Abstract
Background and aims
Obesity-related cardiometabolic risk factors associate with COVID-19 severity and outcomes. Epicardial adipose tissue (EAT) is associated with cardiometabolic disturbances, is a source of proinflammatory cytokines and a marker of visceral adiposity. We investigated the relation between EAT characteristics and outcomes in COVID-19 patients.
Methods and results
This post-hoc analysis of a large prospective investigation included all adult patients (≥18 years) admitted to San Raffaele University Hospital in Milan, Italy, from February 25th to April 19th, 2020 with confirmed SARS-CoV-2 infection who underwent a chest computed tomography (CT) scan for COVID-19 pneumonia and had anthropometric data available for analyses. EAT volume and attenuation (EAT-At, a marker of EAT inflammation) were measured on CT scan. Primary outcome was critical illness, defined as admission to intensive care unit (ICU), invasive ventilation or death. Cox regression and regression tree analyses were used to assess the relationship between clinical variables, EAT characteristics and critical illness.
One-hundred and ninety-two patients were included (median [25th-75th percentile] age 60 years [53–70], 76% men). Co-morbidities included overweight/obesity (70%), arterial hypertension (40%), and diabetes (16%). At multivariable Cox regression analysis, EAT-At (HR 1.12 [1.04–1.21]) independently predicted critical illness, while increasing PaO2/FiO2 was protective (HR 0.996 [95% CI 0.993; 1.00]). CRP, plasma glucose on admission, EAT-At and PaO2/FiO2 identified five risk groups that significantly differed with respect to time to death or admission to ICU (log-rank p < 0.0001).
Conclusion
Increased EAT attenuation, a marker of EAT inflammation, but not obesity or EAT volume, predicts critical COVID-19.
Trial registration
Keywords: COVID-19, SARS-CoV-2, Epicardial adipose tissue, Inflammation, Cardiac injury, Visceral fat
Graphical abstract
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of Coronavirus disease 2019 (COVID-19) [1], drives a profound response in susceptible individuals, leading to hyperinflammation, cytokine storm syndrome and acute cardiac injury that associate with disease severity and outcome [2,3]. Cardiometabolic risk factors such as hypertension, diabetes mellitus and history of ischaemic heart disease (IHD) are highly prevalent among patients with COVID-19, and are associated with increased odds of developing severe disease [4,5]. Despite a high prevalence of obesity among COVID-19 patients [6,7] and the close association of obesity with cardiometabolic risk factors, not all data point towards an association between body mass index (BMI) and critical illness [7,8], suggesting that the relationship between obesity and COVID-19 severity is more complex. Recent observational data indicate that visceral adipose tissue rather than total body mass might play a role in determining the severity of COVID-19 [9,10]. Epicardial adipose tissue (EAT), i.e. the visceral adipose tissue of the heart situated between the myocardium and the visceral layer of the pericardium, is a potential source of inflammatory mediators, including interleukin (IL)-1β, IL-6, and tumour necrosis factor (TNF)-α [11]. In turn, EAT inflammation can act in a paracrine manner to influence the structure and function of neighboring myocardial tissue [11]. The role of EAT as an independent cardiovascular risk factor that associates with fatal and non-fatal cardiovascular events in the general population is well-established [12]. Recently, EAT has also been proposed as a pathophysiological driver of myocardial inflammation in COVID-19 [13], and preliminary data seem to confirm this hypothesis [14]. We aimed to investigate the relationship between EAT volume and attenuation (EAT-At), which reflects overall EAT inflammation and predicts all-cause and cardiac mortality [15], with disease severity in patients hospitalised for COVID-19.
Methods
Study design
This post-hoc analysis was part of the COVID-BioB study, a large observational investigation performed at San Raffaele University Hospital in Milan, Lombardy Region, Italy. The study protocol complies with the Declaration of Helsinki, was approved by the local Hospital Ethics Committee (protocol no. 34/int/2020) and was registered on ClinicalTrials.gov (NCT04318366). Patients able to provide a signed informed consent were consented prior to data collection. The Ethics Committee waived the requirement for obtaining written informed consent for patients who had died or were unreachable after discharge. Full description of patient management and clinical protocols were previously published [16].
Participants
All patients aged ≥18 years admitted to the Emergency Department (ED) at San Raffaele University Hospital with confirmed SARS-CoV-2 infection since February 25th, 2020 to April 19, 2020 were consecutively enrolled in the COVID-BioB study. Confirmed infection was defined as positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasal and/or throat swab together with signs, symptoms, and radiological findings suggestive of COVID-19 pneumonia. For the purpose of this analysis, only hospitalised patients who underwent a CT scan and had COVID-19 characteristic features on CT scan were included. Patients admitted for other reasons and subsequently diagnosed with superimposed SARS-CoV-2 infection were excluded.
Procedures
Data were collected from medical chart review or directly by patient interview and entered in a dedicated electronic case record form (eCRF) specifically developed for the COVID-BioB study. Prior to the analysis, data were cross-checked with medical charts and verified by data managers and clinicians for accuracy. The following variables were extracted for all patients: age, sex, ethnicity, body mass index (BMI, calculated as the ratio of weight in kilograms [kg] divided by height in squared metres), PaO2/FiO2 (calculated as the ratio between the arterial partial pressure of oxygen measured on arterial blood gas analysis and the fraction of inspired oxygen), plasma glucose (mg/dL), estimated glomerular filtration rate (eGFR, as estimated by the CKD-EPI equation and expressed as ml/min/1.73 m2), lymphocyte and neutrophil counts (×109/L), and high-sensitivity C-reactive protein (CRP, mg/dL) on admission, peak CRP and high-sensitivity troponin T levels (ng/L) during hospital stay, troponin T levels measured within 3 days of the CT scan, comorbidities (including history of hypertension, diabetes mellitus, dyslipidaemia, IHD, and malignancy) and clinical outcome (discharge, death or invasive ventilation or admission to intensive care unit, ICU). Myocardial injury was defined as high-sensitivity troponin T levels above the 99th percentile upper reference limit [17] (at our Center, 14 ng/L).
CT scan protocol
CT scans were performed in a dedicated suite easily accessible via assigned elevators and paths, on a 64-slice scanner (LightSpeed V CT, GE Healthcare) in supine position, during inspiratory breath hold. CT scan parameters were as follows: 120 kV tube voltage, automatic tube current modulation 150–550 mA, 0.4 s rotation time, pitch 1.375 mm/rot, 64 × 0.625 mm detector collimation. Images were reconstructed at 1.25- and 3-mm slice thickness with sharp and medium-soft kernel, respectively, for lung and mediastinum evaluation. The lung and mediastinal window width and level were set as 1500/-700 Hounsfield Units (HU) and 350/40 HU, respectively. Mean dose length product was 438 ± 153 mGy cm.
