Abstract
The Northern Bolivian Altiplano is the human fascioliasis hyperendemic area where the highest prevalences and intensities in humans have been reported. Preventive chemotherapy was implemented in the last ten years. Surveillance showed high human infection and re-infection rates in between the annual triclabendazole monodose treatments. A complementary One Health control action was launched to decrease the infection risk. Among the multidisciplinary axes, there is the need to establish animal reservoir species priorities for a more efficient control. Laboratory and field studies were performed for the first time to assess the Fasciola hepatica transmission capacity of the pig and its potential reservoir role. The experimental follow-up of altiplanic pig isolates through altiplanic Galba truncatula snail vector isolates were performed at different miracidial doses and different day/night temperatures. Experiments included egg embryonation, miracidial infectivity, lymnaeid snail infection, intramolluscan larval development, cercarial production, chronobiology of the cercarial shedding, vector survival to infection, metacercarial infectivity of mammal host, and adult stage development. Surveys included the assessment of prevalence, intensity, egg measurements and egg shedding rates in nature. Pig contribution was evaluated by comparing with the main altiplanic reservoirs sheep and cattle. Results demonstrated that the pig assures the whole F. hepatica life cycle and participates in its transmission in this area. The fast egg embryonation, high cercarial production, long multi-wave shedding chronobiological pattern in monomiracidial infections at permanent 20 °C temperature, and the high daily egg outputs per pig are worth mentioning. The high infection risk suggests early infection of freely running piglets and evolutionary long-term adaptation of the liver fluke to this omnivorous mammal, despite its previously evoked resistance or non-suitability. Genetic, physiological and immune similarities with humans may also underlie the parasite adaptation to humans in this area. The pig should be accordingly included for appropriate control measures within a One Health action against human fascioliasis. The pig should henceforth be considered in epidemiological studies and control initiatives not only in fascioliasis endemic areas with human infection risk on other Andean countries, but also in rural areas of Latin America, Africa and Asia where domestic pigs are allowed to run freely.
Keywords: Human fascioliasis hyperendemic, One Health, Pig, Fasciola hepatica, Galba truncatula experimental transmission, Field epidemiology, Reservoir role, Bolivia, Andean countries
Graphical abstract
Highlights
-
•
One Health control action in Northern Bolivian Altiplano human hyperendemic area.
-
•
Experimental study of the transmission capacity of pig isolate of Fasciola hepatica.
-
•
Epidemiological surveys to assess the reservoir role of the pig in the field.
-
•
Need to prioritize the pig in fascioliasis control in the Northern Bolivian Altiplano.
-
•
Pig to be henceforth considered in fascioliasis control initiatives in endemic areas.
1. Background
Fascioliasis is a zoonotic disease caused by the digenean species Fasciola hepatica and F. gigantica. This trematodiasis shows a worldwide distribution due to the introduction of livestock almost everywhere. Thus, F. hepatica is present in Europe, Asia, Africa, the Americas and Oceania, whereas F. gigantica appears widely distributed in parts of Africa and Asia [1].
The World Health Organization (WHO) decided to include fascioliasis in the group of food-borne trematodiases within the list of Neglected Tropical Diseases (NTDs) to which priority should be given [2], based on:
-
-
the human fascioliasis endemic areas where children are the highest infection risk group mainly in low-income countries, including from early postnatal infection [3], and the increasing number of case reports throughout [4];
-
-
the high pathogenicity both at short-term in the acute phase and long-term during the chronic phase [[5], [6], [7], [8]], including severe clinical pictures and sequelae [6,9];
-
-
the immunosuppression effects it induces in the acute phase [10] and the chronic phase [11], and consequent facilitation of coinfections [[12], [13], [14]];
-
-
the problems posed by the diagnosis in human endemic areas and patients [4,15,16];
-
-
the influences of climate change and the impact of anthropogenic modifications of the environment leading to human disease spread and infection increases [[17], [18], [19], [20]];
-
-
the wide variety of human infection sources underlying a great disease heterogeneity depending on the different endemic areas [21];
-
-
and, altogether, its impact on the development of rural communities of low-income countries.
Latin America is a priority region for human fascioliasis control due to the many countries presenting human endemic areas. In South America, the public health problems concern mainly high altitude areas of Andean countries, such as Bolivia, Peru, Chile and Argentina. In areas with high human prevalences and intensities, the availability of a highly efficient and safe drug as triclabendazole [22] has allowed for mass treatment campaigns by means of annual monodose administrations, with the main purpose of decreasing the high burdens linked to the worse pathological effects and morbidity [23,24].
The Northern Bolivian Altiplano is the area where the highest prevalences and intensities in humans have been reported [[25], [26], [27]]. This hyperendemic area is located between the Lake Titicaca and the valley of La Paz city, at 3820–4100 m a.s.l. In this area, control by such a preventive chemotherapy has been applied for more than ten years. Despite successful implementation and lack of cases with secondary effects, periodical surveillance allowed for the detection of new infections and re-infections in children in between the annual campaigns. This led to a PAHO-WHO monographic meeting for an in-depth analysis of the local situation. The problem posed by the resistance to triclabendazole reported in other human and animal endemic areas of the aforementioned Andean countries was also considered [28].
When deciding which further interventions would be appropriate to decrease the infection/re-infection risk not only in the Northern Bolivian Altiplano, but also in other human endemic Andean areas, crucial aspects of the disease merited detailed focus, such as (i) the eurixenous specificity for herbivore mammals but also several omnivore mammals as definitive hosts including domestic as well as sylvatic species together with humans [1], (ii) the oligoxenous specificity for freshwater amphibious snails of the family Lymnaeidae as invertebrate vectors [29], and (iii) the opportunistic capacity of the free-living, encysted infective metacercarial stage for oral infection of the definitive hosts according to a large spectrum of infection sources including both food and drinks [21,30]. Indeed, these characteristics underlie a wide complexity and heterogeneity of transmission patterns [31,32] and epidemiological scenarios [33,34].
A multidisciplinary One Health action was concluded to be the most appropriate subsequent step. This was proposed to be included within new control initiatives to complement the preventive chemotherapy interventions already under way. Indeed, WHO has recently reinforced the strategies to fight against NTDs, human liver fluke diseases included [35]. The Northern Bolivian Altiplano was selected for such a One Health intervention and several implementation axes have been designed accordingly. Among them, one is devoted to assess priorities between the animal reservoirs. Therefore, experimental studies and field surveys are being performed. Sheep, cattle and donkey have already been the focus of such assessments [36,37].
The present study exposes the results obtained in laboratory analyses on altiplanic pig isolates through altiplanic Galba truncatula snail vector isolates, including the experimental follow-up of the egg embryonation, miracidial infectivity, lymnaeid snail infection, intramolluscan larval development, cercarial production, chronobiology of the cercarial shedding, vector survival to infection, metacercarial infectivity of mammal host, and adult stage development. The field studies included the assessment of prevalence, intensity, egg measurements and egg shedding rates in nature. The contribution of the pig to fascioliasis in this area is evaluated by comparing with results of similar studies previously performed under identical methods and techniques on the main altiplanic reservoir species: sheep and cattle [36]. This is the first time that such experimental fascioliasis studies focus on the pig. The epidemiological role of the pig as reservoir in a human endemic area is also assessed for the first time.
2. Material and methods
2.1. Experimental studies
2.1.1. Materials of Fasciola hepatica and lymnaeid snail vector
For the experimental study of the fasciolid egg embryonation, eggs produced by liver flukes naturally infecting domestic pigs from the locality of Batallas, in the Northern Bolivian Altiplano human hyperendemic area, at 3858 m a.s.l. (Fig. 1), were isolated by filtration (filter pore size of 40 μm) and conserved in natural water under complete darkness at 4 °C until starting of the embryogenesis follow up study.
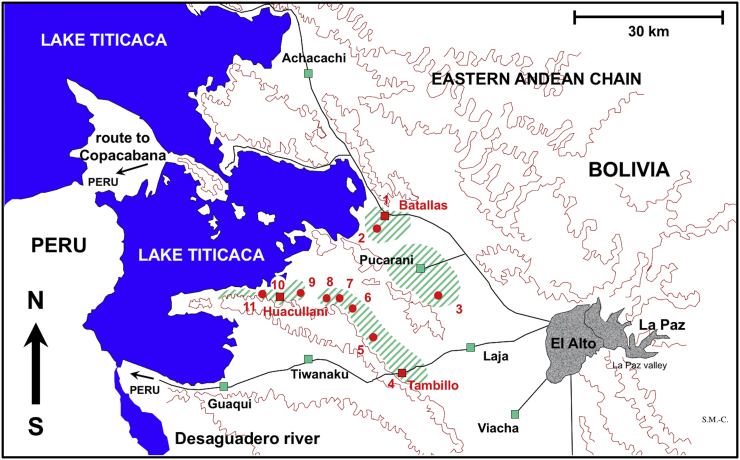
Fig. 1.
Map showing the Northern Bolivian Altiplano human fascioliasis hyperendemic area, at 3820–4100 m altitude, including zones where pigs were surveyed and localities where lymnaeid snail vector specimens of Galba truncatula were collected. Localities: 1) Batallas; 2) Chijipata Alto; 3) Ancocagua; 4) Tambillo; 5) Caleria; 6) Korila; 7) Chiripujo; 8) Lacaya Baja; 9) Chojasihui; 10) Huacullani; 11) Queroni.
For the experimental infection of lymnaeid snail vectors, F. hepatica eggs were similarly isolated from pigs of Batallas and conserved until used for snail infection in the laboratory. Experimental assays were started immediately after each field mission and arrival to the laboratory, and only first laboratory generation specimens of the altiplanic species Galba truncatula [38] were used.
For the experimental infection of Wistar rats, F. hepatica metacercariae were obtained from the aforementioned experimentally infected lymnaeid snails. These metacercariae were stored in natural water in total darkness until required. The storage temperature was 4 °C, according to the usual standards in liver fluke studies [39].
2.1.2. Egg embryogenesis follow up
The egg embryogenesis was experimentally studied under conditions of 90% relative humidity and a photoperiod of 12 h light/12 h darkness in climatic chambers (HPS-1500, VB-0714 and HPS-500 models of Heraeus-Vötsch) [40]. For the follow-up analyses, microscopic observations and counts were made at intervals of every four days under two different temperature patterns:
-
A)
20 °C/20 °C day/night temperature to allow for comparisons with previous studies made on liver fluke isolates from the two main reservoir species sheep and cattle of the same altiplanic endemic area, obtained following the same procedures [36].
-
B)
22 °C/5 °C 12 h day/12 h night temperature to assess the potential influence of the daily varying temperature in this altiplanic endemic area [18].
Egg development was analyzed by differentiation of (i) eggs in the phase of morula, showing vitelline granules and/or spheroidal cells (E.M.), (ii) eggs in the phase of outlined miracidium, in which a miracidial form begins to be observed (E.O.M.), and (iii) eggs in the phase of developed miracidium, in which a fully developed miracidium is observed inside (E.D.M.). Counting did not only include E.M., E.O.M. and E.D.M., but also (iv) degenerated eggs, (v) empty eggs, and (vi) broken eggs. For images showing these different egg stages, see previously published illustrations [37].
For each 4-day study, a total of 33 eggs from each host individual were analyzed between slip and coverslip. Egg counts were noted in percentages independently per observational day.
2.1.3. Experimental infection of snails with miracidia
Only laboratory reared lymnaeid specimens of a size of 4.0–5.0 mm were used to assess snail susceptibility to miracidial infection by the pig isolate. For this purpose, miracidia were obtained by putting fully embryonated eggs under light to force their hatching [41]. Individually isolated snails were exposed to miracidia for 4 h in a small Petri dish containing 2 ml of fresh water. The disappearance of the miracidia was taken as verification of its successful penetration into the snail.
To asses the pig isolate infection capacity, mono- and tri-miracidial infection doses were assayed and subsequent intramolluscan development followed-up at 20 °C/20 °C day/night temperature according to a photoperiod of 12 h light/12 h darkness. Additionally, a mono-miracidial infection dose and subsequent intramolluscan development were analyzed in a 22 °C/5 °C day/night temperature experiments, to reproduce the daily variability of the air temperature on the Altiplano [18], which appears however buffered inside freshwater [42]. All the assays were performed in the aforementioned climatic chambers.
After the infection, snails were returned to 2000 ml containers, at 90% relative humidity, 12 h/12 h light/darkness, and dry lettuce ad libitum, until day 30 post-infection. Afterwards, they were again isolated in Petri dishes under the aforementioned corresponding conditions of temperature to allow daily monitoring of cercarial shedding by individual snails. Lettuce was provided ad libitum to each snail in a Petri dish during both shedding and post-shedding periods until death of the snail. The cercarial shedding was followed by daily counting of metacercariae in each Petri dish [41].
