Abstract
The viral infection caused by SARS-CoV-2 has increased the mortality rate and engaged several adverse effects on the affected individuals. Currently available antiviral drugs have found to be unsuccessful in the treatment of COVID-19 patients. The demand for efficient antiviral drugs has created a huge burden on physicians and health workers. Plasma therapy seems to be less accomplishable due to insufficient donors to donate plasma and low recovery rate from viral infection. Repurposing of antivirals has been evolved as a suitable strategy in the current treatment and preventive measures. The concept of drug repurposing represents new experimental approaches for effective therapeutic benefits. Besides, SARS-CoV-2 exhibits several complications such as lung damage, blood clot formation, respiratory illness and organ failures in most of the patients. Based on the accumulation of data, sulfated marine polysaccharides have exerted successful inhibition of virus entry, attachment and replication with known or unknown possible mechanisms against deadly animal and human viruses so far. Since the virus entry into the host cells is the key process, the prevention of such entry mechanism makes any antiviral strategy effective. Enveloped viruses are more sensitive to polyanions than non-enveloped viruses. Besides, the viral infection caused by RNA virus types embarks severe oxidative stress in the human body that leads to malfunction of tissues and organs. In this context, polysaccharides play a very significant role in providing shielding effect against the virus due to their polyanionic rich features and a molecular weight that hinders their reactive surface glycoproteins. Significantly the functional groups especially sulfate, sulfate pattern and addition, uronic acids, monosaccharides, glycosidic linkage and high molecular weight have greater influence in the antiviral activity. Moreover, they are very good antioxidants that can reduce the free radical generation and provokes intracellular antioxidant enzymes. Additionally, polysaccharides enable a host-virus immune response, activate phagocytosis and stimulate interferon systems. Therefore, polysaccharides can be used as candidate drugs, adjuvants in vaccines or combination with other antivirals, antioxidants and immune-activating nutritional supplements and antiviral materials in healthcare products to prevent SARS-CoV-2 infection.
Keywords: Sulfated polysaccharide, COVID-19, Drug repurposing, SARS-CoV-2, Antivirals, Immunomodulators
Graphical abstract
1. Introduction
The rapid spread of infectious disease in the current pandemic caused by Corona Virus (CoV) is striking the world to a great extent. Basically, COVID-19 is caused by a CoV that are similar to SARS (Severe Acute Respiratory Syndrome). The initial source of SARS-CoV-2 is not exactly identified and remains unclear. On the other hand, reports from genomic analysis of this virus have suggested logical similarity to pangolin CoV and bat CoV genomes [1,2]. SARS-CoV-2 infection is estimated to have an average incubation period of 3–7 days in human and likely varies on individuals [3]. Besides, several investigations have displayed that CoV has high structural complexity and efficient binding ability to host cells [4]. There seems to be no specific drugs to prevent their binding and entry process despite robust investigation globally. Additionally, accurate diagnostics and screening methods are inadequate to employ it for larger population. Fortunately, social distancing and quarantine/self-isolation is the basis for the prevention of virus spread, so far. Currently, the repurposing of antivirals [5,6] has been the possible strategy to prevent the mild and moderate COVID-19 cases [7,8]. Most of the clinical trials involving COVID-19 treatments and vaccines preparations is on the raise, with encouraging results and outcomes. Especially remdesivir is found to improve the clinical symptoms in COVID-19 patients during early treatment. However, the effectiveness of repurposing is still unsuccessful and remains a challenge in severe COVID-19 patients [9,10]. Several countries have started vaccine trials in humans but it requires longer duration to assess their therapeutic efficiency and biocompatibility.
Marine environment is an exceptional treasure for novel bioactive natural products, with diverse structural and chemical features which are generally absent in terrestrial natural products. Marine organisms, especially, have surplus potential drug candidates which have not been largely explored. Thus far, several marine natural products [11] are utilized as antitumor, anti-inflammatory, antibacterial, antifungal [12], antiparasitic and antiviral due to their promising pharmacological significance [13,14]. Moreover, the FDA (The Food and Drug Administration) has approved many marine-derived natural products as therapeutic drugs, which are already in the market and several of them are under different stages of clinical trials [[15], [16], [17]]. Especially, sulfated polysaccharides (PS) from marine seaweeds have a broad spectrum of antiviral activities and distinctive antiviral mechanisms [18]. Herein, we have discussed the possible mechanism of marine sulfated polysaccharides that deserve detailed investigation related to their antiviral effects against SARS-CoV-2. Also, we have summarized the importance of repurposing antivirals especially relating sulfated polysaccharides as an effective antiviral strategy for the prevention and treatment of recent disease outbreak caused by CoV. In light of its importance, this review focuses on the interaction of structural features of sulfated polysaccharides while exhibiting antiviral, immune activation, antithrombotic, anticoagulant and antioxidant activities.
2. SARS-CoV-2: Structure, lifecycle, severity of infection and exigency of potent antiviral drugs
2.1. Viral attachment and entry
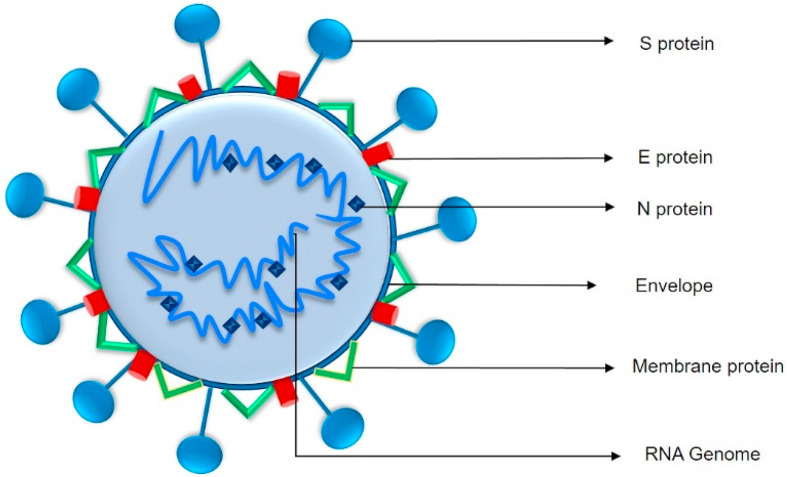
SARS-CoV-2 is an enveloped spherical shape virus with a diameter of 60–140 nm embedded virus particles and their characteristics belong to the Coronaviridae family. The virus is composed of spike protein (S), envelope protein (E), nucleocapsid protein (N) and membrane protein (M) (Fig. 1 ). It has been proposed that spike protein of this virus, predicted to be 8–12 nm in length can bind to ACE2 (Angiotensin-converting enzyme 2) with higher affinity and ensures tight binding process. Mostly virus exploit various cellular events to gain entry into cells with help of host cell receptors. The interaction of these virus particles and cell surface receptors facilitates virus entry. In the case of SARS-CoV-2, the glycosylated S proteins present at the surface facilitates the viral entry via binding to ACE2 host cell receptor [5,19,20]. ACE2 transcripts are mostly distributed in organs such as heart, lungs, kidney, testis, intestine and thus being the target for SARS-CoV-2 infection. However, every virus has its unique entry mechanism by displaying their affinity to cell surface receptors. After attachment, the human transmembrane protease serine 2 (TMPRSS2) enables the spike protein function that processes the SARS-CoV-2 entry into the cells [21,22].
Fig. 1.
Basic structure of SARS-CoV-2: The structure contains four main proteins that encapsulates the viral genomic RNA: the envelope protein (E), membrane glycoprotein (M), spike glycoprotein (S) and nucleocapsid protein (N) [216] (Section 2).
2.2. Virus adsorption and internalization
Following attachment, the virus must enter the cell to release their genome into the cells either through endocytosis or membrane fusion of the viral envelope with the host membrane. Virus entry into host cells is facilitated by the endocytic pathway with various mechanisms [23]. During attachment, the viral spike protein clings to ACE2 resulting in ACE2/SARS-CoV-2 complex and subjected to endocytosis through a clathrin-mediated endocytic pathway (CME) in case of SARS-CoV-2 reported in a recent study [[24], [25], [26]].
2.3. Membrane fusion
Fusion generally happens within acidified endosomes, but some CoVs can accomplish fusion at the plasma membrane. Membrane fusion between viral and host cell membranes in CoV is enabled by the S protein (Fig. 1). CoV S proteins have a hydrophobic “fusion peptide” that are exposed during fusion events. This enveloped virus embarks fusion process either in the plasma membrane or endosomal membrane. The S protein comprises two subunits [27], where S1 at the N-terminus serves for virus binding process while the S2 at the C-terminus responsible for fusion activity. Subsequently, the completion of fusion events accesses the host cell that ensures the release of viral RNA genome (uncoating) and initiates the further viral replication cycle [[28], [29], [30]].
2.4. RNA synthesis, genome translation and replication
After the successful accomplishment of uncoating, CoV RNA synthesis is mediated by the replication-transcription complex (RTC) which is stimulated by nonstructural proteins (nsps) leading to the synthesis of +ve strand genomes and mRNAs. The SARS-CoV-2 genome contains 14 open reading frames (ORFs), led by transcriptional regulatory sequences (TRSs) [31]. Moreover, the viral replication machinery of SARS-CoV-2 includes an array of structural and functional proteins [32]. The translation of main transcriptional units ORF1a and ORF1b from the genomic RNA generates two polyproteins, pp1a and pp1ab that includes sixteen nonstructural proteins such as pp1a (nsp1–11) and pp1ab (nsp1-10, nsp 12–16) which are processed by many viral proteases. Subsequently, these polyproteins are cleaved by viral cysteine proteases nsp3 (papain-like) and nsp5 (chymotrypsin-like proteases) [30,33]. Certainly, these nonstructural proteins together play a supportive role in accommodating viral RTC, modulating intracellular components, the supply of co-factors, RNA synthesis and modification that eventually leads to viral replication [34]. The N protein present in the viral genome involves RNA packaging, virus replication and enable the ribonucleoprotein (RNP) complex formation during virus assembly [35,36]. Additionally, the RNA-dependent RNA polymerase (nsp12), helicase (nsp13) are specifically involved in the sub-genomic replication of SARS-CoV-2 in the host cells. Following replication and subgenomic RNA synthesis, the interaction of viral RNA and structural proteins at the endoplasmic reticulum (ER) and Golgi complex enables the assembly of virions. These virions are released out of the cells via vesicles by the exocytic process as progeny virus [37,38].
2.5. Severity of SARS-CoV-2 infection and exigency of potent antivirals
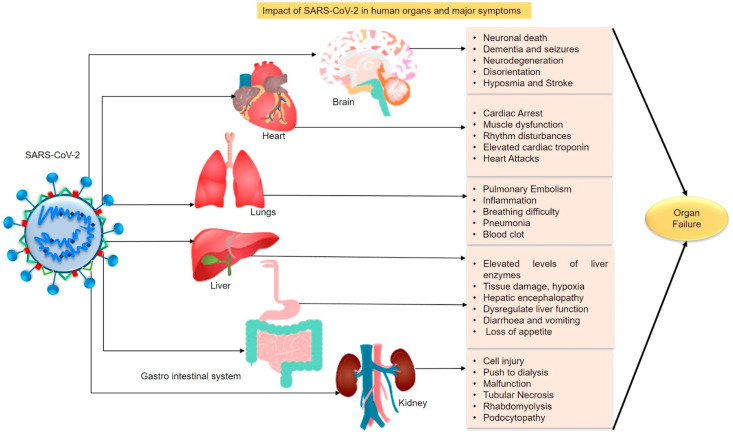
Currently, CoV disease has evolved as a major outbreak and global threat due to its transmission from animal to human and human to human through respiratory droplets that are carried by the infected individuals in their residing environment. The symptoms begin with a cough, fever, fatigue and respiratory illness causes sneezing, breathing difficulty, sore throat and pneumonia in most cases [39]. Several recent reports have declared that SARS-CoV-2 causes damage in multiple organs, in a majority of the patients [[40], [41], [42]]. Fig. 2 depicts the impact of viral infection on the organs and the major symptoms reported so far in COVID-19 patients. These adverse effects lead to irreversible loss of organ function and most often causes organ failure [[43], [44], [45]]. Despite the treatments offered to patients, excess drugs given for the mitigation of multiple symptoms cause organ dysfunction in most cases. Seemingly, the brain, kidney, lungs and heart are suggested to be highly targeted organs in case of SARS-CoV-2 infection (Fig. 2) [[46], [47], [48]]. The virus latches its spike protein directly to the receptors of organs which then initiates inflammation, cell injury, muscular dysfunction, degeneration of small blood vessels and organ malfunction [49]. It has been reported that most of the patients affected by pulmonary embolism with COVID-19 pneumonia have a higher mortality rate [50]. Moreover, neuro-inflammation, dementia, neuronal death were observed in COVID-19 patients. The hyperinflammatory response was observed, in many patients, due to abnormal immune activation induced by a viral infection that eventually leads to a cytokine storm [51,52]. Also, individuals with diabetes, obesity, cancer, liver diseases, cardiovascular complications, hypertension and chronic liver disease are more vulnerable to SARS-CoV-2 infection [[53], [54], [55]]. These comorbidities are the cause for several death cases recorded [[56], [57], [58]]. This pandemic disease is posing stunned challenges all over the world in a short period. Unfortunately, we are lacking suitable antiviral drugs, globally, to combat the adverse effects encountered by COVID-19 affected patients. The need for appropriate treatment is escalating as physicians struggle due to lack of adequate equipment facilities and health workers [59,60]. This persistently added burden on the healthcare investigators to deploy Hydroxychloroquine (HCQ, an antimalarial drug) initially to mitigate the dreadful actions of SARS-CoV-2 [61]. Later, the World Health Organization (WHO) has announced that the clinical trial for HCQ has been suspended due to its ineffectiveness. There is no FDA approved specific drug or standard therapeutic procedure to treat COVID-19 [62]. Besides, the rapid progression of COVID-19 led to the use of antibiotics for the treatment of respiratory and other bacterial infections in patients. However WHO warned about the vulnerability of antimicrobial resistance that can cause more deaths [63,64]. Fortunately, convalescent plasma therapy seems to be successful in treating COVID-19 patients. In this procedure, plasma containing antibodies are collected from recovered individuals and injected into the affected patients. As a result, patients have shown gradual recovery and improvement in their health status. However, lack of donors, quantity and the complete recovery of the patients are being a challenge [65]. To ward off the illness and severity of SARS-CoV-2 infection, efficient and specific drugs are immediately required to prevent and restore the huge loss posed by the global threat. Instead of routine blind dependency over limited action and side effect causing drugs, it is essential to disclose the previously reported structurally efficient antiviral compounds that have extensive scientifically proven data that addresses logical virus inhibiting mechanisms. The underlying theme will be that ‘If Nature poses a problem, resolve the problem using the Nature and its resources’.
Fig. 2.
The impact of SARS-CoV-2 infection on human organs including major symptoms (Section 2).
