Abstract
Background:
Corticotropin-releasing factor (CRF) pathways coordinate behavioral, endocrine, autonomic and visceral responses to stress. Convergent anatomical, molecular, pharmacological and functional experimental evidence supports a key role of brain CRF receptor (CRF-R) signaling in stress-related alterations of gastrointestinal functions. These include the inhibition of gastric acid secretion and gastric-small intestinal transit, stimulation of colonic enteric nervous system and secretory-motor function, increase intestinal permeability, and visceral hypersensitivity. Brain sites of CRF actions to alter gut motility encompass the paraventricular nucleus of the hypothalamus, locus coeruleus complex and the dorsal motor nucleus while those modulating visceral pain are localized in the hippocampus and central amygdala. Brain CRF actions are mediated through the autonomic nervous system (decreased gastric vagal and increased sacral parasympathetic and sympathetic activities). The activation of brain CRF-R2 subtype inhibits gastric motor function while CRF-R1 stimulates colonic secreto-motor function and induces visceral hypersensitivity. CRF signaling is also located within the gut where CRF-R1 activates colonic myenteric neurons, mucosal cells secreting serotonin, mucus, prostaglandin E2, induces mast cell degranulation, enhances mucosal permeability and propulsive motor functions and induces visceral hyperalgesia in animals and humans. CRF-R1 antagonists prevent CRF- and stress-related gut alterations in rodents while not influencing basal state.
Discussion:
These preclinical studies contrast with the limited clinical positive outcome of CRF-R1 antagonists to alleviate stress-sensitive functional bowel diseases such as irritable bowel syndrome.
Conclusion:
The translational potential of CRF-R1 antagonists in gut diseases will require additional studies directed to novel anti-CRF therapies and the neurobiology of brain-gut interactions under chronic stress.
Keywords: CRF peptides, CRF receptor antagonists, irritable bowel syndrome, gut secreto-motor function, stress, visceral pain
1. INTRODUCTION
R. Guillemin and A. Schally demonstrated independently the existence of a corticotropin-releasing factor (CRF) stimulating adrenocorticotropic hormone (ACTH) release from the pituitary in the mid nineteen fifties [1]. After decades of intensive research, Wylie Vale (Guillemin’s former PhD’s student) [2] and his colleagues made a series of milestone discoveries. They identified the structure of CRF as a 41 amino acid peptide [3], the related peptides, urocortins (Ucns) including, Ucn 1 [4], Ucn 2 [5] or stresscopin-related peptide [6], and Ucn 3 [7] or stresscopin [6]. In addition, they cloned the first cognate G-protein coupled receptors, CRF receptor subtype 1 (CRF-R1) [8] and almost simultaneously with Lovenberg et al. [9], the CRF receptor subtype 2 (CRF-R2) [10]. Both CRF receptors are products derived from distinct genes [10] and subject to extensive alternative RNA splicing [11–13]. Radioligand binding studies revealed that CRF is a preferential CRF-R1 agonist with low affinity to CRF-R2, whereas Ucn 1 displays high affinity to both receptor subtypes and Ucn 2 and Ucn 3 are selective ligands for CRF-R2 [14].
The release of CRF from the paraventricular nucleus of the hypothalamus (PVN) into the median eminence plays a primary role in mediating the pituitary ACTH secretion induced by various stressors. This was established by the use of CRF antibody [15], various peptide CRF-R1 and/or CRF-R2 antagonists developed by J. Rivier [16], selective non peptide CRF-R1 antagonists [17] (Table 1), and genetically modified mice [18]. However, reports that CRF ligands and receptors are also expressed outside the hypothalamus [19–21], provided neuroanatomical support for their involvement beyond the endocrine component of the stress response. Early on, compelling reports demonstrated that CRF acutely injected into the lateral brain ventricles of rodents induces behavioral, autonomic and visceral changes mimicking the effects of stress [22–26]. Moreover, CRF ligands and receptors are also expressed in viscera, prominently in the heart and the gut in experimental animals and human subjects [27–31] where they exert biological actions [31–34]. A vast pre-clinical literature also indicates that the dysregulation of CRF/CRF-R1 signaling system contributes to the etiology or the exacerbation of several stress-sensitive diseases such as anxiety/depression, relapse to addiction, and functional bowel diseases [35, 36–39].
Table 1.
List of non-selective CRF receptor antagonists (blocking both CRF-R1 and CRF-R2) and selective CRF-R1 or CRF-R2 antagonists.
| CRF-R1 & CRF-R2 (peptide) | CRF-R1 (Non-Peptide)* | CRF-R2 (Peptide) |
|---|---|---|
| α-Helical CRF9–41 | CP-154, 526 | Anti-sauvagine-30 |
| D-Phe12CRF12–41 | CP-376, 395 | Astressin2-B |
| Astressin | Antalarmin | |
| Astressin-B | NBI-30775 | |
| NBI-27914 | ||
| NBI-35965 | ||
| NBI-30545 | ||
| R317573 | ||
| GW876008 | ||
| NGD 98–2 | ||
| NGD 9002 | ||
| CRA1000 | ||
| DMP696 | ||
| JTC-017 | ||
| Pexacerfont (BMS-562086) |
Cross the blood brain barrier.
This review will focus on the biological actions of CRF/Ucns systems in the brain and the periphery, namely within the gut, to influence gastrointestinal secreto-motor functions and the role of CRF receptor subtypes in stress-related alterations of gut functions and visceral pain. We will also address whether inhibition of the CRF-R1 signaling system will hold promises to curtail stress-sensitive functional bowel disorders namely the irritable bowel syndrome (IBS).
2. BRAIN CRF AND UROCORTINS: ACTIONS TO INFLUENCE GASTRIC FUNCTION
2.1. Brain CRF Peptides Inhibit Gastric Acid Secretion
Two years after the CRF identification, the central action of the peptide to influence gastric acid secretion in rats was the first report indicating that CRF can alter visceral function by acting in the brain [40]. Thereafter, several reports established that CRF or the amphibian CRF-related peptide, sauvagine, injected into the cerebrospinal fluid (CSF) either into the lateral brain ventricle (intracerebroventricular, ICV) or the brainstem at the level of the cisterna magna (intracisternally, IC) induces a dose-related, rapid in onset, and sustained inhibition of gastric acid secretion [40–45]. The inhibitory effect occurred under conditions of stimulated gastric acid secretion induced by either vagal activation (central injection of thyrotropin releasing hormone, TRH, 2-deoxy-D-glucose, pylorus ligation or gastric distention) or peripheral hormone (pentagastrin) while not influencing the acid secretory response to histamine in rats [40–45]. Similarly in conscious dogs, the injection of CRF into the 3rd ventricle inhibits 2-deoxy-D-glucose-, a peptone meal- and pentagastrin- but not histamine-stimulated acid secretion [46, 47] suggesting a conserved mechanism among species. CRF’s inhibitory action is mediated through interaction with brain CRF receptor and not related to leakage of the peptide into the periphery [40] where CRF or sauvagine can also inhibit acid secretion [48]. Further studies showed that Ucn 1 injected ICV reduces gastric acid secretion in rats, an effect that is blocked by ICV astressin2-B, a CRF-R2 antagonist, but not NBI-27914, a CRF-R1 antagonist [49]. This indicates a primary role of brain CRF-R2 in mediating the gastric acid inhibitory effect of Ucn 1.
Responsive sites of CRF action are located in the hypothalamus, namely the PVN, ventromedial (VMH) and lateral (LH) hypothalamic nuclei where peptide microinjection suppresses gastric acid secretion in conscious pylorus-ligated rats [40, 44, 50–51]. By contrast, CRF microinjected into the caudate putamen or dorsomedial frontal cortex, under the same conditions, did not influence acid secretion, showing selectivity to specific hypothalamic nuclei [40, 50, 51]. In the hindbrain, other responsive sites include the locus coeruleus/subcoeruleus (LC/SC) where CRF suppresses the acid response to intravenous (IV) infusion of pentagastrin in rats while CRF microinjected into sites within the vicinity but outside the LC/SC had no effect [52].
The autonomic nervous system (ANS) mediates the inhibitory effect of CRF from the brain to the stomach. In rats, central CRF inhibits gastric vagal efferent discharges [53] and stimulates sympathetic outflow as shown by the rise in circulating levels of epinephrine and norepinephrine in rats and dogs [46, 54]. Ganglionic blockade with chlorisondamine, spinal cord transection at the fifth cervical level, noradrenergic blockade with bretylium, α2-adrenergic blockade with yohimbine and acute adrenalectomy, abolish the inhibitory effect of IC, ICV or LC/SC injections of CRF on gastric acid secretion supporting a primary role of the sympathetic nervous system in rats [40, 41, 52]. It is to note that vagal-dependent mechanisms also contribute to the inhibitory effect of IC but not ICV or LC/SC injections of CRF [40, 41, 52]. This is consistent with the direct access of CRF injected IC to influence neurons in the dorsal motor nucleus of the vagus, which regulate gastric function through efferent axonal projections from their cell bodies to the gastric enteric nervous system [55]. These data combined with the unaltered gastric acid inhibitory response to central injection of CRF and sauvagine by hypophysectomy and peripheral injection of naloxone [40–41] further ascertain that the central action of CRF is mediated by the ANS and unrelated to the activation of the hypothalamic-pituitary-adrenal (HPA) hormones in rats. In dogs, peripheral mediators involve arginine vasopressin as shown by the increased plasma levels of peptide by ICV CRF and the dampening of ICV CRF inhibitory effect by the peripheral injection of a vasopressin receptor antagonist [47, 56]. Although gastrin is a hormone well established to regulate gastric acid secretion, the inhibition of gastric acid secretion induced by central CRF does not involve gastrin in rats and dogs [40, 51].
Early clinical and experimental observations demonstrated that emotional stimuli inhibit gastric acid secretion. Beaumont noticed when his fistulous subject experienced “fear, anger or whatever depresses or disturbs the nervous system”, that gastric secretion was inhibited [57]. Cannon showed that the flight-or-fight response resulted in decreased gastric acid secretion in cats [58]. The involvement of CRF receptor in the brain in acute stress-related inhibition of gastric acid secretion has been demonstrated in rats. The CRF-R1/CRF-R2 antagonist, α-helical CRF9–41 injected IC or ICV prevents brain surgery-and partial restraint-induced reduction of gastric acid secretion while the antagonist injected peripherally has no effect [54, 59]. Moreover, the activation of central CRF signaling pathways is part of the brain mechanisms through which specific anorexic brain peptides inhibit gastric acid secretion. In particular, CSF injection of cocaine- and amphetamine-regulated transcript (CART) or neuromedin U-induced inhibition of gastric acid secretion in pylorus-ligated rats is completely blocked by the pretreatment into the CSF with α-helical CRF9–41 or anti-CRF immunoglobulin G while the inhibitory effect of gastrin-releasing peptide, and interleukin-1ß are not altered [54, 60, 61]. Lastly, the activation of cell bodies into the hypothalamic arcuate nucleus (ARC) by microinjection of kainic acid inhibits pentagastrin-stimulated acid secretion, a response that the peptide CRF-R1/R2 antagonist, astressin microinjected into the PVN prevented [62]. These data indicate that the activation of CRF receptors by endogenous CRF in the PVN reduces gastric acid secretion consistent with the effect of exogenous injection of the peptide at this site [50]. Of importance is the lack of change in basal gastric secretion in rats injected with CRF receptor antagonists into the CSF. This indicates that brain CRF signaling is not involved in the basal regulation of gastric acid secretion [54, 59].
2.2. Brain CRF Stimulates Gastric Bicarbonate Secretion
The first evidence that the CRF in the brain can influence gastric bicarbonate secretion came from observations that the peptide microinjected into hypothalamic nuclei (PVN, LH and VMH) increases the volume of gastric secretion while inhibiting total acid output and acid concentration in conscious rats [50]. Such a response was established to be peptide- and site-specific. Bombesin, calcitonin gene-related peptide, or calcitonin microinjected into these hypothalamic sites have no effect, and CRF and sauvagine injected IC inhibits both gastric secretory volume and acid concentration in rats [40, 43]. Subsequent reports established that CRF microinjected into the VMH stimulated gastric bicarbonate concentration and output and potassium secretion in conscious pylorus-ligated rats [51].
2.3. Brain CRF Peptides Inhibit Gastric Transit and Motility
The central action of CRF to suppress gastric motor function is well established in various experimental conditions. All mammalian CRF ligands (CRF, Ucn 1, Ucn 2, and Ucn 3) and amphibian/fish CRF-related peptides (sauvagine and urotensin I) injected into the CSF at the level of the lateral or 4th ventricle, cisterna magna or L5-L6 spinal cord segments, induce a rapid in onset and long-lasting inhibition of gastric transit of non-caloric or caloric liquid or solid meal in several non-primate mammal species [63–74].
CRF and Ucn 1 alter gastric contractility pattern and the propagation of motor events in various species including rats, dogs and sheep [70, 75–80]. In fed rats, CRF injected ICV induces a long-lasting inhibition of postprandial antral motility [81]. Other studies in fasted rats showed that CRF or Ucn 1 injected IC suppresses vagally stimulated high amplitude gastric contractions evoked by IC TRH agonist or 2-deoxy-D-glucose [82, 83]. In fasted dogs, ICV CRF induces an immediate and sustained disruption of the interdigestive pattern of gastric motility with abolition of the cyclic activity front in the antrum and the occurrence of irregular contractions of small amplitude [84, 85]. Several lines of evidence established that the activation of brain CRF-R2 signaling mediates CRF and Ucns action to impair gastric propagative motor function. The selective CRF-R2 ligand, Ucn 2 injected into the brain ventricle mimicked CRF inhibitory action. In addition, the ICV or IC injection of non-selective peptide, CRF-R1/CRF-R2 antagonists, α-helical CRF9–41, D-Phe12CRF12–41, astressin, or astressin-B and the selective CRF-R2, astressin2-B, blocked ICV or IC injection of CRF-, Ucn 1- and Ucn 2-induced delayed gastric emptying and motility while administration of selective CRF-R1 antagonists, NBI-35965 or NBI-27914 have no effect in rats, mice or dogs [49, 64, 66, 68–71, 73, 77, 81, 86–91].
The PVN and dorsal vagal complex (DVC) are brain responsive sites to microinjection of CRF to inhibit basal gastric emptying or vagally-stimulated gastric contractility while delivering the peptide into the LH, other medial hypothalamic sites, central amygdala (CeA) or LC/SC, had no effect [80, 83, 92–95]. These sites of action express CRF-R2 mRNA [20] and regulate gastric motor function through parasympathetic pathways [55]. In particular, the dorsal and ventral caps of the parvocellular division of the PVN contain neurons that send direct projections to the DVC and spinal cord intermedio-lateral column [96]. Consistent with these neuroanatomical connections, the majority of studies using combined pharmacologic, surgical or electrophysiological approaches to assess the pathways involved in CRF inhibitory action have established the primary involvement of the vagus. Subdiaphragmatic vagotomy prevents ICV or IC CRF and Ucn 1-induced delayed gastric emptying in mice, rats and dogs [64–66, 70, 73, 81, 85, 92, 95, 97] and the alterations of gastric motility in rats or dogs [70, 81, 85, 95]. Electrophysiological recording of efferent activity in the gastric branch of the vagus also shows that CRF, and more prominently sauvagine, injected IC decreases vagal efferent outflow in anesthetizes rats [53]. However, a few reports also indicate the role of the sympathetic nervous system in mediating ICV or IC CRF-induced delayed emptying and the inhibition of postprandial antral and pyloric contractions [65, 72, 78]. In our studies, only IC Ucn 2-induced delayed gastric emptying is mediated by sympathetic pathway and peripheral alpha-adrenergic receptors while the inhibitory action of IC CRF and Ucn 1 is vagal-dependent in rats [73]. CRF injected into the PVN or CSF increases sympathetic outflow to the viscera [54, 98]. It may be speculated that the discrepancies in neuronal pathways are related to differences in peptides (CRF or urocortins) and doses used along with brains sites of injection that may differentially influence sympathetic activity and thereby the modulation of peripheral cholinergic transmission to the stomach.
The end effectors through which alterations of autonomic outflow suppress gastric motor function may involve gut peptides. A few data suggest the contribution of peripheral somatostatin in ICV CRF-induced delayed gastric emptying in rats [99] but not in dogs [76]. The suppression of the circulating prokinetic hormones, motilin in dogs and acyl ghrelin in rats in response to ICV CRF may contribute in the inhibition of gastric propulsive motor function [49, 76]. However, this needs to be ascertained using peripheral injection of motilin and ghrelin antagonists. Other studies clearly established that the effects of centrally injected CRF are not secondary to the stimulation of pituitary-adrenal hormones as shown by the unaltered delayed emptying induced by ICV or IC CRF in hypophysectomized or acute adrenalectomized rodents [64–66, 97].
Support for a role of brain CRF receptor activation in stress-induced inhibition of gastric propulsive motor function comes from consistent reports using pharmacological CRF receptor blockade. The IC, ICV or PVN injection of CRF-R1/CRF-R2 antagonists, α-helical CRF9–41, D-Phe12 CRF12–41, astressin, or astressin-B, abrogate the gastric stasis induced by various stressors [25]. These encompass psychological/physical (swim stress, wrap restraint), visceral (trephination, abdominal surgery, peritoneal irritation induced by intraperitoneal, IP injection of 0.6% acetic acid), immunological (IV or ICV injection of interleukin-1ß), or chemical (ether) [54, 63, 69, 87, 90, 100–102]. All these stressors, including abdominal surgery and immune challenge activate CRF neurons and upregulate CRF mRNA in the PVN [21, 90, 103–110]. However, the respective involvement of CRF and/or Ucns to activate CRF receptors leading to stress-related inhibition of gastric motor function is still largely unexplored and may vary with the type of stressors. A recent study points to a primary role of hypothalamic Ucn 3 in the inhibition of antral contractions in the stomach induced by chronic psychological stress of a communication box paradigm whereby rats receive visual, auditory and olfactory stimuli from rats subjected to foot shock for 1-h/day for 5 days. Under these conditions, there is an upregulation of Ucn 3 gene expression in the hypothalamus, unlike that of Ucn 1 or Ucn 2, and the ICV injection of Ucn 3 antiserum prevented the inhibition of antral motility (although the selectivity of the antiserum to Ucn 3 vs. Ucn 1 or 2 was not mentioned) [79].
