Abstract
Background
Studies suggest Porphyromonas gingivalis (Pg) increased the incidence of oral squamous cell carcinoma (OSCC). However, fimA genotypes distribution of Pg, the origination of Pg in tissue, and its prognostic value are inconclusive. We aimed to investigate the frequency of fimA genotypes in OSCC patients, study the association between Pg and OSCC, and explore the prognostic value of Pg.
Methods
The abundance of Pg in saliva from the OSCC group and the OSCC-free group was analysed by qPCR. The presence of Pg was explored in OSCC tissue and para-cancerous tissue by in situ hybridization. The frequency of fimA genotypes in saliva and OSCC tissue was determined by PCR, then PCR products were sequenced and compared. Clinical data were extracted, and patients followed up for a median period of 23 months. Clinicopathological variables were compared with the abundance of Pg using Pearson Chi-square test or Fisher’s exact test. The disease-free survival (DFS) rate was calculated by Kaplan–Meier method with log-rank tests.
Results
Comparing the OSCC-free group, 95 patients with OSCC showed a high abundance of Pg in saliva (P = 0.033), and OSCC tissue showed strong in situ expression of Pg compared with paired normal tissue. Patients with OSCC showed a dominant distribution of Pg with genotype I + Ib (21.1%), II (31.6%), and IV (21.1%). FimA genotypes detected in saliva were in accordance with those in OSCC tissue, there was, moreover, a significant similarity in amplified Pg fragments. Of the 94 responsive OSCC patients, the recurrence rate was 26.6% (25/94). Overabundance of Pg in saliva showed advanced pathologic staging (P = 0.008), longer disease-free time (P = 0.029) and lower recurrence rate (P = 0.033). The overabundance of Pg in saliva was associated with improved disease-free survival (P = 0.049).
Conclusions
This study indicated that Pg might involve in the pathogenesis of OSCC, Pg carrying fimA I, Ib, II, and IV were prevalent genotypes in patients with OSCC, the provenance of Pg in OSCC tissue might be from the salivary microbial reservoir, and the abundance of Pg in saliva might consider as a favorable potential prognostic indicator in OSCC.
Keywords: Porphyromonas gingivalis, FimA, Genotype, Oral squamous cell carcinoma, Fimbriae, Prognosis
Background
There were approximately 354,864 new cases of lip and oral cavity cancer and 177,384 related deaths worldwide in 2018 [1]. Oral squamous cell carcinoma (OSCC) is the most common malignant disease in the head and neck besides non-melanoma skin cancer. Traditionally, risk factors associated with OSCC included tobacco/alcohol consumption, betel quid chewing, virus infection, dietary factor, vitamin/mineral deficiencies, occupational exposures, and heritable conditions. However, accumulating epidemiologic, clinicopathologic, and molecular studies have proven that oral microbial species played an important role in the carcinogenesis of OSCC [2].
Porphyromonas gingivalis (Pg), an anaerobic Gram-negative bacterium, was associated with a high risk of OSCC in extensive studies [3–9]. However, the effect of Pg infection on the prognosis of OSCC is unclear. Recently, Pg infection in OSCC tissue showed a trend (HR 0.34; P = 0.055 in multivariate regression analyses) towards improved overall survival [10]. Given that salivary microbiota is stable and saliva is easy and non-invasive to collect, saliva is an acceptable biofluid for the evaluation of the oral microbiome [11, 12]. We hypothesized that the abundance of Pg in saliva was associated with a favorable prognosis in patients with OSCC.
Fimbriae are crucial to initial attachment, organization of biofilms, and adhesion that mediate invasion and colonization of cells, although Pg has an arsenal of virulence factors, such as gingipain, lipopolysaccharide, and capsule [13]. The major fimbriae fimA and the minor one mfa1 were two distinct fimbriae expressed in Pg. Six genotypes (types I-V, Ib) of fimA have been identified based on sequence variations. Previous studies have reported a high prevalence of fimA genotype II in periodontitis patients, and fimA genotype Ib, II and, IV are more aggressive [14–17]. However, no study was found evaluating the frequency of fimA genotypes in patients with OSCC.
In this study, we explored the association between Pg and OSCC. Meanwhile, we investigated the prevalence of fimA genotypes in patients with OSCC. To verify the origination of Pg in the OSCC tissue, we compared the frequency of fimA genotypes in saliva and those in OSCC tissues. Furthermore, we compared the amplified Pg fragments from OSCC tissue and those from saliva. The potential prognostic value of Pg in OSCC was also assessed.
Methods
Approval from the institutional review board was obtained at the Hospital of Stomatology Wuhan University before starting the study (2016–60). Informed consent was obtained from each patient.
The inclusion criterion was patients with primary OSCC managed by curative-intent, and a total number of 111 consecutive patients were included from October 2018 and April 2019. The exclusion criteria were patients: (1) received oral prophylaxis in the latest three months (one patient), (2) underwent radiotherapy and/or chemotherapy before surgery (three patients), (3) edentulous (two patients), (4) refused to receive surgery, (5) disagreed to participate in this study (four patients disagreed to the collection of saliva and six patients did not fast overnight before saliva sample collection).