3. Importance and effects of repurposing antivirals
Creating vaccines for CoV will be a perineal challenge until their complete clinical trial outcomes are consistent in all the experiments carried out. As an alternative approach, drug repurposing serves as a valuable strategy to reuse the existing drugs that have been tested already in humans with beneficial therapeutic outcomes. Rapid repurposing of several other drugs including antivirals are initiated all over the world to combat severe complications caused by CoVs [66,67]. Before the use of drugs, it is essential to ascertain and assess the key cellular events of SARS-CoV-2 and their related cellular functions. Targeting any of SARS-CoV-2 cellular events could offer an effective basis for repurposing antivirals [25]. Indeed globally many antivirals are being enrolled in targeting the key cellular events of SARS-CoV-2 in vitro and in vivo [68,69]. Among them, remdesivir has gained more attention due to its phenomenal antiviral strategy against several deadly viruses. Remdesivir is a prodrug of an adenosine analogue that has a potent antiviral activity against several RNA viruses including pneumoviruses, filoviruses, paramyxoviruses, and CoVs [70]. It is a clinically approved viral RdRp inhibitor that is critical for viruses to replicate with the help of RdRp protein. Initially, the remdesivir has been unsuccessful in its first randomized clinical trial conducted for COVID-19. Moreover, side effects such as nausea and elevation of liver enzymes were observed in patients [71,72]. Significantly, both remdesivir and favipiravir were found to be effective against SARS-CoV-2 invitro [73]. Favipiravir is also a clinically approved viral RdRp inhibitor that halts the viral replication. An open-label nonrandomized control study indicated that favipiravir has viral clearance potential in patients. It was observed that few cases experienced diarrhea, nausea, palpitations and liver injury [74]. Another, multicenter randomized controlled study of favipiravir seemed to inhibit the polymerase activity of SARS-CoV-2 [75]. But recent evidence strongly refuses considering favipiravir for COVID-19 treatment due to its carcinogenic and embryotoxic potential [76]. Lopinavir - ritonavir (protease inhibitors) have found to affect the proteolytic process of CoV replication invitro but the therapy was unsuccessful in COVID 19 patients [[77], [78], [79]]. Ribavirin (RdRp inhibitor) is a clinically approved guanine analogue that has the potential to inhibit in vitro viral replication but the dose of the drug has been suggested to be unsuitable for the treatment. So far it has been reported to cause hematological toxicity and liver injury during different treatments [80,81]. Sofosbuvir, a clinically approved anti-hepatitis C virus (HCV), has been recommended for clinical trial against SARS-CoV-2 due to its RdRp inhibiting potential [82]. An HIV protease inhibitor darunavir (DRV) was found to be ineffective against SARS-CoV-2 in their in vitro antiviral activity assessment [83]. According to a recent report, an antiparasitic agent named ivermectin has shown antiviral activity against SARS-CoV-2 in vitro [84]. Interferon Alfa-2B a protein to treat HCV has been found to stimulate the immune response in COVID-19 affected individuals when given in combination with or without arbidol (Umifenovir) [85]. Despite the benefits of these drugs, it remains unclear which antiviral drug can effectively fight SARS-CoV-2 without rendering toxicity and troubling side effects. Currently, some of these drugs are being tested in ongoing clinical trials. However, more confirmatory multinational studies are required to validate their effects in COVID -19 patients. While randomized clinical trials are underway, consistent COVID-19 recovery data by these drugs have to be completely verified. Recently, WHO Solidarity Trial reports suggest that remdesivir, hydroxychloroquine, lopinavir, and interferon beta-1a regimens were not effective in COVID-19 patients [86]. Most of these antiviral agents only have strong in vitro data that they fight against SARS-CoV-2 since their clinical testing is far away to be considered as effective antivirals for COVID-19 [9,87]. Therefore it is essential to hasten the exploration of suitable natural compound based antivirals that are already reported for their absolute virus inhibiting potential. It has been found that sulfated PS can inhibit SARS-CoV-2 in vitro [88]. Hence, natural marine sulfated PS can be investigated against SARS-CoV-2 for their phenomenal biological properties that confer effective antiviral activities from virus entry to the replication process since they are less/non-cytotoxic with diverse structural features.
4. The rationale behind the antiviral activity of marine polysaccharides
Antiviral researches have been on the rise over the last few decades, but lack consistency and depth due to less severity of virus-related infections in humans, apart from fatal ones being HIV (human immunodeficiency virus), Ebola and Dengue (DENV). In general, marine polysaccharides (PS) include a range of marine animal, plant and microbial polysaccharides. Numerous beneficial biomedical applications are obtained from these marine polysaccharides ranging from antiviral, antioxidant, antitumor, immunomodulatory, vaccine preparation, cell/gene therapy, drug delivery to biomaterial synthesis [[89], [90], [91]]. Interestingly, significant antiviral activities against different viruses have been observed by sulfated polysaccharides [92,93]. As per the evidence available in the literature and investigational reports, that the diverse and novel structural features of marine polysaccharide are responsible for broad antiviral activities against several animals and human viruses (HSV- herpes simplex virus, HPV-human papillomavirus, HMPV- Human metapneumovirus, HIV and DENV) with well-known possible mechanisms so far [[94], [95], [96], [97]].
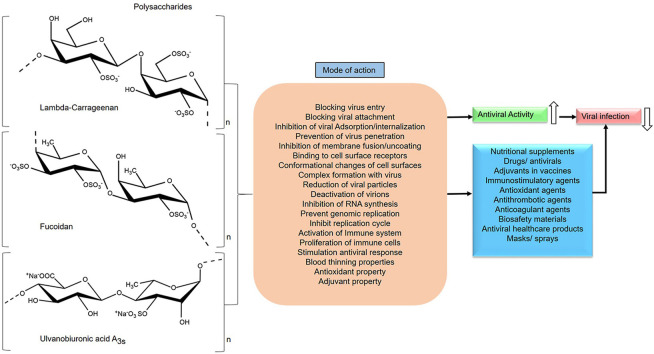
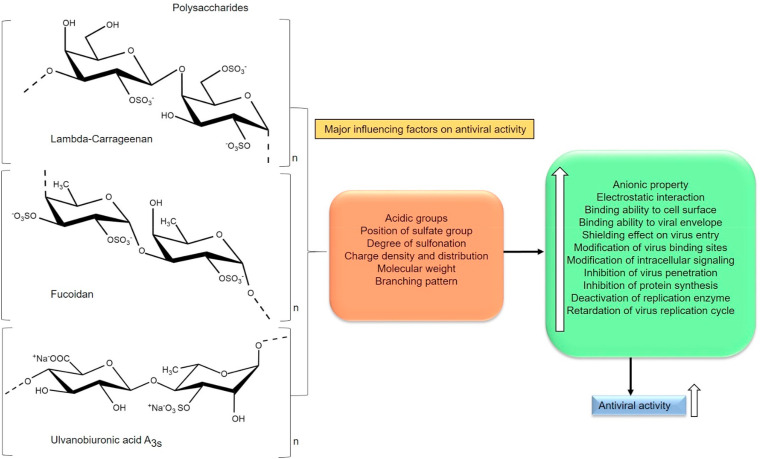
Certain common structural motifs of PS suggests influencing antiviral activity. Based on the data, PS should have the required sugar composition, molecular weight (5–10 kDa), polyanionic nature, aldehyde groups, uronic acid content, carboxyl group, methyl group, phosphates, (>2 SO3 −) sulfate group per sugar residue especially on the exterior surface and branched-chain length to exhibit antiviral activity (Fig. 3 ). A highly charged molecule can interfere with electrostatic interactions between the positively charged region of a viral glycoprotein and the negatively charged heparan sulfate chains of the cell-surface glycoprotein receptor. Moreover, enveloped viruses are more sensitive to polyanionic inhibitors than non-enveloped viruses. Another important factor for prolonged antiviral activity is the slower degradation of polyanions present in the PS [98]. Additionally, sulfate pattern has a greater influence on the antiviral activity of PS reported so far [99,100]. Chemical modification is quite easier in PS whereas the degree of sulfation, acetylation and other modifications can be successfully implemented to amplify the antiviral activity and immune-stimulatory effect. Depolymerization can render the desired molecular weight of PS to penetrate efficiently into the cell that provokes higher antiviral effects, thereby inhibiting the cell to cell spread of the virus [101,102]. Hence, concerning antiviral property, the polyanionic nature (charge density and distribution) of a PS is a crucial factor and the antiviral activity is quantitatively and qualitatively depends on the structural architecture of PS (Fig. 3) [103].
Fig. 3.
Schematic representation of major essential factors influencing the antiviral activity of sulfated polysaccharides, Lambda-carrageenan [217,218], Fucoidan [219,220], Ulvanobiuronicacid A3s [221,222] (Section 4).
5. Structural features of sulfated polysaccharides and their possible antiviral mechanism
To be a passable effective antiviral agent, virus attachment should be prevented, so that the subsequent process of entry and retardation of intracellular events that occur during post entry and replication process is inhibited. Few possible inhibition mechanisms can be postulated based on in vitro analysis. It also depends on the lifecycle of the virus that can enable certain cellular events to invade the host cells. The receptors on the virus have an essential role in the downstream events of virus entry such as signalling, attachment, internalization, endocytosis, uncoating, allostery and replication [[132], [133], [134]].
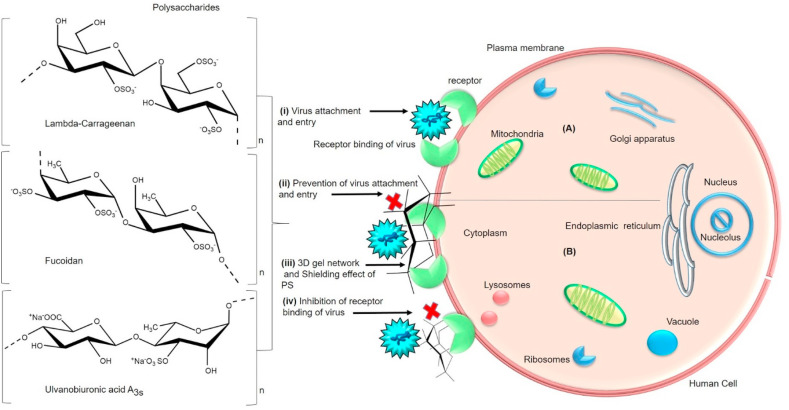
5.1. Shielding effect on viral attachment and entry
The fact is that the anionic nature of PS interferes with the attachment of the virus to its target cells. PS can prevent the binding process of the virus by affording three-dimensional gel network over the cell surface and interacts with the positive domains of cell surface glycoproteins thus providing shielding effect that subsequently prevents binding of the virus to host cells (Fig. 4 ). A sulfated chitosan 36S (58.3 kDa) comprised of β-(1 → 4)-linked N-acetyl-2-amino-2-deoxyD-glucopyranose units with sulfate substitution at C3 and C6 hydroxyl groups have exhibited good anti-HPV activity (Human papillomavirus) and regulated the cellular pathways during viral infection. Especially, PS entered HeLa cells and down-regulated PI3K/Akt/mTOR pathway. Additionally, PS has inhibited HPV infection after adsorption by targeting viral capsid protein and host PI3K/Akt/mTOR pathway in human embryonic kidney cells (293FT), HeLa and HaCaT cells [129]. Recently in a structure-activity relationship study of SARS-CoV-2 and PS obtained from Saccharina japonica brown macroalga, PS fractions were reported to have a significant antiviral effect on HEK293T cells. One of them is a glucuronomannan composed of glucose and mannose as monosaccharides along the backbone of 1, 4-linked β-D-GlcAp residues and 1, 2-linked α-D-Manp residues with a molecular weight of 7.0 k Da and another one called as sulfated galactofucan composed of 21% sulfate, 36% fucose and 10% galactose had a molecular weight of 13.7 kDa with a backbone of 1, 3-linked α-L-Fucp residues sulfated at C4 and C2/C4 and 1, 3-linked α-L-Fucp residues sulfated at C4 and branched with 1, 6-linked β-d-galacto-biose. Both PS have revealed stronger binding activity to SARS-CoV-2 spike glycoproteins (SGPs) pseudotyped particles. The findings showed that strong interactions especially the binding ability of PS to viral glycoproteins were attributed to molecular weight and sulfates present in the PS. Additionally, these PS have been suggested to be good antiviral agents that can target and prevent the attachment of SARS-CoV-2 in the cells [135]. Thus more PS needs to be explored for their reliable structural-activity relationship against SARS-CoV-2 virus.
Fig. 4.
(A) General schematic representation of virus attachment and entry into the host cell- (i) Initial attachment of virus to cell surface receptors takes place through binding process [223,224]. (B) Mode of antiviral action of sulfated polysaccharides (Lambda-carrageenan, Fucoidan, Ulvanobiuronicacid A3s) (ii) The polysaccharide involving in the prevention of virus attachment to host cells by providing (iii) shielding effect that fails the (iv) virus receptor binding activity (Table 1). (Section5.1).
An investigation of PS obtained from marine sponges have exerted potent anti-HIV activity. Especially Erylus discophorus was found to be active high molecular weight (>2000 kDa) PS fraction that has shown anti-HIV-1 activity. Significantly, PS has prevented viral attachment, entry and fusion in Jurkat lymphocytic cell line. The inhibition mechanism is due to the high molecular weight of PS [136]. Thus the molecular weight of PS can provide a shielding effect against viral entry and attachment. Similarly, another sulfated PS derived from Laminaria angustata has prevented HSV-1virus attachment by direct interaction with virus particles. This PS has consisted of sulfated xylogalactofucan and alginic acid fractions with a backbone of (1→3-, 1→4- and 1 → 2)-linked fucopyranosyl residues and molecular mass of 56 ± 5 kDa. The algin consisted of gulo- (55.5%) and mannuronic (44.5%) acid residues with a molecular mass of 32 ± 5 kDa. The findings showed that the addition of sulfate groups to PS has blocked the HSV-1 infection over the cells [110]. Exopolysaccharide from Porphyridium cruentum has exhibited good antiviral activity by preventing the viral entry against HSV-1, Vaccinia virus and Vesicular stomatitis virus in Human Erythroleukemia Cells (HEL). The monosaccharides are glucose (22.5–24 M %) and arabinose (16 M %), mannose, fucose, xylose and rhamnose in minor amounts. Authors suggested that sulfate content, uronic acids and carboxyl groups have enhanced the negative charge of EPS to protect the cells against the virus [112]. Hence the electron-donating/electron-withdrawing functional groups have an essential role in the antiviral activity of PS. In a similar fashion, a λ-carrageenan (Lambda-carrageenan) which are basically sulfated galactans has prevented DENV serotypes entry into human myelomonocytic cells (U937) and the human myelogenous erythroleukemic cells (K562). Significantly, the inhibitory action of carrageenan was stronger and higher during primary infection as well as antibody-dependent infection mediated by Fcγ-RII in both cells. Authors indicated that carrageenan was more active during viral entry than later steps of virus lifecycle [131]. PS are known for their specific interacting ability with virus particles and surfaces (Fig. 4). For example, a fucoidan obtained from Dictyota bartayesiana (DD) and Turbinaria decurrens (TD) has blocked the (HIV-1) viral entry in the PBMC (Peripheral blood mononuclear cells). The inhibitory mechanism was due to binding of PS with the HIV particle and neutralization of positively charged amino acid on the viral envelope glycoprotein (gp120) by their sulfate contents [127]. Hence the structural features of PS have an essential role in the prevention of virus attachment and initial entry process. Therefore the SARS-CoV-2 attachment and entry can be blocked by competitively inhibiting PS interaction mode established so far with other enveloped virus (Fig. 4) (Table 1 ) [137].
Table 1.
