Colombia, South America has one of the world’s highest burdens of Helicobacter pylori infection and gastric cancer. While multidrug antibiotic regimens can effectively eradicate H. pylori, treatment efficacy is being jeopardized by the emergence of antibiotic-resistant H. pylori strains.
KEYWORDS: Colombia, South America, Helicobacter pylori, PCR, adaptive mutations, antibiotic resistance, antimicrobial susceptibility testing, levofloxacin, metronidazole, whole-genome sequencing
ABSTRACT
Colombia, South America has one of the world’s highest burdens of Helicobacter pylori infection and gastric cancer. While multidrug antibiotic regimens can effectively eradicate H. pylori, treatment efficacy is being jeopardized by the emergence of antibiotic-resistant H. pylori strains. Moreover, the spectrum of and genetic mechanisms for antibiotic resistance in Colombia is underreported. In this study, 28 H. pylori strains isolated from gastric biopsy specimens from a high-gastric-cancer-risk (HGCR) population living in the Andes Mountains in Túquerres, Colombia and 31 strains from a low-gastric-cancer-risk (LGCR) population residing on the Pacific coast in Tumaco, Colombia were subjected to antibiotic susceptibility testing for amoxicillin, clarithromycin, levofloxacin, metronidazole, rifampin, and tetracycline. Resistance-associated genes were amplified by PCR for all isolates, and 29 isolates were whole-genome sequenced (WGS). No strains were resistant to amoxicillin, clarithromycin, or rifampin. One strain was resistant to tetracycline and had an A926G mutation in its 16S rRNA gene. Levofloxacin resistance was observed in 12/59 isolates and was significantly associated with N87I/K and/or D91G/Y mutations in gyrA. Most isolates were resistant to metronidazole; this resistance was significantly higher in the LGCR (31/31) group compared to the HGCR (24/28) group. Truncations in rdxA and frxA were present in nearly all metronidazole-resistant strains. There was no association between phylogenetic relationship and resistance profiles based on WGS analysis. Our results indicate H. pylori isolates from Colombians exhibit multidrug antibiotic resistance. Continued surveillance of H. pylori antibiotic resistance in Colombia is warranted in order to establish appropriate eradication treatment regimens for this population.
INTRODUCTION
Helicobacter pylori, a Gram-negative, spiral-shaped, microaerophilic flagellated bacterium, is considered a pathogen that colonizes the human stomach and is the causative agent of acute and chronic gastritis, peptic ulcer disease (10 to 20%), gastric adenocarcinoma (1 to 2%), and gastric lymphoma (<1%) (1–4). In addition, it has more recently been suggested that H. pylori may also be associated with extraintestinal diseases such as immune thrombocytopenic purpura, refractory iron deficiency anemia, and vitamin B12 deficiency (5, 6). Therefore, eradication of H. pylori infection for treatment or prevention of these disorders is commonly prescribed; however, emerging antimicrobial resistance is increasing worldwide and is the main factor for the failure of H. pylori eradication (7).
The prevalence of H. pylori infections approaches 50% worldwide (5), and can be as high as 80 to 90% in developing countries (1, 8). For example, in emerging nations such as Vietnam, India, and Saudi Arabia, or Canadian aboriginal populations, >80% of the populations are infected (9), whereas the infection rate prevalence in industrialized nations is as low as 30% (8, 10). Also noted are differences in the prevalence of infection both within different geographical locations within a country and between countries (8), as well as differences in the antimicrobial-resistance rates (1, 11). One of the highest gastric cancer rates in the world occurs in Colombia, South America, where >80% of the population is estimated to be infected with H. pylori (12–18). Interestingly, the incidence rates of gastric cancer in Colombia differ markedly between high-risk individuals residing in the Andes Mountains in Túquerres (∼150/100,000) compared to those living on the coast in Tumaco, where there is a low risk of gastric cancer (6/100,000) (19, 20).
As a result of the high prevalence rate of H. pylori and its association with serious gastric disorders, different anti-H. pylori treatment regimens have emerged, in some cases depending upon the resistance to clarithromycin (CLR) and metronidazole (MTZ). For example, in one study, >85% of the H. pylori strains in Colombia were resistant to MTZ (21). While first-line therapy, typically a proton pump inhibitor (PPI) plus two of the three antibiotics CLR, MTZ, or amoxicillin (AMX), first proposed in the Maastricht Consensus Report (22), has generally been associated with successful cure rates, the rise of multidrug-resistant (MDR) strains of H. pylori has led to treatment failures. MDR has resulted from multiple antibiotics previously prescribed or, in some cases, indiscriminate use of “over-the-counter” antibiotics. Mutations in the 23S rRNA (A2142G/C, A2143G), 16S rRNA (AGA926-928TTC), and gyrA (QRDR) genes are responsible for CLR, tetracycline (TET), and fluoroquinolone resistance, respectively, as well as mutations in genes frxA/rdxA, rpoB (500 to 545), and pbp1A, conferring resistance to MTZ, rifampin (RIF), and AMX, respectively. Knowledge of these mutations provides guidance to management and treatment options. The aim of this study was to ascertain the prevalence and mechanism of H. pylori antibiotic resistance of six commonly used antibiotics in Colombian populations with high and low risk of gastric cancer, with each population having a ≥90% prevalence of H. pylori infection.
MATERIALS AND METHODS
Study participants, histopathology, and Helicobacter pylori culture.
Individuals from two locations in Colombia with contrasting risks of gastric cancer were invited to participate. The two locations were Tuquerres, in a high-gastric-cancer-risk region (HGCR), and Tumaco, in a low-gastric-cancer-risk region (LGCR). Inclusion criteria were age between 40 and 60 years and dyspeptic symptoms meriting upper gastrointestinal tract endoscopy. Exclusion criteria were use of proton pump inhibitors, H2-receptor antagonists, or antimicrobials in the month before the endoscopy, history of chronic conditions, or a prior gastrectomy. Informed consent was obtained from all participants. The ethics committees of the participating hospitals and the Universidad del Valle in Colombia, and the Institutional Review Board of Vanderbilt University approved all study protocols. Gastric mucosa biopsy samples were obtained from all participants between 2006 and 2010, from the gastric antrum, incisura angularis, and corpus for histopathology. One additional antral biopsy specimen from each participant was frozen in glycerol/thioglycolate for H. pylori culture. Histopathological diagnoses were determined by two pathologists (authors P.C. and M.B.P.), according to internationally accepted criteria (23, 24). H. pylori strains in this study included 37 from individuals from a previous study (25), from which H. pylori isolates were available. Additionally, 22 strains were selected from a total of 271 enrolled participants following a similar protocol. Individuals from both regions were matched by age and sex, selecting those with the least advanced histological lesions (NAG or MAG). However, a few subjects with more advanced lesions were included as there were not enough cases with less advanced lesions to match, mainly in the HGCR area. In total, 59 representative strains of H. pylori were isolated from human gastric antrum biopsy samples; 31 strains were recovered from the samples taken from patients in the LGCR, and 28 strains were recovered from biopsy specimens from patients in the HGCR. Biopsies were homogenized in a sterile tissue grinder containing Brucella broth (BBL, Becton, Dickinson and Co., Sparks, MD) with 20% glycerol. Aliquots (50 μl) were plated onto Glaxo blood agar plates containing vancomycin, bacitracin, naladixic acid, and amphotericin B (26). Plates were incubated microaerobically at 37°C with a gas mixture of 80:10:10 (N2, CO2, H2) for up to 14 days. H. pylori was identified by colony morphology and Gram stain, and confirmed by PCR and 16S rRNA sequencing.
Determination of antimicrobial susceptibility.
