Rapid diagnostic tests (RDTs) for SARS-CoV-2 antigens (Ag) that can be performed at point of care (POC) can supplement molecular testing and help mitigate the COVID-19 pandemic. Deployment of an Ag RDT requires an understanding of its operational and performance characteristics under real-world conditions and in relevant subpopulations.
KEYWORDS: COVID-19, SARS-CoV-2, diagnostic, antigen, point of care
ABSTRACT
Rapid diagnostic tests (RDTs) for SARS-CoV-2 antigens (Ag) that can be performed at point of care (POC) can supplement molecular testing and help mitigate the COVID-19 pandemic. Deployment of an Ag RDT requires an understanding of its operational and performance characteristics under real-world conditions and in relevant subpopulations. We evaluated the Abbott BinaxNOW COVID-19 Ag card in a high-throughput, drive-through, free community testing site in Massachusetts using anterior nasal (AN) swab reverse transcriptase PCR (RT-PCR) for clinical testing. Individuals presenting for molecular testing in two of seven lanes were offered the opportunity to also receive BinaxNOW testing. Dual AN swabs were collected from symptomatic and asymptomatic children (≤18 years of age) and adults. BinaxNOW testing was performed in a testing pod with temperature/humidity monitoring. One individual performed testing and official result reporting for each test, but most tests had a second independent reading to assess interoperator agreement. Positive BinaxNOW results were scored as faint, medium, or strong. Positive BinaxNOW results were reported to patients by phone, and they were instructed to isolate pending RT-PCR results. The paired RT-PCR result was the reference for sensitivity and specificity calculations. Of 2,482 participants, 1,380 adults and 928 children had paired RT-PCR/BinaxNOW results and complete symptom data. In this study, 974/1,380 (71%) adults and 829/928 (89%) children were asymptomatic. BinaxNOW had 96.5% (95% confidence interval [CI], 90.0 to 99.3) sensitivity and 100% (95% CI, 98.6 to 100.0) specificity in adults within 7 days of symptoms and 84.6% (95% CI, 65.1 to 95.6) sensitivity and 100% (95% CI, 94.5 to 100.0) specificity in children within 7 days of symptoms. Sensitivity and specificity in asymptomatic adults were 70.2% (95% CI, 56.6 to 81.6) and 99.6% (95% CI, 98.9 to 99.9), respectively, and in asymptomatic children, they were 65.4% (95% CI, 55.6 to 74.4) and 99.0% (95% CI, 98.0 to 99.6), respectively. By cycle threshold (CT) value cutoff, sensitivity in all subgroups combined (n = 292 RT-PCR-positive individuals) was 99.3% with CT values of ≤25, 95.8% with CT values of ≤30, and 81.2% with CT values of ≤35. Twelve false-positive BinaxNOW results (out of 2,308 tests) were observed; in all 12, the test bands were faint but otherwise normal and were noted by both readers. One invalid BinaxNOW result was identified. Interoperator agreement (positive versus negative BinaxNOW result) was 100% (n = 2,230/2,230 double reads). Each operator was able to process 20 RDTs per hour. In a separate set of 30 specimens (from individuals with symptoms ≤7 days) run at temperatures below the manufacturer’s recommended range (46 to 58.5°F), sensitivity was 66.7% and specificity 95.2%. BinaxNOW had very high specificity in both adults and children and very high sensitivity in newly symptomatic adults. Overall, 95.8% sensitivity was observed with CT values of ≤30. These data support public health recommendations for use of the BinaxNOW test in adults with symptoms for ≤7 days without RT-PCR confirmation. Excellent interoperator agreement indicates that an individual can perform and read the BinaxNOW test alone. A skilled laboratorian can perform and read 20 tests per hour. Careful attention to temperature is critical.
INTRODUCTION
Nucleic acid amplification tests (NAAT) for SARS-CoV-2, the etiologic agent of COVID-19, can be highly sensitive and are being performed at high volumes in centralized laboratories around the world (https://coronavirus.jhu.edu/map.html; https://covid.cdc.gov/covid-data-tracker/#testing_testsperformed). Unfortunately, shortages of testing reagents and logistic barriers (e.g., sample transport) have impacted access to molecular testing and turnaround time for results. The need for decentralized testing options with short turnaround times has led to the development of rapid diagnostic tests (RDTs) for point-of-care (POC) use that detect SARS-CoV-2 nucleocapsid antigen (Ag) in as little as 15 min. As of 26 December 2020, there are 11 Ag RDTs with FDA Emergency Use Authorization (EUA) (https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas#individual-antigen). Most of these tests can be performed by personnel without formal laboratory training in patient care settings that operate minimally under a Clinical Laboratory Improvement Amendments (CLIA) Certificate of Waiver (COW).
Reported clinical sensitivities for Ag RDTs performed at POC in individuals suspected of COVID-19 vary widely, ranging from 74% to 97% compared with reverse transcriptase PCR (RT-PCR) (1–9). Reports of false-negative and false-positive Ag RDT results (10) have raised concerns regarding their use, although a range of settings would benefit from RDT availability (e.g., K-12 schools, nursing homes, community testing centers, and home settings). Gaps in knowledge of how Ag RDTs perform in asymptomatic individuals and children remain.