Sulfated polysaccharide from different sources and their mode of virus inhibition.
| S.No | Name of the Polysaccharide and Organism | Sources | Virus Inhibition mechanism | Type of Virus | References |
|---|---|---|---|---|---|
| 1 | Carrageenan Acanthophora specifira Hydroclathrus clathratus |
Marine red alga Brown alga |
Inhibition of propagation | Herpes simplex virus type 1 (HSV-1) and Rift valley fever virus (RVFV) | [104] |
| 2 | Grateloupia filicina | Marine macroalga | Inhibition of viral entry | Avian Influenza Virus (H9N2 subtype) | [105] |
| 3 | Ascophyllan A-Fucoidan S- Fucoidan Ascophyllum nodosum Fucus vesiculosus F8190 |
Marine brown algae | Inhibition of early step of viral infection | Human immunodeficiency virus (HIV-1) and vesicular stomatitis virus (VSV)-G-pseudotyped HIV-1 | [106] |
| 4 | Sulfated rhamnan Monostroma latissimum |
Marine green alga | Inhibition of invasion, replication and reduction of viral titers | Enterovirus 71 (EV71) | [107] |
| 5 | Sargassum naozhouense | Brown macroalga | Not specified | Herpes simplex virus HSV-1 strain F | [108] |
| 6 | Erylus discophorus | Marine Sponge | Prevention of viral attachment and entry | Human immunodeficiency virus HIV-1 | [109] |
| 7 | Laminaria angustata | Marine brown alga | Inhibition of virus attachment | Herpes simplex virus HSV-1 | [110] |
| 8 | Fucoidan and alginate Eisenia arborea Solieria filiformis |
Brown and red macroalga | Inhibition of virus penetration and reduction of syncytia formation | Measles virus | [111] |
| 9 | Porphyridium cruentum | Marine microalga | Inhibition of virus entry | Vaccinia virus and Vesicular stomatitis virus | [112] |
| 10 | Glucuronorhamnan Monostroma nitidum |
Green macroalga | Prevention of adsorption and blocking of the virus life cycle | Enterovirus 71 (EV71) | [113] |
| 11 |
Grateloupia indica, Scinaia hatei Gracilaria corticata Stoechospermum marginatum Cystoseira indica Caulerpa racemosa |
Red, brown and green macroalgae | Prevention of adsorption and internalization | Dengue virus (DENV) | [114] |
| 12 | Fucoidan Cladosiphon okamuranus |
Brown alga | Inhibition of syncytia formation and cell-to-cell spread of NDV | Newcastle Disease Virus (NDV) | [115] |
| 13 | P-KG03 Gyrodinium impudium |
Marine red microalga | Prevention of viral adsorption and internalization | Influenza type A virus | [99] |
| 14 |
Sphaerococcus coronopifolius Boergeseniella thuyoides |
Marine red algae | Inhibition of replication | HIV and HSV-1 | [116] |
| 15 | Fucosylated chondroitin sulfate (FuCS-1) Thelenota ananas |
Sea cucumber | Blocking entry and replication | HIV strains | [117] |
| 16 | Fucans Ascophyllum nodosum Fucus vesiculosus |
Brown macroalgae | Inhibition of adsorption and blocking fusion events | Influenza A/PR/8/34 virus H1N1 virus |
[118] |
| 17 | Ulvan Enteromorpha compressa |
Green alga | Inhibition of adsorption and penetration | HSV | [119] |
| 18 | Fucoidan Sargassum swartzii |
Marine brown algae | Reverse transcriptase inhibition activity | HIV-1 | [120] |
| 19 | Xylomannan sulfate Sebdenia polydactyla |
Red macroalga | Inhibition of replication and direct virucidal activity | HSV-1 | [121] |
| 20 | Ulvan Ulva pertusa |
Marine green alga | Inhibition of infection and replication | Vesicular stomatitis virus (VSV) | [122] |
| 21 | Calcium spirulan Arthrospira platensis |
Marine blue-green alga | Inhibition of virus entry | HSV-1 HIV-1 |
[123] |
| 22 | Nostoflan Nostoc flagelliforme |
Blue-green alga | Inhibition of virus binding to host cells | HSV-1 and HSV-2, human cytomegalovirus, and influenza A virus | [124] |
| 23 | A1 and A2 Cochlodinium polykrikoides |
Marine red microalga | Not specified | HIV-1, influenza virus types A and B, respiratory syncytial virus types A and B | [125] |
| 24 | Mucopolysaccharide (OKU40) Dinoflagellata Sulfated polysaccharide (OKU41) Pseudomonas |
Marine algae and marine bacteria | Inhibition of virus-cell fusion and viral adsorption. Suppression of syncytium formation. Inhibition of binding of HIV-1 to cells |
HIV-1 and HIV-2 HSV-herpes simplex virus type 1, influenza virus A and B, respiratory syncytial virus and measles virus |
[126] |
| 25 | Fucoidan Dictyota bartayesiana (DD) Turbinaria decurrens |
Marine brown macroalgae | Inhibition of propagation and proliferation | HIV | [127] |
| 26 | Polyguluronate sulfate | Marine brown algae | Inhibition of protein expression and transcription | Hepatitis B virus | [128] |
| 27 | Sulfated chitosan 36S | Artificially synthesized (fungi and shrimps) | Inhibition of viral entry and adsorption | Human papillomavirus | [129] |
| 28 | Iota-Carrageenan | Red macroalga | Prevention of virus binding, entry and replication | Human rhinovirus (HRV) | [130] |
| 29 | Agarans and carrageenan Acanthophora muscoides Gracila riabirdiae Solieria filiformis |
Macroalgae | Inhibition of virus adsorption and early viral replication | HSV-1 and HSV-2 | [103] |
| 30 | λ-carrageenan | Red Macroalgae | Inhibition of virus entry during both primary and antibody-dependent infection | DENV serotypes | [131] |
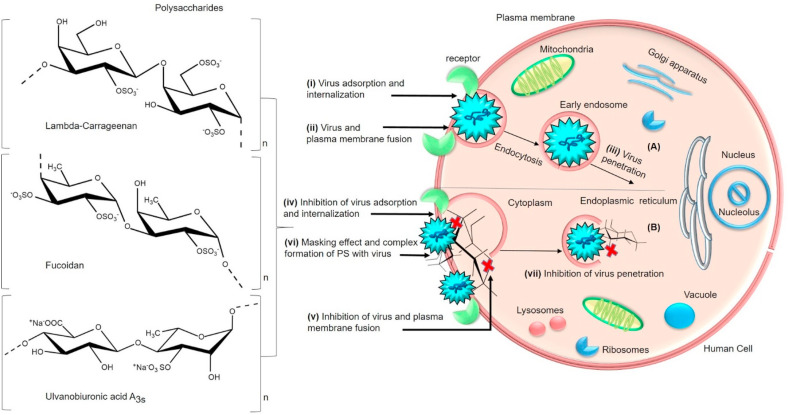
5.2. Prevention of virus adsorption, internalization and penetration
PS can directly enable its virucidal activity by forming a complex with virus parts that are possibly facilitated by the net negative charge of PS. As a result of firm binding to the virus, PS can alter the structure of viral components such as glycoproteins, leading to inactivation of the virus that will otherwise infect host cells [130,138]. The virus poses an electrostatic interaction to make the initial attachment process on the host cells and ensures a stable strong binding. PS can directly interact with the virus receptors and conceals the positive charges of host cells due to their negative charge, especially the sulfate content. Sulfate content and position in the PS has a crucial role in the antiviral activity. Such consistent masking effect eventually inhibits the virus adsorption and penetration into host cells (Fig. 5 ). For instance, heterofucans obtained from Ascophyllum nodosum and Fucus vesiculosus consisted of fucose as major sugar followed by glucuronic acid, mannose, xylose, galactose and glucose with molecular weight ranges from 26 kDa to 2482 kDa. Authors suggested that uronic acids, sulfate and fucose in the PS are responsible for enabling the sufficient degree of ionization that has inhibited the influenza A/PR/8/34 virus. Functional groups of PS blocked the virus adsorption [139,140] by suppressing the viral reverse transcriptase (RT) activity. Collectively the monosaccharides, sulfates and uronic acids have displayed strong antiviral effect by their direct interaction with the virus. Hence the PS can directly interact with virus particles and deactivates them by enabling strong antiviral responses [118]. In the study of PS fractions obtained from a red alga, Lithothamnion muelleri has displayed strong antiviral activity by inhibiting the early steps of HSV-1 replication in Vero cells. The structural analysis revealed that PS fractions are composed of monosaccharides such as galactose, glucose, xylose, mannose, rhamnose, arabinose, uronic acid (4.17–5.07%) and sulfate (8.94–11.70%) with molecular mass ranges from 43 to 60 kDa respectively. Authors conclude that the antiviral effect was effective mainly during adsorption and penetration of the virus [141]. In another example, a sulfated glucuronorhamnan (MWS) obtained from Monostroma nitidum comprised backbone of →3)-α-l-Rhap-(1→, →4)-β-d-GlcpA-(1→ and →2)-α-l-Rhap-(1→ unit. Additionally, rhamnose (88.83%) and glucuronic acid (11.17%) were their main monosaccharide residues. Moreover, their sulfate groups were located at C-4/C-2 of →3)-α-l-Rhap-(1→ and C-4/C-3 of →2)-α-l-Rhap-(1→ unit. The MWS was reported to bind with virus particles to inhibit the EV71 virus adsorption and retard the virus lifecycle by downregulating the host phosphoinositide 3-kinase/protein kinase B signalling pathway in Madin-Darby canine kidney (MDCK) cells [113]. According to the demonstration of several reports, the antiviral effect of PS is mostly contributed by their molecular weight [88,142,143]. This fact is exemplified by the sulfated galactans, sulfated xylomannans and sulfated fucans composed of sulfate, uronic acid and monosaccharides such as galactose, glucose, arabinose and xylose, mannose, rhamnose in different amounts and traces have displayed the inhibition of virus adsorption and internalization. The molecular weight of these polysaccharides fractions was in the range of 30 kDa–60 kDa and suggested to influence the anti-DENV activities. Especially sulfate groups that are present at C2 or C4 position linked to respective backbone of PS have played an essential role in exerting antiviral activity [114]. Hence the position of sulfate has a crucial role in exerting strong antiviral activity.
Fig. 5.
(A) General schematic representation of the virus lifecycle post attachment into the host cell- (i) Virus adsorption and internalization after the attachment to the receptor (ii) initiation of fusion event by the virus with plasma membrane of the host cell via endocytosis and its transportation/translocation to the cytoplasm leading to (iii) virus penetration [225,226]. (B) Mode of antiviral action of sulfated polysaccharides (Lambda-carrageenan, Fucoidan, Ulvanobiuronicacid A3s) (iv) inhibition of virus adsorption and internalization (v) inhibition of membrane fusion (plasma membrane) and (vii) inhibition of virus penetration by the interaction of polysaccharides that confers (vi) masking effect, complex formation and destabilizing of fusion peptides (Table 1). (Section 5.2).
Based on the reports, uronic acid contents in the PS have a significant contribution to biological activities due to the enrichment of carboxylic acids. These anionic groups of uronic acids can elevate the acidic nature of PS [106,144] by enhancing the negative charge that in turn boosts their binding ability to the virus [145]. By this fact, the presence of uronic acids has boosted the antiviral activities of several PS. Fucoidan from marine alga Cladosiphon okamuranus has strongly inhibited the infection and binding of viral strain ThNH-7/93 to BHK-21 cells. The authors confirmed that glucuronic acid residues have played an essential role in the structure-based study on antiviral activity. The rationale is that the positive charge of a few basic amino acid residues on ThNH-7/93 strain has specifically interacted with glucuronic acid residue of the fucoidan. Additionally, fucoidan has shown anti-dengue virus activity by directly binding to the envelope glycoprotein (EGP) on DEN2 which is attributed to their sulfate content. Hence uronic acid and sulfate contents in PS play a crucial role during the interaction with virus particles [101]. For example, a sulfated polysaccharide p-KG03 from gyrodinium impudium composed of homogenous galactose residues with uronic acid and sulfate groups have inhibited mainly viral adsorption and internalization by direct interaction with virus particles [146]. It was reported that Ulvan (SUF1) obtained from Enteromorpha compressa composed of rhamnose, glucuronic and iduronic acid, xylose, glucose, galactose and sulfate(6%) with molecular weight (34 kDa) has exerted strong anti-herpetic activity due to higher sulfate content (22%) achieved by chemical modification. These molecular characteristics have hindered virus adsorption and penetration into human larynx epithelial cells carcinoma (HEp-2) [119]. Therefore, the structural features including monosaccharides, acidic groups, uronic acids and molecular weight of PS are capable of inhibiting events such as endocytosis and virus internalization to prevent further virus penetration in the cells (Fig. 5) (Table 1) [147,148]. Based on these facts and pieces of evidence, PS can interact with SARS-CoV-2 with a similar fashion of complex formation or by direct interaction and prevent their adsorption or internalization into cells.
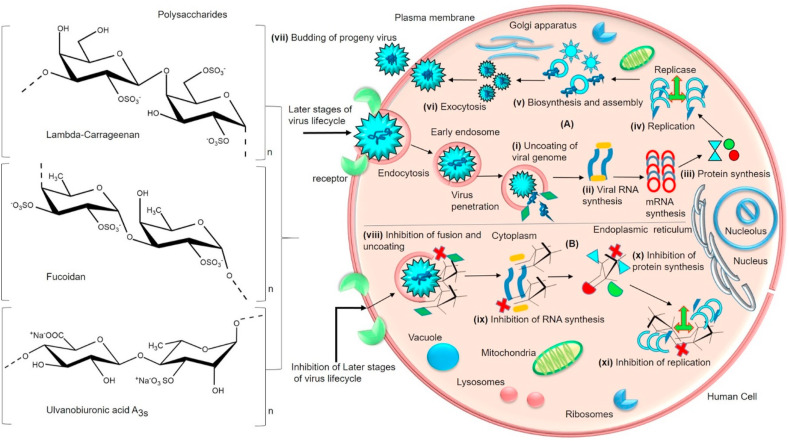
5.3. Inhibition of membrane fusion, virus uncoating, transcription and replication
The virus can internalize itself through host endocytosis which then is transported to secondary organelles and reaches the cytosol. After the internalization, the virus penetrates via membrane fusion and extends its intracellular transport in the cytoplasm (Fig. 6 ). Based on the mounting shreds of evidence, several PS have the potential to hinder the fusion events that occur between viral and host cell membranes. Apparently, PS can inhibit the membrane fusion activity during virus-host cell interaction by interfering with membrane proteins responsible for fusion events. Specifically, PS can bind to fusion proteins and inactivate them by declining their hydrophobic properties. Moreover, PS are capable to bind with sugar groups linked to the polypeptide chains of the virus thereby preventing their penetration [117,[149], [150], [151], [152]]. In a study of sulfated PS (ulvan and fucoidan) obtained from green algae Ulva clathrata and Cladosiphon okamuranus fucoidan have demonstrated the inhibition of fusion in Vero cells. The structure of ulvan consisted of sulfated rhamnose and glucuronic acid, iduronic acid, xylose and glucose, galactose in lower proportions. The antiviral activity has been attributed to high molecular weight 359,800 g mol−1 and the direct interaction of PS to a fusion protein of Newcastle disease virus (NDV). Besides fucoidan composed of sulfate, fucose, glucuronic acid and traces of xylose with a molecular weight of 92.1 kDa. It was reported that sulfate and uronic acids have been essential factors for antiviral activities [153]. A fucoidan obtained from Cladosiphon okamuranus has inhibited syncytia formation by interacting with the viral fusion protein and prevented cell to cell spread of Newcastle Disease Virus [115]. It was observed that curdlan sulfate (CRDS), a sulfated 1→3-β-D glucan (41 kDa) has prevented the entry and attachment of DENV to LLC-MK2 cells and blocked the viral fusion with host vesicular membranes which eventually halted the release of the viral genome into the host cytoplasm. Unfortunately, PS was unable to inhibit replication of DENV sub-genomic replicon. Additionally, PS restricted syncytia formation and cell-to-cell infection efficiently. The authors indicated that PS has interfered with the viral binding and membrane fusion steps that blocked further replication. Sulfate and high molecular weight have been significant attributes for efficient binding and prevention [154]. All of these results suggested that PS can interact with fusion proteins and destabilize the hydrophobic properties that protect the cells from viral infection. Thus PS, when exposed to fusion peptides, can exhibit an anti-fusion effect in the cells (Fig. 6) (Table 1) [155]. Hence PS can interact with fusion peptides of SARS-CoV-2 and inhibit their membrane fusion that orchestrates the viral penetration and genome uncoating process.
Fig. 6.
(A) General schematic representation of later stages of virus lifecycle- (i) partial uncoating of virus particles takes place in the cytosol/nucleus. Initiation of (ii) transcription (iii) translation and (iv) replication resulting in the (v) biosynthesis and assembly of new virions (vi) virus release through exocytosis and (vii) budding of progeny virus [[227], [228], [229]]. (B) Mode of antiviral action of sulfated polysaccharides (Lambda-carrageenan, Fucoidan, Ulvanobiuronicacid A3s) (viii) inhibition of endosomal membrane fusion and virus uncoating (ix) inhibition of RNA genome and (x) protein synthesis. (xi) inhibition and deactivation of enzymes responsible for replication by the interaction of polysaccharides (Table 1). (Section 5.3).
According to various demonstration, the virus genome that is highly condensed by the proteins and membrane bilayers has to be uncoated at the nucleoplasm to replicate in the host cell. In such a case, PS can interfere with the uncoating process and retard the stepwise allosteric process due to their strong polyanionic features. During the interaction, PS can decrease the protein synthesis of the virus in the cells and ensure specific binding to the replicating enzymes which in turn arrests the initiation of replication [[156], [157], [158], [159], [160]]. An investigation of a xylomannan derived from a red seaweed Sebdenia polydactyla has inhibited HSV-1 viral replication in Vero cells. The antiviral effect was attributed to the anionic features and structural characteristics of PS which consisted of 0.6 sulfate groups per monomer unit (mannose and xylose) with the backbone of α-(1 → 3)- linked d-mannopyranosyl residues substituted at position 6 with a single stub of β-d-xylopyranosyl residues and molecular weight of 150 kDa. Besides, the degree of sulfation from 1.0 to 1.6 in the PS fractions have exhibited higher antiviral activity by preventing virions attachment to the cells [121]. Therefore the certain chemical modification can amplify the antiviral capacity of PS.