A total number of 95 patients with OSCC (65 male and 30 female subjects, aged 21–82 years, mean age 55.8) treated in the hospital were included in this study. Pathological staging was stratified in accordance with the eighth edition of the American Joint Committee on Cancer [18]. Except for one patient with bone metastatic OSCC, all patients were M0 category. The control group comprised 39 OSCC-free subjects (21 males and 18 females, aged 33–76 years, mean age 52.6) diagnosed with salivary gland disease, lymphadenopathy, lymphoma, buccal or tongue chronic infection, epulis, ranula, lipoma, lymphoepithelial cyst, and sebaceous gland carcinoma.
Clinical records were retrieved. Assessed clinicopathological variables included age, gender, systematic disease, location of the tumor, size, pathological report, smoking, alcohol consumption, and treatment. Systemic disease included hypertension, diabetes, coronary artery disease, and chronic hepatitis B. Sixty-four patients underwent surgery, twenty-two patients underwent surgery + radiotherapy (IMRT: 76 Grays to 63 Grays) and eight patients underwent surgery + chemo-radiotherapy (Docetaxel, Cisplatin, 5-Fluorouracil). Pathological diagnosis was established by one pathologist and confirmed by another experienced pathologist from the department of pathological at the Hospital of Stomatology Wuhan University.
Patients were followed up from discharge by telephone or clinical assessment. Pathologic confirmation of recurrence was obtained in patients with clinical signs or symptoms. Disease-free survival (DFS) is defined as the time (in months) from the date of discharge to March 2021 or until the date recurrence was diagnosed.
A total of 134 saliva samples were collected between 6 a.m. and 8 a.m. following an overnight fast and refrainment of tooth brushing. Subjects were asked to swish vigorously with 40 mL sterilized double distilled water (bacteria negative in PCR assay) for 1 min, and then to expectorate into another specimen tube [19]. The saliva samples were centrifuged at 14,000 rpm for 15 min, and then the cell pellet was suspended in 1 mL of sterile TE buffer. Saliva samples stored at -80 ºC until testing.
Bacterial DNA was extracted from saliva samples using a commercial DNA extraction kit (DP302, Tiangen, China) according to the manufacturer’s protocol, except adding an enzymatic lysis step with lysozyme (20 mg/ml, 37 ºC, 60 min). The resultant DNA was stored at − 20 ºC until in PCR.
A total of 15 out of 95 fresh-frozen OSCC tissue samples were obtained. DNA was extracted using the Total DNA/RNA/Protein Kit (R6734, Omega Bio-tek, USA) according to the procedure recommended by the manufacturer. Quantification of Pg in saliva samples and detection of fimA genotypes were measured by Real-time quantitative PCR. Amplifications were performed in duplicate on Bio-Rad CFX96 thermal cycler (Bio-Rad Laboratories, USA). The primers, synthesized by Sangon Biotech (Shanghai, China), were listed in Table 1.
Table 1.
Specific oligonucleotides used in this study
| Primer | Sequence (5′-3′) | Annealing temperature (°C) | References |
|---|---|---|---|
| Universal primers | F: TCCTACGGGAGGCAGCAGT | 60 | [20] |
| R: GGACTACCAGGGTATCTAATCCTGTT | |||
| Pg | F: ACCTTACCCGGGATTGAAATG | 58 | [20] |
| R: CAACCATGCAGCACCTACATAGAA | |||
| fimA I | F: CTGTGTGTTTATGGCAAACTTC | 58 | [21] |
| R: AACCCCGCTCCCTGTATTCCGA | |||
| fimA Ib | F: CAGCAGAGCCAAAAACAATCG | 58 | [14] |
| R: TGTCAGATAATTAGCGTCTGC | |||
| fimA II | F: GCATGATGGTACTCCTTTGA | 58 | [22] |
| R: CTGACCAACGAGAACCCACT | |||
| fimA III | F: ATTACACCTACACAGGTGAGGC | 58 | [21] |
| R: AACCCCGCTCCCTGTATTCCGA | |||
| fimA IV | F: CTATTCAGGTGCTATTACCCAA | 58 | [21] |
| R: AACCCCGCTCCCTGTATTCCGA | |||
| fimA V | F: AACAACAGTCTCCTTGACAGTG | 58 | [14] |
| R: TATTGGGGGTCGAACGTTACTGTC | |||
| Pg probe used in ISH | CAATACTCGTATCGCCCGTTATTC-Digoxin | [3] |
Pg: Porphyromonas gingivalis. ISH: In situ hybridization
The reaction mixture of 20 μL was composed of 50 ng saliva DNA template or 2 μL tissue DNA template, 0.4 μM of the specific primers, ChamQTM SYBR® qPCR Master with a final concentration of 1X (Q311, Vazyme, China), and an appropriate dose of sterilized DNase-RNase-free water. The conditions for Real-time quantitative PCR were as follows: 94 ºC for 5 min, then 28 cycles for Pg or 40 cycles for fimA genotypes of 30 s at 94 ºC, 45 s at 58 ºC or 60 ºC, and 1 min at 72 ºC; with a final extension of 10 min at 72 ºC. Melting curves were generated from 60 ºC to 95 ºC and read every 0.5 ºC for 5 s. An average Ct value was obtained. The ΔCt for Pg was determined by subtracting the Ct value of Pg from that of universal primer. The relative abundance of Pg was calculated by the 2−ΔΔCt method.