In symptomatic adults, viral loads in nasopharyngeal (NP) samples peak within the first week of symptom onset and then decrease over a variable time frame; RNA levels in asymptomatically infected adults appear to follow similar kinetics (11, 12). Ag concentrations have been shown in one study to correlate tightly with cycle threshold (CT) values in NP samples from adults and children (13). While NP sampling remains the reference method, anterior nasal (AN) sampling substantially increases testing access and acceptability, and a recent head-to-head study suggested that Ag RDTs performed on paired NP/AN samples yielded similar results (2).
The BinaxNOW COVID-19 Ag card (5) has FDA EUA for AN swab samples and can provide visually read results at POC in 15 min. The potential to use this test at large scale, combined with the lack of data for test performance in asymptomatic adults and in children, motivated us to perform an implementation and performance evaluation in a high-volume, high-prevalence community testing site currently using AN swab RT-PCR for clinical testing.
MATERIALS AND METHODS
Study population.
The study was performed between 26 October and 22 December 2020 (all ages, 26 October to 12 November 2020, and children only from 11 to 22 December 2020) at the Lawrence General Hospital “Stop the Spread” drive-through testing site, which accommodates Massachusetts residents from the surrounding area. No study-specific effort was made to recruit individuals to present to the testing site. Two of seven drive-through lanes were utilized for the study. Verbal consent for dual AN swabbing was obtained from adults and guardians of minors (with verbal assent for ages 7 to 17). Participants were informed that they would be called for positive Ag RDT results only and that positive results would require isolation while waiting for the RT-PCR result. Presence or absence of symptoms (sore throat, cough, chills, body aches, shortness of breath, fever, runny nose, congestion, nausea, vomiting, diarrhea, loss of taste or smell) were recorded for each individual, including the date of symptom onset. Participants whose symptoms started on the day of testing were classified as day 0. The study was reviewed by the Massachusetts Department of Public Health institutional review board (IRB) and deemed not human subject research.
Swab collection procedure.
Cars with consented patients were marked with a glass marker, notifying the specimen collector to collect two AN swabs rather than one. Swab collection details are in Supplemental Methods; in brief, collection involved swabbing both nostrils with each swab, and operators alternated which swab was collected first (for RT-PCR versus BinaxNOW). BinaxNOW swabs were captured in an empty sterile tube and taken to the testing pod by a designated “runner.” The time of sample collection was recorded.
Abbott BinaxNOW performance.
Details of kit storage, quality control, and testing and results reporting procedures are in Supplemental Methods.
RT-PCR assay.
Dry AN swabs (SteriPack sterile polyester spun swab, 3 in. [Lakeland, FL]) were collected per site routine and transported at room temperature to the Broad Institute for testing using the CRSP SARS-CoV-2 real-time reverse transcriptase (RT) PCR diagnostic assay under EUA (14). Details are in Supplemental Methods.
Results reporting.
All positive BinaxNOW results were reported to individuals the same day by Massachusetts Department of Public Health (DPH) epidemiologists. Participants with positive BinaxNOW results were informed that the result was presumptive and that they should maintain isolation for a minimum of 10 days based upon the result but that a confirmatory result would be obtained by RT-PCR and reported in 1 to 2 days. If the RT-PCR result was negative, they could discontinue isolation. RT-PCR results were provided to the patient by the Lawrence General Hospital’s portal or by walk-up to a designated location at the Lawrence General Hospital. RT-PCR results were reported to DPH through routine electronic laboratory reporting mechanisms, and individuals with positive results were referred to local boards of health or the Community Tracing Collaborative for instruction on isolation and contact tracing.
Statistical analysis.
Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) for the BinaxNOW were calculated using the RT-PCR result as the reference. We calculated 95% confidence intervals (CIs) using the Clopper-Pearson method. Analyses utilized Microsoft Excel and GraphPad Prism.
RESULTS
Four different lots of BinaxNOW kits were used for the study. Each operator was able to set up a new test every 2 to 3 min, and two operators were able to manage testing of samples coming from two drive-through lanes. All tests were initiated within 1 h of collection per manufacturers’ instructions. Temperature and humidity in the testing pod (Supplemental Methods) between 7:30 a.m. to 6:00 p.m. ranged from 33.3 to 75.7°F and 27.8 to 81.0%, respectively, in the shipping container and from 67.6 to 81.6°F and 20.8 to 69.0%, respectively, in the trailer. Tests for the main study were not run until the temperature reached 59°F per the manufacturer’s recommendation. Data for tests performed out of the temperature range in the package insert (<59°F) were analyzed separately (below).
Of 2,482 participants (excluding samples tested at <59°F [n = 94], inconclusive RT-PCR results [n = 26], and missing data [n = 54]), 2,308 had paired PCR/BinaxNOW results and complete symptom data, including 829 asymptomatic children, 974 asymptomatic adults, 99 symptomatic children, and 406 symptomatic adults. Symptomatic individuals were further classified as ≤7 days versus >7 days of symptoms. Clinical data for the study population are presented in Table 1 (demographics) and Tables S1 and S2 (symptoms).
TABLE 1.