Lopes et al., [2017] reported that Ulvan from Enteromorpha compressa has inhibited the DNA replication and transcription by downregulating HSV protein synthesis in the human epithelial type 2 cells (HEp-2). Authors indicated that anti-herpes simplex virus activity of PS has interfered with later steps of virus replication [119]. This implies that PS can obstruct the virus replication and suppress the viral nucleic acid synthesis through various modes of inhibition. Investigation of calcium spirulan obtained from Spirulina platensis that was made of rhamnose, ribose, mannose, fructose, galactose, xylose, glucose, glucuronic acid, galacturonic acid, sulfate and calcium with a molecular weight of 2.6 × 105 and 3.1 × 105 kDa has displayed significant antiviral activity. Noticeably PS was more active towards enveloped viruses during inhibition of penetration and replication than non-enveloped viruses. Their results inferred that sulfate content had a major influence on the antiviral effect against HIV-1, HSV-1, and HCMV (human cytomegalovirus) in the cells (HeLa, human embryonic lung (HEL), green monkey kidney-Vero, MDCK), and MT-4 cells) [123]. Another investigation of low molecular-weight sulfated derivative, namely polyguluronate sulfate (PGS) composed of 2, 3-O-disulfated-1, 4-poly-l-guluronic acid with about 1.5 sulfates per sugar residue has inhibited the replication of hepatitis B virus (HBV) and the expression of Hepatitis B antigens (HBsAg and HBeAg) in HepG2.2.15 cells. It was clearly inferred that HBV release and replication in the cells were inhibited through upregulation of NF-κB and Raf/MEK/ERK pathways to enhance the interferon system. Besides, PGS has stimulated the production and secretion of interferon beta (IFN-β) in the cells [128]. In the present study, carrageenan with 21.14% of sulfate derived from Solieria filiformis against Measles virus (MeV), exerted higher antiviral activity by inhibiting post-binding events that take place after the viral adsorption. Besides another PS obtained from Eisenia arborea consisted of fucoidan, alginic acid with 12.85% sulfate and uronic acid has exhibited the best antiviral activity before viral infection. As a result of their study, the authors suggested that both these PS have strong potential to hinder the viral penetration, adsorption and syncytia formation thereby inhibiting the viral replication [111]. These results confirm that sulfated PS tend to occupy the cellular sites where the virus utilizes for their intracellular signalling mediated virus propagation and replication. According to the above-mentioned results, structural features of PS have a significant contribution towards an antiviral activity that can prevent transcription; protein translation; deactivate the replicating enzymes and initiation of replication (Fig. 6) (Table 1) [161]. However, more in vitro and in vivo structure-activity studies should be carried out by exploiting the structurally efficient sulfated PS against SARS-CoV-2.
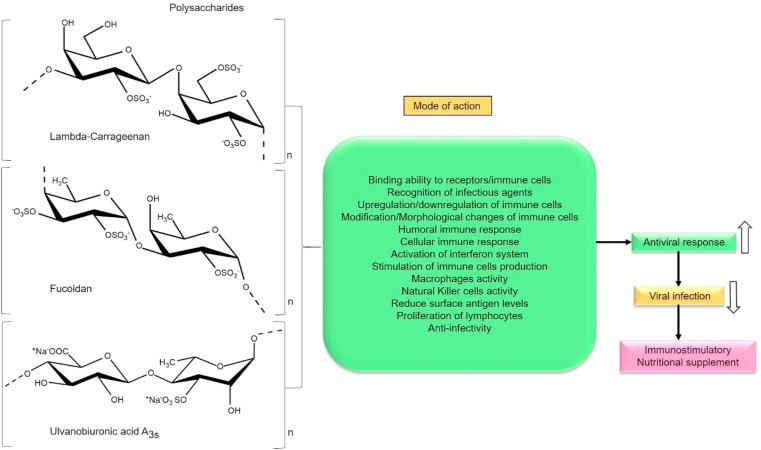
6. Activation of host immune system
Host immune response serves as an essential mechanism against the development of viral infections and recruits immune cells to suppress the infection. Interaction among various immune cells, mediated by a complex network of cytokines and chemokines enforce antiviral effect. The release of cytokines, interferon-gamma (IFN-γ), interferon-alpha (IFN-α), tumor necrosis factor-alpha (TNF-α) can induce an antiviral state in cells [162]. Significantly, CoVs enable immune evasion mechanisms that cause a delay in the activation of interferon systems; supply of immune cells that eventually leads to deregulation of the immune cell function (CD8+ and CD4+ cells) responsible for antiviral effect [163]. As a result of viral infection, the lymphocytes levels are reduced and inflammatory cytokines are higher in COVID-19 patients. This abnormal excess cytokine production leads to the severity of the infection and causes death. The hyperinflammatory state of the host can induce hyperferritinaemia, cytopenia and a higher ratio of other immune cells which in turn embarks severe damages to host cells [164]. Any compound that is capable of regulating (upregulation/downregulation) the immune system either through enhancement or suppression of host responses can be considered as immunomodulators. Based on various studies, PS have a major contribution in activating the immune system. Moreover, they elicit broad immunomodulatory activities through various mechanisms reported so far. Among them, PS can reduce surface antigen levels of virus, stimulate phagocytosis, activate host macrophages, B lymphocytes, T lymphocytes and Natural Killer cells, and enables the secretion of antibodies, cytokines, and complement molecules (Fig. 7 ). Especially PS activates interferon system (type1) of host thus recruits stronger immune reaction against viral infection [[165], [166], [167]]. Sun et al., [2018] investigated the immunomodulatory effects of xylogalactomanans (CLGP4) isolated from green seaweed Caulerpa lentillifera that is composed of xylose, mannose and galactose with a molecular weight of 3877.8 kDa and a minor amount of uronic acids (2.37%) and high sulfate content (21.2%). According to the results, PS exhibited strong immunoregulatory activity by enhancing the proliferation of macrophages, phagocytosis, increased NO production and phosphatase activity in macrophages. Their results suggested that the monosaccharide composition, sulfate content and ultrastructure of PS are involved in the immunostimulation, especially sulfate group had influenced the binding of PS with surface receptors of RAW 264.7 cells through hydrogen bonding and electrostatic interactions [168]. Another example of sulfated PS extracted from red algae Gracilariopsis lemaneiformis composed of uronic acid 6.0%, 3, 6-anhydrogalactose 12.9% and sulfate 29.3% with a molecular weight of 2856 Da has increased T-lymphocytes proliferation and reduced B- lymphocytes proliferation in-vitro. It was inferred that PS can modulate immune cell proliferation that is beneficial for both immune suppression and activation (Fig. 7). Further, the PS has shown good superoxide scavenging due to their electron density at carbon atoms in the heterocyclic ring of PS [169]. The study involved in the assessment of immunologically active PS namely UPP-2 extracted from Undaria pinnatifida which is composed of abundant uronic acid (13.0%), low sulfate content with xylose (64.55%), glucose (23.81%), arabinose (5.90%) and mannose (4.26%) with a molecular weight of 1035.52 kDa that includes the glycosidic linkage of →2)-α-D-Xylp-(1→, →4)-α-D-Glcp-(1→, α-D-Xylp-(1→ and →2,4)-β-D-Xylp-(1 →, it has stimulated the proliferation and pinocytic capacity of RAW264.7 cells and upregulated the mRNA expressions of inducible nitric oxide synthase (iNOS), TNF-α, IL-6 and IL-1β. Additionally, a significant increase in the secretions of nitric oxide (NO), TNF-α and IL-6 were observed. The results suggested that a higher amount of glucose, mannose, xylose and their glycosidic linkage have played an essential role in the immunostimulatory activity of PS. It was inferred that especially monosaccharides have significantly facilitated the binding activity of PS to pattern recognition receptors (PRRs) such as Toll-like receptors [170]. According to these above-mentioned reports, PS can be efficiently recognized by these type of cellular receptors due to their broad structural features to elicit immunomodulatory activities. Antivirals that induce cellular synthesis of interferons have been recommended as an effective strategy to combat COVID-19 [171]. Since PS are a rich source of polyanions, it can stimulate the production of interferons in the host cells thereby promoting immune response against viral infection. Despite these evident reports, many robust investigations of sulfated PS against SARS-CoV-2 are necessary to reveal their immunomodulation potential. Hence PS can be a suitable interferon inducers, natural immunomodulatory drugs or food supplements for the treatment of COVID-19 due to the efficient immune activation and modification of cellular signalling mechanisms that provokes higher antiviral responses in the cells.
Fig. 7.
Schematic representation of sulfated polysaccharides (Lambda-carrageenan, Fucoidan, Ulvanobiuronicacid A3s) influencing various modes of immune activation/modulation (Section 6).
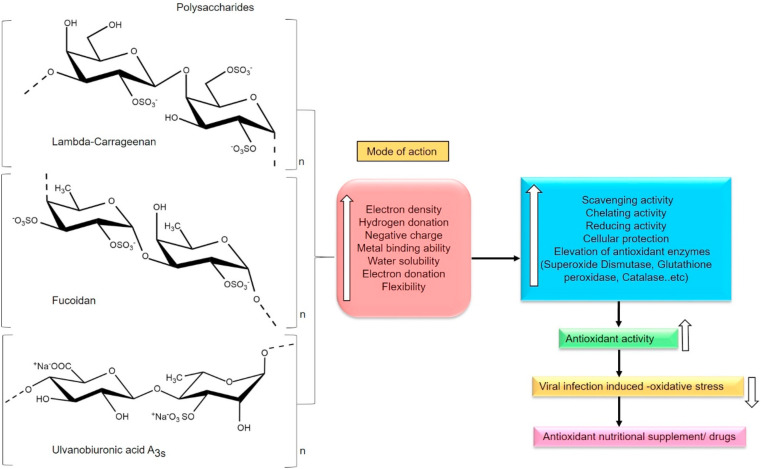
7. Antioxidant defense and protection
ROS (Reactive oxygen species) are byproducts of cellular metabolism. During viral infection, individuals are at higher risk of free radical-induced pathogenicity. Especially, oxygen radicals and nitric oxide can cause oxidative damages to the tissues and reduce the host immune responses drastically. Any drop in the antioxidant levels can weaken the function and solidity of the host immune system. Hence antioxidants are crucial in the viral pathogenesis [172]. More shreds of evidence suggest that patients affected by any type of RNA viruses can undergo chronic oxidative stress. Relatively, several changes can occur in the host antioxidant defense system, especially in both enzymatic and non-enzymatic antioxidants. Moreover, RNA viral infection mediated oxidative stress can induce apoptosis (cell death), loss of immune-related functions, virus replication, increase lipid peroxidation in tissues, loss of body weight, exhaust the micronutrients and elevates free radical generating enzymes. Significantly ROS (as signalling molecules) along with virus provokes severe damages to the proteins, mitochondrial DNA and alters cellular signalling pathways [173,174]. Similarly, SARS-CoV-2 is a positive-sense single-stranded RNA virus, which has a high mutation rate that causes its escape from host immunity and shows drug resistance so far [175]. As per the evidence, SARS-CoV-2 can cause severe oxidative stress especially to the tissues of the vital organs [176]. Marine sulfated PS are good sources of antioxidants for their effective scavenging and chelating potential in various applications. The diverse structural features of PS renders desirable physicochemical properties, efficient biological properties and functional behaviors. The structural features of PS have shown remarkable antioxidant activities and reduced oxidative stress in various diseases thus far. Several of them are utilized as protectants, preservatives, additives, thickeners, stabilizers and emulsifiers in various pharmaceutical and food applications [[177], [178], [179]]. It has been suggested that marine algal antioxidants have potent antiviral activity against several viruses. Moreover, they have the potential to promote cellular survival against different stages of viral infections [180]. Sulfated PS are complex macromolecules that can interact with eukaryotic cellular proteins due to their complex structure that has abundant polyanions. Recently PS represents a new approach for preventing the free radical generation in several biomedical and food industrial applications especially due to their reliable structure-activity relationships [181]. In the study of PS derived from a marine alga Solieria filiformis which is an iota-carrageenan with a molecular weight of 210.9 kDa and high sulfate content has exhibited strong scavenging actions over DPPH and chelating activity of ferrous ion. Besides, PS has protected gastric cells of mice against the ethanol-induced damage; prevented glutathione consume; reduced malondialdehyde (MDA) and hemoglobin levels in gastric mucosa [182]. Another study of three different fractions of PS obtained from marine diatom namely Navicula sp. is composed of glucose, galactose, rhamnose, xylose and mannose with molecular weight 17, 107 and 108 kDa have shown higher DPPH and ABTS scavenging activity. The antioxidant activity was attributed to the molecular weight and sulfate content of PS [183]. It is a well-known fact that PS are good elevators of intracellular antioxidant enzymes and protectors of cells based on the accumulation of several reports (Fig. 8 ). This was inferred from a novel agar-type galactans produced by Gracilaria caudata composed of 3,6-α-l-anhydrogalactose (LA) and β- d-galactose attached to LA and sulfate groups at C-6 of galactose residues with a molecular weight of 116.51 kDa which has exhibited good chelating activities. Additionally, PS has elevated cellular antioxidant enzymes such as Catalase (CAT) and superoxide dismutase (SOD) levels in the rats treated with 2,2′-azobis(2-methylpropionamidine) dihydrochloride (ABAP). The research results suggested that antioxidant potential was due to nucleophilic nature of the free electrons that belongs to hydroxyl and sulfate groups [184]. Another important antioxidant function of PS is to reduce the intracellular ROS generation and protect the cells from ROS damages (Fig. 8). This was inferred from a fucoidan composed of fucose, galactose, mannose, xylose and glucuronic acid with high sulfate content from a marine algae Chnoospora minima which had shown high DPPH and alkyl radical scavenging activities. Significantly, PS has exhibited high AAPH (2,2′-azobis(2-amidinopropane) dihydrochloride and H2O2 scavenging activities in Chang liver cells thereby reducing intracellular ROS production. The antioxidant properties were enhanced by sulfation and influenced by the structural features of PS [185]. In the present study, a sulfated polysaccharide extracted from a red seaweed Porphyra haitanensis that consisted of galactose and 3,6-anhydrogalactose with a backbone of → 4–3,6-anhydro-α-l-galactopyranose-(1 → 3)-β-d-galactopyranose, sulfate 3.8%, hydroxyl groups and molecular weight of 2.5 × 105 Da has displayed effective ABTS (2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) scavenging activity and moderate DPPH activity. However, scavenging abilities are attributed to their structural complexity (Fig. 8) [186]. Hence, PS as potential radical scavengers [187,188] can attenuate the severity during viral infections as antioxidants. Due to their structural features like several electron-donating and electron-withdrawing functional groups, a variety of monosaccharides which are rich in aldehyde groups, uronic acids can provide effective antioxidant activity and reduce intracellular ROS production. Significantly, PS can elevate the intracellular antioxidant status of the host to prevent the tissue injuries caused by lipid peroxidation [[189], [190], [191]]. Collectively these results suggest that PS can reduce oxidative stress, maintain homeostasis and protect the cells. Therefore PS can be a suitable source for the therapeutic application against viral infection-induced oxidative stress either as a drug or antioxidant food supplement. However, more cell line and animal studies are required to investigate the promising antioxidant effects of sulfated PS against viral infection-induced oxidative stress.
Fig. 8.
Schematic representation of sulfated polysaccharides (Lambda-carrageenan, Fucoidan, Ulvanobiuronicacid A3s) influencing their antioxidant activity (Section 7).
