Abstract
Background
Cancer survivors who develop breast cancer as a second malignancy (BCa-2) are common. Yet, little is known about the prognosis of BCa-2 compared to first primary breast cancer (BCa-1).
Methods
Using the Surveillance, Epidemiology, and End Results database, we conducted a population-based cohort study including 883,881 patients with BCa-1 and 36,313 patients with BCa-2 during 1990–2015. Compared with patients with BCa-1, we calculated hazard ratios (HRs) of breast cancer-specific mortality among patients with BCa-2, using multivariable Cox regression.
Results
During the follow-up (median 5.5 years), 114,964 and 3829 breast cancer-specific deaths were identified among BCa-1 and BCa-2 patients, respectively. Patients with BCa-2 had more favorable tumor characteristics and received less intensive treatment e.g., surgery and chemo−/radio-therapy, compared to patients with BCa-1. When adjusting for demographic factors, patients with BCa-2 were at similar risk of breast cancer-specific mortality (HR 1.00, 95% CI 0.97–1.03) compared to patients with BCa-1. However, when additionally controlling for tumor characteristics and treatment modes, BCa-2 patients were at an increased risk of breast cancer-specific mortality (HR 1.11, 95% CI 1.08–1.15). The risk elevation was particularly greater when the first malignancy was lung, bladder, ovarian or blood malignancy (HRs 1.16–1.85), or when the first malignancy was treated with chemotherapy and radiotherapy (HR 1.44, 95% CI 1.28–1.63).
Conclusions
Overall, patients with BCa-2 have worse breast cancer-specific survival, compared with their BCa-1 counterparts, although the risk elevation is mild. High-risk subgroups based on first malignancy’s characteristics may be considered for active clinical management.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08132-9.
Keywords: Second primary breast cancer, Breast cancer-specific mortality, Prognosis, Survival
Background
More people are surviving cancer and with the increased survival comes opportunities for second primaries [1]. Second primary cancer has significantly increased over recent decades, accounting for 8–10% of newly diagnosed cancers in the U.S. and Australia [2, 3]. Breast cancer is the most common type, accounting for almost half of second cancer developed among female cancer survivors in the U.S. [2, 4]. It was recently reported that the incidence of second primary breast cancer has been increased by 600% from 1994 to 2015 in the U.S. [5], although the clinical course has not been studied yet. The vast majority of research attention was devoted to contralateral breast cancer (CBC) [6], however, it only accounts for approximately 3% of primary breast cancers as a second malignancy [3]. It is well-recognized that the prognosis of CBC is inferior to first primary breast cancer [7], whereas little is known about primary breast cancer developed among survivors of non-mammary malignancy (referred to as BCa-2 below).
Emerging evidence suggests that BCa-2 may have different pathogenesis or biological behaviors compared with BCa-1. This is partly attributable to intensive cancer treatment for the first malignancy (e.g., radiotherapy [8] and chemotherapy [1]), as well as certain lifestyle (e.g. smoking [9, 10]) and genetic factors [11, 12] that predisposed the individual to both first and second malignancies. Compared with the general population, high-dose chest radiation or exposure to alkylator or anthracycline was associated with drastically increased risk of subsequent breast cancer among childhood cancer survivors [13–16]. Moreover, the tumor characteristics of BCa-2 appear to differ. Compared with BCa-1, BCa-2 after Hodgkin’s lymphoma (HL) were characterized by early stage, hormone receptor negative status, and more likely to be located in external quadrant of breast [15, 17].
To aid the clinical management of an increasing number of BCa-2, it is important to understand whether the prognosis of BCa-2 is different from BCa-1. Milano et al. [18] reported worse breast cancer-specific survival among HL survivors with localized, but not regional or distant, BCa-2. It is however unclear whether BCa-2 as a whole have a worse disease course than BCa-1. Leveraging the population-based cancer cohort from the Surveillance, Epidemiology, and End Results (SEER) database, we aimed to assess the risk of breast cancer-specific mortality among cancer survivors of non-mammary malignancy who developed BCa-2 as a second malignancy, and identify potential high-risk subgroups due to the associations of first malignancy.
Methods
Study population
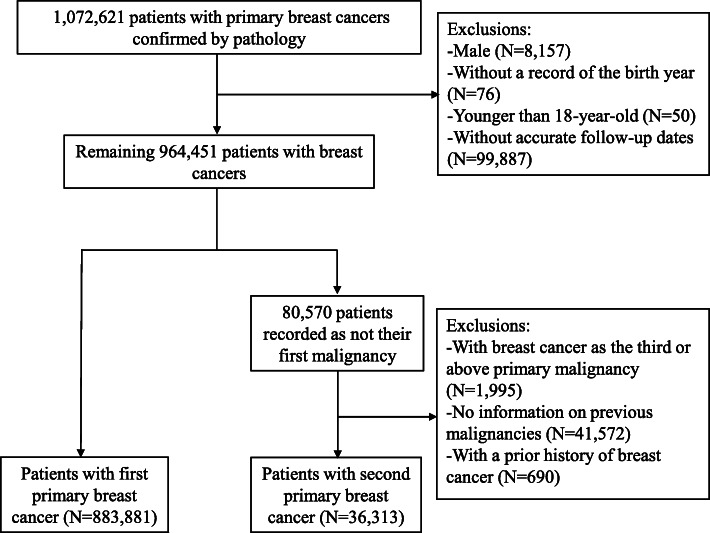
The SEER database contains information on demographic, tumor and clinical characteristics, and follow-up from nine registries (SEER9), in 1973, and expanded to 13 registries (SEER 13), in 1992 and 18 (SEER 18), in 2000 (covering about 28% of the US population) [19]. Considering the data for both the status of estrogen receptor and progesterone receptor was collected from 1990, we conducted a population-based cohort study of patients with primary breast cancer diagnosed between January 1, 1990 and December 31, 2015 in the United States. Similar to our previous study [20], we first identified 1,072,621 patients with pathologically confirmed, primary invasive breast cancer; and then excluded patients who were male (N = 8157), without a record of birth year (N = 76), or younger than 18 years at diagnosis (N = 50). All patients were followed from breast cancer diagnosis until death, occurrence of a subsequent malignancy, or December 31, 2015, whichever occurred first, whereas patients without accurate follow-up (including incomplete dates available, complete dates available but 0 days of survival, or unknown follow-up dates) were excluded (N = 99,887). The exclusions were illustrated in Fig. 1.
Fig. 1.