2.1.4. Laboratory cultures of the snail vector
Galba truncatula has recently been proved to be the only lymnaeid species inhabiting the Northern Bolivian Altiplano hyperendemic area. Sequencing of complete nuclear ribosomal DNA and mitochondrial DNA markers proved moreover its genetic uniformity throughout the whole endemic area [38]. This is an introduced species of European origin which differs from the Neotropical species of the Galba/Fossaria group of lymnaeids which also act as vectors of fascioliasis in South America [43]. Living specimens of G. truncatula were collected in the altiplanic localities of Huacullani and Ancocagua (Fig. 1) and transported under isothermal conditions for their laboratory adaptation to standardized controlled conditions of 90% relatively humidity, 12 h/12 h light/darkness photoperiod, and 20 °C/20 °C day/night temperature in the aforementioned precision climatic chambers. The possible natural infection by fasciolids was always individually verified prior to the launch of laboratory cultures. This was performed by keeping each lymnaeid specimen isolated in a Petri dish containing a small amount of natural water. After 24 h, the presence or absence of motionless metacercarial cysts or moving cercariae was verified in each Petri dish. Non-infected lymnaeids were arranged in standard breeding boxes containing 2000 ml fresh water, to assure locality-pure cultures. The water was changed weekly and lettuce added ad libitum.
2.1.5. Experimental infections of laboratory mammal model
A total of 24 male Wistar rats (Iffa Credo, Barcelona, Spain) aged 4–5 weeks were used throughout. A balanced commercial rodent diet (Panlab Chow A04) and water were provided ad libitum, according to standards previously reported [44].
Wistar rats were infected according to methods previously described [45,46]. A dose of 20 F. hepatica metacercariae per rat was used. Metacercariae were inoculated orally by means of a gastric tube. Animal care, animal health, body condition and well-being were assessed on a weekly basis by means of checking their body weight and the appearance of the fur. Infected animals presented a lower body weight than negative controls at the end of the experiment. No mortality occurred.
Infection prevalence and intensity (number of worms successfully developed in each rat) were established by necropsy 12 weeks after infection. Therefore, animals were humanely euthanized with an overdose of an anesthetic (IsoFlo; Dr. Esteve SA, Barcelona, Spain), and F. hepatica worms were collected under a dissecting microscope, according to methods already outlined before [47]. The bile duct was initially examined for the presence of flukes, followed by the whole liver, although the rest of the organs were also evaluated. The thoracic and abdominal viscera and cavities were examined and thoroughly rinsed with water to assure the recovery of all worms.
2.2. Field surveys of domestic pigs
2.2.1. Host animals studied
Faecal samples from a total of 73 pigs were collected from different localities and zones of the Northern Bolivian Altiplano hyperendemic area (Fig. 1). Animals studied included both sexes and ranged between 1 and 2 years in almost all individuals, except sporadic cases of 5 or 8 months and one specimen of 8 years. The presence of fasciolid adult flukes in the liver was verified in all pig specimens analyzed from the localities of Batallas, Chijipata Alto and Pucarani (Fig. 1). In the Northern Bolivian Altiplano, Aymara inhabitants do not slaughter small piglets, nor do they treat pigs against liver fluke infection.
2.2.2. Stool sample preparation and study
Only coprological methods for qualitative and quantitative analyses were carried out. Faecal samples were placed in numbered plastic bags, transported to the laboratory within the following 5 h, and maintained at 4 °C until examination. From each stool sample a quantity of 5 g was sedimented twice, first with 50 ml of detergent solution (1 ml/1000 cm2) after filtration and second with 50 ml water, and stained with methyl green according to a modified sedimentation test [48,49], before examination under light microscope for F. hepatica eggs. To avoid false positives due to spurious infection, microscopic slides of coprological samples were analyzed by members of the WHO Collaborating Centre on Human Fascioliasis of Valencia, whether in a La Paz laboratory during missions or in the WHO CC of Valencia itself directly after arrival from such a mission when previously impossible in La Paz. As known, an expert microscopist may differentiate the somewhat degenerated aspect of ‘eggs in transit’ from ‘normal eggs’ [4]. The number of eggs shed by a pig was used to estimate the infection intensity and was expressed in eggs per gram of stools (epg).
Egg measurements were carried out using a computer image analysis system (CIAS) on the basis of standardized measurements known to be useful for fasciolid species [50]. Standardized measurements were taken using a microscope and images captured by a digital camera (3CCD color videocamera Sony DXC-930P), which were then analyzed by image analysis software (ImagePro plus version 5.0 for Windows, Media Cybernetics, Silver Spring, Maryland, USA). Egg characteristics studied were: (a) linear measurements: egg length (EL), egg width (EW), and egg perimeter (EPe); (b) areas: egg area (EA); (c) ratios: EL/EW ratio. For each measure, minimum and maximum values, mean and standard deviation were determined.
2.3. Statistical analyses
Both transmission capacity and epidemiological role of the pig isolate were assessed by comparing each characteristic studied with those of the F. hepatica sheep and cattle isolates from the same Northern Bolivian Altiplano. The significance of the results from these comparisons can be analyzed because based on data obtained following the same methods and techniques both in the laboratory experiments and in the field work and which have been reported in another study [36].
Statistical analyses were performed using SPSS Statistics 26. Development egg data (E.M., E.O.M., E.D.M.) were compared by ANOVA test. Statistical comparison of categorical variables was carried out with the Chi-square test and Yates continuity corrected Chi-square test. Means obtained in data from experimental infections of lymnaeid snails and from experimental infections of Wistar rats were compared by non-parametric Kruskal-Wallis test. Fasciolid egg size measurements (EL, EW, EPe, EA and EL/EW) were compared by post-hoc tests (L.S.D., Student–Newman–Keuls and Duncan's tests). Results were considered statistically significant when P < 0.05.
3. Results
3.1. Egg embryonation
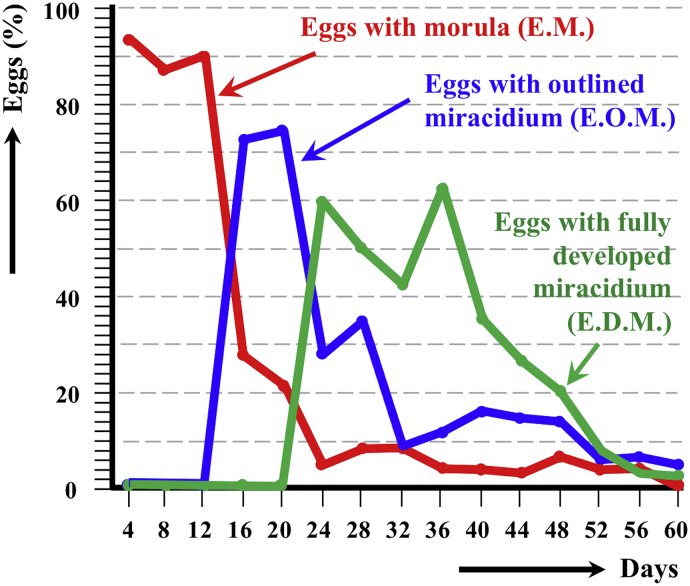
At a 24 h permanent 20 °C, an outlined miracidium form begins to be observed inside eggs at day 16, and the first fully developed miracidium appears at day 24. Eggs including a fully developed miracidium were henceforth observed in each observational day until day 60, although progressively decreasing after a peak on day 36 (Fig. 2).
Fig. 2.
Graph showing the results of the experimental follow-up study of the egg embryonation of the altiplanic pig isolate of Fasciola hepatica, at 4-day study intervals and constant temperature of 20 °C. Curves of the percentages of degenerated, empty and broken eggs are not included.
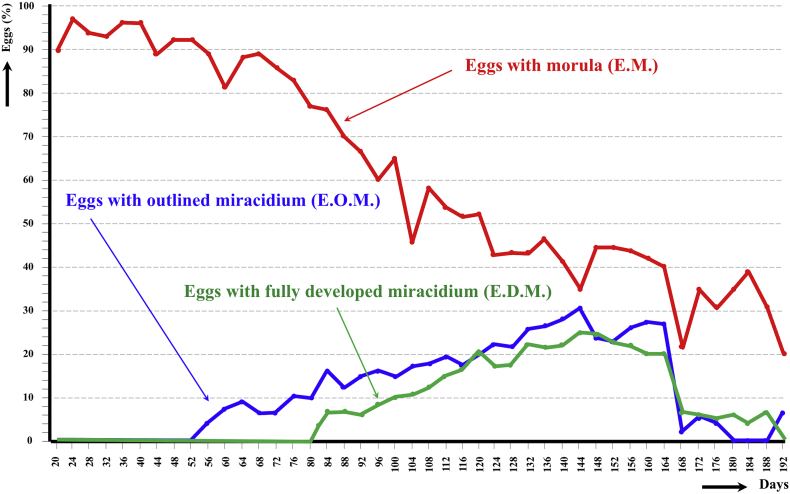
At a varying 22 °C/5 °C day/night temperature, the embryogenesis shows a pronounced slow down. The first outlined miracidium only appeared from day 56, and the first fully developed miracidium only from day 82. Both type of eggs followed gradual increases until a peak on day 144, with a subsequent acrophase until day 164, to drastically decrease thereafter. Exhaustion of fully embryonated eggs was found at day 192 (Fig. 3). When comparing the egg embryonation (E.O.M., E.D.M.) at 22 °C/5 °C day/night temperature of the pig isolate (47.62%, 0.75%), with the egg embryonation at 20 °C (22.62%, 20.47%), statistically significant differences in the average egg embryonation were detected (P < 0.05 in both comparisons).
Fig. 3.
Graph showing the results of the experimental follow-up study of the egg embryonation of the altiplanic pig isolate of Fasciola hepatica, at 4-day study intervals and day/night temperature of 20 °C/5 °C. Curves of the percentages of degenerated, empty and broken eggs are not included.
When comparing the egg embryonation at 20 °C of the pig isolate with the sheep and cattle isolates from the Bolivian Altiplano, no statistically significant differences in the average egg embryonation (E.O.M., E.D.M.) of the pig isolate (22.62%, 20.47%) along 60 days were detected when compared to the sheep isolate (35.51%, 12.31%) and cattle isolate (31.89%,16.11%) (P > 0.05 in both comparisons).
3.2. Snail infectivity and intramolluscan development
Lymnaeid snail infection assays performed with the F. hepatica isolate from altiplanic pig furnished results which, together with experimental characteristics, conditions and number of snails used, are shown in Table 1, including their comparison with the altiplanic sheep and cattle isolates.
Table 1.