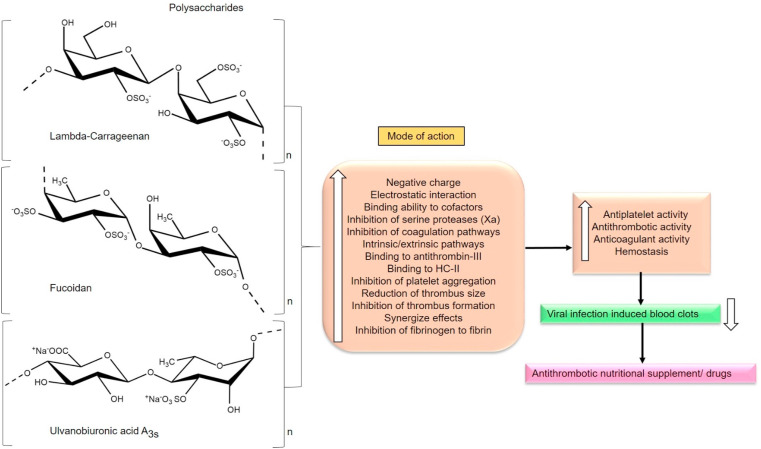
8. Antithrombotics and anticoagulants
Apart from severe lung and blood vessel damages, the autopsies revealed unusual blood clots (micro clots) in the lung region that seem to cause low oxygen levels and can cause death in the COVID-19 patients. Moreover, it has been suggested to use blood thinners to subside the clots for all the COVID-19 patients, including the mild cases [192,193]. The reason for clot formation is still unclear and therefore an area for intense investigation. Recently researchers from Aurora have disclosed that COVID-19 patients are at high risk of blood clot formation that can eventually cause clot-related complications like stroke, venous thrombosis and renal failure. Normal blood flow in the lungs was restricted by the accumulation of blood clots. Due to this fact, oxygen supply to the tissues of the host can be diminished and embarks hypoxic conditions in organs which then leads to oxidative stress and malfunction of organs. However, the use of blood thinners has been discussed [194,195]. On the other hand, platelet aggregation is a critical event in thrombosis and is necessary for effective hemostasis at the sites of vessel/vascular injury. Arterial thrombosis results from the clot formation induced by atherosclerotic plaques, platelet aggregation, and thrombus forming collagen and tissue factors, vessel occlusion and ischemic stroke. Agents that inhibit the events of platelet aggregation and the signalling pathways that are orchestrated by the various aggregating agents can be relied on for use as antiplatelet agents [196].
Literature reveals that marine PS can serve as effective antiplatelet, antithrombotic and anticoagulant agents due to their biofunctional efficacies and similarities related to Heparin (HP), the first approved anticoagulant. PS (sulfated fucans, sulfated galactans and GAGs) can inhibit both venous and arterial thrombosis. The rationale is that anionic PS can interact with cationic proteins (Cofactors) responsible for coagulation cascade resulting in the complex formation due to the electrostatic interaction. Especially, sulfated low molecular weight PS have greater influence over resisting the clot formation (coagulation and platelet aggregation). Some common mechanism of PS can block/inhibit thrombin activity, activate anti-thrombin III, delay the clot formation by either or both intrinsic and extrinsic pathways (Fig. 9 ). Both antithrombin III (ATIII) and Heparin cofactor II (HCII) are two main heparin dependent thrombin inhibitors present in the human plasma. Notably, the anticoagulation activity of PS can be enhanced through modifications such as sulfation, reduction or oxidation [[197], [198], [199]].
Fig. 9.
Schematic representation of sulfated polysaccharides (Lambda-carrageenan, Fucoidan, Ulvanobiuronicacid A3s) influencing their antithrombotic and anticoagulant activity (Section 8).
In the study, PS obtained from a red alga Gelidiella acerosa has exhibited good antithrombotic and antiplatelet effects in the rats by interacting with the blood coagulation system and hemostasis. The structure consisted of β-d-galactose (37.2%), α- Lanhydrogalactose (41.7%) and 6-O-methyl-β-d-galactose (20.9%) and sulfate groups with a molecular weight of 284.8 kDa which is basically a sulfated agaran. The results suggested that sulfate content and structural conformation have influenced the inhibition of thromboplastin-induced thrombus formation without hemorrhage [200]. The serine protease factor Xa has the main role in coagulation and platelet activation. This Xa is an enzyme that serves for both extrinsic and intrinsic coagulation pathways. This Xa combines with factor V to generate prothrombinase (Xa-Va) which then initiates the clot formation via the conversion of prothrombin to thrombin [201]. In such a case, the thrombus formation can be decreased by the complex formation of PS with antithrombin-III that mediates the enzymatic inhibition of coagulation factors (Xa) with possible coagulation pathways. For example, a novel PS (MS-1), a sulfated heterorhamnan extracted from a marine green alga, Monostroma nitidum made up of 4-linked β-d-xylose, 4-/6-linked d-glucose, terminal β-d-glucuronic acid, and 3-/2-linked α-l-rhamnose with a molecular weight of 79.8 kDa has displayed strong anticoagulant activity both in vitro and in vivo. Additionally, PS inhibited thrombus formation in vitro and delivered strong inhibition of coagulation and platelet aggregation in carotid artery thrombosis in vivo. Significantly, PS has synergized the inhibitions of thrombin and coagulation factor Xa by heparin cofactor-II and antithrombin-III respectively. Hence these results confirm that PS has interacted with both the intrinsic and/or common pathways of coagulation; prevented thrombin activity or conversion of fibrinogen to fibrin and inhibited them eventually. As reported by authors, these blood-thinning properties and specific interactions of PS are facilitated by the molecular size, charge density, sulfate position and the linkage pattern of rhamnose residues [202]. According to various demonstration, the PS can delay the coagulation time and reduce the thrombus formation. In their study, Reis et al., [2020] reported that the PS of Ulva lactuca L is an ulvan composed of rhamnose as the major component and other monosaccharides such as glucose, galactose, uronic acid, sulfate groups have possessed good blood-thinning properties. One fraction of PS namely F50U1 had a molecular weight of 185.28 kDa and is enriched with ulvanobioronic acid. The suggested mechanism of in vitro anticoagulant activity of this F50U1 is involved by extending the plasma coagulation time whereas the anti-Xa activity is enabled by the inhibition of factor Xa and IIa activity. The results indicated that the PS fraction has inhibited all the coagulation pathways by their possible interaction. Moreover, the venous thrombus formation was inhibited by the association of PS to the anticoagulant pathway mediated by antithrombin III (Fig. 9) [203]. Altogether, the blood-thinning properties of these PS were attributed to their interaction of negative charges with positive charges of peptide sequences in the coagulation system. Therefore these PS might be a hopeful source to prevent blood clot formation induced by viral infection through various mechanism either as drugs or functional food that benefits the recovery of patients who are vulnerable to intense clot formation.
9. Adjuvants in the vaccine
Vaccination is a powerful weapon against the mortality of the infectious disease and promotes life anticipation. After constant and untiring efforts, several vaccines have been produced against Human PapillomaVirus (HPV), hepatitis C virus (HCV), Chikungunya Virus (CHIKV) and Influenza (Flu) virus. The development of vaccines for CoV has been critical due to their nature and mutation of strains that reside in the host [204]. Adjuvants are the bioactive substance that is added to the vaccine in order to promote immune responses along with the vaccine antigens. The addition of adjuvants enhances the efficacy of vaccines by enabling long term immunological memory and protection of the immune system. Adjuvants (polysaccharides, cytokines, saponins, liposomes etc.) are ingredients that can induce or amplify a stronger immune response along with purified antigens in the vaccines; stimulate subtype antibodies production and complement activation. PS, which function as adjuvants, have promising effects for promoting antigen-specific immune response and enhance host immunity due to their polydispersity. The high molecular weight of PS plays an essential role in eliciting immunomodulation than low molecular weight PS. Reports suggest that sulfated PS have strong immune-stimulatory activity and are suitable as vaccine adjuvants [[205], [206], [207]]. PS are nontoxic, non-mutagenic, biodegradable, and biocompatible that can activate humoral and cellular immunity, elicit mucoadhesion, boosts antigen absorption, enhances residual time at mucosal sites, sustained release, promotes cellular antigen uptake and improves mucosal immunity [[208], [209], [210]]. Sulfated PS can provide a significant approach towards producing therapeutic vaccines due to their desired physicochemical properties and structural features that are readily suitable for modifications. Based on the reports, fucoidan possessed the best adjuvant qualities that can be adopted for future vaccine preparations since enables strong cell-mediated and humoral immune responses [211]. Moreover, these PS can be utilized for carbohydrate-based conjugate vaccine preparations to achieve desirable immunogenicity and efficacy. Carrageenan has enhanced the peptide vaccine potency by stimulating E7-specific CD8+ T cell (antigen-specific) immune response by activating the TLR4 pathway and antitumor activity in the mice vaccinated with human papillomavirus type 16 (HPV-16) [212]. In the assessment of vaccine adjuvant and antitumor effect of λ-carrageenan, it has inhibited tumor progression and increased the production of M1 macrophages, dendritic cells and CD4+CD8+ T lymphocytes in melanoma B16–F10 and mammary cancer 4T1 bearing mice. Additionally, it has stimulated Interleukin 17A (IL17A) and Tumor Necrosis Factor-alpha (TNF-α) in tumor. Their results demonstrated that λ-carrageenan has increased the efficiency of ovalbumin (OVA) based preventative and therapeutic cancer vaccine which stimulated anti-OVA antibody production in mice that was injected by (OVA)-expressing E.G7 cells. Therefore PS can elicit adjuvant effects by immunostimulating cells and increase the production of proinflammatory cytokines [213]. Especially chitosan has been suggested as potential adjuvants [214] for promoting vaccine immune effect that stimulates humoral and cell-mediated immune responses, especially in RNA virus vaccines. Authors reported that chitosan has reduced the respiratory syncytial virus (RSV) infection in mice and enhanced the antigen-specific immune responses [215]. Thus, PS can be a potential adjuvant candidate in the preparation of antiviral vaccines for SARS-CoV-2 infection. However more in vivo studies and verifications are required to achieve effective vaccines against CoVs.
10. Conclusion
Currently, there is no FDA approved drug to prevent deadly SARS-CoV-2 infection and standard treatment procedure is still lacking. Besides oxygen supplement, mechanical ventilation and symptom suppressing clinical management are being primary supportive care for hospitalized COVID-19 cases. Lack of plasma donors, tougher job to convince donors and lesser population recovery from plasma-treated COVID-19 have been a major crisis to accomplish further plasma therapy. Repurposing of previously reported clinically approved antivirals have been under rapid clinical trials. Subsequently, most of the drugs have been found ineffective after their initial clinical trials and reported to have few adverse effects during treatments. Recently, remdesivir, hydroxychloroquine, lopinavir, favipiravir and interferon regimens are considered ineffective antivirals for the mitigation and treatment of COVID-19 due to their incomplete action against the infection and side effects. Researchers are focusing on targetable cellular processes to prevent the virus entry and further replication in the host cells using already approved antivirals. However multiple in vivo and clinical trial data are required to conclude the anti-CoV effects of repurposed drugs. To resolve the drug precariousness, it is essential to entail the existing or already reported natural marine polysaccharides, especially polyguluronate sulfate, chitosan, carrageenans, sulfated galactans, sulfated rhamnans, alginates, fucoidans, fucans, ulvan and related polysaccharides for further investigation on animal studies and clinical trials against SARS-CoV-2. They have potential antiviral effects by interfering with the life cycle of the virus which makes them have a great application prospect in the prevention of SARS-CoV-2 entry and replication. Especially sulfated PS can strongly prevent the virus attachment and entry by devitalizing the viral proteins responsible for efficient binding process either through direct interaction or complex formation. Also, sulfated PS are capable of modifying the structural characteristics and hydrophobic features of viral proteins responsible for fusion, penetration and replication. In accordance with these facts, sulfated PS are biocompatible, non-toxic, chemically modifiable that can amplify the higher antiviral activities through efficient binding abilities and interactive anionic features. Hence the electron-donating/withdrawing functional groups especially sulfate and carboxyl, monosaccharides, uronic acids, high molecular weight, sulfate pattern of PS have a major influence in intensifying the antiviral activities and activation of host immune responses. According to the importance of antiviral and immune-stimulatory effects of PS in combatting viral infections, clarifying the exact mechanism of actions against SARS-CoV-2 could pave the way for new antivirals and treatments. The multifarious activities of sulfated PS can be exploited for COVID-19 treatment as candidate drug, adjuvants in vaccine preparation, combination with other antivirals, nutrition for enhancement of host immunity, blood thinners, and formulation of antioxidant based functional foods and production of biosafety antiviral materials in healthcare products (Gloves, sprays, masks, handling tools).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors acknowledge the host institution for providing the necessary support.