Investigations of brain CRF receptor subtype(s) involved in various stressors-induced inhibition of gastric motor function point to the implication of either CRF-R1 or CRF-R2, depending upon their interoceptive or exteroceptive nature. For instance, the gastric ileus occurring immediately post-surgical stress (abdominal surgery and cecal palpation), is mediated by CRF-R1 as shown by the use of genetically modified CRF-R1 or CRF-R2 knockout mice or ICV injection of CRF-R1 antagonist in rats [111] (unpublished observations). In contrast, acute restraint stress-induced gastric ileus involves CRF-R2 in rats [72]. Additional studies are needed to identify the CRF receptor subtypes involved in other stress models. In particular, it will be important to dissect whether the activation of differential brain circuitries by physical vs. psychological stressors [112] recruits different CRF ligands and receptor subtypes within these pathways and thereby their primary role.
Of interest, is also the evidence that CRF receptors in the brain serve as downstream mechanisms for specific brain peptides known to activate CRF neurons in the PVN. Those include CART, nociceptin/orphanin FQ (N/OFQ), glucagon-like peptide 1 (GLP-1) and des-acyl ghrelin that act in the brain to inhibit gastric motor function and appetite [113–116]. Studies have shown that the injection of α-helical CRF9–41 into the 4th or lateral ventricle or astressin IC prevents respectively the delayed gastric emptying in rats induced by CART injected into the 4th ventricle, ICV N/OFQ and IC GLP-1 [113–115]. The ICV pretreatment with CRF-R1/CRF-R2 antagonist, astressin and the selective CRF-R2 antagonist, anti-sauvagine-30 abolishes ICV injection of des-acyl ghrelin-induced reduction of antral contractions in fasted rats while the CRF-R1 antagonist, NBI-27914 had no effect [116]. However, IC α-helical CRF9–41 and ICV astressin2-B do not alter IC adrenomedullin or ICV nesfatin-1-induced delayed gastric emptying in rats [117, 118] showing peptide specificity.
By contrast, CRF receptor antagonists injected into the CSF do alter neither basal gastric emptying of a liquid non-nutrient or solid nutrient meal nor motility in rats, mice or dogs in the large majority of studies [25, 64, 66, 70]. This indicates that brain CRF signaling is not involved in basal and postprandial regulation of gastric motor function.
3. CENTRAL ACTIONS OF CRF PEPTIDES TO INFLUENCE SMALL INTESTINAL SECRETORY MOTOR FUNCTION
3.1. Central CRF Stimulates Duodenal Bicarbonate Secretion
When injected ICV, CRF is one of the most potent peptides to stimulate duodenal bicarbonate secretion in rats compared with other known stimulants while a number of peptides (ICV bombesin, urotensin, calcitonin, vasoactive intestinal peptide, gastrin, growth hormone-releasing factor, gonadotropin-releasing hormone and β-endorphin) and norepinephrine tested under the same conditions in conscious or anesthetized rats are inactive [119–121]. The ICV injection of α-helical CRF9–41 abolishes both ICV CRF and partial restraint stress-induced three-fold increase in duodenal bicarbonate secretion in rats [120, 122]. These data suggest that the activation of brain CRF receptors during stress promotes duodenal secretory function and may enhance the resistance of the duodenal mucosa similar to what reported in the gastric mucosa [51].
Peripheral mechanisms through which central CRF stimulates duodenal bicarbonate secretion are not mediated by the ANS as shown by the unaltered response by chlorisondamine, vagotomy, blockade of sympathetic nervous system with bretylium and adrenalectomy in rats [120]. Evidence indicates the involvement of the pituitary axis releasing β-endorphin, as hypophysectomy and naloxone prevent the duodenal bicarbonate response. In addition, β-endorphin, unlike ACTH, injected IV at doses reproducing the circulating levels induced by ICV CRF, stimulates duodenal bicarbonate secretion [120]. Lastly, ICV or IV injection of CRF shows similar potency to stimulate duodenal bicarbonate secretion consistent with the activation of the HPA axis by CRF injected centrally or peripherally [120].
3.2. Central CRF Stimulates Ileal Water Absorption
CRF injected ICV stimulates water, chloride and sodium absorption in the ileum of conscious rats [122]. The enhanced ileal water transport is not altered by hypophysectomy, adrenalectomy, vagotomy and naloxone, is blocked by chlorisondamine, bretylium and phentolamine, and mimicked by peripheral injection of norepinephrine [122]. This is consistent with the known action of brain CRF to stimulate the sympathetic noradrenergic outflow to the gut [123]. So far, only one study indicates that brain CRF receptors are involved in restraint-induced increased ileal water absorption in rats as shown by the use of ICV α-helical CRF9–41 before acute restraint stress [122].
3.3. Central CRF Inhibits Small Intestinal Motor Function
The influence of brain CRF on small intestine has been less investigated compared to the proximal (stomach) and distal (colon) segments of the gut. However, existing literature indicates that ICV injection of CRF also inhibits small intestinal transit in rats [54, 65–66]. Further studies to investigate the underlying alterations of small intestinal motility indicate that CRF injected into the brain alters the pattern of contractions that varies in function of the fed or fasted state and species. Under fed conditions, CRF injected ICV after a nutrient meal increases the frequency of proximal duodenal contractions in dogs, while not altering their amplitude and durations [70]. In fed rats, ICV Ucn 1 increases the amplitude of pressure waves in the duodenum [81], while in fed sheep, ICV CRF induces a long-lasting inhibition of duodenal motor activity [75]. In fasted dogs, ICV CRF inhibits the occurrence of migrating motor complex in the duodenum and proximal jejunum [84, 85]. Likewise, in rats the ICV injection of Ucn 1 acts through CRF-R2 to disrupt the fasted pattern of duodenal motor activity and replaces it with a fed pattern resulting in non-propagative irregular spike burst activity monitored in the duodenum and jejunum [81, 124].
In rats and dogs, peripheral mechanisms of actions of ICV CRF and Ucn 1 to inhibit small intestinal transit and disrupt fasted motility involve vagal cholinergic pathway and are independent from the activation of the HPA axis [65, 72, 81, 84, 124]. Collectively, these studies indicate that central CRF induces a vagally mediated suppression of small intestinal transit associated with alterations of coordinated propagative motor activity.
Studies on the implication of CRF receptors in stress-related inhibition of intestinal transit have been limited and yield discrepant results with ICV α-helical CRF9–41 having no effect or reversing acute partial restraint stress-induced slowing of small intestinal transit [54, 63]. Collectively, existing evidence so far is scant to ascertain a role of brain CRF signaling in the alterations of small intestinal motor function by stress.
4. CENTRAL ACTIONS OF CRF PEPTIDES TO INFLUENCE COLONIC FUNCTION
4.1. Activation of Brain CRF-R1 Stimulates Colonic Secreto-Motor Function
A vast literature shows that, opposite to the upper gut, the ICV or IC injection of CRF or Ucn 1 evokes a rapid onset increased motility in the cecum, triggers spike burst activity and pressure wave in the proximal and distal colon, accelerates whole and distal colonic transit, decreases colonic fluid absorption, and induces defecation and diarrhea in freely moving rats, mice or gerbils [63, 65, 71, 87, 93, 125–131]. By contrast, ICV injection of the selective CRF-R2 agonist, Ucn 3 has no effect in mice [71]. The colonic changes induced by ICV CRF or Ucn 1 are blocked by the ICV injection of CRF-R1/CRF-R2 antagonists, α-helical CRF9–41, D-Phe12CRF12–41, and astressin or by selective CRF-R1 antagonists, NBI-35965, NBI-27914, NGD 98–2, and NGD 9002. In contrast, selective CRF-R2 antagonists, astressin2-B or anti-sauvagine, injected ICV are inefficient to block the central effects of CRF or Ucn 1 in rodents [65, 71, 87, 91, 130–133]. Other studies also showed increased release of mucin, prostaglandin E2 (PGE2) and mast cell protease II from the colonic mucosal explants of rats 30 min after ICV injection of CRF, mimicking the effects of immobilization stress [133]. These convergent findings establish that the activation of brain CRF-R1 signaling by CRF or Ucn 1 in the brain stimulates colonic secretory motor function in experimental animals.
4.2. Brain Sites of Action and Peripheral Mechanisms
The PVN and pontine areas, namely the LC/Barrington nucleus complex in rats have been identified as brain nuclei responsive to CRF to induce increased tonic and phasic colonic motility, stimulation of colonic transit and induction of watery fecal output [92, 93, 134–136]. By contrast, microinjection of CRF into the LH and other medial hypothalamic sites, the CeA, and pontine sites adjacent but outside of the boundaries of the LC/SC, have no effect on colonic transit or fecal pellet output [92, 93, 134–136]. Of significance is the demonstration that these hypothalamic and pontine sites are also brain nuclei involved in central CRF-induced CRF-R1 mediated anxiety and depression [137, 138] providing anatomical and biochemical substrata for the early use of defecation in rodents as an index of emotionality [139, 140].
CRF-R1 is the main receptor subtype mediating the pituitary release of ACTH and downstream glucocorticoids [15]. However, peripheral mechanisms involved in the stimulation of colonic secreto-motor function in response to central injection of CRF are not secondary to the activation of the HPA axis and implicate the ANS [65]. Tracing studies identified a proportion of CRF immunoreactive neurons in the Barrington’s nucleus and PVN transynaptically linked to the colon [141, 142]. This provides neuroanatomical support for CRF signaling in the PVN and pontine sites to influence colonic motor functions through the activation of sacral parasympathetic cholinergic outflow. In addition, peripheral ganglionic blockade using chlorisondamine and atropine prevents, and subdiaphragmatic vagotomy reduces, the stimulation of colonic transit, phasic and tonic contractions, and defecation induced by CRF injected IC-, ICV- or into the PVN, while the noradrenergic blockade by bretylium has no effect [65, 92, 135, 143].
Within the colon, effector mechanisms recruited by central CRF relate to the release of serotonin (5-HT) interacting with serotonin receptor type 3 (5-HT3) and type 4 (5-HT4). There is evidence that IC injection of CRF, like restraint stress, increases 5-HT content in the feces harvested in the proximal colon [144]. In addition, convergent pharmacological studies show that several 5-HT3 antagonists such as granisetron, ramosetron, ondansetron and azasetron, or the 5-HT4 antagonist, SB-204070, given orally or into the lumen of the proximal colon, block the stimulation of colonic transit and defecation induced by ICV or IC injection of CRF [127, 131, 144, 145]. Additional mechanisms of ICV CRF-induced defecation may involve the activation of colonic myenteric cholinergic and substance Pergic neurons interacting with neurokinin 1 receptor located on colonic smooth muscles [146].
4.3. Role of Brain CRF-R1 Signaling
CRF-R1 signaling in the brain plays a role in stress-related stimulation of colonic propulsive motor function while not being involved in basal or postprandial regulation of colonic secreto-motor function under non-stress conditions [39, 147]. First, the ICV injection CRF and the high affinity CRF-R1 ligand, Ucn 1 [14] recapitulate the colonic secreto-motor responses to stressors while that of selective CRF-R2 agonists, Ucn 2 and Ucn 3 either have no effect on basal colonic transit or inhibit stimulated colonic contractions in rats [63, 65, 87, 127, 129, 130, 143, 148–150]. However the ICV, IC or PVN injection of α-helical CRF9–41, D-Phe12 CRF12–41, or astressin prevents the reduction of colonic transit time or defecation evoked by acute restraint, water avoidance stress and the increased frequency of colonic spike-bursts induced by conditioned fear in rats [54, 63, 87, 91, 126, 127, 129, 135, 142, 143, 151]. Lastly, several brain penetrant non-peptide CRF-R1 antagonists such as antalarmin, CP-154,526, CRA1000, JTC-017, NBI-27914, NBI-35965, NGD 98–2 and NGD 9002 [17, 130, 152] injected either centrally or peripherally (subcutaneous, IP and for some compounds, orally) dampen various acute stressors-(restraint, water avoidance stress, elevated plus maze, social intruder, morphine withdrawal) or 5 days exposure to heterotypic stressors-induced stimulation of defecation, colonic transit, motility and luminal 5-HT release in rodents [63, 71, 87, 91, 130–132, 153–157]. Likewise, in primates, antalarmin attenuates the defecation occurring in animals exposed to a social intruder [23]. By contrast, the selective CRF-R2 antagonist, astressin2-B injected centrally at doses that block CRF-R2-mediated inhibition of gastric emptying, does not alter restraint stress-related defecation in mice [71]. In addition, CRF-R1 deficient mice display significantly lower defecation in an open field test than their wild-type littermates [158]. Conversely, CRF-overexpressing mice show enhanced defecation to a novel environment [159]. Microinjection of CRF receptor antagonist or CRF oligonucleotide antisense before water avoidance stress into specific brain nuclei point to the PVN and LC/SC nuclei as main sites of CRF/CRF-R1 pathway to stimulate colonic motor function induced by stress while CRF receptors in the CeA are not involved [92, 126, 160–162]. Further supporting the key role of these brain nuclei, ICV injection of CRF-R1/CRF-R2 antagonist, α-helical CRF9–41 before exposure to water avoidance stress curtails the activation of neurons selectively in the PVN and LC neurons in correlation with the decrease in fecal output [126]. In addition, studies used strains of rats with different susceptibility to stress, namely the stress resilient Lewis which has a defective CRF synthesis/release compared to Fischer rats [163]. The Lewis and Fischer rats showed respectively low and high activation of neurons in the PVN and intermediolateral columns of the lumbosacral segment of the spinal cord correlated with the magnitude of defecation [142].
A number of brain peptides influencing food intake and activating CRF neurons in the PVN, such as neuropeptide Y, GLP-1, CART, and ghrelin, also stimulate colonic motor function through activation of brain CRF receptor [164–168]. Other studies showed that CRF receptor located in the PVN mediated the activation of neurons in the ARC-induced stimulation of colonic motor function [144]. Therefore, CRF/CRF-R1 signaling in the PVN induced by various stressors and specific brain peptides is an important convergent signaling pathway that leads to the stimulation of colonic secretory motor function.
5. CENTRAL ACTIONS OF CRF PEPTIDES TO INFLUENCE VISCERAL SENSITIVITY
Recurrent abdominal pain in the absence of detectable organic diseases is the hallmark of irritable bowel syndrome (IBS) [169]. Clinical evidence indicates that IBS is a stress-sensitive disease as various stressors of psychosocial, physical or immune origin and early adverse life events can contribute to the development and/or exacerbation of IBS symptoms [170–172]. Several stress-related animal models developed to reproduce some common features of IBS [173, 174] provided valuable tools to examine the implication of CRF signaling in stress-related visceral hypersensitivity.
5.1. Role of Brain CRF-R1 in Stress-Related Visceral Hypersensitivity
Earlier studies demonstrated that the ICV injection of CRF in rats enhances the pain response to colorectal distension (CRD) mimicking the effects of restraint stress [175]. CRF action is blocked by the ICV injection of α-helical CRF9–41, or the CRF-R1 antagonist, antalarmin given intraperitonerally [175, 176] pointing to brain CRF-R1 activation in the induction of visceral hypersensitivity. The role of this signaling pathway was further established to mediate the hypersensitivity to CRD induced by various stressors in rodents using pharmacological or genetic approach [39]. The selective CRF-R1 antagonists (Table 1) including NBI-35965 [130], CP-154,526 [177–180], NBI-27914 [181], antalarmin [176, 182–184], JTC-017 [155], NBI-30775 [185], DMP696 [186], NGD 98–2 and NGD 9002 [132], inhibit the visceral hyperalgesia-induced by a variety of conditions such as early life adverse events, repeated water avoidance stress [130, 178, 186], chronic high anxiety in the Wistar Kyoto rats [176, 183], colonic inflammation [180], repeated nociceptive activation of colonic mechanoreceptors [132, 155, 176, 178, 182, 183] or post-natal intracolonic injection of acetic acid [181]. Likewise, CRF-R1 knockout mice show a reduction of visceral response to phasic CRD compared to wild type littermate [185]. Collectively these pre-clinical studies support a pivotal role of brain CRF-R1 signaling in the visceral hyperalgesia resulting from different stress context: early life adverse events, repeated psychological stress in adulthood, chronic high anxiety as well as peripherally initiated mechanisms associated with colonic inflammatory event or repeated CRD at nociceptive range.
