Abstract
BK virus (BKV), a human polyomavirus that remains latent in renal epithelial cells, can be reactivated after hematopoietic stem cell transplantation (HSCT) leading to hemorrhagic cystitis. The incidence of BK viremia is higher after Umbilical cord blood transplantation (UCBT) than HSCT from adult donors. Data regarding the role of immune recovery after UCBT in BKV reactivation is lacking. We examined the correlation between the development of BK viremia and immune reconstitution in 27 adult recipients of UCBT. The incidence of BK viremia was 52% and developed most frequently within the first 8 weeks after the transplantation, but persisted in seven patients at 6 months, and three patients at 1-year post UCBT. Detection of BK viremia 1 year after transplant was negatively associated with the number of CD8+ cells (p=0.03) and CD8+CD45RO+ cells (p=0.05) at 6 months, and the number of CD4+ (p=0.03) and CD4+CD45RO+ cells (p=0.03) at 12 months after UCBT. Conversely, BK viremia at 6 and 12 months was positively correlated with the number of T regulatory (Treg) cells at 1 month (p=0.005 and p=0.016, respectively). Because UCB Treg have highly potent immunosuppressive function, our findings indicate that sustained BK viremia in UCBT recipients might be associated with the increase of Treg cells early after transplantation, which mediate impaired and delayed reconstitution of CD4+ and CD8+ T effector cells.
INTRODUCTION
Polyomavirus BK (BKV) is a double-stranded, non-enveloped DNA virus, which usually causes primary infection during childhood and subsequently remains in a latent phase in the kidneys and urinary tract [1, 2]. Immunosuppression can cause re-activation of the virus associated with nephropathy, ureteral stenosis and cystitis in renal transplant recipients [3], and hemorrhagic cystitis (HC) in recipients of hematopoietic stem cell transplantation (HSCT) [4, 5], and is associated with increased morbidity and mortality [6-8]. Previous studies have shown that myeloablative therapy, CMV viremia, chronic and acute graft versus host disease (GVHD), HLA-mismatched donor and impaired immune reconstitution are risk factors for the development of HC in HSCT recipients [9-12].
Umbilical cord blood (UCB) is an alternative source of hematopoietic stem cells for patients who lack suitable matched related or matched unrelated adult donors. The use of two UCB units has shortened the time to engraftment mainly for the myeloid precursors [13] but lymphoid immune reconstitution remains delayed in the UCBT recipients [14]. It was recently reported that UCB transplantation (UCBT) is an independent risk factor for severe BKV disease development [12, 15-17]. Our group observed that the incidence of BKV re-activation in a cohort of double UCBT (dUCBT) was 56% [18], which is higher than reported in previous studies of UCBT [19], further supporting that recipients of UCBT are at higher risk for developing BKV infection. Previously, we examined the immunological mechanisms related to reactivation and clearance of CMV after dUCBT. We observed that clearance of CMV viremia in patients who underwent dUCBT depended on the recovery of CD4+CD45RA+ naïve T cells and thymic reconstitution, which was also significantly associated with overall survival [20]. The exact mechanisms implicated in the increased susceptibility of UCBT recipients to BKV infection along with the critical components of the immune response for its clearance remain unknown. Understanding these mechanisms might be useful for the development of therapeutic interventions to prevent BKV reactivation, as currently there are no effective treatments for BKV infection [21, 22].
The aim of our study is to assess whether the incidence of BK viremia is associated with quantitative and qualitative recovery of the T cell immune response in adult recipients of dUCBT from a single cancer center treated with one protocol of pre-transplant conditioning and post-transplantation immunosuppression.
MATERIALS AND METHODS
This research protocol was approved by the Institution Review Board of the Dana-Farber/Harvard Cancer Center. Written informed consent was obtained from all patients for the correlative laboratory study of immune reconstitution before enrollment and participation in accordance with the Declaration of Helsinki. The trial was prospectively registered at http://www.clinicaltrials.gov (NCT00133367).
Patients
Patients with hematologic malignancies were eligible for inclusion in the study if they lacked a 6/6 or 5/6 HLA-A,-B,-DRB1-matched related donor, a 10/10 HLA-A,-B,-C,-DRB1,-DQ-matched unrelated donor. Eligibility was based on previously established criteria [23].
Selection of UCB units
UCB units were obtained from national and international cord blood banks. Both units were required to be a 4/6 or greater HLA A, HLA B, and HLA DRB1 allele-level match with each other and the patient.