All antimicrobials were purchased from Sigma Chemical Company, St. Louis, MO. Antimicrobial susceptibility to AMX, CLR, levofloxacin (LEV), MTZ, TET, and RIF were tested by agar dilution using a Steers replicator, as recommended by the CLSI guidelines (27). Briefly, each strain was suspended in sterile saline at a density equivalent to a McFarland no.2 to 3 turbidity standard. Using the Steers replicator, approximately 5 μl of each suspension was delivered onto Müeller-Hinton agar plates (MHB; Remel, Lenexa, KS) supplemented with 5% aged defribrinated sheep blood containing 2-fold serial dilutions of each antibiotic. An antibiotic-free plate was inoculated before and after each series of antibiotic plates to confirm viability of the inoculum and observe possible contamination. The plates were incubated under microaerobic conditions at 37°C for 3 days. Isolates were classified as resistant based on the following minimal inhibitory concentration (MIC) breakpoints: >8 μg/ml for MTZ (28); ≥2 μg/ml for RIF (28); ≥1 μg/ml for LEV; ≥2 μg/ml for AMX (28); and ≥2 μg/ml for TET (28). CLR breakpoints were based on CLSI criteria (≤0.25 μg/ml, susceptible; 0.5 μg/ml, intermediate; and ≥1.0 μg/ml, resistant) (29, 30). The MIC is defined as the lowest concentration of antibiotic at which there is a marked decrease in growth relative to the control. H. pylori ATCC 43504 was used as the quality control reference strain. Susceptibility assays were run in duplicate, a minimum of three separate times.
Mutation analysis of resistance genes.
DNA was extracted from H. pylori using a High Pure PCR template preparation kit (Roche Molecular Biochemicals, Indianapolis, IN), according to the manufacturer’s instructions. 16S rRNA and 23S rRNA gene fragments conferring resistance to TET and CLR, respectively, were amplified. The rpoB, gyrA, and rdxA/frxA genes were also amplified for RIF, LEV, and MTZ, respectively. The PCR primer pairs and the nucleotide sequences used in this study are listed in Table 1. An expanded high-fidelity PCR system (Roche Molecular Biochemicals) was used for PCR amplification. The following conditions were used for amplification: 35 cycles of denaturation at 94°C for 1 min, annealing at 55 to 58°C for 1.5 min, and elongation at 72°C for 2 min, followed by an elongation step of 7 min at 72°C. The PCR products were Sanger sequenced (Quintara Biosciences, Allston, MA). The sequence data were analyzed with DNASTAR Lasergene software.
TABLE 1.
PCR primers used to amplify select portions of H. pylori genes
| Antibiotic | Gene | Primer name | Nucleotide sequence | Product size (bp) | Reference |
|---|---|---|---|---|---|
| Metronidazole (MTZ) | rdx | forward primer rdx1 | 5′-GCC ACT CCT TGA ACT TTA ATT TAG G-3′ | 749 | (63) |
| reverse primer rdx4 | 5′-CGT TAG GGA TTT TAT TGT ATG CTA C-3′ | ||||
| frx | forward primer frx1 | 5′-CGA ATT GGA TAT GGC AGC CG-3′ | 913 | (63) | |
| reverse primer frx4 | 5′-TAT GTG CAT ATC CCC TGT AGG -3′ | ||||
| Clarithromycin (CLR) | 23S | forward primer 23SF2 | 5′-CGG TGC TCG AAG GTT AAG AG-3′ | 913 | This study |
| reverse primer 23S R2 | 5′-TTC AGC GGT TAT CAC ATC CA -3′ | ||||
| Levofloxacin (LEV) | gyrA | forward primer gyrA1 | 5′-TTT AGC TTA TTC AAT GAG CGT-3′ | 428 | (64) |
| reverse primer gyrA2 | 5′-GCA GAC GGC TTG GTA GAA TA -3′ | ||||
| Rifampin (RIF) | rpoB | forward primer1369f | 5′-AGG GAC CAC TTG GGC AAT CGT AGG-3′ | 498 | (65) |
| reverse primer1867r | 5′-TAG CGG TCA AAT AAA TCG TCT CAC -3′ | ||||
| Tetracycline (TET) | 16S | forward primer-9F | 5′-GAG TTT GAT YCT GGC TCA G-3′ | 1532 | This study |
| reverse primer-1541R | 5′-AAG GAG GTG WTC CAR CC-3′ | ||||
| Amoxicillin (AMX) | pbp1 | forward primer pbpF1 | 5′-TGC GAA CAC CCT TTT AAA T-3′ | 2385 | (66) |
| reverse primer pbpR1 | 5′-GCG ACA ATA AGA GTG GCA-3′ |
Whole-genome sequencing and analysis.
DNA was extracted from 29 isolates (9 HGCR, 20 LGCR) of the 59 strains using the High Pure PCR product purification kit (Roche Molecular Biochemicals). SMRTbell template libraries were prepared according to the instructions from Pacific Biosciences (PacBio; Menlo Park, CA), following the procedure and checklist for 10 kb template preparation. SMRT sequencing was performed on the PacBio Sequel system. The de novo assembly of the 29 genomes was performed following the instructions of the hierarchical genome assembly process (HGAP), version 4.0. A complete closed contig was obtained for each bacterial genome. The circular genomes were reoriented to a common start site 12 bp 3′ of the nusB (HP0001) gene on the minus strand. Laboratory analyses were performed at the Frederick National Laboratory for Cancer Research, U.S. National Cancer Institute. Genomes were annotated using RAST, hosted by PATRIC (31). Using OrthoFinder, concatenated core gene sequences were determined followed by MAFFT for multi-sequence alignment and FastTree to infer approximate maximum-likelihood phylogenetic trees (32). Chromatiblock was used to produce and visualize whole-genome syntenic alignments (33). The gene sequences for frxA, rdxA, fdxB, gyrA, and gyrB were extracted from assembled contigs using BBMap and translated sequences were aligned using Clustal Omega to build gene trees with the neighbor-joining method. Genomes were analyzed against the Comprehensive Antibiotic Resistance Database to identify additional antibiotic resistance genes (34). MLST 2.0 was used for in silico multilocus sequencing type (MLST) prediction (35). To build a phylogeographic tree, 380 globally distributed H. pylori isolates, belonging to seven ancestral haplogroups, were acquired from PubMLST (36, 37). Concatenated MLSTs were aligned using MAFFT followed by FastTree for the tree inference.
Statistical analysis.
Fisher’s exact tests and kappa coefficients were calculated using GraphPad QuickCalcs (https://www.graphpad.com/quickcalcs/). For Fisher’s exact test, a P value of ≤0.05 was considered statistically significant. For kappa coefficients, a value of <0.4 was considered low agreement, a value of 0.4 to 0.6 was considered moderate agreement, a value of 0.61 to 0.8 was considered substantial agreement, and a value of 0.81 to 1.0 was considered nearly perfect or perfect agreement.
RESULTS
Isolation of H. pylori from Colombian populations.
A total of 59 strains of H. pylori were isolated from human gastric biopsy samples; 31 strains were recovered from patients in the LGCR area, and 28 strains were isolated from patients in the HGCR region. The patients in the LGCR and HGCR groups had similar ages, gender distributions, and gastric pathologies (Table S1 in the supplemental material).
Phenotypic antimicrobial resistance of H. pylori isolates.
All 59 isolates were tested for antibiotic susceptibility to CLR, AMX, MTZ, TET, LEV, and RIF. MTZ resistance was observed in 93.2% (55 of 59) of the H. pylori isolates. MICs for MTZ ranged from 0.125 to 128 μg/ml, with only 4 isolates demonstrating MICs below 8 μg/ml (Table 2). Resistance to LEV was observed in 20.3% (12 of 59) of the H. pylori isolates (MIC range, 0.03 to 32 μg/ml). Only one isolate exhibited resistance to TET. All isolates were susceptible to CLR, AMX, and RIF. The MIC50 and MIC90 for each antibiotic are shown in Table 2. All isolates (31/31) from LGCR patients were resistant to MTZ, compared to 24 of 28 isolates from the HGCR patients (Fisher’s exact test, P value = 0.045) (Table 3). There was no statistical difference in the number of isolates resistant to TET or LEV between the risk groups (Table 3).
TABLE 2.
