Abstract
Concentrated animal feeding operations (CAFOs) present highly efficient means of meeting food demands. CAFOs create unique conditions that can affect the health and environment of animals and humans within and outside operations, leading to potential epidemiological concerns that scale with operational size. One such arena meriting further investigation is their possible contribution to novel influenzas. CAFOs present opportunities for cross-species transmission of influenza as demonstrated by reports of swine flu and avian influenza outbreaks. Conditions and pathways leading to novel influenza strains are complex and require varied prevention and intervention approaches. Current challenges for prevention of respiratory viruses entering or leaving swine and poultry CAFOs are multifaceted and include adherence of personal safety measures, lack of training and safety provisions for personnel, and incomplete standardized federal, state, and/or county regulation and enforcement coverage across agricultural systems. This report acknowledges that any proposed CAFO-associated influenza intervention should be cross-organizational, and no single intervention should be expected to provide full resolution. Proposed interventions affect multiple components of the One Health triad, and include seasonal human influenza immunization, PPE regulation and adherence, alternative waste management, general biosecurity standardization and an industry best practices incentive program. Due to the complexity of this problem, multiple anticipated communication, enforcement, and logistical challenges may hinder the full implementation of proposed solutions. General and operation-specific (swine and poultry) biosecurity practices may mitigate some of the risks associated with influenza virus reassortment across species. Education and advocacy can help protect workers, communities, veterinarians and consumers from CAFO-associated influenza virus. To achieve this, there must be more complete communication between CAFOs, governing agencies, health services, animal services, researchers, and consumers to better explore the potential health outcomes associated with CAFOs.
Keywords: Influenza, CAFO, Swine, Poultry, One Health
1. Introduction
Increasing demands for animal-based protein necessitate innovative production and supply methods. Concentrated animal feeding operations (CAFOs) have proved efficient means of meeting demands, with the United States heavily reliant on CAFOs for cattle, poultry, pork, milk, and egg production. An estimated 99.9% of U.S. poultry and livestock are CAFO-raised [1]. Under current USDA regulations, CAFOs are defined as animal feeding operations contributing some form of discharge to natural or man-made waterways, regardless of size; or housing at least 1000 animal units. Increasing CAFO sizes create unique conditions that can affect the health of animals, humans and the environment within and outside operations [2]. Further research in CAFOs is necessary to better understand the impacts and interactions with human, animal and environmental health.
1.1. Novel influenza viruses in CAFOs
Novel influenzas pose a significant threat to human populations; three pandemics in the 20th century were caused by influenza [3]. Wildlife reservoirs may introduce influenza into CAFO populations [4]. As influenza spreads throughout the CAFO, risk of antigenic shift (described below) increases, increasing the risk of further transmission to new species, including humans. In 2009 at a Mexican village, La Gloria, the world witnessed emergence of a new pandemic influenza, H1N1. Cases spread outside the village; by June 11, 2009, the WHO classified H1N1 a pandemic [5]. Significant risk has been seen in other animals as well. In 2014, an outbreak of highly pathogenic avian influenza (HPAI) is believed to have originated from contact between wild migratory birds and backyard flocks, traveling to larger commercial poultry farms [6]. While no transmission to humans is known to have occurred, thousands of animals were culled, resulting in huge financial losses to the poultry industry.
Novel zoonotic influenza strains may be introduced to human populations through close interactions between animals, CAFO employees, and their communities [7]. Workers can also transmit human adapted influenza strains to animals [4]. Subtle respiratory symptoms of influenza A in swine may be undetected and allow higher probability of animal-animal or animal-human transmissions [8,9].
A core component of novel influenzas is their ability to “jump” from one species to another, following genetic change by background mutation or reassortment (usually referred to as genetic drift and shift). Influenza A surface proteins Hemagglutinin (HA) and neuraminidase (NA) are common sites for antibody binding; small changes in these regions may substantially alter the host range or immune response to the individual strain [10]. This phenomenon is the primary reason vaccines must be reassessed and distributed annually [[10], [11], [12]]. Coinfection of a host with multiple virus strains may result in new viruses with surface proteins from both viruses, allowing for cross-species transmission opportunities, as seen in reports of bird-pig-human swine flu transmission [13,14].
Emergence of novel influenza strains in humans are possible through either of two events: 1) spillover of swine and/or avian influenza strains entering human populations or 2) mixing of human, swine and avian influenza strains within a host [15]. During mixing events, human influenza strains may spillover into swine populations, which subsequently act as mixing vessels for influenza viral segments [16,17]. Swine may be an ideal mixing vessel, because they can be infected by both avian and human influenzas [18]. Reassortment may allow influenzas to evade vaccines, as seen in swine H3N2 transmission to turkeys vaccinated to H1N1 [19]; farmers believed their birds were safe, but birds were still infected by a novel influenza strain. In other cases, animal influenza mutation outpaces the vaccines used against them. Some zoonotic influenzas are so diverse and mutable that vaccinations have low efficacy in preventing transmission and infection among swine [20]. Wildlife may not require direct interaction with livestock for cross-species transmission to occur. Avian influenza shed through feces may contaminate animal food and water sources and infect animals [21]. Though methods and practices may be in place to limit exposure of CAFO animals with local wildlife, proximity and interactions between avian and swine CAFOs may promote novel influenza emergence [22]. Influenza strains may circulate in farm and feral populations for long time periods prior to detection [23]. Strains related to the 2009 H1N1 pandemic were found to have been present in local CAFO populations for up to a decade before transmission to humans [24].