Amplified PCR products of fimA genotype from Real-time quantitative PCR were checked on 2% agarose gel (ST004L, Beyotime, China). This was done using 1X Tris Acetate-EDTA buffer (TAE) from 50X TAE (ST716, Beyotime, China). Gels were stained with 4S GelRed (A616697, Sangon Biotech, China). Image results were captured with the digital imaging system (NuGenius, SYNGENE, UK). One pair of amplified Pg fragments from OSCC tissue and saliva were confirmed following nucleotide sequencing by Sangon Biotech (Shanghai, China) and the correlation of two sequences by aligning two sequences with BLAST (http://www.ncbi.nlm.nih.gov/BLAST) [23].
Among 15 OSCC tissue patients, remaining OSCC tissue and normal tissue adjacent to OSCC from one patient were fixed in 4% paraformaldehyde, paraffin-embedded, and cut into 4 μm sections, which was stained with haematoxylin and eosin, gram and subjected to in situ hybridization (ISH) using Enhanced Sensitive ISH Detection kit I (POD) (MK1030, Boster, China) according to the manufacturer's instructions. The probe is listed in Table 1. Omission of the probe was obtained as the negative controls.
Statistical analysis
Shapiro–Wilk test was used to assess whether or not data were normally distributed. Normally distributed data were analysed by Student’s t test and presented as Mean ± Standard Deviation. The data without normal distribution presented as the median and inter-quartile range (M, Q) and analysed by the Mann–Whitney U test. Categorical variables were analysed by Pearson Chi-square test or Fisher’s exact test. The cutoff point to convert the number of Pg 16S rRNA gene copies into categorical data (low, < 4 and high, ≥ 4) was performed using X-tile software [24]. The Kaplan–Meier log-rank test was performed to compare the DFS. All two-tailed P values < 0.05 were considered significant. All analyses were carried out using IBM SPSS Statistics software (IBM SPSS Statistics V.25.0, USA).
Results
As showed in Table 2, compared with controls matched for gender and age (P > 0.05, respectively), OSCC patients showed an overabundance of Pg in saliva (P < 0.05). To exclude contamination of samples, Pg was also detected in tissues by ISH from one patient. Compared with normal tissue which is adjacent to OSCC, OSCC tissue showed strong in situ expression of Pg (Fig. 1).
Table 2.
Overabundance of Pg in saliva from OSCC patient
| Porphyromonas gingivalis | P | ||
|---|---|---|---|
| Low | High | ||
| OSCC | 84 | 11 | 0.033 |
| OSCC-free | 39 | 0 | |
Fig. 1.
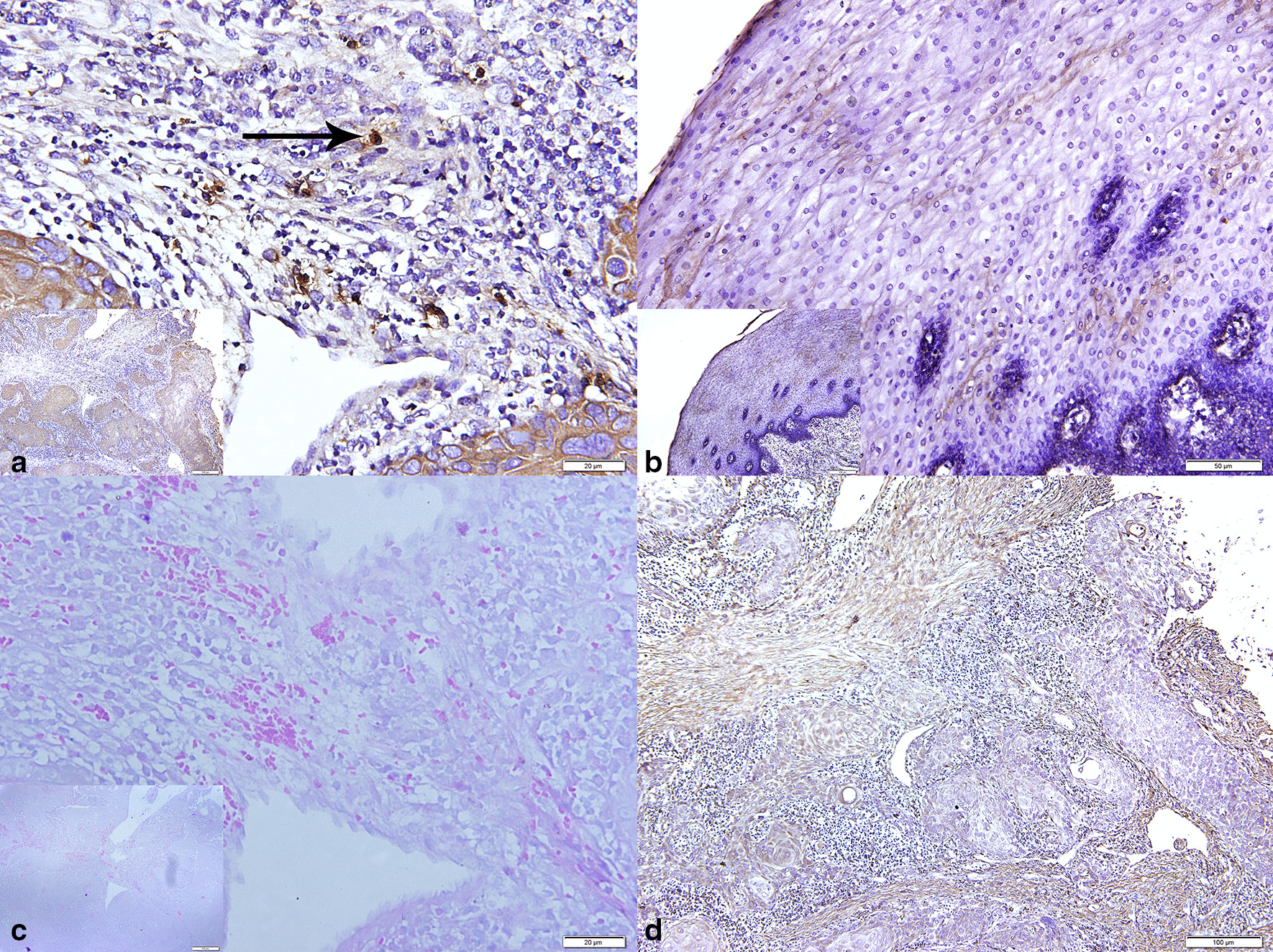
Expressions of Porphyromonas gingivalis in oral squamous cell carcinoma (OSCC) (a), normal tissue adjacent to OSCC (b), and probe-free as negative control (d) by in situ hybridization; Gram staining (c)