Treatment
Patients received the following conditioning regimen before UCBT: fludarabine 30 mg/m2 per day from day −8 through day −3 (total dose of 180 mg/m2), melphalan 100 mg/m2 per day on day −2 only, and rabbit antithymocyte globulin 1.5mg/kg per day on days −7, −5, −3, and −1. Prophylaxis for GVHD consisted of tacrolimus starting on day −3 at a dose of 0.05 mg/kg orally. A loading dose of sirolimus (12 mg) was given on day −3, with subsequent daily doses of 4 mg/day with a target blood level of 3 to 12 ng/mL. In the absence of GVHD, tacrolimus and sirolimus were tapered from day +100 through +180. Patients received filgrastim at 5 ug/kg per day from day +5 until an absolute neutrophil count higher than 2.0 x 109 cells/L was reached for 2 consecutive days.
Sample collection
Peripheral blood samples were collected at the following time-points: immediately before administration of conditioning chemotherapy, 4 weeks, 8 weeks, 100 days, 6 months, 12 months, and 24 months after transplantation. Serum was separated with centrifugation and stored at −80°C. Urine sampling was done if clinically indicated.
Immunophenotyping
Peripheral blood mononuclear cells (PBMCs) were obtained at the above indicated time-points for staining with fluorescence-conjugated monoclonal antibodies for lineage-specific markers and analysis using a BD FACSCanto flow cytometer (BD Biosciences) as previously described [20]. Representative flow cytometry plots of gating are shown in Supplementary Figure 1.
Detection of BKV DNA and Antibody
DNA extraction was performed using the QIAamp® Minelute Virus Spin Kit (Qiagen, CA) as previously [18]. BKV DNA was quantified using Quantitative PCR (qPCR) using a 7300 Real Time PCR System (Applied Biosystems, CA) as previously described [18]. BKV ELISA was used to quantify BKV IgM, IgA, and IgG and results were reported as mean values of duplicates as described before [18].
Clinical monitoring for BKV infection
Medical records were reviewed for covariates associated with the clinical monitoring for BKV infection. Clinical data including creatinine and estimated glomerular filtration rate at each time point of sera collection all bacterial urine culture results, all clinical urine and blood PCR results, clinician notes documenting symptoms of cystitis around and at each time point, onset of GVHD and treatments for BKV infection were recorded.
Statistics
The Wilcoxon rank-sum test was used to test differences between continuous variables, whereas the Fisher exact test was used for categorical measures. Correlation between continuous variables was evaluated by using the Spearman rank test. BKV serostatus and phenotypic markers were included as time-varying covariates. Phenotypic markers were assessed at the above mentioned time-points. BK viremia was assessed in the following intervals: 0 to 4 weeks (1 month), 4 weeks to 100 days (3 months), 100 days to 6 months (6 months) and 6 months to 12 months (12 months). All phenotypic markers analyzed as continuous variables. All tests were two-sided at the significance level of 0.05 and multiple comparisons were not considered. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
RESULTS
Patient Characteristics
A total of 32 patients with hematologic malignancies were enrolled in the study. Four patients were excluded from analysis because of death before day 100, and one patient was excluded because of insufficient number of samples after transplantation. The results presented in this study are based on 27 patients. Median age was 48 years, 52% of patients in this cohort were male and lymphoma was the indication for transplantation in 48% of patients, with acute leukemia (33%), myeloproliferative disease (11%) and chronic leukemia (7%) to be the other indications. Approximately half of the patients (48%) died before the completion of the study and one patient (4%) was lost in the follow up. Seven patients (26%) developed acute GVHD and 2 patients (7%) developed chronic GVHD. The median days to neutrophil and platelet engraftment were 21 and 42 respectively [23].
One hundred forty-six serum samples from 27 patients were available for BKV studies and correlation analysis. None of the patients’ serum was found positive for BKV DNA prior to transplantation, while anti-BKV IgM antibody was found positive in 3 patients (13%) prior to transplantation [18]. The baseline characteristics of the patients are summarized in Table 1.
Table 1.