1.2. Occupational and welfare challenges in CAFOs
CAFOs may be reluctant to share operation procedures [25]. However, this can be justifiable: under the current system, farmers are economically disincentivized to share detailed production information such as feed composition, vaccination schedules, and biosecurity measures not directly required by law, as these are considered trade secrets. Support for novel influenza surveillance is limited as well. To our knowledge, few programs actively support or collect in-depth data on the epidemiology of influenza in animal populations. Additionally, there can and have been massive financial and economic costs for organizations that are tied to a novel influenza. The 2009 H1N1 pandemic resulted in an estimated $1.3 billion in lost sales [26]. The 2014 HPAI outbreak saw even greater losses to the poultry industry, with an estimated $3.3 billion in costs associated with culling, lost production, and disinfection [6]. This may have been a result of poor reporting, fearmongering, poor public health communication, or many other reasons [25]. Lack of financial incentives and harsh market backlash may create unfavorable environments for CAFO-specific disease surveillance.
Occupational exposures present significant opportunities for additional intervention. There can be significant gaps in protocol that overlook employees and present opportunities for animal-human transmission in employees and communities in which they reside [27,28]. Multi-level infection prevention plans may have gaps in any layer of the prevention plan. For example, PPE may be provided to a visitor, however if PPE is not sterilized, visitors may still be exposed. Potential routes of transmission via fomites have been described in reports from the 2014 HPAI outbreak [6]. Lastly, vaccination plans, while effective [29], have yet to see standardized rollout and incentivization to support regular vaccination of workers.
Workers may be more vulnerable and victims of systemic issues. In 2018, the Bureau of Labor Statistics reported that the median annual wage for farmworkers working with animals was $26,560, well below the all-occupation median wage of $38,640 [30]. Generally, employees tend to be lower-income, minority populations [31]. These operations present a multitude of psychologically and physically stressful jobs for the entire community [32]. Psychological and physical stresses related to low socioeconomic status and demanding jobs can subsequently create vulnerabilities to infections and disease [33], though to our knowledge no studies have focused on stress-related influenza in CAFO workers and their communities.
Current regulations target human and environmental factors, but animal welfare has been comparatively neglected. Even though states have animal welfare protections, they are variable and non-standardized [34]. Further, animal welfare laws designed to protect animals from abuse often fail protection against neglect [35]. Aside from moral implications surrounding animal neglect and mistreatment, physiological outcomes manifest from these stressful environments. Like humans, animals experiencing chronic stress become vulnerable to infection and illness [36].
The problems surrounding CAFOs, summarized in Fig. 1, are multifaceted and complex. The closed-door nature of CAFOs creates significant barriers to approaching the above issues from a One Health perspective. There are also not enough incentives and regulations to encourage CAFOs to be more transparent about practices and protocols surrounding influenza outbreaks. Surveillance can provide a better picture of the conditions surrounding a potential outbreak, but surveillance alone cannot prevent future pandemics. This review aims to identify current practices in place, assess their strengths and limitations, and offer potential solutions and recommendations. We hope that the implementation of One Health perspectives opens new avenues for interventions to explore new stakeholders while strengthening existing relationships.
Fig. 1.
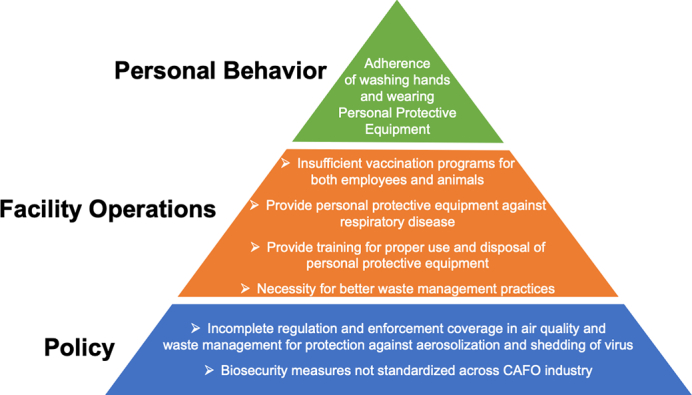
Pyramid diagram of current challenges of preventing respiratory viruses from entering or emerging from swine and poultry CAFOs.
2. Current practices
2.1. Regulations
Currently, regulations for CAFOs are generally decentralized. These facilities have various practices that impact the environment, workers, consumers, and nearby communities in different ways. Therefore, rules are imposed by relevant agencies for each of these areas. Many major federal regulations for CAFOs come from the Environmental Protection Agency (EPA) through the Clean Water Act of 2008 (CWA) under the CAFO rule [37]. The purpose of the act is to establish regulations for pollutant discharge in water systems in the U.S. and ensure quality standards in surface waters [38]. Animal feeding operations (AFOs) were first included in the CWA in 1972 when they were recognized as point-sources for pollution [39]. Any AFO that discharges wastewater or manure into a natural or human-made ditch, regardless of its size, is considered a CAFO by the EPA [31]. CAFOs must possess appropriate permits required by the National Pollutant Discharge Elimination Systems (NPDES), and meet effluent limitations and standards limiting waste discharge into water systems and requiring adherence to best management practices [39].