References
- 1.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Di Jiang R., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam T.T.Y., Jia N., Zhang Y.W., Shum M.H.H., Jiang J.F., Zhu H.C., Tong Y.G., Shi Y.X., Ni X.B., Liao Y.S., Li W.J., Jiang B.G., Wei W., Yuan T.T., Zheng K., Cui X.M., Li J., Pei G.Q., Qiang X., Cheung W.Y.M., Li L.F., Sun F.F., Qin S., Huang J.C., Leung G.M., Holmes E.C., Hu Y.L., Guan Y., Cao W.C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 3.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law S., Leung A.W., Xu C. Severe acute respiratory syndrome (SARS) and coronavirus disease-2019 (COVID-19): from causes to preventions in Hong Kong. Int. J. Infect. Dis. 2020;94:156–163. doi: 10.1016/j.ijid.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazafa A., ur-Rahman K., ul Haq I., Jahan N., Mumtaz M., Farman M., Naeem H., Abbas F., Naeem M., Sadiqa S., Bano S. The broad-spectrum antiviral recommendations for drug discovery against COVID-19. Drug Metabol. Rev. 2020;52:408–424. doi: 10.1080/03602532.2020.1770782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S., Zhi K., Mukherji A., Gerth K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses. 2020;12:486. doi: 10.3390/V12050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Ju L., Zhang J., Wang X. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020:2020. doi: 10.1101/2020.03.17.20037432. 03.17.20037432. [DOI] [Google Scholar]
- 8.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of covid-19 — final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Públic. 2020;44:1. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer A.M.S., Guerrero A.J., Rodríguez A.D., Taglialatela-Scafati O., Nakamura F., Fusetani N. Marine pharmacology in 2014-2015: marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities; affecting the immune and nervous systems, and other miscellaneous. Mar. Drugs. 2019;18:5. doi: 10.3390/md18010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang X., Luo D., Luesch H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019;147:104373. doi: 10.1016/j.phrs.2019.104373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagar S., Kaur M., Minneman K.P. Antiviral lead compounds from marine sponges. Mar. Drugs. 2010;8:2619–2638. doi: 10.3390/md8102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogineni V., Schinazi R.F., Hamann M.T. Role of marine natural products in the genesis of antiviral agents. Chem. Rev. 2015;115:9655–9706. doi: 10.1021/cr4006318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.pdf F. Apps. Exploring the ocean for new drug developments: marine pharmacology. J. Pharm. BioAllied Sci. 2016;8:83–91. doi: 10.4103/0975-7406.171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.T Che C. Marine products as a source of antiviral drug leads. Drug Dev. Res. 1991;23:201–218. doi: 10.1002/ddr.430230302. [DOI] [Google Scholar]
- 17.Pereira F. Have marine natural product drug discovery efforts been productive and how can we improve their efficiency? Expet Opin. Drug Discov. 2019;14:717–722. doi: 10.1080/17460441.2019.1604675. [DOI] [PubMed] [Google Scholar]
- 18.Chen L., Huang G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018;115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 19.Chu H., Chan J.F.-W., Yuen T.T.-T., Shuai H., Yuan S., Wang Y., Hu B., Yip C.C.-Y., Tsang J.O.-L., Huang X., Chai Y., Yang D., Hou Y., Chik K.K.-H., Zhang X., Fung A.Y.-F., Tsoi H.-W., Cai J.-P., Chan W.-M., Ip J.D., Chu A.W.-H., Zhou J., Lung D.C., Kok K.-H., To K.K.-W., Tsang O.T.-Y., Chan K.-H., Yuen K.-Y. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. The Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/s2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., Wang Q., Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U.S.A. 2020;117:11727. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kliche J., Kuss H., Ali M., Ivarsson Y. Cytoplasmic short linear motifs in ACE2 and integrin β3 link SARS-CoV-2 host cell receptors to mediators of endocytosis and autophagy. Sci. Signal. 2021;14:1117. doi: 10.1126/SCISIGNAL.ABF1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amraei R., Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9 doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glebov O.O. Understanding SARS‐CoV‐2 endocytosis for COVID‐19 drug repurposing. FEBS J. 2020:15369. doi: 10.1111/febs.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astuti I. Ysrafil, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes and Metabolic Syndrome: Clin. Res. Rev. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y., Yang C., feng Xu X., Xu W., wen Liu S. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benton D.J., Wrobel A.G., Xu P., Roustan C., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020 doi: 10.1038/s41586-020-2772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y., Du L. SARS-CoV-2 spike protein: a key target for eliciting persistent neutralizing antibodies. Signal Transduction and Targeted Therapy. 2021;6:95. doi: 10.1038/s41392-021-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimoto F.K. The proteins of severe acute respiratory syndrome coronavirus-2 (SARS CoV-2 or n-COV19), the cause of COVID-19. Protein J. 2020;39:198–216. doi: 10.1007/s10930-020-09901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijder E.J., Decroly E., Ziebuhr J. Advances in Virus Research. Academic Press Inc.; 2016. The nonstructural proteins directing coronavirus RNA synthesis and processing; pp. 59–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9:1267. doi: 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raj R. Analysis of non-structural proteins, NSPs of SARS-CoV-2 as targets for computational drug designing. Biochemistry and Biophysics Reports. 2021;25:100847. doi: 10.1016/j.bbrep.2020.100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou R., Zeng R., von Brunn A., Lei J. Structural characterization of the C-terminal domain of SARS-CoV-2 nucleocapsid protein. Molecular Biomedicine. 2020;1:1–11. doi: 10.1186/s43556-020-00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M., He S., Chen X., Huang Z., Zhou Z., Zhou Z., Chen Q., Chen S., Kang S. Structural insight into the SARS-CoV-2 nucleocapsid protein C-terminal domain reveals a novel recognition mechanism for viral transcriptional regulatory sequences. Frontiers in Chemistry. 2021;8:624765. doi: 10.3389/fchem.2020.624765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q., Wu J., Wang H., Gao Y., Liu Q., Mu A., Ji W., Yan L., Zhu Y., Zhu C., Fang X., Yang X., Huang Y., Gao H., Liu F., Ge J., Sun Q., Yang X., Xu W., Liu Z., Yang H., Lou Z., Jiang B., Guddat L.W., Gong P., Rao Z. Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell. 2020;182:417–428. doi: 10.1016/j.cell.2020.05.034. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Malone B., Llewellyn E., Grasso M., Shelton P.M.M., Olinares P.D.B., Maruthi K., Eng E.T., Vatandaslar H., Chait B.T., Kapoor T.M., Darst S.A., Campbell E.A. Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell. 2020;182:1560–1573. doi: 10.1016/j.cell.2020.07.033. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C.-I., Postema P.G., Arbelo E., Behr E.R., Bezzina C.R., Napolitano C., Robyns T., Probst V., Schulze-Bahr E., Remme C.A., Wilde A.A.M. Heart Rhythm; 2020. SARS-CoV-2, COVID-19 and Inherited Arrhythmia Syndromes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T., Du Z., Zhu F., Cao Z., An Y., Gao Y., Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52. doi: 10.1038/s41423-020-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16:1–24. doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nerli R., Sharma M., Ghagane S., Gupta P., Patil S., Shubhashree M., Hiremath M. Acute kidney injury in patients with COVID-19. Indian Journal of Health Sciences and Biomedical Research (KLEU) 2020;13:64. doi: 10.4103/kleuhsj.kleuhsj_116_20. [DOI] [Google Scholar]
- 43.Elezkurtaj S., Greuel S., Ihlow J., Michaelis E., Bischoff P., Kunze C.A., Sinn B.V., Gerhold M., Hauptmann K., Ingold-Heppner B., Miller F., Herbst H., Corman V.M., Martin H., Heppner F.L., Horst D. Causes of Death and Comorbidities in Patients with COVID-19. MedRxiv. 2020:2020. doi: 10.1101/2020.06.15.20131540. 06.15.20131540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sisnieguez C.E.L., Espeche W.G., Salazar M.R. Arterial hypertension and the risk of severity and mortality of COVID-19. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.01148-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. The BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dariya B., Nagaraju G.P. Understanding novel COVID-19: its impact on organ failure and risk assessment for diabetic and cancer patients. Cytokine Growth Factor Rev. 2020;53:43. doi: 10.1016/j.cytogfr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiology. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 49.Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expet Rev. Respir. Med. 2020:1–4. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bompard F., Monnier H., Saab I., Tordjman M., Abdoul H., Fournier L., Sanchez O., Lorut C., Chassagnon G., Revel M. European Respiratory Journal; 2020. Pulmonary embolism in patients with Covid-19 pneumonia; p. 2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thepmankorn P., Bach J., Lasfar A., Zhao X., Souayah S., Chong Z.Z., Souayah N. Cytokine storm induced by SARS-CoV-2 infection: the spectrum of its neurological manifestations. Cytokine. 2021;138:155404. doi: 10.1016/j.cyto.2020.155404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.It's Not Just Lungs: Covid-19 May Damage the Heart, Brain, and Kidneys | Advisory Board Daily Briefing. 2020. https://www.advisory.com/daily-briefing/2020/04/17/organ-damage (n.d.) (accessed June 2, 2020) [Google Scholar]
- 54.Kandasamy M. Perspectives for the use of therapeutic Botulinum toxin as a multifaceted candidate drug to attenuate COVID-19. Medicine in Drug Discovery. 2020;6:100042. doi: 10.1016/j.medidd.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. 2020. COVID-19 and Multiorgan Response, Current Problems in Cardiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.COVID-19 Infection in People with Diabetes – touchENDOCRINOLOGY. 2020. https://www.touchendocrinology.com/insight/covid-19-infection-in-people-with-diabetes/ (n.d.) (accessed June 2, 2020) [Google Scholar]
- 57.Lee L.Y.W., Cazier J.B., Starkey T., Turnbull C.D., Kerr R., Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study, Lancet (London, England) 2020. UK Coronavirus Cancer Monitoring Project Team. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.• Pubpp. 2020. https://www.statista.com/statistics/1110949/common-comorbidities-in-covid-19-deceased-patients-in-italy/ (n.d.) (accessed June 2, 2020) [Google Scholar]
- 59.Liu Q., Luo D., Haase J.E., Guo Q., Wang X.Q., Liu S., Xia L., Liu Z., Yang J., Yang B.X. The experiences of health-care providers during the COVID-19 crisis in China: a qualitative study. The Lancet Global Health. 2020;8:e790–e798. doi: 10.1016/S2214-109X(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peiffer-Smadja N., Lucet J.C., Bendjelloul G., Bouadma L., Gerard S., Choquet C., Jacques S., Khalil A., Maisani P., Casalino E., Descamps D., Timsit J.F., Yazdanpanah Y., Lescure F.X. Challenges and issues about organizing a hospital to respond to the COVID-19 outbreak: experience from a French reference centre. Clin. Microbiol. Infect. 2020;26:669–672. doi: 10.1016/j.cmi.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh R., Vijayan V. Transactions of the Indian National Academy of Engineering; 2020. Chloroquine: A Potential Drug in the COVID-19 Scenario; p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.FDA and WHO Drop Hydroxychloroquine from COVID-19 Treatment List. Drug Discovery And Development; 2020. https://www.labroots.com/trending/drug-discovery-and-development/17914/fda-cancels-hydroxychloroquine (n.d.) accessed July 22, 2020. [Google Scholar]
- 63.WHO Warns Overuse of Antibiotics for Covid-19 Will Cause More Deaths. World news | The Guardian; 2020. https://www.theguardian.com/world/2020/jun/01/who-warns-overuse-of-antibiotics-for-covid-19-will-cause-more-deaths (n.d.) accessed July 22, 2020. [Google Scholar]
- 64.Baron S.A., Devaux C., Colson P., Raoult D., Rolain J.M. Teicoplanin: an alternative drug for the treatment of COVID-19? Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Convalescent Plasma's Success against COVID-19 Continues in New Study - the Wire Science. 2020. https://science.thewire.in/the-sciences/convalescent-plasmas-success-against-covid-19-continues-in-new-study/ n.d. (accessed April 27, 2020) [Google Scholar]
- 66.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat. Biotechnol. 2020;38:379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 67.Cherian S.S., Agrawal M., Basu A., Abraham P., Gangakhedkar R.R., Bhargava B. Perspectives for repurposing drugs for the coronavirus disease 2019. Indian J. Med. Res. 2020;151:160–171. doi: 10.4103/ijmr.IJMR_585_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., Wurtz N., Rolain J.M., Colson P., La Scola B., Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broad Anti-coronaviral Activity of FDA Approved Drugs against SARS-CoV-2 in Vitro and SARS-CoV in Vivo | bioRxiv. 2020. https://www.biorxiv.org/content/10.1101/2020.03.25.008482v2.full (n.d.) accessed July 22, 2020. [Google Scholar]
- 70.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Experimental Virus Drug Remdesivir Failed in Human Trial (Update) 2020. https://medicalxpress.com/news/2020-04-experimental-virus-drug-remdesivir-human.html n.d. (accessed April 27, 2020) [Google Scholar]
- 72.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. 2020. Experimental Treatment with Favipiravir for COVID-19: an Open-Label Control Study, Engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dabbous H.M., Abd-Elsalam S., El-Sayed M.H., Sherief A.F., Ebeid F.F.S., El Ghafar M.S.A., Soliman S., Elbahnasawy M., Badawi R., Tageldin M.A. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch. Virol. 2021;166:949–954. doi: 10.1007/s00705-021-04956-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Kelleni M.T. Tocilizumab, remdesivir, favipiravir, and dexamethasone repurposed for COVID-19: a comprehensive clinical and pharmacovigilant reassessment. SN Comprehensive Clinical Medicine. 2021;2021:1–5. doi: 10.1007/S42399-021-00824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choy K.T., Wong A.Y.L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.H., Huang X., Peiris M., Yen H.L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopinavir/ritonavir . 2020. A Rapid Review of Effectiveness in COVID-19 - CEBM.https://www.cebm.net/covid-19/lopinavir-ritonavir-a-rapid-review-of-the-evidence-for-effectiveness-in-treating-covid/ (n.d.) (accessed July 21, 2020) [Google Scholar]
- 79.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khalili J.S., Zhu H., Mak N.S.A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J. Med. Virol. 2020;92:740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morgenstern B., Michaelis M., Baer P.C., Doerr H.W., Cinatl J. Ribavirin and interferon-β synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem. Biophys. Res. Commun. 2005;326:905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sayad B., Sobhani M., Khodarahmi R. Archives of Medical Research; 2020. Sofosbuvir as repurposed antiviral drug against COVID-19: why were we convinced to evaluate the drug in a registered/approved clinical trial? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Buyck C., Van Loock M., Woodfall B., Ciesek S. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou Q., Chen V., Shannon C.P., Wei X.-S., Xiang X., Wang X., Wang Z.-H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-α2b treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan Z., Karataş Y., Ceylan A.F., Rahman H. COVID-19 and therapeutic drugs repurposing in hand: the need for collaborative efforts. Pharmacien Hospitalier et Clinicien. 2020 doi: 10.1016/j.phclin.2020.06.003. [DOI] [Google Scholar]
- 88.Kwon P.S., Oh H., Kwon S.J., Jin W., Zhang F., Fraser K., Hong J.J., Linhardt R.J., Dordick J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discovery. 2020;6:50. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee Y.E., Kim H., Seo C., Park T., Bin Lee K., Yoo S.Y., Hong S.C., Kim J.T., Lee J. Marine polysaccharides: therapeutic efficacy and biomedical applications. Arch Pharm. Res. (Seoul) 2017;40:1006–1020. doi: 10.1007/s12272-017-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park S., Lee K.W., Lim D.-S., Lee S. The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem Cell. Dev. 2012;21:2204–2211. doi: 10.1089/scd.2011.0521. [DOI] [PubMed] [Google Scholar]