Experimental infections of Galba truncatula lymnaeid snails with Fasciola hepatica pig isolate both from the Northern Bolivian Altiplano human hyperendemic area, and their comparison with results obtained in the F. hepatica sheep and cattle isolates from the same endemic area.
| Host isolate |
Pig |
Sheepa |
Cattlea |
||||||
|---|---|---|---|---|---|---|---|---|---|
| F. hepatica geogr. Origin | Batallas | Batallas | Batallas | Batallas | Batallas | Batallas | Batallas | Kallutaca | Kallutaca |
| Lymnaeid geogr. Origin | Huacullani | Huacullani | Ancocagua | Huacullani | Huacullani | Ancocagua | Huacullani | Viacha | Tambillo |
| Miracidial dose | mono-miracidial | mono-miracidial | tri-miracidial | mono-miracidial | mono-miracidial | bi-miracidial | mono-miracidial | mono-miracidial | tri-miracidial |
| Temperature (12 h day/12 h night) | 20° C/20 °C | 22 °C/5 °C | 20 °C/20 °C | 20 °C/20 °C | 22 °C/5 °C | 20° C/20 °C | 20 °C/20 °C | 22 °C/5 °C | 20 °C/20 °C |
| No. lymnaeids infected | 92 | 46 | 79 | 62 | 50 | 37 | 55 | 21 | 50 |
| No. survivor snails at beginning of shedding (%) | 54 (58.7%) | 43 (93.5%) | 24 (30.4%) | 54 (87.1%) | 41 (82.0%) | 13 (35.1%) | 48 (87.3%) | 13 (61.9%) | 23 (46.0%) |
| No. shedding snails (%) | 16 (29.6%) | 5 (13.7%) | 4 (16.7%) | 28 (51.8%) | 9 (21.9%) | 7 (53.8%) | 12 (25.0%) | 2 (15.4%) | 15 (65.2%) |
| Prepatent period in dpi (mean) | 49–55 (51.1) | 48–59 (53.0) | 44–57 (53.5) | 48–92 (55.6) | 59–93 (69.3) | 41–69 (49.7) | 49–76 (55.5) | 94–95 (94.5) | 43–95 (67.2) |
| Shedding end in dpi (mean) | 69–131 (119.1) | 48–62 (56.6) | 49–71 (59.5) | 52–136 (89.4) | 70–93 (75.7) | 49–87 (67.6) | 58–135 (101.6) | 95–106 (100.5) | 70–117 (89.3) |
| Shedding length in days | 20–83 (70.1) | 1–11 (4.6) | 1–15 (6.7) | 1–88 (34.7) | 1–15 (7.3) | 6–29 (18.9) | 1–85 (47.1) | 1–13 (7.0) | 1–85 (23.1) |
| No. total cercariae shed | 3859 | 204 | 130 | 5542 | 311 | 429 | 3672 | 30 | 762 |
| No. cercariae/snail (mean) | 101–569 (428.8) | 1–131 (40.8) | 19–47 (32.5) | 8–562 (197.9) | 1–155 (34.5) | 15–101 (61.3) | 8–581 (306.0) | 1–29 (15.0) | 2–100 (51.8) |
| Snail survival after shedding end in days | 1–73 (38.7) | 0–83 (21.8) | 0–1 (0.75) | 1–132 (24.5) | 0–34 (5.0) | 1–10 (3.9) | 1–133 (42.3) | 0–25 (12.5) | 1–21 (3.8) |
| Longevity of shedding snails in dpi | 92–181 (157.8) | 59–138 (78.4) | 50–72 (60.2) | 53–192 (113.8) | 72–127 (75.9) | 51–90 (71.4) | 76–268 (143.9) | 106–120 (113.0) | 71–128 (93.1) |
| Longevity of non-shedding snails in dpi | 98–180 (140.3) | 48–153 (98.3) | 31–62 (43.5) | 49–196 (139.1) | 56–143 (83.2) | 44–107 (62.7) | 31–209 (105.4) | 96–161 (132.9) | 32–49 (38.5) |
dpi = days post-infection.
Data from Mas-Coma et al. [36].
When using a monomiracidial dose and temperature of 20 °C/20 °C day/night, the snail infectivity (= % of cercariae shedding snails) proved to be of 29.6%. This is slightly more efficient than the cattle isolate (25.0%), although both at distance from the sheep isolate (51.8%). However, no statistically significant differences in the percentage of snails successfully infected between the pig isolate and the two main reservoirs (P > 0.05) were detected. The mean value of the prepatent period (from infection up to the shedding of the first cercaria) of the pig isolate (51.1 days) was slightly lower than in the sheep (55.6 days) and cattle (55.5 days) isolates. These differences are not statistically significant (P > 0.05). The cercarial shedding period or patent period of the pig isolate (average: 70.1 days) showed a pronouncedly longer mean value than in the sheep (average: 34.7 days) and cattle (average: 47.1 days) isolates. No statistically significant differences in the cercarial shedding period were anyway detected between the pig isolate and the two main reservoirs (P > 0.05). When comparing the high production of cercariae per infected snail of the pig isolate (average: 428.8 cercariae/snail) with that of the sheep (average: 197.9 cercariae/snail) and cattle (average: 306.0 cercariae/snail) isolates, no significant differences were observed (P > 0.05). The assessment of the infection impact on snails was measured by (i) the snail survival after shedding end in days, (ii) the longevity of shedding snails, and (iii) the longevity of experimentally infected non-shedding snails. In the pig isolate, these three characteristics showed mean and range values overlapping those in sheep and cattle isolates, all of them proving to be not statistically different (P > 0.05).
When using a monomiracidial dose and 22 °C/5 °C day/night temperatures, the infectivity rate, the length of the patent period, and the number of cercariae/snail showed values lower than those monomiracidially obtained at 20 °C/20 °C day/night, although no statistical differences was found in their comparison (P > 0.05). Only the prepatent period was similar. The three values assessing the infection impact on snails were also lower, although no statistically different differences were found (P > 0.05). All these values of the pig isolate using a monomiracidial dose and 22 °C/5 °C day/night temperatures showed evident similarities with those obtained with the isolates of the main reservoirs sheep and cattle when using the same monomiracidial dose and same day/night temperatures (Table 1) (P > 0.05).
The results of the pig isolate when using a tri-miracidial dose at 20 °C/20 °C day/night temperature were similar to those obtained using a monomiracidial dose and 22 °C/5 °C day/night temperatures, except for the lower values of the three characteristics assessing the infection impact on snails (Table 1) (P > 0.05).
3.3. Chronobiology of cercarial shedding
The total length of the shedding period of the pig isolate, using a monomiracidial dose and temperature of 20 °C/20 °C day/night, lasted up to 83 days (Fig. 4 A) or 12 weeks (Fig. 4 C), with a mean of 70.1 days (Table 1).
Fig. 4.
Chronobiological patterns of cercarial emergence by altiplanic Galba truncatula monomiracidially infected with the pig isolate of Fasciola hepatica from the Northern Bolivian Altiplano human fascioliasis hyperendemic area: A) shedding period analyzed according to the mean amounts of cercariae shed daily from the day of the emergence of the first cercaria by each snail; B) shedding period analyzed according to the mean amounts of cercariae shed daily from the day of the miracidial infection; prepatent period not shown; C) shedding period analyzed according to the mean amounts of cercariae shed weekly from the day of the emergence of the first cercaria by each snail; D) shedding period analyzed according to the mean amounts of cercariae shed weekly from the day of the miracidial infection; prepatent period not shown.
The chronobiological patterns of cercarial emergence by altiplanic Galba truncatula monomiracidially infected with the altiplanic pig isolate of F. hepatica according to the mean amounts of cercariae shed daily from the day of the emergence of the first cercaria by each snail and from the day of the miracidial infection of each snail are shown in Fig. 4 A and Fig. 4 B, respectively. The daily shedding process appears as a long succession of small waves in which the number of cercariae/snail is high already from the beginning. It reaches the highest peak at day 8, to subsequently follow a gradual quantitative decrease until complete exhaustion at day 84 (Fig. 4 A).
In the chronobiological analysis of the cercarial shedding period from the day of the miracidial infection, the curve shows a similar pattern. It should be highlighted, however, that there are four days in which all snails simultaneously stop their shedding. These days of unanimous lack of cercarial emergence corresponded to days 65, 82, 92 and 119 dpi (Fig. 4 B). Such days without shedding are not observed when the chronobiological curve concerns the daily follow-up from the day of the first cercarial shedding by each snail.
The weekly analysis of the curve of the chronobiological cercarial shedding period from the day of the emergence of the first cercaria by each snail and from the day of the miracidial infection of each snail are shown in Fig. 4 C and Fig. 4 D, respectively. Both curves are similarly regular and allow to show the general shedding trend which appears masked when analyzed by daily values. The highest values are found in the initial two weeks, the highest peak in the second week, and a progressive decrease from the third week until the final exhaustion. The total shedding length of 12 weeks covers from week 8 up to week 19 when the counting is made from the miracidial infection day (Fig. 4 D).
3.4. Mammal host infectivity
The results of the laboratory infection of Wistar rats with experimentally obtained metacercariae of the altiplanic pig isolate are shown in Table 2. The experimental prevalence (= percentage of rats successfully infected) obtained in the metacercarial infections using the pig isolate (45.8%) are lower than those found when using the altiplanic sheep (78.4%) and cattle (75.0%) isolates. However, no statistically significant differences appeared when comparing these infectivity rates (P > 0.05). The infectivity decrease, from 80.0% with 6–8-week-aged metacercariae down to 21.4% with 9–11-week-aged metacercariae, suggests that the age of the metacercariae may influence the infection capacity of the pig isolate more than in the sheep and cattle isolates.
Table 2.
Experimental infections of Wistar rats with experimentally obtained metacercariae of pig isolate from the Northern Bolivian Altiplano human hyperendemic area and comparison with sheep and cattle isolates from the same area.
| Host isolate |
Pig |
Sheepc |
Cattlec |
|||
|---|---|---|---|---|---|---|
| F. hepatica geographical origin | Batallas | Batallas | Batallas | Ancocagua | Kallutaca | Batallas |
| Age of metacercariae | 6–8 weeks | 9–11 weeks | 1 week | 2 weeks | 6 weeks | 8 weeks |
| No. metacercariae inoculated per rat | 20 | 20 | 20 | 20 | 20 | 20 |
| No. inoculated rats | 10 | 14 | 14 | 23 | 4 | 4 |
| No. rats infected (%) | 8 (80.0%) | 3 (21.4%) | 11 (78.6%) | 18 (78.3%) | 4 (100%) | 2 (50.0%) |
| No. flukes recovered per rat (mean) | 1–3 (1.6) | 1–1 (1.0) | 1–8 (3.6) | 1–10 (3.7) | 1–2 (1.7) | 1–2 (1.5) |
| Intensitya | 8.0% | 5.0% | 14.3% | 14.6% | 8.8% | 3.7% |
| Mean % flukes recovered/ratb | 8.0% | 7.0% | 18.2% | 18.6% | 8.8% | 7.5% |
Intensity = total % of flukes recovered = (total No. of flukes recovered/total No. of metacercariae administered in all rats) x 100.
Mean % flukes recovered/rat = Mean % of flukes recovered per infected rat = (flukes recovered/metacercariae administered per infected rat) x 100.
Data from Mas-Coma et al. [36].
Regarding the infection intensity, measured by the number of flukes recovered per metacercariae inoculated and also per rat, the results obtained did not show significant differences when comparing the pig isolate with the sheep and cattle isolates (P > 0.05).
3.5. Prevalence, intensity, egg measurements and shedding rates in nature
Prevalences and intensities (epg) found in the coprological surveys on pigs inhabiting selected zones of the fascioliasis hyperendemic area (Fig. 1) are shown in Table 3. In the Altiplano, pigs are numerous in all zones of the endemic region, as they are usually part of the livestock of Aymara families. According to their number, it appears to be the third domestic animal species in importance in the Northern Bolivian Altiplano, after sheep and cattle. In most zones, pigs run free and can usually be observed in or around freshwater bodies inhabited by Iymnaeid snails (Fig. 5).
Table 3.
Prevalence and intensity of Fasciola hepatica infection found by coprological analyses in pigs of different zones of the Northern Bolivian Altiplano human hyperendemic area and comparison with local prevalences in sheep and cattle from the same zones.
| Locality | Endemic zone | Pig |
Sheepb |
Cattleb |
|||
|---|---|---|---|---|---|---|---|
| Prevalence |
Intensity |
Prevalence |
Prevalence |
||||
| No. analyzed/infected | % | Range (epg) | Mean (epg) | No. analyzed/infected (%) | No. analyzed/infected (%) | ||
| Batallas | Batallas | 10/7 | 70.0a | 4–67 | 25.4 | 135/76 (56.3) | 303/45 (14.8) |
| Chijipata Alto | Batallas | 1/0 | 0.0 | – | – | 35/21 (60.0) | 55/24 (43.6) |
| Ancocagua | Pucarani | 8/5 | 62.5a | 5–37 | 23.8 | 170/97 (57.1) | 195/22 (11.3) |
| Tambillo | Tambillo-Lacaya | 6/1 | 16.7a | 11 | 11.0 | 230/113 (49.1) | 50/2 (4.0) |
| Caleria | Tambillo-Lacaya | 1/0 | 0.0 | – | – | 53/43 (81.1) | 43/3 (7.0) |
| Korila | Tambillo-Lacaya | 2/0 | 0.0 | – | – | 47/41 (87.2) | – |
| Chiripujo | Tambillo-Lacaya | 2/1 | 50.0 | 23 | 23.0 | – | 141/17 (12.0) |
| Lacaya Baja | Tambillo-Lacaya | 7/0 | 0.0 | – | – | 100/64 (64.0) | 43/3 (7.0) |
| Chojasihui | Huacullani | 5/0 | 0.0 | – | – | 100/70 (70.0) | 29/9 (31.0) |
| Huacullani | Huacullani | 27/5 | 18.5a | 1–7 | 2.8 | 120/81 (67.5) | 210/95 (45.2) |
| Queroni | Huacullani | 14/2 | 14.3 | 9–21 | 15.0 | 100/70 (70.0) | – |
| TOTAL | 73/21 | 28.7a | 1–67 | 17.1 | 920/582 (63.7) | 768/148 (19.3) | |
Significantly different vs. sheep and cattle, determined by chi-square test (P < 0.05).