Clinical characteristics of adult and pediatric patients contributing paired samples
| Characteristic | Adults, symptomatic (n = 407) | Adults, asymptomatic (n = 974) | Children, symptomatic (n = 99) | Children, asymptomatic (n = 829) |
|---|---|---|---|---|
| Age in yrs (no.) | ||||
| <7 | NAc | NA | 17 | 244 |
| 7–13 | NA | NA | 42 | 339 |
| 14–18 | NA | NA | 40 | 246 |
| 19–29 | 120 | 212 | NA | NA |
| 30–49 | 189 | 392 | NA | NA |
| 50–69 | 89 | 312 | NA | NA |
| 70 and older | 8 | 58 | NA | NA |
| Sex (% female) | 58.9 | 56.4 | 61.6 | 52.0 |
| Days of symptoms prior to COVID test (median [IQR]) | 3 (2–5)a | NA | 2 (1–4)b | NA |
Range, 0 to 55 days.
Range, 0 to 60 days.
NA, not applicable.
BinaxNOW performance in adults and children (≤18 years).
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) calculations for BinaxNOW results versus RT-PCR results as the reference for each clinical subgroup are presented in Table 2. Tables with data for each subgroup are presented in Table S3.
TABLE 2.
Performance of the Abbott BinaxNOW versus RT-PCR (reference method) for detection of SARS-CoV-2 in anterior nasal swab samples from adult and pediatric (≤18 years of age) patientsa
| Age | Group | No. of patients | Prevalence (%) | Sensitivity (% [95% CI]) | Specificity (% [95% CI]) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|---|
| All | Sx and ASx | 2,308 | 12.7 | 77.4 (72.2–82.1) | 99.4 (99.0–99.7) | 95.0 (91.5–97.1) | 96.8 (96.1–97.4) |
| Pediatric | Sx and ASx | 928 | 14.5 | 69.6 (61.1–77.2) | 99.0 (98.2–99.6) | 92.1 (84.7–96.1) | 95.7 (94.5–96.6) |
| Adult | Sx and ASx | 1,380 | 11.4 | 84.1 (77.4–89.4) | 99.6 (99.1–99.9) | 96.4 (91.7–98.5) | 98.0 (97.1–98.6) |
| Pediatric | ASx | 829 | 12.9 | 65.4 (55.6–74.4) | 99.0 (98.0–99.6) | 90.9 (82.5–95.5) | 95.1 (93.7–96.2) |
| Adult | ASx | 974 | 5.9 | 70.2 (56.6–81.6) | 99.6 (98.9–99.9) | 90.9 (78.8–96.4) | 98.1 (97.3–98.8) |
| Pediatric | Sx ≤7 days | 91 | 28.6 | 84.6 (65.1–95.6) | 100.0 (94.5–100.0) | 100.0 | 94.2 (86.8–97.6) |
| Adult | Sx ≤7 days | 355 | 23.9 | 96.5 (90.0–99.3) | 100.0 (98.6–100.0) | 100.0 | 98.9 (96.7–99.6) |
| Pediatric | Sx >7 days | 8 | 25 | NA | NA | NA | NA |
| Adult | Sx >7 days | 51 | 29.4 | 66.7 (38.4–88.2) | 97.2 (85.5–99.9) | 90.9 (58.3–98.6) | 88.2 (77.4–95.6) |
Sx, symptomatic; ASx, asymptomatic; NA, not applicable.
Sensitivity in adults with symptoms for ≤7 days was 96.5%, similar to that in the BinaxNOW package insert (97.1%) (5). Sensitivity in children with symptoms for ≤7 days was 84.6%. Specificity in both groups was 100%. Relative to symptomatic individuals, sensitivity in asymptomatic adults and children was lower at 70.2% and 65.4%, respectively, while specificity remained high (99.6% and 99.0%, respectively). The anticipated NPV/PPVs with various prevalence, based on the observed sensitivity/specificity in each subgroup, are presented in Table S4.
Discordant analysis and analysis of CT values.
There were 12 false-positive BinaxNOW results across all 2,308 individuals tested; 4 were in asymptomatic adults, 7 in asymptomatic children, and 1 in a symptomatic adult with >7 days of symptoms. All 12 positives were scored as faint bands by both independent readers, and there was nothing unusual noted about band morphology. Sixty-one out of sixty-six false negatives had dual read; 61/61 were scored as negative by both independent readers. There was no apparent correlation between false positives/false negatives and lot or kit number in testing through 12 November. In the pediatric-only phase initiated 11 December, all 6 false-positive samples observed utilized one lot that was not used in the first phase; there was no correlation between false negatives and lot number.