Antimicrobial susceptibilities of H. pylori isolates
| Antimicrobial | MIC range (μg/ml) | MIC50 (μg/ml)a | MIC90 (μg/ml)b | Resistant |
Susceptible |
||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Metronidazole | 0.125–128 | 32 | 64 | 55/59 | 93.2 | 4/59 | 6.8 |
| Clarithromycin | ≤0.015–0.25 | ≤0.015 | 0.03 | 0/59 | 0 | 59/59 | 100 |
| Levofloxacin | 0.03–32 | 0.25 | 16 | 12/59 | 20.3 | 47/59 | 79.6 |
| Rifampin | ≤0.015–1 | 0.25 | 0.5 | 0/59 | 0 | 59/59 | 100 |
| Tetracycline | ≤0.015–2 | 0.125 | 0.5 | 1/59 | 1.7 | 58/59 | 98.3 |
| Amoxicillin | ≤0.015–0.25 | ≤0.015 | 0.06 | 0/59 | 0 | 59/59 | 100 |
MIC50 = the MIC which inhibits 50% of the isolates.
MIC90 = the MIC which inhibits 90% of the isolates.
TABLE 3.
Summary of resistant H. pylori strains in gastric cancer populations in Colombia
| Parameter | MTZ (MIC >8 μg/ml)b | LEV (MIC >1 μg/ml)b | TET (MIC >1 μg/ml)b | |||
|---|---|---|---|---|---|---|
| Risk group | LGCR | HGCR | LGCR | HGCR | LGCR | HGCR |
| Range (μg/ml) | 16–128 | 0.125–128 | 0.06–32 | 0.03–16 | ≤0.015–1 | ≤0.015–2 |
| No. resistant strains | 31/31 | 24/28 | 9/31 | 3/28 | 0/31 | 1/28 |
| % Resistance | 100 | 86 | 29 | 11 | 0 | 4 |
| P valuea | 0.045* | 0.0621 | 0.4746 | |||
Asterisk (*) indicates P value < 0.05 for Fisher’s exact test between the low- and high-risk groups.
LEV, levofloxacin; TET, tetracycline; MTZ, metronidazole.
Genotypic determination of antimicrobial resistance.
Antibiotic resistance-related genes for CLR, AMX, MTZ, TET, LEV, and RIF from all 59 isolates were amplified by PCR, followed by Sanger sequencing to identify mutations previously associated with resistance phenotypes. Additionally, whole-genome sequencing (WGS) was performed on 29 isolates (20 LGCR and 9 HGCR) (Table S2). WGS identified the same resistance mutations as targeted sequencing (i.e., PCR followed by Sanger sequencing) for all genes evaluated in this study.
Consistent with susceptibility to CLR, AMX, and RIF, no previously reported drug resistance-associated mutations were present in the 23S rRNA (38, 39), rpoB (40, 41), or pbp1A (42–44) genes in any of the isolates. The single isolate resistant to TET had a mutation in the 16S rRNA gene at position A926G (Table 4), which has been associated with low-level (1 to 4 μg/ml) resistance (45, 46).
TABLE 4.
H. pylori isolate MIC values and drug resistance-associated mutations for levofloxacin, tetracycline, and metronidazolea
| Isolate IDb | Cancer risk group | LEV | gyrA (LEV) | TET | 16s rRNA (TET) | MTZ | rdxA (MTZ) | frxA (MTZ) |
|---|---|---|---|---|---|---|---|---|
| MT5110 | LGCR | 8–32 | N87I | S | 16–64 | >217 ELONGATED | ||
| MT5118* | LGCR | 8 | N87K | S | 32–64 | |||
| MT5125* | LGCR | 32 | N87I | S | 32–64 | M1V | 73 STOP | |
| MT5127 | LGCR | 4–8 | D91Y | S | 64–128 | 38 STOP | ||
| MT5156* | LGCR | S | N87T | S | 64–128 | 10 STOP | 73 STOP | |
| MT5165* | LGCR | S | S | 16–32 | 86 STOP | 108 STOP | ||
| MT5175* | LGCR | S | S | 16–32 | 145 STOP | |||
| MT5178 | LGCR | S | S | 32–64 | 73 STOP | |||
| MT5179 | LGCR | 32 | N87I | S | 64–128 | 122 STOP | 73 STOP | |
| MT5101* | LGCR | S | S | 64 | M1V | 38 STOP | ||
| MT5105* | LGCR | 8–32 | N87I | S | 32–64 | 73 STOP | ||
| MT5106* | LGCR | S | S | 64 | 38 STOP | |||
| MT5107 | LGCR | S | S | 32–64 | 49 STOP | 38 STOP | ||
| MT5111* | LGCR | S | N87T | S | 64–128 | 82 STOP | 73 STOP | |
| MT5113 | LGCR | S | N87T | S | 64 | 49 STOP | ||
| MT5114* | LGCR | 16 | N87I | S | 64 | 73 STOP | 73 STOP | |
| MT5116* | LGCR | S | S | 64 | 145 STOP | 38 STOP | ||
| MT5117 | LGCR | S | N87T | S | 64–128 | 73 STOP | ||
| MT5119* | LGCR | S | S | 32–64 | 73 STOP | |||
| MT5120* | LGCR | S | S | 64–128 | 167 STOP | |||
| MT5124* | LGCR | 16–32 | N87I | S | 128 | 73 STOP | ||
| MT5126 | LGCR | S | S | 32–64 | 64 STOP | 129 STOP | ||
| MT5131* | LGCR | S | N87T | S | 64 | 26 STOP | 73 STOP | |
| MT5135* | LGCR | S | N87T | S | 32–64 | 73 STOP | ||
| MT5136* | LGCR | S | N87T | S | 64 | 26 STOP | 73 STOP | |
| MT5139 | LGCR | 4 | N87T, D91Y | S | 16–64 | 32 STOP | ||
| MT5155 | LGCR | S | S | 32 | 67 STOP | |||
| MT5176* | LGCR | S | S | 16–64 | ||||
| PZ5004* | LGCR | S | S | 32–64 | 101 STOP | 73 STOP | ||
| PZ5024 | LGCR | S | N87T | S | 32–64 | 211 ELONGATED | 73 STOP | |
| PZ5026* | LGCR | S | N87T | S | 64–128 | 129 STOP | 38 STOP | |
| MT2139 | HGCR | S | S | 64 | 148 STOP | |||
| MT2141* | HGCR | S | S | 64 | 209 STOP | 38 STOP | ||
| MT2158* | HGCR | S | N87T | S | 64–128 | 49 STOP | 73 STOP | |
| MT2164* | HGCR | S | S | 32–64 | 110 STOP | |||
| MT2143 | HGCR | S | S | >32 | 49 STOP | 38 STOP | ||
| MT2171* | HGCR | S | S | S | ||||
| MT2174* | HGCR | S | S | 64–128 | ||||
| MT2102 | HGCR | S | S | S | 38 STOP | |||
| MT2106 | HGCR | S | N87T | S | 32–64 | 148 STOP | ||
| MT2108 | HGCR | S | S | S | 38 STOP | |||
| MT2112 | HGCR | S | S | 16–32 | 10 STOP | |||
| MT2114 | HGCR | S | S | 16–32 | 215 ELONGATED | |||
| MT2115 | HGCR | S | S | 8 | 38 STOP | |||
| MT2118 | HGCR | S | S | 32 | 58 STOP | 73 STOP | ||
| MT2120* | HGCR | S | S | 64 | 157 STOP | 38 STOP | ||
| MT2122* | HGCR | S | S | 32–64 | 38 STOP | |||
| MT2124 | HGCR | 16–32 | N87I | S | 16–64 | 109 STOP | 73 STOP | |
| MT2127 | HGCR | S | S | 64–128 | 38 STOP | |||
| MT2129* | HGCR | S | S | 64 | 51 STOP | 38 STOP | ||
| MT2130 | HGCR | S | S | 16–32 | 38 STOP | |||
| MT2131 | HGCR | S | S | 8–16 | 90 STOP | |||
| MT2133 | HGCR | 2–4 | D91G | S | 32–64 | 38 STOP | ||
| MT2136 | HGCR | S | N87T | S | 64–128 | 38 STOP | ||
| MT2156 | HGCR | S | S | 64 | 38 STOP | |||
| MT2160* | HGCR | 4–8 | D91Y | S | 64 | 29 STOP | 38 STOP | |
| PZ5056 | HGCR | S | 2 | A926G | 32–64 | 157 STOP | 19 STOP | |
| PZ5080 | HGCR | S | S | S | 73 STOP | |||
| PZ5086 | HGCR | S | S | 64–128 | 206 STOP | |||
| 26695 (ATCC 700392) | Reference strain | |||||||
| ATCC 43504 | Reference strain | 72 STOP | 38 STOP |
Phenotypically resistant MIC values (μg/ml) and drug resistance-associated mutations are highlighted in gray shading. Truncated and elongated rdxA and frxA genes are indicated by their length in amino acid residues and “STOP” or “ELONGATED,” respectively. LEV, levofloxacin; TET, tetracycline; MTZ, metronidazole.