5.2. Brain Sites of Action
The CeA, bed nucleus stria terminalis, LC and hippocampus are brain sites through which the activation of CRF/CRF-R1 signaling results in visceral hypersensitivity [39, 155, 162, 187, 188]. A few reports showed that α-helical CRF9–41 microinjected directly into the hippocampus decreases the frequency of abdominal contractions evoked by tonic nociceptive CRD [155]. Other study showed that the CRF-R1 antagonist, CP-376,395 microinjected into the anterolateral bed nucleus stria terminalis reduces the hypersensitivity to CRD induced by repeated water avoidance stress [188]. Additionally, several convergent studies support a role CRF/CRF-R1 in the CeA [162,187]. CRF microinjected into the CeA increased sensitivity to CRD in Wistar rats that was blocked by the intra-CeA microinjection of CP-154,526 [189]. Moreover, the CRF-R1 antagonist, CP-376,395 microinjected into the CeA reduced visceral hyperalgesia to CRD in a high anxiety strain of rats [187]. Neuroanatomical studies further support the role of CRF-R1 signaling in the CeA to modulate visceral pain. CRF is densely expressed in cell bodies of CeA that send axonal projections to basal forebrain and pontine/brainstem including the LC [190, 191]. There is also evidence of upregulation of CRF and CRF-R1 gene expression at these brain sites in rat models of anxiety associated with altered visceral pain [192]. Likewise, CRF gene and peptide expression are upregulated in the CeA by CRD, repeated exposure to water avoidance and other conditions inducing visceral pain or hyperalgesia in rats [193–199].
With regard to the influence of brain CRF-R2 activation on visceral pain, still little is known and further studies are required to establish its role. One report indicates that the CRF-R2 antagonist, astressin2-B microinjected into the anterolateral bed nucleus stria terminalis reduced visceral pain induced at low CRD pressure in rats but had no effect at higher CRD pressure or even enhanced visceral pain in rats submitted to water avoidance stress [188].
5.3. Central and Peripheral Mechanisms of Action
Several possible mechanisms are involved in the prevention of stress- or mechanosensitization-related visceral hyperalgesia induced by CRF-R1 antagonist pretreatment. Previous studies on somatic pain provided molecular, electrophysiological and biochemical evidence that endogenous CRF/CRF-R1 signaling within the CeA participates to the mechanisms of pain sensitization [196, 200, 201]. The laterocapsular division of the CeA known as the “nociceptive amygdala” is the recipient of nociceptive-specific inputs originating from the parabrachial area as part of the spinal-parabrachial-amyloidal pathway [202]. In the CeA, CRF/CRF-R1 facilitates synaptic transmission of pain by switching on silent N-methyl-D-aspartate receptors through protein kinase A-dependent signaling [201, 203–205]. Further studies in genetically modified mice with deletion of CRF-R1 selectively on glutamatergic CeA neurons clearly ascertained that CRF facilitates the excitatory transmission by a CRF-R1-mediated direct action on glutamatergic neurons [205]. Whether similar cellular mechanisms in the CeA are involved in CRF/CRF-R1 mediated visceral hyperalgesia need to be explored. Additional components may involve the inhibition of noradrenergic output from the LC [189, 206–208], the largest of all noradrenergic nuclei [138]. Electrophysiological studies showed that CRF-R1/CRF-R2 antagonist, D-Phe12CRF12–41 or astressin injected ICV or directly into the LC and the selective CRF-R1 antagonist, NBI-35965, injected peripherally, blocked the activation of LC neurons responsive to central injection of CRF and CRD in rats [206–208]. Additionally, the CRF-R1 antagonist, JTC-017 or CP-154,526 reduced CRD-induced noradrenaline release in the hippocampus and CeA, which are sites of rostral efferent projections from the LC [155, 189, 209]. The cortical and limbic rostral noradrenergic efferent projections from the LC are known to induce arousal and anxiogenic responses along with hypervigilance to visceral input that is a common feature in IBS patients [172, 210, 211]. It is to note that the brain sites (CeA, hippocampus, LC, hypothalamus) involved in CRF-R1-induced visceral hypersensitivity are also those implicated in anxiety disorders [212] and may have relevance in the co-morbidity of IBS with anxiety disorder [172, 213].
Among peripheral mechanisms, there is evidence of an important role of intestinal mucosal mast cells. Rats exhibiting visceral hypersensitivity after exposure to crowding stress display an increased number of mast cells in the colon mucosa [214, 215]. The ICV injection of CRF or restraint stress activates colonic mast cells monitored by the increase in colonic release of mast cell protease II, PGE2 and histamine [133, 216]. Additionally, the mast cell stabilizer doxantrazole blocked the visceral hyperalgesia to CRD induced by ICV CRF or restraint [175] and ICV injection of α-helical CRF9–41, abolished acute restraint stress–induced increase of colonic histamine content [216].
6. PERIPHERAL CRF SIGNALING: INFLUENCE ON COLONIC SECRETORY MOTOR FUNCTION
Early on, there was an enormous stride made in the biology of the CRF system to assess its actions and role in the brain due to the widely held consensus that the stress response is coordinated exclusively within the central nervous system [102]. The past few years, the CRF signaling systems within the gut are now emerging as important components of the stress response [27, 33, 217]. In vitro as well as whole animal and human studies indicate that the peripheral CRF signaling systems can modulate gut motility, secretion and barrier functions as well as visceral pain [33, 217]. Moreover, CRF-R1 and CRF-R2 and their ligands are localized throughout the gastrointestinal tract in various cell types including neuronal (the enteric nervous system), endocrine (enterochromaffin cells) and immune (mast cells, eosinophils, T-helper lymphocytes in the lamina propria) in experimental animals and human subjects [28, 218–225]. This provides support for a local role of the CRF systems within the gut as established in other viscera such as the cardiovascular system [226]. In addition, stressors modulate the expression of CRF peptides and CRF receptors within the gastrointestinal tract [28, 220, 227, 228].
6.1. Activation of Peripheral CRF-R1 Stimulates Secreto-Motor Function
Peripheral injections (IP, IV or subcutaneous) of CRF and Ucn 1 are equipotent to central injections to stimulate whole and distal colonic transit, defecation and to induce prominent diarrhea in rats, mice or dogs [63, 65, 127, 130, 133, 154, 222, 229–237]. These functional alterations are associated with an increase of clustered spike-burst propagative activity in the cecum, proximal and distal colon in rodents [154, 233, 235]. In addition, CRF injected IV induces the release of mucin, PGE2 and mast cell protease II in colonic mucosal explants in rats indicative of the stimulation of goblet and mast cells [133].
Peripheral administration of CRF- or Ucn 1-induced the activation of CRF-R1 signaling mediates the acceleration of colonic transit and induction of watery diarrhea as shown by the use of selective CRF agonists and receptor antagonists. The selective CRF-R1 peptide agonists, cortagine, or stressin1 [238, 239] injected IP stimulate colonic contractions distal colonic transit, defecation with a peak response within 30 min post injection, and a rapid onset diarrhea in rodents [154, 219, 222, 229, 231, 235, 238, 240]. Contrasting, the IP or IV injection of Ucn 2 or Ucn 3 has no effect on basal distal colonic transit, colonic contractions and defecation [222, 229, 235, 241]. In addition, astressin or selective CRF-R1 antagonists, NBI-35965, NBI-27914, CP-154,526 given peripherally (IP, SC or orally) block IP CRF, Ucn 1-or cortagine-induced accelerated colonic contractions and distal transit, defecation and diarrhea while the CRF-R2 antagonist given IP has not effect in rats and mice [130, 154, 229, 231, 232, 235, 240].
Peripheral vs. central injections of CRF-induced CRF-R1-mediated stimulation of colonic motor function involve distinct target sites of action. First, the CRF receptor antagonists given centrally (IC astressin or ICV α-helical CRF9–41) do not alter IP or IV CRF-induced stimulation of fecal pellet output and colonic transit in rats [54,154]. Second, IP CRF-induced acceleration of colonic transit is not abolished by ganglion blockade or truncal vagotomy that contrasts with the role of the ANS in centrally injected CRF-induced stimulation of colonic propulsive motor function [65,134]. Further support for direct action within the colon comes from in vitro studies in isolated colonic rat preparations or isolated rat colonic muscle strips where CRF and Ucn 1 increase basal myoelectrical peristaltic activity, phasic contractions and electric field stimulation off-contractions while Ucn 2 has no effect on basal activity [154, 221, 235, 242].
Convergent evidence substantiates that the stimulatory action of peripherally injected CRF, Ucn 1 or cortagine involves the activation of cholinergic and nitrergic neurons in colonic myenteric ganglia promoting peristaltic through interaction with CRF-R1 in rats or guinea pigs [143, 219, 221, 222, 240, 243, 244]. The use of laser-capture microdissection combined with reverse transcriptase polymerase chain reaction or immunohistochemical detection in myenteric whole-mount preparation of the colon reveals that CRF-R1 are primarily expressed at the gene and protein levels on myenteric neurons compared with other layers of the rat or guinea colon [219, 221–222, 243]. In addition, the use of a neuronal blocker, tetrodotoxin, abolishes Ucn 1-evoked phasic contractions in rat colonic smooth muscle strips further supporting the role of enteric nervous system [221]. Other evidence shows that IP CRF or IP cortagine in rats or partial restraint in mice induces Fos expression in CRF-R1 expressing cholinergic and nitrergic myenteric neurons in the colon while Ucn 2 under the same conditions has no effect [219, 222, 240, 245, 246]. Of note, atropine, a muscarinic blocker, does not influence IP CRF-induced neuronal activation in colonic myenteric ganglia, indicating that the Fos response is not secondary to the activation of muscarinic receptors either on the myenteric ganglia (which possess both nicotinic and muscarinic receptors) or on colonic muscles [245]. Electrophysiological recording demonstrated that the administration of CRF or Ucn 1 onto colonic myenteric and submucosal plexus preparations of guinea pig directly excites myenteric and submucosal neurons through CRF-R1 [243, 244, 247].
The colonic CRF/CRF-R1 signaling may have relevance as local effector of the acute stress response. There is evidence that stressors that stimulate colonic secreto-motor function upregulate CRF and CRF-R1 in the colonic mucosa and submucosa plus muscle layers [28, 227]. Pharmacological studies show that peripherally restricted peptide CRF antagonists, α-helical CRF9–41, astressin or astressin-B injected IP or IV abolish or dampen the stimulation of propulsive colonic motor function (distal colonic transit, high amplitude high frequency contractions and defecation) and the colonic release of mucin, PGE2 and mast cell protease II evoked by acute exposure to restraint, water avoidance stress and novel environment in rats or mice [63, 127, 130, 133, 154, 234, 248].
6.2. Peripheral CRF-R2 Inhibits CRF-R1 Stimulatory Action
The inhibitory effect of CRF-R2 was suspected initially by the demonstration that IP CRF (CRF-R1>CRF-R2 agonist) was more potent to stimulate defecation in rats than IP Ucn 1 which has not only high affinity to CRF-R1 but also higher affinity than CRF on CRF-R2 [154]. Subsequent studies showed that the IP injection of the selective CRF-R2 agonist, Ucn 2 induces a CRF-R2-mediated inhibition of colonic contractility and defecation stimulated by IP injection of CRF or Ucn 1 (Ucn 1), restraint stress in rats or CRF overexpression in genetically-modified mice [154, 159, 222, 235]. Likewise, in vitro studies on colonic muscle strips showed that Ucn 2 blocks the amplitude of contractions elevated by CRF [235]. Conversely, rats injected IP with astressin2-B or CRF-R2 knockout mice showed enhanced colonic motility, defecation and diarrhea responses to IP CRF- and Ucn 1- or restraint [159, 222, 235]. These results are consistent with CRF and Ucn 1 activating both CRF receptor subtypes [14], and under conditions of CRF-R2 blockade, only the CRF-R1-mediated stimulatory effect is maintained without the dampening action of CRF-R2. These findings open new insight into the dual mechanisms through which acute stress influences colonic function, engaging not only the colonic CRF-R1-mediated enteric stimulatory pathway, but also CRF-R2 to dampen the response suggestive of an adaptive counter-regulatory role of CRF-R2 in the colonic response to stress [222, 235]. However, in the absence of stress, peripheral CRF-R2 agonists or antagonists do not influence basal colonic motor function [222, 235]. The opposite effects of CRF-R1 and CRF-R2 are also observed at the level of intestinal mucosal barrier function altered by stress. Pigs exposed to early-weaning stress display intestinal barrier dysfunction and hypersecretion that involve CRF-R1 activation whereas activation of peripheral CRF-R2 prevents the intestinal barrier dysfunction induced by early life stress [249].
One target of Ucn 2 inhibitory action involved the colonic myenteric neurons where both CRF-R1 and CRF-R2 are localized [222, 228]. The IP injection of Ucn 2 reduces the neuronal activation evoked by IP CRF and the phosphorylation of extracellular signal regulated kinase (ERK) ½ [222]. The localization of CRF-R2 on nitrergic inhibitory myenteric neurons may also play a role [222].
7. PERIPHERAL ACTIONS OF CRF PEPTIDES TO INFLUENCE VISCERAL SENSITIVITY IN EXPERIMENTAL ANIMALS
7.1. Peripheral CRF-R1 Induces Visceral Hyperalgesia
Recent reports suggest that the activation of CRF/CRF-R1 in the colon is involved in the development of visceral hyperalgesia. Peripheral administration of CRF or CRF-R1 peptide selective agonist, cortagine, induces visceral hypersensitivity to CRD in naïve rats and mice [235, 240, 250] that is abolished by IP, but not ICV, injection of the CRF-R/CRF-R2 antagonist, astressin [235, 240]. The role of endogenous peripheral CRF-R1 signaling is further supported by the demonstration that IP pretreatment with peripherally restricted CRF antagonists, astressin, or α-helical CRF9–41 prevents visceral hyperalgesia induced by repeated water avoidance stress, tonic CRD, 4% acetic acid or maternal separation in rats [179, 235, 250, 251]. In addition, chronic psychosocial stress-induced visceral hyperalgesia in rats up-regulates CRF-R1 gene expression in the colon while not influencing CRF-R2 [215]. Collectively these data support the participation of a peripheral CRF-R1 component in the development of visceral hyperalgesia in rats.
Several mechanisms contribute the visceral hypersensitivity induced by the activation of CRF-R1 in the colon [215, 248]. Alteration of intestinal permeability appears to be a prerequisite for the development of visceral hypersensitivity and nociceptor sensitization in rodents [252, 253]. Consistent evidence showed that peripheral CRF-R1 activation is involved in various stressors-induced mast cell dependent disruption of the intestinal epithelial barrier function [217]. Namely acute or chronic exposure to water avoidance stress, early life adverse event, or sustained overcrowding increased the paracellular and transcellular permeability in the intestine which is mimicked by peripheral injection of CRF in vivo and in vitro [26, 217, 248, 254–257]. The alterations of intestinal permeability evoked by various stressors or IP CRF do not occur under conditions of pretreatment with peripheral injection of peptide CRF receptor antagonists [26, 240, 248, 254, 256–259] and selective CRF-R1 antagonists [26]. The beneficial effect of peptide CRF receptor antagonist is associated with increases in the protein expression of the tight junction protein, occludin, in the distal colon [251]. Additional insights into mechanisms come from the strong link between peripheral CRF receptor activation and colonic mucosal mast cell degranulation. This is supported by the localization of CRF receptors on mucosal mast cells [248, 260], the degranulation of mast cells by peripheral injection of CRF and the blockade of acute stressors-evoked mucosal mast cell degranulation by peripheral injection of peptide CRF receptor antagonists in rats and pigs [26, 133, 216, 217, 248, 251, 253, 254, 261]. The content of mast cells can in turn lead to the release of several preformed (histamine, 5-HT, protease) or newly synthesized compounds (PGE2, cytokines, nerve growth factor, tumor necrosis factor-α) [261] known to influence permeability and also to activate or sensitize sensory afferents [262, 263]. Lastly CRF-R1 are localized on enterochromaffin cells and CRF or Ucn 1 through CRF-R1 activation stimulates 5-HT release [11] which is known to active spinal afferents [264].
7.2. Peripheral CRF-R2 Dampens Visceral Hyperalgesia
Opposite to the visceral hyperalgesia to CRD induced by peripheral injection of selective or preferential CRF-R1 agonist, sauvagine and of the selective CRF-R2 agonist, Ucn 2, inhibit visceral sensitization induced by repeated CRD [182, 235, 241]. Conversely, IP injection of CRF-R2 antagonist, astressin2-B, enhances the visceral response to CRD induced by IP CRF [235]. Ucn 2 inhibitory effect is associated with the reduction of ERK 1/2 activation in the L6/S1 segment of the spinal cord in rats [241]. In addition, in vitro studies showed that Ucn 2 reduces CRD-induced increase in the activity of inferior splanchnic afferent fibers [241] indicating the modulation of the nociceptive visceral input by Ucn 2. However, additional mechanisms may be involved. CRF-R2 is expressed on colonic enterochromaffin and mucosal mast cells, and myenteric cell bodies and fibers [27, 222, 265–267]. Ucns-CRF-R2 activation can modulate transmitter release from entechromaffin cells, mast cells and myenteric neurons stimulated by CRF-R1.
8. CRF PEPTIDES INFLUENCE ON GUT FUNCTION AND VISCERAL SENSITIVITY IN HUMAN SUBJECTS
8.1. Biological Actions of CRF in Healthy Subjects
Similar to rodent reports, CRF/Ucns and CRF receptors are localized in human colonic enteric nervous system, mast cells, macrophages and enterochromaffin cells [29, 30, 267–272]. Functional studies also demonstrated that peripherally injected CRF in healthy human subjects largely reproduces the biological actions observed in experimental animals, namely the increased colonic motility, intestinal permeability, mast cell activation and visceral hypersensitivity [273, 274].