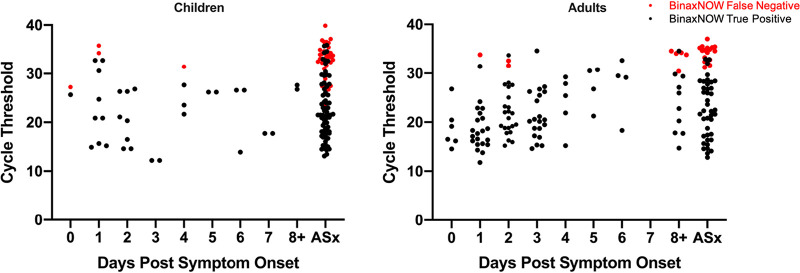
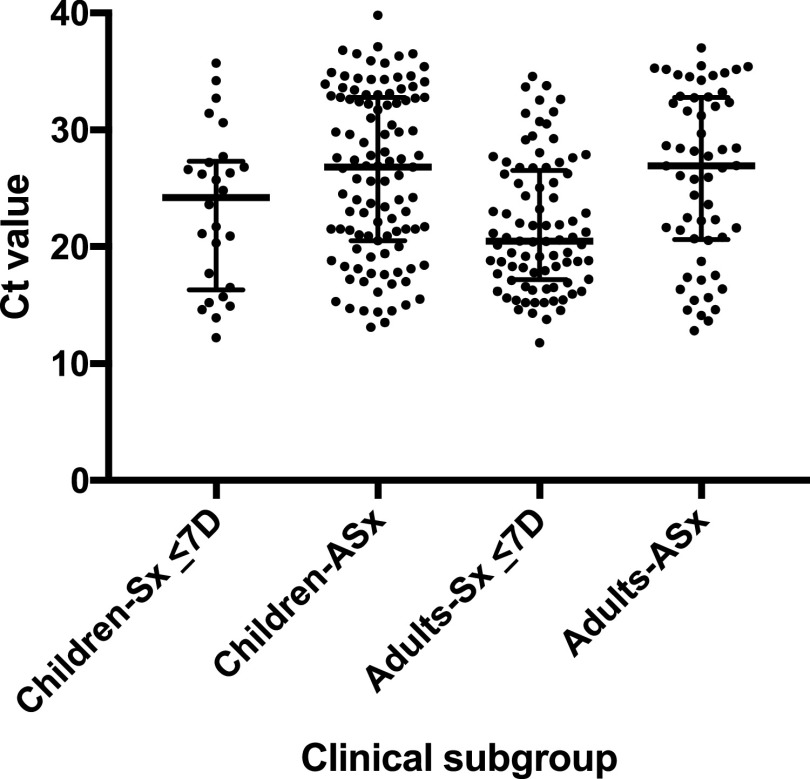
Distributions of CT values for RT-PCR-positive symptomatic (by days post-symptom onset) and asymptomatic children and adults are shown in Fig. 1; false-negative versus true-positive paired BinaxNOW results are indicated for all individuals. As expected, false-negative BinaxNOW results were paired with RT-PCR tests with higher CT values. CT distributions in the four main clinical subgroups are shown in Fig. 2. Median CT values (interquartile range [IQR]) for children and adults symptomatic for ≤7 days were 24.2 (16.3 to 27.3) and 20.5 (17.2 to 26.5), respectively, and for asymptomatic children and adults, they were 26.8 (20.5 to 32.8) and 26.9 (20.6 to 32.8), respectively. Sensitivity was evaluated at three different CT cutoffs, ≤25, ≤30, and ≤35 (Table S5). Sensitivity in all subgroups combined (n = 292 RT-PCR-positive individuals) was 99.3% with CT values of ≤25, 95.8% with CT values of ≤30, and 81.2% with CT values of ≤35. Band strength (1, faint [n = 41]; 2, medium [n = 15]; and 3, strong [n = 170]) as interpreted by the primary reader for the 226 true-positive BinaxNOW tests correlated clearly with CT value, with median (IQR) CT values of 29.7 (27.6 to 32.7), 27.7 (25.5 to 29.1), and 19.5 (16.4 to 22.8), respectively, as shown in Fig. S1. All but 1 (65/66) false-negative BinaxNOW results had a paired RT-PCR CT values of >25, and 9 had CT values of ≤30; the median (IQR) cycle threshold value for these false negatives was 33.7 (32.1 to 34.8). The distribution of the 66 false-negative results among clinical subgroups is shown in the 2 by 2 tables in Table S3.
FIG 1.
Distribution of cycle threshold (CT) values in RT-PCR-positive children and adults by days post-symptom onset. CT values for each RT-PCR-positive individual are shown; red circles, false-negative BinaxNOW results; black circles, true-positive BinaxNOW results. Participants whose symptoms started on the day of testing are indicated as day 0. ASx, asymptomatic.
FIG 2.
Distribution of CT values in RT-PCR-positive individuals. Cycle threshold (CT) values for RT-PCR-positive individuals in each of four clinical subgroups are shown; horizontal bars represent median and IQR. Sx, symptomatic; ASx, asymptomatic; D, days. Median CT values (IQR) in patients who were symptomatic for ≤7 days are 24.2 (16.3 to 27.3) for children and 20.5 (17.2 to 26.5) for adults and in asymptomatic patients were 26.8 (20.5 to 32.8) for children and 26.9 (20.6 to 32.8) for adults.
Operational findings.
A small number of BinaxNOW tests were performed at temperatures below the manufacturer’s recommended temperature range due to difficulty maintaining temperature in the antigen testing location and interest in exploring the effect of low temperature on test performance. These data were analyzed separately from the main study. In 30 specimens run at low temperature (46 to 58.5°F) from individuals (3 children and 27 adults) with symptoms for ≤7 days, sensitivity was 66.7% and specificity 95.2% (Table S6), in comparison to sensitivity of 93.7% and specificity of 100% in individuals with ≤7 days symptoms when testing was performed at >59°F. Relative humidity ranged from 45.7 to 66.4% during the two testing days with low temperatures (≤59°F). For false-positive or false-negative tests performed at >59°F, the humidity range was 27.2 to 62.6% and 22.6 to 61.5%, respectively, mirroring the overall humidity range and distribution.