Asterisk (*) indicate isolates evaluated by PCR and whole-genome sequencing (WGS).
The 428-bp region of the H. pylori quinolone resistance-determining region (QRDR) of the gyrA gene was evaluated from the 12 LEV-resistant strains. All resistant strains had a nonsynonymous mutation occurring at amino acid positions N87 and/or D91 (Table 4), which has been previously reported to confer resistance to fluoroquinolones (47, 48). High-level resistance coincided with N87I or N87K mutations, while low-level resistance was present in isolates with D91G or D91Y mutations. Twelve isolates with N87T mutations were susceptible to LEV. One isolate coharboring N87T and D91Y mutations had low-level LEV resistance. Several reports have described N87T mutations in the gyrA gene of LEV-susceptible H. pylori strains (49–53). The N87T mutation has been associated with hypersensitivity to quinolone agents (53). No mutations associated with quinolones were found in gyrB gene sequences. Mutations in the gyrA gene were significantly associated with LEV resistance (Fisher’s exact test, P < 0.00001). The kappa coefficient equaled 0.543 (95% confidence interval [CI]: 0.337 to 0.748), suggesting moderate agreement between phenotypic and genotypic assays to predict LEV resistance in these isolates. When N87T mutations were ignored, the gyrA genes remained significantly associated with LEV resistance (Fisher’s exact test, P < 0.00001), and the kappa coefficient had a perfect agreement of 1.000 (95% CI: 1.00 to 1.00).
In agreement with the high prevalence of MTZ resistance, variant sequences for the rdxA and frxA genes were frequently present in these isolates (Table 4). Of the 59 isolates, 25 had truncated rdxA genes, yielding predicted products of ≤157 residues versus 210 residues for wild-type rdxA found in the H. pylori type strain ATCC 26695. Two isolates had point mutations changing the start codon from M to V. One isolate lacked a stop codon and had a predicted rdxA product of >217 residues. In addition to truncation events, mutations at positions R16H/C/P, H25R, H53R/A, D59N, L62V, A68T/V/S/N, G98S, G163V/D, V204I, and A206T in the rdxA gene product were present in nearly all isolates (Table S3). These substitutions have been previously associated with MTZ resistance (54–57). Forty-nine isolates had truncated frxA gene sequences yielding predicted products of ≤167 compared to 217 residues of the wild-type frxA in the H. pylori type strain ATCC 26695. Nonsynonymous point mutations were also present in the frxA genes of nearly all isolates (Table S4). All resistant strains had at least one truncated rdxA or frxA gene, except for four isolates (MT5118, MT2174, MT5176, and MT2114). However, mutations in at least one residue previously associated with drug resistance (54, 55) were present in these four strains (Table S3, Table S4). Three of the four susceptible strains had truncated frxA sequences but intact rdxA genes, suggesting inactivation of rdxA may be more important in the mechanism of MTZ resistance in some H. pylori isolates. The fourth susceptible strain had intact genes for both rdxA and frxA. There was no statistical association between rdxA or frxA gene mutations and resistance phenotype by Fisher’s exact tests or kappa coefficients, likely because the tests were underpowered by having only four susceptible strains versus 55 resistant strains for comparison. Dendrograms built from the rdxA, frxA, or the concatenation of both gene sequences did not reveal a clear phylogenetic relationship between gene sequence, risk group, or MIC levels (Fig. S1).
Complete genomes of 29 isolates (9 HGCR and 20 LGCR) were sequenced using PacBio to further evaluate mechanisms of antibiotic resistance. Additional antibiotic resistance genes for CLR, AMX, MTZ, TET, LEV, and RIF were not detected. Whole genome alignments showed extensive variation exists in the chromosomal structures among isolates, including rearrangement in the location of the syntenic blocks encoding of frxA, rdxA, and gyrA genes (Fig. 1). These rearrangements suggest chromosomal instability that may have influenced the emergence of antibiotic resistance in these genes.
FIG 1.
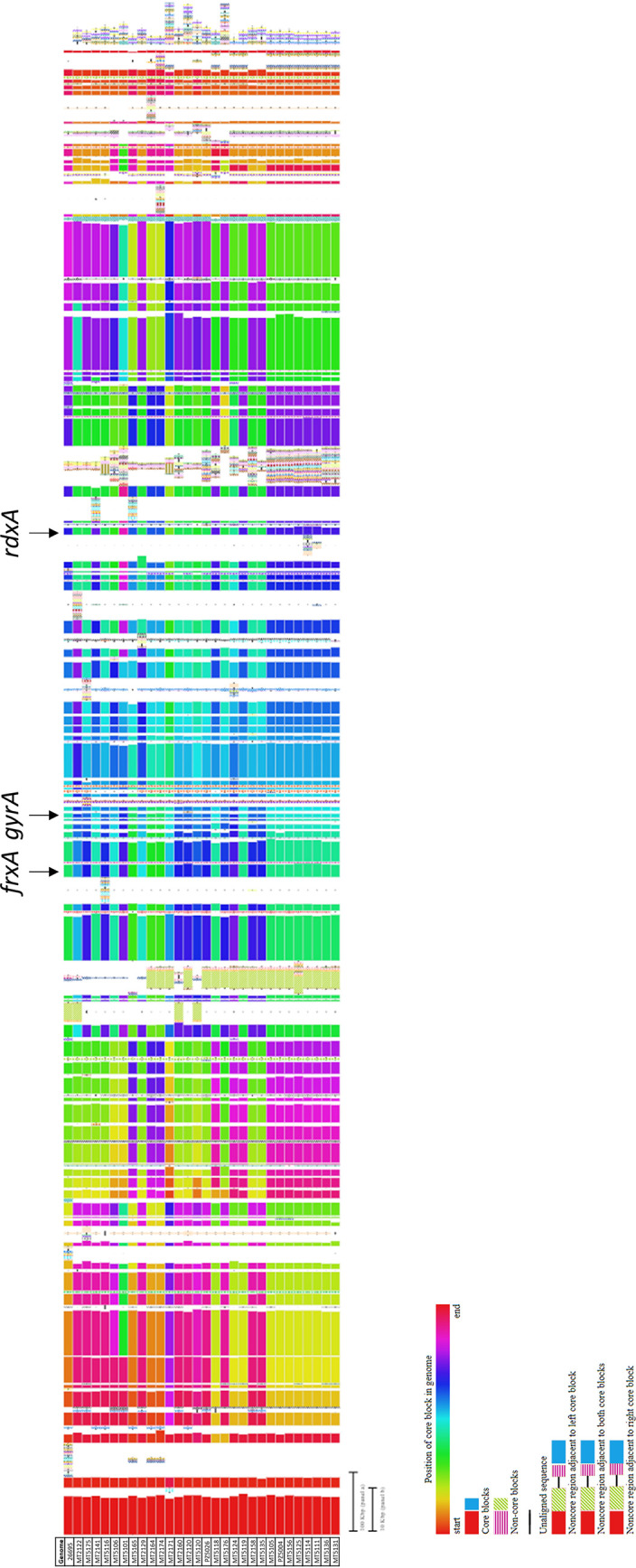
Whole-genome alignments of syntenic blocks of 29 Colombian H. pylori isolates versus the reference strain 26695 (ATCC 700392). The syntenic blocks harboring the frxA, rdxA, and gyrAB genes are indicated.
