expression of whirlin and Cav1.3alpha1 is mutually independent in photoreceptors. Vis. Res 75, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]