Interoperator agreement was excellent, with the two readers agreeing on the positive versus negative results for all 2,230 BinaxNOW tests that had dual reads. The two readers disagreed on the strength of the positive band (faint versus medium versus strong) in 5 cases. Overall, readers noted that detection of faint positive bands required very close observation. While we did observe occasional inconsistent band intensity across the width of some test bands, we did not identify a correlation between that finding and false positivity. One invalid BinaxNOW result was identified during the study (a manufacturing issue whereby plastic covered the test strip, preventing the buffer from making contact with the test strip).
DISCUSSION
The development of Ag RDTs has expanded the options for POC COVID-19 testing and raised critical questions about how these tests could and should be used. Prominent examples of both false-negative and false-positive results (10) on Ag RDTs have raised questions about how well the tests perform in real-world settings, even if performed carefully by trained operators. Major gaps in performance data, including performance in asymptomatic adults and both symptomatic and asymptomatic children, have generated uncertainty about how to optimally deploy Ag RDTs, and emerging comparison studies reiterate that not all Ag RDTs perform equivalently (1, 15). The FDA EUA for the BinaxNOW RDT was based upon 102 subjects, all adults, who had symptomatic COVID-19 with symptom onset within 7 days prior to testing (5). In order to understand how well the BinaxNOW RDT could perform in both symptomatic and asymptomatic adults and children, we implemented the test at a high-volume community testing site already experienced in collecting AN samples for RT-PCR, performing the test with careful attention to sample collection, test performance, results documentation, and quality control. The BinaxNOW was performed by trained laboratory personnel (master’s- to PhD-level laboratorians). This study design allowed us to evaluate performance of the BinaxNOW in a real-world but also best-case scenario and to provide the first data on performance in a large population of children.
We found that test specificity was very high in this study in all populations, while sensitivity was variable. Sensitivity was very high in symptomatic adults within the first 7 days of illness and less so in symptomatic children. Sensitivity in asymptomatic adults and children was even lower, corresponding with the broad viral load distribution observed in this population (likely capturing early and late infections given unknown disease onset). For all groups, sensitivity was highest in individuals with the highest viral loads.
Our study yielded some important operational findings relevant to test implementation. Interoperator agreement on positive/negative results was 100%, confirming that only one person is needed to read each test result. While one skilled operator was able to run 20 tests per hour, two people were needed to run tests in high volume, and additional people were needed to transport samples from the collection site to the testing site. We noticed a substantial impact of low temperature (below the manufacturer’s recommended range) on test accuracy, reinforcing that temperature is a critical parameter for test performance; the instructions for use should be followed closely, and temperature should be monitored for every test performed. Gathering additional data in summer months to confirm that test specificity remains high with elevated environmental temperatures would be prudent.
Our data are consistent with, or show better performance than, those obtained in other field studies of visually read Ag RDTs. A recent European prospective study (1) evaluated visually read Ag RDT test performance at POC versus RT-PCR using nasopharyngeal/oropharyngeal (OP) samples and found that the best-performing Ag RDT (Standard Q COVID-19 Ag; SD Biosensor) was 76.6% sensitive and 99.3% specific, with 100% sensitivity in samples with CT values of <25 and 62.1% sensitivity in samples with CT values of ≥25 (1). Another POC study of the SD Q test (NP swab) versus RT-PCR (NP/OP swab) in symptomatic adults at a drive-through site in the Netherlands (9) yielded Ag RDT sensitivity of 84.9% and specificity of 99.5%, with 95.8% sensitivity in people within 7 days of symptoms and with CT values of <30. A recent study of the Abbott BinaxNOW versus AN swab RT-PCR as performed in adults on a public plaza in San Francisco found that the test had 93.3% sensitivity in samples with CT values of <30 and 99.9% specificity (6). However, achieving this specificity required an off-label reading procedure in which the reader was asked to disregard bands that did not extend across the full width of the strip. We did observe occasional inconsistent band intensity across the width of some test bands but did not identify a correlation between that finding and false positivity. All 12 false-positive tests in our study had faint positive (and otherwise normal) bands scored by both independent readers. The San Francisco study also used two readers for each test, with a tiebreaker read if they disagreed, but did not report data on interoperator agreement. A study of the Abbott PanBio test in symptomatic adults and children in Spain (8) compared POC Ag RDT (NP swab) to RT-PCR (NP swab in 3.0 ml media) and found overall Ag RDT sensitivity of 79.6%, with specificity of 100%. Notably, sensitivity was higher in adults (82.6%) than children (62.5%), and sensitivity in individuals with RT-PCR CT values of <25 (viral load >5.9 log10 copies/ml) was 100%; our own observations are consistent with both findings. The authors speculated that the lower sensitivity in symptomatic children versus adults might be due to more difficulty in pinpointing the date of symptom onset in children, which is also a possibility in our study. However, an alternative explanation is that symptomatic children have a different distribution of viral loads than symptomatic adults, with a higher proportion having lower viral loads (e.g., above CT values of 30) within the first week of symptoms. This hypothesis is supported by distributions of CT values (and antigen concentrations) in NP swab samples from symptomatic adults versus children in a recent study (13), but other studies of this question have yielded mixed results (16–18). Defining viral load distributions and kinetics in symptomatic children remains an important area for research.