Local data from various sources - see references noted in Mas-Coma et al. [36].
Fig. 5.
Pigs in human fascioliasis hyperendemic areas of the Northern Bolivian Altiplano (A-E) and the Peruvian province of Cajamarca (F): A) Freely running piglet close to freshwater collection in Batallas; B) Weekly pig slaughtering at the river in Batallas; C) Pigs inside freshwater collection inhabited by lymnaeid snails in the Pucarani zone (in rainy season); D) Freely running pigs in Huarina, northward from Batallas (in dry season; see grazing cow beside); E) Pig close to small freshwater canal inhabited by lymnaeid snails in Chijipata Alto; F) Pig besides small freshwater stream at a road border in the Cajamarca area. Photographs S. Mas-Coma.
A total of 186 F. hepatica eggs from altiplanic pigs were measured. Results of EL, EW, EPe, EA and EL/EW and their comparative analysis with fasciolid eggs shed by altiplanic sheep and cattle are shown in Table 4. The statistical analysis by post-hoc tests (L.S.D., Student–Newman–Keuls and Duncan's tests) showed that, in the Altiplano, EL, EPe, EA and EL/EW proved to be significantly smaller than the same parameters in the sheep and cattle isolates (P < 0.05).
Table 4.
Comparison of egg measurements (A) and egg shedding (B) between the Fasciola hepatica pig isolate and the sheep and cattle isolates from the Northern Bolivian Altiplano human hyperendemic area.
| Host | Pig | Sheepa | Cattlea | |||
|---|---|---|---|---|---|---|
| A) Egg measurements | ||||||
| No. of eggs studied | 186 | 104 | 168 | |||
| Measurements in μm | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD |
| EL | 73.8–148.6 | 123.8 ± 11.3 | 114.8–151.2 | 130.8 ± 7.1 | 105.3–155.9 | 132.0 ± 10.5 |
| EW | 58.1–82.5 | 71.8 ± 4.3 | 65.5–81.4 | 72.6 ± 3.9 | 61.7–82.5 | 71.1 ± 4.4 |
| EPe | 247.8–360.0 | 313.6 ± 20.92 | 294.2–368.2 | 327.6 ± 15.0 | 270.6–422.9 | 340.0 ± 33.4 |
| EA | 3988.7–8626.9 | 6836.9 ± 820.3 | 5998.2–8608.4 | 7238.0 ± 532.8 | 5286.5–9676.8 | 7170.2 ± 802.5 |
| EL/EW | 1.0–2.1 | 1.7 ± 0.1 | 1.5–2.1 | 1.8 ± 0.1 | 1.6–2.3 | 1.8 ± 0.2 |
| B) Egg shedding | ||||||
| Intensity range (epg) | 1–65 | 3–241 | 1–96 | |||
| Stools/day (kg) | 0.5–3.0 (a) | 1–3 | 15–35 | |||
| No.eggs/animal/day | 500–195,000 | 3000–723,000 | 15,000-3,360,000 | |||
The infection intensity in pigs varied between 1 and 67 epg, with a mean of 17.1 epg (Table 3). Comparisons with intensities in sheep and cattle are neither included nor statistically analyzed in this Table 3, because epg data are not comparable between different animal species, i.e. the pronounced differences in quantities of faeces per day give rise to different dilutions of epg according to host species. However, the daily capacity of each host species to contribute egg contamination of the environment may be appropriately compared. When considering the amount of stools defecated by each domestic animal species per day, the total number of F. hepatica eggs shed with faeces by each species per day in the Bolivian Altiplano could be estimated (Table 4). The range of daily egg output per pig per day proved to be markedly lower than the similarly estimated values for sheep and cattle.
4. Discussion
4.1. Participation of the pig in fascioliasis transmission
In digenean trematodes in whose life cycle there is a low specificity at definitive host level, the finding of natural infections of a mammal species allows to include this host species among the list of animals affected by the disease caused by the trematode, but does a priori not enable to epidemiologically consider it as a reservoir because it may indeed only be a dead end. The infection and development of the adult stage in a definitive host species does not mean that this species participates in the transmission of the parasite. There may even be differences in the viability of the same definitive host species, with geographical populations playing a transmission role whereas others being a blind alley.
To prove that a definitive host species or local geographical strain participates in the transmission of a trematode, there is the need to (i) experimentally assess that the life cycle of the parasite is not blocked at any of the subsequent life cycle larval stages until the infection of another definitive host individual, and (ii) demonstrate that the rates of development in all these life cycle phases is sufficient as to estimate a successful transmission in nature. In Fasciola, this implies appropriate laboratory research on definitive host egg shedding, egg embryonation, miracidial hatching and snail vector infection, complete intramolluscan larval development until cercarial production, cercarial shedding and its characteristics, metacercarial production and definitive host infection, and final adult stage development until mature stage.
4.2. Egg embryonation in altiplanic pig isolate
The miracidium development is temperature-dependent. The pig isolate of altiplanic F. hepatica could be experimentally followed until complete development of the inner miracidium under both experimental conditions assayed of 20 °C/20 °C day/night temperature (Fig. 2) and 22 °C/5 °C day/night temperature (Fig. 3).
In the follow-up at permanent 20 °C (Fig. 2), the first appearance of outlined miracidium and fully developed miracidium at days 16 and 24, respectively, agrees with results obtained with the altiplanic sheep and cattle isolates [36] and also with the 19–27 day range observed in ruminant isolates in southern Europe [51] and in the southern part of Chile [52]. The egg embryonation in the pig isolate differs, however, with the delayed embryonation observed in the donkey isolate from the same Altiplano endemic area [37]. Moreover, the percentage of inner developed miracidia in the pig isolate shows a higher acrophase between days 24 and 36 and a lower percentage at day 60, which indicate a faster miracidium development than in the sheep and cattle isolates.
In the follow-up at varying 22 °C/5 °C day/night temperature (Fig. 3), the process appears delayed, although high percentages of eggs containing a fully developed miracidium were found between days 120 and 164. It should be highlighted that a minimum temperature threshold of 10 °C for the development of the egg of F. hepatica was established early on [53]. At 10 °C, the time needed to reach a 50% of the eggs presenting a fully developed miracidium was 161 days [54]. In another study in southern Europe, a hatching time of 56 days was found at 15 °C [51]. Nevertheless, in studies performed in the open field in the southern part of Chile, F. hepatica proved to be more adapted to cold temperatures, with first hatching times at 101 days at 9.1 °C, 80 days at 10 °C, 57 days at 12.4 °C, 44 days at 12.6 °C, 42 days at 13.8 °C, and 34 days at 15.1 °C [52]. At the very high altitude of 3820–4100 m of the Northern Bolivian Altiplano hyperendemic area, the mean environmental temperature is slightly lower than 10 °C throughout the year in most of the transmission foci, and the substantial variation of temperature within a daily 24-h period allows for the relatively high temperature during sunshine hours to counteract the negative impact of the cold temperature during the night [18]. Recent field studies in altiplanic transmission foci have moreover demonstrated that although the mean environmental temperature was always lower than the fluke development minimum temperature threshold, the water temperature was higher (except during winter), explaining egg embryonation viability during most of the year [42].
4.3. Miracidial infectivity, intramolluscan development and cercarial chronobiology
The monomiracidial infectivity of 29.6% at 20 °C/20 °C of 12 h day/12 h night photoperiod in the altiplanic pig isolate agrees with the range of 14.0–56.8% experimentally found in different G. truncatula populations in France [55,56], as well as with that of 25.0–51.8% detected in altiplanic sheep and cattle isolates [36].
The prepatent period of the pig isolate neither statistically differed from that in the altiplanic sheep and cattle isolates [36], nor from those found in other experimental studies on F. hepatica/G. truncatula: 56–86 days at 15 °C, 48–51 days at 20 °C, and 38 days at 25 °C [[57], [58], [59]].
The length of the patent period of the pig isolate ranged between 20 and 83 days, with a high mean of 70.1 days. This overlaps well with the ranges found in the altiplanic sheep and cattle isolates, although the means in the latter two are pronouncedly shorter [36]. At 20 °C, different mean lengths were experimentally found in monomiracidial infections of F. hepatica/G. truncatula in the European lowlands: 18.6–28.0 days in cattle isolate [60]; 47.3 and 60.4 days in sheep and cattle isolates, respectively [61].
The high cercarial production of the pig isolate (range 101–569, mean 428.8 cercariae/snail) is worth mentioning. This amount is higher than those found in the altiplanic sheep and cattle isolates [36] and those obtained in F. hepatica/G. truncatula in given populations from lowlands: 120.0 cercariae/snail [62]; 300.0 cercariae/snail [58]; 91.7 cercariae/snail [63]. However, higher values have also been found, such as the ranges of 476–544 cercariae/snail [57], and 952–2275 cercariae/snail obtained when infected with many miracidia and providing special food [64].
The lower values in the infectivity rate, the length of the patent period, and the number of cercariae/snail found when using a monomiracidial dose and 22 °C/5 °C day/night temperatures and also when using a tri-miracidial dose at 20 °C/20 °C day/night temperature, coincide with the lower values found under similar doses and conditions in the altiplanic sheep and cattle isolates [36], Previous studies with the same fluke/snail species pair indicate that a higher number of miracidia used for the infections may furnish lower cercarial productions than monomiracidial ones [65].
The chronobiological pattern of cercarial emergence of the pig isolate, using a monomiracidial dose and temperature of 20 °C/20 °C day/night, is markedly similar to the patterns found in altiplanic sheep and cattle isolates under the same conditions, concerning both the extreme length and the gradual progressive decrease after an initial acrophase [66]. The observed four pauses in which cercarial shedding completely stopped by all shedding snails should be highlighted. Indeed, four similar daily pauses were also described in the altiplanic sheep isolate at days 90, 102, 111 and 126 dpi. These wave-distinguishing pauses have been linked to the redial generation replication processes [66].
4.4. Lymnaeid survival and metacercarial infectivity
When using a monomiracidial dose and temperature of 20 °C/20 °C day/night, the snail survival after shedding end and the longevity of shedding snails and infected non-shedding snails in infections by the altiplanic pig isolate are similar than in those obtained when infecting with the altiplanic sheep and cattle isolates under the same conditions [36], and differ from those found in the altiplanic donkey isolate [37]. The results obtained with the altiplanic pig isolate confirm that this longevity is longer in infected lymnaeids from high altitude areas than the same survival period known in F. hepatica/G. truncatula in the lowlands and that this phenomenon is independent on the host isolate of F. hepatica [67].
A special mention depicts the lower values of the three characteristics assessing the infection impact on snails when using a tri-smiracidial dose at 20 °C/20 °C day/night temperature, because they show the pathogenicity of such a miracidial dose in the pig isolate, similarly as observed in the altiplanic sheep and cattle isolates [36].
The experimental infections of Wistar rats with metacercariae of the altiplanic pig isolate allowed to verify its definitive host infectivity (Table 2). Both the prevalences and the intensities fitted well in the results obtained with the altiplanic sheep and cattle isolates [36], and also in the present knowledge when dealing with short-aged metacercariae of ruminant origin [39,68].
4.5. Epidemiological role of the pig
Liver fluke infection prevalences in pigs varied pronouncedly between 0 and 70%, with a mean of 28.7%, in the different zones of the fascioliasis hyperendemic area of the Bolivian Altiplano (Table 3), without any evident quantitative parallelism with the prevalences found in sheep and cattle from the same zones [36].
Intensities, measured by individual epg amount per pig, ranged between 1 and 67 epg (mean 17.7 epg). The intensities found in the Bolivian Altiplano endemic area were 3–241 epg (mean 54.7 epg) in sheep and 1–96 epg (mean 6.5 epg) in cattle [36], No comparison of intensities between these animal species is feasible, because of the different quantities of faeces per day produced by each species, and the consequent different dilution of epg in them.
Eggs of F. hepatica shed by naturally infected pigs of the Bolivian Altiplano prove to be smaller than those found in naturally infected altiplanic sheep and cattle (Table 4). This agrees with present knowledge about the influence of the definitive host species on the size of fasciolid eggs [50,69].
The total number of liver fluke eggs expelled with faeces by a pig per day in the Northern Bolivian Altiplano, according to the amount of stools defecated by a pig per day [70,71], may be estimated to be between 500 and 195,000 eggs/pig/day. This range appears to be relatively high, although lower than similar values of estimates made for sheep and cattle (Table 4) [36] and also donkeys (9000–808,000 eggs/pig/day) [37] in the same Northern Altiplano hyperendemic area.