To evaluate the frequency of different fimA genotypes in patients with OSCC. Amplified PCR products of fimA genotype were checked on 2% agarose gel electrophoresis. The distribution of fimA genotype from the saliva of 95 OSCC patients was listed in Table 3. FimA genotype I and Ib were detected in 20 (21.1%) specimens, genotype II in 30 (31.6%) specimens, genotype III in 4 (4.2%) specimens, genotype IV in 20 (21.1%) specimens, genotype V in 2 (2.1%) specimens. We also found two or more genotypes of fimA from one sample. FimA genotype I, Ib, and II was detected in 1 (1.1%) participant, genotype I, Ib, and III in 2 (2.1%) participants, genotype I, Ib and IV in 1 (1.1%) participant, genotype I, Ib, and V in 1 (1.1%) participant, genotype II and IV in 3 (3.2%) participants, genotype I, Ib, II, and IV in 1 (1.1%) participant. Ten participants showed negative on 2% agarose gel electrophoresis assay. This finding supported the dominant distribution of Pg with genotype I, Ib, II, and IV in saliva from OSCC patients.
Table 3.
Clinicopathological details
| Parameters | Number (%) | Porphyromonas gingivalis | P value | ||
|---|---|---|---|---|---|
| Low | High | ||||
| Age (years) | 55.8 ± 12.7 | 95 (100) | 55.7 ± 12.6 | 56.5 ± 14.6 | 0.860 |
| Gender | Male | 65 (68.4) | 57 | 8 | 0.999 |
| Female | 30 (31.6) | 27 | 3 | ||
| Systemic disease | No | 69 (72.6) | 61 | 8 | 0.999 |
| Yes | 26 (27.4) | 23 | 3 | ||
| Pathologic staging | pStage I + II | 69 (72.6) | 65 | 4 | 0.008 |
| pStage III + IV | 26 (27.4) | 19 | 7 | ||
| Smoking | No | 45 (47.4) | 39 | 6 | 0.612 |
| Yes | 50 (52.6) | 45 | 5 | ||
| Alcohol consumption | No | 57 (60.0) | 50 | 7 | 0.999 |
| Yes | 38 (40.0) | 34 | 4 | ||
| Location | Buccal | 21 (24.2) | 21 | 2 | 0.447 |
| Tongue | 52 (54.7) | 47 | 5 | ||
| Gingiva | 11 (11.6) | 9 | 2 | ||
| Floor of mouth | 5 (5.3) | 4 | 1 | ||
| Hard palate | 4 (4.2) | 3 | 1 | ||
| Differentiation grade | Well | 17 (19.1) | 16 | 1 | 0.735 |
| Moderate | 65 (73.0) | 57 | 8 | ||
| Poor | 7 (7.9) | 6 | 1 | ||
| FimA genotypes | I + Ib | 20 (21.1) | 17 | 3 | 0.795* |
| II | 30 (31.6) | 27 | 3 | ||
| III | 4 (4.2) | 4 | 0 | ||
| IV | 20 (21.1) | 16 | 4 | ||
| V | 2 (2.1) | 2 | 0 | ||
| I + Ib + II | 1 (1.1) | 1 | 0 | ||
| I + Ib + III | 2 (2.1) | 2 | 0 | ||
| I + Ib + IV | 1 (1.1) | 1 | 0 | ||
| I + Ib + V | 1 (1.1) | 1 | 0 | ||
| II + IV | 3 (3.2) | 3 | 0 | ||
| I + Ib + II + IV | 1 (1.1) | 1 | 0 | ||
| Untyped | 10 (10.5) | 9 | 1 | ||
| Treatment† | Surgery | 64 (68.1) | 60 | 4 | – |
| Surgery + RT or CRT** | 30 (31.9) | 23 | 7 | ||
| Outcome† | Recurrence | 24 (25.5) | 25 | 0 | 0.033 |
| Disease-free | 70 (74.5) | 58 | 11 | ||
| Disease-free time (months) | 23, 10 | 94 (98.9) | 22, 13.8 | 27, 8 | 0.029 |
*Chi-square test was used among fimA genotype I + Ib, II, III, IV, V
†One patient dropped out in follow-up
**RT: Radiotherapy, CRT: Chemo-radiotherapy
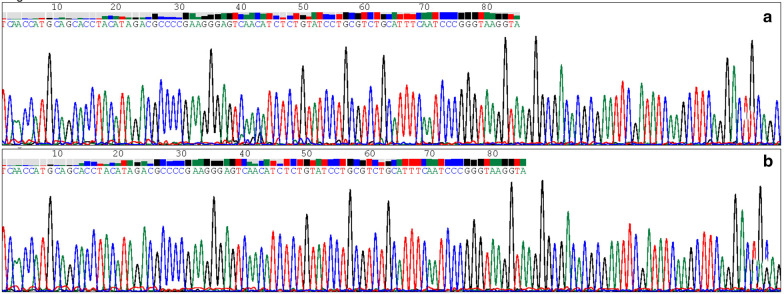
To clarify the homogeneity of Pg between saliva and OSCC tissue, the frequency of fimA genotypes was also detected in fifteen OSCC tissues. Among fifteen patients, the fimA genotypes detected in saliva were in accordance with those in OSCC tissue (Table 4). Besides, amplified Pg fragments from OSCC tissue and those from saliva were examined in one patient, we found a significant correlation in nucleotide similarity (Figs. 2, 3). Collectively, these results implied that Pg in OSCC tissue might originate from the salivary microbial reservoir.
