To the Editor:
Immunization against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is a major step in protecting the healthcare worker beyond standard pandemic precautions and infection control measures.1 Healthcare workers (HCW) are at high risk for SARS-CoV-2 exposure and infection placing burden on the workplace.2,3 Hence, reducing SARS-CoV-2 infection of HCWs serves multiple goals in the occupational health arena. These include protecting the health and safety of the HCW as well as reducing transmission of Coronavirus Disease 2019 (COVID-19) in the clinic and the larger workplace it serves. Furthermore, when HCWs demonstrate appropriate pandemic precautions and health practices, they serve as strategic models for other workers. This modeling represents leadership which can inspire optimism and hope in a workforce beleaguered by the pandemic.
Modern medical practices and public health measures (ie, clean water, antibiotics, and vaccination) have transformed public health and human society,4 and there is good reason to expect that immunizing employees against COVID-19 will similarly transform the workplace burdened by the SARS-CoV-2 pandemic. However, one barrier to this success could be vaccine hesitancy on the part of the HCW as well as the general workforce. Vaccine hesitancy is multidetermined and not amenable to a one-size-fits-all approach. Yet, achieving immunity amongst HCWs is vital for the HCW, the employees they serve, and to communicate confidence and hope for workers whose immunization is pending or in doubt by hesitancy.5–7
With these concerns in mind, we report data from serial immunoassays for anti-SARS-CoV-2 antibodies collected on a group of confirmed COVID-19 naïve occupational HCWs who were immunized by mRNA vaccine. Antibody reactivity and kinetics data were compared with results obtained from employees recovered from confirmed COVID-19 from whom serial antibody immunoassays were obtained. In addition, a group of HCW with prior infection by SARS-CoV-2 were immunized and provided preliminary data on antibody formation. Side effect data from immunization of HCWs were also gathered. These results are discussed with respect to level of immune protection attained and how this achievement may mitigate vaccine hesitancy.
METHODS
HCWs (physicians, psychologists, nurse practitioners, nurses, medical technologists, medical assistants, medical administrative assistants, and technicians; N = 62) were immunized with the Pfizer/BioNTech (B162b2; N = 49) or Moderna (mRNA-1273; N = 13) SARS-CoV-2 vaccine. Vaccines were administered by local county health departments following standardized dosing and protocol of the Food and Drug Administration (FDA) Emergency Use Authorization. Serologic immunoglobulin G (IgG) antibody formation was measured at approximate 2-week, 3-week, 4-week, and 5-week intervals post-immunization. Immunoassay was done with the Beckman-Coulter Access SARS-CoV-2 IgG test which detects the receptor binding domain (RBD) spike protein of the SARS-CoV-2 virus. Laboratory analyses were performed in an on-site high-complexity Clinical Laboratory Improvement Amendments (CLIA) lab. A self-report survey (this survey is available in supplemental table 1 (S1)) designed to assess common SARS-CoV-2 vaccine side effects was administered 2 weeks after the boost dose (week 5 for Pfizer/BioNTech; week 6 for Moderna). In addition to reactive versus non-reactive status, signal to cut off ratios (S/CO) for the IgG assays were examined. Statistical analyses were conducted with JMP (Version 15.2.0, SAS Institute Inc., Cary, NC, 1989–2020) and SPSS (Version 26, IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Armonk, NY) software packages.
SARS-CoV-2 infection was diagnosed by results of real time-polymerase chain reaction (RT-PCR) testing using the Applied Biosystems TaqPath COVID-19 Combo Kit. The occupational health clinic has performed over 46,136 RT-PCR tests on employees over an 11-month period on our large workforce of approximately 5700 employees. The testing strategy involved random surveillance of workers in addition to targeted testing of mission-critical employees and testing of symptomatic and exposed workers. As of the time of this report, 684 workers were confirmed positive for COVID-19 (overall new case positivity rate = 1.49%).