Whole-genome phylogenetic analysis of H. pylori isolates.
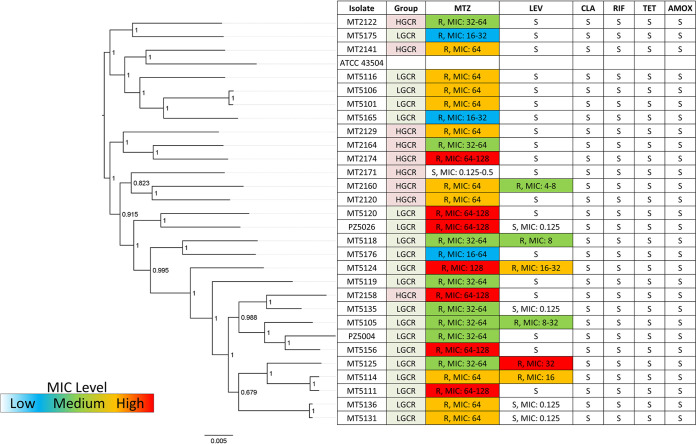
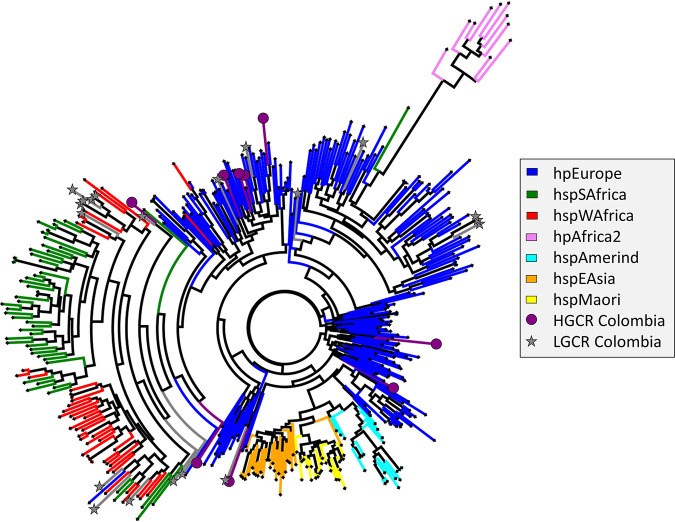
The complete genomes of 29 representative isolates (9 HGCR, 20 LGCR) were also assessed to determine phylogenetic origins. Interestingly, antibiotic resistance profiles for MTZ or LEV did not correlate with whole-genome phylogeny (i.e., the strains with similar resistance levels did not cluster together). Based on a whole-genome phylogenetic tree constructed from multisequence alignment of concatenated core gene sequences, isolates mainly clustered according to risk group (Fig. 2). The H. pylori type strain ATCC 43504 grouped in the HGCR clade. According to multilocus sequence typing, isolates from the LGCR cohort were in general more similar to hspWAfrica, hspSAfrica, and hpEurope strains, while those from HGCR patients associated most closely with hpEurope (Fig. 3), which is similar to the findings by Sablet et al. (58).
FIG 2.
Whole-genome phylogenetic tree constructed from multisequence alignment of concatenated core gene sequences of 29 Colombian H. pylori isolates and the reference strain ATCC 43504. Local support values are indicated on the branches.
FIG 3.
Phylogeographic tree constructed from multisequence alignment of concatenated multilocus sequencing types of 29 Colombian H. pylori isolates with whole-genome sequences. Also included were 380 reference strains with known distinct ancestral haplogroups. Branches are colored according to ancestral haplogroup.
DISCUSSION
In this study, we have determined antibiotic resistance in 59 H. pylori strains cultured from stomach biopsy specimens of Colombian patients from high- and low-risk-gastric-cancer populations. As the prevalence of H. pylori in these populations exceeds 90%, effective use of antibiotics to eradicate infection and mitigate the development of subsequent gastric diseases, including cancer, is critically important. In addition to quantifying phenotypic levels of antibiotic resistance to six antibiotics commonly used in H. pylori eradication modalities (AMX, CLR, LEV, MTZ, RIF, and TET), mutational events in antibiotic resistance-related genes were also identified via PCR and product sequencing, as well as whole-genome sequencing (WGS).
In our study, we used PacBio for WGS of select H. pylori isolates to complement our characterization of antimicrobial resistance. PacBio sequencing technology yields long sequencing reads up to ∼25 kb that enables assembly of complete, continuous bacterial chromosomes and plasmids (59). Conversely, Illumina and other short-read sequencing platforms typically can produce only up to ∼500-bp read lengths and yield incompletely assembled genomes that are fragmented into noncontinuous sequences called contigs (59). While PacBio and short-read sequencing technologies can both provide highly accurate sequence coverage for detection of base pair mutations, contigs produced from short sequencing reads can sometimes result in missing or fragmented genes. Such confounding artifacts are avoided with PacBio, giving this technology a significant advantage for gene detection and sequence analysis. Unlike PCR and Sanger sequencing, WGS analysis is also a time- and cost-effective approach for detecting and evaluating antibiotic-resistant gene profiles. Both PacBio and short-read sequencing technologies have been used to study the evolution, as well as pathogenic mechanisms, of H. pylori (60, 61). While Illumina and Sanger sequencing have also been used to identify mutations in antibiotic-resistance genes in H. pylori (62), our study is the first to our knowledge to use PacBio to study the mechanisms of antimicrobial resistance in this organism. Thus, in addition to detecting specific mutations associated with antibiotic resistance, we were able to analyze how whole-genome phylogenetic relationships, other potential resistance genes, and chromosomal rearrangements may have impacted the antibiotic resistance phenotypes of the HGCR and LGCR Colombian H. pylori isolates.
No drug resistance to CLR, AMX, and RIF, nor drug resistance-associated mutations in the 23S rRNA, rpoB, or pbp1A genes, was observed in any of the 59 isolates. This is in contrast to a systematic review of H. pylori antibiotic resistance in Colombia that reported the prevalence of CLR and AMX resistance to be 16% (7 to 28%) and 6% (2 to 12%), respectively (63). In Latin American countries (Argentina, Brazil, Chile, Colombia, Costa Rica, Cuba, Ecuador, Mexico, Paraguay, Peru, Uruguay, and Venezuela) the prevalence of CLR and AMX resistance is 12% (9 to 16%) and 4% (2 to 8%), respectively (63).
However, in a recent study conducted in patients from Tumaco and Túquerres, Colombia whose H. pylori infection status was 88.7% and 85.4%, respectively, the prevalence of H. pylori resistance to CLR and AMX in patients with dyspepsia symptoms was significantly higher in the LGCR group in Tumaco (20.5% and 22.8%, respectively) than in the high-risk group in Túquerres (3.4% and 5.4%, respectively) (P value <0.05) (17). Of the 74 H. pylori isolates reported in this paper, 57 were cultured from low-risk patients. Of these 57, 28 isolates were resistant to CLR, and 29 were susceptible. Of the 17 H. pylori strains isolated from the high-risk patients, 12 were susceptible to CLR and 5 were resistant. A subset of these isolates was analyzed for mutations noted in the PCR products of the 23S rRNA gene V domain. At least one mutation was noted in the region V domain in 31 (55.3%) of the H. pylori isolates, with 17 (33.3%) resistant and 14 (25%) susceptible to CLR. Interestingly, 9 (16.1%) of the resistant H. pylori isolates did not have mutations in the 23S rRNA region amplified. The authors, by analyzing kappa coefficients, surmised there was no relationship between the presence of mutations and in vitro resistance to CLR (17). Similarly, the authors found there was no statistical relationship to the lack of mutation in the domain V of the 23S rRNA in a particular strain and its in vitro susceptibility to CLR. Nevertheless, the authors concluded that therapeutic failure of eradication treatment (omeprazole 20 mg, CLR 500 mg, and AMX 1,000 mg for 14 days) was associated with punctual mutations of the 23S rRNA gene in CLR-resistant H. pylori (17). While none of the 59 H. pylori isolates from our study had point mutations in the 23S rRNA gene associated with CLR resistance, several of these isolates had identical 23S rRNA mutations found in the CLR-susceptible strains isolated by Matta et al. (17).