Table 4.
The frequency of fimA genotypes in saliva and in oral squamous cell carcinoma tissues
| I + Ib | II | III | IV | V | I + Ib + III | |
|---|---|---|---|---|---|---|
| Saliva | 3 | 6 | 1 | 2 | 2 | 1 |
| Tissue | 3 | 6 | 1 | 2 | 2 | 1 |
Fig. 2.
The PCR product examined Porphyromonas gingivalis in saliva (a) and oral squamous cell carcinoma tissue (b)
Fig. 3.
The homology analysis of Porphyromonas gingivalis detected in saliva and oral squamous cell carcinoma tissue
The deadline for follow-up was March 2021. After a median follow-up period of 23 months (range 3 to 28 months). Ninety-four patients were available for the follow-up visit, but one patient was non-responsive to any form of contact. Recurrence was diagnosed as the endpoint for 25 patients, with a 26.6% (25/94) cumulative recurrence rate (Fig. 4).
Fig. 4.
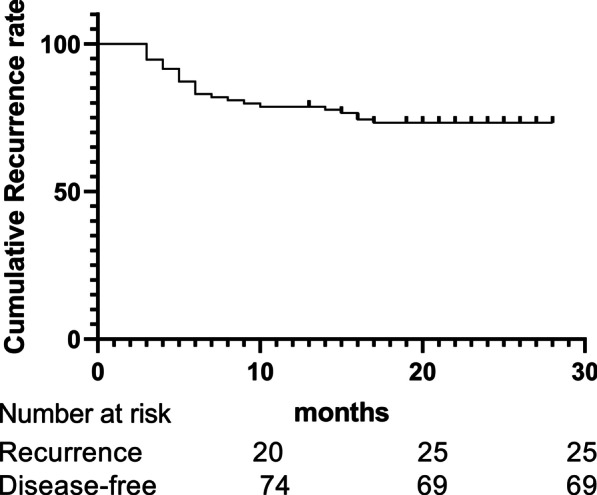
Cumulative recurrence curve of 94 patients
Clinicopathological information of OSCC patients is shown in Table 3. The distribution of clinicopathological outcomes was compared with the abundance of Pg to assess the potential prognostic variables. Patients with the overabundance of Pg in saliva had an advanced pathologic staging than those with a low abundance of Pg (P = 0.008). While, compared with the weak group, patients with the overabundance of Pg in saliva had a longer disease-free time (Z = -2.188, P = 0.029). The overabundance of Pg in saliva was associated with a lower recurrence rate (P = 0.033). However, differences were not statistically significant by age, gender, systemic disease, smoking, alcoholic consumption, location, differentiation grade, and fimA genotypes. Neither single fimA genotype was statistically significantly associated with any clinicopathological parameters.
A total of 94 cases with follow-up data were included in the survival analysis. Univariate analysis showed that the overabundance of Pg was a favorable prognostic factor (Chi-square = 3.86, P < 0.05) (Fig. 5). Statistically, age, gender, FimA genotypes, systemic disease, pathologic staging, smoking, alcoholic consumption, location, differentiation grade, and treatment were not the independent prognostic indicators. Consequently, our results showed the overabundance of Pg was associated with favorable outcomes in patients with OSCC.
Fig. 5.