Recovered confirmed COVID-19 positive employees were offered serial antibody testing. Sera for immunoassay were taken at approximately 14 days and at monthly intervals (30, 60, 90 days, etc) after initial positive RT-PCR result. Recovered COVID-19 employee comparison groups were formed by closely matching mean, median, and range of days since first positive RT-PCR test result with the time intervals at which immunoassay sera were collected on HCWs. Vaccinated HCWs provided self-report ratings of side effect symptoms and perceived global interference in daily activity using a 0 to 4 Likert scale (0 = none; 1 = mild; 2 = moderate; 3 = severe; 4 = extreme). In addition, 10 HCW who had previously tested positive for SARS-CoV-2 infection were also examined for antibody development. All analyses were conducted as internal quality metrics of existing clinic data; the pertinent Institutional Review Board (IRB) determined that these comparisons did not require full IRB review.
Descriptive statistics for age and sex of the vaccinated HCW and the recovered COVID-19 employees were collected and percent antibody reactive status and the IgG geometric mean at each time interval for the sera samples were determined. Table 1 (T1) summarizes these descriptive statistics. Figure 1 (F1) shows the time trend in antibody formation for vaccinated employees and the recovered COVID-19 positive employees.
TABLE 1.
Descriptive Statistics on Recovered COVID-19 and Vaccinated Health Care Worker Employees
| Convalescent | Pfizer | Moderna | Pooled Vaccinated | ||
| Serum 1 | Geometric S/CO | 3.42 | 3.47 | 7.47 | 4.07 |
| 11–17 days | N | 168 | 45 | 12 | 57 |
| Age (Mean) | 44.31 | 43.27 | 44.25 | 43.47 | |
| Age (Std Dev.) | 10.98 | 12.88 | 16.12 | 13.47 | |
| Male | 69% | 24% | 67% | 33% | |
| Reactive | 79.76% | 77.78% | 83.33% | 78.95% | |
| Draw Day (Mean) | 14.54 | 14.10 | 14.17 | 14.10 | |
| Serum 2 | Geometric S/CO | 4.81 | 4.21 | 11.21 | 5.63 |
| 18–23 days | N | 21 | 26 | 11 | 37 |
| Age (Mean) | 45.67 | 46.03 | 45.09 | 45.76 | |
| Age (Std Dev.) | 11 | 13.79 | 16.63 | 14.46 | |
| Male | 71% | 23% | 55% | 32% | |
| Reactive | 95.24% | 80.77% | 100% | 86.49% | |
| Draw Day (Mean) | 20.33 | 20.23 | 21.10 | 20.49 | |
| Serum 3 | Geometric S/CO | 6 | 19.1 | 11.28 | 16.31 |
| 24–29 days | N | 166 | 26 | 11 | 37 |
| Age (Mean) | 45.1 | 46.23 | 45.91 | 46.14 | |
| Age (Std Dev.) | 11.55 | 13.4 | 15.8 | 13.92 | |
| Male | 73% | 27% | 73% | 41% | |
| Reactive | 90.36% | 100% | 100% | 100% | |
| Draw Day (Mean) | 28.29 | 26.65 | 27.73 | 26.98 | |
| Serum 4 | Geometric S/CO | 5.44 | 38.19 | 39.74 | 38.64 |
| 34–44 days | N | 57 | 24 | 10 | 34 |
| Age (Mean) | 46.26 | 46.17 | 45.8 | 45.6 | |
| Age (Std Dev.) | 11.84 | 13.66 | 16.65 | 14.39 | |
| Male | 70% | 25% | 70% | 38% | |
| Reactive | 89.47% | 100% | 100% | 100% | |
| Draw Day (Mean) | 36.44 | 35.75 | 42.7 | 37.79 |
Total healthcare workers who received vaccinations = 62; total N at each blood draw interval varied. S/CO, signal to cut off ratio, SD, standard deviation.
FIGURE 1.
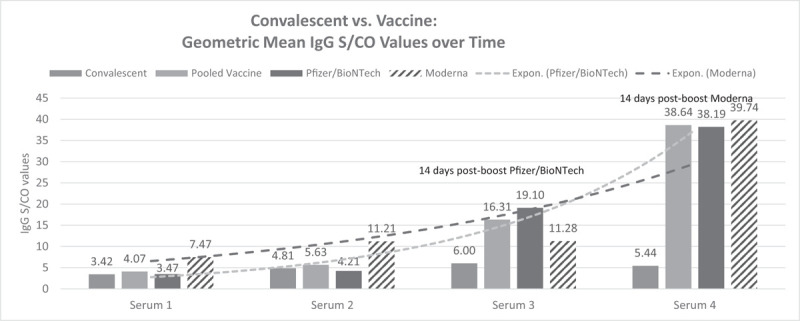
Convalescent COVID-19 employees and vaccinated health care worker IgG antibody responses. Mean day of serum 1 = 14 days; serum 2 = 20 days; serum 3 = 28 days; serum 4 = 38 days. IgG, Immunoglobulin G; S/CO, signal to cut off ratio.