Image analysis
EAT volume was automatically segmented on a single slice at the level of the left main coronary artery on basal non-contrast scan, as previously described and validated [18,19], using a dedicated software (IntelliSpace Portal v.8.0, Philips The Netherlands). Briefly, total thoracic fat was automatically segmented (range between −150 and −70 HU) using the “tissue segmentation” plug-in. Subsequently, an experienced radiologist (D.V., 5 years of experience in chest imaging) manually contoured the pericardium, in order to extract the EAT volume (mm3) and attenuation (HU mean ± standard deviation). CT scan of 50 randomly selected patients were segmented by a second radiologist blinded to the assessment of the first operator (R.L., 4 years of experience in chest imaging), according to the same method as previously described, in order to assess the interobserver reproducibility by calculating the intraclass correlation coefficient (ICC). ICC was excellent for EAT volume (ICC = 0.92 [95% CI 0.87; 0.96]), and good for EAT-At (ICC = 0.84 [95% CI 0.74; 0.91]). Mean time for image analysis was 3.25 ± 0.37 min.
Statistical analyses
Descriptive statistics were obtained for all study variables. Continuous variables were expressed as medians (25th – 75th percentile). Categorical variables were summarised as counts and percentages. Categorical variables were compared by using the Fisher exact test or χ2 test, and continuous variables were compared using the Kruskall–Wallis test. Post hoc pairwise multiple comparison with Bonferroni correction was done using the Dunn test. The correlation between BMI, CRP and EAT volume and attenuation was assessed using Spearman's rank correlation test. Hazard ratios (HRs) for critical COVID-19, defined as death or admission to ICU (primary outcome) were estimated using univariable and multivariable Cox regression analysis. Age, sex and variables significantly associated with critical illness in univariable analyses were included in the multivariable Cox model. Multicollinearity of the independent predictors was assessed by variance inflation factor (VIF), which showed no evidence of multicollinearity (VIFs: age = 1.13; sex = 1.17; CRP = 2.59; PaO2/FiO2 = 1.65; EAT-At = 1.18; plasma glucose = 1.45; Neutrophil count = 2.54; Lymphocytes = 1.24). The proportional hazards assumption was tested using the Schoenfeld's global test (p = 0.063). A classification and regression tree (CART) approach was used to predict critical illness and identify risk groups based on significant predictors [20]. Age, sex, BMI, EAT, EAT-At, PaO2/FiO2, plasma glucose, absolute lymphocyte and neutrophil count, LDH, eGFR and CRP on admission, history of hypertension, diabetes mellitus, dyslipidaemia, coronary artery disease, and malignancy were included in the regression tree analysis. Time-to-event analyses to test differences across risk groups identified by regression tree analysis were performed using the Kaplan–Meier method and the log-rank test. All statistical tests were two-tailed with p < 0.05 considered significant. The accuracy of the risk prediction model was estimated using receiver operating characteristic (ROC) curve and the corresponding area under curve (AUC). Missing data were not imputed. No sample size calculation was performed for the present analysis; the sample size was established by the time window of the study. Statistical analyses were performed using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) and R statistical package (version 4.0.0, R Foundation for Statistical Computing, Vienna, Austria). The analyses were conducted when the final outcomes (death or hospital discharge) were known for all patients.
Results
Study population
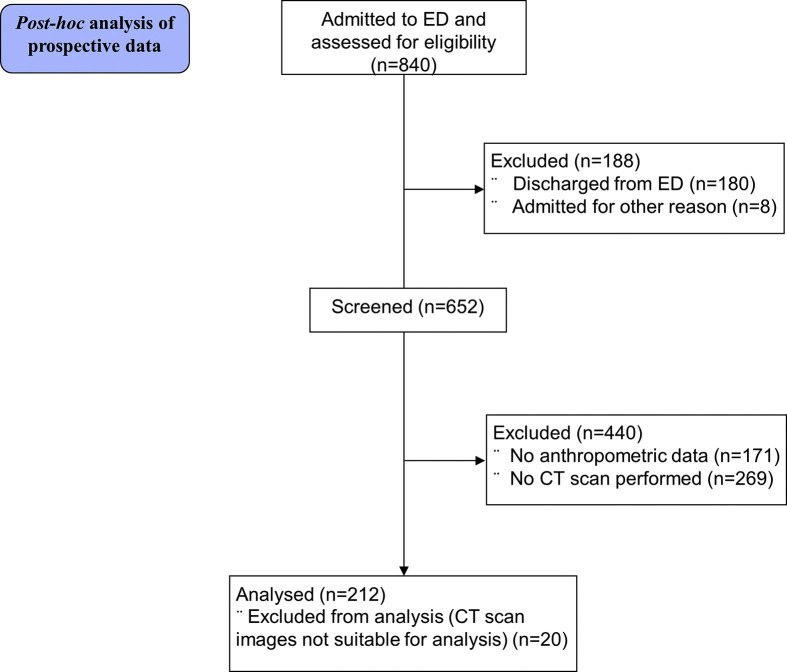
Between February 25th, 2020 and April 19, 2020, 652 patients with confirmed SARS-CoV-2 infection were admitted to San Raffaele University Hospital, of which 212 underwent a CT scan and had clinical and anthropometric data available for analyses (Supplemental Figure 1). Twenty patients with CT images not suitable for analysis (contrast-enhanced CT images and/or post-surgical fibrotic mediastinal alterations) were excluded. A total of 192 patients were included in the present analysis, whose characteristics are presented in Table 1 . Median time from admission to CT scan was 3 (1.0; 6.5) days. The median time of observation was 44 (14; 54) days. The median age was 60 years (53; 70). Most patients were men (76%), of Non-Hispanic ethnicity (88%). Overall, nearly 70% of patients had overweight or obesity. On admission to the ED, 59% met the criteria [21] for acute respiratory distress syndrome (ARDS). The most common comorbidity was arterial hypertension (40%), followed by diabetes mellitus (16%) and dyslipidaemia (11%). Twenty-six (13.5%) patients had died after a median of 21 (14; 35) days in the hospital, 166 (86.5%) had been discharged (median length of hospital stay 19 [12,33] days), and 23% had been admitted to ICU.
Table 1.