When considering these results on natural infections in domestic pigs in the Altiplano, but also other field studies having described F. hepatica infection in wild boars [[72], [73], [74], [75], [76]], the insufficient number of field surveys on fasciolid infection of domestic pigs is surprising, mainly in rural areas of human fascioliasis endemicity where pigs use to run freely (Fig. 5). A prevalence of 20.6% was found in a total of 330 pigs analyzed in the human endemic area of Talca, Chile [77,78]. A few years later, a prevalence of 27.1% was reported in a total of 59 pigs analyzed in the Bolivian Altiplano [79]. More recently, a prevalence of 12.1% was noted in a total of 257 pigs analyzed in the human endemic of the Mantaro valley, Junin, Peru [80]. The liver fluke infection has also been found in pigs in the human endemic area of Cajamarca province, although no prevalence data were reported [81]. Sporadic studies on natural infection of domestic pigs by F. hepatica were made long time ago in Europe [82].
The lack of focus on domestic pigs within field investigations on fascioliasis in the last decades is undoubtedly due to previous studies demonstrating the substantial natural resistance of the pig to infection by Fasciola species in several geographical areas [[83], [84], [85], [86]], or even considering the pig as a non-viable host for F. hepatica in other areas [87]. Additionally, there were several studies on the experimental infection of pigs confirming these assumptions [[88], [89], [90], [91]]. These studies demonstrated that in pigs infected shortly after birth the percentage of metacercariae recovered as adult parasites was comparable with that of very susceptible host species, whereas a considerable resistance was observed in pigs infected already from 8 weeks of age. The increasing resistance with age coincided with the capacity to give rise to a marked fibroblastic reaction in response to the infection, the resulting fibrous tissue acting as a mechanical barrier to the fluke migration in the hepatic parenchyma. It was also argued that the inhibition of migration could also be increased by a stronger immune reaction with the increasing age, although the knowledge on the complex immune mechanisms induced by the liver fluke infection was still in its infancy in that period. That is why the pig was included in the group of early resistance hosts characterized by possessing tissues that are not suitable for the fluke, with a high degree of natural resistance, and with a self-limiting infection without harming the host [92].
In light of this high resistance of the pig to the liver fluke infection, the question arises on how to understand the aforementioned prevalences the pig shows in human fascioliasis endemic areas of South America.
On the one hand, the infection of pigs may be understood by considering that, in such hyperendemic areas, (i) transmission foci are numerous [38], (ii) lymnaeid vector populations are whether yearly permanent or present throughout most seasons [42], and (iii) main reservoirs as sheep and cattle are sufficiently numerous, freely dispersed and with high infection rates [36], as to assure an extensive and permanent environmental contamination by long-time infective liver fluke metacercariae [93]. This implies high infection and re-infection risks [21,24,94,95], which allows for the early infection of freely running piglets (Fig. 5 A).
On the other hand, as a consequence of the opportunity of the liver fluke life cycle for numerous and repeated passages through the pig in such endemic areas along time, a progressive adaptation of the fasciolid parasite to the pig host may evolutionarily have occurred. The great capacity of the fasciolid flukes for an evolutionarily fast adaptation to markedly differentiated environments [96] and phylogenetically separated host species [1] has been highlighted. Indeed, the substantial polymorphism levels found in the very long genome of F. hepatica have been argued to underlie the evolutionary potential for rapid adaptation to changes in host availability, climate change or to even drug or vaccine interventions [97].
Finally, the adaptation of the liver fluke to the pig parallelly focuses on its adaptation to another physiologically close omnivorous mammal as humans. In these endemic areas, human infection appears always manifested by faecal shedding of fasciolid eggs reaching high epg outputs [12,14,98], whereas the liver fluke is known to produce no eggs when infecting humans in several zones where human infection is rare or sporadic [4], as in many developed countries [99].
Indeed, domestic pigs are closely related to humans in terms of anatomy, genetics and physiology. Anatomically, pigs have all of the same thoracic and abdominal organs as humans, although there are small differences in a few organs, as for instance the liver. In the pig, this organ has five lobes including a right lateral, right central, left central, left lateral, and caudate lobes, whereas there are only the four right, left, caudate and quadrate lobes in humans. The genetic and physiological traits of pigs are also similar to those of humans. The similarity in size and physiology between pigs and humans allows pigs to be used for many experimental approaches not feasible in rodents [100]. This makes the pig one of the most useful and versatile animal models for translational research, surgery and procedural training involved in a wide variety of health aspects [101]. There are unique advantages to the use of swine in this setting given that they share with humans similar anatomic and physiologic characteristics involving the cardiovascular, urinary, integumentary and digestive systems [102]. These features have also made this mammal the primary species of interest as organ donors for xenographic procedures [103] and pig-to-primate organ transplantation models are already being used successfully [104].
The domestic pig also represents an excellent animal model for the study of infectious diseases. This animal has a full set of innate and adaptive immune effectors, and responds to the infection of human pathogens similarly as humans do [105]. Most proteins of the immune system share structural and functional similarities with their human counterparts. The porcine immune system closely resembles humans in more than 80% of the analyzed parameters [106]. Most of the immune cell populations identified in humans have been verified to be also present in pigs. The cluster of differentiation, the cell-surface proteins that allow the identification, and the characterization of the various immune cell populations have been described in pigs [107]. Globally functional orthologs for all the cytokines involved in the Th1/Th2/ Th17/Treg paradigm and corresponding cells have been reported in pigs [[108], [109], [110]]. These immunity characteristics emphasize that pigs represent a valuable model to assess knowledge on human infectious diseases.
Moreover, studies on the gut barrier functions have revealed conserved defense mechanisms between pigs and humans, particularly in functional permeability. Interestingly, the pig has additionally proved to be also useful for studying the changes in human microbiota following nutritional interventions [111].
5. Concluding remarks
The experimental follow-up of the development stages of egg, miracidium, lymnaeid snail vector infection, intramolluscan larval development, cercarial production, chronobiology of the cercarial shedding, vector survival to infection, metacercarial infectivity of mammal host, and adult stage development of the altiplanic pig isolate through altiplanic G. truncatula vectors, demonstrates that the pig is a definitive host which assures the viability of the whole life cycle of F. hepatica in the Northern Bolivian Altiplano human hyperendemic area. The field study results on prevalence, intensity, egg measurements and egg shedding rates in nature, further proved that the pig participates in the transmission of F. hepatica in this endemic area (Fig. 5 C-E).
When considering both experimental and field results together, it can be concluded that the pig develops a reservoir role in this endemic area, with a priority weight lower than sheep and cattle [36] but markedly higher than the fourth reservoir animal species to be considered, the donkey [37]. Therefore, this omnivorous mammal species should be accordingly included for appropriate control measures within a One Health action against human fascioliasis. Unfortunately, pigs have never been considered for fasciolicide treatments nor for other infection prevention measures in the Northern Bolivian Altiplano (Fig. 5 B).
This study illustrates the long-term research complexity and difficulties involved in the needed experimental tasks and field activities to appropriately assess the participation of a mammal host species in the transmission and epidemiology in an endemic area of a disease of very high heterogeneity and low definitive host species specificity as fascioliasis. This study demonstrates that when applying a One Health control action against a zoonotic disease caused by a parasite with the wide adaptation capacity of fasciolid flukes, all kind of animals inhabiting the endemic area and presenting a way of life and feeding habits in the open field allowing them for infection by metacercariae should be considered for assessment of potential reservoirs needing control measures. The case of the pig is particularly worth mentioning, because concerning a mammal species which previous analyses showed it to be a non-suitable host for F. hepatica and usually considered out of the spectrum of the typical herbivorous hosts because of its omnivorous nature.
Moreover, within a One Health action there is the need to establish the order of priorities of the control measures, thus enabling the most efficient application and highest impact of the funds available, which for rural areas in low-income countries are usually scarce. It is therefore crucial to clarify the participation rate of a definitive host by comparing with the participation rates of other definitive host species in the same endemic area.
The extreme complexity of a One Health action against fascioliasis is evident when considering that the present study only concerns the One Health intervention axis of the mammal reservoirs, and that in the Northern Bolivian Altiplano the disease is only caused by F. hepatica and transmitted by only genetically uniform G. truncatula. This is opposite to the usual scenarios of human endemic areas including the presence of more than one lymnaeid vector species in Latin America [112], such as in Peru [113], Ecuador and Colombia [114], Venezuela [115], Argentina [32,116], and Chile [117], Additionally, this complexity increases in many regions of Africa and Asia due to the overlap of F. hepatica, F. gigantica and corresponding multiple specific lymnaeid vector species, without forgetting the existence of hybrid intermediate forms in many endemic areas [1].
Personal observations and available information indicate that pigs should play a similar reservoir role in other human endemic areas but also animal endemic areas with human infection risk on other Andean countries, as at least Peru (Fig. 5 F) and Chile and probably also Argentina, Ecuador, Colombia and Venezuela. In Central America, Mexico is a country where the pig may also play an important fascioliasis reservoir role. All in all, not only Latin America but also many regions of Africa and Asia include rural areas where domestic pigs are traditionally running freely.
The at present available knowledge on two other NTDs caused by plathelminth parasites, a cestodiasis and another food-borne trematodiasis, may be of added value for the endeavor of assessing the potential reservoir role of the pig in fascioliasis endemic areas. The pig is the intermediate host in taeniasis and cysticercosis by Taenia solium [118] and the only animal reservoir species in fasciolopsiasis by Fasciolopsis buski [119]. The geographical distribution of taeniasis/cysticercosis in the Americas, Africa and Asia [120] and that of fasciolopsiasis throughout South Central Asia and South East Asia [121] may facilitate the selection of areas for the appropriate epidemiological surveys and make it possible for the design of combined control initiatives.
Ethics statement
All experimental research was performed with the approval of the Evaluation of Projects concerning Animal Research at University of Valencia (Organo Habilitado para la Evaluación de Proyectos de Experimentación Animal de la Universidad de Valencia) (A1263 915,389,140), strictly following the institution's guidelines based on Directive 2010/63/EU. Permission for animal research was additionally obtained from the Servicio de Sanidad y Bienestar Animal, Dirección General de Producción Agraria y Ganadería, Consellería de Presidencia y Agricultura, Pesca, Alimentación y Agua, Generalitat Valenciana, Valencia, Spain (No. 2015/VSC/PEA/00001 tipo 2). Animal ethics guidelines regarding animal care were strictly adhered.
The study was approved by the Comisión de Etica de la Investigación of the Comité Nacional de Bioética, La Paz (Certificate dated 10 September 2007), Comité de Etica y Bioética de la Facultad de Medicina de la Universidad Mayor de San Andres, UMSA, La Paz - COMETICA (Resolución COMETICA No. 03/2019, dated 23 July 2019), and Comité de Revisión Etica (PAHOERC) of the Pan American Health Organization, PAHO, Washington DC (Dictamen Ref. No. 2018-02-0007, dated 10 September 2019).
All investigations were made after permission was obtained from local Aymara community chiefs (jilakatas and mallkus), and with the consent of animal owners.
Consent for publication
All authors read and approved the final version for publication consideration.
Availability of supporting data
The datasets generated for this study are available on request to the corresponding author.
Funding
Studies funded by Project No. 2017/ACDE/001583 de Innovación para el Desarrollo of the Agencia Española de Cooperación Internacional para el Desarrollo (AECID), Ministry of Foreign Affairs and Cooperation, Madrid, Spain; by Project No. RLA5049 of the International Atomic Energy Agency (Animal Production and Health Section, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Department of Nuclear Sciences and Applications, IAEA Headquarters Vienna, Austria); by Health Research Project No. PI16/00520, Subprograma Estatal de Generación de Conocimiento de la Acción Estratégica en Salud (AES) y Fondos FEDER, Plan Estatal de Investigación Científica y Técnica y de Innovación, ISCIII-MINECO, Madrid, Spain; by the Red de Investigación de Centros de Enfermedades Tropicales – RICET (Project No. RD16/0027/0023 of the PN de I + D + I, ISCIII-Subdirección General de Redes y Centros de Investigación Cooperativa RETICS), Ministry of Health and Consumption, Madrid; by Project No. 2016/099 of the PROMETEO Program, Programa de Ayudas para Grupos de Investigación de Excelencia, Generalitat Valenciana, Valencia, Spain; and by Project No. 2017/01 of the V Convocatoria de Proyectos de Cooperación al Desarrollo de la Universidad de Valencia de 2016, Valencia, Spain.