2.2. Compliance
There are several ways the EPA and state authorities (where allowed by the EPA) can inspect CAFOs to monitor compliance. They may check AFOs to determine if they are making unauthorized discharges, which would make the facility a CAFO [39], requiring appropriate permits. Both AFO and CAFO inspections can result from reports or complaints from the community, random selection, or the states' targeting system [39]. However, the USDA and EPA rely on self-audit of CAFOs, which may lead to several hazards if CAFOs are not in compliance [37,39]. Additionally, AFOs that do not fall under the CWA might have increased oversight. State authorities vary in broadness or strictness of rules that go beyond federal requirements. Other federal regulations, like the Clean Air Act (CAA), have driven states to regulate other activities in the agriculture industry that may harm human health. However, these regulations are indirectly applied to CAFOs; the CAA only has an Air Quality Conservation Reference Guide for poultry and livestock operations [40]. No centralized air quality rules have been created for feedlots. Therefore, it would seem there needs to be further examination of potential regulations to protect human or animal health from respiratory illnesses, such as influenza.
There are several challenges as a result of limited regulation. Air pollutants or aerosolization of pathogens are not accounted for in the CWA, the CAA only has guidelines, and no other federal laws are in place to prevent these potential health hazards. Aside from citizen complaint reports, community stakeholders are not engaged in policy and decision-making regarding nearby CAFOs. Historically, the most common resource for affected stakeholders has been legal action. This process does not remain feasible due to right-to-farm laws, which protect farmers from unreasonable regulations before CAFOs were common. This dynamic complicates intervention by public health workers. Currently, the best approach is mediation through local boards of health to resolve the conflicts between affected communities and non-complying CAFOs [41].
2.3. Biosecurity
The USDA has numerous recommendations for animal management that vary for each type of industry. Generally, the ultimate goals of these recommendations are to ensure animal welfare and product quality. However, there are also general biosecurity measures that are universal across all CAFOs, such as the use of personal protective equipment (PPE), and vaccination programs for both employees and animals, as illustrated in Fig. 2 [42,43]. A study of swine farms evaluated by Minnesota veterinarians found that only 9.7% of employees had with certainty been vaccinated against seasonal influenza, and only 7.9% of growing pigs were vaccinated [29]. The industry relies upon the implementation of these company-specific biosecurity measures to prevent the spread of pathogens within their operations, or to other settings, which could be ineffective if there is no compliance [28]. The same Minnesota study found that workers in the swine CAFOs reported specialized footwear as the most commonly used PPE and were less likely to use other PPE like masks and gloves [29]. Additionally, washing hands before and after handling animals was not widely encouraged across swine farms [29]. Neglecting to implement and enforce biosecurity measures could reflect on a significant scale in outbreaks and epizootics, having major detrimental health and economic effects.
Fig. 2.
Structural and operational levels of general biosecurity measures that can be observed across all CAFOs.
3. Proposed interventions
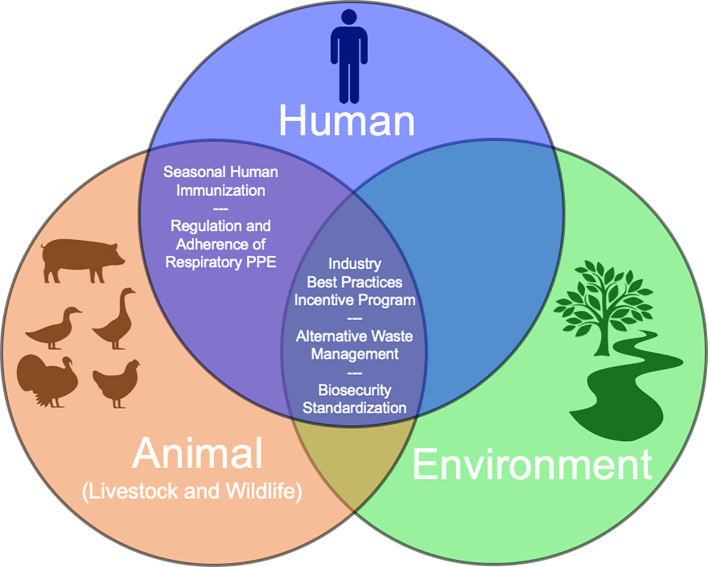
Though this review has outlined existing and potential influenza-related health risks associated with CAFOs, it is important to acknowledge that these agricultural operations fulfill a significant role in food production globally [44]. It also should be strongly noted that this report recognizes the uniqueness of every agricultural system and that some organizations may already be utilizing heightened biosecurity and occupational health procedures. Any proposed CAFO-associated influenza intervention should follow a cross-organizational One Health model, managed by organizations stretched across human, animal, and environmental health with no single intervention expected to provide full resolution. Instead, CAFO-associated influenza prevention should be considered multifaceted, requiring a layered approach of multiple interventions [45,46]. The following proposed interventions affect multiple components of the One Health triad, such as seasonal human influenza immunization (human/animal), regulation and adherence of respiratory PPE (human/animal), alternative waste management (human/animal/environment), biosecurity standardization (human/animal/environment), and an industry best practices incentive program that address emerging pathogens, including novel influenza from poultry and swine in CAFO settings (human/animal/environment) as illustrated in Fig. 3.
Fig. 3.
Diagram of the One Health triad with illustration of where proposed interventions may impact intersections between human, animal, and/or environmental health.