In healthy human volunteers, CRF applied intranasally, a route established to maximize the delivery of peptides or drugs into the brain [275, 276] results in a rise in gastric pH associated with a change in mood while circulating cortisol levels are not altered indicating the recruitment of CRF central pathways to inhibit acid secretion [277]. Other functional studies showed that systemic injection of CRF increases the motility index in the descending colon in both healthy volunteers and more so in IBS patients [274]. Recent investigations also indicate that systemic injection of CRF constricts the small intestine, increases volume in the ascending colon, and induces a sense of distention and bloating in healthy volunteers [278]. There are also convergent reports that systemic bolus injection of CRF lowers pain threshold to repetitive rectal distensions and enhances the intensity of discomfort sensation in healthy human subjects [274, 279, 280]. Also consistent with preclinical data, systemic injection of CRF increases intestinal permeability in a mast cell-dependent fashion as shown by the blockade of CRF action by the mast cell stabilizer disodium cromoglycate [273]. In vitro studies in sigmoid colon biopsies of healthy subjects further established that CRF increases transcellular permeability and uptake of protein antigens through direct activation of subepithelial mast cells where CRF receptors are located [267]. The human enterochromaffin-like cells, the BON subclone 1 cells express CRF-R1, and CRF, unlike Ucn 2, stimulates 5-HT release and up-regulates the expression of tryptophan hydroxylase, the 5-HT synthesizing enzyme, through interaction with CRF-R1 [11]. In view of the prominent role of 5-HT in the pathophysiology of IBS [281], this may represent an important site of action of CRF to increase colonic motility and visceral pain in human subjects in addition of mast cell activation and permeability changes.
8.2. Therapeutic future of CRF Receptor Antagonists in Stress-sensitive Functional Diseases
Irritable bowel syndrome is a functional bowel disease that involves alterations in the bidirectional communication between the brain and the gut and is commonly associated with psychosocial and psychiatric comorbidity [170, 282]. Both experimental and clinical studies in healthy subjects highlight that the activation of central/peripheral CRF-R1 recapitulates important features occurring in IBS patients (anxiogenic/hypervigilance behavior, colonic mast cell activation and serotonin release, stimulation of colonic propulsive motor function, increased intestinal permeability and mucus secretion, watery diarrhea and visceral hypersensitivity) [36]. Compelling preclinical studies also established the pivotal actions of CRF-R1 antagonists in the brain and/or the gut to abolish or reduce the functional gut alterations induced by exogenous administration of CRF /Ucn 1 and endogenously released by stress [39]. These lines of evidence supported the conceptual framework that sustained activation of the CRF-R1 system at central and/or peripheral sites may be part of underlying mechanisms triggering IBS symptoms [36, 39]. Therefore, it raised high expectation regarding the potential translational applications of CRF-R1 antagonists for treating IBS [283–285].
A large number of small molecules CRF-R1 antagonists have been developed and tested in a preclinical setting [17], however, only a few compounds so far have progressed to clinical trials [286]. This setback may be related to the formation of reactive metabolites, suboptimal pharmacokinetics with prominent tissue accumulation due to lipophilicity, long elimination half-life, or high affinity to protein binding [17, 286, 287]. Efforts are still ongoing to overcome these issues as shown by recent disclosure of new compounds [132, 288, 289]. Among those, NBI-30545, NBI-35965, NGD 98–2 and NGD 9002 have the distinct advantage to be orally active and water-soluble [17, 132, 290, 291].
Several double-blind, placebo-controlled clinical studies provided preliminary proof-of-concept that oral administration of CRF-R1 antagonists dampened stress-related biological responses in healthy human subjects. For instance, in a clinical model of generalized anxiety evoked by inhaling 7.5% CO2 for 20 min, the CRF-R1 antagonist R317573 (40 mg once daily) given for 7 days significantly dampened panic symptom score and generalized anxiety compared to placebo [292]. Positron emission tomography studies indicate that the acute administration of R317573 at 30 and 200 mg results in dose-related changes in brain activity in regions (putamen, amygdala, orbital frontal cortex) behaviorally relevant to anxiety and mood regulation [293]. Of relevance, a recent functional magnetic resonance imaging study showed that oral administration of the CRF-R1 antagonist, GW876008 (single dose of 20 or 200 mg) reduced the amygdala activation in response to the anticipation of visceral pain in IBS female patients compared to placebo [294]. Consistent reports established that CRF/CRF-R1 signaling in the CeA is a key component of the neuronal circuitry contributing to anxiety-like behavior providing neuroanatomical and biochemical substrata on mechanisms that drive the existing reciprocal relationship between visceral pain and affective mood [196]. It may be speculated that the overactivity of the CRF/CRF-R1 in the CeA underlies the comorbidity of subset of IBS patients who display hypersensitivity to CRD [295] and mood disorders [296].
A few studies using systemic injection of peripherally restricted, -helical CRF9–41 showed also beneficial effects to reduce electrical rectal stimulation-induced higher colonic motility index, abdominal pain and anxiety in IBS patients compared with healthy subjects [297]. The peptide CRF-R1/CRF-R2 antagonist also normalizes the altered EEG activities in IBS patients under basal and in response to CRD [298].
However, so far these clinical data have not translated yet in therapeutic use of CRF-R1 antagonists. Whether the existing or newly developed CRF-R1 antagonists [17, 132] will progress to show therapeutic benefits in subsets of IBS patients is still to be established. A placebo-controlled, double-blind clinical trial in IBS-diarrhea predominant patients was performed using the CRF-R1 antagonist pexacerfont (BMS-562086). Results showed only a dose-related trend to reduce visceral pain [299]. The suboptimal treatment (changes in ACTH plasma levels under pexacerfont treatment were not assessed), lack of information on the central and peripheral CRF-R1 occupancy by CRF antagonist treatment in the absence of positron emission tomography ligands and potential role of CRF-R1 splice variants (some of which are functional) [11, 300] may be contributing factors. Importantly CRF-R1 antagonists may improve symptoms mainly under conditions of altered brain/gut CRF/CRF-R1 signaling that cannot be identified in the heterogeneous populations of IBS patients enrolled in clinical studies performed so far. Therefore, whether the existing or newly developed CRF-R1 antagonists will provide therapeutic benefits for stress-sensitive diseases including IBS [147] or cyclic vomiting syndrome [301] for a subset of patients is still a work in progress.
CONCLUDING REMARKS
CRF, urocortins and CRF receptors are expressed in both the brain and gut where they exert profound alterations of gastrointestinal functions (Figs. 1, 2). Increasing knowledge has been gained during the past decades to localize CRF sites of action in the brain and the gut and to characterize the CRF receptor subtypes involved in mediating CRF/urocortins actions (Figs. 1, 2 & Table 2). Additionally, changes in the ANS activity induced by brain CRF receptor activation have been delineated and shown to be largely responsible for the alterations of gut function in rodents (Figs. 1, 2). By contrast, the peripheral effects are conveyed by direct actions on enteric nervous system, immune and/or endocrine cells within the gut wall in experimental animals (Figs. 1, 2) and healthy human subjects. Consistent reports established that the activation of central or peripheral CRF signaling during stress plays a key role in stress-related alterations with CRF-R1 driving the stimulation of colonic secretory-motor function and the development of visceral hyperalgesia (Fig. 2 & Table 2). However, CRF-R1 antagonists have not yet hold the promise of therapeutic benefits in clinical setting of stress-sensitive functional bowel disease such as irritable bowel syndrome, in part because of lack of adequate knowledge on the biology of the more than 10 variants of CRF-R1 and brain CRF receptor occupancy of the antagonists used.
Fig. (1).
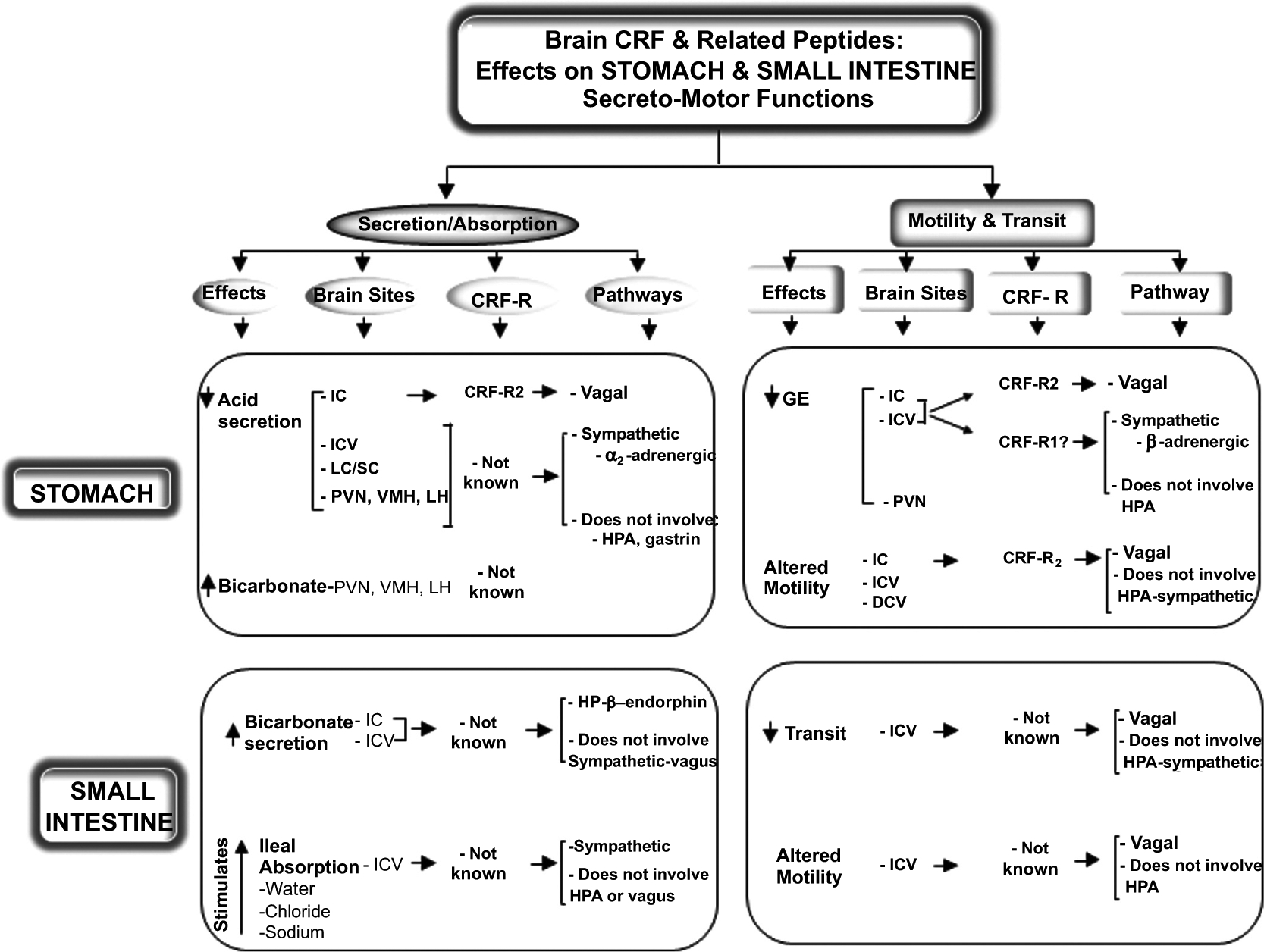
Summary of known influence of CRF and related peptides injected into the brain on gastric and small intestinal functions: CRF receptor mediating the effects, brain sites of action, and neural/hormonal pathways involved.
Fig. (2).
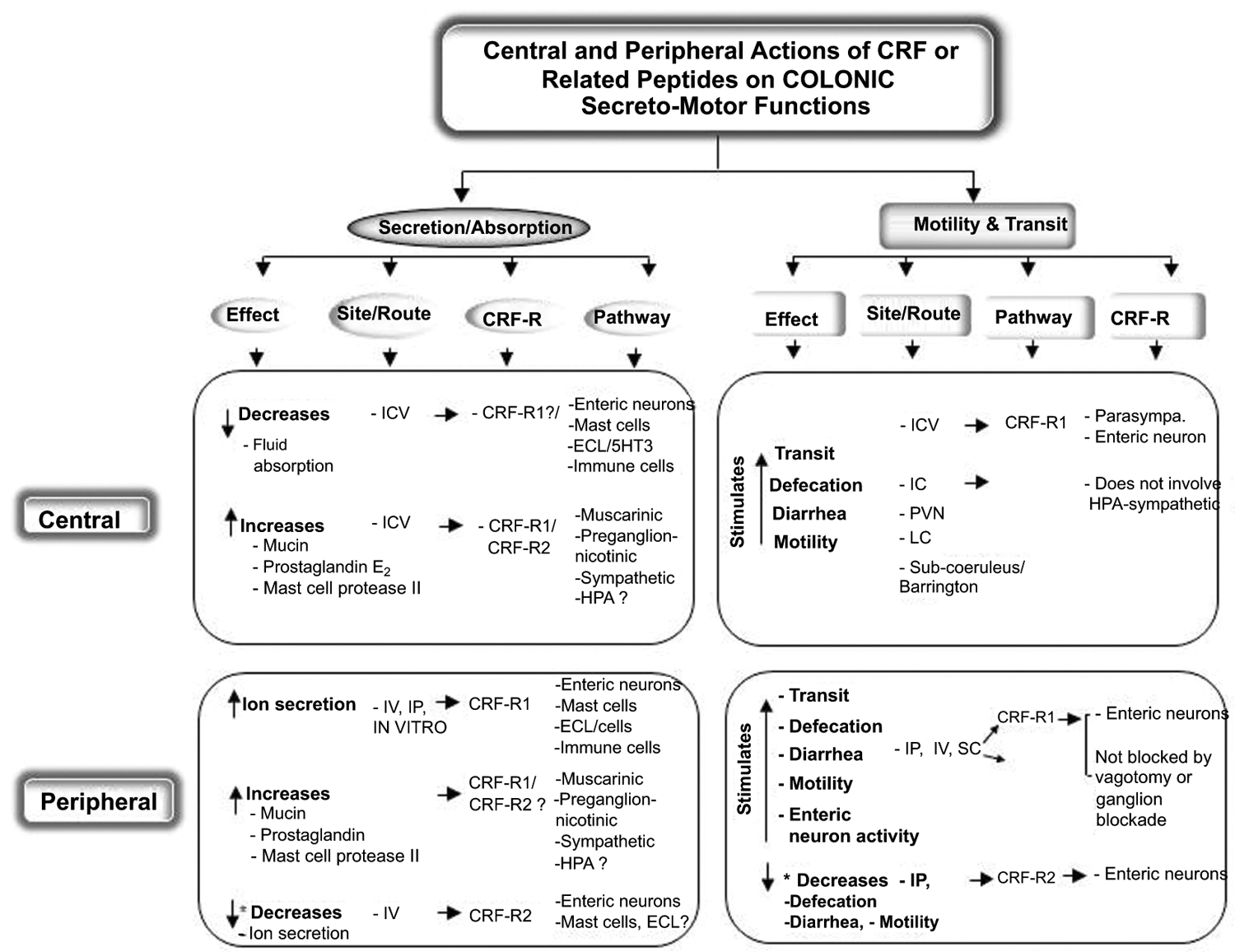
Summary of known influence of CRF and related peptides injected into the brain (central) or peripherally on colonic functions: CRF receptors mediating the effects, sites of action in the brain and the colon and the pathways involved.
Table 2.
Summary of known influence of CRF and related peptides injected into the brain (central) or peripherally on visceral pain of the gut: CRF receptors mediating the effects, sites of action in the brain and the colon and the pathways involved.
| Species | Effect | Route/Site of Action | CRF Receptor | Pathways |
|---|---|---|---|---|
| Rodents | - | - | - | - |
| Brain CRF Urocortin 1 |
Visceral hyperalgesia | ICV LC CeA, BNST, Hippocampus |
CRF-R1 | ↑ NE release in hippocampus and CeA following LC activation -Peripheral recruitment of mast cells? |
| ↓ or ↑ visceral pain | BNST | CRF-R2 | - | |
| Peripheral CRF Urocortin 1 |
Visceral hyperalgesia | IP | CRF-R1 | ↑ Intestinal permeability Mast cells (histamine, proteases) Enterochromaffin cells (5-HT) |
| ↓Visceral hyperalgesia | IP | CRF-R2 | ↓ ERK1/2 activation in L6-S1 ↓ Inferior splanchnic afferent fibers activity - Mast cell activity? - Enterochromaffin cells activation? |
|
| Human | - | - | - | - |
| Peripheral CRF |
Visceral hyperalgesia | IV | - | ↑ Intestinal permeability ↑ Mast cell activation |
ACKNOWLEDGEMENTS
The author’ work was supported by the NIHDDK grants R01 DK-57236 (YT), R01 DK-078676 (MM), DK-41301 (Models of Gastrointestinal Function and Disease, YT, MM), P50 DK-64539 (YT, MM, and ML), K01DK088937 (ML) and VA Merit and Senior Scientist Awards (YT). We thank Dr. Jean River (Salk Institute, La Jolla, CA) for the generous supply of peptide CRF agonists and antagonists and Dr D. Grigoriadis (Neurocrine Biosciences, La Jolla, CA) for generous supply of non-peptide CRF-R1 antagonists used in our studies.