Antibody Level Comparisons
At 2-week comparison, the natural infection group was 79% reactive for IgG antibodies and the pooled Pfizer/BioNTech and Moderna vaccinated HCWs also were 78% reactive. Percent reactive for the recovered infected and vaccinated groups at 3-, 4-, and 5-week immunoassay were as follows: 95% and 86%, 90% and 100%, and 89% and 100%, respectively. Comparisons of the geometric mean IgG ratios for convalescent employees, Pfizer/BioNTech, Moderna, and pooled vaccinated HCW employees at sample collection intervals evidenced meaningful differences. Previously infected employees showed generally stable and moderate geometric mean IgG levels at and beyond 3 weeks post infection, but Pfizer/BioNTech immunized HCWs demonstrated an approximate 3-fold increase in antibody level at 4 weeks (1 week after boost), and Moderna vaccinated HCWs showed over an approximate 7-fold increase in geometric mean IgG level at 5 weeks (2 weeks after boost). The pooled Pfizer/BioNTech and Moderna geometric mean IgG S/CO ratio for vaccinated HCWs approximately 2 weeks after their respective boosts was almost eight times higher than the natural infection group (38.64 vs 5.44 S/CO). Comparison of the distribution of geometric mean IgG ratios for these collection intervals between recovered COVID-19 employees and vaccinated HCWs showed that the vaccinated groups achieved significantly greater mean IgG levels at week 4 and 5 (after their second [boost] dose) by Wilcoxon Signed-Ranks tests: week 4: Z = 3.712, P < 0.0002; week 5: Z = 6.513, P < 0.0001. The group geometric means did not differ at the first two sera samplings (week 2: Z = –0.186, P < 0.853; week 3: Z = –0.525, P < 0.594).
A small group of vaccinated HCWs (N = 10) had previously been infected by SARS-CoV-2. Of these, eight had provided serial antibody levels prior to vaccination; 75% were IgG reactive at the first IgG assay (mean of 30 days post infection). The geometric mean IgG S/CO ratios at an average of 30 and 53 days after infection were 2.13 and 2.96. However, 2 weeks after the first vaccination dose (all Pfizer/BioNTech), their geometric mean was 17.90 S/CO, an approximate 6-fold increase. Of note, one HCW's most recent antibody level (1.61 S/CO) was taken at day 151 from initial infection. At 2-weeks after vaccination, the IgG was 18.5 S/CO, showing a similar strong antibody response to the first vaccination dose.
Side Effect Analyses
Fifty-three of the 57 HCWs who had received their vaccine boost and completed the immunization protocol provided a completed survey (93%). The mean levels of rated interference in daily activity (on a 0 to 4 scale) at dose one and dose two for vaccinated HCWs were 0.23 (SD = 0.69) and 1.34 (SD = 1.43), respectively; this was a statistically significant difference (t = –4.21, 34 df, P < 0.0001) showing side effect interference was greater after the vaccine boost. The difference between mean side effect rating at dose one (0.31 [SD = 0.74]) and dose two (0.74 [SD = .0.60]) nearly achieved conventional statistical difference of P < 0.05 determined by one-way analysis of variance (ANOVA; df 23; F = 3.957; P < 0.06). The two most elevated side effects were pain at injection site (mean dose one = 1.54 [SD = 1.09] and mean dose two = 1.75 [SD = 1.18]) and fatigue (mean dose one = .57 [SD = .96] and mean dose two = 1.58 [SD = 1.34]). The difference between injection site pain level at dose one and two was not significantly significant (t = –1.24, df 35, P < 0.22), but fatigue was rated significantly higher at dose two (t = –4.79, df 35, P < 0.000). Although the mean levels of side effect symptoms and interference in daily activity ratings were generally low, individuals reported a wide range of values. For example, eight persons (20%) reported interference ratings of 3 (severe) or 4 (extreme) after the boost vaccination, 13 persons (37%) reported no interference from experienced side effects, and 3 (9%) persons reported no side effects or interference at all.