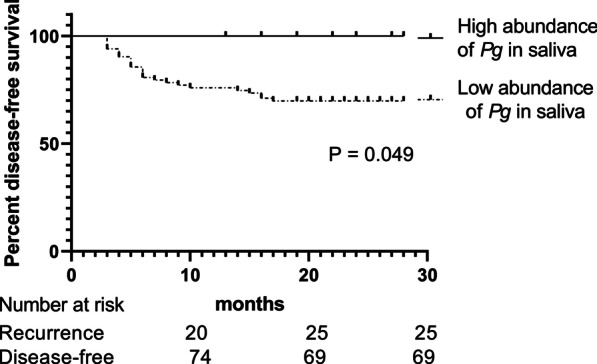
Disease-free survival according to 94 oral squamous cell carcinoma patients at the time of leaving the hospital. The Kaplan–Meier method for recurrence with the log-rank test was used for statistical comparisons
Discussions
With recent breakthroughs in high-throughput genetic-based tools, there has been a hot issue concerning the relationship between the oral microbiome and neoplasms, especially OSCC. Recently accumulating evidence indicated the relationship between Pg and OSCC. Immortalized oral keratinocytes stimulated with Pg led to a more aggressive malignant profile phenotype and contributing to enhanced tumor features [25]. The serum immunoglobulin G antibody against Pg was higher in OSCC patients compared with non-OSCC patients [4]. Pg increased the size and the multiplicity of carcinoma to promote the development of oral cancer [5]. Previous studies have confirmed the association between Pg and OSCC by examining the abundance of Pg in the saliva of patients and unveiled that patients with medium and poor differentiation, overall clinical stage III and stage IV, lymph node metastasis, and shorter overall survival associated with Pg involvement [3, 10]. But in multivariate analyses, colonization of Pg was a favorable prognostic factor, with a strong tendency towards statistical significance (HR 0.340, 95% CI 0.112–1.025, P = 0.055) [10]. Our study also verified the overabundance of Pg from OSCC patients compared with OSCC-free subjects in saliva, and the overabundance of Pg in saliva was more likely to correspond with the advanced pathological stage. Those results are in resonance to previous reports [3]. To exclude contamination of samples, we examined the presence of Pg in OSCC tissue by ISH. Consistent with the previous report [3], there was high enrichment of Pg in OSCC tissue compared with normal tissue adjacent to OSCC. After a median follow-up period of 23 months, the OSCC recurred in 26.6% (25/94) of patients. As the same as our findings, it was reported early-stage patients have a 90–95% survival rate for one year or more, and advanced-stage patients have a 65–70% survival rate [26].
In this study, compared with the weak group, patients with the overabundance of Pg in saliva had a longer disease-free time and lower recurrence rate. Those suggested that Pg may affect the prognosis of OSCC. Most interestingly, we found the overabundance of Pg is a favorable prognostic factor for DFS. Contrary to popular belief, they found that Pg was associated with a higher risk of pancreatic cancer [27, 28], esophageal squamous cell carcinoma [29, 30], and oral squamous cell carcinoma [3, 10]. Meanwhile, Pg was associated with poor overall survival rates in esophageal squamous cell carcinoma [31]. Furthermore, patients with a high level of Pg had the worst prognosis in esophageal squamous cell carcinoma [30]. Besides the population and follow-up period contributed to this prognostic incongruity, the inherent mechanistic also should be taken into consideration.
One of the most vital virulence in Pg has been supposed to the presence of fimbriae, which plays an important role in adhesion, colonization, and invasion to tissues [32]. Most of the studies focused on the distribution of fimA genotypes in periodontitis. However, the frequency of fimA genotypes in OSCC was not clear. FimA genotypes I and Ib could be discriminated by Rsa I enzyme digestion. However, discrimination of genotypes I and Ib seems to be improbable [14]. Besides, there were no differences in the immunological analysis between fimA I and Ib fimbriae [14]. So we consider fimA genotypes I and Ib as a whole.
In this study, the association of fimA genotypes and clinicopathological parameters was not statistically significant. However, the predominantly detected fimA genotypes in OSCC were genotypes I, Ib, II, and IV. Several studies concluded that nucleotide genetic variation was likely associated with virulence. Some reported fimA genotypes Ib, II, and IV are the most virulent fimbriae in periodontitis and assist in adhesion and invasion [14, 33]. It was reported that fimA genotype Ib, II, and IV led to more severe infections and inflammations [34, 35]. Clinical isolation of Pg from chronic periodontitis patients also supported the virulence of fimA genotypes Ib, II, and IV [36]. Different Pg fimA genotype was injected subcutaneously, and Nakano et al. found that the weakest inflammatory response was induced by genotype III [35]. FimA genotype V was the least amount of genotypes in this study. The reason might be the low prevalence (0–29%) of this genotype [37]. Single Pg fimA genotype was determined by more than 70% of OSCC patients, and two or more genotypes were also detected in a subset of the subjects. Approximately 10% of the samples were multiple genotypes in this population, which was less than the results of other studies [14, 17]. Researchers attributed to the limitations of PCR in the discrimination of fimA genotypes and the possibility of classifying new genotypes [38].
Due to the conservative properties of DNA, the bacterial 16S ribosomal DNA allows identification of the genus and species. Analysis of Pg nucleotide sequences in the OSCC tissue and those in the saliva showed a homology of 100%. Moreover, the distribution of fimA genotypes in OSCC tissue is according to those in saliva. Those results support the origination of Pg in OSCC tissues might be from the salivary microbial reservoir [39].
The limitations of this study included the short follow-up period and small sample. Further research will need to be done to elucidate the mechanism of the prognostic role. Except for fimbriae of Pg, important virulence included: encapsulation (K1–K6), gingipains (types A, B, C) as well as lysine-specific types I and II, may also involve in the carcinogenesis of OSCC. Those remain to be uncovered in future studies.
Conclusions
This study found the overabundance of Pg was associated with OSCC, and patients with advanced pathological stage, longer disease-free time, and the lower recurrence rate were related to the overabundance of Pg. Meanwhile, the overabundance of Pg was a favorable prognostic factor in patients with OSCC. Furthermore, there was a dominant distribution of Pg with genotype I, Ib, II, and IV from patients with OSCC, and the origination of Pg in the tumor might be from the salivary microbial reservoir.