LIST OF ABBREVIATIONS
- ACTH
Adrenocorticotropic Hormone
- ANS
Autonomic Nervous System
- ARC
Arcuate
- CART
Cocaine- and Amphetamine-Regulated Transcript
- CeA
Central Amygdala
- CRD
Colorectal Distension
- CRF
Corticotropin Releasing Factor
- CRF-R1
Corticotropin Releasing Factor Receptor Subtype 1
- CRF-R2
Corticotropin Releasing Factor Receptor Subtype 2
- CSF
Cerebrospinal Fluid
- DVC
Dorsal Vagal Complex
- ERK
Extracellular Signal Regulated Kinase
- GLP-1
Glucagon-like Peptide
- HPA
Hypothalamic-Pituitary Axis
- 5-HT
Serotonin
- 5-HT3
Serotonin Receptor Type 3
- 5-HT4
Serotonin Receptor Type 4
- IBS
Irritable Bowel Disease
- IC
Intracisternal(ly)
- ICV
Intracerebroventricular (ly)
- IgG
Immunoglobulin G
- IP
Intraperitoneal
- IV
Intravenous
- LH
Lateral Hypothalamus
- LC/SC
Locus Coeruleus/Subcoeruleus
- NE
Norepinephrine
- N/OFQ
Nociceptin/Orphanin FQ
- PGE2
Prostaglandin E2
- PVN
Paraventricular Nucleus of the Hypothalamus
- TRH
Thyrotropin Releasing Hormone
- Ucns
Urocortin 1, 2 and 3
- VMH
Ventromedial Hypothalamus
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Guillemin R Neuroendocrinology: a short historical review. Ann. N. Y. Acad. Sci, 2011, 1220, 1–5. [DOI] [PubMed] [Google Scholar]
- [2].Bale TL; Chen A Minireview: CRF and Wylie Vale: a story of 41 amino acids and a Texan with grit. Endocrinology, 2012, 153, 2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vale W; Spiess J; Rivier C; Rivier J Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and b-endorphin. Science, 1981, 213, 1394–1397. [DOI] [PubMed] [Google Scholar]
- [4].Vaughan J; Donaldson C; Bittencourt J; Perrin MH; Lewis K; Sutton S; Chan R; Turnbull AV; Lovejoy D; Rivier C; Rivier J; Sawchenko PE; Vale W Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature, 1995, 378, 287–292. [DOI] [PubMed] [Google Scholar]
- [5].Reyes TM; Lewis K; Perrin MH; Kunitake KS; Vaughan J; Arias CA; Hogenesch JB; Gulyas J; Rivier J; Vale WW; Sawchenko PE Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA, 2001, 98, 2843–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hsu SY; Hsueh AJ Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med, 2001, 7, 605–611. [DOI] [PubMed] [Google Scholar]
- [7].Lewis K; Li C; Perrin MH; Blount A; Kunitake K; Donaldson C; Vaughan J; Reyes TM; Gulyas J; Fischer W; Bilezikjian L; Rivier J; Sawchenko PE; Vale WW Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. U.S.A, 2001, 98, 7570–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen R; Lewis KA; Perrin MH; Vale WW Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. U.S.A, 1993, 90, 8967–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lovenberg TW; Liaw CW; Grigoriadis DE; Clevenger W; Chalmers DT; De Souza EB; Oltersdorf T Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U.S.A, 1995, 92, 836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perrin MH; Vale WW Corticotropin releasing factor receptors and their ligand family. Ann. N.Y. Acad. Sci, 1999, 885, 312–328. [DOI] [PubMed] [Google Scholar]
- [11].Wu SV; Yuan PQ; Lai J; Wong K; Chen MC; Ohning GV; Taché Y Activation of Type 1 CRH receptor isoforms induces serotonin release from human carcinoid BON-1N cells: an enterochromaffin cell model. Endocrinology, 2011, 152, 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu SV; Yuan P-Q; Wang L; Peng YL; Chen C-Y; Taché Y Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology, 2007, 148, 1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zmijewski MA; Slominski AT Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim. Pol, 2010, 57, 1–13. [PMC free article] [PubMed] [Google Scholar]
- [14].Hauger RL; Grigoriadis DE; Dallman MF; Plotsky PM; Vale WW; Dautzenberg FM International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol. Rev, 2003, 55, 21–26. [DOI] [PubMed] [Google Scholar]
- [15].Rivier CL; Grigoriadis DE; Rivier JE Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology, 2003, 144, 2396–2403. [DOI] [PubMed] [Google Scholar]
- [16].Rivier JE; Rivier CL Corticotropin-releasing factor peptide antagonists: design, characterization and potential clinical relevance. Front. Neuroendocrinol, 2014, 35, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zorrilla EP; Koob GF Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov. Today, 2010, 15, 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Turnbull AV; Rivier C Corticotropin-releasing factor (CRF) and endocrine response to stress: CRF receptors, binding protein, and related peptides. Proc. Soc. Exp. Biol. Med, 1997, 215, 1–10. [DOI] [PubMed] [Google Scholar]
- [19].Bittencourt JC; Vaughan J; Arias C; Rissman RA; Vale WW; Sawchenko PE Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J. Comp. Neurol, 1999, 415, 285–312. [PubMed] [Google Scholar]
- [20].Bittencourt JC; Sawchenko PE Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J. Neurosci, 2000, 20, 1142–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang L; Goebel-Stengel M; Stengel A; Wu SV; Ohning G; Taché Y Comparison of CRF-immunoreactive neurons distribution in mouse and rat brains and selective induction of Fos in rat hypothalamic CRF neurons by abdominal surgery. Brain Res, 2011, 1415, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bale TL; Vale WW CRF and CRF receptor: Role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol, 2004, 44, 525–557. [DOI] [PubMed] [Google Scholar]
- [23].Habib KE; Weld KP; Rice KC; Pushkas J; Champoux M; Listwak S; Webster EL; Atkinson AJ; Schulkin J; Contoreggi C; Chrousos GP; McCann SM; Suomi SJ; Higley JD; Gold PW Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc. Natl. Acad. Sci. USA, 2000, 97, 6079–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stengel A; Taché Y Corticotropin-releasing factor signaling and visceral response to stress. Exp. Biol. Med. (Maywood.) 2010, 235, 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Taché Y; Bonaz B Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J. Clin. Invest, 2007, 117, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Usui D; Yamaguchi-Shima N; Okada S; Shimizu T; Wakiguchi H; Yokotani K Selective activation of the sympathetic ganglia by centrally administered corticotropin-releasing factor in rats. Auton Neurosci. 2009, 146,111–114. [DOI] [PubMed] [Google Scholar]
- [27].Larauche M; Kiank C; Taché Y Corticotropin releasing factor signaling in colon and ileum: Regulation by stress and pathophysiological implications. J. Physiol Pharmacol, 2009, 60 (Suppl) 7, 33–46. [PMC free article] [PubMed] [Google Scholar]
- [28].Yuan PQ; Wu SV; Wang L; Taché Y Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides, 2010, 31, 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yuan PQ; Wu SV; Elliott J; Anton PA; Chatzaki E; Million M; Taché Y Expression of corticotropin releasing factor receptor type 1 (CRF(1)) in the human gastrointestinal tract and upregulation in the colonic mucosa in patients with ulcerative colitis. Peptides, 2012, 38, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wallon C; Persborn M; Jonsson M; Wang A; Phan V; Lampinen M; Vicario M; Santos J; Sherman PM; Carlson M; Ericson AC; McKay DM; Soderholm JD Eosinophils express muscarinic receptors and corticotropin-releasing factor to disrupt the mucosal barrier in ulcerative colitis. Gastroenterology, 2011, 140, 1597–1607. [DOI] [PubMed] [Google Scholar]
- [31].Adao R; Santos-Ribeiro D; Rademaker MT; Leite-Moreira AF; Bras-Silva C Urocortin 2 in cardiovascular health and disease. Drug Discov. Today, 2015, 20, 906–914. [DOI] [PubMed] [Google Scholar]
- [32].Kiank C; Taché Y; Larauche M Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav. Immun, 2010, 24, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Taché Y; Perdue MH Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol. Mot, 2004, 16(Suppl),1, 1–6. [DOI] [PubMed] [Google Scholar]
- [34].Walczewska J; Dzieza-Grudnik A; Siga O; Grodzicki T The role of urocortins in the cardiovascular system. J. Physiol. Pharmacol, 2014, 65, 753–766. [PubMed] [Google Scholar]
- [35].Zorrilla EP; Logrip ML; Koob GF Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol, 2014, 35, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Taché Y; Brunnhuber S From Hans Selye’s discovery of biological stress to the identification of corticotropin-releasing factor signaling pathways: implication in stress-related functional bowel diseases. Ann. N. Y. Acad. Sci, 2008, 1148, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gravanis A; Margioris AN The corticotropin-releasing factor (CRF) family of neuropeptides in inflammation: potential therapeutic applications. Curr. Med. Chem, 2005, 12, 1503–1512. [DOI] [PubMed] [Google Scholar]
- [38].Holsboer F; Ising M Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur. J. Pharmacol, 2008, 583, 350–357. [DOI] [PubMed] [Google Scholar]
- [39].Taché Y; Million M Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J. Neurogastroenterol. Motil, 2015, 21, 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Taché Y; Goto Y; Gunion MW; Vale W; River J; Brown M Inhibition of gastric acid secretion in rats by intracerebral injection of corticotropin-releasing factor. Science, 1983, 222, 935–937. [DOI] [PubMed] [Google Scholar]
- [41].Druge G; Raedler A; Heiner G; Lenz J Pathways mediating CRF-induced inhibition of gastric acid secretion in rats. Am. J. Physiol, 1989, 256, G214–G219. [DOI] [PubMed] [Google Scholar]
- [42].Brown MR; Fisher LA; Spiess J; Rivier J; Rivier C; Vale W Comparison of the biological actions of corticotropin-releasing factor and sauvagine. Regul. Pept, 1982, 4, 107–114. [DOI] [PubMed] [Google Scholar]
- [43].Lenz HJ; Forquignon I; Druge G; Greten H Effects of neuropeptides on gastric acid and duodenal bicarbonate secretions in freely moving rats. Regul. Pept, 1989, 24, 293–300. [DOI] [PubMed] [Google Scholar]
- [44].Gué M; Yoneda M; Mönnikes H; Junien J-L; Taché Y Central neuropeptide Y and sigma ligand, JO 1784, reverse corticotropin-releasing factor-induced inhibition of gastric acid secretion in rats. Br. J. Pharmacol, 1992, 107, 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Guglietta A; Nardi RV; Lazarus LH Indomethacin (i.c.v.) reverses the inhibitory action of peptides on gastric secretion. Eur. J. Immunol, 1989, 170, 87–90. [DOI] [PubMed] [Google Scholar]
- [46].Lenz HJ; Raedler A; Greten H; Brown MR CRF initiates biological actions within the brain that are observed in response to stress. Am. J. Physiol, 1987, 252, R34–R39. [DOI] [PubMed] [Google Scholar]
- [47].Lenz HJ; Hester SE; Brown MR Corticotropin-releasing factor mechanisms to inhibit gastric acid secretion in conscious dogs. J. Clin. Invest, 1985, 75, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Taché Y; Goto Y; Gunion M; Rivier J; Debas H Inhibition of gastric acid secretion in rats and in dogs by corticotropin-releasing factor. Gastroenterology, 1984, 86, 281–286. [PubMed] [Google Scholar]
- [49].Yakabi K; Noguchi M; Ohno S; Ro S; Onouchi T; Ochiai M; Takabayashi H; Takayama K; Harada Y; Sadakane C; Hattori T Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am. J. Physiol, 2011, 301, E72–E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gunion MW; Taché Y Intrahypothalamic microinfusion of corticotropin-releasing factor inhibits gastric acid secretion but increases secretion volume in rats. Brain Res, 1987, 411, 156–161. [DOI] [PubMed] [Google Scholar]
- [51].Gunion MW; Kauffman GL; Taché Y Intrahypothalamic microinfusion of corticotropin-releasing factor elevates gastric bicarbonate secretion and protects against cold-stress ulceration in rats. Am. J. Physiol, 1990, 258, G152–G157. [DOI] [PubMed] [Google Scholar]
- [52].Mönnikes H; Tebbe J; Bauer C; Lauer G; Arnold R Microinfusion of corticotropin-releasing factor into the locus coeruleus/subcoeruleus nuclei inhibits gastric acid secretion via spinal pathways in the rat. Brain Res., 1996,728,157–165. [DOI] [PubMed] [Google Scholar]
- [53].Kosoyan HP; Wei JY; Taché Y Intracisternal sauvagine is more potent than corticotropin-releasing factor to decrease gastric vagal efferent activity in rats. Peptides 1999, 20, 851–858. [DOI] [PubMed] [Google Scholar]
- [54].Lenz HJ; Raedler A; Greten H; Vale WW; Rivier JE Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology, 1988, 95, 1510–1517. [DOI] [PubMed] [Google Scholar]
- [55].Taché Y The parasympathetic nervous system in the pathophysiology of the gastrointestinal tract. In Handbook of autonomic nervous system in health and disease, Bolis CL, Licinio J, Govoni S, Eds.; Dekker Inc: New York, 2002, pp 463–503. [Google Scholar]
- [56].Lenz HJ; Klapdor R; Hester SE; Webb VJ; Galyean RF; Rivier JE; Brown MR Inhibition of gastric acid secretion by brain peptides in the dog. Role of the autonomic nervous system and gastrin. Gastroenterology, 1986, 91, 905–912. [DOI] [PubMed] [Google Scholar]
- [57].Beaumont W In Experiments and Observations on the Gastric Juice and the Physiology of Digestion, Osler W, Ed.; Dover Publications Inc.: New York, 1833, pp. 1–280. [Google Scholar]
- [58].Cannon WB Bodily changes in pain, hunger, fear and rage; Branford, CT: Boston, 1953. pp. 1–404. [Google Scholar]
- [59].Stephens RL; Yang H; Rivier J; Taché Y Intracisternal injection of CRF antagonist blocks surgical stress-induced inhibition of gastric secretion in the rat. Peptides, 1988, 9, 1067–1070. [DOI] [PubMed] [Google Scholar]
- [60].Mondal MS; Date Y; Murakami N; Toshinai K; Shimbara T; Kangawa K; Nakazato M Neuromedin U acts in the central nervous system to inhibit gastric acid secretion via CRH system. Am. J. Physiol, 2003, 284, G963–G969. [DOI] [PubMed] [Google Scholar]
- [61].Okumura T; Yamada H; Motomura W; Kohgo Y Cocaine-amphetamine-regulated transcript (CART) acts in the central nervous system to inhibit gastric acid secretion via brain corticotropin-releasing factor system. Endocrinology, 2000, 141, 2854–2860. [DOI] [PubMed] [Google Scholar]
- [62].Tebbe JJ; Mronga S; Schäfer MK; Rüter J; Kobelt P; Mönnikes H Stimulation of neurons in rat ARC inhibits gastric acid secretion via hypothalamic CRF1/2- and NPY-Y1 receptors. Am. J. Physiol, 2003,285, G1075–1083. [DOI] [PubMed] [Google Scholar]
- [63].Williams CL; Peterson JM; Villar RG; Burks TF Corticotropin-releasing factor directly mediates colonic responses to stress. Am. J. Physiol, 1987, 253, G582–G586. [DOI] [PubMed] [Google Scholar]
- [64].Taché Y; Maeda-Hagiwara M; Turkelson CM Central nervous system action of corticotropin-releasing factor to inhibit gastric emptying in rats. Am. J. Physiol, 1987, 253, G241–G245. [DOI] [PubMed] [Google Scholar]
- [65].Lenz HJ; Burlage M; Raedler A; Greten H Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology, 1988, 94, 598–602. [DOI] [PubMed] [Google Scholar]
- [66].Sheldon RJ; Qi JA; Porreca F; Fisher LA Gastrointestinal motor effects of corticotropin-releasing factor in mice. Regul. Pept, 1990, 28, 137–151. [DOI] [PubMed] [Google Scholar]
- [67].Riviere PJ; Pascaud X; Chevalier E; Junien JL Fedotozine reversal of peritoneal-irritation-induced ileus in rats: possible peripheral action on sensory afferents. J. Pharmacol. Exp. Ther, 1994, 270, 846–850. [PubMed] [Google Scholar]
- [68].Smedh U; Uvnäs-Moberg K; Grill HJ; Kaplan JM Fourth ventricle injection of corticotropin-releasing factor and gastric emptying of glucose during gastric fill. Am. J. Physiol, 1995, 269, G1000–G1003. [DOI] [PubMed] [Google Scholar]
- [69].Coskun T; Bozkurt A; Alican I; Özkutlu U; Kurtel H; Yegen Ç Pathways mediating CRF-induced inhibition of gastric emptying in rats. Regul. Pept, 1997, 69, 113–120. [DOI] [PubMed] [Google Scholar]
- [70].Lee C; Sarna SK Central regulation of gastric emptying of solid nutrient meals by corticotropin releasing factor. Neurogastroenterol. Mot, 1997, 9, 221–229. [DOI] [PubMed] [Google Scholar]
- [71].Martinez V; Wang L; Rivier J; Grigoriadis D; Taché Y Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J. Physiol, 2004, 556.1, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nakade Y; Tsuchida D; Fukuda H; Iwa M; Pappas TN; Takahashi T Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am. J. Physiol, 2005, 288, R427–R432. [DOI] [PubMed] [Google Scholar]
- [73].Czimmer J; Million M; Taché Y Urocortin 2 acts centrally to delay gastric emptying through sympathetic pathways while CRF and urocortin 1 inhibitory actions are vagal dependent in rats. Am. J. Physiol, 2006, 290, G511–G518. [DOI] [PubMed] [Google Scholar]
- [74].Ushikai M; Asakawa A; Sakoguchi T; Tanaka C; Inui A Centrally administered urocortin 3 inhibits food intake and gastric emptying in mice. Endocrine, 2011, 39, 113–117. [DOI] [PubMed] [Google Scholar]