3.1. Seasonal human immunization
There is currently no mandate for agricultural systems to recommend seasonal influenza vaccination among employees. Though seasonal influenza vaccines are not specific to influenza strains currently circulating among swine and poultry populations, mathematical simulations have shown that vaccine uptake as low as 50% in CAFO employees can mitigate or prevent amplification of a novel influenza within an operation between humans and swine and decrease the number of human influenza cases among employees [29]. An additional study showed seasonal influenza vaccination provided moderate cross-protection against pandemic influenza circulating between 2007 and 2010 [45], potentially reducing severity of novel influenza infecting CAFO employees. System-wide influenza vaccination implementation across agricultural organizations may also reduce the percentage of lost workdays among employees [47], making such a policy economical in addition to protecting workers and livestock. Agricultural systems may provide incentive programs for employees who receive vaccination annually or provide on-site vaccination clinics to increase uptake [48] as seen in healthcare systems for employees [49].
3.2. Regulation and adherence of respiratory PPE
While many reports have illustrated proper PPE use, the need for systemic regulation and compliance of PPE should also be considered and highlighted here. There are no current Occupational Safety and Health Administration (OSHA) standards specific for avian or swine influenza in agriculture workers. This can leave the interpretation of these threats subject to the discretion of CAFOs health risk assessments [50]. The National Pork Board has instructed swine producers and veterinarians to use N-95 respirators for people who have already developed influenza illness and are necessary for operation function [51]. In a survey conducted among immigrant swine workers from a CAFO in Missouri, 92.5% of employees indicated the agricultural system provided respirators as part of their PPE. However, only 30.4–42.9% of employees indicated they use respirators all the time while working [52]. Another survey for PPE use was administered to veterinarians and pork producers across Minnesota and only 2.9% of swine farms reported they always use respiratory PPE [29]. There is a wide disconnect between federal and organizational regulations and personal adherence to respiratory PPE policies with little guidance provided by the U.S. federal government regarding respiratory illnesses, particularly influenza, for the agricultural industry. There is also an uneven implementation of respiratory PPE policies across CAFOs. However, even when such policies are in place, there are adherence concerns for PPE use among employees. This is a systematic challenge that needs rectification to prevent future zoonotic influenza transmission and may be achieved through new policy implementation and education among organizations and their employees. Government agencies may create standardized PPE regulations across agricultural systems with instructions pertaining PPE apparel, how to don and doff required PPE, and when to wear required PPE for protection from swine and avian influenza. Enforcement of such policies would require annual or semi-annual audits determining compliance. Federal and state agencies may also utilize a multi-level award system for successful PPE policy compliance among agricultural systems. At the organization-level, compliance award programs, health education and PPE training may take place at time of hiring with annual or semi-annual follow-up training by trained health education providers from local public health authorities.
3.3. Wastewater management
CAFO manure lagoon systems may potentially be environments conducive to influenza virus reassortment and other pathogen survival and transmission. These waste systems are typically located outside of the facility, exposed to the environment and wildlife [28]. Fecal matter and wastewater traveling from the CAFO facility to manure lagoons can contain influenza virus shed from infected animal hosts. The primary route of influenza transmission among mammalian species is airborne infection via influenza virus aerosolization [53], however spread between avian species [54] and spillover to swine species can be facilitated by fecal-oral route [55]. Migratory waterfowl or feral swine exposed to manure lagoons may be susceptible to influenza infection through influenza virus contaminated open waste systems [20,54,[56], [57], [58], [59]]. Though the lagoon may not directly put human populations at risk of influenza, infected wildlife from contaminated lagoons may spread infection to nearby human and animal communities.
There are alternative waste storage and management techniques available, different from traditional manure lagoons. For example, in 2019, Holzem and Katers evaluated the effectiveness and financial plausibility of an alternative waste management system for dairy CAFOs in Wisconsin [60]. The fecal matter and wastewater from the CAFO facilities were pumped into an anaerobic digester system, producing biogas. The biogas product would flow to a generator, creating electricity that can be sold to local utilities. The remaining waste is separated, with the solid fraction entering a drying system and the liquid fraction entering a two-stage lagoon. The solid fraction of the waste can be stored and used for agriculture crop bedding. The two-stage lagoon separates the liquid waste, creating partitions between nitrogen‑phosphorus nutrient concentrations. The waste liquids are used for land nutrient management. Though this system demands initial substantial financial costs, this alternative would provide a financial pay back in less than 20 years [60]. Similar alternative waste management systems have been adapted for swine manure produced from CAFOs [61]. Replacement of traditional CAFO manure lagoons with advanced alternatives would provide a source of energy, nutrient adjusted fertilizer and prevent the potential exposure of influenza virus to wildlife.
3.4. General biosecurity protocol standardization
The goals of CAFO biosecurity are to address all aspects of infectious agents on the premises. This includes minimizing the impact of endemic pathogens and reducing the emergence of novel pathogens among livestock through policies regarding location of animals, facilities and flow of operations, human access, waste management, and sick animal procedures. There are currently a wide range of self-assessment checklists from multiple government agencies, academic institutions and swine/poultry advocate groups [[62], [63], [64]]. Most self-assessment surveys and guidelines have many overlapping practices, but there is no clear indication which set of guidelines is the gold standard. Inconsistency in biosecurity practices among agricultural systems calls for a universal governing body within the U.S. to manage and support standardization of biosecurity policies. Such biosecurity policies would necessitate consideration of species, production size and type of production system (e.g., closed air, open air, backyard etc.). Regulations need balance between clarity without ambiguous interpretation and yet adaptive such that the regulations are realistic and specific to varying production systems. Efforts toward building trust would need to be made between all governing bodies, poultry/pork producers, and the scientific community to achieve harmonious implementation of any proposed intervention for this complexity, therefore this report proposes that the universal governing body be formulated as a council with members stretched across government, industry and the scientific community.