Author contributions
Conceptualization: SMC, RA, MAV, MDB; Data curation: SMC, IRF, PB, MAV, MDB; Formal analysis: SMC, IRF, PB, MAV, MDB; Funding acquisition: SMC, MAV, MDB; Investigation: SMC, IRF, RA, PB, CMB, PA, MAV, MDB; Methodology: IRF, PB, CMB, PA; Project administration: SMC, RA; Resources: SMC, RA, MAV, MDB; Software: MAV; Supervision: SMC; Validation: SMC, MAV, MDB; Visualization: SMC, RA; Writing - original draft: SMC; Writing - review & editing: SMC, RA, PB, CMB, MAV, MDB.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
Studies of this article have been performed within the framework of the Worldwide Initiative of WHO against Human Fascioliasis (WHO Headquarters, Geneva, Switzerland). One Health initiative designed within the official meeting “Reunión de Análisis con Expertos sobre la Situación Actual y Próximos Pasos para el Control de la Fascioliasis en Bolivia”, organized by PAHO/WHO in Hotel Camino Real, Calacoto, La Paz, on 10-12 November 2014, with the participation of (i) Ministerio de Salud de Bolivia, (ii) Ministerio de Desarrollo Rural y Tierras de Bolivia, (iii) Servicio Departamental de Salud de La Paz (SEDES La Paz), (iv) representatives of the Aymara communities from the Northern Altiplano endemic area, (v) delegates from Perú, (vi) experts and advisers of the Programa Regional de Enfermedades Infecciosas Desatendidas of PAHO/WHO, and from the “WHO Collaborating Centre on Fascioliasis and its Snail Vectors of Valencia, and (vii) other foreign experts.
The authors acknowledge the facilities provided and the collaboration received from the following Bolivian organisms, institutions and centres, as well as their respective representatives or directors: Servicio Departamental de Salud La Paz (SEDES La Paz); Unidad de Epidemiología of the Bolivian Ministry of Health, La Paz; Office of the Pan American Health Organization in La Paz; Dirección Nacional de Producción Pecuaria and the Instituto Nacional de Biología Animal of Chasquipampa-Calacoto both of the Ministerio de Asuntos Campesinos y Agropecuarios (M.A.C.A.) in La Paz; and Granja de Mejoramiento Ganadero de Kallutaca related to the Programa de Fomento Lechero of the Corporación Regional de Desarrollo de La Paz (CORDEPAZ, El Alto).
References
- 1.Mas-Coma S., Valero M.A., Bargues M.D. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009;69:41–146. doi: 10.1016/S0065-308X(09)69002-3. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Department of Control of Neglected Tropical Diseases, World Health Organization, WHO Headquarters; Geneva: 2013. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases; pp. 1–128. [Google Scholar]
- 3.De N.V., Le T.H., Agramunt V.H., Mas-Coma S. Early postnatal and preschool age infection by Fasciola spp.: Report of five cases from Vietnam and worldwide review. Am. J. Trop. Med. Hyg. 2020;103:1578–1589. doi: 10.4269/ajtmh.20-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mas-Coma S., Bargues M.D., Valero M.A. Diagnosis of human fascioliasis by stool and blood techniques: update for the present global scenario. Parasitology. 2014;141:1918–1946. doi: 10.1017/S0031182014000869. [DOI] [PubMed] [Google Scholar]
- 5.Chen M.G., Mott K.E. Progress in assessment of morbidity due to Fasciola hepatica infection: a review of recent literature. Trop. Dis. Bull. 1990;87:R1–R38. [Google Scholar]
- 6.Mas-Coma S., Agramunt V.H., Valero M.A. Neurological and ocular fascioliasis in humans. Adv. Parasitol. 2014;84:27–149. doi: 10.1016/B978-0-12-800099-1.00002-8. [DOI] [PubMed] [Google Scholar]
- 7.Valero M.A., Bargues M.D., Khoubbane M., Artigas P., Quesada C., Berinde L., Ubeira F.M., Mezo M., Hernandez J.L., Agramunt V.H., Mas-Coma S. Higher physiopathogenicity by Fasciola gigantica than by the genetically close F. hepatica: experimental long-term follow-up of biochemical markers. Trans. R. Soc. Trop. Med. Hyg. 2016;110:1. doi: 10.1093/trstmh/trv110. Special Issue, 55–66. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Miguel J., Valero M.A., Reguera-Gomez M., Mas-Bargues C., Bargues M.D., Simon-Martin F., Mas-Coma S. Numerous Fasciola plasminogen-binding proteins may underlie blood-brain barrier leakage and explain neurological disorder complexity and heterogeneity in the acute and chronic phases of human fascioliasis. Parasitology. 2019;146:284–298. doi: 10.1017/S0031182018001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rondelaud D., Dreyfuss G., Vignoles P. Clinical and biological abnormalities in patients after fasciolosis treatment. Méd. Mal. Infect. 2006;36:466–468. doi: 10.1016/j.medmal.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Dalton J.P., Robinson M.W., Mulcahy G., O'Neill S.M., Donnelly S. Immunomodulatory molecules of Fasciola hepatica: candidates for both vaccine and immunotherapeutic development. Vet. Parasitol. 2013;195:272–285. doi: 10.1016/j.vetpar.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Girones N., Valero M.A., Garcia-Bodelon M.A., Chico-Calero M.I., Punzon C., Fresno M., Mas-Coma S. Immune supression in advanced chronic fascioliasis: an experimental study in a rat model. J. Infect. Dis. 2007;195:1504–1512. doi: 10.1086/514822. [DOI] [PubMed] [Google Scholar]
- 12.Esteban J.G., Flores A., Angles R., Strauss W., Aguirre C., Mas-Coma S. A population-based coprological study of human fascioliasis in a hyperendemic area of the Bolivian Altiplano. Tropical Med. Int. Health. 1997;2:695–699. doi: 10.1046/j.1365-3156.1997.d01-356.x. [DOI] [PubMed] [Google Scholar]
- 13.Brady M.T., O'Neill S.M., Dalton J.P., Mills K.H. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect. Immun. 1999;67:5372–5378. doi: 10.1128/iai.67.10.5372-5378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez L.C., Esteban J.G., Bargues M.D., Valero M.A., Ortiz P., Naquira C., Mas-Coma S. Hyperendemic human fascioliasis in Andean valleys: an altitudinal transect analysis in children of Cajamarca province, Peru. Acta Trop. 2011;120:119–129. doi: 10.1016/j.actatropica.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Ubeira F.M., Muiño L., Valero M.A., Periago M.V., Perez-Crespo I., Mezo M., Gonzalez-Warleta M., Romaris F., Paniagua E., Cortizo S., Llova J., Mas-Coma S. MM3-ELISA detection of Fasciola hepatica coproantigens in preserved human stool samples. Am. J. Trop. Med. Hyg. 2009;81:156–162. [PubMed] [Google Scholar]
- 16.Valero M.A., Periago M.V., Perez-Crespo I., Rodriguez E., Perteguer M.J., Garate T., Gonzalez-Barbera E.M., Mas-Coma S. Assessing the validity of an ELISA test for the serological diagnosis of human fascioliasis in different epidemiological situations. Tropical Med. Int. Health. 2012;17:630–636. doi: 10.1111/j.1365-3156.2012.02964.x. [DOI] [PubMed] [Google Scholar]
- 17.Ollerenshaw C.B. The ecology of the liver fluke (Fasciola hepatica) Vet. Rec. 1959;71:957–965. [Google Scholar]
- 18.Fuentes M.V., Valero M.A., Bargues M.D., Esteban J.G., Angles R., Mas-Coma S. Analysis of climatic data and forecast indices for human fascioliasis at very high altitude. Ann. Trop. Med. Parasitol. 1999;93:835–850. [PubMed] [Google Scholar]
- 19.Fuentes M.V., Malone J.B., Mas-Coma S. Validation of a mapping and predicting model for human fasciolosis transmission in Andean very high altitude endemic areas using remote sensing data. Acta Trop. 2001;79:87–95. doi: 10.1016/s0001-706x(01)00106-1. [DOI] [PubMed] [Google Scholar]
- 20.Afshan K., Fortes-Lima C.A., Artigas P., Valero M.A., Qayyum M., Mas-Coma S. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat. Health. 2014;8:317–334. doi: 10.4081/gh.2014.22. [DOI] [PubMed] [Google Scholar]
- 21.Mas-Coma S., Bargues M.D., Valero M.A. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology. 2018;145:1665–1699. doi: 10.1017/S0031182018000914. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi P., Schmitt E.K., Chen C.W., Samantray S., Venishetty V.K., Hughes D. Triclabendazole in the treatment of human fascioliasis: a review. Trans. R. Soc. Trop. Med. Hyg. 2019;113:797–804. doi: 10.1093/trstmh/trz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villegas F., Angles R., Barrientos R., Barrios G., Valero M.A., Hamed K., Grueningr H., Ault S.K., Montresor A., Engels D., Mas-Coma S., Gabrielli A.F. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valero M.A., Periago M.V., Perez-Crespo I., Angles R., Villegas F., Aguirre C., Strauss W., Espinoza J.R., Herrera P., Terashima A., Tamayo H., Engels D., Gabrielli A.F., Mas-Coma S. Field evaluation of a coproantigen detection test for fascioliasis diagnosis and surveillance in human hyperendemic areas of Andean countries. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillyer G.V., Soler de Galanes M., Rodriguez-Perez J., Bjorland J., Silva de Lagrava M., Ramirez Guzman S., Bryan R.T. Use of the falcon assay screening test - enzyme-linked Immunosorbent assay (FAST-ELISA) and the enzyme-linked Immunoelectrotransfer blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am. J. Trop. Med. Hyg. 1992;46:603–609. doi: 10.4269/ajtmh.1992.46.603. [DOI] [PubMed] [Google Scholar]
- 26.Bjorland J., Bryan R.T., Strauss W., Hillyer G.V., McAuley J.B. An outbreak of acute fascioliasis among Aymara Indians in the Bolivian Altiplano. Clin. Infect. Dis. 1995;21:1228–1233. doi: 10.1093/clinids/21.5.1228. [DOI] [PubMed] [Google Scholar]
- 27.Mas-Coma S., Angles R., Esteban J.G., Bargues M.D., Buchon P., Franken M., Strauss W. The Northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Tropical Med. Int. Health. 1999;4:454–467. doi: 10.1046/j.1365-3156.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 28.Fairweather I., Brennan G.P., Hanna R.E.B., Robinson M.W., Skuce P.J. Drug resistance in liver flukes. Int. J. Parasitol. Drugs Drug Resist. 2020;12:39–59. doi: 10.1016/j.ijpddr.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bargues M.D., Mas-Coma S. Reviewing lymnaeid vectors of fascioliasis by ribosomal DNA sequence analyses. J. Helminthol. 2005;79:257–267. doi: 10.1079/joh2005297. [DOI] [PubMed] [Google Scholar]
- 30.Ashrafi K., Valero M.A., Massoud J., Sobhani A.R., Solaymani-Mohammadi S., Conde P., Khoubbane M., Bargues M.D., Mas-Coma S. Plant-borne human contamination by fascioliasis. Am. J. Trop. Med. Hyg. 2006;75:295–302. [PubMed] [Google Scholar]
- 31.Valero M.A., Perez-Crespo I., Khoubbane M., Artigas P., Panova M., Ortiz P., Maco V., Espinoza J.R., Mas-Coma S. Fasciola hepatica phenotypic characterisation in Andean human endemic areas: valley versus altiplanic patterns analysed in liver flukes from sheep from Cajamarca and Mantaro, Peru. Infect. Genet. Evol. 2012;12:403–410. doi: 10.1016/j.meegid.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Bargues M.D., Malandrini J.B., Artigas P., Soria C.C., Velasquez J.N., Carnevale S., Mateo L., Khoubbane M., Mas-Coma S. Human fascioliasis endemic areas in Argentina: multigene characterisation of the lymnaeid vectors and climatic-environmental assessment of the transmission pattern. Parasit. Vectors. 2016;9:306. doi: 10.1186/s13071-016-1589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zumaquero-Rios J.L., Sarracent-Perez J., Rojas-Garcia R., Rojas-Rivero L., Martinez-Tovilla Y., Valero M.A., Mas-Coma S. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla state, Mexico: epidemiology and treatment with nitazoxanide. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashrafi K., Valero M.A., Peixoto R.V., Artigas P., Panova M., Mas-Coma S. Distribution of Fasciola hepatica and F. gigantica in the endemic area of Guilan, Iran: relationships between zonal overlap and phenotypic traits. Infect. Genet. Evol. 2015;31:95–109. doi: 10.1016/j.meegid.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization . A Road Map for Neglected Tropical Diseases 2021–2030. World Health Organization, WHO Headquarters; Geneva: 2020. Ending the neglect to attain the sustainable development goals; pp. 1–47.https://www.who.int/neglected_diseases/Ending-the-neglect-to-attain-the-SDGs--NTD-Roadmap.pdf [Google Scholar]