Patient characteristics.
| Variable | Values | Missing |
|---|---|---|
| Age, years | 60.0 (53.1; 70.0) | 0 |
| Female sex, n (%) | 46 (24.0) | 0 |
| Ethnicity, n (%) | ||
|
171 (89.1) | 0 |
|
21 (10.9) | |
| BMI, kg/m2 | 26.7 (24.2; 29.4) | 0 |
| Normal weight (18.5–24.9 kg/m2) | 61 (31.8) | |
| Overweight (25–29.9 kg/m2) | 90 (46.9) | |
| Obesity (≥30 kg/m2) | 41 (21.3) | |
| PaO2/FiO2 | 281.4 (211.9; 330.4) | 12 |
| ARDS,a n (%) | 105 (59.0) | 12 |
| CRP, mg/dL | 75.7 (38.0; 135.9) | 1 |
| Plasma glucose, mg/dL | 109 (98.0; 129.0) | 7 |
| Haemoglobin, g/dL | 14.0 (12.6, 15.1) | 3 |
| Neutrophil count, ×109/L | 4.8 (3.4; 7.6) | 7 |
| Lymphocyte count, ×109/L | 0.9 (0.7, 1.2) | 7 |
| Platelets, ×109/L | 186 (149.5, 246.0) | 3 |
| eGFR, ml/min/1.73/m2 | 79.7 (62.1; 92.5) | 11 |
| <60 ml/min/1.73/m2 | 37 (20.4) | |
| Arterial hypertension, n (%) | 75 (39.5) | 2 |
| Diabetes mellitus, n (%) | 31 (16.2) | 1 |
| Dyslipidaemia, n (%) | 21 (10.9) | 0 |
| Coronary artery disease, n (%) | 13 (6.8) | 2 |
| Chronic kidney disease, n (%) | 11 (5.8) | 2 |
| Chronic obstructive pulmonary disease, n (%) | 6 (3.2) | 2 |
| Malignancy, n (%) | 7 (3.7) | 2 |
| Outcome, n (%) | ||
|
166 (86.5) | 0 |
|
26 (13.5) | |
| Admitted to ICU, n (%) | 44 (22.9) | 0 |
Continuous variables are expressed as median (25th and 75th percentile). Categorical variables are expressed as absolute values (%). ARDS, acute respiratory distress syndrome; BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate, calculated with the CKD-EPI equation; ICU, intensive care unit; PaO2/FiO2, arterial partial pressure of oxygen measured on arterial blood gas analysis/fraction of inspired oxygen.
ARDS defined according to the Berlin criteria [22]. Percentages are calculated on the actual number of cases.
Epicardial adipose tissue, body mass index and systemic inflammation
Median EAT volume in the whole group was 2510 (1561; 3539) mm3, and median EAT-At was −95.8 (−99.1; −93.0) HU. EAT volume, but not EAT-At, significantly correlated with BMI (Spearman's rho = 0.321, p < 0.001). Conversely, EAT-At, but not EAT volume, significantly correlated with systemic inflammation (Spearman's rho = 0.172, p = 0.019), as estimated by peak CRP levels during hospital stay.
Clinical outcomes
Overall, 59 (30.7%) patients experienced critical illness during study follow-up. All non-critically ill patients were discharged home, after a median of 15 (10; 24.5) days, while only 33 patients (55.9%) with critical illness were eventually discharged, after a median of 47 (33; 66) days (p < 0.001 vs. non-critical illness).
Critically ill patients had worse respiratory function, had higher markers of systemic inflammation, lower lymphocyte count and higher plasma glucose on admission (Supplemental Table 1).
Both BMI and EAT volume were similar between the two groups, while EAT-At was significantly greater in patients with critical illness (Supplemental Table 1). Higher TnT values (either peak values or values measured within 3 days of the CT scan), along with occurrence of myocardial injury were more frequently reported among critically ill patients (Supplemental Table 1).
Predictors of critical illness
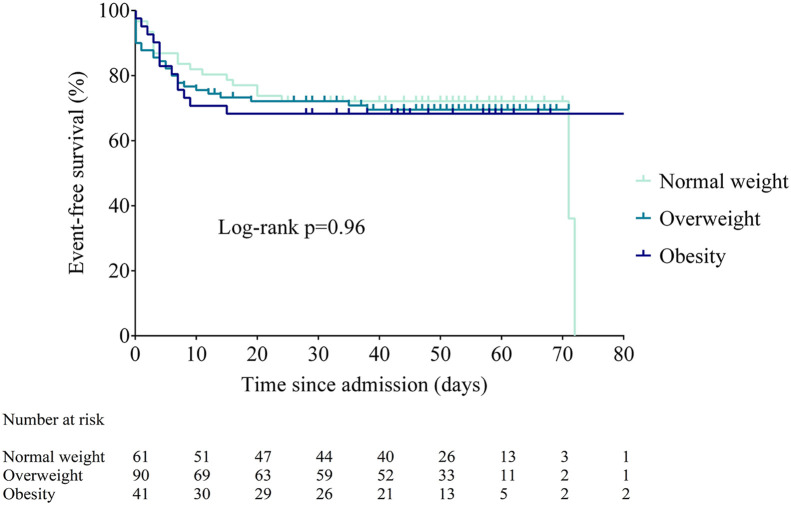
At univariable analyses, higher PaO2/FiO2 (HR 0.993 [95% CI 0.991; 0.996], p < 0.001) and absolute lymphocyte count (HR 0.36 [95% CI 0.18; 0.70], p = 0.003) were protective, whereas higher CRP values (HR 1.005 [95% CI 1.005; 1.010], p < 0.001), plasma glucose on admission (HR 1.005 [95% CI 1.003; 1.008], p < 0.001), neutrophil count (HR 1.18 [95% CI 1.11; 1.26], p < 0.001) and EAT-At (HR 1.09 [1.02; 1.16], p = 0.007) were significantly associated with admission to ICU or invasive ventilation or death. Neither BMI (either as BMI categories or as a continuous variable (Figure 1 and Supplemental Table 2) nor EAT volume predicted critical illness. At multivariable Cox regression analysis adjusted for age and sex, only PaO2/FiO2 (HR 0.996 [95% CI 0.993; 1.00], p = 0.046) and EAT-At (HR 1.12 [1.04; 1.21], p = 0.003) remained independently associated with critical illness (Supplemental Table 2).
Figure 1.
Time to death or ICU admission according to BMI category (NW, normal weight; OW, overweight; OB, obesity).