- [75].Ruckebusch Y; Malbert CH Stimulation and inhibition of food intake in sheep by centrally-administered hypothalamic releasing factors. Life Sci, 1986, 38, 929–934. [DOI] [PubMed] [Google Scholar]
- [76].Buéno L; Fargeas MJ; Gue M; Peeters TL; Bormans V; Fioramonti J Effects of corticotropin-releasing factor on plasma motilin and somatostatin levels and gastrointestinal motility in dogs. Gastroenterology, 1986, 91, 884–889. [DOI] [PubMed] [Google Scholar]
- [77].Chen CY; Million M; Adelson DW; Martinez V; Rivier J; Taché Y Intracisternal urocortin inhibits vagally stimulated gastric motility in rats: role of CRF(2). Br. J. Pharmacol, 2002, 136, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nakade Y; Tsuchida D; Fukuda H; Iwa M; Pappas TN; Takahashi T Restraint stress augments postprandial gastric contractions but impairs antropyloric coordination in conscious rats. Am. J. Physiol, 2006, 290, R616–R624. [DOI] [PubMed] [Google Scholar]
- [79].Ataka K; Nagaishi K; Asakawa A; Inui A; Fujimiya M Alteration of antral and proximal colonic motility induced by chronic psychological stress involves central urocortin 3 and vasopressin in rats. Am. J. Physiol, 2012, 303, G519–G528. [DOI] [PubMed] [Google Scholar]
- [80].Browning KN; Babic T; Toti L; Holmes GM; Coleman FH; Travagli RA Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor. J. Physiol, 2014, 592, 4591–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kihara N; Fujimura M; Yamamoto I; Itoh E; Inui A; Fujimiya M Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am. J. Physiol, 2001, 280, G406–G419. [DOI] [PubMed] [Google Scholar]
- [82].Garrick T; Veiseh A; Sierra A; Weiner H; Taché Y Corticotropin-releasing factor acts centrally to suppress stimulated gastric contractility in the rat. Regul. Pept, 1988, 21, 173–181. [DOI] [PubMed] [Google Scholar]
- [83].Heymann-Monnikes I; Taché Y; Trauner M; Weiner H; Garrick T CRF microinjected into the dorsal vagal complex inhibits TRH analog- and kainic acid-stimulated gastric contractility in rats. Brain. Res, 1991, 554, 139–144. [DOI] [PubMed] [Google Scholar]
- [84].Buéno L; Fioramonti J Effects of corticotropin-releasing factor, corticotropin and cortisol on gastrointestinal motility in dogs. Peptides, 1986, 7, 73–77. [DOI] [PubMed] [Google Scholar]
- [85].Gué M; Fioramonti J; Frexinos J; Alvinerie M; Buéno L Influence of acoustic stress by noise on gastrointestinal motility in dogs. Dig. Dis. Sci, 1987, 32, 1411–1417. [DOI] [PubMed] [Google Scholar]
- [86].Martinez V; Barquist E; Rivier J; Taché Y Central CRF inhibits gastric emptying of a nutrient solid meal in rats: the role of CRF2 receptors. Am. J. Physiol, 1998, 274, G965–G970. [DOI] [PubMed] [Google Scholar]
- [87].Martinez V; Rivier J; Wang L; Taché Y Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J. Pharmacol. Exp. Ther, 1997, 280, 754–760. [PubMed] [Google Scholar]
- [88].Taché Y; Barquist E; Stephens RL; Rivier J Abdominal surgery- and trephination-induced delay in gastric emptying is prevented by intracisternal injection of CRF antagonist in the rat. J. Gastrointest. Motil, 1991, 3, 19–25. [Google Scholar]
- [89].Sütó G; Király A; Taché Y Interleukin-1b inhibits gastric emptying in rats: mediation through prostaglandin and corticotropin-releasing factor. Gastroenterology 1994, 106, 1568–1575. [DOI] [PubMed] [Google Scholar]
- [90].Barquist E; Bonaz B; Martinez V; Rivier J; Zinner MJ; Taché Y Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am. J. Physiol, 1996, 270, R888–R894. [DOI] [PubMed] [Google Scholar]
- [91].Martinez V; Taché Y Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res, 2001, 893, 29–35. [DOI] [PubMed] [Google Scholar]
- [92].Mönnikes H; Schmidt BG; Raybould HE; Taché Y CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am. J. Physiol, 1992, 262, G137–G143. [DOI] [PubMed] [Google Scholar]
- [93].Mönnikes H; Schmidt BG; Tebbe J; Bauer C; Taché Y Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus stimulates colonic motor function in rats. Brain Res, 1994, 644, 101–108. [DOI] [PubMed] [Google Scholar]
- [94].Smedh U; Moran TH The dorsal vagal complex as a site for cocaine- and amphetamine-regulated transcript peptide to suppress gastric emptying. Am. J. Physiol. Regul, 2006, 291, R124–R130. [DOI] [PubMed] [Google Scholar]
- [95].Lewis MW; Hermann GE; Rogers RC; Travagli RA In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J. Physiol, 2002, 543, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Swanson LW; Sawchenko PE Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology, 1980, 31, 410–417. [DOI] [PubMed] [Google Scholar]
- [97].Broccardo M; Improta G Pituitary-adrenal and vagus modulation of sauvagine- and CRF-induced inhibition of gastric emptying in rats. Eur. J. Pharmacol, 1990, 182, 357–362. [DOI] [PubMed] [Google Scholar]
- [98].Kang YM; Zhang AQ; Zhao XF; Cardinale JP; Elks C; Cao XM; Zhang ZW; Francis J Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res. Cardiol, 2011, 106, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Smedh U; Kaplan JM; Uvnas-Moberg K Corticotropin-releasing factor-induced suppression of gastric emptying in the rat is blocked by cyclo(7-aminoheptanoyl-phe-D-TRP-LYS-THR[BZL], an in vivo somatostatin antagonist. Neurosci. Lett, 1999, 260, 41–44. [DOI] [PubMed] [Google Scholar]
- [100].Rivier J; Gulyas J; Corrigan A; Martinez V; Craig AG; Taché Y; Vale W; Rivier C Astressin analogues (corticotropin-releasing factor antagonists) with extended duration of action in the rat. J. Med. Chem, 1998, 41, 5012–5019. [DOI] [PubMed] [Google Scholar]
- [101].Suto G; Kiraly A; Plourde V; Taché Y Intravenous interleukin-1-beta-induced inhibition of gastric emptying: involvement of central corticotropin-releasing factor and prostaglandin pathways in rats. Digestion, 1996, 57, 135–140. [DOI] [PubMed] [Google Scholar]
- [102].Taché Y; Martinez V; Million M; Wang L Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am. J. Physiol, 2001, 280, G173–G177. [DOI] [PubMed] [Google Scholar]
- [103].Bonaz B; Plourde V; Taché Y Abdominal surgery induces Fos immunoreactivity in the rat brain. J. Comp. Neurol, 1994, 349, 212–222. [DOI] [PubMed] [Google Scholar]
- [104].Harbuz MS; Lightman SL Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J. Endocrinol, 1989, 122, 705–711. [DOI] [PubMed] [Google Scholar]
- [105].Rivest S; Rivier C Stress and interleukin-1 beta-induced activation of c-fos, NGFI-B and CRF gene expression in the hypothalamic PVN: comparison between Sprague-Dawley, Fisher-344 and Lewis rats. J. Neuroendocrinol, 1994, 6, 101–117. [DOI] [PubMed] [Google Scholar]
- [106].Gourcerol G; Gallas S; Mounien L; Leblanc I; Bizet P; Boutelet I; Leroi AM; Ducrotte P; Vaudry H; Jegou S Gastric electrical stimulation modulates hypothalamic corticotropin-releasing factor-producing neurons during postoperative ileus in rat. Neuroscience, 2007, 148, 775–781. [DOI] [PubMed] [Google Scholar]
- [107].Rivest S Molecular mechanisms and neural pathways mediating the influence of interleukin-1 on the activity of neuroendocrine CRF motoneurons in the rat. Int. J. Dev. Neurosci, 1995, 13, 135–146. [DOI] [PubMed] [Google Scholar]
- [108].Rivier C Influence of immune signals on the hypothalamic-pituitary axis of the rodent. Front. Neuroendocrinol, 1995, 16, 151–182. [DOI] [PubMed] [Google Scholar]
- [109].Tanaka Y; Makino S; Noguchi T; Tamura K; Kaneda T; Hashimoto K Effect of stress and adrenalectomy on urocortin II mRNA expression in the hypothalamic paraventricular nucleus of the rat. Neuroendocrinology, 2003, 78, 1–11. [DOI] [PubMed] [Google Scholar]
- [110].Korosi A; Schotanus S; Olivier B; Roubos EW; Kozicz T Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res, 2005, 1046, 172–179. [DOI] [PubMed] [Google Scholar]
- [111].Luckey A; Wang L; Jamieson PM; Basa NR; Million M; Czimmer J; Vale W; Taché Y Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology, 2003, 125, 654–659. [DOI] [PubMed] [Google Scholar]
- [112].Ulrich-Lai YM; Herman JP Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci, 2009, 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Smedh U; Moran TH Peptides that regulate food intake: separable mechanisms for dorsal hindbrain CART peptide to inhibit gastric emptying and food intake. Am. J. Physiol, 2003, 284, R1418–R1426. [DOI] [PubMed] [Google Scholar]
- [114].Broccardo M; Scaccianoce S; Del BP; Agostini S; Petrella C; Improta G Nociceptin/orphanin FQ-induced delay in gastric emptying: role of central corticotropin-releasing factor and glucocorticoid receptors. Neurogastroenterol. Motil, 2005, 17, 871–877. [DOI] [PubMed] [Google Scholar]
- [115].Nakade Y; Tsukamoto K; Pappas TN; Takahashi T Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res, 2006, 1111, 117–121. [DOI] [PubMed] [Google Scholar]
- [116].Chen CY; Inui A; Asakawa A; Fujino K; Kato I; Chen CC; Ueno N; Fujimiya M Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology, 2005, 129, 8–25. [DOI] [PubMed] [Google Scholar]
- [117].Martinez V; Cuttitta F; Taché Y Central action of adrenomedullin to inhibit gastric emptying in rats. Endocrinology, 1997, 138, 3749–3755. [DOI] [PubMed] [Google Scholar]
- [118].Stengel A; Goebel M; Wang L; Rivier J; Kobelt P; Monnikes H; Lambrecht NW; Taché Y Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology, 2009, 150, 4911–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lenz HJ; Forquignon I; Druge G; Friemel E; Rivier JE; Raedler A; Greten H Central nervous system effects of hypothalamic peptides on gastric acid and proximal duodenal bicarbonate secretions in rats. Gastroenterology, 1987, 92, 1501 Abstr. [Google Scholar]
- [120].Lenz HJ Regulation of duodenal bicarbonate secretion during stress by corticotropin-releasing factor and b-endorphin. Proc. Natl. Acad. Sci. U. S. A, 1989, 86, 1417–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Flemstrom G; Jedstedt G Stimulation of duodenal mucosal bicarbonate secretion in the rat by brain peptides. Gastroenterology, 1989, 97, 412–420. [DOI] [PubMed] [Google Scholar]
- [122].Lenz HJ Regulation of small intestinal and pancreaticobiliary functions by CRF. Ann. N. Y. Acad. Sci, 1993, 697, 254–259. [DOI] [PubMed] [Google Scholar]
- [123].Brown MR; Fisher LA; Spiess J; Rivier C; Rivier J; Vale W Corticotropin-releasing factor: Action on the sympathetic nervous system and metabolism. Endocrinology, 1982, 111, 928–931. [DOI] [PubMed] [Google Scholar]
- [124].Yin Y; Dong L; Yin D Peripheral and central administration of exogenous urocortin 1 disrupts the fasted motility pattern of the small intestine in rats via the corticotrophin releasing factor receptor 2 and a cholinergic mechanism. J. Gastroenterol. Hepatol, 2008, 23, e79–e87. [DOI] [PubMed] [Google Scholar]
- [125].Jiménez M; Buéno L Inhibitory effects of neuropeptide Y (NPY) on CRF and stress-induced cecal motor response in rats. Life Sci, 1990, 47, 205–211. [DOI] [PubMed] [Google Scholar]
- [126].Bonaz B; Taché Y Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res, 1994, 641, 21–28. [DOI] [PubMed] [Google Scholar]
- [127].Miyata K; Ito H; Fukudo S Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am. J. Physiol, 1998, 274, G827–G831. [DOI] [PubMed] [Google Scholar]
- [128].Gué M; Tekamp A; Tabis N; Junien JL; Buéno L Cholecystokinin blockade of emotional stress- and CRF-induced colonic motor alterations in rats: role of the amygdala. Brain Res, 1994, 658, 232–238. [DOI] [PubMed] [Google Scholar]
- [129].Gué M; Junien JL; Buéno L Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology, 1991, 100, 964–970. [DOI] [PubMed] [Google Scholar]
- [130].Million M; Grigoriadis DE; Sullivan S; Crowe PD; McRoberts JE; Zhou CY; Saunders PR; Maillot C; Mayer AE; Taché Y A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res, 2003, 985, 32–42. [DOI] [PubMed] [Google Scholar]
- [131].Ataka K; Kuge T; Fujino K; Takahashi T; Fujimiya M Wood creosote prevents CRF-induced motility via 5-HT3 receptors in proximal and 5-HT4 receptors in distal colon in rats. Auton. Neurosci, 2007, 133, 136–145. [DOI] [PubMed] [Google Scholar]
- [132].Million M; Zhao JF; Luckey A; Czimmer J; Maynard GD; Kehne J; Hoffman DC; Taché Y The newly developed CRF1-receptor antagonists, NGD 98–2 and NGD 9002, suppress acute stress-induced stimulation of colonic motor function and visceral hypersensitivity in rats. PLoS. One, 2013, 8, e73749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Castagliuolo I; Lamont JT; Qiu B; Fleming SM; Bhaskar KR; Nikulasson ST; Kornetsky C; Pothoulakis C Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am. J. Physiol, 1996, 271, G884–G892. [DOI] [PubMed] [Google Scholar]
- [134].Mönnikes H; Raybould HE; Schmidt B; Taché Y CRF in the paraventricular nucleus of the hypothalamus stimulates colonic motor activity in fasted rats. Peptides, 1993, 14, 743–747. [DOI] [PubMed] [Google Scholar]
- [135].Mönnikes H; Schmidt BG; Taché Y Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology, 1993, 104, 716–723. [DOI] [PubMed] [Google Scholar]
- [136].Mönnikes H; Heymann-Monnikes I; Taché Y CRF in the paraventricular nucleus of the hypothalamus induces dose-related behavioral profile in rats. Brain Res, 1992, 574, 70–76. [DOI] [PubMed] [Google Scholar]
- [137].Koob GF; Heinrichs SC A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res, 1999, 848, 141–152. [DOI] [PubMed] [Google Scholar]
- [138].Itoi K; Sugimoto N The brainstem noradrenergic systems in stress, anxiety and depression. J. Neuroendocrinol, 2010, 22, 355–361. [DOI] [PubMed] [Google Scholar]
- [139].Allain P; Lechat P Action of some psychotropic drugs on emotional defecation in mice. Therapie, 1970, 25, 655–662. [PubMed] [Google Scholar]
- [140].Rouzade-Dominguez ML; Pernar L; Beck S; Valentino RJ Convergent responses of Barrington’s nucleus neurons to pelvic visceral stimuli in the rat: A juxtacellular labelling study. Eur. J. Neurosci, 2003, 18, 3325–3334. [DOI] [PubMed] [Google Scholar]
- [141].Sawchenko PE; Swanson LW Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or the spinal cord in the rat. J. Comp. Neurol, 1982, 205, 260–272. [DOI] [PubMed] [Google Scholar]
- [142].Million M; Wang L; Martinez V; Taché Y Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res, 2000, 877, 345–353. [DOI] [PubMed] [Google Scholar]
- [143].Nakade Y; Fukuda H; Iwa M; Tsukamoto K; Yanagi H; Yamamura T; Mantyh C; Pappas TN; Takahashi T Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am. J. Physiol, 2007, 292, G1037–G1044. [DOI] [PubMed] [Google Scholar]
- [144].Tebbe JJ; Pasat IR; Mönnikes H; Ritter M; Kobelt P; Schafer MK Excitatory stimulation of neurons in the arcuate nucleus intitiates central CRF-dependent stimulation of colonic propulsion in rats. Brain Res, 2005, 1036, 130–138. [DOI] [PubMed] [Google Scholar]
- [145].Haga K; Asano K; Fukuda T; Kobayakawa T The function of 5-HT3 receptors on colonic transit in rats. Obesity Res, 1995, 3, 801S–810S. [DOI] [PubMed] [Google Scholar]
- [146].Okano S; Nagaya H; Ikeura Y; Natsugari H; Inatomi N Effects of TAK-637, a novel neurokinin-1 receptor antagonist, on colonic function in vivo. J. Pharmacol. Exp. Ther 2001, 298, 559–564. [PubMed] [Google Scholar]
- [147].Taché Y; Martinez V; Wang L; Million M CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br. J. Pharmacol, 2004, 141, 1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Chen CY; Doong ML; Li CP; Liaw WJ; Lee HF; Chang FY; Lin HC; Lee SD A novel simultaneous measurement method to assess the influence of intracerebroventricular obestatin on colonic motility and secretion in conscious rats. Peptides, 2010, 31, 1113–1117. [DOI] [PubMed] [Google Scholar]
- [149].Buéno L; Gue M; Delrio C CNS vasopressin mediates emotional stress and CRH-induced colonic motor alterations in rats. Am. J. Physiol, 1992, 262, G427–G431. [DOI] [PubMed] [Google Scholar]
- [150].Hirata T; Keto Y; Nakata M; Takeuchi A; Funatsu T; Akuzawa S; Sasamata M; Miyata K Effects of serotonin 5-HT(3) receptor antagonists on CRF-induced abnormal colonic water transport and defecation in rats. Eur. J. Pharmacol, 2008, 587, 281–284. [DOI] [PubMed] [Google Scholar]
- [151].Funatsu T; Takeuchi A; Hirata T; Keto Y; Akuzawa S; Sasamata M Effect of ramosetron on conditioned emotional stress-induced colonic dysfunction as a model of irritable bowel syndrome in rats. Eur. J. Pharmacol, 2007, 573, 190–195. [DOI] [PubMed] [Google Scholar]
- [152].Seymour PA; Schmidt AW; Schulz DW The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor: a review. CNS. Drug Rev, 2003, 9, 57–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Gabry KE; Chrousos GP; Rice KC; Mostafa RM; Sternberg E; Negrao AB; Webster EL; McCann SM; Gold PW Marked suppression of gastric ulcerogenesis and intestinal responses to stress by a novel class of drugs. Mol. Psychiatry, 2002, 7, 474–483. [DOI] [PubMed] [Google Scholar]