- 91.Novetsky A.P., Keller M.J., Gradissimo A., Chen Z., Morgan S.L., Xue X., Strickler H.D., Fernández-Romero J.A., Burk R., Einstein M.H. In vitro inhibition of human papillomavirus following use of a carrageenan-containing vaginal gel. Gynecol. Oncol. 2016;143:313–318. doi: 10.1016/j.ygyno.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tandon R., Sharp J.S., Zhang F., Pomin V.H., Ashpole N.M., Mitra D., Jin W., Liu H., Sharma P., Linhardt R.J. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. BioRxiv. 2020 doi: 10.1101/2020.06.08.140236. 2020.06.08.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hans N., Malik A., Naik S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: mini review. Bioresource Technology Reports. 2021;13:100623. doi: 10.1016/j.biteb.2020.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuda M., Shigeta S., Okutani K. Antiviral activities of marine pseudomonas polysaccharides and their oversulfated derivatives. Mar. Biotechnol. 1999;1:68–73. doi: 10.1007/PL00011753. [DOI] [PubMed] [Google Scholar]
- 95.Sanniyasi E., Venkatasubramanian G., Anbalagan M.M., Raj P.P., Gopal R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory) Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-47917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Talyshinsky M.M., Souprun Y.Y., Huleihel M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Canc. Cell Int. 2002;2:14–17. doi: 10.1186/1475-2867-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mendes G.S., Duarte M.E.R., Colodi F.G., Noseda M.D., Ferreira L.G., Berté S.D., Cavalcanti J.F., Santos N., Romanos M.T.V. Structure and anti-metapneumovirus activity of sulfated galactans from the red seaweed Cryptonemia seminervis. Carbohydr. Polym. 2014;101:313–323. doi: 10.1016/j.carbpol.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 98.Schandock F., Riber C.F., Röcker A., Müller J.A., Harms M., Gajda P., Zuwala K., Andersen A.H.F., Løvschall K.B., Tolstrup M., Kreppel F., Münch J., Zelikin A.N. Macromolecular antiviral agents against zika, Ebola, SARS, and other pathogenic viruses. Advanced Healthcare Materials. 2017;6:1700748. doi: 10.1002/adhm.201700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim M., Yim J.H., Kim S.-Y., Kim H.S., Lee W.G., Kim S.J., Kang P.-S., Lee C.-K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012;93:253–259. doi: 10.1016/j.antiviral.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 100.McCLURE M.O., Moore J.P., Blanc D.F., Scotting P., Cook G.M.W., Keynes R.J., Weber J.N., Davies D., Weiss R.A. Investigations into the mechanism by which sulfated polysaccharides inhibit HIV infection in vitro. AIDS Res. Hum. Retrovir. 1992;8:19–26. doi: 10.1089/aid.1992.8.19. [DOI] [PubMed] [Google Scholar]
- 101.Hidari K.I.P.J., Takahashi N., Arihara M., Nagaoka M., Morita K., Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008;376:91–95. doi: 10.1016/j.bbrc.2008.08.100. [DOI] [PubMed] [Google Scholar]
- 102.Rothan H.A., Yusof R. Antiviral and virucidal activities of sulphated polysaccharides against Japanese encephalitis virus. BioRxiv. 2020:2020. doi: 10.1101/2020.02.11.944116. 02.11.944116. [DOI] [PubMed] [Google Scholar]
- 103.De Sousa E., Vanderlei O., Gomes Eloy R., Fernandes De Araújo I.W., Gomes Quinderé A.L., Fontes B.P., Mendes G.S., Figueiredo Cavalcanti J., Teresa M., Romanos V., Maria N., Benevides B. Structural features, molecular weight and anti-HSV activity of sulfated polysaccharides from three red seaweeds. Journal of Chemical and Pharmaceutical Research. 2016;8:164–170. Www.Jocpr.Com www.jocpr.com Available Online. accessed July 28, 2020. [Google Scholar]
- 104.Gomaa H.H.A., Elshoubaky G.A. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. International Journal of Current Pharmaceutical Review and Research. 2016;7:34–42. http://impactfactor.org/PDF/IJCPR/7/IJCPR,Vol7,Issue1,Article5.pdf [Google Scholar]
- 105.Song L., Chen X., Liu X., Zhang F., Hu L., Yue Y., Li K., Li P. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar. Drugs. 2016;14:4. doi: 10.3390/MD14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ueno M., Nogawa M., Siddiqui R., Watashi K., Wakita T., Kato N., Ikeda M., Okimura T., Isaka S., Oda T., Ariumi Y. Acidic polysaccharides isolated from marine algae inhibit the early step of viral infection. Int. J. Biol. Macromol. 2019;124:282–290. doi: 10.1016/j.ijbiomac.2018.11.152. [DOI] [PubMed] [Google Scholar]
- 107.Wang S., Wang W., Hao C., Yunjia Y., Qin L., He M., Mao W. Antiviral activity against enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydr. Polym. 2018;200:43–53. doi: 10.1016/J.CARBPOL.2018.07.067. [DOI] [PubMed] [Google Scholar]
- 108.Peng Y., Xie E., Zheng K., Fredimoses M., Yang X., Zhou X., Wang Y., Yang B., Lin X., Liu J., Liu Y. Nutritional and chemical composition and antiviral activity of cultivated seaweed sargassum naozhouense Tseng et Lu. Mar. Drugs. 2013;11:20–32. doi: 10.3390/md11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Esteves A.I.S., Nicolai M., Humanes M., Goncalves J. Sulfated polysaccharides in marine sponges: extraction methods and anti-HIV activity. Mar. Drugs. 2011;9:139–153. doi: 10.3390/md9010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saha S., Navid M.H., Bandyopadhyay S.S., Schnitzler P., Ray B. Sulfated polysaccharides from Laminaria angustata: structural features and in vitro antiviral activities. Carbohydr. Polym. 2012;87:123–130. doi: 10.1016/j.carbpol.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 111.Morán-Santibañez K., Cruz-Suárez L.E., Ricque-Marie D., Robledo D., Freile-Pelegrín Y., Peña-Hernández M.A., Rodríguez-Padilla C., Trejo-Avila L.M. Synergistic effects of sulfated polysaccharides from Mexican seaweeds against Measles virus. BioMed Res. Int. 2016 doi: 10.1155/2016/8502123. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raposo M.F.D.J., De Morais A.M.M.B., De Morais R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014;101:56–63. doi: 10.1016/j.lfs.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 113.Wang S., Wang W., Hou L., Qin L., He M., Li W., Mao W. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: characteristics of its structure and antiviral activity. Carbohydr. Polym. 2020;227:115280. doi: 10.1016/j.carbpol.2019.115280. [DOI] [PubMed] [Google Scholar]
- 114.Pujol C.A., Ray S., Ray B., Damonte E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012;51:412–416. doi: 10.1016/j.ijbiomac.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 115.Elizondo-Gonzalez R., Cruz-Suarez L.E., Ricque-Marie D., Mendoza-Gamboa E., Rodriguez-Padilla C., Trejo-Avila L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012;9:307. doi: 10.1186/1743-422X-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bouhlal R., Haslin C., Chermann J.-C., Colliec-Jouault S., Sinquin C., Simon G., Cerantola S., Riadi H., Bourgougnon N. Antiviral activities of sulfated polysaccharides isolated from sphaerococcus coronopifolius (rhodophytha, gigartinales) and boergeseniella thuyoides (rhodophyta, ceramiales) Mar. Drugs. 2011;9:1187–1209. doi: 10.3390/md9071187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang N., Wu M.Y., Zheng C.B., Zhu L., Zhao J.H., Zheng Y.T. The depolymerized fucosylated chondroitin sulfate from sea cucumber potently inhibits HIV replication via interfering with virus entry. Carbohydr. Res. 2013;380:64–69. doi: 10.1016/j.carres.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 118.Jiao G., Yu G., Wang W., Zhao X., Zhang J., Ewart S.H. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J. Ocean Univ. China. 2012;11:205–212. doi: 10.1007/s11802-012-1906-x. [DOI] [Google Scholar]
- 119.Lopes N., Ray S., Espada S.F., Bomfim W.A., Ray B., Faccin-Galhardi L.C., Linhares R.E.C., Nozawa C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017;102:605–612. doi: 10.1016/j.ijbiomac.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 120.Dinesh S., Menon T., Hanna L.E., Suresh V., Sathuvan M., Manikannan M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016;82:83–88. doi: 10.1016/j.ijbiomac.2015.09.078. [DOI] [PubMed] [Google Scholar]
- 121.Ghosh T., Pujol C.A., Damonte E.B., Sinha S., Ray B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: structural features, chemical modification and antiviral activity. Antivir. Chem. Chemother. 2009;19:235–242. doi: 10.1177/095632020901900603. [DOI] [PubMed] [Google Scholar]
- 122.Chi Y., Zhang M., Wang X., Fu X., Guan H., Wang P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020;157:75–82. doi: 10.1016/j.ijbiomac.2020.04.187. [DOI] [PubMed] [Google Scholar]
- 123.Hayashi T., Hayashi K., Maeda M., Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996;59 doi: 10.1021/NP960017O. [DOI] [PubMed] [Google Scholar]
- 124.Kanekiyo K., Hayashi K., Takenaka H., Lee J.-B., Hayashi T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga nostoc flagelliforme. Biol. Pharm. Bull. 2007;30:1573–1575. doi: 10.1248/bpb.30.1573. [DOI] [PubMed] [Google Scholar]
- 125.Hasui M., Matsuda M., Okutani K., Shigeta S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995;17:293–297. doi: 10.1016/0141-8130(95)98157-T. [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto K., Kodama E., Mori S., Watanabe J., Baba M., Okutani K., Matsuda M., Shigeta S. Antiviral activity of a sulphated polysaccharide extracted from the marine Pseudomonas and marine plant dinoflagellata against human immunodeficiency viruses and other enveloped viruses. Antiviral Chem. Chemother. 1996;7:189–196. doi: 10.1177/095632029600700403. [DOI] [Google Scholar]
- 127.Sanniyasi E., Venkatasubramanian G., Anbalagan M.M., Raj P.P., Gopal R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory) Sci. Rep. 2019;9:12185. doi: 10.1038/s41598-019-47917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu L., Wang W., Zhang X., Zhao X., Yu G. Anti-HBV activity and mechanism of marine-derived polyguluronate sulfate (PGS) in vitro. Carbohydr. Polym. 2016;143:139–148. doi: 10.1016/j.carbpol.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 129.Gao Y., Liu W., Wang W., Zhang X., Zhao X. The inhibitory effects and mechanisms of 3,6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018;198:329–338. doi: 10.1016/j.carbpol.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 130.Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008;5:1–13. doi: 10.1186/1743-422X-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Piccini L.E., Carro A.C., Quintana V.M., Damonte E.B. Antibody-independent and dependent infection of human myeloid cells with dengue virus is inhibited by carrageenan. Virus Res. 2020;290:198150. doi: 10.1016/j.virusres.2020.198150. [DOI] [PubMed] [Google Scholar]
- 132.Helenius A. Virus entry: looking back and moving forward. J. Mol. Biol. 2018;430:1853–1862. doi: 10.1016/j.jmb.2018.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zlotnick A., Mukhopadhyay S. Virus assembly, allostery and antivirals. Trends Microbiol. 2011;19:14–23. doi: 10.1016/j.tim.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:1–22. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jin W., Zhang W., Mitra D., McCandless M.G., Sharma P., Tandon R., Zhang F., Linhardt R.J. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020;163:1649–1658. doi: 10.1016/j.ijbiomac.2020.09.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Esteves A.I.S., Nicolai M., Humanes M., Goncalves J. Sulfated polysaccharides in marine sponges: extraction methods and anti-HIV activity. Mar. Drugs. 2011;9:139–153. doi: 10.3390/md9010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Buck C.B., Thompson C.D., Roberts J.N., Müller M., Lowy D.R., Schiller J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:671–680. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Milewska A., Ciejka J., Kaminski K., Karewicz A., Bielska D., Zeglen S., Karolak W., Nowakowska M., Potempa J., Bosch B.J., Pyrc K., Szczubialka K. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013;97:112–121. doi: 10.1016/j.antiviral.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baranova E.O., Shastina N.S., Shvets V.I. Polyanionic inhibitors of HIV adsorption. Russ. J. Bioorg. Chem. 2011;37:527–542. doi: 10.1134/S1068162011050037. [DOI] [PubMed] [Google Scholar]
- 140.Sosa M.A.G., Fazely F., Koch J.A., Vercellotti S.V., Ruprecht R.M. N-Carboxymethylchitosan-N,O-sulfate as an anti-HIV-1 agent. Biochem. Biophys. Res. Commun. 1991;174:489–496. doi: 10.1016/0006-291X(91)91443-G. [DOI] [PubMed] [Google Scholar]
- 141.Malagoli B.G., Cardozo F.T.G.S., Gomes J.H.S., Ferraz V.P., Simões C.M.O., Braga F.C. Chemical characterization and antiherpes activity of sulfated polysaccharides from Lithothamnion muelleri. Int. J. Biol. Macromol. 2014;66:332–337. doi: 10.1016/j.ijbiomac.2014.02.053. [DOI] [PubMed] [Google Scholar]
- 142.Faccin-Galhardi L.C., Ray S., Lopes N., Ali I., Espada S.F., dos Santos J.P., Ray B., Linhares R.E.C., Nozawa C. Assessment of antiherpetic activity of nonsulfated and sulfated polysaccharides from Azadirachta indica. Int. J. Biol. Macromol. 2019;137:54–61. doi: 10.1016/j.ijbiomac.2019.06.129. [DOI] [PubMed] [Google Scholar]
- 143.Mukherjee S., Ghosh K., Hahn F., Wangen C., Strojan H., Müller R., Anand N., Ali I., Bera K., Ray B., Hutterer C., Marschall M., Ray S. Chemically sulfated polysaccharides from natural sources: assessment of extraction-sulfation efficiencies, structural features and antiviral activities. Int. J. Biol. Macromol. 2019;136:521–530. doi: 10.1016/j.ijbiomac.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 144.El Awady M.E., Nasr Eldin M.A., Ibrahim H.M., Al Bahnasy M.E., Aziz S.H.A. In vitro evaluation of antioxidant, anticancer, and antiviral activities of exopolysaccharide from Streptomyces hirsutus NRC2018 ARTICLE INFO. J. Appl. Pharmaceut. Sci. 2019;9 doi: 10.7324/JAPS.2019.91102. 10–018. [DOI] [Google Scholar]
- 145.Damonte E., Matulewicz M., Cerezo A. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2012;11:2399–2419. doi: 10.2174/0929867043364504. [DOI] [PubMed] [Google Scholar]
- 146.Kim M., Yim J.H., Kim S.Y., Kim H.S., Lee W.G., Kim S.J., Kang P.S., Lee C.K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012;93:253–259. doi: 10.1016/j.antiviral.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 147.Chen M.Z., Xie H.G., Yang L.W., Liao Z.H., Yu J. In vitro anti-influenza virus activities of sulfated polysaccharide fractions from Gracilaria lemaneiformis. Virol. Sin. 2010;25:341–351. doi: 10.1007/s12250-010-3137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang W., Wu J., Zhang X., Hao C., Zhao X., Jiao G., Shan X., Tai W., Yu G. Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci. Rep. 2017;7:1–14. doi: 10.1038/srep40760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song S., Peng H., Wang Q., Liu Z., Dong X., Wen C., Ai C., Zhang Y., Wang Z., Zhu B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food and Function. 2020;11:7415–7420. doi: 10.1039/d0fo02017f. [DOI] [PubMed] [Google Scholar]
- 150.Batinic D., Robey F.A. The V3 region of the envelope glycoprotein of human immunodeficiency virus type 1 binds sulfated polysaccharides and CD4-derived synthetic peptides. J. Biol. Chem. 1992;267:6664–6671. doi: 10.1016/s0021-9258(19)50478-1. [DOI] [PubMed] [Google Scholar]
- 151.Baba M., Schols D., Pauwels R., Nakashima H., De Clercq E. Sulfated polysaccharides as potent inhibitors of HIV-induced syncytium formation: a new strategy towards AIDS chemotherapy. J. Acquir. Immune Defic. Syndr. 1990;3:493–499. doi: 10.1097/00126334-199003050-00005. [DOI] [PubMed] [Google Scholar]
- 152.Hoshino T., Hayashi T., Hayashi K., Hamada J., Lee J.B., Sankawa U. An antivirally active sulfated polysaccharide from Sargassum horneri (Turner) C. Agardh, Biol. Pharm. Bull. 1998;21:730–734. doi: 10.1248/bpb.21.730. [DOI] [PubMed] [Google Scholar]
- 153.Aguilar-Briseño J.A., Cruz-Suarez L.E., Sassi J.F., Ricque-Marie D., Zapata-Benavides P., Mendoza-Gamboa E., Rodríguez-Padilla C., Trejo-Avila L.M. Sulphated polysaccharides from Ulva clathrata and Cladosiphon okamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar. Drugs. 2015;13:697–712. doi: 10.3390/md13020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ichiyama K., Gopala Reddy S.B., Zhang L.F., Chin W.X., Muschin T., Heinig L., Suzuki Y., Nanjundappa H., Yoshinaka Y., Ryo A., Nomura N., Ooi E.E., Vasudevan S.G., Yoshida T., Yamamoto N. Sulfated polysaccharide, curdlan sulfate, efficiently prevents entry/fusion and restricts antibody-dependent enhancement of dengue virus infection in vitro: a possible candidate for clinical application. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Copeland R., Balasubramaniam A., Tiwari V., Zhang F., Bridges A., Linhardt R.J., Shukla D., Liu J. Using a 3-O-sulfated heparin octasaccharide to inhibit the entry of herpes simplex virus type 1. Biochemistry. 2008;47:5774–5783. doi: 10.1021/bi800205t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ghosh T., Chattopadhyay K., Marschall M., Karmakar P., Mandal P., Ray B. Focus on antivirally active sulfated polysaccharides: from structure-activity analysis to clinical evaluation. Glycobiology. 2009;19:2–15. doi: 10.1093/glycob/cwn092. [DOI] [PubMed] [Google Scholar]
- 157.Wang W., Wang S.X., Guan H.S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar. Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bandyopadhyay S.S., Navid M.H., Ghosh T., Schnitzler P., Ray B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry. 2011;72:276–283. doi: 10.1016/j.phytochem.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 159.Shi Q., Wang A., Lu Z., Qin C., Hu J., Yin J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017:453–454. doi: 10.1016/j.carres.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 160.Radonić A., Thulke S., Achenbach J., Kurth A., Vreemann A., König T., Walter C., Possinger K., Nitsche A. Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to Vaccinia virus. J. Antivir. Antiretrovir. 2010;2:51–55. doi: 10.4172/jaa.1000023. [DOI] [Google Scholar]