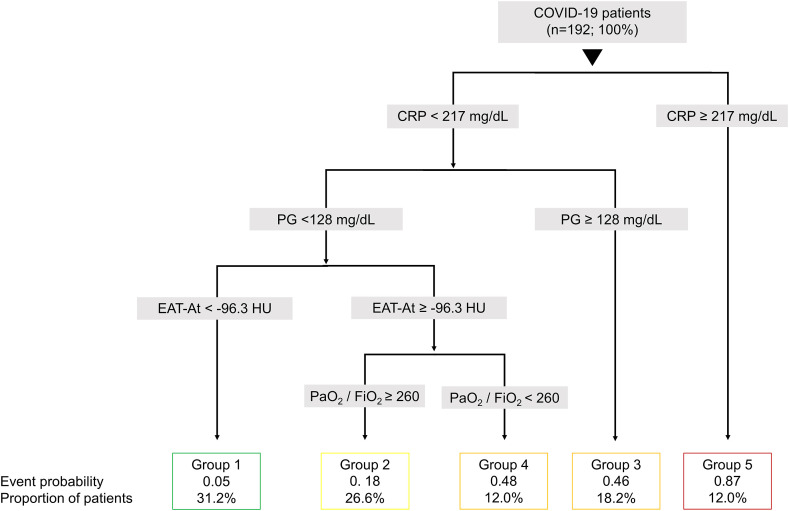
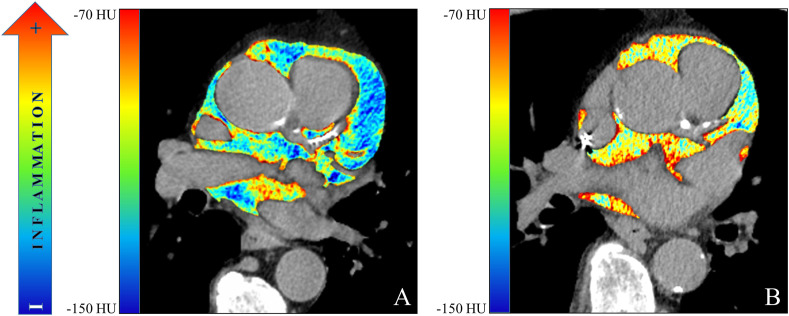
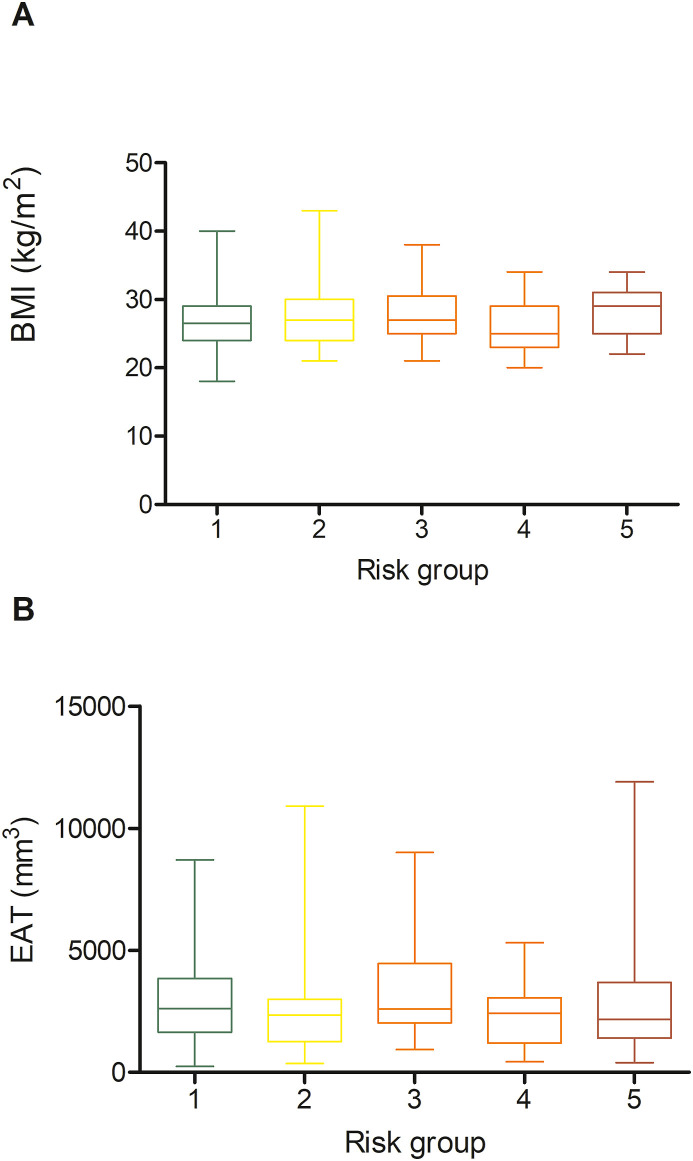
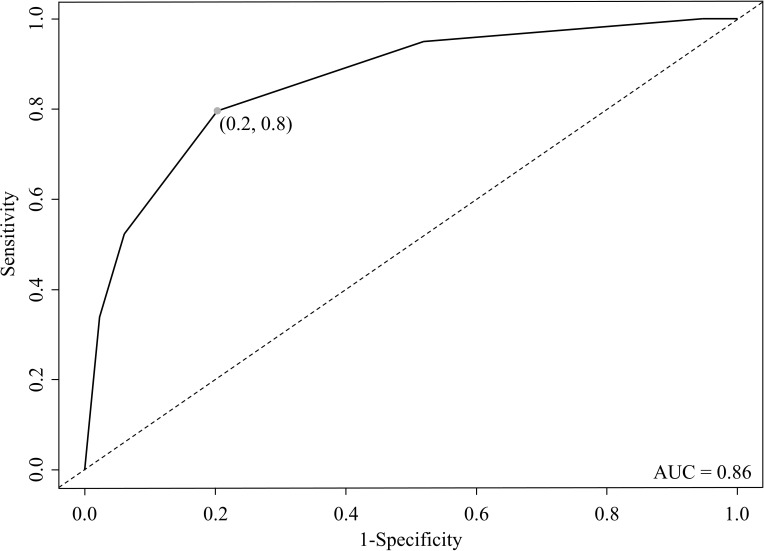
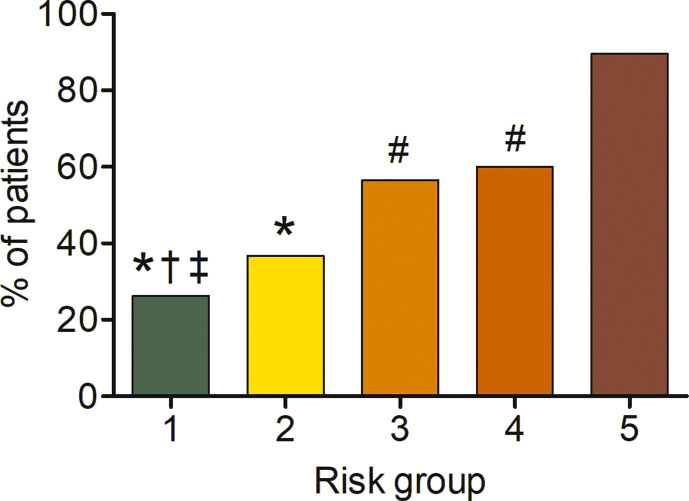
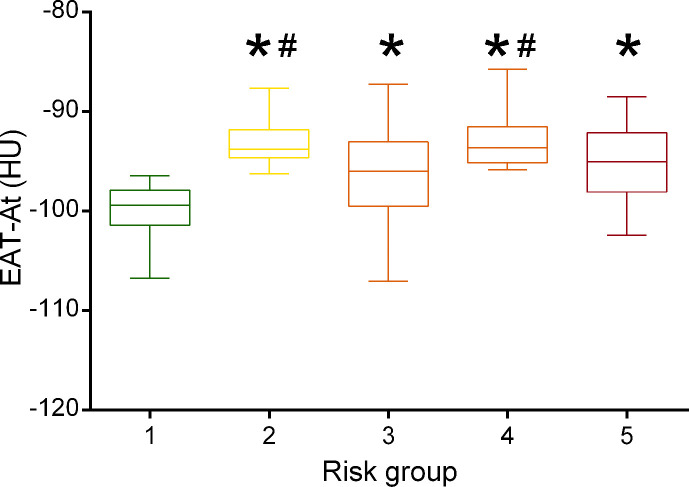
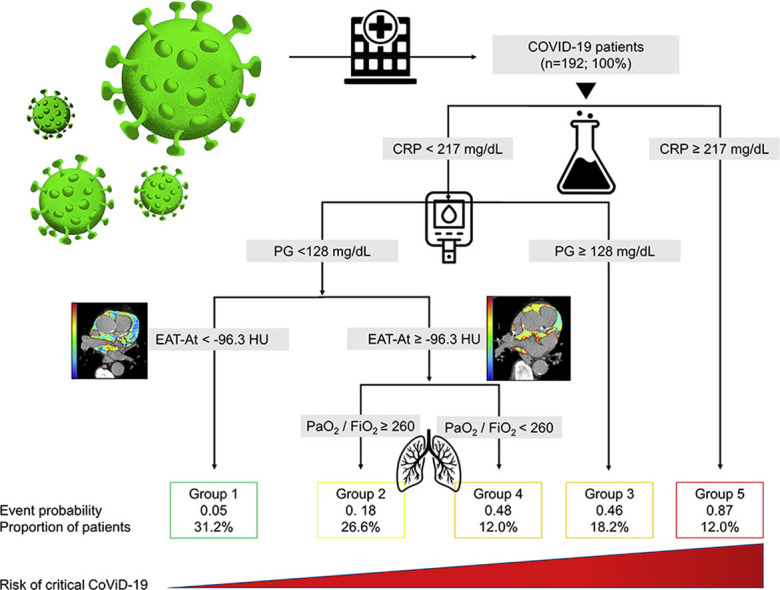
In the CART analysis, CRP, plasma glucose on admission, EAT-At and PaO2/FiO2 emerged as most important predictors of critical illness, allowing patient stratification into five risk groups (Fig. 2 ). The first node was a CRP above or below 217 mg/dL. The probability of critical illness was highest in patients with CRP ≥217 mg/dL (87%, Group 5). For those with a CRP <217 mg/dL, there was an additional node, i.e. plasma glucose on admission above or below 128. The probability of critical illness was greater for those with a plasma glucose ≥128 (46%, Group 3). In those below this value, a EAT-At of - 96.3 HU identified two additional subgroups: the probability of death or ICU admission was lowest (5%) in the subgroup with lower EAT-At (Group 1). A higher adipose tissue attenuation (less negative HU) is suggestive of EAT inflammation. Fig. 3 shows the difference between a patient with EAT-At < −96.3 HU (less inflamed EAT, Fig. 3A) and one with EAT-At ≥ −96.3 HU (more inflamed EAT, Fig. 3B). For those with lower CRP (<217 mg/dL) and lower plasma glucose on admission (<128 mg/dL) but a EAT-At greater or equal to −96.3 HU, the CART analysis identified a further node: a PaO2/FiO2 cutoff of 260, which partitioned patients into groups with lower (18%, Group 2) or higher (48%, Group 4) probability of critical illness (Fig. 2). The ROC curve for the model obtained by CART analysis is showed in Supplemental Figure 2. The AUC was 0.86, the sensitivity and specificity rates were 79.7% and 79.7%, respectively. The incidence of admission to ICU or invasive ventilation or death significantly increased from Group one to Group five (5.3%, 