DISCUSSION
These results are important in several ways. First, our data are consistent with early reports from clinical trials and other studies of the two mRNA vaccines for efficacy and with respect to differential antibody response in vaccinated and recovered COVID-19 patients.8–12 Although studies have used various antibody assays and characterized different aspects of the humoral immune response, results suggest these mRNA vaccines confer strong immunogenicity.13–16 Secondly, measurements of immune response indicators have shown that the second (boost) dose of either Pfizer/BioNTech or Moderna immunization produces robust antibody formation,17 consistent with our findings of a greater than approximate 7-fold increase in geometric mean IgG level relative to that observed in recovered COVID-19 employees. Of note, our study tracked one indicator of humoral immunity (IgG) using an EUA-approved qualitative assay. However, studies have shown that IgG ratios are very highly correlated with assessments of neutralizing antibodies and other aspects of the adaptive immune response (eg, Spearman rho = 0.95),14 and the S/CO value of a qualitative immunoassay can be analyzed non-parametrically and examined as a relative indicator for investigative study (as we did). It is important to conduct further studies of vaccine antibody kinetics and durability with different methods and in diverse peoples, with respect to co-morbidities and covariates, and their performance relative to SARS-CoV-2 variants. Nonetheless, these early results from mRNA vaccination are very promising.18 Third, although individuals report both a range of side effects and severity level (which increase at boost dose), most people experience few, mild, and short-lived side effects.10,11 Thus, tolerability of the vaccine appears quite acceptable. Fourth and very importantly, results from prior studies and the current data collectively provide a platform on which increased acceptance of SARS-CoV-2 vaccination can be built.
Success begets success. HCWs can capitalize on findings documenting robust antibody response and the low side effect profile of mRNA immunization against SARS-CoV-2. This information may be combined with the strong efficacy data to communicate informed, fact-based, positive, and hopeful messages in the workplace. Empirical evidence provides the information base the HCW may use to discuss SARS-CoV-2 vaccination with hesitant workers, but often information is not sufficient to accomplish behavior change. Surveys show vaccination hesitancy is a significant concern nationally and internationally, a potential barrier to achieving herd immunity, and is not rare within HCWs.19–22 A multi-prong approach informed by behavioral science is needed to reduce vaccine hesitancy.23 Health behavior change addresses fears and knowledge gaps as well as leveraging communication and marketing principles,6,24 the power of social modeling,25,26 and peer-based example27,28 to effect change. These efforts are amplified by empathic listening on the part of the health professional.29 Actions speak louder than words in inspiring trust, and the HCW seeking to reduce vaccine hesitancy knows the healing relationship itself is curative.30 Thus, both modeling vaccine acceptance and establishing credibility as an ally of the reluctant individual are important. Effective encouragement proceeds non-judgmentally and makes use of motivational interviewing techniques.31 In point of fact, a few HCWs in our occupational health clinic were vaccine hesitant. Leading by example, providing accessible and fact-based information for knowledge gaps, and relating to the reluctant HCW with interest and a lack of judgment succeeded in increasing our numbers of vaccinated HCWs.
Supplementary Material
Acknowledgments
This work represents the opinions of the authors and not of the U.S. DOE, ORNL, or UT-Battelle, LLC, on work authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-publicaccessplan).
Footnotes
Readers are invited to submit letters for publication in this department. Submit letters online at http://joem.edmgr.com. Choose “Submit New Manuscript.” A signed copyright assignment and financial disclosure form must be submitted with the letter. Form available at www.joem.org under Author and Reviewer information.
Conflicts of Interest: None declared.
Ethical Considerations & Disclosure: The reported data are summary internal quality metrics maintained by ORNL Health Services Division. The Department of Energy-Oak Ridge Institutional Review Board Committee (IRB) determined these data were “not human research since the focus is on quality improvement/assessment of the program using already collected data (August 18, 2020).”
Supplemental digital contents are available for this article.