The flowchart
Ascertainment of BCa-2
In these breast cancer cases (N = 964,451), 80,570 were coded as non-first primary malignancy. Through linkage to the information on previous diagnoses in SEER, we excluded breast cancer cases diagnosed after two primary malignancies of which the clinical course may be very different from BCa-2 (N = 1995; and 41,572 cases without any identifiable diagnosis of the first malignancy (the baseline characteristics and mortality rates were summarized in Supporting Information Table S1 and were comparable to the patients with BCa-2 included in the final analysis). BCa-2 patients with a prior history of breast cancer were also excluded (N = 690) because of the difficulty to determine deaths from BCa-1 or BCa-2. In the present study, BCa-2 is therefore restricted to primary breast cancers subsequent to a non-mammary malignancy. The ten most common sites of first primary malignancies were colon and rectum, corpus and uterus, blood, skin, lung and bronchus, thyroid, ovary, urinary bladder, kidney and cervix uteri (Supporting Information Table S2). Finally, 36,313 BCa-2 and 883,881 BCa-1 were included for analysis.
Ascertainment of mortality
Breast cancer-specific and overall mortality were considered as the primary and secondary outcomes, respectively. Patients registered in the SEER program are followed periodically by linking registries through health care institutions and by directly contacting patients. To ensure the maximal follow-up of patients, personal contacts are also periodically implemented for those who are considered lost to follow-up [20, 21].
SEER uses algorithms to determine the single, disease-specific cause of death utilizing information on death certificates, tumor sequence, tumor site, and comorbidities [21, 22]. We identified deaths due to breast cancer using SEER Cause of Death Recode (code: 26000) [22, 23], of which the quality has been validated [24] in BCa-1.
Demographic, tumor and clinical characteristics
We extracted information on calendar year at diagnosis (1990–1993, 1994–1997, 1998–2001, 2002–2005, 2006–2009, or 2010–2015), age at diagnosis, race (White, Black, Asian, or other), and marital status (non-cohabitation, cohabitation, or unknown). Information on educational level and cost of living was obtained at the county level and classified into low, middle and high groups based on tertiles. We obtained information on tumor stage (localized, regional, distant, or unknown), tumor size (0–2 cm, 2–5 cm, or > 5 cm), histology (ductal, lobular, mixed, or other origins), tumor grade (well, moderately, or poorly differentiated, undifferentiated, or unknown), status of estrogen receptor (ER), progesterone receptor (PR), hormone receptor status (i.e., combining ER and PR status), and human epidermal growth factor receptor 2 (HER2, available from 2010 onward). Molecular subtype (available from 2010 onward) was classified as hormone receptor positive (HR+)/HER2-, HR+/HER2+, hormone receptor negative (HR-)/HER2+, triple negative, or unknown. We also extracted information on treatment, including surgery (mastectomy, lumpectomy, or no/unknown; available from 1998 onward), radiotherapy (yes or no/unknown), and chemotherapy (yes or no/unknown). To better reflect the treatment modes, we further classified treatment into lumpectomy only, mastectomy only, chemo−/radio-therapy, lumpectomy plus chemo−/radio-therapy, mastectomy plus chemo−/radio-therapy, and others.
Statistical analysis
First, we compared tumor and clinical characteristics between patients with BCa-2 and BCa-1 using logistic regression models with adjustment for demographic characteristics and tumor characteristics (only in the analysis of treatment modes).
Next, we calculated the mortality rates and hazard ratios (HRs) of breast cancer-specific and overall mortality among patients with BCa-2, as compared to patients with BCa-1, using Cox regression where we graphically assessed the assumption in a log-log plot. It is known that childhood cancer survivors are at higher risk of breast cancer due to the higher doses of anthracyclines [25]. To illustrate that the associations were not driven by childhood cancer survivors, an additional analysis was performed by excluding women who were aged ≤20 years at the diagnosis of first primary malignancy. To alleviate the concerns that some deaths due to first malignancy were misclassified as due to BCa-2, we performed a sensitivity analysis by restricting to BCa-2 diagnosed > 10 years after the first malignancy, when the first malignancy was presumably cured.
In these analyses, we adjusted for demographic characteristics as Model A, and additionally controlled for tumor characteristics (Model B) and treatment modes (Model C). Age at diagnosis was used as a continuous variable, while other factors were categorized as shown in Tables 1 and 2. In order to show associations independent of tumor characteristics and treatment modes, we only applied Model C in analyses below.
Table 1.
Baseline characteristics of women with primary breast cancer as the first (BCa-1) and second (BCa-2) malignancy: a SEER population-based study in US, 1990–2015
| BCa-1 | BCa-2 | |||
|---|---|---|---|---|
| N | % | N | % | |
| Total number | 883,881 | – | 36,313 | – |
| Year of diagnosis | ||||
| 1990–1993 | 47,727 | 5.4 | 1806 | 5.0 |
| 1994–1997 | 62,863 | 7.1 | 2380 | 6.6 |
| 1998–2001 | 117,368 | 13.3 | 3332 | 9.2 |
| 2002–2005 | 165,528 | 18.7 | 5250 | 14.5 |
| 2006–2009 | 185,587 | 21.0 | 7775 | 21.4 |
| 2010–2015 | 304,808 | 34.5 | 15,770 | 43.4 |
| Age at diagnosis (mean ± SD), years | 60.5 ± 14.0 | 68.9 ± 12.4 | ||
| Race | ||||
| White | 708,820 | 80.2 | 30,929 | 85.2 |
| Black | 97,049 | 11.0 | 3086 | 8.5 |
| Asian | 68,641 | 7.8 | 2127 | 5.9 |
| Other | 9371 | 1.1 | 171 | 0.5 |
| Cohabitation status | ||||
| Non-cohabitation | 359,775 | 40.7 | 17,217 | 47.4 |
| Cohabitation | 484,233 | 54.8 | 17,252 | 47.5 |
| Unknown | 39,873 | 4.5 | 1844 | 5.1 |
| % of High-school education in the county of residence | ||||
| Lowest tertile | 173,646 | 19.6 | 6202 | 17.1 |
| Middle tertile | 303,827 | 34.4 | 10,779 | 29.7 |
| Highest tertile | 406,408 | 46.0 | 19,332 | 53.2 |
| Cost of living adjusted median household income in the county of residence | ||||
| Lowest tertile | 54,584 | 6.2 | 1885 | 5.2 |
| Middle tertile | 116,136 | 13.1 | 4648 | 12.8 |
| Highest tertile | 713,161 | 80.7 | 29,780 | 82.0 |
Abbreviations: N Number, SD Standard deviation
Table 2.