Key considerations for use of Ag RDTs are whether they are able to reliably identify patients with high loads of live virus that may be more at risk for transmitting disease and whether infected individuals missed by Ag RDTs might or might not be infectious to others. Several reports have noted that virus could only rarely be cultured from patient samples with measured viral loads below approximately 1 × 105 RNA copies/ml (16, 19–21). Notably, Albert et al. found that SARS-CoV-2 could not be cultured from specimens from 11 individuals with positive RT-PCR/negative POC Abbott PanBio Ag RDT results, all with <5.9 log10 copies/ml (8), and Igloi et al. found that most culture-positive individuals were also positive by the SD Q assay (9). The overlap between viral culture and Ag RDT percent positivity (versus RT-PCR) has also been described for the BD Veritor system (22). However, virus has been recovered from samples with RNA levels as low as 1.2 × 104 copies/ml (23) and from samples with CT values of 34 to 35 or greater on a range of RT-PCR assays (24–26). Multiple evaluations of POC Ag RDT performance have assessed performance at CT value cutoffs of 25 and 30 (e.g., references 1 and 6), which guided our choice to assess sensitivity of the BinaxNOW at three different CT cutoffs, 25, 30, and 35. For the RT-PCR assay used in this study, CT values of 25, 30, and 35 correspond to approximately 5.4 × 105, 1.7 × 104, and 5.5 × 102 copies/ml, respectively (N. Lennon, personal communication; Supplemental Methods), and we found that patients with CT values of ≤30 were reliably detected (95.8%) by the BinaxNOW, similar to results from Pilarowski et al. (93.3% sensitivity with CT values of <30, corresponding to 1.9 × 104 copies/ml) (6). A recent study of multiple commercial Ag RDTs found that the analytical sensitivity range overlapped with viral load ranges typically observed in the first week of symptoms, also considered to be the period of highest infectiousness (15); consistent with this, we observed that the sensitivity of the BinaxNOW in adults with ≤7 days of symptoms was 96.5%.
Our study had some limitations. We recognize that the comparator in our study was RT-PCR performed on an AN swab, as opposed to an NP swab, which is still considered the reference method by the FDA (https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/faqs-testing-sars-cov-2). While AN swabs have had lower sensitivity than NP swabs in some studies, the sensitivity is highly dependent on the sampling technique and assay used (27). The dry AN swab sampling method used in this study has been shown to have similar sensitivity to paired NP swabs in transport media (14). We also note that a recent comparison study demonstrated that Ag RDT performance with AN swabs was similar to Ag RDT performance with NP swabs (2). We were not able to have all tests read by two independent readers, but did so for the majority of tests. Finally, we recognize that our symptomatic pediatric cohort was relatively small and thus the confidence interval relatively wide, exemplifying the challenges in assessing COVID-19 diagnostics in children. There may be important differences in performance characteristics of Ag tests or in viral dynamics corresponding to ages of children, but there were insufficient symptomatic children with positive tests in the study to stratify by age group.
Current recommendations from the WHO (28) suggest that Ag RDTs be used in symptomatic individuals within the first 5 to 7 days of symptoms, with repeat testing or RT-PCR confirmation of negative Ag RDT results if possible. WHO recommendations include testing during suspected outbreaks, monitoring trends in disease incidence, and screening for early detection/isolation when widespread community transmission exists, but operationalizing testing under these scenarios is complicated by the recommendations for confirmation by RT-PCR of both positive (outbreak) and negative (symptomatic patient) results (28). The Centers for Disease Control and Prevention recommendations for use of antigen tests were recently updated and address use of antigen tests (with and without NAAT confirmation) in various testing scenarios based on data to date (https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html).
Our study data provide the following practical considerations for use of the BinaxNOW test in different populations, taking into account current conditions in Massachusetts as reflected in prevalence at this testing site (asymptomatic, >5 to 10%; symptomatic, >20%).
The test had particularly strong performance in adults with symptoms for ≤7 days, with very high sensitivity (96.5%), specificity (100%), PPV (100%), and NPV (98.9%). In children with symptoms for ≤7 days, specificity (100%) and PPV (100%) were very high, but sensitivity was lower than in adults (84.6% versus 96.5%) though still acceptable per the FDA’s target of ≥80%) (29; https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas); NPV was 94.2%. Collection of additional data in symptomatic children will help further support these conclusions.
In asymptomatic individuals, the BinaxNOW test had very high specificity in both children (99.0%) and adults (99.6%); PPV was the same in children (90.9%) and adults (90.9%). Sensitivity in children (65.4%) and adults (70.2%) was low. Thus, the test does not appear to be optimal for ruling out SARS-CoV-2 infection in asymptomatic adults or children; use in serial testing programs and for testing of contacts of known cases deserves independent study. The FDA does provide guidance for consideration of serial antigen testing if the sensitivity is lower, e.g., 70% (29; https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas). It should be noted that in all groups, BinaxNOW sensitivity followed CT value distribution, with 95.8% sensitivity observed in all individuals with CT values of ≤30 (all 9 false-negative results in individuals with CT ≤30 were in children). Therefore, false-negative BinaxNOW results were largely confined to those perhaps least likely to transmit SARS-CoV-2.