18.8%, 43.8%, 47.6% and 85.7%, respectively; p < 0.001). Neither BMI nor EAT volume differed across risk groups (Fig. 4 A and B). In patients with the lowest risk (Group 1), EAT-At was significantly lower (more negative HU) than in the other risk Groups (p < 0.001, Supplemental Fig. 4). At Cox regression analyses, the risk of critical COVID-19 tended to be higher in Group 2 vs. Group 1 (HR 3.6 [0.98; 13.42], p = 0.053) and was 10- to 41-fold greater in Groups 3 to 5 as compared with Group 1 (HR 10.4 [3.0; 36.5], p < 0.001; 12.0 [3.3; 44.2], p < 0.001 and 41.0 [11.9; 141.3], p < 0.001, respectively).
Figure 2.
The structured risk tree for prediction of critical illness developed by regression tree analysis using age, sex, BMI, EAT attenuation (EAT-At), PaO2/FiO2 (data available for 177 patients), plasma glucose (PG), neutrophil and lymphocyte counts, LDH, eGFR and CRP on admission, history of hypertension, diabetes mellitus, dyslipidaemia, ischaemic heart disease, and malignancy. Risk Groups identified by the regression tree analysis are as follows: Group 1 (lowest risk, 5% probability of critical illness): CRP < 217 mg/dL and PG < 128 mg/dL and EAT-At < −96.3 HU; Group 2 (18% probability of critical illness): CRP < 217 mg/dL and PG < 128 mg/dL and EAT-At ≥ −96.3 HU and PaO2/FiO2 ≥ 260; Group 3 (46% probability of critical illness): CRP < 217 mg/dL and PG ≥ 128 mg/dL; Group 4 (48% probability of critical illness): CRP < 217 mg/dL and PG < 128 mg/dL and EAT-At ≥ −96.3 HU and PaO2/FiO2 < 260; Group 5 (highest risk, 87% probability of critical illness): CRP ≥ 217 mg/dL.
Figure 3.
Colorimetric map of epicardial fat attenuation of (A) a 63-year old man with low EAT attenuation (<96.3 HU) and (B) a 71-year old man with high EAT attenuation (>96.3 HU).
Figure 4.
Body mass index (A) and epicardial adipose tissue (EAT) volume (B) across the five risk groups identified by classification and regression tree analysis.