Baseline characteristics of the patients
| Number of patients | n = 27 (range of %) |
|---|---|
| Median age (range) | 48 (19 – 67) |
| Male sex (percentage) | 14 (52%) |
| Baseline disease | |
| Acute leukemia | 9 (33%) |
| Chronic leukemia | 2 (7%) |
| Lymphoma | 13 (48%) |
| Myeloproliferative disease | 3 (11%) |
| Clinical outcome | |
| Completed study | 13 (48%) |
| Death prior to study completion | 13 (48%) |
| Lost to follow up | 1 (4%) |
| Acute GVHD | 7 (26%) |
| Chronic GVHD | 2 (7%) |
| Median days to neutrophil engraftment | 21 (13 – 70) |
| Median days to platelet engraftment | 42 (25 – 162) |
| Positive anti-BKV IgM antibody | 3 (13%) |
Development of BK viremia and BK infection
Fourteen of twenty seven patients (52%) developed BK viremia ranging from 4.1x103–7.9x106 copies/mL of serum (median 8.9x104 copies). BK viremia was detected as early as four weeks and as late as 8 weeks after dUCBT and median time was 40 days. Overall, the cumulative incidence of BK viremia was highest between 8 weeks and 100 days, since during this period more than 50% of patients were found with BK viremia. The probability of developing BK viremia by day 100 was 0.52 (95% CI, 0.33-0.71) [18]. Table 2 shows the incidence of BK viremia at different time-points during follow up. Eight of 27 patients developed hemorrhagic cystitis (30%) by day 100 and 6 of these patients (75%) had concurrent BK viremia [18]. In 9 of the 15 patients (60%) with detectable serum BKV DNA, urinary BKV PCR was also performed. All 9 patients (100%) had detectable urinary BKV and developed clinical symptoms ranging from dysuria to hemorrhagic cystitis [18].
Table 2.
Incidence of BK viremia at different time-points
| Time Point | Tested (n) | Positive (n, %) |
|---|---|---|
| Pre-Transplant | 24 | 0 |
| 4 weeks | 23 | 8 (35%) |
| 8 weeks | 26 | 14 (54%) |
| 100 days | 26 | 14 (54%) |
| 6 months | 21 | 7 (33%) |
| 1 year | 16 | 3 (19%) |
| 2 years | 8 | 1 (13%) |
BK viremia is negatively correlated with T effector CD4+ and CD8+ cell reconstitution and positively associated with the number of T regulatory cells.
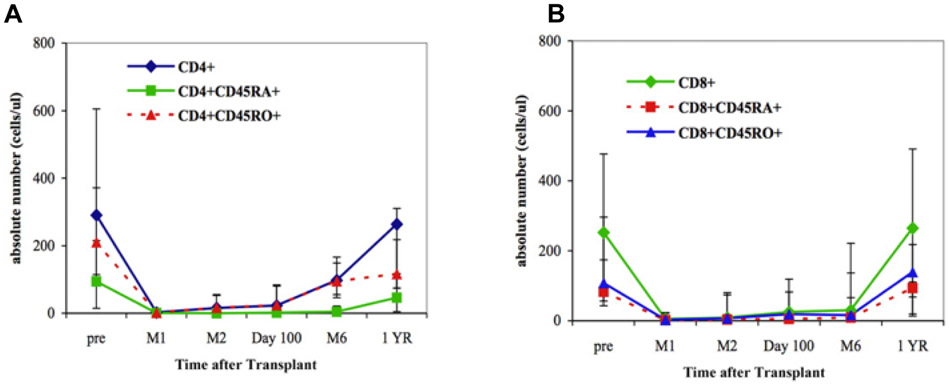
Next, we analyzed all the immune subsets of PBMC for potential correlation with the development of BK viremia. For this purpose, we analyzed CD4+ and CD8+ counts and their correlation to viral outcomes at each time point. We further examined potential correlation between CD4+ and CD8+ counts at each time point, which indicates the kinetics of immune reconstitution, to viremia at 1 year after dUCBT (Table 3a and Table 3b). As we have previously reported [14, 20], median values of CD4+ and CD8+ T cells fell after the transplantation, and subsets of CD8+ cells remained depressed throughout the first 6 months reaching normal median values at 1 year after the transplantation. On the contrary, the subsets of CD4+ cells did not reach normal median values within 1 year after transplantation (Figure 1). We observed a statistically significant inverse correlation between BK viremia and the absolute numbers of CD4+ at 12 months (r=−0.64, p=0.03). We also observed that the absolute numbers of CD8+ at 6 months were inversely correlated with BK viremia at 12 months (r=−0.62, p=0.03).
Table 3a.
Correlations between CD4+ and CD8+ T cell counts and BK viremia at the corresponding time points
| Month(s) Post Transplant |
Absolute CD4+ Count and BK viremia |
Absolute CD8+ count and BK viremia |
|---|---|---|
| 1 month | r = −0.05, P = 0.84 | r = 0.14, P = 0.60 |
| 3 months | r = −0.24, P = 0.28 | r = 0, P = 1 |
| 6 months | r = −0.07, P = 0.78 | r = −0.16, P = 0.50 |
| 12 months | r = −0.64, P = 0.03 | r = −0.41, P = 0.18 |
Table 3b.