Sensitivity (66.7%) was low in adults with symptoms for >7 days, and there were too few children with symptoms for >7 days to draw conclusions about performance. The test is not currently recommended for use in individuals with symptoms for >7 days.
Quality control (QC) testing should be performed per the manufacturer’s recommendations, with weekly repeats of QC samples if the kit has not been fully used. It would be prudent to reevaluate test specificity in warm conditions; careful attention to temperature is critical. A skilled laboratorian can perform and read 20 tests per hour. Excellent interoperator agreement indicates that an individual can perform and read the BinaxNOW test alone.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kerin Milesky (director, Office of Emergency Preparedness, Massachusetts Department of Public Health), Samantha Phillips (director, Massachusetts Emergency Management Agency), and members of the Massachusetts State Fire Services (Bill Pappas, Mike Burnell, Emit Magdeleno, Greg Jackson, and Ed Loader) for assistance with logistical and support assistance at the Lawrence General Hospital Stop the Spread site. We acknowledge the assistance of the members of the Epidemiology Division of the Bureau of Infectious Disease and Laboratory Sciences of the Massachusetts Department of Public Health.
This publication was supported by Cooperative Agreement Number 1U60OE000103, funded by Centers for Disease Control and Prevention through the Association of Public Health Laboratories. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) nor the official views of the Association of Public Health Laboratories.
This work was funded by the Massachusetts Department of Public Health. The community testing site was funded by the Centers for Disease Control and Prevention Building and Enhancing Epidemiology, Laboratory and Health Information Systems Capacity in Massachusetts-Enhancing Detection COVID Supplement (grant number 6 NU50CK000518-01-08). BinaxNOW kits were supplied as part of the federal allocation to state health departments.
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Krüger LJ, Gaeddert M, Köppel L, Brümmer LE, Gottschalk C, Miranda IB, Schnitzler P, Kräusslich HG, Lindner AK, Nikolai O, Mockenhaupt FP, Seybold J, Corman VM, Drosten C, Pollock NR, Cubas-Atienzar AI, Kontogianni K, Collins A, Wright AH, Knorr B, Welker A, de Vos M, Sacks JA, Adams ER, Denkinger CM. 2020. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv 10.1101/2020.10.01.20203836. [DOI] [Google Scholar]
- 2.Lindner AK, Nikolai O, Kausch F, Wintel M, Hommes F, Gertler M, Krüger L, Gaeddert M, Tobian F, Lainati F, Köppel L, Seybold J, Corman VM, Drosten C, Hofmann J, Sacks J, Mockenhaupt F, Denkinger CM. 2020. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected anterior nasal swab versus professional-collected nasopharyngeal swab. Eur Respir J, in press. 10.1183/13993003.03961-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becton Dickinson and Company. 2021. Package insert for the BD VeritorTM system for rapid detection of SARS-CoV-2. https://www.fda.gov/media/139755/download.
- 4.Quidel Corporation. 2020. Package insert for the Sofia SARS antigen FIA test. https://www.fda.gov/media/137885/download.
- 5.Abbott Laboratories. 2020. Package insert for the Abbott BinaxNOW COVID-19 Ag card. https://www.fda.gov/media/141570/download.
- 6.Pilarowski G, Lebel P, Sunshine S, Liu J, Crawford E, Marquez C, Rubio L, Chamie G, Martinez J, Peng J, Black D, Wu W, Pak J, Laurie MT, Jones D, Miller S, Jacobo J, Rojas S, Rojas S, Nakamura R, Tulier-Laiwa V, Petersen M, Havlir DV, DeRisi J. Performance characteristics of a rapid SARS-CoV-2 antigen detection assay at a public plaza testing site in San Francisco. J Infect Dis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Access Bio. 2021. Package insert for the Access Bio CareStart COVID-19 antigen test. https://www.fda.gov/media/142919/download.
- 8.Albert E, Torres I, Bueno F, Huntley D, Molla E, Fernández-Fuentes MÁ, Martínez M, Poujois S, Forqué L, Valdivia A, de la Asunción CS, Ferrer J, Colomina J, Navarro D. 2021. Field evaluation of a rapid antigen test (Panbio COVID-19 Ag rapid test device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect 27:472.e7–472.e10. 10.1016/j.cmi.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglói Z, Velzing J, van Beek J, van de Vijver D, Aron G, Ensing R, Benschop K, Han W, Boelsums T, Koopmans M, Geurtsvankessel C, Molenkamp R. 2020. Clinical evaluation of the Roche/SD Biosensor rapid antigen test with symptomatic, non-hospitalized patients in a municipal health service drive-through testing site. medRxiv 10.1101/2020.11.18.20234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin R. 2020. The challenges of expanding rapid tests to curb COVID-19. JAMA 324:1813. 10.1001/jama.2020.21106. [DOI] [PubMed] [Google Scholar]
- 11.Sethuraman N, Jeremiah SS, Ryo A. 2020. Interpreting diagnostic tests for SARS-CoV-2. JAMA 323:2249–2251. 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 12.Kissler SM, Fauver JR, Mack C, Tai C, Shiue KY, Kalinich C, Jednak S, Ott I, Vogels C, Wohlgemuth J, Weisberger J, DiFiori J, Anderson DJ, Mancell J, Ho D, Grubaugh ND, Grad YH. 2020. Viral dynamics of SARS-CoV-2 infection and the predictive value of repeat testing. medRxiv 10.1101/2020.10.21.20217042. [DOI] [Google Scholar]
- 13.Pollock NR, Savage TJ, Wardell H, Lee R, Mathew A, Stengelin M, Sigal GB. 2020. Correlation of SARS-CoV-2 nucleocapsid antigen and RNA concentrations in nasopharyngeal samples from children and adults using an ultrasensitive and quantitative antigen assay. J Clin Microbiol, in press. 10.1128/JCM.03077-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical Research Sequencing Platform (CRSP), LLC at the Broad Institute of MIT and Harvard. CRSP SARS-CoV-2 real-time reverse transcriptase (RT)-PCR diagnostic assay. https://www.fda.gov/media/139858/download.