Associations of tumor characteristics and treatment modes with primary breast cancer as the second malignancy (BCa-2): a SEER population-based study in US, 1990–2015
| BCa-1 | BCa-2 | ||
| N (%) | N (%) | OR (95% CI) a | |
| Histology | |||
| Ductal | 643,139 (72.8) | 25,487 (70.2) | 1.00 |
| Lobular | 73,112 (8.3) | 3456 (9.5) | 1.01 (0.97–1.05) |
| Mixed | 82,546 (9.3) | 3439 (9.5) | 1.02 (0.98–1.06) |
| Others | 85,084 (9.6) | 3931 (10.8) | 1.04 (1.00–1.08) |
| Tumor grade | |||
| Well differentiated | 165,869 (18.8) | 7817 (21.5) | 1.00 |
| Moderately differentiated | 337,563 (38.2) | 14,526 (40.0) | 0.97 (0.94–1.00) |
| Poorly differentiated | 277,729 (31.4) | 9435 (26.0) | 0.91 (0.88–0.94) |
| Undifferentiated | 9863 (1.1) | 266 (0.7) | 0.86 (0.76–0.98) |
| Tumor size | |||
| 0-2 cm | 506,784 (57.3) | 22,629 (62.3) | 1.00 |
| 2-5 cm | 260,786 (29.5) | 9608 (26.5) | 0.85 (0.83–0.87) |
| > 5 cm | 66,236 (7.5) | 1884 (5.2) | 0.67 (0.64–0.71) |
| Tumor stage | |||
| Local | 547,036 (61.9) | 24,624 (67.8) | 1.00 |
| Regional | 265,617 (30.1) | 8815 (24.3) | 0.86 (0.84–0.88) |
| Distant | 57,578 (6.5) | 2065 (5.7) | 0.79 (0.75–0.83) |
| Molecular subtypesc | |||
| HR+/HER2- | 205,424 (67.4) | 11,106 (70.4) | 1.00 |
| HR+/HER2+ | 30,893 (10.1) | 1283 (8.1) | 0.93 (0.88–0.99) |
| HR−/HER2+ | 13,507 (4.4) | 492 (3.1) | 0.82 (0.75–0.90) |
| Triple negative | 32,172 (10.6) | 1481 (9.4) | 1.00 (0.94–1.05) |
| N (%) | N (%) | OR (95% CI) b | |
| Surgery d | |||
| No | 60,648 (7.8) | 3712 (11.6) | 1.00 |
| Lumpectomy | 404,612 (52.3) | 17,372 (54.1) | 0.75 (0.72–0.79) |
| Mastectomy | 304,812 (39.4) | 10,900 (33.9) | 0.74 (0.71–0.78) |
| Chemotherapy | |||
| No/unknown | 536,470 (60.7) | 27,537 (75.8) | 1.00 |
| Yes | 347,411 (39.3) | 8776 (24.2) | 0.84 (0.81–0.86) |
| Radiotherapy | |||
| No/unknown | 462,215 (52.3) | 21,631 (59.6) | 1.00 |
| Yes | 421,666 (47.7) | 14,682 (40.4) | 0.83 (0.82–0.85) |
| Treatment modesd | |||
| Lumpectomy only | 79,295 (10.3) | 5149 (16.0) | 1.00 |
| Mastectomy only | 138,766 (17.9) | 6648 (20.7) | 0.88 (0.85–0.92) |
| Chemo−/radio-therapy | 27,038 (3.5) | 1038 (3.2) | 1.05 (0.97–1.13) |
| Lumpectomy plus chemo−/radio-therapy | 325,317 (42.1) | 12,223 (38.0) | 0.79 (0.76–0.82) |
| Mastectomy plus chemo−/radio-therapy | 166,046 (21.5) | 4252 (13.2) | 0.74 (0.71–0.78) |
| Otherse | 36,829 (4.8) | 2817 (8.8) | 1.16 (1.10–1.23) |
NOTE. Patients with missing information on tumor stage (N = 14,459, 1.57%), tumor grade (N = 97,126, 10.56%), tumor size (N = 52,267, 5.68%), molecular types (N = 24,220, 7.6%) or surgery (N = 3362, 0.4%) were not included in the corresponding analysis
Abbreviations: BCa-1 Breast cancer as the first malignancy, CI Confidence interval, HR Hormone-receptor, HER2 Human epidermal growth factor receptor 2, N Number; OR Odds ratio
a The models were adjusted for age (continuous) and calendar period at diagnosis, race, cohabitation status, percentile of cost of living and high-school education in county of residence
b The models were additionally adjusted for tumor stage, histology, tumor grade, ER status, PR status, and HER2 status
c Information on HER2 status was available from 2010 onward, and thus the analysis was restricted to patients diagnosed thereafter
d Information on surgery and therapy was available from 1998 onward, and thus the analysis was restricted to patients diagnosed thereafter
e Others included surgery (no/unknown), chemotherapy (no/unknown), and radiotherapy (no/unknown)
To explore the potential associations of first malignancy with BCa-2 patients, we calculated the HRs by characteristics of their first malignancies, including top ten common sites, tumor stage, and chemo−/radiotherapy for first malignancy. Because tumor characteristics and treatment modes of breast cancer might have strong impact on breast cancer specific mortality, we also performed stratified analyses and tested potential interactions between tumor characteristics, treatment modes and BCa-2 using Wald test.
All statistical analyses were conducted using STATA (version 14.1; Stata Corporation). P < 0.05 was considered as the statistical significance. This study was reviewed by the Biomedical Research Ethics Committee at West China Hospital, Sichuan University (reference number 2018–230).
Results
Demographic and clinical characteristics
Compared to patients with BCa-1, patients with BCa-2 were more likely to be diagnosed in recent years (2010–2015), older at diagnosis, and more likely to be white, not cohabitating, and residing in counties with a higher percentage of high-school education attainment (Table 1). Moreover, patients with BCa-2 had more favorable tumor characteristics (i.e., better differentiation, smaller tumor size, less advanced stage, and less likely HER2+), compared to patients with BCa-1 (Table 2). Patients with BCa-2 also received less intensive treatment, including both surgery and chemo−/radio-therapy, after controlling for differential tumor characteristics (Table 2).
Among BCa-2 patients, the most common sites of first malignancy were colon and rectum (17.8%), uterine corpus (16.7%), and blood (10.7%; Supporting Information Fig. S1). The median interval from the first malignancy to BCa-2 diagnosis was 4.75 years.