- [154].Maillot C; Million M; Wei JY; Gauthier A; Taché Y Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology, 2000, 119, 1569–1579. [DOI] [PubMed] [Google Scholar]
- [155].Saito K; Kasai T; Nagura Y; Ito H; Kanazawa M; Fukudo S Corticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distention in rats. Gastroenterology, 2005, 129, 1533–1543. [DOI] [PubMed] [Google Scholar]
- [156].Yoshimoto S; Cerjak D; Babygirija R; Bulbul M; Ludwig K; Takahashi T Hypothalamic circuit regulating colonic transit following chronic stress in rats. Stress, 2012, 15, 227–236. [DOI] [PubMed] [Google Scholar]
- [157].Funada M; Hara C; Wada K Involvement of corticotropin-releasing factor receptor subtype 1 in morphine withdrawal regulation of the brain noradrenergic system. Eur. J. Pharmacol, 2001, 430, 277–281. [DOI] [PubMed] [Google Scholar]
- [158].Bale TL; Picetti R; Contarino A; Koob GF; Vale WW; Lee KF Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J. Neurosci, 2002, 22, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Million M; Wang L; Stenzel-Poore MP; Coste SC; Yuan PQ; Lamy C; Rivier J; Buffington T; Taché Y Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am. J. Physiol, 2006, 292, R1429–R1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Bonaz B; Rivest S Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am. J. Physiol, 1998, 275, R1438–R1449. [DOI] [PubMed] [Google Scholar]
- [161].Reyes BA; Bangasser DA; Valentino RJ; Van Bockstaele EJ Using high resolution imaging to determine trafficking of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Life Sci, 2014, 112, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Taché Y Corticotrophin-releasing factor 1 activation in the central amygdale and visceral hyperalgesia. Neurogastroenterol. Motil, 2015, 27, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sternberg EM; Glowa JR; Smith MA; Calogero AE; Listwak SJ; Aksentijevich S; Chrousos GP; Wilder RL; Gold PW Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Res, 1992, 570, 54–60. [DOI] [PubMed] [Google Scholar]
- [164].Tebbe JJ; Mronga S; Tebbe CG; Ortmann E; Arnold R; Schafer MK Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activation. J. Neuroendocrinol, 2005, 17, 570–576. [DOI] [PubMed] [Google Scholar]
- [165].Mönnikes H; Tebbe J; Bauer C; Grote C; Arnold R Neuropeptide Y in the paraventricular nucleus of the hypothalamus stimulates colonic transit by peripheral cholinergic and central CRF pathways. Neurogastroenterol. Motil, 2000, 12, 343–352. [DOI] [PubMed] [Google Scholar]
- [166].Tebbe JJ; Ortmann E; Schumacher K; Monnikes H; Kobelt P; Arnold R; Schafer MK Cocaine- and amphetamine-regulated transcript stimulates colonic motility via central CRF receptor activation and peripheral cholinergic pathways in fed, conscious rats. Neurogastroenterol. Motil, 2004, 16, 489–496. [DOI] [PubMed] [Google Scholar]
- [167].Junien JL; Gué M; Buéno L Neuropeptide Y and sigma ligand (JO 1784) act through a Gi protein to block the psychological stress and corticotropin-releasing factor-induced colonic motor activation in rats. Neuropharmacology, 1991, 30, 1119–1124. [DOI] [PubMed] [Google Scholar]
- [168].Nakade Y; Tsukamoto K; Iwa M; Pappas TN; Takahashi T Glucagon like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton. Neurosci, 2007, 131, 50–56. [DOI] [PubMed] [Google Scholar]
- [169].Spiegel BM; Bolus R; Harris LA; Lucak S; Chey WD; Sayuk G; Esrailian E; Lembo A; Karsan H; Tillisch K; Talley J; Chang L Characterizing abdominal pain in IBS: guidance for study inclusion criteria, outcome measurement and clinical practice. Aliment. Pharmacol. Ther, 2010, 32, 1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Chang L The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology, 2011, 140, 761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Qin HY; Cheng CW; Tang XD; Bian ZX Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol, 2014, 20, 14126–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Grinsvall C; Tornblom H; Tack J; Van OL; Simren M Psychological factors selectively upregulate rectal pain perception in hypersensitive patients with irritable bowel syndrome. Neurogastroenterol. Motil, 2015, 27, 1772–1782. [DOI] [PubMed] [Google Scholar]
- [173].Larauche M; Mulak A; Taché Y Stress-related alterations of visceral sensation: animal models for irritable bowel syndrome study. J. Neurogastroenterol. Motil, 2011, 17, 213–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Greenwood-Van MB; Prusator DK; Johnson AC Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am. J. Physiol, 2015, 308, G885–G903. [DOI] [PubMed] [Google Scholar]
- [175].Gué M; Del Rio-Lacheze C; Eutamene H; Theodorou V; Fioramonti J; Buéno L Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol. Motil, 1997, 9, 271–279. [DOI] [PubMed] [Google Scholar]
- [176].Greenwood-Van Meerveld B; Johnson AC; Cochrane S; Schulkin J; Myers DA Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol. Motil, 2005, 17, 415–422. [DOI] [PubMed] [Google Scholar]
- [177].Schwetz I; Bradesi S; McRoberts JA; Sablad M; Miller JC; Zhou H; Ohning G; Mayer EA Delayed stress-induced colonic hypoersensitivity in male Wistar rats: role of neurokinin-1 and corticotropin releasing factor-1 receptors. Am. J. Physiol, 2004, 286, G683–G691. [DOI] [PubMed] [Google Scholar]
- [178].Schwetz I; McRoberts JA; Coutinho SV; Bradesi S; Gale G; Fanselow M; Million M; Ohning G; Taché Y; Plotsky PM; Mayer EA Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am. J. Physiol, 2005, 289, G704–G712. [DOI] [PubMed] [Google Scholar]
- [179].Larauche M; Bradesi S; Million M; McLean P; Taché Y; Mayer EA; McRoberts JA Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am. J. Physiol, 2008, 294, G1033–G1040. [DOI] [PubMed] [Google Scholar]
- [180].Saito-Nakaya K; Hasegawa R; Nagura Y; Ito H; Fukudo S Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol. Motil, 2008, 20, 1147–1156. [DOI] [PubMed] [Google Scholar]
- [181].Jia FY; Li XL; Li TN; Wu J; Xie BY; Lin L Role of nesfatin-1 in a rat model of visceral hypersensitivity. World J. Gastroenterol, 2013, 19, 3487–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Million M; Maillot C; Adelson DW; Nozu T; Gauthier A; Rivier C; Chrousos GP; Bayati A; Mattsson H; Taché Y Peripheral injection of sauvagine prevents repeated colorectal distention-induced visceral pain in female rats. Peptides, 2005, 26, 1188–1195. [DOI] [PubMed] [Google Scholar]
- [183].Buckley MM; O’Halloran KD; Rae MG; Dinan TG; o’malley D Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J. Physiol, 2014, 592(23), 5235–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Smith C; Nordstrom E; Sengupta JN; Miranda A Neonatal gastric suctioning results in chronic visceral and somatic hyperalgesia: role of corticotropin releasing factor. Neurogastroenterol. Motil, 2007, 19, 692–699. [DOI] [PubMed] [Google Scholar]
- [185].Trimble N; Johnson AC; Foster A; Greenwood-Van Meerveld B Corticotropin-releasing factor receptor 1-deficient mice show decreased anxiety and colonic sensitivity. Neurogastroenterol. Motil, 2007, 19, 754–760. [DOI] [PubMed] [Google Scholar]
- [186].Bradesi S; Martinez V; Lao L; Larsson H; Mayer EA Involvement of vasopressin 3 receptors in chronic psychological stress-induced visceral hyperalgesia in rats. Am. J. Physiol, 2009, 296, G302–G309. [DOI] [PubMed] [Google Scholar]
- [187].Johnson AC; Tran L; Schulkin J; Greenwood-Van Meerveld B Importance of stress receptor-mediated mechanisms in the amygdala on visceral pain perception in an intrinsically anxious rat. Neurogastroenterol. Motil, 2012, 24, 479–86, e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Tran L; Schulkin J; Greenwood-Van Meerveld B Importance of CRF receptor-mediated mechanisms of the bed nucleus of the stria terminalis in the processing of anxiety and pain. Neuropsychopharmacology, 2014, 39, 2633–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Su J; Tanaka Y; Muratsubaki T; Kano M; Kanazawa M; Fukudo S Injection of corticotropin-releasing hormone into the amygdala aggravates visceral nociception and induces noradrenaline release in rats. Neurogastroenterol. Motil, 2014, 27(1), 30–39. [DOI] [PubMed] [Google Scholar]
- [190].Van Bockstaele EJ; Colago EE; Valentino RJ Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J. Neuroendocrinol, 1998, 10, 743–757. [DOI] [PubMed] [Google Scholar]
- [191].Gray TS Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann. N. Y. Acad. Sci, 1993, 697, 53–60. [DOI] [PubMed] [Google Scholar]
- [192].Bravo JA; Dinan TG; Cryan JF Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int. J. Neuropsychopharmacol, 2011, 14, 666–683. [DOI] [PubMed] [Google Scholar]
- [193].Johnson AC; Tran L; Greenwood-Van MB Knockdown of corticotropin-releasing factor in the central amygdala reverses persistent viscerosomatic hyperalgesia. Transl. Psychiatry, 2015, 5, e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kim SH; Han JE; Hwang S; Oh DH The expression of corticotropin-releasing factor in the central nucleus of the amygdala, induced by colorectal distension, is attenuated by general anesthesia. J. Korean Med. Sci, 2010, 25, 1646–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Tran L; Chaloner A; Sawalha AH; Greenwood Van-Meerveld B Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology 2013, 38, 898–906. [DOI] [PubMed] [Google Scholar]
- [196].Ji G; Fu Y; Ruppert KA; Neugebauer V Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol. Pain, 2007, 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Rouwette T; Klemann K; Gaszner B; Scheffer GJ; Roubos EW; Scheenen WJ; Vissers K; Kozicz T Differential responses of corticotropin-releasing factor and urocortin 1 to acute pain stress in the rat brain. Neuroscience, 2011, 183, 15–24. [DOI] [PubMed] [Google Scholar]
- [198].Greenwood-Van Meerveld B; Johnson AC; Schulkin J; Myers DA Long-term expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus in response to an acute colonic inflammation. Brain Res, 2006, 1071, 91–96. [DOI] [PubMed] [Google Scholar]
- [199].Nishii H; Nomura M; Aono H; Fujimoto N; Matsumoto T Up-regulation of galanin and corticotropin-releasing hormone mRNAs in the key hypothalamic and amygdaloid nuclei in a mouse model of visceral pain. Regul. Pept, 2007, 141, 105–112. [DOI] [PubMed] [Google Scholar]
- [200].Rouwette T; Vanelderen P; Roubos EW; Kozicz T; Vissers K The amygdala, a relay station for switching on and off pain. Eur. J. Pain, 2012, 16, 782–792. [DOI] [PubMed] [Google Scholar]
- [201].Ji G; Neugebauer V Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J. Neurophysiol, 2007, 97, 3893–3904. [DOI] [PubMed] [Google Scholar]
- [202].Neugebauer V; Li W; Bird GC; Han JS The amygdala and persistent pain. Neuroscientist, 2004, 10, 221–234. [DOI] [PubMed] [Google Scholar]
- [203].Fu Y; Neugebauer V Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J. Neurosci, 2008, 28, 3861–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Ji G; Fu Y; Adwanikar H; Neugebauer V Non-pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses. Mol. Pain, 2013, 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Refojo D; Schweizer M; Kuehne C; Ehrenberg S; Thoeringer C; Vogl AM; Dedic N; Schumacher M; von Wolff G; Avrabos C; Touma C; Engblom D; Schutz G; Nave KA; Eder M; Wotjak CT; Sillaber I; Holsboer F; Wurst W; Deussing JM Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science, 2011, 333, 1903–1907. [DOI] [PubMed] [Google Scholar]
- [206].Kosoyan H; Grigoriadis D; Taché Y The CRF1 antagonist, NBI-35965 abolished the activation of locus coeruleus by colorectal distention and intracisternal CRF in rats. Brain Res, 2004, 1056, 85–96. [DOI] [PubMed] [Google Scholar]
- [207].Rouzade-Dominguez ML; Curtis AL; Valentino RJ Role of Barrington’s nucleus in the activation of rat locus coeruleus neurons by colonic distension. Brain Res, 2001, 917, 206–218. [DOI] [PubMed] [Google Scholar]
- [208].Lechner SM; Curtis AL; Brons R; Valentino RJ Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res., 1997, 756, 114–124. [DOI] [PubMed] [Google Scholar]
- [209].Kravets JL; Reyes BA; Unterwald EM; Van Bockstaele EJ Direct targeting of peptidergic amygdalar neurons by noradrenergic afferents: linking stress-integrative circuitry. Brain Struct. Funct, 2015, 220(1), 541–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Naliboff BD; Munakata J; Fullerton S; Gracely RH; Kodner A; Harraf F; Mayer EA Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut, 1997, 41, 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Berridge CW Noradrenergic modulation of arousal. Brain Res. Rev, 2008, 58, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Kehne J; De Lombaert S Non-peptidic CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr. Drug Target CNS. Neurol. Disord, 2002, 1, 467–493. [DOI] [PubMed] [Google Scholar]
- [213].Fond G; Loundou A; Hamdani N; Boukouaci W; Dargel A; Oliveira J; Roger M; Tamouza R; Leboyer M; Boyer L Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiat. Clin. Neurosci, 2014, 264, 651–660. [DOI] [PubMed] [Google Scholar]
- [214].Vicario M; Guilarte M; Alonso C; Yang P; Martinez C; Ramos L; Lobo B; Gonzalez A; Guila M; Pigrau M; Saperas E; Azpiroz F; Santos J Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav. Immun, 2010, 24, 1166–1175. [DOI] [PubMed] [Google Scholar]
- [215].Vicario M; Alonso C; Guilarte M; Serra J; Martinez C; Gonzalez-Castro AM; Lobo B; Antolin M; Andreu AL; Garcia-Arumi E; Casellas M; Saperas E; Malagelada JR; Azpiroz F; Santos J Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology, 2012, 37, 65–77. [DOI] [PubMed] [Google Scholar]
- [216].Eutamene H; Theodorou V; Fioramonti J; Buéno L Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotropin-releasing factor release in rats. J. Physiol, 2003, 553, 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Larauche M Novel insights in the role of peripheral corticotropin-releasing factor and mast cells in stress-induced visceral hypersensitivity. Neurogastroenterol. Motil, 2012, 24, 201–205. [DOI] [PubMed] [Google Scholar]
- [218].Chatzaki E; Crowe PD; Wang L; Million M; Taché Y; Grigoriadis D CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J. Neurochem, 2004, 90, 309–316. [DOI] [PubMed] [Google Scholar]
- [219].Yuan PQ; Million M; Wu SV; Rivier J; Taché Y Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol. Motil, 2007, 19, 923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].o’malley D; Julio-Pieper M; Gibney SM; Gosselin RD; Dinan TG; Cryan JF Differential stress-induced alterations of colonic corticotropin-releasing factor receptors in the Wistar Kyoto rat. Neurogastroenterol. Motil, 2010, 22, 301–311. [DOI] [PubMed] [Google Scholar]
- [221].Kimura T; Amano T; Uehara H; Ariga H; Ishida T; Torii A; Tajiri H; Matsueda K; Yamato S Urocortin I is present in the enteric nervous system and exerts an excitatory effect via cholinergic and serotonergic pathways in the rat colon. Am. J. Physiol, 2007, 293, G903–G910. [DOI] [PubMed] [Google Scholar]
- [222].Gourcerol G; Wu SV; Yuan PQ; Pham H; Miampamba M; Larauche M; Sanders P; Amano T; Mulak A; Im E; Pothoulakis C; Rivier J; Taché Y; Million M Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology, 2011, 140, 1586–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Kawahito Y; Sano H; Kawata M; Yuri K; Mukai S; Yamamura Y; Kato H; Chrousos GP; Wilder RL; Kondo M Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology, 1994, 106, 859–865. [DOI] [PubMed] [Google Scholar]
- [224].O’malley D; Cryan JF; Dinan TG Crosstalk between interleukin-6 and corticotropin-releasing factor modulate submucosal plexus activity and colonic secretion. Brain Behav. Immun, 2013, 30, 115–124. [DOI] [PubMed] [Google Scholar]
- [225].Sand E; Themner-Persson A; Ekblad E Corticotropin releasing factor-distribution in rat intestine and role in neuroprotection. Regul. Pept, 2011, 166, 68–75. [DOI] [PubMed] [Google Scholar]
- [226].Takahashi K Distribution of urocortins and corticotropin-releasing factor receptors in the cardiovascular system. Int. J. Endocrinol, 2012, 2012, 395284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].O’malley D; Dinan TG; Cryan JF Alterations in colonic corticotropin-releasing factor receptors in the maternally separated rat model of irritable bowel syndrome: differential effects of acute psychological and physical stressors. Peptides, 2010, 31, 662–670. [DOI] [PubMed] [Google Scholar]