- 36.Mas-Coma S., Buchon P., Funatsu I.R., Angles R., Artigas P., Valero M.A., Bargues M.D. Sheep and cattle reservoirs in the highest human fascioliasis hyperendemic area: experimental transmission capacity, field epidemiology and control within a one health initiative in Bolivia. Front. Vet. Sci. 2020;7:583204. doi: 10.3389/fvets.2020.583204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mas-Coma S., Buchon P., Funatsu I.R., Angles R., Mas-Bargues C., Artigas P., Valero M.A., Bargues M.D. Donkey fascioliasis within a one health control action: transmission capacity, field epidemiology, and reservoir role in a human hyperendemic area. Front. Vet. Sci. 2020;7:591384. doi: 10.3389/fvets.2020.591384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bargues M.D., Artigas P., Angles R., Osca D., Duran P., Buchon P., Gonzales-Pomar R.K., Pinto-Mendieta J., Mas-Coma S. Genetic uniformity, geographical spread and anthropogenic habitat modifications of lymnaeid vectors found in a one health initiative in the highest human fascioliasis hyperendemic of the Bolivian Altiplano. Parasit. Vectors. 2020;13:171. doi: 10.1186/s13071-020-04045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boray J.C., Enigk K. Laboratory studies on the survival and infectivity of Fasciola hepatica and F. gigantica metacercariae, Zeitsch. Tropenmed. Parasitol. 1964;15:324–331. [PubMed] [Google Scholar]
- 40.Bargues M.D., Artigas P., Khoubbane M., Flores R., Glöer P., Rojas-Garcia R., Ashrafi K., Falkner G., Mas-Coma S. Lymnaea schirazensis, an overlooked snail distorting fascioliasis data: genotype, phenotype, ecology, worldwide spread, susceptibility, applicability. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bargues M.D., Gayo V., Sanchis J., Artigas P., Khoubbane M., Birriel S., Mas-Coma S. DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bargues M.D., Angles R., Coello J., Artigas P., Funatsu I.R., Cuervo P.F., Buchon P., Mas-Coma S. One health initiative in the Bolivian Altiplano human fascioliasis hyperendemic area: lymnaeid biology, population dynamics, microecology and climatic factor influences. Braz. J. Vet. Parasitol. 2021 doi: 10.1590/S1984-29612021014. (in press) [DOI] [PubMed] [Google Scholar]
- 43.Bargues M.D., Artigas P., Sierra R. Mera y, Pointier J.P., Mas-Coma S. Characterisation of Lymnaea cubensis, L. viatrix and L. neotropica n. sp., the main vectors of Fasciola hepatica in Latin America, by analysis of their ribosomal and mitochondrial DNA. Ann. Trop. Med. Parasitol. 2007;101:621–641. doi: 10.1179/136485907X229077. [DOI] [PubMed] [Google Scholar]
- 44.Valero M.A., Panova M., Comes A.M., Fons R., Mas-Coma S. Patterns in size and shedding of Fasciola hepatica eggs by naturally and experimentally infected murid rodents. J. Parasitol. 2002;88:308–313. doi: 10.1645/0022-3395(2002)088[0308:PISASO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 45.Valero M.A., Navarro M., Garcia-Bodelon M.A., Marcilla A., Morales M., Garcia J.E., Hernandez J.L., Mas-Coma S. High risk of bacterobilia in advanced experimental chronic fasciolosis. Acta Trop. 2006;100:17–23. doi: 10.1016/j.actatropica.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Valero M.A., Girones N., Garcia-Bodelon M.A., Periago M.V., Chico-Calero I., Khoubbane M., Fresno M., Mas-Coma S. Anaemia in advanced chronic fasciolosis. Acta Trop. 2008;108:35–43. doi: 10.1016/j.actatropica.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Valero M.A., Santana M., Morales M., Hernandez J.L., Mas-Coma S. Risk of gallstone disease in advanced chronic phase of fascioliasis: an experimental study in a rat model. J. Infect. Dis. 2003;188:787–793. doi: 10.1086/377281. [DOI] [PubMed] [Google Scholar]
- 48.Dennis W.R., Stone W.M., Swanson L.E. A new laboratory and field diagnostic test for fluke ova in feces. J. Am. Vet. Med. Assoc. 1954;124:47–50. [PubMed] [Google Scholar]
- 49.Cheriuyot H.K., Jordan J.E. Potential for the spread of Fasciola hepatica in cattle in Oklahoma. J. Am. Vet. Med. Assoc. 1990;196:1090–1094. [PubMed] [Google Scholar]
- 50.Valero M.A., Panova M., Mas-Coma S. Phenotypic analysis of adults and eggs of Fasciola hepatica by computer image analysis system. J. Helminthol. 2005;79:217–225. doi: 10.1079/joh2005301. [DOI] [PubMed] [Google Scholar]
- 51.Diez-Baños M.A., Rojo-Vázquez F.A. Influencia de la temperatura en el desarrollo de los huevos de Fasciola hepatica. An. Fac. Vet. León. 1976;22:65–75. [Google Scholar]
- 52.Valenzuela G. Estudio epidemiológico acerca del desarrollo de huevos de Fasciola hepatica en el medio ambiente en Valdivia, Chile. Bol. Chil. Parasitol. 1979;34:31–35. [PubMed] [Google Scholar]
- 53.Ross I.C., McKay A.C. The bionomics of Fasciola hepatica in New South Wales and the intermediate host Limnaea brazieri. Bull. Counc. Sc. Ind. Res. Australia. 1929;5:1–63. [Google Scholar]
- 54.Rowcliffe S.A., Ollerenshaw C.B. Observations on the bionomics of the egg of Fasciola hepatica. Ann. Trop. Med. Parasitol. 1960;54:172–181. doi: 10.1080/00034983.1960.11685973. [DOI] [PubMed] [Google Scholar]
- 55.Rondelaud D. Variabilité interpopulationelle de l'infestation fasciolenne chez le molllesque Lymnaea truncatula Müller. Influence du contact préalable de la population avec le parasite. Bull. Soc. Zool. Fr. 1993;118:185–193. [Google Scholar]
- 56.Vignoles P., Dreyfuss G., Rondelaud D. Larval development of Fasciola hepatica in experimental infections: variations with populations of Lymnaea truncatula. J. Helminthol. 2002;76:179–183. doi: 10.1079/JOH2002112. [DOI] [PubMed] [Google Scholar]
- 57.Roberts W.E. Studies on the life-cycle of Fasciola hepatica (Linnaeus) and of its snail host Limnaea (Galba) truncatula (Müller) in the field and under controlled conditions in the laboratory. Ann. Trop. Med. Parasitol. 1950;44:187–206. doi: 10.1080/00034983.1950.11685441. [DOI] [PubMed] [Google Scholar]
- 58.Wilson R.A., Smith G., Thomas M.R. Fascioliasis. In: Anderson R.M., editor. The Population Dynamics of Infectious Diseases: Theory and Applications. Chapman and Hall; London, New York: 1982. pp. 262–319. [Google Scholar]
- 59.Rondelaud D., Barthe D. Les générations rédiennes de Fasciola hepatica L. Premières observations chez des Limnées tronquées en fin de cycle parasitaire. Bull. Soc. Fr. Parasitol. 1986;4:29–38. [Google Scholar]
- 60.Dreyfuss G., Rondelaud D. Fasciola hepatica: a study of the shedding of cercariae from Lymnaea truncatula raised under constant conditions of temperature and photoperiod. Parasite. 1994;4:401–404. doi: 10.1051/parasite/1994014401. [DOI] [PubMed] [Google Scholar]
- 61.Rondelaud D., Dreyfuss G. Fasciola hepatica: the influence of the definitive host on the characteristics of the infection in the snail Lymnaea truncatula. Parasite. 1995;2:275–280. [Google Scholar]
- 62.Hodasi J.K.M. The output of cercariae of Fasciola hepatica by Lymnaea truncatula and the distribution of metacercariae on grass. Parasitology. 1972;63:431–456. doi: 10.1017/s0031182000044644. [DOI] [PubMed] [Google Scholar]
- 63.Audousset J.C., Rondelaud D., Dreyfuss G., Vareille-Morel C. Les émissions cercariennes de Fasciola hepatica L. chez le mollusque Lymnaea truncatula Müller. A propos de quelques observations chronobiologiques. Bull. Soc. Fr. Parasitol. 1989;7:217–224. [Google Scholar]
- 64.Kendall S.B. Nutritional factors affecting the rate of development of Fasciola hepatica in Limnaea truncatula. J. Helminthol. 1993;23:179–190. doi: 10.1017/s0022149x00032491. [DOI] [PubMed] [Google Scholar]
- 65.Dreyfuss G., Vignoles P., Rondelaud D. Fasciola hepatica: the infectivity of cattle-origin miracidia had increased over the past years in Central France. Parasitol. Res. 2007;101:115–160. doi: 10.1007/s00436-007-0580-1. [DOI] [PubMed] [Google Scholar]
- 66.Mas-Coma S., Funatsu I.R., Bargues M.D. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology. 2001;123:S115–S127. doi: 10.1017/s0031182001008034. [DOI] [PubMed] [Google Scholar]
- 67.Bargues M.D., Oviedo J.A., Funatsu I.R., Rodriguez A., Mas-Coma S. Survival of lymnaeid snails from the Bolivian northern Altiplano after the parasitation by different Bolivian isolates of Fasciola hepatica (Linnaeus, 1758) (Trematoda: Fasciolidae) In: Guerra A., Rolán E., Rocha F., editors. Unitas Malacologica. Instituto de Investigaciones Marinas, CSIC; Vigo: 1995. pp. 443–445. [Google Scholar]
- 68.Kimura S., Shimizu A. Studies on the survival and infectivity of Fasciola gigantica metacercariae. Sci. Rep. Fac. Agr. Kobe Univ. 1979;13:347–349. [Google Scholar]
- 69.Valero M.A., Darce N.A., Panova M., Mas-Coma S. Relationships between host species and morphometric patterns in Fasciola hepatica adults and eggs from the northern Bolivian Altiplano hyperendemic region. Vet. Parasitol. 2001;102:85–100. doi: 10.1016/s0304-4017(01)00499-x. [DOI] [PubMed] [Google Scholar]
- 70.Morros Sarda J. Editorial Científico-Médica; Madrid: 1967. Elementos de Fisiología; pp. 1–525. [Google Scholar]
- 71.Gurtler H., Ketz H.A., Kolb E., Schroder L., Seidel H. Las heces. In: Kolb E., editor. Fisiología Veterinaria. Editorial Acribia; Zaragoza: 1979. pp. 1–377. [Google Scholar]
- 72.Barutzki D., Schoierer R., Gothe R. Helminth infections in wild boars in enclosures in southern Germany: species spectrum and infection frequency. Tierarzliche-Praxis. 1990;18:529–534. [PubMed] [Google Scholar]
- 73.Shimalov V.V., Shimalov V.T. Findings of Fasciola hepatica Linnaeus, 1758 in wild animals in Belorussian Polesie. Parasitol. Res. 2000;86:527. doi: 10.1007/s004360050707. [DOI] [PubMed] [Google Scholar]
- 74.Capucchio M.T., Deborah C., Vincenzo D.M., Miriam R., Vincenzo A., Amedeo T., Alessandro M.T., Stefano A., Eleanora S.F., Bruno D., Franco G. Natural trematode infestation in feral Nebrodi black pigs: pathological investigations. Vet. Parasitol. 2009;159:37–42. doi: 10.1016/j.vetpar.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 75.Thompson H., Irvine R.M., Philbey A.W. Fasciola hepatica infection in wild boar in the UK. Vet. Rec. 2009;165:697–698. [PubMed] [Google Scholar]
- 76.Mezo M., González-Warleta M., Castro-Hermida J.A., Manga-Gonzalez M.Y., Peixoto R., Mas-Coma S., Valero M.A. The wild boar (Sus scrofa Linnaeus, 1758) as secondary reservoir of Fasciola hepatica in Galicia (NW Spain) Vet. Parasitol. 2013;198:274–283. doi: 10.1016/j.vetpar.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Alcaino H., Vega F., Klein P., Gorman T., Apt W. Fascioliasis en caballos, cerdos y conejos silvestres de la provincia de Talca, VII Región Chile. Parasitol. Al Día. 1990;14:9–13. [Google Scholar]