Correlations between CD4+ and CD8+ T cell counts on different time points and BK viremia 1 year after transplant
| Month(s) Post Transplant |
Absolute CD4+ Count and BK viremia 1 year after transplant |
Absolute CD8+ count and BK viremia 1 year after transplant |
|---|---|---|
| 1 month | r = 0.35, P = 0.24 | r = 0.20, P = 0.52 |
| 3 months | r = −0.30, P = 0.34 | r = −0.24, P = 0.45 |
| 6 months | r = −0.40, P = 0.17 | r = −0.62, P = 0.03 |
| 12 months | r = −0.64, P = 0.03 | r = −0.41, P = 0.18 |
Figure 1.
Reconstitution of various cell populations after double UCB transplantation. (A) CD4+ T-cell subsets. (B) CD8+ T-cell subsets. The 25th and 75th percentiles are denoted by the error bars; solid symbols (■, ♦, ▲, ●) denote median values. (Adapted from Brown et al. [20]).
By analyzing naïve and effector memory subsets of CD4+ and CD8+ T cells, we found no significant association between BK viremia and absolute number of naïve CD4+CD45RA+ and CD8+CD45RA+ cells (Table 4). On the contrary, BK viremia at 1 year after transplant was inversely correlated with the absolute number of T effector CD8+CD45RO+ cells at 6 months (r=−0.56, p=0.05) and T effector CD4+CD45RO+ cells at 12 months after dUCBT (r=−0.64, p=0.03) (Table 5).
Table 4.
Correlations between naïve CD4+ and CD8+ T cell counts on different time points and BK viremia 1 year after transplant
| Month(s) Post Transplant |
CD4+CD45RA+ and BK viremia 1 year after transplant |
CD8+CD45RA+ and BK viremia 1 year after transplant |
|---|---|---|
| 1 month | r = 0.41, P= 0.19 | r = 0.16, P = 0.61 |
| 3 months | r = −0.41, P = 0.19 | r = −0.26, P = 0.41 |
| 6 months | r =−0.44, P = 0.13 | r = −0.53, P = 0.06 |
| 12 months | r =−0.54, P = 0.07 | r = −0.33, P = 0.30 |
Table 5.
Correlations between effector CD4+ and CD8+ T cell counts on different time points and BK viremia 1 year after transplant.
| Month(s) Post Transplant |
CD4+CD45RO+ and BK viremia 1 year after transplant |
CD8+CD45RO+ and BK viremia 1 year after transplant |
|---|---|---|
| 1 month | r = 0.40, P = 0.18 | r = 0.20, P = 0.52 |
| 3 months | r = −0.33, P = 0.30 | r = −0.30, P = 0.33 |
| 6 months | r = −0.35, P = 0.24 | r = −0.56, P = 0.05 |
| 12 months | r = −0.64, P = 0.03 | r = −0.41, P = 0.18 |
Distinct cellular components of immune reconstitution are correlated with reactivation of BK and CMV reactivation after UCBT.
Our group determined previously that the ability of dUCBT recipients to generate new, post-thymic T cells is associated with clearance of CMV viremia and improved overall survival [20]. Our present studies showed that BK viremia was not associated with the absolute numbers of naïve CD4+CD45RA+ and CD8+CD45RA+ cells but inversely correlated with the numbers of CD4+CD45RO+ and CD8+CD45RO+ T cells (Table 4). These findings suggest that immune surveillance of CMV and BK viremia might be mediated by distinct mechanisms which involve different immune populations. We assessed whether correlation between the development of BK and CMV viremia could be detected. We observed that there was no correlation between BK and CMV viremia in at any time point after dUCBT.
One cell subset that might have an active role in the expansion and immune function of CD4+CD45RO+ and CD8+CD45RO+ T cells is the natural immunosuppressive population of Treg cells. Particularly UCB Treg cells have distinct signaling properties and mediate potent immunosuppressive function [24, 25]. We examined whether a correlation between Treg cell numbers and BK viremia could be detected. The absolute number of Treg cells at 1 month after dUCBT was positively correlated with the development of BK viremia at 6 months (r=+0.65, p=0.005) and 12 months (r=+0.65, p=0.016) (Table 6). Patients with BK viremia at 8 weeks, 100 days and 6 months also had higher CD4+CD25+ counts at 1 month after the dUCBT (p=0.05, p=0.043 and p=0.02 respectively) (Table 6). Because UCB Treg have highly potent immunosuppressive function, these correlations indicate that sustained BK viremia in UCBT recipients might be associated with the increase of Treg cells early after transplantation, which mediate impaired and delayed reconstitution of CD4+ and CD8+ T effector cells.