- 15.Corman VM, Haage VC, Bleicker T, Schmidt ML, Muehlemann B, Zuchowski M, Lei WKJ, Tscheak P, Moencke-Buchner E, Mueller MA, Krumbholz A, Drexler JF, Drosten C. 2020. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests. medRxiv 10.1101/2020.11.12.20230292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones TC, Mühlemann B, Veith T, Biele G, Zuchowski M, Hoffmann J, Stein A, Edelmann A, Corman VM, Drosten C. 2020. An analysis of SARS-CoV-2 viral load by patient age. medRxiv 10.1101/2020.06.08.20125484. [DOI] [Google Scholar]
- 17.Held L. 2020. A discussion and reanalysis of the results reported in Jones et al (2020). https://osf.io/bkuar/. Accessed 3 December 2020.
- 18.Baggio S, L'Huillier AG, Yerly S, Bellon M, Wagner N, Rohr M, Huttner A, Blanchard-Rohner G, Loevy N, Kaiser L, Jacquerioz F, Eckerle I. 2020. SARS-CoV-2 viral load in the upper respiratory tract of children and adults with early acute COVID-19. Clin Infect Dis, in press. 10.1093/cid/ciaa1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perera R, Tso E, Tsang OTY, Tsang DNC, Fung K, Leung YWY, Chin AWH, Chu DKW, Cheng SMS, Poon LLM, Chuang VWM, Peiris M. 2020. SARS-CoV-2 virus culture and subgenomic RNA for respiratory specimens from patients with mild coronavirus disease. Emerg Infect Dis 26:2701–2704. 10.3201/eid2611.203219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallichote E, Quicke K, Sexton N, Young M, Janich A, Gahm G, Carlton EJ, Ehrhart N, Ebel GD. 2020. Longitudinal surveillance for SARS-CoV-2 RNA among asymptomatic staff in five Colorado skilled nursing facilities: epidemiologic, virologic and sequence analysis. medRxiv 10.1101/2020.06.08.20125989. [DOI] [Google Scholar]
- 21.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 22.Pekosz A, Cooper CK, Parvu V, Li M, Andrews JC, Manabe YC, Kodsi S, Leitch J, Gary DS, Roger-Dalbert C. 2020. Antigen-based testing but not real-time PCR correlates with SARS-CoV-2 virus culture. medRxiv 10.1101/2020.10.02.20205708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.L'Huillier AG, Torriani G, Pigny F, Kaiser L, Eckerle I. 2020. Culture-competent SARS-CoV-2 in nasopharynx of symptomatic neonates, children, and adolescents. Emerg Infect Dis 26:2494–2497. 10.3201/eid2610.202403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Dyal JW, Harney J, Chisty Z, Bell JM, Methner M, Paul P, Carlson CM, McLaughlin HP, Thornburg N, Tong S, Tamin A, Tao Y, Uehara A, Harcourt J, Clark S, Brostrom-Smith C, Page LC, Kay M, Lewis J, Montgomery P, Stone ND, Clark TA, Honein MA, Duchin JS, Jernigan JA, Public Health–Seattle and King County and CDC COVID-19 Investigation Team. 2020. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 382:2081–2090. 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singanayagam A, Patel M, Charlett A, Lopez BJ, Saliba V, Ellis J, Ladhani S, Zambon M, Gopal R. 2020. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 25:2001483. 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaafar R, Aherfi S, Wurtz N, Grimaldier C, Hoang VT, Colson P, Raoult D, La SB. 2020. Correlation between 3790 qPCR positives samples and positive cell cultures including 1941 SARS-CoV-2 isolates. Clin Infect Dis, in press. 10.1093/cid/ciaa1491. [DOI] [Google Scholar]
- 27.Lee RA, Herigon JC, Benedetti A, Pollock NR, Denkinger C. 2021. Performance of saliva, oropharyngeal swabs, and nasal swabs for SARS-CoV-2 molecular detection: a systematic review and meta-analysis. J Clin Microbiol 59:e02881-20. 10.1128/JCM.02881-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. 2020. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays. [Google Scholar]
- 29.Food and Drug Administration. 2020. Policy for coronavirus disease-2019 tests during the public health emergency (revised). Immediately in effect guidance for clinical laboratories, commercial manufacturers, and food and drug administration staff. Food and Drug Administration, Washington, DC. https://www.fda.gov/media/135659/download. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.