- 78.Apt W., Aguilera X., Vega F., Alcaino H., Zulantay I., Apt P., Gonzalez V., Retamal C., Rodriguez J., Sandoval J. Prevalencia de fascioliasis en humanos, caballos, cerdos y conejos silvestres en tres provincias de Chile. Bol. Of. Sanit. Panam. 1993;115:405–414. [PubMed] [Google Scholar]
- 79.Mas-Coma S., Rodriguez A., Bargues M.D., Valero M.A., Coello J.R., Angles R. Secondary reservoir role of domestic animals other than sheep and cattle in fascioliasis transmission in the northern Bolivian Altiplano. Res. Rev. Parasitol. 1997;57:39–46. [Google Scholar]
- 80.Gilbert J.T. E.A.P. de Medicina Veterinaria, Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos; Lima: 2015. Prevalencia y evaluación de la carga parasitaria de cerdos criados en los distritos de el Mantaro y San Lorenzo, provincia de Jauja, departamento de Junín, Tesis para optar el Título Profesional de Médico Veterinario; pp. 1–65. [Google Scholar]
- 81.Rodriguez J., Agapito J., Delgado de la Flor I., Barreto T., Hererra-Velit P., Espinoza J.R. FAO/IAEA International Symposium on Sustainable Improvement of Animal Production and Health, Joint FAO/IAEA Programme, International Atomic Energy Agency, Vienna. 8–11, June 2009. Tracking Fasciola hepatica transmission using ND1 and CO1 gene polymorphisms in endemic areas; pp. 393–394. [Google Scholar]
- 82.Dalchow W., Hörchner F., Zuschneid K. Zum Vorkommen und zur Verbreitung von Fasciola hepatica beim Schwein. Berl. Münch. Tierärztl. Wochensch. 1971;84:402–405. [PubMed] [Google Scholar]
- 83.Ashizawa A.H., Nosaka D., Osato K. Pathological studies on fascioliasis. VI. Lesions in pig livers infected with Fasciola. Bull. Fac. Agric. Miyazaki Univ. 1966;13:1–63. [Google Scholar]
- 84.Ross J.G., Dow C., Todd J.R. The pathology of Fasciola hepatica infection in pigs: comparison of the infection in pigs and other hosts. Br. Vet. J. 1967;123:317–321. doi: 10.1016/s0007-1935(17)39909-8. [DOI] [PubMed] [Google Scholar]
- 85.Oshima K., Ito T., Miura S. Tissue reactions against immature liver-flukes invaded in pigs. Jpn. J. Vet. Sci. 1971;33:121–126. doi: 10.1292/jvms1939.33.121. [DOI] [PubMed] [Google Scholar]
- 86.Enigk K., Dey-Hazra A. Mineralstoffgehalt und Enzymaktivität des Blutplasmas sowie Mineralstoffgehalt der Leber beim Schwein während der präpatenten Fasciolose. Deut. Tierärztl. Wochensch. 1973;80:550–554. [PubMed] [Google Scholar]
- 87.Polyakova-Kr'steva O., Gorchilova L. Morphological study of the liver in experimental fascioliasis in pig. Izv. Tsentr. Khelmintol. Lab. Bulg. Akad. Nauk. 1972;15:181–188. (in Russian) [Google Scholar]
- 88.Hörchner F., Dalchow W. Zur experimentellen Fasciola hepatica-Infektion beim Schwein. Berl. Munch. Tierarztl. Wochenschr. 1972;85:184–188. [PubMed] [Google Scholar]
- 89.Nansen P., Andersen S., Harmer E., Riising H.J. Experimental fascioliasis in the pig. Exp. Parasitol. 1972;31:247–254. doi: 10.1016/0014-4894(72)90115-4. [DOI] [PubMed] [Google Scholar]
- 90.Nansen P., Andersen S., Harmer E. The course of an experimental liver fluke infection in the pig. Acta Vet. Scand. 1974;15:138–140. doi: 10.1186/BF03547501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shimizu A., Fukuda K., Kawano J., Kimura S. Pathological changes in the liver of a pig experimentally infected with Japanese Fasciola sp. Sci. Rep. Fac. Agric. Kobe Univ. 1994;21:11–17. [Google Scholar]
- 92.Boray J.C. Experimental fascioliasis in Australia. Adv. Parasitol. 1969;7:95–210. doi: 10.1016/s0065-308x(08)60435-2. [DOI] [PubMed] [Google Scholar]
- 93.Valero M.A., Mas-Coma S. Comparative infectivity of Fasciola hepatica metacercariae from isolates of the main and secondary reservoir animal host species in the Bolivian Altiplano high human endemic region. Folia Parasitol. 2000;47:17–22. doi: 10.14411/fp.2000.004. [DOI] [PubMed] [Google Scholar]
- 94.Valero M.A., Perez-Crespo I., Chillon-Marinas C., Khoubbane M., Quesada C., Reguera-Gomez M., Mas-Coma S., Fresno M., Girones N. Fasciola hepatica reinfection potentiates a mixed Th1/Th2/Th17/Treg response and correlates with the clinical phenotypes of anemia. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valero M.A., Girones N., Reguera-Gomez M., Perez-Crespo I., Lopez-Garcia M.P., Quesada C., Bargues M.D., Fresno M., Mas-Coma S. Impact of fascioliasis reinfection on Fasciola hepatica egg shedding: relationship with the immune-regulatory response. Acta Trop. 2020;209:105518. doi: 10.1016/j.actatropica.2020.105518. [DOI] [PubMed] [Google Scholar]
- 96.Mas-Coma S., Bargues M.D., Valero M.A., Fuentes M.V. Adaptation capacities of Fasciola hepatica and their relationships with human fascioliasis: from below sea level up to the very high altitude. In: Combes C., Jourdane J., editors. Taxonomy, Ecology and Evolution of Metazoan Parasites. Vol. 2. Presses Universitaires de Perpignan; Perpignan: 2003. pp. 81–123. [Google Scholar]
- 97.Cwiklinski K., Dalton J.P., Dufresne P.J., La Course J., Williams D.J.L., Hodgkinson J., Peterson S. The Fasciola hepatica genome: gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biol. 2015;16:71. doi: 10.1186/s13059-015-0632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Esteban J.G., Gonzalez C., Bargues M.D., Angles R., Sanchez C., Naquira C., Mas-Coma S. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Tropical Med. Int. Health. 2002;7:339–348. doi: 10.1046/j.1365-3156.2002.00870.x. [DOI] [PubMed] [Google Scholar]
- 99.Mas-Coma S. Human fascioliasis emergence risks in developed countries: from individual patients and small epidemics to climate and global change impacts. Enf. Emerg. Microbiol. Clín. 2020;38:253–256. doi: 10.1016/j.eimc.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 100.Schook L.B., Collares T.V., Darfour-Oduro K.A., De A.K., Rund L.A., Schachtschneider K.M., Seixas F.K. Unraveling the swine genome: implications for human health. Annu. Rev. Anim. Biosci. 2015;3:219–244. doi: 10.1146/annurev-animal-022114-110815. [DOI] [PubMed] [Google Scholar]
- 101.Kuzmuk K.N., Schook L.B. Pigs as a model for biomedical sciences. In: Rothschild M.F., Ruvinsky A., editors. The Genetics of the Pig. 2nd ed. CAB International; 2011. pp. 426–444. [Google Scholar]
- 102.Swindle M.M., Makin A., Herron A.J., Clubb F.J., Jr., Frazier K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012;49:344–356. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 103.Swindle M.M., Smith A.C. Comparative anatomy and physiology of the pig. Scand. J. Lab. Anim. Sci. 1997;25(Suppl. 1):1–10. [Google Scholar]
- 104.Ibrahim Z., Bush J., Awwad M., Wagner R., Wells K., Cooper D.K.C. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13:488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 105.Meurens F., Summerfield A., Nauwynck H., Saif L., Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dawson H. A comparative assessment of the pig, mouse, and human genomes: Structural and functional analysis of genes involved in immunity and inflammation. In: McAnulty P.A., editor. The Minipig in Biomedical Research. CRC Press, Taylor & Francis Group; 2011. pp. 321–341. [Google Scholar]
- 107.Piriou-Guzylack L., Salmon H. Membrane markers of the immune cells in swine: an update. Vet. Res. 2008;39:54. doi: 10.1051/vetres:2008030. [DOI] [PubMed] [Google Scholar]
- 108.Murtaugh M.P., Craig R.J., Xiao Z., Scamurra R.W., Zhou Y. Species specialization in cytokine biology: is interleukin-4 central to the T(H)1-T(H)2 paradigm in swine? Dev. Comp. Immunol. 2009;33:344–352. doi: 10.1016/j.dci.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 109.Käser T., Gerner W., Saalmüller A. Porcine regulatory T cells: mechanisms and T-cell targets of suppression. Dev. Comp. Immunol. 2011;35:1166–1172. doi: 10.1016/j.dci.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 110.Kiros T.G., van Kessel J., Babiuk L.A., Gerdts V. Induction, regulation and physiological role of IL-17 secreting helper T-cells isolated from PBMC, thymus, and lung lymphocytes of young pigs. Vet. Immunol. Immunopathol. 2011;144:448–454. doi: 10.1016/j.vetimm.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 111.Roura E., Koopmans S.J., Lallès J.P., Le Huerou-Luron I., de Jager N., Schuurman T., Val-Laillet D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016;29:60–90. doi: 10.1017/S0954422416000020. [DOI] [PubMed] [Google Scholar]
- 112.Bargues M.D., Mangold A.J., Muñoz-Antoli C., Pointier J.P., Mas-Coma S. SSU rDNA characterization of lymnaeid snails transmitting human fascioliasis in south and Central America. J. Parasitol. 1997;83:1086–1092. [PubMed] [Google Scholar]
- 113.Bargues M.D., Artigas P., Khoubbane M., Ortiz P., Naquira C., Mas-Coma S. Molecular characterisation of Galba truncatula, Lymnaea neotropica and L. schirazensis from Cajamarca, Peru and their potential role in transmission of human and animal fascioliasis. Parasit. Vectors. 2012;5:174. doi: 10.1186/1756-3305-5-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bargues M.D., Artigas P., Khoubbane M., Mas-Coma S. DNA sequence characterisation and phylogeography of Lymnaea cousini and related species, vectors of fascioliasis in northern Andean countries, with description of L. meridensis n. sp. (Gastropoda: Lymnaeidae) Parasit. Vectors. 2011;4:132. doi: 10.1186/1756-3305-4-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bargues M.D., Gonzalez C., Artigas P., Mas-Coma S. A new baseline for fascioliasis in Venezuela: lymnaeid vectors ascertained by DNA sequencing and analysis of their relationships with human and animal infection. Parasit. Vectors. 2011;4:200. doi: 10.1186/1756-3305-4-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sierra R. Mera y, Agramunt V.H., Cuervo P., Mas-Coma S. Human fascioliasis in Argentina: retrospective overview, critical analysis and baseline for future research. Parasit. Vectors. 2011;4:104. doi: 10.1186/1756-3305-4-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Artigas P., Bargues M.D., Sierra R. Mera y, Agramunt V.H., Mas-Coma S. Characterisation of fascioliasis lymnaeid intermediate hosts from Chile by DNA sequencing, with emphasis on Lymnaea viator and Galba truncatula. Acta Trop. 2011;120:245–257. doi: 10.1016/j.actatropica.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Garcia H.H., Gonzalez A.E., Evans C.A.W., Gilman R.H. Taenia solium cysticercosis. Lancet. 2003;362:547–556. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mas-Coma S., Valero M.A., Bargues M.D. Fasciola and Fasciolopsis. In: Xiao L., Ryan U., Feng Y., editors. Biology of Foodborne Parasites, Food Microbiology Series. Vol. 20. CRC Press - Taylor & Francis Group; Boca Raton: 2015. pp. 371–404. [Google Scholar]
- 120.Coral-Almeida M., Gabriël S., Abatih E.N., Praet N., Benitez W., Dorny P. Taenia solium human cysticercosis: a systematic review of sero-epidemological data from endemic zones around the world. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mas-Coma S. Helminth-Trematode: Fasciolopsis buski. In: Motarjemi Y., Moy G.G., Todd E.C.D., editors. Encyclopedia of Food Safety, Elsevier Major Reference Works, ScienceDirect Online Platform. Vol. 2. Elsevier, Academic Press; Waltham, MA: 2014. pp. 146–157. Hazards Diseas. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.