3.5. Industry best practices incentive program
Presently, there are multiple incentive-assistance programs in place for agricultural producers to combat climate change and increase environmental sustainability [65]. Furthermore, there are best practices programs to address emerging pathogens and antimicrobial resistance in livestock, such as the USDA Process Verified Program One Health Certified [66]. However, neither of these holistic efforts address novel influenza. There may already be partnerships that exist between public health and food industry producers, but there are no publicly documented existing incentive-assistance programs that address the emergence of novel influenza viruses in U.S. agriculture. This review has identified the need for incentive or assistance programs that encourage complete collaboration between pork and poultry food producers and public health efforts, with holistic efforts to prevent all potential infectious diseases including novel influenza virus emergence. This report proposes a two-fold voluntary incentive and assistance program, designed to guide, encourage and provide assistance to CAFOs in planning and implementing best One Health practices in preventing development and spread of novel influenza. This incentive and assistance program aims to increase community and industry participation in One Health efforts and incentivize collaboration between CAFOs and government agencies. Funding of this program would optimistically be the result of joint efforts between federal departments or agencies whose missions are preserving human, animal or environmental health and safety.
The first objective of the proposed program would focus on establishing best practice procedures for CAFOs and include providing guidance and encouraging CAFOs to be stronger stewards of animal, human and environmental health as it pertains to operation function. This report recommends a consortium of government agencies (county, state, and federal), academic institutions, swine/poultry advocacy groups, veterinarians, and pork/poultry producers to collaborate and create a unified best practices “checklist.” The principle aims of the consortium would be organizing sets of guidelines through prioritization of necessities, recommendations and suggestions to secure the health and welfare of animals, humans, and the environment and reducing the risk of novel influenza from entering into or emerging from CAFOs.
The second objective of the proposed program would provide financial assistance to CAFOs for planning and implementing interventions that strengthen One Health infrastructure and achieve the proposed One Health best practices. The program would be available to all eligible CAFOs in the U.S. Eligibility for financial assistance requires the CAFO show willingness to achieve One Health best practices through establishing partnerships with local and/or state health, agricultural, and environmental authorities. Such partnerships would provide CAFOs with technical assistance and planning for human health safety protocols, animal welfare, and environmental health safety.
4. Potential challenges
Due to the complexity of this problem, there are anticipated challenges that may prevent implementation of the proposed solutions. Obstacles may include difficulty navigating government legislation, from lobbying forces and political agendas. Establishing a regulatory standard would require the coordination of federal, state and local jurisdictions. Governing bodies, including government agencies (county, state, and federal) and swine/poultry advocate groups, currently remain siloed, creating communication, enforcement, and logistical issues. Many missing organization-government partnerships may be described by overall lack of One Health perspectives in legislative and regulatory bodies. CAFOs influence humans, animals, and the environment, necessitating interdisciplinary multi-organizational collaborations that may be difficult to form. Furthermore, qualified human resources may be difficult to engage. As of 2019, direct on-farm employment only accounted for 1.3% of U.S. employment [67]. Policy makers, regulators and compliance inspectors may not have necessary training or intricate knowledge of food animal production to develop and implement proposed solutions. It is unclear what expectations are for control and prevention of such a complex issue as novel influenza viruses emerging in CAFOs. Therefore, more research is needed to explore the ecology of novel influenza pathways and their involvement with CAFOs. It is understood that proposed intervention costs may be substantial for all stakeholders, including governing bodies, producers, and consumers. Aging swine/poultry production equipment and facilities may be too expensive to retrofit for producers at any level. Agricultural system costs may trickle down to consumers, increasing the cost for poultry and pork. Finally, transparency between agricultural systems, governing bodies, and the general public may be a challenge leading to limited communication or miscommunication pertaining to the emergence of novel influenza.
5. Conclusion
One Health approaches to identify and implement interventions are necessary to address the complexities and challenges posed by CAFOs, including the potential emergence of novel influenza viruses. There is great need for collaboration between veterinary medicine, diagnostic services, and epidemiology. Furthermore, general and operation-specific (swine and poultry) biosecurity practices may mitigate some of the risks associated with influenza virus reassortment across species. Education and advocacy can help protect workers, communities, and consumers from CAFO associated influenza virus. We must move toward greater transparency and open communication between CAFOs, governing agencies, health services, animal services, researchers, and consumers.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare there is no conflict of interest.
Acknowledgements
The authors would like to thank Dr. Dan Engeljohn from the School of Animal and Comparative Biomedical Sciences, University of Arizona, Tucson, Arizona, USA and Dr. Alexandra Armstrong from the Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, Arizona, USA for reading the manuscript and their comments.