Table 6.
Correlation between regulatory T cell count at 1 month and BK viremia at 1 month, 3 months, 6 months and 12 months after transplant.
| Month(s) Post Transplant | CD4+CD25+ count 1 month post-dUCBT and BK viremia |
|---|---|
| 1 month | r = 0.32, P = 0.21 |
| 3 months | r =0.38, P = 0.10 |
| 6 months | r = 0.65 , P = 0.005 |
| 12 months | r = 0.65, P = 0.016 |
DISCUSSION
In the present study we evaluated the development of BK viremia, which was seen in 52% of our patients by the day 100 after transplant. Interestingly, we found that BK viremia was prevalent in seven patients (33%) at six months and three patients (19%) at 1 year after dUCBT. This finding reflects the impaired and delayed immune reconstitution in dUCBT recipients [20, 26, 27],. We found that BK viremia at 12 months after the transplantation was negatively associated with the total number of CD8+ and CD4+ T cells at 6 and 12 months, respectively. BK viremia at 12 months was also negatively associated with T effector CD8+CD45RO+ and CD4+CD45RO+ cell counts at 6 and 12 months, respectively. Moreover, the development of BK viremia positively correlated with the number of Treg cells at one month after transplantation. The association between the Treg cells count at 1 month after the dUCBT with the persistent BK viremia at later time points suggest an early and sustained immunosuppressive effect of these potent Treg cells which compromises the expansion of effector T cells and correlates with the impaired clearance of BK viremia at later times after transplantation.
Hemorrhagic cystitis associated with BK viremia is a severe complication of HSCT related to significant morbidity and mortality [4]. Previous studies showed that myeloablative conditioning, use of a graft from an unrelated or mismatched donor, CMV viremia, severe acute and chronic GVHD are risk factors for development of BK-associated hemorrhagic cystitis [9, 12, 28, 29]. The incidence of BK viremia has been found to be higher in recipients of dUCBT compared to non-dUCBT allogeneic HSCT recipients [15, 18, 19]. It is known that T cells transferred to the recipient with the UCB are naïve, and fewer compared to HSCT from adult donors leading to decreased risk of GVHD but also to inadequate protection against viral infections such as CMV and EBV [14, 17]. Our group and others have identified a delayed recovery of T cells through thymic reconstitution in UCB recipients [20, 26, 27], which is critical for the clearance of CMV viremia and predictive of the overall survival of these patients [20].
Our present study suggests that immunologic mechanisms associated with the protection from BK viremia and hemorrhagic cystitis are distinct from protection from CMV reactivation. We analyzed the correlations between the numbers of reconstitution of various cellular immune populations and the development of BK viremia. Our data showed that the detection of BK viremia at 1 year after the transplantation is negatively associated with the total number of CD8+ T cells at 6 months and CD4+ T cells at 1 year after the transplantation. These data are consistent with previous reports showing that the incidence of BK viremia and development of BK-associated graft nephropathy are higher in renal transplant recipients with defective CD8+ dependent immune reactivity and abnormal CD4+ T cell function [30, 31]. Of note, Abudayyeh et al. recently showed that apart from decreased CD3+, CD4+, and CD8+ counts, decreased CD56+ counts are also associated with increased incidence of BKV infection in HCT recipients including UCBT recipients [12]. Focusing further on T cells we found that the incidence of BK viremia at 12 months is negatively associated with the number of effector memory CD8+CD45RO+ at 6 months and CD4+CD45RO+ T cells at 12 months after transplantation. On the contrary we did not identify any statistically significant correlation between the BK viremia and number of naïve T cells at any time-point during the follow up. Previous studies have already highlighted the role of CD4+ and CD8+ T cells recognizing BKV specific antigens for the clearance of the infection in recipients of renal transplants and HSCT [32, 33]. Our study is the first to reveal a role of effector T cells for the protection from BK viremia in recipients of UCBT.