Acknowledgements
Thanks to all staff in our laboratory for their kind heart and selfless contribution as well as Pro. Zhongcheng Gong (Oncological Department of Oral and Maxillofacial Surgery, The First Affiliated Hospital of Xinjiang Medical University) for his encouragement.
Abbreviations
- OSCC
Oral squamous cell carcinoma
- Pg
Porphyromonas gingivalis
- ISH
In situ hybridization
Authors’ contributions
QC, Z Shao, Z Shang conceived the study; QC, KL performed the experiments; XZ, LW performed the statistical analysis; QC and Z Shao wrote the initial draft of the manuscript; LT, EJ revised and edited the manuscript; Z Shang finalised the manuscript; All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant 81672666 to Zhengjun Shang). The funding organization was not involved in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript.
Availability of data and materials
The raw data are confidential and cannot readily be shared. Researchers need to obtain permission from the Institutional Review Board and apply for access to the data from The Ethics Committee of Stomatological Hospital, Wuhan University.
Declarations
Ethics approval and consent to participate
Approval from the institutional review board was obtained at the Hospital of Stomatology Wuhan University before starting the study (2016-60). Informed consent was written by each patient. Animal Ethics clearance is not applicable, as this study does not involve any animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Karpiński TM. Role of oral microbiota in cancer development. Microorganisms. 2019;7(1):E20. doi: 10.3390/microorganisms7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang C, Geng F, Shi X, Li Y, Zhang X, Zhao X, et al. The prevalence rate of periodontal pathogens and its association with oral squamous cell carcinoma. Appl Microbiol Biotechnol. 2019;103(3):1393–1404. doi: 10.1007/s00253-018-9475-6. [DOI] [PubMed] [Google Scholar]
- 4.Park DG, Woo BH, Lee BJ, Yoon S, Cho Y, Kim YD, et al. Serum levels of interleukin-6 and titers of antibodies against Porphyromonas gingivalis could be potential biomarkers for the diagnosis of oral squamous cell carcinoma. Int J Mol Sci. 2019;20(11):E2749. doi: 10.3390/ijms20112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu JS, Zheng M, Zhang M, Pang X, Li L, Wang SS, et al. Porphyromonas gingivalis Promotes 4-nitroquinoline-1-oxide-induced oral carcinogenesis with an alteration of fatty acid metabolism. Front Microbiol. 2018;9:2081. doi: 10.3389/fmicb.2018.02081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utispan K, Pugdee K, Koontongkaew S. Porphyromonas gingivalis, lipopolysaccharide-induced macrophages modulate proliferation and invasion of head and neck cancer cell lines. Biomed Pharmacother. 2018;101:988–995. doi: 10.1016/j.biopha.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Groeger S, Jarzina F, Domann E, Meyle J. Porphyromonas gingivalis activates NFκB and MAPK pathways in human oral epithelial cells. BMC Immunol. 2017;18(1):1. doi: 10.1186/s12865-016-0185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo BH, Kim DJ, Choi JI, Kim SJ, Park BS, Song JM, et al. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potentia. Oncotarget. 2017;8(29):46981–46992. doi: 10.18632/oncotarget.16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha NH, Woo BH, Kim DJ, Ha ES, Choi JI, Kim SJ, et al. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015;36(12):9947–9960. doi: 10.1007/s13277-015-3764-9. [DOI] [PubMed] [Google Scholar]
- 10.Wen L, Mu W, Lu H, Wang X, Fang J, Jia Y, et al. Porphyromonas gingivalis promotes oral squamous cell carcinoma progression in an immune microenvironment. J Dent Res. 2020;99(6):666–675. doi: 10.1177/0022034520909312. [DOI] [PubMed] [Google Scholar]
- 11.Rasiah IA, Wong L, Anderson SA, Sissons CH. Variation in bacterial DGGE patterns from human saliva: over time, between individuals and in corresponding dental plaque microcosms. Arch Oral Biol. 2005;50(9):779–787. doi: 10.1016/j.archoralbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Belstrøm D, Holmstrup P, Bardow A, Kokaras A, Fiehn NE, Paster BJ. Temporal stability of the salivary microbiota in oral health. PLoS ONE. 2016;11(1):e0147472. doi: 10.1371/journal.pone.0147472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abreu MGL, Kawamoto D, Mayer MPA, Pascoal VDB, Caiaffa KS, Zuza EP, et al. Frequency of Porphyromonas gingivalis fimA in smokers and nonsmokers after periodontal therapy. J Appl Oral Sci. 2019;27:e20180205. doi: 10.1590/1678-7757-2018-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayala-Herrera JL, Abud-Mendoza C, Gonzalez-Amaro RF, Espinosa-Cristobal LF, Martínez-Martínez RE. Distribution of Porphyromonas gingivalis fimA genotypes in patients affected by rheumatoid arthritis and periodontitis. Acta Odontol Scand. 2018;76(7):520–524. doi: 10.1080/00016357.2018.1469788. [DOI] [PubMed] [Google Scholar]
- 15.Nagano K, Hasegawa Y, Abiko Y, Yoshida Y, Murakami Y, Yoshimura F. Porphyromonas gingivalis FimA fimbriae: fimbrial assembly by fimA alone in the fim gene cluster and differential antigenicity among fimA genotypes. PLoS ONE. 2012;7(9):e43722. doi: 10.1371/journal.pone.0043722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura M, Hamachi T, Fujise O, Maeda K. The prevalence and pathogenic differences of Porphyromonas gingivalis fimA genotypes in patients with aggressive periodontitis. J Periodontal Res. 2010;40(2):147–152. doi: 10.1111/j.1600-0765.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- 17.Amano A, Kuboniwa M, Nakagawa I, Akiyama S, Morisaki I, Hamada S. Prevalence of specific genotypes of Porphyromonas gingivalis fimA and periodontal health status. J Dent Res. 2000;79(9):1664–1668. doi: 10.1177/00220345000790090501. [DOI] [PubMed] [Google Scholar]
- 18.American Joint Committee on Cancer. Manual for Staging of Cancer 8th ed. New York: Springer International Publishing; 2017:563–588.