REFERENCES
- 1.Honein MA, Christie A, Rose DA, et al. Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, december 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koh D. Occupational risks for COVID-19 infection. Occup Med (Lond) 2020; 70:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker MG, Peckham TK, Seixas NS. Estimating the burden of United States workers exposed to infection or disease: a key factor in containing risk of COVID-19 infection. PLoS One 2020; 15:e0232452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotkin S. History of vaccination. Proc Natl Acad Sci U S A 2014; 111:12283–12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok KO, Li KK, Wei WI, Tang A, Wong SY, Lee SS. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: a survey. Int J Nurs Stud 2021; 114:103854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy B, Kumar V, Venkatesh A. Health care workers’ reluctance to take the Covid-19 vaccine: a consumer-marketing approach to identifying and overcoming hesitancy. NEJM Catal Innov Care Deliv 2020; 1:1–10. [Google Scholar]
- 7.Chevallier C, Hacquin AS, Mercier H. COVID-19 vaccine hesitancy: shortening the last mile. Trends Cogn Sci 2021; S1364-6613(21)00033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020; 383:2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020; 586:589–593. [DOI] [PubMed] [Google Scholar]
- 10.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020; 383:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021; 384:80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021; 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature 2020; 586:594–599. [DOI] [PubMed] [Google Scholar]
- 15.Irsara C, Egger AE, Prokop W, et al. Evaluation of four commercial, fully automated SARS-CoV-2 antibody tests suggests a revision of the Siemens SARS-CoV-2 IgG assay. Clin Chem Lab Med 2021; 14:000010151520201758. [DOI] [PubMed] [Google Scholar]
- 16.Narasimhan M, Mahimainathan L, Raj E, et al. Clinical evaluation of the Abbott Alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays in infected, recovered, and vaccinated groups. medRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter PR, Brainard JS. Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose. A reanalysis of a study of’real-world’vaccination outcomes from Israel. Medrxiv 2021; 10.1101/2021.02.05.21251139 [DOI] [Google Scholar]
- 18.Rossman H, Shilo S, Meir T, Gorfine M, Shalit U, Segal E. Patterns of COVID-19 pandemic dynamics following deployment of a broad national immunization program. medRxiv 2021; 10.1101/2021.02.08.21251325 [DOI] [PubMed] [Google Scholar]
- 19.Ernsting A, Schwarzer R, Lippke S, Schneider M. ‘I do not need a flu shot because I lead a healthy lifestyle’: compensatory health beliefs make vaccination less likely. J Health Psychol 2013; 18:825–836. [DOI] [PubMed] [Google Scholar]
- 20.Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine 2016; 34:6700–6706. [DOI] [PubMed] [Google Scholar]
- 21.McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther 2017; 39:1550–1562. [DOI] [PubMed] [Google Scholar]
- 22.Szilagyi PG, Thomas K, Shah MD, et al. National trends in the US public's likelihood of getting a COVID-19 vaccine—April 1 to December 8, 2020. JAMA 2021; 325:396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpp KG, Loewenstein G, Buttenheim AM. Behaviorally informed strategies for a national COVID-19 vaccine promotion program. JAMA 2021; 325:125–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein S, MacDonald NE, Guirguis S. Health communication and vaccine hesitancy. Vaccine 2015; 33:4212–4214. [DOI] [PubMed] [Google Scholar]
- 25.Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health 1998; 13:623–649. [Google Scholar]
- 26.Schwarzer R. Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Appl Psychol 2008; 57:1–29. [Google Scholar]
- 27.Webel AR, Okonsky J, Trompeta J, Holzemer WL. A systematic review of the effectiveness of peer-based interventions on health-related behaviors in adults. Am J Public Health 2010; 100:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson R, Grosshuesch A, Schmidt S, Gray M, Wipfli B. Observational learning and workplace safety: the effects of viewing the collective behavior of multiple social models on the use of personal protective equipment. J Saf Res 2009; 40:383–387. [DOI] [PubMed] [Google Scholar]
- 29.Pollak KI, Alexander SC, Tulsky JA, et al. Physician empathy and listening: associations with patient satisfaction and autonomy. J Am Board Fam Med 2011; 24:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank JD, Frank JB. Persuasion and Healing: A Comparative Study of Psychotherapy. Baltimore, MD: JHU Press; 1993. [Google Scholar]
- 31.Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol 2005; 1:91–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