References
- 1.Reese J. Sentience Institute; 2019. US Factory Farming Estimates.https://www.sentienceinstitute.org/us-factory-farming-estimates (accessed 23 July 2020) [Google Scholar]
- 2.Kellogg R., Moffitt D., Gollehon D. United States Department of Agriculture; 2014. Estimates of Recoverable and non-Recoverable Manure Nutrients based on the Census of Agriculture.https://www.nrcs.usda.gov/wps/portal/nrcs/detail/national/technical/nra/rca/?cid=nrcseprd1360819 (accessed 24 July 2020) [Google Scholar]
- 3.Smith G.J., Bahl J., Vijaykrishna D., Zhang J., Poon L.L., Chen H., Webster R.G., Malik Peiris J.S., Guan Y. Dating the emergence of pandemic influenza viruses. Proc. Natl. Acad. Sci. 2009;106(28):11709–11712. doi: 10.1073/pnas.0904991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollenbeck J.E. Interaction of the role of concentrated animal feeding operations (CAFOs) in emerging infectious diseases (EIDS) Infect. Genet. Evol. 2016;38:44–46. doi: 10.1016/J.MEEGID.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Disease Control and Prevention The 2009 H1N1 Pandemic: Summary Highlights, April 2009 – April 2010. 2010. https://www.cdc.gov/h1n1flu/cdcresponse.htm (accessed 24 July 2020)
- 6.Greene J.L. Update on the highly-pathogenic avian influenza outbreak of 2014–2015. Cong. Res. Ser. 2015;2015:1–8. [Google Scholar]
- 7.Zhou N.N., Senne D.A., Landgraf J.S., Swenson S.L., Erickson G., Rossow K., Liu L., Yoon K.J., Krauss S., Webster R.G. Genetic reassortment of avian, swine, and human influenza A viruses in american pigs. J. Virol. 1999;73(10):8851–8856. doi: 10.1128/jvi.73.10.8851-8856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corzo C.A., Culhane M., Juleen K., Stigger-Rosser E., Ducatez M.F., Webby R.J., Lowe J.F. Active surveillance for influenza A virus among swine, midwestern United States, 2009–2011. Emerg. Infect. Dis. 2013;19(6):954–960. doi: 10.3201/eid1906.121637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers K.P., Olsen C.W., Setterquist S.F., Capuano A.W., Donham K.J., Thacker E.L., Merchant J.A., Gray G.C. Are swine workers in the United States at increased risk of infection with zoonotic influenza virus? Clin. Infect. Dis. 2006;42(1):14–20. doi: 10.1086/498977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholtissek C. Pandemic influenza: antigenic shift. Perspectives Med. Virol. 2002;7:87–100. [Google Scholar]
- 11.Centers for Disease Control and Prevention How the Flu Virus can Change: “drift” and “shift”. 2019. https://www.cdc.gov/flu/about/viruses/change.htm (accessed 18 March 2021)
- 12.Nelson M.I., Holmes E.C. The evolution of epidemic influenza. Nat. Rev. Genet. 2007;8(3):196–205. doi: 10.1038/nrg2053. [DOI] [PubMed] [Google Scholar]
- 13.Taubenberger J.K., Kash J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010;7(6):440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinde V., Bridges C.B., Uyeki T.M., Shu B., Balish A., Xu X., Lindstrom S., Gubareva L.V., Deyde V., Garten R.J., Harris M. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N. Engl. J. Med. 2009;360(25):2616–2625. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 15.Parrish C.R., Holmes E.C., Morens D.M., Park E.C., Burke D.S., Calisher C.H., Laughlin C.A., Saif L.J., Daszak P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008;72(3):457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma W., Kahn R.E., Richt J.A. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J. Mol. Genet. Med. 2008;3(1):158–166. [PMC free article] [PubMed] [Google Scholar]
- 17.Rajao D.S., Vincent A.L., Perez D.R. Adaptation of human influenza viruses to swine. Front. Veterinary Sci. 2019;5:347. doi: 10.3389/fvets.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma W., Kahn R.E., Richt J.A. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J. Mol. Genet. Med. 2009;3(1):158. [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Y.K., Lee J.H., Erickson G., Goyal S.M., Joo H.S., Webster R.G., Webby R.J. H3N2 influenza virus transmission from swine to turkeys, United States. Emerg. Infect. Dis. 2004;10(12):2156–2160. doi: 10.3201/eid1012.040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandbulte M.R., Spickler A.R., Zaabel P.K., Roth J.A. Optimal use of vaccines for control of influenza A virus in swine. Vaccines. 2015;3(1):22–73. doi: 10.3390/vaccines3010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torremorell M., Allerson M., Corzo C., Diaz A., Gramer M. Transmission of influenza A virus in pigs. Transbound. Emerg. Dis. 2012;59:68–84. doi: 10.1111/j.1865-1682.2011.01300.x. [DOI] [PubMed] [Google Scholar]