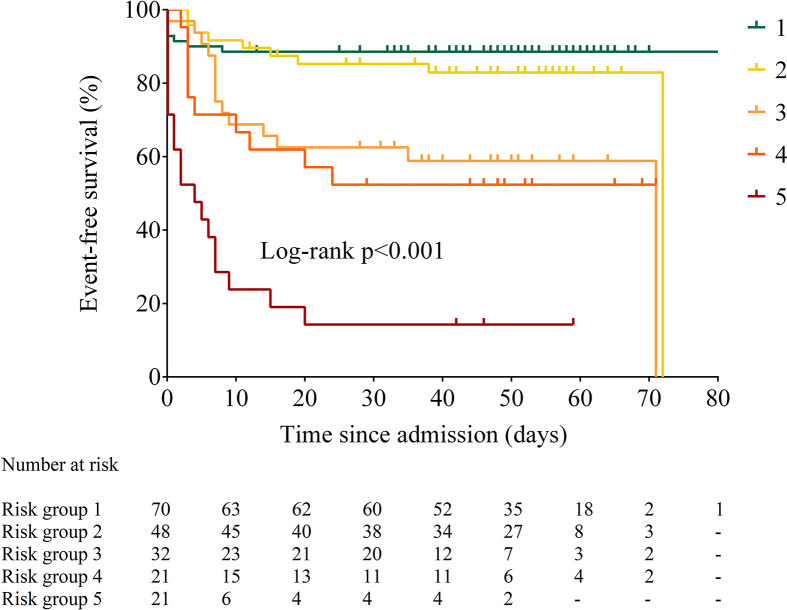
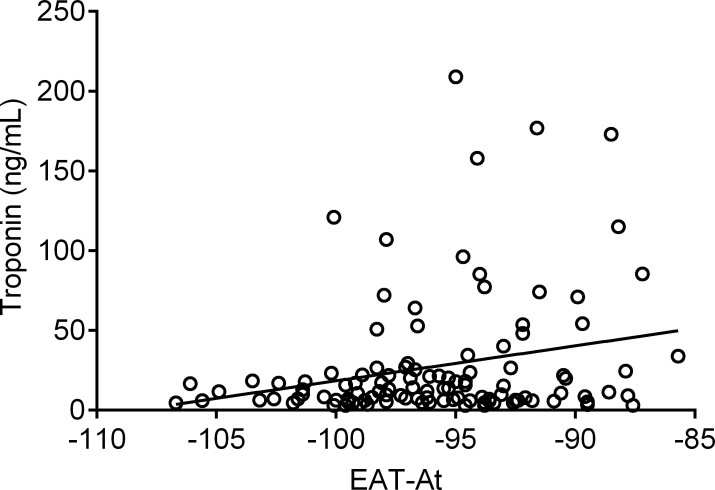
We compared time to death or ICU among groups identified by the CART analysis. Overall, the five risk groups were significantly different with respect to time to death or admission to ICU (log rank p < 0.0001, p for trend <0.0001, Fig. 5 ). Time to event was similar between Groups 3 and 4 (p = 0.069), with significant differences for other pairwise comparisons (p = 0.039 for Group 1 vs. Group 2 and p = 0.001 vs. Groups 3–5; p ≤ 0.01 for Group 2 vs. Groups 3 and 4 and p < 0.001 vs. Group 5; p < 0.01 for Groups 3 and 4 vs. Group 5). The proportion of patients with myocardial injury increased across risk groups (26.3%, 36.7%, 56.5%, 60% and 89.5% from group one to five, respectively; p < 0.001) (Supplemental Fig. 3). Finally, we found a significant– although weak – correlation between EAT-At and troponin T levels (Spearman's rho 0.19, p = 0.049, Supplemental Fig. 5).
Figure 5.
Event-free survival across the five risk groups identified by classification and regression tree analysis.
Discussion
Risk stratification based on CRP, EAT, plasma glucose and respiratory function
In this cohort of patients hospitalised for COVID-19, EAT inflammation (EAT-At) and poor respiratory function (PaO2/FiO2) independently predicted critical illness. These variables, along with plasma glucose on admission and systemic inflammation (CRP) emerged as the most important predictors of critical illness in the CART analysis, which allowed to identify five risk groups with elevated sensitivity and specificity. It is not surprising that other variables compared to the Cox regression analysis turned out to be significant predictors of critical COVID-19 in the CART analysis, as this machine learning approach tests all values relative to a single variable against others to identify the “nodes” that allow splitting patients into risk groups, selecting the optimal sequence of classifications as defined by a hierarchy of prognostic factors and associated cut-points in each subgroup of patients. The five risk groups could readily be used for patient stratification in clinical practice. Patients with the lowest EAT attenuation (more negative values, less inflamed EAT) were at the lowest risk of critical illness (Group 1). Patients with greater systemic inflammation (CRP ≥217 mg/dL) were at the highest risk (Group 5). Of note, despite EAT-At not being a “node” for risk Groups 3 and 5, patients in these groups had significantly higher EAT-At as compared with Group 1 (lowest risk), suggesting a role of EAT inflammation in the development of critical illness even in these patients.
EAT inflammation: trigger or marker of cardiac inflammation?
Increased EAT attenuation on chest CT is a marker of EAT inflammation. Visceral adipose tissue inflammation is the initial and key event triggering whole body metabolic abnormalities [22], and may trigger coronary microvascular inflammation and dysfunction [23]. EAT inflammation could be triggered by SARS-CoV-2 infection. SARS-CoV-2 enters cells via the angiotensin converting enzyme 2 (ACE2) receptor, which is highly expressed in the cardiovascular system, including EAT [24,25]. Loss of ACE2 induces EAT inflammation, cardiac insulin resistance and alterations in cardiac metabolism in murine models of diet-induced obesity [26]. Binding of SARS-CoV-2 to ACE2 reduces surface ACE2 expression [25], possibly leading to EAT inflammation [13]. We found that EAT inflammation weakly -although significantly - correlated with systemic inflammation. EAT-derived inflammatory mediators might have systemic effects [27,28], but it is more likely that during COVID-19 EAT acts locally, as a ‘fuel for cardiac inflammation’ [29], enhancing the release of cytokines such as leptin, IL-1, IL-6 and TNF-α and free fatty acids that may induce lipotoxicity [11,30]. However, both local (e.g., EAT) and systemic inflammation may induce myocardial injury [17]. When systemic inflammation prevails, as in Group 5, myocardial injury might be secondary to an imbalance between myocardial oxygen demand and supply due to increased oxygen consumption during exaggerated systemic inflammation and reduced oxygenation due to respiratory insufficiency [31]. Inflammation-mediated myocardial injury is increasingly reported in patients with troponin increase during COVID-19 [32]. In these patients, increased EAT-At might be a marker of inflammation-mediated myocardial injury. In summary, EAT inflammation could be either a trigger for myocardial inflammation/injury or a marker of myocardial injury induced by systemic inflammation.