Mortality risk in BCa-2
The median (interquartile range) of follow-up was 5.58 (2.33, 10.42) and 3.58 (1.42, 7.17) years for BCa-1 and BCa-2, respectively; and 114,964 and 3829 breast cancer-specific deaths were identified. Compared with BCa-1, BCa-2 were not associated with an increased risk of breast cancer-specific mortality (HR 1.00, 95% CI 0.97–1.03), when only controlling for demographic characteristics (Table 3). However, when accounting for tumor characteristics, we found a higher risk of breast cancer-specific mortality among patients with BCa-2 (HR 1.11, 95% CI 1.08–1.15). Additional adjustment for treatment modes led to similar association (HR 1.11, 95% CI 1.08–1.15). Given the history of a prior malignancy, patients with BCa-2 were unsurprisingly at greater increased risk of overall mortality (HR 1.56, 95% CI 1.54–1.59). Reassuringly, by restricting to BCa-2 diagnosed > 10 years after the first malignancy (i.e., presumably cured from the first malignancy and less likely misclassified for cancer-specific deaths), largely comparable association with breast cancer-specific mortality was noted (HR 1.09, 95% CI 1.02–1.16; Supporting Information Table S3). The overall mortality risk was however smaller but remained elevated compared with BCa-1 (HR 1.25, 95% CI 1.20–1.30).
Table 3.
Hazard ratios (HRs) of cancer-specific and overall mortality among women with primary breast cancer as the second malignancy (BCa-2), compared to women with primary breast cancer as the first malignancy (BCa-1): a SEER population-based study in US, 1990–2015
| BCa-1 N (IR) |
BCa-2 N (IR) |
HR (95% CI)a | HR (95% CI)b | HR (95% CI)c | |
|---|---|---|---|---|---|
| All BCa-2 | |||||
| Breast cancer-specific mortality | 114,964 (1.9) | 3829 (2.1) | 1.00 (0.97–1.03) | 1.11 (1.08–1.15) | 1.11 (1.08–1.15) |
| Overall mortality | 227,860 (3.7) | 13,625 (7.6) | 1.46 (1.44–1.49) | 1.57 (1.54–1.59) | 1.56 (1.54–1.59) |
Abbreviations: CI Confidence interval, HR Hazards ratio, IR Mortality rate per 100 person-years, N Number of deaths
a HR was adjusted for age (continuous) and calendar period at diagnosis, race, cohabitation status, percentile of cost of living and high-school education in county of residence
b HR was additionally adjusted for tumor stage, histology, grade, estrogen receptor status, progesterone receptor status, and human epidermal growth factor receptor 2 status
c HR was additionally adjusted for surgery, radiotherapy, and chemotherapy
In a sensitivity analysis, we observed robust results after excluding childhood cancer survivors who were aged ≤20 years at the first malignancy (Supporting Information Table S4).
Mortality risk by characteristics of the first malignancy
BCa-2 patients with a history of lung, urinary bladder, ovarian, or blood cancer were at a higher risk of breast cancer-specific mortality, whereas no risk increase was noted for patients with a previous thyroid, colorectal, skin, uterine corpus, kidney, or cervical cancer (Table 4). Moreover, stronger associations were found among BCa-2 patients whose first malignancy was at more advanced stage or treated with both chemotherapy and radiotherapy.
Table 4.
Hazard ratios (HRs) of breast cancer-specific mortality among women with primary breast cancer as the second malignancy (BCa-2), by characteristics of the first malignancy, compared to women with primary breast cancer as the first malignancy (BCa-1): a SEER population-based study in US, 1990–2015
| N (%) of patients | N (IR) of deaths | HR (95% CI)a | |
|---|---|---|---|
| By sites of first malignancy | |||
| Colon and Rectum | 6463 (17.8) | 681 (2.1) | 0.95 (0.88–1.03) |
| Uterine corpus | 6057 (16.7) | 610 (1.8) | 0.98 (0.90–1.06) |
| Blood | 3887 (10.7) | 407 (2.3) | 1.16 (1.05–1.28) |
| Skin | 3489 (9.6) | 309 (1.6) | 0.96 (0.86–1.07) |
| Lung and Bronchus | 3092 (8.5) | 418 (4.1) | 1.85 (1.68–2.03) |
| Thyroid | 2722 (7.5) | 207 (1.4) | 0.93 (0.81–1.07) |
| Ovary | 1612 (4.4) | 181 (2.3) | 1.19 (1.02–1.37) |
| Urinary Bladder | 1547 (4.3) | 177 (2.2) | 1.22 (1.05–1.42) |
| Kidney | 1382 (3.8) | 116 (1.9) | 1.01 (0.84–1.21) |
| Uterine cervix | 1123 (3.1) | 132 (2.0) | 1.01 (0.85–1.20) |
| Others | 4939 (13.6) | 591 (2.9) | 1.35 (1.25–1.47) |
| By tumor stage of first malignancy b | |||
| Localized | 20,100 (62.3) | 1906 (1.8) | 0.98 (0.94–1.03) |
| Regional | 7088 (22.0) | 802 (2.4) | 1.20 (1.12–1.29) |
| Distant | 2661 (8.3) | 379 (4.7) | 1.63 (1.47–1.80) |
| Unstaged | 2394 (7.4) | 335 (3.3) | 1.36 (1.22–1.51) |
| By chemotherapy and radiotherapy for first malignancy | |||
| No/unknown | 24,129 (66.4) | 2580 (2.1) | 1.09 (1.05–1.13) |
| Radiotherapy only | 4816 (13.3) | 470 (2.3) | 1.12 (1.03–1.23) |
| Chemotherapy only | 4796 (13.2) | 505 (2.0) | 1.12 (1.02–1.22) |
| Chemotherapy plus radiotherapy | 2572 (7.1) | 274 (2.9) | 1.44 (1.28–1.63) |
Abbreviations: CI Confidence interval, HR Hazards ratio, IR Mortality rate per 100 person-years, N Number of deaths
a HR was adjusted for age (continuous) and calendar period at diagnosis, race, cohabitation status, percentile of cost of living and high-school education in county of residence, tumor stage, histology, tumor grade, estrogen receptor status, progesterone receptor status, and human epidermal growth factor receptor 2 status, surgery, radiotherapy, and chemotherapy
b BCa-2 patients with blood malignancy as first malignancy were removed from this analysis
Mortality risk by characteristics of breast cancer
When comparing BCa-2 to BCa-1 by the clinical characteristics of breast cancer, a stronger association with breast cancer-specific mortality was noted for patients with well-differentiated tumor or local stage, or in those only underwent lumpectomy (P for interaction< 0.05; Table 5). Similar associations were found across age or calendar year groups.