- 22.Gilchrist M.J., Greko C., Wallinga D.B., Beran G.W., Riley D.G., Thorne P.S. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ. Health Perspect. 2007;115(2):313–316. doi: 10.1289/ehp.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin B.E., Sun H., Carrel M., Cunningham F.L., Baroch J.A., Hanson-Dorr K.C., Young S.G., Schmit B., Nolting J.M., Yoon K.J., Lutman M.W. Feral swine in the United States have been exposed to both avian and swine influenza A viruses. Appl. Environ. Microbiol. 2017;83(19) doi: 10.1128/AEM.01346-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith G.J., Vijaykrishna D., Bahl J., Lycett S.J., Worobey M., Pybus O.G., Ma S.K., Cheung C.L., Raghwani J., Bhatt S., Peiris J.M. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459(7250):1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 25.Pappaioanou M., Gramer M. Lessons from pandemic H1N1 2009 to improve prevention, detection, and response to influenza pandemics from a one health perspective. ILAR J. 2010;51(3):268–280. doi: 10.1093/ilar.51.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler D. 2009. Testimony of the National Pork Producers Council on the US Pork Industry Economic Crisis before the US House Committee on Agriculture Subcommittee on Livestock, Dairy, and Poultry. [Google Scholar]
- 27.Schmidt C.W. Swine CAFOs & novel H1N1 flu: separating facts from fears. Environ. Health Perspect. 2009;117(9):394–401. doi: 10.1289/89/ehp.117-a394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaudoin A., Johnson S., Davies P., Bender J., Gramer M. Characterization of influenza A outbreaks in Minnesota swine herds and measures taken to reduce the risk of zoonotic transmission. Zoonoses Public Health. 2012;59(2):96–106. doi: 10.1111/j.1863-2378.2011.01423.x. [DOI] [PubMed] [Google Scholar]
- 29.Saenz R.A., Hethcote H.W., Gray G.C. Confined animal feeding operations as amplifiers of influenza. Vector-Borne & Zoonotic Diseases. 2006;6(4):338–346. doi: 10.1089/vbz.2006.6.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Department of Labor, Bureau of Labor Statistics . JIST Publishing; 2018. Agricultural Workers. Occupational Outlook Handbook. [Google Scholar]
- 31.Dorovskikh A. 2015. Killing for a Living: Psychological and Physiological Effects of Alienation of Food Production on Slaughterhouse Workers; p. 771. Undergraduate Honors Theses. [Google Scholar]
- 32.Dillard J. A slaughterhouse nightmare: psychological harm suffered by slaughterhouse employees and the possibility of redress through legal reform. Geo. J. Poverty L & Pol'y. 2008;15(2):391–408. [Google Scholar]
- 33.Burgard S.A., Lin K.Y. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am. Behav. Sci. 2013;57(8):1105–1127. doi: 10.1177/0002764213487347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mench J.A. Farm animal welfare in the USA: farming practices, research, education, regulation, and assurance programs. Appl. Anim. Behav. Sci. 2008;113(4):298–312. doi: 10.1016/j.applanim.2008.01.009. [DOI] [Google Scholar]
- 35.Mosel A. What about Wilbur? Proposing a federal statute to provide minimum humane living conditions for farm animals raised for food production. U Dayton L Rev. 2001;27(1):133–188. [Google Scholar]
- 36.Moberg G.P. The Biology of Animal Stress. Centre for Agriculture and Bioscience International; New York: 2000. Biological response to stress: implications for animal welfare; pp. 1–21. [Google Scholar]
- 37.US Department of Agriculture, Natural Resources Conservation Service Animal Feeding Operations. 2020. https://www.nrcs.usda.gov/wps/portal/nrcs/main/national/plantsanimals/livestock/afo/ (accessed 24 July 2020)
- 38.US Environmental Protection Agency Summary of the Clean Water Act. 2020. https://www.epa.gov/laws-regulations/summary-clean-water-act (accessed 24 July 2020)
- 39.US Environmental Protection Agency Clean Water Act (CWA) Compliance Monitoring. 2020. https://www.epa.gov/sites/production/files/2015-10/documents/cafo_permitmanual_entire.pdf (accessed 24 July 2020)
- 40.US Department of Agriculture, US Environmental Protection Agency Reference Guide for Poultry and Livestock Production Systems. 2017. https://www.epa.gov/sites/production/files/2017-01/documents/web_placeholder.pdf (accessed 24 July 2020)
- 41.Hribar C. Understanding Concentrated Animal Feeding Operations and their Impact on Communities. 2010. https://www.cdc.gov/nceh/ehs/docs/understanding_cafos_nalboh.pdf (accessed 19 March 2021)
- 42.Torremorell M. The Three Levels of Biosecurity Animals. 2021. https://www.merckvetmanual.com/management-and-nutrition/biosecurity/the-three-levels-of-biosecurity-of-animals (accessed 19 March 2021)
- 43.US Department of Agriculture, Animal and Plant Health Inspection Service Biosecurity. 2020. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/defend-the-flock-program/dtf-biosecurity (accessed 19 March 2021)
- 44.Haley M. US Department of Agriculture; 2020. Livestock, Dairy, and Poultry Outlook: February 2020.https://www.ers.usda.gov/publications/pub-details/?pubid=95916 (accessed 30 May 2020) [Google Scholar]