- 161.Zhao C., Gao L., Wang C., Liu B., Jin Y., Xing Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016;144:382–389. doi: 10.1016/j.carbpol.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 162.Koyama S., Ishii K.J., Coban C., Akira S. Innate immune response to viral infection. Cytokine. 2008;43:336–341. doi: 10.1016/j.cyto.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 163.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience. 2020;19:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhong J., Tang J., Ye C., Dong L. The immunology of COVID-19: is immune modulation an option for treatment? The Lancet Rheumatology. 2020 doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huang L., Shen M., Morris G.A., Xie J. Sulfated polysaccharides: immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019;92:1–11. doi: 10.1016/j.tifs.2019.08.008. [DOI] [Google Scholar]
- 166.Gugliandolo C., Spanò A., Lentini V., Arena A., Maugeri T.L. Antiviral and immunomodulatory effects of a novel bacterial exopolysaccharide of shallow marine vent origin. J. Appl. Microbiol. 2014;116:1028–1034. doi: 10.1111/jam.12422. [DOI] [PubMed] [Google Scholar]
- 167.Tang C., Ding R., Sun J., Liu J., Kan J., Jin C. The impacts of natural polysaccharides on intestinal microbiota and immune responses – a review. Food Funct. 2019;10:2290–2312. doi: 10.1039/C8FO01946K. [DOI] [PubMed] [Google Scholar]
- 168.Sun Y., Gong G., Guo Y., Wang Z., Song S., Zhu B., Zhao L., Jiang J. Purification, structural features and immunostimulatory activity of novel polysaccharides from Caulerpa lentillifera. Int. J. Biol. Macromol. 2018;108:314–323. doi: 10.1016/j.ijbiomac.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 169.Wang X., Zhang Z., Wu Y., Sun X., Xu N. Synthesized sulfated and acetylated derivatives of polysaccharide extracted from Gracilariopsis lemaneiformis and their potential antioxidant and immunological activity. Int. J. Biol. Macromol. 2019;124:568–572. doi: 10.1016/j.ijbiomac.2018.11.244. [DOI] [PubMed] [Google Scholar]
- 170.Yu Y., Zhang Y., Hu C., Zou X., Lin Y., Xia Y., You L. Chemistry and immunostimulatory activity of a polysaccharide from Undaria pinnatifida. Food Chem. Toxicol. 2019;128:119–128. doi: 10.1016/j.fct.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 171.Bagheri A., Moezzi S.M.I., Mosaddeghi P., Nadimi Parashkouhi S., Fazel Hoseini S.M., Badakhshan F., Negahdaripour M. Interferon-inducer antivirals: potential candidates to combat COVID-19. Int. Immunopharm. 2021;91:107245. doi: 10.1016/j.intimp.2020.107245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beck M.A. Antioxidants and viral infections: host immune response and viral pathogenicity. J. Am. Coll. Nutr. 2001;20:384S–388S. doi: 10.1080/07315724.2001.10719172. [DOI] [PubMed] [Google Scholar]
- 173.Reshi M.L., Su Y.C., Hong J.R. RNA viruses: ROS-mediated cell death. International Journal of Cell Biology. 2014:2014. doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akaike T. Role of free radicals in viral pathogenesis and mutation. Rev. Med. Virol. 2001;11:87–101. doi: 10.1002/rmv.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pachetti M., Marini B., Benedetti F., Giudici F., Mauro E., Storici P., Masciovecchio C., Angeletti S., Ciccozzi M., Gallo R.C., Zella D., Ippodrino R. Emerging SARS - CoV - 2 mutation hot spots include a novel RNA - dependent - RNA polymerase variant. J. Transl. Med. 2020:1–9. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muthukumar J., Chidambaram R., Sukumaran S. Sulfated polysaccharides and its commercial applications in food industries—a review. J. Food Sci. Technol. 2020:1–14. doi: 10.1007/s13197-020-04837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reys L.L., Silva S.S., Oliveira C., Lopez‐Cebral R., Neves N.M., Martins A., Oliveira J.M., Silva T.H., Reis R.L. Encyclopedia of Marine Biotechnology. Wiley; 2020. Marine‐origin polysaccharides for tissue engineering and regenerative medicine; pp. 2619–2650. [DOI] [Google Scholar]
- 179.Jönsson M., Allahgholi L., Sardari R.R.R., Hreggviðsson G.O., Nordberg Karlsson E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules. 2020;25:930. doi: 10.3390/molecules25040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sansone C., Brunet C., Noonan D.M., Albini A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants. 2020;9:392. doi: 10.3390/antiox9050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Manlusoc J.K.T., Hsieh C.-L., Hsieh C.-Y., Salac E.S.N., Lee Y.-T., Tsai P.-W. Pharmacologic application potentials of sulfated polysaccharide from marine algae. Polymers. 2019;11:1163. doi: 10.3390/polym11071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sousa W.M., Silva R.O., Bezerra F.F., Bingana R.D., Barros F.C.N., Costa L.E.C., Sombra V.G., Soares P.M.G., Feitosa J.P.A., de Paula R.C.M., Souza M.H.L.P., Barbosa A.L.R., Freitas A.L.P. Sulfated polysaccharide fraction from marine algae Solieria filiformis: structural characterization, gastroprotective and antioxidant effects. Carbohydr. Polym. 2016;152:140–148. doi: 10.1016/j.carbpol.2016.06.111. [DOI] [PubMed] [Google Scholar]
- 183.Fimbres-Olivarria D., Carvajal-Millan E., Lopez-Elias J.A., Martinez-Robinson K.G., Miranda-Baeza A., Martinez-Cordova L.R., Enriquez-Ocaña F., Valdez-Holguin J.E. Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula sp. Food Hydrocolloids. 2018;75:229–236. doi: 10.1016/j.foodhyd.2017.08.002. [DOI] [Google Scholar]
- 184.Alencar P.O.C., Lima G.C., Barros F.C.N., Costa L.E.C., Ribeiro C.V.P.E., Sousa W.M., Sombra V.G., Abreu C.M.W.S., Abreu E.S., Pontes E.O.B., Oliveira A.C., de Paula R.C.M., Freitas A.L.P. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: in vitro and in vivo activities. Food Hydrocolloids. 2019;90:28–34. doi: 10.1016/j.foodhyd.2018.12.007. [DOI] [Google Scholar]
- 185.Shanura Fernando I.P., Asanka Sanjeewa K.K., Samarakoon K.W., Lee W.W., Kim H.-S., Kim E.-A., Gunasekara U.K., Abeytunga D.T.U., Nanayakkara C., De Silva E.D., Lee H.-S., Jeon Y.-J. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. ALGAE. 2017;32:75–86. doi: 10.4490/algae.2017.32.12.1. [DOI] [Google Scholar]
- 186.Khan B.M., Qiu H.-M., Xu S.-Y., Liu Y., Cheong K.-L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020;145:1155–1161. doi: 10.1016/j.ijbiomac.2019.10.040. [DOI] [PubMed] [Google Scholar]
- 187.Karaman M., Janjušević L., Jakovljević D., Šibul F., Pejin B. Anti-hydroxyl radical activity, redox potential and anti-AChE activity of Amanita strobiliformis polysaccharide extract. Nat. Prod. Res. 2019;33:1522–1526. doi: 10.1080/14786419.2017.1422183. [DOI] [PubMed] [Google Scholar]
- 188.Wu S.-C. Solubility of Polysaccharides. InTech; 2017. Antioxidant activity of sulfated seaweeds polysaccharides by novel assisted extraction; pp. 89–108. [DOI] [Google Scholar]
- 189.Andrew M., Jayaraman G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020;487:107881. doi: 10.1016/j.carres.2019.107881. [DOI] [PubMed] [Google Scholar]
- 190.Wang J., Hu S., Nie S., Yu Q., Xie M. 2016. Reviews on Mechanisms of in Vitro Antioxidant Activity of Polysaccharides, Oxidative Medicine and Cellular Longevity. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dore C.M.P.G., das M.G., Faustino Alves C., Pofírio Will L.S.E., Costa T.G., Sabry D.A., de Souza Rêgo L.A.R., Accardo C.M., Rocha H.A.O., Filgueira L.G.A., Leite E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013;91:467–475. doi: 10.1016/j.carbpol.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 192.Yu I.C., Chen R., Li J.J., Li J.J., Drahansky M., Paridah M., Moradbak A., Mohamed A., abdulwahab taiwo Owolabi H., FolaLi, Asniza M., Abdul Khalid S.H., Sharma T., Dohare N., Kumari M., Singh U.K., Khan A.B., Borse M.S., Patel R., Paez A., Howe A., Goldschmidt D., Corporation C., Coates J., Reading F. Intech. i; 2012. Radiological Society of North America. “New Research Highlights Blood Clot Dangers of COVID-19.” ScienceDaily; p. 13.https://www.sciencedaily.com/releases/2020/04/200423143100.htm (accessed April 26, 2020) [DOI] [Google Scholar]
- 193.COVID Patients Are Getting Mysterious and Deadly Blood Clots. 2020. https://futurism.com/neoscope/covid-patients-deadly-blood-clots n.d. (accessed April 26, 2020) [Google Scholar]
- 194.Blood Clotting Abnormalities Reveal COVID-19 Patients at Risk for Thrombotic Events. ScienceDaily; 2020. https://www.sciencedaily.com/releases/2020/05/200515131909.htm accessed June 2, 2020. [Google Scholar]
- 195.They Don't Struggle to Breathe—But COVID-19 Is Starving Them of Oxygen. 2020. https://www.nationalgeographic.com/science/2020/05/they-do-not-struggle-to-breathe-but-coronavirus-starves-them-of-oxygen-cvd/ accessed June 2, 2020. [Google Scholar]
- 196.Hou Y., Carrim N., Wang Y., Gallant R.C., Marshall A., Ni H. Platelets in hemostasis and thrombosis: novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. Journal of Biomedical Research. 2015;29:437–444. doi: 10.7555/JBR.29.20150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carvalhal F., Cristelo R.R., Resende D.I.S.P., Pinto M.M.M., Sousa E., Correia-Da-Silva M. Antithrombotics from the sea: polysaccharides and beyond. Mar. Drugs. 2019;17:170. doi: 10.3390/md17030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ruocco N., Costantini S., Guariniello S., Costantini M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules. 2016;21:1–16. doi: 10.3390/molecules21050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pomin V.H. In: Marine Medicinal Foods. Kim S.-K., editor. Academic Press; 2012. Chapter 12 - structure–function relationship of anticoagulant and antithrombotic well-defined sulfated polysaccharides from marine invertebrates; pp. 195–209. [DOI] [PubMed] [Google Scholar]
- 200.Chagas F.D. da S., Lima G.C., dos Santos V.I.N., Costa L.E.C., de Sousa W.M., Sombra V.G., de Araújo D.F., Barros F.C.N., Marinho-Soriano E., de Andrade Feitosa J.P., de Paula R.C.M., Pereira M.G., Freitas A.L.P. Sulfated polysaccharide from the red algae Gelidiella acerosa: anticoagulant, antiplatelet and antithrombotic effects. Int. J. Biol. Macromol. 2020;159:415–421. doi: 10.1016/j.ijbiomac.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 201.Starling S. Targeting the Xa factor. Nat. Rev. Cardiol. 2017 doi: 10.1038/nrcardio.2017.178. [DOI] [PubMed] [Google Scholar]
- 202.Cao S., He X., Qin L., He M., Yang Y., Liu Z., Mao W. Anticoagulant and antithrombotic properties in vitro and in vivo of a novel sulfated polysaccharide from marine green alga monostroma nitidum. Mar. Drugs. 2019;17:247. doi: 10.3390/md17040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Reis S.E., Andrade R.G.C., Accardo C.M., Maia L.F., Oliveira L.F.C., Nader H.B., Aguiar J.A.K., Medeiros V.P. Influence of sulfated polysaccharides from Ulva lactuca L. upon Xa and IIa coagulation factors and on venous blood clot formation. Algal Research. 2020;45:101750. doi: 10.1016/j.algal.2019.101750. [DOI] [Google Scholar]
- 204.Scientists Develop New Tool to Monitor Coronavirus Mutations - the Week. 2020. https://www.theweek.in/news/health/2020/09/12/Scientists-develop-new-tool-to-monitor-coronavirus-mutations.html accessed September 22, 2020. [Google Scholar]
- 205.Sanina N. Vaccine adjuvants derived from marine organisms. Biomolecules. 2019;9:340. doi: 10.3390/biom9080340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ghendon Y., Markushin S., Krivtsov G., Akopova I. Chitosan as an adjuvant for parenterally administered inactivated influenza vaccines. Arch. Virol. 2008;153:831–837. doi: 10.1007/s00705-008-0047-4. [DOI] [PubMed] [Google Scholar]
- 207.Abdullahi A.Y., Kallon S., Yu X., Zhang Y., Li G. Vaccination with Astragalus and ginseng polysaccharides improves immune response of chickens against H5N1 avian influenza virus. BioMed Res. Int. 2016 doi: 10.1155/2016/1510264. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sun B., Yu S., Zhao D., Guo S., Wang X., Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36:5226–5234. doi: 10.1016/j.vaccine.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 209.He C., Lin H.Y., Wang C.C., Zhang M., Lin Y.Y., Huang F.Y., Lin Y.Z., Tan G.H. Exopolysaccharide from Paecilomyces lilacinus modulates macrophage activities through the TLR4/NF-κB/MAPK pathway. Mol. Med. Rep. 2019;20:4943–4952. doi: 10.3892/mmr.2019.10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li P., Wang F. Polysaccharides: candidates of promising vaccine adjuvants. Drug Discoveries & Therapeutics. 2015;9:88–93. doi: 10.5582/ddt.2015.01025. [DOI] [PubMed] [Google Scholar]
- 211.Kuznetsova T.A., Zaporozhets T.S., Persianova E.V., Khotimchenko Y.S., Besednova N.N. Prospects for the use of sulfated polysaccharides from brown seaweeds as vaccine adjuvants. Russ. J. Mar. Biol. 2016;42:443–450. doi: 10.1134/S1063074016060055. [DOI] [Google Scholar]
- 212.Zhang Y.-Q., Tsai Y.-C., Monie A., Hung C.-F., Wu T.-C. Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine. 2010;28:5212–5219. doi: 10.1016/j.vaccine.2010.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Luo M., Shao B., Nie W., Wei X.-W., Li Y.-L., Wang B.-L., He Z.-Y., Liang X., Ye T.-H., Wei Y.-Q. Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015;5:11062. doi: 10.1038/srep11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xing L., Fan Y.T., Zhou T.J., Gong J.H., Cui L.H., Cho K.H., Choi Y.J., Jiang H.L., Cho C.S. Chemical modification of Chitosan for efficient vaccine delivery. Molecules. 2018;23:229. doi: 10.3390/molecules23020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Muralidharan A., Russell M.S., Larocque L., Gravel C., Sauvé S., Chen Z., Li C., Chen W., Cyr T., Rosu-Myles M., Wang L., Li X. Chitosan alters inactivated respiratory syncytial virus vaccine elicited immune responses without affecting lung histopathology in mice. Vaccine. 2019;37:4031–4039. doi: 10.1016/j.vaccine.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 216.ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. Journal of Pharmaceutical Analysis. 2020;10:313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cotas J., Marques V., Afonso M.B., Rodrigues C.M.P., Pereira L. Antitumour potential of gigartina pistillata carrageenans against colorectal cancer stem cell-enriched tumourspheres. Mar. Drugs. 2020;18 doi: 10.3390/md18010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu B., Ni F., Sun Y., Zhu X., Yin H., Yao Z., Du Y. Insight into carrageenases: major review of sources, category, property, purification method, structure, and applications. Crit. Rev. Biotechnol. 2018;38:1261–1276. doi: 10.1080/07388551.2018.1472550. [DOI] [PubMed] [Google Scholar]
- 219.Senthilkumar K., Ramajayam G., Venkatesan J., Kim S.K., Ahn B.C. Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications. Elsevier; 2017. Biomedical applications of fucoidan, seaweed polysaccharides; pp. 269–281. [DOI] [Google Scholar]
- 220.Wijesinghe W.A.J.P., Jeon Y.J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: a review. Carbohydr. Polym. 2012;88:13–20. doi: 10.1016/j.carbpol.2011.12.029. [DOI] [Google Scholar]
- 221.Cindana Mo’o F.R., Wilar G., Devkota H.P., Wathoni N. Ulvan, a polysaccharide from macroalga Ulva sp.: a review of chemistry, biological activities and potential for food and biomedical applications. Appl. Sci. 2020;10:5488. doi: 10.3390/app10165488. [DOI] [Google Scholar]
- 222.Kidgell J.T., Magnusson M., de Nys R., Glasson C.R.K. Ulvan: a systematic review of extraction, composition and function. Algal Research. 2019;39:101422. doi: 10.1016/j.algal.2019.101422. [DOI] [Google Scholar]
- 223.Ryu W.-S. Molecular Virology of Human Pathogenic Viruses. Elsevier; 2017. Virus life cycle; pp. 31–45. [DOI] [Google Scholar]
- 224.Day P.M., Schelhaas M. Concepts of papillomavirus entry into host cells. Current Opinion in Virology. 2014;4:24–31. doi: 10.1016/J.COVIRO.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cruz-Oliveira C., Freire J.M., Conceição T.M., Higa L.M., Castanho M.A.R.B., Da Poian A.T. Receptors and routes of dengue virus entry into the host cells. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 2015;39:155–170. doi: 10.1093/femsre/fuu004. [DOI] [PubMed] [Google Scholar]
- 226.Douam F., Lavillette D., Cosset F.L. Progress in Molecular Biology and Translational Science. Elsevier B.V.; 2015. The mechanism of HCV entry into host cells; pp. 63–107. [DOI] [PubMed] [Google Scholar]
- 227.Lim Y., Ng Y., Tam J., Liu D. Human coronaviruses: a review of virus–host interactions. Diseases. 2016;4:26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Garmaroudi F.S., Marchant D., Hendry R., Luo H., Yang D., Ye X., Shi J., McManus B.M. Coxsackievirus B3 replication and pathogenesis. Future Microbiol. 2015;10:629–653. doi: 10.2217/fmb.15.5. [DOI] [PubMed] [Google Scholar]
- 229.Matsuoka Y., Matsumae H., Katoh M., Eisfeld A.J., Neumann G., Hase T., Ghosh S., Shoemaker J.E., Lopes T.J.S., Watanabe T., Watanabe S., Fukuyama S., Kitano H., Kawaoka Y. A comprehensive map of the influenza A virus replication cycle. BMC Syst. Biol. 2013;7:1–18. doi: 10.1186/1752-0509-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]