COVID-19, EAT inflammation and myocardial injury
We found a significant, positive correlation between EAT-At and troponin T (Spearman's rho 0.19, p = 0.049), i.e. the more the inflammation (less negative EAT-At), the higher the troponin values (Supplemental Fig. 5). This correlation, however, was weak, probably because of the interplay between local and systemic inflammation contributing to myocardial injury [17]. In fact, the proportion of patients with myocardial injury increased across risk groups, which were identified by both EAT-At and CRP. Few data are available on the relationship between EAT inflammation and troponin T levels, a marker of myocardial injury. Iacobellis and colleagues found an inverse relationship (r = −0.45; P < 0.05) between EAT-At and troponin T levels in 41 COVID-19 patients, while Grodecki and colleagues found no correlation between EAT-At and troponin T in a cohort of 109 COVID-19 patients [14]. It is possible that these discrepancies are due to the smaller sample size of previous studies and, as already mentioned, to systemic factors involved in the pathophysiology of myocardial injury. Of note, the proportion of patients with myocardial injury was high, ranging from 26.3% to 89.5% in the five risk groups, despite a low prevalence of CAD in our cohort. This is in agreement with the report of Iacobellis and colleagues, who found that the degree of EAT-At in patients with critical COVID-19 was similar to that observed in patients with CAD, despite few patients having CAD and/or high coronary calcium content [33]. It should be highlighted, however, that the main aim of our study was to investigate the relationship between EAT characteristics and disease severity in patients hospitalised for COVID-19, and the assessment of the relationship between EAT-At and myocardial injury as assessed by troponin T levels was only exploratory.
EAT inflammation is a better predictor of critical COVID-19 than BMI or EAT volume
The lack of association between obesity or EAT volume with disease severity is also a novel finding. Previous cross-sectional reports suggested obesity as a risk factor for COVID-19 severity, mainly based on a higher prevalence of obesity among patients with severe and critical COVID-19 [6]. The prevalence of obesity was 21% in our cohort, which is nearly twice as high as in the general Italian population (10.9%), and 2.5-fold that in the Lombardy Region (7.9%) [34]. Neither BMI nor obesity (BMI ≥ 30 kg/m2) predicted critical illness. Moreover, neither BMI nor obesity did differ across the five risk groups identified based on CRP, plasma glucose on admission, EAT-At and PaO2/FiO2 (Fig. 4). Having obesity increases the odds of a positive SARS-CoV-2 test (OR 1.41) [35]. However, previous studies demonstrated that obesity is associated with increased risk of ARDS, but lower risk of mortality (the so called “obesity paradox”) [36]. The mechanisms responsible for this “obesity paradox” are yet to be clarified. Similar to BMI, EAT volume - which moderately correlated with BMI - neither predicted critical illness nor differed among groups. This finding is in contrast with those from a smaller study by Grodecki and colleagues in which EAT volume was independently associated with adverse outcomes in patients with COVID-19 in logistic regression models, despite a moderate correlation of BMI, which did not differ between patients with or without clinical deterioration [14]. Consistent with that study, we found that EAT-At was a significant predictor of worse outcomes. However, in our study neither the time-varying analyses nor the CART analysis support a role for obesity or EAT volume in determining the severity of COVID-19. Our findings are in line with recently published data on patients hospitalised with COVID-19 in New York City [7], and with reports of no difference in EAT thickness between patients with or without severe COVID-19 [10,33].
Clinical implications
A clinical implication of our findings is the identification of risk groups based on systemic inflammation (CRP), plasma glucose, EAT inflammation and poor respiratory function, i.e. parameters that may be easily obtained upon hospital admission. A strength of this risk classification is the measurement of EAT-At on non ECG-gated non-contrast CT scans done for the assessment of lung involvement in COVID-19, a reproducible method that does not involve additional radiation exposure for patients. Image analysis took approximately 3 min per patient, and can be done using commercial softwares. The finding that EAT inflammation predicts critical illness in COVID-19 could also inspire downstream research about potential therapeutic implications, as agents commonly used in the management of diabetes mellitus and dyslipidaemia such as pioglitazone and some statins were shown to reduce EAT inflammation [37,38]. Of note, statin use has been associated with reduced in-hospital mortality in COVID-19 patients with diabetes [39].
Concluding remarks
The results of our investigation should be viewed as hypothesis-generating in light of the study design and its limitations. The observational nature of the study does not allow to establish a causative role of EAT inflammation in COVID-19 critical illness. Autopsies or endomyocardial biopsies were not performed, therefore we could not compare CT findings with myo-pericardial histology. Patients with pre-exhisting cardiovascular disease are more likely to have myocardial injury [40]. A limitation of the present analysis is that we relied on medical history to rule out CAD rather than collecting echocardiography parameters or measuring coronary artery calcium (CAC) score, the latter being also an independent predictor of in-hospital mortality in COVID-19 [41]. Our results should be confirmed in larger multicenter studies, and EAT parameters complemented with the measurement of echocardiography parameters, CAC and cytokines such as IL-1β, IL-6, and TNF-α. Strengths of the present study include comprehensive clinical, biochemical and imaging evaluation, the study conduction at a single site, which allowed for homogenous treatment protocols, and the use of multivariable, time-varying analyses to assess the relationship of clinical, biochemical and EAT characteristics with critical illness in COVID-19 patients.
In conclusion, we found that despite being highly prevalent among COVID-19 patients, obesity was not associated with disease severity. Besides recognised predictors of critical illness in COVID-19, i.e. systemic inflammation, hyperglycaemia and poor respiratory function, EAT inflammation was significantly associated with risk of admission to ICU or invasive ventilation or death in our cohort. Future studies should investigate factors associated with increased susceptibility to SARS-CoV-2 infection in obesity, the role of ectopic fat deposition and the mechanisms underlying EAT inflammation and cardiac injury in COVID-19.
Data availability
Data are available upon reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. CC is supported by the European Foundation for the Study of Diabetes Mentorship Programme 2019.
Contributors
CC and AE equally contributed to study design, data acquisition, analysis and interpretation, and writing of the first draft; RDL contributed to data analysis and interpretation; LDF, AP, DV, RL, and VN contributed to data acquisition and interpretation; AR contributed to data analysis and interpretation; AS, EB, MT, AC, GL, GG, AZ, FDC, and FC contributed to the conception of the work; PC contributed to drafting of the work; PRQ contributed to the conception of the work and data interpretation. All authors revised the manuscript critically for important intellectual content and approved it in its final version, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
The authors wish to thank Prof. Dr. Michael Roden, German Diabetes Center (DDZ) - Leibniz Center for Diabetes Research at Heinrich Heine University Düsseldorf, Germany, and Prof. Dr. Angelo A.M. Manfredi, Vita-Salute san Raffaele University and IRCCS San Raffaele Scientific Institute, Milan, Italy, for their insightful comments, and Dr. Luigi Nocera, Vita-Salute san Raffaele University, Milan, Italy for his help with statistical analyses.
Handling Editor: A. Siani
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2021.04.020.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
Supplemental Figure 1. Study flow-chart.
figs2.
Supplemental Figure 2. Receiver operating characteristic (ROC) curve for the model obtained by classification and regresstion tree (CART) analysis.
figs3.
Supplemental Figure 3. Proportion of patients with myocardial injury, as defined by high-sensitivity troponin T levels above the upper limit of normal (14 ng/L) across risk groups. ∗ p<0.001 vs. Group 5; # p<0.05 vs. Group 5; † p<0.05 vs. Group 3; ‡ p<0.05 vs. Group 4.
figs4.