TET, LEV, and MTZ drug-resistant phenotypes and genotypes were detected in the 59 H. pylori strains isolated from the low- and high-risk Colombian populations in our study. Only a single isolate exhibited TET resistance, which was confirmed by a mutation in its 16S rRNA gene at position A926, a putative drug-binding site that has been previously associated with drug resistance in H. pylori (64). A recent systematic review and meta-analysis reported that TET resistance is present in ≤10 to 14% of H. pylori isolates worldwide (65). In this meta-analysis, no studies evaluated TET resistance in Colombia; however, one study conducted in Lima, Peru found that ∼4% of H. pylori isolates exhibited TET resistance (65). A systematic review of Latin American countries, including Colombia, noted the prevalence of TET resistance was 6% (2 to 14%) (63).
In our study, the prevalence of resistance for LEV was 20.3% (12/59 isolates). While 9/30 (30%) H. pylori isolates from the low-risk gastric cancer versus 3/29 (11%) in the high-risk group were resistant to LEV, there was no statistical association between risk group and drug resistance. However, there was a significant association between drug resistance phenotype and the presence of mutations at N87 and/or D91 within the quinolone resistance-determining region (QRDR) of the gyrA gene among all 59 isolates. In agreement with our study, mutations at N87 and D91 have been previously reported to confer high- and low-level fluoroquinolone resistance, respectively (47, 48). Worldwide, more than 15% of H. pylori isolates exhibit LEV resistance (65). Prevalence of LEV resistance ranges from 9 to 36% in South American countries (only data from Argentina, Brazil, and Peru were included in the meta-analysis) (65). In all Latin American countries, the reported prevalence of LEV or ciprofloxacin resistance was estimated to be 15% (6 to 28%) (63).
Nearly all isolates in this study exhibited MTZ resistance. These resistant isolates often contained truncated rdxA and/or frxA genes encoding multiple nonsynonymous point mutations implicated with MTZ resistance in previous studies (54–56). Experimentally, it has been shown that MTZ resistance in isolates coharboring both truncated rdxA and frxA genes compared to strains with a single truncated gene alone had higher levels of resistance (66). Of the 59 strains in this study, 20 were resistant to MTZ and contained truncated rdxA and frxA genes. In Peru and Brazil, the prevalence of MTZ-resistant H. pylori ranged from 40 to 62% (65). A study of Colombians from Pasto, Nariño published in 2001 noted that about 50% of the population had H. pylori strains resistant to MTZ (67). In our study, nearly all H. pylori isolates from Tumaco and Túquerres Colombian populations were resistant to MTZ, suggesting uncontrolled, over-the-counter use of this drug may in large part be responsible for MTZ resistance. The prevalence of MTZ resistance in Tumaco and Túquerres, Colombia found in our study was substantially higher than the 53% (46 to 60%) estimated across Latin America (63).
H. pylori strains evaluated by WGS indicated the isolates from Tumaco and Túquerres originated from different phylogeographic locations. Consistent with the study by Sablet et al. (58), the LGCR isolates were of either European or African origin, while the HGCR isolates were more related to the European phylogeographic origin. Likewise, phylogenetic analysis of core gene sequences indicated that H. pylori isolated from the LGCR and HGCR patients were in general distinct from each other. Interestingly, antibiotic phenotypes did not appear to correlate with phylogenetic origin. However, rdxA mutations previously found to be enriched in H. pylori isolates of the European or African phylogeographic origin (57) were frequently present in the strains from our study (Table S4). While large chromosomal rearrangements were apparent among the genomes, including in the regions harboring gyrAB, frxA, and rdxA, antibiotic resistance likely emerged due to the selective pressures of drug use by patients during attempts to eradicate H. pylori or during antibiotic treatment for other infections.
Interestingly, MTZ resistance was the most frequently observed resistance phenotype and was present at a similar rate, although statistically different, between the LGCR and HCGR populations (31/31 versus 24/28 strains). Resistance to other antibiotics evaluated in our study was not detected or not significantly different between these groups. As mentioned previously, over-the-counter access and use of antibiotics may have driven the selection of antibiotic resistance in H. pylori in these Colombian populations, irrespective of their risk for gastric cancer. This surprising finding reinforces the important clinical ramifications of determining community level information regarding H. pylori resistance rates, specifically regarding the selection of personalized first-line eradication therapies versus nonspecific empirical regimens. The primary determinant with respect to selection of triple therapies versus quadruple therapies is the local resistance rate of H. pylori to CLR. Further, rates of resistance to other antibiotics may influence the selection of bismuth-based quadruple therapy (PPI, bismuth, MTZ, TET) versus non-bismuth-based quadruple therapy (PPI, AMX, CLR, MTZ). Knowledge regarding rates of resistance to antibiotics commonly used in eradication regimens may therefore represent an inflection point in optimizing the care of H. pylori-infected persons.
In conclusion, our study found H. pylori strains isolated from Colombians with low and high risk for gastric cancer exhibited multidrug antibiotic resistance. Furthermore, we found antibiotic resistance phenotypes were associated with mutations in genes previously implicated in H. pylori drug resistance. Thorough detection and characterization of antibiotic resistance in H. pylori strains from Colombia is necessary in order to provide efficacious treatment regimens that will eradicate infection in high-risk patients. Thus, the findings from this study indicate that continued monitoring of antibiotic resistance using susceptibility assays and genotyping is warranted in Colombia due to the high occurrence of H. pylori infection and gastric cancer in this country.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge excellent laboratory assistance from Kristine Jones, Kedest Teshome, and Wen Luo from the Frederick National Laboratory for Cancer Research, U.S. National Cancer Institute.