We also observed that the absolute number of Treg cells at 1 month after the transplantation was positively correlated with BK viremia at 6 and 12 months after dUCBT. It should be noted that our study is based on retrospective analysis of data that were generated when Treg identification was based only on co-expression of CD4, CD25 and Foxp3 in patients’ T cells. However, this finding is important as UCB grafts per se have higher content of Treg cells than adult grafts [17]. Treg cells are involved in maintaining self-tolerance and immune homeostasis and their ability to inhibit the host immune responses may lead to progression of viral infections and neoplastic diseases. Treg cells reduce the protective T cell response against viral replication through their repressive effect on effector cell expansion and cytokine production and by inhibiting the trafficking of CD4+ and CD8+ T cells to the sites of the infection [34-36]. It has been suggested that viral infections may trigger the expansion and activation of Treg cells via several mechanisms including recognition of viral antigens by the Treg TCR or secretion of inflammatory cytokines including IL-2 and TNF-α at the site of the infection [37]. Despite the extensive research regarding the impact of Treg cells on viral replication and progression of viral infections such as HIV and HCV, a role of Treg on the development of BK viremia especially after HSCT has not been identified before. In this context, our findings support the novel hypothesis that Treg cells may be involved in BKV proliferation and sustained BK viremia in recipients of dUCBT, and inhibition of Treg activity might be a promising therapeutic approach to improve this detrimental complication.
Godfrey et al showed previously that UCB is a superior source of Treg cells compared with adult blood [24] while our group showed that UCB Treg can retain their suppressive properties after prolonged in vitro culture in IL-2 [25]. Moreover, Treg cells from UCB are more potent compared to adult Treg in regard to their suppressive effects on T effector cells [24, 25]. Our finding that higher number of Treg cells early after the dUCBT is associated with sustained BK viremia after the transplantation may be related to highly suppressive properties of UCB Treg cells transfer in the UCB grafts, which contribute to the delayed immune reconstitution by inducing prolonged suppression effects leading to impaired T cell expansion. Together, our findings provide an immunological insight why BK viremia is more common in recipients of UCB compared to recipients of adult HSCT. Although these findings are intriguing, the interpretation of these data should be taken with caution due to the relatively small sample size in our clinical trial in which more than one primary endpoint were assessed. However, the strength of our studies is the collection of data from adult recipients of dUCBT from a single cancer center, treated with one protocol of pre-transplant conditioning and after transplantation immunosuppression. Further studies are warranted to establish the role of Treg on the reactivation of BKV and potential other viruses after UCBT.
Supplementary Material
Supplementary Figure 1: Lymphocyte gates for identification of T cell subsets. (A) Within the CD3+ T cell gate, CD4+ and CD8+ T cells were identified by staining with the relevant antibodies. (B-C) Within the CD4+ T cell gate (B) or the CD8+ T cell gate (C), expression of CD45RA and CD45RO was assessed. (D) Within the CD3+ T cell gate, Treg were identified as CD4+ FoxP3+ and Tcon are identified as CD4+ FoxP3−.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Bennett SM, Broekema NM, Imperiale MJ, BK polyomavirus: emerging pathogen, Microbes and infection / Institut Pasteur, 14 (2012) 672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pinto M, Dobson S, BK and JC virus: a review, The Journal of infection, 68 Suppl 1 (2014) S2–8. [DOI] [PubMed] [Google Scholar]
- [3].Hirsch HH, Steiger J, Polyomavirus BK, The Lancet. Infectious diseases, 3 (2003) 611–623. [DOI] [PubMed] [Google Scholar]
- [4].Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R, Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants, The New England journal of medicine, 315 (1986) 230–234. [DOI] [PubMed] [Google Scholar]
- [5].Arthur RR, Shah KV, Charache P, Saral R, BK and JC virus infections in recipients of bone marrow transplants, The Journal of infectious diseases, 158 (1988) 563–569. [DOI] [PubMed] [Google Scholar]
- [6].Sencer SF, Haake RJ, Weisdorf DJ, Hemorrhagic cystitis after bone marrow transplantation. Risk factors and complications, Transplantation, 56 (1993) 875–879. [DOI] [PubMed] [Google Scholar]