- 45.Saunders-Hastings P., Reisman J., Krewski D. Assessing the state of knowledge regarding the effectiveness of interventions to contain pandemic influenza transmission: a systematic review and narrative synthesis. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0168262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qualls N., Levitt A., Kanade N., Wright-Jegede N., Dopson S., Biggerstaff M., Reed C., Uzicanin A., Group CC, Group CC, Levitt A. Community mitigation guidelines to prevent pandemic influenza—United States, 2017. MMWR Recommendations Rep. 2017;66(1):1. doi: 10.15585/mmwr.rr6601a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bridges C.B., Thompson W.W., Meltzer M.I., Reeve G.R., Talamonti W.J., Cox N.J., Lilac H.A., Hall H., Klimov A., Fukuda K. Effectiveness and cost-benefit of influenza vaccination of healthy working adults. JAMA. 2000;284(13):1655–1663. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 48.Tsutsui Y., Benzion U., Shahrabani S., Din G.Y. A policy to promote influenza vaccination: a behavioral economic approach. Health Policy. 2010;97(2–3):238–249. doi: 10.1016/J.HEALTHPOL.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Greene L.R., Cox T., Dolan S., Gray P., Khoury R., Kulich P., Myers F.E., Ludlow C., Streed S. APIC; Washington, DC: 2011. APIC Position Paper: Influenza Vaccination Should Be a Condition of Employment for Healthcare Personnel, unless Medically Contraindicated. [Google Scholar]
- 50.US Department of Labor, Occupational Safety and Health Administration Avian influenza. 2020. https://www.osha.gov/SLTC/avianflu/standards.html (accessed 24 July 2020)
- 51.US Pork Center of Excellence Novel H1N1 Biosecurity Recommendations for Producers. 2009. http://porkgateway.org/resource/novel-h1n1-biosecurity-recommendations-for-producers/ (accessed 24 July, 2020)
- 52.Ramos A.K., Fuentes A., Trinidad N. Perception of job-related risk, training, and use of personal protective equipment (PPE) among Latino Immigrant Hog CAFO workers in Missouri: a pilot study. Saf (Basel, Switzerland) 2016;2(4):25. doi: 10.3390/safety2040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cowling B.J., Ip D.K., Fang V.J., Suntarattiwong P., Olsen S.J., Levy J., Uyeki T.M., Leung G.M., Peiris J.M., Chotpitayasunondh T., Nishiura H. Aerosol transmission is an important mode of influenza A virus spread. Nat. Commun. 2013 Jun;4(1):1–6. doi: 10.1038/ncomms2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinshaw V.S., Webster R.G., Turner B. Water-borne transmission of influenza A viruses? Intervirology. 1979;11(1):66–68. doi: 10.1159/000149014. [DOI] [PubMed] [Google Scholar]
- 55.Webster R.G. Influenza virus: transmission between species and relevance to emergence of the next human pandemic. Viral Zoonoses Food Animal Origin. 1997:105–113. doi: 10.1007/978-3-7091-6534-8_11. [DOI] [PubMed] [Google Scholar]
- 56.Brown J.D., Swayne D.E., Cooper R.J., Burns R.E., Stallknecht D.E. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 2007;51(s1):285–289. doi: 10.1637/7636-042806R.1. [DOI] [PubMed] [Google Scholar]
- 57.Lang A.S., Kelly A., Runstadler J.A. Prevalence and diversity of avian influenza viruses in environmental reservoirs. J. Gen. Virol. 2008;89(Pt 2):509–519. doi: 10.1099/vir.0.83369-0. [DOI] [PubMed] [Google Scholar]
- 58.Anderson B.D., Ma M.J., Wang G.L., Bi Z.Q., Lu B., Wang X.J., Wang C.X., Chen S.H., Qian Y.H., Song S.X., Li M. Prospective surveillance for influenza A virus in Chinese swine farms. Emerging Microb. Infect. 2018;7(1):1–10. doi: 10.1038/s41426-018-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall J.S., Minnis R.B., Campbell T.A., Barras S., DeYoung R.W., Pabilonia K., Avery M.L., Sullivan H., Clark L., McLean R.G. Influenza exposure in United States feral swine populations. J. Wildl. Dis. 2008;44(2):362–368. doi: 10.7589/0090-3558-44.2.362. [DOI] [PubMed] [Google Scholar]
- 60.Holzem R.M., Katers J.F. Life-cycle analysis of advanced manure management systems for a Wisconsin confined animal feeding operation (Cafo) Appl. Eng. Agric. 2019;35(1):51–59. doi: 10.13031/aea.12663. [DOI] [Google Scholar]
- 61.Wang M., Lee E., Zhang Q., Ergas S.J. Anaerobic co-digestion of swine manure and microalgae chlorella sp.: experimental studies and energy analysis. BioEnergy Res. 2016;9(4):1204–1215. doi: 10.1007/s12155-016-9769-4. [DOI] [Google Scholar]
- 62.Environmental Protection Agency, Office of Enforcement and Compliance Assurance Standard Operating Procedure (SOP) Biosecurity Procedures for Visits to Livestock and Poultry Facilities. 2016. https://www.epa.gov/sites/production/files/2016-05/documents/biosecuritysop.pdf (accessed 24 July 2020)
- 63.Levis D., Baker R. University of Nebraska-Lincoln, Extension; 2011. Biosecurity of Pigs and Farm Security. [Google Scholar]
- 64.National Pork Board Biosecurity Management Best Practices. 2019. https://www.pork.org/food-safety/biosecurity-management-best-practices/ (accessed 24 July 2020)
- 65.United States Department of Agriculture, Natural Resources Conservation Service Incentive Programs and Assistance for Producers. 2020. https://www.nrcs.usda.gov/wps/portal/nrcs/detail/national/climatechange/resources/?cid=stelprdb1043608 (accessed 18 August 2020)
- 66.One Health Certification Foundation One Health Certified. 2021. https://onehealthcertified.org/ (accessed 5 March 2021)
- 67.United States Department of Agriculture, Economic Research Service Ag and Food Sectors and the Economy. 2020. https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials/ag-and-food-sectors-and-the-economy/ (accessed 10 March 2021)