Table 5.
Hazard ratios (HRs) of breast cancer-specific mortality among women with primary breast cancer as the second malignancy (BCa-2), stratified clinical characteristics, compared to women with primary breast cancer as the first malignancy (BCa-1): a SEER population-based study in US, 1990–2015
| BCa-1 N (IR) |
BCa-2 N (IR) |
HR (95% CI)a | |
|---|---|---|---|
| Age at diagnosis, years | |||
| 18–44 | 19,010 (2.0) | 164 (2.0) | 1.05 (0.90–1.22) |
| 45–54 | 25,197 (1.6) | 385 (1.7) | 1.05 (0.95–1.16) |
| 55–64 | 25,246 (1.6) | 720 (1.7) | 1.19 (1.11–1.28) |
| 65–74 | 20,535 (1.7) | 978 (1.8) | 1.15 (1.08–1.23) |
| 75–84 | 16,797 (2.5) | 1060 (2.5) | 1.10 (1.04–1.17) |
| ≥ 85 | 8179 (6.0) | 522 (5.0) | 1.01 (0.93–1.11) |
| P for interaction | 0.056 | ||
| Year of diagnosis | |||
| 1990–1993 | 11,532 (1.8) | 344 (2.2) | 1.07 (0.96–1.19) |
| 1994–1997 | 13,760 (1.8) | 393 (1.9) | 0.98 (0.89–1.09) |
| 1998–2001 | 21,259 (1.7) | 516 (1.9) | 1.15 (1.05–1.25) |
| 2002–2005 | 26,642 (1.8) | 740 (2.0) | 1.15 (1.06–1.23) |
| 2006–2009 | 23,333 (2.0) | 890 (2.1) | 1.13 (1.06–1.21) |
| 2010–2015 | 18,438 (2.4) | 946 (2.6) | 1.14 (1.07–1.22) |
| P for interaction | 0.136 | ||
| Histology | |||
| Ductal | 79,321 (1.8) | 2521 (2.0) | 1.13 (1.09–1.18) |
| Lobular | 9222 (1.9) | 393 (2.4) | 1.06 (0.95–1.17) |
| Mixed | 8133 (1.4) | 314 (1.8) | 1.20 (1.08–1.35) |
| Others | 18,288 (3.2) | 601 (3.5) | 1.03 (0.95–1.12) |
| P for interaction | 0.067 | ||
| Tumor grade | |||
| Well differentiated | 5445 (0.5) | 294 (0.7) | 1.43 (1.27–1.61) |
| Moderately differentiated | 31,639 (1.4) | 1149 (1.6) | 1.15 (1.09–1.22) |
| Poorly differentiated | 53,944 (3.0) | 1593 (3.6) | 1.18 (1.12–1.24) |
| Undifferentiated | 2400 (2.9) | 52 (3.1) | 1.07 (0.82–1.41) |
| P for interaction | 0.011 | ||
| Tumor size | |||
| 0–2 cm | 28,594 (0.7) | 1264 (1.0) | 1.29 (1.22–1.36) |
| 2–5 cm | 45,123 (2.7) | 1434 (3.5) | 1.15 (1.09–1.21) |
| > 5 cm | 23,418 (7.4) | 563 (8.6) | 1.25 (1.15–1.36) |
| P for interaction | 0.011 | ||
| Tumor stage | |||
| Local | 27,833 (0.7) | 1293 (1.0) | 1.31 (1.24–1.39) |
| Regional | 48,765 (2.7) | 1398 (3.3) | 1.07 (1.01–1.13) |
| Distant | 33,806 (21.2) | 981 (26.1) | 1.03 (0.97–1.10) |
| P for interaction | < 0.001 | ||
| Molecular subtypesb | |||
| HR+/HER2- | 8076 (1.5) | 447 (1.7) | 1.09 (0.99–1.20) |
| HR+/HER2+ | 1743 (2.3) | 75 (2.6) | 1.38 (1.09–1.74) |
| HR−/HER2+ | 1281 (3.9) | 62 (5.5) | 1.61 (1.25–2.08) |
| Triple negative | 4322 (5.5) | 213 (6.7) | 1.18 (1.03–1.36) |
| P for interaction | 0.020 | ||
| Treatment modesc | |||
| Lumpectomy only | 5816 (1.2) | 349 (1.7) | 1.32 (1.19–1.47) |
| Mastectomy only | 11,190 (1.3) | 510 (1.6) | 1.13 (1.03–1.23) |
| Chemo−/radio-therapy | 12,602 (16.5) | 365 (17.9) | 1.14 (1.03–1.27) |
| Lumpectomy plus chemo/radio-therapy | 17,627 (0.8) | 555 (0.9) | 1.13 (1.03–1.23) |
| Mastectomy plus chemo/radio-therapy | 28,121 (2.8) | 649 (3.3) | 1.13 (1.04–1.22) |
| Othersd | 14,316 (13.9) | 664 (14.1) | 0.99 (0.92–1.07) |
| P for interaction | 0.002 | ||
NOTE. Patients with missing information on tumor stage (N = 14,459, 1.57%), tumor grade (N = 97,126, 10.56%) or size (N = 52,267, 5.68%) or molecular types (N = 24,220, 7.6%) were not included for the corresponding analysis. We added an interaction term between BCa-2 and the risk modifier and reported the statistical significance of the term as P for interaction
Abbreviations: CI Confidence interval, HR+ Hormone-receptor positive, HR- Hormone-receptor negative, HER2 Human epidermal growth factor receptor 2, HR Hazard ratio, IR Mortality rate per 100 person-years, N Number of deaths
a HR was adjusted for age (continuous) and calendar period at diagnosis, race, cohabitation status, percentile of cost of living and high-school education in county of residence, tumor stage, histology, tumor grade, estrogen receptor status, progesterone receptor status, and human epidermal growth factor receptor 2 status, surgery, radio-therapy, and chemo-therapy
b Information on HER2 status was available from 2010 onward, and thus the analysis was restricted to patients diagnosed thereafter
c Information on surgery was available from 1998 onward, and thus the analysis was restricted to patients diagnosed thereafter
d Others included surgery (no/unknown), chemotherapy (no/unknown), and radiotherapy (no/unknown)
Discussion
To the best of our knowledge, this is the first study to assess the mortality risk for BCa-2 as one entity among all cancer survivors. In this population-based cohort study, we found that, although with more favorable tumor characteristics and receiving less intensive treatment, BCa-2 were independently associated with an increased risk of breast cancer-specific mortality, compared with BCa-1. The risk increase was particularly greater when the first malignancy was lung, urinary bladder, ovarian, or blood malignancy, at more advanced stage, or treated with both chemotherapy and radiotherapy. Stronger associations were also found for BCa-2 with favorable characteristics or only treated by lumpectomy.