- [7].Gilis L, Morisset S, Billaud G, Ducastelle-Lepretre S, Labussiere-Wallet H, Nicolini FE, Barraco F, Detrait M, Thomas X, Tedone N, Sobh M, Chidiac C, Ferry T, Salles G, Michallet M, Ader F, High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation, Bone marrow transplantation, 49 (2014) 664–670. [DOI] [PubMed] [Google Scholar]
- [8].Hill JA, Mayer BT, Xie H, Leisenring WM, Huang ML, Stevens-Ayers T, Milano F, Delaney C, Sorror ML, Sandmaier BM, Nichols G, Zerr DM, Jerome KR, Schifer JT, Boeckh M, The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality, Blood, 129 (2017) 2316–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uhm J, Hamad N, Michelis FV, Shanavas M, Kuruvilla J, Gupta V, Lipton JH, Messner HA, Seftel M, Kim DD, The risk of polyomavirus BK-associated hemorrhagic cystitis after allogeneic hematopoietic SCT is associated with myeloablative conditioning, CMV viremia and severe acute GVHD, Bone marrow transplantation, 49 (2014) 1528–1534. [DOI] [PubMed] [Google Scholar]
- [10].Giraud G, Priftakis P, Bogdanovic G, Remberger M, Dubrulle M, Hau A, Gutmark R, Mattson J, Svahn BM, Ringden O, Winiarski J, Ljungman P, Dalianis T, BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts, Bone marrow transplantation, 41 (2008) 737–742. [DOI] [PubMed] [Google Scholar]
- [11].Satyanarayana G, Marty FM, Tan CS, The polyomavirus puzzle: is host immune response beneficial in controlling BK virus after adult hematopoietic cell transplantion?, Transplant infectious disease : an official journal of the Transplantation Society, 16 (2014) 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abudayyeh A, Hamdi A, Abdelrahim M, Lin H, Page VD, Rondon G, Andersson BS, Afrough A, Martinez CS, Tarrand JJ, Kontoyiannis DP, Marin D, Gaber AO, Oran B, Chemaly RF, Ahmed S, Abudayyeh I, Olson A, Jones R, Popat U, Champlin RE, Shpall EJ, Rezvani K, Poor immune reconstitution is associated with symptomatic BK polyomavirus viruria in allogeneic stem cell transplant recipients, Transplant infectious disease : an official journal of the Transplantation Society, 19 (2017). [DOI] [PubMed] [Google Scholar]
- [13].Barker JN, Weisdorf DJ DeFor TE, Blazar BR, McGlave PB, Miller JS, Verfaillie CM, Wagner JE, Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy, Blood, 105 (2005) 1343–1347. [DOI] [PubMed] [Google Scholar]
- [14].Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, Herrera MI, Reynolds CG, Alyea EP, Ho VT, Koreth J, Armand P, Chen YB, Ballen K, Soiffer RJ, Antin JH, Cutler CS, Ritz J, Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation, Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation, 18 (2012) 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rorije NM, Shea MM, Satyanarayana G, Hammond SP, Ho VT, Baden LR, Antin JH, Soiffer RJ, Marty FM, BK virus disease after allogeneic stem cell transplantation: a cohort analysis, Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation, 20 (2014) 564–570. [DOI] [PubMed] [Google Scholar]
- [16].Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J, Cayuela JM, Traineau R, Robin M, Madureira A, Ribaud P, Ferry C, Devergie A, Purtill D, Rabian C, Gluckman E, Toubert A, Socie G, Rocha V, Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases, Transplant infectious disease : an official journal of the Transplantation Society, 13 (2011) 456–465. [DOI] [PubMed] [Google Scholar]
- [17].Brown JA, Boussiotis VA, Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution, Clinical immunology (Orlando, Fla.), 127 (2008) 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Satyanarayana G, Hammond SP, Broge TA Jr., Mackenzie MR, Viscidi R, Politikos I, Koralnik IJ, Cutler CS, Ballen K, Boussiotis V, Marty FM, Tan CS, BK polyomavirus reactivation after reduced-intensity double umbilical cord blood cell transplantation, Transplant immunology, 32 (2015) 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Silva Lde P, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, Han XY, Tarrand J, Ribeiro R, Gulbis A, Shpall EJ, Jones R, Popat U, Walker JA, Petropoulos D, Chiattone A, Stewart J, El-Zimaity M, Anderlini P, Giralt S, Champlin RE, de Lima M, Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type, Haematologica, 95 (2010) 1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brown JA, Stevenson K, Kim HT, Cutler C, Ballen K, McDonough S, Reynolds C, Herrera M, Liney D, Ho V, Kao G, Armand P, Koreth J, Alyea E, McAfee S, Attar E, Dey B, Spitzer T, Soiffer R, Ritz J, Antin JH, Boussiotis VA, Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis, Blood, 115 (2010) 4111–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cesaro S, Hirsch HH, Faraci M, Owoc-Lempach J, Beltrame A, Tendas A, Baltadakis I, Dalle JH, Koc Y, Toporski J, Styczynski J, Yesilipek MA, Heinz W, Caniglia M, Rascon J, Fauser AA, Michallet M, Lopez-Corral L, Neuburger S, Tridello G, Einsele H, Cidofovir for BK virus-associated hemorrhagic cystitis: a retrospective study, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 49 (2009) 233–240. [DOI] [PubMed] [Google Scholar]