This work was supported by the NIH under awards P01-CA028842-29 (to J.G.F. and K.T.W.) and P30-ES002109 (to J.G.F.) and the Intramural Research Program of the National Cancer Institute.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Bauer B, Meyer TF. 2011. The human gastric pathogen Helicobacter pylori and its association with gastric cancer and ulcer disease. Ulcers 2011:1–23. 10.1155/2011/340157. [DOI] [Google Scholar]
- 2.Blaser MJ, Atherton JC. 2004. Helicobacter pylori persistence: biology and disease. J Clin Invest 113:321–333. 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vakil N, Megraud F. 2007. Eradication therapy for Helicobacter pylori. Gastroenterology 133:985–1001. 10.1053/j.gastro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M, Japan Gast Study Group . 2008. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 372:392–397. 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon ATR, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ, European Helicobacter Study Group . 2012. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut 61:646–664. 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 6.Banić M, Franceschi F, Babić Z, Gasbarrini A. 2012. Extragastric manifestations of Helicobacter pylori infection. Helicobacter 17 Suppl 1:49–55. 10.1111/j.1523-5378.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- 7.Lim SG, Park RW, Shin SJ, Yoon D, Kang JK, Hwang JC, Kim SS, Kim JH, Lee KM. 2016. The relationship between the failure to eradicate Helicobacter pylori and previous antibiotics use. Dig Liver Dis 48:385–390. 10.1016/j.dld.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, Vaz CL, Fock M, Fedail S, Cohen H, Malfertheiner P, Vakil N, Hamid S, Goh KL, Wong BCY, Krabshuis J, Le MA, World Gastroenterology Organization . 2011. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis 20:299–304. [PubMed] [Google Scholar]
- 9.Bernstein CN, McKeown I, Embil JM, Blanchard JF, Dawood M, Kabani A, Kliewer E, Smart G, Coghlan G, MacDonald S, Cook C, Orr P. 1999. Seroprevalence of Helicobacter pylori, incidence of gastric cancer, and peptic ulcer-associated hospitalizations in a Canadian Indian population. Dig Dis Sci 44:668–674. 10.1023/A:1026689103952. [DOI] [PubMed] [Google Scholar]
- 10.Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. 2016. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 43:514–533. 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghotaslou R, Leylabadlo HE, Asl YM. 2015. Prevalence of antibiotic resistance in Helicobacter pylori: a recent literature review. World J Methodol 5:164–174. 10.5662/wjm.v5.i3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etemadi A, Safiri S, Sepanlou SG, Ikuta K, Bisignano C, Shakeri R, Amani M, Fitzmaurice C, Nixon M, Abbasi N, Abolhassani H, Advani SM, Afarideh M, Akinyemiju T, Alam T, Alikhani M, Alipour V, Allen CA, Almasi-Hashiani A, Arabloo J, Assadi R, Atique S, Awasthi A, Bakhtiari A, Behzadifar M, Berhe K, Bhala N, Bijani A, Bin Sayeed MS, Bjørge T, Borzì AM, Braithwaite D, Brenner H, Carreras G, Carvalho F, Castañeda-Orjuela CA, Castro F, Chu D-T, Costa VM, Daryani A, Davitoiu DV, Demoz GT, Demis AB, Denova-Gutiérrez E, Dey S, Dianati NM, Djalalinia S, Emamian MH, Farahmand M, Fernandes JC, Fischer F, Foroutan M, Gad MM, GBD 2017 Stomach Cancer Collaborators , et al. 2020. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 5:42–54. 10.1016/S2468-1253(19)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor A, O'Morain CA, Ford AC. 2017. Population screening and treatment of Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol 14:230–240. 10.1038/nrgastro.2016.195. [DOI] [PubMed] [Google Scholar]
- 14.Correa P. 2003. Helicobacter pylori infection and gastric cancer. Cancer Epidemiol Biomarkers Prev 12:238s–241s. [PubMed] [Google Scholar]
- 15.Park JY, Forman D, Waskito LA, Yamaoka Y, Crabtree JE. 2018. Epidemiology of Helicobacter pylori and CagA-positive infections and global variations in gastric cancer. Toxins (Basel) 10:163. 10.3390/toxins10040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierra MS, Cueva P, Bravo LE, Forman D. 2016. Stomach cancer burden in Central and South America. Cancer Epidemiol 44:S62–S73. 10.1016/j.canep.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Matta AJ, Zambrano DC, Pazos AJ. 2018. Punctual mutations in 23S rRNA gene of clarithromycin-resistant Helicobacter pylori in Colombian populations. World J Gastroenterol 24:1531–1539. 10.3748/wjg.v24.i14.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camargo MC, Beltran M, Conde-Glez CJ, Harris PR, Michel A, Waterboer T, Carolina Flórez A, Torres J, Ferreccio C, Sampson JN, Pawlita M, Rabkin CS. 2015. Serological response to Helicobacter pylori infection among Latin American populations with contrasting risks of gastric cancer. Int J Cancer 137:3000–3005. 10.1002/ijc.29678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, Dominguez R, Morgan D. 2013. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control 24:249–256. 10.1007/s10552-012-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correa P, Cuello C, Duque E, Burbano LC, Garcia FT, Bolanos O, Brown C, Haenszel W. 1976. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst 57:1027–1035. 10.1093/jnci/57.5.1027. [DOI] [PubMed] [Google Scholar]
- 21.Fischbach LA, Bravo LE, Zarama GR, Bravo JC, Ojha RP, Ojha PR, Priest EL, Collazos T, Casabon AL, Casabon LA, Guerrero LZ, Singh KP, Singh PK, Correa P. 2009. A randomized clinical trial to determine the efficacy of regimens containing clarithromycin, metronidazole, and amoxicillin among histologic subgroups for Helicobacter pylori eradication in a developing country. Helicobacter 14:100–108. 10.1111/j.1523-5378.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 22.Malfertheiner P, Mégraud F, O’Morain C, Bell D, Bianchi Porro G, Deltenre M, Forman D, Gasbarrini G, Jaup B, Misiewicz JJ, Pajares J, Quina M, Rauws E. 1997. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut 41:8–13. 10.1136/gut.41.1.8. [DOI] [PubMed] [Google Scholar]
- 23.Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. 2000. Gastric dysplasia: the Padova international classification. Am J Surg Pathol 24:167–176. 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Dixon MF, Genta RM, Yardley JH, Correa P. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 20:1161–1181. 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Yang I, Woltemate S, Piazuelo MB, Bravo LE, Yepez MC, Romero-Gallo J, Delgado AG, Wilson KT, Peek RM, Correa P, Josenhans C, Fox JG, Suerbaum S. 2016. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci Rep 6:18594. 10.1038/srep18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. 1999. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res 59:4823–4828. [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically (M07), 11th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Mégraud F, Lehours P. 2007. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 20:280–322. 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2016. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria (M45), 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PubMed] [Google Scholar]
- 30.Chen D, Cunningham SA, Cole NC, Kohner PC, Mandrekar JN, Patel R. 2017. Phenotypic and molecular antimicrobial susceptibility of Helicobacter pylori. Antimicrob Agents Chemother 61:e02530-16. 10.1128/AAC.02530-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, Gerdes S, Henry CS, Kenyon RW, Machi D, Mao C, Nordberg EK, Olsen GJ, Murphy-Olson DE, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Vonstein V, Warren A, Xia F, Yoo H, Stevens RL. 2017. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res 45:D535–D542. 10.1093/nar/gkw1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol 20:238. 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan MJ, van Bakel H. 2020. Chromatiblock: scalable whole-genome visualization of structural differences in prokaryotes. J Open Source Software 5:2451. 10.21105/joss.02451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen A-LV, Cheng AA, Liu S, Min SY, Miroshnichenko A, Tran H-K, Werfalli RE, Nasir JA, Oloni M, Speicher DJ, Florescu A, Singh B, Faltyn M, Hernandez-Koutoucheva A, Sharma AN, Bordeleau E, Pawlowski AC, Zubyk HL, Dooley D, Griffiths E, Maguire F, Winsor GL, Beiko RG, Brinkman FSL, Hsiao WWL, Domselaar GV, McArthur AG. 2020. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 48:D517–D525. 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, Blaser MJ, Graham DY, Vacher S, Perez-Perez GI, Yamaoka Y, Mégraud F, Otto K, Reichard U, Katzowitsch E, Wang X, Achtman M, Suerbaum S. 2003. Traces of human migrations in Helicobacter pylori populations. Science 299:1582–1585. 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 37.Jolley KA, Bray JE, Maiden MCJ. 2018. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res 3:124. 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor DE, Ge Z, Purych D, Lo T, Hiratsuka K. 1997. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother 41:2621–2628. 10.1128/AAC.41.12.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques AT, Vítor JMB, Santos A, Oleastro M, Vale FF. 2020. Trends in Helicobacter pylori resistance to clarithromycin: from phenotypic to genomic approaches. Microb Genom 6:e000344. 10.1099/mgen.0.000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heep M, Rieger U, Beck D, Lehn N. 2000. Mutations in the beginning of the rpoB gene can induce resistance to rifamycins in both Helicobacter pylori and Mycobacterium tuberculosis. Antimicrob Agents Chemother 44:1075–1077. 10.1128/aac.44.4.1075-1077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glocker E, Bogdan C, Kist M. 2007. Characterization of rifampicin-resistant clinical Helicobacter pylori isolates from Germany. J Antimicrob Chemother 59:874–879. 10.1093/jac/dkm039. [DOI] [PubMed] [Google Scholar]