Interestingly, the breast-cancer specific mortality was comparable among patients with BCa-2 and BCa-1 when only accounting for demographic characteristics, likely explained by a mixed effect of poorer prognosis but more favorable tumor features (e.g., less advanced stage) in BCa-2. However, the inferior prognosis of BCa-2 is evident when controlling for tumor characteristics and treatment modes. Indeed, we lacked detailed information on regimens of chemo−/radio-therapy, and therefore were not able to eliminate the residual effect, if any, of differential regimens on BCa-2 prognosis. However, BCa-2 patients received less intensive treatment than their BCa-1 counterparts given the same demographic (e.g., age at diagnosis) and tumor characteristics (e.g., tumor stage and molecular subtype).
Previous studies suggested that BCa-2 subsequent to HL were associated with a higher risk of breast cancer-specific mortality, compared with BCa-1 [15, 18]. In the present study, we also observed worse prognosis in BCa-2 among patients with hematopoietic and lymphoid malignancies, which accounted for 10% of all BCa-2 cases. Importantly, we extend the current knowledge to BCa-2 among other cancer survivors, especially in survivors of lung, ovarian, or bladder cancers. It is conceivable that the worsened mortality among these patients may stem from mutations shared between BCa-2 and other malignancies or undertreatment, if any, of BCa-2 in the face of a competing, presumably the more life-threatening first malignancy. Indeed, BCa-2 with a history of many less fatal cancer types are not at increased risk of breast cancer-specific mortality. Prior cancer is a common exclusion criterion in clinical trials due to concerns that prior cancer may affect trial conduct or outcomes [26]. Our findings therefore may add to the ongoing discussion in support of the broader inclusion of BCa-2 subgroups that were not of poorer prognosis in clinical trials. Moreover, a recent report suggested childhood cancer survivors (age at first primary malignancy ≤20 years) with BCa-2 tended to have an modestly increased, although not statistically significant, risk of breast cancer-specific mortality than individuals with BCa-1 (HR 1.3, 95% CI 0.9–2.0) [27]. In our sensitivity analysis, the increased risk remained the same after excluding childhood cancer survivors. We, therefore, add to the knowledge by revealing the worse prognosis of BCa-2 developed in adulthood cancer survivors.
The major strength of our study is the large-scale population-based prospective cohort of patients with primary breast cancer, which assures minimal biases including selection and surveillance biases. One of the major concerns is that some deaths due to the first malignancy were misclassified as deaths due to breast cancer, which may lead to overestimated associations. However, we performed a sensitivity analysis by restricting to BCa-2 diagnosed > 10 years, i.e., presumably cured, from the first malignancy which yielded similar association with breast cancer-specific mortality. Our findings are therefore unlikely explained by the misclassification (if any) of deaths due to first malignancy. Second, we lacked information on a few factors that may be associated with survival, e.g., performance status [28], body mass index [29] and comorbidities [30], which may differ between individuals with multiple cancers and patients with BCa-1. However, these factors are likely influential through treatment modes which have been carefully addressed in our analyses. Third, the ascertainment of some BCa-2 may be challenged if the first malignancy was metastatic. However, as pathological diagnosis is required for inclusion, it is unlikely that the identified BCa-2 are in fact metastasis from the first primary malignancy. Also, breast metastasis from non-mammary malignancies is rare, accounting for approximately 1.8% of all breast malignancies [31]. In addition, we have shown the increased breast cancer-specific mortality for BCa-2 patients with regional stage of first malignancy. Lastly, the follow-up in our study is relatively short. Future studies with longer follow-up are needed.
Several potential mechanisms may help explain the unfavorable prognosis of BCa-2. First, it is possible that BCa-2 are different from BCa-1 in tumor biology. Similar to a previous study on BCa-2 after HL [15], our data indicate that BCa-2, in general, are characterized by less aggressive tumor at diagnosis, including early stage and smaller size, potentially due to the early detection during the clinical follow-up for the first malignancy. However, as our data suggest that the non-aggressive clinical features did not translate to a better prognosis of BCa-2. Behrens et al. reported [32] the overall frequency of microsatellite alterations in BCa-2 after HL was substantially higher than that in BCa-1, potentially influenced by immunosuppression and radiation exposure due to HL. A line of research also suggests that ionizing radiation may lead to breast carcinogenesis via radiation-induced amplification of proto-oncogene c-MYC [33]. Synergistic effect on a second malignancy has been postulated when the first malignancy was treated with both radiation and chemotherapy [34]. Interestingly, we found greater risk increase of breast cancer-specific mortality in BCa-2 patients who underwent chemotherapy and radiotherapy for the first malignancy. Moreover, genetic susceptibility may contribute to the multiple malignancies in an individual. For example, genetic variants in BRCA locus are associated with breast and ovarian cancers [11]. It is plausible that BCa-2 developed in survivors of ovarian cancer are driven by shared genetic variants and, thus, different from sporadic cases. This may partly explain our findings on the particularly worse prognosis of BCa-2 after ovarian cancer. A recent study also showed that breast cancer developed between two screenings, a known type of worse prognosis, is more likely to have a non-breast malignancy before and after breast cancer diagnosis, potentially through rare deleterious mutations in cancer genes [12]. Further research is warranted to understand mutations leading to BCa-2 after other malignancies.
Second, the clinical management for BCa-2 may be less intensive than BCa-1. This has been acknowledged in previous studies, suggesting that BCa-2 in HL survivors were less treated than BCa-1 with similar tumor characteristics [15, 17]. The present study further confirmed that BCa-2 in cancer survivors were in general less treated, particularly when the first malignancy is considered of poor prognosis. For instance, we found greater risk increase of breast cancer-specific mortality in BCa-2 when the first malignancy was diagnosed at a more advanced stage, even though we exhaustively controlled for treatment modes.
Conclusions
Our findings suggest that, overall, patients with BCa-2 have worse breast cancer-specific survival, compared with their BCa-1 counterparts, although the risk elevation is mild. Active clinical management may be considered for high-risk groups, for example, patients with a prior lung, bladder, ovarian, or blood malignancy. On the other hand, our findings may add to the current discussion in support of broader inclusion of the other BCa-2 subgroups, who have a comparable prognosis with BCa-1, in clinical trials. Future research is also required to understand the potential difference between BCa-2 and BCa-1 regarding tumor biology.