- 19.Fan X, Peters BA, Min D, Ahn J, Hayes RB. Comparison of the oral microbiome in mouthwash and whole saliva samples. PLoS ONE. 2018;13(4):e0194729. doi: 10.1371/journal.pone.0194729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Rawi N, Al-Marzooq F. The relation between periodontopathogenic bacterial levels and resistin in the saliva of obese type 2 diabetic patients. J Diabetes Res. 2017;2017:2643079. doi: 10.1155/2017/2643079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amano A, Nakagawa I, Kataoka K, Morisaki I, Hamada S. Distribution of Porphyromonas gingivalis strains with fimA genotypes in periodontitis patients. J Clin Microbiol. 1999;37(5):1426–1430. doi: 10.1128/jcm.37.5.1426-1430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon JH, Shin SI, Chung JH, Lee SW, Amano A, Lee JY. Development and evaluation of new primers for PCR-based identification of type II fimA of Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2012;64(3):425–428. doi: 10.1111/j.1574-695X.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- 23.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 24.Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 25.Hoppe T, Kraus D, Probstmeier R, Jepsen S, Winter J. Stimulation with Porphyromonas gingivalis enhances malignancy and initiates anoikis resistance in immortalized oral keratinocytes. J Cell Physiol. 2019;234(12):21903–21914. doi: 10.1002/jcp.28754. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi P, Singh A, Chien CY, Warnakulasuriya S. Tobacco related oral cancer. BMJ. 2019;5(365):l2142. doi: 10.1136/bmj.l2142. [DOI] [PubMed] [Google Scholar]
- 27.Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67(1):120–127. doi: 10.1136/gutjnl-2016-312580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62(12):1764–1770. doi: 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters BA, Wu J, Pei Z, Yang L, Purdue MP, Freedman ND, et al. Oral Microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017;77(23):6777–6787. doi: 10.1158/0008-5472.CAN-17-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao SG, Yang JQ, Ma ZK, Yuan X, Zhao C, Wang GC, et al. Preoperative serum immunoglobulin G and A antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer. 2018;18(1):17. doi: 10.1186/s12885-017-3905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao S, Li S, Ma Z, Liang S, Shan T, Zhang M, et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agent Cancer. 2016;11:3. doi: 10.1186/s13027-016-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin X, Wu J, Xie H. Porphyromonas gingivalis minor fimbriae are required for cell-cell interactions. Infect Immun. 2006;74(10):6011–6015. doi: 10.1128/IAI.00797-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Missailidis CG, Umeda JE, Ota-Tsuzuki C, Anzai D, Mayer MP. Distribution of fimA genotypes of Porphyromonas gingivalis in subjects with various periodontal conditions. Oral Microbiol Immunol. 2004;19(4):224–229. doi: 10.1111/j.1399-302X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 34.Amano A, Nakagawa I, Okahashi N, Hamada N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J Periodontal Res. 2004;39(2):136–142. doi: 10.1111/j.1600-0765.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- 35.Nakano K, Kuboniwa M, Nakagawa I, Yamamura T, Nomura R, Okahashi N, et al. Comparison of inflammatory changes caused by Porphyromonas gingivalis with distinct fimA, genotypes in a mouse abscess model. Oral Microbiol Immunol. 2004;19(3):205–209. doi: 10.1111/j.0902-0055.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 36.Enersen M, Olsen I, Kvalheim Ø, Caugant DA. fimA genotypes and multilocus sequence types of Porphyromonas gingivalis from patients with periodontitis. J Clin Microbiol. 2008;46(1):31–42. doi: 10.1128/JCM.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno S, Jaramillo A, Parra B, Botero JE, Contreras A. Porphyromonas gingivalis Fim-A genotype distribution among Colombians. Colomb Med (Cali) 2015;46(3):122–127. [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Chaparro PJ, Rouillon A, Minet J, Lafaurie GI, Bonnaure-Mallet M. fimA genotypes and PFGE profile patterns in Porphyromonas gingivalis isolates from subjects with periodontitis. Oral Microbiol Immunol. 2009;24(5):423–426. doi: 10.1111/j.1399-302X.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 39.Castillo-Rojas G, Cerbón MA, López-Vidal Y. Presence of Helicobacter pylori in a Mexican Pre-Columbian Mummy. BMC Microbiol. 2008;8(1):119. doi: 10.1186/1471-2180-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data are confidential and cannot readily be shared. Researchers need to obtain permission from the Institutional Review Board and apply for access to the data from The Ethics Committee of Stomatological Hospital, Wuhan University.