- [228].Yuan PQ; Wu SV; Pothoulakis C; Taché Y Urocortins and CRF receptor type 2 variants in the male rat colon: gene expression and regulation by endotoxin and anti-inflammatory effect. Am. J. Physiol, 2016, 310(6),G387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Martinez V; Wang L; Rivier JE; Vale W; Taché Y Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J. Pharmacol. Exp. Ther, 2002, 301, 611–617. [DOI] [PubMed] [Google Scholar]
- [230].Tsukamoto K; Nakade Y; Mantyh C; Ludwig K; Pappas TN; Takahashi T Peripherally administered CRF stimulates colonic motility via central CRF receptors and vagal pathways in conscious rats. Am. J. Physiol, 2006, 290, R1537–R1541. [DOI] [PubMed] [Google Scholar]
- [231].Million M; Maillot C; Saunders PR; Rivier J; Vale W; Taché Y Human urocortin II, a new CRF-related peptide, displays selective CRF2-mediated action on gastric transit in rats. Am. J. Physiol, 2002, 282, G34–G40. [DOI] [PubMed] [Google Scholar]
- [232].Saunders PR; Maillot C; Million M; Taché’ Y Peripheral corticotropin-releasing factor induces diarrhea in rats: role of CRF1 receptor in fecal watery excretion. Eur. J. Pharmacol, 2002, 435, 231–235. [DOI] [PubMed] [Google Scholar]
- [233].Maillot C; Wang L; Million M; Taché Y Intraperitoneal corticotropin-releasing factor and urocortin induce Fos expression in brain and spinal autonomic nuclei and long lasting stimulation of colonic motility in rats. Brain Res, 2003, 974, 70–81. [DOI] [PubMed] [Google Scholar]
- [234].Gourcerol G; Wang L; Adelson DW; Larauche M; Taché Y; Million M Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am. J. Physiol, 2009, 296, G992–G1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Nozu T; Takakusaki K; Okumura T A balance theory of peripheral corticotropin-releasing factor receptor type 1 and type 2 signaling to induce colonic contractions and visceral hyperalgesia in rats. Endocrinology, 2014, 155, 4655–4664. [DOI] [PubMed] [Google Scholar]
- [236].Andoh K; Kimura T; Saeki I; Tabata R; Yamazaki S; Eguchi I; Hanazuka M; Horii D; Munt PL; Davis AS; . General pharmacological properties of the human corticotropin-releasing hormone corticorelin (human). Arzneimittelforschun, 1994, 44, 715–726. [PubMed] [Google Scholar]
- [237].Shannon MH; Bihm CC; Short WJ; Burks TF; Williams CL Interactions of oxytocin and vasopressin with CRF on the rat colon. Neuropeptides, 1997, 31, 94–98. [DOI] [PubMed] [Google Scholar]
- [238].Rivier J; Gulyas J; Kunitake K; DiGruccio M; Cantle J; Perrin MH; Donaldson C; Vaughan J; Million M; Gourcerol G; Adelson D; Rivier C; Taché Y; Vale W Stressin1-A, a potent corticotropin releasing factor receptor 1 (CRF1)-selective peptide agonist. J. Med. Chem, 2007, 50, 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Tezval H; Jahn O; Todorovic C; Sasse A; Eckart K; Spiess J Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc. Natl. Acad. Sci. U. S. A, 2004, 101, 9468–9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Larauche M; Gourcerol G; Wang L; Pambukchian K; Brunnhuber S; Adelson DW; Rivier J; Million M; Taché Y Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am. J. Physiol, 2009, 297, G215–G227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Million M; Wang L; Wang Y; Adelson DW; Yuan PQ; Maillot C; Coutinho SV; McRoberts JA; Bayati A; Mattsson H; Wu VS; Wei JY; Rivier J; Vale W; Mayer EA; Taché Y CRF2 receptor activation prevents colorectal distension-induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut, 2006, 55, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Mancinelli R; Azzena GB; Diana M; Forgione A; Fratta W In vitro excitatory actions of corticotropin-releasing factor on rat colonic motility. J. Auton. Pharmacol, 1998, 18, 319–324. [DOI] [PubMed] [Google Scholar]
- [243].Liu S; Gao X; Gao N; Wang X; Fang X; Hu HZ; Wang GD; Xia Y; Wood JD Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J. Comp Neurol, 2005, 481, 284–298. [DOI] [PubMed] [Google Scholar]
- [244].Liu S; Ren W; Qu MH; Bishop GA; Wang GD; Wang XY; Xia Y; Wood JD Differential actions of urocortins on neurons of the myenteric division of the enteric nervous system in guinea pig distal colon. Br. J. Pharmacol, 2010, 159, 222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Miampamba M; Maillot C; Million M; Taché Y Peripheral CRF activates myenteric neurons in the proximal colon through CRF1 receptor in conscious rats. Am. J. Physiol, 2002, 282, G857–G865. [DOI] [PubMed] [Google Scholar]
- [246].Miampamba M; Million M; Yuan P-Q; Larouche M; Taché Y Water avoidance stress activates colonic myenteric neurons in female rats. Neuroreport, 2007, 18, 679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Hanani M; Wood JD Corticotropin-releasing hormone excites myenteric neurons in the guinea-pig small intestin. Eur. J. Pharmacol, 1992, 211, 23–27. [DOI] [PubMed] [Google Scholar]
- [248].Keita AV; Soderholm JD; Ericson AC Stress-induced barrier disruption of rat follicle-associated epithelium involves corticotropin-releasing hormone, acetylcholine, substance P, and mast cells. Neurogastroenterol. Motil, 2010, 22, 770–772. [DOI] [PubMed] [Google Scholar]
- [249].Smith F; Clark JE; Overman BL; Tozel CC; Huang JH; Rivier JE; Blikslager AT; Moeser AJ Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol, 2010, 298, G352–G363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].La JH; Sung TS; Kim HJ; Kim TW; Kang TM; Yang IS Peripheral corticotropin releasing hormone mediates post-inflammatory visceral hypersensitivity in rats. World J. Gastroenterol, 2008, 14, 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].van den Wijngaard RM; Stanisor OI; van Diest SA; Welting O; Wouters MM; de Jonge WJ; Boeckxstaens GE Peripheral alpha-helical CRF (9–41) does not reverse stress-induced mast cell dependent visceral hypersensitivity in maternally separated rats. Neurogastroenterol. Motil, 2012, 24, 274–e111. [DOI] [PubMed] [Google Scholar]
- [252].Ait-Belgnaoui A; Bradesi S; Fioramonti J; Theodorou V; Buéno L Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain, 2005, 113, 141–147. [DOI] [PubMed] [Google Scholar]
- [253].Soderholm JD; Perdue MH II. Stress and intestinal barrier function. Am. J. Physiol, 2001, 280, G7–G13. [DOI] [PubMed] [Google Scholar]
- [254].Barreau F; Cartier C; Leveque M; Ferrier L; Moriez R; Laroute V; Rosztoczy A; Fioramonti J; Buéno L Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J. Physiol, 2007, 580, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Saunders PR; Kosecka U; McKay DM; Perdue MH Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am. J. Physiol, 1994, 267, G794–G799. [DOI] [PubMed] [Google Scholar]
- [256].Santos J; Saunders PR; Hanssen NPM; Yang PC; Yates D; Groot JA; Perdue MH Corticotropin-releasing hormone mimics stress-induced rat colonic epithelial pathophysiology in the rat. Am. J. Physiol, 1999, 277, G391–G399. [DOI] [PubMed] [Google Scholar]
- [257].Teitelbaum AA; Gareau MG; Jury J; Yang PC; Perdue MH Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am. J. Physiol, 2008, 295, G452–G459. [DOI] [PubMed] [Google Scholar]
- [258].Saunders PR; Santos J; Hanssen NP; Yates D; Groot JA; Perdue MH Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig. Dis. Sci, 2002, 47, 208–215. [DOI] [PubMed] [Google Scholar]
- [259].Soderholm JD; Yates DA; Gareau MG; Yang PC; MacQueen G; Perdue MH Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am. J. Physiol, 2002, 283, G1257–G1263. [DOI] [PubMed] [Google Scholar]
- [260].Stead RH; Dixon MF; Bramwell NH; Riddell RH; Bienenstock J Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology, 1989, 97, 575–585. [DOI] [PubMed] [Google Scholar]
- [261].Overman EL; Rivier JE; Moeser AJ CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS. One, 2012, 7, e39935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Sengupta JN Visceral pain: the neurophysiological mechanism. Handb. Exp. Pharmacol, 2009, 31–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Cervero F; Laird JM Understanding the signaling and transmission of visceral nociceptive events. J. Neurobiol, 2004, 61, 45–54. [DOI] [PubMed] [Google Scholar]
- [264].Mawe GM; Coates MD; Moses PL Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment. Pharmacol. Ther, 2006, 23, 1067–1076. [DOI] [PubMed] [Google Scholar]
- [265].von Mentzer B; Murata Y; Ahlstedt I; Lindstrom E; Martinez V Functional CRF receptors in BON cells stimulate serotonin release. Biochem. Pharmacol 2007, 73, 805–813. [DOI] [PubMed] [Google Scholar]
- [266].Theoharides TC; Donelan JM; Papadopoulou N; Cao J; Kempuraj D; Conti P Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol. Sci, 2004, 25, 563–568. [DOI] [PubMed] [Google Scholar]
- [267].Wallon C; Yang PC; Keita AV; Ericson AC; McKay DM; Sherman PM; Perdue MH; Soderholm JD Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut, 2008, 57, 50–58. [DOI] [PubMed] [Google Scholar]
- [268].Wallon C; Soderholm JD Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann. N.Y. Acad. Sci, 2009, 1165, 206–210. [DOI] [PubMed] [Google Scholar]
- [269].Chatzaki E; Anton PA; Million M; Lambropoulou M; Constantinidis T; Kolios G; Taché Y; Grigoriadis DE Corticotropin-releasing factor receptor subtype 2 in human colonic mucosa: down-regulation in ulcerative colitis. World J. Gastroenterol, 2013, 19, 1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Kawahito Y; Sano H; Chrousos GP; Wilder RL; Yuri K; Katawa M; Kato H; Kondo M The production of corticotropin-releasing hormone in normal human colonic mucosal cells (enterochromaffin cells). Gastroenterology, 1993, 104, A1044. [Google Scholar]
- [271].Kempuraj D; Papadopoulou NG; Lytinas M; Huang M; Kandere-Grzybowska K; Madhappan B; Boucher W; Christodoulou S; Athanassiou A; Theoharides TC Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology, 2004, 145, 43–48. [DOI] [PubMed] [Google Scholar]
- [272].Saruta M; Takahashi K; Suzuki T; Fukuda T; Torii A; Sasano H Urocortin 3/stresscopin in human colon: possible modulators of gastrointestinal function during stressful conditions. Peptides, 2005, 26, 1196–1206. [DOI] [PubMed] [Google Scholar]
- [273].Vanuytsel T; van WS; Vanheel H; Vanormelingen C; Verschueren S; Houben E; Salim RS; Tomicronth J; Holvoet L; Farre R; Van OL; Boeckxstaens G; Verbeke K; Tack J Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut, 2014, 63, 1293–1299. [DOI] [PubMed] [Google Scholar]
- [274].Fukudo S; Nomura T; Hongo M Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut 1998, 42, 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Illum L Is nose-to-brain transport of drugs in man a reality? J. Pharm. Pharmacol, 2004, 56, 3–17. [DOI] [PubMed] [Google Scholar]
- [276].Hallschmid M; Benedict C; Born J; Fehm HL; Kern W Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol. Behav, 2004, 83, 55–64. [DOI] [PubMed] [Google Scholar]
- [277].Kern W; Schiefer B; Schwarzenburg J; Stange EF; Born J; Fehm HL Evidence for central nervous effects of corticotropin-releasing hormone on gastric acid secretion in humans. Neuroendocrinology, 1997, 65, 291–298. [DOI] [PubMed] [Google Scholar]
- [278].Pritchard SE; Garsed KC; Hoad CL; Lingaya M; Banwait R; Thongborisute W; Roberts E; Costigan C; Marciani L; Gowland PA; Spiller RC Effect of experimental stress on the small bowel and colon in healthy humans. Neurogastroenterol. Motil, 2015, 27, 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Lembo T; Plourde V; Shui Z; Fullerton S; Mertz H; Taché Y; Sytnik B; Munakata J; Mayer E Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol. Motil, 1996, 8, 9–18. [DOI] [PubMed] [Google Scholar]
- [280].Nozu T; Kudaira M Corticotropin-releasing factor induces rectal hypersensitivity after repetitive painful rectal distention in healthy humans. J. Gastroenterol, 2006, 41, 740–744. [DOI] [PubMed] [Google Scholar]
- [281].Stasi C; Bellini M; Bassotti G; Blandizzi C; Milani S Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech. Coloproctol, 2014, 18, 613–621. [DOI] [PubMed] [Google Scholar]
- [282].Tanaka Y; Kanazawa M; Fukudo S; Drossman DA Biopsychosocial model of irritable bowel syndrome. J. Neurogastroenterol. Motil, 2011, 17, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Taché Y Corticotropin releasing factor receptor antagonists: potential future therapy in gastroenterology? Gut, 2004, 53 (7), 919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Fukudo S Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J. Gastroenterol, 2007, 42 (Suppl) 17, 48–51. [DOI] [PubMed] [Google Scholar]
- [285].Martinez V; Taché Y CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr. Pharm. Des, 2006, 12, 1–18. [DOI] [PubMed] [Google Scholar]
- [286].Williams JP Corticotropin-releasing factor 1 receptor antagonists: a patent review. Expert. Opin. Ther. Pat, 2013, 23, 1057–1068. [DOI] [PubMed] [Google Scholar]
- [287].Chen C Recent advances in small molecule antagonists of the corticotropin-releasing factor type-1 receptor-focus on pharmacology and pharmacokinetics. Curr. Med. Chem, 2006, 13, 1261–1282. [DOI] [PubMed] [Google Scholar]
- [288].Tellew JE; Lanier M; Moorjani M; Lin E; Luo Z; Slee DH; Zhang X; Hoare SR; Grigoriadis DE; St Denis Y; Di Fabio R; Di Modugno E; Saunders J; Williams JP Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg. Med. Chem. Lett, 2010, 20, 7259–7264. [DOI] [PubMed] [Google Scholar]
- [289].Liapakis G; Venihaki M; Margioris A; Grigoriadis D; Gkountelias K Members of CRF family and their receptors: from past to future. Curr. Med. Chem, 2011, 18, 2583–2600. [DOI] [PubMed] [Google Scholar]
- [290].Hodgetts KJ; Ge P; Yoon T; Lombaert SD; Brodbeck R; Gulianello M; Kieltyka A; Horvath RF; Kehne JH; Krause JE; Maynard GD; Hoffman D; Lee Y; Fung L; Doller D Discovery of N-(1-Ethylpropyl)-[3-methoxy-5-(2-methoxy-4-trifluoromethoxyphenyl)-6-methyl-pyra zin-2-yl]amine 59 (NGD 98–2): An Orally Active Corticotropin Releasing Factor-1 (CRF-1) Receptor Antagonist. J. Med. Chem, 2011, 54, 4187–4206. [DOI] [PubMed] [Google Scholar]
- [291].Kehne JH; Cain CK Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: evidence from animal models. Pharmacol. Ther, 2010, 128, 460–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Bailey JE; Papadopoulos A; Diaper A; Phillips S; Schmidt ME; van der AP; Dourish CT; Dawson GR; Nutt DJ Preliminary evidence of anxiolytic effects of the CRF1 receptor antagonist R317573 in the 7.5% CO2 proof-of-concept experimental model of human anxiety. J. Psychopharmacol, 2011, 25(9), 1199–1206. [DOI] [PubMed] [Google Scholar]
- [293].Schmidt ME; Andrews RD; van der AP; Brown T; Mannaert E; Steckler T; de Hoon J; Van Laere K Dose-dependent effects of the CRF(1) receptor antagonist R317573 on regional brain activity in healthy male subjects. Psychopharmacology (Berl), 2010, 208, 109–119. [DOI] [PubMed] [Google Scholar]
- [294].Hubbard CS; Labus JS; Bueller J; Stains J; Suyenobu B; Dukes GE; Kelleher DL; Tillisch K; Naliboff BD; Mayer EA Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J. Neurosci, 2011, 31, 12491–12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Bouin M; Plourde V; Boivin M; Riberdy M; Lupien F; Laganiere M; Verrier P; Poitras P Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology, 2002, 122, 1771–1777. [DOI] [PubMed] [Google Scholar]
- [296].North CS; Hong BA; Alpers DH Relationship of functional gastrointestinal disorders and psychiatric disorders: Implications for treatment. World J. Gastroenterol, 2007, 13, 2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Sagami Y; Shimada Y; Tayama J; Nomura T; Satake M; Endo Y; Shoji T; Karahashi K; Hongo M; Fukudo S Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut, 2004, 53, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Tayama J; Sagami Y; Shimada Y; Hongo M; Fukudo S Effect of alpha-helical CRH on quantitative electroencephalogram in patients with irritable bowel syndrome. Neurogastroenterol. Mot, 2007, 19, 471–483. [DOI] [PubMed] [Google Scholar]
- [299].Sweetser S; Camilleri M; Linker Nord SJ; Burton DD; Castenada L; Croop R; Tong G; Dockens R; Zinsmeister AR Do Corticotropin Releasing Factor-1 Receptors Influence Colonic Transit and Bowel Function in Females with Irritable Bowel Syndrome? Am. J. Physiol. Gastrointest. Liver. Physiol, 2009, 296(6),G1299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Zmijewski MA; Slominski AT CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J. Cell Physiol, 2009, 218, 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Taché Y Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Dig. Dis. Sci, 1999, 44, 79S–86S. [PubMed] [Google Scholar]