Supplemental Figure 4. Epicardial adipose tissue attenuation (EAT-At) across risk groups. More negative values indicate less inflamed EAT. ∗ p<0.001 vs. Group 1; # p<0.05 vs. Group 3.
figs5.
Supplemental Figure 5. Correlation between epicardial adipose tissue attenuation (EAT-At) and high-sensitivity troponin T (Spearman’s rho 0.19, p=0.049). More negative values indicate less inflamed EAT.
Supplemental Table 1. Comparison between non-critical patients and critically ill patients.
Supplemental Table 2. Cox regression univariable and multivariable analyses for critical illness.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhmerov A., Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita K., Ding N., Kou M., Hu X., Chen M., Gao Y. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. Glob Heart. 2020;15:64. doi: 10.5334/gh.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Fang X., Cai Z., Wu X., Gao X., Min J. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C) 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caussy C., Pattou F., Wallet F., Simon C., Chalopin S., Telliam C. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y., Ding L., Zou X., Shen Y., Hu D., Hu X. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity (Silver Spring) 2020;28:2040–2048. doi: 10.1002/oby.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battisti S., Pedone C., Napoli N., Russo E., Agnoletti V., Nigra S.G. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43:e129–e130. doi: 10.2337/dc20-1333. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–2372. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 12.Mahabadi A.A., Berg M.H., Lehmann N., Kalsch H., Bauer M., Kara K. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 13.Malavazos A.E., Goldberger J.J., Iacobellis G. Does epicardial fat contribute to COVID-19 myocardial inflammation? Eur Heart J. 2020;41:2333. doi: 10.1093/eurheartj/ehaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grodecki K., Lin A., Razipour A., Cadet S., McElhinney P.A., Chan C. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metabolism. 2020;115:154436. doi: 10.1016/j.metabol.2020.154436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oikonomou E.K., Marwan M., Desai M.Y., Mancio J., Alashi A., Hutt Centeno E. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rovere-Querini P., Tresoldi C., Conte C., Ruggeri A., Ghezzi S., De Lorenzo R. Biobanking for COVID-19 research. Panminerva Med. 2020 doi: 10.23736/S0031-0808.20.04168-3. [DOI] [PubMed] [Google Scholar]
- 17.Sandoval Y., Januzzi J.L., Jr., Jaffe A.S. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyama N., Goto D., Ito Y.M., Ishimori N., Mimura R., Furumoto T. Single-slice epicardial fat area measurement: do we need to measure the total epicardial fat volume? Jpn J Radiol. 2011;29:104–109. doi: 10.1007/s11604-010-0524-z. [DOI] [PubMed] [Google Scholar]
- 19.Tran T., Small G., Cocker M., Yam Y., Chow B.J. A single slice measure of epicardial adipose tissue can serve as an indirect measure of total epicardial adipose tissue burden and is associated with obstructive coronary artery disease. Eur Heart J Cardiovasc Imaging. 2014;15:423–430. doi: 10.1093/ehjci/jet175. [DOI] [PubMed] [Google Scholar]
- 20.Breiman L, Friedman J, Stone CJ. Classification and regression trees. 1st ed. Boca Raton, FL: Chapman & Hall/CRC Press (Taylor Francis Group).
- 21.Ferguson N.D., Fan E., Camporota L., Antonelli M., Anzueto A., Beale R. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 22.Roden M., Shulman G.I. The integrative biology of type 2 diabetes. Nature. 2019;576:51–60. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 23.Camici P.G., Tschope C., Di Carli M.F., Rimoldi O., Van Linthout S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc Res. 2020;116:806–816. doi: 10.1093/cvr/cvaa023. [DOI] [PubMed] [Google Scholar]
- 24.Couselo-Seijas M., Almenglo C., Agra-Bermejo R., Fernandez A.L., Alvarez E., Gonzalez-Juanatey J.R. Higher ACE2 expression levels in epicardial than subcutaneous stromal cells from patients with cardiovascular disease: diabetes and obesity as possible enhancer. Eur J Clin Invest. 2020 doi: 10.1111/eci.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142:426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 26.Patel V.B., Mori J., McLean B.A., Basu R., Das S.K., Ramprasath T. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 2016;65:85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malavazos A.E., Corsi Romanelli M.M., Bandera F., Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity (Silver Spring) 2020;28:1178–1179. doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L. Obesity accompanying COVID-19: the role of epicardial fat. Obesity (Silver Spring) 2020;28:1367. doi: 10.1002/oby.22867. [DOI] [PubMed] [Google Scholar]
- 29.Kim I.C., Han S. Epicardial adipose tissue: fuel for COVID-19-induced cardiac injury? Eur Heart J. 2020;41:2334–2335. doi: 10.1093/eurheartj/ehaa474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–371. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 31.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esposito A., Palmisano A., Natale L., Ligabue G., Peretto G., Lovato L. Cardiac magnetic resonance characterization of myocarditis-like acute cardiac syndrome in COVID-19. JACC Cardiovasc Imaging. 2020;13:2462–2465. doi: 10.1016/j.jcmg.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iacobellis G., Secchi F., Capitanio G., Basilico S., Schiaffino S., Boveri S. Epicardial fat inflammation in severe COVID-19. Obesity (Silver Spring) 2020;28:2260–2262. doi: 10.1002/oby.23019. [DOI] [PubMed] [Google Scholar]
- 34.Italian Institute of Health Istituto superiore di Sanità I. overweight and obesity, national data. 2015-2018. https://www.epicentro.iss.it/passi/dati/sovrappeso?tab-container-1=tab5 Available at: [Italian]
- 35.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhi G., Xin W., Ying W., Guohong X., Shuying L. "Obesity paradox" in acute respiratory distress syndrome: asystematic review and meta-analysis. PloS One. 2016;11 doi: 10.1371/journal.pone.0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacks H.S., Fain J.N., Cheema P., Bahouth S.W., Garrett E., Wolf R.Y. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care. 2011;34:730–733. doi: 10.2337/dc10-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raggi P., Gadiyaram V., Zhang C., Chen Z., Lopaschuk G., Stillman A.E. Statins reduce epicardial adipose tissue attenuation independent of lipid lowering: a potential pleiotropic effect. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeed O., Castagna F., Agalliu I., Xue X., Patel S.R., Rochlani Y. Statin use and in-hospital mortality in diabetics with COVID-19. J Am Heart Assoc. 2020 doi: 10.1161/JAHA.120.018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giannini F., Toselli M., Palmisano A., Cereda A., Vignale D., Leone R. Coronary and total thoracic calcium scores predict mortality and provides pathophysiologic insights in COVID-19 patients. J Cardiovasc Comput Tomogr. 2021 doi: 10.1016/j.jcct.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Comparison between non-critical patients and critically ill patients.
Supplemental Table 2. Cox regression univariable and multivariable analyses for critical illness.
Data Availability Statement
Data are available upon reasonable request.