- [22].Leung AY, Chan MT, Yuen KY, Cheng VC, Chan KH, Wong CL, Liang R, Lie AK, Kwong YL, Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 40 (2005) 528–537. [DOI] [PubMed] [Google Scholar]
- [23].Cutler C, Stevenson K, Kim HT, Brown J, McDonough S, Herrera M, Reynolds C, Liney D, Kao G, Ho V, Armand P, Koreth J, Alyea E, Dey BR, Attar E, Spitzer T, Boussiotis VA, Ritz J, Soiffer R, Antin JH, Ballen K, Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis, Bone marrow transplantation, 46 (2011) 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB, Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function, Blood, 105 (2005) 750–758. [DOI] [PubMed] [Google Scholar]
- [25].Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, Boussiotis VA, CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy, Blood, 106 (2005) 3068–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, Bryan SG, Kaur I, Martin S, Wieder ED, Worth L, Cooper LJ, Petropoulos D, Molldrem JJ, Champlin RE, Shpall EJ, Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing, Blood, 110 (2007) 4543–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Klein AK, Patel DD, Gooding ME, Sempowski GD, Chen BJ, Liu C, Kurtzberg J, Haynes BF, Chao NJ, T-Cell recovery in adults and children following umbilical cord blood transplantation, Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation, 7 (2001) 454–466. [DOI] [PubMed] [Google Scholar]
- [28].Erard V, Storer B, Corey L, Nollkamper J, Huang ML, Limaye A, Boeckh M, BK virus infection in hematopoietic stem cell transplant recipients: frequency, risk factors, and association with postengraftment hemorrhagic cystitis, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 39 (2004) 1861–1865. [DOI] [PubMed] [Google Scholar]
- [29].Leung AY, Mak R, Lie AK, Yuen KY, Cheng VC, Liang R, Kwong YL, Clinicopathological features and risk factors of clinically overt haemorrhagic cystitis complicating bone marrow transplantation, Bone marrow transplantation, 29 (2002) 509–513. [DOI] [PubMed] [Google Scholar]
- [30].Renner FC, Dietrich H, Bulut N, Celik D, Freitag E, Gaertner N, Karoui S, Mark J, Raatz C, Weimer R, Feustel A, The risk of polyomavirus-associated graft nephropathy is increased by a combined suppression of CD8 and CD4 cell-dependent immune effects, Transplantation proceedings, 45 (2013) 1608–1610. [DOI] [PubMed] [Google Scholar]
- [31].Chakera A, Bennett S, Lawrence S, Morteau O, Mason PD, O'Callaghan CA, Cornall RJ, Antigen-specific T cell responses to BK polyomavirus antigens identify functional antiviral immunity and may help to guide immunosuppression following renal transplantation, Clinical and experimental immunology, 165 (2011) 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schneidawind D, Schmitt A, Wiesneth M, Mertens T, Bunjes D, Freund M, Schmitt M, Polyomavirus BK-specific CD8+ T cell responses in patients after allogeneic stem cell transplant, Leukemia & lymphoma, 51 (2010) 1055–1062. [DOI] [PubMed] [Google Scholar]
- [33].Zhou W, Sharma M, Martinez J, Srivastava T, Diamond DJ, Knowles W, Lacey SF, Functional characterization of BK virus-specific CD4+ cells with cytotoxic potential in seropositive adults, Viral immunology, 20 (2007) 379–388. [DOI] [PubMed] [Google Scholar]
- [34].Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT, CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions, Journal of immunology (Baltimore, Md. : 1950), 172 (2004) 4123–4132. [DOI] [PubMed] [Google Scholar]
- [35].Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, Greenberg PD, Stromnes IM, Iwashiro M, Sakaguchi S, Evans LH, Peterson KE, Yang G, Hasenkrug KJ, Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection, Immunity, 20 (2004) 293–303. [DOI] [PubMed] [Google Scholar]
- [36].Lund JM, Hsing L, Pham TT, Rudensky AY, Coordination of early protective immunity to viral infection by regulatory T cells, Science (New York, N.Y.), 320 (2008) 1220–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Veiga-Parga T, Sehrawat S, Rouse BT, Role of regulatory T cells during virus infection, Immunological reviews, 255 (2013) 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Lymphocyte gates for identification of T cell subsets. (A) Within the CD3+ T cell gate, CD4+ and CD8+ T cells were identified by staining with the relevant antibodies. (B-C) Within the CD4+ T cell gate (B) or the CD8+ T cell gate (C), expression of CD45RA and CD45RO was assessed. (D) Within the CD3+ T cell gate, Treg were identified as CD4+ FoxP3+ and Tcon are identified as CD4+ FoxP3−.