- 42.Paul R, Postius S, Melchers K, Schäfer KP. 2001. Mutations of the Helicobacter pylori genes rdxA and pbp1 cause resistance against metronidazole and amoxicillin. Antimicrob Agents Chemother 45:962–965. 10.1128/AAC.45.3.962-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon YH, Kim JY, Kim N, Park JH, Nam RH, Lee SM, Kim J-W, Kim JM, Park JY, Lee DH. 2017. Specific mutations of penicillin-binding protein 1A in 77 clinically acquired amoxicillin-resistant Helicobacter pylori strains in comparison with 77 amoxicillin-susceptible strains. Helicobacter 22:e12437. 10.1111/hel.12437. [DOI] [PubMed] [Google Scholar]
- 44.Rimbara E, Noguchi N, Kawai T, Sasatsu M. 2008. Mutations in penicillin-binding proteins 1, 2 and 3 are responsible for amoxicillin resistance in Helicobacter pylori. J Antimicrob Chemother 61:995–998. 10.1093/jac/dkn051. [DOI] [PubMed] [Google Scholar]
- 45.Glocker E, Berning M, Gerrits MM, Kusters JG, Kist M. 2005. Real-time PCR screening for 16S rRNA mutations associated with resistance to tetracycline in Helicobacter pylori. Antimicrob Agents Chemother 49:3166–3170. 10.1128/AAC.49.8.3166-3170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawson AJ, Elviss NC, Owen RJ. 2005. Real-time PCR detection and frequency of 16S rDNA mutations associated with resistance and reduced susceptibility to tetracycline in Helicobacter pylori from England and Wales. J Antimicrob Chemother 56:282–286. 10.1093/jac/dki199. [DOI] [PubMed] [Google Scholar]
- 47.Garcia M, Raymond J, Garnier M, Cremniter J, Burucoa C. 2012. Distribution of spontaneous gyrA mutations in 97 fluoroquinolone-resistant Helicobacter pylori isolates collected in France. Antimicrob Agents Chemother 56:550–551. 10.1128/AAC.05243-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore RA, Beckthold B, Wong S, Kureishi A, Bryan LE. 1995. Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of Helicobacter pylori. Antimicrob Agents Chemother 39:107–111. 10.1128/aac.39.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bachir M, Allem R, Benejat L, Tifrit A, Medjekane M, Drici AE-M, Megraud F, Douidi KT. 2018. Molecular detection of mutations involved in Helicobacter pylori antibiotic resistance in Algeria. J Antimicrob Chemother 73:2034–2038. 10.1093/jac/dky167. [DOI] [PubMed] [Google Scholar]
- 50.Lauener FN, Imkamp F, Lehours P, Buissonnière A, Benejat L, Zbinden R, Keller PM, Wagner K. 2019. Genetic determinants and prediction of antibiotic resistance phenotypes in Helicobacter pylori. J Clin Med 8:53. 10.3390/jcm8010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trespalacios AA, Rimbara E, Otero W, Reddy R, Graham DY. 2015. Improved allele-specific PCR assays for detection of clarithromycin and fluoroquinolone resistant of Helicobacter pylori in gastric biopsies: identification of N87I mutation in GyrA. Diagn Microbiol Infect Dis 81:251–255. 10.1016/j.diagmicrobio.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bilgilier C, Stadlmann A, Makristathis A, Thannesberger J, Kastner M-T, Knoflach P, Steiner P, Schöniger-Hekele M, Högenauer C, Blesl A, Datz C, Huber-Schönauer U, Schöfl R, Wewalka F, Püspök A, Mitrovits N, Leiner J, Tilg H, Effenberger M, Moser M, Siebert F, Hinterberger I, Wurzer H, Stupnicki T, Watzinger N, Gombotz G, Hubmann R, Klimpel S, Biowski-Frotz S, Schrutka-Kölbl C, Graziadei I, Ludwiczek O, Kundi M, Hirschl AM, Steininger C, Austrian Helicobacter Study Group of the Austrian Society of Gastroenterology and Hepatology . 2018. Prospective multicentre clinical study on inter- and intrapatient genetic variability for antimicrobial resistance of Helicobacter pylori. Clin Microbiol Infect 24:267–272. 10.1016/j.cmi.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 53.Cattoir V, Nectoux J, Lascols C, Deforges L, Delchier J-C, Megraud F, Soussy C-J, Cambau E. 2007. Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents 29:389–396. 10.1016/j.ijantimicag.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Kim SY, Joo YM, Lee HS, Chung I-S, Yoo Y-J, Merrell DS, Cha J-H. 2009. Genetic analysis of Helicobacter pylori clinical isolates suggests resistance to metronidazole can occur without the loss of functional rdxA. J Antibiot (Tokyo) 62:43–50. 10.1038/ja.2008.6. [DOI] [PubMed] [Google Scholar]
- 55.Solcà NM, Bernasconi MV, Piffaretti JC. 2000. Mechanism of metronidazole resistance in Helicobacter pylori: comparison of the rdxA gene sequences in 30 strains. Antimicrob Agents Chemother 44:2207–2210. 10.1128/aac.44.8.2207-2210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saranathan R, Levi MH, Wattam AR, Malek A, Asare E, Behin DS, Pan DH, Jacobs WRJ, Szymczak WA. 2020. Helicobacter pylori infections in the Bronx, New York: surveying antibiotic susceptibility and strain lineage by whole-genome sequencing. J Clin Microbiol 58:e01591-19. 10.1128/JCM.01591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang S, Wang X, Wise MJ, He Y, Chen H, Liu A, Huang H, Young S, Tay CY, Marshall BJ, Li X, Chua EG. 2020. Mutations of Helicobacter pylori RdxA are mainly related to the phylogenetic origin of the strain and not to metronidazole resistance. J Antimicrob Chemother 75:3152–3155. 10.1093/jac/dkaa302. [DOI] [PubMed] [Google Scholar]
- 58.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, Bravo LE, Sicinschi LA, Delgado AG, Mera RM, Israel DA, Romero-Gallo J, Peek RM, Cover TL, Correa P, Wilson KT. 2011. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut 60:1189–1195. 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teng JLL, Yeung ML, Chan E, Jia L, Lin CH, Huang Y, Tse H, Wong SSY, Sham PC, Lau SKP, Woo PCY. 2017. PacBio but not Illumina technology can achieve fast, accurate and complete closure of the high GC, complex Burkholderia pseudomallei two-chromosome genome. Front Microbiol 8:1448. 10.3389/fmicb.2017.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ailloud F, Didelot X, Woltemate S, Pfaffinger G, Overmann J, Bader RC, Schulz C, Malfertheiner P, Suerbaum S. 2019. Within-host evolution of Helicobacter pylori shaped by niche-specific adaptation, intragastric migrations and selective sweeps. Nat Commun 10:2273. 10.1038/s41467-019-10050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Estibariz I, Ailloud F, Woltemate S, Bunk B, Spröer C, Overmann J, Aebischer T, Meyer TF, Josenhans C, Suerbaum S. 2020. In vivo genome and methylome adaptation of cag-negative Helicobacter pylori during experimental human infection. mBio 11:e01803-20. 10.1128/mBio.01803-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Egli K, Wagner K, Keller PM, Risch L, Risch M, Bodmer T. 2020. Comparison of the diagnostic performance of qPCR, Sanger sequencing, and whole-genome sequencing in determining clarithromycin and levofloxacin resistance in Helicobacter pylori. Front Cell Infect Microbiol 10:596371. 10.3389/fcimb.2020.596371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camargo MC, García A, Riquelme A, Otero W, Camargo CA, Hernandez-García T, Candia R, Bruce MG, Rabkin CS. 2014. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol 109:485–495. 10.1038/ajg.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerrits MM, de Zoete MR, Arents NLA, Kuipers EJ, Kusters JG. 2002. 16S rRNA mutation-mediated tetracycline resistance in Helicobacter pylori. Antimicrob Agents Chemother 46:2996–3000. 10.1128/aac.46.9.2996-3000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. 2018. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology 155:1372–1382.e17. 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwon D-H, El-Zaatari FAK, Kato M, Osato MS, Reddy R, Yamaoka Y, Graham DY. 2000. Analysis of rdxA and involvement of additional genes encoding NAD(P)H flavin oxidoreductase (FrxA) and ferredoxin-like protein (FdxB) in metronidazole resistance of Helicobacter pylori. Antimicrob Agents Chemother 44:2133–2142. 10.1128/aac.44.8.2133-2142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischbach LA, Correa P, Ramirez H, Realpe JL, Collazos T, Ruiz B, Bravo LE, Bravo JC, Casabon AL, Schmidt BA. 2001. Anti-inflammatory and tissue-protectant drug effects: results from a randomized placebo-controlled trial of gastritis patients at high risk for gastric cancer. Aliment Pharmacol Ther 15:831–841. 10.1046/j.1365-2036.2001.00998.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.