Supplementary Information
Additional file 1: Table S1. Baseline characteristics and mortality of women indicated as BCa-2 but no information on the first malignancy in the SEER. Table S2. Site recode definitions of first primary malignancies among patients with second primary breast cancer. Table S3. Hazard ratios (HRs) of breast cancer-specific and overall mortality among women with primary breast cancer as the second malignancy (BCa-2) diagnosed > 10 years after first malignancy, compared to women with primary breast cancer as the first malignancy (BCa-1): a SEER population-based study in US, 1990–2015. Table S4. Hazard ratios (HRs) of breast cancer-specific and overall mortality among adulthood cancer survivors with primary breast cancer as the second malignancy (BCa-2), compared to women with primary breast cancer as the first malignancy (BCa-1): a SEER population-based study in US, 1990–2015. Childhood cancer survivors who were < 20 years old at first primary malignancy (n = 192) were excluded in this analysis. Fig. S1. Ten most common sites of first malignancy among women with primary breast cancer as the second malignancy: a SEER population-based study in US, 1990–2015.
Acknowledgements
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-based database
This work was presented as a poster in the ESMO Breast Cancer Congress on 2-4
May 2019 in Berlin, Germany.
Abbreviations
- BCa-2
Cancer survivors who developed breast cancer as a second malignancy
- BCa-1
Patients with breast cancer as the first malignancy
- HR
Hazard ratio
- CBC
Contralateral breast cancer
- HL
Hodgkin’s lymphoma
- SEER
Surveillance, Epidemiology, and End Results
- ER
Status of estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor 2
- HR+
Hormone receptor positive
- HR
Hormone receptor negative
Authors’ contributions
All authors have read and approved the manuscript. CW and DL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CW, LD, and DL. Acquisition, analysis, or interpretation of data: CW and DL. Drafting of the manuscript: CW, KH and DL. Critical revision of the manuscript for important intellectual content: CW, LD, WH, FF, RT and DL. Statistical analysis: CW and DL. Obtained funding: CW and DL. Administrative, technical, or material support: DL. Study supervision: DL.
Funding
This study was funded by the National Natural Science Foundation of China (grant number: 81872307; to Dr. Lu) and Swedish Research Council (grant number: 2018–00648; to Dr. Lu) and Full-time Postdoc Research and Development Foundation of West China Hospital (grant number: 2019HXBH098; to Dr. Wang). The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Open Access funding provided by Karolinska Institute.
Availability of data and materials
The data is publicly available from the SEER Program (https://seer.cancer.gov/).
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Biomedical Research Ethics Committee at West China Hospital, Sichuan University (reference number 2018–230) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO open. 2017;2(2):e000172. doi: 10.1136/esmoopen-2017-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donin N, Filson C, Drakaki A, Tan HJ, Castillo A, Kwan L, Litwin M, Chamie K. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122(19):3075–3086. doi: 10.1002/cncr.30164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Y, Otahal P, Wills KE, Neil AL, Venn AJ. Temporal trends in the risk of second primary cancers among survivors of adult-onset cancers, 1980 through 2013: an Australian population-based study. Cancer. 2018;124(8):1808–1818. doi: 10.1002/cncr.31247. [DOI] [PubMed] [Google Scholar]
- 4.Chao C, Bhatia S, Xu L, Cannavale KL, Wong FL, Huang PS, Cooper R, Armenian SH. Incidence, risk factors, and mortality associated with second malignant neoplasms among survivors of adolescent and young adult Cancer. JAMA Netw Open. 2019;2(6):e195536. doi: 10.1001/jamanetworkopen.2019.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y, Huang Z, Liao Q, Yu X, Jiang H, He Y, Yao S, Nie S, Liu L. Risk of second primary breast cancer among cancer survivors: implications for prevention and screening practice. PLoS One. 2020;15(6):e0232800. doi: 10.1371/journal.pone.0232800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narod SA. Bilateral breast cancers. Nat Rev Clin Oncol. 2014;11(3):157–166. doi: 10.1038/nrclinonc.2014.3. [DOI] [PubMed] [Google Scholar]
- 7.Vichapat V, Garmo H, Holmberg L, Fentiman IS, Tutt A, Gillett C, Luchtenborg M. Prognosis of metachronous contralateral breast cancer: importance of stage, age and interval time between the two diagnoses. Breast Cancer Res Treat. 2011;130(2):609–618. doi: 10.1007/s10549-011-1618-8. [DOI] [PubMed] [Google Scholar]
- 8.Dracham CB, Shankar A, Madan R. Radiation induced secondary malignancies: a review article. Radiat Oncol J. 2018;36(2):85–94. doi: 10.3857/roj.2018.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabuchi T, Ito Y, Ioka A, Nakayama T, Miyashiro I, Tsukuma H. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: a retrospective cohort study. Ann Oncol. 2013;24(10):2699–2704. doi: 10.1093/annonc/mdt279. [DOI] [PubMed] [Google Scholar]
- 10.Shiels MS, Gibson T, Sampson J, Albanes D, Andreotti G, Beane Freeman L, Berrington de Gonzalez A, Caporaso N, Curtis RE, Elena J, et al. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. J Clin Oncol. 2014;32(35):3989–3995. doi: 10.1200/JCO.2014.56.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehringer G, Kraft P, Pharoah PD, Eeles RA, Chatterjee N, Schumacher FR, Schildkraut JM, Lindstrom S, Brennan P, Bickeboller H, et al. Cross-Cancer genome-wide analysis of lung, ovary, breast, prostate, and colorectal Cancer reveals novel pleiotropic associations. Cancer Res. 2016;76(17):5103–5114. doi: 10.1158/0008-5472.CAN-15-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassmann F, He W, Eriksson M, Gabrielson M, Hall P, Czene K. Interval breast cancer is associated with other types of tumors. Nat Commun. 2019;10(1):4648. doi: 10.1038/s41467-019-12652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson TO. Moskowitz CS, Chou JF, Bradbury AR, Neglia JP, Dang CT, Onel K, Novetsky Friedman D, Bhatia S, Strong LC, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: a report from the childhood cancer survivor study. J Clin Oncol. 2016;34(9):910–918. doi: 10.1200/JCO.2015.62.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia S, Robison L, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, Meadows A. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334(12):745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 15.Veit-Rubin N, Rapiti E, Usel M, Benhamou S, Vinh-Hung V, Vlastos G, Bouchardy C. Risk, characteristics, and prognosis of breast cancer after Hodgkin's lymphoma. Oncologist. 2012;17(6):783–791. doi: 10.1634/theoncologist.2011-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Novetsky Friedman D, Mubdi NZ, Leisenring WM, Stovall M, Hammond S, Smith SA, Henderson TO, Boice JD, Hudson MM, Diller LR, Bhatia S, Kenney LB, Neglia JP, Begg CB, Robison LL, Oeffinger KC. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32(21):2217–2223. doi: 10.1200/JCO.2013.54.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkin EB, Klem ML, Gonzales AM, Ishill NM, Hodgson D, Ng AK, Marks LB, Weidhaas J, Freedman GM, Miller RC, Constine LS, Myrehaug S, Yahalom J. Characteristics and outcomes of breast cancer in women with and without a history of radiation for Hodgkin's lymphoma: a multi-institutional, matched cohort study. J Clin Oncol. 2011;29(18):2466–2473. doi: 10.1200/JCO.2010.32.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milano MT, Li H, Gail MH, Constine LS, Travis LB. Long-term survival among patients with Hodgkin's lymphoma who developed breast cancer: a population-based study. J Clin Oncol. 2010;28(34):5088–5096. doi: 10.1200/JCO.2010.29.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng L, Harethardottir H, Song H, Xiao Z, Jiang C, Wang Q, Valdimarsdottir U, Cheng H, Loo BW, Lu D. Mortality of lung cancer as a second primary malignancy: a population-based cohort study. Cancer Med. 2019;8(6):3269–3277. doi: 10.1002/cam4.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng YJ, Nie XY, Ji CC, Lin XX, Liu LJ, Chen XM, et al. Long-Term Cardiovascular Risk After Radiotherapy in Women With Breast Cancer. J Am Heart Assoc. 2017;6(5):e005633. 10.1161/JAHA.117.005633. [DOI] [PMC free article] [PubMed]
- 22.Dias A, Claudino W, Sinha R, Perez CA, Jain D. Human epidermal growth factor antagonists and cardiotoxicity-a short review of the problem and preventative measures. Crit Rev Oncol Hematol. 2016;104:42–51. doi: 10.1016/j.critrevonc.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Wittstein IS. The sympathetic nervous system in the pathogenesis of Takotsubo syndrome. Heart Fail Clin. 2016;12(4):485–498. doi: 10.1016/j.hfc.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Hu CY, Xing Y, Cormier JN, Chang GJ. Assessing the utility of cancer-registry-processed cause of death in calculating cancer-specific survival. Cancer. 2013;119(10):1900–1907. doi: 10.1002/cncr.27968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrhardt MJ, Howell CR, Hale K, Baassiri MJ, Rodriguez C, Wilson CL, Joshi SS, Lemond TC, Shope S, Howell RM, et al. Subsequent breast Cancer in female childhood Cancer survivors in the St Jude lifetime cohort study (SJLIFE) J Clin Oncol. 2019;37(19):1647–1656. doi: 10.1200/JCO.18.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy CC, Gerber DE, Pruitt SL. Prevalence of prior Cancer among persons newly diagnosed with Cancer: an initial report from the surveillance, epidemiology, and end results program. JAMA Oncol. 2018;4(6):832–836. doi: 10.1001/jamaoncol.2017.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moskowitz CS, Chou JF, Neglia JP, Howell RM, Lisa Diller AP, Leisenring WM, et al. Mortality following breast cancer in survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2019.36(15):suppl.10511. 10.1200/JCO.2018.36.15_suppl.10511.
- 28.West HJ, Jin JO. JAMA Oncology Patient Page. Performance Status in Patients With Cancer. JAMA Oncol. 2015;1(7):998. doi: 10.1001/jamaoncol.2015.3113. [DOI] [PubMed] [Google Scholar]
- 29.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan TH, Gomez SL, Cheng I, Shariff-Marco S, Caan BJ, et al. Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California breast Cancer survivorship consortium (CBCSC) Cancer Epidemiol Biomark Prev. 2015;24(2):361–368. doi: 10.1158/1055-9965.EPI-14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surov A, Fiedler E, Holzhausen HJ, Ruschke K, Schmoll HJ, Spielmann RP. Metastases to the breast from non-mammary malignancies: primary tumors, prevalence, clinical signs, and radiological features. Acad Radiol. 2011;18(5):565–574. doi: 10.1016/j.acra.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Behrens C, Travis L, Wistuba I, Davis S, Maitra A, Clarke E, Lynch C, Glimelius B, Wiklund T, Tarone R, et al. Molecular changes in second primary lung and breast cancers after therapy for Hodgkin’s disease. Cancer Epidemiol Biomark Prev. 2000;9(10):1027–1035. [PubMed] [Google Scholar]
- 33.Wade MA, Sunter NJ, Fordham SE, Long A, Masic D, Russell LJ, Harrison CJ, Rand V, Elstob C, Bown N, Rowe D, Lowe C, Cuthbert G, Bennett S, Crosier S, Bacon CM, Onel K, Scott K, Scott D, Travis LB, May FEB, Allan JM. C-MYC is a radiosensitive locus in human breast cells. Oncogene. 2015;34(38):4985–4994. doi: 10.1038/onc.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan HG, Malmgren JA, Atwood MK. Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990-2005. BMC Cancer. 2011;11(1):260. doi: 10.1186/1471-2407-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline characteristics and mortality of women indicated as BCa-2 but no information on the first malignancy in the SEER. Table S2. Site recode definitions of first primary malignancies among patients with second primary breast cancer. Table S3. Hazard ratios (HRs) of breast cancer-specific and overall mortality among women with primary breast cancer as the second malignancy (BCa-2) diagnosed > 10 years after first malignancy, compared to women with primary breast cancer as the first malignancy (BCa-1): a SEER population-based study in US, 1990–2015. Table S4. Hazard ratios (HRs) of breast cancer-specific and overall mortality among adulthood cancer survivors with primary breast cancer as the second malignancy (BCa-2), compared to women with primary breast cancer as the first malignancy (BCa-1): a SEER population-based study in US, 1990–2015. Childhood cancer survivors who were < 20 years old at first primary malignancy (n = 192) were excluded in this analysis. Fig. S1. Ten most common sites of first malignancy among women with primary breast cancer as the second malignancy: a SEER population-based study in US, 1990–2015.
Data Availability Statement
The data is publicly available from the SEER Program (https://seer.cancer.gov/).